Abstract
Background
Benralizumab is effective in severe eosinophilic asthma (SEA), but suboptimal responses are observed in some patients. Although several factors have been associated with benralizumab response, no cluster analysis has yet been undertaken to identify different responsiveness sub‐phenotypes.
Objective
To identify SEA sub‐phenotypes with differential responsiveness to benralizumab.
Methods
One hundred and five patients diagnosed with SEA who had completed 6 months of benralizumab treatment were included in a hierarchical cluster analysis based on a set of clinical variables that can be easily collected in routine practice (age, age at disease onset, disease length, allergen sensitization status, blood eosinophil count, IgE levels, FEV1% predicted, nasal polyposis, bronchiectasis).
Results
Four clusters were identified: Clusters 2 and 3 included patients with high levels of both IgE and eosinophils (type‐2 biomarkers high), whereas Clusters 1 and 4 included patients with only one type‐2 biomarker at a high level: IgE in Cluster 1 and eosinophils in Cluster 4. Clusters 2 and 3 (both type‐2 biomarkers high) showed the highest response rate to benralizumab in terms of elimination of exacerbations (79% and 80% respectively) compared to Clusters 1 and 4 (52% and 60% respectively). When super‐response (the absence of exacerbation without oral corticosteroid use) was assessed, Cluster 2, including patients with more preserved lung function than the other clusters, but comparable exacerbation rate, oral corticosteroid use and symptom severity, was the most responsive cluster (87.5% of patients).
Conclusions
Our cluster analysis identified benralizumab differential response sub‐phenotypes in SEA, with the potential of improving disease treatment and precision management.
Keywords: biologicals, monoclonal antibodies, observational studies, precision medicine, real‐life
Cluster analysis revealed four different benralizumab responsiveness sub‐phenotypes within the apparently homogeneous severe eosinophilic asthma type‐2 phenotype. Clusters 2 and 3, with both eosinophils and IgE at high level, and distinct clinical features, showed the highest response rate. This approach may potentially identify biomarkers useful to select the right target population.

Key messages.
• Benralizumab is effective in severe eosinophilic asthma, but it is not successful in all patients, and suboptimal responses are reported.
• This study suggests the presence of different clusters within the apparently homogeneous severe eosinophilic asthma Type2‐phenotype.
• These clusters, characterized by differential expression of type‐2 biomarkers and distinct clinical features, show differential benralizumab responsiveness.
1. INTRODUCTION
Benralizumab has been shown to be effective in patients with severe eosinophilic asthma (SEA). 1 , 2 , 3 , 4 Phase 3 randomized controlled trials (RCTs) have demonstrated a significant reduction in exacerbation rate and oral corticosteroid (OCS) use, as well as improvement of lung function, asthma control and quality of life measures. 1 , 2 , 3 , 4 However, as for other biologics, it is evident that not all patients respond equally well. 5 This difference in treatment response is likely multifactorial and related to the heterogeneity of the severe asthma population. 6
Prediction of treatment response is difficult and deals with various problems such as how to define a response, what are clinically relevant outcome measures, and what should be the timing for the evaluation of response. 7 Data from RCTs consistently showed that higher levels of blood eosinophils and a history of frequent exacerbations were the most important baseline characteristics that predicted treatment response to benralizumab. 8 The presence of late‐onset asthma (>18 years), OCS dependency, impaired lung function and nasal polyposis might further increase the chance of a good response. 9
It is recognized that subjects recruited in RCTs are not fully representative of the severe asthma population, as seen in routine practice, since trial populations appear to have a moderate‐to‐severe disease. 1 , 10 , 11 , 12 , 13 Furthermore, patients with fixed‐flow obstruction, smoking history and important medical comorbidities are usually excluded. 14 Consequently, real‐life data on biologics are needed to assess effectiveness and identify clinically relevant predictors, as data generated in clinical trial settings may not be easily translated in a real‐life setting.
In this multi‐centre, observational, retrospective study, by cluster analysis, we sought to characterize different clinical sub‐phenotypes within SEA, aiming at identifying subgroups of patients with differential benralizumab responsiveness.
2. METHODS
2.1. Study design
We performed a retrospective analysis of all patients with SEA who commenced treatment with benralizumab between May 2019 and January 2020 at 12 Italian tertiary referral asthma centres. Informed consent for the retrospective analysis was obtained from all subjects. The involved asthma centres used a shared anonymized database to collect clinical, functional and biological data. Ethical approval was obtained from each local Ethic Committee (coordinator centre: Catania, Italy; document number, 33/2020/PO–14th/April/2020).
2.2. Study population
All patients were reviewed by expert physicians and fulfilled the ATS/ERS definition of severe asthma. 14 All patients met spirometric criteria for asthma. Patients started benralizumab if they had an eosinophil count ≥300/μl in the preceding 12 months and a minimum of 2 exacerbations in the previous year and/or were on maintenance oral corticosteroid (mOCS). 15 Exacerbations were defined as a worsening in asthma control requiring ≥3 days of oral prednisone treatment or increase of prednisone dose if already on mOCS. Baseline exacerbation rate was determined by reviewing patients’ medical records. Patients’ demographic data were collected from the patient's clinical file.
2.3. Outcomes and analysis
Clinical assessment was performed at each scheduled visit. Complete data were collected at T6 and T12 (6 and 12 months after benralizumab respectively). Data collected included number of exacerbations, oral corticosteroid dosage, asthma control test (ACT), 16 mini‐asthma quality of life questionnaire (mini‐AQLQ), 17 Sino‐nasal Outcome Test‐22 (SNOT‐22), 18 spirometry, peripheral blood eosinophil count, total IgE levels, fractional exhaled nitric oxide (FeNO).
Clinical response was defined for each outcome as follows: (1) ≥50% reduction in the exacerbation rate (ER); (2) ≥50% reduction in mean daily OCS dose; (3) ≥0.23L increase of pre‐bronchodilator FEV1 or FEV1≥80% 19 , 20 ; (4) ≥3 point increase in ACT score or ACT score ≥20 21 ; (5) ≥0.5 point increase in mini‐AQLQ score. 1 , 2 , 3 , 4
A further analysis was performed in a subgroup of patients defined as ‘superResponders’, representing subjects who were completely exacerbation‐free and no longer required OCS (ER0/OCS0). Two additional subgroups were also defined within the superResponders: (1) those with ACT score ≥20 (ER0/OCS0/ACT≥20); (2) those with ACT score ≥20 and FEV1≥80% (ER0/OCS0/ACT≥20/FEV1≥80).
