Abstract
Droughts in a warming climate have become more common and more extreme, making understanding forest responses to water stress increasingly pressing. Analysis of water stress in trees has long focused on water potential in xylem and leaves, which influences stomatal closure and water flow through the soil‐plant‐atmosphere continuum. At the same time, changes of vegetation water content (VWC) are linked to a range of tree responses, including fluxes of water and carbon, mortality, flammability, and more. Unlike water potential, which requires demanding in situ measurements, VWC can be retrieved from remote sensing measurements, particularly at microwave frequencies using radar and radiometry. Here, we highlight key frontiers through which VWC has the potential to significantly increase our understanding of forest responses to water stress. To validate remote sensing observations of VWC at landscape scale and to better relate them to data assimilation model parameters, we introduce an ecosystem‐scale analog of the pressure–volume curve, the non‐linear relationship between average leaf or branch water potential and water content commonly used in plant hydraulics. The sources of variability in these ecosystem‐scale pressure‐volume curves and their relationship to forest response to water stress are discussed. We further show to what extent diel, seasonal, and decadal dynamics of VWC reflect variations in different processes relating the tree response to water stress. VWC can also be used for inferring belowground conditions—which are difficult to impossible to observe directly. Lastly, we discuss how a dedicated geostationary spaceborne observational system for VWC, when combined with existing datasets, can capture diel and seasonal water dynamics to advance the science and applications of global forest vulnerability to future droughts.
Keywords: drought response, drought‐induced tree mortality, microwave remote sensing, pressure–volume, vegetation optical depth, vegetation water content, water potential
Changes of vegetation water content (VWC) are linked to a range of tree responses to drought, including fluxes of water and carbon, mortality, flammability, and more, and can be retrieved from microwave remote sensing measurements. We highlight key frontiers through which remotely sensed VWC has the potential to significantly increase our understanding of forest responses to water stress. We argue that separate consideration of diel, seasonal, and decadal timescales can facilitate interpretation of VWC measurements for different process studies, and that VWC observations can be useful for constraining belowground water uptake. To link remotely sensed VWC estimates to plant hydraulic models, the utility and interpretation of ecosystem‐scale pressure‐volume curves are discussed.

1. INTRODUCTION
As the climate warms, droughts are getting hotter, more extreme, and more frequent (Dai, 2013; Touma et al., 2015; Trenberth et al., 2014). Forests respond to drought by reducing photosynthesis and transpiration (Liu et al., 2020; Short Gianotti et al., 2019; Trugman et al., 2018) and exhibiting increased mortality (Adams et al., 2017; Choat et al., 2018). Their response to drought is mediated by the flow and distribution of water in the soil‐plant‐atmosphere continuum (Tyree & Sperry, 1989), but is also affected by stand‐scale processes such as belowground redistribution of water, composition, competition, and demography. These processes have been studied at fine scales (Brodribb et al., 2020; Sperry & Love, 2015), but aggregated processes controlling ecosystem response to droughts remain poorly understood (Allen et al., 2015; Gazol et al., 2018; Levine et al., 2016). Thus, we are challenged to understand forest resilience in response to the major disturbances brought by climate change (Anderegg et al., 2013; Brodribb et al., 2020), including effects on forest ecosystem services such as carbon sequestration (Anderegg et al., 2020) and water cycling (Mastrotheodoros et al., 2020; Tague et al., 2019).
Understanding the aggregated effects of drought on forests is difficult, particularly due to extensive heterogeneity in plant traits (Anderegg, 2015; Skelton et al., 2015), edaphic conditions, and topography (Goulden & Bales, 2019). Remote sensing tools naturally provide aggregate observations across ecosystems at local to global scales. Commonly used retrievals of surface temperature, leaf area, or solar‐induced fluorescence provide information on the consequences of forest drought responses (Deshayes et al., 2006; West et al., 2019), but they do not directly capture the drought stress affecting the trees themselves. These measurements also do not provide information on belowground processes affecting water redistribution and root access, a critical influence on forest drought response (Agee et al., 2021; Hagedorn et al., 2016; Phillips et al., 2016).
Microwave remote sensing of vegetation water content has become an increasingly popular alternative for studying forest responses to drought (Anderegg et al., 2018; Rao et al., 2019; Saatchi et al., 2013; Schroeder et al., 2016). For decades, numerous studies of forest–water interactions have focused on plant water potential. However, recently there has been a renewed interest in the role of vegetation water content (VWC) in influencing water and carbon fluxes, tree mortality, and fire risk (Martinez‐Vilalta et al., 2019; Matheny et al., 2015; Nolan et al., 2020). Microwave remote sensing‐based estimates of VWC (and proxies of VWC) may thus transform studies of forest responses to drought stress. However, because VWC is a relatively new remote sensing product, its optimal interpretation and use, as well as its relation to water stress, has not been comprehensively explored.
Here, we review key frontiers through which remotely sensed observations of VWC can be used to significantly increase our understanding of forest ecosystem response to droughts. We pose questions and raise challenges to maximize the utility of microwave remote sensing for studies of forest drought responses. While we focus on forests, many of the ideas in this paper also apply to other natural biomes, and particularly to detecting water stress in croplands (Steele‐Dunne et al., 2017; Togliatti et al., 2019). In Section 2, we introduce and compare plant water potential and plant water content at the tree scale, including a description of measurement challenges for each. We argue that observations of ecosystem‐scale VWC would inform a number of ecological applications and overcome several existing in situ measurement challenges. The ecosystem‐scale VWC measurements that are feasible from remote sensing are then introduced in Section 3. This section aims to provide some background on the theoretical basis of microwave remote sensing of VWC, in order to clarify how it informs measurement characteristics such as the canopy depth represented. This section also includes a description of challenges for improved estimation of VWC, including how additional in situ observations may help improve estimation accuracy. Having introduced both the in situ and remotely sensed conceptualizations of VWC, Sections 3, 4, 5, 6 of the paper then discuss the ways that remotely sensed VWC can be used to study forest response to droughts. Section 4 discusses how the current generation of remotely sensed VWC estimates can be interpreted at different timescales to provide information about different drought response processes (e.g., disturbance dynamics, canopy dehydration, etc) and the ways VWC datasets can be combined with other observations and plant hydraulic models. Because using VWC to constrain plant hydraulic models requires determining how VWC changes translate to the water potential variables that plant hydraulic models simulate, Section 5 discusses whether VWC observations can be linked to the concept of an ecosystem‐scale water potential, how such a variable could be interpreted and used, and what controls these linkages. Section 6 then discusses a specific component of forest drought response for which VWC observations can be particularly useful: determining belowground processes based on model inversion and the analysis of phase dynamics. Lastly, Section 7 considers the mismatch between available remote sensing data for VWC and the dataset properties that this paper identifies as particularly useful for studies of forest drought response (high spatial resolution, capturing diel variations). It then presents an alternative concept for a new satellite mission to address these data gaps.
2. FOREST DROUGHT RESPONSES—PHYSIOLOGY, POTENTIAL, AND WATER CONTENT
2.1. Water potential gradients influence forest drought response
The movement of water through trees (and other vascular plants) is dictated by a continuum of water potential gradients from the soil through the various plant components to the atmosphere. Variations in the magnitude of the gradient dictate how trees respond to water stress. Under dry conditions, water loss through stomata causes leaf water potential declines. As a result, stomata close, reducing the water loss but also decreasing photosynthesis. Under a drying atmosphere and/or soil, xylem water potential also decreases. At very large negative pressure (tension) in the xylem, embolisms can form, blocking water flow within the xylem vessels (Tyree & Sperry, 1989). Such embolisms reduce conductance to water transport, largely reducing the capacity to transport water from the soil to leaves. High conductance losses can potentially lead to tree mortality (Brodribb, 2009). Long‐term reductions in photosynthesis due to water stress can also make trees more vulnerable to death from some combination of biotic attack and physiological failure (McDowell, 2011; Trugman, Detto, et al., 2018; Wu et al., 2018).
2.2. VWC as an indicator of plant water status
While studies of plant water relations predominantly focus on quantifying water potential variations across the soil‐plant‐atmosphere continuum, there is significant evidence that water content itself can also be an informative metric of water status. For example, plant water storage forms a significant fraction of transpiration (Goldstein et al., 1998; Matheny, Fiorella, et al., 2017; Phillips et al., 2003). In addition, the relative water content (defined by normalizing VWC by its maximum value) also provides a threshold‐based predictor for wilting and mortality under drought (Bartlett et al., 2012; Rao et al., 2019; Sapes et al., 2019). Unlike for leaf water potential, the threshold of relative water content at the wilting point was found to be relatively conservative across species (Bartlett et al., 2012). Lastly, VWC is directly related to live fuel moisture content (Konings et al., 2019; Rao et al., 2020), which is defined as the VWC per unit dry biomass and is a widely used indicator of fire risk. LFMC shows threshold‐like impacts on fire ignition probability (Chuvieco et al., 2011; Dimitrakopoulos & Papaioannou, 2001) and fire size (Argañaraz et al., 2018; Dennison & Moritz, 2009). Overall, improved quantification of VWC will likely contribute to better assessment of forest drought responses including transpiration, mortality, and wildfire risk (Figure 1). Given the large variations of VWC and relative water content within different tree components and across species, this quantification must take into account variations in VWC across vertical and horizontal scales.
FIGURE 1.

Changes in water content drive forest changes at diurnal (inner ring), seasonal (middle ring), and decadal (outer ring) timescales. Across decadal‐scale responses, declines in VWC can lead to mortality and/or fire. VWC will also increase in concert with successional dynamics. Across dry and wet seasons, forest VWC evolves through both phenology and de‐/rehydration. Lastly, VWC has a strong diurnal cycle driven by the diurnal cycle of ET
2.3. Measurement challenges for plant water status
For decades, manual plant water potential observations (e.g., using a pressure chamber, Scholander et al., 1965) have played a central role in our understanding and quantification of tree water status, especially at the site level (Cavender‐Bares & Bazzaz, 2000; Tardieu & Simonneau, 1998; Tyree & Sperry, 1988). However, they are destructive measurements that are particularly challenging in tall forests where access to canopies leaves is limited. Consequently, these data are typically collected at weekly to monthly temporal resolutions, which may be sufficient to resolve dynamics linked to soil water drying, but are too coarse to capture variability in plant water status at diurnal timescales. Continuous, automated measurement of stem and leaf water potential is possible for some species with psychrometry (e.g., Guo et al., 2020), but these instruments are expensive, require substantial maintenance, and their use is not yet widespread. Moreover, unlike observations of water fluxes possible using micrometeorological and sap flux approaches, time series of plant water potential have yet to be collected and standardized in databases and networks, hindering synthesis of information across sites. Finally, even within a site, leaf and stem water potential measurements are also generally limited to individual trees, and scaling to the entire stand can be challenging, particularly in ecosystems with multiple species across multiple edaphic conditions. This difficulty in scaling hinders efforts to harmonize species‐specific observations to those from eddy covariance flux towers (which typically have footprints on the orders of 103–107 m2, Chu et al., 2021). These measurement difficulties also determine the scarcity of information about water potential–water content relationships (usually determined using pressure–volume curves or P‐V curves) across species and tree components. Because P‐V curves are most often determined destructively, information on P‐V curves is relatively abundant only for leaves, while very little is known about the equivalent properties of bark tissues and roots.
Direct measurements of vegetation water content can be less labor intensive and more cost effective, facilitating increased spatial and temporal observation. For wood water content, micron‐scale dendrometers can be automated and used to infer water content after detrending (Peters et al., 2021; Pfautsch et al., 2015). Reflectometry (TDR and FDR) and capacitance‐style sensors can provide automated measurements of dielectric permittivity, which can be directly converted to water content (Holbrook & Sinclair, 1992; Matheny et al., 2015; Wullschleger et al., 1996). However, these sensors are sensitive to differences in wood density, and should be calibrated for use in different species (Matheny et al., 2017). Unlike for woody tree components, there is no commonly used direct, non‐destructive measurement technique to determine leaf water content. In situ spectroscopy is sensitive to leaf water content (Browne et al., 2020; de Jong et al., 2014), but also requires species‐specific calibration. At larger scales, VWC from microwave remote sensing could be used instead of ground measurements.
3. MICROWAVE REMOTE SENSING OF VWC
Several remote sensing techniques allow monitoring VWC or proxy measures of VWC with different levels of precision. These measurements cover a wide range of the electromagnetic spectrum, ranging from optical spectral imaging (Asner et al., 2016; Ustin et al., 2012) to thermal infrared imaging (Jones et al., 2009), to active (radar) and passive (radiometer) microwave sensing (Konings et al., 2019; Vermunt et al., 2020). Microwave frequencies are arguably the most useful for systematic measurement of VWC because of their all‐time observational capabilities during day and night and irrespective of cloud cover, and the ability to penetrate beyond the top few millimeters of the forest canopy. This avoids systematic biases that would occur if only cloud‐free periods can be measured. Observations illustrating the sensitivity of microwave remote sensing observations of VWC at different timescales are shown in Figure 2. However, no current spaceborne system is dedicated to systematically observing VWC and its changes due to water stress. We discuss prospects for a new spaceborne system, with the aim to provide estimates of VWC at sub‐daily temporal resolutions to resolve the dynamic physiological response of vegetation to water stress, in Section 7.
FIGURE 2.
