Abstract
Objective
To systematically review the dental literature for clinical studies reporting on production time, effectiveness and/or costs of additive and subtractive computer‐aided manufacturing (CAM) of implant prostheses.
Materials and methods
A systematic electronic search for clinical studies from 1990 until June 2020 was performed using the online databases Medline, Embase and Cochrane. Time required for the computer‐aided design (CAD) process, the CAM process, and the delivery of the CAD‐CAM prostheses were extracted. In addition, articles reporting on the effectiveness and the costs of both manufacturing technologies were included.
Results
Nine clinical studies were included reporting on subtractive CAM (s‐CAM; 8 studies) and additive CAM (a‐CAM; 1 study). Eight studies reported on the s‐CAM of prosthetic and auxiliary components for single implant crowns. One study applied a‐CAM for the fabrication of an implant bar prototype. Time was provided for the CAD process of implant models (range 4.9–11.8 min), abutments (range 19.7–32.7 min) and crowns (range 11.1–37.6 min). The time for s‐CAM of single implant crown components (abutment/crown) ranged between 8.2 and 25 min. Post‐processing (e.g. sintering) was a time‐consuming process (up to 530 min). At delivery, monolithic/veneered CAD‐CAM implant crowns resulted in additional adjustments chairside (51%/93%) or labside (11%/19%).
Conclusions
No scientific evidence exists on production time, effectiveness and costs of digital workflows comparing s‐CAM and a‐CAM. For both technologies, post‐processing may substantially contribute to the production time. Considering effectiveness, monolithic CAD‐CAM implant crowns may be preferred compared to veneered CAD‐CAM crowns.
Keywords: clinical research, clinical trials, material sciences, prosthodontics
1. INTRODUCTION
Computer‐aided design (CAD) and computer‐aided manufacturing (CAM) technologies used for the fabrication of implant‐supported reconstructions enhanced time efficiency of the laboratory workflow compared to conventional manual techniques (Muhlemann et al., 2018; de Oliveira et al., 2020). Moreover, quality of implant prostheses fabricated by the aid of CAD‐CAM technology meets the standards of prosthetic care (Mello et al., 2019; Muhlemann, Kraus, et al., 2018; Muhlemann et al., 2020).
Subtractive CAM (s‐CAM) is considered the gold standard and referred to computer numeric controlled (CNC) milling of a desired shape out of a prefabricated material block. Limitations of this manufacturing process, however, apply. The waste of material, which includes also unused remnants of the blocks, may reach up to 90% (Strub et al., 2006). Fabrication accuracy applying s‐CAM is influenced by the type of milling device (Bosch et al., 2014; Padros et al., 2020; Zeltner et al., 2017). Moreover, number, size and geometry of milling burs used for s‐CAM seem to influence the surface resolution and represent a limitation that cannot be overcome (Atzeni & Salmi, 2012; Koch et al., 2016; Yara et al., 2005). Therefore, type and strategy of procedural milling determine the time for production, the quality of outcome and the costs for the milled prosthetic components.
Additive CAM (a‐CAM) is referred to three‐dimensional printing (3D printing) and is a process in which the desired object is produced by the deposition of layer upon layer (ISO/ASTM 52900‐15, 2009). From an engineering point of view, a‐CAM has the advantage to overcome the geometric restrictions that are present with s‐CAM and more complex forms may be produced (Torabi et al., 2015). In addition, a‐CAM may reduce waste of material (Zhang, Qiu, et al., 2019; Zhang et al., 2018).
Different a‐CAM technologies exist and are applied in implant dentistry for processing polymers (Revilla‐Leon & Ozcan, 2019), ceramics (Methani et al., 2020; Revilla‐Leon et al., 2020) and metals (Revilla‐Leon et al., 2019; Sun & Zhang, 2012). Applied a‐CAM technology, processed materials, layer thickness and possible post‐processing measures can affect the time of a‐CAM procedures (Kaleli & Ural, 2020; Kessler et al., 2020; Presotto et al., 2019; Sames et al., 2016; Silva et al., 2011).
It is often postulated that a‐CAM is a time‐efficient and cost‐effective manufacturing technology (Revilla‐Leon et al., 2020; Williams et al., 2020). There is no general consent, however, to support or reject the potential advantages of a‐CAM technologies compared to the s‐CAM technologies in terms of fabrication efficiency and effectiveness for fixed and removable implant prostheses. Therefore, the aim of the present systematic review was to systematically assess the dental literature reporting on production time, effectiveness and/or costs of CAD‐CAM implant prostheses involving s‐CAM and a‐CAM.