Patients who did not show a 50% reduction in exacerbation rate or mOCS use were defined as ‘non‐responders’. However, in all the analyses performed in this study, they were included in the ‘non‐superResponders’ group, as opposite to ‘superResponders’.
2.4. Statistics
Data were analysed by means of SPSS software (IBM SPSS). Data were shown as mean and standard deviation (SD) if normally distributed, or median and interquartile range (IQR) if not. To test the normality of distributions, the Shapiro–Wilk test was used. Categorical variables were analysed by Chi‐Square test or Fisher's exact test when appropriate.
To compare the three measurements at T0, T6 and T12, respectively, the Friedman test was used. Alternatively, the Wilcoxon test for paired samples was used when investigating the possible differences between paired medians.
The Ward's hierarchical clustering method (squared Euclidean distance) was used. Moreover, a k‐means clustering was carried out. The optimal number of clusters detected through the hierarchical cluster approach was confirmed as such by means of the Silhouette coefficient and the scree plot. Clusters’ comparisons were performed using one‐way ANOVA test for continuous variables and the Marascuilo test for multiple proportions (independent samples).
The Student's t‐test and the non‐parametric Mann–Whitney U test were used to compare the two unrelated samples corresponding to SuperResponders/Non‐SuperResponders.
Predictive analyses on responders were conducted using logistic regression (Ward's criterion). Variables were selected through the backward elimination process and considered significant at p ≤ .05.
Differences were considered significant at p < .05 and all the values are two sided.
3. RESULTS
A total of 160 patients who commenced benralizumab were included in this analysis. The baseline patient characteristics (68.1% females; mean age of 53.8 ± 13.9 years; BMI, 26.6 ± 5.1) are shown in Table 1. The median peripheral blood eosinophil count was 690 cells/μl (IQR, 460; 975), the median FeNO concentration was 45 ppb (IQR, 24.7; 62.2), the median IgE level was 134 kU/L (IQR, 57; 368). Furthermore, other eosinophilic comorbidities were reported such as nasal polyposis (49.4% of patients) and atopic dermatitis (10.6% of patients). Bronchiectasis was present in 21.9% of cases. Sixty percent of patients were sensitized to inhaled allergens. Despite all patients being prescribed a high dosage of ICS/LABA, with 59.4% of them on mOCS, the control was poor (ACT, 13.6 ± 4.1; AQLQ, 3.4 ± 1.1; mean exacerbations in the 12 months prior to benralizumab, 5 ± 3.4) and the mean pre‐bronchodilator FEV1% predicted was 65.6 ± 21.1.
TABLE 1.
Clinical characteristics of the patients (n = 160) prior to Benralizumab
| Patients | |
|---|---|
| Mean age ± SD, year | 53.8 ± 13.9 |
| Female, n (%) | 109 (68.1) |
| Mean body weight ± SD, Kg | 71.7 ± 15.9 |
| Mean height ± SD, cm | 163.7 ± 9.4 |
| Mean body mass index ± SD, Kg/m2 | 26.6 ± 5.1 |
| Mean age asthma diagnosis ± SD, year | 33.5 ± 14.9 |
| Mean disease length ± SD, year | 20.3 ± 14.3 |
| Atopy (SPT‐positive for perennial/seasonal allergens), n (%) | 96 (60) |
| Relatives with asthma, n (%) | 69 (43.1) |
| Relatives with atopy, n (%) | 76 (47.5) |
| Smoking history | |
| ‐ Current smoker, n (%) | 11 (6.9) |
| ‐ Ex smokers, n (%) | 30 (18.7) |
| ‐ Never smoker, n (%) | 119 (74.4) |
| Peripheral blood eosinophil count (cells/μl), median (IQR) | 690 (460, 975) |
| Total serum IgE level (kU/L), median (IQR) | 134 (57, 368) |
| FeNO 50 (ppb), median (IQR) | 45 (24.7, 62.2) |
| Pulmonary function tests | |
| ‐ Mean FVC ± SD, L | 2.75 ± 1 |
| ‐ Mean FVC ± SD, % predicted | 81.1 ± 20.4 |
| ‐ Mean pre‐bronchodilator FEV1 ± SD, L | 1.83 ± 0.8 |
| ‐ Mean pre‐bronchodilator FEV1 ± SD, % predicted | 65.6 ± 21.1 |
| ‐ Mean reversibility in FEV1, % (range) | 12 (0 to 54) |
| [‐ Patients with reversibility, n (%)] | 84 (52.5) |
| ‐ Mean FEV1/FVC ratio ± SD, % | 66 ± 13.2 |
| ‐ Mean FEF 25–75 ± SD, % predicted | 41.6 ± 23.4 |
| Exacerbations in year prior to benralizumab, n (mean ± SD) | 796 (5 ± 3.4) |
| ‐ emergency room admission, n (%) | 73 (9.2) |
| ‐ hospitalization, n (%) | 36 (4.5) |
| ‐ Intensive care unit, n (%) | 0 (0) |
| Drug use, number of patients | |
| ‐ ICS/LABA, n (%) | 160 (100) |
| ‐ LAMA, n (%) | 111 (69.4) |
| ‐ OCS (steroid dependent), n (%) | 95 (59.4) |
| [‐ Prednisone eq. median dose in the previous 6 months (IQR ) , mg] | 5 (0, 12.5) |
| ‐ Leukotriene receptor antagonist, n (%) | 76 (47.5) |
| ‐ Azithromycin, n (%) | 21 (13.1) |
| ‐ Theophylline, n (%) | 6 (3.7) |
| Comorbidities, number of patients | |
| ‐ Nasal polyposis, n (%) | 79 (49.4%) |
| ‐ EGPA, n (%) | 12 (7.5) |
| ‐ Eosinophilic pneumonia, n (%) | 5 (3.1) |
| ‐ Atopic dermatitis, n (%) | 17 (10.6) |
| ‐ Bronchiectasis, n (%) | 35 (21.9) |
| ‐ Gastroesophageal reflux disease, n (%) | 75 (46.9) |
| ‐ Osteoporosis, n (%) | 39 (24.4) |
| ‐ Anxiety, n (%) | 24 (15) |
| ‐ ASA sensibility, n (%) | 20 (12.5) |
| ‐ OSAS, n (%) | 13 (8.1) |
| ‐ Urticaria, n (%) | 8 (5) |
| ‐ Depression, n (%) | 7 (4.4) |
| Previous monoclonal antibody treatment, n (%) | 8 (5) |
| ‐ omalizumab, n (%) | 5 (3.1) |
| ‐ mepolizumab, n (%) | 5 (3.1) |
| SNOT 22, mean ± SD | 49.4 ± 19.7 |
| ACT score at baseline, mean ± SD | 13.6 ± 4.1 |
| ‐ Patients with ACT ≥20, n (%) | 11 (6.9) |
| AQLQ score at baseline, mean ± SD | 3.4 ± 1.1 |
Abbreviations: ACT, Asthma control test; AQLQ, Asthma quality of life questionnaire; ASA, Acetylsalicylic acid; EGPA, Eosinophilic granulomatosis with polyangiitis; FEF 25–75, Forced expiratory flow at 25–75% of FVC; FeNO, Fractional exhaled nitric oxide; FEV1, Forced expiratory volume in 1 s; FVC, Forced vital capacity; ICS, Inhaled corticosteroids; IQR, Interquartile range; LABA, Long‐acting beta2‐adrenergic agonist; LAMA, Long‐acting muscarinic antagonist; OCS, Oral corticosteroids; OSAS, Obstructive Sleep Apnoea Syndrome; PEF, Peak expiratory flow; SD, Standard deviation; SNOT‐22, Sino‐nasal outcome test; SPT, Skin prick test.
