Abstract
Objective
To assess the survival, failure, and complication rates of veneered and monolithic all‐ceramic implant‐supported single crowns (SCs).
Methods
Literature search was conducted in Medline (PubMed), Embase, and Cochrane Central Register of Controlled Trials until September 2020 for randomized, prospective, and retrospective clinical trials with follow‐up time of at least 1 year, evaluating the outcome of veneered and/or monolithic all‐ceramic SCs supported by titanium dental implants. Survival and complication rates were analyzed using robust Poisson's regression models.
Results
Forty‐nine RCTs and prospective studies reporting on 57 material cohorts were included. Meta‐analysis of the included studies indicated an estimated 3‐year survival rate of veneered‐reinforced glass‐ceramic implant‐supported SCs of 97.6% (95% CI: 87.0%–99.6%). The estimated 3‐year survival rates were 97.0% (95% CI: 94.0%–98.5%) for monolithic‐reinforced glass‐ceramic implant SCs, 96.9% (95% CI: 93.4%–98.6%) for veneered densely sintered alumina SCs, 96.3% (95% CI: 93.9%–97.7%) for veneered zirconia SCs, 96.1% (95% CI: 93.4%–97.8%) for monolithic zirconia SCs and only 36.3% (95% CI: 0.04%–87.7%) for resin‐matrix‐ceramic (RMC) SCs. With the exception of RMC SCs (p < 0.0001), the differences in survival rates between the materials did not reach statistical significance. Veneered SCs showed significantly (p = 0.017) higher annual ceramic chipping rates (1.65%) compared with monolithic SCs (0.39%). The location of the SCs, anterior vs. posterior, did not influence survival and chipping rates.
Conclusions
With the exception of RMC SCs, veneered and monolithic implant‐supported ceramic SCs showed favorable short‐term survival and complication rates. Significantly higher rates for ceramic chipping, however, were reported for veneered compared with monolithic ceramic SCs.
Keywords: biological, complications, fixed dental prostheses, implant crown, meta‐analysis, monolithic, success, survival, systematic review, technical, veneered, zirconia framework
1. INTRODUCTION
Implant‐supported single crowns (SC) are a valid treatment option for the replacement of missing teeth with 5‐year survival rates of more than 90%, as reported in previous systematic reviews (Abou‐Ayash et al., 2017; Jung et al., 2012; Larsson & Wennerberg, 2014; Pjetursson et al., 2018; Rabel et al., 2018). These positive clinical results have resulted in the frequent use of implant‐supported SCs as an alternative to a tooth‐supported multiple‐unit fixed dental prostheses, an approach in line with today's pursuit of tooth conserving procedures.
A more recent factor that could influence the outcomes of the implant‐supported SCs is the material that the crowns are made out of. While in the past metal‐ceramics was predominantly used for the fabrication of the crowns, nowadays, a myriad of all‐ceramic or hybrid‐ceramic materials are available. Metal‐ceramic restorations have dominated the clinical applications and are well documented with high 5‐year SC survival rates of 95.8% (Jung et al., 2012). A systematic review by Jung and co‐workers (Jung et al., 2012) on the clinical outcomes of implant‐supported SCs reported a high 5‐year SC survival rate of 96.3%. From the included 46 studies, only 4 studies investigated all‐ceramic SCs, corresponding to only 10% of the reviewed patients. The studies reported satisfactory clinical outcomes for metal‐ceramic crowns and high mechanical stability.
The first introduced all‐ceramic materials (i.e., feldspathic ceramic, pressed leucite, and alumina‐reinforced glass‐ceramics) could not compete with the mechanical stability of metal‐ceramics in the high load bearing sites in the dental arch. Their indication was, therefore, limited to the esthetically high‐demanding areas in the beginning. The improvements in digital dental technologies offered alternative pathways to the conventional manufacturing processes, which facilitated the use of larger range of ceramic materials namely high‐strength oxide ceramic (zirconia [ZrO2] and alumina [Al2O3]), reinforced glass‐ceramics (lithium disilicate [LiS2] and zirconia‐reinforced lithium silicate [LiSi]), and resin‐matrix‐ceramics (RMC) (resin‐based composites and polymer‐infiltrated ceramic network [PICN]) (Spitznagel et al., 2018). As the digital workflows get more efficient and effective (Mühlemann et al., 2018), the indications for the all‐ceramic restorations were more and more widened.
High strength zirconia frameworks, veneered with ceramics for esthetic purposes (i.e., veneered zirconia), are a well‐documented all‐ceramic alternative to metal‐ceramics for implant‐supported restorations today Pjetursson et al., 2018; Rabel et al., 2018). The systematic review by Pjetursson et al. (2018), comparing veneered zirconia and metal‐ceramic implant‐supported SCs, reported a 97.6% (95% confidence interval (CI): 94.3–99.0) and a 98.3% (95% CI: 96.8–99.1) 5‐year survival rates, respectively, for the two types of SCs. Both, the biological and the technical complication rates of the veneered zirconia and the metal‐ceramic SCs were similar. However, fracture of the veneering layer was the predominant technical problem of both veneered types of restorations (Pjetursson et al., 2018).
Another systematic review compared the outcomes of oxide‐ceramic and glass‐ceramic implant‐supported SCs (Rabel et al., 2018). The authors reported good overall estimated 5‐year survival rates of the all‐ceramic implant‐supported SCs (93% (95% CI: 86.6%–96.4%)), yet again, high rates of veneering ceramic chipping reaching 9.0% (95% CI: 5.4%–14.8%) over a period of 5 years (Rabel et al., 2018).
In order to overcome the technical problems experienced with all types of veneered restorations, more recently, monolithic, that is, un‐veneered, micro‐veneered, or partially veneered, types of restorations were presented (Caramês et al., 2019; Cheng et al., 2019; Rammelsberg et al., 2020). However, a clear distinction between the definitions of monolithic, micro‐veneered, and conventionally veneered designs is lacking. In the present systematic review, micro‐veneered all‐ceramic restorations were defined as minimally veneered (≤0.5 mm) solely in the non‐functional areas, whereas monolithic restorations were considered without any ceramic layer. These monolithic types of restorations may offer two main advantages. Firstly, the increase in the efficiency of the laboratory procedures by enabling a faster fabrication of the restorations (Joda & Brägger, 2014, 2015, 2016). Secondly, improvement in clinical outcomes by reducing the number of ceramic fractures which persist as one of the predominant problems observed with veneered restorations (Pjetursson et al., 2018; Rabel et al., 2018). Still, one should keep in mind that the clinical importance of chipping may vary depending on the location and characteristics of the ceramic fractures.
Yet, systematic reviews analyzing the influence of the crown design (monolithic/ micro‐veneered/ veneered) on the survival rates and the technical complication rates of implant‐supported SCs were not able to indicate significant results due to lack of reports on monolithic and micro‐veneered restorations (Pjetursson et al., 2018; Rabel et al., 2018). Hence, the clinical longevity of those restorations remained to be elucidated. In the meantime, a pronounced amount of short‐term clinical studies on monolithic, micro‐veneered, and veneered all‐ceramic implant restorations has been published.
The aim of the present systematic review, therefore, was to analyses the survival, failure, and the complication rates of monolithic/micro‐veneered, and conventionally veneered of all‐ceramic implant‐supported SCs.
2. MATERIALS AND METHODS
2.1. Study design
This systematic review was designed as an update of two previous systematic reviews (Pjetursson et al., 2018; Rabel et al., 2018).
The study protocol of this systematic review followed the guidelines for Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) (Moher et al., 2009). This report is in compliance with the appropriate EQUATOR (http://www.equator‐network.org) guidelines. Furthermore, to improve searching databases for clinical questions, the PICO framework was applied (Schardt et al., 2007). PICO stands for patient/population (P), intervention (I), comparison (C), and outcome (O). For this systematic review, the “PICO” question was defined as follows:
Population: Partially edentulous patients who received implant‐supported SCs in the anterior and posterior regions.
Intervention: Titanium dental implants restored with monolithic or micro‐veneered all‐ceramic SCs.
Comparison: Titanium dental implants restored with veneered all‐ceramic SCs.
Outcome: Survival, failure, and complication rates of the restorations.
The focus question was “In partially edentulous patients do monolithic all‐ceramic implant‐supported SCs exhibit differences in survival, failure, and complication rates when compared to veneered all‐ceramic implant‐supported SCs?”.
Ethical approval was not required for this systematic review.
2.2. Search strategy
As this systematic review was an update of previous reviews, the search strategies for the present review were adopted from the two respective previous systematic reviews (Pjetursson et al., 2018; Rabel et al., 2018). Two independent searches were conducted in three databases, MEDLINE via PubMed (http://www.ncbi.nlm.nih.gov/pubmed), EMBASE (https://www.embase.com), and the Cochrane Central Register of Controlled Trials (CENTRAL) (http://www.thecochranelibrary.com) by the authors of the corresponding reviews duplicating the same strategy. The search strategies are explained in detail in the previous publications (Pjetursson et al., 2018; Rabel et al., 2018). The electronic search dates are summarized in Figure 1.
FIGURE 1.

Summary of the search terms that were used for two independent electronic literature searches. The blocks are addressing the restoration type, the restoration support and the restoration material
2.3. Search terms
The search terms and the combinations can be seen in Figure 1. Some free‐text terms were additionally tagged with an asterisk as truncation symbol to improve the search sensitivity. The publications found through the searches were imported into a reference management software (EndNote X9, Thomson Reuter, New York, USA).
2.4. Eligibility criteria
The inclusion criteria for the clinical investigations were as follow:
Randomized controlled clinical trials (RCTs) or prospective clinical trials published in the English language.
At least 10 patients included in the study.
A follow‐up time of at least 1 year after inserting the restoration.
Detailed information on the restoration material used.
Restoration type clearly described and data from SCs reported separately from other types of included restorations.
If there are multiple publications on the same patient cohort, only the publication with the longest follow‐up time was included.
SCs, made of ceramic materials, monolithic or veneered, namely zirconia, glass‐ceramics (lithium disilicate and leucite‐reinforced glass‐ceramics), and resin‐matrix ceramics (RMC)
All brands, kinds of titanium dental implants
Sufficient reporting on the detailed clinical outcomes (survival, technical) of SCs.
The clinical studies with following characteristics were excluded.
Not meeting all inclusion criteria
Retrospective studies, retrospective case series, technical reports, and case reports
Studies that pool outcomes of different restoration types and materials
Studies reporting on metal‐ceramic, metal‐resin, polyether ether ketone (PEEK) implant restorations
Studies reporting on ceramic implants
Poor reporting on drop‐outs and number of patients at follow‐up.
2.5. Selection of studies
The records of the two electronic searches were imported to the reference management software, and subsequently, the duplicates were removed. Two reviewers (DK and AL) screened independently the titles, thereafter the abstracts. Disagreements were resolved by discussion, and the articles were then obtained in full text for full‐text screening. In addition, the full texts of the included studies from the Rabel et al., 2018 and Pjetursson et al., 2018 systematic reviews were screened for their eligibility by the reviewers DK and BEP. Furthermore, the excluded studies list and the complete reference lists of those systematic reviews were screened. Full‐text articles were evaluated by two reviewers (DK and BEP) independently, and the selection of the eligible studies was done based on the inclusion/exclusion criteria. The included studies were double checked in terms of study centers, ethical committee approval number, and full author lists by the third reviewer (AL) in order to detect multiple publications that might be reporting on the same patient cohort.
2.6. Data extraction
Two reviewers (DK and BEP) independently extracted the data of the included articles. Authors were calibrated prior to the data extraction in order to establish consistency in the process.
The data extraction tables were created based on the focus question of the systematic review and included both qualitative and quantitative data. The study characteristics as author, year, study setting, study design, mean follow‐up time, total exposure time, total number of included patients, number of patients at the end of the follow‐up period, number of patients dropped‐out, number of implants, abutments, and SCs at the baseline and at the end of the follow‐up period, the number of drop‐outs were recorded. The restoration characteristics and the number of SCs based on retention type (screw retention/cement) and region (anterior/ posterior) were extracted. The material characteristics namely the restoration design (veneered/ micro‐veneered/ monolithic) and abutment, framework, veneering ceramic materials/brands/fabrication methods were recorded.
2.7. Outcome Measures
Crown survival was defined as the SCs remaining in situ, with or without modification, for the entire observation period. The failure therefore in the present systematic review was considered when the SC was reported to be lost, removed, and/or remade. Accordingly, the overall failure rate of SCs includes the number of SCs that were lost and/or needed to be remade due to reasons such as implant loss, ceramic fracture, repeated loss of retention, repeated screw loosening, esthetic, and biological complications. The failures that happened due to ceramic fractures (overall failure rates due to ceramic fracture) were further extracted and then subcategorized as “failure due to core fractures,” “failure due to catastrophic veneer fractures,” and “failure due to abutment fractures” when the data was available. By doing this, the chippings that are repairable and/or polishable were considered as a technical complication and the data were extracted accordingly, as ceramic chippings. Screw loosening and retention loss were other technical complications. Screw‐loosening data were extracted when the loss of torque of the implant abutment screw and/or prosthetic screw reported. Meanwhile, loss of retention was considered in the present systematic review as the technical complications that was due to the cement layer problems either with the intraoral or the extraoral cementation.
Biological outcomes namely significant bone loss as reported >2 mm and soft tissue complications such as peri‐implantitis, peri‐implant mucositis, gingival hyperplasia, fistula, and mucosal recession were extracted.
Following the independent data extraction, the extraction sheets were reviewed for any disagreement, and revision was repeated until all disagreements were resolved. The authors of the articles that were lacking some information yet as judged to be noteworthy were contacted by e‐mail or telephone for additional data.
2.8. Quality assessment of the included studies
DK and AL made the quality assessment of the included studies. The quality assessment for RCTs and non‐randomized studies were performed with the Newcastle–Ottawa Scale (NOS). According to the NOS, studies with scores <5 were considered as low quality, whereas scores with 5–7 were considered as moderate quality and >7 as high quality.
2.9. Statistics
In the present systematic review, failure, and complication rates were calculated by dividing the number of events (failures or complications) in the numerator by the total SC exposure time in the denominator.
The numerator could usually be extracted directly from the publication. The total exposure time was calculated by taking the sum of:
Exposure time of SCs that could be followed for the whole observation time.
Exposure time up to a failure of the SCs that were lost during the observation time.
Exposure time up to the end of observation time for SCs in patients that were lost to follow‐up due to reasons such as death, change in address, refusal to participate, non‐response, chronic illnesses, missed appointments, and work commitments.
For each study, event rates for the SCs were calculated by dividing the total number of events by the total SC exposure time in years. For further analysis, the total number of events was considered to be Poisson distributed for a given sum of SC exposure and Poisson regression were used with a logarithmic link‐function and total exposure time per study as an offset variable (Kirkwood & Sterne, 2003a). To assess heterogeneity of the study‐specific event rates, the Spearman goodness‐of‐fit statistics and associated p‐value were calculated. To reduce the effect of heterogeneity, robust standard errors were calculated to obtain 95% confidence intervals of the summary estimates of the event rates (White 1980, 1982).
The three‐year survival proportions were calculated via the relationship between event rate and survival function S, S (T) = exp (‐T * event rate), by assuming constant event rates (Kirkwood & Sterne, 2003a). The 95% confidence intervals for the survival proportions were calculated by using the 95% confidence limits of the event rates. Multivariable Poisson regression was used to investigate formally whether event rates varied by material utilized, the design of the restoration (monolithic/veneered), and the position of the crowns in the dental arch (anterior/posterior). All analyses were performed using Stata®, version 15.0 (Stata Corp., College Station, TX, USA).
3. RESULTS
3.1. Screening process
The searches resulted in a total of 1633 records (Figure 2). After the duplicate removal, 1194 references were screened by title. Out of these, 243 studies were further screened by abstract, and 163 excluded at the abstract level. Eighty full‐text articles were assessed for eligibility and subsequently 25 studies were identified as eligible for inclusion based on the electronic search. Additional 44 full‐text articles, included in the systematic reviews by Rabel et al. (2018) and Pjetursson et al. (2018), were screened based on the present systematic review's eligibility criteria. Seven additional studies were identified for full‐text assessment after the hand search on the excluded study tables of the aforementioned systematic reviews. Accordingly, from the 51 evaluated full‐text articles, 24 were found to be eligible for inclusion (Figure 2). These articles were added to the 25 previously included full‐text articles. Hence, a total number of 49 studies were included for the qualitative and quantitative analysis in this review (Figure 2).
FIGURE 2.
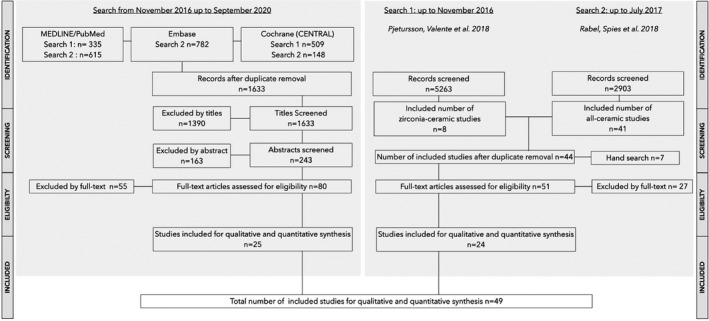
Search strategy—For summary of the excluded full‐text articles see Table S1
The detailed reasons for exclusion of the full‐text articles were given in a supplementary table (Table S1).
3.2. Included studies
The included 49 studies were reporting on 57 material cohorts (Table 1). Four of the included studies (Heierle et al., 2019; Kraus et al., 2019; Paolantoni et al., 2016; Wittneben et al., 2020) reported on SCs made of two different material combinations, and one study included patients restored with implant‐supported SCs made out of five different material combinations (Rammelsberg et al., 2020). Twenty‐four of the included cohorts reported on veneered zirconia abutments or on zirconia SCs supported by titanium or ceramic abutments (n=969), eight cohorts on monolithic or micro‐veneered zirconia implant‐supported SCs (n=394), five cohorts on veneered lithium disilicate or leucite‐reinforced glass‐ceramic implant‐supported SCs (n=110), 14 cohorts on monolithic or micro‐veneered lithium disilicate or leucite‐reinforced glass‐ceramic implant‐supported SCs (n=484), four cohorts on veneered densely sintered alumina implant‐supported SCs (n=128), and two on RMC implant‐supported SCs (n = 75) (Table 1). Twenty of the included studies were RCTs (Table 2; Table S2 ) comparing directly veneered zirconia customized and stock zirconia abutments with cemented ceramic SCs (Wittneben et al., 2020), esthetic outcomes for screw‐retained SCs with or without using provisional crowns for tissue conditioning (Furze et al., 2019), metal‐ceramic and resin‐matrix ceramic SCs (Agustín‐Panadero et al., 2020), monolithic zirconia and porcelain‐fused‐to‐metal (PFM) implant‐supported SCs (Mühlemann et al., 2020), cemented and screw‐retained SCs on customized zirconia abutments (Heierle et al., 2019), 11 mm implants used in combination with sinus floor elevation and 6mm implants without bone augmentation (Guljé et al., 2019b), screw‐retained monolithic zirconia and cemented PFM SCs (Weigl, Saarepera, et al., 2019), submucosal veneered zirconia abutments and non‐veneered zirconia abutments (Laass et al., 2019), immediate, non‐detached glass‐ceramic individualized abutments and dis‐/reconnections (Erhan Çömlekoğlu et al., 2018), monolithic zirconia SCs and short‐span FDPs (Cheng et al., 2019), cemented and screw‐retained zirconia‐based implant‐supported SCs (Kraus et al., 2019), tooth‐ and implant‐supported veneered zirconia single SCs (Cantner et al., 2019), cemented and screw‐retained CAD/CAM zirconia abutments for esthetically located implant‐supported SCs (Amorfini et al., 2018), digital and analog procedures for manufacturing of implant‐supported SCs (Mangano & Veronesi, 2018), customized zirconia and titanium abutments (Bösch et al., 2018), immediately loaded one‐ and two‐piece implants (Bomicke et al., 2017), two‐piece and one‐piece zirconia abutments (Paolantoni et al., 2016), bonding of a RMC restorative material to zirconia stock abutments and zirconia customized abutments (Schepke et al., 2016), zirconia and titanium abutments (Zembic et al., 2013), and zirconia and metal‐ceramic implant‐supported SCs (Hosseini et al., 2011) (Table S2). The remaining 29 studies were prospective cohort studies (Andersson et al., 1998; Cantner et al., 2019; Canullo, 2007; Cheng et al., 2019; Cooper et al., 2016; Fenner et al., 2016; Gierthmuehlen et al., 2020; Guarnieri et al., 2015; Guljé et al., 2019a; Guncu et al., 2016; Henriksson & Jemt, 2003; Hosseini et al., 2011; Hosseini et al., 2013; Joda et al., 2017; Kolgeci et al., 2014; Koller et al., 2020; Linkevicius et al., 2018; Lops et al., 2013; Ma et al., 2019; Meijndert et al., 2020; Nothdurft et al., 2014; Ormianer & Schiroli, 2006; Peron & Romanos, 2020; Pieri et al., 2013; Pol et al., 2020; Rammelsberg et al., 2020; Teichmann et al., 2017; Vandeweghe et al., 2012; Vanlioglu et al., 2012; Weigl, Trimpou, et al., 2019; Zembic et al., 2013; Zembic et al., 2015). As none of the included RCTs address the focused question of the present systematic review, they were addressed as prospective studies and analyzed as such.
