Abstract
Aims
To investigate the epidemiological and prognostic relationship between heart failure with preserved ejection fraction (HFpEF) and left‐sided valve surgery using all‐cause mortality as a primary endpoint.
Methods and results
We studied a total of 973 patients, of whom 673 had undergone left‐sided valve surgery (time from surgery to enrolment 50 ± 30 months after valve surgery) and 300 patients with HFpEF without prior surgery served as control group. Among patients after surgery, 67.4% fulfilled all criteria of HFpEF according to current guideline recommendations, 20.6% had no heart failure (HF), and 12.0% had HF with mid‐range or reduced ejection fraction (HFmrEF/HFrEF). During 83 ± 39 months of follow‐up, a total of 335 (34.4%) patients died. Compared to surgical patients with no subsequent HF, patients with HFpEF and HFmrEF/HFrEF after surgery showed significantly higher all‐cause mortality rates [hazard ratio (HR) 1.80, 95% confidence interval (CI) 1.25–2.57, P = 0.001; and HR 1.86, 95% CI 1.16–2.98, P = 0.010, respectively]. This increased mortality rate was similar to the control HFpEF group without surgery (HR 2.05, 95% CI 1.38–3.02, P < 0.001). Results remained consistent after adjustment for clinical and imaging risk factors and when using the established HFA‐PEFF risk score for HFpEF diagnosis. Notably, only 12.5% of HFpEF patients after surgery were diagnosed with HF despite regular follow‐up visits by board‐certified cardiologists. In contrast, 92.1% of HFmrEF/HFrEF patients after surgery were diagnosed correctly.
Conclusions
Heart failure with preserved ejection fraction following left‐sided valve surgery is highly prevalent, associated with unfavourable outcomes, but rarely recognized.
Keywords: Heart failure with preserved ejection fraction, Left‐sided valve surgery, Mortality
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a clinical condition with similar prevalence and prognosis as heart failure with reduced ejection fraction (HFrEF), showing a 5‐year mortality rate of up to 75%. 1 , 2 Several risk factors have been linked to the incidence of HFpEF, including older age, female sex, obesity, diabetes, coronary artery disease, and atrial fibrillation. 3 , 4 Furthermore, large cohort studies have identified several prognostic markers in an effort to improve risk stratification in these patients, including right ventricular (RV) function, 5 diffuse left ventricular (LV) fibrosis, 6 LV end‐diastolic pressure, 7 and LV systolic function. 8 However, our understanding of the pathophysiology of HFpEF is still incomplete as all large randomized clinical trials have failed to demonstrate a convincing survival benefit for any specific treatment. 9 Identifying different HFpEF phenotypes is of great interest, as large HFpEF cohorts are typically comprised of extremely heterogeneous populations. This has frequently been cited as a reason why large trials have failed to show a morbidity or mortality benefit for any particular pharmacotherapy in this group. 10
Patients with long‐standing left‐sided valve disease share many features with HFpEF patients, including increased diffuse LV fibrosis, elevated LV filling pressures, diastolic dysfunction, and clinical manifestations of heart failure. 11 , 12 We previously demonstrated that even after surgical repair of left‐sided valve lesions, clinical features consistent with HFpEF, including significant tricuspid regurgitation (TR) and RV dysfunction, persist and continue to impact prognosis. 11 Interestingly, similar risk factors have been proposed for poor outcomes in patients after left‐sided valve surgery 11 , 13 , 14 and patients with HFpEF. 5 , 8 From a pathophysiological perspective, patients with HFpEF and those with valvular heart disease share an increase in diffuse myocardial fibrosis which could explain the link between these entities. 15 , 16 However, there are no data on the prevalence and independent prognostic impact of prior valve surgery in patients diagnosed with HFpEF.
Methods
Study setting
All patients were recruited at the Vienna General Hospital, an academic referral centre with a large multidisciplinary interventional valvular heart disease unit and a dedicated programme for HFpEF. Patients were recruited after left‐sided valve surgery during their post cardiac surgery visits in our outpatient clinic from January 2007 to January 2011. Minimum time from valvular surgery to study inclusion was 6 months. Preliminary results from this cohort have previously been published. 11 Patients with severe mitral or aortic valve disease, including prosthesis dysfunction, were excluded. In addition, patients with well‐defined HFpEF free of prior cardiac surgery were also recruited from our dedicated outpatient clinic (December 2010 to January 2018) as a control group to compare characteristics and outcomes with patients after surgery. 5 , 17
All participants provided informed consent and each study was approved by our local institutional review board (EK 424/2007 and 796/2010).
Echocardiography
All patients underwent a full transthoracic echocardiogram performed by a board certified sonographer or cardiologist. LV end‐diastolic and end‐systolic volumes (LVEDV, LVESV) were assessed using Simpson's biplane method, and LV ejection fraction (LVEF) was calculated as (LVEDV – LVESV)/LVEDV*100. Fractional area change (FAC) of the right ventricle on a monoplane 4‐chamber view and the tricuspid annular plane systolic excursion (TAPSE) were used for RV functional assessment, while area–length measurements were used for calculating left atrial volume indexed to body surface area (LAVi). Global longitudinal strain (GLS) of the left ventricle was performed using a 4‐, 3‐, and 2‐chamber view, where more negative values indicate better longitudinal function. EchoPAC (GE Medical Systems) was used for GLS assessment. Additionally, we assessed the ratio between early mitral inflow velocity and mitral annular early diastolic velocity (E/e′) as well as the mitral annular plane systolic excursion (MAPSE) in patients without prior mitral valve surgery. Presence and severity of valvular lesions were classified in concordance with current guidelines, in addition to other standard measures. 18
Definition of heart failure
For this analysis, we defined HFpEF for all patients based on the most recent guideline recommendation comprising of (i) signs and symptoms of HFpEF [e.g. New York Heart Association (NYHA) functional class II or higher], (ii) LVEF ≥50%, (iii) elevated natriuretic peptide levels, and (iv) at least one of the following: LV hypertrophy, left atrial enlargement, or signs of diastolic dysfunction. 1 HFrEF was defined as presence of signs and symptoms of heart failure and an LVEF <40%. HFmrEF was defined as LVEF between 40% to 50% in addition to signs and symptoms of heart failure. 1
In addition, we assessed the HFA‐PEFF score, comprising functional, morphological, and biomarker parameters, where a score ≥5 indicates definite HFpEF, a score between 2–4 warrants further functional testing, and a score ≤1 makes HFpEF unlikely. 19
Outcome definition and follow‐up
We used data from the national death registry to identify deceased patients of the primary outcome of all‐cause mortality. Follow‐up data were obtained from visits in our dedicated outpatient clinic or telephone visits (in case of immobility) at regular 6‐month intervals. Hospital charts and electronical medical records were reviewed to identify whether patients who fulfilled all criteria for the diagnosis of HFpEF, as described above, were in fact diagnosed as heart failure patients.