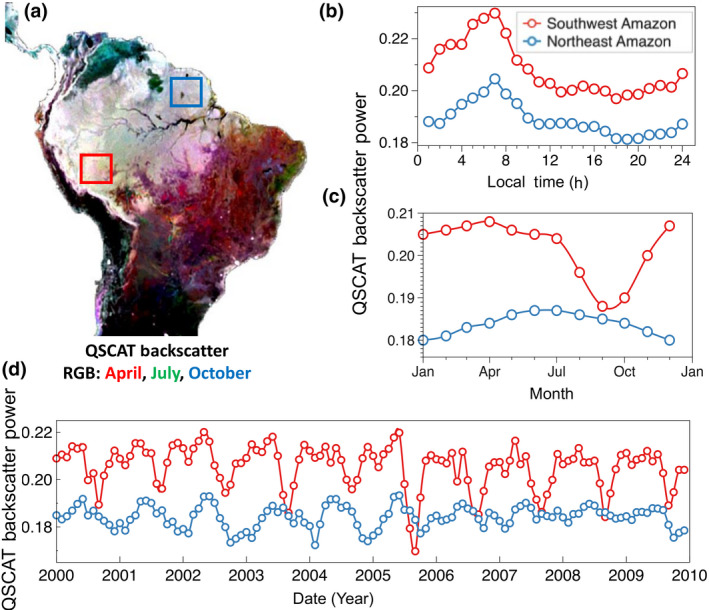
Variations of radar backscatter measurements across the Amazon Basin. Radar backscatter coefficients at Ku‐band are used as a proxy for changes of canopy water content showing: (a) spatial variations as an RGB color composite of QuikSCAT (QSCAT) radar backscatter in the months of April, July, and October capturing regional and seasonal changes, (b) seasonal cycle of QSCAT backscatter averaged across two regions in southwest and northeast of the Amazon, (c) diurnal cycle of the same regions detected by the RapidSCAT satellite observations onboard International Space Station from 2014 to 2016, and (d) time series of QSCAT backscatter capturing seasonal and interannual variations including extreme droughts of 2005 in the southwest of the Amazon
3.1. Theoretical basis
Microwave remote sensing measurements respond directly to changes in VWC due to their sensitivity to the dielectric constant and thus to free water volume (i.e., water that is not chemically bound) in vegetation (including leaves, branches, stems) (Ulaby & El‐rayes, 1987). Depending on the electromagnetic frequency, the depth of penetration of microwaves into the forest canopy may vary (Figure 3). The sensitivity to VWC is expressed as the mass of water per ground area (i.e., in units of kg water/m2) (Schmugge & Jackson, 1992). The main observation of VWC from active and passive microwave remote sensing is through the vegetation optical depth (VOD), which is a measure of how much the VWC attenuates the microwave signal from the soil surface (Frappart et al., 2020; Konings et al., 2019). The theoretical basis for this relationship and typical retrieval approaches are reviewed extensively in Frappart et al. (2020).
FIGURE 3.
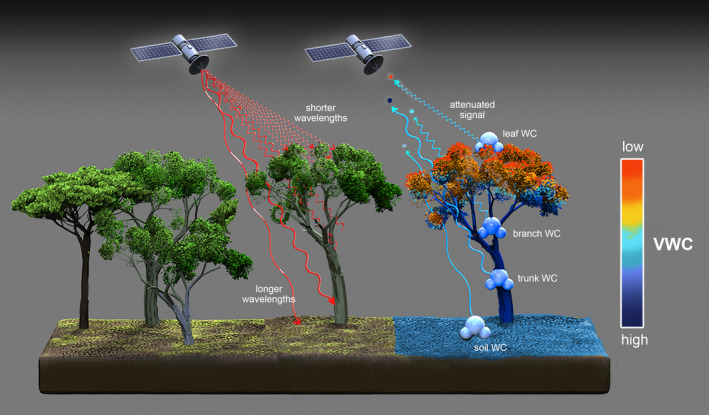
Microwave remote sensing is able to observe water content in forests. The canopy layers represented in each measurement (the penetration depth) varies across different microwave frequency bands (and thus different wavelengths), as show through different red and blue electromagnetic waves. Observations represent deeper areas of the canopy as wavelengths increase (and frequencies decrease) from Ku‐band across X‐, C‐, and L‐bands to P‐band. Higher frequencies are most sensitive to leaves and branches while lower frequencies also have increasing sensitivity to trunks and soils. Red waves represent transmissions on a radar system while blue waves represent the returns, with dots at the end of each wave representing different magnitude backscatter coefficient measurements depending on the water content (colorbar) of the different vegetation components each wavelength is sensitive to. If only the blue waves are considered and the dots are interpreted as measurements of VOD, the figure is representative of a radiometer system instead
Vegetation optical depth is a dimensionless quantity, with higher values indicating more attenuation and hence a larger quantity of VWC. VOD is often assumed to be linearly related to VWC (Jackson & Schmugge, 1991) with a coefficient depending on the frequency of observation, forest type, and structure (height, biomass density, and gap size). Studies comparing microwave sensing to in situ measurements of VWC have been able to establish the linear relation for a wide range of vegetation types (van Emmerik et al., 2015; Jackson & Schmugge, 1991; Sharma et al., 2020). Note that direct retrieval of VWC—rather than a quantity proportional to VWC—has not yet been performed at global scale. Nevertheless, the linear relationship between VOD and VWC has enabled a range of applications such as detection of water stress in forest ecosystems (Frolking et al., 2011; Rao et al., 2019; Saatchi et al., 2013), quantification of the diel cycle of VWC (van Emmerik et al., 2015; Konings, Yu, et al., 2017; Schroeder et al., 2016), and estimation of seasonal changes of VWC related to phenology (Tian et al., 2016; Wang et al., 2020; Xu et al., 2015).
3.2. Challenges and opportunities for estimation of VWC from remote sensing
While applications of microwave vegetation remote sensing are growing rapidly, some long‐standing challenges remain. At ecosystem‐scale resolutions, VWC depends not only on water stress but also on seasonal to interannual changes in biomass (Brandt et al., 2018; Konings et al., 2021; Liu et al., 2015). Changes in relative water content can be disentangled from changes in phenology and biomass by considering diel or other short‐term timescales, as further discussed in Section 4. Nevertheless, this complicates interpretation of VWC observations.
Because microwave remote sensing is sensitive to both VWC and soil moisture, retrieval algorithms are needed to separate these two factors. However, most operational retrieval algorithms represent the VWC as consisting of a homogenous cloud of water droplets, which neglects the roles of vertical and horizontal variations in water content, canopy gaps, surface water from dew and rainfall interception (Xu et al., 2021), etc. Not only do these simplifications risk incurring retrieval or interpretation errors for both existing datasets and future VOD retrievals, they also cause a missed opportunity. Because the sensitivity of microwave observables to these factors varies based on frequency and polarization (Baur et al., 2019), heterogeneity in water content across different heights in the canopy could in theory be accounted for. If overlapping observations at multiple electromagnetic frequencies are available, these could then be combined to determine water content across different heights in the canopy. However, doing so will require more sophisticated electromagnetic models (Saatchi & Moghaddam, 2000; Steele‐Dunne et al., 2017), which in turn require detailed information about tree and forest structure. Recent progress in remote sensing‐derived vegetation structure information may be able to help fill this gap (Dubayah et al., 2020; Quegan et al., 2019; Yu & Saatchi, 2016). A more mechanistic understanding of microwave observations will also offer more synergies with optical and spectroscopic methods (Bohn et al., 2019), which are most sensitive to the upper layers of the canopy and can therefore provide complementary information to deeper microwave observations. In the case of passive microwave observations, it may also enable better accounting for changes in temperature across the canopy, and associated improvements in retrieval accuracy (Parinussa et al., 2016). However, further development of more advanced retrieval approaches will require coordinated field campaigns for calibration and validation, including non‐destructive ground‐based samples of water content such as those in Section 2.3. If the relationship between water content and (leaf or xylem) water potential can be quantified (Section 5), existing water potential measurements—while sparse—could also be used in validation field campaigns. Indeed, given the sparsity of ecosystem VWC measurements, additional validation field campaigns would also be useful for validating existing VOD retrieval methods.
Lastly, existing satellite observations of VOD from radiometers (Du et al., 2017; Konings et al., 2017; Moesinger et al., 2020; Wigneron et al., 2021) and scatterometers (Frolking et al., 2006) can be noisy when only individual measurements are considered. Furthermore, they have coarse spatial resolution (25–50 km) and cannot reliably separate changes of VWC from other disturbance and recovery processes associated with canopy cover and biomass. The applications of these measurements will improve substantially if the spatial resolution of the observations reaches the landscape scale (100–1000 m) (Martínez‐Vilalta & Lloret, 2016).
3.3. Past and future sensors for VWC observations
Despite the challenges above, our ability to monitor VWC dynamics with microwave remote sensing is currently constrained by sensor availability, not by technology. Existing and planned passive microwave observations (e.g., AMSR‐E, SMAP, and CIMR) and scatterometers (e.g., QuikScat and ASCAT) at different electromagnetic frequencies provide long‐term coarse resolution observations to monitor soil and vegetation water status regionally, while less frequent but high‐resolution synthetic aperture radar measurements (e.g., Sentinel‐1 (Torres et al., 2012), NISAR (Kumar et al., 2016)) help to quantifying landscape‐scale variations of VWC. However, the greatest limitation of existing spaceborne measurements from sun‐synchronous orbits is the lack of diel observations of VWC that can be directly linked to the plant physiology and to water and carbon exchange. At such timescales, there is minimal influence from changes in phenology and forest structure on the total ecosystem‐scale VWC (Section 4). Microwave observations from RapidScat onboard the International Space Station (van Emmerik et al., 2017; Konings, Yu, et al., 2017) and from ground‐based tower systems (Holtzman et al., 2021; Monteith & Ulander, 2018; Schneebeli et al., 2011; Vermunt et al., 2020) have demonstrated the feasibility of quantifying the VWC dynamics throughout the day. Thus, the ability to monitor the diel signal of VWC is driven by the orbital choices of the existing sensors, not by limitations of the microwave observations’ intrinsic sensitivity. Section 4 further discusses the ways different timescales of analysis enable study of different aspects of forest drought response.
4. DERIVING PROCESS UNDERSTANDING FROM VWC
4.1. VWC information depends on analysis timescales
Remote measurements of VWC can extend process‐level understanding in forests by leveraging variation in VWC in space and time. VWC integrates processes associated with water storage and fluxes of different forest water reservoirs (leaf, wood, soil) at different timescales. We therefore posit that measuring VWC dynamics can be crucial for understanding, quantifying, and modeling ecological and hydrological processes at roughly three timescales (Figure 4).
FIGURE 4.
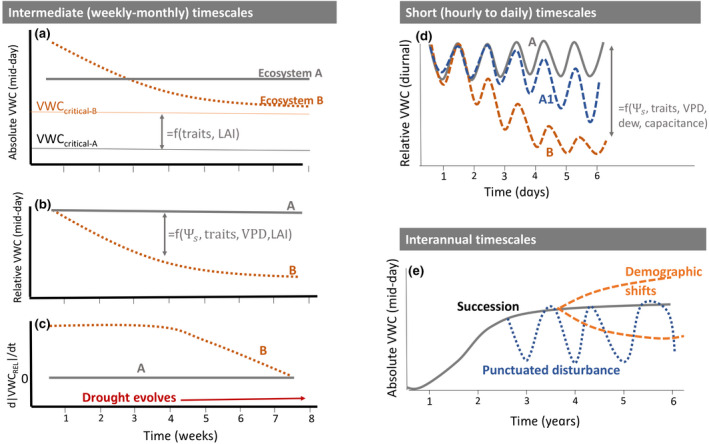
What can vegetation water content tell us about plant stress? The absolute value of VWC (panel a, shown as mid‐day values) is difficult to interpret without context about its maximum and critically limiting values (e.g., the VWCcritical). For example, while Ecosystem B initially has higher absolute VWC than Ecosystem A, its VWCcritica is also higher. When VWC is expressed as a relative value compared to the seasonal maximum (panel b), Ecosystem B emerges as consistently more stressed than A, with the difference between the two reflecting traits, structure, as well as environmental states (soil water potential, VPD). The time derivative of relative VWC (panel C) illustrates that the time change in VWC can be zero for both very stressed and very unstressed ecosystems, but the change in d|VWC|/dt over periods of weeks to months is highly informative of the ecosystem water status. On the right side, panel (d) shows differences in the diurnal amplitude of relative VWC for ecosystems experiencing little stress (A), intermediate (A1), and more severe stress (B). Panel (e) shows long‐term (interannual) changes in absolute VWC attributable to succession, disturbance, and demographic shifts
At the timescale of multiple years to decades (Figure 4e), VWC patterns largely reflect forest biomass and structure (Konings et al., 2019; Liu et al., 2013, 2015). Thus, spatial patterns in VWC can shed light on variation in biomass, canopy structure, biome boundaries, and species‐level traits that influence water content (e.g., wood density (Gentine et al., 2016; Araújo et al., 1999)). The sensitivity of VWC to aboveground biomass can inform disturbance and land‐use change dynamics (Liu et al., 2015; Pugh et al., 2019) and potentially slower, non‐disturbance shifts in demographic rates such as succession or climate‐driven increases in mortality that lead to changes in species composition (Anderegg et al., 2020; Trugman et al., 2020; van Mantgem et al., 2009). Multi‐year averaged measurements of VWC can therefore provide a powerful set of measurements for spatial scaling and quantifying ecological dynamics—particularly those related to biomass, rather than water content or physiology alone per se—at biome, continent, and global scales.
At the timescale of multiple weeks to months (Figure 4a‐c), VWC dynamics may reflect progressive dehydration of multiple tissues of trees associated with changes in soil water potential and xylem function, as well as changes in forest leaf area index caused by leaf shedding or leaf flushing (Frolking et al., 2011; Jones et al., 2011; Konings et al., 2019; Tian et al., 2017). Assuming a threshold‐type response (Section 2.2), VWC may therefore be useful to assess the risk of drought‐induced forest mortality and flammability risk. The slope of VWC curves during prolonged droughts and after post‐drought precipitation events may also be used as metrics to compare community‐level drought resistance and recovery capacity (Asefi‐Najafabady & Saatchi, 2013; Suding & Hobbs, 2009). More gradual slopes likely means that the tree cover has more mechanisms that minimize dehydration, indicating higher resistance.
At diel timescales (Figure 4d), changes in biomass are slow and VWC dynamics reflect the balance of transpiration and root water uptake, as well as redistribution of water through trees (van Emmerik et al., 2017; Konings, Yu, et al., 2017). These timescales are thus most closely affected by root, xylem, and stomatal responses to drying soil and air. As a result, VWC observations at diel timescales are arguably best able to isolate the effects of water stress. VWC variations across the diel cycle are also closely related to belowground processes, which are difficult to disentangle at other timescales (see Section 6). The shape of the diel cycle of VWC can be used to detect water stress (Nelson et al., 2018), before it is detectable through other leaf properties. Therefore, the diel dynamics of VWC also hold promise as an early warning signal for forest risks.
