Abstract
People living within the same household as someone ill with influenza are at increased risk of infection. Here, we use Markov chain Monte Carlo methods to partition the hazard of influenza illness within a cohort into the hazard from the community and the hazard from the household. During the 2013‐2014 influenza season, 49 (4.7%) of the 1044 people enrolled in a community surveillance cohort had an acute respiratory illness (ARI) attributable to influenza. During the 2014‐2015 influenza season, 50 (4.7%) of the 1063 people in the cohort had an ARI attributable to influenza. The secondary attack rate from a household member was 2.3% for influenza A (H1) during 2013‐2014, 5.3% for influenza B during 2013‐2014, and 7.6% for influenza A (H3) during 2014‐2015. Living in a household with a person ill with influenza increased the risk of an ARI attributable to influenza up to 350%, depending on the season and the influenza virus circulating within the household.
Keywords: epidemiology, household transmission, influenza
1. INTRODUCTION
In accepting the family as a semiclosed group one must believe that infections in successive family members within a short period of time are more likely to be related to each other than to have been separately acquired from outside sources. This is essentially an act of faith and nearly impossible to establish in terms of exact probability. –Carol Buck, 1956 1
For almost a century, researchers have prospectively followed households to elucidate many of the intricacies of the natural history of influenza and other respiratory illnesses: the range of severity, the collection of symptoms, the diversity of etiologies, the spatiotemporal trends, the incidence within subgroups, and the transmission within the household. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 Additionally, case‐ascertained household studies have sought to also answer similar questions about influenza by following the household contacts of a case series. 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 To summarize the transmissibility of influenza, studies of both types often report the secondary attack rate (SAR), a descriptive statistic that quantifies the risk of an exposed person becoming ill from an infectious person living within the same household. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 The SAR is the de facto measure of transmissibility of influenza, and trials of antivirals have used SAR to quantify changes in the transmissibility of influenza attributable to a therapeutic regimen given to infectious household members or a prophylactic regimen given to susceptible household members. 13 , 14 , 21 , 66 , 71 , 72 , 111 Here, we estimate the SAR of influenza A and B virus using data from a prospective cohort in New York City during the 2013‐2014 and 2014‐2015 seasons. 55 Counting the number of cases within a household following the index case may overestimate the SAR of seasonal influenza, as household members may be infected outside the home, for example, at work or at school. 37 , 79 , 81 , 93 , 96 , 134 , 135 , 136 , 137 , 138 , 139 To avoid overcounting, we fit a compartmental model that quantifies both community transmission and household transmission of influenza.
2. METHODS
The Mobile Surveillance for Acute Respiratory and Influenza like Illness in the Community (MoSAIC) study was a prospective cohort study enrolling households in New York City to investigate the incidence, etiology, and risk factors for acute respiratory infections. 55 Inclusion in the study required a household with at least three people, of whom at least 1 was a child under 18 years old. Investigators defined an episode of an acute respiratory illness (ARI) as the presence of at least two of the following symptoms: rhinorrhea, congestion, sore throat, cough, and fever. Investigators also included rhinorrhea among children under 1 year of age as an episode of ARI. Enrollees reported possible ARI within their household via text message. After confirming an episode of ARI over the telephone, researchers collected mid‐turbinate nasal swabs from enrollees with an ARI and tested swabs for a panel of 20 viral and bacterial respiratory pathogens using PCR. 140 The institutional review boards of the Columbia University Medical Center (New York, NY) and the Centers for Disease Control and Prevention (Atlanta, GA) approved this study.
Here, we investigated a secondary objective of the study: to characterize the transmission of influenza within households enrolled in the study. We defined a case as an enrollee
who had a confirmed episode of ARI with onset of symptoms between October 5, 2013 and October 5, 2015 and
whose nasal swab was positive for influenza A, influenza A (H1), influenza A (H3), or influenza B by RT‐PCR.
We divided the study period into two influenza seasons. The 2013‐2014 influenza season started October 5, 2013 and ended September 27, 2014, and the 2014‐2015 influenza season started September 28, 2014 and ended October 5, 2015. For the 2013‐2014 and 2014‐2015 seasons, we tabulated the number of households with 0, 1, 2, 3, 4, or 5 cases for
influenza A (H1);
influenza A (H3);
influenza A (H1) or A (H3);
influenza B; and,
influenza A (H1), A (H3), or B.
For each of these season‐virus combination, we considered enrollees at risk of influenza if they (1) were enrolled on or before the onset date of the first case for that season and (2) remained enrolled by the onset date of the last case of the season. Because investigators enrolled households into the cohort on a rolling basis, this time frame ensured all cases were included while also including as many households as possible. As this time frame was different for each season‐virus combination, a different number of households was at risk for each analysis. The attack rate of influenza was the proportion of cases among those at risk. We used Wilson's interval to compute 95% confidence intervals for the attack rate. 141
For the primary analysis of household transmission, we only considered pairs of cases both PCR‐positive for the same type or subtype of influenza. As a secondary analysis of household transmission, we included additional episodes of ARI up to 10 days after the onset date of a PCR‐positive case within the household, regardless of the test results for these additional episodes of ARI. The results from this secondary analysis simplify comparison of our results to previous work which used a syndromic case definition for secondary cases. For both analyses, we separately analyzed cases of influenza A (H1), influenza A (H3), and influenza B to reduce spurious household transmission in the data. We computed 3‐, 5‐, and 7‐day SARs by counting the number of apparent secondary cases among those at risk, as follows. For each household, we defined the index case (or index cases) as the enrollee(s) who met the case definition with the earliest onset date within that household. The enrollees at risk of secondary transmission were those enrollees who were not index cases but lived in a household with at least 1 case. We denoted the length of time at risk in days as r and consider . For our primary analysis, the r‐day secondary cases were those cases with an onset date at least 1 day and at most r days following the date of onset of the index case within their household. For our secondary analysis, we also counted the episodes of ARI regardless of test result within 10 days of the onset of a case when counting secondary cases. The r‐day SAR was the proportion of r‐day secondary cases among those enrollees at risk of secondary transmission. We used Wilson's interval to compute 95% confidence intervals for the r‐day SAR. 141
We continued our analysis of SAR using Markov chain Monte Carlo methods. We modeled the transmission of influenza within our cohort using a transmission model—built on a susceptible‐infectious‐recovered (SIR) compartmental model—modified from the work of Cauchemez and colleagues. 125 Details follow in the remainder of this section.
2.1. Household transmission model
We built a compartmental SIR model for the transmission of influenza into and within a cohort of households. We used the framework that Cauchemez and colleagues developed to analyze data from a case‐ascertained study. 125 They assumed the length of the infectious period followed a gamma distribution and imputed the start and end of each case's infectious period. Additionally, they allowed for different susceptibility and infectiousness for children and adults. However, we needed to reduce the number of parameters in our model because cohort data have substantially fewer cases, and we wanted to avoid technical issues when imputing sparse data which leads to implausible results. 142 So, we assumed the shape parameter of the gamma distribution is 1; the infectious period began with symptom onset; and, we did not estimate separate parameters for children and adults. These simplifications allowed estimation with sparse data from a prospective cohort. In addition to information available from case‐ascertained studies, the prospective cohort has richer information on the hazard from the community over time. So, we also wanted to take advantage of this information in the model.
Now, we make the following assumptions to begin building our model.
The time to infection follows an exponential distribution with a time‐dependent rate parameter.
The time from infection to recovery follows an exponential distribution.
The hazard from the community is proportional to the smoothed hazard within the cohort.
The hazard within the household is proportional to the percent of household members who are infectious.
The overall hazard is the sum of the community hazard and the household hazard.
The observed onset dates are conditionally independent given the first case of the season, the overall hazard within the cohort, the onset dates of other household members, and the model parameters.
The prior probability distribution of our parameters follows a uniform distribution on .
In the remainder of this subsection, we will implement these assumptions to build a posterior, and we will detail our Metropolis algorithm.
2.1.1. Hazard within the cohort
Here, we construct smoothed estimates of the hazard within the cohort for use in our model. In the next subsection, we will use these smoothed estimates when estimating the hazard within each household.
We take the earliest onset date of the season among laboratory confirmed cases as and the last onset date of the season as day . Then, we compute the Kaplan‐Meier estimates of survival for : the probability of not meeting the case definition before time t. 143 We estimate the cummulative hazard for with
We estimate the instantaneous hazard for with
We use LOESS to smooth our estimates of . 144 We name these locally smoothed estimates .
2.1.2. Hazard within the household
The time‐dependent hazard within each household underlays the results from the model. Suppose we follow a cohort of households for T days and document the days on which household members first meet the case definition. For an individual, we take the sample space of the first day when a household member meets the case definition to be
where individuals who do not meet the case definition during followup are assigned an onset date of . Suppose a household has n members. Then, we may write the set of observed onset dates for the household in increasing order as
We write the onset dates in order to simplify our indices. We fit three parameters to our data: , , and .
If h is the instantaneous hazard within the cohort, then is the hazard attributable to community transmission.
If is the proportion of household members who are infectious, then is the hazard attributable to household transmission.
The rate of recovery among infectious individuals is .