Statistical analysis
We report mean and standard deviation for continuous parameters, and total numbers and percent for categorial data. Comparisons between two groups were performed using the Wilcoxon rank sum test and chi‐square test, as appropriate. We used the natural logarithm of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) for all statistical analyses for this parameter. For survival analysis, we used Kaplan–Meier survival curves and the log‐rank test to demonstrate differences in event‐free survival between groups. We used Cox regression models to study the impact of presence and subtype of heart failure after left‐sided valve surgery, with adjustment for age, left atrial size, RV FAC, prior coronary artery bypass grafting, and diabetes, as these have been identified previously as independent predictors of the primary outcome. 11 Patients without heart failure after cardiac surgery were used as a reference group. In addition, we performed a stepwise regression model including all parameters with significant impact on a univariable level (P < 0.10 and 0.05 for removal and addition to the model, respectively). In a separate step, we performed adjustment for age, sex, NT‐proBNP levels, estimated glomerular filtration rate (eGFR), as well as RV FAC.
In an exploratory step, we used the HFA‐PEFF score as metric variable and tested its association with all‐cause mortality adjusting for variables not reflected by the score, including age, sex, RV FAC, and renal function.
We used Stata 15.1 (StataCorp, College Station, TX, USA) for all analyses and set the level of significance to an alpha of 0.05 unless stated otherwise.
Results
Baseline characteristics
Initially, 977 patients were included, of whom four were excluded due to severe mitral regurgitation at study recruitment after surgery. Baseline characteristics of the final cohort (n = 973) are displayed in Table 1 . A total of 415 patients had undergone isolated aortic valve replacement (AVR), 130 isolated mitral valve replacement or repair (MVR/r), and 128 had multiple valve surgery. Out of the 673 patients, 50 ± 30 months after left‐sided valve surgery, 453 (67.3%) fulfilled all criteria to establish the diagnosis of HFpEF based on the European Society of Cardiology (ESC) guidelines, 78 (11.6%) had HFmrEF or HFrEF, and 139 (20.7%) were free of heart failure. The control group consisted of 300 consecutively recruited patients with HFpEF without prior cardiac surgery. HFpEF was found in the majority of patients irrespective of the type of prior cardiac surgery, with highest rates in patients after MVR/r (73.8%), followed by multiple valve surgery (71.1%) and AVR (64.1%). In contrast, HFrEF was most frequently found after isolated AVR (8.4%) and was less prevalent after multiple valve surgery (6.2%) or isolated MVR/r (3.8%). Online supplementary Table S1 displays characteristics at study recruitment of the entire cohort, stratified by whether patients had undergone valvular surgery in the past, and presence of heart failure.
Table 1.
Characteristics at study recruitment of the entire cohort and stratified by whether patients had undergone valvular surgery in the past, including type of surgery
| Total (n = 973) | Missing values | HFpEF (ESC guideline) | Isolated AVR | Isolated MVR/r | Multiple valve surgery | P‐value | |
|---|---|---|---|---|---|---|---|
| (%) | (n = 300) | (n = 415) | (n = 130) | (n = 128) | |||
| Demographics and comorbidities | |||||||
| Age, years | 70.1 (10.7) | 0 | 71.6 (8.3) | 72.3 (9.8) | 65.2 (12.7) | 64.7 (12.7) | <0.001 |
| Male sex | 41.3% | 0 | 29.7% | 50.4% | 46.9% | 33.6% | <0.001 |
| BMI, kg/m2 | 28.0 (5.5) | 0 | 30.3 (6.7) | 27.7 (4.5) | 26.0 (4.4) | 25.7 (4.9) | <0.001 |
| Hyperlipidaemia | 47.5% | 0 | 54.7% | 52.5% | 32.3% | 29.7% | <0.001 |
| Hypertension | 73.9% | 0 | 94.7% | 72.8% | 50.8% | 52.3% | <0.001 |
| SBP, mmHg | 139.8 (21.4) | 7 | 140.5 (21.0) | 138.4 (24.6) | 136.3 (24.0) | 132.1 (21.4) | 0.47 |
| DBP, mmHg | 78.6 (12.5) | 7 | 79.3 (12.5) | 75.6 (12.1) | 74.4 (13.6) | 74.0 (9.2) | 0.11 |
| CAD | 27.6% | 0 | 26.7% | 36.6% | 16.9% | 11.7% | <0.001 |
| CABG | 9.8% | 0 | 0.0% | 18.8% | 9.2% | 3.9% | <0.001 |
| PM/ICD | 11.6% | 0 | 8.3% | 8.7% | 14.8% | 26.5% | <0.001 |
| COPD | 16.5% | 6 | 30.1% | 10.1% | 8.6% | 13.5% | <0.001 |
| Diabetes | 22.4% | 0 | 34.0% | 19.3% | 13.1% | 14.8% | <0.001 |
| Atrial fibrillation | 49.0% | 0 | 55.7% | 42.0% | 46.9% | 57.8% | <0.001 |
| NYHA class | 0 | <0.001 | |||||
| ≤II | 73.00% | 36.50% | 92.00% | 84.30% | 85.70% | ||
| ≥III | 27.00% | 63.50% | 8.00% | 15.60% | 14.30% | ||
