Abstract
Objective
The aim of this narrative review is to explore the relationship between visual snow syndrome (VSS), migraine, and a group of other perceptual disorders.
Background
VSS is characterized by visual snow and additional visual and nonvisual disturbances. The clinical picture suggests a hypersensitivity to internal and external stimuli. Imaging and electrophysiological findings indicate a hyperexcitability of the primary and secondary visual areas of the brain possibly due to an impairment of inhibitory feedback mechanisms. Migraine is the most frequent comorbidity. Epidemiological and clinical studies indicate that other perceptual disorders, such as tinnitus, fibromyalgia, and dizziness, are associated with VSS. Clinical overlaps and parallels in pathophysiology might exist in relation to migraine.
Methods
We performed a PubMed and Google Scholar search with the following terms: visual snow syndrome, entoptic phenomenon, fibromyalgia, tinnitus, migraine, dizziness, persistent postural‐perceptual dizziness (PPPD), comorbidities, symptoms, pathophysiology, thalamus, thalamocortical dysrhythmia, and salience network.
Results
VSS, fibromyalgia, tinnitus, and PPPD share evidence of a central disturbance in the processing of different stimuli (visual, somatosensory/pain, acoustic, and vestibular) that might lead to hypersensitivity. Imaging and electrophysiological findings hint toward network disorders involving the sensory networks and other large‐scale networks involved in the management of attention and emotional processing. There are clinical and epidemiological overlaps between these disorders. Similarly, migraine exhibits a multisensory hypersensitivity even in the interictal state with fluctuation during the migraine cycle. All the described perceptual disorders are associated with migraine suggesting that having migraine, that is, a disorder of sensory processing, is a common link.
Conclusion
VSS, PPPD, fibromyalgia, and chronic tinnitus might lie on a spectrum of perceptual disorders with similar pathophysiological mechanisms and the common risk factor migraine. Understanding the underlying network disturbances might give insights into how to improve these currently very difficult to treat conditions.
Keywords: fibromyalgia, migraine, persistent postural‐perceptual dizziness, sensory processing, tinnitus, visual snow syndrome
Abbreviations
- BOLD
blood‐oxygen‐level‐dependent
- DTI
diffusion tensor imaging
- FDG‐PET
[18F]‐2‐fluoro‐2‐deoxy‐D‐glucose positron emission tomography
- fMRI
functional magnetic resonance imaging
- H‐MR‐spectroscopy
proton magnetic resonance spectroscopy
- PET
positron emission tomography
- PPPD
persistent postural‐perceptual dizziness
- SPECT
single photon emission computed tomography
- VBM
voxel‐based morphometry
- VSS
visual snow syndrome
INTRODUCTION
Visual snow syndrome (VSS) has recently been described as a disorder distinct from migraine and migraine aura, although migraine is its most prevalent comorbidity. 1 Both migraine and VSS are associated with other perceptual disorders such as dizziness (i.e., persistent postural‐perceptual dizziness [PPPD]), fibromyalgia, and chronic tinnitus. 2 , 3 , 4 , 5 , 6 , 7 These conditions have in common that there is clinical evidence suggesting hypersensitivity to certain external and/or internal stimuli. This review aims to explore the common underlying mechanisms and the relation between the disorders.
METHODS
We performed a narrative literature review using Google Scholar and PubMed, searching for articles in English using the following keywords: visual snow syndrome, entoptic phenomenon, fibromyalgia, tinnitus, migraine, dizziness, PPPD, comorbidities, symptoms, pathophysiology, thalamus, thalamocortical dysrhythmia, and salience network. We found articles covering a multitude of topics and extracted information relevant to this review. Abstracts were screened to evaluate whether they gave clinical, pathophysiological, or radiological information on the topic of this review. Furthermore, we examined the literature lists of relevant reviews to identify additional relevant articles.
RESULTS
Visual snow syndrome
Up to 2.2% of the population could be affected by VSS. 8 In a study by Puledda et al. with 1100 patients, it was shown that the symptoms seem to be on a spectrum so that their prevalence might actually be higher than expected in the population. 9
Typically, patients describe visual disturbances with the key symptom visual snow, a continuous static in the entire visual field, palinopsia (trailing and afterimages), enhanced entoptic phenomena, photophobia, and nyctalopia (impaired night vision). 1 , 10 , 11 Up to 40% of patients with VSS report to have had symptoms their whole life. 9 Patients who have later onset or stepwise worsening report correlations with headache. 1 , 7 In most cases, however, there is no identifiable cause. Usually, the visual disturbances are persistent. 7 , 9
The prevalence of migraine in patients with visual snow was reported in up to 72%. 9 Comorbid migraine seems to aggravate the clinical presentation of VSS. 12 Treatment of migraine, however, does not seem to improve VSS. 1
Additionally, there is an association with other “perceptual disorders,” such as tinnitus in 52% 13 to 75%, 9 fibromyalgia in up to 7.1%, dizziness in up to 13.3%, 7 and psychiatric comorbidities, especially depression and anxiety. 7
In most cases, neuro‐ophthalmologic and radiological findings are normal. 7 , 14
The clinical presentation indicates a hypersensitivity to internal and external visual stimuli. For example, entoptic phenomena are produced by structures of the eye and are a normal phenomenon. 15 In VSS, however, these entoptic phenomena are “enhanced” and experienced excessively. 1 , 16
There is evidence suggesting failure of inhibitory processes. Palinopsia, for example, is an abnormal persistence of visual memory. 17 , 18 In this respect, the visual threshold in discrimination tasks was higher in patients with VSS as a sign of lacking inhibitory control. 19 , 20 The same abnormality was reported in patients with migraine in the interictal state. 21 , 22 , 23 Additionally, visually evoked potentials demonstrate prolonged P145‐response pointing toward disturbed processing in the secondary visual areas 24 and a hyperactivation (lack of inhibition during the double pulse adaptation paradigm) in the primary visual cortex. 25
Such involvement of secondary visual areas was also demonstrated in an FDG‐PET study that found hypermetabolism in the right lingual gyrus, 26 which corresponded to a significant increase in lactate concentrations in H‐MR‐spectroscopy in this same cortical area indicating reduced metabolic reserves. 27