Next, we need an estimate of the proportion of infectious household members at day t. For individual , we define characteristic functions
The characteristic function reflects the fact that an individual is not infectious before their onset date. Using these characteristic functions, we define the expected proportion of infectious people in the household at time t as
where is the probability that the ith case in the household is still infectious at time t for . Finally, we may define the time‐dependent hazard within the household:
We summarize the model with Figure 1.
FIGURE 1.
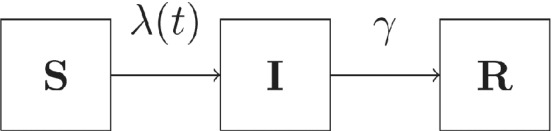
Compartmental SIR model. Susceptible individuals become infectious with a time‐dependent, household‐specific hazard , and recover at a constant rate of
2.1.3. Conditional probability of onset dates
We do not want the first case of the season to contribute to our likelihood. Instead, we want to condition our probability on the first case of the season. We use the symbol to denote the fact that the season's index case is . Recall that are ordered onset dates of a household with n people. Suppose is the onset date of a household member. Because we want to condition our analysis on the season's index case, if , then the household member is an index case for the season. So, if , we take . If , we take the conditional probability of onset on day a as
| (1) |
If , we take the conditional probability of not meeting the case definition during followup as
| (2) |
The integrals in (1) and (2) are integrals of an exponential distribution with a time‐dependent rate parameter . Because is not continuous for cases, is not continuous for households with at least one case. However, is continuous between onset dates. So, we want to rewrite the integrals in (1) and (2) so that we may compute them easily. For a household with d unique onset dates, we order the unique onset dates in increasing order . Then, we define a non‐decreasing sequence of times as . Now, for every we may compute the integral
| (3) |
Using (3) allows us to compute the integral in (1) for each onset date after day 1 and the integral in (2) for household members who do not meet the case definition during followup.
We assume conditional independence of onset dates within a household to estimate the conditional probability of onset dates A
Similarly, we assume conditional independence of onset dates between households in order to compute the conditional probability of the onset dates within the cohort. We index the households with . Then, we label the onset dates within the hth household as . We define the onset dates for the cohort as . The overall conditional probability of is
In summary, we have a conditional probability of the observed onset dates. This is the likelihood we use when estimating our model parameters.
2.1.4. Metropolis algorithm
We use Markov chain Monte Carlo methods to estimate . Specifically, we use a stepwise Metropolis algorithm. 145 We use a uniform prior with a large support for our parameters
Before the algorithm begins, we tune mixing parameters using small chains such that the acceptance of the Metropolis algorithm is about . Before the first iteration of the algorithm, we initialize the parameters using random draws from the uniform distribution on . Then, we compute the conditional probability of the onset dates within the cohort given these parameters and given the date of the first observed case of the season, .
We begin the first iteration of our stepwise algorithm. To begin the first step, we take a random draw from a standard normal distribution and call this value u. Using u and the tuning parameter , we propose a new value for as
As , we have . So, our proposal distribution is symmetric. Next, we decide whether to accept or reject our proposal .
If , then we reject .
-
Otherwise, we compute .
-
–
If , then we accept as the new value of .
-
–Otherwise, we accept as the new value of with probability
-
–
This completes the first step. For the second step, we propose and either accept or reject using the same method as the first step. For the third step, we propose and either accept or reject using the same method as the first step. This completes the first iteration of the algorithm. The algorithm continues for iterations.
2.1.5. Estimates
We assume the parameter values from the Metropolis algorithm are a sample from the posterior probability distribution with density . For each iterate, we compute the community attack rate (CAR) within the cohort by setting , and we have
So, the CAR is the probability of influenza from the community during followup when no household members are infectious. We compute the SAR within a household of size n by setting , and we have
So, the SAR is the probability of influenza from a single infectious household member when no community members are infectious.
For each iterate, we compute the relative attack rate of influenza within a household
with a single infectious member and
susceptible members
vs. within a household
without any infectious people and
of size n
using the formula
So, the denominator of our RR is simply the CAR. The numerator of the RR is the risk of influenza: the competing risk from the community and from a single infectious household member.
We use the median value as our point estimate. We create 95% credible intervals with the 2.5% and 97.5% quantiles.
2.2. Computer software
We used R version 3.5.1“Feather Spray” (Copyright 2018 The R Foundation for Statistical Computing) and GCC version 4.9.3 (Copyright 2015 Free Software Foundation, Inc.) for all computations. We used the R package Rcpp to execute our C++ functions within R. 146
3. RESULTS
The number of households enrolled for our analyses ranged from a low of 230 for influenza A and B in 2013‐2014 to a high of 242 for influenza A (H3) in 2014‐2015 (Table 1). During the 2014‐2015 season, a single enrollee met the case definition twice: once for influenza A (H3) and once for influenza B. Otherwise, enrollees met the case definition at most once per season. The attack rate of influenza A and B was 4.7% during 2013‐2014 and 4.7% during 2014‐2015 seasons (Table 1). Most cases were attributable to community transmission (Tables 1 and 2). Two households had at least 2 cases of influenza A (H1) in 2013‐2014; 8 households had at least 2 cases of influenza A (H3) in 2014‐2015; and 3 household had at least 2 cases of influenza B in 2013‐2014 (Table 1).
TABLE 1.
Number of households with 0, 1, 2, 3, 4, or 5 cases; number of cases; number of people enrolled; and attack rate by virus and season
| Cases in household | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Influenza virus | Season | Households enrolled | 0 | 1 | 2 | 3 | 4 | 5 | Cases | People enrolled | Attack rate % (95% CIa) |
| A and B | 2013‐2014 | 230 | 189 | 34 | 6 | 1 | 49 | 1044 | 4.7 (3.6, 6.2) | ||
| A and B | 2014‐2015 | 238 | 206 | 19 | 10 | 2 | 0 | 1b | 50 | 1063 | 4.7 (3.6, 6.1) |
| B | 2013‐2014 | 234 | 217 | 14 | 2 | 1 | 21 | 1062 | 2.0 (1.3, 3.0) | ||
| B | 2014‐2015 | 238 | 234 | 4 | 4 | 1064 | 0.4 (0.1, 1.0) | ||||
| A | 2013‐2014 | 233 | 207 | 23 | 3 | 29 | 1067 | 2.7 (1.9, 3.9) | |||
| A | 2014‐2015 | 242 | 212 | 18 | 10 | 1 | 1 | 45 | 1088 | 4.1 (3.1, 5.5) | |
| A (H1) | 2013‐2014 | 241 | 223 | 16 | 2 | 20 | 1144 | 1.7 (1.1, 2.7) | |||
| A (H1) | 2014‐2015 | 242c | 242 | 0 | 1088c | 0 (0, 0.4)c | |||||
| A (H3) | 2013‐2014 | 233 | 225 | 8 | 8 | 1067 | 0.7 (0.4, 1.5) | ||||
| A (H3) | 2014‐2015 | 242 | 213 | 21 | 6 | 1 | 1 | 40 | 1088 | 3.7 (2.7, 5.0) | |
Confidence interval.
One enrollee met the case definition twice: once for influenza A (H3) and once for influenza B.
Based on the number of people enrolled in the cohort for the influenza A (H3) analysis during 2014‐2015.
TABLE 2.
Attack rate, community attack rate, and secondary attack rate for seasons and influenza virus with at least one case
| Influenza virus | Season | Attack rate % (95% CIc) | Community attack rate % (95% CIb) | Secondary attack rate % (95% CIb) | Relative attack rate RR (95% CIb) |
|---|---|---|---|---|---|
| A (H1) | 2013‐2014 | 1.7 (1.1, 2.7) | 1.5 (0.9, 2.3) | 2.3 (0.3, 7.7) | 2.6 (1.2, 6.8) |
| A (H3) | 2013‐2014 | 0.7 (0.4, 1.5) | 0.6 (0.3, 1.2) | 0.2 (0.0, 6.8) | 1.3 (1.0, 13.3) |
| A (H3) | 2014‐2015 | 3.7 (2.7, 5.0) | 2.7 (1.8, 3.8) | 7.6 (3.7 13.3) | 3.8 (2.2, 6.5) |
| B | 2013‐2014 | 2.0 (1.3, 3.0) | 1.5 (0.9, 2.4) | 5.3 (1.5, 12.7) | 4.5 (1.9, 10.9) |
| B | 2014‐2015 | 0.4 (1.3, 3.0) | 0.3 (0.1, 0.7) | 0.3 (0.0, 14.3) | 2.3 (1.0, 78.9) |
Note: The relative attack rate is the attack rate of influenza in households with a single case relative to the attack rate of influenza in households with no cases.
Computed for the average household size.
Credible interval.
Confidence interval.
Secondary attack rates varied depending on the season and the circulating virus type (Figure 2 and Table 2). Similarly, the CAR varied by season and circulating virus type (Figure 3 and Table 2). For a household of average size (4.7 people), the SAR of influenza A (H1) was 2.3% during 2013‐2014 (Table 2). Similarly, the SAR of influenza A (H3) was 7.6% during 2014‐2015 for a household of average size (4.5 people). The SAR of influenza B was 5.3% during 2013‐2014 for a household of average size (4.5 people).
