Abstract
Aim
To conduct a systematic review of published studies reporting on the longitudinal impacts of hypoglycaemia on quality of life (QoL) in adults with type 2 diabetes.
Method
Database searches with no restrictions by language or date were conducted in MEDLINE, Cochrane Library, CINAHL and PsycINFO. Studies were included for review if they used a longitudinal design (e.g. cohort studies, randomised controlled trials) and reported on the association between hypoglycaemia and changes over time in patient‐reported outcomes related to QoL.
Results
In all, 20 longitudinal studies published between 1998 and 2020, representing 50,429 adults with type 2 diabetes, were selected for review. A descriptive synthesis following Synthesis Without Meta‐analysis guidelines indicated that self‐treated symptomatic hypoglycaemia was followed by impairments in daily functioning along with elevated symptoms of generalised anxiety, diabetes distress and fear of hypoglycaemia. Severe hypoglycaemic events were associated with reduced confidence in diabetes self‐management and lower ratings of perceived health over time. Frequent hypoglycaemia was followed by reduced energy levels and diminished emotional well‐being. There was insufficient evidence, however, to conclude that hypoglycaemia impacted sleep quality, depressive symptoms, general mood, social support or overall diabetes‐specific QoL.
Conclusions
Longitudinal evidence in this review suggests hypoglycaemia is a common occurrence among adults with type 2 diabetes that impacts key facets in the physical and psychological domains of QoL. Nonetheless, additional longitudinal research is needed—in particular, studies targeting diverse forms of hypoglycaemia, more varied facets of QoL and outcomes assessed using hypoglycaemia‐specific measures.
Keywords: adult, blood glucose, health‐related quality of life, hypoglycaemia, quality of life, systematic review, type 2 diabetes
Novelty statement.
What is already known? Cross‐sectional studies report negative associations between hypoglycaemia and numerous facets of quality of life, but systematic reviews of longitudinal evidence have focused exclusively on health status and emotional well‐being.
What has the study found? Severe hypoglycaemia is associated with reduced diabetes self‐efficacy and perceived health.
Self‐treated hypoglycaemia is followed by impaired daily functioning and elevated symptoms of anxiety, diabetes distress and fear of hypoglycaemia.
Frequent hypoglycaemia is followed by diminished energy and emotional well‐being.
What are the implications of the study? Understanding which specific facets of quality of life are impacted by hypoglycaemia may enable targeted interventions to improve quality of life on an outcome‐by‐outcome basis.
1. INTRODUCTION
For adults with type 2 diabetes, hypoglycaemia is a common side effect of glucose‐lowering medication. An estimated 83% of insulin and 59% of sulfonylurea users experience symptoms of hypoglycaemia annually. 1 Hypoglycaemia can be distressing 2 and may interfere with diabetes self‐management, resulting in greater glucose variability and suboptimal HbA1c. 3 Hypoglycaemia is also associated with heightened risks for long‐term neurological and cardiovascular complications, 4 and poorer quality of life (QoL). 5
Disagreements remain regarding the suitability of specific instruments, 6 but most agree QoL is best conceptualised as a subjective appraisal spanning multiple dimensions of life, particularly facets in the physical, psychological and social domains. 7 Hypoglycaemia has been linked primarily to detriments in the physical domain, including diminished work capacity and sleep quality, 8 and the psychological domain, including elevated anxiety 9 and depression 10 symptoms.
Previous systematic reviews reinforce these findings but present two major limitations. First, evidence linking hypoglycaemia and QoL comes predominantly from cross‐sectional studies. 11 , 12 The lack of longitudinal research makes it difficult to determine whether hypoglycaemia impacts QoL or merely shares an independent association. Second, outcomes in longitudinal studies have typically been assessed using a handful of domain‐specific measures which fall short of capturing the full breadth of QoL (e.g. a single mood questionnaire). In fact, no systematic review to date has investigated the longitudinal impacts of hypoglycaemia on multiple facets of QoL; a single review from 2010 examined longitudinal data from randomised controlled trials (RCTs) but targeted measures related exclusively to emotional well‐being—a single facet of psychological QoL—and health status. 13
This systematic review aimed to summarise evidence from longitudinal studies reporting on the association between hypoglycaemia and changes in QoL among adults with type 2 diabetes. QoL was characterised using a wide scope, with measures considered for inclusion if they were subjective evaluations of (a) generic QoL; (b) facets in the physical, psychological or social domains of generic QoL; or (c) diabetes‐specific or hypoglycaemia‐specific QoL (and constituent domains).
2. METHOD
This systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 14 The protocol is registered on Prospective Register of Systematic Reviews (PROPSERO) under record no. CRD42020154023.
2.1. Search strategy
A database search strategy was developed to identify published studies reporting on the relationship between hypoglycaemia and patient‐reported outcomes (PROs) related to QoL (Table S1). This approach employed a comprehensive set of search terms in four categories: population (e.g. ‘type 2 diabetes’), exposure (e.g. ‘hypoglycaemia’), generic and specific outcomes (e.g. ‘quality of life’, ‘well‐being’) and study design (e.g. ‘randomised controlled trial’, ‘cohort study’). No restrictions were placed on language or date of publication. Searches of MEDLINE, Cochrane Library, CINAHL and PsycINFO were completed on 5 November 2020.
2.2. Screening process
Studies were eligible for inclusion if they (a) sampled adults (18+ years old) with type 2 diabetes; (b) had a longitudinal quantitative design; (c) assessed the occurrence of hypoglycaemia; (d) included PRO(s) related to generic, diabetes‐specific or hypoglycaemia‐specific QoL; and (e) reported on the statistical relationship between hypoglycaemia and changes in PROs over time. Mixed‐population studies (e.g. combined samples of adults with type 1 and type 2 diabetes) were eligible for inclusion if results for adults with type 2 diabetes were reported separately. Outcomes were restricted to standardised PROs to maintain a person‐centred, subjective appraisal of QoL and to maximise validity and reliability of findings, reducing heterogeneity and enabling comparisons across studies.
Records identified through database searches were split between five assessors (AC, AS, KM, MB and MC) who screened titles and abstracts; 15% were double‐screened to ensure consistent application of inclusion criteria. Short‐listed records were full text screened independently by two reviewers (KM and MVJ) as dual screening produces fewer errors than single screening. 15 Studies meeting inclusion criteria were subjected to forward citation searches on Web of Science and backward citation searches. To address publishing and database indexing bias, hand searches were conducted on ClinicalTrials.gov and references from excluded studies deemed to be of interest. As an additional safeguard, 10% of excluded records, chosen at random, were double‐screened by a third reviewer (MCJC). Inconsistencies were discussed between reviewers until consensus was reached.
2.3. Study quality
Quality of included studies was rated by a reviewer (KM) using critical appraisal tools from the Joanna Briggs Institute (JBI) specific to RCTs and cohort studies, 16 and ratings were checked by a second reviewer (MVJ). To provide a concise metric for quantitative comparisons across designs, an overall quality proportion (α q) was calculated for each study using a count score 17 whereby the number of ‘yes’ items was divided by the total number of items on the appraisal tool (‘unclear’ items were counted as half, while ‘not applicable’ items were excluded). Proportions ranged from 0 to 1 and were interpreted in a manner similar to Cronbach's α 18 : α q ≥ 0.9 (excellent), α q ≥ 0.8 (good), α q ≥ 0.7 (acceptable) and α q < 0.7 (poor).
2.4. Extraction and synthesis
One reviewer (KM) extracted data pertaining to study authors, publication year, country, sample size, study design, intervention type, hypoglycaemia measures and frequency, PROs related to QoL, and findings regarding the relationship between hypoglycaemia and changes in PROs. Extractions for measures and results were verified independently by another reviewer (MVJ) and disagreements were resolved by consensus. Due to the diversity of PROs and inconsistent reporting of statistical values, meta‐analysis and subgroup analysis were not possible. Consequently, following Synthesis Without Meta‐analysis (SWiM) guidelines, 19 descriptive synthesis of hypoglycaemic impacts was performed with PROs grouped by QoL domain 7 and specificity to better demarcate the degree of generalisability.
3. RESULTS
3.1. Study selection and characteristics
Database searches identified 12,571 records. Abstract and title screening excluded 12,096 records, and full‐text screening eliminated 460 records, leaving 15 studies 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 that satisfied inclusion criteria. Backward and forward citation searches of these studies identified 3 additional studies, 35 , 36 , 37 and hand searches identified 2 studies, 38 , 39 resulting in 20 total studies selected for extraction and synthesis (Figure 1).
FIGURE 1.
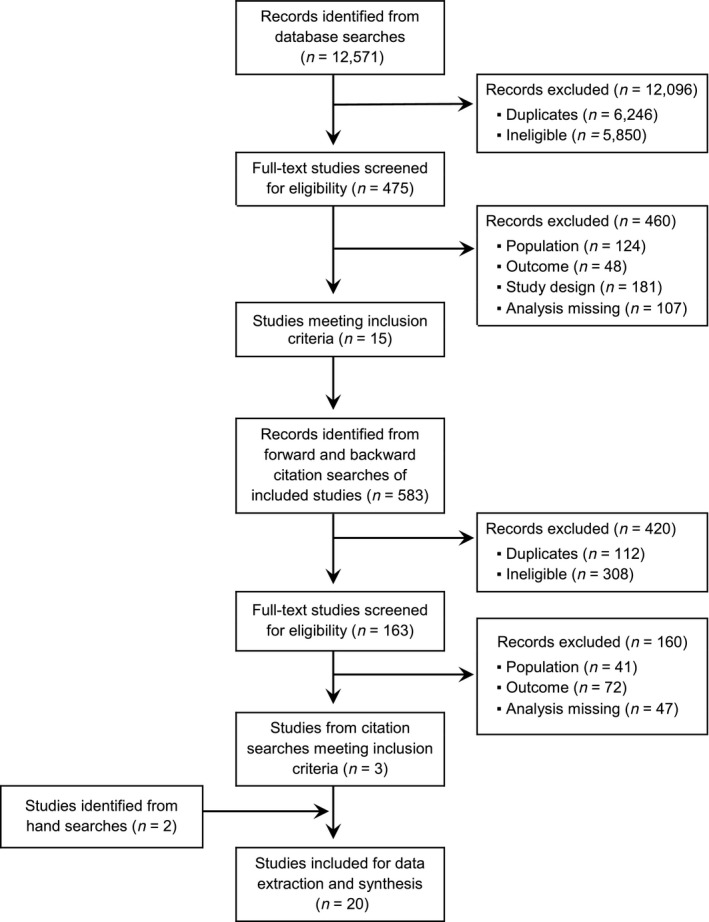
PRISMA flow diagram of the search and screening process
These 20 studies, published between 1998 and 2020, sampled adults with type 2 diabetes (N = 50,429) from more than 20 countries (Table 1). Studies were conducted in Europe (n = 9), North America (n = 5), Asia (n = 3) and other regions worldwide (n = 3). Participants were generally older adults, with a mean age of 60 across studies (range: 55–71). Gender ratios varied greatly between studies (range: 32%–74% female), but overall representation was roughly balanced (M = 45% female). Studies used either cohort (60%) or RCT (40%) designs, and most examined the effectiveness of oral or injectable glucose‐lowering medications (n = 10) or self‐management interventions (n = 6). The remainder had no stated intervention (n = 4).
TABLE 1.
Characteristics and findings of included studies
| Authors (year) | Reference | Design | Country | Sample N (baseline) | Study intervention | Study quality (α q) | Quality of life assessment | Impact of self‐treated symptomatic hypoglycaemia | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Outcome | Direction a | Sig. | |||||||
| Ali et al. (2012) | 35 | RCT | US | 2053 | Intensive versus standard glucose control | 0.58 | SF‐36 | General health | ||
| Physical health |

