ABSTRACT
Ocean acidification, due to the increase of carbon dioxide (CO2) concentration in the atmosphere and its absorption by the oceans, affects many aspects of marine calcifying organisms' biology, including reproduction. Most of the available studies on low pH effects on coral reproduction have been conducted on tropical species under controlled conditions, while little information is reported for either tropical or temperate species in the field. This study describes the influence of decreasing pH on sexual reproduction of the temperate non‐zooxanthellate colonial scleractinian Astroides calycularis, transplanted in four sites along a natural pH gradient at the underwater volcanic crater of Panarea Island (Tyrrhenian Sea, Italy). The average pH values of each site (range: pHTS 8.07–7.40) match different scenarios of the Intergovernmental Panel on Climate Change (IPCC) for the end of the century. After 3 months under experimental conditions, the reproductive parameters of both oocytes and spermaries (abundance, gonadal index, and diameters) seem to be unaffected by low pH. However, a delay in spermary development in the pre‐fertilization period and a persistence of mature oocytes in the fertilization period were observed in the most acidic site. Furthermore, no embryos were found in colonies from the two most acidic sites, suggesting a delay or an interruption of the fertilization process due to acidified conditions. These findings suggest a negative effect of low pH on A. calycularis sexual reproduction. However, long‐term experiments, including the synergistic impact of pH and temperature, are needed to predict if this species will be able to adapt to climate change over the next century.
Carbon dioxide (CO2) is the main greenhouse gas produced by human activities, and over the last decades, its concentration in the atmosphere has followed an exponential growth (Stocker et al. 2013). About 25% of atmospheric CO2 is absorbed by the ocean (Friedlingstein et al. 2020), with a consequent decrease in marine pH and carbonate ions concentration, in a phenomenon known as ocean acidification (Feely et al. 2004). Carbonate ions are essential for the calcification process of several marine organisms, including corals (Kleypas et al. 1999; Al‐Horani et al. 2003; Cohen and McConnaughey 2003). Scleractinian corals (i.e., stony corals) are essential in the maintenance of ecosystems and their biodiversity (Bellwood and Hughes 2001; Wild et al. 2011). Studies on the effects of ocean acidification on calcification of both tropical and temperate corals reveal variable responses, suggesting that the calcification response to increasing ocean acidity may be rather complex (Chan and Connolly 2013; Prada et al. 2017; Teixidó et al. 2020). Since scleractinian corals are among the most sensitive taxa to changes in environmental conditions, they play an essential role in understanding the evolution of life cycles (Harrison 2011) and how these changes may influence the reproductive biology of calcifying marine organisms (Richmond et al. 2018; Shlesinger and Loya 2019; Olischläger and Wild 2020). Recent studies suggest that ocean acidification may impact the synchronicity of sexual reproduction in coral spawning events (Olischläger and Wild 2020; Liberman et al. 2021), affecting the recruitment success and thus threatening the persistence of coral populations (Shlesinger and Loya 2019). Furthermore, multiple studies have shown adverse effects of ocean acidification on coral sexual reproduction and early life history stages, including sperm motility (Morita et al. 2010), fertilization (Albright and Mason 2013), planula development (Suwa et al. 2010), larvae metabolism and survival (Cumbo et al. 2013; Rivest and Hofmann 2014), and recruitment efficiency (Caroselli et al. 2019). However, although most studies on coral reproduction and early life stages found a negative effect of pH, few studies showed controversial results, probably due to different sensitivity of coral species (Foster et al. 2015; Gizzi et al. 2017; Caroselli et al. 2019) or to the lack of a comprehensive reproductive investigation (Fine and Tchernov 2007; Jokiel et al. 2008). Moreover, most of these few studies have been conducted on tropical species under laboratory conditions (Fine and Tchernov 2007; Jokiel et al. 2008; Albright and Langdon 2011), neglecting the full range of field environmental settings (i.e., nutrients, currents, and irradiance) and organism interactions that are difficult or impossible to simulate ex situ. To complement laboratory studies, areas naturally enriched in CO2, such as CO2 vents have been used to investigate the effect of ocean acidification in the field (Hall‐Spencer et al. 2008; Fabricius et al. 2011; Rodolfo‐Metalpa et al. 2011). CO2 vents are not perfect predictors of future ocean conditions owing, among other factors, to their high pH variability that increases in proximity to the vent area (Kerrison et al. 2011; Johnson et al. 2012). pH variance may influence biological processes differently from mean pH values (Dufault et al. 2012; Johnson et al. 2014). Since mean and variance cannot be separated at CO2 vents, these sites do not perfectly match climate change‐induced acidification (Johnson et al. 2012). Nevertheless, previous investigations conducted at the CO2 vent off Panarea Island (Tyrrhenian Sea, Italy) have shown how this site is a valuable natural laboratory for ocean acidification studies (Goffredo et al. 2014; Fantazzini et al. 2015; Wall et al. 2019), thanks to its low depth and its CO2 emissions at ambient temperature which lack toxic compounds (Capaccioni et al. 2007). Recent studies performed at the Panarea CO2 vents report no effect on reproductive parameters in the solitary and non‐zooxanthellate coral Leptopsammia pruvoti transplanted along the pH gradient for 3 months (Gizzi et al. 2017), and in the solitary and zooxanthellate coral Balanophyllia europaea, naturally living along the Panarea pH gradient (Caroselli et al. 2019).
The present study investigated the influence of decreasing seawater pH on the reproductive output of the colonial and non‐zooxanthellate Mediterranean coral Astroides calycularis (Pallas, 1766; Supporting Information Fig. S1) through a transplant experiment along the Panarea pH gradient. A. calycularis is an endemic reef‐forming Mediterranean species (Musco et al. 2017) that typically colonizes vertical walls or caves within the shallow infralittoral (0–15 m depth) (Kruzic et al. 2002), with densities of up to 90% of cover (Goffredo et al. 2010; Prada et al. 2019). Moreover, it can reach 50 m depth and live in both well‐lit and dark habitats, preferring turbulent environments (Kruzic et al. 2002; Grubelić et al. 2004; Ingrosso et al. 2018).
A. calycularis is gonochoric and brooding (Goffredo et al. 2010, 2011; Casado‐Amezua et al. 2013) with a reproductive cycle characterized by a different timing development between oogenesis and spermatogenesis: the oocytes follow a 2‐yr cycle while the spermaries follow an annual cycle (Goffredo et al. 2011). The sexual reproductive process of scleractinians is strongly related to multiple environmental parameters such as sea temperature, day length, solar insulation, lunar irradiance, and wind speed (Gorbunov and Falkowski 2002; Brady et al. 2009; van Woesik 2010). Likewise, the reproductive period of A. calycularis is related to local environmental parameters, showing a delay of 3 months in fertilization timing between Palinuro (Italy, Southern Tyrrhenian Sea) and Punta de la Mona (Spain, Alboran Sea), reflecting the shift in seawater temperature among the two localities (mean ± standard deviation: 22.0 ± 4.3°C in Palinuro and 18.5 ± 4.5°C in Punta de la Mona) (Goffredo et al. 2011; Casado‐Amezua et al. 2013). In Palinuro, fertilization occurs from February to May, and planulation takes place from June to July (Goffredo et al. 2011).