After 6 months of treatment, overall disease control was significantly improved, with reduction in exacerbations and OCS use with a parallel improvement in lung function, ACT and AQLQ scores (Table 2), as well as a reduction in FeNO concentration and eosinophil count (Table 2). The benefit observed after 6 months remained stable for the following months (T12) (Table 2).
TABLE 2.
Outcomes after 6 and 12 months of treatment
| Baseline | T6 | T12 | p | |
|---|---|---|---|---|
| Exacerbations, mean ± SD | 5.0 ± 3.4 | 0.3 ± 0.7 | 0.2 ± 0.5 | <.01 |
| Patients (n) | 160 | 134 | 49 | |
| OCS (prednisone eq.), mg/day, Median(IQR) | 5.0 (0.0–12.5) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | <.01 |
| Patients on OCS (n)* | 95/160 | 24/126 | 4/47 | |
| Pre‐bronchodilator FEV1 | ||||
| ‐ L, mean ± SD | 1.83 ± 0.8 | 2.3 ± 0.8 | 2.25 ± 0.7 | <.01 |
| ‐ %, mean ± SD | 65.6 ± 21.1 | 79.3 ± 21.5 | 76.3 ± 17.8 | |
| Patients (n) | 160 | 103 | 44 | |
| ACT, mean ± SD | 13.6 ± 4.1 | 20.8 ± 4.1 | 21.6 ± 3.3 | <.01 |
| Patients (n) | 160 | 118 | 49 | |
| AQLQ, mean ± SD | 3.4 ± 1.1 | 5.6 ± 1.1 | 5.9 ± 1.0 | <.01 |
| Patients (n) | 160 | 82 | 35 | |
| SNOT 22, mean ± SD | 49.4 ± 19.7 | 34.2 ± 20.5 | 23.5 ± 16.9 | NS |
| Patients (n) | 70 | 63 | 8 | |
| FeNO (ppb), median (IQR) | 45 (24.5, 62.5) | 25.5 (15.0, 41.0) | 25.0 (18.0, 65.0) | <.01 |
| Patients (n) | 80 | 46 | 30 | |
| Eosinophils (cells/μl), median (IQR) | 0.69 (0.46, 0.97) | 0 (0, 0) | 0 (0, 0) | <.01 |
| Patients (n) | 160 | 115 | 44 | |
Abbreviations: ACT, Asthma control test; AQLQ, Asthma quality of life questionnaire; denominator: patients observed at each specific time‐point; FeNO, Fractional exhaled nitric oxide. To compare the 3 measurements in T0, T6 and T12, the Friedman test was used. *numerator: patients for whom the data are available; FEV1, Forced expiratory volume in 1 second; IQR, Interquartile range; OCS, Oral corticosteroids; SD, Standard deviation; SNOT‐22, Sino‐nasal outcome test.
At T6, 111 out of 126 patients (88.1%) were responders, defined as having ≥50% reduction in ER and ≥50% reduction in mean daily OCS dose. As for superResponders, 84 (65.1%) patients remained completely exacerbation free at T6, without requiring mOCS (ER0/OCS0).
Thus, despite a general good response to benralizumab, about one‐third of patients had a suboptimal response, as they did not eliminate either exacerbations or mOCS (non‐SuperResponders).
We, thus, tried to identify sub‐phenotypes of patients with differential responsiveness to benralizumab.
In order to explore/investigate patients’ characteristics in depth, a hierarchical cluster analysis was carried out using a set of variables that can be easily collected in routine practice. They included age, age at disease onset, disease length, family history of atopy, allergen sensitization (Skin Prick Test positive/negative), blood eosinophil count, IgE levels, FEV1% predicted, nasal polyposis, bronchiectasis, number of exacerbations in the previous year. Moreover, a k‐means clustering was carried out and the four clusters detected through the hierarchical cluster approach was confirmed as optimal (Figure S1).
Clustering the 105 available patients at T6 with our hierarchical algorithm (21 out of 126 patients were excluded because of ≥10% of missing data in at least one of the variables used for clustering) revealed four distinct asthma clusters (Table 3). The 12 months time‐point could not be used because the number of patients observed was too small (n = 49).
TABLE 3.