4.2. Complementary measurements to improve VWC interpretation
By combining VWC measurements with complementary field and remote sensing data, we can vastly improve process‐level understanding in forests across multiple timescales. At the timescales of multiple years to decades, data on species composition, forest structure and biomass, demography, and disturbance history could be used to test for species‐, age‐, or disturbance‐dependent responses to drought in forests (Hanson & Weltzin, 2000; Zhang et al., 2018). Disentangling the sources of within‐ and cross‐ecosystem variability in patterns of VWC is facilitated by observations of factors such as soils and topography and canopy structural attributes like leaf area index and vertical architecture. At the intermediate timescales of multiple weeks to a month, soil moisture and meteorological conditions and phenology are the major constraints on VWC dynamics. Additional measurements such as volumetric soil moisture, temperature, precipitation, and vapor pressure deficit, along with phenology‐related data, can provide crucial context. At diel timescales, complementary measurements related to canopy composition and biomass dynamics are less necessary and less useful. Surface soil moisture dynamics and evapotranspiration estimates (or more directly, thermal imagery sensitive to evapotranspiration) may provide complementary information about the water distribution through the soil‐plant‐atmosphere continuum at remote sensing scales. Observations and estimates of canopy water content from dew or rainfall interception will also be useful, to remove these signals from the observed VWC proxies (Binks et al., 2021; Vermunt et al., 2020; Xu et al., 2021). Additionally, analyses based on diel variations in VWC may be constrained based on functional trait data (i.e., hydraulic traits), where available (Trugman et al., 2020).
Beyond complementary datasets, process models are a necessary counterpart to VWC measurements to enable interpretation of VWC patterns and its underlying mechanisms (Xu et al., 2021). Models can also benefit from the constraints that VWC can place upon their interpretation, and thus model‐measurement integration is a win‐win situation. The type of models that can integrate VWC information most effectively are hydraulically enabled (e.g., Christoffersen et al., 2016; Kennedy et al., 2019; Li et al., 2020; Mencuccini et al., 2019), and would be able to simulate water pools (Martinez‐Vilalta et al., 2019). Such models can operate over a range of spatial scales and on timescales that span minutes to years, enabling process understanding of VWC over short to long timescales. Examples of model‐data benefits include the opportunity to examine the role of community‐scale plant water storage and capacitance (simulated via models) in regulating the observed VWC variation, understanding how transpiration rates may drive variation in observed VWC, or understanding belowground controls on water uptake (as further discussed in Section 6). To link models and data, an ecosystem‐scale water release curve could be generated in which VWC is the dependent variable and simulated community‐scale water potential is the independent variable. Such curves are further discussed in the next section.
5. SCALING WATER CONTENT TO ECOSYSTEM SCALE
5.1. An ecosystem‐scale pressure–volume curve
In order to use VWC as a constraint on plant hydraulic models that simulate the dynamics of water potential (Ψ), the relationship between Ψ and VWC must be known at the ecosystem scale. As a thermodynamic property of the water itself (i.e., its free energy), the Ψ can be averaged at any scale and across media, enabling the consideration of an instantaneous Ψ for a cell, a leaf, a shoot, a branch, or a tree (Pallardy et al., 1991; Scholander et al., 1964), or potentially, the whole ecosystem. Given the spatial scale of remote sensing observations, estimating Ψ from orbit would require ecosystem‐scale Ψ‐WC curves (eco Ψ‐WC; pronounced, ecopsych). Such curves can be conceived as large‐scale analogs of tissue‐scale P‐V curves: graphical plots of the relationship between relative or absolute water content and Ψ, commonly constructed by dehydrating leaves or stems (Richter, 1978; Scholander et al., 1964; Tyree & Hammel, 1972). They are also analogous to soil water retention curves (Hillel 2013). The earliest conceptualization of a P‐V curve was applied to twigs and leaves, and recognized the potentially large variability in water retention properties of the constituent living cells, but showed that cellular‐level P‐V curves follow a remarkably similar form to that of the bulk P‐V curve (Tyree & Hammel, 1972). We argue that the scale jump from organ‐level P‐V curves to the canopy is no greater than from cells to organs, and as such, the eco Ψ‐WC concept is more than possible, it is inevitable.
Notably, even for one ecosystem at any moment in time, the eco Ψ‐WC would consist of a family of curves, each dependent on the spatial scale being used to average Ψ and VWC, and on fluxes through the system that influence the relative distribution of water among individual trees, and within trees, among cells and organs. When modeled comprehensively and given enough spatial resolution, the eco Ψ‐WC could enable a full three‐dimensional suite of Ψ‐WC relationships, at a range of scales (per leaf area, ground area, volume, or mass; by canopy layers, plant organs, sizes or species; scanning layers vertically vs. integrating across volume). Across the suite of possible eco Ψ‐WC curves, some may be especially powerful for particular applications, which there are potentially a wide range of (Table 1, see also Section 2). Indeed, the fine‐scale distribution of canopy VWC and Ψ gained from eco Ψ‐WC curves would allow assessing (1) the allocation of water throughout the forest including shifts in storage (e.g., wood swelling (Pfautsch et al., 2015)); (2) water status thresholds for loss of function throughout the ecosystem (Martinez‐Vilalta et al., 2019; Sack et al., 2018; Trueba et al., 2007), (3) the driving forces for water movement; and (4), with knowledge of hydraulic conductances and capacitances, the water flows throughout the ecosystem (Figure 5). Furthermore, there is potential to extract parameters from eco Ψ‐WC curves, analogous to those extracted from leaf P‐V curves, to enable the consideration of how whole ecosystem drought resilience and its determinants shift over the course of the day and seasonally, and how ecosystem‐level drought responses vary across ecosystems of different diversity, climate, or soil type.
TABLE 1.
Applications of remotely sensed vegetation water content, relative water content at ecosystem scale (VWCeco, normalized by its maximum value), water potential (Ψ), and the ecosystem Ψ‐WC curve (eco Ψ‐WC)
| VWC | VWCeco | Ψ | Eco Ψ‐WC |
|---|---|---|---|
|
· Estimation of water distribution throughout the ecosystem and its dynamics with time, and environmental change · Can be directly converted to an ecosystem‐scale live fuel moisture content for fire risk estimation, by dividing VWC by aboveground dry biomass. · VWC may also predict drought‐induced mortality in trees |
·Thresholds for stomatal control, photosynthesis, wood growth, embolism, hydraulic dysfunction, mortality, etc., for a given tree or tissue and potentially ecosystems. |
·Thresholds for stomatal control, photosynthesis, wood growth, embolism, hydraulic dysfunction, mortality, etc., for a given tree or tissue and potentially ecosystems ·Overall driving forces for water flows at landscape, community, and ecosystem scale · Additional signal regarding tissues and belowground soil water potential ·Given known hydraulic conductances and capacitances, estimates of flows through given components of the system at any scale |
· Scaling up phenomena from cells to organs, to plants, to ecosystem · Determination of water allocation throughout the forest including shifts in storage · Transfer function for data assimilation of remotely sensed VWC or VWC validation campaigns · Clarification of the most informative water status thresholds for loss of function throughout the ecosystem · May yield ecosystem Ψ‐WC parameters useful for comparative assessment of drought tolerance and water relations across space and time |
FIGURE 5.
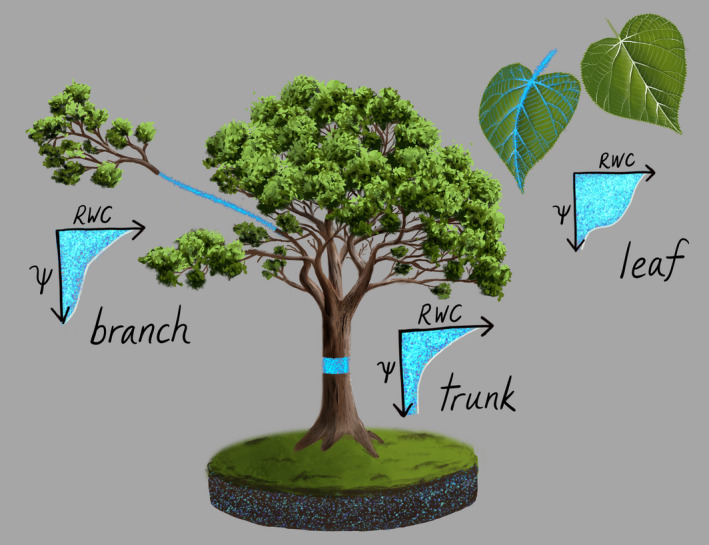
Vertical distributions of tissue‐specific water retention properties (RWC – Ψ curves), biomass, and sensor penetration depth all jointly determine remotely sensed water content and its temporal variation. Several hypothesized curves delineating gradients of capacitance, defined as the change in relative water content relative to that of water potential (C = ΔRWC/ΔΨ) are shown. Therefore, temporal variation in remotely sensed metrics of VWC will be determined not only by temporal variation in Ψ, but by potentially large differences in the exchangeability of water in response to changes in Ψ across different plant tissues, and the response of sensor penetration depth to changes in water content
The time of day considered affects the utility of eco Ψ‐WC curves. At predawn and midday or early afternoon, they may provide especially informative snapshots. Eco Ψ‐WC curves estimated at predawn have the advantages of simplicity and stability because, at equilibrium, flows through the system will not affect the distribution of water, and the root‐zone soil Ψ will also be indicated. Predawn Ψ may best yield certain thresholds for determining ecosystem function (e.g., wood growth, plant maximum hydraulic conductance) (Cabon et al., 2020). By contrast, midday or early afternoon eco Ψ‐WC curves include the near minimum Ψ and WC values, corresponding to the strongest diel drought stress (with the exact time of greatest stress variable depending on ecosystem type and meteorological conditions). Because the ways in which eco Ψ‐WC curves can be used vary by time of day, diurnally variable observations of VWC may be particularly useful for forest drought stress studies analyzing ecosystem‐scale Ψ variations.
5.2. Determining eco Ψ‐WC curves
To derive the eco Ψ‐WC, one must apply a modeling approach to the P‐V curves of tree tissues at given times (considering each tissue's water storage capacity and elastic properties), and scale these up based on forest structure (tree volume; tree sizes; allometries for roots, stems, and leaves; water content distribution) (Figure 5). Furthermore, the model must be dynamic, as Ψ will depend on the flow rate and hydraulic conductances and capacitances throughout the soil‐plant‐atmosphere continuum. While VWC is usually measured as the mass of water in vegetation per unit ground area, the relative water content (RWC), which normalizes tissue water content by the maximum (i.e., turgid) water‐holding capacity of vegetation, better allows comparisons across organs, species, growth stages, and scales, and may thus be the more useful quantity for use in an eco Ψ‐WC curve. To determine relative water content from absolute water content, a saturation water content must be estimated. One approach to measuring canopy RWC is to normalize VWC by its annual or seasonal maximum (resulting in a relative vegetation water content at ecosystem‐scale, VWCeco; Rao et al., 2019) while accounting for changes in aboveground biomass, for example, from leaf abscission during a drought event. This accounting requires ancillary information on phenology. It is also important to consider whether leaf surface water is included in the eco Ψ‐WC curve, or separated during the remote sensing retrieval of VWC (see Section 3.2). Along with tissue‐level scaling (including for components such as bark or roots, for which P‐V curves may not be as readily available as for leaves, see Section 2.3), information about canopy structure and diversity is needed to scale from trees to ecosystems. Structure and diversity information may be derived from a combination of forest inventories and remote sensing, including from ground, airborne, or space‐based lidar.
In order to relate eco Ψ‐WC curves to microwave retrievals of VWC, we need information on the vertical structure of the canopy and the frequency‐dependent penetration depth of VWC observations (Figure 4e–h). Because, at a given electromagnetic frequency, microwaves also pass farther through canopies with less water (Section 3.1), the effective depth that a given VWC measurement represents is likely to vary in space and time, particularly during a drought. To account for penetration depth variations, different eco Ψ‐WC curves be built to apply to specific electromagnetic frequencies. Once created, eco Ψ‐WC curves should then enable linking remotely sensed VWC to models, leading to improved quantification of plant traits, belowground variables (as further discussed below), and other factors affecting ecosystem drought response.
6. INFERRING BELOWGROUND ACTIVITY FROM ABOVEGROUND VWC OBSERVATIONS
A complete description of forest responses to drought requires accounting for several belowground factors, including the regulation of root water uptake, its three‐dimensional distribution, or competition among differing rooting systems, among others (Manoli et al., 2017). However, remote sensing measurements are currently unable to measure soil water or water fluxes in the root‐zone directly. Instead, belowground conditions must be inferred from aboveground information, such as VWC observations. This can be achieved through an inverse approach based on the analysis of aboveground conditions linked to belowground processes. When doing so, the complexity of the belowground mechanisms that are accounted for can span a wide range (Figure 6). Conventional ecohydrological models that seek to infer belowground conditions without considering VWC (assuming a single land surface water pool, e.g., n=1 in Figure 6) implicitly consider root water uptake to be in balance with transpiration (Chitra‐Tarak et al., 2018; Dralle et al., 2020; Fan et al., 2017; Kleidon, 2004), and therefore cannot resolve root water uptake variations that deviate from transpirational demand (Chuang et al., 2006; Hollinger et al., 1994; Phillips et al., 1997). Even non‐linear models that account for, for example, rooting depth changes with soil water content or the influence of biomass are not able to separate out root water uptake from transpiration if they do not account for VWC. We therefore argue that accounting for VWC (n ≥ 2 in Figure 6) is a necessary first step in inferring belowground conditions. If VWC observations across different vegetation layers are available (n ≥ 3 in Figure 6), this will enable greater detail in the inferred belowground conditions, such as soil water content variations across different depth layers and hydraulic redistribution.
FIGURE 6.