A previous study on A. calycularis transplanted along the Panarea CO2 vent showed a marked decrease in net calcification rate and an increase in polyp and tissue mortality, suggesting that this species is particularly sensitive to a decrease in pH, especially in warmer seasons characterized by elevated temperatures (Prada et al. 2017). However, a recent study performed on colonies of A. calycularis naturally living in an acidified environment in Ischia (Italy) showed variable responses in net calcification and growth patterns (Teixidó et al. 2020). While single polyps were characterized by a higher net calcification rate in the low pH sites, the entire colonies maintained the net calcification rate homogeneous, resulting in an equal linear extension rate among the sites (low and normal pH). However, the colonies were smaller and formed by fewer polyps in the low pH sites, leading the authors to hypothesize that the few polyps composing the colony need to invest more energy in calcification, to grow faster and reach the size of sexual maturity (Teixidó et al. 2020).
Considering the results of these previous studies, we expected to find negative effects of low pH also on reproductive output and/or gonadal development.
Materials and methods
Study site
The experimental site is in Bottaro 1, which is a big rock close to Panarea Island (Mediterranean Sea, Aeolian Archipelago, Italy, 38°38′16″N 15°06′37″E; Fig. 1a). Bottaro 1 is characterized by an underwater volcanic crater (20 × 14 m) at 10 m depth that generates constant and continuous CO2 emissions (98–99% CO2) at ambient temperature, creating a natural pH gradient (Capaccioni et al. 2007; Fantazzini et al. 2015; Prada et al. 2017; Fig. 1b). Along this gradient, four sampling sites were selected: the control site (site 1: mean pH total scale [TS] pHTS 8.07), located about 34 m away from the center of the crater; two intermediate pH sites (sites 2 and 3: mean pHTS respectively 7.87 and 7.74), matching the intermediate (Representative Concentration Pathway [RCP] 6.0) and the most pessimistic (RCP 8.5) IPCC scenario for the end of this century; and the extreme pH site (site 4: mean pHTS 7.40) situated in proximity to the vent, matching the most pessimistic IPCC scenario (RCP 8.5) for 2300 (Collins et al. 2013). The experimental site has stable hydrothermal–chemical properties, and among all measured parameters (temperature, salinity, pH, total alkalinity), only the pH differs significantly among the four sites (see Prada et al. 2017 for detailed seawater carbonate chemistry in the four transplantation sites).
Fig. 1.
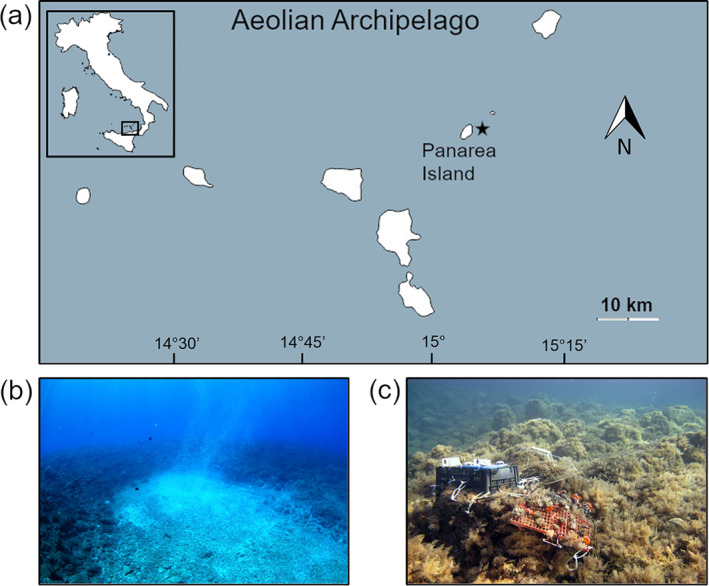
(a) Map of Aeolian Archipelago showing Panarea Island and Bottaro 1 (black star). The inset map shows the location of the Archipelago in the Southern Tyrrhenian Sea. (b) Picture of the underwater crater of Bottaro 1 with the CO2 bubbling. (c) Picture of site 2 (mean pHTS 7.87) showing transplanted A. calycularis colonies fixed with cable ties onto plastic grids.
Sampling and field transplantation
Based on a previous detailed study on the reproductive cycle of this species, two reproductive periods (described in the Definitions section) were selected to identify the key phases and the seasonality of reproductive processes (Goffredo et al. 2011). Concurrently to each reproductive period, 16–24 mature colonies of A. calycularis (colony area > 3–4 cm2; Goffredo et al. 2011) were collected at Pietra Nave, ~ 2 km away from the experimental field, where the species grows naturally at ~ 10 m depth. The collected colonies were fixed with cable ties onto plastic grids and transplanted in the four sites (3–6 colonies for each site; Fig. 1c) to be exposed to experimental conditions for about 3–4 months and then collected for histological analysis. At the beginning of the following reproductive period, new sampled colonies were transplanted for the same period, and so on, in 2.5 subsequent years (from November 2010 to June 2012 for a total of four sampling campaigns).
All the post‐treatment colonies were fixed in a formaldehyde solution (90% seawater and 10% formaldehyde buffered with calcium carbonate) for histological analyses.
Biometric analysis
The length (L, maximum axis of the oral disk), width (w, minimum axis of the oral disk), and height (h, oral‐aboral diameter) of each polyp from each colony were measured with a caliper (± 0.05 mm). The body volume of each polyp (V) was then estimated using the equation: V = (L/2) × (w/2) × h × π (Goffredo et al. 2011). The volume was used to calculate the reproductive parameters analyzed (see the Definitions section).
Histological processing
Each single polyp was separated from the colony by a hammer and chisel and placed inside a bottle. After decalcification in ethylenediaminetetraacetic acid and dehydration in increasing concentration of ethanol (from 80% to 100%), polyps were clarified in histolemon and embedded in paraffin wax (Goffredo et al. 2002). Serial transverse sections were cut at 7 μm intervals along the oral‐aboral axis, from the oral to the aboral pole of the entire polyp. Sections were placed on microscope slides previously washed with 100% ethanol and combined with albumin‐glycerin to bond the sections to the slides. Tissues were then stained with Mayer's hematoxylin and eosin (Goffredo et al. 2002).
