Abstract
New Findings
-
What is the central question of this study?
What is the effect of sub‐maximal aerobic exercise training on signs and symptoms of chronic mountain sickness (CMS) in Andean highlanders?
-
What is the main finding and its importance?
Aerobic exercise training (ET) effectively reduces haematocrit, ameliorates symptoms and improves aerobic capacity in CMS patients, suggesting that a regular aerobic ET programme might be used as a low‐cost non‐invasive/non‐pharmacological management strategy of this syndrome.
Abstract
Excessive erythrocytosis is the hallmark sign of chronic mountain sickness (CMS), a debilitating syndrome associated with neurological symptoms and increased cardiovascular risk. We have shown that unlike sedentary residents at the same altitude, trained individuals maintain haematocrit within sea‐level range, and thus we hypothesise that aerobic exercise training (ET) might reduce excessive haematocrit and ameliorate CMS signs and symptoms. Eight highlander men (38 ± 12 years) with CMS (haematocrit: 70.6 ± 1.9%, CMS score: 8.8 ± 1.4) from Cerro de Pasco, Peru (4340 m) participated in the study. Baseline assessment included haematocrit, CMS score, pulse oximetry, maximal cardiopulmonary exercise testing and in‐office plus 24 h ambulatory blood pressure (BP) monitoring. Blood samples were collected to assess cardiometabolic, erythropoietic, and haemolysis markers. ET consisted of pedalling exercise in a cycloergometer at 60% of for 1 h/day, 4 days/week for 8 weeks, and participants were assessed at weeks 4 and 8. Haematocrit and CMS score decreased significantly by week 8 (to 65.6 ± 6.6%, and 3.5 ± 0.8, respectively, P < 0.05), while and maximum workload increased with ET (33.8 ± 2.4 vs. 37.2 ± 2.0 ml/min/kg, P < 0.05; and 172.5 ± 9.4 vs. 210.0 ± 27.8 W, P < 0.01; respectively). Except for an increase in high‐density lipoprotein cholesterol, other blood markers and BP showed no differences. Our results suggest that reduction of haematocrit and CMS symptoms results mainly from haemodilution due to plasma volume expansion rather than to haemolysis. In conclusion, we show that ET can effectively reduce haematocrit, ameliorate symptoms and improve aerobic capacity in CMS patients, suggesting that regular aerobic exercise might be used as a low‐cost non‐invasive and non‐pharmacological management strategy.
Keywords: Andean highlanders, chronic mountain sickness, excessive erythrocytosis, exercise training, high altitude, Monge´s disease
1. INTRODUCTION
The excessive production of red blood cells (excessive erythrocytosis; EE) is the hallmark feature of chronic mountain sickness (CMS) or Monge's disease, a highly prevalent and incapacitating syndrome in Andean and other high‐altitude populations around the world (Leon‐Velarde et al., 2005). CMS is defined by the presence of EE associated with severe hypoxaemia, neurological sequelae and sleep disorders, also often accompanied by other complications such as pulmonary hypertension and cardio‐cerebrovascular accidents due to adverse changes in blood rheology (Penaloza & Arias‐Stella, 2007; Villafuerte & Corante, 2016). In addition, we and others have shown that EE associates with increased cardiovascular disease risk factors and cardiometabolic disorders such as in‐office and ambulatory hypertension, insulin resistance, dyslipidaemia and metabolic syndrome (Bilo et al., 2020; Corante et al., 2018; De Ferrari et al., 2014; Gonzales & Tapia, 2013; Miele et al., 2016) in various high‐altitude regions across the world (Gonzales & Tapia, 2013; Okumiya et al., 2011, 2010; Sherpa et al., 2011). The long‐term burden of CMS equates to a loss of 3 months of healthy life per year at high altitude (4000 m) (Pei et al., 2012). It is estimated that 5–10% of the world's population living at high‐altitude may develop this condition, and its prevalence increases with altitude and age (Leon‐Velarde et al., 2014; Monge‐C et al., 1989). Above 4300 m in the central Andes of Peru, more than 30% of highlanders by their mid‐50s suffer from EE (Leon‐Velarde et al., 1997; Monge‐C et al., 1992, 1989). CMS signs and symptoms disappear completely when patients descend to sea‐level conditions and following bloodletting or haemodilution at their native altitude of residence, suggesting that the underlying symptoms are secondary to EE. Therefore, treatment strategies for CMS aim to reduce haematocrit and blood viscosity, either by reducing the number of red blood cells or reducing the hypoxic stimulus. However, there is little evidence supporting the safety and efficacy of any long‐term pharmacological or non‐pharmacological option for either and relocation to lower altitudes is unfeasible for the majority of individuals, compromising health, social cohesion and economic wellbeing of patients and their families. Several studies have shown that regular aerobic exercise at 50–60% of maximal effort improves cardio‐respiratory function and O2 transport to tissues which might reduce hypoxaemia‐stimulated erythropoiesis (American College of Sports Medicine, 2010; British Thoracic Society Standards of Care Subcommittee on Pulmonary Rehabilitation, 2001; Reis et al., 1997). Moreover, people that practice exercise regularly have lower Hb concentrations than sedentary people (Mairbaurl, 2013; Telford et al., 2003). This difference is even more pronounced in athletes (Bonilla et al., 2005; Lippi & Sanchis‐Gomar, 2019; Schobersberger et al., 1990; Telford et al., 2003).
Studies in Andean dwellers suggest that physical exercise may reduce haematocrit at high altitude. Schmidt et al. (1990) and Cornolo et al. (2005) have shown that native high‐altitude athletes living and training above 4000 m have sea‐level Hb concentrations in contrast to sedentary individuals living at the same altitude. Moreover, a recent study by our group has shown that exercise training (ET) reduces haematocrit in a rat model of chronic hypoxia‐induced erythrocytosis mainly due to exercise‐induced haemolysis without changes in plasma volume (Macarlupu et al., 2021).
Although exercise fatigue and reduced exercise capacity have been commonly reported in CMS patients, allegedly due to systemic or pulmonary haemodynamic burden and O2‐diffusion impairment as a consequence of excessive haematocrit and blood viscosity (Letcher et al., 1981; Monge, 1943; Ostergaard, 2020; Pratali et al., 2012; Soria et al., 2019; Stuber et al., 2010; Winslow & Monge‐C, 1987), several studies indirectly suggest that adaptive mechanisms exist to maintain O2 transport in the face of a high haematocrit (Juvonen et al., 1991; Lindenfeld et al., 1985). We have previously shown that Andeans diagnosed with mild to moderate CMS can attain normal maximal aerobic capacities compared to healthy Andean controls in Cerro de Pasco (4340 m) (Groepenhoff et al., 2012), and we have also recently confirmed these findings and identified evidence for an adaptive phenotype for O2 transport during exercise with EE (Hansen et al., 2021).
These adaptive characteristics challenge the common belief of reduced tolerance to exercise and suggest that CMS highlanders could benefit from regular moderate aerobic ET. Thus we hypothesise that sub‐maximal aerobic ET can reduce excessive haematocrit and ameliorate CMS signs and symptoms. In addition, we hypothesise that ET can improve aerobic capacity and also reduce cardiovascular and cardiometabolic risk factors in CMS highlanders. Therefore, the primary aim of this study was to assess the effect of an 8‐week sub‐maximal aerobic ET programme on haematocrit and CMS score. Additionally we aimed to investigate changes in aerobic capacity, conventional in‐office and ambulatory blood pressure, together with cardiometabolic, erythropoietic and haemolysis blood markers.
2. METHODS
2.1. Ethical approval
The study was approved by the Institutional Ethics Committee of Universidad Peruana Cayetano Heredia (CIEH‐UPCH approval no. 606‐25‐18, SIDISI no. 100520), and was conducted in accordance with the principles of the Declaration of Helsinki (except for registration in a database). All participants received a detailed explanation of the experimental protocol before consent, and were provided with a consent form in Spanish to be signed before participation in the study.
2.2. Study participants
Ten untrained men with CMS were recruited for the study, and two of them withdrew before the first session of the ET protocol. Thus, we were able to follow eight CMS patients throughout the study (Table 1). Each participant was their own baseline control. All participants were lifelong residents of Cerro de Pasco, Peru (4340 m). Volunteers were excluded if they had a history of pulmonary, cardiovascular or renal disease; were current smokers; work in mining activities; had undergone surgery, blood transfusions or phlebotomies in the previous 6 months; had travelled to lower altitudes (<3000 m) for more than 7 days during the previous 6 months; or had demonstrated abnormal electrocardiogram (ECG) or decreased pulmonary function during screening procedures.
TABLE 1.
Characteristics of study participants
| Characteristic | Value |
|---|---|
| Age (years) | 38.3 ± 12.4 |
| Body weight (kg) | 73.1 ± 12.9 |
| BMI (kg/m2) | 27.5 ± 4.6 |
| Hct (%) | 70.6 ± 5.3 |
| [Hb] (g/dl) | 23.6 ± 1.8 |
| CMS score | 8.8 ± 3.9 |
| (%) | 82.6 ± 3.5 |
| HR (bpm) | 81.9 ± 11.3 |
| SBP (mmHg) | 111.9 ± 8.9 |
| DBP (mmHg) | 77.1 ± 7.0 |
All values are presented as means ± SD. Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; [Hb], haemoglobin concentration; Hct, haematocrit; HR, heart rate; , peripheral oxygen saturation; SBP, systolic blood pressure.