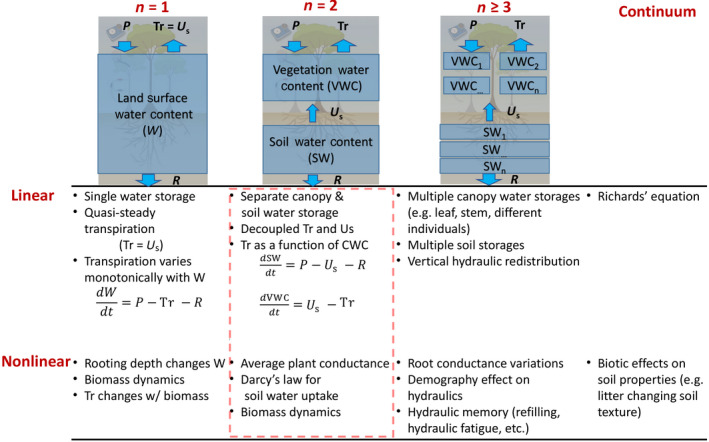
Conceptual diagram showing the complexity of vegetation–soil water dynamics viewed in the dimensionality–nonlinearity plane. The red dashed box shows the near‐term research direction for inferring belowground dynamics enabled by new observations of VWC from remote sensing. A diurnal hysteresis between VWC and soil water cannot be captured with traditional ecohydrological models that consider a single land surface water pool (n=1) and can only be explained with a two (or greater) pool framework (n≥2), which further allows for inference of vegetation water uptake based on the timing and magnitude of the hysteresis. Figure adapted from Strogatz (2015)
Inferring belowground activity from VWC dynamics requires solving the complex interactions between water pools and water fluxes. One approach for doing so relies on building plant hydraulic models and using data assimilation or optimization methods to constrain the parameters and states of these models (Liu et al., 2020; Mirfenderesgi et al., 2016). As with any data assimilation/optimization method, innovations in the assimilation/optimization technique and cost function specification (i.e., which mathematical function is optimized) may further improve the ability to accurately make belowground inferences (Dietze et al., 2011; Trudinger et al., 2007). Ensuring a reasonable balance between model parsimony (e.g., making sure the number of degrees of freedom of the model is not much greater than that of the observations) and model complexity (to ensure realistic dynamics can be captured) is also key for accurate assimilation methods. Ultimately, the accuracy of such inferences likely inherently depends on the relative sensitivity and information content of different observations, including the VWC estimates. Nevertheless, some early applications of data assimilation with remotely sensed VWC estimates show this approach has promise (Liu et al., 2021; Liu et al., 2021). In each of these studies, observations at two times a day were used, but a more complete diel cycle may act as an even stronger constraint.
As an alternative to computationally expensive data assimilation methods, additional information may be gained by considering the phase dynamics of soil and VWC (Figure 6). At any given time, the evolution of both soil and VWC is influenced by sap flow, plant water storage (in particular VWC) and other factors (i.e., precipitation, transpirational losses). The sap flow, in turn, depends on both the soil and vegetation water content (both through the potential gradient between each, and through VWC's influence on xylem conductance). Thus, for each of soil water content and VWC, the evolution of one water pool depends on the value of the other pool at any given amount of time. This state dependence inherently generates a hysteresis across diel timescales, as illustrated in Figure 7 (Lin et al., 2019; Zhang et al., 2014). With increasing data availability of VWC and (surface) soil water dynamics from remote sensing products, analysis of this hysteresis and the phase dynamics more generally can be used for detecting lags and tipping points. A specific example of such a phase dynamical analysis is mathematically illustrated through an analogy with the much‐studied predator–prey (also known as Lotka–Volterra) ecological model (Wangersky, 1978) in the Data S1 (where VWC is the predator in the Lotka–Volterra analogy, which preys on root‐zone water content). Development of simplified mathematical models such as these will enable more sophisticated phase dynamics interpretations using VWC or even multi‐layer VWC datasets, if those become available (Section 3.2). Taken together in a suite of work with data assimilation and inference approaches, VWC analyses can therefore generate significant progress in determining belowground hydrological activity with remote sensing at global scale.
FIGURE 7.
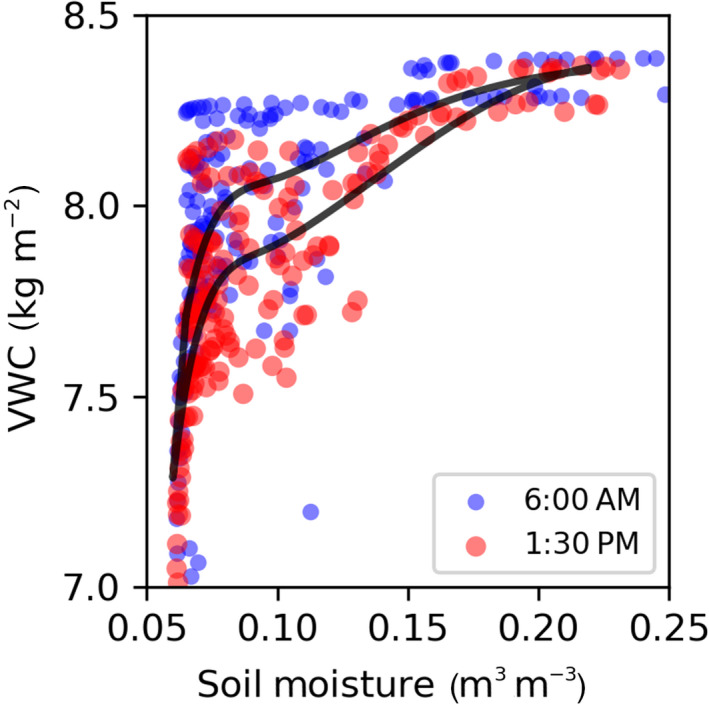
Example phase diagram of simulated dynamics of VWC and root‐zone soil moisture content for a model test bed site in an Amazon moist forest using a hydraulics‐enabled terrestrial biosphere model (ED‐2.2‐hydro, Xu et al., 2021). The diurnal hysteresis (closed curves in black between VWC and soil water cannot be captured with traditional ecohydrological models that consider a single land surface water pool (n=1 in Figure 6). Such hysteresis can only be explained with a two(or more)‐pool framework (n>=2 in Figure 6), which further allows for inference of vegetation water uptake based on the timing and magnitude of the hysteresis
7. THE NEED FOR SPACEBORNE DIEL OBSERVATIONS
In this section, we reflect on the value of current satellite measurements of VWC and then consider what satellite observational strategy might be most useful for understanding how forest ecosystems respond to drought in a changing climate. As discussed in Section 3, existing observations of VWC from space are mainly based on opportunistic analyses of measurements originally designed and used for other science applications. These datasets have been used for many studies of forest drought responses. However, they are all limited by a particular set of measurement characteristics. For example, most of these satellites are in sun‐synchronous orbits, which results in an overpass at each location on earth at a consistent time of the day, which is usually around 6:00 AM and PM or around 1:30 AM and PM, depending on the sensor. The sensor revisits every location on earth every 2–3 days, but only two particular times of day are ever observed. This prevents a full view of the diurnal cycle. In terms of spatial scale, available microwave remote sensing datasets of VWC exist at a range of resolutions (tens of meters to tens of kilometers), depending on the sensor type (radiometers, scatterometer, synthetic aperture radar (SAR)). However, a trade‐off exists. Relatively coarser datasets from radiometers and scatterometers have spatial resolutions of tens of kilometers, but are designed such that they revisit each location every few (2–3) days. By contrast, SAR sensors are able to obtain observations at scales of tens of meters, but only revisit a given location infrequently, often in an irregular fashion and usually averaging only a few (1–3) observations per month in any given location. Some sensor combinations provide observations more frequently, but only over small areas (e.g., Sentinel‐1 over Europe (Torres et al., 2012)). Furthermore, not all SAR instruments measure multiple polarizations in all observing modes. Measurements of different polarizations are required to disentangle the contributions of soil moisture and vegetation to observed backscatter. Additional polarizations may also further be useful to separate VWC from vegetation structural changes. Although VWC has been successfully retrieved from different combinations of possible polarizations (e.g., VV and VH from Sentinel‐1 (Han et al., 2019; Rao et al., 2020; Vreugdenhil et al., 2018), or VV and HH from QuikScat (Oveisgharan et al., 2018)), more research is needed to better understand the relative value of different polarizations and the optimal design of retrieval algorithms.
We recommend, at a high level, alternative satellite observations that can address several scientific measurement requirements to detect forest water stress and response to droughts. First, to predict how transpiration responds to droughts, it is necessary to quantify the influence of vegetation water content on stomatal conductance. This requires measurements of VWC throughout the day because the time of maximum vegetation water stress varies significantly from day to day, and because the shape of the diurnal cycle of VWC allows differentiation between the effects of limitations in root water uptake, transpiration, and redistribution of water storages through the canopy. Therefore, frequent observations of VWC throughout the day, at least every few hours, will allow for quantifying the response of plant stomatal conductance to water stress. Moreover, as discussed in Section 2.2, tree mortality and forest flammability have been shown in a few studies to have a threshold‐like response to declines in VWC. Whether such threshold‐like behavior holds at ecosystem scale and how such thresholds vary across biomes is unknown. Again, observations of the diel cycle will ensure that the periods of maximum VWC stress—where VWC is most likely to decrease below any thresholds—are determined. Beyond capturing the diel cycle, the revisit times between days of observations should also be sufficiently small so as to ensure periods of maximum stress are observed. Additionally, the temporal observations of VWC must extend across seasonal cycles of water availability to allow separation of the impacts of long‐term droughts from climatological seasonality, and must include multiple years of observations to capture both episodic climate extremes, interannual variability of forest canopy dynamics, and long‐term gradual climate stress. A long enough interannual observational record across large (e.g., continental to global) scales would enable determination of the factors driving how well and how fast biomes adapt to shifts in climate and seasonality.
The needs identified above—for diel observations with frequent revisit and over an extended period of time—suggest that a geostationary platform (rather than the typical sun‐synchronous orbits previously used for microwave satellites) would be needed to better quantify forest responses to drought. Furthermore, for each of the above applications, observational datasets would be particularly useful if they were able to distinguish landscape‐scale (e.g., kilometer‐scale) spatial variations, to allow detection of landscape‐scale variations of forest ecosystem response to water stress that depend on edaphic conditions, topography, forest structure, and land use history. At the high altitude of geostationary orbits, passive microwave radiometer systems cannot provide the required spatial resolutions. However, recent developments in radar technology, particularly at X‐ and Ku‐band frequencies (1–3 cm wavelength) provide an excellent opportunity for relatively high‐spatial resolution observations from a geostationary platform (Rodríguez et al., 2019; Xiao et al., 2020). Alternatively, a collection of smallsats (cubesats or other similar size classes) with different daily observation times could also potentially achieve the required temporal and spatial resolution (e.g., the Capella Space constellation (Stringham et al., 2019)). Each individual satellite could be placed in a sun‐synchronous orbit, but combining observations from multiple instruments would still enable observations of the full diurnal cycle. This approach has been successfully used to increase observational frequency by the Cyclone Global Navigation Satellite System (CYGNSS) (Ruf et al., 2019). Given the multiple potential approaches for developing spaceborne observations of VWC at diurnal cycles (although note the remaining technical challenges associated with retrieval algorithms, see Section 3.2), further research is needed to determine the best technological solution. Using the relatively high X‐band or Ku‐band frequencies will enable higher spatial resolution and reduce the effect of soil moisture on the radar backscatter observations, increasing retrieval accuracy. RapidScat radar observations at Ku‐band have previously been shown to successfully capture diel dynamics of VWC (van Emmerik et al., 2017; Konings, Yu, et al., 2017). Furthermore, at X‐band, cross‐comparison with existing sun‐synchronous datasets at times of near overlap (Du et al., 2017; Moesinger et al., 2020) could be used for calibration of the VWC retrieval algorithm. If technologically feasible, multiple electromagnetic frequencies could also be combined in a single observing platform to enable determination of VWC across multiple layers of the canopy (Section 3.2). We note that a geostationary platform would not be able to observe the entire globe, but continental coverage can nevertheless probe forest behavior across a range of biomes. A focus on the Americas may be particularly useful given the diversity of biomes and vegetation types spanned by this region and the relatively larger number of available field measurements compared to many other regions. Diel microwave observations of the Americas could also benefit from existing and future geostationary measurements covering the same area, such as solar‐induced fluorescence from the Geostationary Carbon Cycle Observatory (GeoCARB, Moore et al., 2018) and land surface temperature from the Geostationary Operational Environmental Satellites (Khan et al., 2021). A satellite mission with the above characteristics could be used not only to address the specific hypotheses discussed in the previous paragraph, but could also be used for a number of operational applications, such as predicting crop yields in the face of water stress, or improving fire risk models. The satellite design proposals above are summarized in Table 2.
TABLE 2.
Relationship between science and application goals and instrument functional requirements (as driven by the measurement requirements and science and application objectives necessary to meet the science and application goals) for a proposed set of new satellite observations
| Science and application goals | Science and applications hypotheses | Measurement requirements | Instrument functional requirements | |
|---|---|---|---|---|
|
Science question: How do forest ecosystems respond to droughts in a changing climate? |
There is a water content threshold beyond which tree mortality and flammability increase and productivity decline | Science requirements | ||
| Landscape‐scale VWC of forest ecosystems at 1σ < 1‐kg/m2 accuracy | Radar reflectivity at spatial resolutions of 1–3 km |
X‐band, Ku‐band, or multiple frequency (Ku‐ & L‐band) scatterometer or SAR Multiple polarization (HH, VV, HV) geostationary platform or collection of smallsats that provides observations several times a day Large swath to cover North and South Americas (50oN ‐ 50oS) at 1–3 day repeat cycle |
||
| Major resistance to water flux in forests is determined by changes in top‐canopy water content and its link to available soil water. | Diel changes of VWC at relative accuracy of 1σ < 10% | Radar reflectivity during day and night at multiple times throughout the day | ||
| Available soil water and the atmospheric environment will drive how well and how fast biomes adapt to climate change and shifts in seasonality | Seasonal changes of VWC at 1σ < 10% relative accuracy | Radar reflectivity at 1–3 day repeat cycle over minimum 3–5 years | ||
|
Application goal: Forecasting wildfires in forests and impacts of droughts on agriculture systems |
VWC determines fire fuel risk and drought resilience of crops | Application requirements | ||
| Daily to interstorm changes of VWC at 1σ < 10% relative accuracy | Radar reflectivity at 1–3 km spatial resolution |
X‐ or Ku‐band Multiple polarizations (HH, VV, HV) 1–3 day repeat cycle < 1‐km spatial resolution |
||
8. SUMMARY
We described the potential benefits of spatially extensive and frequent microwave remote sensing‐based VWC measurements for studying forest responses to drought, including for prediction of mortality and fire risk. Although such data have been increasingly used to characterize forests and their water relations, we identified several technical and scientific developments which could significantly accelerate the utility of these data. Specific recommendations include:
Analysis methods that consider VWC at different timescales depending on the ecosystem process of interest: multiple years to decades for forest biomass and structure, multiple weeks to months for changes in leaf area and multi‐day trends of relative water content, and diel for changes in relative water content due to plant water uptake, redistribution, and loss.