Cyto‐histometric analysis
Cyto‐histometric analysis was performed on histological samples with a light microscope NIKON Eclipse 80i using the image analysis software NIKON NIS‐Elements D 3.1. The maximum and minimum diameter of oocytes (in nucleated sections) and spermaries were measured and classified into developmental stages, according to previous studies on gametogenesis of this species (Goffredo et al. 2010, 2011). The presence of embryos in the gastrovascular cavity was recorded and their maturation stage was identified (Goffredo et al. 2010). The size of each reproductive element and each embryo was determined as the mean of the two diameters (Goffredo et al. 2002).
Definitions
Reproductive output was defined by six parameters: (1) oocyte and spermary abundance, both defined as the number of reproductive elements per body volume unit (100 mm3); (2) gonadal index, defined as the percentage of body volume occupied by oocytes and spermaries (the volume of each reproductive element was estimated as described in the subsection “Gonadal index” of Goffredo et al. 2002); (3) oocyte and spermary diameter, defined as the average of the maximum and minimum diameter of oocytes in nucleated sections and spermaries; (4) fertility, defined as the number of embryos per body volume unit (100 mm3) (Marchini et al. 2015); (5) embryonal index, defined as the percentage of body volume occupied by embryos; and (6) embryo diameter, defined as the average of the maximum and minimum diameter of embryos.
Based on the developing stages of gametes and the presence of embryos in the coelenteric cavity of female polyps, samples of the four sampling campaigns were divided into two gonadal activity periods: (1) pre‐fertilization period, characterized by the presence of two stocks of oocytes (i.e., small oocytes recruited after the previous fertilization period and larger oocytes) and the beginning of spermary development (corresponding to the two sampling campaigns: November 2010–March 2011 and November 2011–March 2012) and (2) fertilization period, characterized by the presence of two stocks of oocytes (i.e., small oocytes and large mature oocytes ready to be fertilized), mature spermaries (V maturation stage) ready to be released and embryos in the coelenteric cavity of female polyps (corresponding to the two sampling campaigns: March 2011–June 2011 and March 2012–June 2012).
Statistical analysis
A one‐way permutation multivariate ANOVA (PERMANOVA) (Anderson 2001) based on Euclidean distances was performed with 999 permutations to test differences among sites in the oocyte size distribution, spermary maturation stage distribution, and in the reproductive parameters (oocyte and spermary abundance, gonadal index, and diameter). For a small sample size, the Monte Carlo correction of p‐value was used. All the statistical analyses were performed with the software Primer 6 (Quest Research Limited).
Results
Both the pre‐fertilization and fertilization periods were characterized by both gametogenetic and sexually inactive polyps, while only the fertilization period showed embryogenetic polyps (Supporting Information Table S1). All gametogenetic polyps analyzed were gonochoric. In 2012, most of the polyps of the pre‐fertilization period were inactive due to the quiescent phase of male polyps (Supporting Information Table S1).
Pre‐fertilization period
Size‐frequency distribution of oocytes showed no significant differences among sites (PERMANOVA, p [Monte Carlo] = 0.073; Fig. 2a). Polyps from all sites presented an abundant stock of small oocytes (diameter < 400 μm) and a second stock characterized by larger diameters (> 400 μm; Fig. 2a). Oocyte abundance, gonadal index, and diameter were not significantly different among sites (PERMANOVA, abundance p (MC) = 0.428, gonadal index p (MC) = 0.354, diameter p = 0.313; Fig. 3a; Supporting Information Table S2).
Fig. 2.
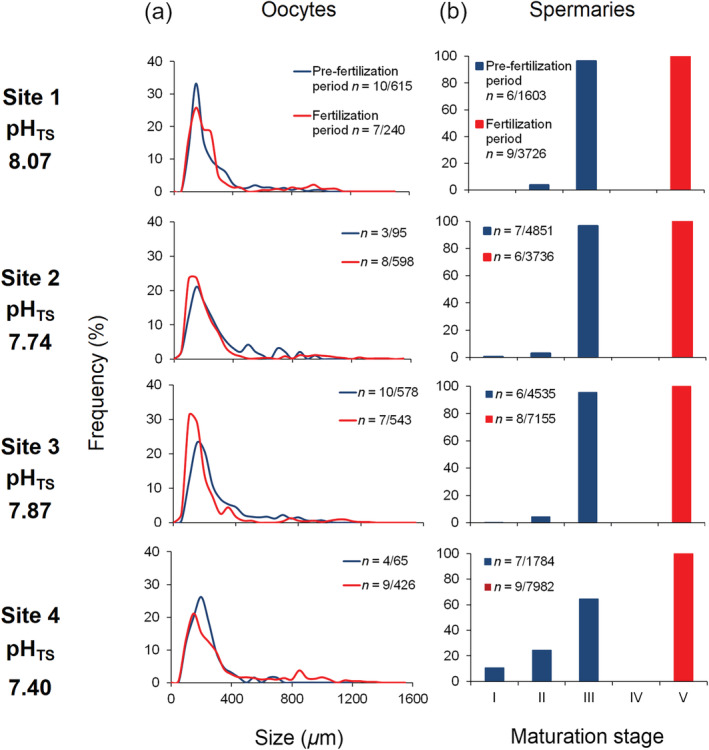
Oocyte size and spermary maturation stage distributions. (a) Distribution of oocyte size during the pre‐fertilization (blue line) and fertilization (red line) periods. n = number of polyps/oocytes. (b) Distribution of the five spermary maturation stages during the pre‐fertilization (blue histogram bars) and fertilization (red histogram bars) periods.
Fig. 3.
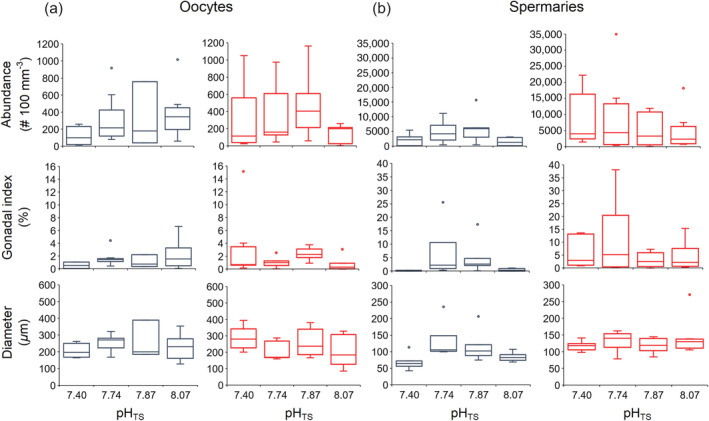
Oocyte and spermary reproductive parameters. Boxplots of oocyte (a) and spermary (b) abundance, gonadal index, and diameter in the pre‐fertilization (blue) and fertilization (red) periods. The box indicates the 25th and 75th percentiles, and the line within the box marks the median. Whisker length is equal to 1.5× interquartile range (IQR). Circles represent outliers.