| NT‐proBNP, pg/mL | 3101 (3822) | 0 | 1579 (2413) | 3938 (4500) | 3643 (3743) | 3472 (3144) | <0.001 |
| Ln (NT‐proBNP) | 7.3 (1.3) | 0 | 6.8 (1.0) | 7.6 (1.4) | 7.6 (1.4) | 7.5 (1.3) | <0.001 |
| eGFR, mL/min/1.73 m2 | 63.7 (19.8) | 0 | 61.3 (22.2) | 65.0 (17.8) | 65.2 (20.6) | 63.2 (18.7) | 0.072 |
| Heart failure diagnostics | |||||||
| Guideline HFpEF diagnosis | <0.001 | ||||||
| HFpEF | 77.4% | 100.0% | 64.1% | 73.8% | 71.1% | ||
| HFmrEF | 3.4% | 0.0% | 4.6% | 3.8% | 7.0% | ||
| HFrEF | 4.9% | 0.0% | 8.4% | 3.8% | 6.2% | ||
| HFA‐PEFF score | 5.0 (1.3) | 5.5 (0.9) | 4.6 (1.3) | 4.8 (1.4) | 5.0 (1.2) | <0.001 | |
| Definite HFpEF (≥5) | 64.9% | 85.7% | 51.1% | 58.5% | 67.2% | ||
| Medication | |||||||
| Beta‐blocker | 74.2% | 7 | 73.2% | 86.4% | 66.7% | 85.7% | 0.34 |
| ACEi/ARB | 31.2% | 6 | 32.4% | 36.4% | 11.1% | 21.4% | 0.21 |
| Oral anticoagulation | 54.5% | 5 | 58.9% | 49.3% | 50.3% | 65.4% | 0.057 |
| Diuretic | 74.0% | 5 | 82.1% | 63.0% | 72.0% | 92.9% | <0.001 |
| MRA | 50.4% | 5 | 65.0% | 31.2% | 50.6% | 78.6% | 0.003 |
| Echo parameters | |||||||
| LVEDVi, mL/m2 | 49.6 (11.7) | 0 | 44.7 (12.3) | 50.2 (10.1) | 53.2 (12.5) | 54.7 (10.5) | <0.001 |
| RVEDD, mm | 34.9 (6.4) | 0 | 36.3 (7.2) | 34.0 (5.7) | 34.3 (5.5) | 35.6 (6.6) | <0.001 |
| IVS, mm | 12.7 (2.5) | 0 | 12.9 (2.5) | 13.1 (2.5) | 12.6 (2.0) | 11.7 (2.2) | 0.007 |
| LVEF, % | 55.6 (8.6) | 0 | 59.0 (7.7) | 54.8 (9.2) | 56.2 (7.2) | 55.2 (8.1) | <0.001 |
| LV GLS, % | −14.2 (4.0) | 5 | −15.2 (3.8) | −13.1 (4.3) | −13.3 (3.7) | −13.7 (3.8) | <0.001 |
| RV FAC, % | 45.4 (11.3) | 10 | 45.5 (12.7) | 47.1 (9.9) | 43.7 (10.8) | 41.9 (10.6) | <0.001 |
| TAPSE, mm | 17.0 (5.5) | 8 | 19.4 (6.3) | 16.4 (4.8) | 15.9 (5.1) | 15.0 (3.9) | <0.001 |
| LAVi, mL/m2 | 38.4 (6.5) | 0 | 38.9 (4.5) | 36.4 (5.4) | 39.4 (7.0) | 42.9 (9.7) | <0.001 |
| MAPSE, mm | 13.7 (3.8) | 8 | 13.3 (3.0) | 14.6 (4.5) | 0.12 | ||
| E/e′ | 13.4 (5.3) | 10 a | 12.9 (5.2) | 16.1 (5.8) | 0.06 | ||
| E | 0.99 (0.30) | 6 a | 0.99 (0.30) | 0.99 (0.23) | 0.13 | ||
| e′ | 0.08 (0.02) | 10 a | 0.08 (0.02) | 0.07 (0.02) | 0.64 | ||
| Peak TR velocity, m/s | 2.97 (0.54) | 15 | 3.14 (0.69) | 2.86 (0.46) | 2.96 (0.44) | 3.05 (0.53) | <0.001 |
| TR severity | 0 | <0.001 | |||||
| Mild | 77.00% | 79.00% | 79.90% | 82.80% | 57.50% | ||
| Moderate | 14.50% | 10.90% | 15.40% | 8.60% | 27.40% | ||
| Severe | 8.50% | 10.00% | 4.70% | 8.60% | 15.10% |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AVR, aortic valve replacement; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; E/e′, ratio between early mitral inflow velocity and mitral annular early diastolic velocity; eGFR, estimated glomerular filtration rate; ESC, European Society of Cardiology; FAC, fractional area change; GLS, global longitudinal strain; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter‐defibrillator; IVS, interventricular septum; LAVi, left atrial volume indexed to body surface area; Ln, natural logarithm; LV, left ventricular; LVEDVi, left ventricular end‐diastolic volume indexed to body surface area; LVEF, left ventricular ejection fraction; MAPSE, mitral annular plane systolic excursion; MRA, mineralocorticoid receptor antagonist; MVr, mitral valve repair; MVR, mitral valve replacement; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PM, pacemaker; RV, right ventricular; RVEDD, right ventricular end‐diastolic diameter; SBP, systolic blood pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Percent of missing values refer to patients who did not had undergone mitral valve surgery.
Table 1 further illustrates differences in baseline characteristics stratified by type of valvular surgery. HFpEF patients without cardiac surgery and patients after AVR were significantly older than MVR/r patients (71.6 ± 8.3 and 72.3 ± 9.8 years old vs. 65.2 ± 12.7, P < 0.001), and patients without surgery had a more unfavourable cardiometabolic risk profile when compared to patients after AVR and MVR/r (hypertension: 94.7% vs. 72.8% and 50.8%; diabetes 34% vs. 19.3% and 13.1%; body mass index: 30.3 ± 6.7 vs. 27.7 ± 4.5 and 26.0 ± 4.4; P < 0.001 for all). Atrial fibrillation was present in 55.7% of patients with HFpEF and no cardiac surgery and 57.8% after multiple valve surgery, which was significantly higher when compared to patients after AVR (42.0%) or MVR/r (46.9%; P < 0.001 across all). A total of 74% were on diuretic treatment, which was highest in multiple valve surgery patients (92.9%), followed by HFpEF without prior surgery (82.1%), MVR/r (72.0%), and was lowest in patients after AVR (63.0%; P < 0.001 across all).