|
NS | ||||||||
| Mental health |

|
NS | ||||||||
| Briggs et al. (2017) | 20 | RCT | Worldwide | 16,492 | Saxagliptin versus placebo | 0.77 | EQ‐5D | General health |
 b
b
|
<0.05 |
| de Sonnaville et al. (1998) | 36 | Cohort | Netherlands | 237 | Insulin versus oral medication | 1.00 | POMS | Negative mood |
 c
c
|
NS |
| Genovese et al. (2013) | 21 | Cohort | Italy | 1046 | Vildagliptin + metformin | 0.90 | WPAI | Work/school impairment | ||
| Activity impairment |

|
0.001 | ||||||||
| Goddijn et al. (1999) | 22 | Cohort | Netherlands | 99 | Insulin versus oral medication | 1.00 | RAND‐36 | General health | ||
| Mental health |

|
NR | ||||||||
| Haluzik et al. (2018) | 23 | Cohort | Eastern Europe | 6369 | None | 0.93 | Targeted question | Work impairment |

|
NA |
| Jódar et al. (2020) | 24 | RCT | Worldwide | 3297 | Semaglutide versus placebo | 0.77 | SF‐36 | General health | ||
| Physical health |

|
<0.01 | ||||||||
| Mental health |

|
0.08 | ||||||||
| Malanda et al. (2011) | 25 | Cohort | UK | 453 | G‐Meter versus standard monitoring | 1.00 | EQ‐5D | General health |

|
0.23 |
| W‐BQ12 | Emotional well‐being |

|
0.82 | |||||||
| Positive mood |

|
0.47 | ||||||||
| Negative mood |

|
0.92 | ||||||||
| Energy level |

|
0.46 | ||||||||
| IPQ‐R | Illness perceptions | |||||||||
| Illness control |

|
NS | ||||||||
| Emotional distress |

|
0.24 | ||||||||
| Menard et al. (2007) | 26 | RCT | Canada | 72 | Intensive versus standard care | 0.88 | DQOL | DQOL |

|
NS |
| Mitchell et al. (2013) | 27 | Cohort | UK | 1329 | None | 0.85 | HFS‐II | Fear of hypoglycaemia |
 d
d
|
<0.001 |
| Nauck et al. (2019) | 28 | RCT | Worldwide | 3014 | Liraglutide versus placebo | 0.85 | EQ‐5D | General health |
 b
b
|
<0.001 |
| EQ‐VAS | General health |
 b
b
|
0.98 | |||||||
| Nicolucci et al. (2011) | 29 | RCT | Italy | 238 | Telephone versus standard care | 0.58 | W‐BQ22 | Emotional well‐being |

|
0.002 |
| Positive mood |

|
NS | ||||||||
| Depressive symptoms |

|
NS | ||||||||
| Anxiety symptoms |

|
0.007 | ||||||||
| Energy level |

|
0.003 | ||||||||
| SF‐36 | General health | |||||||||
| Physical health |

|
NS | ||||||||
| Mental health |

|
0.004 | ||||||||
| Pathan et al. (2018) | 38 | Cohort | Southeast Asia | 2594 | None | 0.93 | Targeted question | Work impairment |

|
NA |
| Peyrot et al. (2008) | 30 | RCT | US | 211 | Pramlintide versus placebo | 0.85 | DDS | Diabetes distress |

|
NS |
| Pichayapinyo et al. (2018) | 31 | Cohort | Thailand | 35 | Telephone assistance | 0.85 | DDS | Diabetes distress |

|
NS |
| SEDS | Diabetes self‐efficacy |

|
NS | |||||||
| PHQ‐8 | Depressive symptoms |

|
NS | |||||||
| SSQ | Social support |

|
NS | |||||||
| PROMIS | General health | |||||||||
| Sleep disturbance |

|
NS | ||||||||
| Polonsky et al. (2018) | 39 | Cohort | US | 424 | None | 0.90 | WHO‐5 | Emotional well‐being |

|
NS |
| GAD | Anxiety symptoms |

|
<0.01 | |||||||
| PHQ‐8 | Depressive symptoms |

|
NS | |||||||
| DDS | Diabetes distress |

|
<0.05 | |||||||
| HFS‐II | Fear of hypoglycaemia | |||||||||
| Hypoglycaemia worry |

|
<0.01 | ||||||||
| Ritter et al. (2016) | 37 | RCT | US | 1674 | Efficacy care versus placebo | 0.77 | DSES | Diabetes self‐efficacy |

|
0.026 |
| Torre et al. (2018) | 32 | Cohort | Portugal | 1303 | DPP‐4 versus GLP‐1 vs.SGLT2 | 0.90 | EQ‐5D | General health |

