Abstract
Background
Dementia is prevalent and underdiagnosed in the dialysis population. We aimed to develop and validate a simple dialysis dementia scoring system to facilitate identification of individuals who are at high risk for dementia.
Methods
We applied a retrospective, nested case‐control study design using a national dialysis cohort derived from the National Health Insurance Research Database in Taiwan. Patients aged between 40 and 80 years were included and 2940 patients with incident dementia were matched to 29,248 non‐dementia controls. All subjects were randomly divided into the derivation and validation sets with a ratio of 4:1. Conditional logistic regression models were used to identify factors contributing to the risk score. The cutoff value of the risk score was determined by Youden's J statistic and the graphic method.
Results
The dialysis dementia risk score (DDRS) finally included age and 10 comorbidities as risk predictors. The C‐statistic of the model was 0.71 (95% confidence interval [CI] 0.70–0.72). Calibration revealed a strong linear relationship between predicted and observed dementia risk (R 2 = 0.99). At a cutoff value of 50 points, the high‐risk patients had an approximately three‐fold increased risk of having dementia compared to those with low risk (odds ratio [OR] 3.03, 95% CI 2.78–3.31). The DDRS performance, including discrimination (C‐statistic 0.71, 95% CI 0.69–0.73) and calibration (p value of Hosmer−Lemeshow test for goodness of fit = 0.18), was acceptable during validation. The OR value (2.82, 95% CI 2.37–3.35) was similar to those in the derivation set.
Conclusion
The DDRS system has the potential to serve as an easily accessible screening tool to determine the high‐risk groups who deserve subsequent neurological evaluation in daily clinical practice.
Keywords: dementia, dialysis, nested case‐control study, risk prediction model, screening
To construct a prediction tool for dementia tailored for dialysis patients, we conducted a retrospective, nested case‐control study and matched 2940 patients with incident dementia with 29,248 non‐dementia controls. All subjects were divided into the derivation and validation sets in a 4:1 ratio. The dialysis dementia risk score (DDRS) contains age and 10 comorbidities as predictors and the C‐statistic was 0.71 (95% CI 0.70–0.72) with good calibration (R 2 = 0.99) in the derivation set. The performance was similar in the validation set. Patients with DDRS ≥ 50 points had an approximately three‐fold increased risk of having dementia compared to those with DDRS < 50 points.

INTRODUCTION
Dementia is a syndrome characterized by impairment in various cognitive domains, leading to decline in independence and daily functions [1]. It is estimated that currently approximately 50 million people have dementia worldwide, and the number is projected to rise to 130 million by 2050 [2], becoming a huge burden on caregivers and the healthcare system [3, 4]. Since the kidney and brain have similar microvascular structures and share many common risk factors for vascular injury, patients with chronic kidney disease (CKD) are susceptible to a wide range of neurological disorders, including dementia [5, 6, 7]. In addition, kidney failure itself has been proven to be one of the etiologies for developing dementia [5]. Therefore, it is not surprising that moderate to severe cognitive impairment is highly prevalent in the dialysis population as compared to an age‐matched population [8]. Because the dialysis procedures and the treatments for concomitant comorbidities have already consumed substantial healthcare expenditure, the presence of dementia in dialysis patients will lead to loss of productivity and further stress the social and healthcare systems [9, 10, 11].
Most nephrologists and dialysis staff are not trained to recognize cognitive changes in patients with CKD/kidney failure, and thus cognitive impairment and dementia are likely to be underdiagnosed in this population [8, 12, 13]. Recognizing dementia is pivotal to improving clinical care, including tailored communication and education, evaluating self‐care ability, medical decisions, management of behavioral and psychological symptoms, providing mental and social support for caregivers, and setting goal of care and end‐of‐life care planning according to the associated morbidities and mortality rates [12, 14, 15]. The construction of a simple screening tool to identify dialysis patients probably with dementia on the basis of obtainable clinical parameters will alert primary care physicians to detect these patients, especially when these parameters are successfully incorporated into the hospital information system. Direct application of the dementia scoring systems which have been developed primarily for the general population [16, 17, 18] in the dialysis population might not be practical because the weights of potential indicators are quite different from those in the general population [5]. This study was aimed at developing and validating an easy‐to‐use scoring system that incorporates predictors readily available in general practice that can be used to stratify dialysis patients into low‐ and high‐risk groups and determine the necessity for referral for further neurocognitive tests/imaging studies.
METHODS
Data source
The data in this study were retrieved from the National Health Insurance Research Database (NHIRD) in Taiwan. The National Health Insurance (NHI) program is a nationwide healthcare insurance program. It provides nearly every type of medical service for beneficiaries and covers more than 99% of Taiwan's entire population (more than 25 million residents) [19]. The detailed information about these claim data is then maintained in the NHIRD after linking with each individual's demographic profile (birth date, sex, place of residence). The diagnoses in this database are defined based on the International Classification of Disease, Ninth Edition (ICD‐9) codes. To avoid claim errors, the National Health Insurance Administration performs random inspections of the claim data, and medical institutions are fined heavily if these data are not found to be compatible with the diagnoses. This procedure undoubtedly ensures the accuracy of the diagnoses in the NHIRD and many of the major illnesses, such as stroke, diabetes, hypertension, and hyperlipidemia, have been validated to prove accuracy [20]. This study was conducted after approval by the Institutional Review Board (IRB) of the National Cheng Kung University Hospital (B‐EX‐108‐024). Since personal identification information is encrypted before releasing the data to researchers, informed consent was able to be waived from the IRB of the institute.
Identification of the study population and study design
A retrospective, population‐based, nested case‐control study design was applied in this study. The study population was derived from a specific cohort consisting of all patients with kidney failure registered in the Catastrophic Illness Certificate Database who had initiated dialysis therapy for more than three consecutive months during the period from 1 January 1998, to 31 December 2010. Any patient with kidney failure is certificated for catastrophic illness if kidney failure is regarded to be in an irreversible status. Before approval, this process is reviewed by expert nephrologists according to the relevant medical information, including underlying etiologies attributable to kidney disease, indications of initiating dialysis, laboratory data, and sonographic reports, to evaluate the necessity of long‐term dialysis. In addition, patients with kidney failure certificated for catastrophic illnesses can be waived from copayment when accessing medical services. These two processes not only improve diagnostic accuracy but also facilitate the adherence of these patients to the NHI program.