Only 12.5% of all patients with HFpEF after cardiac surgery were labelled as heart failure patients, despite regular follow‐ups by board certified internists and/or cardiologists.
The HFA‐PEFF score was 4.6 ± 1.3 in patients after AVR, 4.8 ± 1.4 after MVR/r, and 5.0 ± 1.2 after multiple valve surgery. The HFpEF control group (based on ESC guideline definition) presented with an HFA‐PEFF score of 5.5 ± 0.9. Using an HFA‐PEFF score ≥5 to define definite HFpEF, rates of definite HFpEF were 85.7% in the surgery‐free control group, 51.1% after AVR, 58.5% after MVR/r, and 67.2% after multiple valve surgery. Hence, a total of 55.6% of all patients after left‐sided surgery presented with an HFA‐PEFF score ≥5.
Table 2 presents data on patients with an HFA‐PEFF score ≥5 (n = 580) stratified by type of valvular surgery, after exclusion of HFmrEF and HFrEF patients. Notably, patients after valvular surgery and a high HFA‐PEFF score presented with similar peak TR velocities (P = 0.28), and E/e′ (P = 0.063, MVR/r patients excluded) but distinctly different risk profiles in terms of their comorbidities. HFpEF patients without prior surgery were more obese (P < 0.001) more often had arterial hypertension (P < 0.001), and were in more advanced NYHA functional class (P < 0.001). Compared to HFpEF patients without prior surgery, those after cardiac surgery had markedly higher natriuretic peptide levels (P < 0.001). Left atrial size, however, was similar between HFpEF patients without surgery and patients with HFpEF after AVR (LAVi: 39.3 ± 4.4 vs. 38.4 ± 5.2 mL/m2) whereas MVR/r and multiple valve surgery patients presented with significantly more dilated left atria (LAVi: 41.2 ± 6.2 and 44.4 ± 10.1 mL/m2, P < 0.001 across all). LV longitudinal function was similar across all groups with slightly worse function in patients after surgery (LV GLS for all: −14.6 ± 3.9; P = 0.048 across all).
Table 2.
Characteristics at study recruitment of patients with a HFA‐PEFF score ≥5, stratified by whether patients had undergone valvular surgery in the past, including type of surgery
| Total (n = 580) | HFpEF (ESC guideline) | Isolated AVR | Isolated MVR/r | Multiple valve surgery | P‐value | |
|---|---|---|---|---|---|---|
| (n = 257) | (n = 181) | (n = 67) | (n = 75) | |||
| Demographics and co‐morbidities | ||||||
| Age, years | 71.8 (9.9) | 71.9 (8.1) | 75.4 (8.2) | 67.9 (12.2) | 66.1 (12.5) | <0.001 |
| Male sex | 33.1% | 28.4% | 35.4% | 44.8% | 33.3% | 0.069 |
| BMI, kg/m2 | 28.4 (5.7) | 30.1 (6.6) | 27.7 (4.3) | 26.7 (4.3) | 25.8 (5.4) | <0.001 |
| Hyperlipidaemia | 47.8% | 54.1% | 49.7% | 35.8% | 32.0% | 0.001 |
| Hypertension | 81.2% | 94.9% | 80.7% | 58.2% | 56.0% | <0.001 |
| SBP, mmHg | 139.6 (21.1) | 140.0 (20.6) | 138.9 (25.1) | 138.1 (23.7) | 133.6 (24.1) | 0.81 |
| DBP, mmHg | 78.2 (12.3) | 78.9 (12.3) | 75.3 (11.1) | 74.1 (13.1) | 71.7 (9.3) | 0.089 |
| CAD | 27.9% | 25.7% | 41.4% | 17.9% | 12.0% | <0.001 |
| CABG | 8.6% | 0.0% | 24.9% | 3.0% | 4.0% | <0.001 |
| PM/ICD | 12.9% | 7.4% | 11.7% | 22.8% | 29.7% | <0.001 |
| COPD | 20.0% | 30.5% | 11.1% | 9.1% | 15.1% | <0.001 |
| Diabetes | 24.0% | 31.1% | 19.3% | 14.9% | 18.7% | 0.004 |
| Atrial fibrillation | 55% | 62% | 45% | 48% | 58% | <0.001 |
| NYHA class | <0.001 | |||||
| ≤II | 63.6% | 36.6% | 88.9% | 78.4% | 80.8% | |
| ≥III | 36.5% | 63.4% | 11.1% | 21.5% | 19.2% | |
| NT‐proBNP, pg/mL | 2799 (3751) | 1671 (2543) | 3678 (4666) | 4049 (4456) | 3521 (3057) | <0.001 |
| eGFR, mL/min/1.73 m2 | 62.0 (19.9) | 61.7 (22.4) | 60.5 (15.6) | 62.9 (22.1) | 66.0 (17.8) | 0.25 |
| Heart failure diagnostics | ||||||
| HFA‐PEFF score | 5.8 (0.4) | 5.9 (0.3) | 5.7 (0.5) | 5.8 (0.4) | 5.7 (0.4) | <0.001 |
| Echo parameters | ||||||
| LVEDVi, mL/m2 | 48.6 (12.1) | 44.9 (12.4) | 48.5 (9.4) | 54.8 (13.4) | 55.1 (11.0) | <0.001 |
| RVEDD, mm | 35.5 (6.6) | 36.6 (7.0) | 34.1 (6.0) | 34.7 (5.5) | 36.1 (6.7) | <0.001 |
| IVS, mm | 12.7 (2.4) | 12.8 (2.5) | 12.9 (2.5) | 12.6 (2.0) | 11.8 (2.2) | 0.13 |
| LVEF, % | 58.2 (5.6) | 59.6 (7.5) | 57.5 (5.1) | 58.5 (4.9) | 58.2 (4.6) | 0.046 |
| LV GLS, % | −14.6 (3.9) | −15.2 (3.8) | −13.7 (4.5) | −13.5 (2.9) | −14.0 (3.8) | 0.048 |