|
NS |
| EQ‐VAS | General health |

|
NS | |||||||
| Wieringa et al. (2018) | 33 | Cohort | Netherlands | 911 | Insulin glargine | 1.00 | WHO‐5 | Emotional well‐being |
 c
c
|
0.30 |
| HFS‐II | Fear of hypoglycaemia | |||||||||
| Hypoglycaemia worry |
 e
e
|
<0.001 | ||||||||
| Yang et al. (2014) | 34 | Cohort | China | 8578 | Biphasic insulin aspart 30/70 | 0.80 | EQ‐VAS | General health |
 b
b
|
<0.001 |
Abbreviations: DDS, Diabetes Distress Scale; DQOL, Diabetes Quality of Life measure; DSES, Diabetes Self‐Efficacy Scale; EQ‐5D, EuroQol 5‐Dimension health status instrument; EQ‐VAS, EuroQol Visual Analogue Scale; GAD, General Anxiety Disorder scale; HFS‐II, Hypoglycaemia Fear Survey version II; IPQ‐R, Illness Perception Questionnaire Revised; NA, not applicable; NR, not reported; NS, not significant (no p‐value reported); PHQ‐8, Patient Health Questionnaire 8‐item; POMS, Profile of Mood States; PROMIS, Patient‐Reported Outcomes Measurement Information System; RAND‐36, RAND Corporation 36‐item health survey; RCT, randomised controlled trial; SEDS, Self‐Efficacy for Diabetes Scale; SF‐36, Medical Outcomes Study Short Form 36‐item health survey; SSQ, Social Support Questionnaire; W‐BQ12 and W‐BQ22, Well‐Being Questionnaire 12‐item and 22‐item; WHO‐5, World Health Organisation 5‐item well‐being index; WPAI, Work Productivity and Activity Impairment.
Increases and decreases in outcomes are depicted with upward ( ) and downward arrows (
) and downward arrows ( ), respectively; associations of unknown direction are depicted with a dash (
), respectively; associations of unknown direction are depicted with a dash ( ); black and grey symbols indicate significant and non‐significant or untested effects, respectively.
); black and grey symbols indicate significant and non‐significant or untested effects, respectively.
Reflects impact of severe hypoglycaemic events only.
Direction and significance of impact was similar for severe hypoglycaemic events.
Reflects impact of any hypoglycaemic event.
Direction of impact for severe hypoglycaemic events was similar but not significant, p = 0.23
3.2. Study quality
Quality proportions (Table 1) revealed that study quality was excellent (n = 9) or good (n = 3) for cohort studies (0.80 ≤ α q ≤ 1.00). Quality was more diverse for RCTs (0.58 ≤ α q ≤ 0.88), with studies rating as good (n = 3), acceptable (n = 3), or poor (n = 2). Apart from two studies, 29 , 35 however, quality was acceptable or better for both cohort studies (Mα q = 0.92) and RCTs (Mα q = 0.75), and differences in quality were largely attributable to omissions in reporting as few RCTs provided information about blinding (20%) or reliability statistics for measures (25%). These criteria were deemed less relevant as hypoglycaemia was not a component of study interventions, and thus was unaffected by blinding, and nearly all studies relied on PROs with established validity and reliability. Consequently, included studies were interpreted as having good quality given the quasi‐experimental nature of the variables under review (see Tables S2 and S3 for item‐by‐item appraisals).
Regarding hypoglycaemic impacts on QoL, evidence across studies was limited by the narrow scope of QoL instruments and the lack of hypoglycaemia‐specific measures. Furthermore, only three studies explicitly controlled for the intervention 25 , 28 , 29 and none controlled for diabetes education. Reporting of statistical values also varied widely, with most studies lacking standardised effect sizes and other values necessary for clear interpretation and meta‐analysis.
3.3. Frequency and severity of hypoglycaemia
All studies assessed hypoglycaemia using self report, except for one RCT that used clinical records. 20 Recall periods for hypoglycaemia ranged from 1 to 36 months, though recall in most studies (70%) was 6 months or longer. Reporting on hypoglycaemic severity was absent in four studies. 21 , 24 , 29 , 37 Remaining studies described the severity of hypoglycaemia using classifications comparable to those given by Malanda et al. 25 : Grade 1, asymptomatic episodes accompanied by glucose measurements below 4.0 mmol/L (72 mg/dl); Grade 2, self‐treated symptomatic episodes; Grade 3, severe events requiring assistance; and Grade 4, severe events requiring hospitalisation.
Nearly, all studies (90%) reported on the occurrence of hypoglycaemia during the study period (Table 2). A few studies provided episode frequencies, but most (75%) categorised participants based on whether or not they had experienced hypoglycaemia. In studies where asymptomatic and symptomatic episodes were considered together, hypoglycaemia was common, affecting 28%–54% of participants over a 1‐month period. 23 , 27 Symptomatic self‐treated and severe hypoglycaemia were less common. Over a 2‐year period, studies reported 23%–86% of participants experienced at least one self‐treated episode 32 , 39 and 1%–23% experienced at least one severe event. 32 , 39
TABLE 2.
Hypoglycaemic episodes reported in included studies
| Authors (year) | Reference | Measure characteristics | Occurrence of hypoglycaemia a | ||
|---|---|---|---|---|---|
| Type of record | Recall period (months) | Severity classification | |||
| Ali et al. (2012) | 35 | Yes/no | 12 | Grade 3 | 2.8% reported 1+ episodes |
| Briggs et al. (2017) | 20 | Yes/no | 12 | Grade 4 | 0.5% hospitalised for 1+ events |
| de Sonnaville et al. (1998) | 36 | Frequency | 1 | Grade 3 | 4.4 events per person‐year |
| Genovese et al. (2013) | 21 | Frequency | 12 | NR | NR |
| Goddijn et al. (1999) | 22 | Yes/no | 1 | Grade 2 | 6.4% and 19.1% reported 1+ episodes (at baseline and 1 year, respectively) |
| Haluzik et al. (2018) | 23 | Yes/no | 1 | Grades 1–2 | 57.0% and 53.7% reported 1+ episodes (at baseline and 1 month, respectively) |
| Grade 3 | 6.7% and 7.6% reported 1+ events (at baseline and 1 month, respectively) | ||||
| Jódar et al. (2020) | 24 | Yes/no | 26 | NR | 22.1% reported 1+ episodes |
| Malanda et al. (2011) | 25 | Yes/no | 12 | Grade 1 | 25.2% reported 1+ episodes |
| Grade 2 | 17.7% reported 1+ episodes | ||||
| Menard et al. (2007) | 26 | Yes//no | 12 | Grade 2 | 20.8% reported 1+ episodes |
| Frequency | Grade 2 | 21.6 episodes per person‐year | |||
| Mitchell et al. (2013) | 27 | Yes/no | 1 | Grades 1–3 | 27.5% reported 1+ episodes |
| Nauck et al. (2019) | 28 | Yes/no | 36 | Grade 3 | 42.3% reported 1+ events |
| Nicolucci et al. (2011) | 29 | Frequency | 1 | NR | 7.2, 19.2 and 21.6 episodes per person‐year (at 1, 5 and 6 months, respectively) |
| Pathan et al. (2018) | 38 | Yes/no | 1 | Grade 2–3 | 40.6% and 97.3.1% reported 1+ episodes (at baseline and 1 month, respectively) |
| Grade 3 | 52.2% and 76.9% reported 1+ events (at baseline b and 1 month, respectively) | ||||
| Frequency | Grade 2–3 | 12.2 and 22.6 episodes per person‐year (at baseline and 1 month, respectively) | |||
| Grade 3 | 2.2 and 12.2 events per person‐year (at baseline b and 1 month, respectively) | ||||
| Peyrot et al. (2008) | 30 | Frequency | 16 | Grade 2 | 2.3 episodes per person‐year |
| Pichayapinyo et al. (2019) | 31 | Severity | 3 | Grade 2 | NR |
| Polonsky et al. (2018) | 39 | Yes/no | 24 | Grade 2 | 86% reported 1+ episodes |
| Grade 3 | 41% reported 1+ events | ||||
| Ritter et al. (2016) | 37 | Symptom severity | 6 | NR | NR |
| Torre et al. (2019) | 32 | Yes/no | 26 | Grade 2–3 | 22.8% reported 1+ episodes |
| Grade 3 | <1% reported 1+ events | ||||
| Wieringa et al. (2018) | 33 | Yes/no | 3 | Grade 2–3 | 37.2%, 42.5% and 43.5% reported 1+ episodes (at baseline, 3 and 6 months, respectively) |
| Grade 3 | 3.1%, 4.3% and 5.6% reported 1+ events (at baseline, 3 and 6 months, respectively) | ||||
| Yang et al. (2014) | 34 | Frequency | 6 | Grade 2 | 2.17 and 1.54 episodes per person‐year (at baseline and 6 months, respectively) |
| Grade 3 | 0.15 and 0.0 events per person‐year (at baseline and 6 months, respectively) | ||||
Grade 1, asymptomatic hypoglycaemia; Grade 2, self‐treated symptomatic hypoglycaemia; Grade 3; severe hypoglycaemia requiring assistance; Grade 4, severe hypoglycaemia requiring hospitalisation.
Abbreviation: NR, not reported.
Unless otherwise stated, values reflect occurrence of hypoglycaemia during the study period. Occurrence of hypoglycaemia at baseline is presented when reported by the study.
Recall period was 6 months for baseline occurrence of severe (Grade 3) hypoglycaemic events.
3.4. Impact of hypoglycaemia on generic QoL