2.3. Preliminary screening, haematocrit and Qinghai CMS score
Clinical examination was performed during a preliminary screening session and general health information was collected. During this session, an ECG (Quark C12x, Cosmed, Albano Laziale, Italy) and spirometry (Pony FX, Cosmed, Albano Laziale, Italy) were performed, and pulse O2 saturation () and heart rate (HR) were measured using a Nellcor N‐560 oximeter (Nellcor Puritan Bennet Inc., Pleasanton, CA, USA), and systolic and diastolic blood pressure (SBP and DBP, respectively) using a validated oscilometric device (UA‐767Plus, A&D, Tokyo, Japan; Verdecchia et al., 2004).
For screening purposes, haematocrit was determined from duplicate micro‐centrifuged blood samples obtained from a fingertip capillary blood draw. Participants with haematocrit ≥63% (equivalent to [Hb] ≥ 21 g/dl) were classified as individuals with EE according to the international consensus for high‐altitude chronic diseases and Qinghai score for CMS questionnaires that were used (Leon‐Velarde et al., 2005). CMS score determines the absence or presence and severity of the syndrome, and is based on the occurrence of EE and the presence and severity of the following signs and symptoms: headache, shortness of breath or palpitations, sleep disturbances, paresthesia, cyanosis, dilated veins, and tinnitus. CMS was diagnosed if the score was ≥6 (Leon‐Velarde et al., 2005).
2.4. Blood samples
Two 9‐ml blood samples were taken from the antecubital vein of all participants under fasting conditions on the morning of the preliminary cardiopulmonary exercise test (CPET) at baseline and after 4 and 8 weeks of ET. Blood samples were collected in clot‐activator and EDTA‐coated tubes. A micro‐capillary sample was obtained from the latter to determine venous haematocrit by centrifugation at 16,000 g for 10 min. Samples for blood analyses were centrifuged at 2750 g for 20 min to obtain serum or plasma, which were immediately stored at −20°C, and then at −80°C for storage and later analysis. Glucose, insulin, lipid profile, erythropoietin (EPO), and iron profile were measured in serum samples obtained after 10–12 h of fasting (MedLab Clinical Laboratory, Lima, Peru) at baseline and after 8 weeks of ET. The concentration of free plasma haptoglobin as a proxy for haemolysis was measured by enzyme‐linked immunosorbent assay according to the manufacturer's recommendations (Human Haptoglobin ELISA Kit ab108856, Abcam, Cambridge, UK) at baseline and after 4 and 8 weeks of ET. The homeostatic model assessment for insulin resistance (HOMA‐IR) index was calculated using fasting glucose and insulin values (HOMA2 Calculator v2.2.3, University of Oxford).
2.5. Ambulatory blood pressure monitoring
Ambulatory blood pressure monitoring (ABPM) is currently recognised by international guidelines as a key instrument for out‐of‐office blood pressure (BP) measurement and in the diagnosis and management of hypertension (Whelton et al., 2018; Williams et al., 2018). Available evidence indicates that ABPM may be superior to conventional office BP measurements allowing a more accurate assessment of the actual daily life BP (Bilo et al., 2020; Parati et al., 2008).
Twenty‐four‐hour ABPM was performed using a validated oscillometric device (TM‐2430; A & D; Palatini et al., 1998) applied on the non‐dominant arm at baseline and after 8 weeks of ET. Measurements took place every 15 min during daytime (06.00–23.00 h) and every 20 min during night‐time (23.00–06.00 h). Participants were asked to stay still during the recordings and keep a standardised activity journal. Valid ABPM recordings were those with at least 70% of expected readings available and which did not contain two or more consecutive hours without valid readings. Variables obtained from the recordings were systolic, diastolic and mean daytime (awake), night‐time (sleep) and 24‐h blood pressure. ABPM thresholds for hypertension were 135/85 mmHg for daytime, 120/70 mmHg for night‐time and 130/80 mmHg for 24 h (Whelton et al., 2018; Williams et al., 2018).
2.6. Cardiopulmonary exercise test
Participants performed a maximal cardiopulmonary exercise test (CPET) in Cerro de Pasco, Peru (4340 m) according to the guidelines of the American Thoracic Society/American College of Chest Physicians (2003). Earlobe and finger oximeter probes (Nellcor N‐560 oximeter, Nellcor Puritan Bennet Inc.) were used to obtain measurements throughout the test. A tight‐fitting silicone oro‐nasal facemask (V2 series 7450 TM, Hans Rudolph, Shawnee, KS, USA) was secured and connected to an ergoespirometer‐metabolic system (Quark CPET, Cosmed, Albano Laziale, Italy). CPET was performed on a cycle ergometer (Ergomedic 828E, Monark Exercise AB, Vansbro, Sweden) with continuous breath‐by‐breath measurements of respiratory parameters to determine O2 consumption (), CO2 production (), minute ventilation (), and end‐tidal and ( and , respectively). Twelve‐lead ECG and HR were recorded continuously (Quark C12x, Cosmed, Albano Laziale, Italy). SBP and DBP during exercise was measured non‐invasively using sphyngomanometry at rest and then during the final minute of each workload increment. Participants performed a maximal preliminary CPET with a step‐incremental protocol at the beginning of the study to determine their aerobic capacity or peak (). In a later session, a baseline five‐step CPET at 25%, 50%, 75% and 100% of each participant's pre‐determined was performed. All measurements were repeated after 4 and 8 weeks of ET.
2.7. Sub‐maximal aerobic exercise training
The training scheme consisted of sessions every other day with 2 days in a row weekly (4 days/week) to complete 32 sessions in 8 weeks. The protocol allowed one session/week as the maximum number of absences. Sub‐maximal aerobic ET consisted of pedalling exercise in a cycle‐ergometer at 60% of for 1 h/day. After 4 weeks, a maximal CPET was performed to readjust 60% of for the following 4 weeks. Training sessions were supervised by research personnel to ensure a proper workload (load and cadence) and duration. Each participant had a resting period of ∼48 h before performing CPET, and the day of the test counted as a training session.
, HR and in‐office SBP and DBP were measured in duplicate after 5 min of rest once the participant was seated on the cycle ergometer before each session and immediately after. No modification of diet or additional exercise activity was advised.
2.8. Sample size and statistical analysis
Assuming comparable differences and corresponding large effect sizes previously observed in haematocrit (η 2 = 0.72) and CMS score (η 2 > 1) in studies of CMS management with acetazolamide (Richalet et al., 2008), our primary end‐outcome variables after 8 weeks of ET in the present study required a sample size of eight participants in order to achieve a power of 0.80 at P < 0.05.
After testing for normality of data, repeated‐measures one‐way ANOVA or Friedman's test followed by Tukey's or Dunn's multiple comparisons, respectively, were performed. In the case of ABPM and blood markers, comparisons (baseline vs. 8 weeks) were made using Student's paired t‐test or Wilcoxon matched‐pairs signed rank test. All statistical tests were performed using GraphPad Prism version 9.1.2 for Windows (GraphPad Software, San Diego, CA, USA). Values are presented as means ± SD throughout.
3. RESULTS
3.1. General clinical screening
Table 1 shows the general characteristics of study participants. All showed typical CMS haematocrit and Qinghai score values (Leon‐Velarde et al., 2005), as well as marked hypoxaemia.
3.2. Sub‐maximal aerobic training sessions
Compliance to the training schedule, defined as the frequency of attendance to supervised ET sessions, had a rate of 96.8% (3.8 ± 0.27 sessions/week or 30.3 ± 0.97 out of 32 sessions/8 weeks). Before and after each ET session, and HR at rest remained unchanged throughout the full 8‐week ET period compared to baseline measurements (P = 0.94). After 4 weeks, training workload was adjusted from its baseline value of 100 ± 15 to 118 ± 15 W to maintain 60% of maximal effort. There was no significant change in body weight or body mass index.
3.3. Haematocrit and Qinghai CMS score
Baseline venous haematocrit (70.6 ± 5.3%) decreased significantly by 5% and 7% (P < 0.05) on week 4 (to 66.8 ± 5.9%) and 8 (to 65.6 ± 6.6%), respectively, while Qinghai CMS score also showed a significant reduction to 4.8 ± 1.3 and 3.5 ± 0.8, respectively (P < 0.05) (Figure 1).
FIGURE 1.
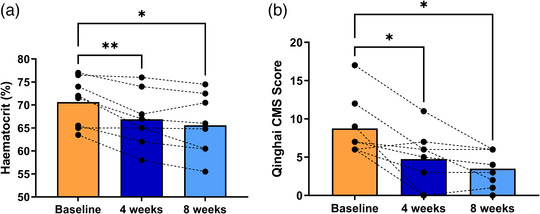
Effect of aerobic exercise training on haematocrit and CMS score. (a) Haematocrit at baseline and after 4 and 8 weeks of ET in highlanders with CMS. (b) The same comparison for the Qinghai CMS score. Bars represent means, and repeated measures of each participant are connected by dashed lines. Changes in haematocrit and CMS score were assessed employing repeated measures one‐way ANOVA and Tukey's multiple comparison test. *P < 0.05, **P < 0.01 vs. baseline
3.4. Cardiopulmonary exercise test
Ambient conditions during CPET sessions were stable with barometric pressure of 456 ± 0.3 mmHg, mean room temperature of 20 ± 0.3°C and relative humidity of 61 ± 0.7%.