| RV FAC, % | 44.5 (11.7) | 45.2 (12.6) | 45.7 (9.8) | 41.8 (12.1) | 41.2 (10.7) | 0.012 |
| TAPSE, mm | 17.5 (5.7) | 19.5 (6.3) | 16.4 (4.5) | 15.7 (5.1) | 14.9 (3.4) | <0.001 |
| LAVi, mL/m2 | 39.9 (6.2) | 39.3 (4.4) | 38.4 (5.2) | 41.2 (6.2) | 44.4 (10.1) | <0.001 |
| MAPSE, mm | 13.8 (3.5) | 13.3 (3.1) | 15.4 (3.8) | 0.013 | ||
| E/e′ | 13.1 (5.1) | 12.9 (5.2) | 14.5 (4.2) | 0.063 | ||
| E | 0.99 (0.30) | 0.99 (0.30) | 0.98 (0.24) | 0.16 | ||
| e′ | 0.08 (0.02) | 0.08 (0.02) | 0.08 (0.02) | 0.94 | ||
| Peak TR velocity, m/s | 3.12 (0.55) | 3.16 (0.69) | 3.08 (0.46) | 3.06 (0.39) | 3.20 (0.52) | 0.28 |
| TR severity | <0.001 | |||||
| Mild | 73.0% | 78.5% | 68.5% | 75.0% | 55.2% | |
| Moderate | 17.0% | 11.0% | 23.6% | 13.9% | 32.7% | |
| Severe | 10.1% | 10.5% | 7.8% | 11.1% | 12.2% |
AVR, aortic valve replacement; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; E/e′, ratio between early mitral inflow velocity and mitral annular early diastolic velocity; eGFR, estimated glomerular filtration rate; ESC, European Society of Cardiology; FAC, fractional area change; GLS, global longitudinal strain; HFpEF, heart failure with preserved ejection fraction; ICD, implantable cardioverter‐defibrillator; IVS, interventricular septum; LAVi, left atrial volume indexed to body surface area; LV, left ventricular; LVEDVi, left ventricular end‐diastolic volume indexed to body surface area; LVEF, left ventricular ejection fraction; MAPSE, mitral annular plane systolic excursion; MVr, mitral valve repair; MVR, mitral valve replacement; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PM, pacemaker; RV, right ventricular; RVEDD, right ventricular end‐diastolic diameter; SBP, systolic blood pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Outcome analysis
Out of 973 patients, 335 (34.4%) died during follow‐up (83.0 ± 39.5 months). Patients with HFpEF and HFrEF showed a similar risk of all‐cause mortality, which was significantly higher than the risk of those patients who did not fulfil heart failure criteria based on ESC guidelines (P < 0.001), and was comparable to that of the control HFpEF group (Figures 1 and 2 ). When compared to patients after cardiac surgery without heart failure and after adjustment for previously reported risk factors, 11 those with HFpEF after surgery were at significantly higher risk for the primary outcome [adjusted hazard ratio (HR) 1.58, 95% confidence interval (CI) 1.03–2.41, P = 0.035] which was similar to the HFpEF control group without cardiac surgery (adjusted HR 1.76, 95% CI 1.11–2.77, P = 0.015). There was no significant difference between patients with HFmrEF/HFrEF after surgery as compared to post surgery patients free of heart failure in the adjusted analysis (n = 81; adjusted HR 1.41, 95% CI 0.82–2.42, P = 0.211).
Figure 1.
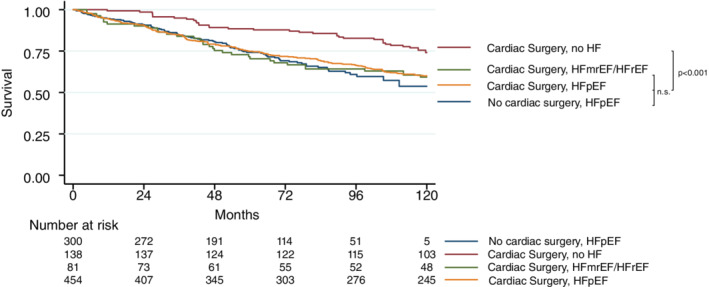
Kaplan–Meier estimates demonstrating lower survival rates in individuals who were diagnosed with heart failure with preserved ejection fraction (HFpEF) or heart failure with mid‐range/reduced ejection fraction (HFmrEF/HFrEF) after left‐sided valve surgery when compared to those free of heart failure (HF) after left‐sided valve surgery and a HFpEF control group free of any cardiac surgery (log‐rank, P ≤ 0.001).
Figure 2.
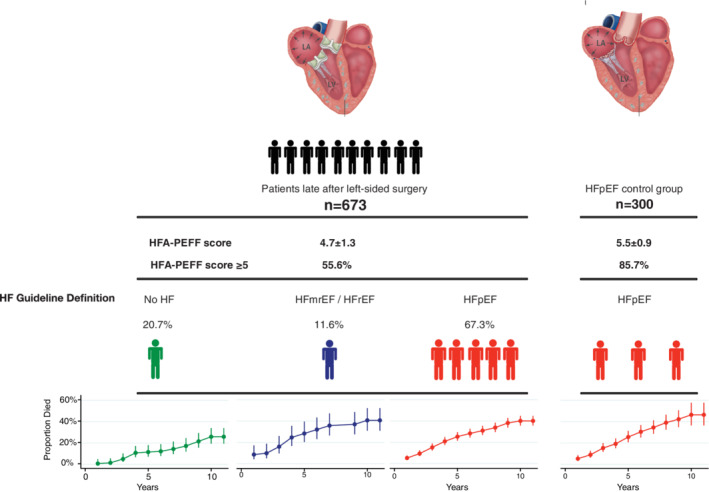
After left‐sided cardiac surgery, heart failure with preserved ejection fraction (HFpEF) was observed in 67.3% of patients and shared similar mortality rates, as compared with a well‐defined HFpEF cohort free of cardiac surgery. HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Similar results were retrieved when using a stepwise approach including all parameters with a significant impact on survival on a univariable level (Table 3 ). In that analysis, HFpEF patients after cardiac surgery and the HFpEF control group were at higher risk for all‐cause death when compared to surgery patients free of heart failure (adjusted HR 1.79, 95% CI 1.18–2.72, P = 0.006, and adjusted HR 1.69, 95% CI 1.08–2.65, P = 0021, respectively), whereas HFrEF patients after surgery were at higher risk but did not reach the level of significance (n = 81; adjusted HR 1.51, 95% CI 0.88–2.58, P = 0.133).