By taking the incidence of dementia as the outcome variable, we first excluded individuals with missing or extreme age or gender values, inconsistent death dates, dementia diagnosed before identification of kidney failure, or renal transplantation before the initiation of maintenance dialysis therapy to construct the retrospective dialysis cohort (Figure 1). To estimate the effects of potential covariates on the risk of dementia, a nested case‐control design was applied in this study. Incident dementia was defined as the diagnosis (Alzheimer's disease [ICD‐9: 331.0], vascular [ICD‐9: 290.4], or unspecified dementia [ICD‐9: 290.0–290.3, 294.1, 331.1, and 331.2]) recorded once or more during inpatient care or twice or more during ambulatory care with a minimum interval of >30 days within 1 year. Any patient defined as having incident dementia during the follow‐up period after enrollment was selected as a case subject. For each case subject, other individuals without any diagnosis of dementia on the date of incident dementia of the case subject were classified as candidates for the control group. In other words, the date of incident dementia was the index date for each case and the corresponding control. Up to 10 control subjects for each case were randomly selected using the incidence‐density sampling method (Figure S1). In addition, the incidence of dementia increased incrementally after the age of 40 years in our study population, and a previous study suggested that patients aged 80 years or older qualify for cognitive screening because age alone is enough to result in an increased risk of dementia. We therefore confined our study population to those aged between 40 and 80 years [5, 16]. With a control‐to‐case ratio of 10, we selected 29,248 non‐dementia controls for the 2940 patients with incident dementia. All the case subjects with their corresponding controls were randomly allocated into the derivation and validation sets at a 4:1 ratio. The derivation set was used to generate a prediction model, and the validation set was used for validation of the established prediction model.
FIGURE 1.
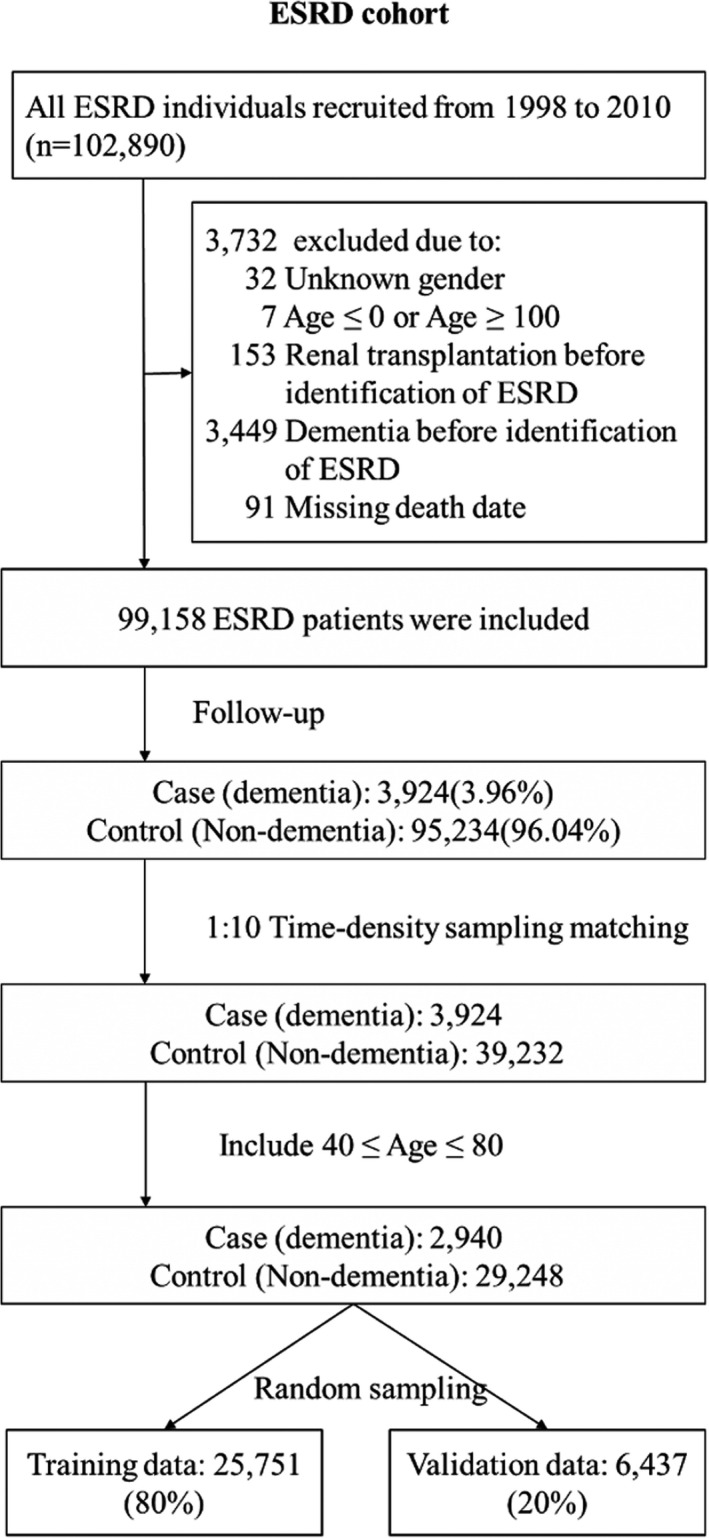
Flow chart of the construction of the dialysis cohort for developing the dialysis dementia risk scoring system
Model construction used to estimate the DDRS
We selected age, sex, dialysis vintage, and various comorbidities listed in Table 1, which have been proven to be risk factors for dementia in previous studies as candidates for predictors [21, 22, 23]. Age was calculated by the difference between the birthday and the index date. Dialysis vintage was calculated from the date at which maintenance dialysis therapy was initiated to the index date of the case and control subjects. An individual was considered to have the selected comorbidity if he or she had these diagnosis ICD‐9 codes at least once during inpatient care or twice during ambulatory care 30 days apart within 1 year before the index date (Table S1).