Table 2 shows CPET measurements together with and BP values at rest before the incremental exercise protocol and during peak exercise at baseline and after 4 and 8 weeks.
TABLE 2.
Cardiopulmonary exercise test
| Parameter | Baseline | 4 weeks | 8 weeks |
|---|---|---|---|
| Rest | |||
| (ml/kg/min) | 4.55 ± 0.6 | 5.05 ± 0.9 | 4.69 ± 1.0 |
| (ml/kg/min) | 4.62 ± 0.7 | 5.21 ± 1.1 | 4.58 ± 1.2 |
| (l/kg/min) | 0.17 ± 0.03 | 0.19 ± 0.1 | 0.17 ± 0.03 |
| (mmHg) | 31.2 ± 2.3 | 31.5 ± 1.8 | 29.5 ± 2.2 |
| (mmHg) | 53.6 ± 3.3 | 53.2 ± 2.3 | 54.2 ± 1.1 |
| R f (bpm) | 17.6 ± 2.1 | 17.5 ± 2.4 | 17.1 ± 2.7 |
| HR (bpm) | 81.9 ± 11.3 | 77.7 ± 8.5 | 72.1 ± 2.8 |
| (%) | 84.3 ± 2.8 | 85.2 ± 3.3 | 85.4 ± 2.1 |
| SBP (mmHg) | 115.8 ± 10.8 | 110.1 ± 11.1 | 109.8 ± 14.6 |
| DBP (mmHg) | 70.6 ± 8.1 | 67.5 ± 7.1 | 66.1 ± 9.6 |
| Peak exercise | |||
| (ml/kg/min) | 33.8 ± 6.7 | 36.4 ± 5.4 | 37.2 ± 5.6* |
| (ml/kg/min) | 40.7 ± 7.4 | 42.2 ± 5.7 | 42.6 ± 5.8 |
| (l/kg/min) | 1.8 ± 0.5 | 1.9 ± 0.5 | 2.0 ± 0.4 |
| (mmHg) | 24.8 ± 4.1 | 24.4 ± 2.8 | 23.6 ± 2.5 |
| (mmHg) | 63.3 ± 3.0 | 63.0 ± 2.0 | 63.1 ± 1.5 |
| R f (bpm) | 52.3 ± 8.8 | 57.4 ± 9.6 | 56.6 ± 6.8 |
| HR (bpm) | 153.4 ± 22.4 | 164.9 ±19.1 | 169.5 ± 16.5* |
| (%) | 80.1 ± 3.5 | 81.8 ± 3.9 | 79.6 ± 2.4 |
| SBP (mmHg) | 156.3 ± 17.5 | 140.0 ± 10.4** | 148.3 ± 15.8 |
| DBP (mmHg) | 89.3 ± 2.9 | 81.4 ± 5.9* | 86.0 ± 4.8 |
| Workload (W) | 172.5 ± 26.6 | 198.8 ± 27.5*** | 210.0 ± 27.8** |
All values are presented as means ± SD. Differences were assessed using repeated measures one‐way ANOVA or Friedman tests followed by Tukey's or Dunn's multiple comparisons, respectively.
* P < 0.05, ** P < 0.01 vs. baseline. Abbreviations : , CO2 production; , O2 consumption; , pulmonary ventilation; , end‐tidal CO2 partial pressure; , end‐tidal O2 partial pressure; R f, respiratory rate; HR, heart rate; , peripheral oxygen saturation; SBP, systolic blood pressure; DBP, diastolic blood pressure.
After 4 weeks, a maximal exercise test was repeated to readjust 60% of for the following 4 weeks. increased after 8 weeks (33.8 ± 2.4 vs. 37.2 ± 2.0 ml/min/kg, P < 0.05; Figure 2a), and maximum baseline workload (172.5 ± 9.4 W) also increased significantly at week 4 and 8 (198.9 ± 9.7 and 210.0 ± 27.8 W, P < 0.01, respectively; Figure 2b). Peak HR (HRpeak) showed an increase at week 8 compared to baseline values (Table 2 and Figure 2c). While at rest SBP and DBP showed no change over 8 weeks of ET, both showed a significant fall at peak exercise at week 4 (P < 0.05), but no statistically significant reduction after 8 weeks.
FIGURE 2.
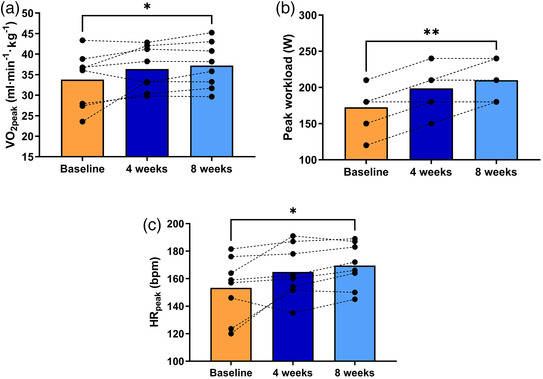
Effect of exercise training on aerobic capacity, peak workload and peak heart rate. (a) Aerobic capacity () at baseline and after 4 and 8 weeks of ET in highlanders with CMS. (b) The corresponding measurements of baseline peak workload (Wpeak) and its change after 4 and 8 weeks of ET. Individual values overlap due to similar workload of participants at each time point. (c) The same comparison of baseline, 4 and 8 weeks for peak HR (HRpeak). Bars represent means, and repeated measures of each participant are connected by dashed lines. Changes in , workload and HR at peak exercise were assessed employing repeated measures one‐way ANOVA and Tukey's multiple comparison test. *P < 0.05, **P < 0.01 vs. baseline
3.5. Ambulatory blood pressure monitoring
ABPM results are shown in Table 3. ABPM parameters showed no differences after 8 weeks of aerobic ET compared to baseline measurements.
TABLE 3.
Ambulatory blood pressure monitoring
| Parameter | Baseline | 8 weeks |
|---|---|---|
| 24 h | ||
| SBP (mmHg) | 115.3 ± 6.6 | 120.1 ± 9.2 |
| DBP (mmHg) | 71.7 ± 3.7 | 74.4 ± 3.5 |
| MAP (mmHg) | 85.9 ± 4.3 | 89.1 ± 5.3 |
| HR (bpm) | 76.7 ± 5.5 | 76.1 ± 5.5 |
| Daytime | ||
| SBP (mmHg) | 117.0 ± 5.8 | 123.0 ± 10.8 |
| DBP (mmHg) | 73.4 ± 4.0 | 76.4 ± 5.1 |
| MAP (mmHg) | 87.7 ± 3.9 | 91.9 ± 6.5 |
| HR (bpm) | 76.4 ± 8.5 | 75.9 ± 7.8 |
| Sleep | ||
| SBP (mmHg) | 105.4 ± 11.8 | 104.4 ± 13.7 |
| DBP (mmHg) | 63.4 ± 7.4 | 65.1 ± 6.0 |
| MAP (mmHg) | 77.0 ± 8.5 | 77.9 ± 8.4 |
| HR (bmp) | 72.9 ± 9.2 | 71.6 ± 10.0 |
All values are presented as means ± SD. Comparisons were made using paired t‐test or Wilcoxon matched‐pairs signed rank test. Abbreviations: DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial blood pressure; SBP, systolic blood pressure.
3.6. Cardiometabolic risk, erythropoietic and haemolysis blood markers
Average glycaemia, insulinaemia or HOMA‐IR index values, as well as EPO and iron profile markers, showed no differences after 8 weeks of ET. Lipid profile analyses showed no differences except for an increase in high‐density lipoprotein cholesterol (HDL‐C) at the end of the study (Table 4).
TABLE 4.
Cardiometabolic risk and erythropoietic blood markers
| Cardiometabolic risk blood markers | Baseline | 8 weeks |
|---|---|---|
| Glucose (mg/dl) | 101.8 ± 28.5 | 96.4 ± 18.4 |
| Insulin (μIU/ml) | 10.3 ± 6.7 | 10.4 ± 9.5 |
| HOMA‐IR (%) | 1.5 ± 0.8 | 1.4 ± 1.1 |
| Total cholesterol (mg/dl) | 166.1 ± 45.9 | 161.4 ± 49.2 |
| HDL‐C (mg/dl) | 39.0 ± 9.3 | 43.8 ± 9.9* |
| LDL‐C (mg/dl) | 82.6 ± 49.9 | 89.2 ± 45.6 |
| Triglycerides (mg/dl) | 222.8 ± 160.3 | 142.4 ± 62 |
| Erythropoietic blood markers | ||
|---|---|---|
| EPO (mIU/ml) | 14.31 ± 8.0 | 20.43 ± 14.6 |
| Iron (mg/dl) | 108.4 ± 29.6 | 120.7 ± 38.1 |
| Ferritin (mg/dl) | 89.3 ± 64.9 | 112.5 ± 90.1 |
| Transferrin (mg/dl) | 305.1 ± 30.0 | 302.9 ± 25.8 |
All values are presented as means ± SD. Comparisons were made using paired t‐test or Wilcoxon matched‐pairs signed rank test.
P < 0.05 vs. baseline. Abbreviations: EPO, erythropoietin; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment for insulin resistance; LDL‐C, low‐density lipoprotein cholesterol.
At baseline, free haptoglobin showed a negative correlation with haematocrit (r = 0.72, P < 0.05, Figure 3a) which lost statistical significance after 4 (r = −0.65, P = 0.08) and 8 (r = −0.51, P = 0.20) weeks of ET once haematocrit decreased. Free plasma haptoglobin concentration showed a non‐significant trend to increase from baseline to week 8 (P = 0.07, Figure 3b).
