Abstract
Aim
To evaluate the effect of empagliflozin on uric acid (UA) levels, antigout medication and gout episodes in the EMPA‐REG OUTCOME trial (NCT01131676).
Materials and methods
A total of 7020 patients with type 2 diabetes (T2D) were randomized to either empagliflozin (10 or 25 mg) or placebo. The effects of empagliflozin versus placebo on UA concentration were assessed using mixed linear models. A composite outcome of new prescription of antigout medication or gout episode was studied with Cox proportional hazards models.
Results
Empagliflozin reduced serum UA levels versus placebo: week 52 adjusted mean treatment difference = −0.37 (95% confidence interval [CI] −0.42, −0.31) mg/dL; this was more pronounced in patients with baseline UA ≥ 7.0 mg/dL versus <7.0 mg/dL: week 52 adjusted mean treatment difference = −0.56 (95% CI −0.68, −0.43) and −0.30 (95% CI −0.37, −0.24) mg/dL, respectively. Among 6607 patients not taking antigout medications at baseline, 5.2% had a gout episode or initiated antigout treatment versus 3.6% in the placebo and empagliflozin groups, respectively: hazard ratio 0.67 (95% CI 0.53, 0.85; P = 0.001). Both components of the composite outcome contributed to the reduction with empagliflozin in the composite. Risk reduction was similar with both empagliflozin doses.
Conclusions
Empagliflozin reduced UA levels and the composite of gout episodes or prescription of antigout medication. These clinically important findings expand the utility of empagliflozin as a potential antigout treatment in patients with T2D, beyond its well‐established cardio‐renal benefits.
Keywords: antigout treatment, empagliflozin, gout, type 2 diabetes, uric acid
1. INTRODUCTION
Elevated uric acid (UA) levels are frequently found in patients with type 2 diabetes (T2D) and have been associated with increased cardiovascular risk. 1 Additionally, elevated UA levels may lead to gouty arthritis, nephrolithiasis, tubule‐interstitial fibrosis, and progression of kidney disease. 2 , 3 , 4 , 5 , 6 Most UA is produced from the metabolism of purines, and it is eliminated by the kidney. 7 Sodium‐glucose cotransporter 2 (SGLT2) inhibitors decrease UA levels by increasing urinary excretion and, possibly, via the reduction of reactive oxygen species leading to a decrease in the activity of the enzyme xanthine‐oxidase (which catalyses the oxidation of xanthine to UA). 8 , 9 , 10 , 11
The UA‐lowering capacity of SGLT2 inhibitors potentially expands the role of these agents in glucose‐lowering and improving cardio‐renal outcomes of patients, including certain patients who do not have T2D, such as those with heart failure and chronic kidney disease. 12 , 13 , 14 , 15 It is possible that some of the mechanisms by which SGLT2 inhibitors improve cardiovascular outcomes also lower UA levels, notably the reduction of oxidative stress. 16 Moreover, through a reduction in UA, a reduction in gout events is of high clinical relevance as gout is the most common form of inflammatory arthritis, may lead to significant symptoms (which can be debilitating and prolonged) and even joint deformity. 17 In the Canagliflozin Cardiovascular Assessment Study (CANVAS) Programme, integrating data from CANVAS and CANVAS‐Renal, canagliflozin reduced the number of gout episodes compared with placebo in patients with T2D. 18 Whether these effects can be externally replicated and expanded to other SGLT2 inhibitors is worth studying.
In this regard, we aimed to study the effect of empagliflozin on UA levels, prescription of an antigout medication and gout episodes in patients with T2D enrolled in the EMPA‐REG OUTCOME trial (a cardiovascular outcome event trial in T2D patients).
2. MATERIALS AND METHODS
2.1. Study design
The design of EMPA‐REG OUTCOME (ClinicalTrials.gov: NCT01131676) has been described previously. 19 Briefly, the study population comprised patients with T2D, established atherosclerotic cardiovascular disease, glycated haemoglobin (HbA1c) 7.0% to 9.0% for drug‐naïve patients and 7.0% to 10.0% for patients on glucose‐lowering therapy, and an estimated glomerular filtration rate (eGFR; determined by the Modification of Diet in Renal Disease equation) ≥30 mL/min/1.73 m2. Patients were randomized 1:1:1 to receive empagliflozin 10 mg, empagliflozin 25 mg, or placebo in addition to standard of care. The primary outcome was a composite of time to first event of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. A total of 7020 patients were randomized and treated (pooled empagliflozin, n = 4687; empagliflozin 10 mg/d, n = 2345; empagliflozin 25 mg/d, n = 2342; and placebo, n = 2333). The median on‐treatment period was 2.6 years.
The trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines, and was approved by local authorities. An independent ethics committee or institutional review board approved the clinical protocol at every participating site. All patients provided written informed consent, and the trial protocol was approved by the ethics committee at each site.