TABLE 1.
Clinical characteristics of the dementia and control subjects used to develop the dialysis dementia risk scoring system
| Variable | Derivation data | Validation data | ||||
|---|---|---|---|---|---|---|
| Dementia patients (n = 2352) | Control subjects (n = 23,399) | P value | Dementia patients (n = 588) | Control subjects (n = 5849) | P value | |
| Age, mean (SD) | 69.87 (7.79) | 69.74 (7.89) | 0.4552 | 69.85 (7.98) | 69.54 (8.02) | 0.3678 |
| Sex, male n (%) | 986 (41.92) | 10,325 (44.13) | 0.0401 | 244 (41.50) | 2,541 (43.44) | 0.3638 |
| Dialysis vintage (year), mean (SD) | 2.74 (2.44) | 3.68 (2.72) | <0.0001 | 2.79 (2.58) | 3.67 (2.67) | <0.0001 |
| Comorbidities, n (%) | ||||||
| Diabetes mellitus | 1705 (72.49) | 13,084 (55.92) | <0.0001 | 435 (73.98) | 3,254 (55.63) | <0.0001 |
| Stroke | 1235 (52.51) | 6,854 (29.29) | <0.0001 | 303 (51.53) | 1,718 (29.37) | <0.0001 |
| Anemia | 1775 (75.47) | 17,110 (73.12) | 0.0142 | 452 (76.87) | 4,282 (73.21) | 0.0550 |
| Heart failure | 1329 (56.51) | 11,848 (50.63) | <0.0001 | 311 (52.89) | 2,975 (50.86) | 0.3484 |
| Hypertension | 2265 (96.30) | 21,691 (92.70) | <0.0001 | 567 (96.43) | 5,403 (92.37) | 0.0003 |
| Hyperlipidemia | 576 (24.49) | 4834 (20.66) | <0.0001 | 141 (23.98) | 1,174 (20.07) | 0.0251 |
| Coronary artery disease | 1510 (64.20) | 13,447 (57.47) | <0.0001 | 363 (61.73) | 3,376 (57.72) | 0.0600 |
| Peripheral vascular disease | 740 (31.46) | 7181 (30.69) | 0.4386 | 175 (29.76) | 1,741 (29.77) | 0.9984 |
| Depression | 633 (26.91) | 2548 (10.89) | <0.0001 | 150 (25.51) | 645 (11.03) | <0.0001 |
| Obstructive sleep apnea | 22 (0.94) | 228 (0.97) | 0.8540 | 11 (1.87) | 61 (1.04) | 0.0688 |
| Insomnia | 1169 (49.70) | 9189 (39.27) | <0.0001 | 274 (46.60) | 2,316 (39.6) | 0.0010 |
| Alcoholism | 30 (1.28) | 312 (1.33) | 0.8152 | 17 (2.89) | 78 (1.33) | 0.0028 |
| Traumatic brain injury | 364 (15.48) | 2344 (10.02) | <0.0001 | 91 (15.48) | 588 (10.05) | <0.0001 |
| Parkinson's disease | 403 (17.13) | 1220 (5.21) | <0.0001 | 93 (15.82) | 314 (5.37) | <0.0001 |
| Myocardial infarction | 338 (14.37) | 2790 (11.92) | 0.0005 | 80 (13.61) | 720 (12.31) | 0.3640 |
| Atrial fibrillation | 289 (12.29) | 2732 (11.68) | 0.3795 | 69 (11.73) | 697 (11.92) | 0.8967 |
| Hyperthyroidism | 50 (2.13) | 501 (2.14) | 0.9611 | 9 (1.53) | 114 (1.95) | 0.4799 |
| Hypothyroidism | 92 (3.91) | 632 (2.70) | 0.0007 | 24 (4.08) | 159 (2.72) | 0.0580 |
Abbreviation: SD, standard deviation.
The variables significantly associated with risk for dementia in the univariate conditional logistic regression analysis (p value < 0.05) in the derivation set were selected to construct the final multivariate conditional logistic regression model. Since hemodialysis (HD) and peritoneal dialysis (PD) conferred a similar risk of dementia in our study and others, dialysis modality was not selected as one of the parameters in the final model [24]. A score for each variable was then created by dividing the regression coefficient (i.e., β) for each variable in the final model by the β of age and rounding to the nearest integer. A total score to predict risk of dementia was calculated from adding the scores of all variables. To examine the linear relationship between age and risk of dementia, we constructed a multivariate logistic regression model treating age as an ordered categorical variable (Figure S2). The results of the trend test revealed a significantly linear relationship between age and risk of dementia (p value < 0.0001), which supported the inclusion of age as a continuous variable in the model.
Discrimination, calibration, and validation of the DDRS
The C‐statistic was used as the indicator of discrimination for the predictive risk‐scoring system. Both Youden's J statistic and the graphic method were applied to facilitate the determination of the cutoff point used to differentiate between the low‐ and high‐risk groups. Using this cutoff point, the sensitivity and specificity were calculated accordingly. For assessing the calibration, we first divided the study population into 10 risk groups according to their predicted risks of dementia, and then these data were plotted against their corresponding observed risks for dementia in each group. Finally, validation of the scoring algorithm was performed using the data in the validation set. Discrimination and calibration were evaluated in the same manner as that used for the derivation set.
Statistical analysis
Continuous variables were compared using the Student's t test, and comparisons of differences between categorical variables were analyzed using the Chi‐square test or Fisher's exact test. The potential problem of multicollinearity between independent variables was evaluated based on the variance inflation factors. While sensitivity analyses were conducted, between‐model differences in the C‐statistic values were compared using DeLong's test. All statistical analyses were performed using SAS, version 9.4 (SAS Institute). A p value <0.05 was considered to be statistically significant.
RESULTS
Baseline characteristics of the national dialysis cohort
The initial distribution of HD and PD in this national dialysis cohort was 93.72% and 6.28% and it finally shifted to 98.33% and 1.67%, respectively, after the matching process. Table 1 shows the differences in clinical characteristics between the case (n = 2940) and control subjects (n = 29,248) in the derivation and validation sets. Among the study subjects in the derivation set, the dementia patients had a higher prevalence of the comorbidities listed in Table 1, with the exception of peripheral vascular disease, obstructive sleep apnea, alcoholism, atrial fibrillation, and hyperthyroidism. Additionally, the dementia patients were slightly female‐dominant and had a shorter dialysis vintage.
