Abstract
Virus threatens life health seriously. The accurate early diagnosis of the virus is vital for clinical control and treatment of virus infection. Aptamers are small single-stranded oligonucleotides (DNAs or RNAs). In this review, we summarized aptasensors for virus detection in recent years according to the classification of the viral target protein, and illustrated common detection mechanisms in the aptasensors (colorimetry, fluorescence assay, surface plasmon resonance (SPR), surface-enhanced raman spectroscopy (SERS), electrochemical detection, and field-effect transistor (FET)). Furthermore, aptamers against different target proteins of viruses were summarized. The relationships between the different biomarkers of the viruses and the detection methods, and their performances were revealed. In addition, the challenges and future directions of aptasensors were discussed. This review will provide valuable references for constructing on-site aptasensors for detecting viruses, especially the SARS-CoV-2.
Keywords: Aptamer, Detection, Virus, Aptasensor
Graphical abstract

1. Introduction
The outbreak of viruses, such as influenza virus [1], Ebola virus [2], hepatitis B virus (HBV) [3], Dengue virus [4], human immunodeficiency virus (HIV) [5], Zika virus [6], as well as the ongoing SARS-CoV-2 [7], posed a tremendous menace to the safety of humans and animals around the world. Early diagnosis is imperative to prevent and control a global pandemic. The conventional diagnostic methods for viruses include virus isolation culture [8], real-time quantitative polymerase chain reaction (RT-qPCR) [9], serological methods [10], antigen testing, enzyme-linked immunosorbent assay (ELISA) [11], etc. These methods are generally effective but arduous, time-consuming, and cost-ineffective. Hence, effective strategies are urgently needed to diagnose various viruses rapidly, accurately, and sensitively.
Aptamers are small single-stranded oligonucleotides (DNAs or RNAs) in the range of 10–100 nucleotides (nt), which are attained through the systematic evolution of ligands by exponential enrichment (SELEX) procedure [12,13]. They show selectivity and affinity to assorted targets, including metal ions [14,15], proteins [16], viruses [17], bacteria [18], whole cells [19], etc. Thus, they are also called “chemical antibodies”, and show advantages over antibodies, including fast synthesis speed, low manufacturing cost, good stability, etc [20]. Up till now, aptamers have been widely applied in sensing [21], food safety [22], environment monitoring [23,24], basic research fields, and even acting as drugs for disease treatment and preventing infection of viruses [[25], [26], [27], [28]].
The biosensor is an analytical strategy that integrates the biological recognition mechanism with the physical-chemical transduction. In the aptamer-based biosensor (Aptasensor), the aptamer serves as the recognition element of the biosensor to recognize and bind the target with high specificity and affinity, avoiding the interference of other biological matrixes. The transducer then converts and outputs the biological signal from the interaction between the target and the aptamer [29]. Up to now, many aptasensors have been applied for detecting viruses, including HIV [30], Zika virus [31], influenza virus [32], Dengue virus [33], Norovirus (NoV) [34], human papillomaviruses [35], as well as SARS-CoV-2 virus [36], etc. In this review, we reviewed and summarized aptasensors of viral detection in recent years according to the classification of viral target proteins. The relationships between the different biomarkers of the viruses and the detection methods, and their performances were revealed. The common detection mechanisms in aptasensors were illustrated, including colorimetry, fluorescence assay, surface plasmon resonance (SPR), surface-enhanced Raman spectroscopy (SERS), electrochemical detection, and field-effect transistor (FET). Moreover, the challenges and future perspectives for the aptasensor were further discussed. This review expects a deeper grasp of aptamer-based sensing platforms for virus detection and offers ideas and inspirations to develop novel aptasensors.
2. Detection mechanisms
In recent years, various novel strategies, including SERS, SPR, etc., have been used for the construction of aptasensors. In this section, we summarized and described the common detection mechanisms of aptasensors for virus detection, including colorimetry, fluorescence assay, SPR, SERS, electrochemical detection, and field-effect transistor (FET).
2.1. Colorimetric detection
Colorimetric detection can be divided into nanomaterial-assisted and enzyme-based colorimetric assays. Through direct observation by naked eyes or using spectrophotometers, the change of color can be measured to evaluate the concentration of targets, offering the advantage of easy-to-perform, without the utilization of large-scale instruments [37,38]. In the nanomaterial-assisted colorimetric sensor, nanomaterials utilize their optical properties to participate in signal conversion. Typically, nanoparticles in the solution are adsorbed by aptamers to prevent aggregation. However, when targets are added, nanoparticles aggregate and produce a color change due to the specific interaction of aptamers and targets. The principle of a typical nanomaterial-assisted aptasensor is shown in Fig. 1 A. The gold nanoparticle (AuNP) is most widely utilized due to its unique optical properties [39]. Recently, Basso et al. [33] used a hybrid nanomaterial formed by coupling magnetic nanoparticles γ-Fe2O3 to aptamer-modified AuNPs on the surface to develop a new colorimetric aptasensor. Upon the binding of aptamers-viruses, the gold nanoparticle coating increased and the surface area of the laser interaction reduced, consequently generating the change of color. The aptasensor used aptamers instead of traditional antibodies, which could exclude the interference of other similar viruses (such as Zika, yellow fever virus, etc.) and achieved specific binding to the Dengue virus. And it utilized the optical characteristics of hybrid nanoparticles to enable visual detection of the Dengue virus and reduced detection time and cost. In addition, the enzyme-assisted colorimetric aptasensor commonly has a sandwich form of aptamer 1-target-aptamer 2. The enzyme-labeled aptamer 2 catalyzes the chromogen reagent to produce a color change and achieve target detection. The principle of a typical enzyme-assisted colorimetric is shown in Fig. 1A.
Fig. 1.
(A) The principles of typical colorimetric-based aptasensors, including nanomaterial-assisted (Top) and enzymes-based colorimetric assays (Bottom). (B) The principles of typical labeled (Top) and label-free (Bottom) fluorescence aptasensors. (C) The principle of typical SPR aptasensors. (D) The principle of typical SERS-based aptasensors. (E) The principles of typical electrochemical aptasensors without (Left) and with enzyme (Right). (F) The principle of typical FET-based aptasensors.
2.2. Fluorescence detection
The fluorophore works as the signal output element. According to whether the fluorophore is labeled with the aptamer, fluorescence detection is mainly parted into labeled and label-free [40]. For the labeled aptasensor, fluorophore-labeled aptamers act as capture nanoprobes in sensors. They can be absorbed on the surface of quenchers (such as graphene, graphdiyne, transition metal sulfides, etc.), resulting in fluorescence quenching. Nevertheless, when viruses are added, fluorescence recovery is achieved due to the interaction of aptamers and viruses. The principle of typical labeled fluorescence aptasensors is shown in Fig. 1B. The fluorescence resonance energy transfer (FRET)-based aptasensor is a typical labeled aptasensor. Suh and coworkers [41] reported a FRET-based aptasensor that allows one-step quantitative detection of HBV. However, the labeling process is time-consuming, complex to perform, and labor-intensive. Thus, the label-free aptasensor has attracted increasing attention in recent years. For the label-free aptasensor, the free fluorescent agent acts as the indicator of the sensor. It had nearly no fluorescence signal in the absence of targets. Only when targets are added, a stable G-quadruplex complex was formed between aptamers and targets. At the same time, the complex can bind the fluorescent agent and excite the fluorescence emission. The principle of a typical label-free fluorescence aptasensor is shown in Fig. 1B.
2.3. Surface plasmon resonance (SPR)
The SPR-based sensor is an optical sensing device that utilizes the sensitivity of surface plasmon, a particular type of electromagnetic field, to change the refractive index [42]. In a typical SPR-based aptasensor, aptamers are usually immobilized on a gold layer as recognition probes [43,44]. When other conditions are constant, the changes in the SPR angle are dependent on the interaction of aptamers and targets [45]. The principle of a typical SPR-based aptasensor is shown in Fig. 1C. However, conventional SPR-based sensors have limitations in selectivity and sensitivity compared to labeled fluorescence detection. Recently, Lee et al. [34] reported a new aptamer-based sandwich platform with gold nanorod-enhanced SPR for detecting Norovirus capsid protein. In the aptasensor, a pair of aptamers were used to recognize and bind to the target to form a sandwich complex, which improved the selectivity of the sensor and reduced non-specific binding. Furthermore, a significant signal amplification was achieved due to the large refractive index changes associated with gold nanorods. The aptasensor could detect Norovirus capsid proteins at the attomolar range. And the study has shown that using gold nanorod particles makes the sensitivity 105 times higher than without gold nanorod particles.
2.4. Surface-enhanced Raman spectroscopy (SERS)
Raman spectroscopy is a scattering spectrum that provides the “unique chemical fingerprint” of molecules. In a typical SERS-based aptasensor, Raman reporter molecules modified aptamers are usually immobilized on a nanostructure metal-dielectric substrate to recognize and capture viruses. Under laser irradiation, the energy of photons changes due to the interaction of aptamers and viruses, and consequently achieving targets quantitation. The principle of a SERS-based aptasensor is shown in Fig. 1D.
Since aptamers were firstly applied in SERS by Halas, Moskovits, and their coworkers [46,47], scientists have shown intense interest in SERS-based aptasensors [[48], [49], [50]]. Negri et al. [51,52] first proposed a label-free SERS-based platform for detecting influenza virus nucleoproteins. In this platform, Ag nanorods were used as the active substrates, and polyvalent aptamers were immobilized on Ag nanorods. The SERS signal was then attained through the intrinsic SERS spectrum of aptamer-nucleoprotein complexes. However, this method based on the intrinsic SERS spectrum suffers from poor reproducibility and practicality. Recently, Raman mapping methods for precise quantification of targets on SERS substrates have been developed to address this issue [53].
2.5. Electrochemical detection
The electrochemical aptasensor evaluates the concentration of interesting targets by measuring the change of current response or electrical resistivities from the redox reaction between targets and aptamers fixed on the electrode surface of the sensor [54,55]. Generally, they were classified into three types: aptasensors with enzymes, aptasensors without enzymes, and field-effect transistors (FET) [56]. For the aptasensor without enzyme, capturing probes are immobilized on electrodes. The interaction of aptamers and targets directly results in changes in the electrical signal. However, for the aptasensor with enzyme, the generation of signal needs the aid of enzymes. The principles of typical electrochemical aptasensor without enzyme and with enzyme are shown in Fig. 1E. In a reported electrochemical aptasensor with enzyme, the ConA-GOx-AuNPs complexes were used to output the signal [57]. The ConA-GOx-AuNPs complexes were prepared by binding concanavalin A (ConA), glucose oxidase (GOx), and gold nanoparticles (AuNPs). Through enzymatic catalysis of glucose oxidase in a particular solution, the chemical signal was transformed into an electrochemical signal. The platform successfully achieved virus detection with only one bare electrode, with a detection limit of 8 × 10−4 HAU in a 200 μL sample.
2.6. Field-effect transistor (FET)
The FET is a voltage-controlled semiconductor apparatus. The FET-based aptasensor immobilizes aptamers on the gate electrode surface. The voltage of the gate electrode regulates the magnitude of the drain current. Thus, the binding of aptamers and target molecules on the gate electrode results in a change in drain current [58]. The principle of a typical FET-based aptasensor is shown in Fig. 1F. The FET-based aptasensor offers excellent performances, including sensitive measurements, portable instrumentation, low cost, high speeds, etc. [59]. In particular, it has great potential for viral point-of-care detection [32].
3. Viral proteins and their detection
Viral antigen detection is a dominant method in on-site detection, and it's significant for the effective prevention of the virus (see Table 1). Many viral proteins, including envelope proteins (HBV, influenza, Ebola, Dengue, SARS-CoV-2, etc.), capsid proteins (HCV, Norovirus, HPV, etc.), nucleoproteins (influenza, SARS-CoV-2, etc.), and functional proteins (HIV), have been used as biomarkers for the diagnosis of different viruses (Fig. 2 ). The functions and characteristics of these proteins were briefly summarized (Table 2 ). The corresponding aptamers for these biomarkers were also screened by the SELEX method (Table 3 ). In this section, the virus detection is divided into detection of envelope protein, capsid protein, nucleoprotein, and functional protein according to the target proteins recognized by aptamers in aptasensors for detecting the virion or antigen.
Table 1.
Detection assays: characteristics, advantages, and disadvantages.
| Detection Methods | Characteristics | Advantages | Disadvantages | Refs |
|---|---|---|---|---|
| Colorimetry |
|
|
|
[56] |
| Fluorescence assay |
|
|
|
[40] |
| Surface plasmon resonance (SPR) |
|
|
|
[60] |
| Surface-enhanced Raman spectroscopy (SERS) |
|
|
|
[61,62] |
| Electrochemical detection |
|
|
|
[63] |
| Field-effect transistor (FET) |
|
|
|
[64] |
Fig. 2.
(A) The typical structure of viruses and (B) the biosensing platforms based on aptamers for virus detection.
Table 2.
Viral proteins: functions and characteristics.
| Viral proteins | Functions | Characteristics | Refs |
|---|---|---|---|
| Envelop protein |
|
|
[65,66] |
| Capsid protein |
|
|
[67] |
| Nucleoprotein |
|
|
[68] |
| Functional protein |
|
|
[69] |
Table 3.
Aptamers against different target proteins of viruses.