Maturation stage/frequency distribution of spermaries showed significant differences among sites (PERMANOVA, p (MC) = 0.011; Fig. 2b): site 1, site 2, and site 3 were characterized by a low percentage of spermaries at stage of maturation II and a majority of spermaries at stage of maturation III, while site 4 (pHTS 7.40) was characterized by a higher percentage of spermaries at stages of maturation I and II (Fig. 2b). Spermary abundance, gonadal index, and diameter did not show significant differences among sites (PERMANOVA, abundance p (MC) = 0.072, gonadal index p (MC) = 0.147, diameter p = 0.268; Fig. 3b; Supporting Information Table S3).
Only four sexually active samples from 2012 have been detected and included in the analysis.
Fertilization period
Size‐frequency distribution of oocytes showed significant differences among sites (PERMANOVA, p (MC) = 0.048; Fig. 2a). All sites were characterized by the presence of an abundant stock of small oocytes (diameter < 400 μm) and a less abundant stock of larger oocytes (diameter > 400 μm; Fig. 2a). Site 4 (pHTS 7.40) was characterized by a higher percentage of large oocytes than the other sites (Fig. 2a). Oocyte abundance, gonadal index, and diameter did not show significant differences among sites (PERMANOVA, abundance p (MC) = 0.286, gonadal index p (MC) = 0.341, diameter p = 0.172; Fig. 3a; Supporting Information Table S2).
Spermary maturation stage distribution was homogeneous among sites (PERMANOVA, p (MC) = 0.483; Fig. 2b) since all sites were characterized by spermaries at the stage of maturation V (Fig. 2b). Spermary abundance, gonadal index, and diameter did not show significant differences among sites (PERMANOVA, abundance p (MC) = 0.644, gonadal index p (MC) = 0.327, diameter p = 0.322; Fig. 3b; Supporting Information Table S3).
Embryos were found in the coelenteric cavity of five female polyps in site 1 (pHTS 8.07) and two female polyps in site 2 (pHTS 7.87; Fig. 4; Supporting Information Table S4).
Fig. 4.
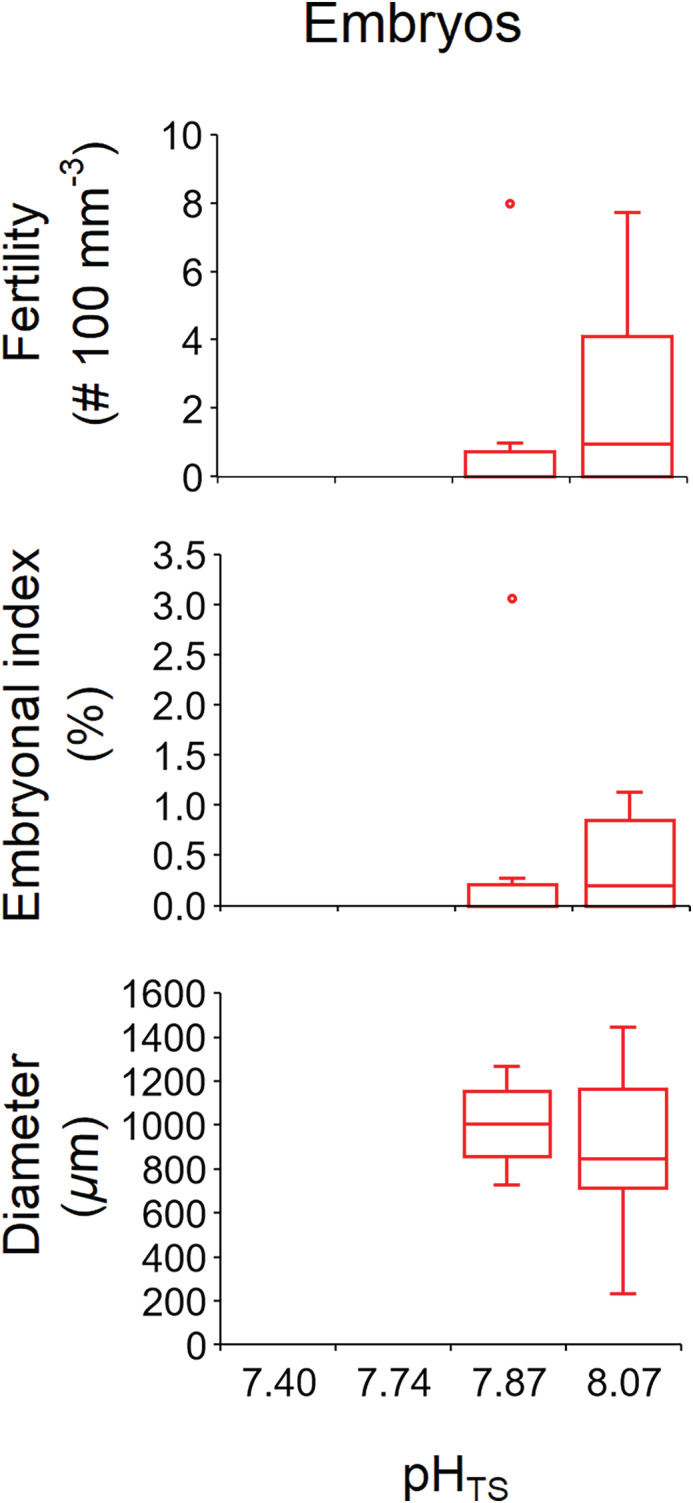
Embryos reproductive parameters. Boxplot of fertility, embryonal index, and embryo diameter in the fertilization period. The box indicates the 25th and 75th percentiles, and the line within the box marks the median. Whisker length is equal to 1.5× interquartile range (IQR). Circles represent outliers.
In 2012, the polyps transplanted in this reproductive period were lost due to a storm.
Discussion
With increasing acidification, in both pre‐fertilization and fertilization periods, the reproductive parameters of both oocytes and spermaries (abundance, gonadal index, and diameters) did not show statistical differences among sites (Fig. 3). However, a larger sample size could increase the analysis robustness that perhaps could reveal significant differences among sites.