FIGURE 2.
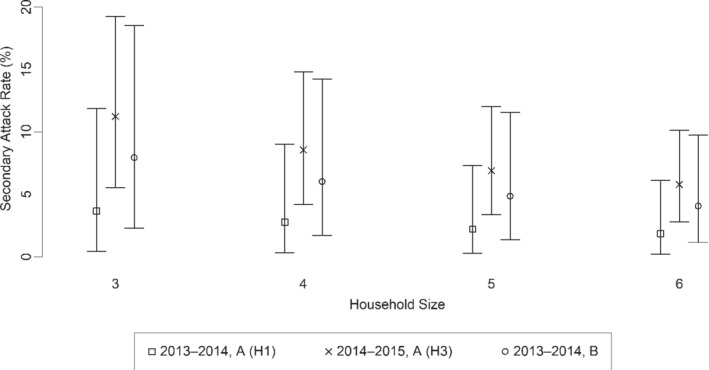
The estimated secondary attack rate by household size for influenza by selected type and subtype during 2013‐2014 and 2014‐2015. Vertical bars represent 95% credible intervals
FIGURE 3.
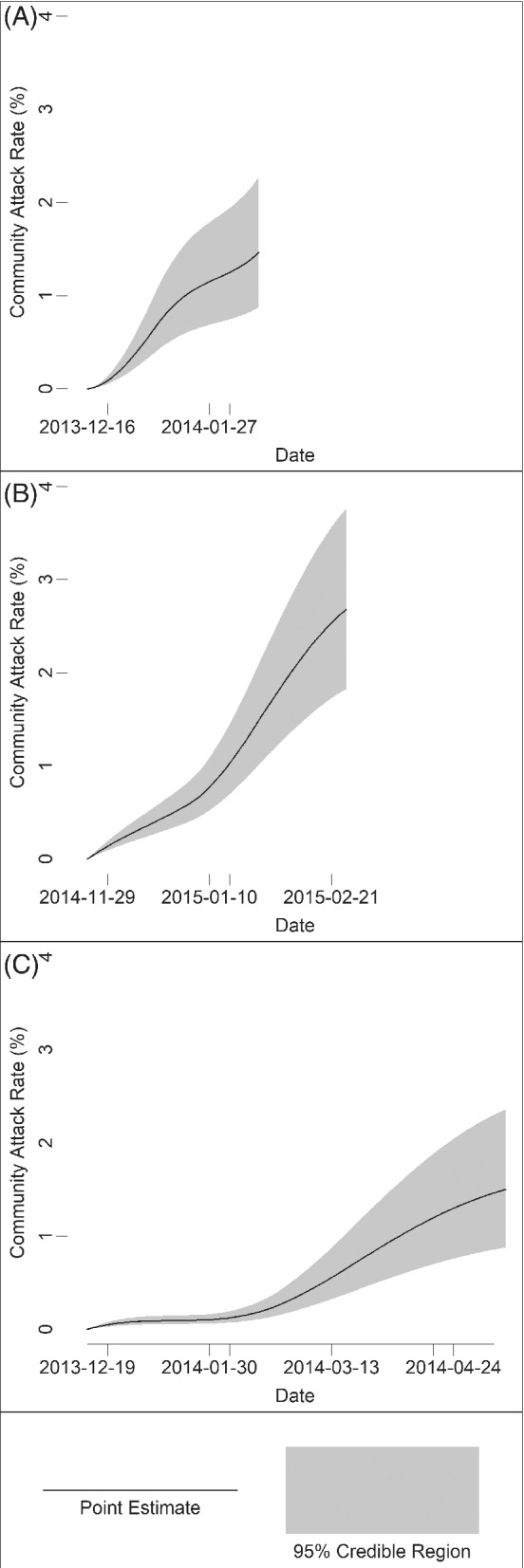
The estimated community attack rate over the followup period of (A) influenza A (H1) during 2013‐2014, (B) influenza A (H3) during 2014‐2015, and (C) influenza B during 2013‐2014. The black lines represent point estimates, and the shaded areas represent 95% credible regions
For an average‐sized household during 2013‐2014, if a household member became infectious with influenza A (H1), then the attack rate of influenza for the other household members increased 160% (Table 2). Similarly, the attack rate increased by 280% during 2014‐2015 if a household member became infectious with influenza A (H3). The attack rate increased by 350% during 2013‐2014 if a household member became infectious with influenza B.
The estimates of the SAR from the transmission model differed from the 3‐day, 5‐day, and 7‐day r‐day SAR (Table 3). As expected, estimates of SAR from the analysis, which also included episodes of ARI regardless of test result, were higher than when limited to only laboratory‐confirmed cases (Table 4).
TABLE 3.
Comparing secondary attack rate of PCR confirmed influenza from the transmission model with 3‐, 5‐, and 7‐day r‐day secondary attack rate
| r‐day secondary attack rate | |||||
|---|---|---|---|---|---|
| Influenza virus | Season | Transmission model secondary attack rate % (95% CIb) | 3‐day % (95% CIc) | 5‐day % (95% CIc) | 7‐day % (95% CIc) |
| A (H1) | 2013‐2014 | 2.3 (0.3, 7.7) | 2.5 (0.7, 8.8) | 2.5 (0.7, 8.8) | 2.5 (0.7, 8.8) |
| A (H3) | 2013‐2014 | 0.2 (0.0, 6.8) | 0.0 (0.0, 12.9) | 0.0 (0.0, 12.9) | 0.0 (0.0, 12.9) |
| A (H3) | 2014‐2015 | 7.6 (3.7 13.3 | 7.4 (3.8, 13.9) | 9.2 (5.1, 16.2) | 9.2 (5.1, 16.2) |
| B | 2013‐2014 | 5.3 (1.5, 12.7) | 3.4 (1.0, 11.7) | 5.2 (1.8, 14.1) | 6.9 (2.7, 16.4) |
| B | 2014‐2015 | 0.3 (0.0, 14.3) | 0.0 (0.0, 24.2) | 0.0 (0.0, 24.2) | 0.0 (0.0, 24.2) |
Computed for the average household size.
Credible interval
Confidence interval
TABLE 4.
Comparing secondary attack rate of episodes of ARI regardless of test results from the transmission model with 3‐, 5‐, 7‐, and 10‐day r‐day secondary attack rate
| r‐day secondary attack rate | ||||||
|---|---|---|---|---|---|---|
| Influenza virus | Season | Transmission model secondary attack rate % (95% CIb) | 3‐day % (95% CIc) | 5‐day % (95% CIc) | 7‐day % (95% CIc) | 10‐day % (95% CIc) |
| A (H1) | 2013‐2014 | 5.7 (2.0, 12.2) | 6.3 (2.7, 14.0) | 6.3 (2.7, 14.0) | 6.3 (2.7, 14.0) | 6.3 (2.7, 14.0) |
| A (H3) | 2013‐2014 | 0.2 (0.0, 6.8) | 0.0 (0.0, 12.9) | 0.0 (0.0, 12.9) | 0.0 (0.0, 12.9) | 0 (0.0, 12.9) |
| A (H3) | 2014‐2015 | 13.5 (8.6, 19.7) | 12.0 (7.2, 19.5) | 16.7 (10.8, 24.8) | 17.6 (11.6, 25.8) | 19.4 (13.1, 27.9) |
| B | 2013‐2014 | 6.5 (2.1, 14.0) | 5.2 (1.8, 14.1) | 6.9 (2.7, 16.4) | 8.6 (3.7, 18.6) | 8.6 (3.7, 18.6) |
| B | 2014‐2015 | 5.6 (0.2, 26.0) | 8.3 (1.5, 35.4) | 8.3 (1.5, 35.4) | 8.3 (1.5, 35.4) | 8.3 (1.5, 35.4) |
Computed for the average household size.
Credible interval.
Confidence interval.
Estimates of model parameters are in Table 5. The 95% credible intervals of and were wide, especially for those analyses with no apparent secondary cases. The point estimates and 95% credible intervals for scale the overall hazard within the cohort, which we present for those analyses with at least one apparent secondary case (Figure 3).
TABLE 5.
