Abstract
Aims
To evaluate, through exploratory post hoc subgroup analyses, the efficacy and safety of oral semaglutide versus comparators in Japanese patients enrolled in the global PIONEER 1, 3, 4 and 8 clinical trials.
Materials and Methods
Patients were randomized to once‐daily oral semaglutide 3, 7 or 14 mg or comparator (placebo, sitagliptin 100 mg or liraglutide 1.8 mg). Change from baseline in glycated haemoglobin (HbA1c) and body weight, and proportions of patients attaining HbA1c <7.0% (53 mmol/mol) and body weight loss ≥5%, were analysed at week 26 for all Japanese patients in each trial separately using the treatment policy estimand (regardless of treatment discontinuation or rescue medication use). Adverse events (AEs) were analysed descriptively.
Results
Reductions in HbA1c from baseline in Japanese patients were 1.0% to 1.2% (11.3 mmol/mol to 13.3 mmol/mol) and 1.4% to 1.7% (15.7 mmol/mol to 18.3 mmol/mol) for oral semaglutide 7 mg and 14 mg, respectively. HbA1c reductions were similar or greater than with comparators. Body weight reductions were 1.0% to 2.7% and 3.7% to 4.7% for oral semaglutide 7 mg and 14 mg, respectively, and were generally greater with oral semaglutide than comparators. As expected, the main class of AEs was gastrointestinal, and these AEs comprised most commonly mild‐to‐moderate constipation, nausea and diarrhoea.
Conclusions
Oral semaglutide appears efficacious and well tolerated in Japanese patients across the type 2 diabetes spectrum.
Keywords: Glucagon‐like peptide‐1 receptor agonist, Japanese, oral semaglutide, PIONEER, post hoc, subgroup analyses
1. INTRODUCTION
Oral semaglutide is a once‐daily tablet comprising the glucagon‐like peptide‐1 (GLP‐1) receptor agonist (GLP‐1RA) semaglutide, co‐formulated with an absorption enhancer sodium N‐(8‐[2‐hydroxybenzoyl] amino) caprylate to enable oral administration. 1 Oral semaglutide is approved for use in Japan, 2 Europe 3 and the United States 4 for the treatment of adults with insufficiently controlled type 2 diabetes (T2D). GLP‐1RAs are usually recommended for use in combination with other glucose‐lowering therapies (generally including metformin). 5 , 6 , 7 However, oral semaglutide is also indicated as an adjunct to diet and exercise to improve glycaemic control when metformin is considered inappropriate due to intolerance or contraindications, and, in this circumstance, can be given either as monotherapy or in combination with other medicinal products for the treatment of diabetes. 2 , 3 , 4
The efficacy and safety of oral semaglutide were investigated in the randomized, phase 3a Peptide InnOvatioN for Early diabEtes tReatment (PIONEER) clinical trials, of which eight were global studies, 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 and two were conducted in Japan. 16 , 17 The PIONEER programme demonstrated that oral semaglutide was well tolerated, with a safety profile similar to that of other GLP‐1RAs. Oral semaglutide was efficacious in reducing glycated haemoglobin (HbA1c) and body weight when given to a broad spectrum of patients, and when compared against placebo and a variety of glucose‐lowering therapies. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17
The pathophysiology of T2D in East Asian populations differs from that of Western populations, with patients tending to have greater impairment of insulin secretion; Japanese patients also generally have a lower body mass index (BMI) than their Western counterparts. 18 , 19 , 20 Therefore, it is necessary to specifically characterize the response to treatments for T2D in East Asian populations, including in Japanese patients. In addition to PIONEER 9 and 10, 16 , 17 four global trials within the oral semaglutide clinical programme—PIONEER 1, 3, 4 and 8 8 , 10 , 11 , 15 —enrolled Japanese patients. Enrolment in these trials was stratified by Japanese ethnicity but subgroup analyses of outcomes in these patients were not prespecified. In the present paper, we report the results of exploratory post hoc analyses of the Japanese patients in PIONEER 1, 3, 4 and 8 to further characterize the efficacy and safety of oral semaglutide in this population.
2. MATERIALS AND METHODS
2.1. Individual trial designs
The full methodologies of the PIONEER 1, 3, 4 and 8 trials have been reported previously. The trials are registered on ClinicalTrials.gov (NCT02906930, NCT02607865, NCT02863419, NCT03021187), and the trial designs are summarized in Table 1. 8 , 10 , 11 , 15 In brief, Japanese patients were aged ≥20 years (compared with ≥18 years for participants from most other countries), had been diagnosed with T2D for ≥90 days before screening, had a baseline HbA1c of 7.0% to 10.5% (53 mmol/mol to 91 mmol/mol, depending on the trial) and, with the exception of PIONEER 1, were receiving stable doses of background glucose‐lowering medication as allowed by the individual trial designs. 8 , 10 , 11 , 15 Background medication was metformin with or without a sulphonylurea in PIONEER 3, 10 metformin with or without a sodium‐glucose cotransporter‐2 inhibitor in PIONEER 4 11 and basal, premixed or basal‐bolus insulin with or without metformin in PIONEER 8 (only those Japanese patients on basal insulin could receive metformin). 15 Patients enrolled in PIONEER 1 were managed by diet and exercise alone in addition to study treatment. 8
TABLE 1.
| Trial (N) | Treatment arms | Key inclusion criteria | Trial duration | Primary/secondary efficacy endpoint |
|---|---|---|---|---|
| PIONEER 1 (global: 703; Japan: 116) |
|
|
|
|
| PIONEER 3 (global: 1864; Japan: 207) |
|
|
|
|
| PIONEER 4 (global: 711; Japan: 75) |
|
|
|
|
| PIONEER 8 (global: 731; Japan: 194) |
|
|
|
|
Abbreviations: HbA1c, glycated haemoglobin; N, number of randomized patients in the specified patient population; s.c., subcutaneous; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulphonylurea.
Basal, basal‐bolus or premixed insulin.