TABLE 1.
Information on materials of SCs, abutment and implants, manufacturing/processing techniques of SCs and abutments of the included studies
| Study |
Restoration veneered (v) micro‐ veneered (micro‐v) monolithic (m) |
Ceramic‐core Abutment (Ab) framework (f) Material |
Brand | Manufacturing method |
Veneering Material |
Brand | Processing method |
Abutment Material |
Brand | Manufacturing Method |
Retention Screw‐ retained [s) Cemented (c) |
Cement type | Cement Brand | Implant | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Material | Implant Brand | ||||||||||||||
| Veneered Zr SC | |||||||||||||||||
| Meijndert et al. | 2020 | v | Ab & f | Zr | nr | nr | Fluorapatite GC | IPS e.max Ceram, Ivoclar | nr | Zr | nr | nr | s & c | GIC | Fuji Plus, GC Europe | Ti | Straumann |
| Rammelsberg et al. Group A | 2020 | v | f | Zr | nr | nr | nr | nr | nr | Zr & Ti & LDS & Au | nr | Stock & customized | nr | nr | nr | Ti | Straumann & Nobel Biocare |
| Wittneben et al. Group B | 2020 | v | Ab | Zr | CARES, Straumann | CAD/CAM | Fluorapatite GC | IPS e.max Ceram, Ivoclar | hand‐layered | Zr | CARES, Straumann | centCAD/CAM | s | na | na | Ti | Straumann |
| Cantner et al. | 2019 | v | f | Zr | IPS e.max ZirCAD, Ivoclar | CAD/CAM | LdS | IPS e.max Press, Ivoclar | press | Zr | IPS e.max ZirCAD, Ivoclar | CAD/CAM | s & c | Resin & GIC | Multilink Implant & Fuji Plus & Ketac Cem | Ti | Camlog |
| Furze et al. | 2019 | v | Ab | Zr | CARES, Straumann | CAD/CAM | nr | nr | nr | Zr | CARES, Straumann | centCAD/CAM | s | na | na | Ti | Straumann |
| Guljé et al. | 2019a | v | f | Zr | nr | nr | nr | nr | nr | Ti | Atlantis, Dentsply Sirona | centCAD/CAM | c | nr | nr | Ti | Astra Tech |
| Guljé et al. | 2019b | v | f | Zr | nr | nr | nr | nr | nr | Ti | Atlantis, Dentsply Sirona | centCAD/CAM | c | nr | nr | Astra Tech | |
| Heierle et al. Group A | 2019 | v | Ab | Zr | CARES, Straumann | CAD/CAM | nr | nr | nr | Zr | CARES, Straumann | centCAD/CAM | s | na | na | Ti | Straumann |
| Ma et al. | 2019 | v | Ab | Zr | CER‐ZR, Southern Implants | nr | GC | Zirox, Wieland Dental | Hand‐layered | Zr | CER‐ZR, Southern Implants | CAD/CAM | s | na | na | Ti | Southern Implants |
| Kraus et al. Group A | 2019 | v | Ab | Zr | Atlantis, Dentsply Sirona | centCAD/CAM | feldsphatic | Creation ZI‐F, Willi Geller | nr | Zr | Atlantis, Dentsply Sirona | centCAD/CAM | s | na | na | Ti | Astra Tech |
| Weigl et al. | 2019b | v | f | Zr | n.r. | CAD/CAM | GC | nr | nr | Zr | Ankylos CERCON Balance | CAD/CAM | c | Provisional | RelyX Temp NE, 3 M ESPE | Ti | Ankylos |
| Amorfini et al. | 2018 | v | Ab & f | Zr | CARES, Straumann | centCAD/CAM | nr | nr | nr | Zr & Ti | CARES & synOcta, Straumann | centCAD/CAM & stock | s & c | GIC | RelyX Luting, 3 M ESPE | Ti | Straumann |
| Bösch et al. | 2018 | v | f | Zr | CARES, Straumann | centCAD/CAM | GC | nr | nr | Zr & Ti | CARES & synOcta, Straumann | centCAD/CAM & stock | s | na | na | Ti | Straumann |
| Bömicke et al. | 2017 | v | f | Zr | Procera, Nobel | centCAD/CAM | silicate ceramic | NobelRondo Zirconia, Nobel | Hand‐layered | Ti | Nobel Replace, Nobel | Stock | c | GIC | Ketac Cem, 3 M ESPE | Ti | Nobel Biocare |
| Güncü et al. | 2016 | v | f | Zr | Lava, 3 M ESPE | CAD/CAM | leucite‐reinforced feldspathic | VM9 (VITA Zahnfabrik) | Hand‐layered | Ti | TiDesign, Astra Tech AB | nr | c | Resin‐modGIC | Fuji Plus, GC Europe | Ti | Astra Tech® |
| Paolantoni Group B | 2016 | v | f | Zr | ART Anchorage, Thommen | nr | nr | nr | nr | Zr | ART Anchorage, Thommen | CAD/CAM | s | nr | nr | Ti | Thommen Medical |
| Kolgeci et al. | 2014 | v | f | Zr | Procera, Nobel | centCAD/CAM | nr | NobelRondo & Creation & Cerabien | nr | Zr & Ti | nr | nr | s & c | Resin | Panavia F, Kuraray Noritake | Ti | Nobel Biocare |
| Nothdurft et al. | 2014 | v | f | Zr | CERCON base, DeguDent | CAD/CAM | Silicate ceramic | Ceramkiss, Degudent | nr | Zr | CERCON, DENTSPLY | Stock & CAD/CAM | c | Resin‐modGIC | GC FujiCEM | Ti | XiVE (Dentsply Friadent) |
| Hosseini et al. | 2013 | v | f | Zr | Procera, Nobel | centCAD/CAM | Leucite GC & fluorapatite GC | IPS Empress 2 & IPS e.max Ceram | nr | Zr & Ti | nr | nr | c | Resin | Panavia, Kuraray Noritake | Ti | Astra Tech, Dentsply Implants |
| Lops et al. | 2013 | v | f | Zr | Lava, 3 M ESPE | CAD/CAM | nr | nr | nr | Zr | ZirDesign, Astra | Stock | c | nr | TempBond Clear, Kerr Dental | Ti | Astra Tech, Dentsply Implants) |
| Pieri et al. | 2013 | v | f | Zr | nr | CAD/CAM | nr | nr | nr | Zr & Ti | nr | Stock & customized | c | nr | nr | Ti | Astra Tech & Xive (Dentsply Implants) |
| Zembic et al. | 2013 | v | Ab & f | Zr | Procera, Nobel | centCAD/CAM | nr | nr | nr | Zr | Procera, Nobel | centCAD/CAM | s & c | Resin & Resin‐modGIC | Panavia & RelyX Unicem & Ketac Cem | Ti | Branemark RP, Nobel |
| Vandeweghe et al. | 2012 | v | Ab | Zr | CER‐ZR45, Southern Implants | CAD/CAM | nr | nr | nr | Zr | CER‐ZR45, Southern Implants | CAD/CAM | s | na | na | Ti | nr |
| Hosseini et al. | 2011 | v | f | Zr | Procera, Nobel & KaVo, Kavo | CAD/CAM | feldsphatic & fluorapatite GC | HeraCeram & IPS e.max Ceram | nr | Zr | ZirDesign, Astra | Stock & CAD/CAM | c | resin & ZP | Panavia & DeTrey Zinc | Ti | Astra Tech (Dentsply Implants) |
| Monolithic Zr SCs | |||||||||||||||||
| Mühlemann et al. | 2020 | m | f | Zr | CARES, Straumann | centCAD/CAM | na | na | na | Ti | Variobase, Straumann | Stock | s | Resin | Multilink Hybrid Abutment, Ivoclar | Roxolid | Straumann |
| Rammelsberg et al. Group B | 2020 | micro‐v | f | Zr | nr | CAD/CAM | nr | nr | na | Zr & Ti & LDS & Au | nr | nr | nr | nr | nr | Ti | Straumann & Nobel Biocare |
| Rammelsberg et al. Group C | 2020 | m | f | Zr | nr | CAD/CAM | na | na | na | Zr & Ti & LDS & Au | nr | nr | nr | nr | nr | Ti | Straumann & Nobel Biocare |
| Cheng et al. | 2019 | micro‐v | f | Zr | Ceramil Zi & Zolid, Amann Girrbach | CAD/CAM | nr | nr | nr | Ti | synOcta & Variobase, Straumann | Stock | s & c | Resin | Premier Implant & RelyX Unicem | Ti | Straumann |
| Koenig et al. | 2019 | m | f | Zr | Lava Plus, 3 M ESPE | CAD/CAM | na | na | na | Ti | Medentika | nr | s & c | Resin | RelyX Ultimate & Multilink Hybrid | Ti | Nobel Biocare & Straumann |
| Pol et al. | 2019 | m | f | Zr | Procera, Nobel | centCAD/CAM | na | na | na | Ti & Zr | Procera Hybrid, Nobel | nr | s | nr | nr | Ti | Nobel Biocare |
| Weigl et al. | 2019a | m | f | Zr | nr | CAD/CAM | na | na | na | Ti | Titanium base, Ankylos | Stock | s | ZP | RelyX Temp NE, 3 M Espe | Ti | Ankylos (Dentsply Implants) |
| Mangano & Veronesi | 2018 | m | f | Zr | Katana, Kuraray Noritake | CAD/CAM | na | na | na | Zr | Leone | Stock | c | nr | nr | Ti | Exacone |
| Veneered LiSi2 and Leucite SCs | |||||||||||||||||
| Heierle et al. Group B | 2019 | v | f | LdS | IPS e.max press, Ivoclar | nr | nr | nr | nr | Zr | CARES, Straumann | centCAD/CAM | c | Resin | RelyX Unicem, 3 M Espe | Ti | Straumann |
| Laass et al. | 2019 | v | f | LdS | IPS e.max Press, Ivoclar | Press | nr | nr | nr | Zr | Atlantis, Dentsply Sirona | centCAD/CAM | c | Resin | Panavia 21, Kuraray Noritake | Ti | Astra Tech (Dentsply Implants) |
| Kraus et al. Group B | 2019 | v | f | LdS | E.max, Ivoclar | nr | Fluorapatite GC | IPS e.max Ceram, Ivoclar | nr | Zr | Atlantis, Dentsply Sirona | centCAD/CAM | c | Resin | Panavia 21, Kuraray Noritake | Ti | Astra Tech (Dentsply Implants) |
| Teichmann et al. | 2017 | v | f | LdS | IPS e.max Press, Ivoclar | Press | Fluorapatite GC | IPS Eris, Ivoclar | hand‐layered | Al2O3 & Ti & Zr | Alumina & Titanium, Nobel | stock | c | GIC & Resin | Ketac‐Cem & Variolink II | Ti | Nobel Biocare & Steri‐Oss |
| Zembic et al. | 2015 | v | f | leucite | Empress I, Ivoclar Vivadent, | Press | nr | nr | nr | Zr | Metoxit, Thayngen | stock | c | Resin | Panavia TC, Kuraray Noritake | Ti | Nobel Biocare |
| Monolithic LiSi2 and Leucite SCs | |||||||||||||||||
| Gierthmuehlen et al. | 2020 | m | f | LdS | IPS e.max Press, Ivoclar | Press | na | na | na | Ti | nr | nr | s | Resin | Multilink Implant, Ivoclar | Ti | Nobel Biocare & Xive (Dentsply Sirona) |
| Koller et al. | 2020 | m | f | LdS | IPS e.max CAD, Ivoclar | CAD/CAM | na | na | na | Ti | Ziterion | nr | s | Resin | Multilink, Ivoclar | Ti & Y‐TZP | Ziterion |
| Rammelsberg et al. Group D | 2020 | micro‐v | f | LdS | nr | nr | na | na | na | Zr & Ti & LDS & Au | nr | nr | nr | nr | nr | Ti | Straumann & Nobel Biocare |
| Rammelsberg et al. Group E | 2020 | m | f | LdS | nr | nr | na | na | na | Zr & Ti & LDS & Au | nr | nr | nr | nr | nr | Ti | Straumann & Nobel Biocare |
| Wittneben et al. Group A | 2020 | micro‐v | f | Fluorapatite | IPS e.max ZirPress, Ivoclar | Press | Fluorapatite GC | IPS e.max Ceram, Ivoclar | hand‐layered | Zr | IPS e.max, Ivoclar | stock | s | nr | nr | Ti | Straumann |
| Cömlekoglu et al. | 2018 | m | f | Leucite | Empress CAD, Ivoclar | CAD/CAM | na | na | na | Ti & LiSi2 | E.max CAD, Ivoclar | CAD/CAM | c | Resin | Variolink II, Ivoclar | Ti | Camlog |
| Linkevicius et al. | 2018 | m | f | LdS | IPS e.max, Ivoclar | CAD/CAM | na | na | na | Ti | nr | nr | s | Resin | LinkAce,GC | Ti | MIS Implant |
| Joda et al. | 2017 | m | f | LdS | IPS e.max CAD, Ivoclar | centCAD/CAM | na | na | na | Ti | Variobase, Straumann | Stock | s | Resin | Multilink Implant, Ivoclar | Ti | Straumann |
| Cooper et al. | 2016 | m & micro‐v | f | LdS | E.max, Ivoclar | Press | nr | nr | nr | Zr | Atlantis, Dentsply | centCAD/CAM | c | Resin | RelyX Unicem, 3 M ESPE | Ti | Astra Tech (Dentsply Sirona) & Nobel Biocare & BIOMET 3i |
| Paolantoni et al. Group A | 2016 | m | f | LdS | Empress II, Ivoclar | Press | na | na | na | Zr | ART, Thommen | nr | c | Resin | Relyx Unicem 2, 3 M ESPE | Ti | Thommen Medical |
| Peron & Romanos | 2016 | m | f | LdS | nr | nr | na | na | na | Ti | nr | nr | s & c | nr | nr | Ti | Zimmer Dental |
| Guarnieri | 2015 | m | f | Leucite | IPS Empress, Ivoclar | Press | na | na | na | Zr | nr | nr | nr | nr | nr | Ti | BioHorizons |
| Vanlioglu et al. | 2012 | m | f | Leucite | Empress II, Ivoclar | Press | na | na | nr | Zr | Zirkohnzahn, Steger | nr | c | Resin | Variolink II, Ivoclar | Ti | Astra Tech, Dentsply Sirona & Straumann |
| Canullo et al. | 2007 | m | f | LiSi2 | Generic Pentrol | Press | na | na | nr | Ti & Zr | ProUnic, Impladent | nr | c | nr | Nimetic‐Cem, 3 M ESPE | Ti | TSA implants, Impladent |
| Bi‐layered Al2O3 | |||||||||||||||||
| Fenner et al. | 2016 | v | Ab & f | Al2O3 | Procera, Nobel | centCAD/CAM | nr | nr | nr | Al2O3 | synOcta In‐Ceram, Straumann | nr | s & c | Resin | Panavia, Kuraray Noritake | Ti | Straumann |
| Ormianer et al. | 2006 | v | f | AlZr | PureForm, Zimmer Dental | stock & customized | nr | Vitadur Alpha, Vident | hand‐layered | Ti | nr | nr | c | GIC & Resin | Ketac‐Cem & Panavia | Ti | Zimmer Dental |
| Henrikson & Jemt | 2003 | v | Ab & f | Al2O3 | Procera, Nobel | centCAD/CAM | nr | nr | nr | Al2O3 | Procera, Nobel | centCAD/CAM | s & c | nr | nr | Ti | Nobel Biocare |
| Andersson et al. | 1998 | v | f | Al2O3 | CeraOne, Nobel | nr | nr | nr | nr | Ti | nr | nr | c | nr | nr | nr | nr |
| RNC | |||||||||||||||||
|
Augustín‐Pandero et al. Schepke et al. |
2020 | m | f | RMC | Lava Ultimate, 3 M ESPE | CAD/CAM | na | na | na | Ti | nr | nr | c | Resin | RelyX Ultimate, 3 M ESPE | Ti | Kohno Straight implants, Sweden & Martina |
| 2016 | m | f | RMC | Lava Ultimate, 3 M ESPE | CAD/CAM | na | na | na | Zr | ZirDesign & ATLANTIS, Dentsply | centCAD/CAM | c | Resin | RelyX Ultimate, 3 M ESPE | Ti | Astra Tech, Dentsply Sirona | |
Abbreviations: Al2O3, alumina; Au, gold; GC, glass‐ceramics; GIC, glass‐ionomer cement; LdS, lithium disilicate; nr, not reported; na, not applicable; RMC, resinmatrix ceramic; Ti, titanium; Zr, zirconia; ZP, zinc–phosphate cement.
TABLE 2.