Baseline characteristics of the clusters
| Cluster | 1 (n = 23) | 2 (n = 19) | 3 (n = 35) | 4 (n = 28) | p |
|---|---|---|---|---|---|
| Female, (%) | 73.9 | 68.4 | 60.0 | 67.9 | N.S. |
| Mean age ± SD, year | 57.6 ± 9.9 | 34.9 ± 8.5 | 55.1 ± 12.2 | 62 ± 8.7 | <.01 |
| Mean age at onset ± SD, year | 46.3 ± 8.4 | 23.8 ± 10 | 26.9 ± 10.3 | 44.9 ± 11.9 | <.01 |
| Mean disease length ± SD, year | 11.3 ± 6.8 | 11.1 ± 9.6 | 28.3 ± 11.5 | 17 ± 9.8 | <.01 |
| Mean body mass index ± SD, Kg/m2 | 26.8 ± 5.6 | 28.3 ± 6.8 | 26.3 ± 4.2 | 26.3 ± 5.1 | N.S. |
| Relatives with atopy, n (%) | 43.5 | 47.4 | 85.7 | 25.0 | <.05 |
| SPT‐positive (perennial/seasonal allergens) | 100 | 78.9 | 97.1 | 7.1 | <.05 |
| Smoking history | N.S. | ||||
| ‐ Current smoker, n (%) | 8.7 | 0 | 2.9 | 0 | |
| ‐ Ex smokers, n (%) | 21.7 | 21.1 | 25.7 | 25 | |
| ‐ Never smoker, n (%) | 69.6 | 78.9 | 71.4 | 75.0 | |
| Peripheral blood eosinophil count (cells/μl), median (IQR) | 470 (390, 788) | 990 (830, 2100) | 700 (540, 900) | 745 (700, 840) | <.05 |
| Median FeNO 50, ppb ± IQR | 43.6 (35, 55) | 55 (35.4, 78) | 33.5 (14, 59) | 47.5 (42, 68) | N.S. |
| Total serum IgE level (kU/L), median (IQR) | 181 (93, 359) | 225 (131, 990) | 231 (185, 457) | 68 (50, 85) | <.01 |
| Mean pre‐bronchodilator FEV1% predicted ± SD | 55.7 ± 17.9 | 79.3 ± 17.8 | 54.1 ± 20 | 62.2 ± 15.5 | <.01 |
| Mean exacerbation number in the year prior to benralizumab, ± SD | 4.0 ± 2.1 | 3.6 ± 1.5 | 8.4 ± 3.9 | 4.1 ± 2.6 | <.01 |
| Drug use in the 6 months prior to benralizumab, | |||||
| ‐ LAMA, patients % | 60.9 | 47.4 | 82.9 | 82.1 | N.S. |
| ‐ OCS, patients % | 43.5 | 52.6 | 71.4 | 64.3 | N.S. |
| ‐ Mean prednisone eq. dose ± SD | 5.9 ± 8.8 | 5.5 ± 6.9 | 11.1 ± 10.4 | 7.21 ± 9.1 | N.S. |
| ‐ Azithromycin, % | 13 | 0 | 22.9 | 21.4 | <.05 |
| Previous allergen immunotherapy – AIT, patients (%) | 30.4 | 15.8 | 20 | 17.9 | N.S. |
| Comorbidities, number of patients | |||||
| ‐ Nasal polyposis (%) | 34.8 | 47.4 | 65.7 | 60.7 | N.S. |
| ‐ Bronchiectasis (%) | 17.4 | 21.1 | 42.9 | 17.9 | N.S. |
| SNOT 22, mean ± SD | 44.3 ± 18.6 | 50.9 ± 20.7 | 55.1 ± 21.8 | 42.38 ± 17.6 | N.S. |
| ACT score at baseline, mean ± SD | 12.8 ± 4.9 | 14.3 ± 4.7 | 12.8 ± 3.9 | 13.8 ± 3.4 | N.S. |
| AQLQ score at baseline, mean ± SD | 3.6 ± 0.9 | 3.8 ± 0.7 | 3.3 ± 1.2 | 3.7 ± 1.2 | N.S. |
Abbreviations: ACT, Asthma control test; AQLQ, Asthma quality of life questionnaire; FeNO, Fractional exhaled nitric oxide; FEV1, Forced expiratory volume in 1 s; FVC, Forced vital capacity; IQR, Interquartile range; LAMA, Long‐acting muscarinic antagonist; OCS, Oral corticosteroids;SD, Standard deviation; SNOT‐22, Sino‐nasal outcome test; SPT, Skin prick test.
Cluster 1 (less eosinophilic, allergic [eosinophils ↓, IgE ↑], older, low nasal polyps frequency, impaired lung function) included 23 patients characterized by the high percentage of allergic sensitization (SPT‐positive for both perennial and/or seasonal allergens, 100%) who underwent allergen immunotherapy (AIT) (30.4%) and the lowest peripheral median eosinophil count (470 cells/μl). This cluster included patients with the oldest disease onset (46.3 ± 8.4 years).
Cluster 2 (type‐2 biomarkers high [eosinophils ↑, IgE ↑], young, preserved lung function), including 19 patients, was characterized by the youngest age of patients under treatment and the youngest patients’ age at disease onset. Furthermore, patients in Cluster 2 showed the highest level of type‐2 biomarkers, such as peripheral blood eosinophil count, FeNO and IgE levels. The lack of statistical significance in FeNO levels between the groups might be due to the high number of missing observations for this variable. Patients in this subgroup featured also less functional impairment (FEV1% predicted, 79.3%) and were never treated with azithromycin. 22
Cluster 3 (type‐2 biomarkers high [eosinophils ↑, IgE ↑], older, exacerbating, nasal polyps, bronchiectasis, impaired lung function), including 35 patients, was characterized by the longest mean disease duration, the highest mean exacerbation number in the year prior to benralizumab (8.4 ± 3.9) and the highest frequency of nasal polyps and bronchiectasis (these latter features were not statistically different between the clusters).
Cluster 4 (eosinophilic, non‐allergic [eosinophils ↑, IgE ↓], older, nasal polyps, impaired lung function) included 28 patients, mainly non‐allergic (SPT‐positive, 7.1%) and without a family history of atopy (25%) with the lowest total IgE levels (median = 68 kU/L) and old age at disease onset (44.9 ± 11.9 years).
These four clusters showed differential benralizumab responsiveness after 6 months of treatment. (Figure 1). Cluster 2 (n = 19) and Cluster 3 (n = 35) included the greater percentage of patients who remained exacerbation free after benralizumab (ER0) (Figure 1,A). Furthermore, Cluster 2 showed overall the strongest effect of benralizumab with the highest rate of super‐response according also to all the 3 remaining criteria (ER0/OCS0; ER0/OCS0/ACT≥20; ER0/OCS0/ACT≥20/FEV1≥80%) (Figure 1A,B,C,D).
FIGURE 1.