Patients were randomized 1:1:1:1 in a double‐blind, parallel‐group fashion to receive once‐daily oral semaglutide 3, 7 or 14 mg or matched placebo for 26 weeks in PIONEER 1 8 and 52 weeks in PIONEER 8. 15 PIONEER 3 was a double‐blind, double‐dummy, parallel‐group trial in which patients were randomized 1:1:1:1 to oral semaglutide 3, 7 or 14 mg or sitagliptin 100 mg once daily for 78 weeks. 10 In PIONEER 4, which was a double‐blind, double‐dummy trial, patients were randomized 2:2:1 to either oral semaglutide 14 mg, subcutaneous liraglutide 1.8 mg or placebo once daily for 52 weeks. 11 In order to mitigate potential gastrointestinal side effects, patients receiving oral semaglutide started at the 3 mg dose, which was then escalated in 4‐week increments in those assigned to the 7 mg or 14 mg doses to reach the randomized dose. 8 , 10 , 11 , 15
The main efficacy outcomes were change in HbA1c (primary efficacy outcome) and body weight (confirmatory secondary outcome) from baseline to 26 weeks of treatment. Changes from baseline in HbA1c and body weight were also recorded at the end of planned treatment. Other efficacy endpoints recorded at week 26 and at the end of planned treatment included the proportion of patients reaching HbA1c <7.0% (53 mmol/mol) and body weight loss ≥5%. Adverse events (AEs; including AEs leading to trial product discontinuation, and events of hypoglycaemia) were recorded descriptively from randomization up to the end of the trials (in‐trial period). 8 , 10 , 11 , 15
2.2. Subgroup analysis methodology
These post hoc subgroup analyses were exploratory in nature and were not prespecified in the protocols of the trials involved. For the purpose of these analyses, individuals enrolled in each trial were stratified according to whether they were of Japanese ethnicity or not. All of the Japanese patients from each trial were included in the subgroup analyses. Data were not pooled across trials because of differences in the designs and patient populations of each study.
As described in detail elsewhere, 21 two estimands were defined to address two scientific questions relating to efficacy. Under the treatment policy estimand, which was the primary estimand in the four trials, data were analysed from all randomized patients, regardless of premature discontinuation of randomized treatment or rescue medication use. For the secondary estimand—the trial product estimand—data were analysed from all randomized patients under the assumption that patients did not use rescue medication or discontinue their randomized treatment. The results of the present subgroup analyses are reported for the treatment policy estimand.
2.3. Statistical analyses
Continuous efficacy endpoints were analysed for the treatment policy estimand using a pattern mixture model with multiple imputation to impute missing data at weeks 26, 52 and 78, depending on the endpoint and the length of each trial. All data collected at week 26, 52 or 78, irrespective of premature discontinuation of the trial product or initiation of additional glucose‐lowering medication (during the in‐trial observation period), were included in the statistical analyses.
It was assumed that the missing‐data mechanism was missing‐at‐random within the groups used for the imputation. Imputation of missing data was done using an analysis of covariance (ANCOVA) model within groups defined by randomized treatment and treatment status (on‐treatment without use of rescue medication; premature discontinuation of trial product or initiation of rescue medication) at week 26. Imputation of missing week 52 or week 78 data was carried out within groups defined by randomized treatment, and treatment status at week 26 and at week 52 or 78 (except PIONEER 4, where treatment status at week 26 was excluded due to a low number of patients in the groups used for imputation). After imputation, each of the 1000 imputed, complete datasets was analysed using an ANCOVA model, with treatment, strata (PIONEER 3, 4 and 8) and interaction between strata (PIONEER 8) as categorical fixed effects, and the baseline value as a covariate. Rubin's rule 22 was used to combine the 1000 analysis results to draw inference.
Efficacy data were reported as changes from baseline for continuous variables (change from baseline in HbA1c and body weight) with estimated treatment differences (ETDs) and 95% confidence intervals, and proportions of patients achieving discrete efficacy targets (HbA1c <7.0% [53 mmol/mol] and body weight ≥5%); P values were not calculated as these subgroup analyses were not prespecified and not controlled for multiplicity. Safety data (incidence of AEs) were analysed descriptively. All analyses were performed using SAS Version 9.4 M2.
3. RESULTS
3.1. Participants
The disposition and key baseline characteristics of Japanese patients in PIONEER 1, 3, 4 and 8 are shown in Table 2. The incidence of treatment discontinuation with oral semaglutide ranged from 2% to 19%, and rescue medication was used by 0% to 27% of those who completed oral semaglutide treatment, depending on trial and randomized dose. Across treatment arms and trials, 57% to 75% of Japanese patients were male, mean baseline HbA1c was 7.9% to 8.3% (63 mmol/mol to 67 mmol/mol), mean body weight was in the range 66 kg to 80 kg and BMI was 25 kg/m2 to 28 kg/m2. Approximately 46% of Japanese patients in PIONEER 8 were receiving background basal insulin, and 26% were receiving basal insulin plus metformin, at randomization. Of the remaining patients, approximately 30% were receiving basal‐bolus insulin and 23% were receiving premixed insulin (Table S1).
TABLE 2.