Studies examined hypoglycaemic impacts using 11 PROs related to generic QoL. Relevant PROs almost exclusively targeted facets within the physical and psychological domains. Impacts on each PRO are summarised in Table 1, and detailed statistical results are provided in Table 3.
TABLE 3.
Relative size of impact of hypoglycaemia in included studies
| Authors (year) a | Reference | Analysis | Quantified impact on measures of quality of life |
|---|---|---|---|
| Ali et al. (2012) | 35 | Linear regression | No; 1+ severe hypoglycaemic events did not lead to changes in mental or physical health; compared to those reporting no events, those reporting severe events experienced a decline in SF‐36 Mental Component scores, B = –2.14, and a rise in SF‐36 Physical Component scores, B = 1.05, though none of these impacts were significant, ps > 0.05 |
| Briggs et al. (2017) | 20 | Linear regression | Yes; 1+ severe hypoglycaemic events led to a decrease in general health; following hospitalisation for hypoglycaemia, EQ‐5D Utility Index scores dropped (M Δ = –0.019, SE Δ = 0.024), p < 0.05 |
| Haluzik et al. (2018) | 23 | NA (targeted question) | Yes; 1+ hypoglycaemic episodes impaired attendance at school or work; during the 1‐month study period, 2.5% of participants reported taking leave from school or work (M = 2.8 days), 2.7% reported arriving late and 4.9% reported leaving early as a direct consequence of hypoglycaemia |
| Jódar et al. (2020) | 24 | Linear regression | Mixed; 1+ hypoglycaemic episodes (of unspecified severity) led to larger improvements in physical health, but not mental health, following intervention; change in SF‐36 Physical Component scores was larger and more positive for those reporting hypoglycaemic episodes (M Δ = 1.04, SE Δ = 2.4) compared to those reporting no episodes (M Δ = 0.5, SE Δ = 0.2), p < 0.01, while change in SF‐36 Mental Component scores did not differ between those reporting episodes (M Δ = –0.5, SE Δ = 0.4) and those who did not (M Δ = 0.3, SE Δ = 0.2), p = 0.08 |
| Malanda et al. (2011) | 25 | ANCOVA (adjusted for gender, age, education, diabetes duration, intervention) | Mixed; 1+ asymptomatic hypoglycaemic episodes led to increased perceived control over diabetes; pairwise comparisons showed changes in IPQ‐R Control subscale scores were larger and more positive for those reporting only asymptomatic episodes (M Δ = 1.04, SD Δ = 2.4), compared to those reporting self‐treated symptomatic episodes (M Δ = –0.3, SD Δ = 2.7), p = 0.007, d = 0.54, or no episodes (M Δ = –0.09, SD Δ = 3.3), p = 0.009, d = 0.37. There was no difference between those reporting symptomatic or no episodes, p > 0.05. Experiencing 1+ hypoglycaemic episodes did not affect general health, general well‐being or diabetes distress; three‐way comparisons for those reporting no episodes, only asymptomatic episodes or self‐treated symptomatic episodes showed no changes in EQ‐5D (M Δ = –0.04, 0.01, –0.03, SD Δ = 0.2, 0.2, 0.2), p = 0.23, W‐BQ12 (M Δ = –0.27, 0.03, 0.16, SD Δ = 5.2, 4.7, 4.0), p = 0.82 or IPQ‐R Emotion subscale scores (M Δ = 0.39, –0.27, –0.79, SD Δ = 3.6, 3.6, 3.9), p = 0.24 |
| Nauck et al. (2019) | 28 | Linear regression (adjusted for gender, region, CVR, intervention) | Yes; 1+ severe hypoglycaemic events (requiring assistance or confirmed plasma glucose <3.1 mmol/L [56 mg/dl]) led to a decrease in general health; compared to those reporting no events, those reporting severe events experienced a drop in EQ‐5D Utility Index scores (M Δ = –0.018, SE Δ = 0.004), p < 0.001, but no change in VAS scores (M Δ = –0.009, SE Δ = 0.351), p = 0.98 |
| Nicolucci et al. (2011) | 29 | Linear regression (adjusted for gender, age, HbA1c, weight, intervention) | Yes; 4+ hypoglycaemic episodes (of unspecified severity) led to a decrease in general well‐being and energy, and smaller improvements in mental health following intervention; compared to those reporting no episodes, those reporting 3+ episodes showed a drop in W‐BQ22 total, B = –5.41 (SE = 1.72), p = 0.002, and W‐BQ22 Energy subscale scores, B = –1.45 (SE = 0.49), p = 0.003, and smaller improvements in SF‐36 Mental Component scores, B = –5.03 (SE = 1.72), p = 0.004. Those reporting 1–3 (but not 4+) hypoglycaemic episodes showed a drop in W‐BQ22 Anxiety subscale scores, B = –1.76 (SE = 0.65), p = 0.007 |
| Pathan et al. (2018) | 38 | NA (targeted question) | Yes; 1+ hypoglycaemic episodes impaired attendance at school or work; during the 1‐month study period, 3.2% of participants reported taking leave from school or work, 2.1% reported arriving late and 2.8% reported leaving early as a direct consequence of hypoglycaemia |
| Pichayapinyo et al. (2019) | 31 | Pearson's correlation | No; more frequent symptoms of hypoglycaemia did not lead to changes in depression, sleep disturbance, social support, diabetes distress or diabetes self‐efficacy; those reporting more symptoms showed a rise in PHQ‐8 total, r = 0.18, and PROMIS Sleep Disturbance subscale scores, r = 0.16, as well as a drop in SSQ total, r = –0.12, DDS total, r = –0.06, and SEDS total scores, r = –0.04, though none of these impacts were significant, ps > 0.05 |
| Polonsky et al. (2018) | 39 | ANCOVA b (adjusted for gender, age, insulin status) | Mixed; 1+ self‐treated symptomatic hypoglycaemic episodes led to increased anxiety, diabetes distress and hypoglycaemic worry, but no change in depression or general well‐being; compared to those reporting no episodes, those reporting self‐treated episodes showed a rise in GAD total, β = 0.16, p < 0.01, DDS total, β = 0.12, p < 0.05, and HFS‐II worry subscale scores, β = 0.18, p < 0.01, but no change in PHQ‐8 total, β = 0.09, p > 0.05 or WHO‐5 total scores, β = 0.02, p > 0.05 |
| Torre et al. (2019) | 32 | Linear regression b | No; 1+ self‐treated hypoglycaemic episodes or severe hypoglycaemic events did not lead to minimally important changes in general health; following self‐treated episodes or severe events, there was a non‐significant rise in EQ‐5D utility index, β = 0.29, p = 0.15, and EQ‐5D Visual Analogue Scale scores, β = 0.11, p = 0.57 |
| Wieringa et al. (2018) | 33 | GEE (adjusted for gender, age, education, diabetes duration, HbA1c, BMI, and number of complications) | Mixed; 2+ self‐treated symptomatic hypoglycaemic episodes led to increased hypoglycaemic worry, but no change in general well‐being; compared to those reporting no episodes, those reporting 2+ self‐treated episodes showed a rise in HFS‐II Worry subscale scores, B = 1.33 (SE = 0.06), p < 0.001, but no change in WHO‐5 totals, B = −0.79 (SE = 0.95), p = 0.30. Experiencing 2+ severe hypoglycaemic events led to no change in hypoglycaemic worry or general well‐being; compared to those reporting no events, those reporting 2+ severe events showed no change in HFS‐II Worry subscale, B = 1.13 (SE = 0.12), p = 0.23, or WHO‐5 total scores, B = −1.63 (SE = 1.58), p = 0.31. A non‐significant interaction between time and hypoglycaemic events across all analyses suggested these impacts did not change over time |
| Yang et al. (2014) | 34 | Linear regression (adjusted for gender, age, BMI, diabetes duration, insulin history, HbA1c) | Mixed; 1+ severe hypoglycaemic events led to smaller improvements in general health following intervention; compared to those reporting no events, those reporting 1+ severe events showed smaller increases in EQ‐5D Visual Analogue Scale scores, B = 6.96, p < 0.001. When self‐treated symptomatic episodes and severe events were combined, this effect was no longer significant; following any hypoglycaemic episode, EQ‐5D Visual Analogue Scale scores did not change, B = 0.02, p = 0.96 |
Abbreviations: ANCOVA, Analysis of Covariance; CVR, Cardiovascular Risk; DDS, Diabetes Distress Scale; EQ‐5D, EuroQol 5‐Dimension health status instrument; GAD, General Anxiety Disorder scale; GEE, Generalised Estimating Equations; HFS‐II, Hypoglycaemia Fear Survey version II; IPQ‐R, Illness Perception Questionnaire Revised; PHQ‐8, Patient Health Questionnaire 8‐item; PROMIS, Patient‐Reported Outcomes Measurement Information System; SEDS, Self‐Efficacy for Diabetes Scale; SF‐36, Medical Outcomes Study Short Form 36‐item health survey; SSQ, Social Support Questionnaire; W‐BQ12 and W‐BQ22; Well‐Being Questionnaire 12‐item and 22‐item; WHO‐5, World Health Organisation 5‐item well‐being index.
Studies which did not report estimates of effect size were omitted from this table.
To facilitate comparisons across studies, OR and 95% CI values were converted to β and p, respectively.
3.4.1. Health status