Construction, discrimination, and calibration of the DDRS model
After selection of the covariates significantly associated with risk of dementia in the univariate conditional logistic regression model, only age and 10 comorbidities were retained in the final multivariate conditional logistic regression model (Table 2). Table 2 shows the odds ratio (OR) associated with each predictor as well as the points assigned to it. Age was assigned as 0 points at age 40 years and increased one point per year up to 40 points at age 80 years. After conversion of the coefficient ratios to points, the point values could be interpreted as an increased risk of being older by that number of years. For instance, a 40‐year‐old patient with diabetes has an equivalent risk of dementia to that of a 47‐year‐old individual without any of the comorbidities listed in Table 2. Parkinson's disease, depression, and stroke were the top three highest‐risk comorbidities for dementia in descending order, and anemia and hyperlipidemia were the lowest two, which were only assigned one point. Therefore, the range of DDRS could range from 0 (age 40 years without any other predictor) to 107 points (age 80 years with all the other predictors).
TABLE 2.
Predictors for risk of dementia with associated odds ratios and derived risk scores from a national dialysis cohort
| Predictor | β coefficient | aOR (95% CI) | P value | Points |
|---|---|---|---|---|
| Age | 0.06 | 1.06 (1.05–1.08) | <0.0001 | Age (years) −40 |
| Comorbid conditions | ||||
| Diabetes mellitus | 0.41 | 1.50 (1.38–1.64) | <0.0001 | 7 |
| Stroke | 0.74 | 2.09 (1.93–2.26) | <0.0001 | 12 |
| Anemia | 0.06 | 1.07 (0.98–1.17) | 0.1593 | 1 |
| Hypertension | 0.18 | 1.19 (0.99–1.44) | 0.0715 | 3 |
| Hyperlipidemia | 0.05 | 1.06 (0.96–1.16) | 0.2571 | 1 |
| Depression | 0.80 | 2.23 (2.02–2.45) | <0.0001 | 13 |
| Insomnia | 0.20 | 1.23 (1.13–1.33) | <0.0001 | 3 |
| Traumatic brain injury | 0.35 | 1.42 (1.28–1.58) | <0.0001 | 6 |
| Parkinson's disease | 1.05 | 2.87 (2.55–3.22) | <0.0001 | 17 |
| Hypothyroidism | 0.24 | 1.27 (1.03–1.56) | 0.0266 | 4 |
| C‐statistic | 0.71 (0.70–0.72) | |||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
Discrimination of the final model based on the C‐statistic (95% confidence interval [CI]) was acceptable in this study: 0.71 (95% CI 0.70–0.72) [25]. Calibration of this final model also showed a strong linear relationship between predicted and actual dementia risk (R 2 = 0.99; Figure 2). When setting 50 points as the cutoff value determined by Youden's J statistic and the graphic method (Figure 3), we could stratify dialysis patients into low‐risk and high‐risk groups (Table 3) with a sensitivity of 63.48% (95% CI 61.53%–65.42%), a specificity of 64.13% (95% CI 63.15%–65.10%), and an OR of 3.03 (95% CI 2.78–3.31), respectively.
FIGURE 2.
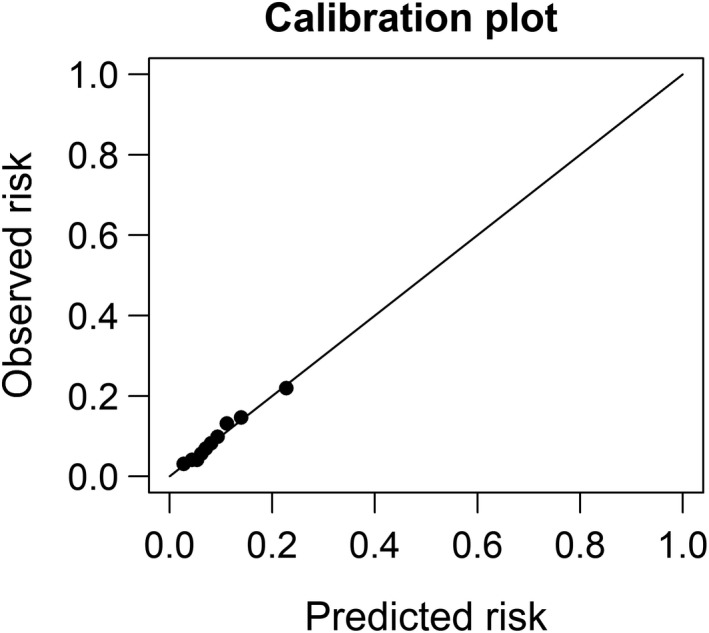
The relationship between predicted and observed risk of dementia in a national dialysis cohort
FIGURE 3.
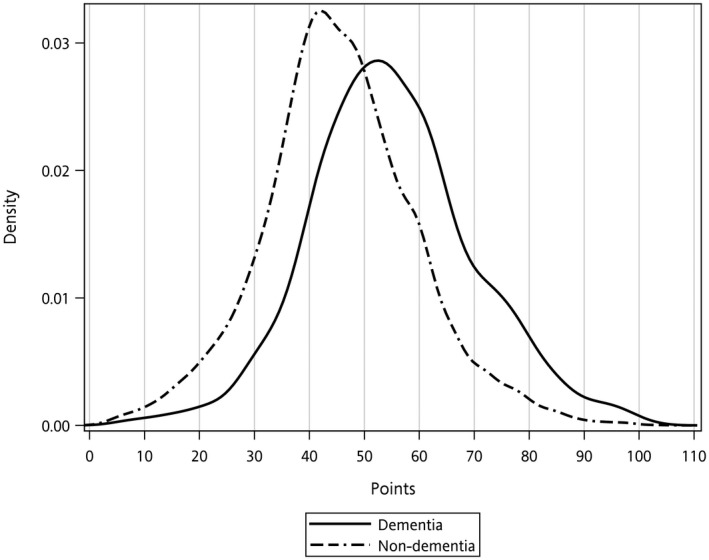
The distribution of dementia risk score points for dialysis patients with and without dementia in the national dialysis cohort
TABLE 3.