Disposition (A) and baseline characteristics (B) of Japanese patients in PIONEER 1, 3, 4 and 8
| (A) | |||||
|---|---|---|---|---|---|
| Treatment completers | |||||
| Trial (background regimen) | Treatment group | Patients, N | Without rescue medication, n (%) | With rescue medication, n (%) | Premature trial product discontinuations |
| PIONEER 1 (diet and exercise) | Oral semaglutide 3 mg | 29 | 27 (93.1) | 0 | 2 (6.9) |
| Oral semaglutide 7 mg | 29 | 28 (96.6) | 0 | 1 (3.4) | |
| Oral semaglutide 14 mg | 28 | 26 (92.9) | 0 | 2 (7.1) | |
| Placebo | 30 | 22 (73.3) | 6 (20.0) | 2 (6.7) | |
| PIONEER 3 (metformin ± SU) | Oral semaglutide 3 mg | 52 | 35 (67.3) | 14 (26.9) | 3 (5.8) |
| Oral semaglutide 7 mg | 52 | 42 (80.8) | 5 (9.6) | 5 (9.6) | |
| Oral semaglutide 14 mg | 51 | 43 (84.3) | 0 | 8 (15.7) | |
| Sitagliptin 100 mg | 52 | 43 (82.7) | 7 (13.5) | 2 (3.8) | |
| PIONEER 4 (metformin ± SGLT2i) | Oral semaglutide 14 mg | 31 | 28 (90.3) | 0 | 3 (9.7) |
| Liraglutide 1.8 mg | 29 | 27 (93.1) | 1 (3.4) | 1 (3.4) | |
| Placebo | 15 | 10 (66.7) | 4 (26.7) | 1 (6.7) | |
| PIONEER 8 (insulin ± metformin) | Oral semaglutide 3 mg | 49 | 38 (77.6) | 10 (20.4) | 1 (2.0) |
| Oral semaglutide 7 mg | 48 | 33 (68.8) | 8 (16.7) | 7 (14.6) | |
| Oral semaglutide 14 mg | 47 | 33 (70.2) | 5 (10.6) | 9 (19.1) | |
| Placebo | 50 | 26 (52.0) | 22 (44.0) | 2 (4.0) | |
| (B) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial (background regimen) | Treatment group | Patients, N | Female, n (%) | Age, years | Diabetes duration, years | HbA1c, % | HbA1c, mmol/mol | Body weight, kg | BMI, kg/m2 |
| PIONEER 1 (diet and exercise) | Oral semaglutide 3 mg | 29 | 9 (31.0) | 61 ± 10 | 7.4 ± 6.1 | 7.9 ± 0.7 | 63.2 ± 7.8 | 67.4 ± 14.6 | 24.9 ± 3.9 |
| Oral semaglutide 7 mg | 29 | 12 (41.4) | 61 ± 9 | 8.1 ± 6.6 | 8.0 ± 0.7 | 64.4 ± 7.6 | 67.4 ± 13.5 | 25.3 ± 4.4 | |
| Oral semaglutide 14 mg | 28 | 12 (42.9) | 59 ± 9 | 6.7 ± 4.3 | 8.0 ± 0.6 | 63.9 ± 6.9 | 67.4 ± 12.2 | 25.4 ± 4.3 | |
| Placebo | 30 | 12 (40.0) | 59 ± 9 | 8.8 ± 6.3 | 8.0 ± 0.7 | 64.5 ± 7.5 | 66.3 ± 11.7 | 25.4 ± 4.4 | |
| PIONEER 3 (metformin ± SU) | Oral semaglutide 3 mg | 52 | 21 (40.4) | 58 ± 10 | 10.7 ± 7.2 | 8.1 ± 1.0 | 65.1 ± 10.4 | 71.4 ± 17.9 | 26.3 ± 4.7 |
| Oral semaglutide 7 mg | 52 | 13 (25.0) | 58 ± 10 | 10.5 ± 6.8 | 8.3 ± 0.9 | 67.1 ± 9.4 | 72.6 ± 15.8 | 26.3 ± 4.2 | |
| Oral semaglutide 14 mg | 51 | 18 (35.3) | 58 ± 11 | 11.7 ± 8.0 | 8.3 ± 0.9 | 66.8 ± 9.3 | 71.5 ± 15.5 | 26.6 ± 4.5 | |
| Sitagliptin 100 mg | 52 | 21 (40.4) | 57 ± 10 | 10.8 ± 7.2 | 8.1 ± 0.8 | 64.6 ± 8.2 | 73.1 ± 13.4 | 27.4 ± 4.4 | |
| PIONEER 4 (metformin ± SGLT2i) | Oral semaglutide 14 mg | 31 | 8 (25.8) | 54 ± 10 | 9.7 ± 6.4 | 8.0 ± 0.7 | 63.5 ± 7.1 | 79.9 ± 24.4 | 28.4 ± 7.1 |
| Liraglutide 1.8 mg | 29 | 10 (34.5) | 54 ± 10 | 8.5 ± 5.4 | 8.1 ± 0.6 | 64.8 ± 6.4 | 77.7 ± 14.6 | 28.1 ± 4.5 | |
| Placebo | 15 | 5 (33.3) | 53 ± 10 | 8.7 ± 5.0 | 8.0 ± 0.8 | 64.5 ± 8.8 | 72.7 ± 8.4 | 26.6 ± 3.4 | |
| PIONEER 8 (insulin ± metformin) | Oral semaglutide 3 mg | 49 | 19 (38.8) | 61 ± 11 | 17.7 ± 9.5 | 8.0 ± 0.6 | 63.4 ± 6.9 | 67.6 ± 12.6 | 25.3 ± 3.3 |
| Oral semaglutide 7 mg | 48 | 16 (33.3) | 64 ± 11 | 17.5 ± 8.9 | 8.0 ± 0.6 | 64.4 ± 6.6 | 67.0 ± 13.4 | 25.1 ± 3.6 | |
| Oral semaglutide 14 mg | 47 | 16 (34.0) | 61 ± 10 | 15.4 ± 8.8 | 8.3 ± 0.7 | 67.8 ± 7.3 | 69.6 ± 11.5 | 25.8 ± 3.7 | |
| Placebo | 50 | 12 (24.0) | 60 ± 11 | 16.5 ± 8.7 | 8.1 ± 0.8 | 65.1 ± 8.4 | 69.7 ± 14.6 | 25.6 ± 4.2 |
Note: Data are mean ± standard deviation unless specified.
Abbreviations: BMI, body mass index; HbA1c, glycated haemoglobin; n, number of patients meeting criterion; N, number of randomized patients; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulphonylurea.
3.2. Efficacy outcomes
3.2.1. Glycaemic efficacy
The change from baseline in HbA1c for Japanese patients across the trials and treatment timepoints is shown in Figure 1. Reductions from baseline were in the range of 0.5% to 0.9% (5.6 mmol/mol to 9.4 mmol/mol) for oral semaglutide 3 mg, 1.0% to 1.2% (11.3 mmol/mol to 13.3 mmol/mol) for oral semaglutide 7 mg and 1.4% to 1.7% (15.7 mmol/mol to 18.3 mmol/mol) for oral semaglutide 14 mg at 26 weeks, and remained generally similar at 52 weeks (PIONEER 3, 4 and 8) and 78 weeks (PIONEER 3). Based on ETDs (not controlled for multiplicity), HbA1c reductions with oral semaglutide 3 mg and 7 mg were greater than with placebo (PIONEER 1 and 8) and were similar to sitagliptin 100 mg for oral semaglutide 7 mg (PIONEER 3) at all timepoints evaluated; changes with oral semaglutide 14 mg were greater than with placebo, sitagliptin 100 mg and liraglutide 1.8 mg (Figure S1).
FIGURE 1.