2.2. Gout definition and UA measurements
A history of gout at baseline was defined based on a diagnosis of gout being recorded in the trial record, a gout episode occurring prior to first study drug intake, or a drug for the management of gout being included in the baseline medication record (ATC3 code “antigout preparations” including allopurinol, benzbromarone, colchicine, febuxostat, or probenecid, among others).
Serum UA concentration was measured at a central laboratory using frozen serum samples stored at −70°C or below and collected at baseline, weeks 12, 28, 52 and 66, and every 14 weeks thereafter up to week 206.
We used two approaches to identify suspected gout episodes following previous reports. 18 , 20 First, we searched the adverse events database for records including permutations of the term “gout” (ie, gout, gouty arthritis, and gouty tophus). Second, we searched the concomitant medications database for recorded post‐randomization prescription of a medication used for the management of gout (ATC3 code “antigout preparations” including allopurinol, benzbromarone, febuxostat, or probenecid or colchicine). An event of new antigout medication based on the initiation of a drug for the management of gout was defined only if there was no documented use of such drug at baseline. Patients with a history of gout were included in the analysis.
2.3. Outcomes
The main outcome of interest for the present analysis was a composite of the time to first occurrence of either an adverse event contributing to the gout episode or the “de novo” initiation on antigout medication. The individual components of the main outcome of interest and the impact of empagliflozin on the serum UA levels over time were also studied.
While all patients of the treated set were considered at risk for the component of gout episode based on adverse event reporting, only patients without antigout medication at baseline were at risk for the event of new antigout medication and the composite outcome.
2.4. Statistical analyses
All analyses were undertaken in patients that were treated with at least one dose of study drug. Treatment effects on serum UA levels were evaluated using a mixed‐effect model for repeated measures analyses with visit time (weeks) as the repeated measure. The model included baseline HbA1c plus the baseline UA value as linear covariates, along with their interaction with visit time. Additionally, fixed, categorical effects of treatment at each visit, baseline eGFR, baseline body mass index (BMI) and geographic region were included, along with an effect for “last projected visit based on dates of randomization and trial closure” to account for each patient's theoretical ability to attain longer weeks in the study depending on the date of randomization. Incidence rates per 1000 patient‐years of new onset of gout or initiation of antigout medication during the follow‐up were calculated in the placebo and empagliflozin groups, separately. The treatment effect of empagliflozin versus placebo on the outcomes of interest was assessed by Cox proportional hazards models with terms for age, sex, geographic region, baseline BMI, baseline HbA1c, baseline eGFR, and treatment (empagliflozin or placebo). Additionally, a treatment‐by‐subgroup interaction was tested to explore whether the treatment effect could vary by baseline subgroups of interest. The studied subgroups were: baseline UA levels (<6.0 vs ≥6.0 mg/dL and <7.0 vs ≥7.0 mg/dL) prevalent chronic kidney disease (CKD; defined by <60 mL/min/1.73 m2 or urine‐albumin‐creatinine ratio >300 mg/g creatinine [yes vs. no]). In further analyses, we evaluated the odds of patients with UA levels ≥6.0 mg/dL and ≥7.0 mg/dL at baseline to reach UA levels <6.0 and <7.0 mg/dL, respectively, at week 52 using multivariable logistic regression analysis.
Finally, we assessed whether the effect of empagliflozin on gout events could have been “mediated” by the lower use of diuretics in empagliflozin versus placebo using a Cox proportional hazards model accounting for diuretic use as a time‐varying covariate. The resulting hazard ratio (HR) for the treatment effect (empagliflozin vs. placebo), adjusted for time‐varying diuretic use, was compared with the HR obtained from the main primary model, and the percentage mediation was calculated as ln(HR)‐ln(HRc))/ln(HR)*100%. If the inclusion of the time‐varying covariate diuretic use in the primary model results in an attenuation of the strength of the association between treatment and gout events, this could be interpreted as the effect of the drug on gout events being mediated by diuretic use. To qualify as a mediator, treatment should affect diuretic use, and diuretic use should be associated with the risk of gout events.Estimates of cumulative incidence function were corrected for death as a competing risk. 21 The analyses were performed in an “on‐treatment” manner, outcomes were assessed based on adverse events or antigout medication that occurred during treatment or within 7 days after the last dose of study drug. Serum UA levels were considered on‐treatment up until 3 days after the last dose of study drug.