2. MATERIAL AND METHODS
2.1. Protocol development and eligibility criteria
A protocol was developed and registered in PROSPERO (CRD42020195942). Conducting and reporting was performed according to the Preferred Reporting Items for Systematic Review and Meta‐analyses (PRISMA) statement (Liberati et al., 2009; Moher et al., 2009).
2.2. Focused question
What are the production time, the effectiveness and costs for s‐CAM and a‐CAM technologies applied in the digital workflow for the fabrication of fixed and removable implant prostheses?
2.3. PICO
The following PICO terms were used:
P Population: patients receiving fixed or removable implant prostheses
I Intervention: fabrication of fixed or removable implant prostheses applying a‐CAM technologies
C Comparison: fabrication of fixed or removable implant prostheses applying s‐CAM
Outcome: production time (primary outcome), effectiveness (number of prostheses in need of chairside/laboratory adjustments at delivery) and costs (secondary outcomes)
2.4. Search strategy
Three online databases Medline (PubMed), Embase and Cochrane Central Register of Controlled Trials (CENTRAL) were screened for eligible studies. The search was limited to clinical studies published from 1 January 1990 to June 2020 in the dental literature and in English language. An additional hand search was performed by screening the reference list of all included full‐text articles.
2.4.1. Search protocol
The following search terms were used:
For identifying the “population”:
Implants
[MeSH terms]: Dental implants OR Dental Implants, Single‐Tooth OR Dental Implantation OR Dental Implantation, Endosseous
OR
[Text Words]: “implant*”
OR
[Emtree terms]: tooth implant OR single tooth implant OR tooth implantation
Reconstructions/dentures
[MeSH terms]: Dental Prosthesis OR Dental Prosthesis, Implant‐Supported OR denture, fixed partial OR dentures, fixed partial OR Crowns OR Dentures OR Overdenture OR Overdentures OR Dental restoration, Permanent OR Tooth, Artificial OR Dental abutments
OR
[Text Words]: “prosth*" OR "replacement*" OR "reconstruction*" OR "restoration*" OR "suprastructure*" OR “restoration” OR "crown*" OR "fixed dental prosthes*" OR "fixed partial denture*" OR "bridge*" OR "full‐arch*" OR "framework*" OR "bar*" OR “denture*” OR “abutment” OR “attachment”
OR
[Emtree terms]: tooth prosthesis OR implant‐supported denture OR tooth crown OR fixed partial denture OR fixed dental prosthesis OR denture OR overlay denture OR dental restoration OR dental abutment
For identifying the “intervention/comparison”:
[MeSH terms]: Dental Technology OR Computer‐Aided Design OR Computer‐Aided Manufacturing OR Manufacturing, Computer Aided OR Design, Computer Aided OR Printing, Three Dimensional OR Printings, Three‐Dimensional OR Three‐Dimensional Printings OR 3‐Dimensional Printing OR 3 Dimensional Printing OR 3‐Dimensional Printings OR Printing, 3‐Dimensional OR Printings, 3‐Dimensional OR 3‐D Printing OR 3 D Printing OR 3‐D Printings OR Printing, 3‐D OR Printings, 3‐D OR Three‐Dimensional Printing OR Three Dimensional Printing OR 3D Printing OR 3D Printings OR Printing, 3D OR Printing, 3D OR Stereolithography OR Hot Melt Extrusion Technology
OR
[Text Words]: “digital” OR “virtual” OR “intraoral impression” OR “IOS” OR “scan” OR “techn*” OR “CAD” OR “CAM” OR “computer” OR “manufactur* OR “design” OR “fabricat*” OR “produc*” OR “additive” OR “print*” OR “3D” OR “stereolithography” OR “SLA” OR “digital light processing” OR “direct light processing” OR “DLP” OR “material jetting” OR “MJ” OR “direct inkjet printing” OR “inkjet printing” OR aerosol jet printing” OR “material extrusion” OR “ME” OR “fused deposition of ceramic” OR “FDC” OR “multiphase jet solidification” OR “MJS” OR “extrusion free forming” OR “solid free forming” OR “EFF” OR “robocasting” OR “direct ink writing” OR “DIW” OR “direct‐write assembly” OR “DWA” OR “microrobotic deposition” OR “µRD” OR “three‐dimensional fibre deposition” OR “micropen” OR “power bed fusion” OR “PBF” OR “selective laser sintering” OR “SLS” OR “powder” OR “slurry coating” OR “aerosol‐assisted spray deposition” OR “selective laser melting” OR “SLM” OR “slurry spraying” OR “ring blade” OR “direct energy deposition” OR “direct laser cladding” OR “hybrid fused deposition modeling” OR “sheet lamination modelling” OR “laminated object modelling” OR “LOM” OR “traditional sheet lamination” OR “sheet lamination” OR computer‐aided manufacturing of laminated engineering materials” OR “binder jetting” OR “3D printers” OR binder jetting of dry powder agglomerates” OR Slurry‐based 3D printing” OR “subtractive” OR “mill*” OR “cut*” OR “CNC” OR “trajectory” OR “bur”