Development of Ψ‐WC curves analogous to branch‐scale pressure–volume curves, to relate ecosystem‐scale VWC to an effective canopy‐scale water potential
Data assimilation and optimization methods that can use integrate VWC into plant hydraulic models for determination of xylem and stomatal traits as well as belowground activity
Investigation of phase dynamics for characterization of belowground activity
Development of retrieval algorithms that account for surface water and vertical variations of VWC within the canopy, rather than retrieving only the vertically integrated, average water content.
The approaches above should greatly accelerate the use of VWC for forest drought responses studies even with existing datasets. Nevertheless, to make the most progress, additional field campaigns are necessary for validating (multi‐layer) VWC retrieval algorithms in a wide range of data types, for testing eco Ψ‐WC curves, and for improved understanding of how VWC‐based thresholds for fluxes, mortality, and fire risk scale across the ecosystem. Finally, a new geostationary mission concept providing diurnally variable measurements of VWC, which can be integrated with existing measurements, would dramatically expand the scope of available forest drought response studies.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Data S1
ACKNOWLEDGMENTS
We are grateful to Kara Skye Gibson for assisting Victor Leshyk with the creation of Figures 1, 3, and 5. We also thank our many collaborators for discussions. The outlines of this work were developed in the “Sensing Forest Water Dynamics from Space: Towards Predicting the Earth System Response to Droughts” study, which was initiated and supported by the W.M. Keck Institute for Space Studies. LS was supported by NSF grant DEB‐2017949. AGK was supported by NASA Terrestrial Ecology award 80NSSC18K0715. EA was supported as part of the Next Generation Ecosystem Experiments‐Tropics, funded by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research. The research carried out at the Jet Propulsion Laboratory, California Institute of Technology, was under a contract with the National Aeronautics and Space Administration. Oak Ridge National Laboratory is managed by UT‐Battelle, LLC, for the U.S. DOE under contract DE‐AC05‐100800OR22725.
Konings, A. G. , Saatchi, S. S. , Frankenberg, C. , Keller, M. , Leshyk, V. , Anderegg, W. R. L. , Humphrey, V. , Matheny, A. M. , Trugman, A. , Sack, L. , Agee, E. , Barnes, M. L. , Binks, O. , Cawse‐Nicholson, K. , Christoffersen, B. O. , Entekhabi, D. , Gentine, P. , Holtzman, N. M. , Katul, G. G. , … Zuidema, P. A. (2021). Detecting forest response to droughts with global observations of vegetation water content. Global Change Biology, 27, 6005–6024. 10.1111/gcb.15872
Data Availability Statement
QuikScat data shown in Figure 2 are available from the NASA PODAAC at https://podaac.jpl.nasa.gov/QuikSCAT.
REFERENCES
- Adams, H. D. , Zeppel, M. J. B. , Anderegg, W. R. L. , Hartmann, H. , Landhäusser, S. M. , Tissue, D. T. , Huxman, T. E. , Hudson, P. J. , Franz, T. E. , Allen, C. D. , Anderegg, L. D. L. , Barron‐Gafford, G. A. , Beerling, D. J. , Breshears, D. D. , Brodribb, T. J. , Bugmann, H. , Cobb, R. C. , Collins, A. D. , Dickman, L. T. , … McDowell, N. G. (2017). A multi‐species synthesis of physiological mechanisms in drought‐induced tree mortality. Nature Ecology & Evolution, 1(9), 1285–1291. 10.1038/s41559-017-0248-x [DOI] [PubMed] [Google Scholar]
- Agee, E. , He, L. , Bisht, G. , Couvreur, V. , Shahbaz, P. , Meunier, F. , Gough, C. M. , Matheny, A. M. , Bohrer, G. , & Ivanov, V. (2021). Root lateral interactions drive water uptake patterns under water limitation. Advances in Water Resources, 151, 103896, 10.1016/j.advwatres.2021.103896 [DOI] [Google Scholar]
- Allen, C. D. , Breshears, D. D. , & McDowell, N. G. (2015). On underestimation of global vulnerability to tree mortality and forest die‐off from hotter drought in the Anthropocene. Ecosphere, 6(8), art129. 10.1890/ES15-00203.1 [DOI] [Google Scholar]
- Anderegg, W. R. L. (2015). Spatial and temporal variation in plant hydraulic traits and their relevance for climate change impacts on vegetation. New Phytologist, 205(3), 1008–1014. 10.1111/nph.12907 [DOI] [PubMed] [Google Scholar]
- Anderegg, W. R. L. , Kane, J. M. , & Anderegg, L. D. L. (2013). Consequences of widespread tree mortality triggered by drought and temperature stress. Nature Climate Change, 3(1), 30–36. 10.1038/nclimate1635 [DOI] [Google Scholar]
- Anderegg, W. R. L. , Konings, A. G. , Trugman, A. T. , Yu, K. , Bowling, D. R. , Gabbitas, R. , Karp, D. S. , Pacala, S. , Sperry, J. S. , Sulman, B. N. , & Zenes, N. (2018). Hydraulic diversity of forests regulates ecosystem resilience during drought. Nature, 561(7724), 538–541. 10.1038/s41586-018-0539-7 [DOI] [PubMed] [Google Scholar]
- Anderegg, W. R. L. , Trugman, A. T. , Badgley, G. , Anderson, C. M. , Bartuska, A. , Ciais, P. , Cullenward, D. , Field, C. B. , Freeman, J. , Goetz, S. J. , Hicke, J. A. , Huntzinger, D. , Jackson, R. B. , Nickerson, J. , Pacala, S. , & Randerson, J. T. (2020). Climate‐driven risks to the climate mitigation potential of forests. Science, 368(6497), eaaz7005. 10.1126/science.aaz7005 [DOI] [PubMed] [Google Scholar]
- Araújo, T. , Higuchi, N. , de Carvalho, A. , & Júnior, J. (1999). Comparison of formulae for biomass content determination in a tropical rain forest site in the state of Pará. Brazil. Forest Ecology and Management, 117(1), 43–52. 10.1016/S0378-1127(98)00470-8 [DOI] [Google Scholar]
- Argañaraz, J. P. , Landi, M. A. , Scavuzzo, C. M. , & Bellis, L. M. (2018). Determining fuel moisture thresholds to assess wildfire hazard: A contribution to an operational early warning system. PLoS One, 13(10), e0204889. 10.1371/journal.pone.0204889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asefi‐Najafabady, S. , & Saatchi, S. (2013). Response of African humid tropical forests to recent rainfall anomalies. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1625), 20120306. 10.1098/rstb.2012.0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asner, G. P. , Brodrick, P. G. , Anderson, C. B. , Vaughn, N. , Knapp, D. E. , & Martin, R. E. (2016). Progressive forest canopy water loss during the 2012–2015 California drought. Proceedings of the National Academy of Sciences, 113(2), E249–E255. 10.1073/pnas.1523397113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett, M. K. , Scoffoni, C. , & Sack, L. (2012). The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta‐analysis: Drivers of plant drought tolerance. Ecology Letters, 15(5), 393–405. 10.1111/j.1461-0248.2012.01751.x [DOI] [PubMed] [Google Scholar]
- Baur, M. J. , Jagdhuber, T. , Feldman, A. F. , Akbar, R. , & Entekhabi, D. (2019). Estimation of relative canopy absorption and scattering at L‐, C‐ and X‐bands. Remote Sensing of Environment, 233, 111384. 10.1016/j.rse.2019.111384 [DOI] [Google Scholar]
- Binks, O. , Finnigan, J. , Coughlin, I. , Disney, M. , Calders, K. , Burt, A. , Vicari, M. B. , da Costa, A. L. , Mencuccini, M. , & Meir, P. (2021). Canopy wetness in the Eastern Amazon. Agricultural and Forest Meteorology, 297, 108250. 10.1016/j.agrformet.2020.108250 [DOI] [Google Scholar]
- Bohn, N. , Kuester, T. , Segl, K. , & Guanter, L. (2019). A coupled retrieval of columnar water vapor and canopy water content from spaceborne hyperspectral measurements. In 2019 10th Workshop on Hyperspectral Imaging and Signal Processing: Evolution in Remote Sensing (WHISPERS) (pp. 1–7). IEEE. 10.1109/WHISPERS.2019.8920860 [DOI] [Google Scholar]
- Brandt, M. , Wigneron, J.‐P. , Chave, J. , Tagesson, T. , Penuelas, J. , Ciais, P. , Rasmussen, K. , Tian, F. , Mbow, C. , Al‐Yaari, A. , Rodriguez‐Fernandez, N. , Schurgers, G. , Zhang, W. , Chang, J. , Kerr, Y. , Verger, A. , Tucker, C. , Mialon, A. , Rasmussen, L. V. , … Fensholt, R. (2018). Satellite passive microwaves reveal recent climate‐induced carbon losses in African drylands. Nature Ecology & Evolution, 2(5), 827–835. 10.1038/s41559-018-0530-6 [DOI] [PubMed] [Google Scholar]
- Brodribb, T. J. (2009). Xylem hydraulic physiology: The functional backbone of terrestrial plant productivity. Plant Science, 177(4), 245–251. 10.1016/j.plantsci.2009.06.001 [DOI] [Google Scholar]
- Brodribb, T. J. , Powers, J. , Cochard, H. , & Choat, B. (2020). Hanging by a thread? Forests and Drought. Science, 368(6488), 261–266. 10.1126/science.aat7631 [DOI] [PubMed] [Google Scholar]
- Browne, M. , Yardimci, N. T. , Scoffoni, C. , Jarrahi, M. , & Sack, L. (2020). Prediction of leaf water potential and relative water content using terahertz radiation spectroscopy. Plant Direct, 4(4), e00197. 10.1002/pld3.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabon, A. , Peters, R. L. , Fonti, P. , Martínez‐Vilalta, J. , & Cáceres, M. D. (2020). Temperature and water potential co‐limit stem cambial activity along a steep elevational gradient. New Phytologist, 226(5), 1325–1340. 10.1111/nph.16456 [DOI] [PubMed] [Google Scholar]
- Cavender‐Bares, J. , & Bazzaz, F. A. (2000). Changes in drought response strategies with ontogeny in Quercus rubra: implications for scaling from seedlings to mature trees. Oecologia, 124(1), 8–18. 10.1007/PL00008865 [DOI] [PubMed] [Google Scholar]
- Chitra‐Tarak, R. , Ruiz, L. , Dattaraja, H. S. , Mohan Kumar, M. S. , Riotte, J. , Suresh, H. S. , McMahon, S. M. , & Sukumar, R. (2018). The roots of the drought: Hydrology and water uptake strategies mediate forest‐wide demographic response to precipitation. Journal of Ecology, 106(4), 1495–1507. 10.1111/1365-2745.12925 [DOI] [Google Scholar]
- Choat, B. , Brodribb, T. J. , Brodersen, C. R. , Duursma, R. A. , López, R. , & Medlyn, B. E. (2018). Triggers of tree mortality under drought. Nature, 558(7711), 531–539. 10.1038/s41586-018-0240-x [DOI] [PubMed] [Google Scholar]
- Christoffersen, B. O. , Gloor, M. , Fauset, S. , Fyllas, N. M. , Galbraith, D. R. , Baker, T. R. et al (2016). Linking hydraulic traits to tropical forest function in a size‐structured and trait‐driven model (TFS vol 1‐Hydro). Geoscientific Model Development, 9(11), 4227–4255. 10.5194/gmd-9-4227-2016 [DOI] [Google Scholar]
- Chu, H. , Luo, X. , Ouyang, Z. , Chan, W. S. , Dengel, S. , Biraud, S. C. , Torn, M. S. , Metzger, S. , Kumar, J. , Arain, M. A. , Arkebauer, T. J. , Baldocchi, D. , Bernacchi, C. , Billesbach, D. , Black, T. A. , Blanken, P. D. , Bohrer, G. , Bracho, R. , Brown, S. , … Zona, D. (2021). Representativeness of Eddy‐Covariance flux footprints for areas surrounding AmeriFlux sites. Agricultural and Forest Meteorology, 301–302, 108350. 10.1016/j.agrformet.2021.108350 [DOI] [Google Scholar]
- Chuang, Y.‐L. , Oren, R. , Bertozzi, A. L. , Phillips, N. , & Katul, G. G. (2006). The porous media model for the hydraulic system of a conifer tree: Linking sap flux data to transpiration rate. Ecological Modelling, 191(3), 447–468. 10.1016/j.ecolmodel.2005.03.027 [DOI] [Google Scholar]
- Chuvieco, E. , Aguado, I. , & Dimitrakopoulos, A. P. (2011). Conversion of fuel moisture content values to ignition potential for integrated fire danger assessment. Canadian Journal of Forest Research, 10.1139/x04-101 [DOI] [Google Scholar]
- Dai, A. (2013). Increasing drought under global warming in observations and models. Nature Climate Change, 3(1), 52–58. 10.1038/nclimate1633 [DOI] [Google Scholar]
- de Jong, S. M. , Addink, E. A. , & Doelman, J. C. (2014). Detecting leaf‐water content in Mediterranean trees using high‐resolution spectrometry. International Journal of Applied Earth Observation and Geoinformation, 27, 128–136. 10.1016/j.jag.2013.09.011 [DOI] [Google Scholar]
- Dennison, P. E. , & Moritz, M. A. (2009). Critical live fuel moisture in chaparral ecosystems: A threshold for fire activity and its relationship to antecedent precipitation. International Journal of Wildland Fire, 18(8), 1021. 10.1071/WF08055 [DOI] [Google Scholar]