| Viruses | Targets | Aptamer sequences | Affinity (Kd) | Refs |
|---|---|---|---|---|
| Hepatitis B virus | HBsAg | 5′-GGG AAT TCG AGC TCG GTA CCG GCA CAA GCA TAT GGA CTC CTC TGA ACC TAC GAT GTA GTA CCT GCA GGC ATG CAA GCT TGG-3′ | – | [76] |
| HBeAg | 5′-TTT TTT GGT CAG ATG AGG CTT GGT GAT CGT GCC CAG GCC ATA TGA GCA AGG AAC CCC TAT GCG TGC T-3′ | 1.2 nM | [179] | |
| HBcAg | 5′-AGC AGC ACA GAG GTC AGA TGA GGC CTG GTG ATC GTG CCC AGG CCA TAT GAG CAA GGA ACC CCT ATG CGT GCT ACC GTG AA-3′ | 0.2 nM | [181] | |
| Influenza virus H5N1 |
HA | 5′-GTG TGC ATG GAT AGC ACG TAA CGG TGT AGT AGA TAC GTG CGG GTA GGA AGA AAG GGA AAT AGT TGT CCT GTT GTT GCC ATG TGT ATG TGG G-3′ | 4.65 nM | [84] |
| H1N1 | 5′-TAC TGC ACA CGA CAC CGA CTG TCA CCA TCA CCT CGG CGC AGT GCT GGA ACT CCC CGA CTG C-3′ | 19.2 nM | [100] | |
| Influenza A | Nucleoprotein | 5′-TAG GGA AGA GAA GGA CAT ATG ATT GGC TTG CAT GCT GGA CTT CCT ACT GGT TTT TGA CTA GTA CAT GAC CAC TTG A-3′ | 77.6 ± 5.9 nM | [182] |
| Influenza B | 5′-ATT ATG GCG TAT TGC AGC GTT CTG GTT GGT GGT GGG CAG GTG GTG GTA CTG CGC TGC AGC TTG TTG GTG AGG TAA CGG CT-3′ | 0.97 ± 0.6 nM | [157] | |
| Influenza A (H1N1) | Whole virus particle | 5′-GCA ATG GTA CTT CCA TTC GAC CTC TGT AAC AGC CAC GAA AAC CCT ATA TCA AAA GTG CAC GCT ACT TTG CTA A-3′ | 23.98 ± 6.39 nM | [183] |
| Avian influenza (H5N2) | 5′-CGT ACG GAA TTC GCT AGC TGA TGG TGT GGC GGG GGG CGG CCT GGG GCG GGC CGC CGA TGG GAT CCG AGC TCC ACG TG-3′ | 6.913 × 105 EID50/mL | [184] | |
| Avian influenza (H5N1) | 5′-CGT ACG GTC GAC GCT AGC TAA CGG TGT GGC CCG GGG GTA CAG CGC ACT CAC GTG GAG CTC GGA TCC-3′ | 7 × 104 EID50/mL | [185] | |
| SARS-CoV-2 | Envelop protein: Receptor binding domain (RBD) S1 subunit |
5′-TGG GAG CCT GGG ACA TAG TGG GGA AAG AGG GGA AGA GTG GGT CT-3 | 2 nM | [108] |
| 5′-CAG CAC CGA CCT TGT GCT TTG GGA GTG CTG GTC CAA GGG CGT TAA TGG ACA-3′ | 5.8 ± 0.8 nM | [106] | ||
| 1.8 ± 0.4 nM | [107] | |||
| 2.7 ± 0.2 nM | ||||
| N-terminal domain | 5′-TCG CTC TTT CCG CTT CTT CGC GGT CAT TGT GCA TCC TGA CTG ACC CTA AGG TGC GAA CAT CGC CCG CGT AAG TCC GTG TGT GCG AA-3 | 80 nM | [105] | |
| Nucleocapsid | 5′-GCT GGA TGT CGC TTA CGA CAA TAT TCC TTA GGG GCA CCG CTA CAT TGA CAC ATC CAG C-3′ | 0.49 ± 0.05 nM | [36] | |
| 5′-GCT GGA TGT CAC CGG ATT GTC GGA CAT CGG ATT GTC TGA GTC ATA TGA CAC ATC CAG C-3′ | 0.70 ± 0.06 nM | |||
| SARS-CoV | Nucleocapsid protein | 5′-GCA ATG GTA CGG TAC TTC CGG ATG CGG AAA CTG GCT AAT TGG TGA GGC TGG GGC GGT CGT GCA GCA AAA GTG CAC GCT ACT TTG CTA A-3′ | 4.93 ± 0.30 nM | [186] |
| 5′-GGG AGA GCG GAA GCG UGC UGG GCC UGU CGG UUC GCU GUC UUG CUA CGU UAC GUU ACA CGG UUG GCA UAA CCC AGA GGU CGA UGG AUC CCC CC-3′ | 1.65 ± 0.41 nM | [187] | ||
| Envelope Glycoprotein E2 | 5′-GCG GAA TTC TAA TAC GAC TCA CTA TAG GGA ACA GTC CGA GCC GAA TGA GGA ATA ATC TAG CTC CTT CGC TGA GGG TCA ATG CGT CAT A-3′ | 1.05 ± 1 nM | [188] | |
| Norovirus | Capsid protein | 5′-GCT AGC GAA TTC CGT ACG AAG GGC GAA TTC CAC ATT GGG CTG CAG CCC GGG GGA TCC-3′ | picomolar range | [130] |
| 5′-GTC TGT AGT AGG GAG GAT GGT CCG GGG CCC CGA GAC GAC GTT ATC AGG C-3′ | – | [189] | ||
| Human papillomavirus | L1 capsid | 5′-GGG AAC GGG AAC AAA AGC UGC ACA GGU UAC CCC CGC UUG GGU CUC CCU AUA GUG AGU CGU AUU ATT TTT-3′ | 0.05 pM | [190] |
| E7 protein | 5′-GGG AGG ACG AUG CGG AAG CAT CAA GGG TGA TCG TTT GAC CCT CCC CAG ACG ACU CGC CCG A-3′ | – | [191] | |
| HIV | Tat protein | 5′-ACG AAG CUU GAU CCC GUU UGC CGG UCG AUC GCU UCG A-3′ | 120 ± 13 pM | [192] |
| 5′-GAA GCU UGA UCC CGA A-3′ (Strand 1) 5′-UCG GUC GAU CGC UUC AUA A-3′ (Strand 2) |
0.5 nM | [172] | ||
| Dengue virus | Envelop protein domain III (ED3) | 5′-GGG AAG ATC TCG ACC AGA AGG CAC CGG GCA GGA CGT CCG GGG TCC TCG GGG GGC TAT GTG CGT CTA CAT GGA TCC TCA-3′ | 200 nM | [193] |
| Zika virus | NS1 | 5′-GAT AGA ATT CGA GCT CGG GCA CTA GGT TGC AGG GGA CTG CTC GGG ATT GCG GAT CAA CCT AGT TGC TTC TCT CGT ATG ATG CGG GTC GAC AAG CTT TAA T-3′ | 24 pM | [177] |
| Ebola virus | Type I transmembrane protein | 5′-AGC AGC ACA GAG GTC AGA TGG CCG CAG ACA GAA ACA CAA ACA GTA TAC AGC TAT CTC GCT TGG ACC TCG CAT AAT ATA CGC CTA TGC GTG CTA CCG TGA A-3′ | 4.1 ± 0.9 nM | [120] |
3.1. Envelop protein
Many types of virus particles exist not as naked nucleocapsids, but as nucleocapsids surrounded by lipid membranes. These membranes contain various virus-encoded proteins known as envelope proteins, such as hepatitis B surface antigen (HBsAg), hemagglutinin (HA), neuraminidase (NA), SARS-CoV-2 spike glycoprotein, etc. Envelope proteins, as external proteins, are often used as recognition sites for aptasensors.
3.1.1. Hepatitis B surface antigen (HBsAg)
Despite significant efforts, HBV infection remains a primary global human disease with high morbidity and mortality rates [70]. It is one of the leading causes of severe liver diseases, such as liver cancer [71]. Clinically, HBsAg, HBsAb, HBeAg, HBeAb, and HBcAb are important biomarkers for HBV diagnosis. Among them, HBsAg, an envelope glycoprotein, is the first serological marker in the blood and the most crucial biomarker of HBV infection (Fig. 3 A) [72]. Thus, the detection of HBsAg is a vital tool for diagnosing HBV infection [[73], [74], [75]].
Fig. 3.
(A) The molecular diagram of HBsAg. (B) The growth mechanism of polypyrrole nanowires during electro-polymerization. (C) Schematic diagram of MCNF surface aptamer conjugation. (D) Schematic diagram of the MCNF-based FET aptasensor. Reproduced with permission from Ref. [79]. Copyright 2018, American Chemical Society.
Currently, several aptamer-based sensors have been developed to detect HBsAg, including chemiluminescent [76,77], fluorescent [41], electrochemical aptasensors [78], etc. Cho and coworkers [79] reported a new nanomaterial-based FET platform for the non-invasive detection of HBsAg. The multidimensional conductive nanofilm (MCNF) was formed by combining vertically oriented carboxylic polypyrrole nanowires (CPPyNW) with a graphene layer. It offered a large active surface region for the immobilization of probes and consequently improved the decoration quantity of capturing aptamers and the combining affinity (Fig. 3 B, C). When the interaction of aptamers and targets, aptamers underwent significant changes in morphology and reduced the aptamer's negative gating effect due to the folding of the aptamer. Therefore, the source-drain current (ISD) was reduced and the detection of the target was achieved (Fig. 3D). The MCNF-based FET aptasensor realized sensitive and rapid HBsAg detection in artificial biological samples and processed good durability and flexibility. In another study, Mohsin et al. [78] proposed a composite nanomaterial (rGO-AuNPs)-based electrochemical aptasensor with an extremely low detection limit. In this platform, the methylene blue (MB) acted as a response indicator. When HBsAg was added, MB was released from the aptamer due to the change in the structure of the aptamer, causing the electrochemical signal became weaken. As far as we know, this aptasensor possesses the lowest detection limit for detecting HBsAg in recently reported cases. However, these aptasensors have not been tested in real clinical samples, their practical application value is worth further exploration.
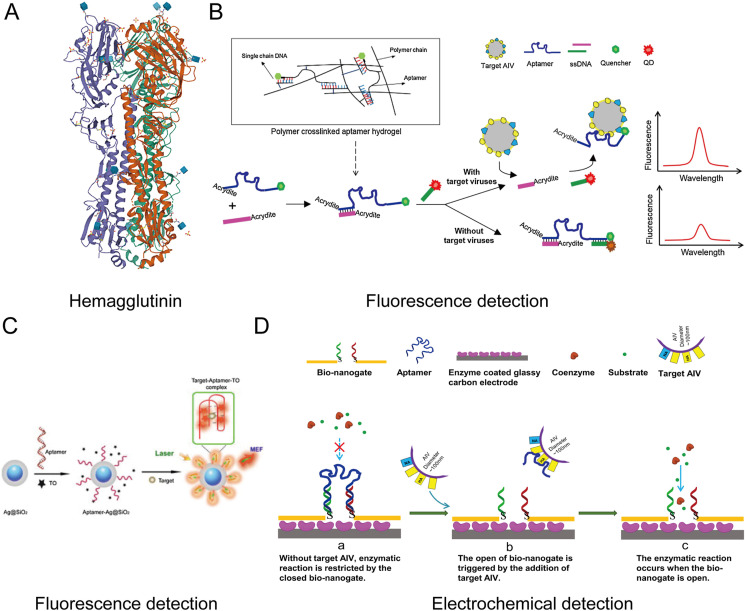
3.1.2. Hemagglutinin protein (HA)
Influenza virus is a highly contagious virus that has long threatened the health of humans and animals, manifested as headache, fatigue, chills, loss of appetite, and other systemic symptoms [80]. Although the mortality rate of influenza is generally low, the high infection rate makes influenza pandemics and epidemics major health concerns. Two envelop glycoproteins, hemagglutinin (HA) and neuraminidase (NA), are present on the surface of viruses and play essential roles in infecting host cells and in emerging influenza pandemics [81,82]. And HA is also one of the main biomarkers for detection because it occupies approximately 80% of the membrane of influenza viruses (Fig. 4 A). Therefore, based on HA-specific aptamers as recognition probes, aptasensors have become promising detection tools of influenza viruses [83].
Fig. 4.
(A) The molecular diagram of HA of H5N1. (B) Schematic diagram of the hydrogel-based QDs aptasensor. Reproduced with permission from Ref. [89]. Copyright 2016, Elsevier. (C) Schematic diagram of the aptamer-Ag@SiO2 sensor. Reproduced with permission from Ref. [91]. Copyright 2015, Elsevier. (D) Schematic diagram of the designed bio-nanogate and its controlled enzymatic reaction for virus detection. Reproduced with permission from Ref. [92]. Copyright 2015, Elsevier.
In 2013, Li et al. screened a specific aptamer against the HA protein of avian influenza virus (AIV) H5N1, which is summarized in Table 3 [84]. Then, utilizing the selected aptamer as the recognition probe, the team continued to report a series of aptasensors for the detection of AIV, including SPR [44], quartz crystal microbalance (QCM) [[85], [86], [87]], and electrochemical impedance spectroscopy (EIS) aptasensors [55,57,88], etc. Some of the aptasensors are summarized in Table 4 . Besides, they also firstly introduced hydrogel-based aptasensors for AIV detection [85,89].
Table 4.
Summary of aptasensors applied in influenza virus detection.
| Virus | Subtype | Recognition sites | Detection techniques | Limit of detection (LOD) | Linear ranges | Detection Time | Refs. |
|---|---|---|---|---|---|---|---|
| Avian Influenza Virus | H5N1 | HA | Field-effect transistor | 5.9 pM | 10 pM-10 nM | – | [32] |
| H5N1 | Cyclic voltammetry | 1 pM | 1 pM-100 nM | 2 h | [54] | ||
| H5N1 | LSPR | 1 pM | 1 pM-100 nM | 10 min | [43] | ||
| H5N1 | QCM | 0.0128 HAU | 0.0128–0.64 HAU | 30 min | [85] | ||
| H5N1 | Fluorescence detection | 0.4 HAU | 0.4–32 HAU | 30 min | [89] | ||
| H5N1 | Impedance | 8 × 10−4 HAU/200 μL | 0.001–1 HAU | 2 h | [57] | ||
| H5N1 | QCM | 2−4 HAU/50 μL | 2−4-24 HAU/50 μL | 10 min | [87] | ||
| H5N1 | Impedance | 0.25 HAU | 0.25–16 HAU/50 μL | 110 min | [55] | ||
| H5N1 | Electrochemical detection | 2−9 HAU | 2−10-22 HAU | <1 h | [92] | ||
| H5N1 | SPR | 0.128 HAU | 0.128–1.28 HAU | 1.5 h | [44] | ||
| H5N1 | QCM | 1 HAU | 1-4 HAU | 1 h | [86] | ||
| H5N1 | Fluorescence detection | 2 ng/ml | 2–100 ng/mL | 30 min | [91] | ||
| Influenza A | H1N1 | HA | SERS | 97 PFU/mL | 10-10000 PFU/mL | 20 min | [53] |
| H3N2 | SERS | 10−4 HAU | 2.5 × 10−5-1.3 HAU/mL | 12 min | [94] | ||
| H3N2 | Colorimetry | 11.16 μg/mL | 12–240 μg/mL | – | [199] | ||
| H1N1 | Cyclic voltammetry | 3.7 PFU/mL | 10-104 PFU/mL | – | [100] | ||
| H1N1 | Cyclic voltammetry/electrochemical impedance spectroscopy (EIS) | 10 pM | 10 pM-100 nM | – | [98] | ||
| Swine Influenza Virus | H1N1 | Electrochemical detection | 10 fM | 10 fM-100 pM | – | [29] | |
| Influenza B | – | Nucleoprotein | Colorimetry | 0.16 pg/mL | 0.1 pg/ml-1 μg/ml | – | [157] |
| Influenza A | Colorimetry | 0.3 pg/mL | |||||
| Enzyme-linked oligonucleotide assay (ELONA) | 1.02 ng/mL | 1–300 ng/mL | – | [182] | |||
| Lateral Flow Strip | 0.26 pg/mL | 0.01–10 ng/mL | 10 min | [158] | |||
| Avian influenza viruses | Electrochemical detection | 1.13 nM | 2 nM-2 μM | – | [156] | ||
| Influenza A | H1N1 | Whole virus particles | Fluorescence detection | 0.032 HAU | 32–3.2 × 10−3 HAU | 40 min | [196] |
| Fluorescence detection | 0.032 HAU | 0.0032–32 HAU | 30 min | [194] | |||
| Impedance | 0.9 pg/μL | – | – | [200] | |||
| Influenza A Influenza B |
H1N1, H3N2 | Fluorescence detection | 3.2 HAU | – | 20 min | [195] | |
| – | |||||||
| Avian influenza virus | H5N2 | Lateral Flow Strip | 1.27 × 105 EID50/mL | 6 × 105–1 × 107 EID50/ml | – | [184] | |
| Avian influenza virus | H5N1 | SPR | 200 EID50/mL | 1 × 103–1 × 105 EID50/mL | – | [185] |
Hydrogels are extremely hydrophilic cross-linked network structures that have been confirmed to be applied in the sensing field [90]. Wang and Li [85] firstly reported a hydrogel-based aptasensor using the QCM as a signal converter. In the hydrogel, the HA-specific aptamer acted as capturing probes and hybridized with ssDNA to form the cross-linker. In the absence of targets, the hydrogel maintained a state of shrink. However, upon the binding of aptamers and targets, the hydrogel abruptly swelled and was monitored by the QCM platform. This QCM platform achieved sensitive analysis, but it's susceptible to the environment. Xu et al. [89] developed another hydrogel-based quantum dots (QDs) aptasensor. The stimuli-responsive hydrogel was designed by introducing two decorated ssDNA sequences and the HA-specific aptamer to the polyacrylamide backbones (Fig. 4B). Fluorescence recovery/quenching of QDs acted as a signal indicator in response to the binding/dissociation of aptamers and targets. This fluorescent aptasensor can successfully detect AIV H5N1 within 30 min. And it had excellent potential for the on-site fast detection of AIV.
A novel guanine-richen fluorescent aptasensor was also reported [91]. The composite nanoparticle (Ag@SiO2) with a core-shell structure served as a reaction platform to amplify the fluorescence signal. The free fluorescent agent (Thiazole Orange, TO) acted as the indicator of the sensor. It had nearly no fluorescence signal in the absence of targets. When HA proteins were added, the stable G-quadruplex complex was formed between aptamers and proteins. Meanwhile, the TO was embedded into the complex and excited the fluorescence (Fig. 4C). The platform does not require the covalent labeling of aptamers with fluorophores and has very low background noise. This is also a major advantage of these label-free aptasensors. Another study reported a label-free bio-nanogate to combine enzymatic reactions, developing a bifunctional bio-nanogate-based electrochemical aptasensor [92]. The multifunctional nanostructure can specifically respond to AIV H5N1 virions and regulate catalytic reactions for virus detection with a detection limit of 2− 9 HAU (hemagglutination units) (Fig. 4D). The aptasensor can be used for directly detecting intact AIV H5N1 virions and exclude interference from other AIV subtypes such as H1N1, H2N2, H4N8, and H7N2.