OR
[Emtree terms]: dental technology OR computer aided design OR computer aided design/computer aided manufacturing OR dental CAD/CAM system OR stereolithography OR three dimensional printing OR fused deposition modeling OR selective laser sintering OR hot melt extrusion OR robocasting OR powder OR selective laser melting OR fused deposition modelling OR digital light processing OR electrophoretic deposition OR milling
For identifying the “outcome”:
[MeSH terms]: Clinical Effectiveness OR Effectiveness, Clinical OR Treatment Effectiveness OR Effectiveness, Treatment OR Treatment Efficacy OR Clinical Efficacy OR Efficacy, Clinical OR Time Management OR Cost Effectiveness OR Effectiveness, Cost OR Cost Benefit OR Costs and Benefits OR Benefits and Costs OR Cost‐Effectiveness Analysis OR Analysis, Cost‐Effectiveness OR Cost Effectiveness Analysis
OR
[Text Words]: "efficien*" OR “effect*” OR ”time" OR "effort*" OR "cost*" OR "money*" OR "finance*" OR "economic*" OR "deliver*" OR “remake” OR “chairside” OR “adjust*” OR “intervention”
OR
[Emtree terms]: productivity OR clinical effectiveness OR therapy OR time OR time management OR exercise OR cost OR cost effectiveness analysis OR money OR finance OR economic aspect OR dental CAD/CAM system
2.4.2. Final search strategy
(Implants AND Reconstructions/Dentures) AND (Intervention/Comparison OR outcome).
2.4.3. Inclusion criteria
Clinical studies with a minimal number of five patients and studies conducted in the dental laboratory with at least five clinical cases were included.
2.5. Exclusion criteria
In vitro and preclinical studies, interviews, charts, case reports with less than five patients and reports based on questionnaires were excluded from this systematic review. Studies on provisional or interim prostheses were not included.
2.6. Selection of publications
Two reviewers (SM and JH) performed the screening independently. Titles and abstracts were evaluated for suitability. In case of disagreement, the decision was made by discussion between all authors. Full‐text articles of selected abstracts were acquired, and the final selection based on inclusion/exclusion criteria was made. For the final inclusion, the Material and Methods, Results and Discussion of the full‐text articles were assessed and double‐checked by two reviewers (SM and JH). Again, in case of disagreement during the evaluation, consensus was attained by discussions between all authors. As a measure of agreement, Cohen's kappa coefficient was calculated for abstract and full‐text screening.
2.7. Data extraction and method of analysis
The following parameters were extracted and recorded from the selected full texts (Table 1): author(s), year of publication, study design, number of patients/cases, mean age, implant system, prosthesis design (fixed, removable), number of prostheses, type and system of digitalization (conventional/IOS), CAD system, CAM system, type of CAM (subtractive/additive), location of CAM and finalization of prosthesis (point‐of‐care, dental laboratory). Authors were contacted for clarification in case the included studies were lacking information to properly extract the data.
TABLE 1.
Description of included studies
| Author/Year | Study design | Subjects n | Mean age | Implant system | Design prosthesis | Number of prosthesis | Digital fabrication | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Data acquisition | Laboratory scanner | CAD system | CAM system | Type of CAM | Location of CAM | Finalization of prosthesis | |||||||