Summary of parameter estimates from the household transmission models
| Season | Virus | Case definition | (95% CI) | (95% CI) | (95% CI) |
|---|---|---|---|---|---|
| 2013‐2014 | A (H1) | PCR confirmed | 0.82 (0.48, 1.29) | 3.3 (0.04, 139) | 33 (0.7, 845) |
| 2013‐2014 | A (H3) | PCR confirmed | 0.80 (0.33, 1.58) | 0.16 (0.01, 39) | 59 (0.1, 886) |
| 2014‐2015 | A (H1) | PCR confirmed | 0.73 (0.49, 1.03) | 0.23 (0.08, 0.56) | 0.65 (0.29, 1.29) |
| 2013‐2014 | B | PCR confirmed | 0.73 (0.42, 1.16) | 0.06 (0.01, 0.22) | 0.24 (0.06, 0.63) |
| 2014‐2015 | B | PCR confirmed | 0.60 (0.13, 1.63) | 0.20 (0.01, 78) | 46.9 (0.09, 878)) |
| 2013‐2014 | A (H1) | ARI episode | 0.72 (0.43, 1.11) 11.0 | (0.3, 282) | 40.5 (1.4, 845) |
| 2013‐2014 | A (H3) | ARI episode | 0.80 (0.33, 1.58) 0.16 | (0.01, 41) | 59.3 (0.1, 886) |
| 2014‐2015 | A (H1) | ARI episode | 0.60 (0.41, 0.83) 0.29 | (0.14, 0.54) | 0.44 (0.25, 0.74) |
| 2013‐2014 | B | ARI episode | 0.70 (0.41, 1.11) | 0.09 (0.01, 0.29) | 0.30 (0.09, 0.71) |
| 2014‐2015 | B | ARI episode | 0.47 (0.10, 1.29) | 4.9 (0.03, 386) | 24.3 (0.2, 828) |
4. DISCUSSION
We found an attack rate of 4.7% for influenza illness each season and a SAR of 2.3% for influenza A (H1) in 2013‐2014, 5.3% for influenza B in 2013‐2014, and 7.6% for influenza A (H3) 2014‐2015. When the first person in an average sized household was infected with influenza, the attack rate increased up to 350% for the rest of the household, depending on the season and virus circulating in the home. Our results are qualitatively similar to published estimates of the attack rate and SAR of PCR‐confirmed influenza infections from household cohort studies in the United States. The Flu Watch cohort study reported the attack rate was on average 4% per season for the 2006‐2007 through 2008‐2009 seasons. 53 The Household Influenza Vaccine Effectiveness (HIVE) study reported SARs of 2.9% for influenza A (H1), 7.7% for influenza B, and 15.3% for influenza A (H3) during the 2010‐2011 season and reported an attack rate of 8.7%. 18 For the 2014‐2015 season, the HIVE study reported a SAR of 17% for influenza A (H3), a SAR of 6% for influenza B, and an attack rate of 12%. 23
When we included episodes of ARI regardless of test results, our estimates of SAR often increased substantially. For example, the estimate of SAR for influenza A (H3) during 2014‐2015 increased from 7.6% to 13.5% by including additional episodes of ARI regardless of test result. While we know that including all episodes of ARI likely overestimates the SAR, the opposite is true when we restricted analysis to laboratory‐confirmed secondary cases. The more inclusive definition of a secondary case allows comparison with previously published work from case ascertained household studies. The earliest reports from the United States on the household transmission of pandemic H1N1 2009 influenza reported a SAR of 13% and 27.3%. 76 , 147 Subsequent reports of estimates of the SAR ranged from 3.5% to 22.8%; and, our analogous estimate of SAR of influenza A (H1) during 2013‐2014 was 5.7%, which is in the middle of this range. 81 , 82 , 85 , 93 , 94 , 148
As accurate counts of the number of secondary cases are rarely available, researchers rely on statistical methods to estimate SARs. If we relied only on counting apparent secondary cases, then neither the 3‐, nor 5‐, nor 7‐day SAR could capture quantitatively similar estimates as our method. In effect, shortening the time at risk for these SARs corrects for over counting, but specifying the optimal time interval is difficult. Specifically, the number of uncounted cases attributable to household transmission must equal the number of over counted cases attributable to community transmission. While the 3‐, 5‐, or 7‐day SAR was similar to that from the transmission model for influenza A (H1) during 2013‐2014, neither the 3‐, 5‐, nor 7‐ day SAR was similar to the SAR from the transmission model for both influenza A (H3) during 2014‐2015 and influenza B during 2013‐2014 (Table 3). As risk from the community relative to the risk from the household increases, this bias increases. Using the model‐based approach obviates choosing the appropriate length of time at risk for household transmission, in exchange for a more cumbersome process of estimation. Additionally, the model‐based approach provides credible intervals for the CAR and the SAR, while simultaneously adjusting for uncertainty in both.
Our results may not be generalizable. In order to participate in this study, households must have at least one child. The dynamics of influenza transmission in homes without children may be qualitatively different than what we present here. 76 In order to participate in this study, households must have at least 3 people. Smaller households account for about 60% of households in the United States (U.S. Census Bureau, 2012‐2016 American Community Survey 5‐Year Estimates). Our case definition relied on molecular evidence of influenza virus in a nasal swab as detected by RT‐PCR using a benchtop system. 140 While highly specific, our methods may not have detected every infection with influenza virus among enrollees with an ARI. Our study did not capture asymptomatic infections with influenza, which would violate our assumptions that enrollees who never met the case definition remained susceptible for the entire influenza season. Although we analyzed the data separately for cases of influenza A (H1), influenza A (H3), and influenza B, more than one influenza virus may have circulated in some households. For example, most of the circulating influenza A (H3) viruses belonged to the 3C.2a genetic group during the 2014‐2015 season in New York, but influenza A (H3) viruses from other genetic groups also circulated within the state. 148
Estimates of the transmissibility of influenza, measured by SAR within a household, ranges widely over recent history. The estimates we present here reinforce the variation of the expected transmissibility of influenza viruses circulating in the population from historical data (Table S3). Future data about this cohort may enable analyses which account for differential susceptibility within the cohort, for example, by extending the compartmental model to allow for immunity. The changing susceptibility of the population to the circulating influenza viruses could explain part of the observed dynamics of the transmissibility of influenza. Quantifying this relationship could strengthen public health messaging about preventing influenza.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Data S1: Supporting information
ACKNOWLEDGEMENTS
We would like to thank the anonymous reviewers of this manuscript for their thoughtful remarks. We thank Dr. Lisa Saiman and Dr. Elaine L. Larson for critically reviewing this article. This study was funded by grant U01IP000618 from the Centers for Disease Control and Prevention. We thank the other members of the MoSAIC study team: Hilbania Diaz, Dr. Yaritza Castellanos de Belliard, Dr. Maria Morban, Othanya Garcia, and Liqun Wang. Investigators from the Centers for Disease Control and Prevention took part in the design and conduct of the study, the analysis and interpretation of the data, and the review and approval of the manuscript. The findings and conclusions presented in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Dahlgren FS, Foppa IM, Stockwell MS, Vargas CY, LaRussa P, Reed C. Household transmission of influenza A and B within a prospective cohort during the 2013‐2014 and 2014‐2015 seasons. Statistics in Medicine. 2021;40(28):6260–6276. 10.1002/sim.9181
Abbreviations: ARI, acute respiratory illness; CAR, community attack rate; MoSAIC, Mobile Surveillance for Acute Respiratory and Influenza‐like Illness in the Community; RR, risk ratio; RT‐PCR, reverse transcription polymerase chain reaction; SAR, secondary attack rate
DATA AVAILABILITY STATEMENT
The data are not available due to privacy or ethical restrictions.