Recently, several SERS-based aptasensors have also been reported [93,94]. Kukushkin et al. [94] developed a sandwich-type SERS-based detection platform with high specificity for multiple types of influenza viruses. In this platform, the primary aptamer, influenza viruses, and the secondary aptamer labeled with Raman-active molecules formed a sandwich system for detection (Fig. 5 A). Then, through the interaction of secondary aptamers-viruses and the measurement of a Raman spectrometer, the increased SERS signal was attained. The platform has a limit of detection of 10−4 HAU and a detection time of only 12 min. In addition, scientists reported another SERS-based imaging aptasensor based on Raman mapping methods to improve the reproducibility of conventional SERS-based aptasensors [53]. The 3D nanostructure substrate was introduced in this sensor. Capture probes were immobilized on the 3D nanostructure, hybridizing with Cy3-labeled Raman reporter to form a detection platform (Fig. 5B). When aptamers bind to A/H1N1 virions, aptamers produced a conformational change and were released from the hybrid complex. Subsequently, the decrease of Raman peak intensity was used to detect targets quantitatively. The aptasensor provides a reliable and ultrasensitive platform for viral detection. However, the preparation of 3D nanostructured substrates requires sophisticated equipment and cumbersome operations. These SERS-based aptasensors were capable of detecting intact virus particles. Therefore, unprocessed clinical samples can be directly detected in practical applications, thereby reducing the risk of infection for operators.
Fig. 5.
(A) Scheme of the sandwich-type SERS-based aptasensor for AIV detection. Reproduced with permission from Ref. [94]. Copyright 2019, Plos One. (B) Schematic diagram of the 3D nanostructure-based SERE aptasensor. Reproduced with permission from Ref. [53]. Copyright 2020, Elsevier. (C) Schematic diagram of the label-free FET for AIV detection. Reproduced with permission from Ref. [32]. Copyright 2020, American Chemical Society.
The label-free FET sensor is a promising assay for real-time virus detection due to its cost-effective, easy operation, hand-held readout system, and rapid signal response [95,96]. Jae Kwon et al. [32] firstly reported a FET-based aptasensor to monitor the HA protein in chicken serum. DNA aptamers were immobilized on the sensing part of the FET transducer to capture the HA protein (Fig. 5C). When HA proteins were added, the electrical properties of the FET transducer produced changes due to the specific binding of aptamer-target. This aptasensor has a dynamic range of 10 pM to 10 nM and a low detection limit of 5.9 pM. More importantly, due to its easy-to-perform, cost-effectiveness, sensitivity, and portability, the FET aptasensor is likely to be developed as a convenient detection kit.
These aptasensors mentioned above all improved the detection performance by introducing different substrates or signal transducers, such as nanowell-based electrode surface [87], gold nanoparticle-based transducers [55], and core-shell nanoparticles [91], etc. However, most of them, especially electrochemical aptasensors, involve complex labeling and immobilization processes [92,97]. Thus, to simplify fabrication, a multifunctional DNA 3 way-Junction (3 WJ) structure was proposed to integrate target recognition, signal acquisition, and immobilization [43,54]. The HPR-DNAzyme with hemin was incorporated into one of the fragments of 3 WJ, and the 3 WJ structure was immobilized on the porous Au nanoparticle (pAuNPs) modified Au electrode. When HA detection fragments interacted with HA proteins, the enhanced redox property of 3 WJ was detected via cyclic voltammetry. Recently, they even reported a multifunctional DNA 4-way junction (4 WJ) [98]. In the sensor, the 4 WJ combined with carboxyl-MoS2 as a sensing platform. Its sensing performances were demonstrated via electrochemical analysiscyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS)).
The HA-specific aptasensor is also served for subtyping influenza viruses detection [99,100]. Bhardwaj et al. [100] reported an aptamer against the HA-stabilized stem, which possessed better binding properties than the influenza virus antibodies. Using the aptamer as a recognition element, an electrochemical aptasensor for rapid influenza virus typing was successfully developed. Moreover, its subtyping speed was faster than the standard ELISA and nucleic acid-based methods.
3.1.3. SARS-CoV-2 spike glycoprotein (S protein)
Published structural biology data indicates that coronaviruses, including SARS-CoV and SARS-CoV-2, contain four main structural proteins: the nucleocapsid (N), spike (S), envelope (E), and membrane (M) proteins [101,102]. The S protein is located on the surface of the virion and mediates entry into host cells through the binding of the receptor-binding domain (RBD) and ACE2 receptor [103,104]. Thus, these make it a key target for the vaccination, diagnosis, and treatment of SARS-CoV-2 (Fig. 6 A).
Fig. 6.
(A) The molecular diagram of the spike protein of SARS-CoV-2. (B) The scheme of the electrochemical aptasensor exploited binding-induced conformational change. Reproduced with permission from Ref. [109]. Copyright 2021, American Chemical Society. (C) The secondary structures of dimeric aptamers DSA1N1, DSA1N5, and DSA5N5 and the schematic illustration of the bivalent aptasensor, Cov-eChip. Reproduced with permission from Ref. [114]. Copyright 2021, Wiley Online Library. (D) Stepwise fabrication of the screen-printed carbon electrode-based EIS detection platform. Reproduced with permission from Ref. [115]. Copyright 2022, Elsevier.
Several related aptamers have been reported against the RBD, S1 subunit, and N-terminal domain of SARS-CoV-2 S protein [[105], [106], [107]]. Some of which are listed in Table 3. Song and coworkers [106] firstly screened and acquired two specific aptamers, CoV2-RBD-1C and CoV2-RBD-4C, through the ACE2 receptor competition-based SELEX procedure. Li et al. [107] reported two other aptamers based on the S1 subunit, MSA1, and MSA5. Compared with the above two aptamers, they possessed higher affinity and wider specificity for original SARS-CoV-2 and variants of the virus (Delta and Alpha variants). Moreover, a novel G-quadruplex DNA aptamer was also reported [108]. The aptamer was selected by the talon bead-based SELEX, and it was characterized thoroughly via diverse biophysical, biochemical, and in silico techniques. Finally, through nasopharyngeal swab specimens (N = 232) detection, 91% sensitivity and 98% specificity were attained, which displayed a commensurate capability with the RT-PCR analysis and the practical application value. All of these aptamers have been utilized for the construction of sensors, including electrochemical [109,110], fluorescent [111], SPR [112], SERS sensors [113], etc.
Many researchers have been working on developing the novel electrochemical aptasensor for SARS-CoV-2 detection due to its great potential for ultrasensitive rapid detection at the point of care [109,110]. Idili et al. [109] reported a reagentless, quantitative, and quick electrochemical aptasensor for the S protein detection. In the sensor, redox indicators modified aptamers were immobilized on gold electrodes for capturing viral proteins. When S proteins were added, the electrochemical signal was produced by the interaction-induced conformational change of aptamers (Fig. 6B). This sensor was capable of one-step detecting the SARS-CoV-2 in biological fluids (serum and artificial saliva) efficiently and rapidly. But it acquired a lower signal gain due to the influence of the matrix on the conformational change of aptamers, which limited its practical application.
As one of the most sensitive transduction approaches, electrochemical impedance spectroscopy (EIS) has been widely applied for sensing. Zhang et al. [114] first reported a bivalent aptamer (DSA1N5) nanoprobe-based EIS aptasensor, Cov-eChip, to detect original SARS-CoV-2, Delta, and Alpha variants of this virus in unprocessed saliva. In this sensor, the DSA1N5 bivalent aptamer was engineered by ligating two monomers (MSA1 and MSA5), and it was immobilized on gold electrodes to generate an electrochemical impedance signal (Fig. 6C). Upon binding the virion to the gold electrodes, the electrochemical impedance increased due to the hindered reaction of Fe2+/Fe3+. The Cov-eChip aptasensor allowed ultrasensitive and rapid detection for SARS-CoV-2 and key variants within 10 min. More importantly, it was used to detect actual clinical saliva samples with a clinical sensitivity of 80.5% and specificity of 100%. As far as we know, it is the first research that integrated multivalent aptamers with the electrochemical test for ultrasensitive rapid virus detection. In another study, Abrego-Martinez et al. [115] reported a sensitive, fast, and user-friendly screen-printed carbon electrode-based EIS detection platform (Fig. 6D). Aptamers functionalized with disulfide were immobilized on gold nanoparticles to capture targets. Photo-induced force microscopy (PiFM) further characterized the formed aptamer-virion complex. Then, through the EIS measurement, the detection of SARS-CoV-2 was achieved with an incubation time of 40 min. This aptasensor exhibited excellent sensing performance, having a less detection time than the standard diagnostic tests, a lower limit of detection than PCR, a high sensing performace for a SARS-CoV-2 pseudovirus, and great potential for practical application.
Several optical aptasensors have also been reported with good detection performance [112,116,117]. Aithal et al. [116] utilized aptamer functionalized gold nanoparticles as nanoprobes to develop a colorimetric aptasensor. These original AuNPs (20 nm) have a plasmon resonance peak at 520 nm wavelength in colloidal solutions. With the increase of nanoparticle size, the peak shifts to higher wavelengths due to the rise of scattering and absorption. Thus, based on this principle, the detection signals were acquired by adding coagulant, MgCl2 salt solution. In the absence of viral particles, the coagulant promoted nanoparticle agglomeration and shifted the peak towards higher wavelengths. However, upon the binding of nanoprobes and sufficient proteins, there was no agglomeration of nanoparticles (Fig. 7 A). This aptasensor detected 16 nM spike proteins. And the color change could be point-of-care detection within 75 min via a commercial absorbance reader. Additionally, Pramanik et al. [117] proposed a new nanoparticle surface energy transfer (NSET) strategy to construct optical aptasensors. The detection platform consisted of Rhodamine 6G (Rh-6G) dye-modified aptamers combined with gold nanostars (GNSs), and the fluorescent signal was attained via the distance-dependent nanoparticle surface energy transfer (NSET) (Fig. 7B). In the absence of S protein, Rh-6G was attached to aptamer-wrapped gold nanoparticles to result in fluorescence quenching. However, upon the virus-aptamer binding, the fluorescence signal got recovered.
Fig. 7.
(A) Schematic diagram of the detection principle of SARS-COV-2. (a) The agglomeration of AuNPs; (b) Aptamers bind with S proteins to resist agglomeration; (c) The plasmon absorbance spectra of nanoprobes. Reproduced with permission from Ref. [116]. Copyright 2022, Elsevier. (B) Schematic diagram of nanoparticle surface energy transfer (NSET) based aptasensors. Reproduced with permission from Ref. [117]. Copyright 2021, American Chemical Society. (C) Schematic diagram of the 3D nano-popcorn plasmonic substrate-based SERE aptasensor. Reproduced with permission from Ref. [118]. Copyright 2021, American Chemical Society. (D) The schematic diagram of the proposed glucometer-based point-of-care aptasensor. Reproduced with permission from Ref. [119]. Copyright 2020, Elsevier.
Moreover, SERS-based aptasensors have also been reported for detecting SARS-CoV-2. Chen et al. [118] reported an Au nano-popcorn substrate-based SERS aptasensor. In this sensor, capturing probes were immobilized on the three-dimensional (3D) nano-popcorn plasmonic substrate, hybridizing with Cy3-labeled aptamers to form a detection platform (Fig. 7C). When aptamers and virion were binding, aptamers occurred conformational changes and were released from the substrate. Meantime, the decrease of Raman peak intensity was used to quantify the number of viruses. This assay successfully detected SARS-CoV-2 in artificial synthetic samples. However, the advanced 3D nano-popcorn SERS substrate preparation needed more than 7 h. Thus, Zavyalova et al. [113] proposed a colloidal solution-based SERS platform for detecting SARS-CoV-2 and distinguishing it from other respiratory viruses. In colloidal solution, silver nanoparticles interacted with the various viral surface proteins and aggregated on virion. However, upon the presence of SARS-CoV-2, substantial aptamers labeled with Raman reporter molecules were specifically bound to target viruses and consequently blocking the interaction of silver nanoparticles, resulting in a SERS intensity increase. This method does not require a complex substrate as a reaction platform and is sensitive, fast (7 min), and simple (one step).
Instead of developing a new sensing device, Singh et al. [119] achieved on-site diagnosis of the virus in saliva utilizing a commercial glucometer as signal readouts. S protein-specific aptamers were conjugated to invertase via an antisense oligonucleotide strand to form an aptamer-antisense-invertase complex. The complex was bound with magnetic beads (Fig. 7D). In the presence of targets, the antisense strand was released from beads and separated. Then the enzyme catalyzed sucrose into detectable glucose via a commercial glucometer. This aptasensor provided a low-cost ($3.20/test), rapid (within 1 h), and sensitive method for large-scale screening of SARS-CoV-2 infection.
3.1.4. Other envelope proteins
Also, envelope proteins of Dengue [33], Ebola [120], Zika virus [121,122], hepatitis C virus (HCV) [123] were acted as interesting targets in aptasensors. Recently, Hong et al. [120] reported an integrated chip for selecting aptamers and detecting the Ebola virus. The detection chip consisted of reaction, separation, and signal attainment zones. A fiber optical spectrometer acquired the detection signal. This aptasensor successfully detected the Ebola virus via a one-step assay (Fig. 8 A). Similarly, another highly integrated microfluidic device was reported by Saraf et al. [121], for the multiplex analysis of viruses such as Zika and Chikungunya. The detection mechanism depended on the construction of aptamer1-antigen-AuNP labeled aptamer2 sandwiches. Upon adding silver reagents, the colorimetric signal was attained via a silver staining technique. These highly integrated aptasensors improved the security of operators by reducing contact and showed great potential for point-of-care diagnosis.
Fig. 8.
(A) The integrated chip for selecting aptamers and detecting the Ebola virus. a) Magnetism-controlled selection chip; b) Highly integrated magnetism-controlled detection chip. Reproduced with permission from Ref. [120]. Copyright 2019, American Chemical Society. (B) The star-shaped DNA nano-scaffolds-based aptasensor for detecting Dengue virus. Reproduced with permission from Ref. [124]. Copyright 2020, Nature Chemistry.
In addition, Kwon and coworkers [124] proposed a new multivalent spatial pattern recognition for viral sensing and inhibition. They designed star-shaped DNA nano-scaffolds based on the Dengue virus envelope protein domain Ⅲ (ED3) distribution, acting as a template to show multiple binding motifs with a precise spatial pattern-recognition ability (Fig. 8B). When aptamers bound to envelope proteins domainⅢ(ED3) on the surface of virions, fluorescence recovery was achieved. In the study, the multivalent aptamers exhibited much more excellent viral detection and inhibition capabilities than monovalent aptamers. Meanwhile, this multivalent spatial recognition mode provided a new strategy for improving the stability and affinity of aptamers.
3.2. Capsid protein
The capsid is the protein coat of the virus that protects its genes from damage caused by the extracellular environment. The capsid together with the packaged viral genome makes up the nucleocapsid. The presence of a single capsid protein cannot spatially encapsulate the complete viral nucleic acid. Thus, the capsid protein is often assembled into a hollow structure in a symmetrical form to package the viral nucleic acid. Some viral capsid proteins can be used as biomarkers for viral diagnoses, such as Norovirus, hepatitis C virus (HCV), human papillomavirus (HPV), etc.
3.2.1. Norovirus capsid protein
Norovirus (NoV), a foodborne pathogen, can cause sporadic and epidemic acute viral gastroenteritis [125]. Its outbreaks frequently occur in public places such as restaurants, schools, hospitals, nursing homes, etc. [126]. Currently, RT-qPCR and ELISA are standard methods for NoV diagnosis [127,128]. But these methods cannot meet the requirements of on-site detection. Moreover, noroviruses possess high resistance to common disinfection and a low dose of infection (<100 virions) [129]. These make the development of early point-of-care detection necessary for preventing and controlling disease outbreaks.
Utilizing the norovirus capsid protein as a diagnostic marker (Fig. 9 A), electrochemical aptasensors have been proposed to provide a promising alternative for norovirus detection [[130], [131], [132]]. Chand et al. [133] reported a microfluidic chip-based electrochemical aptasensor. Aptamers tagged with ferrocene molecules were immobilized on graphene-gold nanoparticle composites. Upon the binding of aptamers and viruses, the electrochemical signal from the ferrocene decreased due to the conformational changes of aptamers. This microfluidic chip could detect norovirus in a range of 100 pM to 3.5 nM within 35 min. Moreover, this microfluidic chip also contained a microbeads-based microfiltration zone for filtering and enriching noroviruses from the complex matrix. Thus, the platform achieved one-step sample purification and detection.
Fig. 9.
(A) The molecular diagram of the capsid protein of murine norovirus (MNV-1). (B) Reaction pathway of guanine chemiluminescence and the schematic diagram of the chemiluminescent aptasensor. Reproduced with permission from Ref. [135]. Copyright 2018, Elsevier. (C) Schematic diagram of the nanozyme-based colorimetric aptasensor. Reproduced with permission from Ref. [38]. Copyright 2019, American Chemical Society. (D) Schematic diagram of the plasmonic nanoplatform for HCVcoreAg. Reproduced with permission from Ref. [37]. Copyright 2020, Elsevier.
