SUMMARY
Ozone (O3) is a damaging air pollutant to crops. As one of the most reactive oxidants known, O3 rapidly forms other reactive oxygen species (ROS) once it enters leaves through stomata. Those ROS in turn can cause oxidative stress, reduce photosynthesis, accelerate senescence, and decrease crop yield. To improve and adapt our feed, fuel, and food supply to rising O3 pollution, a number of Free Air Concentration Enrichment (O3‐FACE) facilities have been developed around the world and have studied key staple crops. In this review, we provide an overview of the FACE facilities and highlight some of the lessons learned from the last two decades of research. We discuss the differences between C3 and C4 crop responses to elevated O3, the possible trade‐off between productivity and protection, genetic variation in O3 response within and across species, and how we might leverage this observed variation for crop improvement. We also highlight the need to improve understanding of the interaction between rising O3 pollution and other aspects of climate change, notably drought. Finally, we propose the use of globally modeled O3 data that are available at increasing spatial and temporal resolutions to expand upon the research conducted at the limited number of global O3‐FACE facilities.
Keywords: air pollution, free air concentration enrichment, oxidative stress response, genetic variation
Significance Statement
Ozone is a damaging air pollutant and potent greenhouse gas with both direct and indirect effects on crops. This review summarizes results from global ozone Free Air Concentration Enrichment (O3‐FACE) experiments and highlights the potential to use remotely sensed data for improved understanding of the impacts of ozone on crops.
INTRODUCTION
Ozone (O3) is a damaging air pollutant and potent greenhouse gas with both direct and indirect effects on vegetation and human health (Ainsworth et al., 2012; DeLang et al., 2021; Monks et al., 2015; Wedow et al., 2021a). As one of the most reactive oxidants known (Audran et al., 2018), O3 forms in the lower atmosphere from chemical reactions of precursor gases including nitrogen oxides (NOx), carbon monoxide, and volatile organic compounds. Ozone is lost by chemical destruction in the atmosphere and deposition to surfaces, including vegetation (Schultz et al., 2017). Local O3 pollution levels and the atmospheric lifetime of O3 vary with the relative concentrations of precursor molecules from local and long‐range sources and meteorological conditions such as radiation, temperature, and atmospheric humidity (Goldberg et al., 2015; Lefohn et al., 2018; Young et al., 2013). The global average lifetime of O3 is approximately 25 days in the troposphere (Young et al., 2013) and decreases to under 5 days in the summertime at the surface boundary layer (Schultz et al., 2017). Maps of global O3 pollution are based on surface observations (Cooper et al., 2014; Schultz et al., 2017), atmospheric chemistry models (e.g., Brauer et al., 2016), and most recently model‐data fusion approaches (DeLang et al., 2021; Figure 1(a)). Current O3 concentrations are highest in East Asia, South‐Central Asia, central Africa, and western North America and lowest in Oceania (Figure 1(a)). In China and India, O3 pollution has been exceptionally high in recent years. Summer monsoons influence the seasonality of O3 pollution in Asia in addition to increasing anthropogenic, biogenic, and biomass burning emissions (Gao et al., 2020). While peak O3 concentrations in Europe and North America have decreased since the 1980s in response to emission reduction legislation and strategies, O3 exposure is increasing globally with significant impacts for human health (DeLang et al., 2021).
Figure 1.
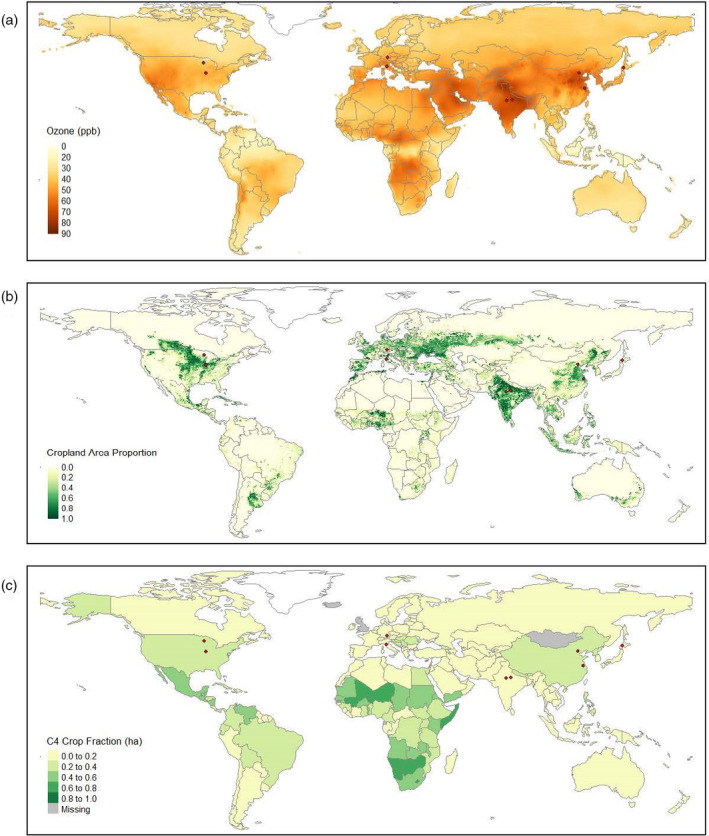
(a) 2017 yearly tropospheric O3 distribution in parts per billion (ppb) at 0.1° resolution, calculated from combined surface O3 observations and a composite of nine atmospheric chemistry models (DeLang et al., 2021). (b) Global distribution of agricultural cropland in the year 2000 derived from remote land‐cover and agricultural inventory data (Ramankutty et al. 2000). (c) The fraction of total harvested area in hectares (ha) comprising C4 crop production in each country, obtained from 2010–2019 average global crop production data from the Food and Agriculture Organization of the United States (FAO). Red dots indicate the location of O3‐FACE experimental sites.
Ozone has caused 0.4 W m−2 of radiative forcing since the industrial revolution (Myhre et al., 2013), thereby contributing to surface warming, which indirectly impacts rates of photosynthesis, transpiration, and respiration. Ozone directly damages plants by entering leaves through the stomata and reacting in the aqueous apoplast to produce other reactive oxygen species (ROS) that subsequently accelerate senescence and at high enough concentrations lead to programmed cell death (Ainsworth, 2017; Hasan et al., 2021). Regions of high O3 pollution (Figure 1(a)) correspond with global croplands (Figure 1(b)) resulting in significant negative effects on crop productivity (Fischer, 2019; Mills et al., 2018; Tai et al., 2021). Fischer (2019) estimated that O3 pollution currently reduces wheat (Triticum aestivum) yields by more than 20% in India. Ozone pollution has been estimated to constrain global soybean (Glycine max) and maize (Zea mays) yields to a similar extent as nutrient stress, heat stress, or aridity stress (Mills et al., 2018). The estimated magnitude of global O3 impacts on crop yields varies with assumptions about O3 flux through stomata into leaves and the degree of detoxification within leaves. Still, conservative estimates suggest that current O3 pollution reduces yield in major staple crops by 3.6% in maize, 2.6% in rice (Oryza sativa), 6.7% in soybean, and 7.2% in wheat (Tai et al., 2021).
To adapt agriculture to O3 pollution, better understanding of the most vulnerable crops and growing regions along with mechanistic understanding of O3 impacts on crops is needed. Manipulative experiments have tested the impacts of elevated O3 concentrations ([O3]) on crops, and for nearly 20 years, O3 Free Air Concentration Enrichment (O3‐FACE) experiments have enabled testing of vegetation responses to elevated [O3] under fully open, field conditions (Table 1). Many of these experiments focused on forest responses to elevated [O3] with only facilities in the Midwest U.S., China, India and Italy having studied crop responses to elevated [O3]. Both C3 and C4 crops including major grain and oilseed crops have been studied (Table 1). While manipulative O3‐FACE experiments have tested responses to increased [O3], other approaches are used to estimate how current background O3 impacts crop productivity. Cumulative metrics for O3 exposure have been used along with assumptions about minimum thresholds for damage to estimate crop yield losses to O3 (e.g., Avnery et al., 2011; Mauzerall and Wang, 2001; Mills et al., 2007; Van Dingenen et al., 2009). This approach is relatively simple and supported by improved global monitoring, remote sensing, and modeling of [O3]. However, it is widely recognized that O3 exposure is not always consistent with uptake into plants and the times and places of greatest O3 exposure do not always coincide with greatest uptake of O3 through stomata (Musselman et al., 2006). Therefore, O3 flux‐based models that consider environmental effects on stomatal conductance and subsequent O3 flux into vegetation are used in combination with statistical relationships between O3 uptake and crop yield loss to more accurately approximate the magnitude and cost of O3 pollution to crop production (e.g., Emberson et al., 2000; Mills et al., 2018; Pleijel et al., 2007). The effects of O3 on vegetation can also be incorporated into mechanistic crop and even earth‐system models (Emberson et al., 2018; Lombardozzi et al., 2018). In this review, we describe the global O3‐FACE facilities that have been used to study crop responses to O3 pollution and discuss some of the key lessons learned for adapting crops to O3 pollution. We then discuss the potential for taking advantage of greater spatial resolution in both O3 metrics and crop performance indicators to improve understanding of O3 impacts on crops.
Table 1.
Description of global ozone Free Air Concentration Enrichment (O3‐FACE) facilities. Superscripts identify reference manuscripts that tested different target [O3] within a given O3‐FACE facility. Bold font indicates C4 species tested in O3‐FACE facilities.