Study, patient, and restoration characteristics of the included studies
| Study | Patient | Implants | SCs | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Design | Setting | Initial (n) | End of follow‐up (n) | Drop‐out (n) | Drop‐out (%) | Mean age (y) | Initial (n) | Failed (n) | Initial (n) | SCs anterior (n) | SCs posterior (n) | End of follow‐up (n) | Drop‐out (n) | Screw‐Retained (n) | Cemented (n) |
| Veneered Zr SCs | |||||||||||||||||
| Meijndert et al. | 2020 | Pro | U | 60 | 50 | 10 | 17 | 36,9 | 60 | 0 | 60 | 54 | 6 | 10 | 33 | 27 | |
| Rammelsberg et al. Group A | 2020 | Pro | U | 404 | nr | nr | 23 | 57,8 | 92 | 3 | 92 | nr | nr | nr | nr | nr | nr |
| Wittneben et al. Group B | 2020 | RCT | U | 20 | 20 | 0 | 0 | nr | 20 | 0 | 20 | nr | nr | 20 | nr | 20 | 0 |
| Cantner et al. | 2019 | Pro | PP | 118 | 105 | 13 | 11 | nr | 114 | 0 | 114 | 0 | 114 | nr | nr | 53 | 61 |
| Furze et al. | 2019 | RCT | PP | 19 | 19 | 0 | 0 | 53,4 | 19 | 0 | 19 | 19 | 0 | 19 | 0 | 19 | 0 |
| Guljé et al. | 2019a | RCT | U & PP | 38 | 36 | 2 | 5 | 49 | 41 | 1AF | 41 | 0 | 41 | 39 | nr | 0 | 41 |
| Guljé et al. | 2019b | Pro | U & PP | 21 | 21 | 0 | 0 | 57,3 | 31 | 0 | 31 | 0 | 31 | 31 | 0 | 0 | 31 |
| Heierle et al. Group A | 2019 | RCT | U | 34 | 27 | 7 | 21 | nr | 17 | nr | 17 | 17 | 0 | 13 | nr | 17 | 0 |
| Kraus et al. Group A | 2019 | RCT | U | 44 | 40 | 4 | 9 | 51,4 | 24 | 0 | 24 | 7 | 17 | 20 | 2 | 24 | 0 |
| Ma et al. | 2019 | Pro | U | 27 | 16 | 9 | 33 | 47,1 | 28 | 2BF | 26 | nr | nr | nr | 9 | 26 | 0 |
| Weigl et al. | 2019 | RCT | U | 21 | 21 | 0 | 0 | 44 | 21 | 0 | 21 | 0 | 21 | 21 | nr | 0 | 21 |
| Amorfini et al. | 2018 | RCT | U & PP | 40 | 30 | 10 | 25 | 48 | 32 | 0 | 32 | 17 | 15 | 30 | 2 | 16 | 16 |
| Bösch et al. | 2018 | RCT | PP | 29 | 29 | 0 | 0 | 43,7 | 13 | 0 | 13 | 3 | 9 | 12 | 0 | 13 | 0 |
| Bömicke et al. | 2017 | RCT | U | 38 | 35 | 3 | 8 | 52,9 | 38 | 1BF | 38 | 0 | 38 | nr | 3 | 0 | 38 |
| Güncü et al. | 2016 | Pro | U & PP | 24 | 24 | 0 | 0 | 44,1 | 23 | 0 | 24 | 0 | 24 | 24 | 0 | 0 | 24 |
| Paolantoni et al. Gruppe B | 2016 | RCT | U | 65 | 65 | 0 | 0 | 53 | 45 | 0 | 45 | 45 | 0 | 45 | 0 | 45 | 29 |
| Kolgeci et al. | 2014 | Pro | PP | 137 | 127 | 10 | 7 | 62.5 | 289 | 2 | 120 | nr | nr | 115 | nr | 108 | 12 |
| Nothdurft et al. | 2014 | Pro | U | 24 | 23 | 1 | 4 | nr | 39 | 0 | 39 | 0 | 39 | 37 | 2 | 0 | 39 |
| Hosseini et al. | 2013 | Pro | U | 59 | 57 | 2 | 3 | 27,9 | 61 | 0 | 61 | 49 | 12 | nr | 2 | 0 | 61 |
| Lops et al. | 2013 | Pro | U | 85 | 81 | 4 | 5 | 54 | 38 | 0 | 38 | na | 37 | 37 | 2 | 0 | 38 |
| Pieri et al. | 2013 | Pro | U | 29 | 29 | 0 | 0 | 45,3 | 29 | 0 | 29 | 29 | 0 | 29 | 0 | 0 | 29 |
| Zembic et al. | 2013 | RCT | U | 22 | 18 | 4 | 18 | 41,3 | 12 | 1AF | 12 | 2 | 10 | nr | nr | 2 | 10 |
| Vandeweghe et al. | 2012 | Pro | U | 14 | 14 | 0 | 0 | 55 | 15 | 0 | 15 | 5 | 10 | 15 | 0 | 15 | 0 |
| Hosseini et al. | 2011 | RCT | U | 36 | 36 | 0 | 0 | 28,1 | 38 | 0 | 38 | 0 | 38 | 38 | 0 | 0 | 38 |
| Monoithic Zr SCs | |||||||||||||||||
| Mühlemann et al. | 2020 | RCT | U | 39 | 33 | 0 | 6 | 57,7 | 39 | 1AF | 39 | 0 | 39 | 38 | 0 | 39 | 0 |
| Rammelsberg et al. Group B | 2020 | Pro | U | 404 | nr | nr | 23 | 57,8 | 42 | 1 | 42 | nr | nr | nr | nr | nr | nr |
| Rammelsberg et al. Group C | 2020 | Pro | U | 404 | nr | nr | 23 | 57,8 | 152 | 0 | 152 | nr | nr | nr | nr | nr | nr |
| Cheng et al. | 2019 | RCT | U | 20 | 20 | 0 | 0 | 48,1 | 36 | 1 | 36 | nr | nr | 36 | 0 | 11 | 25 |
| Koenig et al. | 2019 | Pro | U | 47 | 44 | 3 | 0 | 54,3 | 48 | 2 | 48 | 0 | 48 | nr | nr | 44 | 4 |
| Pol et al. | 2019 | Pro | U | 30 | 30 | 0 | 0 | 53 | 30 | 0 | 30 | 0 | 30 | 30 | 0 | 30 | 0 |
| Weigl et al. | 2019 | RCT | U | 22 | 22 | 0 | 0 | 43 | 22 | 0 | 22 | 0 | 22 | 22 | 0 | 22 | 0 |
| Mangano & Veronesi | 2018 | RCT | PP | 25 | 25 | 0 | 0 | 51,6 | 25 | 0 | 25 | 0 | 25 | 24 | 0 | 0 | 25 |
| Veneered LiSi2 and Leucite SCs | |||||||||||||||||
| Heierle et al. Group B | 2019 | RCT | U | 34 | 27 | 7 | 21 | nr | 17 | nr | 17 | 17 | 0 | 14 | nr | 0 | 17 |
| Kraus et al. Group B | 2019 | RCT | U | 44 | 40 | 4 | 9 | 51,4 | 20 | 1AF | 20 | 4 | 16 | 16 | 2 | 0 | 20 |
| Laass et al. | 2019 | RCT | U | 20 | 16 | 4 | 20 | 46 | 20 | 0 | 20 | 10 | 10 | 16 | nr | 0 | 20 |
| Teichmann et al. | 2017 | Pro | U | 14 | 12 | 2 | 14 | 40,7 | 32 | 0 | 22 | 10 | 7 | 17 | 15 | 0 | 17 |
| Zembic et al. | 2015 | Pro | U | 27 | 16 | 11 | 41 | nr | 54 | 0 | 54 | 24 | 7 | 31 | 23 | 31 | 0 |
| Monolithic LiSi2 and Leucite SCs | |||||||||||||||||
| Gierthmuehlen et al. | 2020 | Pro | U | 28 | 27 | 1 | 4 | 49,9 | 45 | 0 | 45 | 0 | 45 | 44 | 1 | 45 | 0 |
| Koller et al. | 2020 | Pro | U | 22 | 22 | 0 | 0 | 46 | 15 | 1AF | 15 | 2 | 13 | 14 | 0 | 0 | 15 |
| Rammelsberg et al. Group D | 2020 | Pro | U | 404 | nr | nr | 23 | 57,8 | 3 | 2 | 3 | nr | nr | nr | nr | nr | nr |
| Rammelsberg et al. Group E | 2020 | Pro | U | 404 | nr | nr | 23 | 57,8 | 7 | 0 | 7 | nr | nr | nr | nr | nr | nr |
| Wittneben et al. Group A | 2020 | RCT | U | 20 | 19 | 1 | 5 | nr | 20 | 0 | 20 | nr | nr | 18 | nr | 0 | 20 |
| Cömlekoglu et al. | 2019 | RCT | U | 16 | 16 | 0 | 0 | 36.1 | 32 | 0 | 32 | 32 | 0 | nr | nr | 0 | 32 |
| Linkevicius et al. | 2018 | Pro | PP | 56 | 55 | 1 | 2 | 47,3 | 56 | 0 | 56 | 0 | 55 | 55 | 1 | 56 | 0 |
| Joda et al. | 2017 | Pro | U | 44 | 44 | 0 | 0 | 58,1 | 50 | 0 | 50 | 0 | 50 | 50 | 0 | 50 | 0 |
| Cooper et al. | 2016 | Pro | U & PP | 141 | 110 | 31 | 22 | 45 | 128 | 2 | 128 | 95 | 33 | 128 | 0 | 0 | 128 |
| Paolantoni Gruppe A et al. | 2016 | RCT | U | 65 | 65 | 0 | 0 | 53 | 29 | 0 | 29 | 29 | 0 | 29 | 0 | 0 | 29 |
| Peron & Romanos et al. | 2016 | Pro | PP | 25 | 25 | 0 | 0 | 43,3 | 26 | 0 | 26 | 5 | 21 | 26 | 0 | 24 | 2 |
| Guarnieri | 2015 | Pro | PP | 21 | 21 | 0 | 0 | 34 | 21 | 1BF | 20 | 20 | 0 | 20 | 0 | nr | nr |
| Vanlioglu et al. | 2012 | Pro | U | 12 | 12 | 0 | 0 | 33,2 | 23 | 0 | 23 | 23 | 0 | 23 | 0 | 0 | 23 |
| Canullo et al. | 2007 | Pro | PP | 25 | 25 | 0 | 0 | 52,3 | 30 | 0 | 30 | 16 | 14 | 30 | 0 | 0 | 30 |
| Vennered Al2O3 | |||||||||||||||||
| Fenner et al. | 2016 | Pro | U | 36 | 29 | 7 | 19 | 48 | 13 | nr | 17 | nr | nr | 13 | 4 | nr | nr |
| Ormianer et al. | 2006 | Pro | PP | 18 | 18 | 0 | 0 | 42,2 | 22 | 0 | 22 | 14 | 8 | 22 | 0 | 0 | 22 |
| Henrikson & Jamt | 2003 | Pro | U | 20 | 19 | 1 | 5 | 29 | 24 | 0 | 24 | 24 | 0 | 23 | 1 | 11 | 13 |
| Andersson et al. | 1998 | Pro | U | 57 | 53 | 4 | 7 | 31,8 | 55 | 0 | 65 | nr | nr | 55 | 5 | 0 | 62 |
| RNC SCs | |||||||||||||||||
| Augustín‐Pandero et al. | 2020 | RCT | U | 42 | 32 | 10 | 24 | nr | 25 | 1 | 25 | 0 | 25 | nr | 0 | 25 | |
| Schepke et al. | 2016 | RCT | U | 50 | 50 | 0 | 0 | 47,7 | 50 | 0 | 50 | 0 | 50 | 7 | 0 | 0 | 50 |
Abbreviations: AF, failed implant after loading; BF, failed implant before loading; nr, not reported; na, not applicable; Pro, prospective clinical study; PP, private practice setting; RCT, randomized controlled trial; U, university setting.
The studies reporting on veneered zirconia implant‐supported SCs were published between 2011 and 2020 (median 2018), for monolithic zirconia implant‐supported SCs the studies were published between 2018 and 2020 (median 2019), for veneered‐reinforced glass‐ceramic implant‐supported SCs were published between 2015 and 2019 (median 2019), for monolithic‐reinforced glass‐ceramic implant‐supported SCs were published between 2007 and 2020 (median 2017.5), for densely sintered alumina implant‐supported SCs were published between 1998 and 2016 (median 2004.5) and the two studies reporting on RMC implant‐supported SCs were published in 2016 and 2020.
The average age of the patients included in the different studies ranged from 27.9 to 62.5 years. The proportion of patients who could not be followed for the entire study period was available for all included studies and ranged from 0% to 41% (median 4%), and only two of the included studies had a drop‐out proportion of more than 25% (Table 2).
From the 969 included veneered zirconia implant‐supported SCs, 55.4% were cement‐retained and 44.6% screw‐retained. The respected percentages of the 394 included monolithic zirconia implant‐supported SCs were 27% for cemented and 73% for screw‐retained. From the 110 included veneered‐reinforced glass‐ceramic implant‐supported SCs, 70.5% were cemented and 29.5% were screw‐retained, from the 484 included monolithic‐reinforced glass‐ceramic implant‐supported SCs, 61.5% were cemented and 38.5% were screw‐retained, from the 128 included densely sintered alumina implant‐supported SCs, 90.1% were cemented and 9.9% were screw‐retained, and all the included RMC SCs were cemented (Table 2).
Evaluating the overall distribution of the implant‐supported SCs in the oral cavity, 37% of the included SCs were located in the anterior area and 63% in the posterior area. For the veneered zirconia SCs, this distribution was 34% anterior and 66% posterior, for monolithic zirconia SCs, it was 22% anterior and 78% posterior, for veneered‐reinforced glass‐ceramic SCs, it was 62% anterior and 38% posterior, and for monolithic‐reinforced glass‐ceramic SCs, it was 49% anterior and 51% posterior. Finally, for densely sintered alumina implant‐supported SCs, the distribution of the restorations was 83% anterior and 17% posterior, and all of the included RMC SCs were inserted in the posterior area (Table 2).
Thirty‐four of the included studies were conducted in an institutional environment, such as university or specialized implant clinics, 10 in private practice setting, and the remaining five studies were a cooperation between universities and private practices (Table 2).
3.3. Survival and failure rates
Twenty‐three studies reporting on 952 SCs with a mean follow‐up time of 3.8 years provided data on the survival of veneered zirconia implant‐supported SCs, 8 studies including 394 SCs with a mean follow‐up time of 1.6 years provided data on monolithic zirconia implant‐supported SCs, 4 studies reporting on 93 SCs with a mean follow‐up time of 8.1 years provided data on veneered‐reinforced glass‐ceramic implant‐supported SCs, 13 studies including 452 SCs with a mean follow‐up time of 2.6 years provided data on monolithic‐reinforced glass‐ceramic implant‐supported SCs, 4 studies reporting on 128 crowns with a mean follow‐up time of 3.7 years provided data on densely sintered alumina implant‐supported SCs, and 2 studies including 75 crowns with a mean follow‐up time of 1.8 years provided data on RMC SCs (Table 3).
TABLE 3.
Annual failure rates and 3‐year survival of all‐ceramic implant‐supported single crowns (SCs).
| Study | Year of publication | Total no. of crowns | Mean follow‐up time | No. of failures | Total crown exposure time | Estimated annual failure rate* (per 100 SC years) | Estimated survival after 3 years* (in percent) |
|---|---|---|---|---|---|---|---|
| Veneered zirconia SCs | |||||||
| Wittneben et al. Group B | 2020 | 20 | 3 | 2 | 60 | 3.3% | 90.5% |
| Meijndert et al. | 2020 | 60 | 4.5 | 1 | 271 | 0.4% | 98.9% |
| Rammelsberg et al. Group A | 2020 | 92 | 5.3 | 10 | 488 | 2.0% | 94.0% |
| Furze et al. | 2019 | 19 | 3 | 0 | 57 | 0% | 100% |
| Weigl et al. | 2019b | 21 | 1 | 0 | 21 | 0% | 100% |
| Ma et al. | 2019 | 26 | 4 | 2 | 105 | 1.9% | 94.4% |
| Guljé et al. | 2019a | 41 | 4.6 | 3 | 188 | 1.6% | 95.3% |
| Guljé et al. | 2019b | 31 | 5 | 0 | 155 | 0% | 100% |
| Kraus et al. Group A | 2019 | 24 | 2.5 | 4 | 61 | 6.3% | 82.1% |
| Canter et al. | 2019 | 114 | 3.5 | 0 | 399 | 0% | 100% |
| Amorfini et al. | 2018 | 32 | 9.9 | 1 | 316 | 0.3% | 99.1% |
| Bösch et al. | 2018 | 13 | 1.5 | 1 | 19 | 5.1% | 85.4% |
| Bömicke et al. | 2017 | 38 | 2.2 | 8 | 85 | 9.0% | 75.4% |
| Güncü et al. | 2016 | 24 | 3.9 | 2 | 94 | 2.1% | 93.8% |
| Paolantoni et al. Group B | 2016 | 45 | 4 | 2 | 180 | 1.1% | 96.7% |
| Kolgeci L et al. | 2014 | 120 | 3.2 | 5 | 385 | 1.3% | 96.2% |
| Nothdurft et al. | 2014 | 39 | 2.9 | 2 | 116 | 1.7% | 95.0% |
| Hosseini et al. | 2013 | 61 | 3.1 | 1 | 189 | 0.5% | 98.4% |
| Lops et al. | 2013 | 38 | 4.9 | 0 | 185 | 0% | 100% |
| Pieri et al. | 2013 | 29 | 5 | 0 | 145 | 0% | 100% |
| Zembic et al. | 2013 | 12 | 4.7 | 1 | 56 | 1.8% | 94.8% |
| Vandeweghe et al. | 2012 | 15 | 1 | 1 | 15 | 6.4% | 81.9% |
| Hosseini et al. | 2011 | 38 | 1.1 | 0 | 43 | 0% | 100% |
| Total | 952 | 3.8 | 46 | 3633 | |||
| Summary estimate (95% CI)* | 1.27% (0.77%−2.10%) | 96.3% (93.9%−97.7%) | |||||
| Monolithic Zirconia SCs | |||||||
| Rammelsberg et al. Group B | 2020 | 42 | 2.2 | 1 | 92 | 1.1% | 96.8% |
| Rammelsberg et al. Group C | 2020 | 152 | 1.8 | 2 | 274 | 0.7% | 97.8% |
| Mühlemann et al. | 2020 | 39 | 1.0 | 1 | 39 | 2.5% | 92.6% |
| Koenig et al. | 2019 | 48 | 1.9 | 2 | 93 | 2.1% | 93.8% |
| Weigl et al. | 2019a | 22 | 1.0 | 0 | 22 | 0% | 100% |
| Pol et al. | 2019 | 30 | 1.0 | 0 | 30 | 0% | 100% |
| Cheng et al. | 2019 | 36 | 1.0 | 1 | 36 | 2.7% | 92.0% |
| Mangano & Veronesi | 2018 | 25 | 1.0 | 1 | 25 | 3.9% | 88.7% |
| Total | 394 | 1.6 | 8 | 611 | |||
| Summary estimate (95% CI)* | 1.31% (0.76%−2.27%) | 96.1% (93.4%−97.8%) | |||||
| Veneered‐reinforced glass‐ceramic SCs | |||||||
| Laass et al. | 2019 | 20 | 4.5 | 1 | 89 | 1.1% | 96.7% |
| Kraus et al. Group B | 2019 | 20 | 2.7 | 4 | 53 | 7.3% | 79.7% |
| Teichmann et al. | 2017 | 22 | 11.9 | 1 | 262 | 0.4% | 98.9% |
| Zembic et al. | 2015 | 31 | 11.3 | 0 | 350 | 0% | 100% |
| Total | 93 | 8.1 | 6 | 754 | |||
| Summary estimate (95% CI)* | 0.80% (0.14%−4.64%) | 97.6% (87.0%−99.6%) | |||||
| Monolithic‐reinforced glass‐ceramic SCs | |||||||
| Koller et al. | 2020 | 15 | 6.7 | 1 | 101 | 1.0% | 97.1% |
| Gierthmuehlen et al. | 2020 | 45 | 1.1 | 0 | 49 | 0% | 100% |
| Wittneben et al. Group A | 2020 | 20 | 2.8 | 1 | 56 | 1.8% | 94.8% |
| Rammelsberg et al. Group D | 2020 | 3 | 4.3 | 2 | 13 | 14.3% | 63.0% |
| Rammelsberg et al. Group E | 2020 | 7 | 4.5 | 1 | 32 | 3.1% | 91.1% |
| Linkevicius et al. | 2018 | 56 | 1.0 | 0 | 56 | 0% | 100% |
| Joda et al. | 2017 | 50 | 2.0 | 0 | 100 | 0% | 100% |
| Cooper et al. | 2016 | 128 | 2.4 | 2 | 307 | 0.6% | 98.1% |
| Paolantoni et al. Group A | 2016 | 29 | 4.0 | 3 | 116 | 2.6% | 92.5% |
| Peron & Romanos | 2016 | 26 | 1.1 | 2 | 29 | 6.7% | 81.3% |
| Guarnieri et al. | 2015 | 20 | 5.0 | 0 | 100 | 0% | 100% |
| Vanlioglu et al. | 2012 | 23 | 5.0 | 0 | 115 | 0% | 100% |
| Canullo | 2007 | 30 | 3.3 | 0 | 100 | 0% | 100% |
| Total | 452 | 2.6 | 12 | 1174 | |||
| Summary estimate (95% CI)* | 1.02% (0.51%−2.05%) | 97.0% (94.0%−98.5%) | |||||
| Veneered densely sintered alumina SCs | |||||||
| Fenner et al. | 2016 | 17 | 7.2 | 0 | 122 | 0% | 100% |
| Ormianer et al. | 2006 | 22 | 1.5 | 1 | 33 | 3.0% | 91.3% |
| Henriksson & Jemt | 2003 | 24 | 1.0 | 0 | 24 | 0% | 100% |
| Andersson et al. | 1998 | 65 | 4.5 | 4 | 295 | 1.3% | 96.0% |
| Total | 128 | 3.7 | 5 | 474 | |||
| Summary estimate (95% CI)* | 1.05% (0.49%−2.29%) | 96.9% (93.4%−98.6%) | |||||
| Resin‐matrix ceramic SCs | |||||||
| Augustín‐Pandero et al. | 2020 | 25 | 3.9 | 7 | 98 | 6.9% | 80.7% |
| Schepke et al. | 2016 | 50 | 0.7 | 43 | 35 | 57.7% | 7.6% |
| Total | 75 | 1.8 | 55 | 133 | |||
| Summary estimate 95% CI)* | 33.8% (4.36%−261.6%) | 36.3% (0.04%−87.7%) | |||||
C.I. stands for “confidence interval.”
Based on robust Poisson regression.