Table 3.
Cox regression demonstrating the association of variables at study recruitment with all‐cause mortality
| Univariable | Multivariable a | |||
|---|---|---|---|---|
| Crude HR (95% CI) | P‐value | Adjusted HR (95% CI) | P‐value | |
| Clinical data | ||||
| Age | 1.06 (1.05–1.08) | <0.001 | 1.05 (1.02–1.08) | 0.003 |
| Male sex | 0.88 (0.70–1.09) | 0.236 | ||
| BMI | 0.99 (0.97–1.11) | 0.342 | ||
| Atrial fibrillation | 1.46 (1.18–1.88) | 0.001 | ||
| Diabetes | 1.67 (1.31–2.12) | <0.001 | 1.82 (1.02–3.23) | 0.044 |
| Hypertension | 1.91 (1.45–2.51) | <0.001 | ||
| Hyperlipidaemia | 1.17 (0.95–1.45) | 0.150 | ||
| CAD | 1.68 (1.34–2.10) | <0.001 | ||
| COPD | 1.90 (1.47–2.46) | <0.001 | ||
| NYHA class | 1.59 (1.40–1.80) | <0.001 | 1.53 (1.07–2.21) | 0.021 |
| CABG | 1.72 (1.28–2. 30) | <0.001 | ||
| eGFR | 0.98 (0.97–0.99) | <0.001 | ||
| NT‐proBNP (log) | 1.14 (1.05–1.24) | 0.002 | ||
| Imaging data | ||||
| LVEDVi | 0.99 (0.98–1‐004) | 0.220 | ||
| RVEDD | 1.04 (1.02–1.06) | <0.001 | ||
| LAVi | 1.05 (1.03–1.06) | <0.001 | 1.05 (1.02–1.08) | 0.001 |
| RA diameter | 1.03 (1.02–1.04) | <0.001 | ||
| LVEF | 0.99 (0.98–1.04) | 0.190 | ||
| RV FAC | 0.97 (0.96–0.98) | <0.001 | 0.97 (0.95–0.99) | 0.044 |
| TR ≥ moderate | 1.89 (1.34–2.67) | <0.001 | ||
| Peak TR velocity | 2.13 (1.67–2.72) | <0.001 | ||
| HF/surgery group | ||||
| Surgery, no HF | Reference | Reference | ||
| Surgery, HFmrEF/HFrEF b | 1.86 (1.16–2.98) | 0.010 | 1.51 (0.88–2.58) | 0.133 a |
| Surgery, HFpEF | 1.80 (1.25–2.57) | 0.001 | 1.79 (1.18–2.72) | 0.006 |
| HFpEF control | 2.05 (1.38–3.02) | <0.001 | 1.69 (1.08–2.65) | 0.021 |
BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; FAC, fractional area change; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; LAVi, left atrial volume indexed to body surface area; LVEDVi, left ventricular end‐diastolic volume indexed to body surface area; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; RV, right ventricular; RVEDD, right ventricular end‐diastolic diameter; TR, tricuspid regurgitation.
Multivariable analysis is based on a stepwise approach including variables with significant impact (P < 0.005) on outcome at a univariable level.
Note limited number (n = 81) of patients with HFmrEF/HFrEF.
In a separate model adjusting for age, sex, NT‐proBNP levels, eGFR, and RV FAC, results remained consistent (adjusted HR for HFpEF following surgery and HFpEF without surgery: 1.51, 95% CI 1.04–2.45, P = 0.038, and 1.67, 95% CI 1.05–2.64, P = 0.029, respectively, when compared to patients after cardiac surgery without heart failure). Similarly, the HFA‐PEFF score, when adjusted for age, sex, RV FAC and eGFR, was significantly associated with all‐cause mortality (adjusted HR 1.16, 95% CI 1.03–1.31, P = 0.015).
Discussion
Our understanding of the pathogenesis of HFpEF, which affects millions of patients worldwide and is associated with significant morbidity and mortality, is still limited. Several HFpEF phenotypes have been proposed, however, a subgroup benefitting from a specific treatment has not yet been identified. We report two main findings: (i) after left‐sided heart valve surgery, two thirds of patients present with HFpEF late after surgery, and (ii) while HFpEF in these patients significantly impacts survival, it is rarely diagnosed.
The pathophysiology of HFpEF is incompletely understood but diffuse myocardial fibrosis has been proposed as one of the key mechanisms. Indeed, patients with HFpEF were found to have increased extracellular matrix when compared to controls, which also has independent prognostic information. 15 Furthermore, cardiometabolic alterations and female sex were identified as risk factors for the development of HFpEF. 3 , 4 Similarly, extracellular matrix alterations are well characterized in patients with left‐sided valvular heart disease. These may impact systolic and – especially – diastolic function. Impaired ventricular relaxation was described as early as the 1980s in patients with aortic stenosis. 20 More recent advances in cardiovascular imaging, such as parametric mapping on cardiovascular magnetic resonance (CMR) imaging, have allowed non‐invasive quantification of extracellular alterations in the myocardium. Indeed, Treibel et al. 16 demonstrated that myocardial fibrosis, quantified using CMR, persists even after AVR in patients with severe aortic stenosis. Similarly, diffuse myocardial fibrosis could be detected in patients with severe mitral regurgitation 21 and was also proposed as a marker for prognostication in this group. 22 Our finding of high filling pressures as suggested by echocardiography and also high natriuretic peptide levels in patients after surgery may be explained by irreversible remodelling prior to surgery.