As the effect of empagliflozin on UA levels and gout outcomes was similar with 10 or 25 mg/d, the main analyses are presented with both empagliflozin‐treated groups combined. Sensitivity analyses with separate doses are presented in the Supporting Information. All analyses were performed at the nominal alpha level of 0.05, without correction for multiple hypothesis testing. All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. RESULTS
3.1. Baseline characteristics
From the 7020 patients treated in the trial, 413 (5.9%) were and 6607 (94.1%) were not taking any antigout medication at baseline. Patients on such therapies were older (mean 65.3 vs. 63.0 years), more often men (86.4% vs. 70.5%), had a higher BMI (mean 32.5 vs. 30.5 kg/m2), had a longer T2D duration (60.5% vs. 56.9% with >10 years T2D duration), had worse renal function (mean eGFR 60.0 vs. 74.9 mL/min/1.73 m2), had higher urinary albumin‐to‐creatinine ratio (median 30.9 vs 16.8 mg/g), more frequently had a history of heart failure (13.3% vs. 9.9%), and were more frequently being treated with diuretics (36.3% vs. 14.2% for loop diuretics and 25.7% vs. 20.9% for thiazides; Table 1).
TABLE 1.
Baseline patients' characteristics by baseline use of antigout medication
| Characteristic | Patients not on antigout medication (n = 6607) | Patients on antigout medication (n = 413) |
|---|---|---|
| Age, years | 63.0 (8.7) | 65.3 (7.8) |
| Female sex, n (%) | 1948 (29.5) | 56 (13.6) |
| Race, n (%) | ||
| White | 4757 (72.0) | 324 (78.5) |
| Black | 342 (5.2) | 15 (3.6) |
| Asian | 1448 (21.9) | 69 (16.7) |
| Others | 60 (0.9) | 5 (1.2) |
| BMI, kg/m2 | 30.5 (5.2) | 32.5 (5.3) |
| Time since T2D diagnosis, n (%) | ||
| ≤1 year | 166 (2.5) | 14 (3.4) |
| >1‐5 years | 1031 (15.6) | 52 (12.6) |
| >5‐10 years | 1649 (25.0) | 97 (23.5) |
| >10 years | 3761 (56.9) | 250 (60.5) |
| Uric acid, mg/dL | 5.9 (1.6) | 6.4 (1.7) |
| eGFR, mL/min/1.73 m2 | 74.9 (21.3) | 60.0 (18.4) |
| Heart failure, n (%) | 651 (9.9) | 55 (13.3) |
| Hypertension, n (%) | 6024 (91.2) | 395 (95.6) |
| Median (IQR) UACR, mg/g creatinine | 16.8 (6.2‐69.8) | 30.9 (8.8‐127.3) |
| HbA1c, % | 8.1 (0.9) | 8.0 (0.8) |
| FPG, mg/dL | 153 (44) | 154 (42) |
| SBP, mmHg | 135 (17) | 136 (17) |
| DBP, mmHg | 77 (10) | 76 (10) |
| Cholesterol, mg/dL | 163 (44) | 155 (39) |
| Triglycerides, mg/dL | 169 (128) | 188 (114) |
| Loop diuretics, n (%) | 938 (14.2) | 150 (36.3) |
| Thiazide diuretics, n (%) | 1381 (20.9) | 106 (25.7) |
Note: Data are mean ± SD, unless otherwise stated.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; SBP, systolic blood pressure; T2D, type 2 diabetes; UACR, urine albumin‐creatinine ratio.
Placebo and empagliflozin groups pooled for all variables.
3.2. Empagliflozin effect on UA levels
At 12 weeks, the mean serum UA level was modestly lower in empagliflozin‐treated patients versus placebo, and this difference was maintained in all subsequent timepoints; at week 52 the adjusted mean treatment difference was −0.37 (95% confidence interval [CI] −0.42, −0.31) mg/dL (Figure 1 and Table S1). The treatment effect differences versus placebo were comparable between both empagliflozin doses (10 vs. 25 mg; Figure S1 and Table S2). The adjusted mean treatment differences in serum UA were somewhat more pronounced among patients with baseline UA levels ≥6.0 mg/dL than in patients with baseline UA levels <6.0 mg/dL throughout the study period up until 3 years from baseline (eg, treatment difference at week 52: −0.46 [95% CI −0.55, −0.38] mg/dL vs. −0.28 [95% CI −0.36, −0.20] mg/dL, respectively). A similar pattern was seen for patients with baseline UA ≥ 7.0 mg/dL versus those with baseline UA < 7.0 mg/dL (eg, at week 52: −0.56 [95% CI −0.68, −0.43] mg/dL vs. −0.30 [−0.37, −0.24] mg/dL, respectively [Figures S2, S3 and Table S3]). Treatment differences of pooled empagliflozin versus placebo at week 52 were similar in prevalent CKD versus non‐prevalent CKD patients: −0.38 (95% CI −0.45, −0.31) versus −0.34 (95% CI −0.44, −0.23 [Table S3]).
FIGURE 1.