The meta‐analysis revealed an estimated annual failure rate of 0.80% (95% CI: 0.14%–4.64%) (Figure 3), translating into a 3‐year survival rate of 97.6% (95% CI: 87.0%–99.6%) (Table 3) for veneered‐reinforced glass‐ceramic implant‐supported SCs, annual failure rate of 1.02% (95% CI: 0.51%–2.05%) (Figure 4) and 3‐year survival rate of 97.0% (95% CI: 94.0%–98.5%) for monolithic‐reinforced glass‐ceramic implant‐supported SCs, annual failure rate of 1.05% (95% CI: 0.49%–2.29%) (Figure 5) and 3‐year survival rate of 96.9% (95% CI: 93.4%–98.6%) for densely sintered alumina implant‐supported SCs, annual failure rate of 1.27% (95% CI: 0.77%–2.10%) (Figure 6) and 3‐year survival rate of 96.3% (95% CI: 93.9%–97.7%) for veneered zirconia implant‐supported SCs, annual failure rate of 1.31% (95% CI: 0.76%–2.27%) (Figure 7) and 3‐year survival rate of 96.1% (95% CI: 93.4%–97.8%) for monolithic zirconia implant‐supported SCs, and annual failure rate of 33.8% (95% CI: 4.36%–261.6%) (Figure 8) and 3‐year survival rate of 36.3% (95% CI: 0.04%–87.7%) for RMC implant‐supported SCs (Table 3). Investigating formally the relative failure rates of different types of implant‐supported SCs, when the monolithic zirconia SCs were taken as reference, there was no statistically significant difference between the SC materials with the exception of the RMC SCs with an annual failure rate of 33.8% (Table 4).
FIGURE 3.
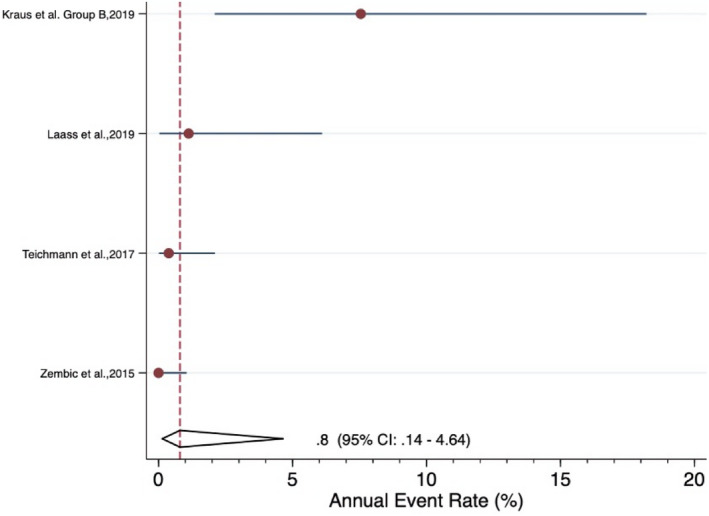
Forrest plot for the annual failure rate of veneered‐reinforced glass‐ceramic implant‐supported SCs
FIGURE 4.
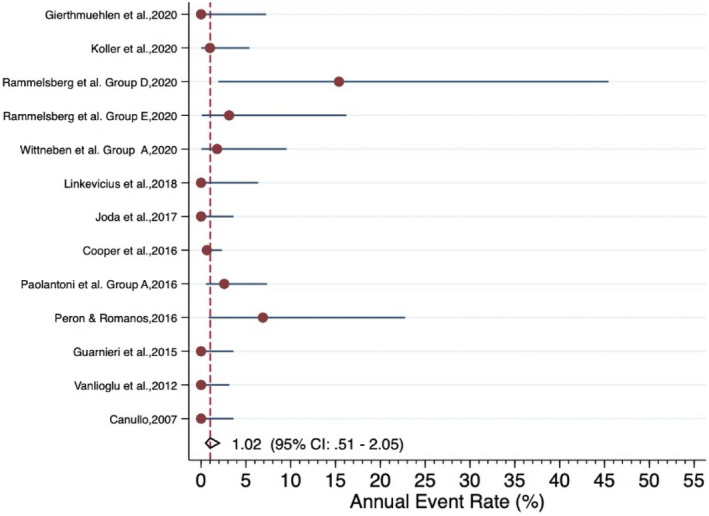
Forrest plot for the annual failure rate of monolithic‐reinforced glass‐ceramic implant‐supported SCs
FIGURE 5.
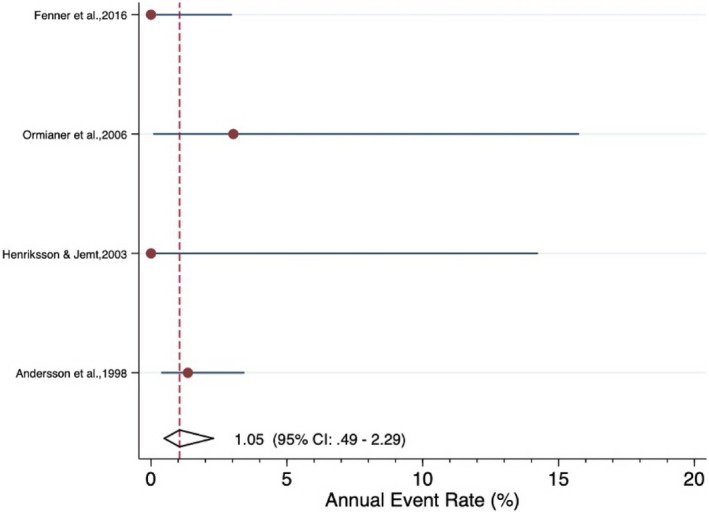
Forrest plot for the annual failure rate of veneered densely sintered alumina implant‐supported SCs
FIGURE 6.
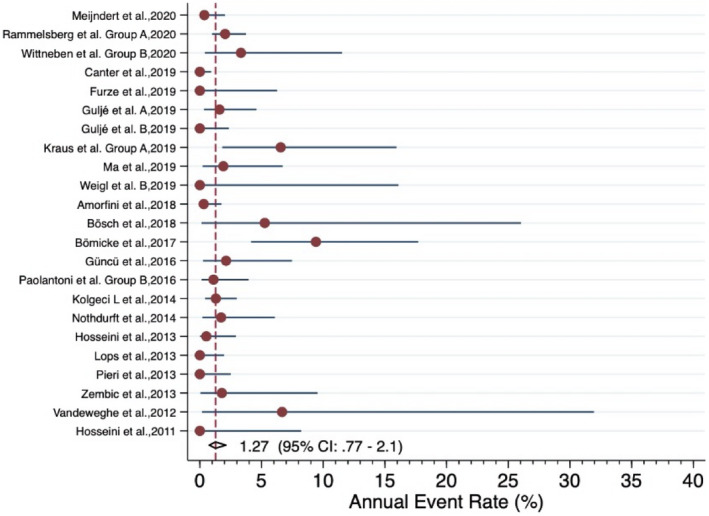
Forrest plot for the annual failure rate of veneered zirconia implant‐supported SCs
FIGURE 7.
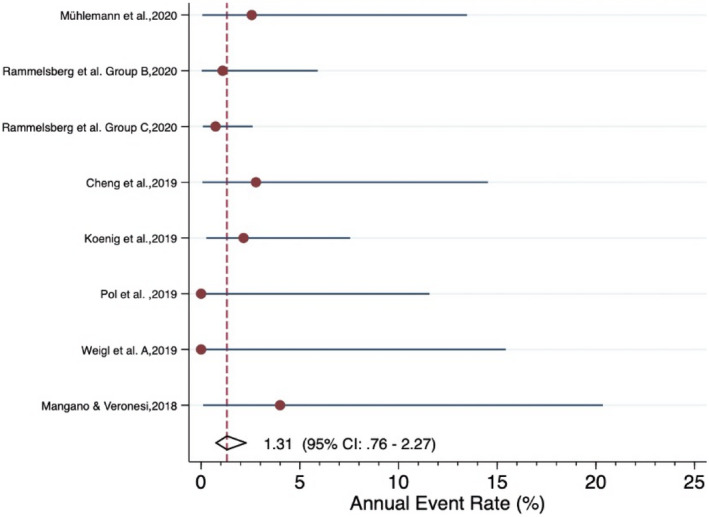
Forrest plot for the annual failure rate of monolithic zirconia implant‐supported SCs
FIGURE 8.
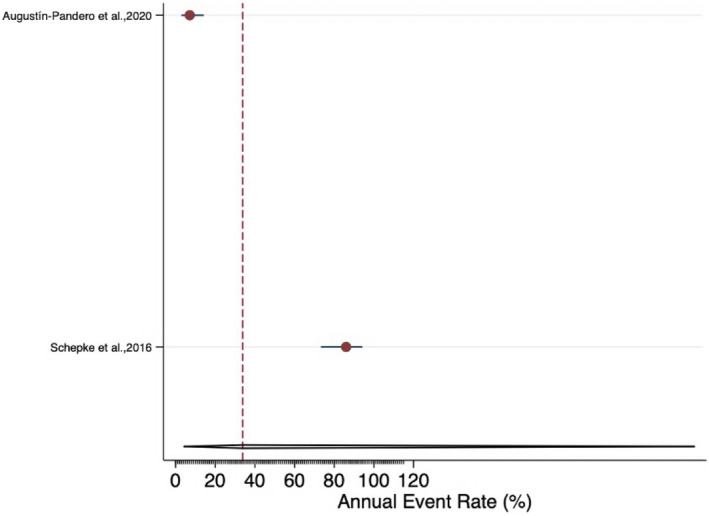
Funnel plot for the annual failure rate of resin‐matrix ceramic implant‐supported SCs
TABLE 4.
Summary of annual failure rates, relative failure rates, and survival estimates for SCs with implant‐supported monolithic zirconia crowns as reference
| Type of SCs | Total number of SCs | Total SCs exposure time | Mean SCs follow‐up time | Estimated annual failure rate* (95% CI) | 3‐year survival summary estimate* (95% CI) | Relative failure rate** (95% CI) | p‐value** |
|---|---|---|---|---|---|---|---|
| Monolithic zirconia SCs | 394 | 611 | 1.6 | 1.31% (0.76%−2.27%) | 96.1% (93.4%−97.8%) | 1.00 (Ref.) | |
| Monolithic‐reinforced glass‐ceramic SCs | 452 | 1174 | 2.6 | 1.02% (0.51%−2.05%) | 97.0% (94.0%−98.5%) | 0.78 (0.33–1.83) | p = 0.568 |
| Veneered zirconia SCs | 952 | 3633 | 3.8 | 1.27% (0.77%−2.1%) | 96.3% (93.9%−97.7%) | 0.97 (0.47–1.98) | p = 0.927 |
| Veneered‐reinforced glass‐ceramic SCs | 93 | 754 | 8.1 | 0.80% (0.14%−4.64%) | 97.6% (87.0%−99.6%) | 0.61 (0.12–3.09) | p = 0.548 |
| Veneered densely sintered alumina SCs | 128 | 474 | 3.7 | 1.05% 0.49%−02.29%) | 96.9% (93.4%−98.6%) | 0.81 (0.34–1.89) | p = 0.619 |
| Resin Nano Ceramic SCs | 75 | 133 | 1.8 | 33.8% (4.36%−261.6%) | 36.3% (0.04%−87.7%) | 25.8 (5.48–121.56) | p < 0.0001 |
C.I. stands for “confidence interval.”
Based on robust Poisson regression.
Based on multivariable robust Poisson regression including all types of SCs.
Investigating the number of implant‐supported SCs that failed due to ceramic fractures such as catastrophic fracture of the veneering material, fracture of the core or fracture of the ceramic abutment, and the lowest fracture rate was reported for densely sintered alumina implant‐supported SCs. None of the 128 included SCs were lost due to fractures over an average observation period of 3.7 years. The annual fracture rate for monolithic zirconia SCs was 0.58%, for monolithic‐reinforced glass‐ceramic SCs was 0.60%, for veneered‐reinforced glass‐ceramic SCs was 0.62%, and for veneered zirconia SCs was 0.98%. RMC SCs, however, showed statistically significantly higher (p < 0.0001) annual fracture rate or 6.08% (Table 5).
TABLE 5.
Summary of annual failure rates due to ceramic fractures, relative failure rates and failure estimate for SCs with implant‐supported monolithic zirconia crowns as reference.
| Type of SCs | Total number of SCs | Total SCs exposure time | Mean SCs follow‐up time | Estimated annual failure rate* (95% CI) | 3‐year failure summary estimate* (95% CI) | Relative failure rate** (95% CI) | p‐value** |
|---|---|---|---|---|---|---|---|
| Monolithic zirconia SCs | 346 | 518 | 1.5 | 0.58% (0.26%−1.31%) | 1.72% (0.77%−3.84%) | 1.00 (Ref.) | |
| Monolithic‐reinforced glass‐ceramic SCs | 449 | 1161 | 2.6 | 0.60% (0.19%−1.89%) | 1.79% (0.57%−5.52%) | 1.04 (0.27–3.98) | p = 0.953 |
| Veneered zirconia SCs | 892 | 3362 | 3.8 | 0.98% (0.55%−1.76%) | 2.90% (1.63%−5.14%) | 1.69 (0.65–4.40) | p = 0.278 |
| Veneered‐reinforced glass‐ceramic SCs | 110 | 801 | 7.3 | 0.62% (0.17%−2.27%) | 1.86% (0.51%−6.58%) | 1.08 (0.27–4.33) | p = 0.916 |
| Veneered densely sintered alumina SCs | 128 | 474 | 3.7 | 0% (0%−9.27%) | 0% (0%−22.57%) | 1.67−7 (3.34−8–8.40−7) | p < 0.0001 |
| Resin Nano Ceramic SCs | 75 | 133 | 1.8 | 6.08% (5.97%−6.19%) | 16.68% (16.41%−16.95%) | 25.8 (5.48–121.56) | p < 0.0001 |
C.I. stands for “confidence interval.”
Based on robust Poisson regression.
Based on multivariable robust Poisson regression including all types of SCs.
Meta‐analysis, comparing the overall failure rates and fracture rates of monolithic and veneered implant‐supported SCs (Table 6), monolithic and veneered zirconia implant‐supported SCs (Table 7), monolithic and veneered‐reinforced glass‐ceramic implant‐supported SCs (Table 8), veneered zirconia and veneered‐reinforced glass‐ceramic implant‐supported SCs (Table 9), and monolithic zirconia and monolithic‐reinforced glass‐ceramic implant‐supported SCs (Table 10), did not reveal any statistical significant differences between the materials compared. Furthermore, the overall failure rates and number of failures due to ceramic fractures were analyzed in relation to the position of the implant‐supported SCs in the mouth (anterior vs. posterior) for monolithic and veneered zirconia ceramic SCs and for monolithic and veneered‐reinforced glass‐ceramic implant‐supported SCs (Table 11). With the exception of monolithic‐reinforced glass‐ceramic reporting no failure due to ceramic fractures in the posterior area, the location of the SCs in the dental arch did not significantly influence the failure or fracture rates for any of the crown materials evaluated (Table 11). Meta‐analysis evaluating the number of SCs that failed due to fracture of the core material concluded with low annual failure rates ranging from 0% to 0.25% with the exception of RMC with an annual failure rate of 6.08% (Table 12). The same applied for SCs that were lost due to fracture of implant abutment with an annual failure rate ranging between 0% and 0.5% (Table 12).
TABLE 6.
Comparison of annual failure and complication rates for veneered and monolithic implant‐supported SCs
| Failures/Complications | n studies |
Veneered Estimated annual failure rate* (95% CI) |
n studies |
Monolithic Estimated annual failure rate* (95% CI) |
p‐value |
|---|---|---|---|---|---|
| Overall failure rate | 27 | 1.18* (0.72–1.94) | 21 | 1.12* (0.70–1.78) | p = 0.869 |
| Overall failure rate due to ceramic fractures | 27 | 0.91* (0.53–1.56) | 19 | 0.60* (0.26–1.35) | p = 0.386 |
| Failures due to core fractures | 26 | 0.14* (0.04–0.42) | 17 | 0.22* (0.03–1.54) | p = 0.662 |
| Failures due to catastrophic veneer fractures | 27 | 0.60* (0.28–1.29) | 19 | 0.32* (0.10–1.02) | p = 0.371 |
| Failures due to abutment fractures | 28 | 0.28* (0.11–0.69) | 19 | 0.13* (0.02–0.72) | p = 0.426 |
| Overall complication rate | 14 | 3.92* (2.34–6.52) | 9 | 1.83* (0.97–3.45) | p = 0.061 |
| Ceramic chippings | 24 | 1.65* (0.90–3.01) | 18 | 0.39* (0.14–1.10) | p = 0.017 |
| Screw loosening | 16 | 0.51* (0.23–1.17) | 12 | 0.27* (0.08–0.94) | p = 0.394 |
| Loss of retention | 13 | 0.15* (0.05–0.43) | 9 | 0.94* (0.21–4.22) | p = 0.045 |
| Soft tissue complications | 15 | 2.58* (1.25–5.27) | 4 | 1.24* (0.60–2.56) | p = 0.138 |
| Bone loss >2 mm | 12 | 0.39* (0.17–0.89) | 7 | 0.62* (0.17–2.22) | p = 0.530 |
C.I. stands for “confidence interval.”
Based on robust Poisson regression.
TABLE 7.
Comparison of annual failure and complication rates for veneered and monolithic zirconia implant‐supported SCs
| Failures/Complications | n studies |
Veneered Estimated annual failure rate* (95% CI) |
n studies |
Monolithic Estimated annual failure rate* (95% CI) |
p‐value |
|---|---|---|---|---|---|
| Overall failure rate | 23 | 1.27* (0.77–2.10) | 8 | 1.31* (0.76–2.27) | p = 0.928 |
| Overall failure rate due to ceramic fractures | 22 | 0.98* (0.55–1.76) | 7 | 0.57* (0.26–1.31) | p = 0.282 |
| Failures due to core fractures | 21 | 0.17* (0.06–0.53) | 5 | 0* (0–11.90) | p < 0.0001 |
| Failures due to catastrophic veneer fractures | 22 | 0.71* (0.33–1.55) | 7 | 0.19* (0.02–2.03) | p = 0.275 |
| Failures due to abutment fractures | 23 | 0.23* (0.07–0.76) | 7 | 0.39* (0.13–1.16) | p = 0.517 |
| Overall complication rate | 11 | 4.63* (2.67–8.02) | 2 | 3.64* (0.43–30.85) | p = 0.777 |
| Ceramic chippings | 19 | 1.84* (0.93–3.64) | 7 | 0.39* (0.07–2.00) | p = 0.071 |
| Screw loosening | 14 | 0.53* (0.20–1.43) | 3 | 2.27* (0.80–6.42) | p = 0.030 |
| Loss of retention | 10 | 0.20* (0.08–0.54) | 3 | 4.55* (1.41–14.66) | p < 0.0001 |
| Soft tissue complications | 13 | 2.77* (1.26–6.07) | 1 | 4.00* (0.10–20.35) | p = 0.356 |
| Bone loss >2 mm | 11 | 0.31* (0.10–0.95) | 3 | 1.00* (0.16–6.07) | p = 0.530 |
C.I. stands for “confidence interval.”
Based on robust Poisson regression.
TABLE 8.
Comparison of annual failure and complication rates for veneered and monolithic‐reinforced glass‐ceramic implant‐supported SCs.
| Failures/Complications | n studies |
Veneered Estimated annual failure rate* (95% CI) |
n studies |
Monolithic Estimated annual failure rate* (95% CI) |
p‐value |
|---|---|---|---|---|---|
| Overall failure rate | 4 | 0.80* (0.14–4.64) | 13 | 1.02* (0.51–2.05) | p = 0.775 |
| Overall failure rate due to ceramic fractures | 5 | 0.62* (0.17–2.26) | 12 | 0.60* (0.19–1.89) | p = 0.967 |
| Failures due to core fractures | 5 | 0* (0–4.16) | 12 | 0.25* (0.03–1.81) | p < 0.0001 |
| Failures due to catastrophic veneer fractures | 5 | 0.13* (0.01–1.38) | 12 | 0.38* (0.10–1.49) | p = 0.402 |
| Failures due to abutment fractures | 5 | 0.50* (0.12–2.02) | 12 | 0* (0–7.52) | p < 0.0001 |
| Overall complication rate | 3 | 2.64* (0.94–7.44) | 7 | 1.72* (0.83–3.54) | p = 0.459 |
| Ceramic chippings | 5 | 1.00* (0.66–1.51) | 11 | 0.40* (0.10–1.55) | p = 0.196 |
| Screw loosening | 2 | 0.46* (0.21–1.01) | 9 | 0.10* (0.01–0.74) | p = 0.149 |
| Loss of retention | 3 | 0* (0–3.94) | 6 | 0.25* (0.06–1.07) | p < 0.0001 |
| Soft tissue complications | 2 | 1.59* (0.64–3.94) | 3 | 1.10* (0.41–2.92) | p = 0.527 |
| Bone loss >2mm | 1 | 0.76* (0.09–2.73) | 4 | 0.53* (0.08–3.31) | p = 0.682 |
C.I. stands for “confidence interval”
Based on robust Poisson regression.