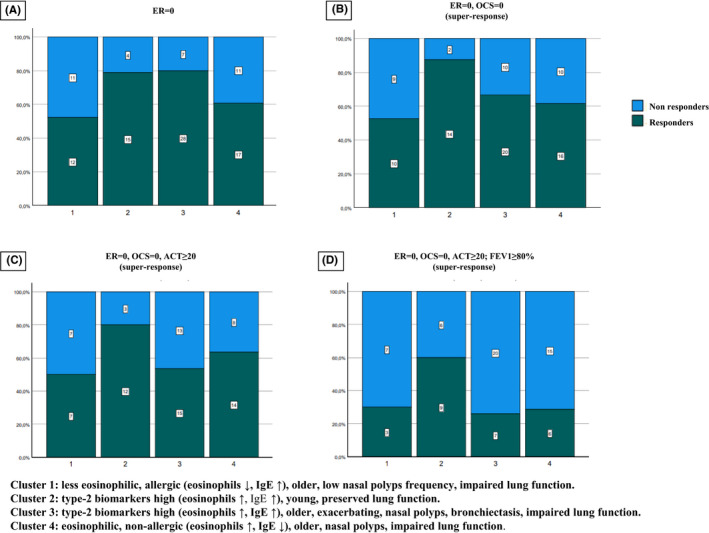
Benralizumab responsiveness of the clusters. The y‐axis reports the percentage of patients who responded and who did not respond according to the four criteria used: A, Elimination of exacerbations (ER = 0); B, elimination of exacerbation and OCS (ER = 0, OCS = 0); C, elimination of exacerbation and OCS, with ACT score ≥20 (ER = 0, OCS = 0, ACT≥20); D, elimination of exacerbation and OCS, with ACT score ≥20 and FEV1≥80% (ER = 0, OCS = 0, ACT≥20, FEV1≥80). The number of patients for each subgroup (responders/non‐responders) are reported within the columns
Ten patients out of the 105 patients included in the cluster analysis were also affected by EGPA or eosinophilic pneumonia. In order to assess benralizumab responsiveness in a purer SEA population, a further analysis after the exclusion of these patients was performed. As shown in Figure S2, the rate of superResponders ER0/OCS0 in Cluster 3 increased from 66% to 74%.
Baseline variables associated with super‐response (ER0, OCS0) at T6 were also explored comparing superResponders and non‐superResponders, regardless of their cluster distribution. The chance of gaining a super‐response (ER0, OCS0) was significantly associated with a lower rate of OCS dependency, lower leukotriene receptor antagonists (LTRA) use and the presence of nasal polyposis (Table 4). However, multivariate logistic regression analysis confirmed only nasal polyposis, as independent predictor of super‐response. Older age, younger disease onset and the absence of azithromycin treatment were also identified as independently associated with the chance of being a superResponders (ER0/OCS0) (Table 5). Thus, three out of the four independent predictors identified by logistic regression analysis, namely age, age at disease onset and azithromycin use, were also differently expressed in the clusters. This indirectly confirms the validity of the clustering process. FeNO was not entered in the logistic regression model due to the high number of missing data.
TABLE 4.
Baseline characteristics of superResponders (no OCS use and no exacerbations during the first 6 months after benralizumab; ER0 = /OCS = 0) and non‐superResponders after 6 months of benralizumab treatment
| Non‐Super Responders (N = 45) | superResponders (N = 84) | p | |
|---|---|---|---|
| Mean age ± SD, year | 54.2 ± 13.6 | 53.0± 14.0 | NS |
| Female, n (%) | 28 (62.2) | 49 (70.2) | NS |
| Mean body weight ± SD, Kg | 73.6 ± 17.6 | 70.7 ± 15.1 | NS |
| Mean height ± SD, cm | 163.6 ± 10.0 | 164.3 ± 9.4 | NS |
| Mean body mass index ± SD, Kg/m2 | 27.1 ± 4.8 | 25.9 ± 5.6 | NS |
| Mean age at onset ± SD, year | 37.7 ± 13.7 | 34.1 ± 14.2 | NS |
| Mean disease length ± SD, year | 16.3 ± 10.1 | 19.0 ± 12.1 | NS |
| Atopy n (%) | 19 (42.2) | 42 (50.0) | NS |
| Smoking history | |||
| ‐ Current smoker, n (%) | 2(4.4) | 2(2.4) | NS |
| ‐ Ex smokers, n (%) | 13 (28.9) | 14 (16.7) | |
| ‐ Never smoker, n (%) | 30 (66.7) | 68 (81.0) | |
| Median Peripheral blood eosinophil count, cells/μl (IQR) | 680 (470, 920) | 747 (495, 1052) | NS |
| Median total serum IgE level, kU/L (IQR) | 114.0 (41–404) | 132.5 (66–472.5) | NS |
| ‐ Mean pre‐bronchodilator FEV1 ± SD, L | 1.7 ± 0.7 | 1.9 ± 0.8 | NS |
| ‐ Mean pre‐bronchodilatorFEV1 ± SD, %predicted | 60.8 ± 20.7 | 60.9 ± 20.6 | NS |
| ‐ Median FeNO 50, ppb (IQR) | 47.3 (23–59) | 49.0; (35–78) | NS |
| Exacerbation rate in year prior to benralizumab, n (mean ± SD) | 5.1 ± 4.2 | 5.0 ± 2.9 | NS |
| Drug use, number of patients | |||
| ‐ LAMA, n (%) | 36(80.0) | 58(69.0) | NS |
| ‐ OCS, n (%) | 33 (73.3) | 50 (59.5) | . 01 |
| ‐ Prednisone eq. dose in the previous 6 months, median(IQR) | 5 (0, 13) | 5 (0, 13) | NS |
| ‐ Leukotriene receptor antagonist, n (%) | 26 (57.8) | 39 (46.4) | . 005 |
| ‐ Azithromycin, n (%) | 13 (28.9) | 8 (9.5) | NS |
| ‐ Salbutamol daily puffs in the month before benralizumab (mean ± SD) | 2.7 ± 2.5 | 2.2 ± 2 | NS |
| ‐Immunotherapy, n (%) | 7 (15.6) | 24 (28.6) | NS |
| Comorbidities, number of patients | |||
| ‐ Nasal polyposis, n (%) | 18 (42.9) | 50 (64.9) | . 020 |
| ‐ Bronchiectasis, n (%) | 13 (28.9) | 16 (19.0) | NS |
| SNOT 22, mean ± SD | 49.4 ± 25 | 50.3 ± 16.9 | NS |
| ACT score at baseline, mean ± SD | 13.1 ± 4.5 | 13.8 ± 3.9 | NS |
| AQLQ score at baseline, mean ± SD | 3.5 ± 0.8 | 3.2 ± 1.3 | NS |
Abbreviations: ACT, Asthma control test; AQLQ, Asthma quality of life questionnaire; FeNO, Fractional exhaled nitric oxide; FEV1, Forced expiratory volume in 1 second; FVC, Forced vital capacity; IQR, Interquartile range; LAMA, Long‐acting muscarinic antagonist; OCS, Oral corticosteroids;SD, Standard deviation; SNOT‐22, Sino‐nasal outcome test; SPT, Skin prick test.