In functional magnetic resonance imaging (fMRI), the so‐called blood‐oxygen‐level‐dependent (BOLD) signal is measured, that is, the diamagnetic properties of the oxygenated hemoglobin in the blood vessels as an indirect measure for the metabolic activity of a brain area. 28 , 29 Such functional MRI data demonstrated a widespread hyperconnectivity of the primary (V1) and secondary visual cortices within and beyond the visual system. Furthermore, there seem to be disturbances in the salience network and the dorsal and ventral attention network, as well as in the visual precortical pathways such as visual cortico‐striatal loop and the thalamocortical projections. 30 The latter take part in visual learning and the selection of relevant visual information. 30 , 31 , 32
The functional abnormalities could be reflected by structural changes such as an increase in gray matter volume in the primary and secondary visual cortices (including the right lingual‐fusiform gyrus junction and the left primary and secondary visual cortices). 26 , 33
Migraine
Migraine affects about 15% of the population. 34 Having migraine is characterized by a predisposition to recurrent headache attacks of the migrainous phenotype. 35 Migraine can be seen as a sensory gating disorder with a cycling devolution. 36 This can be measured in the form of sensory thresholds, which reach their minimal turning point in the ictal phase. 37 However, even in the interictal state, hypersensitivity to multimodal stimuli persists. 37 , 38 The pathogenesis is probably a multifactorial process with a significant genetic component. 39 , 40 , 41
An MRI study by Karsan et al. demonstrated hyperperfusion of the hypothalamus, anterior cingulate cortex, midbrain, and limbic areas before the onset of nitroglycerine‐triggered migraine attacks. 42 In studies by Schulte et al. with consecutive fMRI scans over 30 days, hypothalamic activity as a response to trigeminal nociceptive stimulation and the pain‐related hypothalamic functional connectivity to the spinal trigeminal nuclei were increased during the preictal phase. 43 , 44 An early hypothalamic involvement in the premonitory phase and during the headache phase was also shown in PET‐studies. 45 , 46 The hypothalamus as a driving force of the migraine cycle would also explain the periodicity of the disease manifestation and the triggering through internal homeostatic and external deviations. 43 , 47 , 48
Anatomically, the hypothalamus is closely linked to the thalamus, 49 sympathetic and parasympathetic brainstem nuclei, 50 and the trigeminovascular system. 51 The thalamus is a central relay station 52 , 53 , 54 projecting to widespread areas of the cortex including sensory areas. 55 , 56 , 57 Some groups have highlighted the role of the trigeminovascular system and its connections to the parasympathetic efferents leading to peripheral nociceptor activation 58 , 59 or the cortex, since the cortical spreading depression correlates with headache onset and aura. 60 In MRI studies, there were structural gray and white matter volume alterations (VBM and DTI) in widespread cortical areas including sensory areas, the prefrontal cortex, the cerebellum, the brainstem, the insula, and the anterior cingulate cortex. 61 , 62 , 63 , 64 , 65 , 66 Functional studies of the interictal state revealed alterations in several functional networks including the default mode network, 67 the dorsal attention network, the salience network, 68 and the visual cortex. 69
Fibromyalgia
Fibromyalgia is regarded as the classical centralized pain syndrome in the sense of a sensory‐processing disorder. 70 , 71 About 5% of the population are affected. 70 , 72
Fibromyalgia is per definition a chronic and persistent disease. The main symptoms are widespread pain in several parts of the body, typically characterized as “musculoskeletal,” and additional cognitive symptoms, often described as “brain fog”. 73 , 74
Similar to migraine, patients affected by fibromyalgia have a general hypersensitivity to painful stimuli. An fMRI study by Gracely et al. showed that less than half the stimulus intensity is needed to evoke a BOLD response in brain areas previously shown to be involved in pain processing in patients with fibromyalgia compared with healthy controls, suggesting central augmentation of the peripheral stimuli, turning neutral signals into “unpleasant” ones. 75
This phenomenon might extend to the other senses as well like auditory perception 76 and smell. 77 , 78 Beyond that, migraine, tinnitus, and dizziness are common comorbidities in fibromyalgia. 79 , 80 , 81
Similar to patients with VSS, tinnitus, and PPPD, patients with fibromyalgia report triggers such as stress, trauma, or environmental changes associated with the onset of the syndrome and flares. 82 , 83 , 84 The widespread pain symptoms with the additional cognitive symptoms indicate a central pathology involving an excessive activation of the pain system and/or an impaired antinociceptive system. 85 , 86 In this respect, repetitive mechanical stimulation leads to excessive temporal summation for fibromyalgia patients at lower stimulus intensities and frequencies, as well as more pronounced painful after‐sensations compared with healthy controls suggesting a lack of pain inhibition. 87 , 88
In patients with fibromyalgia, Wagner et al. demonstrated a specific and significantly more expansive BOLD response to painful stimuli in brain areas implicated in pain processing including the bilateral insula, the secondary somatosensory cortex, and the thalamus. 89 Other workgroups found in addition increased activity in the prefrontal cortex, the cerebellum, and the primary somatosensory cortex. 90 , 91 , 92 , 93 There was reduced connectivity between the somatosensory cortex and increased connectivity between the somatosensory cortex and the bilateral anterior insula. 94 Structural imaging showed total gray matter volume decrease in fibromyalgia in one study 95 and widespread regional decreases in the cingulate, insular and medial frontal and prefrontal cortices, parahippocampal gyri, thalamus, and pons. 90 , 96 , 97 , 98
Tinnitus
Tinnitus is an acoustic misperception occurring in the absence of an external acoustical source. 99 It is often associated with hyperacusis 100 and has severe impact on quality of life. 101 Tinnitus has a high prevalence in the general population, between 11.9% and 30.3%. 102 It is clearly more prevalent in patients affected by VSS, 1 fibromyalgia, 81 and migraine. 6 Patients with complete resections of the auditory nerve can still have persistent tinnitus. This points toward a central component. 103 However, hearing loss is a trigger for the development of tinnitus 104 , 105 , 106 potentially via sensory deafferentation and development of a lack of inhibitory input resulting in cortical hyperexcitability. 107 , 108 Functional MRI studies of patients with tinnitus showed an increase in sound‐evoked BOLD signal in the inferior colliculus, auditory midbrain, thalamus, and primary auditory cortex. 109 , 110 , 111 A 15O‐H2O positron emission tomography study indicated a decrease in regional blood flow over the auditory cortex after the application of lidocaine correlating with tinnitus loudness. 112