- Deshayes, M. , Guyon, D. , Jeanjean, H. , Stach, N. , Jolly, A. , & Hagolle, O. (2006). The contribution of remote sensing to the assessment of drought effects in forest ecosystems. Annals of Forest Science, 63(6), 579–595. 10.1051/forest:2006045 [DOI] [Google Scholar]
- Dietze, M. C. , Vargas, R. , Richardson, A. D. , Stoy, P. C. , Barr, A. G. , Anderson, R. S. , Arain, M. A. , Baker, I. T. , Black, T. A. , Chen, J. M. , Ciais, P. , Flanagan, L. B. , Gough, C. M. , Grant, R. F. , Hollinger, D. , Izaurralde, R. C. , Kucharik, C. J. , Lafleur, P. , Liu, S. , … Weng, E. (2011). Characterizing the performance of ecosystem models across time scales: A spectral analysis of the North American Carbon Program site‐level synthesis. Journal of Geophysical Research: Biogeosciences, 116(G4), G04029. 10.1029/2011JG001661 [DOI] [Google Scholar]
- Dimitrakopoulos, A. P. , & Papaioannou, K. K. (2001). Flammability assessment of mediterranean forest fuels. Fire Technology, 37(2), 143–152. 10.1023/A:1011641601076 [DOI] [Google Scholar]
- Dralle, D. N. , Jesse Hahm, W. , Rempe, D. M. , Karst, N. , Anderegg, L. D. L. , Thompson, S. E. , Dawson, T. E. , & Dietrich, W. E. (2020). Plants as sensors: Vegetation response to rainfall predicts root‐zone water storage capacity in Mediterranean‐type climates. Environmental Research Letters, 15(10), 104074. 10.1088/1748-9326/abb10b [DOI] [Google Scholar]
- Du, J. , Kimball, J. S. , Jones, L. A. , Kim, Y. , Glassy, J. , & Watts, J. D. (2017). A global satellite environmental data record derived from AMSR‐E and AMSR2 microwave Earth observations. Earth System Science Data, 9(2), 791–808. 10.5194/essd-9-791-2017 [DOI] [Google Scholar]
- Dubayah, R. , Blair, J. B. , Goetz, S. , Fatoyinbo, L. , Hansen, M. , Healey, S. , Hofton, M. , Hurtt, G. , Kellner, J. , Luthcke, S. , Armston, J. , Tang, H. , Duncanson, L. , Hancock, S. , Jantz, P. , Marselis, S. , Patterson, P. L. , Qi, W. , & Silva, C. (2020). The Global Ecosystem Dynamics Investigation: High‐resolution laser ranging of the Earth’s forests and topography. Science of Remote Sensing, 1, 100002. 10.1016/j.srs.2020.100002 [DOI] [Google Scholar]
- Fan, Y. , Miguez‐Macho, G. , Jobbágy, E. G. , Jackson, R. B. , & Otero‐Casal, C. (2017). Hydrologic regulation of plant rooting depth. Proceedings of the National Academy of Sciences, 114(40), 10572–10577. 10.1073/pnas.1712381114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frappart, F. , Wigneron, J.‐P. , Li, X. , Liu, X. , Al‐Yaari, A. , Fan, L. , Wang, M. , Moisy, C. , Le Masson, E. , Aoulad Lafkih, Z. , Vallé, C. , Ygorra, B. , & Baghdadi, N. (2020). Global monitoring of the vegetation dynamics from the Vegetation Optical Depth (VOD): A review. Remote Sensing, 12(18), 2915. 10.3390/rs12182915 [DOI] [Google Scholar]
- Frolking, S. , Milliman, T. , McDonald, K. , Kimball, J. , Zhao, M. , & Fahnestock, M. (2006). Evaluation of the SeaWinds scatterometer for regional monitoring of vegetation phenology. Journal of Geophysical Research, 111(D17), D17302. 10.1029/2005JD006588 [DOI] [Google Scholar]
- Frolking, S. , Milliman, T. , Palace, M. , Wisser, D. , Lammers, R. , & Fahnestock, M. (2011). Tropical forest backscatter anomaly evident in SeaWinds scatterometer morning overpass data during 2005 drought in Amazonia. Remote Sensing of Environment, 115(3), 897–907. 10.1016/j.rse.2010.11.017 [DOI] [Google Scholar]
- Gazol, A. , Camarero, J. J. , Vicente‐Serrano, S. M. , Sánchez‐Salguero, R. , Gutiérrez, E. , de Luis, M. , Sangüesa‐Barreda, G. , Novak, K. , Rozas, V. , Tíscar, P. A. , Linares, J. C. , Martín‐Hernández, N. , Martínez del Castillo, E. , Ribas, M. , García‐González, I. , Silla, F. , Camisón, A. , Génova, M. , Olano, J. M. , … Galván, J. D. (2018). Forest resilience to drought varies across biomes. Global Change Biology, 24(5), 2143–2158. 10.1111/gcb.14082 [DOI] [PubMed] [Google Scholar]
- Gentine, P. , Guérin, M. , Uriarte, M. , McDowell, N. G. , & Pockman, W. T. (2016). An allometry‐based model of the survival strategies of hydraulic failure and carbon starvation. Ecohydrology, 9(3), 529–546. 10.1002/eco.1654 [DOI] [Google Scholar]
- Goldstein, G. , Andrade, J. L. , Meinzer, F. C. , Holbrook, N. M. , Cavelier, J. , Jackson, P. , & Celis, A. (1998). Stem water storage and diurnal patterns of water use in tropical forest canopy trees. Plant, Cell and Environment, 21(4), 397–406. 10.1046/j.1365-3040.1998.00273.x [DOI] [Google Scholar]
- Goulden, M. L. , & Bales, R. C. (2019). California forest die‐off linked to multi‐year deep soil drying in 2012–2015 drought. Nature Geoscience, 12(8), 632–637. 10.1038/s41561-019-0388-5 [DOI] [Google Scholar]
- Guo, J. S. , Hultine, K. R. , Koch, G. W. , Kropp, H. , & Ogle, K. (2020). Temporal shifts in iso/anisohydry revealed from daily observations of plant water potential in a dominant desert shrub. New Phytologist, 225(2), 713–726. 10.1111/nph.16196 [DOI] [PubMed] [Google Scholar]
- Hagedorn, F. , Joseph, J. , Peter, M. , Luster, J. , Pritsch, K. , Geppert, U. , Kerner, R. , Molinier, V. , Egli, S. , Schaub, M. , Liu, J.‐F. , Li, M. , Sever, K. , Weiler, M. , Siegwolf, R. T. W. , Gessler, A. , & Arend, M. (2016). Recovery of trees from drought depends on belowground sink control. Nature Plants, 2(8), 1–5. 10.1038/nplants.2016.111 [DOI] [PubMed] [Google Scholar]
- Han, D. , Liu, S. , Du, Y. , Xie, X. , Fan, L. , Lei, L. , Li, Z. , Yang, H. , & Yang, G. (2019). Crop water content of winter wheat revealed with sentinel‐1 and sentinel‐2 imagery. Sensors, 19(18), 4013. 10.3390/s19184013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, P. J. , & Weltzin, J. F. (2000). Drought disturbance from climate change: Response of United States forests. Science of the Total Environment, 262(3), 205–220. 10.1016/S0048-9697(00)00523-4 [DOI] [PubMed] [Google Scholar]
- Hillel, D. (2013). Fundamentals of soil physics. Academic press. [Google Scholar]
- Holbrook, N. M. , & Sinclair, T. R. (1992). Water balance in the arborescent palm, Sabal palmetto. II. Transpiration and stem water storage. Plant, Cell & Environment, 15(4), 401–409. 10.1111/j.1365-3040.1992.tb00990.x [DOI] [Google Scholar]
- Hollinger, D. Y. , Kelliher, F. M. , Schulze, E.‐D. , & Köstner, B. M. M. (1994). Coupling of tree transpiration to atmospheric turbulence. Nature, 371(6492), 60–62. 10.1038/371060a0 [DOI] [Google Scholar]
- Holtzman, N. M. , Anderegg, L. D. L. , Kraatz, S. , Mavrovic, A. , Sonnentag, O. , Pappas, C. , Cosh, M. H. , Langlois, A. , Lakhankar, T. , Tesser, D. , Steiner, N. , Colliander, A. , Roy, A. , & Konings, A. G. (2021). L‐band vegetation optical depth as an indicator of plant water potential in a temperate deciduous forest stand. Biogeosciences, 18(2), 739–753. 10.5194/bg-18-739-2021 [DOI] [Google Scholar]
- Jackson, T. , Bindlish, R. , Hoonyol, L. , & Sukyoung, H. (2012). Radar vegetation index for estimating the vegetation water content of rice and soybean. IEEE Geoscience and Remote Sensing Letters, 9(4), 564–568. 10.1109/LGRS.2011.2174772 [DOI] [Google Scholar]
- Jackson, T. J. , & Schmugge, T. J. (1991). Vegetation effects on the microwave emission of soils. Remote Sensing of Environment, 36(3), 203–212. 10.1016/0034-4257(91)90057-D [DOI] [Google Scholar]
- Jones, H. G. , Serraj, R. , Loveys, B. R. , Xiong, L. , Wheaton, A. , & Price, A. H. (2009). Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Functional Plant Biology, 36(10–11), 978–989. 10.1071/FP09123 [DOI] [PubMed] [Google Scholar]
- Jones, M. O. , Jones, L. A. , Kimball, J. S. , & McDonald, K. C. (2011). Satellite passive microwave remote sensing for monitoring global land surface phenology. Remote Sensing of Environment, 115(4), 1102–1114. 10.1016/j.rse.2010.12.015 [DOI] [Google Scholar]
- Kennedy, D. , Swenson, S. , Oleson, K. W. , Lawrence, D. M. , Fisher, R. , Lola da Costa, A. C. , & Gentine, P. (2019). Implementing plant hydraulics in the community land model, version 5. Journal of Advances in Modeling Earth Systems, 11(2), 485–513. 10.1029/2018MS001500 [DOI] [Google Scholar]
- Khan, A. M. , Stoy, P. C. , Douglas, J. T. , Anderson, M. , Diak, G. , Otkin, J. A. , Hain, C. , Rehbein, E. M. , & McCorkel, J. (2021). Reviews and syntheses: Ongoing and emerging opportunities to improve environmental science using observations from the Advanced Baseline Imager on the Geostationary Operational Environmental Satellites. Biogeosciences, 18(13), 4117–4141. 10.5194/bg-18-4117-2021 [DOI] [Google Scholar]
- Kleidon, A. (2004). Global datasets of rooting zone depth inferred from inverse methods. Journal of Climate, 17(13), 2714–2722. [DOI] [Google Scholar]
- Konings, A. G. , Holtzman, N. M. , Rao, K. , Xu, L. , & Saatchi, S. S. (2021). Interannual variations of vegetation optical depth are due to both water stress and biomass changes. Geophysical Research Letters, 48(16), e2021GL095267. 10.1029/2021GL095267 [DOI] [Google Scholar]
- Konings, A. G. , Piles, M. , Das, N. , & Entekhabi, D. (2017). L‐band vegetation optical depth and effective scattering albedo estimation from SMAP. Remote Sensing of Environment, 198, 460–470. 10.1016/j.rse.2017.06.037 [DOI] [Google Scholar]
- Konings, A. G. , Rao, K. , & Steele‐Dunne, S. C. (2019). Macro to micro: Microwave remote sensing of plant water content for physiology and ecology. New Phytologist, 223(3), 1166–1172. 10.1111/nph.15808 [DOI] [PubMed] [Google Scholar]
- Konings, A. G. , Yu, Y. , Xu, L. , Yang, Y. , Schimel, D. S. , & Saatchi, S. S. (2017). Active microwave observations of diurnal and seasonal variations of canopy water content across the humid African tropical forests. Geophysical Research Letters, 44(5), 2290–2299. 10.1002/2016GL072388 [DOI] [Google Scholar]
- Kumar, R. , Rosen, P. , & Misra, T. (2016). NASA‐ISRO synthetic aperture radar: science and applications. In Earth Observing Missions and Sensors: Development, Implementation, and Characterization IV (Vol. 9881, p. 988103). International Society for Optics and Photonics. 10.1117/12.2228027 [DOI] [Google Scholar]
- Levine, N. M. , Zhang, K. E. , Longo, M. , Baccini, A. , Phillips, O. L. , Lewis, S. L. , Alvarez‐Dávila, E. , Segalin de Andrade, A. C. , Brienen, R. J. W. , Erwin, T. L. , Feldpausch, T. R. , Monteagudo Mendoza, A. L. , Nuñez Vargas, P. , Prieto, A. , Silva‐Espejo, J. E. , Malhi, Y. , & Moorcroft, P. R. (2016). Ecosystem heterogeneity determines the ecological resilience of the Amazon to climate change. Proceedings of the National Academy of Sciences, 113(3), 793–797. 10.1073/pnas.1511344112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Yang, Z.‐L. , Matheny, A. M. , Zheng, H. , Swenson, S. C. , Lawrence, D. M. , Barlage, M. , Yan, B. , McDowell, N. G. , & Leung, L. R. (2020). Representation of plant hydraulics in the Noah‐MP land surface model: Model development and multi‐scale evaluation. Journal of Advances in Modeling Earth Systems, 13(4), e2020MS002214. 10.1029/2020MS002214 [DOI] [Google Scholar]
- Lin, C. , Gentine, P. , Frankenberg, C. , Zhou, S. , Kennedy, D. , & Li, X. (2019). Evaluation and mechanism exploration of the diurnal hysteresis of ecosystem fluxes. Agricultural and Forest Meteorology, 278, 107642. 10.1016/j.agrformet.2019.107642 [DOI] [Google Scholar]
- Liu, Y. Y. , Evans, J. P. , McCabe, M. F. , de Jeu, R. A. M. , van Dijk, A. I. J. M. , Dolman, A. J. , & Saizen, I. (2013). Changing climate and overgrazing are decimating mongolian steppes. PLoS One, 8(2), e57599. 10.1371/journal.pone.0057599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Holtzman, N. M. , & Konings, A. G. (2021). Global ecosystem‐scale plant hydraulic traits retrieved using model–data fusion. Hydrology and Earth System Sciences, 25(5), 2399–2417. 10.5194/hess-25-2399-2021 [DOI] [Google Scholar]