REFERENCES
- 1. Buck C. Acute upper respiratory infections in families. Am J Hyg. 1956;63(1):1‐12. 10.1093/oxfordjournals.aje.a119786 [DOI] [PubMed] [Google Scholar]
- 2. Lidwell OM, Sommerville T. Observations on the incidence and distribution of the common cold in a rural community during 1948 and 1949. J Hyg (Lond). 1951;49(4):365‐381. 10.1017/s0022172400066699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Badger GF, Dingle JH, Feller AE, Hodges RG, Jordan J, Rammelkamp J. A study of illness in a group of Cleveland families. IV. the spread of respiratory infections within the home. Am J Hyg. 1953;58(2):174‐178. 10.1093/oxfordjournals.aje.a119598 [DOI] [PubMed] [Google Scholar]
- 4. Brimblecombe FSW, Cruickshank R, Masters PL, Reid DD, Stewart GT. Family studies of respiratory infections. Br Med J. 1958;1(5063):119‐128. 10.1136/bmj.1.5063.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall CE, Cooney MK, Fox JP. The seattle virus watch. IV. comparative epidemiologic observations of infections with influenza A and B viruses, 1965–1969, in families with young children. Am J Epidemiol. 1973;98(5):365‐380. 10.1093/oxfordjournals.aje.a121566 [DOI] [PubMed] [Google Scholar]
- 6. Foy HM, Cooney MK, Allan I. Longitudinal studies of types A and B influenza among Seattle schoolchildren and families, 1968–1974. J Infect Dis. 1976;134(4):362‐369. 10.1093/infdis/134.4.362 [DOI] [PubMed] [Google Scholar]
- 7. Jennings LC, Miles JAR. A study of acute respiratory disease in the community of Port Chalmers. II. influenza A/Port Chalmers/1/73: intrafamilial spread and the effect of antibodies to the surface antigens. J Hyg (Lond). 1978;81(1):67‐75. 10.1017/s0022172400053778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The Royal College of General Practitioners. The Public Health Laboratory Service . Long‐term study of influenza in families. J R Coll Gen Pract. 1981;31(227):351‐356. [PMC free article] [PubMed] [Google Scholar]
- 9. Fox JP, Cooney MK, Hall CE, Foy HM. Influenzavirus infections in Seattle families, 1975–1979. II. pattern of infection in invaded households and relation of age and prior antibody to occurrence of infection and related illness. Am J Epidemiol. 1982;116(2):228‐242. 10.1093/oxfordjournals.aje.a113408 [DOI] [PubMed] [Google Scholar]
- 10. Longini J, Koopman JS, Monto AS, Fox JP. Estimating household and community transmission parameters for influenza. Am J Epidemiol. 1982;115(5):736‐751. 10.1093/oxfordjournals.aje.a113356 [DOI] [PubMed] [Google Scholar]
- 11. Frank AL, Taber LH, Glezen WP, Geyer EA, McIlwain S, Paredes A. Influenza B virus infections in the community and the family. the epidemics of 1976–1977 and 1979–1980 in Houston, Texas. Am J Epidemiol. 1983;118(3):313‐325. 10.1093/oxfordjournals.aje.a113638 [DOI] [PubMed] [Google Scholar]
- 12. Longini J, Monto AS. Efficacy of virucidal nasal tissues in interrupting familial transmission of respiratory agents. a field trial in Tecumseh, Michigan. Am J Epidemiol. 1988;128(3):639‐644. 10.1093/oxfordjournals.aje.a115011 [DOI] [PubMed] [Google Scholar]
- 13. Hayden FG, Gubareva LV, Monto AS, et al. Inhaled Zanamivir for the prevention of influenza in families. N Engl J Med. 2000;343(18):1282‐1289. 10.1056/NEJM200011023431801 [DOI] [PubMed] [Google Scholar]
- 14. Hayden FG, Belshe R, Villanueva C, et al. Management of influenza in households: a prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis. J Infect Dis. 2004;189(3):440‐449. 10.1086/381128 [DOI] [PubMed] [Google Scholar]
- 15. Klick B, Nishiura H, Ng S, et al. Transmissibility of seasonal and pandemic influenza in a cohort of households in Hong Kong in 2009. Epidemiology. 2011;22(6):793‐796. 10.1097/EDE.0b013e3182302e8e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nukiwa‐Souma N, Burmaa A, Kamigaki T, et al. Influenza transmission in a community during a seasonal influenza A(H3N2) outbreak (2010–2011) in Mongolia: a community‐based prospective cohort study. PLoS One. 2012;7(3):e33046. 10.1371/journal.pone.0033046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohmit SE, Petrie JG, Malosh RE, et al. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis. 2013;56(10):1363‐1369. 10.1093/cid/cit060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petrie JG, Ohmit SE, Cowling BJ, et al. Influenza transmission in a cohort of households with children: 2010‐2011. PLoS One. 2013;8(9):e75339. 10.1371/journal.pone.0075339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cauchemez S, Ferguson NM, Fox A, et al. Determinants of influenza transmission in South East Asia: insights from a household cohort study in Vietnam. PLoS Pathog. 2014;10(8):e1004310. 10.1371/journal.ppat.1004310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thai PQ, Mai LQ, Welkers MRA, et al. Pandemic H1N1 virus transmission and shedding dynamics in index case households of a prospective Vietnamese cohort. J Infect. 2014;68(6):581‐590. 10.1016/j.jinf.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fry AM, Goswami D, Nahar K, et al. Effects of oseltamivir treatment of index patients with influenza on secondary household illness in an urban setting in Bangladesh: secondary analysis of a randomised, placebo‐controlled trial. Lancet Infect Dis. 2015;15(6):654‐662. 10.1016/S1473-3099(15)70041-1 [DOI] [PubMed] [Google Scholar]
- 22. Judd MC, Emukule GO, Njuguna H, et al. The role of HIV in the household introduction and transmission of influenza in an urban slum, Nairobi, Kenya, 2008–2011. J Infect Dis. 2015;212(5):740‐744. 10.1093/infdis/jiv106 [DOI] [PubMed] [Google Scholar]
- 23. Petrie JG, Malosh RE, Cheng CK, et al. The household influenza vaccine effectiveness study: lack of antibody response and protection following receipt of 2014–2015 influenza vaccine. Clin Infect Dis. 2017;65(10):1644‐1651. 10.1093/cid/cix608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buchwald AG, Tamboura B, Haidara FC, et al. Maternal influenza vaccination and the risk of laboratory‐confirmed influenza among household contacts under the age of five in Mali. Am J Trop Med Hyg. 2019;100(1):159‐164. 10.4269/ajtmh.18-0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malosh RE, Noppert GA, Zelner J, Martin ET, Monto AS. Social patterning of acute respiratory illnesses in the Household Influenza Vaccine Evaluation (HIVE) study 2014–2015. Epidemiol Infect. 2019;147:e185. 10.1017/S0950268819000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ranjeva S, Subramanian R, Fang VJ, et al. Age‐specific differences in the dynamics of protective immunity to influenza. Nat Commun. 2019;10(1):1660. 10.1038/s41467-019-09652-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sydenstricker E. The incidence of illness in a general population group. General results of a morbidity study from December 1, 1921, through March 31, 1924, in Hagerstown, MD. Public Health Rep. 1925;40(7):279‐293.19314969 [Google Scholar]
- 28. Sydenstricker E. A study of illness in a general population group. Hagerstown morbidity studies no. I: the method of study and general results. Public Health Rep. 1926;41(39):2069‐2088. [Google Scholar]
- 29. Van Volkenburgh VA, Frost WH. Acute minor respiratory diseases prevailing in a group of families residing in Baltimore, Maryland, 1928–1930. Prevalence, distribution and clinical description of observed cases. Am J Epidemiol. 1933;17(1):122‐153. 10.1093/oxfordjournals.aje.a117900 [DOI] [Google Scholar]
- 30. Dingle JH, Badger GF, Feller AE, Hodges RG, Jordan J, Rammelkamp J. A study of respiratory infections in families. Trans Assoc Am Phys. 1949;62:99‐104. [Google Scholar]
- 31. Tucher D, Coulter JE, Downes J. Incidence of acute respiratory illness among males and females at specific ages; study no. 5. Milbank Mem Fund Q. 1952;30(1):42‐60. [PubMed] [Google Scholar]
- 32. Badger GF, Dingle JH, Feller AE, Hodges RG, Jordan J, Rammelkamp J. A study of illness in a group of Cleveland families. V. Introductions and secondary attack rates as indices of exposure to common respiratory diseases in the community. Am J Hyg. 1953;58(2):179‐182. 10.1093/oxfordjournals.aje.a119599 [DOI] [PubMed] [Google Scholar]
- 33. Dingle JH, Badger GF, Feller AE, Hodges RG, Jordan J, Rammelkamp J. A study of illness in a group of Cleveland families. I. Plan of study and certain general observations. Am J Hyg. 1953;58(1):16‐30. 10.1093/oxfordjournals.aje.a119587 [DOI] [PubMed] [Google Scholar]
- 34. Jordan J, Badger GF, Dingle JH. A study of illness in a group of Cleveland families. XVI. The epidemiology of influenza, 1948‐1953. Am J Hyg. 1958;68(2):169‐189. 10.1093/oxfordjournals.aje.a119961 [DOI] [PubMed] [Google Scholar]
- 35. Dunn FL, Carey DE, Cohen A, Martin JD. Epidemiologic studies of Asian influenza in a Louisiana parish. Am J Hyg. 1959;70:351‐371. 10.1093/oxfordjournals.aje.a120083 [DOI] [PubMed] [Google Scholar]
- 36. Philip RN, Bell JA, Davis DJ, et al. Epidemiologic studies on influenza in familial and general population groups, 1951–1956. II. Characteristics of occurrence. Am J Hyg. 1961;73:123‐137. 10.1093/oxfordjournals.aje.a120171 [DOI] [PubMed] [Google Scholar]
- 37. Dingle JH, Badger GF, Jordan J. Illness in the Home. A Study of 25,000 Illnesses in a Group of Cleveland Families. Cleveland: The Press of Western Reserve University; 1964. [Google Scholar]