The conscious perception and distress of tinnitus seems to be influenced by the connectivity patterns detected using fMRI resting state in the anterior cingulate cortex and left precuneus, the posterior cingulate cortex, and right medial prefrontal cortex. 113 The limbic and auditory systems interact at the thalamic level and modulate the perception of auditory signals. 114
Persistent postural‐perceptual dizziness
PPPD, formerly called phobic postural vertigo, space‐motion discomfort, visual vertigo, or chronic subjective dizziness, is a perceptual disorder, which makes up to 15%–20% of patients presenting in neuro‐otologic centers. 115 , 116 In the population, symptoms probably lie on a spectrum. 117
Patients typically describe diffuse dizziness, waxing, and waning over longer periods (hours), aggravated by upright posture and moving stimuli. The disorder is typically triggered by an acute or episodic vertigo condition, for example benign paroxysmal positional vertigo. Per definition, it is a chronic disorder lasting more than 3 months. 116 There is a link between central‐type vertigo in migraine 118 and an increased prevalence of migraine in patients fulfilling the criteria of PPPD. 119 Individuals with increased PPPD symptoms also show increased sensitivity across a range of sensory modalities including touch, taste, smell, and audition. 119 , 120 PPPD probably results from an overreliance on visual and postural stimuli and reduced input from the central vestibular system. 116
A seed‐based fMRI study demonstrated an increased functional connectivity between the thalamus, occipital, and cerebellar areas, as well as between the associative visual cortex and the middle frontal gyrus and precuneus. 121 Reduced BOLD response to sound‐evoked vestibular stimulation was shown in the multisensory vestibular cortical regions in the insula and increased visual cortical activity correlating with symptom severity. 122 Similarly, a SPECT study demonstrated insular and frontal hypoperfusion and cerebellar hyperperfusion. 123 In MRI, gray matter volume decreases in the temporal cortex (V5, perisylvian vestibular cortex), cingulate cortex, hippocampus, prefrontal cortex, insula, caudate nucleus, and cerebellum were demonstrated 124 , 125 including the regions of the multisensory vestibular network. 126
Depression, anxiety, “brain fog,” and sleep
All the above‐described perceptual disorders are associated with mood disorders, especially anxiety and depression. 7 , 127 , 128 , 129 , 130 Additionally sleep disturbances in the sense of insomnia, abnormal sleep architecture, and sleep fragmentation are well known in fibromyalgia 131 , 132 but have also been reported in patients with tinnitus 133 and migraine. 134 We found no literature on this topic concerning VSS and PPPD.
Cognitive symptoms such as declines in memory and mental alertness, the so‐called “brain fog,” are reported in about 50% of patients with fibromyalgia 135 , 136 and are frequent in VSS as well. 1 , 7
SUMMARY AND DISCUSSION
There are many overlaps between VSS, fibromyalgia, tinnitus, and PPPD. Patients affected by these disorders exhibit a hypersensitivity to external and internal stimuli in the sense of perceptional disorders. Reduced sensory thresholds often exceed the predominantly affected sensory modality. Fittingly, these distinct disorders are frequently associated with one another.
The defining symptoms of VSS, tinnitus, PPPD, and fibromyalgia, that is, visual or acoustic noise, vertigo, and somatosensory discomfort, might be more prevalent within the general population than expected. These disorders might therefore be regarded as extremes of a spectrum between normal and pathologic with the diagnostic criteria representing a cutoff. The suspected underlying pathophysiology might be network disorders involving a disbalance between inhibitory and excitatory connections resulting in a hyperactive or disinhibited state of primary and/or secondary perceptual brain areas. Networks involved in the management of attention (salience network) and the regulation of emotions (limbic system) might be affected as well.
Migraine is a sensory gating disorder with a periodic course probably driven by hypothalamic fluctuations as a reaction to homeostatic and external changes. Importantly, patients exhibit decreased sensory thresholds in all sensory domains even interictally.
Our hypothesis is, therefore, that migraine constitutes a risk factor for the development of the other persistent perceptual disorders. In other words, having migraine might be the common link between the difficult to treat perceptual disorders presented in Figure 1.
FIGURE 1.
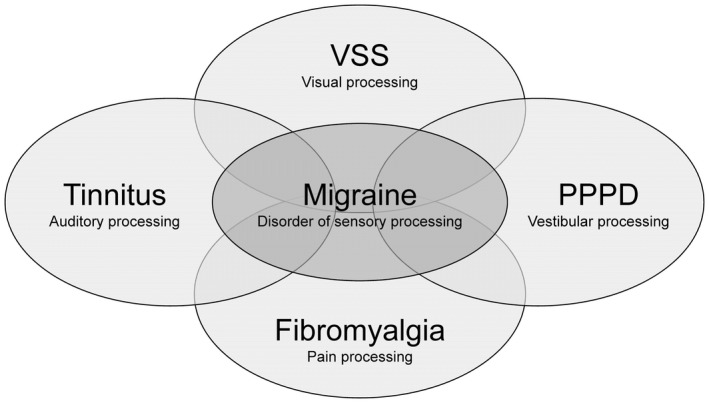
Migraine is comorbid with several chronic and difficult to treat disorders of sensory processing, such as visual snow syndrome (VSS), chronic tinnitus, persistent postural‐perceptual dizziness (PPPD), and fibromyalgia. Having migraine involves recurrent headache attacks of migrainous phenotype, as well as interictal difficulties during multimodal sensory processing. Migraine might be a common link to processing disorders of more specific modalities, such as the visual (i.e., VSS), vestibular (PPPD), auditory (tinnitus), and pain system (fibromyalgia). This might also partly explain the clinical overlap of these disorders and why they are often related to each other.
This is supported by the clinical presentation of patients described in detail above and by the neuroanatomical correlates. The anterior cingulate gyrus and the insula, both of which are components of the salience network, 137 seem to be affected in all the mentioned perceptual disorders. There is evidence that this network is also relevant in the pathophysiology of depression and anxiety. 138 , 139 The salience network is involved in the direction of attention and the switch between other large‐scale brain networks such as the default mode network, which is usually active during the resting state, and the central execute network, which is active during tasks. 140 It seems to play a crucial role in the evaluation and prioritization of stimuli and perceptual decision‐making. 141 Therefore, an impairment of these functions might modulate symptom perception and might even explain the link with the psychiatric symptoms.