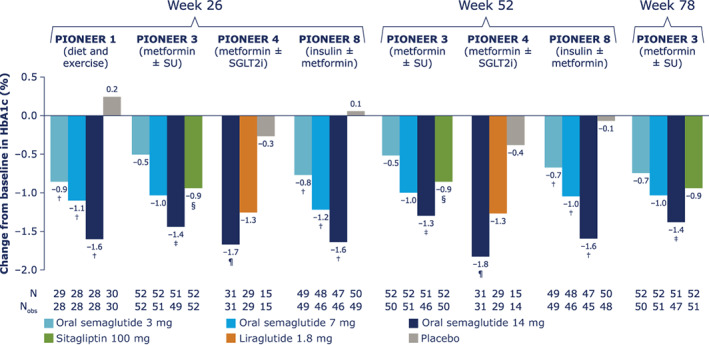
Change from baseline in glycated haemoglobin (HbA1c) for Japanese patients in PIONEER 1, 3, 4 and 8. Data from the in‐trial observation period were included in the statistical analysis. The efficacy endpoints were analysed for the treatment policy estimand using a pattern mixture model with multiple imputation to impute missing data for the landmark visits. Imputation of missing data was done using an analysis of covariance (ANCOVA) model within groups defined by randomized treatment and treatment status (on‐treatment without use of rescue medication; premature discontinuation of trial product or initiation rescue medication). After imputation, each of the 1000 imputed, complete datasets were analysed using an ANCOVA model with treatment, strata (PIONEER 3, 4 and 8) and interaction between strata (PIONEER 8) as categorical fixed effects and the baseline value as a covariate. Rubin's rule was used to combine the 1000 analysis results to draw inference. †Upper limit of 95% confidence interval (CI) for estimated treatment difference (ETD) for oral semaglutide 3, 7 and 14 mg versus placebo <0. ‡Upper limit of 95% CI for ETD for oral semaglutide 14 mg versus sitagliptin <0. §Lower limit of 95% CI for ETD for oral semaglutide 3 mg versus sitagliptin ≥0. ¶Upper limit of 95% CI for ETD for oral semaglutide 14 mg versus liraglutide and placebo <0. N, number of randomized patients contributing to the analyses; Nobs, number of patients with observations; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulphonylurea
The proportions of Japanese patients achieving HbA1c <7.0% (53 mmol/mol) increased with oral semaglutide dose (Table S2). At week 26, 37% to 57%, 41% to 61% and 69% to 86% of patients receiving oral semaglutide 3, 7 and 14 mg, respectively, had HbA1c <7.0% (53 mmol/mol) compared with ≤20% of patients with placebo, 48% with sitagliptin 100 mg and 72% with liraglutide 1.8 mg. These proportions remained broadly similar for oral semaglutide 7 mg and 14 mg, and comparators, at week 52 (PIONEER 3, 4 and 8) and week 78 (PIONEER 3).
3.2.2. Body weight
The percentage change from baseline in body weight in Japanese patients across the trials and treatment timepoints is shown in Figure 2. Body weight reductions from baseline were 0.6% to 1.6% (absolute reduction 0.3 kg to 0.9 kg) for oral semaglutide 3 mg, 1.0% to 2.7% (0.6 kg to 2.3 kg) for oral semaglutide 7 mg and 3.7% to 4.7% (2.4 kg to 3.1 kg) for oral semaglutide 14 mg at week 26. In the trials lasting longer than 26 weeks, weight loss tended to increase in the oral semaglutide 14 mg groups between week 26 and the end of treatment, whereas there was little further change with comparator treatments. A similar picture was evident when looking at absolute body weight changes from baseline (in kg) (Figure S2). Based on ETDs (not controlled for multiplicity), body weight reductions as a proportion of baseline weight were generally greater with oral semaglutide 7 mg and 14 mg than with placebo, sitagliptin 100 mg and liraglutide 1.8 mg (Figure S3).
FIGURE 2.
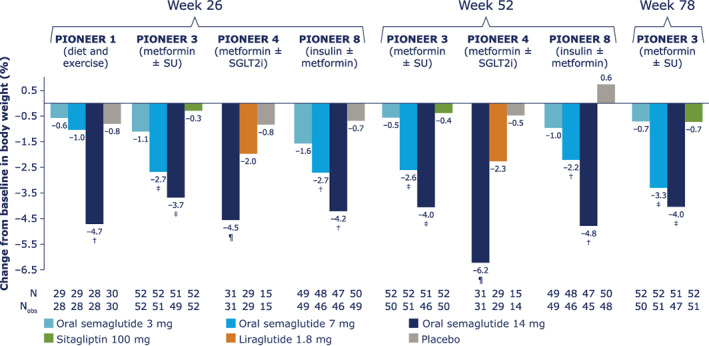
Change from baseline in body weight (%) for Japanese patients in PIONEER 1, 3, 4 and 8. Data from the in‐trial observation period were included in the statistical analysis. The efficacy endpoints were analysed for the treatment policy estimand using a pattern mixture model with multiple imputation to impute missing data for the landmark visits. Imputation of missing data was done using an analysis of covariance (ANCOVA) model within groups defined by randomized treatment and treatment status (on‐treatment without use of rescue medication; premature discontinuation of trial product or initiation rescue medication). After imputation, each of the 1000 imputed, complete datasets were analysed using an ANCOVA model with treatment, strata (PIONEER 3, 4 and 8) and interaction between strata (PIONEER 8) as categorical fixed effects and the baseline value as a covariate. Rubin's rule was used to combine the 1000 analysis results to draw inference. †Upper limit of 95% confidence interval (CI) for estimated treatment difference (ETD) for oral semaglutide 3, 7 and 14 mg versus placebo <0. ‡Upper limit of 95% CI for ETD for oral semaglutide 7 and 14 mg versus sitagliptin <0. ¶Upper limit of 95% CI for ETD for oral semaglutide 14 mg versus liraglutide and placebo <0. N, number of randomized patients contributing to the analyses; Nobs, number of patients with observations; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulphonylurea
The proportions of Japanese patients achieving body weight loss ≥5% increased with oral semaglutide dose (Table S3). At week 26, 7% to 14%, 7% to 24% and 33% to 43% of patients receiving oral semaglutide 3, 7 and 14 mg, respectively, had lost ≥5% of their baseline body weight, compared with 0% to 13% receiving placebo, 4% receiving sitagliptin 100 mg and 17% of patients receiving liraglutide 1.8 mg. At later timepoints, the proportions of patients receiving oral semaglutide 7 mg and 14 mg who had ≥5% body weight loss had increased to 24% to 28% and 40% to 48%, respectively, whereas the proportions were 2% to 7% with placebo, 6% to 14% with sitagliptin 100 mg and remained at 17% with liraglutide 1.8 mg.
3.3. Safety outcomes
The rates of AEs, AEs leading to trial product discontinuation and hypoglycaemia in Japanese patients are shown in Table 3.