| Facility name | Location | Operational | Ecosystem | Plant species | Target ozone concentration | References |
|---|---|---|---|---|---|---|
| China FACE | Jiangdu, Jiangsu, China | 2007–2012 | Crop |
Sativa oryza Triticum aestivum |
1.5× ambient [O3] | Tang et al., 2011 |
| China O3‐FACE | Yanqing, Beijing, China | 2018–present | Forest |
Populus deltoides Populus euramericana |
1.5× ambient [O3] | Xu et al., 2021 |
| KROFEX | Freising, Germany | 2000–2007 | Forest |
Fagus sylvatica Picea abies |
2× ambient [O3] | Werner and Fabian, 2002 |
| FAOCE | New Delhi, India | 2016–present | Crop |
Cicer arietinum Triticum aestivum Zea mays |
60–70 ppb | Yadav et al., 2019 |
| India O3‐FACE | Lucknow, Uttar Pradesh, India | 2018–present | Forest | Leucaena leucocephala | +20 ppb above ambient [O3] | Singh et al., 2021 |
| FO3X | Florence, Italy | 2015–present | Crop/forest |
Passiflora edulis Phaseolus vulgaris Phoenix dactylifera Populus spp. Punica granatum Quercus spp. Saccharum spp. |
1.2–1.4× ambient [O3] | Paoletti et al. 2017 |
| Sapporo Forest | Sapporo, Hokkaido, Japan | 2011–present | Forest |
Betula platyphylla Fagus crenata Larix kaempferi Quercus mongolica Salix sachalinensis |
60–70 ppba 2× ambient [O3]b |
Watanabe et al., 2013 a Agathokleous et al., 2017 b |
| Tsukuba FACE | Tsukuba, Japan | 2011–2019 | Forest | Betula platyphylla | 2× ambient [O3] | Kitao et al., 2021 |
| Aspen FACE | Rhinelander, WI, USA | 1998–2009 | Forest |
Acer saccharum Betula papyrifera Populus tremuloides |
60–100 ppb | Dickson et al., 2000 |
| SoyFACE | Savoy, IL, USA | 2002–present | Crop |
Glycine max Panicum virgatum Sorghum bicolor Zea mays |
1.2–2× ambient [O3]a, b 60‐200 ppbc |
Morgan et al. 2004 a Gillespie et al., 2012 b Betzelberger et al., 2012c |
O3‐FACE EXPERIMENTS
FACE technology was originally developed to investigate plant responses to elevated [CO2] and [O3] in settings more natural than growth chambers or open top chambers (Lewin et al., 1994; Figure 2). FACE plots operate by releasing or blowing air enriched with O3 into the wind which then increases [O3] across an experimental plot (varying from approximately 2 m to 30 m in diameter). A sensor in the center of each O3‐FACE plot continuously monitors [O3] in real‐time and relays the information to a proportional‐integral‐derivative control system which automatically adjusts O3 output to maintain a desired setpoint. While exposing plants to elevated [O3] in growth chambers, greenhouses, or open‐top chambers (OTCs) is simpler due to the ease of fumigation control in an enclosed system, plants grown in these controlled conditions often show different responses compared to plants grown in an open‐field setting (De Graaf et al., 2006; Poorter et al., 2016). A meta‐analysis of O3‐FACE and OTC experiments found that the relative yield loss of rice and wheat to increasing [O3] was greater in FACE experiments than OTC experiments, while the opposite was true for soybean (Feng et al., 2018). Notable differences between enclosed systems and open‐air growing environments are light attenuation, humidity or vapor pressure deficit, water availability, wind conditions, and constraints on root growth. These microenvironmental factors in the growing environment can lead to confounding experimental results, at times under‐ or overestimating the effect of the treatment (Ainsworth and Long, 2005; McLeod and Long, 1999).
Figure 2.

Image of an elevated O3 plot at the SoyFACE facility. Soybean and C4 grass species are grown at 100 ppb ozone. A retractable awning is located inside of the plot and is released to capture rainfall and study the interaction of elevated O3 pollution and drought stress.
O3‐FACE experiments mitigate many of these concerns by creating an environment which is as close to natural conditions as possible, only altering the concentration of the target gas and leaving the remainder of the environment unaltered. Despite these improvements, O3‐FACE technology still has several experimental limitations. The accuracy of fumigation control is dependent upon wind speed, so when wind speed is very low, control is poor. Also, because of the rapid reactions of O3 on wet surfaces, most facilities do not fumigate plants when leaves are wet due to rain or dew. O3 also varies diurnally in response to light levels, precursor pollutant concentrations, and other environmental conditions (Heath et al., 2009). Some O3‐FACE experiments track the diurnal variations in [O3] and scale to a fold‐change factor above the ambient [O3], while other experiments at O3‐FACE facilities raise the elevated treatment to a particular threshold and maintain the set concentration throughout the day (Table 1). The former treatment would more accurately simulate a natural environment by varying throughout the day (Heath et al., 2009), while the latter fixed treatment would ensure season‐long concentrations above the thresholds for sensitivity (Fuhrer et al., 1997). Another limitation of O3‐FACE is the expense of setting up the infrastructure for fumigation.
To date, O3‐FACE experiments have investigated crop and forest ecosystems. Studies conducted at China FACE (Jiangdu, Jiangsu, China), FAOCE (New Delhi, India), and Soybean FACE (SoyFACE; Savoy, IL, USA) investigated annual cropping systems, focusing on locally relevant crops. Maize and soybean have been the major focus at SoyFACE, while rice and wheat have been investigated in China and India. The O3‐FACE (Beijing, China), KROFEX (Freising, Germany), Sapporo Forest FACE (Sapporo, Japan), and Aspen FACE (Rhinelander, WI, USA) facilities investigated forest responses to elevated [O3] (Table 1). Free air O3 eXposure (FO3X), an O3‐FACE site in Florence, Italy, investigates both crop and forest species. Historically, far fewer O3‐FACE facilities have been in operation at any point than CO2 FACE facilities (for a comprehensive list of CO2 FACE facilities see https://facedata.ornl.gov/global_face.html). Of the 10 O3‐FACE facilities established, six are currently in operation (Table 1). While CO2 FACE facilities have been built in the Northern and Southern Hemispheres, all O3‐FACE facilities are in the Northern Hemisphere (Figure 1). For this review, we will focus on the O3‐FACE facilities largely investigating crop species, namely China FACE, SoyFACE, FO3X, and FAOCE.
SoyFACE
The SoyFACE research facility (soyface.illinois.edu) is situated in one of the most productive maize and soybean regions in the United States and is the longest‐running FACE facility investigating crop responses to elevated [CO2] and [O3] (Table 1). SoyFACE began in 2002 and has 24 FACE plots approximately 20 m in diameter, each capable of augmenting the local atmospheric concentrations of trace gases including O3, CO2, and the combination of both (Figure 2). Because of the prominence of maize and soybean as feed, fuel, and oil crops, these species have been the primary focus of research to date, but work at SoyFACE has also deepened our understanding of how other crops, like snap bean (Phaseolus vulgaris; Burkey et al., 2012), sorghum (Sorghum bicolor L.; Li et al., 2021), and switchgrass (Panicum virgatum; Li et al., 2019a), respond to elevated [O3].
The early SoyFACE O3 experiments were conducted with limited numbers of soybean genotypes, and the elevated O3 treatment was set at 1.2× ambient [O3]. The primary findings from this early research were: (i) young leaves showed little measurable differences in gas exchange and other photosynthetic parameters under the elevated O3 treatment (Morgan et al., 2004); (ii) significant differences in leaf photosynthetic traits appeared during reproductive development in older leaves (Morgan et al., 2004); (iii) total leaf area, plant height, stem diameter, and aboveground biomass were all significantly negatively affected by chronic O3 exposure (Morgan et al., 2006); (iv) elevated [O3] increased the rate of senescence (Dermody et al., 2008); and (v) significant decreases in soybean yields were observed in elevated [O3] and reached as high as 25% (Morgan et al., 2006). Decreased yield was driven primarily by reduced seed size and number (Morgan et al., 2006). However, the extent of the O3‐induced yield loss varied substantially across growing seasons, with a 15% decrease in 2002 and a non‐significant 4% decrease in 2004 (Christ et al., 2006).
Another set of O3 experiments at SoyFACE aimed to quantify genetic variation in soybean sensitivity to elevated [O3] (2× ambient [O3]) and to establish more nuanced exposure thresholds (ambient and eight set points ranging from 40 ppb to 200 ppb) (Betzelberger et al., 2010, 2012). In accordance with previous research, Betzelberger et al. (2010) reported significant differences under 2× ambient [O3] for leaf area index, photosynthesis, stomatal conductance, and chlorophyll content across 10 soybean varieties, with greater effects of elevated [O3] observed during reproductive development. Yield reductions varied from 8% to 37% across the different cultivars. While it had been previously known that U.S. and Canadian soybean germplasm displayed different sensitivities to O3 pollution (Burkey and Carter, 2009), this was one of the first experiments to obtain field‐grown estimates of yield sensitivity across multiple mid‐maturity group soybeans under an elevated [O3] treatment. Betzelberger et al. (2012) later tested the dose response of seven soybean genotypes, and found linear reductions in harvest index, light interception efficiency, and photosynthetic capacity, all of which cumulatively influence seed yield. That study also discovered a linear reduction in seed yield of 37–39 kg ha−1 per ppb cumulative O3 exposure over 40 ppb (Betzelberger et al., 2012).
More recent experiments at SoyFACE have tested the response of maize (Choquette et al., 2019, 2020; Yendrek et al., 2017a, 2017b) and other C4 plants, specifically sorghum and switchgrass (Li et al., 2019a, 2021), to a step increase in elevated [O3] (100 ppb). Yendrek et al. (2017a,b) grew over 200 inbred and hybrid maize lines from 2013–2015 and directly measured and modeled multiple leaf traits including instantaneous gas exchange (A and g s), the rate limiting steps of C4 photosynthesis (V pmax and V max), chlorophyll content, specific leaf area, and leaf nitrogen content. Similar to soybean, many of these traits in both inbred and hybrid lines showed significant O3‐induced decreases. Genotype by O3 treatment interactions were also significant with large differences in the O3 response across different maize lines. For example, photosynthesis in the leaf subtending the ear was reduced under elevated [O3] by 0% to 59% during grain fill. Further study revealed that lower photosynthesis was associated with reduced Rubisco content and activity, but not activation state (Choquette et al., 2020).
Sorghum and switchgrass, two proposed bioenergy crops, showed greater tolerance to elevated [O3] at SoyFACE. Switchgrass, like maize, showed significant decreases in A, V max, and PSII maximum efficiency, but maintained nutrient composition, leaf area, and total biomass in elevated [O3] (Li et al., 2019a). Ten varieties of bioenergy sorghum also appeared to be robust against elevated [O3] with significant decreases only found transiently for photosynthetic capacity, while total aboveground biomass remained largely unaffected (Li et al., 2021).
China FACE and FAOCE
Two O3‐FACE systems have been established in Asia to perform crop research, China FACE and FAOCE (Table 1). These O3‐FACE facilities are important because of increasing O3 pollution in Asia in recent years (Kunchala et al., 2021; Li et al., 2019b). The China O3‐FACE facility was established in 2007 in Jiangsu, China, where rice and wheat cultivation has been in practice for over 1000 years (Shi et al., 2009). The O3‐FACE plots were 14‐m diameter octagons and both rice and wheat were exposed to elevated [O3] of approximately 25–50% above ambient (Tang et al., 2011). Five Chinese rice cultivars have been measured at China FACE and elevated [O3] at 50% above ambient significantly reduced rice yields by 15–17.5% in two sensitive cultivars, but had no effect on two tolerant cultivars (Shi et al., 2009; Wang et al., 2012a). Further investigation of two contrasting cultivars attributed yield loss to a decrease in the number of spikelets per panicle in elevated [O3] (Wang et al., 2012b). Another study tested the effects of plant density on the response of rice to elevated [O3] and failed to detect any effect of plant density on O3 response (Peng et al., 2018). The deleterious effects of elevated O3 on rice grain quality were found in a sensitive rice hybrid Shanyou 63 (Wang et al., 2012a).
In wheat, a 25% increase in background O3 concentration resulted in 10–35% decrease in yield in four Chinese cultivars (Zhu et al., 2011). Genetic variation in O3 response in both rice and wheat (Shi et al., 2009; Zhu et al., 2011) were reported at the China FACE experiment, and further experiments suggested that antioxidative enzymes play a key role in determining sensitivity (Feng et al., 2011, 2016). In wheat, allocation to biomass belowground was reduced in elevated [O3], and soil CO2 and N2O emissions increased in response to elevated [O3] in a sensitive cultivar (Kou et al., 2018), but not in a resistant cultivar (Kou et al., 2015). Additionally, elevated [O3] altered the soil microbial food web, with the soil biota changing more in response to elevated [O3] in an O3‐tolerant wheat variety compared to a sensitive line (Li et al., 2012). Specifically, soil microbial communities in the rhizosphere of O3‐tolerant wheat shifted towards consumption of easily degradable carbon sources, while consumption of more complex carbon sources was associated with the microbial communities in the rhizosphere of O3‐sensitive wheat (Bao et al., 2015). Changes in the microbial community associated with specific wheat cultivars were suggested to alter their plant growth, nutrient uptake, and subsequent response to O3 pollution.
