ABSTRACT
Aims
To determine the effects of oestrogen or oestrogen deprivation on vaginal wound healing. Impaired wound healing following prolapse surgery may increase the risk of recurrent prolapse in the future. Vaginal oestrogen therapy may improve wound healing, hereby possibly improving surgical outcomes.
Methods
A systematic search of OVID MEDLINE, OVID Embase, and Web of Science was conducted up to January 28, 2020. We included original studies comparing wound healing‐related outcomes of oestrogen exposed subjects (female animals and women) to hypo‐oestrogenic subjects after vaginal surgery. Data on wound healing‐related outcome measures were extracted. For each individual comparison, the standardised mean difference (Hedges' g; SMD) and 95% confidence interval (CI) were calculated.
Results
Of the 1474 studies reviewed, 14 studies were included for review, and 11 provided data for meta‐analysis. Oestrogen improves neovascularisation (SMD: 1.13, 95% CI: 0.67–1.60), microscopic wound closure (SMD: 0.98, 95% CI: 0.66–1.29), collagen synthesis (SMD: 1.08, 95% CI: 0.42–1.74), and tissue strength (SMD: 1.26, 95% CI: 0.53–1.99) in animals. Oestrogen increases granulation (SMD: 1.67, 95% CI: 0.54–2.79) and accelerates macroscopic wound closure (SMD: 1.82, 95% CI: 1.22–2.42) in women and animals. Oestrogen decreases the inflammatory response (SMD: −0.58, 95% CI: −1.14 to −0.02) in women and animals and reduces levels of transforming growth factor (TGF)‐β1 (SMD: −1.68, 95% CI: −2.52 to −0.83) in animals. All results were statistically significant.
Conclusions
Oestrogen therapy has a positive effect on vaginal wound healing. Future studies should determine whether oestrogen therapy has the potential to improve surgical outcomes.
Keywords: collagen synthesis, oestrogen, granulation, inflammatory response, neovascularisation, pelvic organ prolapse, re‐epithelialisation, TGF‐β1, tissue strength, vaginal surgery, wound contraction, wound healing
Abbreviations
- CI
confidence interval
- CMA
Comprehensive Meta‐Analysis
- H&E
hematoxylin and eosin
- IQR
interquartile range
- PDGF
platelet‐derived growth factor
- POP
pelvic organ prolapse
- PRISMA
Reporting Items for Systematic Reviews and Meta‐Analyses
- PROSPERO
prospective register of systematic reviews
- SD
standard deviation
- SE
standard error
- SMD
standardised mean difference
- SUI
stress urinary incontinence
- SYRCLE
SYstematic Review Center for Laboratory animal Experimentation
- TGF‐β1
transforming growth factor‐β1
1. INTRODUCTION
Postmenopausal women with pelvic floor pathology may benefit from vaginal oestrogen therapy. A reduction of symptoms has been described in women with vaginal atrophy, stress urinary incontinence (SUI), overactive bladder, and recurrent cystitis when prescribed vaginal oestrogens. 1 , 2 , 3 The suggested underlying mechanism is that oestrogen thickens the vaginal wall and urothelium, and improves vascularisation of the pelvic floor. 4 In addition, oestrogen therapy may play an important role in the outcome of vaginal surgery, such as the surgical treatment of pelvic organ prolapse (POP).
Surgery for POP is associated with high recurrence rates of up to 50%. 5 This stresses the need for improvement of the outcome of vaginal POP surgery. It is thought that the outcome of POP surgery may be negatively affected by impaired wound healing in postmenopausal, hypo‐oestrogenic women. Although vaginal wound closure often occurs without problems, tissue quality may be poor, thereby negatively affecting the outcome of surgery. It is hypothesised that treatment with oestrogen may enhance vaginal wound healing, resulting in improved tissue quality and tissue strength, which is essential for long‐term surgical outcome.
Oestrogen and its derivatives are known to have a positive effect on cutaneous wound healing and are important for vascularisation, oxygenation, tissue regeneration, epidermal function, matrix deposition, and inflammatory response. 6 , 7 , 8 , 9 , 10 With regard to POP surgery, wound healing is essential to re‐establish tissue integrity and to restore functional, strong tissue to keep the pelvic organs in place and avoid recurrence of POP. Besides POP surgery, other vaginal surgeries such as surgery for urinary incontinence, and vaginal fistula surgery may also benefit from improved wound healing.
To date, not much is known about the effects of oestrogen on vaginal wound healing specifically and the effects of promoted wound healing on the (long‐term) outcome of vaginal surgery. Therefore, as a first step, we systematically reviewed the literature on the effects of oestrogen on all phases of wound healing after vaginal surgery, providing an overview of available evidence from both animal and human studies. Improved understanding of these effects may provide further opportunities to develop oestrogen‐related therapies to improve the outcome of vaginal surgery.
2. METHODS
This systematic review and meta‐analysis were reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 11 Furthermore, this review was conducted in collaboration with SYstematic Review Center for Laboratory animal Experimentation (SYRCLE) and has been registered in the prospective register of systematic reviews (PROSPERO; CRD42019156601).