TABLE 9.
Comparison of annual failure and complication rates for veneered‐reinforced glass‐ceramic and veneered zirconia implant‐supported SCs
| Failures/Complications | n studies |
Veneered Zir Estimated annual failure rate* (95% CI) |
n studies |
Veneered LDS Estimated annual failure rate* (95% CI) |
p‐value |
|---|---|---|---|---|---|
| Overall failure rate | 23 | 1.27* (0.77–2.10) | 4 | 0.80* (0.14–4.64) | p = 0.577 |
| Overall failure rate due to ceramic fractures | 22 | 0.98* (0.55–1.76) | 5 | 0.62* (0.17–2.26) | p = 0.449 |
| Failures due to core fractures | 21 | 0.17* (0.06–0.53) | 5 | 0* (0–4.16) | p < 0.0001 |
| Failures due to catastrophic veneer fractures | 22 | 0.71* (0.33–1.55) | 5 | 0.13* (0.01–1.38) | p = 0.141 |
| Failures due to abutment fractures | 23 | 0.23* (0.07–0.76) | 5 | 0.50* (0.12–2.02) | p = 0.382 |
| Overall complication rate | 11 | 4.63* (2.67–8.02) | 3 | 2.64* (0.94–7.44) | p = 0.287 |
| Ceramic chippings | 19 | 1.84* (0.93–3.64) | 5 | 1.00* (0.66–1.51) | p = 0.123 |
| Screw loosening | 14 | 0.53* (0.20–1.43) | 2 | 0.46* (0.21–1.01) | p = 0.797 |
| Loss of retention | 10 | 0.20* (0.08–0.54) | 3 | 0* (0–3.94) | p < 0.0001 |
| Soft tissue complications | 13 | 2.77* (1.26–6.07) | 2 | 1.59* (0.64–3.94) | p = 0.289 |
| Bone loss >2 mm | 11 | 0.31* (0.10–0.95) | 1 | 0.76* (0.09–2.73) | p = 0.114 |
C.I. stands for “confidence interval”.
*Based on robust Poisson regression.
TABLE 10.
Comparison of annual failure and complication rates for monolithic‐reinforced glass‐ceramic or monolithic zirconia implant‐supported SCs.
| Failures/Complications | n studies | Monolithic Zir Estimated annual failure rate* (95% CI) | n studies | Monolithic LDS Estimated annual failure rate* (95% CI) | p‐value |
|---|---|---|---|---|---|
| Overall failure rate | 8 | 1.31* (0.76–2.27) | 13 | 1.02* (0.51–2.05) | p = 0.574 |
| Overall failure rate due to ceramic fractures | 7 | 0.57* (0.26–1.31) | 12 | 0.60* (0.19–1.89) | p = 0.954 |
| Failures due to core fractures | 5 | 0* (0–11.90) | 12 | 0.25* (0.03–1.81) | p < 0.0001 |
| Failures due to catastrophic veneer fractures | 7 | 0.19* (0.02–2.03) | 12 | 0.38* (0.10–1.49) | p = 0.614 |
| Failures due to abutment fractures | 7 | 0.39* (0.13–1.16) | 12 | 0* (0–7.52) | p < 0.0001 |
| Overall complication rate | 2 | 3.64* (0.43–30.85) | 7 | 1.72* (0.83–3.54) | p = 0.403 |
| Ceramic chippings | 7 | 0.39* (0.07–2.00) | 11 | 0.40* (0.10–1.55) | p = 0.975 |
| Screw loosening | 3 | 2.27* (0.80–6.42) | 9 | 0.10* (0.01–0.74) | p = 0.005 |
| Loss of retention | 3 | 4.55* (1.41–14.66) | 6 | 0.25* (0.06–1.07) | p = 0.001 |
| Soft tissue complications | 1 | 4.00* (0.10–20.35) | 3 | 1.10* (0.41–2.92) | p = 0.006 |
| Bone loss >2 mm | 3 | 1.00* (0.16–6.07) | 4 | 0.53* (0.08–3.31) | p = 0.591 |
C.I. stands for “confidence interval.”
*Based on robust Poisson regression.
TABLE 11.
Annual overall failure rates, annual ceramic fracture rates and ceramic chipping rates according to the position in the dental arch (anterior and posterior)
| Failures complications | Number of studies | Anterior | Number of studies | Posterior | p‐value |
|---|---|---|---|---|---|
| Estimated annual failure rate (95% CI) | Estimated annual failure rate (95% CI) | ||||
| Overall failure rate | 14 | 1.08* (0.45–2.62) | 24 | 1.45* (0.69–3.09) | p = 0.610 |
| Overall failure rate due to ceramic fractures | 14 | 0.46* (0.15–1.47) | 24 | 0.65* (0.20–2.10) | p = 0.678 |
| Ceramic chippings | 8 | 0.77* (0.37–1.58) | 20 | 1.28* (0.52–3.17) | p = 0.371 |
| Monolithic zirconia | |||||
| Overall failure rate | 0 | n.r. | 4 | 1.72* (0.61–4.87) | n.a. |
| Overall failure rate due to ceramic fractures | 0 | n.r. | 4 | 0.86* (0.11–6.79) | n.a. |
| Ceramic chippings | 0 | n.r. | 4 | 0.86* (0.11–6.79) | n.a. |
| Veneered zirconia | |||||
| Overall failure rate | 6 | 1.93* (0.49–7.61) | 12 | 1.51* (0.55–4.17) | p = 0.770 |
| Overall failure rate due to ceramic fractures | 6 | 0.72* (0.22–2.35) | 12 | 0.87* (0.23–3.22) | p = 0.838 |
| Ceramic chippings | 3 | 1.19* (0.45–3.12) | 10 | 1.70* (0.64–4.52) | p = 0.583 |
| Monolithic LDS | |||||
| Overall failure rate | 5 | 1.04* (0.33–3.34) | 5 | 0.70* (0.08–6.28) | p = 0.739 |
| Overall failure rate due to ceramic fractures | 5 | 0.63* (0.10–4.06) | 5 | 0* (0–7.08) | p < 0.0001 |
| Ceramic chippings | 3 | 0* | 4 | 0* | n.a. |
| Veneered LDS | |||||
| Overall failure rate | 3 | 0.25* (0.01–4.67) | 3 | 1.96* (0.38–10.18) | p = 0.179 |
| Overall failure rate due to ceramic fractures | 3 | 0* | 3 | 0* | n.a. |
| Ceramic chippings | 2 | 1.28* (0.88–1.86) | 2 | 0* (0–4.46) | p < 0.0001 |
n.r. stands for "not reported"; C.I. stands for “confidence interval.”
Based on robust Poisson regression.
TABLE 12.
Overview of biological and technical complications of different types of implant‐supported SCs
| Complications Failures | Number of abutments or SCs | Estimated annual failure/complication rates (95% CI) | Number of abutments or SCs | Estimated annual failure/complication rates (95% CI) | Number of abutments or SCs | Estimated annual failure/complication rates (95% CI) | Number of abutments or SCs | Estimated annual failure/complication rates (95% CI) | Number of abutments or SCs | Estimated annual failure/complication rates (95% CI) | Number of abutments or SCs | Estimated annual failure/complication rates (95% CI) | Number of abutments or SCs | Estimated annual failure/complication rates (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Overall results Estimated annual failure rate* (95% CI) |
Monolithic zirconia SCs Estimated annual failure rate* (95% CI) |
Monolithic‐reinforced glass‐ceramic SCs Estimated annual failure rate* (95% CI) |
Veneered zirconia SCs Estimated annual failure rate* (95% CI) |
Veneered‐reinforced glass‐ceramic SCs Estimated annual failure rate* (95% CI) |
Veneered densely sintered alumina SCs Estimated annual failure rate* (95% CI) |
Resin‐matrix ceramic SCs Estimated annual failure rate* (95% CI) |
||||||||
| Overall complication rate | 888 | 4.21* (2.65–6.60) | 55 | 3.57* (0.43–26.55) | 348 | 1.70* (0.83–3.48) | 317 | 4.52* (2.63–7.71) | 71 | 2.61* (0.93–7.17) | 22 | 14.1* (5.0–27.3) | 75 | 15.54* (12.6–19.1) |
| Overall failures due to ceramic fractures | 1783 | 0.75* (0.43–1.28) | 194 | 0.41* (0.05–3.51) | 449 | 0.60* (0.19–1.87) | 892 | 0.77* (0.39–1.53) | 110 | 0.12* (0.01–1.37) | 63 | 0* (0–5.60) | 75 | 6.08* (5.97–6.19) |
| Failure due to core fractures | 1674 | 0.32* (0.13–0.79) | 152 | 0* (0–11.2) | 474 | 0.25* (0.03–1.79) | 800 | 0.17* (0.06–0.53) | 110 | 0* (0–4.0) | 63 | 0* (0–5.60) | 75 | 5.90* (5.80–6.01) |
| Failure due to abutment fractures | 1941 | 0.23* (0.10–0.50) | 346 | 0.39* (0.13–1.16) | 423 | 0* (0–5.96) | 924 | 0.23* (0.07–0.76) | 110 | 0.50* (0.12–2.00) | 63 | 0* (0–5.60) | 75 | 0* (0–5.25) |
| Ceramic chippings | 1725 | 1.25* (0.69–2.26) | 346 | 0.39* (0.07–1.98) | 373 | 0.40* (0.10–1.54) | 743 | 1.82* (0.93–3.57) | 110 | 1.00* (0.66–1.50) | 128 | 0.64* (0.28–1.48) | 25 | 0* (0–3.6) |
| Screw loosening | 1153 | 0.44* (0.23–0.82) | 88 | 2.25* (0.80–6.22) | 413 | 0.10* (0.01–0.73) | 473 | 0.53* (0.20–1.42) | 51 | 0.45* (0.21–1.00) | 128 | 0.42* (0.13–1.36) | 0 | n.a. |
| Loss of retention | 829 | 1.97* (0.49–7.87) | 54 | 4.44* (1.40–13.63) | 195 | 0.25* (0.06–1.07) | 443 | 0.20* (0.08–0.54) | 40 | 0* (0–3.8) | 22 | 0* (0–10) | 75 | 28.19* (4.95–88.47) |
| Soft tissue complications | 822 | 2.58* (1.43–4.65) | 25 | 3.9* (0.1–18.4) | 192 | 1.1* (0.4–2.9) | 513 | 2.73* (1.25–5.89) | 42 | 1.57* (0.64–3.86) | 24 | 11.8* (2.6–27.6) | 25 | 6.9* (2.9–13.2) |
| Bone loss >2 mm | 614 | 0.52* (0.28–0.98) | 100 | 1.00* (0.16–5.89) | 117 | 0.52* (0.08–3.26) | 350 | 0.31* (0.10–0.94) | 22 | 0.8* (0.1–2.7) | 0 | n.r. | 25 | 2.0* (0.2–6.9) |
n.a. stands for "not available"; n.r. stands for "not reported"; C.I. stands for “confidence interval.”
Based on robust Poisson regression.
3.4. Overall complication rate
Twenty‐six of the included studies, reporting on 888 implant‐supported SCs, evaluated the total number of complications or the number of restorations free of all complications. The overall annual complication rate for the 888 SCs was 4.2%, ranging from 1.7% to 15.5% (Table 12). The annual complication rate of 1.7% was reported for monolithic‐reinforced glass‐ceramic SCs, 2.6% for veneered‐reinforced glass‐ceramics SCs, 3.6% for monolithic zirconia SCs, 4.5% for veneered zirconia SCs, and 14.1% for densely sintered alumina SCs. The highest annual complication rate (15.5%) was reported for RMC implant‐supported SCs (Table 12). Meta‐analysis comparing the overall complication rate of monolithic and veneered‐reinforced glass‐ceramic SCs and monolithic and veneered zirconia ceramic SCs did not reveal any statistically significant difference (Tables 6, 7, 8, 9, 10). However, the overall annual complication rate of 3.9% for the veneered SCs was tendentially higher than the annual complication rate of 1.8% for monolithic SCs (p = 0.061) (Table 6).
3.5. Technical complications
Forty‐five studies, reporting on 1725 implant‐supported SCs, analyzed the incidence of ceramic chipping of the ceramic surface. The estimated average annual chipping rate was 1.25%, ranging from 0% to 1.82%. No surface chippings were reported for RMC SCs, but they showed the annual core fracture rate of 5.90% (Table 12). Veneered SCs generally showed higher annual ceramic chipping rates than monolithic SCs (Tables 6, 7, 8), and meta‐analysis formally comparing the annual chipping rates for veneered SCs (1.65%), and monolithic SCs (0.39%) concluded a statistically significant difference (p = 0.017) between the two crown designs (Table 6). The location of the implant‐supported SC in the dental arch, anterior vs. posterior, did not significantly influence the annual chipping rate (Table 11).
Thirty‐two studies with 1153 implant‐supported SCs reported an annual rate of 0.44% for loosening of the abutment or prosthetic screws (Table 12). The highest screw‐loosening rate (2.25%) was reported for monolithic zirconia SCs. The difference between the screw‐loosening rates of monolithic zirconia SCs and all the other SCs types reached statistically significant difference (p < 0.02) (Tables 7, 10, 12).
Twenty‐five studies with 829 cemented implant‐supported SCs reported an annual complication rate of 1.97% for loss of retention (Table 12). The highest annual rate of retention loss (28.19%) was reported for RMC SCs. This problem was mainly related to one study (Schepke et al., 2016), in which majority of the resin‐matrix ceramic SCs were remade out of different restorative material due to cementation failures. The second‐highest rate of loss of retention, 4.44%, was reported for monolithic zirconia implant‐supported SCs. This result was also related to one study (Koenig et al., 2019) where 7 out of 48 SCs lost retention and the authors reported changing the cementation protocol during the study period due to this problem.
3.6. Biological complications
Peri‐implant mucosal lesions or soft tissue complications were reported in various ways by different authors. Twenty‐one of the included studies with 822 implant‐supported SCs reported a mean overall annual rate for soft tissue complication of 2.58%, ranging from 1.1% to 11.8% (Table 12). The lowest annual soft tissue complication rate of 1.1% was reported for monolithic‐reinforced glass‐ceramic SCs, followed by veneered‐reinforced glass‐ceramics SCs (1.57%), veneered zirconia SCs (2.73%), and monolithic zirconia SCs (3.9%). Significantly (p < 0.0001) higher soft tissue complication rates, 6.9% and 11.8% respectively, were reported for RMC and densely sintered alumina SCs (Table 12).
Twenty of the included studies reported on the number of implants with significant (> 2 mm) bone loss. The way bone loss is evaluated on radiographs and reported seems to be more standardized than the soft tissue evaluation. The reported incidence of annual rate of bone loss ranged only from 0.31% to 2% with an average annual complication rate of 0.52% (Table 12).
3.7. Quality assessment of the included studies
The quality assessment of the included RCTs and prospective studies was conducted with the Newcastle–Ottawa Scale (NOS) for cohort investigations (Table 13). Most of the studies were judged to have moderate‐to‐high methodological quality (NOS Score 6, 7, or 8 points from 8). Two studies lacked reporting on conflict of interest (Cooper et al., 2016; Vanlioglu et al., 2012). Therefore, methodological quality was judged to be moderate in some of the studies (NOS Score 6‐7/9). A maximum score of eight stars (NOS) could be assigned to the investigations that were succeed by 8 criteria as follows: (1) representativeness of cases, (2) ascertainment of exposure, (3) demonstration outcome of interest not present at start of study, (4–5) comparability in age of the patients and implants location, (6) assessment of outcome, (7) follow‐up long enough, and (8) adequacy of follow‐up (Table 13).
TABLE 13.
Quality assessment based on Newcastle–Ottawa Scale (NOS)
| Study | Selection | Comparability | Outcome | Score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Representativeness of cases | Selection of controls (RCT ‐ control group of exposure from the same cohort) | Ascertainment of exposure | Demonstration outcome of interest not present at start of study | Age | Location | Assessment of outcome | Follow‐up long enough | Adequacy of follow‐up | Total |
| Wittneben et al. Group B | 2020 | * | * | * | * | * | * | * | 7 | ||
| Meijndert et al. | 2020 | * | * | * | * | * | * | * | * | 8 | |
| Rammelsberg et al. Group A | 2020 | * | * | * | * | * | * | * | * | 8 | |
| Furze et al. | 2019 | * | * | * | * | * | * | * | 7 | ||
| Weigl et al. | 2019 | * | * | * | * | * | * | 6 | |||
| Heierle et al. Group A | 2019 | * | * | * | * | * | * | 6 | |||
| Ma et al. | 2019 | * | * | * | * | * | * | * | * | 8 | |
| Guljé et al. (RCT) | 2019 | * | * | * | * | * | * | * | * | 8 | |
| Guljé et al. (PRO) | 2019 | * | * | * | * | * | * | * | * | 8 | |
| Kraus et al. Group A | 2019 | * | * | * | * | * | * | * | 7 | ||
| Canter et al. | 2019 | * | * | * | * | * | * | * | * | 8 | |
| Amorfini et al. | 2018 | * | * | * | * | * | * | * | * | 8 | |
| Bösch et al. | 2018 | * | * | * | * | * | * | * | * | 8 | |
| Bömicke et al. | 2017 | * | * | * | * | * | * | * | * | 8 | |
| Güncü et al. | 2016 | * | * | * | * | * | * | * | * | 8 | |
| Paolantoni et al. Gruppe B | 2016 | * | * | * | * | * | * | * | * | 8 | |
| Kolgeci et al. | 2014 | * | * | * | * | * | * | * | * | 8 | |
| Nothdurft et al. | 2014 | * | * | * | * | * | * | * | 7 | ||
| Hosseini et al. | 2013 | * | * | * | * | * | * | * | * | 8 | |
| Lops et al. | 2013 | * | * | * | * | * | * | * | * | 8 | |
| Pieri et al. | 2013 | * | * | * | * | * | * | * | 7 | ||
| Zembic et al. | 2013 | * | * | * | * | * | * | * | * | 8 | |
| Vandeweghe et al. | 2012 | * | * | * | * | * | * | * | 7 | ||
| Hosseini et al. | 2011 | * | * | * | * | * | * | * | 7 | ||
| Rammelsberg et al. Group B | 2020 | * | * | * | * | * | * | * | 7 | ||
| Rammelsberg et al. Group C | 2020 | * | * | * | * | * | * | * | 7 | ||
| Mühlemann et al. | 2020 | * | * | * | * | * | * | * | 7 | ||
| Koenig et al. | 2019 | * | * | * | * | * | * | * | 7 | ||
| Weigl et al. | 2019 | * | * | * | * | * | * | * | 7 | ||
| Pol et al. | 2019 | * | * | * | * | * | * | * | 7 | ||
| Cheng et al. | 2019 | * | * | * | * | * | * | * | 7 | ||
| Mangano & Veronesi | 2018 | * | * | * | * | * | * | * | 7 | ||
| Laass et al. | 2019 | * | * | * | * | * | * | * | * | 8 | |
| Heierle et al. Group B | 2019 | * | * | * | * | * | * | 6 | |||
| Kraus et al. Group B | 2019 | * | * | * | * | * | * | * | 7 | ||
| Teichmann et al. | 2017 | * | * | * | * | * | * | * | * | 8 | |
| Zembic et al. | 2015 | * | * | * | * | * | * | * | * | 8 | |
| Koller et al. | 2020 | * | * | * | * | * | * | * | 7 | ||
| Gierthmuehlen et al. | 2020 | * | * | * | * | * | * | * | * | 8 | |
| Wittneben et al. Group A | 2020 | * | * | * | * | * | * | * | 7 | ||
| Rammelsberg et al. Group D | 2020 | * | * | * | * | * | * | * | * | 8 | |
| Rammelsberg et al. Group E | 2020 | * | * | * | * | * | * | * | * | 8 | |
| Cömlekoglu et al. | 2019 | * | * | * | * | * | * | * | 7 | ||
| Linkevicius et al. | 2018 | * | * | * | * | * | * | 6 | |||
| Joda et al. | 2017 | * | * | * | * | * | * | 6 | |||
| Cooper et al. | 2016 | * | * | * | * | * | * | * | 7 | ||
| Paolantoni Gruppe A et al. | 2016 | * | * | * | * | * | * | * | * | 8 | |
| Peron & Romanos et al. | 2016 | * | * | * | * | * | * | * | 7 | ||
| Guarnieri | 2015 | * | * | * | * | * | * | * | * | 8 | |
| Vanlioglu et al. | 2012 | * | * | * | * | * | * | 6 | |||
| Canullo et al. | 2007 | * | * | * | * | * | * | * | * | 8 | |
| Fenner et al. | 2016 | * | * | * | * | * | * | * | 7 | ||
| Ormianer et al. | 2006 | * | * | * | * | * | * | * | 7 | ||
| Henrikson & Jamt | 2003 | * | * | * | * | * | * | * | 7 | ||
| Andersson et al. | 1998 | * | * | * | * | * | * | * | * | 8 | |
| Augustín‐Pandero et al. | 2020 | * | * | * | * | * | * | * | * | 8 | |
| Schepke et al. | 2016 | * | * | * | * | * | * | 6 | |||
Asterisk (*) means criteria is fulfilled and every asterisk stands for one score.