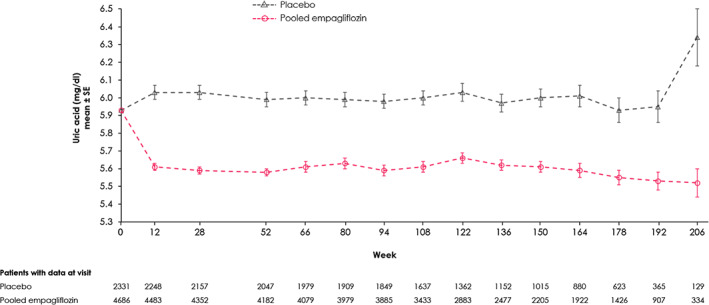
Effect of empagliflozin (10 and 25 mg doses pooled) versus placebo on uric acid (UA) levels (mg/dL) over time (on‐treatment). Mixed‐effect model repeated measures analyses included the continuous fixed effects of baseline UA and baseline glycated haemoglobin at each visit and discrete fixed effects for treatment at each visit, sex, region, baseline estimated glomerular filtration rate, baseline body mass index and last projected visit
The number of patients with a serum UA level < 6.0 mg/dL at all time points during the trial was higher in empagliflozin than in placebo. For example, at week 52 there were 21.8% in the empagliflozin versus 11.1% in the placebo group who reached UA level < 6.0 mg/dL, odds ratio [OR] 2.36 (95%CI 1.89, 2.95). Similar results were obtained for reaching a level <7.0 mg/dL at week 52: 28.5% in empagliflozin versus 15.8% in placebo, OR 2.22 (95% CI 1.69, 2.92; Figure S4).
3.3. Effect of empagliflozin on gout
Among the 6607 patients not taking antigout medications at baseline, 5.2% experienced a gout episode or initiated an antigout treatment in the placebo group versus 3.6% in the pooled empagliflozin group, corresponding to incidence rates of 21.6 versus 14.1 events per 1000 patient‐years, and an HR of 0.67 (95% CI 0.53, 0.85; P = 0.001 [Figures 2 and 3]). The risk reduction was similar with either 10 or 25 mg empagliflozin, and in patients with baseline UA levels below or greater than 6.0 and 7.0 mg/dL (interaction P > 0.1 for all; Figures S5, S6 and Table S4). Both components of the outcome of interest contributed to the reduction seen with empagliflozin on the composite endpoint: gout episodes HR 0.81 (95% CI 0.56, 1.16) and new initiation of antigout medication HR 0.63 (95% CI 0.49, 0.82; Figure 3 ).The effect of empagliflozin on new onset of gout or antigout medication is not attributed to an increased use of diuretics during the on‐treatment follow‐up in empagliflozin versus placebo, as the proportion of the treatment effect mediated by use of loop diuretics, thiazides or both was minor, not exceeding 11% (Table S5).
FIGURE 2.
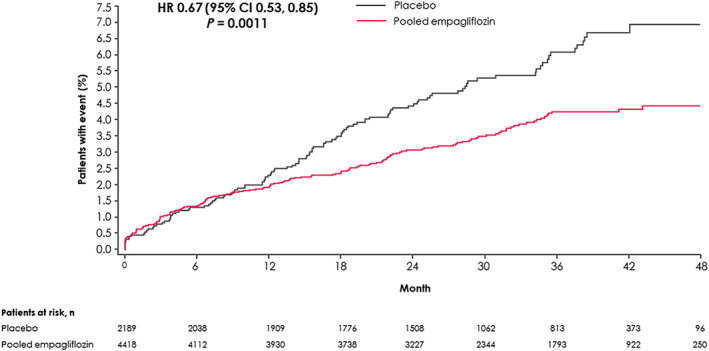
Time to new onset of gout or antigout medication during on‐treatment phase, for patients not on antigout medication at baseline. Hazard ratio (HR) based on Cox proportional hazards model with terms for age, sex, geographic region, baseline glycated haemoglobin, baseline estimated glomerular filtration rate, baseline body mass index and treatment. CI, confidence interval
FIGURE 3.
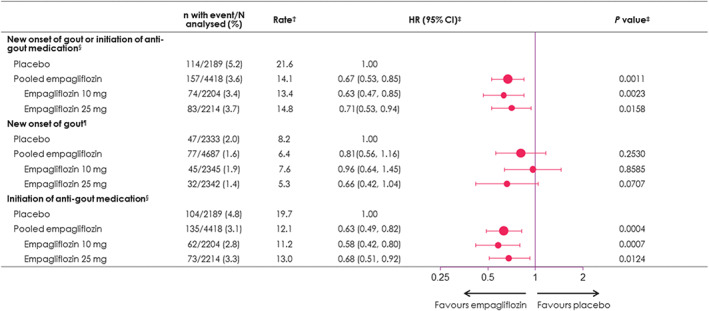
Effect of empagliflozin on new onset of gout or initiation of antigout medication during follow‐up (on‐treatment). †Per 1000 patient‐years. ‡Based on Cox regression with terms for age, sex, geographic region, baseline body mass index, baseline glycated haemoglobin, baseline estimated glomerular filtration rate, and treatment. §Analysis performed in patients not on antigout medication at baseline (n = 6607). ¶Analysis performed in all patients (n = 7020). CI, confidence interval; HR, hazard ratio
4. DISCUSSION
Our analysis from EMPA‐REG OUTCOME shows that, compared with placebo, empagliflozin reduced UA levels as well as gout or the need for antigout medication. These findings are of clinical importance as they could potentially expand the clinical utility of empagliflozin in patients with T2D, beyond the well‐established cardio‐renal benefits, 22 as a potential additional tool in the therapeutic options for gout. Similar findings were seen with canagliflozin in the CANVAS programme, 18 which suggests that the reduction in gout‐related episodes in patients with T2D is a SGLT2 inhibitor class effect. Furthermore, the replication of the findings in different studies and their consistency across subgroups supports a clinically meaningful antigout effect of SGLT2 inhibitors.