TABLE 5.
Logistic regression predicting chance of super‐response (ER0/OCS0: no OCS use and no exacerbations during the first 6 months after benralizumab)
| Variable | Code | β | Adj. OR | 95%CI | p‐value |
|---|---|---|---|---|---|
| Patient's age | 0.039 | 1.04 | 1.01; 1.07 | .001 | |
| Age at disease onset | −0.44 | 0.96 | 0.92; 0.99 | .049 | |
| CRwNP | 0: No (ref.)/1: Yes | 0.858 | 2.36 | 1.07, 5.21 | .034 |
| Azithromycin use | 0: No (ref.)/1: Yes | −1.818 | 0.16 | 0.05; 0.52 | .002 |
Abbreviation: CRwNP, Chronic rhino‐sinusitis with nasal polyps.
4. DISCUSSION
To investigate differential benralizumab responses among patients with severe eosinophilic asthma and the various factors that contribute to them, we performed a cluster analysis based on a set of clinical variables and inflammatory biomarkers that can be easily collected in the routine practice. This approach identified four distinct clusters within adult‐onset SEA patients with variable benralizumab responsiveness.
Patients with SEA experience frequent exacerbations. These patients often depend on the chronic use of OCS or use OCS during exacerbations, with increased risk of OCS‐related adverse events. Therefore, especially in real‐life settings, when assessing responsiveness to biologics, the elimination of OCS should be considered as a critical outcome. Thus, using a response definition as either 50% reduction in exacerbations (with consequent reduction of OCS) or 50% reduction in mOCS dose for OCS‐dependent patients might be not completely adequate. According to this definition of response we observed an 88.1% response rate in our cohort, showing that benralizumab is highly effective. However, this percentage decreased to 65,1% when responders were considered as those patients who eliminated both exacerbations and OCS use (superResponders, ER0/OCS0). Thus, about one‐third of patients had a suboptimal response, despite what appeared to be an appropriate eosinophilic phenotype, with a median peripheral eosinophil count of 690 cell/μl (IQR, 460; 975) in the year prior to benralizumab.
Our findings showed that distinct characteristics related both to the level of inflammation (showed by the combination of two inflammatory biomarkers, namely eosinophil count and IgE levels) and to disease history and comorbidities may contribute to differential benralizumab responsiveness observed in the four clusters.
Clusters 2 and 3 showed the highest response rate (compared to Clusters 1 and 4) when response was assessed as elimination of exacerbations (ER0) (Figure 1,A). These clusters included patients with high levels of both peripheral blood eosinophils and IgE (Table 3). In contrast, Clusters 1 and 4, characterized by only one type‐2 biomarker high (IgE only for Cluster 1, and eosinophils only for Cluster 4) showed a lower ER0 response rate (Figure 1A). Notably, ER0 response rate was comparable between Clusters 2 and 3, (around 80%) despite Cluster 2 containing subjects with fewer exacerbations (3.6 ± 1.5 exacerbations/patient), while Cluster 3 contained patients with the most exacerbations in the previous year (8.4 ± 1.5 exacerbations/patient) (Table 1). Collectively these data may suggest that elimination of exacerbations is correlated with a high level of type‐2 inflammation, which can be assessed as the presence of two biomarkers (namely eosinophils and IgE) at high level (Clusters 2 and 3). The pooled analysis of the SIROCCO and CALIMA studies showed a correlation between enhanced benralizumab efficacy in exacerbation reduction and increased peripheral eosinophil count, as a biomarker of type‐2 inflammation. 8 However, our data show that increased eosinophil levels alone are not sufficient to identify highly ER0 responsive sub‐phenotypes. In fact, Cluster 4, characterized by high eosinophil count (but low IgE level), showed a lower ER0 response rate. Univariate analysis and logistic regression analysis seem to support this consideration, as eosinophil counts were not independently associated with a super‐response (Table 4 and Table 5). Thus, a combination of type‐2 biomarkers (eosinophils and IgE) seems more sensitive in identifying subjects with higher chance of exacerbation reduction.
Cluster 2 showed overall the strongest effect of benralizumab. In fact, Cluster 2 was also the most responsive cluster when super‐response was assessed, using the three pre‐specified super‐response criteria (ER0/OCS0; ER0/OCS0/ACT≥20; ER0/OCS0/ACT≥20/FEV1≥80%) (Figure 1B,C,D).
However, when a pure asthmatic population was analysed, after the exclusion of patients with EGPA and eosinophilic pneumonia, the ER0/OCS0 super‐response rate in Cluster 3 was increased, (it did not reach Cluster 2 super‐response rate, but it was higher than the super‐response rate of Clusters 1 and 4), confirming that patients with both type‐2 biomarkers high tend to have a better response, despite their different severity at baseline (Figure S2).
Notably, although lung function of Cluster 2 patients was more preserved (therefore, this might have been considered a less severe group at baseline), exacerbation number, OCS use, ACT, AQLQ and SNOT‐22 were comparable to that of Clusters 1 and 4. Thus, patients in Cluster 2 showed a clinical picture similar to that of Clusters 1 and 4 (comparable severity).
The different super‐response rate between Clusters 2 and 3 (Figure 1B,C,D), despite their comparable ER0 response rate (Figure 1A) deserves some considerations.
Although Clusters 2 and 3 are both characterized by high type‐2 inflammation (high levels of eosinophils and IgE) and comparable age at onset, the higher frequency of nasal polyps and bronchiectasis in Cluster 3 might identify a different sub‐phenotype. Furthermore, patients in Cluster 2 never used azithromycin, whereas Cluster 3 featured the highest proportion of patients who had been treated with azithromycin, probably because of their highest exacerbation rate. 22 Finally, the lower lung function of subjects in Cluster 3 could be the result of a longer disease with repeated exacerbations, since patients in Cluster 3 have a longer disease duration than those in Cluster 2 (Table 3). Thus, besides the high level of type‐2 biomarkers, clinical features and disease duration or age at onset may also contribute to identify bernalizumab super‐responsiveness sub‐phenotypes. In other words, it is possible that Clusters 2 and 3 included similar patient populations (as suggested by their comparable levels of inflammatory markers) that received treatment at difference phases of their disease, as Cluster 3 included patients with longer disease duration (who commenced treatment at older age) (Table 3). The importance of age at onset was confirmed by logistic regression analysis (Table 5). In conclusion, older age at onset and the presence of only one inflammatory biomarker at high level (either blood eosinophil or IgE) are characteristics shared by the two lower response Clusters 1 and 4, according to all four response criteria. In contrast, younger age at onset and high levels of the two inflammatory biomarkers characterize the highest ER0 response rate of Clusters 2 and 3 (Table 3; Figure 1A).