| Di Fiore et al. (2018) | prospective cohort study with a crossover design | 10 | na | na | fixed | 10 | Cerec Omnicam | CEREC Biogeneric | CEREC MC XL, Sirona |
Subtractive (monolithic crown) |
point‐of‐care | point‐of‐care | |
| Joda and Bragger (2014) | prospective cohort study | 6 | na | Straumann Tissue Level | fixed | 3 | iTero, Align Technology | Straumann CARES | Straumann CARES |
subtractive (monolithic crown) |
implant manufacturer | dental laboratory | |
| 3 | subtractive (customized abutment, monolithic crown) | ||||||||||||
| Joda and Bragger (2014) | prospective cohort study with a crossover design | 20 | 55.4y | Straumann Tissue Level | fixed | 20 | iTero, Align Technology | Straumann CARES | Straumann CARES | subtractive (customized abutment, crown coping) | implant manufacturer | dental laboratory | |
| Joda and Bragger (2016) | Randomized controlled trial | 20 | 55.4y | Straumann Tissue Level | fixed | 10 | iTero, Align Technology | Straumann CARES | Straumann CARES | subtractive (monolithic crown) | implant manufacturer | dental laboratory | |
| 10 | subtractive (customized abutment, model) | ||||||||||||
| Mangano, Margiani, et al. (2019) | retrospective cohort study | 25 | 51.1 | Exacone, Leone Implants | fixed | 40 | CS 3,600, Carestream Dental | Exocad DentalCAD, Exocad | Roland DWX−50, Roland Easy Shape | subtractive (abutment and monolithic crown | dental laboratory | dental technician | |
| Mangano and Veronesi (2018) | randomized controlled clinical trial | 50 | 52.6 | Exacone, Leone Implants | fixed | 25 | CS 3,600, Carestream Dental | Exocad DentalCAD, Exocad | Roland DWX−50, Roland Easy Shape | subtractive | dental laboratory | dental technician | |
| Mangano, Mangano, et al. (2019) | prospective cohort study | 15 | 68.8 | BTSafe, BTK | removable | 15 | CS 3,600, Carestream Dental | Exocad DentalCAD, Exocad | 3500PD | additive (prototype bar) | dental laboratory | ||
| Roland DWX−51, Roland Easy Shape | subtractive (PEEK bar) | dental laboratory | |||||||||||
| Pan et al. (2019) | prospective cohort study with a crossover design | 40 | 45.1y | Straumann Tissue Level and Bone Level | fixed | 40 | Trios, 3Shape | 3Shape Designer | Zentotec Select Hybrid | subtractive (monolithic crown) | dental laboratory | dental laboratory | |
| 40 | conventional | D2000, 3Shape | |||||||||||
| Zhang, Qiu, et al. (2019) | Randomized controlled trial | 33 | 46.8y | CAMLOG SCREW‐LINE, Camlog Biotechnologies AG | fixed | 17 | Cerec Omnicam | CEREC | CEREC MC XL Premium |
subtractive (monolithic crown) |
point‐of‐care | point‐of‐care (dentist, dental technician) | |
| 16 | conventional | Trios lab, 3Shape | Trios lab, 3Shape | centralized manufacture | subtractive (crown coping) | unknown | |||||||
2.8. Evaluation of quality
The methodological quality of the included studies was assessed independently by two reviewers (SM and JH) using Cochrane Collaboration's tool for assessing risk of bias (Higgins & Green, 2011). For non‐randomized studies, modified risk assessment tool was used. In case of disagreement, the decision was made by discussion between all authors.
3. RESULTS
3.1. Search
The details of the search strategy are illustrated in Figure 1. The electronic search identified a total of 7,482 titles. After the evaluation of titles, 7,219 studies were discarded and 20 duplicates were removed. Following the screening of 243 abstracts, 34 studies were selected for detailed reading of the full text (Cohen's kappa coefficient = 0.78). Finally, 8 studies met the inclusion criteria (Cohen's kappa coefficient = 0.72). One study was added through the manual search, resulting in an overall number of 9 included articles.
FIGURE 1.
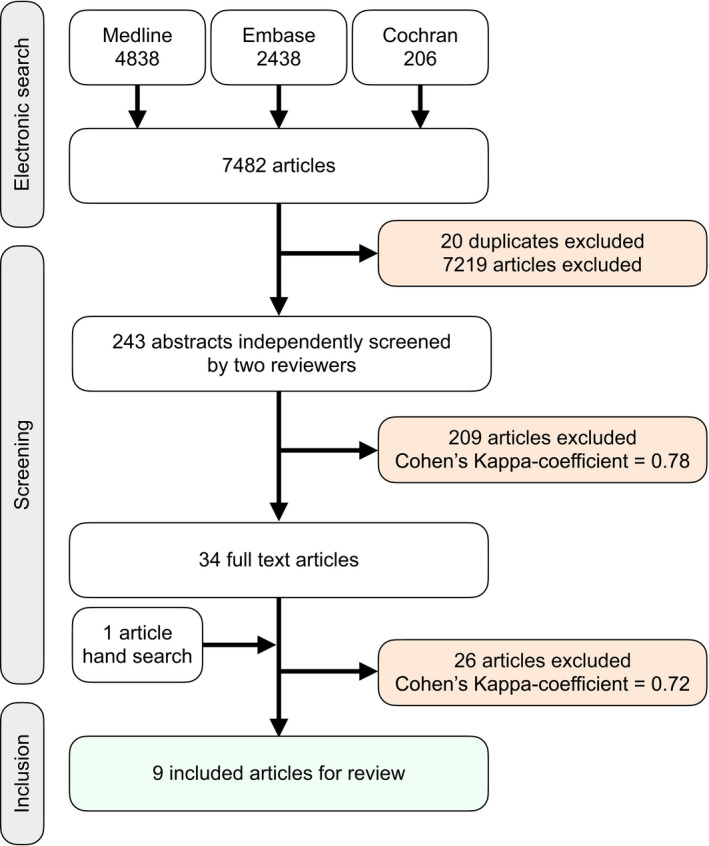
Flow diagram and selection process
The reasons for exclusion of studies are depicted in Table S1: description of a CAM process without information on time and/or effectiveness (n = 14), no detailed data on fabrication method (1), no CAM fabrication (1), no implant reconstruction (n = 1), outcomes limited to clinical follow‐up (6), interview (n = 1), interim prosthesis (n = 1), and full text not in English language (n = 1).