Sensitivity and specificity of the dementia risk scores among dialysis patients when applied to high‐ and low‐risk groups
| Optimal cutoff value | Derivation data | Validation data | ||||||
|---|---|---|---|---|---|---|---|---|
| Risk | With dementia | Without dementia | Total | Risk | With dementia | Without dementia | Total | |
| 50 | High | 1493 | 8394 | 9887 | High | 356 | 2063 | 2419 |
| Low | 859 | 15,005 | 15,864 | Low | 232 | 3786 | 4018 | |
| Total | 2352 | 23,399 | 25,751 | Total | 588 | 5849 | 6437 | |
| Sensitivity | 63.48% (95% CI 61.53%–65.42%) | 60.54% (95% CI 56.59%–64.49%) | ||||||
| Specificity | 64.13% (95% CI 63.15%–65.10%) | 64.73% (95% CI 62.77%–66.68%) | ||||||
| Odds ratio | 3.03 (2.78–3.31) | 2.82 (2.37–3.35) | ||||||
Abbreviation: CI, confidence interval.
Validation of the DDRS system
When applied to the validation set, the DDRS model also had acceptable discrimination (C‐statistic 0.71, 95% Cl 0.69–0.73) and good calibration (p value of the Hosmer−Lemeshow test for goodness of fit = 0.180). The sensitivity (60.54% [95% CI 56.59%–64.50%]), specificity (64.73% [95% CI 62.77%–66.68%]), and OR (2.82, 95% CI 2.37–3.35) were similar to those in the derivation set.
Sensitivity analysis
To validate whether the proposed DDRS model was specific for predicting dementia, we performed a sensitivity analysis by applying it to another two illnesses, lung cancer (ICD‐9 codes: 162−163) and hepatocellular carcinoma (ICD‐9 code: 155), which are unrelated to dementia. The C‐statistics for the lung cancer and hepatocellular carcinoma models were all 0.63, and the C‐statistics between these two models and the DDRS model were statistically different (all p values < 0.05). The results of the sensitivity analyses supported the specificity of the DDRS model.
DISCUSSION
Through the retrospective nested case‐control study design, we developed and validated a simple risk scoring system for dementia screening in a dialysis population. To the best of our knowledge, this scoring system is the first one to be specifically designed for identifying dementia patients in the dialysis population. The validation results revealed that the model was robust with satisfactory discrimination and calibration. The two‐step strategy is now adopted for dementia diagnosis worldwide, and is recommended to be integrated in clinical practice in guidelines [26]. The first step is to provide an initial brief screening, usually by a non‐specialist. The second step is to perform a subsequent comprehensive diagnostic workup for those identified as high‐risk patients by dementia specialists. Such a strategy is proven to increase new diagnoses of dementia by a factor of two to three [27] and might be particularly useful in populations with a high prevalence of dementia, such as the dialysis population. Due to the nature of low cost, no labor‐consuming and automatically continuous update, DDRS has the potential to be the choice for a first‐step screening tool when it is integrated into the hospital information system. Despite its relative low sensitivity and specificity for identifying dementia patients, DDRS might serve as a prototype model which could be further improved by including more parameters (clinical features and/or biomarkers), just as the evolution of CHADS2 to CHAD2DS2‐VASc. In addition, the change of the cutoff value to accommodate the clinical service capacity is an alternative way to apply this scoring system (Figure S3). More research is still warranted to evaluate cost‐effectiveness, potential benefits and harms, and acceptability to patients and clinicians before clinical implementation.
Cerebrovascular disease and CKD have considerably overlapping risk factors, and microvascular disease is undoubtedly a major contributing factor to cognitive impairment in the CKD population. In addition to these traditional vascular risk factors, nephrogenic (albuminuria, anemia, uremia, inflammation, oxidative stress, vascular calcification, and impaired amyloid clearance and its associated synaptopathy [28, 29]) and dialysis‐associated risk factors (intradialytic hypotension and hyperviscosity, etc.) may also play roles in pathogenesis of cognitive impairment [13]. A screening tool or scoring system for dementia in the end‐stage renal disease population therefore should be tailored to these patients.
To the best of our knowledge, the DDRS system proposed in this work is the first one specifically designed to quantify dementia risk in a dialysis population. Therefore, we are unable to compare the performance of our model with others. There are screening models developed for dementia risk prediction in the general population [30, 31]. The performances of these models differed in the key parameters they used, including cognitive tests, health and vascular risk indices, multifactorial models (combining demographic and neuropsychological measures with health or genetic variables), multistage screening, or multi‐dementia subtype preclinical grouping [30, 31]. In general, their C‐statistics, sensitivities, and specificities varied in the ranges 0.49–0.91, 20%–93%, and 35.7%–94%, respectively, which are comparable with our results [30, 31]. In addition, the C‐statistic of our model (0.71) is also comparable with other widely used risk indices, including the Dementia Screening Indicator (0.68–0.78) [16], ARO all‐cause mortality risk score for dialysis patients (0.68–0.79) [32], the CHA2DS2‐VASc (0.66–0.79) [33], and the Framingham coronary heart disease prediction risk scores (0.79–0.83) [34]. To improve the performance of this model, a prospective regular collection of the 11 components of this scoring system and/or adding other potential candidate parameters might facilitate construction of a better model by using either the mixed‐effect model, generalized estimating equation model, or methods of machine/deep learning.
Low education level is an important risk factor for dementia [16, 18], and previous risk prediction systems applied in general populations always include education level as one of their predictors [16, 18, 31]. To clarify the relationship between education level and the 11 predictors in the DDRS model, we constructed a multivariate logistic regression model using a local dialysis patient database (n = 293) and treated education level (> or ≤6 years) as the dependent variable and the 11 predictors as independent variables. The C‐statistic of the logistic regression model was 0.85, which indicated that the combination of age and 10 comorbidities could serve as good surrogate markers of education level. Thus, absence of education level in our model might not be a major limitation of our study. Since there are complex pathways between education and dementia, there might be variance in dementia that is explained by education but not explained by comorbidities (e.g. through cognitive reserve). More research is still needed to evaluate whether the inclusion of education in our model can improve the performance of prediction or not.