TABLE 3.
On‐treatment adverse events (AEs) (A) and most frequent gastrointestinal AEs (B) in Japanese patients in PIONEER 1, 3, 4 and 8
| (A) | Treatment group | Patients, N | Any AE, n (%) | Serious AE, n (%) | Severity, n (%) | AE leading to trial product discontinuation, n (%) | Severe or BG‐confirmed a symptomatic hypoglycaemia, n (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Trial (background regimen) | Mild | Moderate | Severe | ||||||
| PIONEER 1 (diet and exercise) | Oral semaglutide 3 mg | 29 | 15 (51.7) | 0 | 15 (51.7) | 1 (3.4) | 0 | 0 | 0 |
| Oral semaglutide 7 mg | 29 | 16 (55.2) | 0 | 15 (51.7) | 2 (6.9) | 0 | 1 (3.4) | 0 | |
| Oral semaglutide 14 mg | 28 | 18 (64.3) | 0 | 17 (60.7) | 3 (10.7) | 0 | 2 (7.1) | 0 | |
| Placebo | 30 | 12 (40.0) | 1 (3.3) | 10 (33.3) | 3 (10.0) | 0 | 1 (3.3) | 0 | |
| PIONEER 3 (metformin ± SU) | Oral semaglutide 3 mg | 52 | 39 (75.0) | 4 (7.7) | 36 (69.2) | 9 (17.3) | 1 (1.9) | 2 (3.8) | 0 |
| Oral semaglutide 7 mg | 52 | 46 (88.5) | 7 (13.5) | 42 (80.8) | 11 (21.2) | 3 (5.8) | 5 (9.6) | 1 (1.9) | |
| Oral semaglutide 14 mg | 51 | 43 (84.3) | 6 (11.8) | 42 (82.4) | 10 (19.6) | 3 (5.9) | 8 (15.7) | 2 (3.9) | |
| Sitagliptin 100 mg | 52 | 47 (90.4) | 2 (3.8) | 45 (86.5) | 7 (13.5) | 2 (3.8) | 1 (1.9) | 2 (3.8) | |
| PIONEER 4 (metformin ± SGLT2i) | Oral semaglutide 14 mg | 31 | 28 (90.3) | 1 (3.2) | 28 (90.3) | 3 (9.7) | 1 (3.2) | 1 (3.2) | 1 (3.2) |
| Liraglutide 1.8 mg | 29 | 26 (89.7) | 1 (3.4) | 26 (89.7) | 0 | 1 (3.4) | 1 (3.4) | 1 (3.4) | |
| Placebo | 15 | 10 (66.7) | 0 | 10 (66.7) | 1 (6.7) | 0 | 0 | 0 | |
| PIONEER 8 (insulin ± metformin) | Oral semaglutide 3 mg | 49 | 39 (79.6) | 7 (14.3) | 39 (79.6) | 9 (18.4) | 1 (2.0) | 1 (2.0) | 13 (26.5) |
| Oral semaglutide 7 mg | 48 | 44 (91.7) | 5 (10.4) | 44 (91.7) | 9 (18.8) | 0 | 4 (8.3) | 10 (20.8) | |
| Oral semaglutide 14 mg | 47 | 41 (87.2) | 0 | 37 (78.7) | 7 (14.9) | 1 (2.1) | 8 (17.0) | 11 (23.4) | |
| Placebo | 50 | 44 (88.0) | 6 (12.0) | 42 (84.0) | 8 (16.0) | 2 (4.0) | 1 (2.0) | 14 (28.0) | |
| (B) | Treatment group | Patients, N | Any GI AE, n (%) | Most common GI AEs, n (%) b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trial (background regimen) | Nausea | Diarrhoea | Constipation | Vomiting | Abdominal distension | Abdominal discomfort | Abdominal pain upper | |||
| PIONEER 1 (diet and exercise) | Oral semaglutide 3 mg | 29 | 6 (20.7) | 2 (6.9) | 1 (3.4) | 0 | 0 | 0 | 1 (3.4) | 1 (3.4) |
| Oral semaglutide 7 mg | 29 | 2 (6.9) | 1 (3.4) | 0 | 0 | 1 (3.4) | 0 | 0 | 0 | |
| Oral semaglutide 14 mg | 28 | 9 (32.1) | 5 (17.9) | 0 | 0 | 1 (3.6) | 3 (10.7) | 2 (7.1) | 1 (3.6) | |
| Placebo | 30 | 4 (13.3) | 0 | 0 | 1 (3.3) | 0 | 0 | 0 | 1 (3.3) | |
| PIONEER 3 (metformin ± SU) | Oral semaglutide 3 mg | 52 | 23 (44.2) | 3 (5.8) | 6 (11.5) | 4 (7.7) | 2 (3.8) | 1 (1.9) | 5 (9.6) | 1 (1.9) |
| Oral semaglutide 7 mg | 52 | 25 (48.1) | 8 (15.4) | 8 (15.4) | 3 (5.8) | 2 (3.8) | 0 | 2 (3.8) | 2 (3.8) | |
| Oral semaglutide 14 mg | 51 | 33 (64.7) | 8 (15.7) | 11 (21.6) | 8 (15.7) | 6 (11.8) | 3 (5.9) | 7 (13.7) | 1 (2.0) | |
| Sitagliptin 100 mg | 52 | 20 (38.5) | 1 (1.9) | 3 (5.8) | 3 (5.8) | 1 (1.9) | 0 | 3 (5.8) | 2 (3.8) | |
| PIONEER 4 (metformin ± SGLT2i) | Oral semaglutide 14 mg | 31 | 14 (45.2) | 1 (3.2) | 0 | 7 (22.6) | 2 (6.5) | 0 | 3 (9.7) | 0 |
| Liraglutide 1.8 mg | 29 | 13 (44.8) | 6 (20.7) | 5 (17.2) | 4 (13.8) | 2 (6.9) | 1 (3.4) | 1 (3.4) | 0 | |
| Placebo | 15 | 4 (26.7) | 0 | 1 (6.7) | 2 (13.3) | 1 (6.7) | 0 | 0 | 2 (13.3) | |
| PIONEER 8 (insulin ± metformin) | Oral semaglutide 3 mg | 49 | 17 (34.7) | 5 (10.2) | 1 (2.0) | 4 (8.2) | 2 (4.1) | 1 (2.0) | 2 (4.1) | 1 (2.0) |
| Oral semaglutide 7 mg | 48 | 29 (60.4) | 8 (16.7) | 5 (10.4) | 10 (20.8) | 3 (6.3) | 3 (6.3) | 6 (12.5) | 3 (6.3) | |
| Oral semaglutide 14 mg | 47 | 27 (57.4) | 9 (19.1) | 7 (14.9) | 4 (8.5) | 6 (12.8) | 1 (2.1) | 2 (4.3) | 5 (10.6) | |
| Placebo | 50 | 21 (42.0) | 3 (6.0) | 7 (14.0) | 4 (8.0) | 2 (4.0) | 1 (2.0) | 1 (2.0) | 1 (2.0) | |
Abbreviations: AE, adverse event; BG, blood glucose; GI, gastrointestinal; n, number of patients with at least one event; N, number of randomized patients; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulphonylurea.