2.1. Information sources and search strategy
An information (J. L.) specialist performed a systematic search in OVID MEDLINE, OVID Embase, and Web of Science from inception to January 28, 2020, using controlled vocabulary (i.e., MeSH‐terms) and text words to retrieve both animal and human studies on the effect of oestrogen on vaginal wound healing. The following concepts were searched: oestrogen or oestrogen‐deficient animal models combined with either (1) vaginal surgery or (2) vagina (disorders) AND wounds/wound healing. Studies on malignancy, reviews, editorials, and conference abstracts were excluded. There were no restrictions on language or date. Reference lists and citing articles of identified relevant papers were crosschecked for additional relevant studies using Web of Science and the search strategy was adapted in case of additional relevant records. Identified studies were imported and deduplicated using Endnote (version X9.3.3, Clarivate Analytics). See Supporting Information Appendix 1 for the complete search strategy.
2.2. Eligibility criteria
We included original studies performed in female animals (all species) and women. Studies were eligible if they compared (1) wound healing‐related outcomes in (2) oestrogen‐exposed subjects to hypo‐oestrogenic subjects after (3) vaginal surgery. The definition and assessment of wound healing are diversely reported due to its complex and dynamic process. In order not to restrict this systematic review to predefined wound‐related outcomes, we included all outcomes that authors reported as wound healing‐related parameters and outcomes. Vaginal surgery included POP surgery, SUI surgery, hysterectomy, episiotomy during delivery, vaginal fistula surgery, and experimental wounding. Oestrogens considered were (ethinyl‐) oestradiol, oestriol, oestrone, epioestriol, oestetrol, dienoestrol, oestradiol congeners, and physiologic oestrogens. There were no restrictions in administration route, dosage, and frequency. Use of diethylstilbenediol was excluded from eligibility due to its carcinogenic effects. 12
Original studies concerned interventional animal studies and clinical studies using the following design: randomised controlled trial, cohort, case‐control, and case series of more than 10 cases. We excluded studies for the following reason: (1) lack of a hypo‐oestrogenic control group; (2) no vaginal wound; (3) no evaluation of wound healing; (4) wounds related to complications of vaginal surgery (e.g., mesh exposure and erosion); (5) no full‐text available.
2.3. Study selection
Two reviewers (E. V., A. K.) independently screened all retrieved studies for eligibility using Rayyan web‐tool. 13 Studies were initially screened by title and abstract after which selected papers underwent full review to make the final selection. Reasons for excluding articles were recorded. Discrepancies were resolved by discussion between the two reviewers (E. V., A. K.) for consensus. Any unresolved discrepancies were adjudicated by a third reviewer (Z. G.).
2.4. Data extraction
From the studies included, both reviewers (E. V., A. K.) independently extracted the following data—using an in‐house developed spreadsheet‐based on MS Office Excel: animal species, strain, age at the beginning of the study, parity, method of vaginal surgery, surgical site, wound closure, description of treatment and control group, oestrogen type, oestrogen dosage, frequency of oestrogen administration, the timing of oestrogen administration relative to vaginal surgery, duration of oestrogen administration, route of oestrogen administration, the timing of data collection, wound healing‐related outcome measures, therapy compliance, sample size in treatment and control group, number of subjects excluded for statistical analysis, and reason for subject exclusion. Bibliographic details such as author, journal, year of publication, original language, and country where the study was conducted were also recorded.
For all outcomes, number of events or mean, standard deviation (SD) or standard error (SE), and total number of subjects per group were recorded. When data were only presented graphically, they were measured using a digital screen ruler (ImageJ) 14 by two independent reviewers (E. V., A. K.). In case of relevant missing data, the authors were contacted.
2.5. Quality assessment
Two reviewers independently scored the included studies on risk of bias (E. V., A. K.). Scoring was performed using predesigned characteristic forms based on the Cochrane risk of bias tool for human studies and the SYRCLE risk of bias tool for animal studies (Figure S1). To overcome the problem of judging too many items as “unclear risk of bias” because reporting of experimental details on methods and materials in animal studies is generally very poor, 15 , 16 we added two items on reporting: reporting of any measure of randomisation, reporting of any measure of blinding.
2.6. Meta‐analysis
We performed a meta‐analysis for each outcome with a minimum of two reporting studies. For all continuous outcome measures, SD was calculated if only the SE was reported (SD = SE ×√n). Studies were excluded from meta‐analysis if not all outcome data (mean, SD, and n) could be obtained. We calculated the standardised mean difference (SMD) and 95% confidence interval (95% CI) for each separate intervention‐control comparison group with Hedges' g correction. 17 In case of multiple experimental groups within one study, correction for multiple use of the control group was performed (number of animals in control group divided by the number of times the control group is used; rounded up to the next whole number). In case multiple similar outcomes were reported from the same cohort of animals or women, we either extracted a single outcome (based on hierarchy of preferred outcome measures) or combined more than one outcome to provide a single outcome statistic (“nested outcome”; each outcome weighted by multiplication by the inverse of the variance for that outcome, summed for all outcomes and divided by the sum of the weights). 18 When sample sizes were presented as a range, the lowest number was used for the meta‐analysis. Despite anticipated heterogeneity, the individual effect sizes were pooled to obtain an overall SMD with Hedges' g correction, with 95% CI. We used the random‐effects model, which takes the precision of individual studies and the variation between studies into account and weights each study accordingly. Meta‐analyses were performed using Comprehensive Meta‐Analysis (CMA version 3.0). Forest plots were used to display the mean overall effect sizes. Subgroup analyses were planned for the following study characteristics: type of surgical procedure; route of oestrogen administration, oestrogen dosing, oestrogen type, treatment duration, and timing of oestrogen treatment related to surgery (pre‐/peri‐/postsurgery).