The proposed DDRS model, in which only age is considered as unmodifiable, may lay the ground for initiating specific intervention studies aimed at reducing the risk of dementia in the dialysis population. In the general population, clinical evidence suggests that dementia risk or declines in cognitive function can indeed be ameliorated through therapeutic and preventive interventions in some of the comorbidities included in our DDRS system [35]. Although poor glucose control has been proven to be associated with greater declines in cognitive function [36], evidence from randomized trials, including ACCORD MIND and ACCORDION MIND, revealed that intensive glucose control does not ameliorate declines in cognitive function [37, 38]. However, some small pilot studies showed that several specific medications or formulas could better preserve cognitive function or brain volume, or improve disease biomarker levels [39, 40, 41]. The SPRINT MIND study revealed that in adults with hypertension, treating systolic blood pressure <120 mm Hg as compared with <140 mm Hg is associated with lower risk of mild cognitive impairment, with no significant interaction with CKD status [42]. Since the SPRINT MIND study did not enroll patients with an estimated glomerular filtration rate of less than 20 mL/min/1.73 m2, caution is required in the application of the study results to dialysis patients. Depression and hypothyroidism are known to be associated with increased risk of dementia and are regarded as reversible illnesses contributing to dementia [43].
The key strength of this study is the application of a nationwide database to derive the predictors for risk prediction. The nature of the reimbursement data‐originated database leads to very few missing data in the NHIRD, as shown in our study (Figure 1). The high coverage, high accessibility, and low copayment results in high adherence of beneficiaries to the NHI program, which greatly minimizes potential selection and information biases.
Several limitations should also be addressed. First, some risk factors for dementia, such as low educational attainment, obesity, physical inactivity, social isolation, and uremic specific risk factors, such as dialysis adequacy and uremic toxins, are not included in the NHIRD and could not be incorporated into this scoring system. Previous evidence suggests that physical inactivity and social isolation increases the risk of dementia, possibly through an increase in the risk of diabetes, hypertension, stroke, or depression [44, 45, 46, 47]. Therefore, these selected illnesses could serve as surrogate markers and at least partly explain the effects of the above factors for risk of dementia absent in our model. As regards the role of dialysis adequacy in risk of dementia, recent studies including one small randomized controlled trial failed to prove the link between dialysis adequacy and cognitive impairment [48, 49, 50]. Conversely, several uremic toxins are found to be correlated with impaired cognitive function in dialysis patients [51]. Further large‐scale clinical studies are still needed to clarify the issues. Second, the diagnosis of dementia and comorbidities totally depends on the claim data from the NHI program rather than a standardized assessment of all members in the cohort, which may increase the uncertainty related to the diagnosis. The potential of missing coding or coding errors may result in misclassifications. Nevertheless, such issues would only bias the association toward the null effect and thus underestimate the predictive power of our scoring system. Furthermore, the accuracy of diagnostic codes for most of the key variables in our scoring system (i.e., acute ischemic stroke, acute myocardial infarction, hypertension, diabetes mellitus, hyperlipidemia, Parkinson's disease, and depression disorder) and the outcome variable, dementia, were previously validated [52, 53, 54, 55, 56, 57]. Most of the sensitivities, specificities, and positive predictive values of these variables were above 86.4%, which suggested a high accuracy of diagnostic codes for these variables, and largely limited the potential disease misclassification bias in our study. Third, the severity of comorbidities may be associated with different degrees of risk of developing dementia [58, 59], and we did not assign different score points in different stratifications of severity of comorbidities. Since the severity of comorbidities should comprise variables with dynamic changes (e.g., HbA1c, blood pressure level) or may be subjective because of the difficulty in terms of quantification (e.g., severity of insomnia or depression), the inclusion of these variables when modeling a scoring system may be too complicated to be easily applicable under clinical conditions. Forth, the false‐negative rate of DDRS is high (36.52%). Among patients whose DDRS values are <50, physicians should always take dementia into consideration if dialysis patients present any symptoms of memory impairment interfering with the activities of daily living, unexplained decline of function or personal hygiene, poor compliance to medical therapies, or new onset of psychiatric symptoms. In such circumstances, screening instruments for cognitive impairment including the Montreal Cognitive Assessment or Mini‐Mental State Examination can be applied for ascertainment. Additional labor‐intensive study or neurologist referral should be considered if there is high suspicion after the above two‐step screening process. Fifth, although the results of validation in one‐fifth of the national dialysis cohort were excellent, the score performance remains unclear without external validation. All these estimates were mainly derived from an Asian population, which might limit the generalizability of the DDRS to other racial/ethnic groups, and the optimal cutoff value of the DDRS system might vary in different countries/regions.
In conclusion, to efficiently identify dementia patients in a dialysis population we proposed a DDRS system for primary care physicians that demonstrated acceptable discrimination and calibration performance. Dialysis patients whose DDRS score is ≥50 could be regarded as at high risk for dementia and should potentially be considered for subsequent cognitive function screening. The inclusion of only age and 10 comorbidities in the DDRS makes it possible to easily integrate it into clinical programs, which could in turn facilitate clinical decision‐making and improve dementia care quality and preventive strategies in the dialysis population.
CONFLICT OF INTEREST
None of the authors declare any conflicts of interest.