3.2. Description of studies
All characteristics of the included studies are described in Table 1. Eight included studies applied s‐CAM technologies for the fabrication of single implant crowns in the posterior area. One included study used a combination of a‐CAM and s‐CAM technologies for the fabrication of an implant bar serving as retention for a maxillary overdenture. No studies were identified reporting on the CAM fabrication of multi‐unit fixed implant prostheses considering the primary and secondary outcomes.
None of the three randomized controlled clinical studies was primarily assessing production time, effectiveness and costs comparing s‐CAM and a‐CAM. Consequently, a total of 9 prospective clinical studies reporting on 11 patient cohorts were identified. Five studies reported on the production time using s‐CAM technologies for the fabrication of models (2), customized abutments (3), monolithic crowns (4) and crown cores (2). Time for the CAD process was reported in all five studies, whereas the time for the manufacturing process was evaluated in two and the post‐processing/finalization of the prostheses in 4 out of these five studies (Table 2). One study reported on the total working time applying a s‐CAM process without further specification and details. Therefore, that study was excluded from the analysis (Joda & Bragger, 2014). Effectiveness and fabrication costs (secondary outcomes) were reported in 8 and 3 of the included studies, respectively.
TABLE 2.
Primary and secondary outcomes of included studies (na, not applicable)
| Manufacturing time | Effectiveness | Costs | |||
|---|---|---|---|---|---|
| Preparation | CAM | Post‐processing | |||
| Di Fiore et al. (2018) | n.a. | n.a. | n.a. | Time for chairside adjustments | n.a. |
| Joda and Bragger (2014) | n.a. | n.a. | n.a. | Number of crowns in need of chairside | Total laboratory costs |
| Joda and Bragger (2015) |
CAD model CAD customized abutment CAD crown coping |
n.a. |
Ceramic veneering glazing and polishing |
Time for chairside adjustments | n.a. |
| Joda and Bragger (2016) |
CAD model CAD monolithic crown |
n.a. |
Assembly of components polishing |
Time for chairside adjustments | Total laboratory costs |
| CAD customized abutment |
Veneering glazing and polishing |
||||
| Mangano, Margiani, et al. (2019) | n.a. | n.a. | n.a. | Number of crowns in need of chairside and laboratory adjustments | n.a. |
| Mangano and Veronesi (2018) |
CAD customized abutment CAD monolithic crown |
Subtractive |
Sintering assembly of components staining |
n.a. |
Customized abutment Monolithic crown staining |
| Mangano, Mangano, et al. (2019) | n.a. | n.a. | n.a. | Number of 3D‐printed prototype bars with passive fit | n.a. |
| Pan et al. (2019) | CAD monolithic crown | n.a. | n.a. |
Time for chairside adjustments Number of crowns in need of chairside and laboratory adjustments |
n.a. |
| Zhang, Qiu, et al. (2019) | CAD monolithic crown | subtractive | Fitting and staining |
Time for chairside adjustments Number of crowns in need of chairside and laboratory adjustments |
n.a. |
| CAD crown coping |
Sintering veneering |
||||
3.3. Risk of bias in included studies
Table 3 describes the risk of bias assessment of the nine included studies. For the double‐blind self‐controlled clinical study (Pan et al., 2019), the risk of bias was rated low in all categories, whereas for the three randomized controlled clinical trials, a high risk of performance bias and an unclear risk of detection bias were estimated. Considering the primary and secondary outcomes, attrition bias was rated low in all included studies.
TABLE 3.