A meta‐analysis of 62 clinical trials including a total of 34 941 T2D patients showed a consistent effect of SGLT2 inhibitors in reducing UA levels. The SGLT2 inhibitors (empagliflozin, dapagliflozin, canagliflozin, tofogliflozin, luseogliflozin or ipragliflozin) rapidly reduced UA concentrations by 0.60 mg/dL, an effect that remained stable for up to 2 years of follow‐up. 11 The mechanisms by which SGLT2 inhibitors lower UA levels are not completely elucidated, but SGLT2 inhibitors may reduce glucose and urate reabsorption in the proximal tubule. 8 However, while this mechanism may explain the relatively small reduction in UA levels, it does not explain the major reduction in gout episodes seen with SGLT2 inhibitors. The relative reduction of UA with SGLT2 inhibitors is only approximately 6% to 7%, which is substantially less than that observed with traditional antigout medications such as allopurinol or febuxostat. 23 However, the apparent relative risk reduction in gout episodes of 30% to 40% with SGLT2 inhibitors is at least similar to that seen with traditional antigout medications and consistent across baseline UA level, presence or absence of gout history and chronic kidney disease. 24 , 25 , 26 Furthermore, antigout treatments such as allopurinol or febuxostat may precipitate acute gout flares early after their introduction, which is not known to happen with SGLT2 inhibitors. In fact, the apparent reduction in gout episodes seen with SGLT2 inhibitors is only comparable to other agents that were not designed specifically for UA reduction. For example, compared with placebo, the interleukin‐1β antagonist canakinumab reduced the incidence of gout episodes by 50% without affecting UA levels. 27 Similar approximately 50% reductions in gout events were seen with the lipid‐lowering drug fenofibrate, which also reduced UA levels by 20% (a smaller reduction than that achieved by allopurinol or febuxostat, but higher than that achieved with SGLT2 inhibitors). 20 It is thought that fenofibrate lowers UA through the inhibition of the renal organic anion transporter URAT1 (SLC22A12), 28 a mechanism that also drives the UA‐lowering properties of losartan. 29 , 30
Given the mismatch between UA lowering (most marked with allopurinol or febuxostat) and gout prevention (most marked with SGLT2 inhibitors, canakinumab and fenofibrate), it is likely that mechanisms not directly related to UA lowering might be responsible for the reduction of gout episodes with SGLT2 inhibitors, canakinumab and fenofibrate. Canakinumab is a potent anti‐interleukin‐1β agent, and its anti‐inflammatory properties can offer protection from gout. 27 Fenofibrate is a peroxisome proliferator‐activated receptor alpha activator which can also display anti‐inflammatory effects beyond the above‐mentioned UA‐lowering effects. 31 SGLT2 inhibitors have also been shown to display anti‐inflammatory properties, including the inhibition of interleukin‐1β. 32 , 33 , 34 Furthermore, SGLT2 inhibitors reduce oxidative stress via enhancement of the SIRT‐1 signalling pathway, which leads to downregulation of xanthine oxidase. 35 It should be noted that the SIRT‐1 pathway is the main driver of increased gluconeogenesis and ketogenesis by SGLT2 inhibitors, which might play some role in preventing cardiovascular events and improving survival 10 , 36 ; thus, potentially linking the UA‐lowering and antigout properties of SGLT2 inhibitors with their benefits in the cardiovascular and renal systems. Reinforcing this hypothesis is the finding that the effect of empagliflozin on preventing gout episodes was not mediated by the reduced use in diuretics; therefore, a direct effect of SGLT2 inhibitors is much more plausible, as also supported by previously published mediation analysis from the EMPA‐REG OUTCOME trial. 16
Some limitations of the present study should be acknowledged. Gout was not a prespecified endpoint in the trial and was captured based on the adverse event and concomitant therapy forms and the events were not adjudicated. Therefore, some event misclassification might have occurred, however, it is not expected that any misclassification would have occurred differentially between the empagliflozin and placebo groups. Data on the reasons for the prescription of concomitant medicines during the trial were described with limited detail. Data on the urinary elimination of urate were not available; therefore, we could not ascertain if the blood UA lowering with empagliflozin could be correlated with the urinary elimination. Finally, this is a post hoc study, which increases the risk of chance findings resulting from the multiple testing. However, the consistency of the findings in two independent cohorts and across several subgroups increases the robustness of the results.