The thalamus is a central component of all sensory networks. 142 , 143 , 144 , 145 It receives input from afferent sensory pathways and projects to widespread primary and secondary sensory areas of the cortex. The thalamus is part of feedback mechanisms (loops) implicated in the “filtering” and partly “suppression” of sensory input. 32 , 146 The above‐described imaging findings indicate an involvement of the thalamus in all the perception disorders discussed in this paper. The concept of a “thalamocortical dysrhythmia” has been discussed as the neuronal correlate of VSS, 147 fibromyalgia, 148 tinnitus, 149 and several other neurologic and psychiatric disorders. This term has been used because of the detection of abnormal electroencephalographic oscillatory patterns, that is, reduced alpha and increased theta power over certain cortical areas of the brain, which might indicate a disturbance in thalamocortical interactions. 150
This review focuses on disorders chosen based on striking similarities and clinical associations but does not claim to be exhaustive. Other perceptual symptoms might be present in the affected patients and would support our concept of an underlying, common network disorder. Whether the link between perceptual disorders is a common predisposition, such as having migraine, or a genetic link remains to be investigated.
CONCLUSION
VSS, PPPD, fibromyalgia, and chronic tinnitus might lie on a spectrum of perceptual disorders. Understanding the similarities in these network disorders and the role of migraine as a possible risk factor might move forward the quest for successful treatment of these often debilitating and difficult to treat disorders.
CONFLICT OF INTEREST
AK: No conflicts. CJS: CJS received scientific support, travel support, and/or honoraria from Novartis, Eli Lilly, TEVA Pharmaceuticals, Lundbeck, Allergan, Almirall, Amgen, MindMed, and Grünenthal. He received research grants from the German Migraine and Headache Society, Eye on Vision Foundation, and Baasch‐Medicus Foundation.
AUTHOR CONTRIBUTIONS
Study concept and design: Antonia Klein, Christoph J. Schankin. Acquisition of data: Antonia Klein, Christoph J. Schankin. Analysis and interpretation of data: Antonia Klein, Christoph J. Schankin. Drafting of the manuscript: Antonia Klein, Christoph J. Schankin. Revising it for intellectual content: Antonia Klein, Christoph J. Schankin. Final approval of the completed manuscript: Antonia Klein, Christoph J. Schankin.
ACKNOWLEDGEMENTS
Open Access Funding provided by Universitat Bern.
Klein A, Schankin CJ. Visual snow syndrome, the spectrum of perceptual disorders, and migraine as a common risk factor: A narrative review. Headache. 2021;61:1306–1313. 10.1111/head.14213
Funding information
CJS was funded by the Baasch‐Medicus Foundation.
REFERENCES
- 1. Schankin CJ, Maniyar FH, Digre KB, Goadsby PJ. ‘Visual snow’ – a disorder distinct from persistent migraine aura. Brain. 2014;137(5):1419‐1428. 10.1093/brain/awu050. [DOI] [PubMed] [Google Scholar]
- 2. Cho SJ, Sohn JH, Bae JS, Chu MK. Fibromyalgia among patients with chronic migraine and chronic tension‐type headache: a multicenter prospective cross‐sectional study. Headache. 2017;57(10):1583‐1592. [DOI] [PubMed] [Google Scholar]
- 3. de Tommaso M, Sciruicchio V. Migraine and central sensitization: clinical features, main comorbidities and therapeutic perspectives. Curr Rheumatol Rev. 2016;12(2):113‐126. [DOI] [PubMed] [Google Scholar]
- 4. Bisdorff A. Migraine and dizziness. Curr Opin Neurol. 2014;27(1):105‐110. [DOI] [PubMed] [Google Scholar]
- 5. Farri A, Enrico A, Lacilla M, Sartoris A. Tinnitus during headache: clinical‐instrumental evaluation. Acta Otorhinolaryngol Ital. 1999;19(2):70‐75. [PubMed] [Google Scholar]
- 6. Hwang J‐H, Tsai S‐J, Liu T‐C, Chen Y‐C, Lai J‐T. Association of tinnitus and other cochlear disorders with a history of migraines. JAMA Otolaryngol Head Neck Surg. 2018;144(8):712‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehta DG, Garza I, Robertson CE. Two hundred and forty‐eight cases of visual snow: a review of potential inciting events and contributing comorbidities. Cephalalgia. 2021;41(9):1015‐1026. [DOI] [PubMed] [Google Scholar]
- 8. Kondziella D, Olsen MH, Dreier JP. Prevalence of visual snow syndrome in the UK. Eur J Neurol. 2020;27(5):764‐772. [DOI] [PubMed] [Google Scholar]
- 9. Puledda F, Schankin C, Goadsby PJ. Visual snow syndrome. A clinical and phenotypical description of 1100 cases. Neurology. 2020;94(6):e564‐e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Puledda F, Schankin C, Digre K, Goadsby PJ. Visual snow syndrome: what we know so far. Curr Opin Neurol. 2018;31(1):52‐58. [DOI] [PubMed] [Google Scholar]
- 11. Viana M, Puledda F, Goadsby PJ. Visual snow syndrome: a comparison between an Italian and British population. Eur J Neurol. 2020;27(10):2099‐2101. [DOI] [PubMed] [Google Scholar]
- 12. Schankin CJ, Maniyar FH, Sprenger T, Chou DE, Eller M, Goadsby PJ. The relation between migraine, typical migraine aura and “visual snow”. Headache: J Headache Pain. 2014;54(6):957‐966. 10.1111/head.12378. [DOI] [PubMed] [Google Scholar]
- 13. van Dongen RM, Waaijer LC, Onderwater GLJ, Ferrari MD, Terwindt GM. Treatment effects and comorbid diseases in 58 patients with visual snow. Neurology. 2019;93(4):e398‐e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoo YJ, Yang HK, Choi JY, Kim JS, Hwang JM. Neuro‐ophthalmologic findings in visual snow syndrome. J Clin Neurol. 2020;16(4):646‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friedman B. Observations on entoptic phenomena. Arch Ophthalmol. 1942;28(2):285‐312. [Google Scholar]
- 16. Sinclair SH, Azar‐Cavanagh M, Soper K, Tuma R, Mayrovitz H. Investigation of the source of the blue field entoptic phenomenon. Invest Ophthalmol Vis Sci. 1989;30(4):668‐673. [PubMed] [Google Scholar]
- 17. Gersztenkorn D, Lee AG. Palinopsia revamped: a systematic review of the literature. Surv Ophthalmol. 2014;60:1‐35. [DOI] [PubMed] [Google Scholar]