Some optical aptasensors have also been reported for point-of-care diagnosis of noroviruses, including colorimetric detection [38], SPR [34], fluorescence detection [134,135],etc. Weng et al. [134] reported an easy-to-fabricate, simple, and cost-effective paper-based microfluidic device. The 6-FAM labeled aptamer acted as capture probes and graphite-based nanomaterials (GO, multi-walled carbon nanotubes, etc.) acted as quenchers. Based on the fluorescence quencher/recovery principle, the detection of norovirus was achieved. In another study, Kim et al. [135] proposed a novel strategy based on the principle of intra-chemiluminescent resonance energy transfer (Intra-CRET). The aptamer probe was modified with 5 extra guanines and fluorescent dye (fluorescein, 6-FAM). In the absence of targets, the interaction of guanines with 3,4,5-trimethoxylphenylglyoxal (TMPG) formed high-energy intermediates. Then, 6-FAM obtained energy from the high-energy intermediate and excited fluorescence emission. However, when the NoV was added, the aptamer-virus complex cannot emit light (Fig. 9B). The aptasensor can quantitatively measure norovirus capsid proteins in various samples with excellent stability and accuracy.
Most of the methods mentioned above can accomplish highly specific and sensitive detection of norovirus. But they still cannot reach the lower end of the ID50. The lowest known detection limit is 30 viruses/mL to date [38]. Weerathunge et al. combined the aptamer with gold nanoparticles (AuNPs) with nanozyme activity through electrostatic interactions to fabricate a colorimetric aptasensor (Fig. 9C). Upon the interaction of aptamers and noroviruses, aptamers were desorbed from AuNPs-aptamer nanoconjugates and the active sites were exposed to catalyze chromogen reagent to produce a blue product. Although this detection method has high detection sensitivity, it is also easily affected by the reaction environment, which limits its practical application.
3.2.2. Hepatitis C core antigen (HCVcoreAg)
HCV is a single-stranded, enveloped, and positive-sense RNA virus. Early diagnosis of HCV infection is urgent because there is a global health burden and no vaccine available. HCVcoreAg, an nucleocapsid protein, possesses the most conservative primary sequence in all HCV proteins [136]. Moreover, the HCVcoreAg is present in the blood 10–15 days after infection, which is much earlier than relevant antibodies [137,138]. Thus, the HCVcoreAg is a crucial serum marker in the clinical detection of HCV infection.
Due to the unique physical and chemical properties, diverse nanomaterials from metal to carbon-based materials have been widely developed and applied for sensing fields [139,140]. Significantly, the high surface-to-volume ratio makes them suitable substrates for the immobilization of probes. Recently, Li et al. [37] reported a plasmonic nanoplatform composed of catalytic hairpin assembly (CHA) amplification, polystyrene (PS) nanofibrous membrane, and AuNPs signal readouts (Fig. 9D). This platform realized naked-eye enzyme-free detection of the HCVcoreAg based on the HCVcoreAg-triggered CHA amplification reaction.
Chitosan, a polysaccharide polymer, has been used as an immobilization matrix for biosensors due to its good adhesion, excellent membrane-forming ability, high water permeability, easy chemical modification, etc. [141,142]. Ghanbari and Roushani [143] took advantages of chitosan and multi-walled carbon nanotubes, developing a multi-walled carbon nanotubes-chitosan nanoplatform (MWCNTs-Chit) based electrochemical aptasensor. Meanwhile, this aptasensor combined the hybrid molecular imprinting (MIP) method to achieve HCVcoreAg detection. This novel aptasensor was reported for the first time. And according to an analogous principle, they also constructed the graphene quantum dots (GQD) modified glassy carbon electrodes (GCE) based nanoplatform [144]. Some of which are summarized in Table 5 .
Table 5.
Summary of aptasensors applied in virus detection.
| Virus | Recognition sites | Detection techniques | Limit of detection (LOD) | Linear ranges | Detection Time | Refs. |
|---|---|---|---|---|---|---|
| Hepatitis B virus | HBsAg | Field-effect transistor | 10 aM | 10 aM-0.1 μM | 5 s | [79] |
| Cyclic voltammetry | 0.0014 fg/mL | 0.125–2.0 fg/mL | – | [78] | ||
| Chemiluminescent | 0.05 ng/mL | 1–225 ng/mL | – | [77] | ||
| Chemiluminescent | 0.1 ng/mL | 1–200 ng/mL | – | [76] | ||
| FRET | 2.5 pM | 50–250 pM | – | [41] | ||
| SARS-CoV-2 | Spike protein | FET | 10 fM | 10 fM-10 pM | – | [201] |
| Electrochemical detection | 1 ag/mL | 1 ag/mL-1 pg/mL | – | [110] | ||
| Nanoparticle surface energy transfer (NSET) | 130 fg/mL | 100 fg/mL-10 pg/ml | 10 min | [117] | ||
| SERS | 1 fM | 1 fM-1μM | – | [111] | ||
| Glucometer | 4.38 pM | 1–500 pM | 60 min | [119] | ||
| SARS-CoV | Nucleocapsid | Colorimetry | 1 ng/mL | – | 15 min | [36] |
| Glucometer | 5.76 pM | 1–500 pM | – | [119] | ||
| Proximity ligation assay (PLA) | 37.5 pg/mL | 50–5000 pg/mL | 120 min | [162] | ||
| Fluorescence detection | 2 pg/mL | – | – | [187] | ||
| Fluorescence detection | 0.1 pg/mL | 60 min | [202] | |||
| Hepatitis C virus | HCVcoreAg | Electrochemical detection | 2 fM | 0.2 fM-0.2 pM | 17 min | [139] |
| Colorimetry | 0.1 fg/mL | 0.1–10 pg/mL | – | [37] | ||
| Electrochemical detection | 1.67 fg/mL | 5.0 fg/mL-1.0 pg/mL | – | [143] | ||
| Impedance | 3.3 pg/mL | 10–70 pg/mL and 10–70 pg/mL | – | [144] | ||
| AFM | 0.1 pM | 0.1–100 pM | – | [146] | ||
| Lateral Flow Strip | 10 pg/mL | 10–1000 pg/mL | 10 min | [203] | ||
| Norovirus | Capsid | Colorimetry | 30 viruses/mL | 200-10000 viruses/mL | 10 min | [38] |
| Square wave voltammetry | 10 aM | 20–120 aM | 60 min | [130] | ||
| SPR | 70 aM | 70–200 aM | – | [34] | ||
| Fluorescence detection | 3.3 ng/mL | 13 ng/mL-13 μg/mL | 10 min | [134] | ||
| 4.4 ng/mL | ||||||
| Differential pulse voltammograms | 100 pM | 100 pM-3.5 nM | 35 min | [133] | ||
| Intra chemiluminescent resonance transfer (Intra-CRET) | 80 ng/mL | 0.16–10 μg/mL | – | [135] | ||
| Electrochemical detection | 1.44 μg/mL | 0.01–1000 μg/mL | – | [131] | ||
| HPV | L1 capsid | laser desorption ionization mass spectrometry (LDI MS) | 58.8 pg/mL | 2–80 ng/mL | – | [152] |
| Differential pulse voltammetry | 0.1 ng/mL | 0.2–2 ng/mL | – | [151] | ||
| Cyclic voltammetry | 490 pM | 10–80 nM | – | [204] | ||
| HIV | Tat portein | SPRe-TIRE | 1.11 pM | 1.0–500 nM | – | [171] |
| Spectroscopic ellipsometry (SE) | 115 pM | 1.0–500 nM | ||||
| Colorimetry | 10 nM | 10–150 nM | – | [173] | ||
| FET | 0.6 nM | 0.6 nM-1.0 uM | – | [175] | ||
| Dengue virus | NS1 protein | Cyclic voltammetry | 0.3 ng/mL | 3–160 ng/mL | – | [176] |
| Envelop protein domain III (ED3) | Fluorescence detection | 1 × 102 p.f.u.ml−1 | 1 × 102–1 × 106 p.f.u.ml−1 | – | [124] | |
| Ebola virus | Type I transmembrane protein | Fluorescence detection | 4.2 ng/mL | 5–150 ng/mL | – | [120] |
| Zika virus | NS1 protein | ELISA | 0.1 ng/mL | 0.1–1 ng/mL | – | [177] |
| Envelop protein | Colorimetry | 1 pM | 1pM-1 nM | – | [121] |
Also, the group of Pleshakova [[145], [146], [147]] firstly reported aptamer-based atomic force microscopy (AFM) analysis methods in recent years. This method enables efficient label-free detection of proteins at the single-molecule level in sample solutions. However, the technique is commonly time-consuming, requires specialized instruments and technicians, and has rarely been reported for the detection of other viruses.
3.2.3. Human papillomavirus L1 protein
The human papillomavirus (HPV) is a nonenveloped virus with a diameter of 52–55 nm [148]. Since HPV is associated with the occurrence of cervical cancer, it has become a vital indicator for clinical cancer diagnosis. The L1 capsid protein is one of the main biomarkers for detection because it occupies approximately 80% of total capsid protein [149,150]. Thus, developing effective methods for HPV L1 protein detection is crucial.
Chekin et al. [151] reported an electrochemical aptasensor for the L1 protein detection. The glassy carbon electrode modified with composite nanomaterials (prGO-MoS2 hybrids) as electrochemical transducers provided a large reaction area for aptamers binding. Direct differential pulse voltammetry (DPV) analysis realized highly sensitive detection with a 0.1 ng/mL limit detection. Recently, Zhu and coworkers [152] proposed a new laser desorption/ionization time-of-flight mass spectrometry (LDI-TOF MS) assay. This assay relied on the competitive non-covalent interaction between L1 protein-specific aptamers and melamines, realizing the quantitative detection of L1 proteins in clinical and vaccine samples. Some other aptasensors are summarized in Table 5.
3.3. Nucleoprotein
The nucleoprotein is an internal protein that binds to the viral nucleic acid to form ribonucleoprotein (RNP) particles. It usually plays an important role in virus replication, translation, and packaging. Viral nucleoproteins are generally highly conserved. Therefore, it is often used as a recognition site for aptasensors.
3.3.1. Influenza virus nucleoprotein
The influenza virus nucleoprotein (NP) is much more than an RNA-binding protein. Still, it plays multiple crucial functions throughout the virus life cycle, including transcription, replication, and packaging of the virus (Fig. 10 A) [153]. Compared with the HA, which could generate “antigenic drift” and “antigenic shift,” the nucleoprotein is minor subject to mutations [154,155].
Fig. 10.
(A) The molecular diagram of the nucleoprotein of influenza A virus (H1N1). (B) Mechanisms of a self-calibrating dual-electrode aptasensor. Reproduced with permission from Ref. [156]. Copyright 2020, Elsevier. (C) Schematic diagram of AuNPs-based lateral flow assay. Reproduced with permission from Ref. [158]. Copyright 2019, American Chemical Society. (D) Schematic diagram of the liposome-based heterogeneous sandwich-type colorimetric aptasensor. Reproduced with permission from Ref. [157]. Copyright 2020, Elsevier.
Recently, Lee et al. [156] proposed a dual-electrode electrochemical aptasensor with the capability of self-calibrating to detect the nucleoprotein of avian influenza virus. The redox-active indicator (MB) was loaded into the pores of 3D nanostructured porous silica film (3DNRE) to form reaction and reference electrodes (Fig. 10B). The reaction electrode was modified with the nucleoprotein-specific aptamer to capture targets, resulting in redox current changes. However, the reference electrode was decorated with random sequences (Aptcon-MB@3DNRE) to self-correct under complex conditions. The platform was found to sensitively detect AIV NPs. And the study has proved that the proposed dual-electrode aptasensor owns higher stability than conventional single-electrode aptasensors.
Several heterogeneous sandwich-type aptasensors have also been reported recently [[157], [158], [159]]. Kang et al. [158] firstly reported a single-stranded DNA binding protein (RPA 70A) conjugated AuNPs-based lateral flow assay for detecting the nucleoprotein. In this sensor, based on the nonspecific binding of RPA70A and ssDNA, multiple AuNPs were attached to an aptamer to overcome the low sensitivity question caused by the small number of AuNPs (Fig. 10C). This aptasensor achieved visual detection at a low femtomolar range and had excellent potential for point-of-care disease diagnosis. Similarly, they utilized the nonspecific binding of RPA70A and ssDNA and developed another liposome-based heterogeneous sandwich-type colorimetric aptasensor [157]. The horseradish peroxidase was encapsulated into the liposome, and the RPA70A was attached to the surface of the liposome. Through catalyzing signal amplification, the thrombocytopenia syndrome virus was successfully detected. However, due to the instinct features of liposomes, the designed aptasensor was challenging to store. Kang and coworkers [157] also devised another colorimetric aptasensor, relying on the binding of biotin-horseradish peroxidase and streptavidin-fusion RPA70A (Fig. 10D). The specificity, universality, stability, and sensitivity of the newly proposed aptasensor got demonstrated.
3.3.2. SARS-CoV-2 nucleocapsid protein
Besides spike glycoprotein as a diagnostic marker, the nucleocapsid protein, a multifunctional RNA-binding protein necessary for transcription and replication of viral RNA, is also a promising biomarker for diagnosing and treating SARS-CoV-2 (Fig. 11 A) [160].
Fig. 11.
(A) The molecular diagram of the nucleoprotein of SARS-CoV-2. (B) a)The fabrication of the Au@Pt/MIL-53 nanoprobes; b)the schematic illustration of the dual-aptamer electrochemical biosensor. Reproduced with permission from Ref. [161]. Copyright 2021, Elsevier. (C) Scheme of aptamer-assisted proximity ligation strategy for SARS-CoV-2 nucleocapsid protein. Reproduced with permission from Ref. [162]. Copyright 2020, Royal Society of Chemistry. (D) The protein-induced fluorescence enhancement (PIFE)-based biosensor for SARS-CoV-2 detection, scheme illustration of the mix-and-read 1-min assay, and the principle of PIFE. Reproduced with permission from Ref. [163]. Copyright 2021, Royal Society of Chemistry. (E) the fabrication of Nb2C-SH QDs and the schematic diagram of the Nb2C-SH QD-based SPR aptasensor. Reproduced with permission from Ref. [164]. Copyright 2021, Springer.
Zhang et al. [36] successfully selected several high-affinity DNA aptamers specific to SARS-CoV-2 nucleocapsid protein. Some of which are listed in Table 3. Recently, Tian and coworkers [161] reported a dual-probe electrochemical aptasensor based on the Au@Pt nanoparticles and enzymes modified metal-organic frameworks (Au@Pt/MIL-53) (Fig. 11B). First, two recognition probes (N48 and N61) were immobilized on the electrode to capture nucleocapsid protein. Then, the Au@Pt nanoparticles were decorated on the MIL-53 metal-organic frameworks to form the Au@Pt/MIL-53 nanomaterial composites. The nanomaterial composites decorated by hemin/G-quadruplex DNAzyme and horseradish peroxidase (HRP) acted as nanoprobes of the sensor for signal amplification. When nucleocapsid proteins and nanoprobes were added, the sandwich structure was formed in the PBS solution containing HQ and H2O2 and the amplifying DPV signal was acquired. This aptasensor achieved highly sensitive detection of SARS-CoV-2 nucleocapsid protein.
Liu et al. [162] reported a novel proximity ligation aptasensor. In this platform, two aptamers linked to different sites of the same protein were used as recognition probes. Upon the interaction of aptamers and proteins, the DNA junction region came close, thus initiating the qPCR amplification (Fig. 11C). The qPCR signal was shown through the cycle threshold (Ct) value, and the system could detect the lowest concentration was 37.5 pg/mL. Lee et al. [163] proposed another novel protein-induced fluorescence enhancement (PIFE) based aptasensor to allow mix-and-read, one-step, and 1-min detection of SARS-CoV-2 nucleocapsid protein. In the PIFE strategy, the excited state of Cy3 can exist as a trans or cis isomer, but only the trans had a significant fluorescence quantum yield. In close to a protein, Cy3 preferentially stays in its trans conformation state, resulting in increased fluorescence. Cy3-labeled aptamers were used as recognition probes in the sensor based on the phenomenon. When nucleocapsid proteins were added to the buffer, Cy3 approached the nucleocapsid protein due to the specific binding between aptamers and nucleocapsid proteins, which increased fluorescence (Fig. 11D). The aptasensor as a mix-and-read detection strategy for SARS-CoV-2 had excellent potential for point-of-care diagnostics in clinical settings.