In the pre‐fertilization period also, the oocyte size‐frequency distribution did not show differences among sites, suggesting that the oogenesis of A. calycularis was unaffected by the decrease in pH in this period. The same result was found in a parallel study on the non‐zooxanthellate but solitary coral L. pruvoti transplanted together with A. calycularis colonies of the present study (Gizzi et al. 2017) and in populations of the zooxanthellate solitary coral B. europaea naturally living along the Panarea gradient (Caroselli et al. 2019). Similar results were also found in mesocosm experiments, where a normal gametogenesis was found in the tropical zooxanthellate coral Montipora capitata after 6 months at pH 7.8 (Jokiel et al. 2008) and in the zooxanthellate species Oculina patagonica and Madracis pharensis after 12 months at pH 7.4 (Fine and Tchernov 2007). In the same period (pre‐fertilization), spermatogenesis seemed more sensitive to the decrease in pH, showing a delay in spermary development in the extreme pH site (site 4, pHTS 7.4), where polyps were characterized by a higher amount of early spermary maturation stages (I and II) than the other sites (Fig. 2b). A delay in gametogenesis was found in the sea urchin Hemicentrotus pulcherrimus after 9 months at pH 7.9 (Kurihara et al. 2013) and in the oyster Crassostrea virginica after 5 weeks at pH 7.1 (Boulais et al. 2017). A delay in spermatogenesis could postpone the release of sperms in the water column, which not only may retard the fertilization but, if it is not accompanied by a parallel delay in oogenesis, could also reduce or impair fertilization (e.g., if the sperms are released in the water when only a few or no mature oocytes are available). A recent study suggests that ocean acidification may impact coral spawning events (Olischläger and Wild 2020), based on the lack of reproductive synchrony highlighted in the last years by several coral species in the Red Sea (Shlesinger and Loya 2019). The synchrony breakdown reduces the probability of successful fertilization, leading to a dearth of new recruits and thus threatening the future of their populations (Shlesinger and Loya 2019).
During the fertilization period, oocyte size‐frequency distribution showed a more abundant stock of large mature oocytes (diameter > 400 μm) in the most acidic site (site 4, pHTS 7.4; Fig. 2a). The persistence of large oocytes may suggest a delay in the fertilization process at lower pH, perhaps caused by a slowdown in the release in the water column of sperms not yet fully mature. However, spermary production (abundance and gonadal index) and their diameter were unaffected by decreasing pH (Fig. 3b; Supporting Information Table S3), as well as the spermary development, since all male polyps analyzed in this period hosted only mature spermaries (V stage of maturation; Fig. 2b). An impairment in spermary development could explain the persistence of mature oocytes in polyps exposed to lower pH. Spermaries could have reached the last maturation stage, but spermatozoa might not be ready to be released, possibly causing the delay in the fertilization process. In the tropical coral Acropora palmata exposed to pCO2 levels projected for the end of this century, a reduction in fertilization success of 12% was observed due to decreased sperm concentration (Albright et al. 2010). The embryogenesis analysis supported a delay, or even a block, in the fertilization process of A. calycularis with increasing acidity. Embryos were found only in site 1 (control, pHTS 8.1) and site 2 (pHTS 7.9), while no embryos were found in the two most acidic sites (site 3 and site 4, respectively, pHTS 7.7 and 7.4; Fig. 4; Supporting Information Table S4). A reduction in embryo production (fertility) was found in the tropical corals A. tenuis and A. palmata, exposed in aquaria to pH levels expected for near‐future IPCC projections (Albright et al. 2010; Albright and Mason 2013). Another possible explanation for the delay or block in fertilization of A. calycularis may be related to sperm motility. A reduction in sperm motility impaired by the acidified environment was found in several marine organisms (Havenhand et al. 2008; Shi et al. 2017; Boulais et al. 2018), including corals: an aquarium experiment revealed that 69% of sperms of the scleractinian coral Acropora digitifera were motile at pH 8.0, while at pH 7.8 the percentage dropped to 46%, and at pH 7.7 to 20% (Morita et al. 2010). Moreover, decreasing pH may alter chemical signals secreted by oocytes to activate sperm flagellar motility (Morita et al. 2006). These studies suggest that sperms could lose their ability to move in a low pH environment, failing to reach the oocytes and thus impairing the fertilization process.
Since sexual reproduction is a costly energy process, corals can reallocate the energy to other essential life processes such as calcification and growth (Langdon and Atkinson 2005; Silverman et al. 2007). A parallel study on the net calcification rate of A. calycularis and L. pruvoti was performed in specimens transplanted along the same natural pH gradient at Panarea (Prada et al. 2017). While the solitary coral L. pruvoti invests more energy to maintain a constant reproduction (Gizzi et al. 2017) at the expense of net calcification (Prada et al. 2017), the colonial A. calycularis showed the most severe effects of low pH, manifesting a high mortality rate, a decreased net calcification rate (Prada et al. 2017) and an affected reproduction (present study). Colonial corals generally grow faster than solitary corals (Chadwick and Loya 1990), and fast‐growing corals (including A. calycularis) may be more sensitive to acidification than solitary slow‐growing corals such as L. pruvoti because of their higher requirement of carbonate ions (Rodolfo‐Metalpa et al. 2010). For this reason, A. calycularis may need to allocate more energy towards growth compared to L. pruvoti at the expense of its reproduction. In a previous study on the hsp70 transcriptional response to heat stress, conducted in different Mediterranean coral species, including L. pruvoti and A. calycularis, the latter resulted in the most sensitive non‐zooxanthellate species, showing the highest hsp70 fold changes (Franzellitti et al. 2018). However, a recent study on A. calycularis naturally living in an acidified environment in Ischia (Italy) showed variability in net calcification rate at polyp and colony level that could suggest a long‐term adaptation in the acidified environment. Colonies from low pH sites calcified and extended at the same rate as those at ambient pH but were composed of fewer polyps, which invested more energy to calcify more and extend their skeleton faster, probably to reach the size of sexual maturity (Teixidó et al. 2020). Since polyps exposed to low pH allocate more energy in the calcification process, less energy could be available for their reproduction that could be affected.
Most studies investigating the effect of pH on coral reproduction, including the current one, assess a short‐term impact, which does not consider the whole gametogenic cycle and thus reduces the potential effects that could be found on different time scales. Studying the reproduction in populations that naturally live in acidified environments or starting the exposure of decreased pH before the gametogenic cycle begins could reveal more “delays” in the gametogenesis or even an initial block of the entire gametogenic process in the most acidic sites. Further studies, including long‐term effects, are needed to elucidate the fine‐scale mechanisms that make this species particularly susceptible to ocean acidification.
In conclusion, the reproductive output of A. calycularis seemed negatively affected by pH levels predicted for the end of the century and beyond, showing a delay in spermary development coupled with the persistence of mature oocytes in the most acidic site (pHTS 7.4) and the lack of embryos in the two most acidic sites (i.e., site 3, pHTS 7.7 and site 4, pHTS 7.4). The delay or block in the fertilization process is likely caused by one or the combination of pre‐fertilization problems, including the lack of reproductive synchrony between sperms release and available mature oocytes, reduced sperm motility, and the alteration of oocyte‐sperm chemical signaling in acidified conditions. The high sensitivity to low pH found in A. calycularis, even in short‐term experiments of 3–4 months, may be due to its fast growth rate, which could leave less energy available for sexual reproduction. These findings suggest that ocean acidification has the potential to impact A. calycularis, threatening the future of this endemic reef‐forming Mediterranean species.