- 18. White OB, Clough M, McKendrick AM, Fielding J. Visual snow: visual misperception. J Neuroophthalmol. 2018;38(4):514‐521. [DOI] [PubMed] [Google Scholar]
- 19. McKendrick AM, Chan YM, Tien M, et al. Behavioral measures of cortical hyperexcitability assessed in people who experience visual snow. Neurology. 2017;88(13):1243‐1249. [DOI] [PubMed] [Google Scholar]
- 20. Eren O, Eggert T, Ruscheweyh R, Straube A, Schankin C. Visual contrast threshold at 15 Hz is able to confirm visual snow syndrome in individual patients (S20.005). Neurology. 2019;92(suppl 15):S20.005. [Google Scholar]
- 21. Shepherd A. Visual contrast processing in migraine. Cephalalgia. 2000;20(10):865‐880. [DOI] [PubMed] [Google Scholar]
- 22. McKendrick AM, Vingrys AJ, Badcock DR, Heywood JT. Visual dysfunction between migraine events. Invest Ophthalmol Vis Sci. 2001;42(3):626‐633. [PubMed] [Google Scholar]
- 23. Battista J, Badcock DR, McKendrick AM. Center‐surround visual motion processing in migraine. Invest Ophthalmol Vis Sci. 2010;51(11):6070‐6076. [DOI] [PubMed] [Google Scholar]
- 24. Eren O, Rauschel V, Ruscheweyh R, Straube A, Schankin CJ. Evidence of dysfunction in the visual association cortex in visual snow syndrome. Ann Neurol. 2018;84(6):946‐949. [DOI] [PubMed] [Google Scholar]
- 25. Luna S, Lai D, Harris A. Antagonistic relationship between VEP potentiation and gamma power in visual snow syndrome. Headache. 2018;58(1):138‐144. [DOI] [PubMed] [Google Scholar]
- 26. Schankin CJ, Maniyar FH, Chou DE, Eller M, Sprenger T, Goadsby PJ. Structural and functional footprint of visual snow syndrome. Brain. 2020;143(4):1106‐1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Puledda F, Ffytche D, Lythgoe DJ, et al. Insular and occipital changes in visual snow syndrome: a BOLD fMRI and MRS study. Ann Clin Transl Neurol. 2020;7(3):296‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ogawa S, Lee T‐M, Nayak AS, Glynn P. Oxygenation‐sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14(1):68‐78. [DOI] [PubMed] [Google Scholar]
- 29. Zborowski M, Ostera GR, Moore LR, Milliron S, Chalmers JJ, Schechter AN. Red blood cell magnetophoresis. Biophys J. 2003;84(4):2638‐2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Puledda F, O'Daly O, Schankin C, Ffytche D, Williams SC, Goadsby PJ. Disrupted connectivity within visual, attentional and salience networks in the visual snow syndrome. Hum Brain Mapp. 2021;42(7):2032‐2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lakatos P, O'Connell MN, Barczak A. Pondering the Pulvinar. Neuron. 2016;89(1):5‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peters SK, Dunlop K, Downar J. Cortico‐striatal‐thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front Syst Neurosci. 2016;10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Puledda F, Bruchhage M, O'Daly O, Ffytche D, Williams SCR, Goadsby PJ. Occipital cortex and cerebellum gray matter changes in visual snow syndrome. Neurology. 2020;95(13):e1792‐e1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steiner TJ, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. J Headache Pain. 2013;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1‐211. [DOI] [PubMed] [Google Scholar]
- 36. Goadsby PJ, Holland PR, Martins‐Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peng K‐P, May A. Migraine understood as a sensory threshold disease. Pain. 2019;160(7):1494‐1501. [DOI] [PubMed] [Google Scholar]
- 38. Peng K‐P, May A. Redefining migraine phases—a suggestion based on clinical, physiological, and functional imaging evidence. Cephalalgia. 2020;40(8):866‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nyholt DR, Borsook D, Griffiths LR. Migrainomics—identifying brain and genetic markers of migraine. Nat Rev Neurol. 2017;13(12):725‐741. [DOI] [PubMed] [Google Scholar]
- 40. Mulder EJ, van Baal C, Gaist D, et al. Genetic and environmental influences on migraine: a twin study across six countries. Twin Res. 2003;6(5):422‐431. [DOI] [PubMed] [Google Scholar]
- 41. de Boer I, van den Maagdenberg AMJM, Terwindt GM. Advance in genetics of migraine. Curr Opin Neurol. 2019;32(3):413‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karsan N, Bose P, Zelaya FO, Goadsby PJ, eds. Alterations in regional cerebral blood (rCBF) during nitroglycerin (NTG) triggered migraine headache assessed using arterial spin‐labelled (ASL) functional magnetic resonance imaging (fMRI). Cephalalgia. 2017;37(1S):28 (EP‐01‐005). [Google Scholar]
- 43. Schulte LH, Mehnert J, May A. Longitudinal neuroimaging over 30 days: temporal characteristics of migraine. Ann Neurol. 2020;87(4):646‐651. [DOI] [PubMed] [Google Scholar]
- 44. Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain. 2016;139(7):1987‐1993. [DOI] [PubMed] [Google Scholar]
- 45. Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ. Brain activations in the premonitory phase of nitroglycerin‐triggered migraine attacks. Brain. 2014;137(Pt 1):232‐241. [DOI] [PubMed] [Google Scholar]
- 46. Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G. Hypothalamic activation in spontaneous migraine attacks. Headache. 2007;47(10):1418‐1426. [DOI] [PubMed] [Google Scholar]
- 47. Schulte LH, May A. Of generators, networks and migraine attacks. Curr Opin Neurol. 2017;30(3):241‐245. [DOI] [PubMed] [Google Scholar]
- 48. Puledda F, Messina R, Goadsby PJ. An update on migraine: current understanding and future directions. J Neurol. 2017;264(9):2031‐2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kagan R, Kainz V, Burstein R, Noseda R. Hypothalamic and basal ganglia projections to the posterior thalamus: possible role in modulation of migraine headache and photophobia. Neuroscience. 2013;248:359‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moulton EA, Becerra L, Johnson A, Burstein R, Borsook D. Altered hypothalamic functional connectivity with autonomic circuits and the locus coeruleus in migraine. PLoS ONE. 2014;9(4):e95508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arbab MA, Wiklund L, Svendgaard NA. Origin and distribution of cerebral vascular innervation from superior cervical, trigeminal and spinal ganglia investigated with retrograde and anterograde WGA‐HRP tracing in the rat. Neuroscience. 1986;19(3):695‐708. [DOI] [PubMed] [Google Scholar]