4. DISCUSSION
The present systematic review and meta‐analysis showed that the implant‐supported SCs made of different all‐ceramic materials with veneered or monolithic designs have similar 3‐year survival rates and low annual failure rates with the exception of RMC SCs. The main technical problem leading to failure of the SCs was identified as ceramic fractures, that is, catastrophic veneer fracture, core fracture and ceramic abutment fracture for the veneered and monolithic‐reinforced glass‐ceramic, and zirconia restorations, whereas RMC SCs predominantly failed due to core fractures and repeated loss of retention.
The monolithic design of the implant‐supported SCs was revealed to influence significantly the annual ceramic chipping rates (p = 0.017), monolithic zirconia, and monolithic‐reinforced glass‐ceramic SCs showed lower annual ceramic chipping rate than veneered ones. The monolithic zirconia implant‐supported SCs, however, demonstrated more frequently the loss of retention (fracture of the luting cement) and screw loosening compared with veneered zirconia SCs. Moreover, the anterior/posterior position of the SC showed no influence on the survival and complication rates for any prosthetic material and crown design.
The previously reported high short‐term (3 to 5 years) survival rates for zirconia, reinforced glass‐ceramic, and alumina implant‐supported SCs (Pjetursson et al., 2018; Rabel et al., 2018) is affirmed by the present systematic review. Moreover, the result by Rabel et al. (2018) reporting no statistical difference in terms of survival rates between the oxide ceramics and the glass‐ceramics is in accordance with the present systematic review's findings. RMC SCs on the contrary showed unfavorable 3‐year estimated survival rate of 36.3%, and the difference was statistically significant (p<0.0001). This result thought to be due to the fact that only two of the included studies investigated RMC SCs in which one reported repeated loss of retention concerning the majority of the SCs (43 out of 50 SCs) in a short follow‐up period (Schepke et al., 2016). Eventually, the investigators replaced all initially included SCs with lithium disilicate SCs (Schepke et al., 2018). In the second study, Augustin‐Pandero and co‐workers (Agustín‐Panadero et al., 2020) reported an annual failure rate of 6.9%, and the main reason for failures was crown material fractures.
Since the first introduction of dental implants, clinical outcomes of implant‐supported restorations improved significantly thanks to the positive learning curve in implant dentistry (Pjetursson et al., 2014), improvements and innovations in biomaterials and better handling/processing of the restorative materials (Larsson & Wennerberg, 2014). Even though chipping of the veneering ceramic still remains as one of the important concerns for implant‐supported SCs, reported to be observed less and less in the more recent publications (Larsson & Wennerberg, 2014). Due to the short follow‐up period of monolithic zirconia restorations (mean follow‐up:1.6 year), in the present meta‐analysis, 3‐year estimation for the survival and complications rates was done. Interestingly, the chipping rates for all‐ceramic SCs were notably lower than reported by Rabel et al. (2018) which reported 5‐year chipping rates of 11.8% for veneered zirconia, 3.5% for veneered‐reinforced glass‐ceramic, and 6% for monolithic‐reinforced glass‐ceramic. No significant difference was detected among the material groups in the same systematic review, whereas the 3‐year estimated chipping rates based on present meta‐analysis were 5.4% for veneered zirconia SCs, 3% for veneered‐reinforced glass‐ceramic and 1.2% for monolithic‐reinforced glass‐ceramic, with no statistically significant difference. The chipping rate differences between the two meta‐analysis can be explained by the quality of the included studies, as the retrospective studies were excluded in the present meta‐analysis and the improved handling of the restorative materials namely zirconia over the years. Moreover, in the present systematic review, the failure due to ceramic fractures was separately reported while the ceramic chipping that was considered as technical complication was solely the ceramic fractures that are repairable and/or polishable. However, a distinction as major chipping/minor chipping/surface roughness when it comes to chipping as a technical complication was not made due to lack of uniformity throughout the included studies regarding the definitions. This approach shows a difference when compared to the previous systematic review by Rabel et al. (2018).
In the present systematic review, the data obtained through included clinical studies allowed to make a direct comparison between the prosthetic materials, that is, zirconia and reinforced‐glass‐ceramic that are available both monolithic/micro‐veneered and veneered crown designs. The statistical analysis comparing directly the monolithic and veneered implant‐supported SCs was done based on material groups that have both monolithic and veneered designs, hence the alumina and RMC SCs were excluded from this analysis (Table 6, 7, 8, 9, 10, 11).
Based on the present meta‐analysis, the loss of retention was observed as an important technical complication. Loss of retention was significantly higher for overall analysis of monolithic compared veneered SCs, which can be due to a single study reporting 7 loss of retention events by Koenig et al on 48 monolithic zirconia SCs while the other studies remained eventless in this aspect. Furthermore, according to an in vitro study by Pitta et al. (2020) the cementation protocol and cement preference plays an important role on the mechanical stability of the SCs supported by titanium bases (Pitta et al., 2020).
Monolithic zirconia and monolithic‐reinforced glass‐ceramics, as they enable the complete digital work‐flow, are becoming more and more widely used for implant‐supported SCs. Accordingly, identifying the predominant reasons for their failure and complication is important. The failure due to core fracture was significantly higher for the monolithic‐reinforced glass‐ceramics (p < 0.0001), whereas monolithic zirconia SCs failed more due to abutment fracture (p < 0.0001), which can be explained by the mechanical properties as higher stiffness of zirconia which resulted of the transfer of the forces to less strong components of implant‐crown assembly. Loss of retention was similarly higher for monolithic zirconia SCs, which can be explained with the same mechanism.
The influence of anterior‐posterior position of the SCs was analyzed in terms of annual failure rate, annual failure rate due to ceramic fracture, and ceramic chipping. The difference between anterior SCs and posterior SCs did not reach statistical significance for either overall prosthetic materials or any specific prosthetic material except for reinforced glass‐ceramic SCs. Posterior monolithic‐reinforced glass‐ceramics showed significantly less annual overall failure rate due to ceramic fracture than anterior ones, 0% and 0.63% (p < 0.0001), respectively. As none of the monolithic zirconia studies included anterior SCs, this analysis was not possible for monolithic zirconia material. This finding is not in accordance with the systematic review by Rabel et al. (2018), in which the posterior all‐ceramic implant‐supported SCs demonstrated significantly higher 5‐year chipping rate than anterior SCs. This difference can be explained by the difference in statistical analysis approaches. In the meta‐analysis by Rabel et al. (2018), the comparison between anterior and posterior SCs was done based on pooled data from all included prosthetic all‐ceramic materials, whereas in the present systematic review, the analysis was done both separately for each material that has both a veneered and monolithic design as well as for the overall materials namely monolithic and veneered zirconia and reinforced glass‐ceramics.
In the present systematic review, only the studies investigated SCs supported by titanium dental implants were included. SCs supported by zirconia implants reported by Rabel et al. (2018) to be more prone to technical complications namely chipping rate. Accordingly, the rationale behind the exclusion of the studies that investigated zirconia implant‐supported SCs was to avoid any cofounding factor that might influence the clinical behavior of different prosthetic materials.
The scientific evidence procured by this systematic review is based on studies that were assessed as moderate‐to‐high quality based on Newcastle–Ottawa scale. All included studies were either RCTs (n = 20) or prospective studies (n = 29) therefore at lower risk of bias compared with retrospective studies (Papageorgiou et al., 2015). As none of the included RCTs were directly addressing the focus question of the present SR, they were considered as prospective cohort studies rather than RCTs and therefore assessed by the Newcastle–Ottawa scale for quality assessment and not with the Cochrane Risk of Bias Tool that is designed for RCTs. However, the included studies were predominantly small and this might introduce small‐study effects (Cappelleri et al., 1996).
5. CONCLUSIONS
Based on the data identified by this systematic review, veneered and monolithic implant‐supported ceramic SCs showed high short‐term survival rates and low complication rates. Significantly higher rates of ceramic chipping were reported for veneered SCs when compared to monolithic SCs, with the exception of RMC SCs. The location of the implant‐supported ceramic SCs, anterior vs. posterior, did not influence survival and chipping rates. However, conclusions on the long‐term clinical performance of the presently evaluated type of restorations should not be drawn based on short‐to‐medium term clinical studies included in the present systematic review.
CONFLICT OF INTEREST
The authors have no specific conflict of interest related to the present systematic review.
Supporting information
Tables S1‐S2
ACKNOWLEDGMENTS
This systematic review was performed in the context of the virtual EAO Consensus Conference 2021. The authors are grateful to Professor Marcel Zwahlen, Institute of Social and Preventive Medicine, University of Bern, Switzerland, for his help to prepare the statistical analysis. Open Access funding provided by Universite de Geneve.
Pjetursson, B. E. , Sailer, I. , Latyshev, A. , Rabel, K. , Kohal, R.‐J. , & Karasan, D. (2021). A systematic review and meta‐analysis evaluating the survival, the failure, and the complication rates of veneered and monolithic all‐ceramic implant‐supported single crowns. Clinical Oral Implants Research, 32, 254–288. 10.1111/clr.13863
References
REFERENCES
- Agustín‐Panadero, R. , Soriano‐Valero, S. , Labaig‐Rueda, C. , Fernández‐Estevan, L. , & Solá‐Ruíz, M. F. (2020). Implant‐supported metal‐ceramic and resin‐modified ceramic crowns: A 5‐year prospective clinical study. Journal of Prosthetic Dentistry, 124(1), 46–52.e42. 10.1016/j.prosdent.2019.07.002. [DOI] [PubMed] [Google Scholar]
- Amorfini, L. , Storelli, S. , Mosca, D. , Scanferla, M. , & Romeo, E. (2018). Comparison of cemented vs screw‐retained, customized computer‐aided design/computer‐assisted manufacture zirconia abutments for esthetically located single‐tooth implants: A 10‐year randomized prospective study. International Journal of Prosthodontics, 31(4), 359–366. 10.11607/ijp.5305. [DOI] [PubMed] [Google Scholar]
- Andersson, B. , Odman, P. , Lindvall, A. M. , & Brånemark, P. I. (1998). Cemented single crowns on osseointegrated implants after 5 years: results from a prospective study on CeraOne. International Journal of Prosthodontics, 11(3), 212–218. [PubMed] [Google Scholar]
- Bomicke, W. , Gabbert, O. , Koob, A. , Krisam, J. , & Rammelsberg, P. (2017). Comparison of immediately loaded flapless‐placed one‐piece implants and flapped‐placed conventionally loaded two‐piece implants, both fitted with all‐ceramic single crowns, in the posterior mandible: 3‐year results from a randomised controlled pilot trial. European Journal of Oral Implantology, 10(2), 179–195. 10.1002/central/CN-01418624/full. [DOI] [PubMed] [Google Scholar]
- Bösch, A. , Jung, R. E. , Sailer, I. , Goran, B. , Hämmerle, C. H. , & Thoma, D. S. (2018). Single‐tooth replacement using dental implants supporting all‐ceramic and metal‐based reconstructions: Results at 18 months of loading. International Journal of Periodontics & Restorative Dentistry, 38(2), 173–179. 10.11607/prd.2846. [DOI] [PubMed] [Google Scholar]
- Cantner, F. , Cacaci, C. , Mücke, T. , Randelzhofer, P. , Hajtó, J. , & Beuer, F. (2019). Clinical performance of tooth‐ or implant‐supported veneered zirconia single crowns: 42‐month results. Clin Oral Investig, 23(12), 4301–4309. 10.1007/s00784-019-02878-0. [DOI] [PubMed] [Google Scholar]
- Canullo, L. (2007). Clinical outcome study of customized zirconia abutments for single‐implant restorations. International Journal of Prosthodontics, 20(5), 489–493. [PubMed] [Google Scholar]
- Cheng, C. W. , Chien, C. H. , Chen, C. J. , & Papaspyridakos, P. (2019). Randomized controlled clinical trial to compare posterior implant‐supported modified monolithic zirconia and metal‐ceramic single crowns: one‐year results. Journal of Prosthodontics, 28(1), 15–21. 10.1111/jopr.12767. [DOI] [PubMed] [Google Scholar]
- Cooper, L. F. , Stanford, C. , Feine, J. , & McGuire, M. (2016). Prospective assessment of CAD/CAM zirconia abutment and lithium disilicate crown restorations: 2.4 year results. Journal of Prosthetic Dentistry, 116(1), 33–39. 10.1016/j.prosdent.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Erhan Çömlekoğlu, M. , Nizam, N. , & Çömlekoğlu, M. D. (2018). Immediate definitive individualized abutments reduce peri‐implant bone loss: A randomized controlled split‐mouth study on 16 patients. Clinical Oral Investigations, 22(1), 475–486. 10.1007/s00784-017-2136-9. [DOI] [PubMed] [Google Scholar]
- Fenner, N. , Hammerle, C. H. , Sailer, I. , & Jung, R. E. (2016). Long‐term clinical, technical, and esthetic outcomes of all‐ceramic vs. titanium abutments on implant supporting single‐tooth reconstructions after at least 5 years. Clinical Oral Implants Research, 27(6), 716–723. 10.1111/clr.12654. [DOI] [PubMed] [Google Scholar]
- Furze, D. , Byrne, A. , Alam, S. , Brägger, U. , Wismeijer, D. , & Wittneben, J. G. (2019). Influence of the fixed implant‐supported provisional phase on the esthetic final outcome of implant‐supported crowns: 3‐year results of a randomized controlled clinical trial. Clinical Implant Dentistry and Related Research, 21(4), 649–655. 10.1111/cid.12796. [DOI] [PubMed] [Google Scholar]
- Gierthmuehlen, P. C. , Berger, L. , & Spitznagel, F. A. (2020). Monolithic screw‐retained lithium disilicate implant crowns: Preliminary data of a prospective cohort study. International Journal of Prosthodontics, 33(3), 272–276. 10.11607/ijp.6684. [DOI] [PubMed] [Google Scholar]
- Guarnieri, R. , Ceccherini, A. , & Grande, M. (2015). Single‐tooth replacement in the anterior maxilla by means of immediate implantation and early loading: clinical and aesthetic results at 5 years. Clin Implant Dent Relat Res, 17(2), 314–326. 10.1111/cid.12111. [DOI] [PubMed] [Google Scholar]
- Guljé, F. L. , Raghoebar, G. M. , Vissink, A. , & Meijer, H. J. A. (2019a). Single crown restorations supported by 6‐mm implants in the resorbed posterior mandible: A five‐year prospective case series. Clin Implant Dent Relat Res, 21(5), 1017–1022. 10.1111/cid.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guljé, F. L. , Raghoebar, G. M. , Vissink, A. , & Meijer, H. J. A. (2019b). Single crowns in the resorbed posterior maxilla supported by either 11‐mm implants combined with sinus floor elevation or 6‐mm implants: a 5‐year randomised controlled trial. International Journal of Oral Implantology, 12(3), 315–326. 10.1002/central/CN-01988813/full. [DOI] [PubMed] [Google Scholar]
- Guncu, M. B. , Cakan, U. , Aktas, G. , Guncu, G. N. , & Canay, S. (2016). Comparison of implant versus tooth‐supported zirconia‐based single crowns in a split‐mouth design: a 4‐year clinical follow‐up study. Clinical Oral Investigations, 20(9), 2467–2473. 10.1007/s00784-016-1763-x. [DOI] [PubMed] [Google Scholar]
- Heierle, L. , Wolleb, K. , Hämmerle, C. H. , Wiedemeier, D. B. , Sailer, I. , & Thoma, D. S. (2019). Randomized controlled clinical trial comparing cemented versus screw‐retained single crowns on customized zirconia abutments: 3‐year results. International Journal of Prosthodontics, 32(2), 174–176. 10.11607/ijp.6080. [DOI] [PubMed] [Google Scholar]
- Henriksson, K. , & Jemt, T. (2003). Evaluation of custom‐made procera ceramic abutments for single‐implant tooth replacement: a prospective 1‐year follow‐up study. International Journal of Prosthodontics, 16(6), 626–630. [PubMed] [Google Scholar]
- Hosseini, M. , Worsaae, N. , Schiodt, M. , & Gotfredsen, K. (2011). A 1‐year randomised controlled trial comparing zirconia versus metal‐ceramic implant supported single‐tooth restorations. European Journal Oral Implantology, 4(4), 347–361. [PubMed] [Google Scholar]
- Hosseini, M. , Worsaae, N. , Schiødt, M. , & Gotfredsen, K. (2013). A 3‐year prospective study of implant‐supported, single‐tooth restorations of all‐ceramic and metal‐ceramic materials in patients with tooth agenesis. Clinical Oral Implants Research, 24(10), 1078–1087. 10.1111/j.1600-0501.2012.02514.x. [DOI] [PubMed] [Google Scholar]
- Joda, T. , Ferrari, M. , & Bragger, U. (2017). Monolithic implant‐supported lithium disilicate (LS2) crowns in a complete digital workflow: A prospective clinical trial with a 2‐year follow‐up. Clinical Implant Dentistry and Related Research, 19(3), 505–511. 10.1111/cid.12472. [DOI] [PubMed] [Google Scholar]
- Koenig, V. , Wulfman, C. , Bekaert, S. , Dupont, N. , Le Goff, S. , Eldafrawy, M. , Vanheusden, A. , & Mainjot, A. (2019). Clinical behavior of second‐generation zirconia monolithic posterior restorations: Two‐year results of a prospective study with Ex vivo analyses including patients with clinical signs of bruxism. Journal of Dentistry, 91, 10.1016/j.jdent.2019.103229. [DOI] [PubMed] [Google Scholar]
- Kolgeci, L. , Mericske, E. , Worni, A. , Walker, P. , Katsoulis, J. , & Mericske‐Stern, R. (2014). Technical complications and failures of zirconia‐based prostheses supported by implants followed up to 7 years: a case series. International Journal of Prosthodontics, 27(6), 544–552. 10.11607/ijp.3807. [DOI] [PubMed] [Google Scholar]