- 38. Fox JP, Elveback LR, Spigland I, Frothingham TE, Sevens DA, Huger M. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. I. Overall plan, methods of collecting and handling information and a summary report of specimens collected and illnesses observed. Am J Epidemiol. 1966;83(3):389‐412. 10.1093/oxfordjournals.aje.a120594 [DOI] [Google Scholar]
- 39. Spigland I, Fox JP, Elveback LR, et al. The Virus Watch program: a continuing surveillance of viral infections in metropolitan New York families. II. Laboratory methods and preliminary report on infections revealed by virus isolation. Am J Epidemiol. 1966;83(3):413‐435. 10.1093/oxfordjournals.aje.a120595 [DOI] [PubMed] [Google Scholar]
- 40. Elveback LR, Fox JP, Ketler A, Brandt CD, Wassermann FE, Hall CE. The Virus Watch program: a continuing surveillance of viral infections in metropolitan New York families. III. Preliminary report on association of infections with disease. Am J Epidemiol. 1966;83(3):436‐454. 10.1093/oxfordjournals.aje.a120596 [DOI] [PubMed] [Google Scholar]
- 41. Hall CE, Brandt CD, Frothingham TE, Spigland I, Cooney MK, Fox JP. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. IX. A comparison of infections with several respiratory pathogens in New York and New Orleans families. Am J Epidemiol. 1971;94(4):367‐385. 10.1093/oxfordjournals.aje.a121332 [DOI] [PubMed] [Google Scholar]
- 42. Monto AS, Napier JA, Metzner HL. The Tecumseh study of respiratory illness. I. Plan of study and observations on syndromes of acute respiratory disease. Am J Epidemiol. 1971;94(3):269‐279. 10.1093/oxfordjournals.aje.a121320 [DOI] [PubMed] [Google Scholar]
- 43. Monto AS, Cavallaro JJ. The Tecumseh study of respiratory illness. II. Patterns of occurrence of infection with respiratory pathogens, 1965–1969. Am J Epidemiol. 1971;94(3):280‐289. 10.1093/oxfordjournals.aje.a121321 [DOI] [PubMed] [Google Scholar]
- 44. Fox JP, Hall CE, Cooney MK, Luce RE, Kronmal RA. The Seattle virus watch. II. Objectives, study population and its observation, data processing and summary of illnesses. Am J Epidemiol. 1972;96(4):270‐285. 10.1093/oxfordjournals.aje.a121458 [DOI] [PubMed] [Google Scholar]
- 45. Cooney MK, Hall CE, Fox JP. The Seattle virus watch. III. Evaluation of isolation methods and summary of infections detected by virus isolations. Am J Epidemiol. 1972;96(4):286‐305. 10.1093/oxfordjournals.aje.a121459 [DOI] [PubMed] [Google Scholar]
- 46. Monto AS, Higgins MW, Ross HW. The Tecumseh study of respiratory illness. VIII. Acute infection in chronic respiratory disease and comparison groups. Am Rev Respir Dis. 1975;111(1):27‐36. 10.1164/arrd.1975.111.1.27 [DOI] [PubMed] [Google Scholar]
- 47. Frank AL, Taber LH, Glezen WP, Paredes A, Couch RB. Reinfection with influenza A (H3N2) virus in young children and their families. J Infect Dis. 1979;140(6):829‐836. 10.1093/infdis/140.6.829 [DOI] [PubMed] [Google Scholar]
- 48. Taber LH, Paredes A, Glezen WP, Couch RB. Infection with influenza A/Victoria virus in Houston families, 1976. J Hyg (Lond). 1981;86(3):303‐313. 10.1017/s0022172400069059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Monto AS, Koopman JS, Longini J. Tecumseh study of illness. XIII. Influenza infection and disease, 1976‐1981. Am J Epidemiol. 1985;121(6):811‐822. 10.1093/oxfordjournals.aje.a114052 [DOI] [PubMed] [Google Scholar]
- 50. Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect. 1993;110(1):145‐160. 10.1017/s0950268800050779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carrat F, Sahler C, Rogez S, et al. Influenza burden of illness: estimates from a national prospective survey of household contacts in France. Arch Intern Med. 2002;162(16):1842‐1848. 10.1001/archinte.162.16.1842 [DOI] [PubMed] [Google Scholar]
- 52. Horby P, Mai LQ, Fox A, et al. The epidemiology of interpandemic and pandemic influenza in Vietnam, 2007–2010: the Ha Nam household cohort study I. Am J Epidemiol. 2012;175(10):1062‐1074. 10.1093/aje/kws121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hayward AC, Fragaszy EB, Bermingham A, et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med. 2014;2(6):445‐454. 10.1016/S2213-2600(14)70034-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Monto AS, Malosh RE, Petrie JG, Thompson MG, Ohmit SE. Frequency of acute respiratory illnesses and circulation of respiratory viruses in households with children over 3 surveillance seasons. J Infect Dis. 2014;210(11):1792‐1799. 10.1093/infdis/jiu327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stockwell MS, Reed C, Vargas CY, et al. MoSAIC: mobile surveillance for acute respiratory infections and influenza‐like illness in the community. Am J Epidemiol. 2014;180(12):1196‐1201. 10.1093/aje/kwu303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Delabre RM, Lapidus N, Salez N, Mansiaux Y, de Lamballerie X, Carrat F. Risk factors of pandemic influenza A/H1N1 in a prospective household cohort in the general population: results from the CoPanFlu‐France cohort. Influenza Other Respir Viruses. 2015;9(1):43‐50. 10.1111/irv.12294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mansiaux Y, Salez N, Lapidus N, et al. Causal analysis of H1N1pdm09 influenza infection risk in a household cohort. J Epidemiol Community Health. 2015;69(3):272‐277. 10.1136/jech-2014-204678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ip DKM, Lau LLH, Chan KH, et al. The dynamic relationship between clinical symptomatology and viral shedding in naturally acquired seasonal and pandemic influenza virus infection. Clin Infect Dis. 2016;62(4):431‐437. 10.1093/cid/civ909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smithgall M, Vargas CY, Reed C, et al. Influenza vaccine effectiveness in a low‐income, urban community cohort. Clin Infect Dis. 2016;62(3):358‐360. 10.1093/cid/civ867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vargas CY, Wang L, de Belliard YC, et al. Pilot study of participant‐collected nasal swabs for acute respiratory infections in a low‐income, urban population. Clin Epidemiol. 2016;8:1‐5. 10.2147/CLEP.S95847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zachariah P, Whittier S, Reed C, et al. Community ‐and hospital laboratory‐based surveillance for respiratory viruses. Influenza Other Respir Viruses. 2016;10(5):361‐366. 10.1111/irv.12387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Petrie JG, Eisenberg MC, Ng S, et al. Application of an individual‐based transmission hazard model for estimation of influenza vaccine effectiveness in a household cohort. Am J Epidemiol. 2017;186(12):1380‐1388. 10.1093/aje/kwx217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tinoco YO, Azziz‐Baumgartner E, Uyeki TM, et al. Burden of influenza in 4 ecologically distinct regions of Peru: household active surveillance of a community cohort, 2009–2015. Clin Infect Dis. 2017;65(9):1532‐1541. 10.1093/cid/cix565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ainslie KEC, Haber MJ, Malosh RE, Petrie JG, Monto AS. Maximum likelihood estimation of influenza vaccine effectiveness against transmission from the household and from the community. Stat Med. 2018;37(6):970‐982. 10.1002/sim.7558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hope Simpson RE, Sutherland I. Does influenza spread within the household? Lancet. 1954;266(6814):721‐726. 10.1016/s0140-6736(54)92126-1 [DOI] [PubMed] [Google Scholar]
- 66. U.S. National Communicable Disease Center Respiratory Viral Diseases Unit, U.S. National Communicable Disease Center Laboratory Branch Respiratory Virology Unit, United States Public Health Service Office of the Surgeon General, Advisory Committee on Immunization Practice, World Health Organization International Influenza Center for the Americas . Influenza‐respiratory disease surveillance report number 85. MMWR Morb Mortal Wkly Rep. 1969;18(25):22‐32. [Google Scholar]
- 67. Hope‐Simpson RE. First outbreak of Hong Kong influenza in a general practice population in Great Britain. A field and laboratory study. Br Med J. 1970;3(5714):74‐77. 10.1136/bmj.3.5714.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hope‐Simpson RE. Epidemic mechanisms of type A influenza. J Hyg (Lond). 1979;83(1):11‐26. 10.1017/s002217240002578x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Katz JM, Lim W, Bridges CB, et al. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti‐H5 antibody among household and social contacts. J Infect Dis. 1999;180(6):1763‐1770. 10.1086/315137 [DOI] [PubMed] [Google Scholar]
- 70. Hurwitz ES, Haber M, Chang A, et al. Effectiveness of influenza vaccination of day care children in reducing influenza‐related morbidity among household contacts. JAMA. 2000;284(13):1677‐1682. 10.1001/jama.284.13.1677 [DOI] [PubMed] [Google Scholar]
- 71. Welliver R, Monto AS, Carewicz O, et al. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA. 2001;285(6):748‐754. 10.1001/jama.285.6.748 [DOI] [PubMed] [Google Scholar]
- 72. Monto AS, Pichichero ME, Blanckenberg SJ, et al. Zanamivir prophylaxis: an effective strategy for the prevention of influenza types A and B within households. J Infect Dis. 2002;186(11):1582‐1588. 10.1086/345722 [DOI] [PubMed] [Google Scholar]
- 73. Neuzil KM, Hohlbein C, Zhu Y. Illness among schoolchildren during influenza season: effect on school absenteeism, parental absenteeism from work, and secondary illness in families. Arch Pediatr Adolesc Med. 2002;156(10):986‐991. 10.1001/archpedi.156.10.986 [DOI] [PubMed] [Google Scholar]