In conclusion, in the EMPA‐REG OUTCOME trial, empagliflozin reduced UA levels, gout episodes or the need for antigout medication. These clinically important findings expand the clinical utility of empagliflozin as a potential antigout treatment in patients with T2D, beyond the well‐established cardio‐renal benefits. The replication of similar findings with canagliflozin in the CANVAS programme suggest that these findings represent an SGLT2 inhibitor class effect. The mechanism behind this apparent effect is deserving of further investigation, especially since the apparent reduction in gout episodes is more than would be predicted by the modest reduction in UA.
CONFLICT OF INTEREST
J.P.F. is a consultant for Boehringer Ingelheim. S.E.I. reports membership on scientific/research advisory boards for Boehringer Ingelheim, AstraZeneca, Intarcia, Lexicon, Janssen, Sanofit, Merck & Co. and Novo Nordisk, has received research supplies to Yale University from Takeda, and has participated in medical educational projects, for which unrestricted funding from Boehringer Ingelheim, Eli Lilly, and Merck & Co. was received by Yale University. C.W. has received honoraria for consultancy and lecturing from AstraZeneca, Bayer, BI, GlaxoSmithKline, Eli Lilly and Company, Merck Sharp & Dome, Mundipharma, Sanofi Genzyme and Takeda. B.Z. received consulting fees from Boehringer Ingelheim, Novo Nordisk and Eli Lilly. M.M., T.M. and D.S. are Boehringer Ingelheim employees.
AUTHOR CONTRIBUTIONS
J.P.F. conceived the idea. J.P.F. and D.S. drafted the manuscript. M.M. provided the statistical expertise. All authors critically reviewed the manuscript, provided feedback and approved the final submitted version.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14559.
Supporting information
Table S1. Adjusted mean changes in uric acid levels (mg/dL) over time (on‐treatment) in the empagliflozin (pooled) versus placebo group
Table S2. Adjusted mean changes of uric acid levels (mg/dL) over time (on‐treatment) in the empagliflozin (10 mg and 25 mg separately) versus placebo group
Table S3. Adjusted mean changes of uric acid (UA) levels (mg/dL) over time (on‐treatment) in the empagliflozin (10 mg and 25 mg pooled) versus placebo group in patients with UA levels < vs ≥6.0 mg/dL and < vs ≥7.0 mg/dL at baseline and by prevalent chronic kidney disease at baseline
Table S4. Effects of empagliflozin on new onset of gout or initiation of antigout medication during follow‐up (on‐treatment) by baseline uric acid level
Table S5. Mediation analysis to study the influence of new initiation of diuretics on the treatment effect of empagliflozin on new onset of gout or initiation of antigout medication during follow‐up (on‐treatment)
Figure S1. Effect of empagliflozin (10 and 25 mg) versus placebo on uric acid levels (mg/dL) over time (on‐treatment)
Figure S2. Effect of empagliflozin (10 and 25 mg pooled) versus placebo on uric acid (UA) levels (mg/dL) over time (on‐treatment) in participants with baseline UA ≥6.0 mg/dL versus <6.0 mg/dL
Figure S3. Effect of empagliflozin (10 and 25 mg pooled) versus placebo on uric acid (UA) levels (mg/dL) over time (on‐treatment) in participants with baseline UA ≥7.0 mg/dL vs <7.0 mg/dL
Figure S4. Proportion of patients with serum uric acid levels <6.0 mg/dL and < 7.0 mg/dL during on treatment phase
Figure S5. Time to new onset of gout or antigout medication during on‐treatment phase (for patients not on antigout medication at baseline) in patients with uric acid levels < versus ≥6.0 mg/dL at baseline
Figure S6. Time to new onset of gout or antigout medication during on‐treatment phase (for patients not on antigout medication at baseline) in patients with uric acid levels < versus ≥7.0 mg/dL at baseline
ACKNOWLEDGMENTS
The authors thank the investigators, coordinators and patients who participated in this trial. The EMPA‐REG OUTCOME trial was funded by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance. Boehringer Ingelheim was involved in the design and conduct of the study and in the concept, execution and interpretation of this analysis. Editorial assistance, limited to the preparation of tables and figures and supported financially by Boehringer Ingelheim, was provided by Paul Lidbury of Elevate Scientific Solutions.
Ferreira JP, Inzucchi SE, Mattheus M, et al. Empagliflozin and uric acid metabolism in diabetes: A post hoc analysis of the EMPA‐REG OUTCOME trial. Diabetes Obes Metab. 2022;24(1):135-141. doi:10.1111/dom.14559
Funding information Boehringer Ingelheim
DATA AVAILABILITY STATEMENT
The sponsor of the EMPA‐REG OUTCOME Trial (Boehringer Ingelheim) is committed to responsible sharing of clinical study reports, related clinical documents, and patient level clinical study data. Researchers are invited to submit inquiries via the following website: https://trials.boehringer-ingelheim.com.