- Liu, Y. , Konings, A. G. , Kennedy, D. , & Gentine, P. (2021). Global coordination in plant physiological and rooting strategies in response to water stress. Global Biogeochemical Cycles, 35(7), e2020GB006758. 10.1029/2020GB006758 [DOI] [Google Scholar]
- Liu, Y. , Kumar, M. , Katul, G. G. , Feng, X. , & Konings, A. G. (2020). Plant hydraulics accentuates the effect of atmospheric moisture stress on transpiration. Nature Climate Change, 10(7), 691–695. 10.1038/s41558-020-0781-5 [DOI] [Google Scholar]
- Liu, Y. Y. , van Dijk, A. I. J. M. , de Jeu, R. A. M. , Canadell, J. G. , McCabe, M. F. , Evans, J. P. , & Wang, G. (2015). Recent reversal in loss of global terrestrial biomass. Nature Climate Change, 5(5), 470–474. 10.1038/nclimate2581 [DOI] [Google Scholar]
- Manoli, G. , Huang, C.‐W. , Bonetti, S. , Domec, J.‐C. , Marani, M. , & Katul, G. (2017). Competition for light and water in a coupled soil‐plant system. Advances in Water Resources, 108, 216–230. 10.1016/j.advwatres.2017.08.004 [DOI] [Google Scholar]
- Martinez‐Vilalta, J. , Anderegg, W. R. L. , Sapes, G. , & Sala, A. (2019). Greater focus on water pools may improve our ability to understand and anticipate drought‐induced mortality in plants. New Phytologist, 223(1), 22–32. 10.1111/nph.15644 [DOI] [PubMed] [Google Scholar]
- Martínez‐Vilalta, J. , & Lloret, F. (2016). Drought‐induced vegetation shifts in terrestrial ecosystems: The key role of regeneration dynamics. Global and Planetary Change, 144, 94–108. 10.1016/j.gloplacha.2016.07.009 [DOI] [Google Scholar]
- Mastrotheodoros, T. , Pappas, C. , Molnar, P. , Burlando, P. , Manoli, G. , Parajka, J. , Rigon, R. , Szeles, B. , Bottazzi, M. , Hadjidoukas, P. , & Fatichi, S. (2020). More green and less blue water in the Alps during warmer summers. Nature Climate Change, 10(2), 155–161. 10.1038/s41558-019-0676-5 [DOI] [Google Scholar]
- Matheny, A. M. , Bohrer, G. , Garrity, S. R. , Morin, T. H. , Howard, C. J. , & Vogel, C. S. (2015). Observations of stem water storage in trees of opposing hydraulic strategies. Ecosphere, 6(9), art165. 10.1890/ES15-00170.1 [DOI] [Google Scholar]
- Matheny, A. M. , Fiorella, R. P. , Bohrer, G. , Poulsen, C. J. , Morin, T. H. , Wunderlich, A. , Vogel, C. S. , & Curtis, P. S. (2017). Contrasting strategies of hydraulic control in two codominant temperate tree species. Ecohydrology, 10(3), e1815. 10.1002/eco.1815 [DOI] [Google Scholar]
- Matheny, A. M. , Garrity, S. R. , & Bohrer, G. (2017). The calibration and use of capacitance sensors to monitor stem water content in trees. Journal of Visualized Experiments, 130, e57062. 10.3791/57062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell, N. G. (2011). Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiology, 155(3), 1051–1059. 10.1104/pp.110.170704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencuccini, M. , Manzoni, S. , & Christoffersen, B. (2019). Modelling water fluxes in plants: From tissues to biosphere. New Phytologist, 222(3), 1207–1222. 10.1111/nph.15681 [DOI] [PubMed] [Google Scholar]
- Mirfenderesgi, G. , Bohrer, G. , Matheny, A. M. , Fatichi, S. , de Moraes Frasson, R. P. , & Schäfer, K. V. R. (2016). Tree level hydrodynamic approach for resolving aboveground water storage and stomatal conductance and modeling the effects of tree hydraulic strategy: Stomatal Conductance Parameterization. Journal of Geophysical Research: Biogeosciences, 121(7), 1792–1813. 10.1002/2016JG003467 [DOI] [Google Scholar]
- Moesinger, L. , Dorigo, W. , de Jeu, R. , van der Schalie, R. , Scanlon, T. , Teubner, I. , & Forkel, M. (2020). The global long‐term microwave Vegetation Optical Depth Climate Archive (VODCA). Earth System Science Data, 12(1), 177–196. 10.5194/essd-12-177-2020 [DOI] [Google Scholar]
- Monteith, A. R. , & Ulander, L. M. H. (2018). Temporal survey of P‐ and L‐Band polarimetric backscatter in boreal forests. IEEE Journal of Selected Topics in Applied Earth Observations and Remote Sensing, 11(10), 3564–3577. 10.1109/JSTARS.2018.2814825 [DOI] [Google Scholar]
- Moore III, B. , Crowell, S. M. R. , Rayner, P. J. , Kumer, J. , O'Dell, C. W. , O'Brien, D. , Utembe, S. , Polonsky, I. , Schimel, D. , & Lemen, J. (2018). The potential of the Geostationary Carbon Cycle Observatory (GeoCarb) to provide multi‐scale constraints on the carbon cycle in the Americas. Frontiers in Environmental Science, 6, 10.3389/fenvs.2018.00109 [DOI] [Google Scholar]
- Nelson, J. A. , Carvalhais, N. , Migliavacca, M. , Reichstein, M. , & Jung, M. (2018). Water‐stress‐induced breakdown of carbon–water relations: indicators from diurnal FLUXNET patterns. Biogeosciences (Online), 15(8), 2433–2447. 10.5194/bg-15-2433-2018 [DOI] [Google Scholar]
- Nolan, R. H. , Blackman, C. J. , de Dios, V. R. , Choat, B. , Medlyn, B. E. , Li, X. , Bradstock, R. A. , & Boer, M. M. (2020). Linking forest flammability and plant vulnerability to drought. Forests, 11(7), 779. 10.3390/f11070779 [DOI] [Google Scholar]
- Oveisgharan, S. , Haddad, Z. , Turk, J. , Rodriguez, E. , & Li, L. (2018). Soil moisture and vegetation water content retrieval using QuikSCAT data. Remote Sensing, 10(4), 636. 10.3390/rs10040636 [DOI] [Google Scholar]
- Pallardy, S. G. , Pereira, J. S. , & Parker, W. C. (1991). Measuring the state of water in tree systems. Techniques and Approaches in Forest Tree Ecophysiology, 28–76. [Google Scholar]
- Parinussa, R. , de Jeu, R. , van der Schalie, R. , Crow, W. , Lei, F. , & Holmes, T. (2016). A quasi‐global approach to improve day‐time satellite surface soil moisture anomalies through the land surface temperature input. Climate, 4(4), 50. 10.3390/cli4040050 [DOI] [Google Scholar]
- Peters, R. L. , Steppe, K. , Cuny, H. E. , De Pauw, D. J. W. , Frank, D. C. , Schaub, M. , Rathgeber, C. B. K. , Cabon, A. , & Fonti, P. (2021). Turgor – a limiting factor for radial growth in mature conifers along an elevational gradient. New Phytologist, 229(1), 213–229. 10.1111/nph.16872 [DOI] [PubMed] [Google Scholar]
- Pfautsch, S. , Hölttä, T. , & Mencuccini, M. (2015). Hydraulic functioning of tree stems—fusing ray anatomy, radial transfer and capacitance. Tree Physiology, 35(7), 706–722. 10.1093/treephys/tpv058 [DOI] [PubMed] [Google Scholar]
- Phillips, N. , Nagchaudhuri, A. , Oren, R. , & Katul, G. (1997). Time constant for water transport in loblolly pine trees estimated from time series of evaporative demand and stem sapflow. Trees, 11(7), 412–419. 10.1007/s004680050102 [DOI] [Google Scholar]
- Phillips, N. G. , Ryan, M. G. , Bond, B. J. , McDowell, N. G. , Hinckley, T. M. , & Čermák, J. (2003). Reliance on stored water increases with tree size in three species in the Pacific Northwest. Tree Physiology, 23(4), 237–245. 10.1093/treephys/23.4.237 [DOI] [PubMed] [Google Scholar]
- Phillips, R. P. , Ibáñez, I. , D’Orangeville, L. , Hanson, P. J. , Ryan, M. G. , & McDowell, N. G. (2016). A belowground perspective on the drought sensitivity of forests: Towards improved understanding and simulation. Forest Ecology and Management, 380, 309–320. 10.1016/j.foreco.2016.08.043 [DOI] [Google Scholar]
- Pugh, T. A. M. , Arneth, A. , Kautz, M. , Poulter, B. , & Smith, B. (2019). Important role of forest disturbances in the global biomass turnover and carbon sinks. Nature Geoscience, 12(9), 730–735. 10.1038/s41561-019-0427-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quegan, S. , Le Toan, T. , Chave, J. , Dall, J. , Exbrayat, J.‐F. , Minh, D. H. T. , Lomas, M. , D'Alessandro, M. M. , Paillou, P. , Papathanassiou, K. , Rocca, F. , Saatchi, S. , Scipal, K. , Shugart, H. , Smallman, T. L. , Soja, M. J. , Tebaldini, S. , Ulander, L. , Villard, L. , & Williams, M. (2019). The European Space Agency BIOMASS mission: Measuring forest above‐ground biomass from space. Remote Sensing of Environment, 227, 44–60. 10.1016/j.rse.2019.03.032 [DOI] [Google Scholar]
- Rao, K. , Anderegg, W. R. L. , Sala, A. , Martínez‐Vilalta, J. , & Konings, A. G. (2019). Satellite‐based vegetation optical depth as an indicator of drought‐driven tree mortality. Remote Sensing of Environment, 227, 125–136. 10.1016/j.rse.2019.03.026 [DOI] [Google Scholar]
- Rao, K. , Williams, A. P. , Flefil, J. F. , & Konings, A. G. (2020). SAR‐enhanced mapping of live fuel moisture content. Remote Sensing of Environment, 245, 111797. 10.1016/j.rse.2020.111797 [DOI] [Google Scholar]
- Richter, H. (1978). A diagram for the description of water relations in plant cells and organs. Journal of Experimental Botany, 29(5), 1197–1203. 10.1093/jxb/29.5.1197 [DOI] [Google Scholar]
- Rodríguez, E. , Bourassa, M. , Chelton, D. , Farrar, J. T. , Long, D. , Perkovic‐Martin, D. , & Samelson, R. (2019). The winds and currents mission concept. Frontiers in Marine Science, 6, 438. 10.3389/fmars.2019.00438 [DOI] [Google Scholar]
- Ruf, C. S. , Chew, C. , Lang, T. , Morris, M. G. , Nave, K. , Ridley, A. , & Balasubramaniam, R. (2018). A New paradigm in earth environmental monitoring with the CYGNSS small satellite constellation. Scientific Reports, 8(1), 8782. 10.1038/s41598-018-27127-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatchi, S. , Asefi‐Najafabady, S. , Malhi, Y. , Aragao, L. E. O. C. , Anderson, L. O. , Myneni, R. B. , & Nemani, R. (2013). Persistent effects of a severe drought on Amazonian forest canopy. Proceedings of the National Academy of Sciences, 110(2), 565–570. 10.1073/pnas.1204651110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatchi, S. S. , & Moghaddam, M. (2000). Estimation of crown and stem water content and biomass of boreal forest using polarimetric SAR imagery. IEEE Transactions on Geoscience and Remote Sensing, 38(2), 697–709. 10.1109/36.841999 [DOI] [Google Scholar]
- Sack, L. , John, G. P. , & Buckley, T. N. (2018). ABA accumulation in dehydrating leaves is associated with decline in cell volume, Not Turgor Pressure. Plant Physiology, 176(1), 489–495. 10.1104/pp.17.01097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapes, G. , Roskilly, B. , Dobrowski, S. , Maneta, M. , Anderegg, W. R. L. , Martinez‐Vilalta, J. , & Sala, A. (2019). Plant water content integrates hydraulics and carbon depletion to predict drought‐induced seedling mortality. Tree Physiology, 39(8), 1300–1312. 10.1093/treephys/tpz062 [DOI] [PubMed] [Google Scholar]
- Schmugge, T. J. , & Jackson, T. J. (1992). A dielectric model of the vegetation effects on the microwave emission from soils. IEEE Transactions on Geoscience and Remote Sensing, 30(4), 757–760. 10.1109/36.158870 [DOI] [Google Scholar]
- Schneebeli, M. , Wolf, S. , Kunert, N. , Eugster, W. , & Mätzler, C. (2011). Relating the X‐band opacity of a tropical tree canopy to sapflow, rain interception and dew formation. Remote Sensing of Environment, 115(8), 2116–2125. 10.1016/j.rse.2011.04.016 [DOI] [Google Scholar]
- Scholander, P. F. , Bradstreet, E. D. , Hemmingsen, E. A. , & Hammel, H. T. (1965). Sap pressure in vascular plants: Negative hydrostatic pressure can be measured in plants. Science, 148(3668), 339–346. 10.1126/science.148.3668.339 [DOI] [PubMed] [Google Scholar]
- Scholander, P. F. , Hammel, H. T. , Hemmingsen, E. A. , & Bradstreet, E. D. (1964). Hydrostatic pressure and osmotic potential in leaves of mangroves and some other plants. Proceedings of the National Academy of Sciences, 52(1), 119–125. 10.1073/pnas.52.1.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, R. , McDonald, K. C. , Azarderakhsh, M. , & Zimmermann, R. (2016). ASCAT MetOp‐A diurnal backscatter observations of recent vegetation drought patterns over the contiguous U.S.: An assessment of spatial extent and relationship with precipitation and crop yield. Remote Sensing of Environment, 177, 153–159. 10.1016/j.rse.2016.01.008 [DOI] [Google Scholar]
- Sharma, A. , Lang, R. H. , Kurum, M. , O'neill, P. E. , & Cosh, M. H. (2020). L‐Band radar experiment and modeling of a corn canopy over a full growing season. IEEE Transactions on Geoscience and Remote Sensing, 58(8), 5821–5835. 10.1109/TGRS.2020.2971539 [DOI] [Google Scholar]