- 74. Viboud C, Boëlle PY, Cauchemez S, et al. Risk factors of influenza transmission in households. Br J Gen Pract. 2004;54(506):684‐689. [PMC free article] [PubMed] [Google Scholar]
- 75. Cowling BJ, Fung ROP, Cheng CKY, et al. Preliminary findings of a randomized trial of non‐pharmaceutical interventions to prevent influenza transmission in households. PLoS One. 2008;3(5):e2101. 10.1371/journal.pone.0002101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cauchemez S, Donnelly CA, Reed C, et al. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med. 2009;361(27):2619‐2627. 10.1056/NEJMoa0905498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Odaira F, Takahashi H, Toyokawa T, et al. Assessment of secondary attack rate and effectiveness of antiviral prophylaxis among household contacts in an influenza A(H1N1)v outbreak in Kobe, Japan, May–June 2009. Eurosurveillance. 2009;14(35):19320. [PubMed] [Google Scholar]
- 78. van Boven M, Donker T, van der Lubben M, et al. Transmission of novel influenza A(H1N1) in households with post‐exposure antiviral prophylaxis. PLoS One. 2010;5(7):e11442. 10.1371/journal.pone.0011442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Calatayud L, Kurkela S, Neave PE, et al. Pandemic (H1N1) 2009 virus outbreak in a school in London, April‐May 2009: an observational study. Epidemiol Infect. 2010;138(2):183‐191. 10.1017/S0950268809991191 [DOI] [PubMed] [Google Scholar]
- 80. Cowling BJ, Chan KH, Fang VJ, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med. 2010;362(23):2175‐2184. 10.1056/NEJMoa0911530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. France AM, Jackson M, Schrag S, et al. Household transmission of 2009 influenza A (H1N1) virus after a school‐based outbreak in New York City, April–May 2009. J Infect Dis. 2010;201(7):984‐992. 10.1086/651145 [DOI] [PubMed] [Google Scholar]
- 82. Goldstein E, Cowling BJ, O'Hagan JJ, et al. Oseltamivir for treatment and prevention of pandemic influenza A/H1N1 virus infection in households, Milwaukee, 2009. BMC Infect Dis. 2010;10:211. 10.1186/1471-2334-10-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Komiya N, Gu Y, Kamiya H, et al. Household transmission of pandemic 2009 influenza A (H1N1) virus in Osaka, Japan in May 2009. J Infect. 2010;61(4):284‐288. 10.1016/j.jinf.2010.06.019 [DOI] [PubMed] [Google Scholar]
- 84. Looker C, Carville K, Grant K, Kelly H. Influenza A (H1N1) in Victoria, Australia: a community case series and analysis of household transmission. PLoS One. 2010;5(10):e13702. 10.1371/journal.pone.0013702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Morgan OW, Parks S, Shim T, et al. Household transmission of pandemic (H1N1) 2009, San Antonio, Texas, USA, April–May 2009. Emerg Infect Dis. 2010;16(4):631‐637. 10.3201/eid1604.091658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ng S, Cowling BJ, Fang VJ, et al. Effects of oseltamivir treatment on duration of clinical illness and viral shedding and household transmission of influenza virus. Clin Infect Dis. 2010;50(5):707‐714. 10.1086/650458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Papenburg J, Baz M, Hamelin MÈ, et al. Household transmission of the 2009 pandemic A/H1N1 influenza virus: elevated laboratory‐confirmed secondary attack rates and evidence of asymptomatic infections. Clin Infect Dis. 2010;51(9):1033‐1041. 10.1086/656582 [DOI] [PubMed] [Google Scholar]
- 88. Pedroni E, Garcia M, Espinola V, et al. Outbreak of 2009 pandemic influenza A(H1N1), Los Lagos, Chile, April‐June 2009. Eurosurveillance. 2010;15(1):19456. 10.2807/ese.15.01.19456-en [DOI] [PubMed] [Google Scholar]
- 89. Sikora C, Fan S, Golonka R, et al. Transmission of pandemic influenza A (H1N1) 2009 within households: Edmonton. Canada J Clin Virol. 2010;49(2):90‐93. 10.1016/j.jcv.2010.06.015 [DOI] [PubMed] [Google Scholar]
- 90. Suess T, Buchholz U, Dupke S, et al. Shedding and transmission of novel influenza virus A/H1N1 infection in households—Germany, 2009. Am J Epidemiol. 2010;171(11):1157‐1164. 10.1093/aje/kwq071 [DOI] [PubMed] [Google Scholar]
- 91. Carcione D, Giele CM, Goggin LS, et al. Secondary attack rate of pandemic influenza A(H1N1) 2009 in Western Australian households, 29 May–7 August 2009. Eurosurveillance. 2011;16(3):19765. [PubMed] [Google Scholar]
- 92. Chang LY, Chen WH, Lu CY, et al. Household transmission of pandemic (H1N1) 2009 virus. Taiwan Emerg Infect Dis. 2011;17(10):1928‐1931. 10.3201/eid1710.101662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Doyle TJ, Hopkins RS, Transmission Investigation Team . Low secondary transmission of 2009 pandemic influenza A (H1N1) in households following an outbreak at a summer camp: relationship to timing of exposure. Epidemiol Infect. 2011;139(1):45‐51. 10.1017/S095026881000141X [DOI] [PubMed] [Google Scholar]
- 94. Jackson ML, France AM, Hancock K, et al. Serologically confirmed household transmission of 2009 pandemic influenza A (H1N1) virus during the first pandemic wave—New York City, April–May 2009. Clin Infect Dis. 2011;53(5):455‐462. 10.1093/cid/cir437 [DOI] [PubMed] [Google Scholar]
- 95. Van Gemert C, Hellard M, McBryde ES, et al. Intrahousehold transmission of pandemic (H1N1) 2009 virus, Victoria, Australia. Emerg Infect Dis. 2011;17(9):1599‐1607. 10.3201/eid1709.101948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Leung YH, Li MP, Chuang SK. A school outbreak of pandemic (H1N1) 2009 infection: assessment of secondary household transmission and the protective role of oseltamivir. Epidemiol Infect. 2011;139(1):41‐44. 10.1017/S0950268810001445 [DOI] [PubMed] [Google Scholar]
- 97. Nishiura H, Oshitani H. Household transmission of influenza (H1N1‐2009) in Japan: age‐specificity and reduction of household transmission risk by Zanamivir treatment. J Int Med Res. 2011;39(2):619‐628. 10.1177/147323001103900231 [DOI] [PubMed] [Google Scholar]
- 98. Pebody RG, Harris R, Kafatos G, et al. Use of antiviral drugs to reduce household transmission of pandemic (H1N1) 2009. United Kingdom Emerg Infect Dis. 2011;17(6):990‐999. 10.3201/eid/1706.101161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Savage R, Whelan M, Johnson I, et al. Assessing secondary attack rates among household contacts at the beginning of the influenza A (H1N1) pandemic in Ontario, Canada, April‐June 2009: a prospective, observational study. BMC Public Health. 2011;11:234. 10.1186/1471-2458-11-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Simmerman JM, Suntarattiwong P, Levy J, et al. Findings from a household randomized controlled trial of hand washing and face masks to reduce influenza transmission in Bangkok. Thailand Influenza Other Respir Viruses. 2011;5(4):256‐267. 10.1111/j.1750-2659.2011.00205.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vargas‐Leguas H, Caylà JA, Ballester I, et al. Factores asociados a la transmisión a los convivientes de gripe (H1N1) 2009. Rev Esp Salud Pública. 2011;85(1):57‐62. 10.1590/S1135-57272011000100007 [DOI] [PubMed] [Google Scholar]
- 102. Behnaz F, Mohammadzadeh M, Sadeghian M. Household transmission of 2009 H1N1 influenza virus in Yazd. Iran J Infect Public Health. 2012;5(4):275‐280. 10.1016/j.jiph.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 103. Hirotsu N, Wada K, Oshitani H. Risk factors of household transmission of pandemic (H1N1) 2009 among patients treated with antivirals: a prospective study at a primary clinic in Japan. PLoS One. 2012;7(2):e31519. 10.1371/journal.pone.0031519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. House T, Inglis N, Ross JV, et al. Estimation of outbreak severity and transmissibility: influenza A(H1N1)pdm09 in households. BMC Med. 2012;10:117. 10.1186/1741-7015-10-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Janjua NZ, Skowronski DM, Hottes TS, et al. Transmission dynamics and risk factors for pandemic H1N1‐related illness: outbreak investigation in a rural community of British Columbia. Canada Influenza Other Respir Viruses. 2012;6(3):e54‐e62. 10.1111/j.1750-2659.2012.00344.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kim CY, Breiman RF, Cosmas L, et al. Secondary household transmission of 2009 pandemic influenza A (H1N1) virus among an urban and rural population in Kenya, 2009–2010. PLoS One. 2012;7(6):e38166. 10.1371/journal.pone.0038166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. McCaw JM, Howard PF, Richmond PC, et al. Household transmission of respiratory viruses – assessment of viral, individual and household characteristics in a population study of healthy Australian adults. BMC Infect Dis. 2012;12:345. 10.1186/1471-2334-12-345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mohamed AG, BinSaeed AA, Al‐Habib H, Al‐Saif H. Communicability of H1N1 and seasonal influenza among household contacts of cases in large families. Influenza Other Respir Viruses. 2012;6(3):e25‐e29. 10.1111/j.1750-2659.2011.00308.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Udompornwattana S, Srajai K, Suwan P, et al. The clinical features, risk of prolonged hospitalization and household infections of hospitalized children for pandemic 2009 influenza A (H1N1) virus infection in Thailand. J Med Assoc Thail. 2012;95(3):403‐411. [PubMed] [Google Scholar]