The FAOCE was installed in 2016 in New Delhi, India, and analyzed the response of yield and grain quality to the combination of elevated [O3] and [CO2] in wheat, maize, and chickpea (Cicer arietinum) (Singh et al., 2021; Yadav et al., 2019, 2021). The FACE rings at FAOCE were 5 m in diameter and were divided into halves or quartiles to study different genotypes. Similar to results from China FACE, significant negative impacts of elevated O3 (70 ppb) on photosynthesis and yield in two wheat cultivars were observed in a FAOCE study in India (Yadav et al., 2019). Chickpea was also sensitive to O3 and growth at approximately 60 ppb decreased leaf area index, pod number per plant, pod and seed mass, and total yield (Singh et al., 2021).
FO3X
FO3X, located near Florence, Italy, is the only O3‐FACE facility in a Mediterranean climate and has been used to study a wide variety of plant species (Paoletti et al., 2017; Table 1). The construction of the FO3X experimental plots differs from SoyFACE and China FACE in that O3 is delivered in a three‐dimensional structure with piping above and on all four sides of a 5 × 5 × 2 m box (Paoletti et al., 2017). Plants are grown in pots, which allows for a mixture of different species to be investigated within the FACE rings with a range of other treatments, but has the drawback of restriction of root growth and access to the natural rhizosphere. Both woody crops (pomegranate [Punica granatum], date palm [Phoenix dactylifera], passion fruit [Passiflora edulis]) and herbaceous crops (snap bean, sugarcane [Saccharum spp.]) have been studied at the FO3X facility (Cotrozzi et al., 2020; Pellegrini et al., 2021). The foliar application of agrochemicals as a protective measure against O3 stress was studied in snap bean. Ethylenediurea and cytokinin applications minimized many of the negative effects of elevated [O3] on foliar injury, photosynthesis, and stomatal conductance (Zhang et al., 2018). Crosstalk between salinity and O3 stress was measured in pomegranate, which was sensitive to O3. Studies focusing on sugarcane varieties found that exposure to elevated O3 reduced aboveground biomass and photosynthetic capacity relative to ambient [O3] (Moura et al., 2018). Susceptibility to O3 varied between sugarcane varieties with varieties showing early reductions in stomatal conductance having less long‐term O3‐induced decreases in biomass. There are trade‐offs in all FACE experiments, especially when studying perennial crops for a single season, and long‐term experiments are needed to fully understand how these crops respond to elevated [O3].
LESSONS LEARNED FROM FACE EXPERIMENTS
C3 versus C4
Previous studies mostly focused on how elevated [O3] affected plant growth, development, and productivity in C3 species because of a lower ratio of stomatal conductance to photosynthesis in C4 species hypothesized to limit O3 flux into the leaf (Treshow and Anderson, 1989). Early studies in open‐top chambers also suggested C4 species were less sensitive (Heagle, 1989). The global importance of C4 plants, which contribute approximately 25% of global terrestrial primary production (Sage et al., 1999; Still et al., 2003), has led to a re‐evaluation of their sensitivity to elevated [O3]. Most C4 plants are grasses, including important crops such as maize and sorghum, and are widely distributed from the tropics to the temperate regions (Still et al., 2003). Maize is one of the most important food sources in the world, providing more than 30% of the food calories to over 4.5 billion people in 94 developing countries (Palacios‐Rojas et al., 2020; Shiferaw et al., 2011). Other C4 crops, including switchgrass and miscanthus are considered as major sources of bioenergy and ethanol production in North America (Heaton et al., 2008; Schmer et al., 2008), while sugarcane is a primary energy crop in South America (Manochio et al., 2017). C4 crops are most abundant in North and South America, East Asia, and Africa (Figure 1(c); Still et al., 2003), where the yearly peak O3 concentrations can exceed 60 ppb or more (Figure 1(a)). It is estimated that background O3 concentrations reduced maize yields by 10% in the United States between approximately 1980 and 2010 (McGrath et al., 2015) and by 6.1% worldwide (Mills et al., 2018). However, more recent empirical estimates suggest that other pollutants, namely particulate matter and nitrogen dioxide, are more damaging to maize than O3 (Lobell and Burney, 2021). Clearly, a better understanding of O3 effects on plant physiology and production in C4 plants is essential to predict how O3 may threaten global food and energy security and provide a new perspective on vegetation responses to O3.
So far, only four C4 crops have been studied for O3 response using O3‐FACE facilities (Table 1). Research from SoyFACE showed elevated O3 concentrations (approximately 100 ppb) significantly reduced leaf photosynthetic capacity in maize (Choquette et al., 2019, 2020; Sorgini et al., 2019; Wedow et al., 2021b; Yendrek et al., 2017a,b), but had little or no effect on photosynthesis and biomass yield in switchgrass (Li et al., 2019a) and sorghum (Li et al., 2021). These studies also reported considerable genotypic variation in O3 sensitivity among maize and sorghum lines (Choquette et al., 2019; Li et al., 2021; Yendrek et al., 2017a,b). In a study at the FO3X facility, Moura et al. (2018) exposed two potted sugarcane genotypes to three levels of O3 concentrations and found a significant reduction in plant biomass at increased [O3]. Overall, the existing FACE studies have shown interspecific and genotypic variation in O3 response among C4 crops like maize and sugarcane, but there is also evidence that many genotypes of C4 species are more tolerant to relatively high concentrations of chronic O3 (Li et al., 2021).
Given that leaf anatomical features differ between C4 and C3 species, a key question is, do C4 and C3 species have different mechanisms of response to O3? Ozone is thought to instantly react with molecules within the intercellular air space after entry through stomata to produce a variety of ROS (Figure 3). In C3 leaves, ROS can directly damage mesophyll cells and chloroplasts where photosynthesis occurs (Figure 3(a)) (Ainsworth, 2017). In the O3‐FACE experiments, many C3 plants show decreased Rubisco activity and content in elevated [O3], often associated with accelerated senescence (Betzelberger et al., 2010; Feng et al., 2011; Morgan et al., 2004). However, in C4 leaves, there is a physical separation between the initial assimilation of atmospheric CO2 in mesophyll cells via phosphoenolpyruvate (PEP) carboxylase and the photosynthetic carbon reduction cycle. After initial assimilation in mesophyll cells, the fixed carbon is then decarboxylated and refixed in the bundle sheath cells where the photosynthetic carbon reduction cycle occurs (Hatch, 1987; Sage, 2004). By surrounding bundle sheath cells, mesophyll cells may protect bundle sheath cells from oxidative damage caused by ROS and prolong the ROS diffusion pathway from the intercellular air space to the bundle sheath cells (Figure 3(b)). A greenhouse study of four sugarcane hybrids suggested that PEP carboxylase was more sensitive than Rubisco to O3 (Grantz et al., 2012), again providing some support for the hypothesis that C4 species may respond differently to elevated O3. However, in the O3‐FACE experiments to date, there is inconclusive evidence for greater sensitivity of PEP carboxylase to O3. Gas exchange studies of maize varieties reported that both maximum PEP carboxylase activity and Rubisco activity were significantly reduced by elevated [O3] (Choquette et al., 2020), and to a greater degree as leaves aged. Similarly, carboxylation efficiency was reduced by elevated [O3] in sugarcane after 75–85 days of exposure (Moura et al., 2018). In switchgrass and sorghum, the effects of O3 on photosynthesis were less consistent, and neither species showed reduced biomass at elevated [O3] (Li et al., 2019a, 2021). While some C4 species appear to be more tolerant to elevated [O3] than many C3 species, others appear to be similarly affected with reduced photosynthetic capacity, especially in aging leaves. O3‐FACE experiments have only scratched the surface of investigating C4 species and future studies are needed to quantitatively characterize how C4 and C3 species respond to elevated [O3] and to determine the anatomical, physiological, and genetic bases for variation in O3 tolerance.
Figure 3.
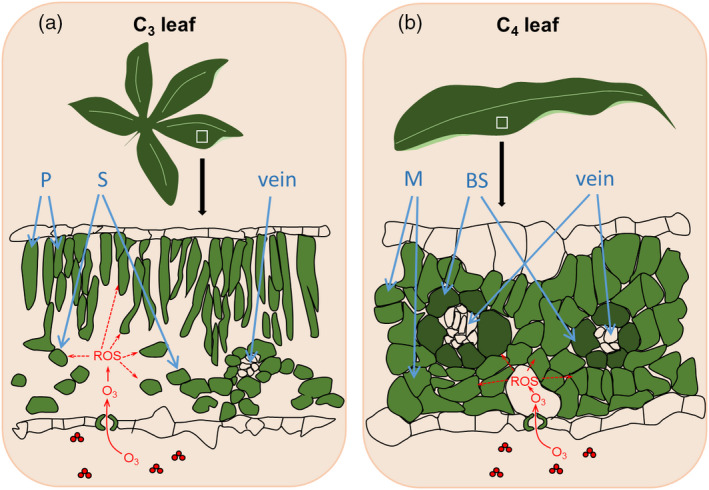
Representative cross‐sections of a typical C3 (a) and C4 (b) leaf. (a) C3 leaves generally have two types of mesophyll cells: palisade and spongy. Palisade mesophyll cells, located below the adaxial surface, are elongated cells containing many chloroplasts, which absorb a major portion of the light energy used for photosynthesis. Spongy mesophyll cells are close to the abaxial surface and composed of rounded cells with few chloroplasts. (b) C4 leaves typically exhibit Kranz anatomy, in which mesophyll cells surround bundle sheath cells and bundle sheath cells further surround vascular bundles. Phosphoenolpyruvate (PEP) carboxylase is localized in the mesophyll cells, but Rubisco and many other Calvin cycle enzymes are found in the bundle sheath cells. In both C3 and C4 leaves, O3 diffuses through stomata into the leaf intercellular airspaces and instantly forms a variety of reactive oxygen species (ROS), including hydroxyl radical (•OH), hydrogen peroxide (H2O2), superoxide radicals (O2 •−), singlet oxygen (1O2), and nitric oxide (NO). In the C3 leaf (a), ROS can directly damage both palisade and spongy mesophyll cells, leading to reductions in photosynthesis. In the C4 leaf (b), the surrounding mesophyll cells may protect bundle sheath cells from direct ROS damage and maintain photosynthetic capacity under O3 stress. P, palisade mesophyll cell; S, spongy mesophyll cell; M, mesophyll cell; BS, bundle sheath cell.