The most common outcome, perceived health, was assessed in 45% of studies. Although frequently interpreted as a global assessment of generic or health‐related QoL, PROs assessing health status measured facets exclusively in the physical and psychological domains.
The EuroQol 5‐Dimension (EQ‐5D) health instrument was the most frequently adopted PRO in studies targeting health status (n = 5). While the full EQ‐5D includes four physical health items and one mental health item, all studies save one 25 used the EQ‐5D utility index, a summed total of all items rated against a normed sample, 20 , 28 , 32 or the EuroQoL Visual Analogue Scale (EQ‐VAS), a single item rating general health on a scale from 1 to 100. 32 , 34
Three studies focusing on severe events found EQ‐5D utility index 20 , 28 and EQ‐VAS scores 34 were negatively associated with hypoglycaemia; compared to those reporting no events, those experiencing at least one severe event had lower ratings of perceived health. In contrast, two cohort studies showed that self‐treated hypoglycaemia was not associated with EQ‐5D totals 25 or EQ‐VAS scores. 27
The Medical Outcomes Study Short Form 36‐item (SF‐36) survey, a broad health status measure comprised of Mental and Physical Component scales, was adopted solely by RCTs (n = 3). A 12‐month study examining the impact of severe hypoglycaemia revealed no significant declines in either physical or mental health among those experiencing severe events. 35 Two studies which did not specify hypoglycaemic severity likewise reported no decline in physical health among those experiencing hypoglycaemia over a 1‐month 29 or 26‐month 24 period. One of these studies did find that treatment‐related improvements in mental health were blunted among those experiencing three or more hypoglycaemic episodes, 29 but the other reported no change in Mental Component scores. 24 Moreover, the latter was consistent with a study examining mental health using a subscale of the RAND Corporation 36‐item (RAND‐36) survey—a measure that parallels the SF‐36—which found no change in mental health following self‐treated hypoglycaemia. 22
Taken as a whole, findings from health status studies (n = 9) indicated perceptions of health worsened among those experiencing hypoglycaemic over a period of 6–36 months, but only for events severe enough to require assistance. Furthermore, this impact was only evident when health status was assessed using the EQ‐5D. Findings regarding self‐treated hypoglycaemia were less clear, with EQ‐5D studies reporting no impact, and SF‐36 studies reporting mixed results for mental health.
3.4.2. Facets in the physical domain of QoL
Energy and sleep
Two glucose monitoring studies examined energy level using the Energy subscale of the Well‐Being Questionnaire (W‐BQ), with one reporting no change in energy among those experiencing one or more asymptomatic or self‐treated symptomatic hypoglycaemic episodes over a 12‐month period, 25 and the other reporting a decline in energy following four or more episodes of unspecified severity within 30 days. 29 A small‐sample study 31 examined sleep quality using a subscale of the Patient‐Reported Outcomes Measurement Information System (PROMIS), revealing a weak, non‐significant correlation between more frequent symptoms of hypoglycaemia and greater sleep disturbance (see Table 3 for effect sizes).
Everyday functioning
Two studies assessed impairment in the workplace among participants from Eastern Europe 23 and Southeast Asia. 38 Over a 30‐day period, approximately 3% of adults with type 2 diabetes reported having been absent from work, and 2%–5% reported having left work early, as a direct consequence of hypoglycaemia. Impairment during non‐work activities (e.g. shopping) was examined in one study using the Activity Impairment subscale of the Work Productivity and Activity Impairment questionnaire, 21 revealing that an increase in the frequency of hypoglycaemia over a 12‐month period was followed by greater impairments in everyday functioning than those who experienced no change or a decrease.
3.4.3. Facets in the psychological domain of QoL
Mood
The most commonly assessed facet within the psychological domain of QoL, mood, was examined in 25% of studies. Three studies investigated general mood using the Profile of Mood States survey 36 or the Positive and Negative Mood subscales of the 12‐item 25 and 22‐item 29 W‐BQ. These studies provided no evidence for an association between hypoglycaemia and general mood, regardless of mood valence (i.e. positivity or negativity) or hypoglycaemic severity.
Symptoms of mood disorders were examined in three studies. One study using the broad Anxiety subscale of the 22‐item W‐BQ found that those experiencing one, two or three hypoglycaemic episodes (but not four or more) reported reduced anxiety. 29 However, a study using the narrower General Anxiety Disorder scale reported that those who experienced at least one self‐treated hypoglycaemic episode in 24 months developed elevated anxiety symptoms. 39 Three studies assessed depressive symptoms using either the 8‐item Patient Health Questionnaire 31 , 39 or the Depression subscale of the 22‐item W‐BQ. 29 Unlike anxiety, results from these studies showed no relationship between self‐treated hypoglycaemia and changes in depressive symptoms, regardless of whether assessments were made over 1, 3 or 24 months.
Emotional well‐being
Four studies examined general emotional well‐being. Two studies using the World Health Organisation 5‐item well‐being index found that, regardless of whether hypoglycaemia was self‐treated or severe, emotional well‐being remained unchanged. 33 , 39 Likewise, a study employing the 12‐item W‐BQ found no relationship between self‐treated events and changes in general emotional well‐being over a 12‐month period. 25 Conversely, an RCT using the long‐form 22‐item W‐BQ reported a significant drop in emotional well‐being among those who experienced four or more hypoglycaemic episodes during a 1‐month period. 29
3.4.4. Facets in the social domain of QoL
A single study assessed an outcome outside the physical and psychological domains of QoL. This small‐sample study used the Social Support Questionnaire, reporting a weak, non‐significant correlation between more frequent hypoglycaemia symptoms and declines in social support over 3 months. 31
3.5. Impact of hypoglycaemia on diabetes‐specific QoL
Studies in this review employed five PROs related to diabetes‐specific QoL. However, only one study 26 utilised a global assessment, the Diabetes Quality of Life (DQOL) survey, for which participants rated areas commonly impacted by diabetes spanning the physical, psychological and social domains. Results showed changes in DQOL totals were not associated with self‐treated hypoglycaemic episodes over 12 months. Remaining studies (n = 7) examined the impact of hypoglycaemia on PROs related to facets of psychological diabetes‐specific QoL.
3.5.1. Diabetes distress
Using the Diabetes Distress Scale (DDS), a large‐sample study with a high incidence of hypoglycaemia reported greater elevations in diabetes distress among those experiencing self‐treated or severe hypoglycaemia over a 3‐month period. 39 However, no impact on DDS totals was reported by studies with a small sample 31 or lower incidence of hypoglycaemia. 30 Similarly, a study assessing diabetes distress using the Emotion subscale of the revised Illness Perception Questionnaire (IPQ‐R) reported no change in distress following asymptomatic or self‐treated symptomatic hypoglycaemia. 25
3.5.2. Diabetes self‐efficacy
Diabetes self‐efficacy refers to a person's confidence in their ability to manage important aspects of diabetes, especially glycaemic control. 40 This outcome was assessed by one study 31 using the Self‐Efficacy for Diabetes Scale and another 25 using the Control subscale of the IPQ‐R, both of which reported no change in diabetes self‐efficacy following self‐treated symptomatic hypoglycaemia experienced over 3 or 24 months, respectively. The latter study also reported increased self‐efficacy among those who experienced only asymptomatic hypoglycaemia. 25 Gains in perceived control over diabetes represented a small improvement compared to those not experiencing hypoglycaemia, and a moderate improvement compared to those experiencing self‐treated symptomatic episodes. Lastly, a study examining the impact of severe hypoglycaemia found that scores on the Diabetes Self‐Efficacy Scale dropped among those who experienced at least one severe event in 6 months. 37
3.6. Impact of hypoglycaemia on hypoglycaemia‐specific QoL