Risk of bias assessment. For non‐randomized studies, selection bias was not applicable (na)
| References | Selection bias, sequence generation | Selection bias, allocation concealment | Performance bias (blinding of participants and personnel) | Detection bias (blinding of outcome assessment) | Attrition bias (loss of patients to follow‐up) | Selective reporting bias (selective revealing or suppression of information) |
|---|---|---|---|---|---|---|
| Di Fiore et al. (2018) | na | na | High | High | Low | High |
| Joda and Brägger (2014) | na | na | High | Unclear | Low | Low |
| Joda and Brägger (2015) | na | na | High | Unclear | Low | High |
| Joda and Brägger (2016) | High | High | High | Unclear | Low | High |
| Mangano and Veronesi (2018) | Low | Low | High | Unclear | Low | High |
| Mangano, Mangano, et al. (2019) | na | na | High | High | Low | High |
| Mangano, Margiani, et al. (2019) | na | na | High | High | Low | Low |
| Pan et al. (2019) | na | na | Low | Low | Low | Low |
| Zhang, Qiu, et al. (2019) | Low | Low | High | Unclear | Low | Low |
The color shades indicates high risk (red), unclear risk (yellow), low risk (green).
3.4. Primary outcome: Production time
3.4.1. Digital workflow applying centralized CAM
In three studies, centralized s‐CAM was involved, whereas the CAD process and the finalization of the prostheses were based in the dental laboratory. Two studies reported on a calibrated workflow (Straumann CARES) using a laboratory CAD software and centralized s‐CAM provided by the implant manufacturer (Joda & Bragger, 2015, 2016), while one study used a laboratory CAD software (3Shape Designer) and a unspecified third‐party provider for centralized s‐CAM (Zhang et al., 2019).
The time for the virtual design (CAD) of (a) an implant model ranged between 4.9 min (Joda & Bragger, 2015) and 11.8 min (Joda & Bragger, 2016), (b) an abutment from 19.7 min (Joda & Bragger, 2016) up to 32.7 min (Joda & Bragger, 2015), (c) a monolithic crown accounted for 22.3 min (monolithic crown) (Joda & Bragger, 2016) and (d) a crown coping between 11.1 min (Zhang, Tian, et al., 2019) and 37.6 min (Joda & Bragger, 2015) (Table 4).
TABLE 4.
Manufacturing time for single implant crowns by means of s‐CAM
| Preparation task | Time (min) | s‐CAM | Time (min) | Post‐processing | Time(min) | |
|---|---|---|---|---|---|---|
| Digital workflow applying centralized s‐CAM | ||||||
| Joda and Bragger (2015) |
CAD model CAD customized abutment CAD crown coping |
4.9 32.7 37.6 |
n.a. |
Ceramic Veneering glazing and polishing |
71.4 11.5 |
|
| Joda and Bragger (2016) |
CAD model CAD monolithic crown |
11.8 22.3 |
n.a. |
Assembly of components Polishing |
5.0 15.4 |
|
| CAD customized abutment | 19.7 |
Veneering Glazing and polishing |
74.7 28.8 |
|||
| Zhang, Qiu, et al. (2019) | CAD crown coping | 11.1 | Milling zirconia crown coping | 8.2 |
Sintering Veneering |
530.0 89.9 |
| Digital workflow applying laboratory s‐CAM | ||||||
| Mangano and Veronesi (2018) |
CAD customized abutment CAD monolithic crown |
30.0 10.0 |
Milling zirconia abutment/crown | 25.0 |
Sintering assembly of Components staining |
480.0 10.0 10.0 |
| Pan et al. (2019) | CAD monolithic crown | 12.6 | n.a. | n.a. | ||
| Digital workflow applying chairside s‐CAM | ||||||
| Zhang, Qiu, et al. (2019) | CAD monolithic crown | 28.9 | Milling lithium disilicate crown | 21.4 | Fitting and staining | 40.2 |
Production time for the centralized s‐CAM process was reported in one study (Zhang, Tian, et al., 2019). The milling of the crown coping (zirconia) accounted for 8.2 min and the sintering lasted for 530 min. No information on delivery times of these centrally manufactured products to the dental laboratory was available.
The time needed for the finalization of the CAM products in the dental laboratory depended on the type of prosthesis. The veneering was most time‐consuming and accounted for 71.4 min (Joda & Bragger, 2015), 74.7 min (Joda & Bragger, 2016) and 89.9 min (Zhang, Tian, et al., 2019). Glazing/polishing ranged between 11.5 min (Joda & Bragger, 2015) and 28.8 min (Joda & Bragger, 2016). For the monolithic design, the assembly of the prosthetic components took 5.0 min followed by the polishing procedure accounting to 15.4 min (Joda & Bragger, 2016).
3.4.2. Digital workflow applying laboratory CAM
In two studies, the entire fabrication process was located in the dental laboratory. The laboratory CAD software was applied for the virtual design of a monolithic crown (3Shape Designer) (Pan et al., 2019), and an implant crown consisting of a customized abutment and a monolithic crown (Exocad DentalCAD) (Mangano & Veronesi, 2018).
The time for the CAD of a monolithic zirconia crown ranged between 10.0 min (Mangano & Veronesi, 2018) and 12.6 min (Pan et al., 2019), while the customization of the abutment took 30.0 min (Mangano & Veronesi, 2018) (Table 4).