Protection versus productivity during O3 stress
FACE studies have revealed several hallmarks of growth under elevated [O3], mainly decreased photosynthetic carbon assimilation and accelerated senescence that ultimately leads to reduced biomass production and yield (Bernacchi et al., 2006; Burkey et al., 2012; Feng et al., 2011; Morgan et al., 2006; Singh et al., 2021). Plants chronically exposed to elevated [O3] face the challenge of maintaining development and productivity while protecting against O3‐induced oxidative damage. The phytotoxicity of O3 is generally thought to be initiated by oxidative stress caused by the formation of ROS upon O3 entry into the intercellular space (Grimes et al., 1983). Decreasing stomatal conductance to limit O3 entry into the leaf and increasing antioxidant capacity to alleviate O3‐induced oxidative stress are mechanisms for protecting against O3 damage, but both have potential costs on plant productivity (Bechtold et al., 2018). Multiple O3‐FACE studies have indicated trade‐offs between biochemical protection and crop productivity under elevated [O3]. Studies conducted at SoyFACE have investigated this trade‐off in soybean at metabolomic, transcriptional, and proteomic levels.
Betzelberger et al. (2010) found a negative correlation between productivity (photosynthesis or seed yield) and total antioxidant capacity in soybean exposed to twice the ambient [O3], suggesting a trade‐off between carbon gain and antioxidant metabolism under O3 stress. A later study by Betzelberger et al. (2012) investigated soybean growth under a range of elevated [O3] and again found a linear decrease in photosynthetic carbon assimilation, biomass, and yield under elevated [O3], while a linear increase in total antioxidant capacity was observed. Further, during reproductive growth a negative linear response to increasing [O3] was found in the levels of glucose, fructose, sucrose, total protein, and total starch in soybean leaves. At the metabolite level, this demonstrates a trade‐off between protection and productivity whereby increasing antioxidant capacity and metabolism may divert resources away from primary metabolism. Gillespie et al. (2012) correlated O3‐induced reductions in photosynthesis and increases in antioxidant capacity with transcriptional reprogramming of antioxidant, chlorophyll, and respiratory metabolism in soybean. Most notable among the transcriptional responses to elevated [O3] was increased abundance of transcripts associated with these three metabolic processes. Increasing respiratory capacity could help meet the energy and carbon demand for increases in antioxidant and chlorophyll metabolism to protect against O3‐induced oxidative stress. Consistent with this shift in energy demand under O3 stress, Galant et al. (2012) found notable changes in the O3 proteome associated with reductions in photosynthetic carbon assimilation. Changes in the abundance and oxidation of proteins involved in photosynthesis, antioxidant pathways, and carbon metabolism were found, including an increase in abundance and oxidation of proteins involved in sugar mobilization and conversion of starch to sugar.
Though not studied as robustly as in soybean, interesting relationships between protection and productivity during season‐long O3 exposure have been found in other species as well. For instance, changes in activity of enzymes involved in ROS metabolism and detoxification (namely catalase [CAT], superoxide dismutase [SOD], and ascorbate peroxidase [APX]) have been found under chronic O3 exposure in wheat (Feng et al., 2016) and sugarcane (Moura et al., 2018), and these changes were linked to differential photosynthetic and yield sensitivities to O3 among genotypes. Wedow et al. (2021b) investigated differential sensitivity between two inbred maize lines (B73 and Mo17) and the hybrid cross (B73 x Mo17) in relation to the accumulation of defense metabolites in leaf tissue. Senescence was accelerated in the hybrid compared to the inbred lines under elevated [O3] and O3‐induced reductions in photosynthetic carbon assimilation during grain filling were greater in the hybrid line, leading to significantly greater yield loss. The greater negative response to O3 in the hybrid line was exacerbated as leaves aged and associated with an increase in the accumulation of phytosterols and α‐tocopherol, metabolites that serve protective roles against oxidative stress, again demonstrating a negative correlation between productivity and protection under O3 stress. Together this research has provided noteworthy insight into the complex interactions that coordinate the O3 stress response in multiple crop species and the mechanisms involved in the physiological and biochemical balance between defense, growth, and productivity of crops during O3 stress.
Utilizing genetic variation
The O3‐FACE experiments discussed in this review have allowed researchers to observe different sensitivities to elevated [O3] within and across the four major staple crop species – maize (Yendrek et al., 2017b), rice (Shi et al., 2009), wheat (Feng et al., 2018), and soybean (Betzelberger et al., 2010). This same variation in O3 response may potentially help adapt our major crops to be less susceptible to current and future O3 concentrations because genetic variation is the foundation of evolutionary adaptation as well as plant breeding. With O3‐induced reductions in yield across all four staple crops, a concerted effort should be made to breed and select crops for O3 tolerance (Mills et al., 2018). This is especially true because it is believed that indirect selection of O3‐tolerant varieties by plant breeders has not occurred due to the substantial temporal and regional variation in ground‐level [O3] (Ainsworth et al., 2008; Biswas et al., 2009). Some of the relevant literature suggests that modern varieties of these staple crops appear more sensitive (Pleijel et al., 2006; Osborne et al., 2016) and current weather patterns leave crops more prone to O3‐induced yield losses (Mills et al., 2018; Ronan et al., 2020); however, this hypothesis has not been substantiated universally (Pleijel et al., 2018).
To date, efforts have been made to leverage variation in O3 sensitivity to better understand the mechanisms involved and map genomic regions associated with O3 tolerance (Frei, 2015). The logistical hurdles of screening large mapping populations under elevated [O3] are a major challenge of this research. Ideally, selection for plant improvement is performed where the crops are grown (Poorter et al., 2016; Rapacz et al., 2015; Rouphael et al., 2018), and FACE technology has allowed for more realistic simulations of future atmospheric environments. However, the size of mapping populations with meaningful statistical power from either traditional linkage mapping or genome‐wide association studies usually exceed the space available in O3‐FACE plots. Yet, genetic studies have successfully mapped leaf damage in elevated [O3] in maize to chromosome 2 (161 Mb) using chromosome segment substitution line (CSSL) populations (Sorgini et al., 2019). Choquette et al. (2019) also estimated heritability and combining abilities for leaf traits associated with O3 damage using a half‐diallel design with 45 F1 hybrid crosses from a diverse set of 10 maize inbred accessions. These two experiments show that meaningful genetic mapping studies can identify mechanisms of the O3 response within O3‐FACE systems.
Additionally, creative techniques have been developed to rapidly survey large populations or selectively chosen representative germplasm to target O3 response traits in younger plants in growth chambers or glasshouses with increased O3 concentrations (Burkey and Carter, 2009; Manigbas et al. 2010; Ueda et al., 2015; Burton et al., 2016; Begum et al., 2020). Many of these glasshouse studies are short‐term experiments measuring and genetically mapping O3‐induced leaf damage. While unable to measure several cumulative traits negatively impacted by O3, including early senescence and yield, these studies provide an important framework to further our understanding of the genetic controls of O3 susceptibility (Mashaheet et al., 2020; Waldeck et al., 2017; Whaley et al., 2015).
Performing experiments at O3‐FACE facilities and in controlled environments has resulted in exciting advances in mapping O3 tolerance traits in rice (Frei, 2015). Frei et al. (2008) mapped significant quantitative trait loci (QTLs) for leaf bronzing in a traditional linkage mapping population using a susceptible and a tolerant variety of rice for population development. Upon confirming the location of the QTL in a CSSL population from the same parental varieties, breeding lines were developed by introgressing two O3‐tolerant QTLs from the tolerant parent into the sensitive parent (Wang et al., 2014). The breeding lines outperformed the sensitive parent during a season‐long O3 treatment, demonstrating less severe leaf bronzing and decreased O3‐induced reductions in total biomass and yield while also maintaining higher photosynthetic rates and chlorophyll content. Ideally, the results from these controlled glasshouse and chamber studies would be validated in the field using FACE technology. Additionally, greater understanding of genetic mechanisms of plant O3 response will inform transgenic approaches for improving O3 tolerance.
Interactions with other climate change factors
Rising tropospheric [O3] is coincident with greater drought and temperature stress as well as rising atmospheric [CO2]. The interaction between drought stress and O3 stress is not well understood, and potential overlap exists between the signaling pathways involved in O3 and drought stress responses (Wilkinson and Davies, 2010). Experiments imposing both drought and O3 stress on tree species have shown mixed results (Cotrozzi, 2021). In some experiments severe drought events increased plant sensitivity to O3 (Pollastrini et al., 2014), while in others drought ameliorated adverse effects of O3 (Cotrozzi, 2021; Li et al., 2015). O3‐FACE studies are well situated to create experimental conditions with interactive stressors in a realistic setting. At the FO3X facility, where plants are grown in pots, irrigation is used to vary the levels of drought stress (Hoshika et al., ; Pellegrini et al., 2019). Ongoing research at SoyFACE is conducted to investigate the interactive effects of drought and O3 stress in soybean (Figure 2). Drought experiments are being conducted using large awnings to exclude rainfall and reduce water availability in sections of the FACE ring (Gray et al., 2016) (Figure 2). The interactive drought and O3 experiments at FO3X and SoyFACE are currently the only research investigating the ways drought and O3 stresses interact in field‐grown crops. In rainfed regions, large‐scale drought experiments could be conducted using rain‐out shelters designed to have retractable roofs and walls to precisely control rainfall amounts on plants (Kant et al., 2016). It is feasible to envision a system whereby O3 is pumped into these buildings while all walls and roofs are open, allowing for mixing in a relatively open‐air system similar to FACE studies. This would allow for larger‐scale studies to occur in rainfed environments, where a major constraint on current drought studies is the restricted size of the awnings.
Another major stressor to crop systems is increasing temperatures, both through season‐long warming and high temperature stress events, both of which are expected to occur at frequencies in the future (Dai, 2011; Fuhrer, 2009). As with drought stress, increasing temperatures are expected to coincide with higher tropospheric [O3], posing the potential for these stresses to interact and alter the plant’s response to the cumulative stress (Suzuki et al., 2014). To date, interactive studies of season‐long warming, heat waves, and altered [CO2] have been conducted at CO2 FACE sites (Köhler et al., 2017, 2018; Thomey et al., 2019), but none have been conducted with crops investigating the interaction between heat and O3 stress. Lee et al. (2020) investigated interactive effects in Brassica juncea using growth chambers, finding that the O3‐induced decrease in biomass is greater under high temperature stress. In another interactive chamber experiment conducted by Hansen et al. (2019), three varieties of spring wheat were grown and exposed to varying levels of O3, CO2, and temperature. They found that O3 stress reduced yield more noticeably at lower temperature, and that at higher temperatures the yield reduction was largely attributable to the effects of heat stress. Given that results from growth chamber experiments are often different from those of FACE studies (McLeod and Long, 1999), finding a way to integrate both O3 fumigation and heating arrays could provide greater insight into how these two stressors may interact under future climate conditions.
Unlike temperature and drought stress, elevated [CO2] has the potential to mitigate the negative impacts of O3 exposure (Ainsworth and Long, 2021; Suzuki et al., 2014). Bernacchi et al. (2006) and Dermody et al. (2008) included a combination treatment of elevated [CO2] and [O3] and showed that elevated [CO2] ameliorated many of the negative effects of chronic O3 stress in part because stomatal conductance is significantly lower in elevated [CO2], and therefore O3 flux is reduced. At FAOCE, researchers found that the interactive effect of elevated [O3] and [CO2] compensated for O3 damage in wheat and chickpea by maintaining yields similar to those in ambient conditions (Singh et al., 2021; Yadav et al., 2019). Work in maize at the same research facility found that elevated [CO2] helped to maintain yield when the plant was exposed to elevated O3 (Yadav et al., 2021). The FACE experiments overall provide evidence that elevated [CO2] partially mitigates the negative effects of O3 pollution.