REFERENCES
- 1. Verma S, Ji Q, Bhatt DL, et al. Association between uric acid levels and cardio‐renal outcomes and death in patients with type 2 diabetes: a subanalysis of EMPA‐REG OUTCOME. Diabetes Obes Metab. 2020;22(7):1207‐1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yuan H, Yu C, Li X, et al. Serum uric acid levels and risk of metabolic syndrome: a dose‐response meta‐analysis of prospective studies. J Clin Endocrinol Metab. 2015;100(11):4198‐4207. [DOI] [PubMed] [Google Scholar]
- 3. Zoppini G, Targher G, Chonchol M, et al. Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care. 2012;35(1):99‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim IY, Lee DW, Lee SB, Kwak IS. The role of uric acid in kidney fibrosis: experimental evidences for the causal relationship. Biomed Res Int. 2014;2014:638732‐638739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bailey CJ. Uric acid and the cardio‐renal effects of SGLT2 inhibitors. Diabetes Obes Metab. 2019;21(6):1291‐1298. [DOI] [PubMed] [Google Scholar]
- 6. Clarson LE, Chandratre P, Hider SL, et al. Increased cardiovascular mortality associated with gout: a systematic review and meta‐analysis. Eur J Prev Cardiol. 2015;22(3):335‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8‐14. [DOI] [PubMed] [Google Scholar]
- 8. Chino Y, Samukawa Y, Sakai S, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014;35(7):391‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McNally JS, Saxena A, Cai H, Dikalov S, Harrison DG. Regulation of xanthine oxidoreductase protein expression by hydrogen peroxide and calcium. Arterioscler Thromb Vasc Biol. 2005;25(8):1623‐1628. [DOI] [PubMed] [Google Scholar]
- 10. Packer M. Cardioprotective effects of Sirtuin‐1 and its downstream effectors: potential role in mediating the heart failure benefits of SGLT2 (sodium‐glucose cotransporter 2) inhibitors. Circ Heart Fail. 2020;13:e007197. [DOI] [PubMed] [Google Scholar]
- 11. Zhao Y, Xu L, Tian D, et al. Effects of sodium‐glucose co‐transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta‐analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20(2):458‐462. [DOI] [PubMed] [Google Scholar]
- 12. McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta‐analysis. JAMA Cardiol. 2020;6:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31‐39. [DOI] [PubMed] [Google Scholar]
- 14. Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2019;7(11):845‐854. [DOI] [PubMed] [Google Scholar]
- 15. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐reduced and DAPA‐HF trials. Lancet. 2020;396:819‐829. [DOI] [PubMed] [Google Scholar]
- 16. Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME Trial. Diabetes Care. 2018;41(2):356‐363. [DOI] [PubMed] [Google Scholar]
- 17. Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649‐662. [DOI] [PubMed] [Google Scholar]
- 18. Li J, Badve S, Zhou Z. The effects of canagliflozin on gout in type 2 diabetes: a post‐hoc analysis of the CANVAS program. Lancet Rheumatol. 2019;1(4):E220–E228. [DOI] [PubMed] [Google Scholar]
- 19. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 20. Waldman B, Ansquer JC, Sullivan DR, et al. Effect of fenofibrate on uric acid and gout in type 2 diabetes: a post‐hoc analysis of the randomised, controlled FIELD study. Lancet Diabetes Endocrinol. 2018;6(4):310‐318. [DOI] [PubMed] [Google Scholar]
- 21. Beyersmann J, Allignol A, Schumacher M. Competing Risks and Multistate Models with R. Springer; 2011. [DOI] [PubMed] [Google Scholar]
- 22. Ferreira JP, Kraus BJ, Zwiener I, et al. Cardio/kidney composite end points: a post hoc analysis of the EMPA‐REG OUTCOME trial. J Am Heart Assoc. 2021;10:e020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cutolo M, Cimmino MA, Perez‐Ruiz F. Potency on lowering serum uric acid in gout patients: a pooled analysis of registrative studies comparing febuxostat vs. allopurinol. Eur Rev Med Pharmacol Sci. 2017;21(18):4186‐4195. [PubMed] [Google Scholar]
- 24. Schumacher HR Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28‐week, phase III, randomized, double‐blind, parallel‐group trial. Arthritis Rheum. 2008;59(11):1540‐1548. [DOI] [PubMed] [Google Scholar]
- 25. Taylor TH, Mecchella JN, Larson RJ, Kerin KD, Mackenzie TA. Initiation of allopurinol at first medical contact for acute attacks of gout: a randomized clinical trial. Am J Med. 2012;125(11):1126‐1134.e7. [DOI] [PubMed] [Google Scholar]