Additionally, a niobium carbide MXene quantum dots (Nb2C QDs)-based SPR aptasensor was also reported by Chen et al. [164]. Through a solvothermal assay, the thiol group functionalized Nb2C QDs (Nb2C-SH QDs) nanoparticles were fabricated, and they were uniformly bound to the gold substrate of SPR via covalent binding. Subsequently, nucleocapsid protein-specific aptamers were immobilized on this surface by electrostatic adsorption, etc (Fig. 11E). When nucleocapsid proteins were added, the aptamer-virus interaction resulted in aptamer conformational variation, which issued in an increase in the contact area or distance between the aptamer and the SPR chip, thus changing the SPR signal. This aptasensor had a low LOD and it showed good stability and specificity in complex matrices.
3.4. Functional protein
Some functional proteins such as enzymes can also be used as biomarkers for virus detection.
3.4.1. HIV trans-activator of transcription (HIV Tat)
HIV is a lentivirus that belongs to the subgroup retrovirus. The HIV trans-activator of transcription (Tat) is the earliest protein to present in blood [165]. It plays a vital role in the HIV life cycle and has become an attractive biomarker in detecting HIV infection [166]. Several aptasensors have been reported to detect HIV Tat protein, including QCM [167], SPR [168], FET [169,170], etc.
Recently, researchers have proposed new strategies to construct aptasensors for HIV detection. Caglayan and coworkers [171] firstly reported two spectrophotometric ellipsometry-based aptasenosors for HIV diagnosis, including the spectroscopic ellipsometry (SE) and the SPR-enhanced total internal reflection ellipsometry (SPRe-TIRE). Through monitoring the changes of delta (Δ) angle, the detection of Tat proteins was realized. Among them, the SPRe-TIRE aptasensor has a lower LOD than the SE aptasensor.
For the first time, Yamamoto-Fujita and Kumar [172] proposed the pair split aptamer of the Tat protein. Based on this pair split aptamer, Fatin et al. [173] reported a split aptamer colorimetric aptasensor. This aptasensor utilized the SPR mechanism of AuNPs. Through the coordinated assembly and disassembly of split aptamers on AuNPs, a color change from red to purple was observed with the naked eye. Similarly, the pair split aptamer was utilized in field-effect transistor (FET) based aptasensors [170,174,175]. Fatin et al. [175] proposed a multiwall carbon nanotube (MWCNTs)-modified FET aptasensor to diagnose HIV infection. Multiwall carbon nanotubes with treatment of acid oxidation formed functionated-MWCNTs, and worked as an immobilization interface of aptamers against HIV-1 Tat. This nanomaterial-based FET aptasensor showed more excellent performance, with a limit detection of 600 pM.
3.4.2. Other functional protein
The NS1, a nonstructural protein of Dengue [176] and Zika [177], the e antigen of hepatitis B (HBeAg) [178,179], the HIV reverse transcriptase, the HPV E7 protein [180], etc., were also acted as interesting targets for the detection of viruses.
Rashid et al. [176] reported a label-free electrochemical aptasensor for detecting the NS1 protein of the Dengue virus. The principle of this aptasensor was the polyethyleneimine (PEI) and aptamer-assisted dispersion-aggregation of AuNPs, leading to changes in electrochemical signals. This aptasensor had enhanced sensitivity without the time-consuming and tedious immobilization procedure. Lee et al. [177] screened a pair of Zika NS1-specific aptamer and developed an ELISA platform for detecting the Zika virus. In the ELISA assay, they respectively utilized an aptamer-aptamer pair and aptamer-antibody pair as capture/detection agents. They showed the aptasensor contained aptamer-antibody (LOD of 0.1 ng/mL) pair possessed higher sensitivity in the buffer.
4. Virus particles and their detection
In the above-mentioned aptasensors for virus detection, the aptamer has a specific recognition site for virus particles (envelope protein, capsid protein, nucleoprotein, or functional protein). Most of these aptamers use soluble purified proteins as targets and are obtained by traditional SELEX procedures. However, with the development of SELEX technology, it is also possible to screen specific aptamers using intact virions as targets. These aptamers can specifically recognize and bind to virions, but their binding sites are unknown. In this section, we reviewed the application of these aptamers in virus detection.
Lee et al. have developed integrated microfluidic devices for virus particle detection in recent years. In 2014, they utilized an integrated microfluidic system for successfully screening an influenza A/H1N1 virus-specific aptamer in a highly efficient and automated assay [183]. Subsequently, several integrated microfluidic-based aptasensors were reported [[194], [195], [196]]. A novel integrated microfluidic aptasensor based on a single universal aptamer was proposed to detect three kinds of viruses (H1N1, H3N2, and influenza B) [195]. The capability of multiple identities of the biosensor relied on the conformational changes of a single probe in different ion environments. Through the level of fluorescence intensity to differentiate different types of influenza viruses. Moreover, they firstly proposed an ELISA-like assay-based structure-free digital microfluidic platform [196].
In the sensor, the magnetic microbeads acted as a driver enveloped into comparatively hydrophilic droplet SETs (surface energy traps). On the super-hydrophobic surface, the SETs were driven via electromagnetic forces. Meanwhile, the magnetic microbeads were also used as substrates for ELISA-like assay, and the fluorescent signals were amplified via the tyramide signal amplification (TSA) assay. The platform has great potential for rapid diagnosis with a limit detection of 0.032 HUA and detection time of 40 min.
Also, the team of Gu reported an immobilization-free SELEX procedure to screen cognate pair of aptamers with different binding sites against virus particles, including whole avian influenza viruses [184,185] and bovine viral diarrhea virus type 1 [197], and developed sandwich-type SPR and lateral flow strip assays for viruses detection. J3APT and JH4APT are aptamer pairs specific to the H5N2 virus. Kim and coworkers [184] utilized them to construct a sandwich-type platform that realized the on-site diagnosis of the H5N2 virus. This sensor showed comparable sensitivity to commercial kits for quick detection of the influenza A virus.
5. Discussion and perspective
Up to now, various optical and electrochemical aptasensors have been successfully developed for virus detection. However, few of them can be applied in clinical diagnosis. On the one hand, the performance of sensors is not enough to meet clinic diagnosis, including sensitivity, repeatability, and simplicity. To improve the detection performance of aptasensors, several ways should be taken: 1) utilizing nanomaterials as immobilization substrates, which provides a larger surface area for immobilizing aptamers, such as gold nanoparticles [36,176], multi-walled carbon nanotubes [134,143], reduced graphene oxide [180], etc. 2) forming microfluidic chip which can achieve on-site sensor for one-step sample purification and detection [120,133,196]. 3) proposing novel strategies and developing novel aptasensors [171], etc.
On the other hand, aptamers' binding affinity and stability need to be improved. Almost all aptasensors used monovalent aptamers as capture probes. The selectivity and affinity of monovalent aptamers are easily subjected to sample conditions, including temperature, pH, ionic strength, interference, viscosity, etc [198]. Several strategies could be taken to address this issue: 1) Using multivalent spatial recognition patterns [114,124]. Generally, multivalent aptamers exhibit more excellent recognition and binding abilities than monovalent aptamers. The binding affinity and stability of aptamers can be improved by linking two or more monomers to construct bivalent or multivalent aptamers. 2) Modification of aptamers, including chemical modification of aptamer nucleotides, insertion of unnatural nucleotides into aptamers, capping of aptamer ends, etc. 3) Further optimization of SELEX procedure to improve aptamer binding affinity, such as Cell-SELEX, microfluidics technology, slow off-rate modified aptamer technology, etc.
Recently, an integrated platform that combined a DNA aptamer-based diagnostic test (AptameX) and its digital health passporting app Teman SehatTM (“Health Buddy”) was successfully developed by Achiko Limited for low-cost, rapid, saliva-based, and user-friendly detection of ongoing SARS-CoV-2. The pilot program has been launched in Indonesia. This platform makes testing affordable to workplaces and communities. It brings the cost of frequent testing down to around the cost of a simple lunch while the reliable day-to-day management of the test results becomes a simple process. More importantly, this is the first commercially available aptamer-based virus detection kit, indicating aptamers' practical value in clinical applications.
6. Conclusion
In this review, aptasensors of viral detection were summarized for the first time according to the classification of the viral target protein, and usual detection mechanisms were illustrated, including colorimetry, fluorescence assay, SPR, SERS, electrochemical detection, and FET. The relationships between the different biomarkers of the viruses and the detection methods and their performances were revealed. This review will provide valuable references for constructing point-of-care aptasensors for the detection of viruses.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the Hunan Provincial Natural Science Foundation for Distinguished Young Scholars (2022JJ10086), Innovation-Driven Project of Central South University (2020CX048), the National Natural Science Foundation of China (81301258), the Natural Science Foundation of Hunan Province (2019JJ60071, 2020JJ4680), the Natural Science Foundation of Changsha (kq2202131), the Shenghua Yuying Project of Central South University, the Postgraduate Innovation Project of Central South University (2020zzts819, 2020zzts408, 2020zzts409, 2021zzts0977, 2021zzts0979), and the Open-End Fund for the Valuable and Precision Instruments of Central South University.
References
- 1.Shortridge K.F., Zhou N.N., Guan Y., Gao P., Ito T., Kawaoka Y., Kodihalli S., Krauss S., Markwell D., Murti K.G. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- 2.Yamayoshi S., Kawaoka Y. Ebolavirus’s foibles. Cell. 2017;169:773. doi: 10.1016/j.cell.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Al-Sadeq D.W., Taleb S.A., Zaied R.E., Fahad S.M., Smatti M.K., Rizeq B.R., Al Thani A.A., Yassine H.M., Nasrallah G.K. Hepatitis B virus molecular epidemiology, host-virus interaction, coinfection, and laboratory diagnosis in the MENA Region: an update. Pathogens. 2019;8:63. doi: 10.3390/pathogens8020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peeling R.W., Artsob H., Pelegrino J.L., Buchy P., Cardosa M.J., Devi S., Enria D.A., Farrar J., Gubler D.J., Guzman M.G. Evaluation of diagnostic tests: dengue. Nat. Rev. Microbiol. 2010;8:S30. doi: 10.1038/nrmicro2459. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz S.A., Nair M.P. Current concepts in human immunodeficiency virus infection and AIDS. Clin. Diagn. Lab. Immunol. 1999;6:295. doi: 10.1128/cdli.6.3.295-305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hills S.L., Fischer M., Petersen L.R. Epidemiology of Zika virus infection. J. Infect. Dis. 2017;216:S868. doi: 10.1093/infdis/jix434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed.: Atenei Parmensis. 2020;91:157. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C.-G., Lee K.-M., Hsiao M.-J., Yang S.-L., Huang P.-N., Gong Y.-N., Hsieh T.-H., Huang P.-W., Lin Y.-J., Liu Y.-C. Culture-based virus isolation to evaluate potential infectivity of clinical specimens tested for COVID-19. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.01068-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ro Y.T., Ticer A., Carrion R., Jr., Patterson J.L. Rapid detection and quantification of Ebola Zaire virus by one-step real-time quantitative reverse transcription-polymerase chain reaction. Microbiol. Immunol. 2017;61:130. doi: 10.1111/1348-0421.12475. [DOI] [PubMed] [Google Scholar]
- 10.Comin A., Toft N., Stegeman A., Klinkenberg D., Marangon S. Serological diagnosis of avian influenza in poultry: is the haemagglutination inhibition test really the ‘gold standard. Influenza Respiratory Virus. 2013;7:257. doi: 10.1111/j.1750-2659.2012.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai S.-C., Huang Y.-Y., Shu P.-Y., Chang S.-F., Hsieh P.-S., Wey J.-J., Tsai M.-H., Ben R.-J., Hsu Y.-M., Fang Y.-C. Development of an enzyme-linked immunosorbent assay for rapid detection of dengue virus (DENV) NS1 and differentiation of DENV serotypes during early infection. J. Clin. Microbiol. 2019;57 doi: 10.1128/JCM.00221-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellington A.D., Szostak J.W. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 13.Tuerk C., Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Sci. Technol. Humanit. 1990;249:505. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 14.Sekhon S.S., Lee S.-H., Lee K.-A., Min J., Lee B.-T., Kim K.-W., Ahn J.-Y., Kim Y.-H. Defining the copper binding aptamotif and aptamer integrated recovery platform (AIRP) Nanoscale. 2017;9:2883. doi: 10.1039/c6nr09408b. [DOI] [PubMed] [Google Scholar]
- 15.Qu H., Csordas A.T., Wang J., Oh S.S., Eisenstein M.S., Soh H.T. Rapid and label-free strategy to isolate aptamers for metal ions. ACS Nano. 2016;10:7558. doi: 10.1021/acsnano.6b02558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai Y., Deng Y., Yang G., Li S., Zhang C., Liu X. Molecular imprinting polymers electrochemical sensor based on AuNPs/PTh modified GCE for highly sensitive detection of carcinomaembryonic antigen. J. Biomed. Nanotechnol. 2018;14:1688. doi: 10.1166/jbn.2018.2617. [DOI] [PubMed] [Google Scholar]
- 17.Lee N., Wang C., Park J. User-friendly point-of-care detection of influenza A (H1N1) virus using light guide in three-dimensional photonic crystal. RSC Adv. 2018;8 doi: 10.1039/c8ra02596g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das R., Dhiman A., Kapil A., Bansal V., Sharma T.K. Aptamer-mediated colorimetric and electrochemical detection of Pseudomonas aeruginosa utilizing peroxidase-mimic activity of gold NanoZyme. Anal. Bioanal. Chem. 2019;411:1229. doi: 10.1007/s00216-018-1555-z. [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Zhang Y., Chen Y., Hong S., Sun Y., Sun N., Pei R. In vitro selection of DNA aptamers against renal cell carcinoma using living cell-SELEX. Talanta. 2017;175:235. doi: 10.1016/j.talanta.2017.07.049. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Lai B.S., Juhas M. Recent advances in aptamer discovery and applications. Molecules. 2019;24:941. doi: 10.3390/molecules24050941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalirirad S., Steckl A.J. Lateral flow assay using aptamer-based sensing for on-site detection of dopamine in urine. Anal. Biochem. 2020;596 doi: 10.1016/j.ab.2020.113637. [DOI] [PubMed] [Google Scholar]