Fear of hypoglycaemia was the sole hypoglycaemia‐specific impact uncovered in this review. This outcome was assessed by three studies, all using the Hypoglycaemia Fear Survey version II (HFS‐II). One study 27 found those who experienced any hypoglycaemia over a 1‐month period displayed increased HFS‐II totals. Similarly, two studies reported increased HFS‐II Worry subscale scores among those experiencing at least one self‐treated episode over 3 months 33 and 24 months. 39 Finally, a study assessing the impact of severe events reported a trend towards elevated HFS‐II Worry subscale scores, although this effect was non‐significant. 33 Together, these findings suggest self‐treated hypoglycaemia was followed by increased fear and worry concerning hypoglycaemia, though impacts for severe events were less clear.
4. DISCUSSION
Findings from 20 longitudinal studies included in this systematic review demonstrate that, among people with type 2 diabetes, hypoglycaemia can have a detrimental impact on key facets within the physical and psychological domains of QoL. Specific impacts, however, vary widely depending upon frequency and severity of hypoglycaemia, as well as choice of QoL measure and the facet(s) and domain(s) targeted.
The largest body of evidence concerns health status, which broadly captures facets across the physical and psychological domains of generic QoL. Multiple studies corroborate that perceived health is negatively affected by severe hypoglycaemia (i.e. events requiring third‐party assistance). However, evidence regarding the impact of self‐treated hypoglycaemia is inconclusive. Studies using the EQ‐5D, a brief instrument emphasising physical QoL, consistently report that self‐treated hypoglycaemia has no impact on perceived health. In contrast, studies using the more comprehensive SF‐36 provide mixed results; hypoglycaemia appears to worsen perceived mental health in some studies but not others. Conclusions in this review are partially consistent with EQ‐5D research in type 1 diabetes 5 and EQ‐5D and SF‐36 clinical studies in type 2 diabetes, 13 all of which report that perceived physical and mental health is negatively affected by both severe and self‐treated hypoglycaemia.
Regarding facets in the physical domain of QoL, approximately 2%–5% of adults with type 2 diabetes report difficulty performing everyday tasks both in and out of the workplace each month as a direct consequence of hypoglycaemia—impacts similar to those reported by adults with type 1 diabetes. 23 , 38 Hypoglycaemia also leads to diminished energy, but only among those experiencing frequent episodes (four or more per month). Again, this impact matches type 1 diabetes research. 41 Sleep quality may be affected as well, though evidence here is inconclusive. Findings from one small‐sample study 31 reveal a non‐significant trend towards progressively disturbed sleep among those experiencing more frequent symptoms of hypoglycaemia. However, significant impacts on sleep reported in large‐sample cross‐sectional studies in type 2 diabetes, 11 as well as laboratory studies in type 1 diabetes, 42 lend support to the notion that symptomatic hypoglycaemia lowers sleep quality.
Regarding facets in the psychological domain of QoL, there is little evidence to suggest hypoglycaemia has long‐term impacts on general mood. Findings from several studies in this review indicate mood positivity and negativity remain unchanged following self‐treated or severe hypoglycaemia. Evidence from multiple studies also reveals no impact on emotional well‐being, as changes are comparable for those experiencing severe, self‐treated and no hypoglycaemia. Nonetheless, the impact of hypoglycaemia on emotional well‐being may be dose dependent. Findings from one study 29 suggest that those with frequent episodes (four or more per month) experience declines in emotional well‐being, matching conclusions from research in type 1 diabetes 5 and clinical studies in type 2 diabetes. 13
Measures of mood disorders reveal nuanced impacts. According to one study, reductions in anxiety are experienced by those reporting one, two or three hypoglycaemic episodes of unspecified severity (but not four or more) during a 1‐month period. 29 However, this counter‐intuitive finding may be due to habituation to hypoglycaemia, or a masking effect caused by the psychotherapeutic component of the intervention which produced improvements in nearly all mood‐related outcomes in the study. Indeed, results from a non‐intervention study in this review 39 suggest self‐treated hypoglycaemia exacerbates symptoms of generalised anxiety. In contrast, results from several longitudinal studies demonstrate that self‐treated hypoglycaemia has no impact on depressive symptoms. Type 1 diabetes research reveals a similar pattern, with evidence suggesting hypoglycaemia is associated with increased symptoms of generalised anxiety 43 but not depression. 44 Conclusions in this review are somewhat consistent with earlier systematic reviews in type 2 diabetes which report increased symptoms of anxiety and depression in some, but not all, cross‐sectional studies. 11 , 12
Evidence concerning impacts outside the physical and psychological domains of QoL is sparse. One study in this review examined a social outcome, reporting a weak, non‐significant correlation between more frequent hypoglycaemic symptoms and decreased social support. 31 This analysis was underpowered, however, and given cross‐sectional evidence in type 1 diabetes research linking hypoglycaemia to reduced participation in social activities, 45 large‐sample longitudinal confirmatory studies are needed.
A considerable body of evidence demonstrates that, among adults with type 2 diabetes, hypoglycaemia affects diabetes‐specific QoL. However, as with generic QoL, targeted measures are more informative. A study utilising a global assessment spanning the physical, psychological and social domains 26 reveals no impact on overall diabetes‐specific QoL for those experiencing self‐treated hypoglycaemia. Meanwhile, evidence from a large‐sample study with a high incidence of hypoglycaemia indicates self‐treated and severe hypoglycaemia are followed by elevations in diabetes distress, 39 matching research in type 1 diabetes. 5
Impacts on diabetes self‐efficacy are complex. Multiple studies suggest confidence in self management of diabetes is diminished by severe hypoglycaemia but unaffected by self‐treated episodes. Intriguingly, one study in this review reports that asymptomatic hypoglycaemia leads to modest gains in diabetes self‐efficacy relative to those without hypoglycaemia. 25 Taken together, these results suggest that experiencing only symptom‐free hypoglycaemia may enhance diabetes self‐efficacy while experiencing severe episodes may undermine a person's confidence in their ability to manage diabetes. Nevertheless, given limited research in this area, additional research is needed to confirm these impacts in adults with type 1 and type 2 diabetes.
Finally, regarding hypoglycaemia‐specific QoL, several studies conclude that self‐treated hypoglycaemia increases fear of hypoglycaemia—an outcome linked to other facets of generic QoL, including anxiety and poor sleep quality. 46 Similar impacts on fear of hypoglycaemia are reported in research in type 1 5 and type 2 diabetes. 13 Conversely, one study in this review reports no change in fear of hypoglycaemia following severe events. 33 One explanation for this lack of effect may be that a focus on avoiding hyperglycaemia, coupled with minimisation of the consequences of hypoglycaemia, may result in both reduced fear and more frequent severe events. 47 A second explanation is that, unlike self‐treated episodes which are managed alone, assistance rendered during a severe event may increase a person's confidence in their support network, reducing uncertainties about future hypoglycaemia. Indeed, this explanation is supported by studies linking perceived lack of social support to greater fear of hypoglycaemia. 48
4.1. Limitations and strengths