FIGURE 3.
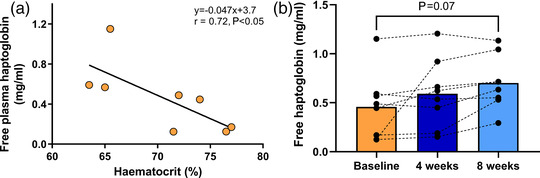
Effect of exercise training on free plasma haptoglobin. (a) The significant inverse correlation between free plasma haptoglobin and haematocrit in highlanders with CMS at baseline. (b) Free plasma haptoglobin at baseline and after 4 and 8 weeks of ET with a tendency to increase in the latter (P = 0.07). Bars represent means, and repeated measures of each participant are connected by dashed lines. Levels of free plasma haptoglobin were assessed employing repeated measures one‐way ANOVA and Tukey's multiple comparison test
4. DISCUSSION
We showed that sub‐maximal aerobic ET for 8 weeks reduces haematocrit and ameliorates CMS signs and symptoms. Despite some evidence regarding diminished exercise capacity (Winslow & Monge‐C, 1987; Winslow et al., 1985), or exercise being counterproductive for highlanders with CMS (Pratali et al., 2012; Soria et al., 2019; Stuber et al., 2010), we show that an exercise programme at 60% of maximal effort for 4 days a week during 8 weeks is a potential management approach for CMS.
Bloodletting and haemodilution have been the traditional management strategies for CMS at high altitude (Klein, 1983; Sedano et al., 1988; Winslow & Monge‐C, 1987; Winslow et al., 1985). Interestingly, signs and symptoms resume within hours when haematocrit is reduced by these methods despite environmental hypoxia, showing that CMS symptomatology is secondary to EE. Pharmacological treatment strategies have been used to reduce haematocrit either by reducing the erythropoietic stimulus or erythropoiesis itself. These interventions included angiotensin converting enzyme inhibitors (Plata et al., 2002; Vargas et al., 1996), dopaminergic antagonists (Leon‐Velarde et al., 2003) and ventilatory stimulants such as medroxyprogesterone (Kryger et al., 1978) and almitrine (Villena et al., 1985). However, only a few studies have shown evidence for safety and efficacy in the treatment of CMS. The most recent and longer‐term randomised controlled trials with clinical significance used acetazolamide, a systemic carbonic anhydrase inhibitor, as a potential treatment of the syndrome. Two randomised, double‐blind, placebo‐controlled studies assessed the safety and efficacy of acetazolamide treatment for up to 6 months in CMS patients in Cerro de Pasco, Peru (Richalet et al., 2008; Richalet et al., 2005). Results showed that acetazolamide increased , decreased serum EPO and decreased haematocrit by 5%. Treatment also decreased pulmonary vascular resistance, increased nocturnal and reduced sleep‐disordered breathing episodes. Overall, acetazolamide reduced hypoventilation, blunted erythropoiesis and improved pulmonary circulation without adverse effects throughout the duration of the trials. Although its implementation as a treatment appears efficient and safe, longer trials would be required to assess any development of tolerance or potential long‐term consequences of the chronic inhibition of carbonic anhydrase in the different organs and systems. For this reason, non‐pharmacological approaches are an interesting avenue to explore, especially in resource‐constrained populations.
Native highlanders who exercise regularly, or highly trained highlander athletes, have significantly lower haematocrit values compared to the sedentary population at the same altitude. We showed that 8‐week submaximal aerobic ET reduced haematocrit by 7% and ameliorated CMS signs and symptoms significantly, reducing CMS score down to values usually observed in the healthy highlander population. Our results paralleled those obtained with acetazolamide treatment, but without the improvement on . In addition, we did not detect any significant change in or . The reduction of haematocrit might be explained by the expansion of plasma volume (PV) and the consequent haemodilution, as this has been well documented in both cross‐sectional and longitudinal endurance ET studies (Convertino, 2007; Schmidt & Prommer, 2008). PV expansion can account for nearly all of the ET‐induced hypervolaemia up to 2–4 weeks; after this time expansion may be distributed equally between plasma and red cell volumes (Convertino, 2007). Hypervolaemia may provide larger vascular volume and filling pressure for greater cardiac stroke volume, and thus cardiac output () during exercise. This, together with greater ET‐induced O2 extraction and increased muscle mitochondrial oxidative capacity (Skattebo et al., 2020) might also contribute to the increased aerobic capacity after 8 weeks of ET despite decreased haematocrit and unchanged . In addition, we have recently shown that aerobic capacity is supported by adrenergic and non‐adrenergic vasoconstriction of non‐active skeletal muscle in individuals with CMS, which likely aids in central redistribution of blood volume during exercise (Hansen et al., 2021). Also, heightened α‐adrenergic signalling restrains vasodilatation within active skeletal muscle to better match O2 delivery (Hansen et al., 2021).
Interestingly, lower haematocrit (lower Hb concentration) might also contribute to increased O2 diffusion and O2 extraction between muscle microcirculatory vessels and mitochondria (Wagner, 1996). When Hb concentration is reduced, time to diffusive equilibration in these vascular beds is shortened. Piiper & Scheid (1981) showed that the compound constant D/(β) determined the degree of diffusion equilibration to be expected in muscle tissue, where D is muscle diffusion coefficient and β is the (average) slope of the O2–Hb dissociation curve. As β must decrease when total Hb concentration decreases, D/(β) must rise, even taking into account a modest increase in , diffusion equilibration would take less time. As a result muscle O2 extraction increases when Hb concentration is lower, and overall O2 transport is improved, counterbalancing the effect of decreased arterial O2 content.
ET‐induced PV and blood volume (BV) expansion together with the reduction of haematocrit are also associated with increased serum EPO levels. Montero et al. (2017) studied the changes in PV, BV and overnight fasting haematological markers before and at 2, 4 and 8 weeks of supervised ET consisting of three to four 60‐min (50–60% of maximal power output) cycloergometry sessions per week for 8 weeks. Increases in PV and BV were observed in week 2 (+16%) and remained stable through week 8 (+14%). Total red blood cell volume (RBCV) increased by 6% in week 4 and 12% by week 8, while overall BV increased by 13% from week 0 to 8. Hematocrit decreased 3% (−7%) in week 2 and remained somewhat reduced at week 8 (−3%). This reduction is slightly less to what we found in our study after 4 (−5%) and 8 weeks (−7%) of ET possibly because of the much higher haematocrit of CMS highlanders which might favour haemolysis due to mechanical stress. Montero and colleagues also showed that overnight‐fasting EPO concentration increased by 25% in week 2, and was no longer different from baseline (week 0) at week 4 and 8. Our results shows that serum EPO exhibited no significant differences at week 4 and 8 compared to baseline, suggesting that we might have lost the earlier window (i.e., 2 weeks) where ET‐induced plasma expansion and serum EPO are maximal. Nevertheless, the fact that EPO remains unaltered during 8 weeks of training suggest that the erythropoietic stimulus is minor. Overall, despite ET‐induced increased RBCV, plasma expansion overrides any increase in red blood cell mass and results in reduced haematocrit. In the case of CMS highlanders this reduction can be magnified by ET‐induced mechanical haemolysis due to excessive haematocrit levels (Macarlupu et al., 2021; Telford et al., 2003). In fact, we have recently shown that haemolysis might be an additional mechanism for ET‐induced haematocrit reduction in a rat model of high‐altitude erythrocytosis (Macarlupu et al., 2021). Haemolysis due to mechanical stress might lead to a reduction in haematocrit without significant modification of PV or total blood volume (Schobersberger et al., 1990; Selby & Eichner, 1986; Telford et al., 2003). This mechanism is particularly expected to be relevant in impact sports such as running. However, there is also evidence of haemolysis induced by exercise in disciplines where impact is reduced or absent (Lippi & Sanchis‐Gomar, 2019; Schobersberger et al., 1990; Selby & Eichner, 1986). Haemolysis would occur to a greater degree when blood shows a higher viscosity, and therefore it would be favoured when haematocrit is excessive. As haptoglobin binds and combines with free plasma Hb, our finding of an inverse relationship of free haptoglobin and haematocrit at baseline suggests a steady‐state background haemolysis due to EE. However, this relationship is lost after 4 and 8 weeks of ET once haematocrit decreased, and total plasma free haptoglobin showed a trend to rise suggesting a marginal increased haemolysis consequent to ET.
One other important effect of ET is the reduction of BP. Studies at sea‐level have shown that aerobic ET for 8 weeks (30‐min session; 3 times/week at 80–90% of ventilatory threshold) decreases 24‐h SBP and DBP, with the largest reduction mainly at night‐time (Carvalho et al., 2009). However, our results show no reduction in office‐based BP or ABPM at rest after 8 weeks of aerobic ET. This lack of change might be explained by a possible concurrent effect of the autonomic characteristics of CMS highlanders, the ET‐induced expansion of PV and the ET‐induced drop in peripheral vascular resistance (Fagard, 2006). We have recently shown that in comparison with healthy Andean highlanders, CMS individuals show lower basal sympathetic vasoconstrictor drive with enhanced cardiovagal baroreflex gain (Moore et al., 2006; Simpson et al., 2021), which might compensate for the haemodynamic consequences of EE and a possible ET‐induced expanded blood volume. Such changes appear to be adaptive physiological responses to the elevated haematocrit, which allow BP homeostasis to be maintained. We have also shown that Andeans with mild‐to‐moderate erythrocytosis have a substantial reduction in the peripheral vascular resistance given the prevailing sympathetic activity, which is likely related to their lower basal vascular tone. Ultimately, vascular phenotypic adaptations in CMS highlanders, in both conduit artery and downstream arterioles, allow the maintenance of local and total vascular resistances, resting BP and vascular shear patterns (Hansen et al., 2021). This effect is probably augmented by ET as we observed a significant drop in SBP and DBP at peak exercise after 4 weeks. Nevertheless, the expression of this phenotype is possibly modest and might be lost over time (Bilo et al., 2020; Corante et al., 2018).