- 22.Xu L., Zhang Z., Zhang Q., Li P. Mycotoxin determination in foods using advanced sensors based on antibodies or aptamers. Toxins. 2016;8:239. doi: 10.3390/toxins8080239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McConnell E.M., Nguyen J., Li Y. Aptamer-based biosensors for environmental monitoring. Front. Chem. 2020;8:434. doi: 10.3389/fchem.2020.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunha I., Biltes R., Sales M.G.F., Vasconcelos V. Aptamer-based biosensors to detect aquatic phycotoxins and cyanotoxins. Sensors. 2018;18:2367. doi: 10.3390/s18072367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun M., Liu S., Song T., Chen F., Zhang J., Huang J., Wan S., Lu Y., Chen H., Tan W. Vol. 143. 2021. Spherical neutralizing aptamer inhibits SARS-CoV-2 infection. [DOI] [PubMed] [Google Scholar]
- 26.Devi A., Chaitanya N.S. Designing of peptide aptamer targeting the receptor-binding domain of spike protein of SARS-CoV-2: an in silico study. Mol. Divers. 2021;26:157. doi: 10.1007/s11030-020-10171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parashar N.C., Poddar J., Chakrabarti S., Parashar G. Repurposing of SARS-CoV nucleocapsid protein specific nuclease resistant RNA aptamer for therapeutics against SARS-CoV-2. Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ladju R.B., Pascut D., Massi M.N., Tiribelli C., Sukowati C.H. Aptamer: a potential oligonucleotide nanomedicine in the diagnosis and treatment of hepatocellular carcinoma. Oncotarget. 2018;9:2951. doi: 10.18632/oncotarget.23359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F., Gopinath S.C., Lakshmipriya T. Aptamer-antibody complementation on multiwalled carbon nanotube-gold transduced dielectrode surfaces to detect pandemic swine influenza virus. Int. J. Nanomed. 2019;14:8469. doi: 10.2147/IJN.S219976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng X., Wang C., Gao Y., Li J., Wen W., Zhang X., Wang S. Applying strand displacement amplification to quantum dots-based fluorescent lateral flow assay strips for HIV-DNA detection. Biosens. Bioelectron. 2018;105:211. doi: 10.1016/j.bios.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 31.Dolai S., Tabib-Azar M. Microfabricated nano-gap tunneling current Zika virus sensors with single virus detection capabilities. IEEE Sensor. J. 2020;20:8597. [Google Scholar]
- 32.Kwon J., Lee Y., Lee T., Ahn J.H. Aptamer-based field-effect transistor for detection of avian influenza virus in chicken serum. Anal. Chem. 2020;92:5524. doi: 10.1021/acs.analchem.0c00348. [DOI] [PubMed] [Google Scholar]
- 33.Basso C.R., Crulhas B.P., Magro M., Vianello F., Pedrosa V.A. A new immunoassay of hybrid nanomater conjugated to aptamers for the detection of dengue virus. Talanta. 2019;197:482. doi: 10.1016/j.talanta.2019.01.058. [DOI] [PubMed] [Google Scholar]
- 34.Kim S., Lee S., Lee H.J. An aptamer-aptamer sandwich assay with nanorod-enhanced surface plasmon resonance for attomolar concentration of norovirus capsid protein. Sensor. Actuator. B Chem. 2018;273:1029. [Google Scholar]
- 35.Lv Q., Wang Y., Su C., Lakshmipriya T., Gopinath S.C.B., Pandian K., Perumal V., Liu Y. Human papilloma virus DNA-biomarker analysis for cervical cancer: signal enhancement by gold nanoparticle-coupled tetravalent streptavidin-biotin strategy. Int. J. Biol. Macromol. 2019;134:354. doi: 10.1016/j.ijbiomac.2019.05.044. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L., Fang X., Liu X., Ou H., Zhang H., Wang J., Li Q., Cheng H., Zhang W., Luo Z. Discovery of sandwich type COVID-19 nucleocapsid protein DNA aptamers. Chem. Commun. 2020;56 doi: 10.1039/d0cc03993d. [DOI] [PubMed] [Google Scholar]
- 37.Li X., Yin C., Wu Y., Zhang Z., Jiang D., Xiao D., Fang X., Zhou C. Plasmonic nanoplatform for point-of-care testing trace HCV core protein. Biosens. Bioelectron. 2020;147 doi: 10.1016/j.bios.2019.111488. [DOI] [PubMed] [Google Scholar]
- 38.Weerathunge P., Ramanathan R., Torok V.A., Hodgson K., Xu Y., Goodacre R., Behera B.K., Bansal V. Ultrasensitive colorimetric detection of murine norovirus using NanoZyme aptasensor. Anal. Chem. 2019;91:3270. doi: 10.1021/acs.analchem.8b03300. [DOI] [PubMed] [Google Scholar]
- 39.Palomino-Vizcaino G., Valencia Reséndiz D.G., Benítez-Hess M.L., Martínez-Acuña N., Tapia-Vieyra J.V., Bahena D., Díaz-Sánchez M., García-González O.P., Alvarez-Sandoval B.A., Alvarez-Salas L.M. Effect of HPV16 L1 virus-like particles on the aggregation of non-functionalized gold nanoparticles. Biosens. Bioelectron. 2018;100:176. doi: 10.1016/j.bios.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Feng C., Dai S., Wang L. Optical aptasensors for quantitative detection of small biomolecules: a review. Biosens. Bioelectron. 2014;59:64. doi: 10.1016/j.bios.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Suh S.K., Song S., Oh H.B., Hwang S.H., Hah S.S. Aptamer-based competitive binding assay for one-step quantitation of hepatitis B surface antigen. Analyst. 2014;139:4310. doi: 10.1039/c4an00619d. [DOI] [PubMed] [Google Scholar]
- 42.Homola J., Piliarik M. vol. 4. Springer; 2006. p. 45. (Surface Plasmon Resonance (SPR) Sensors). [Google Scholar]
- 43.Lee T., Kim G.H., Kim S.M., Hong K., Kim Y., Park C., Sohn H., Min J. Label-free localized surface plasmon resonance biosensor composed of multi-functional DNA 3 way junction on hollow Au spike-like nanoparticles (HAuSN) for avian influenza virus detection. Colloids Surf. B Biointerfaces. 2019;182 doi: 10.1016/j.colsurfb.2019.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai H., Wang R., Hargis B., Lu H., Li Y. A SPR aptasensor for detection of avian influenza virus H5N1. Sensors. 2012;12 doi: 10.3390/s120912506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen H.H., Park J., Kang S., Kim M. Surface plasmon resonance: a versatile technique for biosensor applications. Sensors. 2015;15 doi: 10.3390/s150510481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barhoumi A., Zhang D., Halas N.J. Correlation of molecular orientation and packing density in a dsDNA self-assembled monolayer observable with surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 2008;130 doi: 10.1021/ja804367c. [DOI] [PubMed] [Google Scholar]
- 47.Kim N.H., Lee S.J., Moskovits M. Aptamer-mediated surface-enhanced Raman spectroscopy intensity amplification. Nano Lett. 2010;10:4181. doi: 10.1021/nl102495j. [DOI] [PubMed] [Google Scholar]
- 48.Xu X., Ma X., Wang H., Wang Z. Aptamer based SERS detection of Salmonella typhimurium using DNA-assembled gold nanodimers. Microchim. Acta. 2018;185:1. doi: 10.1007/s00604-018-2852-0. [DOI] [PubMed] [Google Scholar]
- 49.Díaz-Amaya S., Lin L.-K., Deering A.J., Stanciu L.A. Aptamer-based SERS biosensor for whole cell analytical detection of E. coli O157: H7. Anal. Chim. Acta. 2019;1081:146. doi: 10.1016/j.aca.2019.07.028. [DOI] [PubMed] [Google Scholar]
- 50.Boushell V., Pang S., He L. Aptamer-based SERS detection of lysozyme on a food-handling surface. J. Food Sci. 2017;82:225. doi: 10.1111/1750-3841.13582. [DOI] [PubMed] [Google Scholar]
- 51.Negri P., Kage A., Nitsche A., Naumann D., Dluhy R.A. Detection of viral nucleoprotein binding to anti-influenza aptamers via SERS. Chem. Commun. 2011;47:8635. doi: 10.1039/c0cc05433j. [DOI] [PubMed] [Google Scholar]
- 52.Negri P., Chen G., Kage A., Nitsche A., Naumann D., Xu B., Dluhy R.A. Direct optical detection of viral nucleoprotein binding to an anti-influenza aptamer. Anal. Chem. 2012;84:5501. doi: 10.1021/ac202427e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen H., Park S.G., Choi N., Moon J.I., Dang H., Das A., Lee S., Kim D.G., Chen L., Choo J. SERS imaging-based aptasensor for ultrasensitive and reproducible detection of influenza virus A. Biosens. Bioelectron. 2020;167 doi: 10.1016/j.bios.2020.112496. [DOI] [PubMed] [Google Scholar]
- 54.Lee T., Park S.Y., Jang H., Kim G.H., Lee Y., Park C., Mohammadniaei M., Lee M.H., Min J. Fabrication of electrochemical biosensor consisted of multi-functional DNA structure/porous au nanoparticle for avian influenza virus (H5N1) in chicken serum. Mater Sci. Eng. Biol. Appl. 2019;99:511. doi: 10.1016/j.msec.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Karash S., Wang R., Kelso L., Lu H., Huang T.J., Li Y. Rapid detection of avian influenza virus H5N1 in chicken tracheal samples using an impedance aptasensor with gold nanoparticles for signal amplification. J. Virol. Method. 2016;236:147. doi: 10.1016/j.jviromet.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 56.Zou X., Wu J., Gu J., Shen L., Mao L. Application of aptamers in virus detection and antiviral therapy. Front. Microbiol. 2019;10:1462. doi: 10.3389/fmicb.2019.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu Y., Callaway Z., Lum J., Wang R., Lin J., Li Y. Exploiting enzyme catalysis in ultra-low ion strength media for impedance biosensing of avian influenza virus using a bare interdigitated electrode. Anal. Chem. 2014;86:1965. doi: 10.1021/ac402550f. [DOI] [PubMed] [Google Scholar]
- 58.Park K.-Y., Kim M.-S., Choi S.-Y. Fabrication and characteristics of MOSFET protein chip for detection of ribosomal protein. Biosens. Bioelectron. 2005;20:2111. doi: 10.1016/j.bios.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 59.Park J., Nguyen H.H., Woubit A., Kim M. Applications of field-effect transistor (FET)-type biosensors. Appl. Sci. Coveregence Technol. 2014;23:61. [Google Scholar]
- 60.Maddali H., Miles C.E., Kohn J., O'Carroll D.M. Optical biosensors for virus detection: prospects for SARS-CoV-2/COVID-19. Chembiochem : Euro J. Chem. Biol. 2021;22:1176. doi: 10.1002/cbic.202000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lim D.K., Jeon K.S., Hwang J.H., Kim H., Kwon S., Suh Y.D., Nam J.M. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat. Nanotechnol. 2011;6:452. doi: 10.1038/nnano.2011.79. [DOI] [PubMed] [Google Scholar]
- 62.Wang P., Sun Y., Li X., Wang L., Xu Y., He L., Li G. Recent advances in dual recognition based surface enhanced Raman scattering for pathogenic bacteria detection: a review. Anal. Chim. Acta. 2021;1157 doi: 10.1016/j.aca.2021.338279. [DOI] [PubMed] [Google Scholar]
- 63.Golichenari B., Nosrati R., Farokhi-Fard A., Faal Maleki M., Gheibi Hayat S.M., Ghazvini K., Vaziri F., Behravan J. Electrochemical-based biosensors for detection of Mycobacterium tuberculosis and tuberculosis biomarkers. Crit. Rev. Biotechnol. 2019;39:1056. doi: 10.1080/07388551.2019.1668348. [DOI] [PubMed] [Google Scholar]
- 64.Syu Y.-C., Hsu W.-E., Lin C.-T. Field-effect transistor biosensing: devices and clinical applications. ECS J. Solid State Sci. Technol. 2018;7:Q3196. [Google Scholar]
- 65.Krupovic M., Koonin E.V. Multiple origins of viral capsid proteins from cellular ancestors. Proc. Natl. Acad. Sci. U. S. A. 2017;114 doi: 10.1073/pnas.1621061114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spencer K.A., Osorio F.A., Hiscox J.A. Recombinant viral proteins for use in diagnostic ELISAs to detect virus infection. Vaccine. 2007;25:5653. doi: 10.1016/j.vaccine.2007.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Byk L.A., Gamarnik A.V. Properties and functions of the dengue virus capsid protein. Annu Rev. Virol. 2016;3:263. doi: 10.1146/annurev-virology-110615-042334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu W.W., Sun Y.H., Panté N. Nuclear import of influenza A viral ribonucleoprotein complexes is mediated by two nuclear localization sequences on viral nucleoprotein. Virol. J. 2007;4:49. doi: 10.1186/1743-422X-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fukushi S., Tani H., Yoshikawa T., Saijo M., Morikawa S. Serological assays based on recombinant viral proteins for the diagnosis of arenavirus hemorrhagic fevers. Viruses. 2012;4:2097. doi: 10.3390/v4102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ho J.K., Jeevan-Raj B., Netter H.J. Hepatitis B virus (HBV) subviral particles as protective vaccines and vaccine platforms. Viruses. 2020;12:126. doi: 10.3390/v12020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lavanchy D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J. Clin. Virol. 2005;34:S1. doi: 10.1016/s1386-6532(05)00384-7. [DOI] [PubMed] [Google Scholar]
- 72.Shepard C.W., Simard E.P., Finelli L., Fiore A.E., Bell B.P. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol. Rev. 2006;28:112. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y.Y., Liang X.S. Progression and status of antiviral monitoring in patients with chronic hepatitis B: from HBsAg to HBV RNA. World J. Hepatol. 2018;10:603. doi: 10.4254/wjh.v10.i9.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin T.M., Chen C.J., Wu M.M., Yang C.S., Chen J.S., Lin C.C., Kwang T.Y., Hsu S.T., Lin S.Y., Hsu L.C. Hepatitis B virus markers in Chinese twins. Anticancer Res. 1989;9:737. [PubMed] [Google Scholar]
- 75.Zhevachevsky N.G., Nomokonova N.Y., Beklemishev A.B., Belov G.F. Dynamic study of HBsAg and HBeAg in saliva samples from patients with hepatitis B infection: diagnostic and epidemiological significance. J. Med. Virol. 2000;61:433. doi: 10.1002/1096-9071(200008)61:4<433::aid-jmv4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 76.Xi Z., Huang R., Li Z., He N., Wang T., Su E., Deng Y. Selection of HBsAg-specific DNA aptamers based on carboxylated magnetic nanoparticles and their application in the rapid and simple detection of hepatitis B virus infection. ACS Appl. Mater. Interfaces. 2015;7 doi: 10.1021/acsami.5b01180. [DOI] [PubMed] [Google Scholar]
- 77.Xi Z., Gong Q., Wang C., Zheng B. Highly sensitive chemiluminescent aptasensor for detecting HBV infection based on rapid magnetic separation and double-functionalized gold nanoparticles. Sci. Rep. 2018;8:9444. doi: 10.1038/s41598-018-27792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mohsin D.H., Mashkour M.S., Fatemi F. Design of aptamer-based sensing platform using gold nanoparticles functionalized reduced graphene oxide for ultrasensitive detection of Hepatitis B virus. Chem. Pap. 2020:17. [Google Scholar]
- 79.Cho K.H., Shin D.H., Oh J., An J.H., Lee J.S., Jang J. Multidimensional conductive nanofilm-based flexible aptasensor for ultrasensitive and selective HBsAg detection. ACS Appl. Mater. Interfaces. 2018;10 doi: 10.1021/acsami.8b09918. [DOI] [PubMed] [Google Scholar]
- 80.Monto A.S., Gravenstein S., Elliott M., Colopy M., Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch. Intern. Med. 2000;160:3243. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 81.Peng Y., Wang D., Wang J., Li K., Tan Z., Shu Y., Jiang T. A universal computational model for predicting antigenic variants of influenza A virus based on conserved antigenic structures. Sci. Rep. 2017;7 doi: 10.1038/srep42051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu C.Y., Lin C.W., Tsai T.I., Lee C.D., Chuang H.Y., Chen J.B., Tsai M.H., Chen B.R., Lo P.W., Liu C.P., Shivatare V.S., Wong C.H. Influenza A surface glycosylation and vaccine design. Proc. Natl. Acad. Sci. U. S. A. 2017;114:280. doi: 10.1073/pnas.1617174114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomas J.K., Noppenberger J. Avian influenza: a review. Am. J. Health Syst. Pharm. 2007;64:149. doi: 10.2146/ajhp060181. [DOI] [PubMed] [Google Scholar]
- 84.Wang R., Zhao J., Jiang T., Kwon Y.M., Lu H., Jiao P., Liao M., Li Y. Selection and characterization of DNA aptamers for use in detection of avian influenza virus H5N1. J. Virol Methods. 2013;189:362. doi: 10.1016/j.jviromet.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 85.Wang R., Li Y. Hydrogel based QCM aptasensor for detection of avian influenzavirus. Biosens. Bioelectron. 2013;42:148. doi: 10.1016/j.bios.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 86.Brockman L. Vol. 2. 2013. QCM aptasensor for rapid and specific detection of avian influenza virus; p. 97. [Google Scholar]