The time for the s‐CAM of the zirconia abutment/crown by means of a 5‐axis milling machine (Roland DWX‐50) was 25 min followed by 480 min of sintering (Mangano & Veronesi, 2018). In the second study, the manufacturer of the laboratory‐based s‐CAM device was provided (Zenotec Select Hybrid), but no time recordings were performed (Pan et al., 2019).
The final assembly of the hybrid abutment (cementation of customized zirconia abutment to titanium base) and the characterization of the monolithic crown was 10 min each (Mangano & Veronesi, 2018).
3.4.3. Digital workflow applying chairside CAM
One study reported on a point‐of‐care CAD‐CAM system (Cerec) in a chairside workflow (Zhang, Tian, et al., 2019). The CAD of a monolithic crown took 28.9 min and the s‐CAM of lithium disilicate by means of a point‐of‐care milling device (Cerec MC XL, Sirona Dentsply) was 21.4 min. Post‐processing included fitting and staining and amounted to a total of 40.2 min (Table 4).
3.5. Secondary outcomes
3.5.1. Effectiveness
Five studies reported on the effectiveness for monolithic implant crowns fabricated by means of s‐CAM (Di Fiore et al., 2018; Joda & Bragger, 2016; Mangano et al., 2019; Pan et al., 2019; Zhang, Tian, et al., 2019)). At delivery, the time for chairside adjustments ranged from no intervention needed (Joda & Bragger, 2016) to 1.9 min (Di Fiore et al., 2018), 8.4 min (Zhang, Tian, et al., 2019), up to a range between 11.4 and 12.3 min (Pan et al., 2019). Three studies reported time for adjustments at delivery of veneered implant crowns consisting of CAM products. The chairside time ranged between 2.6 min (Joda & Bragger, 2015), 3.3 min (Joda & Bragger, 2016) and 12.3 min (Zhang, Tian, et al., 2019).
Five studies provided frequency distributions on the number of monolithic implant crowns in need of chairside or laboratory adjustments (Joda & Bragger, 2014, 2016; Mangano, Margiani, et al., 2019; Pan et al., 2019; Zhang, Tian, et al., 2019). Out of a total of 136 (96 model‐free and 40 model‐based) laboratory‐made monolithic implant crowns, 50.7% (range 0% to 80%) and 11.0% (range 0%–13.8%) needed chairside adjustments and laboratory interventions (redo or adjustment), respectively. Of model‐free crowns, 43.8%/10.4% needed chairside/laboratory adjustments, whereas of model‐based crowns, 70%/12.5% needed chairside/laboratory adjustments. In a chairside workflow, 15 out of 17 (88.2%) monolithic lithium disilicate implant crowns were adapted chairside to be delivered, whereas one crown needed to be redone in a subsequent laboratory workflow (Zhang, Tian, et al., 2019). For veneered CAD‐CAM implant crowns, 24/26 (92.3%) needed chairside interventions and additional laboratory veneering was needed in 5/26 (19.2%) (Joda & Bragger, 2016; Zhang, Tian, et al., 2019).
One clinical study evaluated the effectiveness of the fabrication of a milled polyether‐ether‐ketone (PEEK) implant bar fixed on 4 implants in the maxilla (Mangano et al., 2019). The clinical protocol involved the try‐in of a 3D‐printed resin bar and demonstrated that 3 out of 15 (20%) resin bars did not have a sufficient fit or adaptation. In these 3 cases, data acquisition and processing were repeated. Finally, all milled PEEK bars could be successfully delivered without remake.
3.5.2. Costs
Laboratory costs for implant crowns were documented in three studies. Based on a laboratory tariff system, the total costs ranged between CHF 505.85 (Joda & Bragger, 2016) and CHF 785 (Joda & Bragger, 2014) for monolithic implant crowns. One study provided detailed costs for the CAM products: EUR 50 for a hybrid abutment, EUR 80 for a monolithic zirconia crown and additional EUR 30 for the characterization of the crown (Mangano & Veronesi, 2018).
4. DISCUSSION
The present systematic review revealed (a) production time, effectiveness and costs to be thoroughly documented for fabrication workflows involving s‐CAM, (b) production time for the implant crowns to range substantially depending on the prosthesis design and the applied digital workflow, (c) the laboratory fabrication of monolithic implant crowns to result in the least number of chairside and labside interventions and (d) a lack of clinical scientific evidence on production time for fabrication workflows applying a‐CAM.