Findings in this systematic review were limited by the characteristics of included studies (e.g. heterogenous measures, inadequate statistical information) and by the inclusion criteria of the review itself. Searching different databases (e.g. JSTOR, PsycExtra) and relaxing the search strategy to include qualitative study designs, measures other than PROs, and additional terms for specific QoL‐related outcomes may alter the scope of hypoglycaemic impacts detected. This review also has several strengths. First and foremost, although studies did not experimentally induce hypoglycaemia, focusing on longitudinal studies provides evidence that draws one step closer to forming causal inferences about the impact of hypoglycaemia on changes in QoL. Second, this review had no restrictions on language and included studies conducted internationally across Europe, North America and Asia, the majority of which recruited large and diverse samples. Finally, in comparison to earlier systematic reviews of clinical trials, this review presents findings for a wider range of outcomes, detailing impacts for 17 different QoL‐related measures in the physical, psychological and social domains.
4.2. Recommendations for future research
Gaps in understanding identified in this review suggest five directions for future investigations. First, more research differentiating the effects of symptomatic and asymptomatic hypoglycaemia is needed. Second, continuous glucose monitoring is important for improving accuracy and consistency in measuring hypoglycaemia, and for capturing moment‐to‐moment fluctuations in blood glucose and QoL outcomes, particularly energy and mood. 49 Third, impaired functioning at work is a commonly reported consequence of hypoglycaemia, but statistical evidence supporting this effect is needed. Fourth, effect sizes suggest hypoglycaemia has a negative impact on sleep quality and social support, but evidence from longitudinal studies with large samples is lacking. Fifth, PROs targeting facets across all domains of QoL, particularly the social domain, and hypoglycaemia‐specific QoL measures are currently underutilised.
5. CONCLUSIONS
Evidence from longitudinal studies demonstrates hypoglycaemia is relatively common among adults with type 2 diabetes, and its impacts on QoL parallel those experienced by adults with type 1 diabetes. Severe hypoglycaemia in type 2 diabetes is associated with reduced confidence in diabetes self‐management and lower ratings of perceived health. Self‐treated symptomatic hypoglycaemia is followed by reductions in physical QoL, including impaired functioning at work and in daily life. Self‐treated hypoglycaemia is also associated with declines in psychological QoL, including elevated symptoms of generalised anxiety, diabetes distress and fear of hypoglycaemia. Frequent hypoglycaemia is followed by diminished energy and emotional well‐being. Nevertheless, available evidence is insufficient to establish whether hypoglycaemia impacts sleep quality, depressive symptoms, general mood, social support or overall diabetes‐specific QoL. Further longitudinal research is needed targeting more varied facets of QoL, particularly in the social domain. Research delineating impacts on individual facets of QoL based on hypoglycaemic severity will also be important for improving existing measures, 50 developing new hypoglycaemia‐specific measures which better capture the breadth of QoL and designing targeted interventions aimed at improving QoL on an outcome‐by‐outcome basis.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Helen Buckley Woods for performing the initial database searches, Anna Cantrell for helping with study selection, Katie Sworn for helping with data extraction, and Mette Valdersdorf Jensen and Manon C. J. Coolen for helping with record screening and verification of extracted data.
Matlock KA, Broadley M, Hendrieckx C, et al. Hypo‐RESOLVE consortium . Changes in quality of life following hypoglycaemia in adults with type 2 diabetes: a systematic review of longitudinal studies. Diabet Med. 2022;39:e14706. doi: 10.1111/dme.14706
Funding information
This study was funded by the EU Innovative Medicines Initiative 2 (IMI2) Joint Undertaking (JU) under grant agreement no. 777460. The JU receives support from European Union's Horizon 2020 Research and Innovation Programme, European Federation of Pharmaceutical Industries and Associations (EEPIA), T1D Exchange, JDRF, International Diabetes Federation (IDF), and the Leona M. and Harry B. Helmsley Charitable Trust. Jane Speight and Christel Hendrieckx are supported by core funding to the Australian Centre for Behavioural Research in Diabetes (ACBRD) provided by a collaboration between Diabetes Victoria and Deakin University.
REFERENCES
- 1. Williams SA, Shi L, Brenneman SK, Johnson JC, Wegner JC, Fonseca V. The burden of hypoglycemia on healthcare utilization, costs, and quality of life among type 2 diabetes mellitus patients. J Diabetes Complications. 2012;26(5):399‐406. doi: 10.1016/j.jdiacomp.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 2. Polonsky WH, Fisher L, Hessler D, Edelman SV. Identifying the worries and concerns about hypoglycemia in adults with type 2 diabetes. J Diabetes Complications. 2015;29(8):1171‐1176. doi: 10.1016/j.jdiacomp.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 3. Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299‐1307. doi: 10.2147/PPA.S106821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410‐1418. doi: 10.1056/NEJMoa1003795 [DOI] [PubMed] [Google Scholar]
- 5. Rossi MC, Nicolucci A, Ozzello A, et al. Impact of severe and symptomatic hypoglycemia on quality of life and fear of hypoglycemia in type 1 and type 2 diabetes. Results of the Hypos‐1 observational study. Nutr Metab Cardiovasc Dis. 2019;29(7):736‐743. doi: 10.1016/j.numecd.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 6. Speight J, Holmes‐Truscott E, Hendrieckx C, Skovlund S, Cooke D. Assessing the impact of diabetes on quality of life: what have the past 25 years taught us? Diabet Med. 2020;37(3):483‐492. doi: 10.1111/dme.14196 [DOI] [PubMed] [Google Scholar]
- 7. World Health Organisation (WHO) . WHOQOL‐BREF: Introduction, Administration, Scoring and Generic Version of the Assessment: Field Trial Version, December 1996. World Health Organisation; 1996. https://apps.who.int/iris/handle/10665/63529 (accessed 03 March 2021). [Google Scholar]
- 8. Kalra S, Mukherjee JJ, Venkataraman S, et al. Hypoglycemia: the neglected complication. Indian J Endocrinol Metab. 2013;17(5):819‐834. doi: 10.4103/2230-8210.117219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aucoin M, Bhardwaj S. Generalized anxiety disorder and hypoglycemia symptoms improved with diet modification. Case Rep Psychiatry. 2016;2016:1–4. doi: 10.1155/2016/7165425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pan A, Lucas M, Sun Q, et al. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med. 2010;170(21):1884–1891. doi: 10.1001/archinternmed.2010.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dumber D, Upendra S. Systematic literature review: effect of hypoglycemia on quality of life among patient with diabetes mellitus. Indian J Forensic Med Toxicol. 2020;14(4):3997‐3999. doi: 10.37506/ijfmt.v14i4.12266 [DOI] [Google Scholar]
- 12. Hendrieckx C, Ivory N, Singh H, Frier BM, Speight J. Impact of severe hypoglycaemia on psychological outcomes in adults with type 2 diabetes: a systematic review. Diabet Med. 2019;36(9):1082‐1091. doi: 10.1111/dme.14067 [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y, Wieffer H, Modha R, Balar B, Pollack M, Krishnarajah G. The Burden of hypoglycemia in type 2 diabetes: a systematic review of patient and economic perspectives. J Clin Outcomes Manag. 2010;17:547‐557. https://www.mdedge.com/jcomjournal (accessed 24 November 2020). [Google Scholar]
- 14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waffenschmidt S, Knelangen M, Sieben W, Bühn S, Pieper D. Single screening versus conventional double screening for study selection in systematic reviews: a methodological systematic review. BMC Med Res Methodol. 2019;19(1):1–9. doi: 10.1186/s12874-019-0782-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. JBI. 2020. doi: 10.46658/JBIMES-20-01 [DOI] [Google Scholar]
- 17. Glasgow MJ, Edlin R, Harding JE. Comparison of risk‐of‐bias assessment approaches for selection of studies reporting prevalence for economic analyses. BMJ Open. 2020;10(9):e037324. doi: 10.1136/bmjopen-2020-037324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. George D, Mallery P. SPSS for Windows Step by Step: A Simple Guide and Reference. 11.0 Update. 4th ed. Allyn and Bacon; 2003. [Google Scholar]