- 87.Wang R., Wang L., Callaway Z.T., Lu H., Huang T.J., Li Y. A nanowell-based QCM aptasensor for rapid and sensitive detection of avian influenza virus. Sensor. Actuator. B Chem. 2017;240:934. [Google Scholar]
- 88.Lum J., Wang R., Hargis B., Tung S., Bottje W., Lu H., Li Y. An impedance aptasensor with microfluidic chips for specific detection of H5N1 avian influenza virus. Sensors. 2015;15 doi: 10.3390/s150818565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu L., Wang R., Kelso L.C., Ying Y., Li Y. A target-responsive and size-dependent hydrogel aptasensor embedded with QD fluorescent reporters for rapid detection of avian influenza virus H5N1. Sensor. Actuator. B Chem. 2016;234:98. [Google Scholar]
- 90.Döring A., Birnbaum W., Kuckling D. Responsive hydrogels–structurally and dimensionally optimized smart frameworks for applications in catalysis, micro-system technology and material science. Chem. Soc. Rev. 2013;42:7391. doi: 10.1039/c3cs60031a. [DOI] [PubMed] [Google Scholar]
- 91.Pang Y., Rong Z., Wang J., Xiao R., Wang S. A fluorescent aptasensor for H5N1 influenza virus detection based-on the core-shell nanoparticles metal-enhanced fluorescence (MEF) Biosens. Bioelectron. 2015;66:527. doi: 10.1016/j.bios.2014.10.052. [DOI] [PubMed] [Google Scholar]
- 92.Wang R., Xu L., Li Y. Bio-nanogate controlled enzymatic reaction for virus sensing. Biosens. Bioelectron. 2015;67:400. doi: 10.1016/j.bios.2014.08.071. [DOI] [PubMed] [Google Scholar]
- 93.Gribanyov D., Zhdanov G., Olenin A., Lisichkin G., Gambaryan A., Kukushkin V., Zavyalova E. SERS-based colloidal aptasensors for quantitative determination of influenza virus. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22041842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kukushkin V.I., Ivanov N.M., Novoseltseva A.A., Gambaryan A.S., Yaminsky I.V., Kopylov A.M., Zavyalova E.G. Highly sensitive detection of influenza virus with SERS aptasensor. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park S., Choi J., Jeun M., Kim Y., Yuk S.S., Kim S.K., Song C.S., Lee S., Lee K.H. Detection of avian influenza virus from cloacal swabs using a disposable well gate FET sensor. Adv. Healthc Mater. 2017;6 doi: 10.1002/adhm.201700371. [DOI] [PubMed] [Google Scholar]
- 96.Ahn J.H., Im M., Park T.J., Lee S.Y., Choi Y.K. Label-free and real-time detection of avian influenza using nanowire field effect transistors. J. Biomed. Nanotechnol. 2015;11:1640. doi: 10.1166/jbn.2015.1501. [DOI] [PubMed] [Google Scholar]
- 97.Fu Y., Wang T., Bu L., Xie Q., Li P., Chen J., Yao S. A post-labeling strategy based on dye-induced peeling of the aptamer off single-walled carbon nanotubes for electrochemical aptasensing. Chem. Commun. 2011;47:2637. doi: 10.1039/c0cc05188h. [DOI] [PubMed] [Google Scholar]
- 98.Park J.A., Kim J., Kim S.M., Sohn H., Park C., Kim T.-H., Lee J.-H., Lee M.-H., Lee T. Fabrication of electrochemical influenza virus (H1N1) biosensor composed of multifunctional DNA four-way junction and molybdenum disulfide hybrid material. Materials. 2021;14:343. doi: 10.3390/ma14020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee J.M., Kim J., Ryu I., Woo H.M., Lee T.G., Jung W., Yim S., Jeong Y.J. An aptamer-based electrochemical sensor that can distinguish influenza virus subtype H1 from H5. J. Microbiol. Biotechnol. 2017;27:2037. doi: 10.4014/jmb.1708.08015. [DOI] [PubMed] [Google Scholar]
- 100.Bhardwaj J., Chaudhary N., Kim H., Jang J. Subtyping of influenza A H1N1 virus using a label-free electrochemical biosensor based on the DNA aptamer targeting the stem region of HA protein. Anal. Chim. Acta. 2019;1064:94. doi: 10.1016/j.aca.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 101.Neuman B.W., Adair B.D., Yoshioka C., Quispe J.D., Orca G., Kuhn P., Milligan R.A., Yeager M., Buchmeier M.J. Supramolecular architecture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy. J. Virol. 2006;80:7918. doi: 10.1128/JVI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walls A.C., Xiong X., Park Y.-J., Tortorici M.A., Snijder J., Quispe J., Cameroni E., Gopal R., Dai M., Lanzavecchia A. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell. 2019;176:1026. doi: 10.1016/j.cell.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kacherovsky N., Yang L.F., Dang H.V., Cheng E.L., Cardle I.I., Walls A.C., McCallum M., Sellers D.L., DiMaio F., Salipante S.J. Discovery and characterization of spike N-terminal domain-binding aptamers for rapid SARS-CoV-2 detection. Angew. Chem. 2021;133 doi: 10.1002/anie.202107730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Song Y., Song J., Wei X., Huang M., Sun M., Zhu L., Lin B., Shen H., Zhu Z., Yang C. Discovery of aptamers targeting the receptor-binding domain of the SARS-CoV-2 spike glycoprotein. Anal. Chem. 2020;92:9895. doi: 10.1021/acs.analchem.0c01394. [DOI] [PubMed] [Google Scholar]
- 107.Li J., Zhang Z., Gu J., Stacey H.D., Ang J.C., Capretta A., Filipe C.D.M., Mossman K.L., Balion C., Salena Bruno J., Yamamura D., Soleymani L., Miller M.S., Brennan John D., Li Y. Diverse high-affinity DNA aptamers for wild-type and B.1.1.7 SARS-CoV-2 spike proteins from a pre-structured DNA library. Nucleic Acids Res. 2021;49:7267. doi: 10.1093/nar/gkab574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gupta A., Anand A., Jain N., Goswami S., Anantharaj A., Patil S., Singh R., Kumar A., Shrivastava T., Bhatnagar S., Medigeshi G.R., Sharma T.K. A novel G-quadruplex aptamer-based spike trimeric antigen test for the detection of SARS-CoV-2. Mol. Ther. Nucleic Acids. 2021;26:321. doi: 10.1016/j.omtn.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Idili A., Parolo C., Alvarez-Diduk R., Merkoçi A. Rapid and efficient detection of the SARS-CoV-2 spike protein using an electrochemical aptamer-based sensor. ACS Sens. 2021;6:3093. doi: 10.1021/acssensors.1c01222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zakashansky J.A., Imamura A.H., Salgado D.F., Mercieca H.C.R., Aguas R.F., Lao A.M., Pariser J., Arroyo-Currás N., Khine M. Detection of the SARS-CoV-2 spike protein in saliva with Shrinky-Dink© electrodes. Anal. Methods. 2021;13:874. doi: 10.1039/d1ay00041a. [DOI] [PubMed] [Google Scholar]
- 111.Stanborough T., Given F.M., Koch B., Sheen C.R., Stowers-Hull A.B., Waterland M.R., Crittenden D.L. Optical detection of CoV-SARS-2 viral proteins to sub-picomolar concentrations. ACS Omega. 2021;6:6404. doi: 10.1021/acsomega.1c00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cennamo N., Pasquardini L., Arcadio F., Lunelli L., Vanzetti L., Carafa V., Altucci L., Zeni L. SARS-CoV-2 spike protein detection through a plasmonic D-shaped plastic optical fiber aptasensor. Talanta. 2021;233 doi: 10.1016/j.talanta.2021.122532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zavyalova E., Ambartsumyan O., Zhdanov G., Gribanyov D., Gushchin V., Tkachuk A., Rudakova E., Nikiforova M., Kuznetsova N., Popova L., Verdiev B., Alatyrev A., Burtseva E., Ignatieva A., Iliukhina A., Dolzhikova I., Arutyunyan A., Gambaryan A., Kukushkin V. SERS-based aptasensor for rapid quantitative detection of SARS-CoV-2. Nanomaterials. 2021;11:1394. doi: 10.3390/nano11061394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Z., Pandey R., Li J., Gu J., White D., Stacey H.D., Ang J.C., Steinberg C.-J., Capretta A., Filipe C.D.M., Mossman K., Balion C., Miller M.S., Salena B.J., Yamamura D., Soleymani L., Brennan J.D., Li Y. High-affinity dimeric aptamers enable the rapid electrochemical detection of wild-type and B.1.1.7 SARS-CoV-2 in unprocessed saliva. Angew. Chem. Int. Ed. 2021;60 doi: 10.1002/anie.202110819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abrego-Martinez J.C., Jafari M., Chergui S., Pavel C., Che D., Siaj M. Aptamer-based electrochemical biosensor for rapid detection of SARS-CoV-2: nanoscale electrode-aptamer-SARS-CoV-2 imaging by photo-induced force microscopy. Biosens. Bioelectron. 2022;195 doi: 10.1016/j.bios.2021.113595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aithal S., Mishriki S., Gupta R., Sahu R.P., Botos G., Tanvir S., Hanson R.W., Puri I.K. SARS-CoV-2 detection with aptamer-functionalized gold nanoparticles. Talanta. 2022;236 doi: 10.1016/j.talanta.2021.122841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pramanik A., Gao Y., Patibandla S., Mitra D., McCandless M.G., Fassero L.A., Gates K., Tandon R., Ray P.C. Aptamer conjugated gold nanostar-based distance-dependent nanoparticle surface energy transfer spectroscopy for ultrasensitive detection and inactivation of corona virus. J. Phys. Chem. Lett. 2021;12:2166. doi: 10.1021/acs.jpclett.0c03570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen H., Park S.-G., Choi N., Kwon H.-J., Kang T., Lee M.-K., Choo J. Sensitive detection of SARS-CoV-2 using a SERS-based aptasensor. ACS Sens. 2021;6:2378. doi: 10.1021/acssensors.1c00596. [DOI] [PubMed] [Google Scholar]
- 119.Singh N.K., Ray P., Carlin A.F., Magallanes C., Morgan S., Laurent L.C., Aronoff-Spencer E., Hall D.A. Hitting the diagnostic sweet spot: point-of-care SARS-CoV-2 salivary antigen testing with an off-the-shelf glucometer. medRxiv. 2020;180 doi: 10.1016/j.bios.2021.113111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hong S.L., Xiang M.Q., Tang M., Pang D.W., Zhang Z.L. Ebola virus aptamers: from highly efficient selection to application on magnetism-controlled chips. Anal. Chem. 2019;91:3367. doi: 10.1021/acs.analchem.8b04623. [DOI] [PubMed] [Google Scholar]
- 121.Saraf N., Villegas M., Willenberg B.J., Seal S. Multiplex viral detection platform based on a aptamers-integrated microfluidic channel. ACS Omega. 2019;4:2234. doi: 10.1021/acsomega.8b03277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bosak A., Saraf N., Willenberg A., Kwan M.W.C., Alto B.W., Jackson G.W., Batchelor R.H., Nguyen-Huu T.D., Sankarapani V., Parks G.D., Seal S., Willenberg B.J. Aptamer-gold nanoparticle conjugates for the colorimetric detection of arboviruses and vector mosquito species. RSC Adv. 2019;9 doi: 10.1039/c9ra02089f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park J.H., Jee M.H., Kwon O.S., Keum S.J., Jang S.K. Infectivity of hepatitis C virus correlates with the amount of envelope protein E2: development of a new aptamer-based assay system suitable for measuring the infectious titer of HCV. Virology. 2013;439:13. doi: 10.1016/j.virol.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 124.Kwon P.S., Ren S., Kwon S.J., Kizer M.E., Kuo L., Xie M., Zhu D., Zhou F., Zhang F., Kim D., Fraser K., Kramer L.D., Seeman N.C., Dordick J.S., Linhardt R.J., Chao J., Wang X. Designer DNA architecture offers precise and multivalent spatial pattern-recognition for viral sensing and inhibition. Nat. Chem. 2020;12:26. doi: 10.1038/s41557-019-0369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Teunis P.F., Moe C.L., Liu P., Miller S.E., Lindesmith L., Baric R.S., Le Pendu J., Calderon R.L. Norwalk virus: how infectious is it? J. Med. Virol. 2008;80:1468. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- 126.Patel M.M., Hall A.J., Vinjé J., Parashar U.D. Noroviruses: a comprehensive review. J. Clin. Virol. 2009;44:1. doi: 10.1016/j.jcv.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 127.Jiang X., Wang M., Graham D.Y., Estes M.K. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 1992;66:6527. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vinjé J., Vennema H., Maunula L., von Bonsdorff C.H., Hoehne M., Schreier E., Richards A., Green J., Brown D., Beard S.S., Monroe S.S., de Bruin E., Svensson L., Koopmans M.P. International collaborative study to compare reverse transcriptase PCR assays for detection and genotyping of noroviruses. J. Clin. Microbiol. 2003;41:1423. doi: 10.1128/JCM.41.4.1423-1433.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ahmed S.M., Hall A.J., Robinson A.E., Verhoef L., Premkumar P., Parashar U.D., Koopmans M., Lopman B.A. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect. Dis. 2014;14:725. doi: 10.1016/S1473-3099(14)70767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Giamberardino A., Labib M., Hassan E.M., Tetro J.A., Springthorpe S., Sattar S.A., Berezovski M.V., DeRosa M.C. Ultrasensitive norovirus detection using DNA aptasensor technology. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hwang H.J., Ryu M.Y., Park C.Y., Ahn J., Park H.G., Choi C., Ha S.-D., Park T.J., Park J.P. High sensitive and selective electrochemical biosensor: label-free detection of human norovirus using affinity peptide as molecular binder. Biosens. Bioelectron. 2017;87:164. doi: 10.1016/j.bios.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 132.Kitajima M., Wang N., Tay M.Q., Miao J., Whittle A.J. Development of a MEMS-based electrochemical aptasensor for norovirus detection. Micro & Nano Lett. 2016;11:582. [Google Scholar]
- 133.Chand R., Neethirajan S. Microfluidic platform integrated with graphene-gold nano-composite aptasensor for one-step detection of norovirus. Biosens. Bioelectron. 2017;98:47. doi: 10.1016/j.bios.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 134.Weng X., Neethirajan S. Aptamer-based fluorometric determination of norovirus using a paper-based microfluidic device. Microchim. Acta. 2017;184:4545. [Google Scholar]
- 135.Kim B., Chung K.W., Lee J.H. Non-stop aptasensor capable of rapidly monitoring norovirus in a sample. J. Pharm. Biomed. Anal. 2018;152:315. doi: 10.1016/j.jpba.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 136.Hadziyannis E., Minopetrou M., Georgiou A., Spanou F., Koskinas J. Is HCV core antigen a reliable marker of viral load? An evaluation of HCV core antigen automated immunoassay. Ann. Gastroenterol. 2013;26:146. [PMC free article] [PubMed] [Google Scholar]
- 137.Buket C.A., Ayşe A., Selçuk K., Süleyman Ö., Emel S. Comparison of HCV core antigen and anti-HCV with HCV RNA results. Afr. Health Sci. 2014;14:816. doi: 10.4314/ahs.v14i4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Soliman H.A., Hozayen W.G., Mahmoud A.M., Abo-Seif M.A., Fayed N.A. Significance of the hepatitis C virus core antigen testing as an alternative marker for hepatitis diagnosis in Egyptian patients. Eur. Rev. Med. Pharmacol. Sci. 2015;19:2240. [PubMed] [Google Scholar]
- 139.Malsagova K.A., Pleshakova T.O., Galiullin R.A., Shumov I.D., Kozlov A.F., Romanova T.S., Popov V.P., Glukhov A.V., Konev V.A., Archakov A.I., Ivanov Y.D. Nanowire aptamer-sensitized biosensor chips with gas plasma-treated surface for the detection of hepatitis C virus core antigen. Coatings. 2020;10 [Google Scholar]
- 140.Camilli L., Passacantando M. Advances on sensors based on carbon nanotubes. Chemosensors. 2018;6:62. [Google Scholar]
- 141.Zhang M., Smith A., Gorski W. Carbon nanotube− chitosan system for electrochemical sensing based on dehydrogenase enzymes. Anal. Chem. 2004;76:5045. doi: 10.1021/ac049519u. [DOI] [PubMed] [Google Scholar]
- 142.Sun J.-Y., Huang K.-J., Zhao S.-F., Fan Y., Wu Z.-W. Direct electrochemistry and electrocatalysis of hemoglobin on chitosan-room temperature ionic liquid-TiO2-graphene nanocomposite film modified electrode. Bioelectrochemistry. 2011;82:125. doi: 10.1016/j.bioelechem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 143.Ghanbari K., Roushani M. A nanohybrid probe based on double recognition of an aptamer MIP grafted onto a MWCNTs-Chit nanocomposite for sensing hepatitis C virus core antigen. Sensor. Actuator. B Chem. 2018;258:1066. [Google Scholar]