2.7. Sensitivity analyses and publication bias analyses
To assess the robustness of our findings, we performed multiple sensitivity analyses in CMA. First, we analysed the human and animal data separately to justify our decision to combine human and animal data in one meta‐analysis. Second, we investigated the effect of a possible interaction based on the timing of the data collection. In the sensitivity analysis of all outcome measures, data collected at the time of vaginal surgery (T0) was added to the analyses. Third, we investigated the effect of a possible interaction based on the administration route of oestrogen therapy. Therefore, vaginally administered oestrogen was excluded in the sensitivity analysis.
To detect publication bias, funnel plots were created and evaluated on symmetry when more than 10 studies per outcome measure were present, using Duval and Tweedie's trim and fill analysis and Egger's regression analysis for small‐study effects. Because SMDs may cause funnel plot distortion we plotted the SMD against a sample size‐based precision estimate (1/√(n)). 19 Heterogeneity was assessed using I 2.
3. RESULTS
3.1. Study selection
The systematic literature search yielded 1474 unique references (Figure 1). After title and abstract screening, 35 studies met our selection criteria. After studying the full‐text articles, 14 studies (12 animal studies and two human studies) remained. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 From 14 studies, 11 studies were included in the meta‐analyses which included a total of 889 female animals and 197 women in 134 comparisons across eight outcomes.
Figure 1.
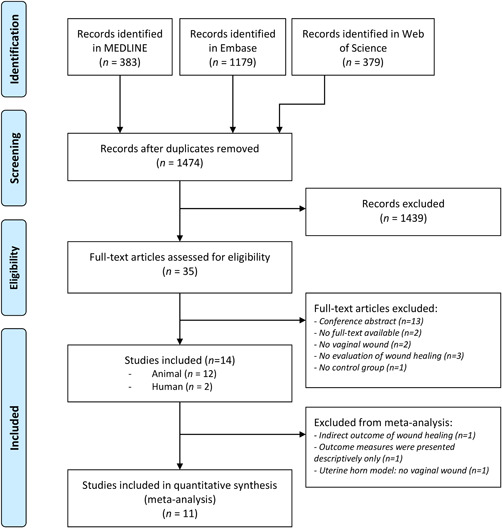
PRISMA flowchart
3.2. Study characteristics
A summary of the included studies is provided in Table S1. Study characteristics varied considerably. The included studies consisted of two human studies, comprising 197 women, and 12 studies comprising 795 rats, 378 rabbits, 24 guinea pigs, and four canines. More than 10 different surgical techniques were used, and vaginal wounds varied in size and location. In six studies the vaginal wound was closed with at least one absorbable suture, whereas in seven studies the wound was not closed. One study included a wound located at the uterine horn, serving as a model for wound healing in the genital tract. 30 Also type of oestrogen, administration route, dosage, frequency, timing, and duration of oestrogen therapy varied greatly between studies. In two studies, oestrogen was administered preoperatively, in six studies postoperatively, and in five studies oestrogen was administered both pre‐ and postoperatively. The median duration of oestrogen therapy was 14.3 days (interquartile range [IQR]: 6.8–19.3). Five studies included physiologic oestrogen as part of oestrogen therapy; 11 studies used exogenous oestrogens, which were administered subcutaneously (five studies), systemically (three studies); and vaginally (four studies). Reported wound healing‐related outcome measures included inflammatory response, neovascularisation, granulation formation, wound closure (defined as microscopic and macroscopic re‐epithelialisation), collagen synthesis, transforming growth factor (TGF)‐β1, and tissue strength (Table S2).
Three studies of this systematic review were not included for meta‐analyses: (1) Rodriguez et al. 25 was excluded because it reported only on fibulin‐5, which is a matricellular glycoprotein that promotes elastogenesis and inhibits the matrix degrading protein, MMP‐9. For this systematic review, this outcome measure was too indirect to represent wound healing. (2) Gale et al. 26 was excluded because all outcome measures were presented descriptively. (3) Schlaff et al. 30 was excluded from meta‐analysis because it evaluated the biomechanical properties of the uterus. Although the study states that the uterine horn model represents the genital tract, we find that the biomechanical properties from a uterus are too different from a vaginal wall to include this in the meta‐analysis. (4) The rat cohort of Cretti et al. 24 could also not be included in the meta‐analyses because SDs were not reported. Therefore, only the data from the rabbit cohort were included.