AUTHOR CONTRIBUTIONS
Tsai‐Chieh Ling: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (lead); Methodology (lead); Software (lead); Visualization (lead); Writing‐original draft (lead). Chiung‐Chih Chang: Conceptualization (lead); Data curation (supporting); Formal analysis (lead); Funding acquisition (lead); Investigation (lead); Methodology (lead); Software (lead); Visualization (lead); Writing‐original draft (lead). Chung‐Yi Li: Formal analysis (lead); Investigation (lead); Methodology (lead); Software (equal); Supervision (equal); Writing‐review & editing (lead). Junne‐Ming Sung: Conceptualization (supporting); Formal analysis (supporting); Investigation (equal); Methodology (equal); Validation (equal). Chien‐Yao Sun: Formal analysis (supporting); Investigation (supporting); Methodology (equal); Visualization (equal). Kuen‐Jer James Tsai: Formal analysis (supporting); Investigation (equal); Methodology (equal); Visualization (supporting). Ya‐Yun Cheng: Formal analysis (equal); Investigation (supporting); Methodology (supporting); Software (supporting). Jia‐Ling Wu: Formal analysis (supporting); Investigation (supporting); Methodology (supporting); Software (lead). Yi‐Ting Kuo: Investigation (supporting); Methodology (supporting). Yu‐Tzu Chang: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Funding acquisition (lead); Investigation (lead); Methodology (lead); Project administration (lead); Resources (lead); Software (lead); Supervision (lead); Validation (lead); Visualization (lead); Writing‐review & editing (lead).
Supporting information
Ling T‐C, Chang C‐C, Li C‐Y, et al. Development and validation of the dialysis dementia risk score: A retrospective, population‐based, nested case‐control study. Eur J Neurol. 2022;29:59–68. doi: 10.1111/ene.15123
Tsai‐Chieh Ling and Chiung‐Chih Chang contributed equally to the work.
Funding information
This research was partly supported by grants NCKUH‐10902065 and NCKUH‐11002022 from the National Cheng‐Kung University Hospital, Tainan, Taiwan and MOST 106‐2314‐B‐006‐027 and MOST 110‐2634‐F‐006‐020 from the Ministry of Science and Technology, Taipei, Taiwan to Yu‐Tzu Chang and CMRPG8J0523, CMRPG8J0842, and CMRPG8K1531 from Chang Gung Memorial Hospital to Chiung‐Chih Chang
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the Ministry of Health and Welfare, R.O.C. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the Health and Welfare Data Science Center (https://dep.mohw.gov.tw/dos/cp‐5119‐59201‐113.html) with the permission of the Ministry of Health and Welfare, R.O.C.
REFERENCES
- 1. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goek ON, Prehn C, Sekula P, et al. Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrol Dial Transplant. 2013;28:2131‐2138. [DOI] [PubMed] [Google Scholar]
- 3. Cheng ST. Dementia caregiver burden: a research update and critical analysis. Curr Psychiatry Rep. 2017;19:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El‐Hayek YH, Wiley RE, Khoury CP, et al. Tip of the iceberg: assessing the global socioeconomic costs of Alzheimer's disease and related dementias and strategic implications for stakeholders. J Alzheimers Dis. 2019;70:323‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuo YT, Li CY, Sung JM, et al. Risk of dementia in patients with end‐stage renal disease under maintenance dialysis‐a nationwide population‐based study with consideration of competing risk of mortality. Alzheimers Res Ther. 2019;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krishnan AV, Pussell BA, Kiernan MC. Neuromuscular disease in the dialysis patient: an update for the nephrologist. Semin Dial. 2009;22:267‐278. [DOI] [PubMed] [Google Scholar]
- 7. Tilki HE, Akpolat T, Coskun M, Stalberg E. Clinical and electrophysiologic findings in dialysis patients. J Electromyogr Kinesiol. 2009;19:500‐508. [DOI] [PubMed] [Google Scholar]
- 8. Murray AM, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216‐223. [DOI] [PubMed] [Google Scholar]
- 9. Klarenbach SW, Tonelli M, Chui B, Manns BJ. Economic evaluation of dialysis therapies. Nat Rev Nephrol. 2014;10:644‐652. [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives ‐ a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247‐259. [DOI] [PubMed] [Google Scholar]
- 11. Chang YT, Hwang JS, Hung SY, et al. Cost‐effectiveness of hemodialysis and peritoneal dialysis: a national cohort study with 14 years follow‐up and matched for comorbidities and propensity score. Sci Rep. 2016;6:30266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen SC, Hung CC, Kuo MC, et al. Association of dyslipidemia with renal outcomes in chronic kidney disease. PLoS ONE. 2013;8:e55643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurella Tamura M, Yaffe K. Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int. 2011;79:14‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kallenberg MH, Kleinveld HA, Dekker FW, et al. Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD‐a systematic review. Clin J Am Soc Nephrol. 2016;11:1624‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Zwieten A, Wong G, Ruospo M, et al. Prevalence and patterns of cognitive impairment in adult hemodialysis patients: the COGNITIVE‐HD study. Nephrol Dial Transplant. 2018;33:1197‐1206. [DOI] [PubMed] [Google Scholar]
- 16. Barnes DE, Beiser AS, Lee A, et al. Development and validation of a brief dementia screening indicator for primary care. Alzheimers Dement. 2014;10:656‐665 e651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnes DE, Covinsky KE, Whitmer RA, Kuller LH, Lopez OL, Yaffe K. Predicting risk of dementia in older adults: the late‐life dementia risk index. Neurology. 2009;73:173‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population‐based study. Lancet Neurol. 2006;5:735‐741. [DOI] [PubMed] [Google Scholar]
- 19. Zarit SH, Reever KE, Bach‐Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649‐655. [DOI] [PubMed] [Google Scholar]
- 20. Hsieh CY, Su CC, Shao SC, et al. Taiwan's National Health Insurance Research Database: past and future. Clin Epidemiol. 2019;11:349‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late‐life dementia. Nat Rev Neurol. 2009;5:649‐658. [DOI] [PubMed] [Google Scholar]
- 22. de Bruijn RF, Ikram MA. Cardiovascular risk factors and future risk of Alzheimer's disease. BMC Med. 2014;12:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455‐532. [DOI] [PubMed] [Google Scholar]
- 24. Lin YT, Wu PH, Kuo MC, et al. Comparison of dementia risk between end stage renal disease patients with hemodialysis and peritoneal dialysis–a population based study. Sci Rep. 2015;5:8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. US Renal Data System . USRDS 2018. Annual data report: atlas of chronic kidney disease and end‐stage renal disease in the United States, 2018. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- 26. Pink J, O'Brien J, Robinson L, Longson D, Guideline C. Dementia: assessment, management and support: summary of updated NICE guidance. BMJ. 2018;361:k2438. [DOI] [PubMed] [Google Scholar]