- 144.Ghanbari K., Roushani M., Azadbakht A. Ultra-sensitive aptasensor based on a GQD nanocomposite for detection of hepatitis C virus core antigen. Anal. Biochem. 2017;534:64. doi: 10.1016/j.ab.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 145.Pleshakova T.O., Kaysheva A.L., Shumov I.D., Ziborov V.S., Bayzyanova J.M., Konev V.A., Uchaikin V.F., Archakov A.I., Ivanov Y.D. Detection of hepatitis C virus core protein in serum using aptamer-functionalized AFM chips. Micromachines. 2019;10:129. doi: 10.3390/mi10020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pleshakova T.O., Kaysheva A.L., Bayzyanova J., Anashkina А S., Uchaikin V.F., Ziborov V.S., Konev V.A., Archakov A.I., Ivanov Y.D. The detection of hepatitis c virus core antigen using afm chips with immobolized aptamers. J. Virol. Method. 2018;251:99. doi: 10.1016/j.jviromet.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 147.Pleshakova T.O., Kaysheva A.L., Bayzyanova J.M., Anashkina A.S., Uchaikin V.F., Shumov I.D., Ziborov V.S., Konev V.A., Archakov A.I., Ivanov Y.D. Advantages of aptamers as ligands upon protein detection by AFM-based fishing. Anal. Methods. 2017;9:6049. [Google Scholar]
- 148.Handisurya A., Schellenbacher C., Kirnbauer R. Diseases caused by human papillomaviruses (HPV) JDDG J. der Deutschen Dermatol. Gesellschaft. 2009;7:453. doi: 10.1111/j.1610-0387.2009.06988.x. [DOI] [PubMed] [Google Scholar]
- 149.Horvath C.A., Boulet G.A., Renoux V.M., Delvenne P.O., Bogers J.-P.J. Mechanisms of cell entry by human papillomaviruses: an overview. Virol. J. 2010;7:1. doi: 10.1186/1743-422X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Woodman C.B., Collins S.I., Young L.S. The natural history of cervical HPV infection: unresolved issues. Nat. Rev. Cancer. 2007;7:11. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 151.Chekin F., Bagga K., Subramanian P., Jijie R., Singh S.K., Kurungot S., Boukherroub R., Szunerits S. Nucleic aptamer modified porous reduced graphene oxide/MoS2 based electrodes for viral detection: application to human papillomavirus (HPV) Sensor. Actuator. B Chem. 2018;262:991. [Google Scholar]
- 152.Zhu L., Han J., Wang Z., Yin L., Zhang W., Peng Y., Nie Z. Competitive adsorption on gold nanoparticles for human papillomavirus 16 L1 protein detection by LDI-MS. Analyst. 2019;144:6641. doi: 10.1039/c9an01612k. [DOI] [PubMed] [Google Scholar]
- 153.Portela A., Digard P. The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication. J. Gen. Virol. 2002;83:723. doi: 10.1099/0022-1317-83-4-723. [DOI] [PubMed] [Google Scholar]
- 154.Kim J., Kwon J.H., Jang J., Lee H., Kim S., Hahn Y.K., Kim S.K., Lee K.H., Lee S., Pyo H., Song C.S., Lee J. Rapid and background-free detection of avian influenza virus in opaque sample using NIR-to-NIR upconversion nanoparticle-based lateral flow immunoassay platform. Biosens. Bioelectron. 2018;112:209. doi: 10.1016/j.bios.2018.04.047. [DOI] [PubMed] [Google Scholar]
- 155.Krammer F., Smith G.J.D., Fouchier R.A.M., Peiris M., Kedzierska K., Doherty P.C., Palese P., Shaw M.L., Treanor J., Webster R.G., García-Sastre A. Influenza Nat. Rev. Dis Primer. 2018;4:3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee I., Kim S.E., Lee J., Woo D.H., Lee S., Pyo H., Song C.S., Lee J. A self-calibrating electrochemical aptasensing platform: correcting external interference errors for the reliable and stable detection of avian influenza viruses. Biosens. Bioelectron. 2020;152 doi: 10.1016/j.bios.2020.112010. [DOI] [PubMed] [Google Scholar]
- 157.Kang J., Yeom G., Jang H., Park C.J., Kim M.G. Highly sensitive and universal detection strategy based on a colorimetric assay using target-specific heterogeneous sandwich DNA aptamer. Anal. Chim. Acta. 2020;1123:73. doi: 10.1016/j.aca.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 158.Kang J., Yeom G., Jang H., Oh J., Park C.J., Kim M.G. Development of replication protein A-conjugated gold nanoparticles for highly sensitive detection of disease biomarkers. Anal. Chem. 2019;91 doi: 10.1021/acs.analchem.9b01827. [DOI] [PubMed] [Google Scholar]
- 159.Yeom G., Kang J., Jang H., Nam H.Y., Kim M.G., Park C.J. Development of DNA aptamers against the nucleocapsid protein of severe fever with thrombocytopenia syndrome virus for diagnostic application: catalytic signal amplification using replication protein A-conjugated liposomes. Anal. Chem. 2019;91 doi: 10.1021/acs.analchem.9b03210. [DOI] [PubMed] [Google Scholar]
- 160.Kang S., Yang M., Hong Z., Zhang L., Huang Z., Chen X., He S., Zhou Z., Zhou Z., Chen Q. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm. Sin. B. 2020;10:1228. doi: 10.1016/j.apsb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tian J., Liang Z., Hu O., He Q., Sun D., Chen Z. An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochim. Acta. 2021;387 [Google Scholar]
- 162.Liu R., He L., Hu Y., Luo Z., Zhang J. A serological aptamer-assisted proximity ligation assay for COVID-19 diagnosis and seeking neutralizing aptamers. Chem. Sci. 2020;11 doi: 10.1039/d0sc03920a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee J.M., Kim C.R., Kim S., Min J., Lee M.-H., Lee S. Mix-and-read, one-minute SARS-CoV-2 diagnostic assay: development of PIFE-based aptasensor. Chem. Commun. 2021;57 doi: 10.1039/d1cc04066a. [DOI] [PubMed] [Google Scholar]
- 164.Chen R., Kan L., Duan F., He L., Wang M., Cui J., Zhang Z., Zhang Z. Surface plasmon resonance aptasensor based on niobium carbide MXene quantum dots for nucleocapsid of SARS-CoV-2 detection. Mikrochim. Acta. 2021;188:316. doi: 10.1007/s00604-021-04974-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li W., Li G., Steiner J., Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotox. Res. 2009;16:205. doi: 10.1007/s12640-009-9047-8. [DOI] [PubMed] [Google Scholar]
- 166.Johri M.K., Mishra R., Chhatbar C., Unni S.K., Singh S.K. Tits and bits of HIV Tat protein. Expet Opin. Biol. Ther. 2011;11:269. doi: 10.1517/14712598.2011.546339. [DOI] [PubMed] [Google Scholar]
- 167.Minunni M., Tombelli S., Gullotto A., Luzi E., Mascini M. Development of biosensors with aptamers as bio-recognition element: the case of HIV-1 Tat protein. Biosens. Bioelectron. 2004;20:1149. doi: 10.1016/j.bios.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 168.Tombelli S., Minunni M., Luzi E., Mascini M. Aptamer-based biosensors for the detection of HIV-1 Tat protein. Bioelectrochemistry. 2005;67:135. doi: 10.1016/j.bioelechem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 169.Kawarada H., Ruslinda A.R. Diamond electrolyte solution gate FETs for DNA and protein sensors using DNA/RNA aptamers. Phys. Status Solidi A. 2011;208:2005. [Google Scholar]
- 170.Rahim Ruslinda A., Tanabe K., Ibori S., Wang X., Kawarada H. Effects of diamond-FET-based RNA aptamer sensing for detection of real sample of HIV-1 Tat protein. Biosens. Bioelectron. 2013;40:277. doi: 10.1016/j.bios.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 171.Caglayan M.O., Üstündağ Z. Spectrophotometric ellipsometry based Tat-protein RNA-aptasensor for HIV-1 diagnosis. Spectrochim. Acta Mol. Biomol. Spectrosc. 2020;227 doi: 10.1016/j.saa.2019.117748. [DOI] [PubMed] [Google Scholar]
- 172.Yamamoto-Fujita R., Kumar P.K.R. Aptamer-derived nucleic acid oligos: applications to develop nucleic acid chips to analyze proteins and small ligands. Anal. Chem. 2005;77:5460. doi: 10.1021/ac050364f. [DOI] [PubMed] [Google Scholar]
- 173.Fatin M.F., Rahim Ruslinda A., Gopinath S.C.B., Arshad M.K.M., Hashim U., Lakshmipriya T., Tang T.-H., Kamarulzaman A. Co-ordinated split aptamer assembly and disassembly on Gold nanoparticle for functional detection of HIV-1 tat. Process Biochem. 2019;79:32. [Google Scholar]
- 174.Zhu C., Yang G., Li H., Du D., Lin Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015;87:230. doi: 10.1021/ac5039863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fatin M.F., Rahim Ruslinda A., Gopinath S.C.B., Arshad M.K.M. High-performance interactive analysis of split aptamer and HIV-1 Tat on multiwall carbon nanotube-modified field-effect transistor. Int. J. Biol. Macromol. 2019;125:414. doi: 10.1016/j.ijbiomac.2018.12.066. [DOI] [PubMed] [Google Scholar]
- 176.Rashid S., Nawaz M.H., Marty J.L., Hayat A. Label free ultrasensitive detection of NS1 based on electrochemical aptasensor using polyethyleneimine aggregated AuNPs. Microchem. J. 2020;158 [Google Scholar]
- 177.Lee K.H., Zeng H. Aptamer-based ELISA assay for highly specific and sensitive detection of Zika NS1 protein. Anal. Chem. 2017;89 doi: 10.1021/acs.analchem.7b02862. [DOI] [PubMed] [Google Scholar]
- 178.Huang R., Xi Z., Deng Y., He N. Fluorescence based Aptasensors for the determination of hepatitis B virus e antigen. Sci. Rep. 2016;6 doi: 10.1038/srep31103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu Y., Le C., Tyrrell D.L., Le X.C., Li X.F. Aptamer binding assay for the E antigen of hepatitis B using modified aptamers with G-quadruplex structures. Anal. Chem. 2020;92:6495. doi: 10.1021/acs.analchem.9b05740. [DOI] [PubMed] [Google Scholar]
- 180.Aspermair P., Mishyn V., Bintinger J., Happy H., Bagga K., Subramanian P., Knoll W., Boukherroub R., Szunerits S. Reduced graphene oxide-based field effect transistors for the detection of E7 protein of human papillomavirus in saliva. Anal. Bioanal. Chem. 2020;413:779. doi: 10.1007/s00216-020-02879-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu Y., Wang C., Li F., Shen S., Tyrrell D.L.J., Le X.C., Li X.-F. DNase-Mediated single-cycle selection of aptamers for proteins blotted on a membrane. Anal. Chem. 2012;84:7603. doi: 10.1021/ac302047e. [DOI] [PubMed] [Google Scholar]
- 182.Kang J., Yeom G., Ha S.-J., Kim M.-G. Development of a DNA aptamer selection method based on the heterogeneous sandwich form and its application in a colorimetric assay for influenza A virus detection. New J. Chem. 2019;43:6883. [Google Scholar]
- 183.Lai H.-C., Wang C.-H., Liou T.-M., Lee G.-B. Influenza A virus-specific aptamers screened by using an integrated microfluidic system. Lab Chip. 2014;14:2002. doi: 10.1039/c4lc00187g. [DOI] [PubMed] [Google Scholar]
- 184.Kim S.H., Lee J., Lee B.H., Song C.S., Gu M.B. Specific detection of avian influenza H5N2 whole virus particles on lateral flow strips using a pair of sandwich-type aptamers. Biosens. Bioelectron. 2019;134:123. doi: 10.1016/j.bios.2019.03.061. [DOI] [PubMed] [Google Scholar]
- 185.Nguyen V.T., Seo H.B., Kim B.C., Kim S.K., Song C.S., Gu M.B. Highly sensitive sandwich-type SPR based detection of whole H5Nx viruses using a pair of aptamers. Biosens. Bioelectron. 2016;86:293. doi: 10.1016/j.bios.2016.06.064. [DOI] [PubMed] [Google Scholar]
- 186.Cho S.-J., Woo H.-M., Kim K.-S., Oh J.-W., Jeong Y.-J. Novel system for detecting SARS coronavirus nucleocapsid protein using an ssDNA aptamer. J. Biosci. Bioeng. 2011;112:535. doi: 10.1016/j.jbiosc.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ahn D.G., Jeon I.J., Kim J.D., Song M.S., Han S.R., Lee S.W., Jung H., Oh J.W. RNA aptamer-based sensitive detection of SARS coronavirus nucleocapsid protein. Analyst. 2009;134:1896. doi: 10.1039/b906788d. [DOI] [PubMed] [Google Scholar]
- 188.Chen F., Hu Y., Li D., Chen H., Zhang X.L. CS-SELEX generates high-affinity ssDNA aptamers as molecular probes for hepatitis C virus envelope glycoprotein E2. PLoS One. 2009;4 doi: 10.1371/journal.pone.0008142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Beier R., Pahlke C., Quenzel P., Henseleit A., Boschke E., Cuniberti G., Labudde D. Selection of a DNA aptamer against norovirus capsid protein VP1. FEMS Microbiol. Lett. 2014;351:162. doi: 10.1111/1574-6968.12366. [DOI] [PubMed] [Google Scholar]
- 190.Leija-Montoya A.G., Benítez-Hess M.L., Toscano-Garibay J.D., Alvarez-Salas L.M. Characterization of an RNA aptamer against HPV-16 L1 virus-like particles. Nucleic Acid Therapeut. 2014;24:344. doi: 10.1089/nat.2013.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gourronc F.A., Rockey W.M., Thiel W.H., Giangrande P.H., Klingelhutz A.J. Identification of RNA aptamers that internalize into HPV-16 E6/E7 transformed tonsillar epithelial cells. Virology. 2013;446:325. doi: 10.1016/j.virol.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yamamoto R., Katahira M., Nishikawa S., Baba T., Taira K., Kumar P.K. A novel RNA motif that binds efficiently and specifically to the Ttat protein of HIV and inhibits the trans-activation by Tat of transcription in vitro and in vivo. Gene Cell. 2000;5:371. doi: 10.1046/j.1365-2443.2000.00330.x. [DOI] [PubMed] [Google Scholar]
- 193.Chen H.L., Hsiao W.H., Lee H.C., Wu S.C., Cheng J.W. Selection and characterization of DNA aptamers targeting all four serotypes of dengue viruses. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tseng Y.-T., Wang C.-H., Chang C.-P., Lee G.-B. Integrated microfluidic system for rapid detection of influenza H1N1 virus using a sandwich-based aptamer assay. Biosens. Bioelectron. 2016;82:105. doi: 10.1016/j.bios.2016.03.073. [DOI] [PubMed] [Google Scholar]
- 195.Wang C.H., Chang C.P., Lee G.B. Integrated microfluidic device using a single universal aptamer to detect multiple types of influenza viruses. Biosens. Bioelectron. 2016;86:247. doi: 10.1016/j.bios.2016.06.071. [DOI] [PubMed] [Google Scholar]
- 196.Lu P.H., Ma Y.D., Fu C.Y., Lee G.B. A structure-free digital microfluidic platform for detection of influenza a virus by using magnetic beads and electromagnetic forces. Lab Chip. 2020;20:789. doi: 10.1039/c9lc01126a. [DOI] [PubMed] [Google Scholar]
- 197.Park J.W., Jin Lee S., Choi E.J., Kim J., Song J.Y., Bock Gu M. An ultra-sensitive detection of a whole virus using dual aptamers developed by immobilization-free screening. Biosens. Bioelectron. 2014;51:324. doi: 10.1016/j.bios.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 198.Sadeghi A.S., Ansari N., Ramezani M., Abnous K., Mohsenzadeh M., Taghdisi S.M., Alibolandi M. Optical and electrochemical aptasensors for the detection of amphenicols. Biosens. Bioelectron. 2018;118:137. doi: 10.1016/j.bios.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 199.Chen C., Zou Z., Chen L., Ji X., He Z. Functionalized magnetic microparticle-based colorimetric platform for influenza A virus detection. Nanotechnology. 2016;27 doi: 10.1088/0957-4484/27/43/435102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bai C., Lu Z., Jiang H., Yang Z., Liu X., Ding H., Li H., Dong J., Huang A., Fang T., Jiang Y., Zhu L., Lou X., Li S., Shao N. Aptamer selection and application in multivalent binding-based electrical impedance detection of inactivated H1N1 virus. Biosens. Bioelectron. 2018;110:162. doi: 10.1016/j.bios.2018.03.047. [DOI] [PubMed] [Google Scholar]
- 201.Farrow T., Laumier S., Sandall I., Zalinge H.v. 2020. Silicon Thin Film Transistor-Based Aptamer Sensor for COVID-19 Detection. Research Square. [Google Scholar]
- 202.Roh C., Jo S.K. Quantitative and sensitive detection of SARS coronavirus nucleocapsid protein using quantum dots-conjugated RNA aptamer on chip. J. Chem. Technol. Biotechnol. 2011;86:1475. doi: 10.1002/jctb.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang C., Zhang L., Shen X. Development of a nucleic acid lateral flow strip for detection of hepatitis C virus (HCV) core antigen. Nucleos Nucleot. Nucleic Acids. 2013;32:59. doi: 10.1080/15257770.2013.763976. [DOI] [PubMed] [Google Scholar]
- 204.Tran L.D., Nguyen D.T., Nguyen B.H., Do Q.P., Le Nguyen H. Development of interdigitated arrays coated with functional polyaniline/MWCNT for electrochemical biodetection: application for human papilloma virus. Talanta. 2011;85:1560. doi: 10.1016/j.talanta.2011.06.048. [DOI] [PubMed] [Google Scholar]