- 52. Noseda R, Kainz V, Borsook D, Burstein R. Neurochemical pathways that converge on thalamic trigeminovascular neurons: potential substrate for modulation of migraine by sleep, food intake, stress and anxiety. PLoS ONE. 2014;9(8):e103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Burstein R, Jakubowski M, Garcia‐Nicas E, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68(1):81‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35(17):6619‐6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Froesel M, Cappe C, Ben HS. A multisensory perspective onto primate pulvinar functions. Neurosci Biobehav Rev. 2021;125:231‐243. [DOI] [PubMed] [Google Scholar]
- 56. Sherman SM, Guillery RW. Exploring the Thalamus and its Role in Cortical Function. MIT Press; 2006. [Google Scholar]
- 57. White EL. Thalamocortical synaptic relations: a review with emphasis on the projections of specific thalamic nuclei to the primary sensory areas of the neocortex. Brain Res Rev. 1979;1(3):275‐311. [DOI] [PubMed] [Google Scholar]
- 58. Ruthirago D, Julayanont P, Kim J. Chapter 7.2—Translational correlation: migraine. In: Conn PM, ed. Conn's Translational Neuroscience. Academic Press; 2017:159‐165. [Google Scholar]
- 59. Burstein R, Jakubowski M. Unitary hypothesis for multiple triggers of the pain and strain of migraine. J Comp Neurol. 2005;493(1):9‐14. [DOI] [PubMed] [Google Scholar]
- 60. Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8(2):136‐142. [DOI] [PubMed] [Google Scholar]
- 61. Ashina S, Bentivegna E, Martelletti P, Eikermann‐Haerter K. Structural and functional brain changes in migraine. Pain Ther. 2021;10(1):211‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Valfrè W, Rainero I, Bergui M, Pinessi L. Voxel‐based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48(1):109‐117. [DOI] [PubMed] [Google Scholar]
- 63. Qin Z, He X‐W, Zhang J, et al. Structural changes of cerebellum and brainstem in migraine without aura. J Headache Pain. 2019;20(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Palm‐Meinders IH, Arkink EB, Koppen H, et al. Volumetric brain changes in migraineurs from the general population. Neurology. 2017;89(20):2066‐2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jin C, Yuan K, Zhao L, et al. Structural and functional abnormalities in migraine patients without aura. NMR Biomed. 2013;26(1):58‐64. [DOI] [PubMed] [Google Scholar]
- 66. Schmitz N, Arkink EB, Mulder M, et al. Frontal lobe structure and executive function in migraine patients. Neurosci Lett. 2008;440(2):92‐96. [DOI] [PubMed] [Google Scholar]
- 67. Tessitore A, Russo A, Giordano A, et al. Disrupted default mode network connectivity in migraine without aura. J Headache Pain. 2013;14(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Niddam DM, Lai K‐L, Fuh J‐L, Chuang C‐YN, Chen W‐T, Wang S‐J. Reduced functional connectivity between salience and visual networks in migraine with aura. Cephalalgia. 2016;36(1):53‐66. [DOI] [PubMed] [Google Scholar]
- 69. Vincent M, Pedra E, Mourão‐Miranda J, Bramati IE, Henrique AR, Moll J. Enhanced interictal responsiveness of the migraineous visual cortex to incongruent bar stimulation: a functional MRI visual activation study. Cephalalgia. 2003;23(9):860‐868. [DOI] [PubMed] [Google Scholar]
- 70. Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311(15):1547‐1555. [DOI] [PubMed] [Google Scholar]
- 71. Clauw DJ, Crofford LJ. Chronic widespread pain and fibromyalgia: what we know, and what we need to know. Best Pract Res Clin Rheumatol. 2003;17(4):685‐701. [DOI] [PubMed] [Google Scholar]
- 72. Branco JC, Bannwarth B, Failde I, et al. Prevalence of fibromyalgia: a survey in five European countries. Semin Arthritis Rheum. 2010;39(6):448‐453. [DOI] [PubMed] [Google Scholar]
- 73. Wolfe F, Clauw DJ, Fitzcharles M‐A, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38(6):1113‐1122. [DOI] [PubMed] [Google Scholar]
- 74. Wolfe F, Clauw DJ, Fitzcharles M‐A, et al., eds. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319‐329. [DOI] [PubMed] [Google Scholar]
- 75. Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46(5):1333‐1343. [DOI] [PubMed] [Google Scholar]
- 76. McDermid AJ, Rollman GB, McCain GA. Generalized hypervigilance in fibromyalgia: evidence of perceptual amplification. Pain. 1996;66(2):133‐144. [DOI] [PubMed] [Google Scholar]
- 77. Ceko M, Bushnell MC, Gracely RH. Neurobiology underlying fibromyalgia symptoms. Pain Res Treat. 2012;2012:585419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schweinhardt P, Sauro KM, Bushnell MC. Fibromyalgia: a disorder of the brain? Neuroscientist. 2007;14(5):415‐421. [DOI] [PubMed] [Google Scholar]
- 79. Marcus DA, Bernstein C, Rudy TE. Fibromyalgia and headache: an epidemiological study supporting migraine as part of the fibromyalgia syndrome. Clin Rheumatol. 2005;24(6):595‐601. [DOI] [PubMed] [Google Scholar]
- 80. Whealy M, Nanda S, Vincent A, Mandrekar J, Cutrer FM. Fibromyalgia in migraine: a retrospective cohort study. J Headache Pain. 2018;19(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bayazıt YA, Gürsoy S, Özer E, Karakurum G, Madenci E. Neurotologic manifestations of the fibromyalgia syndrome. J Neurol Sci. 2002;196(1):77‐80. [DOI] [PubMed] [Google Scholar]
- 82. Vincent A, Whipple MO, Rhudy LM. Fibromyalgia flares: a qualitative analysis. Pain Med. 2016;17(3):463‐468. [DOI] [PubMed] [Google Scholar]
- 83. Buskila D, Atzeni F, Sarzi‐Puttini P. Etiology of fibromyalgia: the possible role of infection and vaccination. Autoimmun Rev. 2008;8(1):41‐43. [DOI] [PubMed] [Google Scholar]
- 84. Buskila D, Neumann L, Vaisberg G, Alkalay D, Wolfe F. Increased rates of fibromyalgia following cervical spine injury: a controlled study of 161 cases of traumatic injury. Arthritis Rheum. 1997;40:446‐452. [DOI] [PubMed] [Google Scholar]