A compensatory or offsetting effect on HR might be also taking place during ET. Our results show that although not statistically significant, HR at rest shows a trend of continuous reduction after 8 weeks of ET compared to baseline values. Normally, ET induces a reduction in HR and the effect occurs after only a few months, with about three training sessions per week (Genovesi et al., 2007; Whyte et al., 2008; Zavorsky, 2000). Meta‐analyses on the effect of ET over HR indicate that endurance training decreases the resting HR between 4 and 6 bmp (Huang et al., 2005; Reimers et al., 2018). Similarly, HRpeak is reduced following regular aerobic exercise in sedentary adults and endurance athletes. The overall effect of aerobic training on HRpeak is moderate as it can be altered by 3–7% (Zavorsky, 2000). It is possible that severe hypoxaemia and basal enhanced sympathetic activity in CMS highlanders prevent the full reduction of resting HR in CMS and may lead to augment HR at peak exercise.
Lastly, aerobic ET is also associated to improved cardiometabolic risk profile (Lin et al., 2015). In previous studies we have identified independent associations between EE and 24 h ambulatory hypertension including systolic–diastolic and isolated diastolic hypertension (Bilo et al., 2020; Corante et al., 2018), and a significant proportion of masked hypertension, the latter linked to increased cardiovascular morbidity and mortality in lowlanders (Pierdomenico & Cuccurullo, 2011). We have also identified independent associations between EE, insulin resistance, hyperglycaemia, and dyslipidaemia. These findings agree with other studies at high altitude in the Peruvian Andes that have identified independent relationships between EE and hypertension, hypertriglyceridaemia (Gonzales & Tapia, 2013; Jefferson et al., 2002; Leon‐Velarde & Arregui, 1994) and metabolic syndrome (De Ferrari et al., 2014). Several RCTs have shown reductions in blood triglycerides, glucose and insulin, and increases in HDL‐C as the most common biomarker changes following ET programmes (Lin et al., 2015). Circulating HDL‐C levels are usually significantly increased with ET in a manner that is dependent on the duration and intensity of the training plan (Durstine et al., 2001; Kodama et al., 2007). Our results showed a significant increase in HDL‐C, a trend to triglyceride reduction and no changes in glycaemic control markers. Studies with training plans from 4 to 104 weeks with mild or moderate intensity reported changes in some cardiometabolic marker levels (Lin et al., 2015), such as an increase in HDL‐C even as early as 4 weeks of ET (Banz et al., 2003; Cox et al., 1993; Haskell, 1984; Kraus et al., 2002; LeMura et al., 2000). On the other hand, other blood lipid, as well as glycaemic control markers, frequently tend to improve over a period ≥12 weeks of ET (Cho et al., 2011; Libardi et al., 2012; Watkins et al., 2003) suggesting that possibly the length of our study was not enough to observe such changes.
4.1. Limitations
One main limitation of our study is that although it is difficult to maintain a group of participants training for 8 weeks, the protocol might have been controlled with the inclusion of CMS highlanders without ET and/or a group of non‐CMS highlanders subjected to a similar ET programme. Either of the two comparisons would have been interesting and informative. However, we believe that this does not preclude the conclusions of our study as each participant was their own baseline control and the main aim of the study was to investigate whether ET can reduce haematocrit and alleviate symptoms in CMS highlanders.
The inclusion of male highlanders only is an additional limitation of our study. CMS has a very low prevalence in pre‐menopausal women (Azad et al., 2021; Leon‐Velarde et al., 1997; Leon‐Velarde et al., 2001), and therefore forming comparable groups covering the same age range to avoid any confounding effects of age, and of menopause itself, would have been difficult. For these and cultural reasons, women were not included in the study and therefore our findings cannot be extrapolated to female CMS highlanders.
4.2. Conclusions
In conclusion, we show that sub‐maximal aerobic ET can effectively reduce haematocrit and ameliorate symptoms in CMS patients. In addition, despite the common belief of reduced tolerance to exercise in CMS highlanders, we show that ET can successfully improve aerobic capacity and exercise workload in these patients maintaining BP homeostasis and improving cardiovascular disease risk markers such as HDL‐C. Overall, our results suggest that a regular aerobic ET might be used as a low‐cost non‐invasive and non‐pharmacological practical management strategy for CMS.
COMPETING INTERESTS
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
J.L.M. and F.C.V. conceived and designed the research; R.F.‐M., G.V.‐G., J.L.M. and F.C.V. performed the experiments and analysed the data; J.L.M. and F.C.V. interpreted results of the experiments, prepared the figures and drafted the manuscript; J.L.M., R.F.‐M., G.V.‐G., J.‐P.R., N.V. and F.C.V. edited and revised the manuscript. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Supporting information
Statistical Summary Document
ACKNOWLEDGMENTS
This work was supported by a Wellcome Trust grant 107544/Z/15/Z to F.C.V. J.L.M. was supported by a scholarship from the Franco‐Peruvian Doctoral School for Life Sciences (EDFPCV) funded by the Institut de Recherché pour le Développement (IRD), the French Embassy in Perú, and Universidad Peruana Cayetano Heredia (UPCH).
Macarlupú, J. L. , Vizcardo‐Galindo, G. , Figueroa‐Mujíca, R. , Voituron, N. , Richalet, J.‐P. , & Villafuerte, F. C. (2021). Sub‐maximal aerobic exercise training reduces haematocrit and ameliorates symptoms in Andean highlanders with chronic mountain sickness. Experimental Physiology, 106, 2198–2209. 10.1113/EP089975
Edited by: Lauro Vianna
Linked articles: This article is highlighted in a Viewpoint article by Teixeira & Lang. To read this paper, visit https://doi.org/10.1113/EP090070.
DATA AVAILABILITY STATEMENT
Datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- American College of Sports Medicine (2010). ACSM'S Guidelines for Exercise Testing and Prescription (8th ed.), Wolters Kluwer/Lippincott Williams & Wilkins. [Google Scholar]
- American Thoracic Society/American College of Chest Physicians . (2003). ATS/ACCP Statement on cardiopulmonary exercise testing. American Journal of Respiratory and Critical Care Medicine, 167(2), 211–277. 10.1164/rccm.167.2.211 [DOI] [PubMed] [Google Scholar]
- Azad, P. , Villafuerte, F. C. , Bermudez, D. , Patel, G. , & Haddad, G. G. (2021). Protective role of estrogen against excessive erythrocytosis in Monge's disease. Experimental & Molecular Medicine, 53(1), 125–135. 10.1038/s12276-020-00550-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banz, W. J. , Maher, M. A. , Thompson, W. G. , Bassett, D. R. , Moore, W. , Ashraf, M. , Keefer, D. J. , &. Zemel, M. B. (2003). Effects of resistance versus aerobic training on coronary artery disease risk factors. Experimental Biology and Medicine, 228(4), 434–440. 10.1177/153537020322800414 [DOI] [PubMed] [Google Scholar]
- Bilo, G. , Acone, L. , Anza‐Ramirez, C. , Macarlupu, J. L. , Soranna, D. , Zambon, A. , Vizcardo‐Galindo, G. , Pengo, M. F. , Villafuerte, F. C. , & Parati, G. (2020). Office and ambulatory arterial hypertension in highlanders. HIGHCARE‐ANDES highlanders study. Hypertension, 76(6), 1962–1970. 10.1161/HYPERTENSIONAHA.120.16010 [DOI] [PubMed] [Google Scholar]
- Bonilla, J. , Narvaez, R. , & Chuaire, L. (2005). Sports as a cause of oxidative stress and hemolysis. Colombia Médica, 36(4), 215–280. [Google Scholar]
- British Thoracic Society Standards of Care Subcommittee on Pulmonary Rehabilitation . (2001). Pulmonary rehabilitation. Thorax, 56(11), 827–834. 10.1136/thorax.56.11.827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho, V. O. , Ciolac, E. G. , Guimaraes, G. V. , & Bocchi, E. A. (2009). Effect of exercise training on 24‐hour ambulatory blood pressure monitoring in heart failure patients. Congestive Heart Failure, 15(4), 176–180. 10.1111/j.1751-7133.2009.00093.x [DOI] [PubMed] [Google Scholar]