- Koller, M. , Steyer, E. , Theisen, K. , Stagnell, S. , Jakse, N. , & Payer, M. (2020). Two‐piece zirconia versus titanium implants after 80 months: Clinical outcomes from a prospective randomized pilot trial. Clinical Oral Implants Research, 31(4), 388–396. 10.1111/clr.13576. [DOI] [PubMed] [Google Scholar]
- Kraus, R. D. , Epprecht, A. , Hämmerle, C. H. F. , Sailer, I. , & Thoma, D. S. (2019). Cemented vs screw‐retained zirconia‐based single implant reconstructions: a 3‐year prospective randomized controlled clinical trial. Clinical Implant Dentistry and Related Research, 21(4), 578–585. 10.1111/cid.12735. [DOI] [PubMed] [Google Scholar]
- Laass, A. , Sailer, I. , Hüsler, J. , Hämmerle, C. H. , & Thoma, D. S. (2019). Randomized controlled clinical trial of all‐ceramic single‐tooth implant reconstructions using modified zirconia abutments: Results at 5 years after loading. International Journal of Periodontics & Restorative Dentistry, 39(1), 17–27. 10.11607/prd.3792. [DOI] [PubMed] [Google Scholar]
- Linkevicius, T. , Linkevicius, R. , Alkimavicius, J. , Linkeviciene, L. , Andrijauskas, P. , & Puisys, A. (2018). Influence of titanium base, lithium disilicate restoration and vertical soft tissue thickness on bone stability around triangular‐shaped implants: A prospective clinical trial. Clinical Oral Implants Research, 29(7), 716–724. 10.1111/clr.13263. [DOI] [PubMed] [Google Scholar]
- Lops, D. , Bressan, E. , Chiapasco, M. , Rossi, A. , & Romeo, E. (2013). Zirconia and titanium implant abutments for single‐tooth implant prostheses after 5 years of function in posterior regions. International Journal of Oral and Maxillofacial Implants, 28(1), 281–287. 10.11607/jomi.2668. [DOI] [PubMed] [Google Scholar]
- Ma, S. , Tawse‐Smith, A. , Brown, S. D. K. , & Duncan, W. (2019). Immediately restored single implants in the aesthetic zone of the maxilla using a novel design: 5‐year results from a prospective single‐arm clinical trial. Clinical Implant Dentistry and Related Research, 21(2), 344–351. 10.1111/cid.12733. [DOI] [PubMed] [Google Scholar]
- Mangano, F. , & Veronesi, G. (2018). Digital versus analog procedures for the prosthetic restoration of single implants: a randomized controlled trial with 1 year of follow‐up. Biomed Research International, 2018, 5325032. 10.1155/2018/5325032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijndert, C. M. , Raghoebar, G. M. , Santing, H. J. , Vissink, A. , & Meijer, H. J. A. (2020). Performance of bone‐level implants with conical connections in the anterior maxilla: A 5‐year prospective cohort study. Clinical Oral Implants Research, 31(2), 173–180. 10.1111/clr.13553. [DOI] [PubMed] [Google Scholar]
- Mühlemann, S. , Lakha, T. , Jung, R. E. , Hämmerle, C. H. F. , & Benic, G. I. (2020). Prosthetic outcomes and clinical performance of CAD‐CAM monolithic zirconia versus porcelain‐fused‐to‐metal implant crowns in the molar region: 1‐year results of a RCT. Clinical Oral Implants Research, 31(9), 856–864. 10.1111/clr.13631. [DOI] [PubMed] [Google Scholar]
- Nothdurft, F. P. , Nonhoff, J. , & Pospiech, P. R. (2014). Pre‐fabricated zirconium dioxide implant abutments for single‐tooth replacement in the posterior region: success and failure after 3 years of function. Acta Odontologica Scandinavica, 72(5), 392–400. 10.3109/00016357.2013.863970. [DOI] [PubMed] [Google Scholar]
- Ormianer, Z. , & Schiroli, G. (2006). Maxillary single‐tooth replacement utilizing a novel ceramic restorative system: results to 30 months. Journal of Oral Implantology, 32(4), 190–199. 10.1563/805.1. [DOI] [PubMed] [Google Scholar]
- Paolantoni, G. , Marenzi, G. , Blasi, A. , Mignogna, J. , & Sammartino, G. (2016). Findings of a four‐year randomized controlled clinical trial comparing two‐piece and one‐piece zirconia abutments supporting single prosthetic restorations in maxillary anterior region. BioMed Research International, 2016, 8767845. 10.1155/2016/8767845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peron, C. , & Romanos, G. (2020). Immediate provisionalization of single narrow implants in fresh extraction sockets and healed sites: clinical and radiographic outcomes of 2 years follow‐up. International Journal of Periodontics & Restorative Dentistry, 40(3), 417–424. 10.11607/prd.4622. [DOI] [PubMed] [Google Scholar]
- Pieri, F. , Aldini, N. N. , Marchetti, C. , & Corinaldesi, G. (2013). Esthetic outcome and tissue stability of maxillary anterior single‐tooth implants following reconstruction with mandibular block grafts: A 5‐year prospective study. International Journal of Oral and Maxillofacial Implants, 28(1), 270–280. 10.11607/jomi.2560. [DOI] [PubMed] [Google Scholar]
- Pol, C. W. P. , Raghoebar, G. M. , Maragkou, Z. , Cune, M. S. , & Meijer, H. J. A. (2020). Full‐zirconia single‐tooth molar implant‐supported restorations with angulated screw channel abutments: A 1‐year prospective case series study. Clinical Implant Dentistry and Related Research, 22(1), 138–144. 10.1111/cid.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammelsberg, P. , Lorenzo Bermejo, J. , Kappel, S. , Meyer, A. , & Zenthofer, A. (2020). Long‐term performance of implant‐supported metal‐ceramic and all‐ceramic single crowns. Journal of Prosthodontic Research, 64(3), 332–339. 10.1016/j.jpor.2019.09.006. [DOI] [PubMed] [Google Scholar]
- Schepke, U. , Meijer, H. J. , Vermeulen, K. M. , Raghoebar, G. M. , & Cune, M. S. (2016). Clinical bonding of resin nano ceramic restorations to zirconia abutments: A case series within a randomized clinical trial. Clinical Implant Dentistry and Related Research, 18(5), 984–992. 10.1111/cid.12382. [DOI] [PubMed] [Google Scholar]
- Teichmann, M. , Gockler, F. , Weber, V. , Yildirim, M. , Wolfart, S. , & Edelhoff, D. (2017). Ten‐year survival and complication rates of lithium‐disilicate (Empress 2) tooth‐supported crowns, implant‐supported crowns, and fixed dental prostheses. Journal of Dentistry, 56, 65–77. 10.1016/j.jdent.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Vandeweghe, S. , Cosyn, J. , Thevissen, E. , Van den Berghe, L. , & De Bruyn, H. (2012). A 1‐year prospective study on Co‐Axis implants immediately loaded with a full ceramic crown. Clinical Implant Dentistry and Related Research, 14(Suppl 1), e126–138. 10.1111/j.1708-8208.2011.00391.x. [DOI] [PubMed] [Google Scholar]
- Vanlioglu, B. A. , Evren, B. , Yildiz, C. , Uludamar, A. , & Ozkan, Y. K. (2012). Internal and marginal adaptation of pressable and computer‐aided design/computer‐assisted manufacture onlay restorations. International Journal of Prosthodontics, 25(3), 262–264. [PubMed] [Google Scholar]
- Weigl, P. , Saarepera, K. , Hinrikus, K. , Wu, Y. , Trimpou, G. , & Lorenz, J. (2019). Screw‐retained monolithic zirconia vs. cemented porcelain‐fused‐to‐metal implant crowns: a prospective randomized clinical trial in split‐mouth design. Clinical Oral Investigations, 23(3), 1067–1075. 10.1007/s00784-018-2531-x. [DOI] [PubMed] [Google Scholar]
- Weigl, P. , Trimpou, G. , Grizas, E. , Hess, P. , Nentwig, G. H. , Lauer, H. C. , & Lorenz, J. (2019). All‐ceramic versus titanium‐based implant supported restorations: Preliminary 12‐months results from a randomized controlled trial. Journal of Advanced Prosthodontics, 11(1), 48–54. 10.4047/jap.2019.11.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittneben, J. G. , Gavric, J. , Sailer, I. , Buser, D. , & Wismeijer, D. (2020). Clinical and esthetic outcomes of two different prosthetic workflows for implant‐supported all‐ceramic single crowns‐3 year results of a randomized multicenter clinical trail. Clinical Oral Implants Research, 31(5), 495–505. 10.1111/clr.13586. [DOI] [PubMed] [Google Scholar]
- Zembic, A. , Bösch, A. , Jung, R. E. , Hämmerle, C. H. , & Sailer, I. (2013). Five‐year results of a randomized controlled clinical trial comparing zirconia and titanium abutments supporting single‐implant crowns in canine and posterior regions. Clinical Oral Implants Research, 24(4), 384–390. 10.1111/clr.12044. [DOI] [PubMed] [Google Scholar]
- Zembic, A. , Philipp, A. O. , Hammerle, C. H. , Wohlwend, A. , & Sailer, I. (2015). Eleven‐year follow‐up of a prospective study of zirconia implant abutments supporting single all‐ceramic crowns in anterior and premolar regions. Clinical Implant Dentistry and Related Research, 17(Supplement 2), e417–e426. 10.1111/cid.12263. [DOI] [PubMed] [Google Scholar]
Further references
- Abou‐Ayash, S. , Strasding, M. , Rücker, G. , & Att, W. (2017). Impact of prosthetic material on mid‐ and long‐term outcome of dental implants supporting single crowns and fixed partial dentures: A systematic review and meta‐analysis. European Journal of Oral Implantology, 10(Suppl 1), 47–65. [PubMed] [Google Scholar]
- Caramês, J. , Marques, D. , Malta Barbosa, J. , Moreira, A. , Crispim, P. , & Chen, A. (2019). Full‐arch implant‐supported rehabilitations: A prospective study comparing porcelain‐veneered zirconia frameworks to monolithic zirconia. Clinical Oral Implants Research, 30(1), 68–78. 10.1111/clr.13393. [DOI] [PubMed] [Google Scholar]
- Cappelleri, J. C. , Ioannidis, J. P. , Schmid, C. H. , de Ferranti, S. D. , Aubert, M. , Chalmers, T. C. , & Lau, J. (1996). Large trials vs meta‐analysis of smaller trials: How do their results compare? Journal of the American Medical Association, 276, 1332–1338. 10.1001/jama.1996.03540160054033. [DOI] [PubMed] [Google Scholar]
- Joda, T. , & Brägger, U. (2014). Complete digital workflow for the production of implant‐supported single‐unit monolithic crowns. Clinical Oral Implants Research, 25(11), 1304–1306. 10.1111/clr.12270. [DOI] [PubMed] [Google Scholar]
- Joda, T. , & Brägger, U. (2015). Time‐efficiency analysis comparing digital and conventional workflows for implant crowns: a prospective clinical crossover trial. International Journal of Oral & Maxillofacial Implants, 30(5), 1047–1053. 10.11607/jomi.3963. [DOI] [PubMed] [Google Scholar]
- Joda, T. , & Brägger, U. (2016). Time‐efficiency analysis of the treatment with monolithic implant crowns in a digital workflow: A randomized controlled trial. Clinical Oral Implants Research, 27(11), 1401–1406. 10.1111/clr.12753. [DOI] [PubMed] [Google Scholar]
- Jung, R. E. , Zembic, A. , Pjetursson, B. E. , Zwahlen, M. , & Thoma, D. S. (2012). Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow‐up of 5 years. Clinical Oral Implants Research, 23(Suppl 6), 2–21. 10.1111/j.1600-0501.2012.02547.x. [DOI] [PubMed] [Google Scholar]
- Kirkwood, B. R. & Sterne, J. A. C. (2003. a). Poisson regression Essential medical statistics, 249–262). Oxford: Blackwell Science Ltd. [Google Scholar]
- Larsson, C. , & Wennerberg, A. (2014). The clinical success of zirconia‐based crowns: A systematic review. International Journal of Prosthodontics, 27(1), 33–43. 10.11607/ijp.3647. [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology, 62(10), 1006–1012. 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Mühlemann, S. , Kraus, R. D. , Hämmerle, C. H. F. , & Thoma, D. S. (2018). Is the use of digital technologies for the fabrication of implant‐supported reconstructions more efficient and/or more effective than conventional techniques: A systematic review. Clinical Oral Implants Research, 29(Suppl 18), 184–195. 10.1111/clr.13300. [DOI] [PubMed] [Google Scholar]
- Papageorgiou, S. N. , Xavier, G. M. , & Cobourne, M. T. (2015). Basic study design influences the results of orthodontic clinical investigations. Journal of Clinical Epidemiology, 68(12), 1512–1522. 10.1016/j.jclinepi.2015.03.008. Epub 2015 Mar 27. PMID: 25910911. [DOI] [PubMed] [Google Scholar]
- Pitta, J. , Hjerppe, J. , Burkhardt, F. , Fehmer, V. , Mojon, P. , & Sailer, I. (2020). Mechanical stability and technical outcomes of monolithic CAD/CAM fabricated abutment‐crowns supported by titanium bases: An in vitro study. Clinical Oral Implants Research, 32(2), 222–232. 10.1111/clr.13693. [DOI] [PubMed] [Google Scholar]
- Pjetursson, B. E. , Asgeirsson, A. G. , Zwahlen, M. , & Sailer, I. (2014). Improvements in implant dentistry over the last decade: comparison of survival and complication rates in older and newer publications. International Journal of Oral & Maxillofacial Implants, 29(Suppl), 308–324. 10.11607/jomi.2014suppl.g5.2. [DOI] [PubMed] [Google Scholar]
- Pjetursson, B. E. , Valente, N. A. , Strasding, M. , Zwahlen, M. , Liu, S. , & Sailer, I. (2018). A systematic review of the survival and complication rates of zirconia‐ceramic and metal‐ceramic single crowns. Clinical Oral Implants Research, 29(Suppl 16), 199–214. 10.1111/clr.13306. [DOI] [PubMed] [Google Scholar]
- Rabel, K. , Spies, B. C. , Pieralli, S. , Vach, K. , & Kohal, R. J. (2018). The clinical performance of all‐ceramic implant‐supported single crowns: A systematic review and meta‐analysis. Clinical Oral Implants Research, 29(Suppl 18), 196–223. 10.1111/clr.13337. [DOI] [PubMed] [Google Scholar]
- Schardt, C. , Adams, M. B. , Owens, T. , Keitz, S. , & Fontelo, P. (2007). Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Medical Informatics and Decision Making, 7, 16. 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel, F. A. , Boldt, J. , & Gierthmuehlen, P. C. (2018). CAD/CAM ceramic restorative materials for natural teeth. Journal of Dental Research, 97(10), 1082–1091. 10.1177/0022034518779759. [DOI] [PubMed] [Google Scholar]
References of excluded studies
- Abduo, J. , Lee, C. L. , Sarfarazi, G. , Xue, B. , Judge, R. , & Darby, I. (2020). Encode protocol versus conventional protocol for single implant restoration: a prospective 2‐year follow‐up randomized controlled trial. Journal of Oral Implantology, 10.1563/aaid-joi-D-19-00150. [DOI] [PubMed] [Google Scholar]
- Alayan, J. , & Ivanovski, S. (2019). Biological and technical outcomes of restored implants after maxillary sinus augmentation‐Results at 1‐year loading. Clinical Oral Implants Research, 30(9), 849–860. 10.1111/clr.13489. [DOI] [PubMed] [Google Scholar]
- Anitua, E. , Saracho, J. , Almeida, G. Z. , Duran‐Cantolla, J. , & Alkhraisat, M. H. (2017). Frequency of prosthetic complications related to implant‐borne prosthesis in a sleep disorder unit. Journal of Oral Implantology, 43(1), 19–23. 10.1563/aaid-joi-D-16-00100. [DOI] [PubMed] [Google Scholar]
- Balmer, M. , Spies, B. C. , Kohal, R. J. , Hämmerle, C. H. , Vach, K. , & Jung, R. E. (2020). Zirconia implants restored with single crowns or fixed dental prostheses: 5‐year results of a prospective cohort investigation. Clinical Oral Implants Research, 31(5), 452–462. 10.1111/clr.13581. [DOI] [PubMed] [Google Scholar]
- Balmer, M. , Spies, B. C. , Vach, K. , Kohal, R. J. , Hämmerle, C. H. F. , & Jung, R. E. (2018). Three‐year analysis of zirconia implants used for single‐tooth replacement and three‐unit fixed dental prostheses: A prospective multicenter study. Clinical Oral Implants Research, 29(3), 290–299. 10.1111/clr.13115. [DOI] [PubMed] [Google Scholar]
- Barwacz, C. A. , Stanford, C. M. , Diehl, U. A. , Cooper, L. F. , Feine, J. , McGuire, M. , & Scheyer, E. T. (2018). Pink esthetic score outcomes around three implant‐abutment configurations: 3‐year results. International Journal of Oral & Maxillofacial Implants, 33(5), 1126–1135. 10.11607/jomi.6659. [DOI] [PubMed] [Google Scholar]
- Becker, J. , John, G. , Becker, K. , Mainusch, S. , Diedrichs, G. , & Schwarz, F. (2017). Clinical performance of two‐piece zirconia implants in the posterior mandible and maxilla: a prospective cohort study over 2 years. Clinical Oral Implants Research, 28(1), 29–35. 10.1111/clr.12610. [DOI] [PubMed] [Google Scholar]
- Beekmans, D. G. , Beekmans, B. R. , & Cune, M. S. (2017). Pink and white esthetics of a new zirconia implant: a 6‐month to 8‐year follow‐up. International Journal of Periodontics & Restorative Dentistry, 37(4), 511–518. 10.11607/prd.2705. [DOI] [PubMed] [Google Scholar]
- Bergenblock, S. , Andersson, B. , Fürst, B. , & Jemt, T. (2012). Long‐term follow‐up of ceraone single‐implant restorations: An 18‐year follow‐up study based on a prospective patient cohort. Clinical Implants Dentistry and Related Research, 14, 471–479. 10.1111/j.1708-8208.2010.00290.x. [DOI] [PubMed] [Google Scholar]
- Branzen, M. , Eliasson, A. , Arnrup, K. , & Bazargani, F. (2015). Implantsupported single crowns replacing congenitally missing maxillary lateral incisors: A 5‐year follow‐up. Clinical Implant Dentistry and Related Research, 17, 1134–1140. 10.1111/cid.12233. [DOI] [PubMed] [Google Scholar]
- Bonde, M. J. , Stokholm, R. , Isidor, F. , & Schou, S. (2010). Outcome of implant‐supported single‐tooth replacements performed by dental students. A 10‐year clinical and radiographic retrospective study. European Journal of Oral Implantology, 3, 37–46. [PubMed] [Google Scholar]
- Cacaci, C. , Cantner, F. , Mücke, T. , Randelzhofer, P. , Hajtó, J. , & Beuer, F. (2017). Clinical performance of screw‐retained and cemented implant‐supported zirconia single crowns: 36‐month results. Clinical Oral Investigations, 21(6), 1953–1959. 10.1007/s00784-016-1982-1. [DOI] [PubMed] [Google Scholar]
- Cannizzaro, G. , Felice, P. , Trullenque‐Eriksson, A. , Lazzarini, M. , Velasco‐Ortega, E. , & Esposito, M. (2018). Immediate vs early loading of 6.6 mm flapless‐placed single implants: 9 years after‐loading report of a split‐mouth randomised controlled trial. European Journal of Oral Implantology, 11(2), 163–173. 10.1002/central/CN-01655781/full. [DOI] [PubMed] [Google Scholar]
- Chrcanovic, B. R. , Kisch, J. , & Larsson, C. (2019). Retrospective clinical evaluation of implant‐supported single crowns: Mean follow‐up of 15 years. Clinical Oral Implants Research, 30(7), 691–701. 10.1111/clr.13454. [DOI] [PubMed] [Google Scholar]
- Chu, S. J. , Ostman, P. O. , Nicolopoulos, C. , Yuvanoglu, P. , Chow, J. , Nevins, M. , & Tarnow, D. P. (2018). Prospective multicenter clinical cohort study of a novel macro hybrid implant in maxillary anterior postextraction sockets: 1‐year results. International Journal of Periodontics & Restorative Dentistry, 38(Supplement), s17–s27. 10.11607/prd.3987. [DOI] [PubMed] [Google Scholar]
- Cionca, N. , Müller, N. , & Mombelli, A. (2015). Two‐piece zirconia implants supporting all‐ceramic crowns: A prospective clinical study. Clinical Oral Implants Research, 26, 413–418. 10.1111/clr.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis, P. , Passarelli, P. C. , Gasparini, G. , Boniello, R. , D'Amato, G. , & De Angelis, S. (2020). Monolithic CAD‐CAM lithium disilicate versus monolithic CAD‐CAM zirconia for single implant‐supported posterior crowns using a digital workflow: A 3‐year cross‐sectional retrospective study. Journal of Prosthetic Dentistry, 123(2), 252–256. 10.1016/j.prosdent.2018.11.016. [DOI] [PubMed] [Google Scholar]