Data from RCTs and meta‐analyses on biologics in severe asthma showed that there is limited evidence for improvement in health‐related quality‐of‐life scores (e.g. ACT, AQLQ) and lung function, which may not meet clinically detectable levels. 23 We confirm in this series that the proportion of patients achieving a clinically meaningful symptom reduction (ACT) and improvement of lung function (FEV1%) is generally small (Figure 1C,D). However, a high proportion of patients with a clinically meaningful improvement of ACT and FEV1 was shown in Cluster 2. Notably, ACT at baseline of Cluster 2 patients was comparable to that of patients included in the other clusters.
4.1. Limitations and strengths
Despite the relatively small patient number for a cluster analysis, the hierarchical clustering method used appeared to be sensitive in identifying clusters with differential benralizumab responsiveness. In particular, Cluster 2 seems to be well differentiated from the other clusters, as response is always the highest according to all the four criteria used.
This analysis has the inherent limitations of all the retrospective studies. In particular, the high number of missing data prevented the use of some variables, namely FeNO, ACT or AQLQ, in the generation of clusters. 24 , 25 This might have influenced the composition of the cluster themselves. Validation/replication of our data in different patient populations will be useful to confirm and expand our findings, particularly for importance of FeNO as an inflammatory biomarker. 26 , 27 , 28
Our data confirmed that IgE alone are not predictive of response to bernalizumab. However, high levels of IgE seem to be predictive of response if associated with high levels of eosinophils.
We speculated that the association of different type‐2 biomarkers (such as IgE, eosinophils, FeNO) might identify more responsive subgroups. In this light, an important limitation of this study is that FeNO was not available for the analysis owing to numerous missing data. Notably, Cluster 2, the most responsive one, besides high levels of IgE and eosinophils, showed also the highest FeNO levels, but unfortunately this biomarker could not be considered in our analysis.
Patients started treatment with benralizumab if they complained of persistent respiratory symptoms despite regular treatment with high dosages of ICS/LABA, with or without LRTA or LAMA or OCS. However, since this is a real‐life study, no measure of drug compliance before entering the study was carried out.
We are confident that patients’ data collection was quite accurate, as all patients were examined regularly in the outpatient clinic of the centres involved in the study. However, as for all the real‐life studies, it is possible that a limited amount of information was missed since some patients might have accessed different healthcare settings during their exacerbations.
The 6 months evaluation time‐point could be considered not fully adequate to assess exacerbation rate and other outcomes. However, the effects of benralizumab remained stable in the following months (T12) (Table 2), suggesting that results observed after 6 months of benralizumab are representative of later time‐points.
Considering the general prevalence of depression in asthmatic patients (11%–13%), it is possible that depression was underestimated in this series (4%). 29 , 30 It could be related to some drawbacks of the Beck Depression Inventory (BDI), used for diagnosis of depression in this real‐life series (e.g. the BDI has no safeguards against faking, lying or variable response sets; the BDI is extremely sensitive to differences in the instructions given to an examinee). 31
Although we used univariate and logistic regression analyses to indirectly confirm cluster analysis results, the discrepancies reported are not surprising, given that these analyses compare different subgroups. For example, we did not find statistically significant differences in nasal polyps frequency between the clusters, whereas, logistic regression analysis identified nasal polyposis as an independent predictor of super‐response (ER0/OCS0), as expected. 9 This means that the proportion of patients with nasal polyps is greater among responders than non‐responders within each cluster, but the difference in nasal polyp frequency among the clusters is not statistically significant. Therefore, this variable, although useful as an independent predictor of response, cannot be used to differentiate clusters from each other.
In summary, we identified four differential benralizumab responsiveness clusters in severe eosinophilic asthma, according to inflammatory biomarker expression and clinical variables. These clusters might reflect differing inflammatory pathways, but this needs to be shown definitively.
Given the multifactorial nature of clinical responses, machine learning approaches would be preferable to classic analysis methods. The use of federated databases or registries would be desirable in order to obtain the large amount of data necessary to enable such approaches. 32
CONFLICTS OF INTEREST
We declare that we have no conflicts of interest.
COLLABORATORS
Aikaterini Detoraki (Allergology and Immunology Unit, University "Federico II" of Naples, Naples, Italy). Carmen Durante (Department of Biomedicine and Internal and Specialistic Medicine, University of Palermo, Palermo, Italy). Maria Pia Foschino Barbaro (Department of Medical and Surgical Sciences, Institute of Respiratory Diseases, University of Foggia, Foggia, Italy). Santi Nolasco (Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy). Pietro Impellizzeri (Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy). Angelantonio Maglio (Department of Medicine, Surgery and Dentistry, University of Salerno, Salerno, Italy). Eustachio Nettis (Department of Emergency and Organ Transplantation, School and Chair of Allergology and Clinical Immunology, University of Bari ‐ Aldo Moro, Bari, Italy). Stefania Principe (Department of Biomedicine and Internal and Specialistic Medicine, University of Palermo, Palermo, Italy). Carla Maria Irene Quarato (Department of Medical and Surgical Sciences, Institute of Respiratory Diseases, University of Foggia, Foggia, Italy). Andrea Tanga (Department of Economics and Finance, University of Bari ‐ Aldo Moro, Bari, Italy). Massimo Triggiani (Department of Medicine, Surgery and Dentistry, University of Salerno, Salerno, Italy). Carolina Vitale (Department of Medicine, Surgery and Dentistry, University of Salerno, Salerno, Italy).
Supporting information
Fig S1
Fig S2
Supplementary Material
ACKNOWLEDGEMENTS
DDB developed the concept of this study. CC, CR, CP, AB, MFC, CCa, GEC, DC, MD, GP, SP, NS, GS, NR, GP GV, AV, NC contributed to data collection. AD and NR did the statistical analyses. The first draft of the manuscript was written by DDB and thoroughly revised by LM. All authors approved the final version of the manuscript. Open Access Funding provided by Universita degli Studi di Bari Aldo Moro within the CRUI‐CARE Agreement. [Correction added on 17 May 2022, after first online publication: CRUI funding statement has been added.]