- 85. Bradley LA. Pathophysiology of fibromyalgia. Am J Med. 2009;122(suppl 12):S22‐S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Russell IJ, Vaeroy H, Javors M, Nyberg F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35(5):550‐556. [DOI] [PubMed] [Google Scholar]
- 87. Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102(1):87‐95. [DOI] [PubMed] [Google Scholar]
- 88. Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind‐up) in patients with fibromyalgia syndrome. Pain. 2001;91:165‐175. [DOI] [PubMed] [Google Scholar]
- 89. Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI‐based neurologic signature of physical pain. N Eng J Med. 2013;368(15):1388‐1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, Meeus M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum. 2014;44(1):68‐75. [DOI] [PubMed] [Google Scholar]
- 91. Jensen KB, Loitoile R, Kosek E, et al. Patients with fibromyalgia display less functional connectivity in the brain's pain inhibitory network. Mol Pain. 2012;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pujol J, López‐Solà M, Ortiz H, et al. Mapping brain response to pain in fibromyalgia patients using temporal analysis of FMRI. PLoS ONE. 2009;4(4):e5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Burgmer M, Pogatzki‐Zahn E, Gaubitz M, Wessoleck E, Heuft G, Pfleiderer B. Altered brain activity during pain processing in fibromyalgia. Neuroimage. 2009;44(2):502‐508. [DOI] [PubMed] [Google Scholar]
- 94. Kim J, Loggia ML, Cahalan CM, et al. The somatosensory link in fibromyalgia: functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheum. 2015;67(5):1395‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27(15):4004‐4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schmidt‐Wilcke T, Luerding R, Weigand T, et al. Striatal grey matter increase in patients suffering from fibromyalgia—a voxel‐based morphometry study. Pain. 2007;132:S109‐S116. [DOI] [PubMed] [Google Scholar]
- 97. Burgmer M, Gaubitz M, Konrad C, et al. Decreased gray matter volumes in the cingulo‐frontal cortex and the amygdala in patients with fibromyalgia. Psychosom Med. 2009;71(5):566‐573. [DOI] [PubMed] [Google Scholar]
- 98. Fallon N, Alghamdi J, Chiu Y, Sluming V, Nurmikko T, Stancak A. Structural alterations in brainstem of fibromyalgia syndrome patients correlate with sensitivity to mechanical pressure. Neuroimage Clin. 2013;3:163‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Baguley D, McFerran D, Hall D. Tinnitus. Lancet. 2013;382(9904):1600‐1607. [DOI] [PubMed] [Google Scholar]
- 100. Schecklmann M, Landgrebe M, Langguth B, TRI Database Study Group . Phenotypic characteristics of hyperacusis in tinnitus. PLoS ONE. 2014;9(1):e86944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nondahl DM, Cruickshanks KJ, Dalton DS, et al. The impact of tinnitus on quality of life in older adults. J Am Acad Audiol. 2007;18(3):257‐266. [DOI] [PubMed] [Google Scholar]
- 102. McCormack A, Edmondson‐Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70‐79. [DOI] [PubMed] [Google Scholar]
- 103. Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med. 2002;347(12):904‐910. [DOI] [PubMed] [Google Scholar]
- 104. Satar B, Kapkin O, Ozkaptan Y. Evaluation of cochlear function in patients with normal hearing and tinnitus: a distortion product otoacoustic emission study. Kulak Burun Bogaz Ihtis Derg. 2003;10(5):177‐182. [PubMed] [Google Scholar]
- 105. Noreña AJ, Farley BJ. Tinnitus‐related neural activity: theories of generation, propagation, and centralization. Hear Res. 2013;295:161‐171. [DOI] [PubMed] [Google Scholar]
- 106. Noreña AJ. Revisiting the cochlear and central mechanisms of tinnitus and therapeutic approaches. Audiol Neurotol. 2015;20(suppl 1):53‐59. [DOI] [PubMed] [Google Scholar]
- 107. Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. Am J Otolaryngol. 1999;20(6):351‐362. [DOI] [PubMed] [Google Scholar]
- 108. Parra LC, Pearlmutter BA. Illusory percepts from auditory adaptation. J Acoust Soc Am. 2007;121(3):1632‐1641. [DOI] [PubMed] [Google Scholar]
- 109. Melcher JR, Sigalovsky IS, Guinan JJ Jr, Levine RA. Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol. 2000;83(2):1058‐1072. [DOI] [PubMed] [Google Scholar]
- 110. Melcher JR, Levine RA, Bergevin C, Norris B. The auditory midbrain of people with tinnitus: abnormal sound‐evoked activity revisited. Hear Res. 2009;257(1):63‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gu JW, Halpin CF, Nam E‐C, Levine RA, Melcher JR. Tinnitus, diminished sound‐level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol. 2010;104(6):3361‐3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Reyes SA, Salvi RJ, Burkard RF, et al. Brain imaging of the effects of lidocaine on tinnitus. Hear Res. 2002;171(1):43‐50. [DOI] [PubMed] [Google Scholar]
- 113. Chen Y‐C, Chen H, Bo F, et al. Tinnitus distress is associated with enhanced resting‐state functional connectivity within the default mode network. Neuropsychiatr Dis Treat. 2018;14:1919‐1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic‐auditory interactions in tinnitus. Neuron. 2010;66(6):819‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Dieterich M, Staab JP, Brandt T. Chapter 37—Functional (psychogenic) dizziness. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology. Vol. 139. Elsevier; 2016:447‐468. [DOI] [PubMed] [Google Scholar]
- 116. Staab JP, Eckhardt‐Henn A, Horii A, et al. Diagnostic criteria for persistent postural‐perceptual dizziness (PPPD): consensus document of the committee for the Classification of Vestibular Disorders of the Bárány Society. J Vestib Res. 2017;27:191‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Powell G, Derry‐Sumner H, Rajenderkumar D, Rushton S, Sumner P. Persistent postural perceptual dizziness is on a spectrum in the general population. Neurology. 2020;94(18):e1929‐e1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Furman JM, Marcus DA, Balaban CD. Migrainous vertigo: development of a pathogenetic model and structured diagnostic interview. Curr Opin Neurol. 2003;16(1):5‐13. [DOI] [PubMed] [Google Scholar]