Nevertheless, further investigations, including long‐term experiments and the synergic effect of pH with temperature, are needed to better understand how A. calycularis will respond to future environmental conditions projected for the coming decades.
Conflict of interest
None declared.
Supporting information
Appendix S1: Supporting Information
Acknowledgments
The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007–2013)/ERC grant agreement no. 249930 – CoralWarm: Corals and global warming: the Mediterranean versus the Red Sea (www.coralwarm.eu). F.G. was supported by a post‐doctoral research fellowship granted by ARDITI in the framework of project RAGES [ARDITI‐RAGES‐2019‐001]. E.C. was supported by the Alma Idea Grant of the University of Bologna for the project “STRAMICRO.” The Scientific Diving School (www.sdseducational.org) gave logistical support for fieldwork. F. Sesso and B. Basile gave previous support for fieldwork on Panarea Island. The experiment complied with current Italian law. Open Access Funding provided by Universita degli Studi di Bologna within the CRUI‐CARE Agreement. [Correction added on 25 May 2022, after first online publication: CRUI funding statement has been added.]
Associate editor: Bradley Eyre
Contributor Information
Chiara Marchini, Email: chiara.marchini2@unibo.it.
Francesca Gizzi, Email: francesca.gizzi@mare-centre.pt.
Thomas Pondrelli, Email: thomas.pondrelli@studio.unibo.it.
Lisa Moreddu, Email: lisa.moreddu@marinesciencegroup.org.
Luca Marisaldi, Email: l.marisaldi@pm.univpm.it.
Francesco Montori, Email: francesco.montori2@studio.unibo.it.
Valentina Lazzari, Email: valentina.lazzari@marinesciencegroup.org.
Valentina Airi, Email: valentina.airi2@unibo.it.
Erik Caroselli, Email: erik.caroselli@unibo.it.
Fiorella Prada, Email: fiorella.prada2@unibo.it.
Giuseppe Falini, Email: giuseppe.falini@unibo.it.
Zvy Dubinsky, Email: zvykalmog@gmail.com.
Stefano Goffredo, Email: s.goffredo@unibo.it.
References
- Al‐Horani, F. A. , Al‐Moghrabi S. M., and de Beer D.. 2003. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis . Mar. Biol. 142: 419–426. doi: 10.1007/s00227-002-0981-8 [DOI] [Google Scholar]
- Albright, R. , and Langdon C.. 2011. Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides . Glob. Chang. Biol. 17: 2478–2487. doi: 10.1111/j.1365-2486.2011.02404.x [DOI] [Google Scholar]
- Albright, R. , and Mason B.. 2013. Projected near‐future levels of temperature and pCO2 reduce coral fertilization success. PLoS One 8: e56468. doi: 10.1371/journal.pone.0056468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright, R. , Mason B., Miller M., and Langdon C.. 2010. Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata . Proc. Natl. Acad. Sci. USA 107: 20400–20404. doi: 10.1073/pnas.1007273107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, M. J. 2001. A new method for non‐parametric multivariate analysis of variance. Austral Ecol. 26: 32–46. doi: 10.1046/j.1442-9993.2001.01070.x [DOI] [Google Scholar]
- Bellwood, D. R. , and Hughes T. P.. 2001. Regional‐scale assembly rules and biodiversity of coral reefs. Science 292: 1532–1535. doi: 10.1126/science.1058635 [DOI] [PubMed] [Google Scholar]
- Boulais, M. , Chenevert K. J., Demey A. T., Darrow E. S., Robison M. R., Roberts J. P., and Volety A.. 2017. Oyster reproduction is compromised by acidification experienced seasonally in coastal regions. Sci. Rep. 7: 1–9. doi: 10.1038/s41598-017-13480-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulais, M. , and others. 2018. pH controls spermatozoa motility in the Pacific oyster (Crassostrea gigas). Biol. Open. 7: 3. doi: 10.1242/bio.031427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady, A. K. , Hilton J. D., and Vize P. D.. 2009. Coral spawn timing is a direct response to solar light cycles and is not an entrained circadian response. Coral Reefs. 28: 677–680. doi: 10.1007/s00338-009-0498-4 [DOI] [Google Scholar]
- Capaccioni, B. , Tassi F., Vaselli O., Tedesco D., and Poreda R.. 2007. Submarine gas burst at Panarea Island (southern Italy) on 3 November 2002: A magmatic versus hydrothermal episode. J. Geophys. Res. Solid Earth. 112: B05201. doi: 10.1029/2006JB004359 [DOI] [Google Scholar]
- Caroselli, E. , and others. 2019. Low and variable pH decreases recruitment efficiency in populations of a temperate coral naturally present at a CO2 vent. Limnol. Oceanogr. 64: 1059–1069. doi: 10.1002/lno.11097 [DOI] [Google Scholar]
- Casado‐Amezua, P. , Gasparini G., and Goffredo S.. 2013. Phenological and morphological variations in the Mediterranean orange coral Astroides calycularis between two distant localities. Fortschr. Zool. 116: 159–167. doi: 10.1016/j.zool.2012.10.005 [DOI] [PubMed] [Google Scholar]
- Chadwick, N. E. , and Loya Y.. 1990. Regeneration after experimental breakage in the solitary reef coral Fungia granulosa Klunzinger, 1879. J. Exp. Mar. Bio. Ecol. 142: 221–234. doi: 10.1016/0022-0981(90)90093-R [DOI] [PubMed] [Google Scholar]
- Chan, N. C. S. , and Connolly S. R.. 2013. Sensitivity of coral calcification to ocean acidification: A meta‐analysis. Glob. Chang. Biol. 19: 282–290. doi: 10.1111/gcb.12011 [DOI] [PubMed] [Google Scholar]
- Cohen, A. L. , and McConnaughey T. A.. 2003. Geochemical perspectives on coral mineralization. Rev. Mineral. Geochem. 54: 151–187. doi: 10.2113/0540151 [DOI] [Google Scholar]
- Collins, M. , and others. 2013. Long‐term climate change: Projections, commitments and irreversibility, p. 1029–1136. In Stocker T. F. and others. [eds.], Climate change 2013‐ the physical science basis: Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge Univ. Press. [Google Scholar]
- Cumbo, V. R. , Fan T. Y., and Edmunds P. J.. 2013. Effects of exposure duration on the response of Pocillopora damicornis larvae to elevated temperature and high pCO2 . J. Exp. Mar. Biol. Ecol. 439: 100–107. doi: 10.1016/j.jembe.2012.10.019 [DOI] [Google Scholar]