3.3. Quality assessment
The results of the risk of bias assessment of the 14 included studies are shown in Figure S1. In total, 64% of the studies reported randomisation at any level; 57% reported blinding at any level, but none of the studies reported on blinding of the outcome assessor. In 29% of the studies, incomplete outcome data was adequately addressed. Figure S1 shows that many items were scored as ‘‘unclear risk of bias,” which indicates poor reporting of bias, mainly in scientific publications of animal studies. Assessment of the risk of publication bias was not assessed because of too few studies per outcome measure.
3.4. Meta‐analysis
3.4.1. Inflammatory response
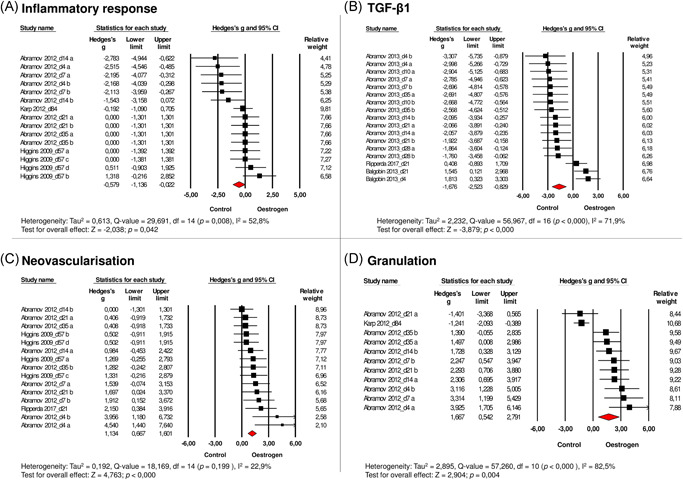
Fourteen comparisons from three studies were included in the meta‐analysis regarding the effect of oestrogen on the inflammatory response after vaginal surgery. The inflammatory response was defined as the presence of neutrophils in hematoxylin and eosin (H&E) stained sections or scored by naked eye inspection (from none to severe inflammation or yes/no presence of micro‐inflammation). The inflammatory response in the oestrogen‐treated group was significantly reduced compared to the control group (SMD: −0.58; [−1.14 to −0.02], n = 15, Figure 2A). The inflammatory response was measured in rabbits and women. From the three included studies, one study administered oestrogen therapy vaginally.
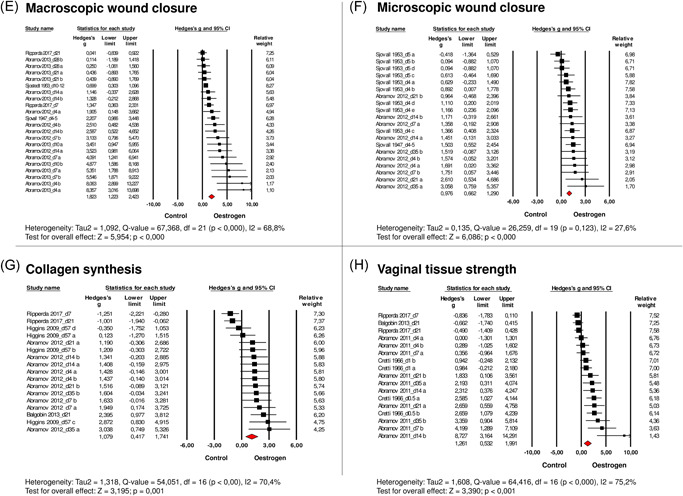
Figure 2.
Effects of oestrogen on (A) the inflammatory response, (B) TGF‐β1, (C) neovascularisation, (D) granulation tissue, (E) macroscopic wound closure, (F) on microscopic wound closure, (G) collagen synthesis, (H) vaginal tissue strength after vaginal wounding. Forest plots display the standardised mean difference (SMD) (Hedges' g), 95% confidence interval, and relative weight of the individual studies. The diamond indicates the global estimate and its 95% confidence interval. Study names contain the moment of data collection in days after surgery (d)
3.4.2. TGF‐β1
TGF‐β1 was measured in three studies (16 comparisons), which were included in the meta‐analysis. Measurement of TGF‐β1 was performed in rabbits, rats, and guinea pigs of which one study administered oestrogen vaginally. Meta‐analysis revealed that oestrogen reduces TGF‐β1 gene transcription after vaginal surgery (SMD: −1.68 [−2.52 to −0.83], n = 17, Figure 2B).
3.4.3. Neovascularisation
Fourteen comparisons from three studies that measured the effect of oestrogen on neovascularisation after vaginal wounding were included. These three studies were performed in rabbits and rats of which one study administered oestrogen vaginally. Meta‐analysis revealed increased vascularisation in animals following oestrogen administration (SMD: 1.13 [0.67–1.60], n = 15, Figure 2C).
3.4.4. Granulation tissue
The effect of oestrogen on granulation tissue after vaginal surgery was analysed in two studies (10 comparisons), performed in rabbits and women of which one study used vaginally administered oestrogen. Meta‐analysis demonstrated that oestrogen increased the amount of granulation tissue after vaginal surgery (SMD: 1.67 [0.54–2.79], n = 11, Figure 2D).
3.4.5. Wound closure (macroscopic re‐epithelialisation)
Overall analysis of the five studies investigating macroscopic wound closure showed that wounds recover faster under influence of oestrogen (SMD: 1.82 [1.22–2.42, n = 22, Figure 2E). Macroscopic wound closure was assessed in rabbits, rats, and women of which one study administered oestrogen vaginally.