Following the concept of increased stiffening of the left ventricle by diffuse fibrosis, another key pathomechanistic feature of HFpEF is the development of postcapillary pulmonary hypertension. Although pulmonary vascular resistance may remain moderately elevated, long‐standing alterations of RV afterload lead to higher incidence of severe TR in HFpEF cohorts. 23 Similarly, RV afterload is elevated both in aortic and mitral valve disease, and both valvular lesions are accompanied by TR in more advanced stages.
The definition of HFpEF is of critical importance in the context of our study cohort as the current guideline definition rely on increased left atrial size and natriuretic peptide levels which may have to be interpreted differently in patients who have undergone cardiac surgery in their past. Especially in patients long after mitral valve surgery, high rates of HFpEF are likely due to left atrial enlargement in these patients. However, even when applying the more advanced HFpEF definition based on the HFA‐PEFF score, our key findings remained unchanged.
Of note, a decrease of longitudinal LV function has been described following open‐heart surgery which may contribute to our findings. 24 In our cohort, MAPSE was not different between subgroups and LV GLS was comparable across groups with a high HFA‐PEFF score. Also, postoperative systolic blood pressure plays a crucial role for regression of LV remodelling. 25 In our cohort, blood pressure levels were comparable across all subgroups.
As we have previously shown, 11 a substantial proportion of patients present with significant TR much later after left‐sided valve surgery. In the present cohort of patients after left heart valve surgery, at least moderate TR was found in one out of five patients. Although our cohort after left‐sided valve surgery lacked invasive haemodynamic assessments, presence of at least moderate TR and sonography‐derived systolic pulmonary artery pressures demonstrated similar features with HFpEF.
Our findings that two out of three patients after left‐sided valve surgery present with HFpEF late after surgery, which is an independent harbinger of risk, may reflect the persistence of diffuse fibrosis resulting in diastolic dysfunction and may be the key link between these two common disease entities.
Patients with HFpEF after cardiac surgery and those without prior cardiac surgery share many features but our study also highlights important differences between the two entities. The younger age, less female predominance, but higher natriuretic peptide levels despite similar RV function in post‐surgery HFpEF patients suggest that these may represent different phenotypes of a heterogeneous disease. The almost identical event rates between HFpEF and HFrEF after cardiac surgery and the HFpEF control group further strengthen our findings. The lack of statistical significance for patients with HFrEF after surgery may be due to a limited sample size of that specific group and may also be influenced by optimal medical treatment.
Although limited data on long‐term prognostication are available in patients late after left‐sided valve surgery, several studies report similar predictors of outcome when compared to large HFpEF cohorts. These include RV dysfunction 5 , 11 and LV GLS 8 , 26 despite preserved LVEF. These previous reports are consistent with results of this study.
Future outlook
Our findings are novel as they suggest high prevalence of HFpEF following left‐sided valve surgery. Physicians treating patients after left‐sided valve surgery should especially be aware of the high rate of HFpEF among these patients, and the association with poor outcomes. Although at the time of this publication specific treatment for these patients is lacking, this remains an active area of research with several large scale trials focusing on therapeutic options for the HFpEF population such as the phase 3 DELIVER study.
Limitations
Several limitations merit comment. Our study comprises two well‐defined cohorts of patients after left‐sided valve surgery and HFpEF in a large single centre setting. While a centre specific selection bias must be taken into account, our homogeneous patient population allowed for steady recruitment and consistent work‐up throughout the enrolment process. Preoperative data, including atrial fibrillation, were not available to assess all components of the HFA‐PEFF score prior to surgery. In patients after cardiac surgery, no stress or invasive testing was available in uncertain cases when applying the HFA‐PEFF score (score 2–4), which would better explain discrepant cases of HFpEF using different definitions. Also, data on long‐term use of vitamin K antagonists are not available, which could contribute to arterial stiffness and diastolic dysfunction. 27 Our dataset furthermore lacks information on pre‐ and postoperative conduction defects and use of biventricular pacing, which may impact results. Lastly, routine invasive haemodynamic assessment and CMR imaging was not available in all patients after left‐sided valve surgery to definitely report on the incidence of post‐capillary pulmonary hypertension or changes in extracellular volume in these patients, respectively.
Conflict of interest: none declared.
Supporting information
Table S1. Characteristics at study recruitment of the entire cohort and stratified by whether patients had undergone valvular surgery in the past, and presence of heart failure.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 2. Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart failure with preserved, borderline, and reduced ejection fraction: 5‐year outcomes. J Am Coll Cardiol 2017;70:2476–2486. [DOI] [PubMed] [Google Scholar]
- 3. Gong FF, Jelinek MV, Castro JM, Coller JM, McGrady M, Boffa U, Shiel L, Liew D, Wolfe R, Stewart S, Owen AJ, Krum H, Reid CM, Prior DL, Campbell DJ. Risk factors for incident heart failure with preserved or reduced ejection fraction, and valvular heart failure, in a community‐based cohort. Open Heart 2018;5:e000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, Gardin JM, Hillege HL, Ji F, Kop WJ, Lau ES, Lee DS, Sadreyev R, van Gilst WH, Wang TJ, Zanni MV, Vasan RS, Allen NB, Psaty BM, van der Harst P, Levy D, Larson M, Shah SJ, de Boer RA, Gottdiener JS, Ho JE. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail 2018;6:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aschauer S, Kammerlander AA, Zotter‐Tufaro C, Ristl R, Pfaffenberger S, Bachmann A, Duca F, Marzluf BA, Bonderman D, Mascherbauer J. The right heart in heart failure with preserved ejection fraction: insights from cardiac magnetic resonance imaging and invasive haemodynamics. Eur J Heart Fail 2016;18:71–80. [DOI] [PubMed] [Google Scholar]