- Dufault, A. M. , Cumbo V. R., Fan T. Y., and Edmunds P. J.. 2012. Effects of diurnally oscillating pCO2 on the calcification and survival of coral recruits. Proc. R. Soc. B Biol. Sci. 279: 2951–2958. doi: 10.1098/rspb.2011.2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius, K. E. , and others. 2011. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Chang. 1: 165–169. doi: 10.1038/nclimate1122 [DOI] [Google Scholar]
- Fantazzini, P. , and others. 2015. Gains and losses of coral skeletal porosity changes with ocean acidification acclimation. Nat. Commun. 6: 1–7. doi: 10.1038/ncomms8785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feely, R. A. , Sabine C. L., Lee K., Berelson W., Kleypas J., Fabry V. J., and Millero F. J.. 2004. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305: 362–366. doi: 10.1126/science.1097329 [DOI] [PubMed] [Google Scholar]
- Fine, M. , and Tchernov D.. 2007. Scleractinian coral species survive and recover from decalcification. Science 315: 1811–1811. doi: 10.1126/science.1137094 [DOI] [PubMed] [Google Scholar]
- Foster, T. , Gilmour J. P., Chua C. M., Falter J. L., and McCulloch M. T.. 2015. Effect of ocean warming and acidification on the early life stages of subtropical Acropora spicifera . Coral Reefs 34: 1217–1226. doi: 10.1007/s00338-015-1342-7 [DOI] [Google Scholar]
- Franzellitti, S. , and others. 2018. Transcriptional response of the heat shock gene hsp70 aligns with differences in stress susceptibility of shallow‐water corals from the Mediterranean Sea. Mar. Environ. Res. 140: 444–454. doi: 10.1016/j.marenvres.2018.07.006 [DOI] [PubMed] [Google Scholar]
- Friedlingstein, P. , and others. 2020. Global carbon budget 2020. Earth Syst. Sci. Data 12: 3269–3340. doi: 10.5194/essd-12-3269-2020 [DOI] [Google Scholar]
- Gizzi, F. , De Mas L., Airi V., Caroselli E., Prada F., Falini G., Dubinsky Z., and Goffredo S.. 2017. Reproduction of an azooxanthellate coral is unaffected by ocean acidification. Sci. Rep. 7: 1–8. doi: 10.1038/s41598-017-13393-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffredo, S. , Arnone S., and Zaccanti F.. 2002. Sexual reproduction in the Mediterranean solitary coral Balanophyllia europaea (Scleractinia, Dendrophylliidae). Mar. Ecol. Prog. Ser. 229: 83–94. doi: 10.3354/meps229083 [DOI] [Google Scholar]
- Goffredo, S. , Gasparini G., Marconi G., Putignano M. T., Pazzini C., Airi V., and Zaccanti F.. 2011. Sexual reproduction in the Mediterranean endemic orange coral Astroides calycularis (Scleractinia: Dendrophylliidae). Bull. Mar. Sci. 87: 589–604. doi: 10.5343/bms.2010.1068 [DOI] [Google Scholar]
- Goffredo, S. , Gasparini G., Marconi G., Putignano M. T., Pazzini C., and Zaccanti F.. 2010. Gonochorism and planula brooding in the mediterranean endemic orange coral Astroides calycularis (Scleractinia: Dendrophylliidae). Morphological aspects of gametogenesis and ontogenesis. Mar. Biol. Res. 6: 421–436. doi: 10.1080/17451000903428488 [DOI] [Google Scholar]
- Goffredo, S. , and others. 2014. Biomineralization control related to population density under ocean acidification. Nat. Clim. Chang. 4: 593–597. doi: 10.1038/nclimate2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunov, M. Y. , and Falkowski P. G.. 2002. Photoreceptors in the cnidarian hosts allow symbiotic corals to sense blue moonlight. Limnol. Oceanogr. 47: 309–315. doi: 10.4319/lo.2002.47.1.0309 [DOI] [Google Scholar]
- Grubelić, I. , Antolić B., Despalatović M., Grbec B., and Paklar G. B.. 2004. Effect of climatic fluctuations on the distribution of warm‐water coral Astroides calycularis in the Adriatic Sea: New records and review. J. Mar. Biol. Assoc. U.K. 84: 599–602. doi: 10.1017/S0025315404009609h [DOI] [Google Scholar]
- Hall‐Spencer, J. M. , and others. 2008. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454: 96–99. doi: 10.1038/nature07051 [DOI] [PubMed] [Google Scholar]
- Harrison, P. L. 2011. Sexual reproduction of scleractinian corals, p. 59–85. In Dubinsky Z. and Stambler N. [eds.], Coral reefs: An ecosystem in transition. Springer. doi: 10.1007/978-94-007-0114-4_6 [DOI] [Google Scholar]
- Havenhand, J. N. , Buttler F. R., Thorndyke M. C., and Williamson J. E.. 2008. Near‐future levels of ocean acidification reduce fertilization success in a sea urchin. Curr. Biol. 18: R651–R652. doi: 10.1016/j.cub.2008.06.015 [DOI] [PubMed] [Google Scholar]
- Ingrosso, G. , and others. 2018. Mediterranean bioconstructions along the Italian coast. Adv. Mar. Biol. 79: 61–136. doi: 10.1016/bs.amb.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Johnson, M. D. , Moriarty V. W., and Carpenter R. C.. 2014. Acclimatization of the crustose coralline alga Porolithon onkodes to variable pCO2 . PLoS One 9: e87678. doi: 10.1371/journal.pone.0087678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, V. R. , Russell B. D., Fabricius K. E., Brownlee C., and Hall‐Spencer J. M.. 2012. Temperate and tropical brown macroalgae thrive, despite decalcification, along natural CO2 gradients. Glob. Chang. Biol. 18: 2792–2803. doi: 10.1111/j.1365-2486.2012.02716.x [DOI] [PubMed] [Google Scholar]
- Jokiel, P. L. , Rodgers K. S., Kuffner I. B., Andersson A. J., Cox E. F., and Mackenzie F. T.. 2008. Ocean acidification and calcifying reef organisms: A mesocosm investigation. Coral Reefs 27: 473–483. doi: 10.1007/s00338-008-0380-9 [DOI] [Google Scholar]
- Kerrison, P. , Hall‐Spencer J. M., Suggett D. J., Hepburn L. J., and Steinke M.. 2011. Assessment of pH variability at a coastal CO2 vent for ocean acidification studies. Estuar. Coast. Shelf Sci. 94: 129–137. doi: 10.1016/j.ecss.2011.05.025 [DOI] [Google Scholar]
- Kleypas, J. A. , Buddemeier R. W., Archer D., Gattuso J. P., Langdon C., and Opdyke B. N.. 1999. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284: 118–120. doi: 10.1126/science.284.5411.118 [DOI] [PubMed] [Google Scholar]