3.4.6. Wound closure (microscopic re‐epithelialisation)
Microscopic wound closure was also accelerated under the administration of oestrogen, which was evaluated in three studies that included 19 comparisons (SMD: 0.98 [0.66–1.29], n = 20, Figure 2F). Re‐epithelialisation was assessed in rabbits and rats. None of the studies administered oestrogen therapy vaginally.
3.4.7. Collagen synthesis
Sixteen comparisons from four studies concerned the effect of oestrogen on collagen synthesis after vaginal wounding. These studies were performed in rabbits, rats, and guinea pigs of which one study administered oestrogen therapy vaginally. Analysis showed that oestrogen increased the amount of newly formed collagen after vaginal surgery (SMD: 1.08 [0.42–1.74], n = 17, Figure 2G).
3.4.8. Biomechanical tissue strength
Sixteen comparisons from four studies concerning the effects of oestrogen on biomechanical tissue strength of a vaginal wound were included in the meta‐analysis. In three studies, vaginal tissue strength was calculated from the slope of the linear portion of the stress–strain curves. Biomechanical tests were performed in rabbits, rats, and guinea pigs of which one study administered oestrogen vaginally. Results revealed an increased tissue strength in oestrogen‐treated animals (SMD: 1.26 [0.53–1.99], n = 17, Figure 2H). The effect size of all outcome measures is shown in Figure 3.
Figure 3.
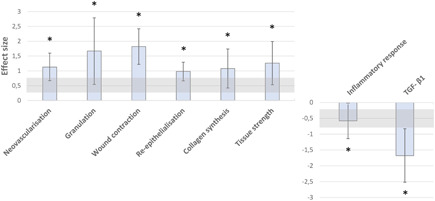
Effect size (Hedges' g, SMD) with 95% confidence intervals. A Hedges' g of 0.2 could be interpreted as a small effect size; a Hedges' g of ≥ 0.8 could be interpreted as large effect size (gray range). *Significant difference
3.4.9. Subgroup analyses and sensitivity analysis
Subgroup analyses of all wound healing‐related outcomes could not be conducted because the number of included studies was too limited. The first sensitivity analysis on animal data only was performed for the outcome measures macroscopic wound closure, granulation tissue, and inflammatory response. This sensitivity analysis showed no large difference in the overall effect size. In other words, the conclusions regarding these outcomes remain the same whether or not humans are included as well. The second sensitivity analysis (with an added time point) showed that the overall effect size slightly decreased in all outcome measures but remained significant compared to the control (hypo‐oestrogen) group. The third sensitivity analysis (vaginal oestrogen administration excluded) was performed for all outcome measures except microscopic wound closure and showed no difference in the overall effect size.
4. DISCUSSION
4.1. Main findings
This systematic review and meta‐analysis report on the effects of oestrogen on different domains of vaginal wound healing in women and animals. We provide evidence that oestrogen therapy has a positive effect on vaginal wound healing by improving macroscopic wound closure and optimising granulation tissue in women and animals, and by improving neovascularisation, microscopic wound closure, collagen synthesis, and tissue strength in animals. In addition, oestrogen may decrease the inflammatory response and reduce levels of TGF‐β1 in women and animals.
4.2. Strengths and limitations
This is the first systematic review and meta‐analysis addressing the effects of oestrogen on vaginal wound healing. Both animal and human studies were systematically appraised and retrieved through an extensive literature search, without language and date restrictions. This study was conducted in collaboration with the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE), an institution highly experienced in the conduct of systematic reviews and meta‐analyses of animal studies.
Nevertheless, there are limitations that may affect the generalisability and validity of our findings. First, the high variation between studies and high heterogeneity in the study results is a limitation. Since the number of included studies was limited, it was not possible to perform subgroup analyses to differentiate between (1) vaginal and systemic oestrogen, (2) oestrogen administration; (3) frequencies, doses, timing, and duration of treatment, (4) type of surgical procedure, and (5) animal species and women. Although we accounted for heterogeneity by using a random‐effects model rather than a fixed‐effects model for meta‐analysis, this may have affected the certainty of evidence. In addition, the quality of evidence may also be affected by the small number of included studies. Furthermore, there may be a risk of overestimation of the effect sizes due to publication bias. However, publication bias could not be assessed because of the small number of included studies. Also, many included studies reported incompletely on important methodological details such as randomisation and blinding. This hampered the reliable risk of bias assessment and may reduce the reliability of our conclusions. Another limitation is that both vaginal and systemic oestrogens were used in the included studies, whereas in clinical practice, low‐dose vaginal oestrogen is preferred over systemic oestrogen, in particular, because systemic oestrogens are associated with an increased risk of thrombosis and endometrial cancer, breast cancer, and ovarian cancer. 34 , 35 , 36 Furthermore, a Cochrane review on oestrogen therapy for urinary incontinence demonstrated that systemic administration of oestrogen increased incontinence, whereas vaginally administered oestrogen actually improved symptoms of incontinence. 3 Nevertheless, the sensitivity analysis showed no difference in the overall effect size when data on vaginally administered oestrogen was excluded. An evaluation of species and type of surgery as well as type, dosage, frequency, timing, and duration of oestrogen therapy with regard to the effect on wound healing and subsequent surgical outcomes is an essential element of future research.