Di Bona D, Crimi C, D’Uggento AM, et al. Effectiveness of benralizumab in severe eosinophilic asthma: Distinct sub‐phenotypes of response identified by cluster analysis. Clin Exp Allergy. 2022;52:312–323. 10.1111/cea.14026
Linked article: Maniscalco M. Clin Exp Allergy 2022; 52:359‐360.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bleecker ER, FitzGerald JM, Chanez P, et al. SIROCCO study investigators. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high‐dosage inhaled corticosteroids and long‐acting β2‐agonists (SIROCCO): a randomised, multicentre, placebo‐controlled phase 3 trial. Lancet. 2016;388(10056):2115‐2127. [DOI] [PubMed] [Google Scholar]
- 2. FitzGerald JM, Bleecker ER, Nair P, et al. CALIMA study investigators. Benralizumab, an anti‐interleukin‐5 receptor α monoclonal antibody, as add‐on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2016;388(10056):2128‐2141. [DOI] [PubMed] [Google Scholar]
- 3. Nair P, Wenzel S, Rabe KF, et al. ZONDA Trial Investigators. oral glucocorticoid‐sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448‐2458. [DOI] [PubMed] [Google Scholar]
- 4. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add‐on therapy on health‐related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double‐blind, placebo controlled, parallel‐group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390‐400. [DOI] [PubMed] [Google Scholar]
- 5. Bel EH, Ten Brinke A. New anti‐eosinophil drugs for asthma and COPD: targeting the trait! Chest. 2017;152(6):1276‐1282. [DOI] [PubMed] [Google Scholar]
- 6. Bel EH. Clinical phenotypes of asthma. Curr Opin Pulm Med. 2004;10(1):44‐50. [DOI] [PubMed] [Google Scholar]
- 7. Kroes JA, Zielhuis SW, van Roon EN, Ten Brinke A. Prediction of response to biological treatment with monoclonal antibodies in severe asthma. Biochem Pharmacol. 2020;179:113978. [DOI] [PubMed] [Google Scholar]
- 8. FitzGerald JM, Bleecker ER, Menzies‐Gow A, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med. 2018;6(1):51‐64. [DOI] [PubMed] [Google Scholar]
- 9. Bleecker ER, Wechsler ME, FitzGerald JM, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. 2018;52(4):1800936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Price D, Bateman ED, Chisholm A, et al. Complementing the randomized controlled trial evidence base. evolution not revolution. Ann Am Thorac Soc. 2014;11(Suppl. 2):S92‐S98. [DOI] [PubMed] [Google Scholar]
- 11. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe osinophilic asthma. N Engl J Med. 2014;371(13):1198‐1207. [DOI] [PubMed] [Google Scholar]
- 12. Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid‐dependent severe asthma. N Engl J Med. 2018;378(26):2475‐2485. [DOI] [PubMed] [Google Scholar]
- 13. Brown T, Jones T, Gove K, et al. Randomised controlled trials in severe asthma: selection by phenotype or stereotype. Eur Respir J. 2018;52(6):1801444. [DOI] [PubMed] [Google Scholar]
- 14. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation, and treatment of severe asthma [published correction appears in. Eur Respir J. 2014;43(4):1216. Dosage error in article text] [published correction appears in Eur Respir J. 2018 Jul 27;52(1):]. Eur Respir J. 2014;43(2):343‐373. [DOI] [PubMed] [Google Scholar]
- 15. Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention, 2020. Available from: www.ginasthma.org [Google Scholar]
- 16. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59‐65. [DOI] [PubMed] [Google Scholar]
- 17. Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the mini asthma quality of life questionnaire. Eur Respir J. 1999;14(1):32‐38. [DOI] [PubMed] [Google Scholar]
- 18. Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22‐item sinonasal outcome test. Clin Otolaryngol. 2009;34(5):447‐454. [DOI] [PubMed] [Google Scholar]
- 19. Agache I, Song Y, Rocha C, et al. Efficacy and safety of treatment with dupilumab for severe asthma: a systematic review of the EAACI guidelines‐Recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1058‐1068. [DOI] [PubMed] [Google Scholar]
- 20. Santanello NC, Zhang J, Seidenberg B, Reiss TF, Barber BL. What are minimal important changes for asthma measures in a clinical trial? Eur Respir J. 1999;14:23‐27. [DOI] [PubMed] [Google Scholar]
- 21. Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553‐558. [DOI] [PubMed] [Google Scholar]
- 22. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2017;390(10095):659‐668. [DOI] [PubMed] [Google Scholar]
- 23. Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti‐IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9(9):CD010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pelaia C, Crimi C, Benfante A, et al. Therapeutic effects of benralizumab assessed in patients with severe eosinophilic asthma: real‐life evaluation correlated with allergic and non‐allergic phenotype expression. J Asthma Allergy. 2021;22(14):163‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Di Bona D, Minenna E, Albanesi M, Nettis E, Caiaffa MF, Macchia L. Benralizumab improves patient reported outcomes and functional parameters in difficult‐to‐treat patients with severe asthma: data from a real‐life cohort. Pulm Pharmacol Ther. 2020;64:101974. [DOI] [PubMed] [Google Scholar]
- 26. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486‐2496. [DOI] [PubMed] [Google Scholar]
- 27. Yang D, Huang T, Liu B, Du Z, Liu C. Dupilumab in patients with uncontrolled asthma: type 2 biomarkers might be predictors of therapeutic efficacy. J Asthma. 2020;57(1):79‐81. [DOI] [PubMed] [Google Scholar]
- 28. Heffler E, Terranova G, Chessari C, et al. Point‐of‐care blood eosinophil count in a severe asthma clinic setting. Ann Allergy Asthma Immunol. 2017;119(1):16‐20. [DOI] [PubMed] [Google Scholar]
- 29. Choi HG, Kim JH, Park JY, Hwang YI, Jang SH, Jung KS. Association between asthma and depression: a national cohort study. J Allergy Clin Immunol Pract. 2019;7:1239‐1245. [CrossRef]. [DOI] [PubMed] [Google Scholar]
- 30. Akula M, Kulikova A, Khan DA, Brown ES. The relationship between asthma and depression in a community‐based sample. J Asthma. 2018;55:1271‐1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Butcher JN, Taylor J, Fekken GC. Chapter 4.14. Objective personality assessment with adults. In: Bellack AS, Hersen M, eds. Comprehensive Clinical Psychology, Vol. 4. New York, NY: Elsevier Science Ltd; 2000:403‐429. [Google Scholar]
- 32. Papadopoulos NG, Barnes P, Canonica GW, et al. The evolving algorithm of biological selection in severe asthma. Allergy. 2020;75(7):1555‐1563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