- Kruzic, P. , Zibrowius H., and Pozar‐Domac A.. 2002. Actiniaria and Scleractinia (Cnidaria, Anthozoa) from the Adriatic Sea (Croatia): First records, confirmed occurrences and significant range extensions of certain species. Ital. J. Zool. 69: 345–353. doi: 10.1080/11250000209356480 [DOI] [Google Scholar]
- Kurihara, H. , Yin R., Nishihara G. N., Soyano K., and Ishimatsu A.. 2013. Effect of ocean acidification on growth, gonad development and physiology of the sea urchin Hemicentrotus pulcherrimus . Aquat. Biol. 18: 281–292. doi: 10.3354/ab00510 [DOI] [Google Scholar]
- Langdon, C. , and Atkinson M. J.. 2005. Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J. Geophys. Res. Oceans 110: 1–16. doi: 10.1029/2004JC002576 [DOI] [Google Scholar]
- Liberman, R. , Fine M., and Benayahu Y.. 2021. Simulated climate change scenarios impact the reproduction and early life stages of a soft coral. Mar. Environ. Res. 163: 105215. doi: 10.1016/j.marenvres.2020.105215 [DOI] [PubMed] [Google Scholar]
- Marchini, C. , and others. 2015. Annual reproductive cycle and unusual embryogenesis of a temperate coral in the Mediterranean Sea. PLoS One 10: e0141162. doi: 10.1371/journal.pone.0141162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, M. , Nishikawa A., Nakajima A., Iguchi A., Sakai K., Takemura A., and Okuno M.. 2006. Eggs regulate sperm flagellar motility initiation, chemotaxis and inhibition in the coral Acropora digitifera, A. gemmifera and A. tenuis . J. Exp. Biol. 209: 4574–4579. doi: 10.1242/jeb.02500 [DOI] [PubMed] [Google Scholar]
- Morita, M. , Suwa R., Iguchi A., Nakamura M., Shimada K., Sakai K., and Suzuki A.. 2010. Ocean acidification reduces sperm flagellar motility in broadcast spawning reef invertebrates. Zygote 18: 103–107. doi: 10.1017/S0967199409990177 [DOI] [PubMed] [Google Scholar]
- Musco, L. , Prada F., D'Anna G., Galasso N. M., Pipitone C., Fernández T. V., and Badalamenti F.. 2017. Turning casualty into opportunity: Fragmenting dislodged colonies is effective for restoring reefs of a Mediterranean endemic coral. Ecol. Eng. 98: 206–212. doi: 10.1016/j.ecoleng.2016.10.075 [DOI] [Google Scholar]
- Olischläger, M. , and Wild C.. 2020. How does the sexual reproduction of marine life respond to ocean acidification? Diversity 12: 241. doi: 10.3390/d12060241 [DOI] [Google Scholar]
- Prada, F. , and others. 2017. Ocean warming and acidification synergistically increase coral mortality. Sci. Rep. 7: 1–10. doi: 10.1038/srep40842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada, F. , and others. 2019. Anthropogenic impact is negatively related to coral health in Sicily (Mediterranean Sea). Sci. Rep. 9: 1–14. doi: 10.1038/s41598-019-49713-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond, R. H. , Tisthammer K. H., and Spies N. P.. 2018. The effects of anthropogenic stressors on reproduction and recruitment of corals and reef organisms. Front. Mar. Sci. 5: 226. doi: 10.3389/fmars.2018.00226 [DOI] [Google Scholar]
- Rivest, E. B. , and Hofmann G. E.. 2014. Responses of the metabolism of the larvae of Pocillopora damicornis to ocean acidification and warming. PLoS One 9: e96172. doi: 10.1371/journal.pone.0096172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodolfo‐Metalpa, R. , and others. 2011. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Clim. Chang. 1: 308–312. doi: 10.1038/nclimate1200 [DOI] [Google Scholar]
- Rodolfo‐Metalpa, R. , Martin S., Ferrier‐Pagès C., and Gattuso J. P.. 2010. Response of the temperate coral Cladocora caespitosa to mid‐ and long‐term exposure to pCO2 and temperature levels projected for the year 2100 AD. Biogeosciences 7: 289–300. doi: 10.5194/bg-7-289-2010 [DOI] [Google Scholar]
- Shi, W. , and others. 2017. Effects of reduced pH and elevated pCO2 on sperm motility and fertilisation success in blood clam, Tegillarca granosa . N. Z. J. Mar. Freshw. Res. 51: 543–554. doi: 10.1080/00288330.2017.1296006 [DOI] [Google Scholar]
- Shlesinger, T. , and Loya Y.. 2019. Breakdown in spawning synchrony: A silent threat to coral persistence. Science 365: 1002–1007. doi: 10.1126/science.aax0110 [DOI] [PubMed] [Google Scholar]
- Silverman, J. , Lazar B., and Erez J.. 2007. Effect of aragonite saturation, temperature, and nutrients on the community calcification rate of a coral reef. J. Geophys. Res. Oceans 112: C05004. doi: 10.1029/2006JC003770 [DOI] [Google Scholar]
- Stocker, T. F. , and others. [eds.]. 2013. Climate change 2013 ‐ the physical science basis: Working Group I Contribution to the IPCC Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ. Press, p. 1535. [Google Scholar]
- Suwa, R. , Nakamura M., Morita M., Shimada K., Iguchi A., Sakai K., and Suzuki A.. 2010. Effects of acidified seawater on early life stages of scleractinian corals (genus Acropora). Fish. Sci. 76: 93–99. doi: 10.1007/s12562-009-0189-7 [DOI] [Google Scholar]
- Teixidó, N. , and others. 2020. Ocean acidification causes variable trait shifts in a coral species. Glob. Change Biol. 26: 6813–6683. doi: 10.1111/gcb.15372 [DOI] [PubMed] [Google Scholar]
- van Woesik, R. 2010. Calm before the spawn: Global coral spawning patterns are explained by regional wind fields. Proc. Royal Soc. B. 277: 715–722. doi: 10.1098/rspb.2009.1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall, M. , and others. 2019. Linking internal carbonate chemistry regulation and calcification in corals growing at a Mediterranean CO2 vent. Front. Mar. Sci. 6: 699. doi: 10.3389/fmars.2019.00699 [DOI] [Google Scholar]
- Wild, C. , and others. 2011. Climate change impedes scleractinian corals as primary reef ecosystem engineers. Mar. Freshw. Res. 62: 205–215. doi: 10.1071/MF10254 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