- 6. Mascherbauer J, Marzluf BA, Tufaro C, Pfaffenberger S, Graf A, Wexberg P, Panzenbock A, Jakowitsch J, Bangert C, Laimer D, Schreiber C, Karakus G, Hulsmann M, Pacher R, Lang IM, Maurer G, Bonderman D. Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging 2013;6:1056–1065. [DOI] [PubMed] [Google Scholar]
- 7. Mascherbauer J, Zotter‐Tufaro C, Duca F, Binder C, Koschutnik M, Kammerlander AA, Aschauer S, Bonderman D. Wedge pressure rather than left ventricular end‐diastolic pressure predicts outcome in heart failure with preserved ejection fraction. JACC Heart Fail 2017;5:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kammerlander AA, Dona C, Nitsche C, Koschutnik M, Schonbauer R, Duca F, Zotter‐Tufaro C, Binder C, Aschauer S, Beitzke D, Loewe C, Hengstenberg C, Bonderman D, Mascherbauer J. Feature tracking of global longitudinal strain by using cardiovascular MRI improves risk stratification in heart failure with preserved ejection fraction. Radiology 2020;296:290–298. [DOI] [PubMed] [Google Scholar]
- 9. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Dungen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP; PARAGON‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 10. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 11. Kammerlander AA, Marzluf BA, Graf A, Bachmann A, Kocher A, Bonderman D, Mascherbauer J. Right ventricular dysfunction, but not tricuspid regurgitation, is associated with outcome late after left heart valve procedure. J Am Coll Cardiol 2014;64:2633–2642. [DOI] [PubMed] [Google Scholar]
- 12. Benfari G, Noni M, Onorati F, Cerrito LF, Pernigo M, Vinco G, Cameli M, Mandoli GE, Borio G, Geremia G, Zivelonghi C, Abbasciano R, Mazzali G, Zamboni M, Faggian G, Rossi A, Ribichini FL. Effects of aortic valve replacement on left ventricular diastolic function in patients with aortic valve stenosis. Am J Cardiol 2019;124:409–415. [DOI] [PubMed] [Google Scholar]
- 13. Ghoreishi M, Evans CF, DeFilippi CR, Hobbs G, Young CA, Griffith BP, Gammie JS. Pulmonary hypertension adversely affects short‐ and long‐term survival after mitral valve operation for mitral regurgitation: implications for timing of surgery. J Thorac Cardiovasc Surg 2011;142:1439–1452. [DOI] [PubMed] [Google Scholar]
- 14. Hwang JW, Kim SM, Park SJ, Cho EJ, Kim EK, Chang SA, Lee SC, Choe YH, Park SW. Assessment of reverse remodeling predicted by myocardial deformation on tissue tracking in patients with severe aortic stenosis: a cardiovascular magnetic resonance imaging study. J Cardiovasc Magn Reson 2017;19:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duca F, Kammerlander AA, Zotter‐Tufaro C, Aschauer S, Schwaiger ML, Marzluf BA, Bonderman D, Mascherbauer J. Interstitial fibrosis, functional status, and outcomes in heart failure with preserved ejection fraction: insights from a prospective cardiac magnetic resonance imaging study. Circ Cardiovasc Imaging 2016;9:e005277. [DOI] [PubMed] [Google Scholar]
- 16. Treibel TA, Kozor R, Schofield R, Benedetti G, Fontana M, Bhuva AN, Sheikh A, Lopez B, Gonzalez A, Manisty C, Lloyd G, Kellman P, Diez J, Moon JC. Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J Am Coll Cardiol 2018;71:860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zotter‐Tufaro C, Mascherbauer J, Duca F, Koell B, Aschauer S, Kammerlander AA, Panzenboeck A, Sadushi‐Kolici R, Bangert C, Laimer D, Ristl R, Lang IM, Bonderman D. Prognostic significance and determinants of the 6‐min walk test in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2015;3:459–466. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 19. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske‐Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 20. Diver DJ, Royal HD, Aroesty JM, McKay RG, Ferguson JJ, Warren SE, Lorell BH. Diastolic function in patients with aortic stenosis: influence of left ventricular load reduction. J Am Coll Cardiol 1988;12:642–648. [DOI] [PubMed] [Google Scholar]
- 21. Edwards NC, Moody WE, Yuan M, Weale P, Neal D, Townend JN, Steeds RP. Quantification of left ventricular interstitial fibrosis in asymptomatic chronic primary degenerative mitral regurgitation. Circ Cardiovasc Imaging 2014;7:946–953. [DOI] [PubMed] [Google Scholar]
- 22. Kitkungvan D, Yang EY, El Tallawi KC, Nagueh SF, Nabi F, Khan MA, Nguyen DT, Graviss EA, Lawrie GM, Zoghbi WA, Bonow RO, Quinones MA, Shah DJ. Prognostic implications of diffuse interstitial fibrosis in asymptomatic primary mitral regurgitation. Circulation 2019;140:2122–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mascherbauer J, Kammerlander AA, Zotter‐Tufaro C, Aschauer S, Duca F, Dalos D, Winkler S, Schneider M, Bergler‐Klein J, Bonderman D. Presence of isolated tricuspid regurgitation should prompt the suspicion of heart failure with preserved ejection fraction. PLoS One 2017;12:e0171542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gozdzik A, Letachowicz K, Grajek BB, Plonek T, Obremska M, Jasinski M, Gozdzik W. Application of strain and other echocardiographic parameters in the evaluation of early and long‐term clinical outcomes after cardiac surgery revascularization. BMC Cardiovasc Disord 2019;19:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Imanaka K, Kohmoto O, Nishimura S, Yokote Y, Kyo S. Impact of postoperative blood pressure control on regression of left ventricular mass following valve replacement for aortic stenosis. Eur J Cardiothorac Surg 2005;27:994–999. [DOI] [PubMed] [Google Scholar]
- 26. Alashi A, Khullar T, Mentias A, Gillinov AM, Roselli EE, Svensson LG, Popovic ZB, Griffin BP, Desai MY. Long‐term outcomes after aortic valve surgery in patients with asymptomatic chronic aortic regurgitation and preserved LVEF: impact of baseline and follow‐up global longitudinal strain. JACC Cardiovasc Imaging 2020;13(1 Pt 1):12–21. [DOI] [PubMed] [Google Scholar]
- 27. Hashmath Z, Lee J, Gaddam S, Ansari B, Oldland G, Javaid K, Mustafa A, Vasim I, Akers S, Chirinos JA. Vitamin K status, warfarin use, and arterial stiffness in heart failure. Hypertension 2019;73:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics at study recruitment of the entire cohort and stratified by whether patients had undergone valvular surgery in the past, and presence of heart failure.