- Cho, J. K. , Lee, S. H. , Lee, J. Y. , & Kang, H. S. (2011). Randomized controlled trial of training intensity in adiposity. International Journal of Sports Medicine, 32(6), 468–475. 10.1055/s-0031-1271789 [DOI] [PubMed] [Google Scholar]
- Convertino, V. A. (2007). Blood volume response to physical activity and inactivity. American Journal of the Medical Sciences, 334(1), 72–79. 10.1097/MAJ.0b013e318063c6e4 [DOI] [PubMed] [Google Scholar]
- Corante, N. , Anza‐Ramirez, C. , Figueroa‐Mujica, R. , Macarlupu, J. L. , Vizcardo‐Galindo, G. , Bilo, G. , Parati, G. , Gamboa, J. L. , León‐Velarde, F. , & Villafuerte, F. C. (2018). Excessive erythrocytosis and cardiovascular risk in Andean highlanders. High Altitude Medicine & Biology, 19(3), 221–231. 10.1089/ham.2017.0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornolo, J. , Brugniaux, J. V. , Macarlupu, J. L. , Privat, C. , Leon‐Velarde, F. , & Richalet, J. P. (2005). Autonomic adaptations in Andean trained participants to a 4220‐m altitude marathon. Medicine and Science in Sports and Exercise, 37(12), 2148–2153. 10.1249/01.mss.0000179901.19280.85 [DOI] [PubMed] [Google Scholar]
- Cox, K. L. , Puddey, I. B. , Morton, A. R. , Beilin, L. J. , Vandongen, R. , & Masarei, J. R. (1993). The combined effects of aerobic exercise and alcohol restriction on blood pressure and serum lipids: A two‐way factorial study in sedentary men. Journal of Hypertension, 11(2), 191–201. 10.1097/00004872-199302000-00012 [DOI] [PubMed] [Google Scholar]
- De Ferrari, A. , Miranda, J. , & Gilman, R. (2014). Prevalence, clinical profile, iron status, and subject‐specific traits for excessive erythrocytosis in Andean adults living permanently at 3825 meters above sea level. Chest, 146(5), 1327–1336. 10.1378/chest.14-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstine, J. L. , Grandjean, P. W. , Davis, P. G. , Ferguson, M. A. , Alderson, N. L. , & DuBose, K. D. (2001). Blood lipid and lipoprotein adaptations to exercise: A quantitative analysis. Sports Medicine, 31(15), 1033–1062. 10.2165/00007256-200131150-00002 [DOI] [PubMed] [Google Scholar]
- Fagard, R. H. (2006). Exercise is good for your blood pressure: effects of endurance training and resistance training. Clinical and Experimental Pharmacology & Physiology, 33(9), 853–856. 10.1111/j.1440-1681.2006.04453.x [DOI] [PubMed] [Google Scholar]
- Genovesi, S. , Zaccaria, D. , Rossi, E. , Valsecchi, M. G. , Stella, A. , & Stramba‐Badiale, M. (2007). Effects of exercise training on heart rate and QT interval in healthy young individuals: Are there gender differences? Europace, 9(1), 55–60. 10.1093/europace/eul145 [DOI] [PubMed] [Google Scholar]
- Gonzales, G. F. , & Tapia, V. (2013). [Association of high altitude‐induced hypoxemia to lipid profile and glycemia in men and women living at 4,100m in the Peruvian Central Andes]. Endocrinología y Nutrición, 60(2), 79–86. [DOI] [PubMed] [Google Scholar]
- Groepenhoff, H. , Overbeek, M. J. , Mule, M. , van der Plas, M. , Argiento, P. , Villafuerte, F. C. , Beloka, S. , Faoro, V. , Macarlupu, J. L. , Guenard, H. , de Bisschop, C. , Martinot, J. B. , Vanderpool, R. , Penaloza, D. , & Naeije, R. (2012). Exercise pathophysiology in patients with chronic mountain sickness. Chest, 142(4), 877–884. 10.1378/chest.11-2845 [DOI] [PubMed] [Google Scholar]
- Hansen, A. B. , Moralez, G. , Amin, S. B. , Simspon, L. L. , Hofstaetter, F. , Anholm, J. D. , Gasho, C. , Stembridge, M. , Dawkins, T. G. , Tymko, M. M. , Ainslie, P. N. , Villafuerte, F. , Romero, S. A. , Hearon, C. M., Jr & Lawley, J. S. (2021). Global Reach 2018: The adaptive phenotype to life with chronic mountain sickness and polycythaemia. Journal of Physiology, 599(17), 4021–4044. 10.1113/JP281730 [DOI] [PubMed] [Google Scholar]
- Haskell, W. L. (1984). The influence of exercise on the concentrations of triglyceride and cholesterol in human plasma. Exercise and Sport Sciences Reviews, 12, 205–244. [PubMed] [Google Scholar]
- Huang, G. , Shi, X. , Davis‐Brezette, J. A. , & Osness, W. H. (2005). Resting heart rate changes after endurance training in older adults: A meta‐analysis. Medicine and Science in Sports and Exercise, 37(8), 1381–1386. 10.1249/01.mss.0000174899.35392.0c [DOI] [PubMed] [Google Scholar]
- Jefferson, J. A. , Escudero, E. , Hurtado, M. E. , Kelly, J. P. , Swenson, E. R. , Wener, M. H. , Burnier, M. , Maillard, M. , Schreiner, G. F. , Schoene, R. B. , Hurtado, A. , & Johnson, R. J. (2002). Hyperuricemia, hypertension, and proteinuria associated with high‐altitude polycythemia. American Journal of Kidney Diseases, 39(6), 1135–1142. 10.1053/ajkd.2002.33380 [DOI] [PubMed] [Google Scholar]
- Juvonen, E. , Ikkala, E. , Fyhrquist, F. , & Ruutu, T. (1991). Autosomal dominant erythrocytosis caused by increased sensitivity to erythropoietin. Blood, 78(11), 3066–3069. 10.1182/blood.V78.11.3066.3066 [DOI] [PubMed] [Google Scholar]
- Klein, H. (1983). Isovolemic hemodilution in high‐altitude polycythemia. Proceedings of the International Symposium on Acclimatization, Adaptation, and Tolerance to High Altitude. National Institutes of Health. [Google Scholar]
- Kodama, S. , Tanaka, S. , Saito, K. , Shu, M. , Sone, Y. , Onitake, F. , Suzuki, E. , Shimano, H. , Yamamoto, S. , Kondo, K. , Ohashi, Y. , Yamada, N. , & Sone, H. (2007). Effect of aerobic exercise training on serum levels of high‐density lipoprotein cholesterol: A meta‐analysis. Archives of Internal Medicine, 167(10), 999–1008. 10.1001/archinte.167.10.999 [DOI] [PubMed] [Google Scholar]
- Kraus, W. E. , Houmard, J. A. , Duscha, B. D. , Knetzger, K. J. , Wharton, M. B. , McCartney, J. S. , Bales, C. W. , Henes, S. , Samsa, G. P. , Otvos, J. D. , Kulkarni, K. R. , & Slentz, C. A. (2002). Effects of the amount and intensity of exercise on plasma lipoproteins. New England Journal of Medicine, 347(19), 1483–1492. 10.1056/NEJMoa020194 [DOI] [PubMed] [Google Scholar]
- Kryger, M. , McCullough, R. E. , Collins, D. , Scoggin, C. H. , Weil, J. V. , & Grover, R. F. (1978). Treatment of excessive polycythemia of high altitude with respiratory stimulant drugs. American Review of Respiratory Disease, 117(3), 455–464. 10.1164/arrd.1978.117.3.455 [DOI] [PubMed] [Google Scholar]
- LeMura, L. M. , von Duvillard, S. P. , Andreacci, J. , Klebez, J. M. , Chelland, S. A. , & Russo, J. (2000). Lipid and lipoprotein profiles, cardiovascular fitness, body composition, and diet during and after resistance, aerobic and combination training in young women. European Journal of Applied Physiology, 82(5–6), 451–458. 10.1007/s004210000234 [DOI] [PubMed] [Google Scholar]
- Leon‐Velarde, F. , & Arregui, A. (1994). Desadaptacion a la vida en las grandes alturas. Lima: Institut Français d'études andines (IFEA). [Google Scholar]
- Leon‐Velarde, F. , Gamboa, J. , Gamboa, A. , Rivera‐Ch, M. , Macarlupu, J. L. , & Monge‐C, C. (2003). Domperidone: a possible strategy for Chronic Mountain Sickness therapy. In Viscor G., Ricart A., & Leal C., (Eds.), Health and height (pp. 57–65). Barcelona: Universitat de Barcelona. [Google Scholar]
- Leon‐Velarde, F. , Maggiorini, M. , Reeves, J. T. , Aldashev, A. , Asmus, I. , Bernardi, L. , Ge, R. L. , Hackett, P. , Kobayashi, T. , Moore, L. G. , Penaloza, D. , Richalet, J. P. , Roach, R. , Wu, T. , Vargas, E. , Zubieta‐Castillo, G. , & Zubieta‐Calleja, G. (2005). Consensus statement on chronic and subacute high altitude diseases. High Altitude Medicine & Biology, 6(2), 147–157. 10.1089/ham.2005.6.147 [DOI] [PubMed] [Google Scholar]
- Leon‐Velarde, F. , Ramos, M. A. , Hernandez, J. A. , De Idiaquez, D. , Munoz, L. S. , Gaffo, A. , Córdova, S. , Durand, D. , & Monge, C. (1997). The role of menopause in the development of chronic mountain sickness. American Journal of Physiology, 272(1 Pt 2), R90–R94. 10.1152/ajpregu.1997.272.1.R90 [DOI] [PubMed] [Google Scholar]