- 110. Azman AS, Stark JH, Althouse BM, et al. Household transmission of influenza A and B in a school‐based study of non‐pharmaceutical interventions. Epidemics. 2013;5(4):181‐186. 10.1016/j.epidem.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 111. Kashiwagi S, Watanabe A, Ikematsu H, et al. Laninamivir octanoate for post‐exposure prophylaxis of influenza in household contacts: a randomized double blind placebo controlled trial. J Infect Chemother. 2013;19(4):740‐749. 10.1007/s10156-013-0622-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Remschmidt C, Stöcker P, de Heiden AM, et al. Preventable and non‐preventable risk factors for influenza transmission and hygiene behavior in German influenza households, pandemic season (H1N1) 2009/2010. Influenza Other Respir Viruses. 2013;7(3):418‐425. 10.1111/j.1750-2659.2012.00407.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Casado I, Martínez‐Baz I, Burgui R, et al. Household transmission of influenza A(H1N1)pdm09 in the pandemic and post‐pandemic seasons. PLoS One. 2014;9(9):e108485. 10.1371/journal.pone.0108485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Qiu C, Yuan S, Tian D, et al. Epidemiologic report and serologic findings for household contacts of three cases of influenza A (H7N9) virus infection. J Clin Virol. 2014;59(2):129‐131. 10.1016/j.jcv.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cheung DH, Tsang TK, Fang VJ, et al. Association of oseltamivir treatment with virus shedding, illness, and household transmission of influenza viruses. J Infect Dis. 2015;212(3):391‐396. 10.1093/infdis/jiv058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Iyengar P, Van Mollendorf C, Tempia S, et al. Case‐ascertained study of household transmission of seasonal influenza — South Africa. J Infect. 2015;71(5):578‐586. 10.1016/j.jinf.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ram PK, DiVita MA, Jannat KEK, et al. Impact of intensive handwashing promotion on secondary household influenza‐like illness in rural Bangladesh: findings from a randomized controlled trial. PLoS One. 2015;10(6):e0125200. 10.1371/journal.pone.0125200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yang Y, Zhang Y, Fang L, et al. Household transmissibility of avian influenza A (H7N9) virus, China, February to May 2013 and October 2013 to March 2014. Euro Surveill. 2015;20(10):21056. 10.2807/1560-7917.es2015.20.10.21056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ng S, Lopez R, Kuan G, et al. The timeline of influenza virus shedding in children and adults in a household transmission study of influenza in Managua. Nicaragua Pediatr Infect Dis J. 2016;35(5):583‐586. 10.1097/INF.0000000000001083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Weaver AM, Khatun‐e‐Jannat K, Cercone E, et al. Household‐level risk factors for secondary influenza‐like illness in a rural area of Bangladesh. Tropical Med Int Health. 2017;22(2):187‐195. 10.1111/tmi.12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gordon A, Tsang TK, Cowling BJ, et al. Influenza transmission dynamics in urban households, Managua, Nicaragua, 2012–2014. Emerg Infect Dis. 2018;24(10):1882‐1888. 10.3201/eid2410.161258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Valley‐Omar Z, Iyengar P, von Mollendorf C, et al. Intra‐host and intra‐household diversity of influenza A viruses during household transmissions in the 2013 season in 2 peri‐urban communities of South Africa. PLoS One. 2018;13(5):e0198101. 10.1371/journal.pone.0198101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cohen C, Tshangela A, Valley‐Omar Z, et al. Household transmission of seasonal influenza from HIV‐infected and HIV‐uninfected individuals in South Africa, 2013–2014. J Infect Dis. 2019;219(10):1605‐1615. 10.1093/infdis/jiy702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hirotsu N, Saisho Y, Hasegawa T. The effect of neuraminidase inhibitors on household transmission in Japanese patients with influenza A and B infection: a prospective, observational study. Influenza Other Respir Viruses. 2019;13(2):123‐132. 10.1111/irv.12590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Cauchemez S, Carrat F, Viboud C, Valleron AJ, Boelle PY. A Bayesian MCMC approach to study transmission of influenza: application to household longitudinal data. Stat Med. 2004;23(22):3469‐3487. 10.1002/sim.1912 [DOI] [PubMed] [Google Scholar]
- 126. Halloran ME, Hayden FG, Yang Y, Longini J, Monto AS. Antiviral effects on influenza viral transmission and pathogenicity: observations from household‐based trials. Am J Epidemiol. 2006;165(2):212‐221. 10.1093/aje/kwj362%J [DOI] [PubMed] [Google Scholar]
- 127. Donnelly CA, Finelli L, Cauchemez S, et al. Serial intervals and the temporal distribution of secondary infections within households of 2009 pandemic influenza A (H1N1): implications for influenza control recommendations. Clin Infect Dis. 2011;52(Suppl 1):S123‐S130. 10.1093/cid/ciq028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Poon LLM, Chan KH, Chu DKW, et al. Viral genetic sequence variations in pandemic H1N1/2009 and seasonal H3N2 influenza viruses within an individual, a household and a community. J Clin Virol. 2011;52(2):146‐150. 10.1016/j.jcv.2011.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang D, Liu W, Yang P, et al. Factors associated with household transmission of pandemic (H1N1) 2009 among self‐quarantined patients in Beijing. China PLoS One. 2013;8(10):e77873. 10.1371/journal.pone.0077873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tsang TK, Cauchemez S, Perera RAPM, et al. Association between antibody titers and protection against influenza virus infection within households. J Infect Dis. 2014;210(5):684‐692. 10.1093/infdis/jiu186%J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lau MS, Cowling BJ, Cook AR, Riley S. Inferring influenza dynamics and control in households. Proc Natl Acad Sci. 2015;112(29):9094‐9099. 10.1073/pnas.1423339112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tsang TK, Cowling BJ, Fang VJ, et al. Influenza a virus shedding and infectivity in households. J Infect Dis. 2015;212(9):1420‐1428. 10.1093/infdis/jiv225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Tsang TK, Fang VJ, Chan KH, et al. Individual correlates of infectivity of influenza a virus infections in households. PLoS One. 2016;11(5):1‐11. 10.1371/journal.pone.0154418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Armstrong C, Hopkins R. An epidemiological study of the 1920 epidemic of influenza in an isolated rural community. Public Health Rep. 1921;36(29):1671‐1702. 10.2307/4576063 [DOI] [Google Scholar]
- 135. Frost WH, Gover M. The incidence and time distribution of common colds in several groups kept under continuous observation. Public Health Rep. 1932;47(36):1815‐1841. 10.2307/4580544 [DOI] [Google Scholar]
- 136. Monto AS, Davenport FM, Napier JA, Francis J. Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. J Infect Dis. 1970;122(1–2):16‐25. 10.1093/infdis/122.1-2.16 [DOI] [PubMed] [Google Scholar]
- 137. Fairchok MP, Martin ET, Chambers S, et al. Epidemiology of viral respiratory tract infections in a prospective cohort of infants and toddlers attending daycare. J Clin Virol. 2010;49(1):16‐20. 10.1016/j.jcv.2010.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Johnson S, Ihekweazu C, Hardelid P, et al. Seroepidemiologic study of pandemic (H1N1) 2009 during outbreak in boarding school. Eng Emerg Infect Dis. 2011;17(9):1670. 10.3201/eid1709.100761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Martin ET, Fairchok MP, Stednick ZJ, Kuypers J, Englund JA. Epidemiology of multiple respiratory viruses in childcare attendees. J Infect Dis. 2013;207(6):982‐989. 10.1093/infdis/jis934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Poritz MA, Blaschke AJ, Byington CL, et al. FilmArray, an automated nested multiplex PCR system for multi‐pathogen detection: development and application to respiratory tract infection. PLoS One. 2011;6(10):e26047. 10.1371/journal.pone.0026047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22(158):209‐212. 10.1080/01621459.1927.10502953 [DOI] [Google Scholar]
- 142. O'Neill PD, Balding DJ, Becker NG, Eerola M, Mollison D. Analyses of infectious disease data from household outbreaks by Markov chain Monte Carlo methods. J Royal Stat Soc Ser C (Appl Stat). 2000;49(4):517‐542. 10.1111/1467-9876.00210 [DOI] [Google Scholar]
- 143. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457‐481. 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 144. Cleveland WS, Grosse E, Shyu WM. Local Regression Models. Pacific Grove, CA: Wadsworth and Brooks/Cole; 1992. [Google Scholar]
- 145. Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E. Equation of state calculations by fast computing machines. J Chem Phys. 1953;21(6):1087‐1092. 10.1063/1.1699114 [DOI] [Google Scholar]
- 146. Eddelbuettel D, François R. Rcpp: seamless C and C++ integration. J Stat Softw. 2011;40(8):1‐18. 10.18637/jss.v040.i08 [DOI] [Google Scholar]
- 147. Yang Y, Sugimoto JD, Halloran ME, et al. The transmissibility and control of pandemic influenza A (H1N1) virus. Science. 2009;326(5953):729‐733. 10.1126/science.1177373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Flannery B, Zimmerman RK, Gubareva LV, et al. Enhanced genetic characterization of influenza A(H3N2) viruses and vaccine effectiveness by genetic group, 2014–2015. J Infect Dis. 2016;214(7):1010‐1019. 10.1093/infdis/jiw181 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting information
Data Availability Statement
The data are not available due to privacy or ethical restrictions.