PRIORITIZING CROPS FOR ADAPTION TO OZONE POLLUTION
The O3‐FACE experiments provided key insights into mechanisms of crop responses to O3 pollution and helped define thresholds and dose response functions. However, O3‐FACE experiments cover a very limited geographical range and number of crop species studied (Figure 1; Table 1). Other approaches for prioritizing which crops and regions most urgently require O3 adaptation are needed. One alternative to experimental studies is to use statistical models of historical data using long‐term records of yield and air pollution (Burney and Ramanathan, 2014; Fishman et al., 2010; Hong et al., 2020; Lobell and Burney, 2021; McGrath et al., 2015). This is similar to the approach used to estimate the global burden of human disease from air pollution (Cohen et al., 2017). By combining estimates of O3 exposure from satellite data, chemical transport models and surface measurements with epidemiological studies to specify theoretical minimum risk exposure levels and relative risks to populations, Cohen et al. (2017) estimated that O3 pollution caused 254 000 deaths and 4.1 million disability‐adjusted life years in 2015.
A similar statistical approach to link O3 exposure to crop yield loss has been used by Burney and Ramanathan (2014) to test air pollution impacts on wheat and rice yields in India. They examined the relationship between O3 precursor emissions (NOx, VOCs), rather than O3 itself, and estimated that wheat yields were reduced by 33% by air pollution and rice yields by 23%. Data from the U.S. Environmental Protection Agency’s Air Quality System were used to test O3 impacts on U.S. maize and soybean yields (McGrath et al., 2015) and perennial crop yields in California (Hong et al., 2020). In these studies, the effects of temperature and precipitation were modeled along with O3 effects, and it was estimated that from 1980 to present, O3 has reduced rainfed U.S. maize and soybean yields by 10% and 5% (McGrath et al., 2015) and cost roughly $1 billion per year to Californian perennial agriculture (Hong et al., 2020). Another recent analysis suggested that improvements to air quality in the U.S. since 1999 have contributed to roughly 20% of yield gains over the past two decades and currently particulate matter pollution and nitrogen dioxide are more damaging to crop yields than O3 pollution (Lobell and Burney, 2021). Such statistical models require a long‐term record of O3 data and make assumptions about how O3 concentrations vary spatially between sensors. The lack of O3 monitoring and long‐term yield records in many parts of the world have prohibited this approach from identifying key regions for adaptation to O3 pollution. However, advances in modeling, machine learning, and data fusion now produce high‐resolution surface O3 datasets that could be used for statistical modeling of crop responses to O3 (DeLang et al., 2021). Additionally, high‐resolution remotely sensed estimates of crop photosynthesis (Bodesheim et al., 2018) could be used in combination with surface O3 datasets to test O3 impacts during a growing season, and not just on end‐of‐season crop yield.
The statistical approach described above assumes that yield loss is associated with cumulative exposure to O3 above a certain threshold and does not mechanistically account for environmental variation in stomatal conductance or genetic variation in O3 detoxification that would alter the effective O3 flux (Clifton et al., 2020). The notion of a phytotoxic O3 dose above a threshold ‘y’ (PODy) and its incorporation into models accounts for both environmental variation in stomatal conductance and detoxification capacity of leaves (Musselman et al., 2006; Mills et al., 2011). PODy values have been estimated for major 2011crop species and their use in global scale crop damage assessments suggests significant effects of O3 on yields (Emberson, 2020). Wheat has been identified as particularly vulnerable to O3, with yields in China 6–15% lower because of O3 pollution, and Indian wheat yields 8–22% lower (Tang et al., 2013). Yet, even PODy approaches to quantify O3 impacts fail to account for seasonal variation in detoxification capacity, and there is likely significant variation even within species for the threshold value (e.g., Feng et al., 2011). Therefore, greater mechanistic understanding of O3 stress and its interaction with other environmental variables is critical to incorporate into process‐based models (Emberson, 2020).
CONCLUSION
Ozone remains a damaging air pollutant in many crop growing regions and new approaches are needed to understand and adapt agriculture to O3 stress. Combining the power of field experiments with high‐spatial resolution atmospheric and yield datasets has the potential to improve our understanding of the mechanisms of the O3 response and to enable prioritization of the crops and regions of greatest need. Future O3‐FACE experiments can combine O3 fumigation with other abiotic stresses, including drought, high temperature, nutrient deficiency, and elevated [CO2]. These interactions are currently poorly understood at all scales, from molecular mechanisms to crop productivity. Satellites provide high‐temporal and spatial resolution crop yield datasets that can be coupled with improved models of air quality to test how current pollution levels and mixtures of pollutants are impacting crop yields around the globe. Both of these approaches are useful for guiding future crop adaptation strategies.
ACKNOWLEDGMENTS
We thank Dr. Jason West for kindly providing the ozone data for Figure 1(a). This work was supported in part by the DOE Center for Advanced Bioenergy and Bioproducts Innovation (U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research under Award Number DE‐SC0018420). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
REFERENCES
- Agathokleous, E. , Vanderstock, A. , Kita, K. & Koike, T. (2017) Stem and crown growth of Japanese larch and its hybrid F1 grown in two soils and exposed to two free‐air O3 regimes. Environmental Science and Pollution Research, 24, 6634–6647. [DOI] [PubMed] [Google Scholar]
- Ainsworth, E.A. (2017) Understanding and improving global crop response to ozone pollution. The Plant Journal, 90, 886–897. [DOI] [PubMed] [Google Scholar]
- Ainsworth, E.A. & Long, S.P. (2005) What have we learned from 15 years of free‐air CO2 enrichment (FACE)? A meta‐analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2 . New Phytologist, 165, 351–372. [DOI] [PubMed] [Google Scholar]
- Ainsworth, E.A. & Long, S.P. (2021) 30 years of free‐air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Global Change Biology, 27, 27–49. [DOI] [PubMed] [Google Scholar]
- Ainsworth, E.A. , Rogers, A. & Leakey, A.D.B. (2008) Targets for crop biotechnology in a future high‐CO2 and high‐O3 world. Plant Physiology, 147, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth, E.A. , Yendrek, C.R. , Sitch, S. , Collins, W.J. & Emberson, L.D. (2012) The effects of tropospheric ozone on net primary productivity and implications for climate change. Annual Review of Plant Biology, 63, 637–661. [DOI] [PubMed] [Google Scholar]
- Audran, G. , Marque, S.R.A. & Santelli, M. (2018) Ozone, chemical reactivity and biological functions. Tetrahedron, 74, 6221–6261. [Google Scholar]
- Avnery, S. , Mauzerall, D.L. , Liu, J. & Horowitz, L.W. (2011) Global crop yield reductions due to surface ozone exposure: 1. Year 2000 crop production losses and economic damage. Atmospheric Environment, 45, 2284–2296. [Google Scholar]
- Bao, X. , Yu, J. , Liang, W. , Lu, C. , Zhu, J. & Li, Q. (2015) The interactive effects of elevated ozone and wheat cultivars on soil microbial community composition and metabolic diversity. Applied Soil Ecology, 87, 11–18. [Google Scholar]
- Bechtold, U. , Ferguson, J.N. & Mullineaux, P.M. (2018) To defend or to grow: lessons from Arabidopsis C24. Journal of Experimental Botany, 69, 2809–2821. [DOI] [PubMed] [Google Scholar]
- Begum, H. , Alam, M.S. , Feng, Y. , Koua, P. , Ashrafuzzaman, M.D. , Shrestha, A. et al. (2020) Genetic dissection of bread wheat diversity and identification of adaptive loci in response to elevated tropospheric ozone. Plant, Cell and Environment, 43, 2650–2665. [DOI] [PubMed] [Google Scholar]
- Bernacchi, C.J. , Leakey, A.D.B. , Heady, L.E. , Morgan, P.B. , Dohleman, F.G. , McGrath, J.M. et al. (2006) Hourly and seasonal variation in photosynthesis and stomatal conductance of soybean grown at future CO2 and ozone concentrations for 3 years under fully open‐air field conditions. Plant, Cell and Environment, 29, 2077–2090. [DOI] [PubMed] [Google Scholar]
- Betzelberger, A.M. , Gillespie, K.M. , McGrath, J.M. , Koester, R.P. , Nelson, R.L. & Ainsworth, E.A. (2010) Effects of chronic elevated ozone concentration on antioxidant capacity, photosynthesis and seed yield of 10 soybean cultivars. Plant, Cell and Environment, 33, 1569–1581. [DOI] [PubMed] [Google Scholar]
- Betzelberger, A.M. , Yendrek, C.R. , Sun, J. , Leisner, C.P. , Nelson, R.L. , Ort, D.R. et al. (2012) Ozone exposure response for U.S. soybean cultivars: linear reductions in photosynthetic potential, biomass, and yield. Plant Physiology, 160, 1827–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas, D.K. , Xu, H. , Yang, J.C. , Li, Y.G. , Chen, S.B. , Jiang, C.D. et al. (2009) Impacts of methods and sites of plant breeding on ozone sensitivity in winter wheat cultivars. Agriculture, Ecosystems & Environment, 134, 168–177. [Google Scholar]
- Bodesheim, P. , Jung, M. , Gans, F. , Mahecha, M.D. & Reichstein, M. (2018) Upscaled diurnal cycles of land–atmosphere fluxes: a new global half‐hourly data product. Earth System Science Data, 10, 1327–1365. [Google Scholar]
- Brauer, M. , Freedman, G. , Frostad, J. , van Donkelaar, A. , Martin, R.V. , Dentener, F. et al. (2016) Ambient air pollution exposure estimation for the global burden of disease 2013. Environmental Science and Technology, 50, 79–88. [DOI] [PubMed] [Google Scholar]
- Burkey, K.O. , Booker, F.L. , Ainsworth, E.A. & Nelson, R.L. (2012) Field assessment of a snap bean ozone bioindicator system under elevated ozone and carbon dioxide in a free air system. Environmental Pollution, 166, 167–171. [DOI] [PubMed] [Google Scholar]