- Short Gianotti, D. J. , Rigden, A. J. , Salvucci, G. D. , & Entekhabi, D. (2019). Satellite and station observations demonstrate water availability’s effect on continental‐scale evaporative and photosynthetic land surface dynamics. Water Resources Research, 55(1), 540–554. 10.1029/2018WR023726 [DOI] [Google Scholar]
- Skelton, R. P. , West, A. G. , & Dawson, T. E. (2015). Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proceedings of the National Academy of Sciences, 112(18), 5744–5749. 10.1073/pnas.1503376112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry, J. S. , & Love, D. M. (2015). What plant hydraulics can tell us about responses to climate‐change droughts. New Phytologist, 207(1), 14–27. 10.1111/nph.13354 [DOI] [PubMed] [Google Scholar]
- Steele‐Dunne, S. C. , McNairn, H. , Monsivais‐Huertero, A. , Judge, J. , Liu, P.‐W. , & Papathanassiou, K. (2017). Radar remote sensing of agricultural canopies: A review. IEEE Journal of Selected Topics in Applied Earth Observations and Remote Sensing, 10(5), 2249–2273. 10.1109/JSTARS.2016.2639043 [DOI] [Google Scholar]
- Stringham, C. , Farquharson, G. , Castelletti, D. , Quist, E. , Riggi, L. , Eddy, D. , & Soenen, S. (2019). The capella X‐band SAR constellation for rapid imaging. In Proc. IEEE Int. Geosci. Remote Sens. Symp. (IGARSS) (pp. 9248–9251). 10.1109/IGARSS.2019.8900410 [DOI] [Google Scholar]
- Strogatz, S. H. (2015). Nonlinear dynamics and chaos: with applications to physics, biology, chemistry, and engineering, 2nd ed. Westview Press, a member of the Perseus Books Group. [Google Scholar]
- Suding, K. N. , & Hobbs, R. J. (2009). Threshold models in restoration and conservation: a developing framework. Trends in Ecology & Evolution, 24(5), 271–279. 10.1016/j.tree.2008.11.012 [DOI] [PubMed] [Google Scholar]
- Tague, C. L. , Moritz, M. , & Hanan, E. (2019). The changing water cycle: The eco‐hydrologic impacts of forest density reduction in Mediterranean (seasonally dry) regions. Wires Water, 6(4), e1350. 10.1002/wat2.1350 [DOI] [Google Scholar]
- Tardieu, F. , & Simonneau, T. (1998). Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. Journal of Experimental Botany, 49(Special_Issue), 419–432. 10.1093/jxb/49.Special_Issue.419 [DOI] [Google Scholar]
- Tian, F. , Brandt, M. , Liu, Y. Y. , Rasmussen, K. , & Fensholt, R. (2017). Mapping gains and losses in woody vegetation across global tropical drylands. Global Change Biology, 23(4), 1748–1760. 10.1111/gcb.13464 [DOI] [PubMed] [Google Scholar]
- Tian, F. , Brandt, M. , Liu, Y. Y. , Verger, A. , Tagesson, T. , Diouf, A. A. , Rasmussen, K. , Mbow, C. , Wang, Y. , & Fensholt, R. (2016). Remote sensing of vegetation dynamics in drylands: Evaluating vegetation optical depth (VOD) using AVHRR NDVI and in situ green biomass data over West African Sahel. Remote Sensing of Environment, 177, 265–276. 10.1016/j.rse.2016.02.056 [DOI] [Google Scholar]
- Togliatti, K. , Hartman, T. , Walker, V. A. , Arkebauer, T. J. , Suyker, A. E. , VanLoocke, A. , & Hornbuckle, B. K. (2019). Satellite L–band vegetation optical depth is directly proportional to crop water in the US Corn Belt. Remote Sensing of Environment, 233, 111378. 10.1016/j.rse.2019.111378 [DOI] [Google Scholar]
- Torres, R. , Snoeij, P. , Geudtner, D. , Bibby, D. , Davidson, M. , Attema, E. , Potin, P. , Rommen, B. Ö. , Floury, N. , Brown, M. , Traver, I. N. , Deghaye, P. , Duesmann, B. , Rosich, B. , Miranda, N. , Bruno, C. , L'Abbate, M. , Croci, R. , Pietropaolo, A. , … Rostan, F. (2012). GMES Sentinel‐1 mission. Remote Sensing of Environment, 120, 9–24. 10.1016/j.rse.2011.05.028 [DOI] [Google Scholar]
- Touma, D. , Ashfaq, M. , Nayak, M. A. , Kao, S.‐C. , & Diffenbaugh, N. S. (2015). A multi‐model and multi‐index evaluation of drought characteristics in the 21st century. Journal of Hydrology, 526, 196–207. 10.1016/j.jhydrol.2014.12.011 [DOI] [Google Scholar]
- Trenberth, K. E. , Dai, A. , van der Schrier, G. , Jones, P. D. , Barichivich, J. , Briffa, K. R. , & Sheffield, J. (2014). Global warming and changes in drought. Nature Climate Change, 4(1), 17–22. 10.1038/nclimate2067 [DOI] [Google Scholar]
- Trudinger, C. M. , Raupach, M. R. , Rayner, P. J. , Kattge, J. , Liu, Q. , Pak, B. , Reichstein, M. , Renzullo, L. , Richardson, A. D. , Roxburgh, S. H. , Styles, J. , Wang, Y. P. , Briggs, P. , Barrett, D. , & Nikolova, S. (2007). OptIC project: An intercomparison of optimization techniques for parameter estimation in terrestrial biogeochemical models. Journal of Geophysical Research: Biogeosciences, 112(G2), G02027. 10.1029/2006JG000367 [DOI] [Google Scholar]
- Trueba, S. , Pan, R. , Scoffoni, C. , John, G. P. , Davis, S. D. , & Sack, L. (2019). Thresholds for leaf damage due to dehydration: Declines of hydraulic function, stomatal conductance and cellular integrity precede those for photochemistry. New Phytologist, 223(1), 134–149. 10.1111/nph.15779 [DOI] [PubMed] [Google Scholar]
- Trugman, A. T. , Anderegg, L. D. L. , Shaw, J. D. , & Anderegg, W. R. L. (2020). Trait velocities reveal that mortality has driven widespread coordinated shifts in forest hydraulic trait composition. Proceedings of the National Academy of Sciences, 117(15), 8532–8538. 10.1073/pnas.1917521117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trugman, A. T. , Detto, M. , Bartlett, M. K. , Medvigy, D. , Anderegg, W. R. L. , Schwalm, C. , Schaffer, B. , & Pacala, S. W. (2018). Tree carbon allocation explains forest drought‐kill and recovery patterns. Ecology Letters, 21(10), 1552–1560. 10.1111/ele.13136 [DOI] [PubMed] [Google Scholar]
- Trugman, A. T. , Medvigy, D. , Mankin, J. S. , & Anderegg, W. R. L. (2018). Soil moisture stress as a major driver of carbon cycle uncertainty. Geophysical Research Letters, 45(13), 6495–6503. 10.1029/2018GL078131 [DOI] [Google Scholar]
- Tyree, M. T. , & Hammel, H. T. (1972). The measurement of the turgor pressure and the water relations of plants by the pressure‐bomb technique. Journal of Experimental Botany, 23(1), 267–282. 10.1093/jxb/23.1.267 [DOI] [Google Scholar]
- Tyree, M. T. , & Sperry, J. S. (1988). Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress?: Answers from a model. Plant Physiology, 88(3), 574–580. 10.1104/pp.88.3.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree, M. T. , & Sperry, J. S. (1989). Vulnerability of Xylem to Cavitation and Embolism. Annual Review of Plant Physiology and Plant Molecular Biology, 40(1), 19–36. 10.1146/annurev.pp.40.060189.000315 [DOI] [Google Scholar]
- Ulaby, F. T. , & El‐rayes, M. A. (1987). Microwave dielectric spectrum of vegetation ‐ Part II: Dual‐dispersion model. IEEE Transactions on Geoscience and Remote Sensing, GE‐25(5), 550–557. 10.1109/TGRS.1987.289833 [DOI] [Google Scholar]
- Ustin, S. L. , Riaño, D. , & Hunt, E. R. (2012). Estimating canopy water content from spectroscopy. Israel Journal of Plant Sciences, 60(1–2), 9–23. 10.1560/IJPS.60.1-2.9 [DOI] [Google Scholar]
- van Emmerik, T. , Steele‐Dunne, S. C. , Judge, J. , & van de Giesen, N. (2015). Impact of diurnal variation in vegetation water content on radar backscatter from maize during water stress. IEEE Transactions on Geoscience and Remote Sensing, 53(7), 3855–3869. 10.1109/TGRS.2014.2386142 [DOI] [Google Scholar]
- van Emmerik, T. , Steele‐Dunne, S. , Paget, A. , Oliveira, R. S. , Bittencourt, P. R. L. , de Barros, F. V. , & van de Giesen, N. (2017). Water stress detection in the Amazon using radar. Geophysical Research Letters, 44(13), 6841–6849. 10.1002/2017GL073747 [DOI] [Google Scholar]
- van Mantgem, P. J. , Stephenson, N. L. , Byrne, J. C. , Daniels, L. D. , Franklin, J. F. , Fule, P. Z. , Harmon, M. E. , Larson, A. J. , Smith, J. M. , Taylor, A. H. , & Veblen, T. T. (2009). Widespread increase of tree mortality rates in the Western United States. Science, 323(5913), 521–524. 10.1126/science.1165000 [DOI] [PubMed] [Google Scholar]
- Vermunt, P. C. , Khabbazan, S. , Steele‐Dunne, S. C. , Judge, J. , Monsivais‐Huertero, A. , Guerriero, L. , & Liu, P.‐W. (2020). Response of subdaily L‐Band backscatter to internal and surface canopy water dynamics. IEEE Transactions on Geoscience and Remote Sensing, 59(9), 7322–7337. 10.1109/TGRS.2020.3035881 [DOI] [Google Scholar]
- Vreugdenhil, M. , Wagner, W. , Bauer‐Marschallinger, B. , Pfeil, I. , Teubner, I. , Rüdiger, C. , & Strauss, P. (2018). Sensitivity of sentinel‐1 backscatter to vegetation dynamics: An Austrian case study. Remote Sensing, 10(9), 1396. 10.3390/rs10091396 [DOI] [Google Scholar]
- Wang, X. , Dannenberg, M. P. , Yan, D. , Jones, M. O. , Kimball, J. S. , Moore, D. J. P. , Leeuwen, W. J. D. , Didan, K. , & Smith, W. K. (2020). Globally consistent patterns of asynchrony in vegetation phenology derived from optical, microwave, and fluorescence satellite data. Journal of Geophysical Research: Biogeosciences, 125(7), e05732. 10.1029/2020JG005732 [DOI] [Google Scholar]
- Wangersky, P. J. (1978). Lotka‐Volterra population models. Annual Review of Ecology and Systematics, 9(1), 189–218. 10.1146/annurev.es.09.110178.001201 [DOI] [Google Scholar]
- West, H. , Quinn, N. , & Horswell, M. (2019). Remote sensing for drought monitoring & impact assessment: Progress, past challenges and future opportunities. Remote Sensing of Environment, 232, 111291. 10.1016/j.rse.2019.111291 [DOI] [Google Scholar]
- Wigneron, J.‐P. , Li, X. , Frappart, F. , Fan, L. , Al‐Yaari, A. , De Lannoy, G. , Liu, X. , Wang, M. , Le Masson, E. , & Moisy, C. (2021). SMOS‐IC data record of soil moisture and L‐VOD: Historical development, applications and perspectives. Remote Sensing of Environment, 254, 112238. 10.1016/j.rse.2020.112238 [DOI] [Google Scholar]
- Wu, X. , Liu, H. , Li, X. , Ciais, P. , Babst, F. , Guo, W. , Zhang, C. , Magliulo, V. , Pavelka, M. , Liu, S. , Huang, Y. , Wang, P. , Shi, C. , & Ma, Y. (2018). Differentiating drought legacy effects on vegetation growth over the temperate Northern Hemisphere. Global Change Biology, 24(1), 504–516. 10.1111/gcb.13920 [DOI] [PubMed] [Google Scholar]
- Wullschleger, S. D. , Hanson, P. J. , & Todd, D. E. (1996). Measuring stem water content in four deciduous hardwoods with a time‐domain reflectometer. Tree Physiology, 16(10), 809–815. 10.1093/treephys/16.10.809 [DOI] [PubMed] [Google Scholar]
- Xiao, P. , Liu, B. , & Guo, W. (2020). ConGaLSAR: A constellation of geostationary and low earth orbit synthetic aperture radar. IEEE Geoscience and Remote Sensing Letters, 17(12), 2085–2089. 10.1109/LGRS.2019.2962574 [DOI] [Google Scholar]
- Xu, L. , Saatchi, S. S. , Yang, Y. , Myneni, R. B. , Frankenberg, C. , Chowdhury, D. , & Bi, J. (2015). Satellite observation of tropical forest seasonality: Spatial patterns of carbon exchange in Amazonia. Environmental Research Letters, 10(8), 084005. 10.1088/1748-9326/10/8/084005 [DOI] [Google Scholar]
- Xu, X. , Konings, A. G. , Longo, M. , Feldman, A. , Xu, L. , Saatchi, S. , Wu, D. , Wu, J. , & Moorcroft, P. (2021). Leaf surface water, not plant water stress, drives diurnal variation in tropical forest canopy water content. New Phytologist, 231(1), 122–136. 10.1111/nph.17254 [DOI] [PubMed] [Google Scholar]
- Yu, Y. , & Saatchi, S. (2016). Sensitivity of L‐Band SAR backscatter to aboveground biomass of global forests. Remote Sensing, 8(6), 522. 10.3390/rs8060522 [DOI] [Google Scholar]
- Zhang, Q. , Manzoni, S. , Katul, G. , Porporato, A. , & Yang, D. (2014). The hysteretic evapotranspiration—Vapor pressure deficit relation. Journal of Geophysical Research: Biogeosciences, 119(2), 125–140. 10.1002/2013JG002484 [DOI] [Google Scholar]
- Zhang, T. , Niinemets, Ü. , Sheffield, J. , & Lichstein, J. W. (2018). Shifts in tree functional composition amplify the response of forest biomass to climate. Nature, 556(7699), 99–102. 10.1038/nature26152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
QuikScat data shown in Figure 2 are available from the NASA PODAAC at https://podaac.jpl.nasa.gov/QuikSCAT.