Severe according to the American Diabetes Association classification and/or confirmed by a BG value <3.1 mmol/L (<56 mg/dL), and symptoms consistent with hypoglycaemia.
≥10% in any treatment group in any trial.
The overall incidence of AEs was similar between oral semaglutide 7 mg and 14 mg and active comparators; most were mild or moderate in severity, and <10% and ≤17% of patients receiving oral semaglutide 7 mg and 14 mg, respectively, discontinued their treatment owing to AEs. The proportions of patients receiving oral semaglutide who had serious AEs were generally low and similar to comparators. The most common class of AEs associated with oral semaglutide was gastrointestinal—most commonly constipation (in up to 23% of patients with oral semaglutide 14 mg), diarrhoea (up to 22% of patients with oral semaglutide 14 mg) and nausea (up to 19% of patients with oral semaglutide 14 mg). There was no clustering of AEs in any system organ class.
The incidence of severe (according to the American Diabetes Association definition) or blood‐glucose‐confirmed (<3.1 mmol/L or 56 mg/dL) symptomatic hypoglycaemia was low across treatment arms in PIONEER 1, 3 and 4 (<4% of patients in any treatment group); as anticipated, the incidence was higher in patients receiving background insulin in PIONEER 8 (21% to 27% with oral semaglutide and 28% with placebo; Table S4).
4. DISCUSSION
Subgroups of Japanese patients in PIONEER 1, 3, 4 and 8 had a higher proportion of males (57% to 75% vs. 51% to 54%), a similar baseline HbA1c (7.9% to 8.3% [63 mmol/mol to 68 mmol/mol] vs. 8.0% to 8.3% [64 mmol/mol to 68 mmol/mol]), and a lower body weight (66 kg to 80 kg vs. 86 kg to 94 kg) and BMI (25 to 28 kg/m2 vs. 31 to 33 kg/m2) than the overall populations of each trial. 8 , 10 , 11 , 15 In these exploratory post hoc analyses, ETDs for HbA1c reductions in Japanese patients appeared greater with oral semaglutide 3, 7 and 14 mg compared with placebo, similar between oral semaglutide 7 mg and sitagliptin 100 mg, and greater with oral semaglutide 14 mg compared with sitagliptin and liraglutide 1.8 mg. ETDs between oral semaglutide and comparators appeared generally similar for Japanese subgroups compared with the overall populations in these trials. 8 , 10 , 11 , 15 Compared with the overall trial populations, 8 , 10 , 11 , 15 Japanese patients receiving oral semaglutide had similar (1.0% to 1.2% [11 mmol/mol to 13 mmol/mol] vs. 0.9% to 1.2% [10 mmol/mol to 13 mmol/mol] for oral semaglutide 7 mg) or slightly greater (1.4% to 1.7% [16 mmol/mol to 18 mmol/mol] vs. 1.2% to 1.4% [13 mmol/mol to 15 mmol/mol] for oral semaglutide 14 mg) reductions from baseline in HbA1c at week 26.
Proportional (%) and absolute (kg) body weight reductions from baseline at week 26 appeared slightly less in Japanese patients than in the overall populations for oral semaglutide 7 mg (1.0% to 2.7%/0.6 kg to 2.3 kg vs. 2.3% to 2.9%/2.2 kg to 2.4 kg). 8 , 10 , 11 , 15 Weight loss appeared similar as a proportion of baseline weight for oral semaglutide 14 mg (3.7% to 4.7% vs. 3.4% to 4.8%) in Japanese and global patients, respectively, albeit with smaller absolute reductions from baseline (2.8 kg to 3.1 kg vs. 3.1 kg to 4.4 kg), at 26 weeks. 8 , 10 , 11 , 15 ETDs between oral semaglutide and comparators were generally similar for Japanese subgroups compared with the overall populations in these trials. 8 , 10 , 11 , 15
In the prospective, Japanese PIONEER 9 and 10 trials, week 26 reductions from baseline in HbA1c (1.6% to 1.7% [17 mmol/mol to 18 mmol/mol] and 1.8% to 2.0% [20 mmol/mol to 22 mmol/mol] for oral semaglutide 7 mg and 14 mg) were greater than those for Japanese patients in PIONEER 1, 3, 4 and 8, whereas absolute body weight reductions (1.0 kg to 1.1 kg and 2.2 kg to 2.4 kg for oral semaglutide 7 mg and 14 mg in PIONEER 9 and 10, respectively) were slightly less. 16 , 17 However, it should be noted that PIONEER 9 and 10 were prospective trials, 16 , 17 whereas the current subgroup analyses are retrospective and involve relatively small numbers of Japanese patients in each treatment group per trial.
Possible explanations for differences in HbA1c and absolute body weight reductions observed in Japanese patients receiving oral semaglutide compared with their global counterparts may relate in part to pathophysiological differences of T2D associated with Japanese ethnicity. Greater β‐cell dysfunction, but with less obesity and insulin resistance, are characteristic features of Japanese patients compared with global populations. 18 , 19 , 20 Furthermore, Japanese people may also accumulate visceral fat more easily than Caucasians, 23 potentially leading to greater insulin resistance at a lower BMI. 18 Incretin‐based therapies such as GLP‐1RAs are believed to exert their glucose‐lowering effect in part by preserving β‐cell function, 18 , 19 , 20 and their effect on glucose control may thus be greater in Japanese relative to Caucasian patients. In addition, lower baseline body weight and BMI reported for the Japanese patients compared with global patients in these studies may also be a factor in the lower absolute body weight reductions observed with oral semaglutide and comparators. 8 , 10 , 11 , 15 , 16 , 17 Nevertheless, the present study was exploratory and included low numbers of Japanese patients, and there is a need for further studies to better understand the response to GLP‐1RAs and other glucose‐lowering treatments in Japanese populations.