Finally, authors were contacted regarding discrepancies, but unfortunately, they did not respond to confirm or refute our assumptions. Regarding the reported outcome on macroscopic wound closure, it was noted that two studies had an identical study set‐up and were performed by the same research group. 20 , 22 To prevent that this cohort was weighed twice; only the data from one study was included in the meta‐analysis. Furthermore, a study reported two data time points in the result section which were not included in the methods section. 21 We included all time points as reported in the result section and assumed the group sizes were identical to the other groups. One study did not report the individual group sizes. 24 Therefore, group sizes were calculated by dividing the total group size by the number of experimental groups and the number of data time points.
4.3. Interpretation
4.3.1. Inflammatory phase of wound healing
This phase includes haemostasis and inflammation, characterised by platelet accumulation followed by cytokine signalling, after which platelet‐derived growth factor (PDGF) and TGF‐β are released, which is chemotactic for neutrophils migrating into the wound bed. 37 Our meta‐analysis showed a reduced inflammatory response, including reduced levels of TGF‐β1 in subjects treated with oestrogen. This anti‐inflammatory effect of oestrogen is also (partially) seen in other studies evaluating the effect of oestrogen on cutaneous wound healing. Two literature reviews on this topic illustrated either no effect or a reduced inflammatory response. 6 , 7 Reduction was expressed by dampening purulent inflammation, decreasing neutrophil numbers, promoting alternative macrophage polarisation (promoting a shift from M1 to M2 subtypes), and reducing the expression of pro‐inflammatory cytokines. 7 It is hypothesised that there is an optimum dose at which oestrogen is beneficial, 38 , 39 which may also apply to the process of healing, resulting in inconsistent effects or even over‐inhibitions of inflammation when an excessive amount of oestrogen is administered.
4.3.2. Proliferative phase of wound healing
The proliferative phase of wound healing is marked by angiogenesis, fibroplasia (formation of granulation tissue matrix), re‐epithelialisation, wound contraction, and collagen synthesis. Our meta‐analysis showed that oestrogen improved these proliferation‐related outcomes. This is also supported by evidence from the literature showing accelerated cutaneous wound healing after oestrogen treatment in aged women and hormone‐deprived animals. 40 , 41 , 42 , 43 Several studies addressed the underlying mechanisms and illustrated that oestrogenic compounds play a prominent role in promoting the healing processes by accelerating re‐epithelialization and promoting collagen deposition, granulation tissue formation, and wound contraction. 9 , 44 , 45 , 46 Moreover, Trenti et al. 47 demonstrated that oestrogen is a key factor in promoting endothelial healing and angiogenesis. Angiogenesis plays an important role in the supply of oxygen and nutrients to fibroblasts and catalyses the hydroxylases for collagen synthesis. 48 Therefore, angiogenesis is imperative for accelerating wound healing as well as for wound strength.
4.3.3. Maturation and remodelling phase of wound healing
The main feature of the maturation and remodelling phase is to organise collagen deposition in a well‐mannered network. 49 As the phase progresses, the tensile strength of the wound gradually increases. In case of matrix deposition failure, the wound's strength will be greatly compromised. Increased tissue strength following oestrogen therapy was also seen in our meta‐analysis. When compared to cutaneous wound healing, Ashcroft et al. 40 also showed increased strength of cutaneous wounds in elderly women treated with oestrogen. In perspective to surgery for POP, maintenance of tissue integrity and tissue strength is essential to keep pelvic organs in place and may therefore also prevent recurrence of POP.
5. CONCLUSIONS AND IMPLICATIONS
This systematic review and meta‐analysis provide evidence that oestrogen therapy has a beneficial effect on vaginal wound healing, improving tissue quality and tissue strength after surgery. Consequently, it could be hypothesised that long‐term surgical outcomes may improve in postmenopausal women when treated with oestrogen, but this needs further investigation.
The outcomes of this study justify further research evaluating the effect of oestrogen‐induced improved vaginal wound healing on surgical outcomes, such as recurrence rates of POP, vaginal health, quality of life, and sexual function. Although the optimal dosage, frequency, and duration of perioperative oestrogen therapy cannot be disclosed based upon this study, we suggest using low‐dose vaginal oestrogen since this is preferred over systematic oestrogen in clinical practice. 34 , 35 , 36 Furthermore, we suggest prescribing oestrogens until 1 year postoperatively, since this comprises the last phase of wound healing; the maturation phase. In this phase, newly formed tissue gains strength and flexibility. Collagen fibres reorganise (collagen III, which was produced in the proliferative phase, is now replaced by the stronger collagen I), tissue remodels and matures and there is an overall increase in tensile strength. 10 We think this could be essential to prevent the recurrence of POP after surgery.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Eva V. Vodegel, Arnoud W. Kastelein, Charlotte H. J. R. Jansen, Sandra E. Zwolsman, Jan‐Paul W. R. Roovers, Carlijn R. Hooijmans, and Zeliha Guler were responsible for the conception and design of this study. Eva V. Vodegel, Arnoud W. Kastelein, and Jacqueline Limpens were responsible for the data collection and data management. Eva V. Vodegel, Arnoud W. Kastelein, and Charlotte H. J. R. Jansen were responsible for conducting the analyses. All authors were involved in the interpretation of the data. Eva V. Vodegel drafted the initial manuscript, which was reviewed and critically revised by all authors. All authors approved the final manuscript as submitted.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENT
Project: “Synthesis of Evidence, more evidence with less animals” from ZonMW (Project number: 114024902) for funding the collaboration with SYRCLE (SYstematic Review Center for Laboratory animal Experimentation).