- Degidi, M. , Nardi, D. , Gianluca, S. , & Piattelli, A. (2018). The conometric concept: a 5‐year follow‐up of fixed partial monolithic zirconia restorations supported by cone‐in‐cone abutments. International Journal of Periodontics & Restorative Dentistry, 38(3), 363–371. 10.11607/prd.3130. [DOI] [PubMed] [Google Scholar]
- Dierens, M. , De Bruyn, H. , Kisch, J. , Nilner, K. , Cosyn, J. , & Vandeweghe, S. (2016). Prosthetic survival and complication rate of single implant treatment in the periodontally healthy patient after 16 to 22 years of follow‐up. Clinical Implants Dentistry and Related Research, 18, 117–128. 10.1111/cid.12266. [DOI] [PubMed] [Google Scholar]
- Eisner, B. , Naenni, N. , Hüsler, J. , Hämmerle, C. , Thoma, D. , & Sailer, I. (2018). Three‐year results of a randomized controlled clinical trial using submucosally veneered and unveneered zirconia abutments supporting all‐ceramic single‐implant crowns. International Journal of Periodontics & Restorative Dentistry, 38(5), 645–652. 10.11607/prd.3669. [DOI] [PubMed] [Google Scholar]
- Ekfeldt, A. , Fürst, B. , & Carlsson, G. E. (2017). Zirconia abutments for single‐tooth implant restorations: a 10‐ to 11‐year follow‐up study. Clinical Oral Implants Research, 28(10), 1303–1308. 10.1111/clr.12975. [DOI] [PubMed] [Google Scholar]
- Fürhauser, R. , Mailath‐Pokorny, G. , Haas, R. , Busenlechner, D. , Watzek, G. , & Pommer, B. (2017). Immediate restoration of immediate implants in the esthetic zone of the maxilla via the copy‐abutment technique: 5‐year follow‐up of pink esthetic scores. Clinical Implant Dentistry and Related Research, 19(1), 28–37. 10.1111/cid.12423. [DOI] [PubMed] [Google Scholar]
- Gallucci, G. O. , Grutter, L. , Nedir, R. , Bischof, M. , & Belser, U. C. (2011). Esthetic outcomes with porcelain‐fused‐to‐ceramic and all‐ceramic single‐implant crowns: A randomized clinicaltrial. Clinical Oral Implants Research, 22, 62–69. 10.1111/j.1600-0501.2010.01997.x. [DOI] [PubMed] [Google Scholar]
- Gamper, F. B. , Benic, G. I. , Sanz‐Martin, I. , Asgeirsson, A. G. , Hämmerle, C. H. F. , & Thoma, D. S. (2017). Randomized controlled clinical trial comparing one‐piece and two‐piece dental implants supporting fixed and removable dental prostheses: 4‐ to 6‐year observations. Clinical Oral Implants Research, 28(12), 1553–1559. 10.1111/clr.13025. [DOI] [PubMed] [Google Scholar]
- Glauser, R. , Sailer, I. , Wohlwend, A. , Studer, S. , Schibli, M. , & Schärer, P. (2004). Experimental zirconia abutments for implant‐supported single‐tooth restorations in esthetically demanding regions: 4‐year results of a prospective clinical study. International Journal of Prosthodontics, 17, 285–290. [PubMed] [Google Scholar]
- Hsu, K. W. , Shen, Y. F. , & Wei, P. C. (2017). Compatible CAD‐CAM titanium abutments for posterior single‐implant tooth replacement: A retrospective case series. Journal of Prosthetic Dentistry, 117(3), 363–366. 10.1016/j.prosdent.2016.07.023. [DOI] [PubMed] [Google Scholar]
- Hsu, K. W. , Wei, P. C. , Chen, Y. L. , & Liou, E. J. (2019). Retrospective and clinical evaluation of aftermarket CAD/CAM titanium abutments supporting posterior splinted prostheses and single crowns. International Journal of Oral & Maxillofacial Implants, 34(5), 1161–1168. 10.11607/jomi.7365. [DOI] [PubMed] [Google Scholar]
- Kniha, K. , Bock, A. , Peters, F. , Heitzer, M. , Modabber, A. , Kniha, H. , Hölzle, F. , & Möhlhenrich, S. C. (2020). Aesthetic aspects of adjacent maxillary single‐crown implants‐influence of zirconia and titanium as implant materials. International Journal of Oral and Maxillofacial Surgery, 49(11), 1489–1496. 10.1016/j.ijom.2020.04.003. [DOI] [PubMed] [Google Scholar]
- Kniha, K. , Schlegel, K. A. , Kniha, H. , Modabber, A. , Hölzle, F. , & Kniha, K. (2018). Evaluation of peri‐implant bone levels and soft tissue dimensions around zirconia implants‐a three‐year follow‐up study. International Journal of Oral and Maxillofacial Surgery, 47(4), 492–498. 10.1016/j.ijom.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Kohal, R. J. , Spies, B. C. , Bauer, A. , & Butz, F. (2018). One‐piece zirconia oral implants for single‐tooth replacement: Three‐year results from a long‐term prospective cohort study. Journal of Clinical Periodontology, 45(1), 114–124. 10.1111/jcpe.12815. [DOI] [PubMed] [Google Scholar]
- Kohal, R. J. , Spies, B. C. , Vach, K. , Balmer, M. , & Pieralli, S. (2020). A prospective clinical cohort investigation on zirconia implants: 5‐year results. Journal of Clinical Medicine, 9(8), 2585. 10.3390/jcm9082585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidis, I. , Trikka, D. , Gasparatos, S. , & Mitsias, M. (2018). Clinical outcomes of monolithic zirconia crowns with CAD/CAM technology. A 1‐year follow‐up prospective clinical study of 65 patients. International Journal of Environmental Research and Public Health, 15(11), 2523. 10.3390/ijerph15112523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner, H. , Mouhyi, J. , Admakin, O. , & Mangano, F. (2020). Artificial intelligence in fixed implant prosthodontics: a retrospective study of 106 implant‐supported monolithic zirconia crowns inserted in the posterior jaws of 90 patients. BMC Oral Health, 20(1), 80. 10.1186/s12903-020-1062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, J. , Giulini, N. , Holscher, W. , Schwiertz, A. , Schwarz, F. , & Sader, R. (2019). Prospective controlled clinical study investigating long‐term clinical parameters, patient satisfaction, and microbial contamination of zirconia implants. Clinical Implant Dentistry and Related Research, 21(2), 263–271. 10.1111/cid.12720. [DOI] [PubMed] [Google Scholar]
- Lops, D. , Bressan, E. , Chiapasco, M. , Rossi, A. , & Romeo, E. (2013). Zirconia and titanium implant abutments for single‐tooth implant prostheses after 5 years of function in posterior regions. International Journal of Oral and Maxillofacial Implants, 28, 281–287. 10.11607/jomi.2668. [DOI] [PubMed] [Google Scholar]
- Malo, P. , de Araujo Nobre, M. , Lopes, A. , Ferro, A. , & Gravito, I. (2015). Single‐tooth rehabilitations supported by dental implants used in an immediate‐provisionalization protocol: Report on long‐term outcome with retrospective follow‐up. Clinical Implants Dentistry and Related Research, 17(Suppl 2), e511–e519. 10.1111/cid.12278. [DOI] [PubMed] [Google Scholar]
- Mangano, F. , Margiani, B. , & Admakin, O. (2019). A novel full‐digital protocol (SCAN‐PLAN‐MAKE‐DONE(®)) for the design and fabrication of implant‐supported monolithic translucent zirconia crowns cemented on customized hybrid abutments: A retrospective clinical study on 25 patients. International Journal of Environmental Research and Public Health, 16(3), 10.3390/ijerph16030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni, S. M. , Baldoni, E. , Duvina, M. , Pisano, M. , De Riu, G. , & Tallarico, M. (2018). Immediate non‐occlusal versus delayed loading of mandibular first molars. Five‐year results from a randomised controlled trial. European Journal of Oral Implantology, 11(4), 409–418. 10.1002/central/CN-02084164/full. [DOI] [PubMed] [Google Scholar]
- Meloni, S. M. , Baldoni, E. , Pisano, M. , Tullio, A. , De Riu, G. , & Tallarico, M. (2018). 1‐year results from a split‐mouth randomised controlled pilot trial comparing implants with 0.75 mm of machined collar placed at bone level or supracrestally. European Journal of Oral Implantology, 11(3), 353–359. 10.1002/central/CN-02084235/full. [DOI] [PubMed] [Google Scholar]
- Monaco, C. , Caldari, M. , & Scotti, R. (2015). Clinical evaluation of zirconia‐based restorations on implants: A retrospective cohort study from the AIOP clinical research group. International Journal of Prosthodontics, 28, 239–242. [DOI] [PubMed] [Google Scholar]
- Montemezzi, P. , Ferrini, F. , Pantaleo, G. , Gherlone, E. , & Capparè, P. (2020). Dental implants with different neck design: A prospective clinical comparative study with 2‐year follow‐up. Materials (Basel), 13(5), 1029. 10.3390/ma13051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedir, R. , Nurdin, N. , Huynh‐Ba, G. , & Bischof, M. (2019). Change in crown‐to‐implant ratio of implants placed in grafted and nongrafted posterior maxillary sites: A 5‐year prospective randomized study. International Journal of Oral and Maxillofacial Implants, 34(5), 1231–1236. 10.11607/jomi.6766. [DOI] [PubMed] [Google Scholar]
- Nejatidanesh, F. , Moradpoor, H. , & Savabi, O. (2016). Clinical outcomes of zirconia‐based implant‐and tooth‐supportedsingle crowns. Clinical Oral Investigation, 20, 169–178. 10.1007/s00784-015-1479-3. [DOI] [PubMed] [Google Scholar]
- Nilsson, A. , Johansson, L. , Lindh, C. , & Ekfeldt, A. (2017). One‐piece internal zirconia abutments for single‐tooth restorations on narrow and regular diameter implants: A 5‐year prospective follow‐up study. Clinical Implant Dentistry and Related Research, 19(5), 916–925. 10.1111/cid.12515. [DOI] [PubMed] [Google Scholar]
- Noelken, R. , Moergel, M. , Kunkel, M. , & Wagner, W. (2018). Immediate and flapless implant insertion and provisionalization using autogenous bone grafts in the esthetic zone: 5‐year results. Clinical Oral Implants Research, 29(3), 320–327. 10.1111/clr.13119. [DOI] [PubMed] [Google Scholar]
- Payer, M. , Arnetzl, V. , Kirmeier, R. , Koller, M. , Arnetzl, G. , & Jakse, N. (2013). Immediate provisional restoration of single‐piece zirconia implants: A prospective case series ‐results after 24 months of clinical function. Clinical Oral Implants Research, 24, 569–575. 10.1111/j.1600-0501.2012.02425.x [DOI] [PubMed] [Google Scholar]
- Payer, M. , Heschl, A. , Koller, M. , Arnetzl, G. , Lorenzoni, M. , & Jakse, N. (2015). All‐ceramic restoration of zirconia two‐piece implants–a randomized controlled clinical trial. Clinical Oral Implants Research, 26, 371–376. 10.1111/clr.12342. [DOI] [PubMed] [Google Scholar]
- Prati, C. , Zamparini, F. , Pirani, C. , Montebugnoli, L. , Canullo, L. , & Gandolfi, M. G. (2020). A multilevel analysis of platform‐switching flapless implants placed at tissue level: 4‐year prospective cohort study. International Journal of Oral & Maxillofacial Implants, 35(2), 330–341. 10.11607/jomi.7541. [DOI] [PubMed] [Google Scholar]
- Raes, F. , Cosyn, J. , Crommelinck, E. , Coessens, P. , & De Bruyn, H. (2011). Immediate and conventional single implant treatment in the anterior maxilla: 1‐year results of a case series on hard and soft tissue response and aesthetics. Journal of Clinical Periodontology, 38, 385–394. 10.1111/j.1600-051X.2010.01687.x. [DOI] [PubMed] [Google Scholar]
- Sailer, I. , Zembic, A. , Jung, R. E. , Siegenthaler, D. , Holderegger, C. , & Hämmerle, C. H. (2009). Randomized controlled clinical trial of customized zirconia and titanium implant abutments for canine and posterior single‐tooth implant reconstructions: Preliminary results at 1 year of function. Clinical Oral Implants Research, 20, 219–225. 10.1111/j.1600-0501.2008.01636.x. [DOI] [PubMed] [Google Scholar]
- Schepke, U. , Lohbauer, U. , Meijer, H. J. , & Cune, M. S. (2018). Adhesive failure of lava ultimate and lithium disilicate crowns bonded to zirconia abutments: a prospective within‐patient comparison. International Journal of Prosthodontics, 31(3), 208–210. 10.11607/ijp.5617. [DOI] [PubMed] [Google Scholar]
- Schlegel, K. A. , Kniha, H. , Modabber, A. , Holzle, F. , & Kniha, K. (2018). Evaluation of peri‐implant bone levels and soft tissue dimensions around zirconia implants‐a three‐year follow‐up study. International Journal of Oral and Maxillofacial Surgery, 47(4), 492–498. 10.1016/j.ijom.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Schlegel, K. A. , Kniha, H. , Modabber, A. , Neukam, F. , & Kniha, K. (2019). Papilla‐crown height dimensions around zirconium dioxide implants in the esthetic area: a 3‐year follow‐up study. Journal of Prosthodontics, 28(2), e694–e698. 10.1111/jopr.12766. [DOI] [PubMed] [Google Scholar]
- Schnider, N. , Forrer, F. A. , Bragger, U. , & Hicklin, S. P. (2018). Clinical performance of one‐piece, screw‐retained implant crowns based on hand‐veneered CAD/CAM zirconia abutments after a mean follow‐up period of 2.3 years. International Journal of Oral & Maxillofacial Implants, 33(1), 188–196. 10.11607/jomi.5929. [DOI] [PubMed] [Google Scholar]
- Sinjari, B. , D'Addazio, G. , Santilli, M. , D'Avanzo, B. , Rexhepi, I. , Scarano, A. , Traini, T. , Piattelli, M. , & Caputi, S. (2020). A 4 year human, randomized, radiographic study of scalloped versus non‐scalloped cemented implants. Materials, 13(9), 2190. 10.3390/ma13092190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino, R. , Galasso, L. , Tete, S. , De Simone, G. , & Zarone, F. (2012). Clinical evaluation of 209 all‐ceramic single crowns cemented on natural and implant‐supported abutments with different luting agents: A 6‐year retrospective study. Clinical Implants Dentistry and Related Research, 14, 184–197. 10.1111/j.1708-8208.2009.00251.x. [DOI] [PubMed] [Google Scholar]
- Spies, B. C. , Balmer, M. , Jung, R. E. , Sailer, I. , Vach, K. , & Kohal, R. J. (2017). All‐ceramic, bi‐layered crowns supported by zirconia implants: three‐year results of a prospective multicenter study. Journal of Dentistry, 67, 58–65. 10.1002/central/CN-01625550/full. [DOI] [PubMed] [Google Scholar]
- Spies, B. C. , Balmer, M. , Jung, R. E. , Sailer, I. , Vach, K. , & Kohal, R. J. (2019). All‐ceramic single crowns supported by zirconia implants: 5‐year results of a prospective multicenter study. Clinical Oral Implants Research, 30(5), 466–475. 10.1111/clr.13433. [DOI] [PubMed] [Google Scholar]
- Spies, B. C. , Kohal, R. J. , Balmer, M. , Vach, K. , & Jung, R. E. (2017). Evaluation of zirconia‐based posterior single crowns supported by zirconia implants: preliminary results of a prospective multicenter study. Clinical Oral Implants Research, 28(5), 613–619. 10.1111/clr.12842. [DOI] [PubMed] [Google Scholar]
- Spies, B. C. , Pieralli, S. , Vach, K. , & Kohal, R. J. (2017). CAD/CAM‐fabricated ceramic implant‐supported single crowns made from lithium disilicate: Final results of a 5‐year prospective cohort study. Clinical Implant Dentistry and Related Research, 19(5), 876–883. 10.1111/cid.12508. [DOI] [PubMed] [Google Scholar]
- Spies, B. C. , Stampf, S. , & Kohal, R.‐J. (2015). Evaluation of zirconia‐based all‐ceramic single crowns and fixed dental prosthesis on zirconia implants: 5‐year results of a prospective cohort study. Clinical Implant Dentistry and Related Research, 17(5), 1014–1028. 10.1111/cid.12203. [DOI] [PubMed] [Google Scholar]
- Tartaglia, G. M. , Sidoti, E. , & Sforza, C. (2015). Seven‐year prospective clinical study on zirconia‐based single crowns and fixed dental prostheses. Clinical Oral Investigation, 19, 1137–1145. 10.1007/s00784-014-1330-2. [DOI] [PubMed] [Google Scholar]
- Thoma, D. S. , Brandenberg, F. , Fehmer, V. , Buchi, D. L. , Hämmerle, C. H. , & Sailer, I. (2016). Randomized controlled clinical trial of all‐ceramic single tooth implant reconstructions using modified zirconia abutments: Radiographic and prosthetic results at 1 year of loading. Clinical Implants Dentistry and Related Research, 18, 462–472. 10.1111/cid.12333. [DOI] [PubMed] [Google Scholar]
- Toia, M. , Galli, S. , Cecchinato, D. , Wennerberg, A. , & Jimbo, R. (2019). Clinical evidence of osseospeed EV implants: A retrospective study and characterization of the newly introduced system. International Journal of Periodontics and Restorative Dentistry, 39(6), 863–874. 10.11607/prd.2549. [DOI] [PubMed] [Google Scholar]
- Vanlioglu, B. A. , Kahramanoglu, E. , Yildiz, C. , Özkan, Y. , & Kulak‐Özkan, Y. (2014). Esthetic outcome evaluation of maxillary anterior single‐tooth bone‐level implants with metal or ceramic abutments and ceramic crowns. International Journal of Oral and Maxillofacial Implants, 29, 1130–1136. [DOI] [PubMed] [Google Scholar]
- Velasco‐Ortega, E. , Wojtovicz, E. , España‐Lopez, A. , Jimenez‐Guerra, A. , Monsalve‐Guil, L. , Ortiz‐Garcia, I. , & Serrera‐Figallo, M. A. (2018). Survival rates and bone loss after immediate loading of implants in fresh extraction sockets (single gaps). A clinical prospective study with 4 year follow‐up. Medicina Oral Patología Oral Y Cirugia Bucal, 23(2), e230–e236. 10.4317/medoral.21651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigolo, P. , Gracis, S. , Carboncini, F. , & Mutinelli, S. (2016). Internal‐ vs external‐connection single implants: A retrospective study in an Italian population treated by certified prosthodontists. International Journal of Oral and Maxillofacial Implants, 31, 1385–1396. 10.11607/jomi.4618 [DOI] [PubMed] [Google Scholar]
- Wang, J. , Lerman, G. , Bittner, N. , Fan, W. , Lalla, E. , & Papapanou, P. N. (2020). Immediate versus delayed temporization at posterior single implant sites: A randomized controlled trial. Journal of Clinical Periodontology, 47(10), 1281–1291. 10.1111/jcpe.13354. [DOI] [PubMed] [Google Scholar]
- Weerapong, K. , Sirimongkolwattana, S. , Sastraruji, T. , & Khongkhunthian, P. (2019). Comparative study of immediate loading on short dental implants and conventional dental implants in the posterior mandible: a randomized clinical trial. International Journal of Oral & Maxillofacial Implants, 34(1), 141–149. 10.11607/jomi.6732. [DOI] [PubMed] [Google Scholar]
- Wittneben, J. G. , Gavric, J. , Belser, U. C. , Bornstein, M. M. , Joda, T. , Chappuis, V. , & Bragger, U. (2017). Esthetic and clinical performance of implant‐supported all‐ceramic crowns made with prefabricated or CAD/CAM zirconia abutments. Journal of Dental Research, 96(2), 163–170. 10.1177/0022034516681767. [DOI] [PubMed] [Google Scholar]
- Worni, A. , Katsoulis, J. , Kolgeci, L. , Worni, M. , & Mericske‐Stern, R. (2017). Monolithic zirconia reconstructions supported by teeth and implants: 1‐to 3‐year results of a case series. Quintessence International, 48, 459–467. [DOI] [PubMed] [Google Scholar]
- Worni, A. , Kolgeci, L. , Rentsch‐Kollar, A. , Katsoulis, J. , & Mericske‐Stern, R. (2015). Zirconia‐based screw‐retained prostheses supported by implants: A retrospective study on technical complications and failures. Clinical Implants Dentistry and Related Research, 17, 1073–1081. 10.1111/cid.12214. [DOI] [PubMed] [Google Scholar]
- Zarone, F. , Sorrentino, R. , Vaccaro, F. , Russo, S. , & De Simone, G. (2005). Retrospective clinical evaluation of 86 Procera AllCeram anterior single crowns on natural and implant‐supported abutments. Clinical Implants Dentistry and Related Research, 7(Suppl 1), S95–S103. 10.1111/j.1708-8208.2005.tb00081.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S2


