Objective
Infants are at risk for developing severe COVID-19 illness1 and are a source of virus spread.2 Recent studies have demonstrated reduction of SARS-CoV-2 positive tests in infants3 and COVID-19 infant hospitalizations following maternal COVID-19 vaccination.4 BNT162b2 (Pfizer/BioNTech) messenger RNA (mRNA) COVID-19 vaccination during the second trimester of pregnancy was associated with high neonatal SARS-CoV-2 immunoglobulin G (IgG) levels at birth.5 Our aim was to evaluate SARS-CoV-2 IgG levels in infants of up to 6 months of age following maternal vaccination during the second trimester of pregnancy.
Study Design
This prospective cohort study, performed between September 2021 and January 2022, included infants at the age of 3 to 6 months of mothers vaccinated with the second BNT162b2 mRNA COVID-19 vaccine. The second dose was received 3 weeks following the first dose according to the standard established for Israel at the time, during the second trimester of pregnancy, and women were not previously diagnosed with COVID-19 (based on self-reported information). All infants had a SARS-CoV-2 IgG antibody level measurement at birth collected by umbilical cord sampling. None of the infants were reported to have a COVID-19 infection during the study period. Following recruitment, we obtained venous blood from each infant, which was assessed by SARS-CoV-2 IgG II Quant (Abbott Laboratories, Chicago, IL), a 2-step chemiluminescent microparticle immunoassay used for the quantitative determination of IgG antibodies. Correlations between infant antibody titers, fetomaternal and infant characteristics, and the time interval from maternal vaccination to the infant follow-up antibody test were analyzed.
Results
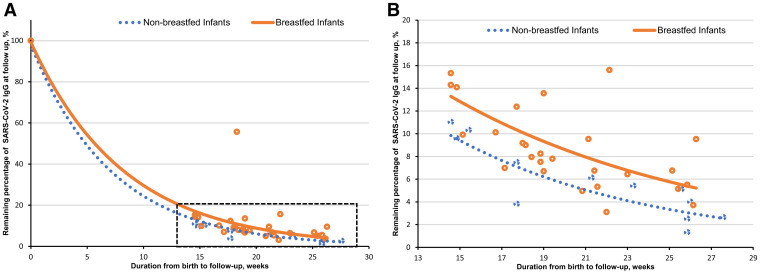
Antibody levels were measured for 40 infants. The median (range) level of IgG antibodies at birth was 2790.3 (350.1–13,405.0) AU/mL and declined to a median (range) of 199 (18.4–904.3) AU/mL at a median (range) age of 19.2 (14.6–27.6) weeks. Three of 40 (7.5%) infants had a negative (<50 AU/mL) antibody test at a median (range) age of 26.1 (21.5–26.1) weeks. No differences were found between the different clinical and demographic characteristics of breastfed and nonbreastfed infants. The median (range) level of SARS-CoV-2 IgG levels at follow-up was higher in the 28 breastfed infants (232.0 [105.7–904.3] AU/mL) than in the 12 nonbreastfed infants (145.3 [18.4–575.5] AU/mL) (P=.02). Multivariable analysis revealed that infant SARS-CoV-2 IgG antibody titers at follow-up were positively correlated with SARS-CoV-2 IgG levels at birth and breastfeeding, yet negatively correlated with time passed from maternal second vaccine dose. For each week that passed since maternal second vaccine dose, SARS-CoV-2 IgG antibody levels decreased by 5.8% (95% confidence interval [CI], −8.6 to −3.9; P<.001). Breastfeeding was significantly and independently associated with higher SARS-CoV-2 IgG levels (absolute difference, 75.1%; 95% CI, 28.4–138.7; P=.001). Moreover, the median (interquartile range) remaining percentage of SARS-CoV-2 IgG antibodies from birth to follow-up was significantly higher in breastfed infants than in nonbreastfed infants (8% [6.5–11.8] vs 5.3% [2.9–9.1]; P=.021) (Figure ).
Figure.
Remaining percentage of SARS-CoV-2 IgG antibodies at follow-up in infants
Correlation between the remaining percentage of SARS-CoV-2 IgG antibodies at follow-up and duration from birth for breastfed and nonbreastfed infants. A, From 100% SARS-CoV-2 IgG antibodies at birth to remaining percentage at follow-up. B, Focus on relevant time period of infant follow-up tests; breastfed infants: r=−0.62; 95% CI, −0.80 to −0.31; P<.001; nonbreastfed infants: r=−0.84; 95% CI, −0.95 to −0.50; P=.001.
CI, confidence interval; Ig, immunoglobulin.
Kugelman. SARS-CoV-2 immunoglobulin G antibody levels in infants following messenger RNA COVID-19 vaccination during pregnancy. Am J Obstet Gynecol 2022.
Conclusion
Our findings suggest that maternal COVID-19 vaccination during pregnancy may possibly provide protection from COVID-19 in early infancy, with SARS-CoV-2 IgG antibody levels enhanced by breastfeeding and sustained at least until 6 months of age.
Footnotes
The authors report no conflicts of interest.
No funding was received for this study.
References
- 1.Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 2.Alsohime F., Temsah M.H., Al-Nemri A.M., Somily A.M., Al-Subaie S. COVID-19 infection prevalence in pediatric population: etiology, clinical presentation, and outcome. J Infect Public Health. 2020;13:1791–1796. doi: 10.1016/j.jiph.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlsen E.Ø., Magnus M.C., Oakley L., et al. Association of COVID-19 vaccination during pregnancy with incidence of SARS-CoV-2 infection in infants. JAMA Intern Med. 2022 doi: 10.1001/jamainternmed.2022.2442. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halasa N.B., Olson S.M., Staat M.A., et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months - 17 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264–270. doi: 10.15585/mmwr.mm7107e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kugelman N., Nahshon C., Shaked-Mishan P., et al. Maternal and neonatal SARS-CoV-2 immunoglobulin G antibody levels at delivery after receipt of the BNT162b2 messenger RNA COVID-19 vaccine during the second trimester of pregnancy. JAMA Pediatr. 2022;176:290–295. doi: 10.1001/jamapediatrics.2021.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]