Safety outcomes for Japanese patients receiving oral semaglutide were generally consistent with those observed in the global populations in the PIONEER 1, 3, 4 and 8 trials, with mild or moderate gastrointestinal AEs representing the most common events. 8 , 10 , 11 , 15 Compared with global populations in the studies, Japanese patients receiving oral semaglutide reported more constipation (eg, 23% vs. 8% for oral semaglutide 14 mg in PIONEER 4 11 ) but less nausea (3% vs. 20% in PIONEER 4 11 ); this may be partly attributable to differences in reporting of AEs between different regions. The proportions of Japanese and global patients who discontinued treatment due to AEs were similar. As with the global populations, there were few events of severe or blood‐glucose‐confirmed hypoglycaemia in Japanese patients in PIONEER 1, 3 and 4, and a higher incidence of such events in PIONEER 8 (as expected in patients receiving insulin). 8 , 10 , 11 , 15 Overall safety findings were consistent with observations in PIONEER 9 and 10 16 , 17 and the known safety profile of GLP‐1RAs 24 (including with liraglutide in PIONEER 4 11 ).
When considering the outcomes for Japanese patients, it should be noted that these were exploratory post hoc subgroup analyses of global trials that were not prespecified in the study designs and included relatively few Japanese patients; thus, statistical comparisons were not powered to show treatment differences within the Japanese subpopulations. The results should thus be regarded as indicative only.
In conclusion, oral semaglutide appears efficacious and well tolerated in Japanese patients across the T2D spectrum.
CONFLICT OF INTEREST
E.A. has received honoraria for lectures from AstraZeneca, Daiichi Sankyo, Kowa Company, Merck Sharp and Dohme (MSD), Novo Nordisk Pharma, Ono Pharmaceutical, Sanofi and Sumitomo Dainippon Pharma, and scholarship grants from Astellas Pharma, Bayer, Daiichi Sankyo, Eli Lilly Japan, Kowa Company, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Novartis Pharma, Novo Nordisk Pharma, Sanofi, Sumitomo Dainippon Pharma and Takeda Pharmaceutical. Y.T. reports receiving honoraria for serving on advisory boards for MSD, Boehringer Ingelheim, Tanabe‐Mitsubishi, Daiichi Sankyo, Novo Nordisk, Eli Lilly, Sanofi, Astellas Pharma, AstraZeneca and Teijin, speaker's fees from MSD, Ono, Boehringer Ingelheim, Takeda, Tanabe‐Mitsubishi, Daiichi Sankyo, Sanwa Kagaku Kenkyusho, Novo Nordisk, Eli Lilly, Sanofi, Dainippon‐Sumitomo, Shionogi, Bayer Yakuhin, Astellas and AstraZeneca, and research support from MSD, Ono, Boehringer Ingelheim, Novartis, Takeda, Daiichi Sankyo, Sanwa Kagaku Kenkyusho, Novo Nordisk, Eli Lilly, Sanofi, Dainippon‐Sumitomo, Shionogi, Astellas Pharma and AstraZeneca. H.W. reports receiving honoraria (eg, lecture fees) from Mitsubishi Tanabe Pharma, Sumitomo Dainippon Pharma, Sanwa Kagaku Kenkyusho, Takeda, Sanofi, Kowa, MSD, Boehringer Ingelheim, Novo Nordisk and Eli Lilly, research support from Takeda, Boehringer Ingelheim, Kissei Pharma, Novo Nordisk, Mitsubishi Tanabe Pharma, Lifescan Japan, Dainippon‐Sumitomo, Kyowa‐Kirin and MSD, and endowed courses supported by Boehringer Ingelheim, Kowa, MSD, Mitsubishi Tanabe Pharma, Ono Pharmaceutical, Sanwa Kagaku Kenkyusho, Soiken, Takeda and Dainippon Sumitomo Pharma. S.D., E.C., and H.H. are employees of Novo Nordisk. E.C. holds shares in Novo Nordisk. T.K. reports receiving honoraria (eg, lecture fees) from MSD Corporation, Daiichi Sankyo Co., Ltd, Takeda Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Kowa Pharmaceutical Co., Ltd, Astellas Pharma Inc., Ono Pharmaceutical Co., Ltd, AstraZeneca K.K., Sumitomo Dainippon Pharma Co., Ltd, Sanofi K.K., Eli Lilly Japan K.K., Nippon Boehringer Ingelheim Co., Ltd, Sanwa Kagaku Kenkyusho Co., Ltd, Kyowa Hakko Kirin Co., Ltd, Taisho Pharmaceutical Co., Ltd and Novo Nordisk Pharma Ltd, research funding (endowed departments by commercial entities) from Asahi Mutual Life Insurance Company, Takeda Pharmaceutical Co., Ltd, TERUMO Corporation, MSD Corporation, Novo Nordisk Pharma Ltd and Nippon Boehringer Ingelheim Co., Ltd, and scholarship donation from MSD Corporation, Daiichi Sankyo Co., Ltd, Novo Nordisk Pharma Ltd, Sanofi K.K., Takeda Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceutical Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Eli Lilly Japan K.K. and Kyowa Hakko Kirin Co., Ltd.
AUTHOR CONTRIBUTIONS
Conception and design of the study: Srikanth Deenadayalan and Erik Christiansen. Generation, collection, assembly, analysis and/or interpretation of data: Hiroshi Horio performed the generation, collection and analysis of the data, and all authors were involved in interpreting the data. Drafting and revision of the manuscript: all authors. Approval of the final version of the manuscript: all authors.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14536.
Supporting information
Table S1 Background insulin regimens for Japanese patients in PIONEER 8
Table S2 Proportions of Japanese patients in PIONEER 1, 3, 4 and 8 achieving HbA1c <7.0%
Table S3 Proportions of Japanese patients in PIONEER 1, 3, 4 and 8 achieving body weight loss ≥5%
Table S4 Proportion of Japanese patients with hypoglycaemic episodes in PIONEER 8
Figure S1 Estimated treatment differences for change from baseline in HbA1c (%) in Japanese patients in PIONEER 1, 3, 4 and 8
Figure S2 Change from baseline in body weight (kg) (A) and estimated treatment differences (B) for Japanese patients in PIONEER 1, 3, 4 and 8
Figure S3 Estimated treatment differences for change from baseline in body weight (%) in Japanese patients in PIONEER 1, 3, 4 and 8
ACKNOWLEDGEMENTS
The trials discussed herein were funded by Novo Nordisk A/S, Denmark. We gratefully thank the patients taking part in these trials, the investigators, all trial site staff and all Novo Nordisk employees involved in the trials. In addition, we would like to thank Stephen Purver of Axis, a division of Spirit Medical Communications Group Limited, for medical writing and editorial assistance (funded by Novo Nordisk A/S), and Yuiko Yamamoto of Novo Nordisk for reviewing the manuscript.