- Burkey, K.O. & Carter, T.E. (2009) Foliar resistance to ozone injury in the genetic base of US and Canadian soybean and prediction of resistance in descendent cultivars using coefficient of parentage. Field Crops Research, 111, 207–217. [Google Scholar]
- Burney, J. & Ramanathan, V. (2014) Recent climate and air pollution impacts on Indian agriculture. Proceedings of the National Academy of Sciences of the United States of America, 111, 16319–16324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, A.L. , Burkey, K.O. , Carter, T.E. Jr , Orf, J. & Cregan, P. (2016) Phenotypic variation and identification of quantitative trait loci for ozone tolerance in a Fiskeby III 9 Mandarin (Ottawa) soybean population. TAG Theoretical and Applied Genetics, 129, 1113–1125. [DOI] [PubMed] [Google Scholar]
- Choquette, N.E. , Ainsworth, E.A. , Bezodis, W. & Cavanagh, A.P. (2020) Ozone tolerant maize hybrids maintain Rubisco content and activity during long‐term exposure in the field. Plant, Cell and Environment, 43, 3033–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquette, N.E. , Ogut, F. , Wertin, T.M. et al. (2019) Uncovering hidden genetic variation in photosynthesis of field‐grown maize under ozone pollution. Global Change Biology, 25, 4327–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ, M.M. , Ainsworth, E.A. , Nelson, R. , Schurr, U. & Walter, A. (2006) Anticipated yield loss in field‐grown soybean under elevated ozone can be avoided at the expense of leaf growth during early reproductive growth stages in favourable environmental conditions. Journal of Experimental Botany, 57, 2267–2275. [DOI] [PubMed] [Google Scholar]
- Clifton, O.E. , Fiore, A.M. , Massman, W.J. , Baublitz, C.B. , Coyle, M. , Emberson, L. et al. (2020) Dry deposition of ozone over land: processes, measurement, and modeling. Reviews of Geophysics, 58, e2019RG000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, A.J. , Brauer, M. , Burnett, R. , Anderson, H.R. , Frostad, J. , Estep, K. et al. (2017) Estimates and 25‐year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet, 389, 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, O.R. , Parrish, D.D. , Ziemke, J. et al. (2014) Global distribution and trends of tropospheric ozone: an observation‐based review. Elementa Science Anthropocene, 2, 000029. [Google Scholar]
- Cotrozzi, L. (2021) The effects of tropospheric ozone on oaks: a global meta‐analysis. Science of the Total Environment, 756, 143795. [DOI] [PubMed] [Google Scholar]
- Cotrozzi, L. , Lorenzini, G. , Nali, C. , Pellegrini, E. , Saponaro, V. , Hoshika, Y. et al. (2020) Hyperspectral reflectance of light‐adapted leaves can predict both dark‐ and light‐adapted Chl fluorescence parameters, and the effects of chronic ozone exposure on Date Palm (Phoenix dactylifera). International Journal of Molecular Sciences, 21, 6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, A. (2011) Drought under global warming: a review. Wiley Interdisciplinary Reviews‐Climate Change, 2, 45–65. [Google Scholar]
- De Graaf, M.A. , Van Groenigen, K.J. , Six, J. , Hungate, B. & Van Kessel, C. (2006) Interaction between plant growth and soil nutrient cycling under elevated CO2: a meta‐analysis. Global Change Biology, 12, 2077–2091. [Google Scholar]
- DeLang, M.N. , Becker, J.S. , Chang, K.‐L. , Serre, M.L. , Cooper, O.R. , Schultz, M.G. et al. (2021) Mapping yearly fine resolution global surface ozone through the Bayesian maximum entropy data fusion of observations and model output for 1990–2017. Environmental Science and Technology, 55, 4389–4398. [DOI] [PubMed] [Google Scholar]
- Dermody, O. , Long, S.P. , McConnaughay, K. & DeLucia, E.H. (2008) How do elevated CO2 and O3 affect the interception and utilization of radiation by a soybean canopy? Global Change Biology, 14, 556–564. [Google Scholar]
- Dickson, R.E. , Lewin, K.F. , Isebrands, J.G. , Coleman, M.D. , Heilman, W.E. , et al. (2000) Forest atmosphere carbon transfer and storage (FACTS‐II) the aspen Free‐air CO2 and O3 Enrichment (FACE) project: an overview. Gen Tech. Rep. NC‐214. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Research Station.
- Emberson, L. (2020) Effects on ozone on agriculture, forests and grasslands. Philosophical Transactions of the Royal Society A, 378, 20190327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson, L.D. , Ashmore, M.R. , Cambridge, H.M. , Simpson, D. & Tuovinen, J.P. (2000) Modelling stomatal ozone flux across Europe. Environmental Pollution, 109, 403–413. [DOI] [PubMed] [Google Scholar]
- Emberson, L.D. , Pleijel, H. , Ainsworth, E.A. et al. (2018) Ozone effects on crops and consideration in crop models. European Journal of Agronomy, 100, 19–34. [Google Scholar]
- Feng, Z. , Pang, J. , Kobayashi, K. , Zhu, J. & Ort, D.R. (2011) Differential responses in two varieties of winter wheat to elevated ozone concentration under fully open‐air field conditions. Global Change Biology, 17, 580–591. [Google Scholar]
- Feng, Z. , Wang, L. , Pleijel, H. , Zhu, J. & Kobayashi, K. (2016) Differential effects of ozone on photosynthesis of winter wheat among cultivars depend on antioxidative enzymes rather than stomatal conductance. Science of the Total Environment, 572, 404–411. [DOI] [PubMed] [Google Scholar]
- Feng, Z. , Uddling, J. , Tang, H. , Zhou, J. & Kobayashi, K. (2018) Comparison of crop yield sensitivity to ozone between open‐top chamber and free‐air experiments. Global Change Biology, 24, 2231–2238. [DOI] [PubMed] [Google Scholar]
- Fischer, T. (2019) Wheat yield losses in India due to ozone and aerosol pollution and their alleviation: a critical review. Outlook on Agriculture, 48, 181–189. [Google Scholar]
- Fishman, J. , Creilson, J.K. , Parker, P.A. , Ainsworth, E.A. , Vining, G.G. , Szarka, J. et al. (2010) An investigation of widespread ozone damage to the soybean crop in the upper Midwest determined from ground‐based and satellite measurements. Atmospheric Environment, 44, 2248–2256. [Google Scholar]
- Frei, M. (2015) Breeding of ozone resistant rice: relevance, approaches and challenges. Environmental Pollution, 197, 144–155. [DOI] [PubMed] [Google Scholar]
- Frei, M. , Tanaka, J.P. & Wissuwa, M. (2008) Genotypic variation in tolerance to elevated ozone in rice: dissection of distinct genetic factors linked to tolerance mechanisms. Journal of Experimental Botany, 59, 3741–3752. [DOI] [PubMed] [Google Scholar]
- Fuhrer, J. (2009) Ozone risk for crops and pastures in present and future climates. Naturwissenschaften, 96, 173–194. [DOI] [PubMed] [Google Scholar]
- Fuhrer, J. , Skärby, L. & Ashmore, M.R. (1997) Critical levels for ozone effects on vegetation in Europe. Environmental Pollution, 97, 91–106. [DOI] [PubMed] [Google Scholar]
- Galant, A. , Koester, R.P. , Ainsworth, E.A. , Hicks, L.M. & Jez, J.M. (2012) From climate change to molecular response: redox proteomics of ozone‐induced responses in soybean. New Phytologist, 194, 220–229. [DOI] [PubMed] [Google Scholar]
- Gao, M. , Gao, J. , Zhu, B. , Kumar, R. , Lu, X. , Song, S. et al. (2020) Ozone pollution over China and India: seasonality and sources. Atmospheric Chemistry and Physics, 20, 4399–4414. [Google Scholar]
- Gillespie, K.M. , Xu, F. , Richter, K.T. , McGrath, J.M. , Markelz, R.J.C. , Ort, D.R. et al. (2012) Greater antioxidant and respiratory metabolism in field‐grown soybean exposed to elevated O3 under both ambient and elevated CO2 . Plant, Cell and Environment, 35, 169–184. [DOI] [PubMed] [Google Scholar]
- Goldberg, D.L. , Vinciguerra, T.P. , Hosley, K.M. , Loughner, C.P. , Canty, T.P. , Salawitch, R.J. et al. (2015) Evidence for an increase in the ozone photochemical lifetime in the eastern United States using a regional air quality model. Journal of Geophysical Research: Atmospheres, 120, 12778–12793. [Google Scholar]
- Grantz, D.A. , Vu, H.B. , Tew, T.L. & Veremis, J.C. (2012) Sensitivity of gas exchange parameters to ozone in diverse C4 sugarcane hybrids. Crop Science, 52, 1270–1280. [Google Scholar]
- Gray, S.B. , Dermody, O. , Klein, S.P. , Locke, A.M. , McGrath, J.M. , Paul, R.E. et al. (2016) Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nature Plants, 2, 1–8. [DOI] [PubMed] [Google Scholar]
- Grimes, H.D. , Perkins, K.K. & Boss, W.F. (1983) Ozone degrades into hydroxyl radical under physiological conditions: a spin trapping study. Plant Physiology, 72, 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, E.M.Ø. , Hauggaard‐Nielsen, H. , Launay, M. , Rose, P. & Mikkelsen, T.N. (2019) The impact of ozone exposure, temperature and CO2 on the growth and yield of three spring wheat varieties. Environmental and Experimental Botany, 168, 103868. [Google Scholar]
- Hasan, M.M. , Rahman, M.A. , Skalicky, M. , Alabdallah, N.M. , Waseem, M. , Jahan, M.S. et al. (2021) Ozone induced stomatal regulations, MAPK and phytohormone signaling in plants. International Journal of Molecular Sciences, 22, 6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch, M.D. (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica Et Biophysica Acta, 895, 81–106. [Google Scholar]
- Heagle, A.S. (1989) Ozone and crop yield. Annual Review of Phytopathology, 27, 397–423. [Google Scholar]
- Heath, R.L. , Lefohn, A.S. & Musselman, R.C. (2009) Temporal processes that contribute to nonlinearity in vegetation responses to ozone exposure and dose. Atmospheric Environment, 43, 2919–2928. [Google Scholar]
- Heaton, E.A. , Dohleman, F.G. & Long, S.P. (2008) Meeting US biofuel goals with less land: the potential of Miscanthus. Global Change Biology, 14, 2000–2004. [Google Scholar]
- Hong, C. , Mueller, N.D. , Burney, J.A. , Zhang, Y. , AghaKouchak, A. , Moore, F.C. et al. (2020) Impacts of ozone and climate change on yields of perennial crops in California. Nature Food, 1, 166–172. [Google Scholar]
- Hoshika, Y. , Moura, B. & Paoletti, E. (2018) Ozone risk assessment in three oak species as affected by soil water availability. Environmental Science and Pollution Research, 25, 8125–8136. [DOI] [PubMed] [Google Scholar]
- Kant, S. , Thoday‐Kennedy, E. , Joshi, S. , Vakani, J. , Hughes, J. , Maphosa, L. et al. (2016) Automated rainout shelter’s design for well‐defined water stress field phenotyping of crop plants. Crop Science, 57, 327–331. [Google Scholar]