- 19. Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta‐analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. doi: 10.1136/bmj.l6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Briggs AH, Bhatt DL, Scirica BM, et al. Health‐related quality‐of‐life implications of cardiovascular events in individuals with type 2 diabetes mellitus: a subanalysis from the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)‐TIMI 53 trial. Diabetes Res Clin Pract. 2017;130:24‐33. doi: 10.1016/j.diabres.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 21. Genovese S, Tedeschi D. Effects of vildagliptin/metformin therapy on patient‐reported outcomes: work productivity, patient satisfaction, and resource utilization. Adv Ther. 2013;30(2):152‐164. doi: 10.1007/s12325-013-0001-z [DOI] [PubMed] [Google Scholar]
- 22. Goddijn PP, Bilo HJG, Feskens EJM, Groenier KH, Van der Zee KI, De Jong BM. Longitudinal study on glycaemic control and quality of life in patients with type 2 diabetes mellitus referred for intensified control. Diabet Med. 1999;16(1):23‐30. doi: 10.1046/j.1464-5491.1999.00002.x [DOI] [PubMed] [Google Scholar]
- 23. Haluzik M, Kretowski A, Strojek K, et al. Perspectives of patients with insulin‐treated type 1 and type 2 diabetes on hypoglycemia: results of the HAT observational study in Central and Eastern European countries. Diabetes Ther. 2018;9(2):727‐741. doi: 10.1007/s13300-018-0388-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jódar E, Michelsen M, Polonsky W, et al. Semaglutide improves health‐related quality of life versus placebo when added to standard of care in patients with type 2 diabetes at high cardiovascular risk (SUSTAIN 6). Diabetes Obes Metab. 2020;22(8):1339‐1347. doi: 10.1111/dom.14039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malanda UL, Bot SD, French DP, et al. Experience of hypoglycaemia is associated with changes in beliefs about diabetes in patients with type 2 diabetes. Diabet Med. 2011;28(11):1395‐1400. doi: 10.1111/j.1464-5491.2011.03340.x [DOI] [PubMed] [Google Scholar]
- 26. Menard J, Payette H, Dubuc N, Baillargeon JP, Maheux P, Ardilouze JL. Quality of life in type 2 diabetes patients under intensive multitherapy. Diabetes Metab. 2007;33(1):54‐60. doi: 10.1016/j.diabet.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 27. Mitchell BD, Vietri J, Zagar A, Curtis B, Reaney M. Hypoglycaemic events in patients with type 2 diabetes in the United Kingdom: associations with patient‐reported outcomes and self‐reported HbA1c. BMC Endocr Disord. 2013;13(1):1‐9. doi: 10.1186/1472-6823-13-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nauck MA, Buse JB, Mann JF, et al. Health‐related quality of life in people with type 2 diabetes participating in the LEADER trial. Diabetes Obes Metab. 2019;21(3):525‐532. doi: 10.1111/dom.13547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nicolucci A, Del Prato S, Vespasiani G; ELEONOR Study Group . Optimizing insulin glargine plus one injection of insulin glulisine in type 2 diabetes in the ELEONOR study: similar effects of telecare and conventional self‐monitoring of blood glucose on patient functional health status and treatment satisfaction. Diabetes Care. 2011;34(12):2524‐2526. doi: 10.2337/dc11-0900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peyrot M, Rubin RR, Polonsky WH. Diabetes distress and its association with clinical outcomes in patients with type 2 diabetes treated with pramlintide as an adjunct to insulin therapy. Diabetes Technol Ther. 2008;10(6):461‐466. doi: 10.1089/dia.2008.0031 [DOI] [PubMed] [Google Scholar]
- 31. Pichayapinyo P, Saslow LR, Aikens JE, et al. Feasibility study of automated interactive voice response telephone calls with community health nurse follow‐up to improve glycaemic control in patients with type 2 diabetes. Int J Nurs Pract. 2019;25(6):e12781. doi: 10.1111/ijn.12781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Torre C, Guerreiro J, Longo P, Raposo JF, Leufkens H, Martins AP. Health‐related quality of life in adults with type 2 diabetes mellitus starting with new glucose lowering drugs: an inception cohort study. Primary Care Diabetes. 2019;13(3):221‐232. doi: 10.1016/j.pcd.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 33. Wieringa TH, de Wit M, Twisk JW, Snoek FJ. Does hypoglycaemia affect the improvement in QoL after the transition to insulin in people with type 2 diabetes? J Endocrinol Invest. 2018;41(2):249‐258. doi: 10.1007/s40618-017-0744-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang W, Zhuang X, Li Y, et al. Improvements in quality of life associated with biphasic insulin aspart 30 in type 2 diabetes patients in China: results from the A1chieve observational study. Health Qual Life Outcomes. 2014;12(1):1–7. doi: 10.1186/s12955-014-0137-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ali MK, Feeney P, Hire D, et al. Glycaemia and correlates of patient‐reported outcomes in ACCORD trial participants. Diabet Med. 2012;29(7):e67‐e74. doi: 10.1111/j.1464-5491.2011.03532.x [DOI] [PubMed] [Google Scholar]
- 36. de Sonnaville JJ, Snoek FJ, Colly LP, Devillé W, Wijkel D, Heine RJ. Well‐being and symptoms in relation to insulin therapy in type 2 diabetes. Diabetes Care. 1998;21(6):919‐924. doi: 10.2337/diacare.21.6.919 [DOI] [PubMed] [Google Scholar]
- 37. Ritter PL, Lorig K, Laurent DD. Characteristics of the Spanish‐and English‐language self‐efficacy to manage diabetes scales. Diabetes Educ. 2016;42(2):167‐177. doi: 10.1177/0145721716628648 [DOI] [PubMed] [Google Scholar]
- 38. Pathan F, Goh SY, Rudijanto A, Gadekar A, Jain A, Nicodemus N Jr. Hypoglycaemia among insulin‐treated patients with diabetes: Southeast Asia cohort of IO HAT Study. J ASEAN Fed Endocr Soc. 2018;33(1):28‐36. doi: 10.15605/jafes.033.01.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Polonsky WH, Fisher L, Hessler D. The impact of non‐severe hypoglycemia on quality of life in patients with type 2 diabetes. J Diabetes Complications. 2018;32(4):373‐378. doi: 10.1016/j.jdiacomp.2018.01.014 [DOI] [PubMed] [Google Scholar]
- 40. Dehghan H, Charkazi A, Kouchaki GM, et al. General self‐efficacy and diabetes management self‐efficacy of diabetic patients referred to diabetes clinic of Aq Qala, North of Iran. J Diabetes Metab Disord. 2017;16(1):1–5. doi: 10.1186/s40200-016-0285-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. King P, Kong MF, Parkin H, Macdonald IA, Tattersall RB. Well‐being, cerebral function, and physical fatigue after nocturnal hypoglycemia in IDDM. Diabetes Care. 1998;21(3):341‐345. doi: 10.2337/diacare.21.3.341 [DOI] [PubMed] [Google Scholar]
- 42. Farabi SS. Type 1 diabetes and sleep. Diabetes Spectr. 2016;29(1):10‐13. doi: 10.2337/diaspect.29.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Al Hayek AA, Robert AA, Braham RB, Issa BA, Al Sabaan FS. Predictive risk factors for fear of hypoglycemia and anxiety‐related emotional disorders among adolescents with type 1 diabetes. Med Princ Pract. 2015;24(3):222‐230. doi: 10.1159/000375306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hessler DM, Fisher L, Polonsky WH, et al. Diabetes distress is linked with worsening diabetes management over time in adults with type 1 diabetes. Diabet Med. 2017;34(9):1228‐1234. doi: 10.1111/dme.13381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nicolucci A, Pintaudi B, Rossi MC, et al. The social burden of hypoglycemia in the elderly. Acta Diabetol. 2015;52(4):677‐685. doi: 10.1007/s00592-015-0717-0 [DOI] [PubMed] [Google Scholar]
- 46. Suteau V, Saulnier PJ, Wargny M, et al. Association between sleep disturbances, fear of hypoglycemia and psychological well‐being in adults with type 1 diabetes mellitus, data from cross‐sectional VARDIA study. Diabetes Res Clin Pract. 2020;160:Article 107988. doi: 10.1016/j.diabres.2019.107988 [DOI] [PubMed] [Google Scholar]
- 47. Singh H, Gonder‐Frederick L, Schmidt K, et al. Assessing hyperglycemia avoidance in people with type 1 diabetes. Diabetes Manag. 2014;4(3):263‐271. doi: 10.2217/dmt.14.3 [DOI] [Google Scholar]
- 48. Anarte MT, Carreira M, Machado A, et al. Identification of risk factors for suffering fear of hypoglycemia in type 1 diabetes mellitus patients. Scand J Psychol. 2014;55(6):554‐557. doi: 10.1111/sjop.12158 [DOI] [PubMed] [Google Scholar]
- 49. Ehrmann D, Schmitt AJ, Rubertus P, Kulzer B, Hermanns N. Can mood and energy levels be predicted by preceding glucose values? Combining ecological momentary assessment (EMA) and continuous glucose monitoring (CGM). Diabetes. 2020;69(suppl 1):783‐P. doi: 10.2337/db20-783-P [DOI] [Google Scholar]
- 50. Carlton J, Leaviss J, Pouwer F, et al. The suitability of patient‐reported outcome measures used to assess the impact of hypoglycaemia on quality of life in people with diabetes: a systematic review using COSMIN methods. Diabetologia. 2021;64:1213‐1225. doi: 10.1007/s00125-021-05382-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