- 27. McCarten JR, Anderson P, Kuskowski MA, McPherson SE, Borson S, Dysken MW. Finding dementia in primary care: the results of a clinical demonstration project. J Am Geriatr Soc. 2012;60:210‐217. [DOI] [PubMed] [Google Scholar]
- 28. Martorana A, Di Lorenzo F, Belli L, et al. Cerebrospinal fluid Abeta42 levels: when physiological become pathological state. CNS Neurosci Ther. 2015;21:921‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng Y, Tian DY, Wang YJ. Peripheral clearance of brain‐derived Abeta in Alzheimer's disease: pathophysiology and therapeutic perspectives. Transl Neurodegener. 2020;9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stephan BC, Kurth T, Matthews FE, Brayne C, Dufouil C. Dementia risk prediction in the population: are screening models accurate? Nat Rev Neurol. 2010;6:318‐326. [DOI] [PubMed] [Google Scholar]
- 31. Tang EY, Harrison SL, Errington L, et al. Current developments in dementia risk prediction modelling: an updated systematic review. PLoS ONE. 2015;10:e0136181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Floege J, Gillespie IA, Kronenberg F, et al. Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int. 2015;87:996‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263‐272. [DOI] [PubMed] [Google Scholar]
- 34. D'Agostino RB, Sr. , Grundy S, Sullivan LM, Wilson P, Group CHDRP . Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180‐187. [DOI] [PubMed] [Google Scholar]
- 35. Serrano‐Pozo A, Growdon JH. Is Alzheimer's disease risk modifiable? J Alzheimers Dis. 2019;67:795‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yaffe K, Falvey C, Hamilton N, et al. Diabetes, glucose control, and 9‐year cognitive decline among older adults without dementia. Arch Neurol. 2012;69:1170‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Launer LJ, Miller ME, Williamson JD, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open‐label substudy. Lancet Neurol. 2011;10:969‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murray AM, Hsu FC, Williamson JD, et al. ACCORDION MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia. 2017;60:69‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luchsinger JA, Perez T, Chang H, et al. Metformin in amnestic mild cognitive impairment: results of a pilot randomized placebo controlled clinical trial. J Alzheimers Dis. 2016;51:501‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Claxton A, Baker LD, Hanson A, et al. Long‐acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early‐stage Alzheimer's disease dementia. J Alzheimers Dis. 2015;44:897‐906. [DOI] [PubMed] [Google Scholar]
- 42. Group SMIftSR , Williamson JD, Pajewski NM, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence‐based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143‐1153. [DOI] [PubMed] [Google Scholar]
- 44. Jenum AK, Anderssen SA, Birkeland KI, et al. Promoting physical activity in a low‐income multiethnic district: effects of a community intervention study to reduce risk factors for type 2 diabetes and cardiovascular disease: a community intervention reducing inactivity. Diabetes Care. 2006;29:1605‐1612. [DOI] [PubMed] [Google Scholar]
- 45. Willey JZ, Moon YP, Paik MC, Boden‐Albala B, Sacco RL, Elkind MS. Physical activity and risk of ischemic stroke in the Northern Manhattan Study. Neurology. 2009;73:1774‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Win S, Parakh K, Eze‐Nliam CM, Gottdiener JS, Kop WJ, Ziegelstein RC. Depressive symptoms, physical inactivity and risk of cardiovascular mortality in older adults: the Cardiovascular Health Study. Heart. 2011;97:500‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673‐2734. [DOI] [PubMed] [Google Scholar]
- 48. Kurella Tamura M, Larive B, Unruh ML, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network trials. Clin J Am Soc Nephrol. 2010;5:1429‐1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giang LM, Weiner DE, Agganis BT, et al. Cognitive function and dialysis adequacy: no clear relationship. Am J Nephrol. 2011;33:33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kurella Tamura M, Vittinghoff E, Hsu CY, et al. Loss of executive function after dialysis initiation in adults with chronic kidney disease. Kidney Int. 2017;91:948‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kurella Tamura M, Chertow GM, Depner TA, et al. Metabolic profiling of impaired cognitive function in patients receiving dialysis. J Am Soc Nephrol. 2016;27:3780‐3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20:236‐242. [DOI] [PubMed] [Google Scholar]
- 53. Hsieh CY, Chen CH, Li CY, Lai ML. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc. 2015;114:254‐259. [DOI] [PubMed] [Google Scholar]
- 54. Cheng CL, Lee CH, Chen PS, Li YH, Lin SJ, Yang YH. Validation of acute myocardial infarction cases in the National Health Insurance Research Database in Taiwan. J Epidemiol. 2014;24:500‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sung SF, Hsieh CY, Lin HJ, Chen YW, Yang YH, Li CY. Validation of algorithms to identify stroke risk factors in patients with acute ischemic stroke, transient ischemic attack, or intracerebral hemorrhage in an administrative claims database. Int J Cardiol. 2016;215:277‐282. [DOI] [PubMed] [Google Scholar]
- 56. Liu CC, Li CY, Lee PC, Sun Y. Variations in incidence and prevalence of Parkinson's disease in Taiwan: a population‐based nationwide study. Parkinsons Dis. 2016;2016:8756359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu CS, Kuo CJ, Su CH, Wang SH, Dai HJ. Using text mining to extract depressive symptoms and to validate the diagnosis of major depressive disorder from electronic health records. J Affect Disord. 2020;260:617‐623. [DOI] [PubMed] [Google Scholar]
- 58. Rawlings AM, Sharrett AR, Albert MS, et al. The association of late‐life diabetes status and hyperglycemia with incident mild cognitive impairment and dementia: the ARIC study. Diabetes Care. 2019;42:1248‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Walker KA, Sharrett AR, Wu A, et al. Association of midlife to late‐life blood pressure patterns with incident dementia. JAMA. 2019;322:535‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Ministry of Health and Welfare, R.O.C. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the Health and Welfare Data Science Center (https://dep.mohw.gov.tw/dos/cp‐5119‐59201‐113.html) with the permission of the Ministry of Health and Welfare, R.O.C.