- 26. Dalbeth N, Saag KG, Palmer WE, et al. Effects of febuxostat in early gout: a randomized, double‐blind, placebo‐controlled study. Arthritis Rheumatol. 2017;69(12):2386‐2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Solomon DH, Glynn RJ, MacFadyen JG, et al. Relationship of interleukin‐1β blockade with incident gout and serum uric acid levels: exploratory analysis of a randomized controlled trial. Ann Intern Med. 2018;169(8):535‐542. [DOI] [PubMed] [Google Scholar]
- 28. Uetake D, Ohno I, Ichida K, et al. Effect of fenofibrate on uric acid metabolism and urate transporter 1. Intern Med. 2010;49(2):89‐94. [DOI] [PubMed] [Google Scholar]
- 29. Nakanishi T, Ohya K, Shimada S, Anzai N, Tamai I. Functional cooperation of URAT1 (SLC22A12) and URATv1 (SLC2A9) in renal reabsorption of urate. Nephrol Dial Transplant. 2013;28(3):603‐611. [DOI] [PubMed] [Google Scholar]
- 30. Würzner G, Gerster JC, Chiolero A, et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001;19(10):1855‐1860. [DOI] [PubMed] [Google Scholar]
- 31. Lee JW, Bajwa PJ, Carson MJ, et al. Fenofibrate represses interleukin‐17 and interferon‐gamma expression and improves colitis in interleukin‐10‐deficient mice. Gastroenterology. 2007;133(1):108‐123. [DOI] [PubMed] [Google Scholar]
- 32. Heerspink HJL, Perco P, Mulder S, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62(7):1154‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mancini SJ, Boyd D, Katwan OJ, et al. Canagliflozin inhibits interleukin‐1β‐stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP‐activated protein kinase‐dependent and ‐independent mechanisms. Sci Rep. 2018;8(1):5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maayah ZH, Ferdaoussi M, Takahara S, Soni S, Dyck JRB. Empagliflozin suppresses inflammation and protects against acute septic renal injury. Inflammopharmacology. 2021;29(1):269‐279. [DOI] [PubMed] [Google Scholar]
- 35. Huang XF, Li HQ, Shi L, Xue JY, Ruan BF, Zhu HL. Synthesis of resveratrol analogues, and evaluation of their cytotoxic and xanthine oxidase inhibitory activities. Chem Biodivers. 2008;5(4):636‐642. [DOI] [PubMed] [Google Scholar]
- 36. Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC‐1alpha and SIRT1. Nature. 2005;434(7029):113‐118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Adjusted mean changes in uric acid levels (mg/dL) over time (on‐treatment) in the empagliflozin (pooled) versus placebo group
Table S2. Adjusted mean changes of uric acid levels (mg/dL) over time (on‐treatment) in the empagliflozin (10 mg and 25 mg separately) versus placebo group
Table S3. Adjusted mean changes of uric acid (UA) levels (mg/dL) over time (on‐treatment) in the empagliflozin (10 mg and 25 mg pooled) versus placebo group in patients with UA levels < vs ≥6.0 mg/dL and < vs ≥7.0 mg/dL at baseline and by prevalent chronic kidney disease at baseline
Table S4. Effects of empagliflozin on new onset of gout or initiation of antigout medication during follow‐up (on‐treatment) by baseline uric acid level
Table S5. Mediation analysis to study the influence of new initiation of diuretics on the treatment effect of empagliflozin on new onset of gout or initiation of antigout medication during follow‐up (on‐treatment)
Figure S1. Effect of empagliflozin (10 and 25 mg) versus placebo on uric acid levels (mg/dL) over time (on‐treatment)
Figure S2. Effect of empagliflozin (10 and 25 mg pooled) versus placebo on uric acid (UA) levels (mg/dL) over time (on‐treatment) in participants with baseline UA ≥6.0 mg/dL versus <6.0 mg/dL
Figure S3. Effect of empagliflozin (10 and 25 mg pooled) versus placebo on uric acid (UA) levels (mg/dL) over time (on‐treatment) in participants with baseline UA ≥7.0 mg/dL vs <7.0 mg/dL
Figure S4. Proportion of patients with serum uric acid levels <6.0 mg/dL and < 7.0 mg/dL during on treatment phase
Figure S5. Time to new onset of gout or antigout medication during on‐treatment phase (for patients not on antigout medication at baseline) in patients with uric acid levels < versus ≥6.0 mg/dL at baseline
Figure S6. Time to new onset of gout or antigout medication during on‐treatment phase (for patients not on antigout medication at baseline) in patients with uric acid levels < versus ≥7.0 mg/dL at baseline
Data Availability Statement
The sponsor of the EMPA‐REG OUTCOME Trial (Boehringer Ingelheim) is committed to responsible sharing of clinical study reports, related clinical documents, and patient level clinical study data. Researchers are invited to submit inquiries via the following website: https://trials.boehringer-ingelheim.com.