- 119. Powell G, Derry‐Sumner H, Shelton K, et al. Visually‐induced dizziness is associated with sensitivity and avoidance across all senses. J Neurol. 2020;267(8):2260‐2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Holle D, Schulte‐Steinberg B, Wurthmann S, et al. Persistent postural‐perceptual dizziness: a matter of higher, central dysfunction? PLoS ONE. 2015;10(11):e0142468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Van Ombergen A, Heine L, Jillings S, et al. Altered functional brain connectivity in patients with visually induced dizziness. Neuroimage Clin. 2017;14:538‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Riccelli R, Passamonti L, Toschi N, et al. Altered insular and occipital responses to simulated vertical self‐motion in patients with persistent postural‐perceptual dizziness. Front Neurol. 2017;8:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Na S, Im JJ, Jeong H, et al. Cerebral perfusion abnormalities in patients with persistent postural‐perceptual dizziness (PPPD): a SPECT study. J Neural Transm. 2019;126(2):123‐129. [DOI] [PubMed] [Google Scholar]
- 124. Wurthmann S, Naegel S, Schulte Steinberg B, et al. Cerebral gray matter changes in persistent postural perceptual dizziness. J Psychosom Res. 2017;103:95‐101. [DOI] [PubMed] [Google Scholar]
- 125. Nigro S, Indovina I, Riccelli R, et al. Reduced cortical folding in multi‐modal vestibular regions in persistent postural perceptual dizziness. Brain Imaging Behav. 2019;13(3):798‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Brandt T. Vestibular cortex: its locations, functions, and disorders. In: Vertigo. New York, NY: Springer; 2003:219‐231. [DOI] [PubMed] [Google Scholar]
- 127. Kleykamp BA, Ferguson MC, McNicol E, et al. The prevalence of psychiatric and chronic pain comorbidities in fibromyalgia: an ACTTION systematic review. Semin Arthritis Rheum. 2021;51(1):166‐174. [DOI] [PubMed] [Google Scholar]
- 128. Zöger S, Svedlund J, Holgers K‐M. Relationship between tinnitus severity and psychiatric disorders. Psychosomatics. 2006;47(4):282‐288. [DOI] [PubMed] [Google Scholar]
- 129. Frediani F, Villani V. Migraine and depression. Neurol Sci. 2007;28(2):S161‐S165. [DOI] [PubMed] [Google Scholar]
- 130. Staab JP, Ruckenstein MJ. Which comes first? Psychogenic dizziness versus otogenic anxiety. Laryngoscope. 2003;113(10):1714‐1718. [DOI] [PubMed] [Google Scholar]
- 131. Roizenblatt S, Neto NSR, Tufik S. Sleep disorders and fibromyalgia. Curr Pain Headache Rep. 2011;15(5):347‐357. [DOI] [PubMed] [Google Scholar]
- 132. Spaeth M, Rizzi M, Sarzi‐Puttini P. Fibromyalgia and sleep. Best Pract Res Clin Rheumatol. 2011;25(2):227‐239. [DOI] [PubMed] [Google Scholar]
- 133. Attanasio G, Russo FY, Roukos R, Covelli E, Cartocci G, Saponara M. Sleep architecture variation in chronic tinnitus patients. Ear Hear. 2013;34(4):503‐507. [DOI] [PubMed] [Google Scholar]
- 134. Bertisch SM, Li W, Buettner C, et al. Nightly sleep duration, fragmentation, and quality and daily risk of migraine. Neurology. 2020;94(5):e489‐e496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kravitz HM, Katz RS. Fibrofog and fibromyalgia: a narrative review and implications for clinical practice. Rheumatol Int. 2015;35(7):1115‐1125. [DOI] [PubMed] [Google Scholar]
- 136. Katz RS, Heard AR, Mills M, Leavitt F. The prevalence and clinical impact of reported cognitive difficulties (fibrofog) in patients with rheumatic disease with and without fibromyalgia. J Clin Rheumatol. 2004;10(2):53‐58. [DOI] [PubMed] [Google Scholar]
- 137. Chiong W, Wilson SM, D’Esposito M, et al. The salience network causally influences default mode network activity during moral reasoning. Brain. 2013;136(6):1929‐1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Pannekoek JN, Veer IM, van Tol M‐J, et al. Resting‐state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. Eur Neuropsychopharmacol. 2013;23(3):186‐195. [DOI] [PubMed] [Google Scholar]
- 139. Manoliu A, Meng C, Brandl F, et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter‐network connectivity in major depressive disorder. Front Hum Neurosci. 2014;7:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Goulden N, Khusnulina A, Davis NJ, et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage. 2014;99:180‐190. [DOI] [PubMed] [Google Scholar]
- 141. Lamichhane B, Dhamala M. The salience network and its functional architecture in a perceptual decision: an effective connectivity study. Brain Connect. 2015;5(6):362‐370. [DOI] [PubMed] [Google Scholar]
- 142. Usrey WM, Alitto HJ. Visual functions of the thalamus. Annu Rev Vis Sci. 2015;1:351‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Dieterich M, Brandt T. The bilateral central vestibular system: its pathways, functions, and disorders. Ann N Y Acad Sci. 2015;1343(1):10‐26. [DOI] [PubMed] [Google Scholar]
- 144. Hu B. Functional organization of lemniscal and nonlemniscal auditory thalamus. Exp Brain Res. 2003;153(4):543‐549. [DOI] [PubMed] [Google Scholar]
- 145. Dostrovsky JO. Role of thalamus in pain. Prog Brain Res. 2000;129:245‐257. [DOI] [PubMed] [Google Scholar]
- 146. Kondo HM, Kashino M. Involvement of the thalamocortical loop in the spontaneous switching of percepts in auditory streaming. J Neurosci. 2009;29(40):12695‐12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Lauschke JL, Plant GT, Fraser CL. Visual snow: a thalamocortical dysrhythmia of the visual pathway? J Clin Neurosci. 2016;28:123‐127. [DOI] [PubMed] [Google Scholar]
- 148. Lim M, Kim JS, Kim DJ, Chung CK. Increased low‐ and high‐frequency oscillatory activity in the prefrontal cortex of fibromyalgia patients. Front Hum Neurosci. 2016;10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. De Ridder D, Vanneste S, Langguth B, Llinas R. Thalamocortical dysrhythmia: a theoretical update in tinnitus. Front Neurol. 2015;6:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Llinás R, Urbano FJ, Leznik E, Ramírez RR, van Marle HJF. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28(6):325‐333. [DOI] [PubMed] [Google Scholar]