Vodegel EV, Kastelein AW, Jansen CHJR, et al. The effects of oestrogen on vaginal wound healing: a systematic review and meta‐analysis. Neurourology and Urodynamics. 2022;41:115‐126. 10.1002/nau.24819
Preliminary data of this study was awarded the best abstract of Session 1 at the 13th Annual Congress of the European Urogynaecological Association 2020 (EUGA 2020).
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article and from the corresponding author upon reasonable request.
REFERENCES
- 1. Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Sys Rev. 2016;8:Cd001500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weber MA, Kleijn MH, Langendam M, Limpens J, Heineman MJ, Roovers JP. Local oestrogen for pelvic floor disorders: a systematic review. PLoS One. 2015;10(9):e0136265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cody JD, Jacobs ML, Richardson K, Moehrer B, Hextall A. Oestrogen therapy for urinary incontinence in post‐menopausal women. Cochrane Database Sys Rev. 2012;10:Cd001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krause M, Wheeler TL, Snyder TE, Richter HE. Local effects of vaginally administered estrogen therapy: a review. J Pelvic Med Surg. 2009;15(3):105‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Denman MA, Gregory WT, Boyles SH, Smith V, Edwards SR, Clark AL. Reoperation 10 years after surgically managed pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2008;198(5):555.e551‐555. [DOI] [PubMed] [Google Scholar]
- 6. Calvin M. Oestrogens and wound healing. Maturitas. 2000;34(3):195‐210. [DOI] [PubMed] [Google Scholar]
- 7. Horng HC, Chang WH, Yeh CC, et al. Estrogen effects on wound healing. Int J Mol Sci. 2017;18(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mukai K, Urai T, Asano K, Nakajima Y, Nakatani T. Evaluation of Effects of topical estradiol benzoate application on cutaneous wound healing in ovariectomized female mice. PLoS One. 2016;11(9):e0163560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35‐43. [DOI] [PubMed] [Google Scholar]
- 11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1‐e34. [DOI] [PubMed] [Google Scholar]
- 12. Marselos M, Tomatis L. Ethylstilboestrol: I, Pharmacology, toxicology and carcinogenicity in humans. Eur J Cancer. 1992;28a(6‐7):1182‐1189. [DOI] [PubMed] [Google Scholar]
- 13. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan‐a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ImageJ [Computer Program]. 1997–2020. https://imagej.nih.gov/ij/
- 15. Kilkenny C, Parsons N, Kadyszewski E, et al. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One. 2009;4(11):e7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Avey MT, Moher D, Sullivan KJ, et al. The devil is in the details: incomplete reporting in preclinical animal research. PLoS One. 2016;11(11):e0166733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hedges LV, Olkin I. Statistical Methods for Meta‐Analysis. Academic Press; 1985. [Google Scholar]
- 18. Vesterinen HM, Sena ES, Egan KJ, et al. Meta‐analysis of data from animal studies: a practical guide. J Neurosci Methods. 2014;221:92‐102. [DOI] [PubMed] [Google Scholar]
- 19. Zwetsloot PP, Van Der Naald M, Sena ES, et al. Standardized mean differences cause funnel plot distortion in publication bias assessments. eLife. 2017;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abramov Y, Golden B, Sullivan M, Goldberg RP, Sand PK. Vaginal incisional wound healing in a rabbit menopause model: a histologic analysis. International Urogynecology Journal. 2012;23(12):1763‐1769. [DOI] [PubMed] [Google Scholar]
- 21. Abramov Y, Hirsch E, Ilievski V, Goldberg RP, Sand PK. Transforming growth factor beta1 gene expression during vaginal wound healing in a rabbit menopause model. Bjog‐an International Journal of Obstetrics and Gynaecology. 2013;120(2):251‐256. [DOI] [PubMed] [Google Scholar]
- 22. Abramov Y, Webb AR, Botros SM, Goldberg RP, Ameer GA, Sand PK. Effect of bilateral oophorectomy on wound healing of the rabbit vagina. Fertil Steril. 2011;95(4):1467‐1470. [DOI] [PubMed] [Google Scholar]
- 23. Balgobin S, Montoya TI, Shi H, et al. Estrogen alters remodeling of the vaginal wall after surgical injury in guinea pigs. Biol Reprod. 2013;89(6):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cretti A. The influence of oestradiol and progesterone on wound healing. [Polish]. Roczn Akad Med Marchlewskiego. 1966:14. [PubMed] [Google Scholar]
- 25. Florian‐Rodriguez M, Chin K, Hamner J, Acevedo J, Keller P, Word RA. Effect of protease inhibitors in healing of the vaginal wall. Sci Rep. 2019;9(1):12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gale CL, Muram D, Adamec TA. The effects of exogenous estrogen on wound healing in dogs. Adolesc Pediatr Gynecol. 1993;6(3):160‐163. [Google Scholar]