- Kitao, M. , Agathokleous, E. , Yazaki, K. , Komatsu, M. , Kitaoka, S. & Tobita, H. (2021) Growth and photosynthetic responses of seedlings of japanese white birch, a fast‐growing pioineer species, to free‐air elevated O3 and CO2 . Forests, 12, 675. [Google Scholar]
- Köhler, I.H. , Huber, S.C. , Bernacchi, C.J. & Baxter, I.R. (2018) Increased temperatures may safeguard the nutritional quality of crops under future elevated CO2 concentrations. The Plant Journal, 97, 872–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler, I.H. , Ruiz‐Vera, U.M. , VanLoocke, A. , Thomey, M.L. , Clemente, T. , Long, S.P. et al. (2017) Expression of cyanobacterial FBP/SBPase in soybean prevents yield depression under future climate conditions. Journal of Experimental Botany, 68, 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou, T.J. , Cheng, X.H. , Zhu, J.G. & Xie, Z.B. (2015) The influence of ozone pollution on CO2, CH4, and N2O emissions from a Chinese subtropical rice–wheat rotation system under free‐air O3 exposure. Agriculture, Ecosystems & Environment, 204, 72–81. [Google Scholar]
- Kou, T. , Hang, X. , Lam, S.K. , Chen, D. & He, J. (2018) Ozone pollution increases CO2 and N2O emissions in ozone‐sensitive wheat system. Agronomy Journal, 110, 496–502. [Google Scholar]
- Kunchala, R.K. , Singh, B.B. , Karumuri, B.K. , Attada, R. , Seelanki, V. & Kumar, K.N. (2021) Understanding the spatiotemporal variability and trends of surface ozone over India. Environmental Science and Pollution Research. 10.1007/s11356-021-16011-w [DOI] [PubMed] [Google Scholar]
- Lee, J.K. , Woo, S.Y. , Kwak, M.J. , Park, S.H. , Kim, H.D. , Lim, Y.J. et al. (2020) Effects of elevated temperature and ozone in Brassica juncea L.: growth, physiology, and ROS accumulation. Forests, 11. [Google Scholar]
- Lefohn, A.S. , Malley, C.S. , Smith, L. et al. (2018) Tropospheric ozone assessment report: Global ozone metrics for climate change, human health and crop/ecosystem research. Elementa Sciences Anthropocene, 6, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin, K.F. , Hendry, G.R. , Nagy, J. & Lamorte, R.L. (1994) Design and application of a free‐air carbon‐dioxide enrichment facility. Agricultural & Forest Meteorology, 70, 15–29. [Google Scholar]
- Li, K. , Jacob, D.J. , Liao, H. , Shen, L. , Zhang, Q. & Bates, K.H. (2019b) Anthropogenic drivers of 2013–2017 trends in summer surface ozone in China. Proceedings of the National Academy of Sciences of the United States of America, 116, 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Manning, W.J. , Tong, L. & Wang, X. (2015) Chronic drought stress reduced but not protected Shantung maple (Acer truncatum Bunge) from adverse effects of ozone (O3) on growth and physiology in the suburb of Beijing, China. Environmental Pollution, 201, 34–41. [DOI] [PubMed] [Google Scholar]
- Li, Q. , Bao, X. , Lu, C. , Zhang, X. , Zhu, J. , Jiang, Y. et al. (2012) Soil microbial food web responses to free‐air ozone enrichment can depend on the ozone‐tolerance of wheat cultivars. Soil Biology & Biochemistry, 47, 27–35. [Google Scholar]
- Li, S. , Courbet, G. , Ourry, A. & Ainsworth, E.A. (2019a) Elevated ozone concentration reduces photosynthetic carbon gain but does not alter leaf structural traits, nutrient composition or biomass in switchgrass. Plants, 8, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Moller, C.A. , Mitchell, N.G. , Lee, D. & Ainsworth, E.A. (2021) Bioenergy sorghum maintains photosynthetic capacity in elevated ozone concentrations. Plant, Cell and Environment, 44, 729–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobell, D.B. & Burney, J.A. (2021) Cleaner air has contributed one‐fifth of US maize and soybean yield gains since 1999. Environmental Research Letters, 16, 074049. [Google Scholar]
- Lombardozzi, D.L. , Bonan, G.B. , Levis, S. & Lawrence, D.M. (2018) Changes in wood biomass and crop yields in response to projected CO2, O3, nitrogen deposition, and climate. JGR Biogeosciences, 123, 3262–3282. [Google Scholar]
- Manigbas, N.L. , Park, D.‐S. , Park, S.‐K. , Kim, S.‐M. , Hwang, W.‐H. , Kang, H.‐W. , Yi, G et al. (2010) Development of a fast and reliable ozone screening method in rice (Oryza sativa L.). Journal of Plant Breeding and Crop Science, 2, 251–258. [Google Scholar]
- Manochio, C. , Andrade, B.R. , Rodriguez, R.P. & Moraes, B.S. (2017) Ethanol from biomass: A comparative overview. Renewable and Sustainable Energy Reviews, 80, 743–755. [Google Scholar]
- Mashaheet, A.M. , Burkey, K.O. , Saitanis, C.J. , Abdelrhim, A.S. , Rafiullah, & Marshall, D.S. (2020) Differential ozone responses identified among key rust‐susceptible wheat genotypes. Agronomy, 10, 1853. [Google Scholar]
- Mauzerall, D.L. & Wang, X. (2001) Protecting agricultural crops from the effects of tropospheric ozone exposure: reconciling science and standard setting in the United States, Europe, and Asia. Annual Review of Environment and Resources, 26, 237–268. [Google Scholar]
- McGrath, J.M. , Betzelberger, A.M. , Wang, S. , Shook, E. , Zhu, X.‐G. , Long, S.P. et al. (2015) An analysis of ozone damage to historical maize and soybean yields in the United States. Proceedings of the National Academy of Sciences of the United States of America, 112, 14390–14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod, A.R. & Long, S.P. (1999) Free air carbon dioxide enrichment (FACE) in global change research: a review. Advances in Ecological Research, 28, 1–55. [Google Scholar]
- Mills, G. , Buse, A. , Gimeno, B. , Bermejo, V. , Holland, M. , Emberson, L. et al. (2007) A synthesis of AOT40‐based response functions and critical levels of ozone for agricultural and horticultural crops. Atmospheric Environment, 41, 2630–2643. [Google Scholar]
- Mills, G. , Pleijel, H. , Braun, S. , Buker, P. , Bermejo, V. , Calvo, E. et al. (2011) New stomatal flux‐based critical levels for ozone effects on vegetation. Atmospheric Environment, 45, 5064–5068. [Google Scholar]
- Mills, G. , Sharps, K. , Simpson, D. , Pleijel, H. , Frei, M. , Burkey, K. et al. (2018) Closing the global ozone yield gap: quantification and co‐benefits for multistress tolerance. Global Change Biology, 24, 4869–4893. [DOI] [PubMed] [Google Scholar]
- Monks, P.S. , Archibald, A.T. , Colette, A. , Cooper, O. , Coyle, M. , Derwent, R. et al. (2015) Tropospheric ozone and its precursors from the urban to the global scale from air quality to short‐lived climate forcer. Atmospheric Chemistry and Physics, 15, 8889–8973. [Google Scholar]
- Morgan, P.B. , Bernacchi, C.J. , Ort, D.R. & Long, S.P. (2004) An in vivo analysis of the effect of season‐long open‐air elevation of ozone to anticipated 2050 levels on photosynthesis in soybean. Plant Physiology, 135, 2348–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, P.B. , Mies, T.A. , Bollero, G.A. , Nelson, R.L. & Long, S.P. (2006) Season‐long elevation of ozone concentration to projected 2050 levels under fully open‐air conditions substantially decreases the growth and production of soybean. New Phytologist, 170, 333–343. [DOI] [PubMed] [Google Scholar]
- Moura, B.B. , Hoshika, Y. , Silveira, N.M. , Marcos, F.C.C. , Machado, E.C. , Paoletti, E. et al. (2018) Physiological and biochemical responses of two sugarcane genotypes growing under free‐air ozone exposure. Environmental and Experimental Botany, 153, 72–79. [Google Scholar]
- Musselman, R.C. , Lefohn, A.S. , Massman, W.J. & Heath, R.L. (2006) A critical review and analysis of the use of exposure‐and flux‐based ozone indices for predicting vegetation effects. Atmospheric Environment, 40, 1869–1888. [Google Scholar]
- Myhre, G. , Shindell, D. , Bréon, F.‐M. et al. (2013) Anthropogenic and natural radiative forcing. In: Stocker, T.F. , Qin, D. , Plattner, G.‐K. , Tignor, M. , Allen, S.K. , Boschung, J. , Nauels, A. , Xia, Y. , Bex, V. & Midgley, P.M. (Eds.) Climate change 2013: the physical science basis. Contribution of Working Group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press, pp. 659–740. [Google Scholar]
- Osborne, S.A. , Mills, G. , Hayes, F. , Ainsworth, E.A. , Büker, P. & Emberson, L. (2016) Has the sensitivity of soybean cultivars to ozone pollution increased with time? An analysis of published dose‐response data. Global Change Biology, 22, 3097–3111. [DOI] [PubMed] [Google Scholar]
- Palacios‐Rojas, N. , McCulley, L. , Kaeppler, M. , Titcomb, T. J. , Gunaratna, N. S. , Lopez‐Ridaura, S. et al. (2020) Mining maize diversity and improving its nutritional aspects within agro‐food systems. Comprehensive Reviews in Food Science and Food Safety, 19, 1809–1834. [DOI] [PubMed] [Google Scholar]
- Paoletti, E. , Materassi, A. , Fasano, G. , Hoshika, Y. , Carriero, G. , Silaghi, D. et al. (2017) A new‐generation 3D ozone FACE (Free Air Controlled Exposure). Science of the Total Environment, 575, 1407–1414. [DOI] [PubMed] [Google Scholar]
- Pellegrini, E. , Cotrozzi, L. , Neri, L. , Baraldi, R. , Carrari, E. , Nali, C. et al. (2021) Stress markers and physiochemical responses of the Mediterranean shrub Phillyrea angustifolia under current and future drought and ozone scenarios. Environmental Research, 201, 111615. [DOI] [PubMed] [Google Scholar]
- Pellegrini, E. , Hoshika, Y. , Dusart, N. et al. (2019) Antioxidative responses of three oak species under ozone and water stress conditions. Science of the Total Environment, 647, 390–399. [DOI] [PubMed] [Google Scholar]
- Peng, B. , Wang, Y. , Zhu, J. , Wang, Y. & Yang, L. (2018) Effects of ozone stress on rice growth and yield formation under different planting densities ‐ a face study. International Journal of Agriculture and Biology, 20, 2599–2605. [Google Scholar]
- Pleijel, H. , Broberg, M.C. , Uddling, J. & Mills, G. (2018) Current surface ozone concentrations significantly decrease wheat grown, yield and quality. Science of the Total Environment, 613–614, 687–692. [DOI] [PubMed] [Google Scholar]
- Pleijel, H. , Danielsson, H. , Emberson, L. , Ashmore, M.R. & Mills, G. (2007) Ozone risk assessment for agricultural crops in Europe: further development of stomatal flux and flux‐response relationships for European wheat and potato. Atmospheric Environment, 41, 3022–3040. [Google Scholar]