- Leon‐Velarde, F. , Rivera‐Ch, M. , Huicho, L. , & Villafuerte, F. C. (2014). Chronic mountain sickness. In Swenson E. R. & Bartsch P. (Eds.), High altitude human adaptation to hypoxia. New York: Springer. [Google Scholar]
- Leon‐Velarde, F. , Rivera‐Chira, M. , Tapia, R. , Huicho, L. , & Monge, C. C. (2001). Relationship of ovarian hormones to hypoxemia in women residents of 4,300 m. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 280(2), R488–R493. 10.1152/ajpregu.2001.280.2.R488 [DOI] [PubMed] [Google Scholar]
- Letcher, R. L. , Chien, S. , Pickering, T. G. , Sealey, J. E. , & Laragh, J. H. (1981). Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects. Role of fibrinogen and concentration. American Journal of Medicine, 70(6), 1195–1202. 10.1016/0002-9343(81)90827-5 [DOI] [PubMed] [Google Scholar]
- Libardi, C. A. , De Souza, G. V. , Cavaglieri, C. R. , Madruga, V. A. , & Chacon‐Mikahil, M. P. (2012). Effect of resistance, endurance, and concurrent training on TNF‐alpha, IL‐6, and CRP. Medicine and Science in Sports and Exercise, 44(1), 50–56. 10.1249/MSS.0b013e318229d2e9 [DOI] [PubMed] [Google Scholar]
- Lin, X. , Zhang, X. , Guo, J. , Roberts, C. K. , McKenzie, S. , Wu, W. C. , Liu, S. , & Song, Y. (2015). Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: A systematic review and meta‐analysis of randomized controlled trials. Journal of the American Heart Association, 4(7), e002014. 10.1161/JAHA.115.002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenfeld, J. , Weil, J. V. , Travis, V. L. , & Horwitz, L. D. (1985). Hemodynamic response to normovolemic polycythemia at rest and during exercise in dogs. Circulation Research, 56(6), 793–800. 10.1161/01.res.56.6.793 [DOI] [PubMed] [Google Scholar]
- Lippi, G. , & Sanchis‐Gomar, F. (2019). Epidemiological, biological and clinical update on exercise‐induced hemolysis. Annals of Translational Medicine, 7(12), 270. 10.21037/atm.2019.05.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarlupu, J. L. , Marchant, D. , Jeton, F. , Villafuerte, F. , Richalet, J. P. , & Voituron, N. (2021). Effect of exercise training in rats exposed to chronic hypoxia: Application for Monge's disease. Physiological Reports, 9(7), e14750. 10.14814/phy2.14750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairbaurl, H. (2013). Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Frontiers in Physiology, 4, 332. 10.3389/fphys.2013.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele, C. H. , Schwartz, A. R. , Gilman, R. H. , Pham, L. , Wise, R. A. , Davila‐Roman, V. G. , Jun, J. C. , Polotsky, V. Y. , Miranda, J. J. , Leon‐Velarde, F. , & Checkley, W. (2016). Increased cardiometabolic risk and worsening hypoxemia at high altitude. High Altitude Medicine & Biology, 17(2), 93–100. 10.1089/ham.2015.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge, C. (1943). Chronic mountain sickness. Physiological Reviews, 23(2), 166–184. [Google Scholar]
- Monge‐C, C. , Arregui, A. , & Leon‐Velarde, F. (1992). Pathophysiology and epidemiology of chronic mountain sickness. International Journal of Sports Medicine, 13(Suppl 1), S79–S81. 10.1055/s-2007-1024603 [DOI] [PubMed] [Google Scholar]
- Monge‐C, C. , Leon‐Velarde, F. , & Arregui, A. (1989). Increasing prevalence of excessive erythrocytosis with age among healthy high‐altitude miners. New England Journal of Medicine, 321(18), 1271. 10.1056/NEJM198911023211819 [DOI] [PubMed] [Google Scholar]
- Montero, D. , Breenfeldt‐Andersen, A. , Oberholzer, L. , Haider, T. , Goetze, J. P. , Meinild‐Lundby, A. K. , & Lundby, C. (2017). Erythropoiesis with endurance training: dynamics and mechanisms. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 312(6), R894–R902. 10.1152/ajpregu.00012.2017 [DOI] [PubMed] [Google Scholar]
- Moore, J. P. , Claydon, V. E. , Norcliffe, L. J. , Rivera‐Ch, M. C. , Leon‐Velarde, F. , Appenzeller, O. , & Hainsworth, R. (2006). Carotid baroreflex regulation of vascular resistance in high‐altitude Andean natives with and without chronic mountain sickness. Experimental Physiology, 91(5), 907–913. 10.1113/expphysiol.2005.033084 [DOI] [PubMed] [Google Scholar]
- Okumiya, K. , Sakamoto, R. , Fukutomi, E. , Kimura, Y. , Ishimoto, Y. , Chen, W. L. , Ishikawa, M. , Hozo, R. , Otsuka, K. , Matsubayashi, K. , Wada, T. , Inamura, T. , Lazo, M. , Lu, J. P. , & Garcia, P. J. (2011). Strong association between polycythemia and glucose intolerance in older adults living at high altitudes in the Andes. Journal of the American Geriatrics Society, 59(10), 1971–1973. 10.1111/j.1532-5415.2011.03610_8 [DOI] [PubMed] [Google Scholar]
- Okumiya, K. , Sakamoto, R. , Kimura, Y. , Ishimoto, Y. , Wada, T. , Ishine, M. , Nakajima, S. , Hozo, R. , Ge, R. L. , Norboo, T. , Otsuka, K. , & Matsubayashi, K. (2010). Strong association between polycythemia and glucose intolerance in elderly high‐altitude dwellers in Asia. Journal of the American Geriatrics Society, 58(3), 609–611. 10.1111/j.1532-5415.2010.02753.x [DOI] [PubMed] [Google Scholar]
- Ostergaard, L. (2020). Blood flow, capillary transit times, and tissue oxygenation: the centennial of capillary recruitment. Journal of Applied Physiology, 129(6), 1413–1421. 10.1152/japplphysiol.00537.2020 [DOI] [PubMed] [Google Scholar]
- Palatini, P. , Frigo, G. , Bertolo, O. , Roman, E. , Da Corta, R. , & Winnicki, M. (1998). Validation of the A&D TM‐2430 device for ambulatory blood pressure monitoring and evaluation of performance according to subjects' characteristics. Blood Pressure Monitoring, 3(4), 255–260. [PubMed] [Google Scholar]
- Parati, G. , Omboni, S. , Palatini, P. , Rizzoni, D. , Bilo, G. , Valentini, M. , Rosei, E. A. , & Mancia, G. (2008). Italian society of hypertension guidelines for conventional and automated blood pressure measurement in the office, at home and over 24 hours. High Blood Pressure & Cardiovascular Prevention, 15(4), 283–310. 10.2165/0151642-200815040-00008 [DOI] [PubMed] [Google Scholar]
- Pei, T. , Li, X. , Tao, F. , Xu, H. , You, H. , Zhou, L. , Liu, Y. , & Gao, Y. (2012). Burden of disease resulting from chronic mountain sickness among young Chinese male immigrants in Tibet. BMC Public Health, 12, 401. 10.1186/1471-2458-12-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penaloza, D. , & Arias‐Stella, J. (2007). The heart and pulmonary circulation at high altitudes: healthy highlanders and chronic mountain sickness. Circulation, 115(9), 1132–1146. 10.1161/CIRCULATIONAHA.106.624544 [DOI] [PubMed] [Google Scholar]
- Pierdomenico, S. D. , & Cuccurullo, F. (2011). Prognostic value of white‐coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. American Journal of Hypertension, 24(1), 52–58. 10.1038/ajh.2010.203 [DOI] [PubMed] [Google Scholar]
- Piiper, J. , & Scheid, P. (1981). Model for capillary‐alveolar equilibration with special reference to O2 uptake in hypoxia. Respiration Physiology, 46(3), 193–208. 10.1016/0034-5687(81)90121-3 [DOI] [PubMed] [Google Scholar]
- Plata, R. , Cornejo, A. , Arratia, C. , Anabaya, A. , Perna, A. , Dimitrov, B. D. , Remuzzi, G. , & Ruggenenti, P. (2002). Angiotensin‐converting‐enzyme inhibition therapy in altitude polycythaemia: a prospective randomised trial. Lancet, 359(9307), 663–666. 10.1016/s0140-6736(02)07812-1 [DOI] [PubMed] [Google Scholar]
- Pratali, L. , Rimoldi, S. F. , Rexhaj, E. , Hutter, D. , Faita, F. , Salmon, C. S. , Villena, M. , Sicari, R. , Picano, E. , Allemann, Y. , Scherrer, U. , & Sartori, C. (2012). Exercise induces rapid interstitial lung water accumulation in patients with chronic mountain sickness. Chest, 141(4), 953–958. 10.1378/chest.11-0084 [DOI] [PubMed] [Google Scholar]
- Reimers, A. K. , Knapp, G. , & Reimers, C. D. (2018). Effects of exercise on the resting heart rate: A systematic review and meta‐analysis of interventional studies. Journal of Clinical Medicine, 7(12). 10.3390/jcm7120503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis, A. L. , Carlin, B. W. , Carrieri‐Kohlman, V. , … Skolnick, J . (1997). Pulmonary rehabilitation: joint ACCP/AACVPR evidence‐based guidelines. Chest, 112(5), 1363–1396. 10.1378/chest.112.5.1363 [DOI] [PubMed] [Google Scholar]