Araki E, Terauchi Y, Watada H, et al. Efficacy and safety of oral semaglutide in Japanese patients with type 2 diabetes: A post hoc subgroup analysis of the PIONEER 1, 3, 4 and 8 trials. Diabetes Obes Metab. 2021;23(12):2785‐2794. doi: 10.1111/dom.14536
Funding information Novo Nordisk A/S, Denmark.
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request. Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. Access request proposals can be found at novonordisk‐trials.com. Data will be made available after research completion, and approval of the product and product use in the European Union and the USA. Individual participant data will be shared in data sets in a de‐identified/anonymized format.
REFERENCES
- 1. Buckley ST, Bækdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon‐like peptide‐1 receptor agonist. Sci Transl Med. 2018;10(467):eaar7047. 10.1126/scitranslmed.aar7047 [DOI] [PubMed] [Google Scholar]
- 2. Pharmaceutical and Medical Devices Agency, Japan. Rybelsus® prescribing information 2020. https://www.pmda.go.jp/files/000208192.pdf. Accessed 14 September 2021.
- 3. European Medicines Agency. Rybelsus® summary of product characteristics 2020. https://www.ema.europa.eu/en/documents/product-information/rybelsus-epar-product-information_en.pdf. Accessed 13 September 2021.
- 4. US Food and Drug Administration. Rybelsus® prescribing information 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf. Accessed 13 September 2021.
- 5. Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int. 2020;11(3):165‐223. 10.1007/s13340-020-00439-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61(12):2461‐2498. 10.1007/s00125-018-4729-5 [DOI] [PubMed] [Google Scholar]
- 7. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487‐493. 10.2337/dci19-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial comparing the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724‐1732. 10.2337/dc19-0749 [DOI] [PubMed] [Google Scholar]
- 9. Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42(12):2272‐2281. 10.2337/dc19-0883 [DOI] [PubMed] [Google Scholar]
- 10. Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321:1466‐1480. 10.1001/jama.2019.2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double‐blind, phase 3a trial. Lancet. 2019;394(10192):39‐50. 10.1016/S0140-6736(19)31271-1 [DOI] [PubMed] [Google Scholar]
- 12. Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo‐controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):515‐527. 10.1016/S2213-8587(19)30192-5 [DOI] [PubMed] [Google Scholar]
- 13. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841‐851. 10.1056/NEJMoa1901118 [DOI] [PubMed] [Google Scholar]
- 14. Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open‐label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):528‐539. 10.1016/S2213-8587(19)30194-9 [DOI] [PubMed] [Google Scholar]
- 15. Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019;42(12):2262‐2271. 10.2337/dc19-0898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamada Y, Katagiri H, Hamamoto Y, et al. Dose‐response, efficacy, and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): a 52‐week, phase 2/3a, randomised, controlled trial. Lancet Diabetes Endocrinol. 2020;8(5):377‐391. 10.1016/S2213-8587(20)30075-9 [DOI] [PubMed] [Google Scholar]
- 17. Yabe D, Nakamura J, Kaneto H, et al. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open‐label, randomised, active‐controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2020;8(5):392‐406. 10.1016/S2213-8587(20)30074-7 [DOI] [PubMed] [Google Scholar]
- 18. Yabe D, Seino Y, Fukushima M, Seino S. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015;15(6):602. 10.1007/s11892-015-0602-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yabe D, Seino Y. Type 2 diabetes via β‐cell dysfunction in East Asian people. Lancet Diabetes Endocrinol. 2016;4(1):2‐3. 10.1016/S2213-8587(15)00389-7 [DOI] [PubMed] [Google Scholar]
- 20. Seino Y, Kuwata H, Yabe D. Incretin‐based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7(suppl 1):102‐109. 10.1111/jdi.12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aroda VR, Saugstrup T, Buse JB, Donsmark M, Zacho J, Davies MJ. Incorporating and interpreting regulatory guidance on estimands in diabetes clinical trials: the PIONEER 1 randomized clinical trial as an example. Diabetes Obes Metab. 2019;21(10):2203‐2210. 10.1111/dom.13804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Little RJA, Rubin DB. Statistical analysis with missing data. New York: John Wiley and Sons, Inc.; 1987. [Google Scholar]
- 23. Tanaka S, Horimai C, Katsukawa F. Ethnic differences in abdominal visceral fat accumulation between Japanese, African‐Americans, and Caucasians: a meta‐analysis. Acta Diabetol. 2003;40(suppl 1):S302‐S304. 10.1007/s00592-003-0093-z [DOI] [PubMed] [Google Scholar]
- 24. Htike ZZ, Zaccardi F, Papamargaritis D, et al. Efficacy and safety of glucagon‐like peptide‐1 receptor agonists in type 2 diabetes: a systematic review and mixed‐treatment comparison analysis. Diabetes Obes Metab. 2017;19(4):524‐536. 10.1111/dom.12849 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Background insulin regimens for Japanese patients in PIONEER 8
Table S2 Proportions of Japanese patients in PIONEER 1, 3, 4 and 8 achieving HbA1c <7.0%
Table S3 Proportions of Japanese patients in PIONEER 1, 3, 4 and 8 achieving body weight loss ≥5%
Table S4 Proportion of Japanese patients with hypoglycaemic episodes in PIONEER 8
Figure S1 Estimated treatment differences for change from baseline in HbA1c (%) in Japanese patients in PIONEER 1, 3, 4 and 8
Figure S2 Change from baseline in body weight (kg) (A) and estimated treatment differences (B) for Japanese patients in PIONEER 1, 3, 4 and 8
Figure S3 Estimated treatment differences for change from baseline in body weight (%) in Japanese patients in PIONEER 1, 3, 4 and 8
Data Availability Statement
Data are available upon reasonable request. Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. Access request proposals can be found at novonordisk‐trials.com. Data will be made available after research completion, and approval of the product and product use in the European Union and the USA. Individual participant data will be shared in data sets in a de‐identified/anonymized format.