- Pleijel, H. , Eriksen, A.B. , Danielsson, H. , Bondesson, N. & Sellden, G. (2006) Differential ozone sensitivity in an old and a modern Swedish wheat cultivar ‐ grain yield and quality, leaf chlorophyll and stomatal conductance. Environmental and Experimental Botany, 56, 63–71. [Google Scholar]
- Pollastrini, M. , Desotgiu, R. , Camin, F. , Ziller, L. , Gerosa, G. , Marzuoli, R. et al. (2014) Severe drought events increase the sensitivity to ozone on poplar clones. Environmental and Experimental Botany, 100, 94–104. [Google Scholar]
- Poorter, H. , Fiorani, F. , Pieruschka, R. , Wojciechowski, T. , van der Putten, W.H. , Kleyer, M. et al. (2016) Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytologist, 212, 838–855. [DOI] [PubMed] [Google Scholar]
- Ramankutty, N. , Evan, A.T. , Monfreda, C. , Foley, J.A. (2008) Farming the planet: 1. Geographic distribution of global agricultural lands in the year 2000. Cycles, 22, 1003. [Google Scholar]
- Rapacz, M. , Sasal, M. & Wójcik‐Jagła, M. (2015) Direct and indirect measurements of freezing tolerance: advantages and limitations. Acta Physiologiae Plantarum, 37, 157. 10.1007/s11738-015-1907-7. [DOI] [Google Scholar]
- Ronan, A.C. , Ducker, J.A. , Schnell, J.L. & Holmes, C.D. (2020) Have improvements in ozone air quality reduced ozone uptake into plants? Elementa: Science of the Anthropocene, 8, 10.1525/elementa.399. [DOI] [Google Scholar]
- Rouphael, Y. , Spíchal, L. , Panzarová, K. , Casa, R. & Colla, G . (2018) High‐throughput plant phenotyping for developing novel biostimulants: from lab to field or from field to lab? Frontiers in Plant Science, 9, 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage, R.F. (2004) The evolution of C4 photosynthesis. New Phytologist, 161, 341–370. [DOI] [PubMed] [Google Scholar]
- Sage, R.F. , Li, M.R. & Monson, R.K. (1999) The taxonomic distribution of C4 photosynthesis. In: Sage, R.F. & Monson, R.K. (Eds). C4 plant biology. San Diego, CA, USA: Academic Press, pp. 551–584. [Google Scholar]
- Schmer, M.R. , Vogel, K.P. , Mitchell, P.B. & Perrin, R.K. (2008) Net energy of cellulosic ethanol from switchgrass. Proceedings of the National Academy of Sciences of the United States of America, 105, 464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, M.G. , Schröder, S. , Lyapina, O. , Cooper, O.R. , Galbally, I. , Petropavlovskikh, I. et al. (2017) Tropospheric ozone assessment report: database and metrics data of global surface ozone observations. Elementa: Science of the Anthropocene, 5, 58. [Google Scholar]
- Shi, G. , Yang, L. , Wang, Y. et al. (2009) Impact of elevated ozone concentration on yield of four Chinese rice cultivars under fully open‐sir field conditions. Agriculture, Ecosystems & Environment, 131, 178–184. [Google Scholar]
- Shiferaw, B. , Prasanna, B.M. , Hellin, J. & Bänziger, M. (2011) Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Security, 3, 307–327. [Google Scholar]
- Singh, R. , Mukherjee, J. , Sehgal, V.K. , Krishnan, P. , Das, D.K. , Dhakar, R.K. et al. (2021) Interactive effect of elevated tropospheric ozone and carbon dioxide on radiation utilisation, growth and yield of chickpea (Cicer arietinum L.). International Journal of Biometeorology, 10.1007/s00484-021-02150-9. [DOI] [PubMed] [Google Scholar]
- Sorgini, C.A. , Barrios‐Perez, I. , Brown, P.J. & Ainsworth, E.A. (2019) Examining genetic variation in maize inbreds and mapping oxidative stress response QTL in B73‐Mo17 nearly isogenic lines. Frontiers in Sustainable Food Systems, 3, 51. [Google Scholar]
- Still, C.J. , Berry, J.A. , Collatz, G.J. & DeFries, R.S. (2003) Global distribution of C3 and C4 vegetation: carbon cycle implications. Global Biogeochemical Cycles, 17(1), 1006. [Google Scholar]
- Suzuki, N. , Rivero, R.M. , Shulaev, V. , Blumwald, E. & Mittler, R. (2014) Abiotic and biotic stress combinations. New Phytologist, 203, 32–43. [DOI] [PubMed] [Google Scholar]
- Tai, A.P.K. , Sadiq, M. , Pang, J.Y.S. , Yung, D.H.Y. & Feng, Z. (2021) Impacts of surface ozone pollution on global crop yields: comparing different ozone exposure metrics and incorporating co‐effects of CO2 . Frontiers in Sustainable Food Systems, 5, 534616. [Google Scholar]
- Tang, H. , Liu, G. , Han, Y. , Zhu, J. & Kobayashi, K. (2011) A system for free‐air ozone concentration elevation with rice and wheat: control performance and ozone exposure regime. Atmospheric Environment, 45, 6276–6282. [Google Scholar]
- Tang, H. , Takigawa, M. , Liu, G. , Zhu, J. & Kobayashi, K. (2013) A projection of ozone‐induced wheat production loss in China and India for the years 2000 and 2020 with exposure‐based and flux‐based approaches. Global Change Biology, 19, 2739–2752. [DOI] [PubMed] [Google Scholar]
- Thomey, M.L. , Slattery, R.A. , Köhler, I.H. , Bernacchi, C.J. & Ort, D.R. (2019) Yield response of field‐grown soybean exposed to heat waves under current and elevated [CO2]. Global Change Biology, 25, 4352–4368. [DOI] [PubMed] [Google Scholar]
- Treshow, M. & Anderson, F.K. (1989) Plant stress from air pollution. Chichester, England; New York: Wiley. [Google Scholar]
- Ueda, Y. , Frimpong, F. , Qi, Y. , Matthus, E. , Wu, L. , Holler, S. et al. (2015) Genetic dissection of ozone tolerance in rice (Oryza sativa L.) by a genome‐wide association study. Journal of Experimental Botany, 66, 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dingenen, R. , Raes, F. , Krol, M.C. , Emberson, L. & Cofala, J. (2009) The global impact of O3 on agricultural crop yields under current and future air quality legislation. Atmospheric Environment, 43, 604–618. [Google Scholar]
- Waldeck, N. , Burkey, K. , Carter, T. , Dickey, D. , Song, Q.J. & Taliercio, E. (2017) RNA‐Seq study reveals genetic responses of diverse wild soybean accessions to increased ozone levels. BMC Genomics, 18, 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Yang, L. , Han, Y. , Zhu, J. , Kobayashi, K. , Tang, H. et al. (2012a) The impact of elevated tropospheric ozone on grain quality of hybrid rice: a free‐air gas concentration enrichment (FACE) experiment. Field Crops Research, 129, 81–89. [Google Scholar]
- Wang, Y. , Yang, L. , Holler, M. , Zaisheng, S. , Pariasca‐Tanaka, J. , Wissuwa, M. et al. (2014) Pyramiding of ozone tolerance QTLs OzT8 and OzT9 confers improved tolerance to season‐long ozone exposure in rice. Environmental and Experimental Botany, 104, 26–33. [Google Scholar]
- Wang, Y. , Yang, L. , Kobayashi, K. , Zhu, J. , Chen, C.P. , Yang, K. et al. (2012b) Investigations on spikelet formation in hybrid rice as affected by elevated tropospheric ozone concentration in China. Agriculture, Ecosystems & Environment, 150, 63–71. [Google Scholar]
- Watanabe, M. , Hoshika, Y. , Inada, N. , Wang, X. , Mao, Q. & Koike, T. (2013) Photosynthetic traits of Siebold's beech and oak saplings grown under free air ozone exposure in northern Japan. Environmental Pollution, 174, 50–56. [DOI] [PubMed] [Google Scholar]
- Wedow, J.M. , Ainsworth, E.A. & Li, S. (2021a) Plant biochemistry influences tropospheric ozone formation, destruction, deposition and response. Trends in Biochemical Sciences, 10.1016/j.tibs.2021.06.007. [DOI] [PubMed] [Google Scholar]
- Wedow, J.M. , Burroughs, C.H. , Acosta, L.R. , Leakey, A.D.B. & Ainsworth, E.A. (2021b) Age‐dependent increase in α‐tocopherol and phytosterols in maize leaves exposed to elevated ozone pollution. Plant Direct, 5, e00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, H. & Fabian, P. (2002) Free‐air fumigation of mature trees. Environmental Science and Pollution Research, 9, 117–121. [DOI] [PubMed] [Google Scholar]
- Whaley, A. , Sheridan, J. , Safari, S. , Burton, A. , Burkey, K. & Schlueter, J. (2015) RNA‐seq analysis reveals genetic response and tolerance mechanisms to ozone exposure in soybean. BMC Genomics, 16, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, S. & Davies, W.J. (2010) Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant, Cell and Environment, 33, 510–525. [DOI] [PubMed] [Google Scholar]
- Xu, J. , Huang, X. , Wang, N. , Li, Y. & Ding, A. (2021) Understanding ozone pollution in the Yangtze River Delta of eastern China from the perspective of diurnal cycles. Science of the Total Environment, 752, 141928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, A. , Bhatia, A. , Yadav, S. , Kumar, V. & Sing, B. (2019) The effects of elevated CO2 and elevated O3 exposure on plant growth, yield and quality of grains of two wheat cultivars grown in north India. Heliyon, 5, e02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, A. , Bhatia, A. , Yadav, S. , Singh, A. , Tomer, R. , Harit, R. et al. (2021) Growth, yield and quality of maize under ozone and carbon dioxide interaction in North West India. Aerosol and Air Quality Research, 21(2), 200194. [Google Scholar]
- Yendrek, C.R. , Erice, G. , Montes, C.M. , Tomaz, T. , Sorgini, C.A. , Brown, P.J. et al. (2017a) Elevated ozone reduces photosynthetic carbon gain by accelerating leaf senescence of inbred and hybrid maize in a genotype‐specific manner. Plant, Cell and Environment, 40, 3088–3100. [DOI] [PubMed] [Google Scholar]
- Yendrek, C.R. , Tomaz, T. , Montes, C.M. , Cao, Y. , Morse, A.M. , Brown, P.J. et al. (2017b) High‐throughput phenotyping of maize leaf physiological and biochemical traits using hyperspectral reflectance. Plant Physiology, 173, 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, P.J. , Archibald, A.T. , Bowman, K.W. , Lamarque, J.‐F. , Naik, V. , Stevenson, D.S. et al. (2013) Pre‐industrial to end 21st century projections of tropospheric ozone from the Atmospheric Chemistry and Climate Model Intercomparison Project (ACCMIP). Atmospheric Chemistry and Physics, 13, 2063–2090. [Google Scholar]
- Zhang, L. , Hoshika, Y. , Carrari, E. , Burkey, K.O. & Paoletti, E. (2018) Protecting the photosynthetic performance of snap bean under free air ozone exposure. Journal of Environmental Science, 66, 31–40. [DOI] [PubMed] [Google Scholar]
- Zhu, X. , Feng, Z. , Sun, T. , Liu, X. , Tang, H. , Zhu, J. et al. (2011) Effects of elevated ozone concentration on yield of four Chinese cultivars of winter wheat under fully open‐air field conditions. Global Change Biology, 17, 2697–2706. [Google Scholar]