- Richalet, J. P. , Rivera, M. , Bouchet, P. , Chirinos, E. , Onnen, I. , Petitjean, O. , Bienvenu, A. , Lasne, F. , Moutereau, S. , & Leon‐Velarde, F. (2005). Acetazolamide: A treatment for chronic mountain sickness. American Journal of Respiratory and Critical Care Medicine, 172(11), 1427–1433. 10.1164/rccm.200505-807OC [DOI] [PubMed] [Google Scholar]
- Richalet, J. P. , Rivera‐Ch, M. , Maignan, M. , Privat, C. , Pham, I. , Macarlupu, J. L. , Petitjean, O. , & Leon‐Velarde, F. (2008). Acetazolamide for Monge's disease: Efficiency and tolerance of 6‐month treatment. American Journal of Respiratory and Critical Care Medicine, 177(12), 1370–1376. 10.1164/rccm.200802-196OC [DOI] [PubMed] [Google Scholar]
- Schmidt, W. , Dahners, H. W. , Correa, R. , Ramirez, R. , Rojas, J. , & Boning, D. (1990). Blood gas transport properties in endurance‐trained athletes living at different altitudes. International Journal of Sports Medicine, 11(1), 15–21. 10.1055/s-2007-1024755 [DOI] [PubMed] [Google Scholar]
- Schmidt, W. , & Prommer, N. (2008). Effects of various training modalities on blood volume. Scandinavian Journal of Medicine & Science in Sports, 18(Suppl 1), 57–69. 10.1111/j.1600-0838.2008.00833.x [DOI] [PubMed] [Google Scholar]
- Schobersberger, W. , Tschann, M. , Hasibeder, W. , Steidl, M. , Herold, M. , Nachbauer, W. , & Koller, A. (1990). Consequences of 6 weeks of strength training on red cell O2 transport and iron status. European Journal of Applied Physiology and Occupational Physiology, 60(3), 163–168. 10.1007/BF00839152 [DOI] [PubMed] [Google Scholar]
- Sedano, O. , Pastorelli, J. , Gomez, A. , & Flores, V. (1988). “Sangre roja” aislada vs hemodilución isovolémica inducida en Mal de Montaña Cronico. V Congreso Nacional. X Curso Internacional de Medicina Interna, Lima. [Google Scholar]
- Selby, G. B. , & Eichner, E. R. (1986). Endurance swimming, intravascular hemolysis, anemia, and iron depletion. New perspective on athlete's anemia. American Journal of Medicine, 81(5), 791–794. 10.1016/0002-9343(86)90347-5 [DOI] [PubMed] [Google Scholar]
- Sherpa, L. Y. , Deji Stigum, H. , Chongsuvivatwong, V. , Luobu, O. , Thelle, D. S. , Nafstad, P. , & Bjertness, E. (2011). Lipid profile and its association with risk factors for coronary heart disease in the highlanders of Lhasa, Tibet. High Altitude Medicine & Biology, 12(1):57–63. 10.1089/ham.2010.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, L. L. , Meah, V. L. , Steele, A. R. , Gasho, C. , Howe, C. A. , Dawkins, T. G. , Busch, S. A. , Oliver, S. J. , Moralez, G. , Lawley, J. S. , Tymko, M. M. , Vizcardo‐Galindo, G. A. , Figueroa‐Mujíca, R. J. , Villafuerte, F. C. , Ainslie, P. N. , Stembridge, M. , Steinback, C. D. , & Moore, J. P. (2021). Global REACH 2018: Andean highlanders, chronic mountain sickness and the integrative regulation of resting blood pressure. Experimental Physiology, 106(1), 104–116. 10.1113/EP088473 [DOI] [PubMed] [Google Scholar]
- Skattebo, O. , Bjerring, A. W. , Auensen, M. , Sarvari, S. I. , Cumming, K. T. , Capelli, C. , & Hallen, J. (2020). Blood volume expansion does not explain the increase in peak oxygen uptake induced by 10 weeks of endurance training. European Journal of Applied Physiology, 120(5), 985–999. 10.1007/s00421-020-04336-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria, R. , Egger, M. , Scherrer, U. , Bender, N. , & Rimoldi, S. F. (2019). Pulmonary arterial pressure at rest and during exercise in chronic mountain sickness: A meta‐analysis. European Respiratory Journal, 53(6). 10.1183/13993003.02040-2018 [DOI] [PubMed] [Google Scholar]
- Stuber, T. , Sartori, C. , Schwab, M. , Jayet, P. Y. , Rimoldi, S. F. , Garcin, S. , Thalmann, S. , Spielvogel, H. , Salmòn, C. S. , Villena, M. , Scherrer, U. , & Allemann, Y. (2010). Exaggerated pulmonary hypertension during mild exercise in chronic mountain sickness. Chest, 137(2), 388–392. 10.1378/chest.09-1355 [DOI] [PubMed] [Google Scholar]
- Telford, R. D. , Sly, G. J. , Hahn, A. G. , Cunningham, R. B. , Bryant, C. , & Smith, J. A. (2003). Footstrike is the major cause of hemolysis during running. Journal of Applied Physiology, 94(1), 38–42. 10.1152/japplphysiol.00631.2001 [DOI] [PubMed] [Google Scholar]
- Vargas, M. , Leon‐Velarde, F. , Monge, C. C. , Orozco, E. , & Rey, L. (1996). Enalapril in the treatment of chronic mountain sickness. Wilderness & Environmental Medicine, 7(2), 193–194. 10.1580/1080-6032(1996)007[0193:ltte]2.3.co;2 [DOI] [PubMed] [Google Scholar]
- Verdecchia, P. , Angeli, F. , Poeta, F. , Reboldi, G. P. , Borgioni, C. , Pittavini, L. , & Porcellati, C. (2004). Validation of the A&D UA‐774 (UA‐767Plus) device for self‐measurement of blood pressure. Blood Pressure Monitoring, 9(4), 225–229. 10.1097/00126097-200408000-00008 [DOI] [PubMed] [Google Scholar]
- Villafuerte, F. C. , & Corante, N. (2016). Chronic mountain sickness: Clinical aspects, etiology, management, and treatment. High Altitude Medicine & Biology, 17(2), 61–69. 10.1089/ham.2016.0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena, M. , Vargas, E. , Guenard, H. , Nallar, N. , Tellez, W. , & Spielvogel, H. (1985). Double‐blind study on the action of almitrine in patients with polycythemia of high altitude. Bulletin Europeen De Physiopathologie Respiratoire, 21(2), 165–170. [PubMed] [Google Scholar]
- Wagner, P. D. (1996). A theoretical analysis of factors determining VO2MAX at sea level and altitude. Respiration Physiology, 106(3), 329–343. 10.1016/s0034-5687(96)00086-2 [DOI] [PubMed] [Google Scholar]
- Watkins, L. L. , Sherwood, A. , Feinglos, M. , Hinderliter, A. , Babyak, M. , Gullette, E. , Waugh, R. , & Blumenthal, J. A. (2003). Effects of exercise and weight loss on cardiac risk factors associated with syndrome X. Archives of Internal Medicine, 163(16), 1889–1895. 10.1001/archinte.163.16.1889 [DOI] [PubMed] [Google Scholar]
- Whelton, P. K. , Carey, R. M. , Aronow, W. S. , Casey, D. E., Jr. , Collins, K. J. , Dennison Himmelfarb, C. , DePalma, S. M. , Gidding, S. , Jamerson, K. A. , Jones, D. W. , MacLaughlin, E. J. , Muntner, P. , Ovbiagele, B. , Smith, S. C. Jr , Spencer, C. C. , Stafford, R. S. , Taler, S. J. , Thomas, R. J. , Williams, K. A., Sr. , … Wright, J. T., Jr. (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension, 71(6), e13–e115. 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- Whyte, G. P. , George, K. , Shave, R. , Middleton, N. , & Nevill, A. M. (2008). Training induced changes in maximum heart rate. International Journal of Sports Medicine, 29(2), 129–133. 10.1055/s-2007-965783 [DOI] [PubMed] [Google Scholar]
- Williams, B. , Mancia, G. , Spiering, W. , Agabiti Rosei, E. , Azizi, M. , Burnier, M. , Clement, D. L. , Coca, A. , de Simone, G. , Dominiczak, A. , Kahan, T. , Mahfoud, F. , Redon, J. , Ruilope, L. , Zanchetti, A. , Kerins, M. , Kjeldsen, S. E. , Kreutz, R. , Laurent, S. , Desormais, I. (2018). 2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal, 39(33), 3021–3104. 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- Winslow, R. , & Monge‐C, C. (1987). Hypoxia, polycythemia, and chronic mountain sickness . Baltimore: Johns Hopkins University Press. [Google Scholar]
- Winslow, R. M. , Monge, C. C. , Brown, E. G. , Klein, H. G. , Sarnquist, F. , Winslow, N. J. , & McKneally, S. S. (1985). Effects of hemodilution on O2 transport in high‐altitude polycythemia. Journal of Applied Physiology, 59(5), 1495–1502. 10.1152/jappl.1985.59.5.1495 [DOI] [PubMed] [Google Scholar]
- Zavorsky, G. S. (2000). Evidence and possible mechanisms of altered maximum heart rate with endurance training and tapering. Sports Medicine, 29(1), 13–26. 10.2165/00007256-200029010-00002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Summary Document
Data Availability Statement
Datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