- 27. Higgins EW, Rao A, Baumann S, et al. Effect of estrogen replacement on the histological response to polypropylene mesh implanted in the rabbit vagina model. Journal of Pelvic Medicine and Surgery. 2009;15:63. [DOI] [PubMed] [Google Scholar]
- 28. Karp DR, Jean‐Michel M, Johnston Y, Suciu G, Aguilar VC, Davila GW. A randomized clinical trial of the impact of local estrogen on postoperative tissue quality after vaginal reconstructive surgery. Female Pelvic Med Reconstr Surg. 2012;18(4):211‐215. [DOI] [PubMed] [Google Scholar]
- 29. Ripperda C, Maldonado PA, Acevedo JF, Keller P, Word RA. Vaginal estrogen: a dual edged sword in postoperative healing of the vaginal wall. Female Pelvic Medicine and Reconstructive Surgery. 2016;22:S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schlaff WD, Cooley BC, Shen W, Gittlesohn AM, Rock JA. A rat uterine horn model of genital tract wound healing. Fertil Steril. 1987;48(5):866‐872. [DOI] [PubMed] [Google Scholar]
- 31. Sjostedt S. The effect of diethylstilbenediol on the healing of wounds in the human vagina. Acta Endocrinol (Copenh). 1953;12(3):260‐263. [DOI] [PubMed] [Google Scholar]
- 32. Sjovall A. The influence of oestrogen upon the healing of vaginal wounds in rats. Acta Obstet Gynecol Scand. 1947;27(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 33. Sjovall A. The influence of oestrogens upon the healing of vaginal wounds in rats. Acta Endocrinol (Copenh). 1953;12(3):249‐259. [DOI] [PubMed] [Google Scholar]
- 34. Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. The Cochrane database of systematic reviews. 2006;(4):Cd001500. [DOI] [PubMed] [Google Scholar]
- 35. Krause M, Wheeler TL 2nd, Richter HE, Snyder TE. Systemic effects of vaginally administered estrogen therapy: a review. Female Pelvic Med Reconstr Surg. 2010;16(3):188‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Crandall C. Vaginal estrogen preparations: a review of safety and efficacy for vaginal atrophy. J Womens Health (Larchmt). 2002;11(10):857‐877. [DOI] [PubMed] [Google Scholar]
- 37. Childs DR, Murthy AS. Overview of wound healing and management. Surg Clin North Am. 2017;97(1):189‐207. [DOI] [PubMed] [Google Scholar]
- 38. Shahrad P, Marks R. A pharmacological effect of oestrone on human epidermis. Br J Dermatol. 1977;97(4):383‐386. [DOI] [PubMed] [Google Scholar]
- 39. Brincat M, Versi E, Moniz CF, Magos A, de Trafford J, Studd JW. Skin collagen changes in postmenopausal women receiving different regimens of estrogen therapy. Obstet Gynecol. 1987;70(1):123‐127. [PubMed] [Google Scholar]
- 40. Ashcroft GS, Greenwell‐Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol. 1999;155(4):1137‐1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brufani M, Rizzi N, Meda C, et al. Novel locally active estrogens accelerate cutaneous wound healing‐part 2. Sci Rep. 2017;7(1):2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hardman MJ, Emmerson E, Campbell L, Ashcroft GS. Selective estrogen receptor modulators accelerate cutaneous wound healing in ovariectomized female mice. Endocrinology. 2008;149(2):551‐557. [DOI] [PubMed] [Google Scholar]
- 43. Midgley AC, Morris G, Phillips AO, Steadman R. 17β‐estradiol ameliorates age‐associated loss of fibroblast function by attenuating IFN‐γ/STAT1‐dependent miR‐7 upregulation. Aging cell. 2016;15(3):531‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Emmerson E, Hardman MJ. The role of estrogen deficiency in skin ageing and wound healing. Biogerontology. 2012;13(1):3‐20. [DOI] [PubMed] [Google Scholar]
- 45. Thornton MJ. Estrogens and aging skin. Dermatoendocrinol. 2013;5(2):264‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mukai K, Nakajima Y, Urai T, et al. 17β‐Estradiol administration promotes delayed cutaneous wound healing in 40‐week ovariectomised female mice. Int Wound J. 2016;13(5):636‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trenti A, Tedesco S, Boscaro C, Trevisi L, Bolego C, Cignarella A. Estrogen, angiogenesis, immunity and cell metabolism: solving the puzzle. Int J Mol Sci. 2018;19(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gantwerker EA, Hom DB. Skin: histology and physiology of wound healing. Facial Plast Surg Clin North Am. 2011;19(3):441‐453. [DOI] [PubMed] [Google Scholar]
- 49. Broughton G, 2nd , Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(7 Suppl):12s‐34s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article and from the corresponding author upon reasonable request.