A recent systematic review demonstrated the implementation of digital technologies to substantially increase time efficiency in the laboratory fabrication of implant prostheses compared to manual techniques (Muhlemann, Kraus, et al., 2018). The present systematic search provides data on time required for all steps of the digital workflow involving s‐CAM technology including the CAD process, the CAM production and the laboratory finalization of the CAD‐CAM products. In addition, time for s‐CAM is documented in chairside (point‐of‐care), laboratory and centralized workflows. No clinical study, however, evaluated production time for digital workflows involving a‐CAM. Therefore, it remains unclear whether fabrication workflows with a‐CAM are more efficient compared to workflows with s‐CAM.
Time for CAD varied greatly among the included studies. In general, the number of prosthetic/auxiliary components and the need for customization influenced production time. The calculated time differences for the CAD process among the included studies were further affected by (a) the heterogeneity of the study design, (b) the applied software systems and (c) even the software version (Haddadi et al., 2018; Shim et al., 2015). The digital systems are constantly improving and, for example, resulted in a 70% more efficient CAD process for a monolithic implant crown between 2015 (37.6 min) (Joda & Bragger, 2015) and 2019 (12.0 min) (Pan et al., 2019).
The restoration materials processed by s‐CAM were zirconia and lithium disilicate (Mangano & Veronesi, 2018; Zhang, Tian, et al., 2019). Production time of single implant components by means of s‐CAM ranged between 8.2 and 25 min. Time differences may be attributed to (a) the different s‐CAM systems applied (e.g. 4‐axis versus 5‐axis milling devices) and (b) the different restoration materials processed.
The present systematic review revealed that post‐processing is a time‐consuming process. Sintering of zirconia may take more than 8 hr (Mangano & Veronesi, 2018) compared to lithium disilicate accounting for 40.2 min (Zhang, Tian, et al., 2019). Consequently, zirconia is not the material of choice for chairside restorations. Recent in vitro studies, however, demonstrated that speed sintering of zirconia may allow to overcome this limitation while still maintaining its favourable mechanical properties (Jerman et al., 2020; Michailova et al., 2020).
The costs of CAD‐CAM technology are rather high when data are compared to manual fabrication in the dental laboratory (Muhlemann, Kraus, et al., 2018). For example, one included study reported on the costs of prosthetic components (between EUR 50 and 80) processed by means of s‐CAM (Mangano & Veronesi, 2018). It remains unknown though whether these costs covered all expenses including the salary of the operator, the restoration material, any consumables (e.g. milling burs) and the costs for the amortization of the CAM device. Still, the possibility of using services offered by external parties may allow accessing cost‐effective CAD‐CAM products.
None of the included studies reported on the production time when using a‐CAM. Until recently, a‐CAM technologies were too expensive to be applied in the fabrication workflow of implant prostheses, thereby explaining the limited number of clinical studies. Moreover, the development of materials (a) to be processed by means of a‐CAM and (b) to be successfully applied in implant prosthetics is still in its early phase (Revilla‐Leon et al., 2019; Revilla‐Leon, Meyer, et al., 2020; Revilla‐Leon & Ozcan, 2019).
The production time by means of a‐CAM depends on the respective technology and the printer settings applied. Generally, an increase in layer thickness results in a faster production of the object (prosthetic or auxiliary component). Such an increase in layer thickness (i.e. a faster production) negatively affects the surface resolution (Kaleli & Ural, 2020; Kaleli et al., 2019) and the mechanical properties of the restoration (Shim et al., 2020). One has to bear in mind that the capacity of the a‐CAM technology (Kessler et al., 2020) and the properties of the processed a‐CAM material (Sames et al., 2016) may limit the layer thickness, thereby controlling the production time. Finally, additional programming (nesting) may be indicated for an ideal orientation of the virtual object relative to the printing direction to improve the marginal and internal adaptation of the prosthesis (Shim et al., 2020).
A‐CAM involves post‐processing procedures. The removal of supporting structures depends on the a‐CAM technology applied and consequently influences the production time. In case of the material jetting technology, the co‐deposited uncured material serves as a supporting structure and its removal is achieved by a fast cleaning process. In contrast, complex supporting structures are crucial to prevent a collapse of the object during the manufacturing process when stereolithography (SLA) or digital light processing (DLP) technologies are applied (Presotto et al., 2019; Silva et al., 2011). Moreover, the object (prosthetic/auxiliary component) can be chemically and/or mechanically treated, and curing cycles may be added. The need, type and time for post‐processing procedures depend on the a‐CAM technology and the manufacturer's recommendations.
The thorough search performed in the present systematic review resulted in only one clinical study applying a digital workflow involving a‐CAM. A‐CAM technology was used for the fabrication of an auxiliary component as part of the fabrication workflow for an implant‐retained bar (Mangano, Mangano, et al., 2019). A prototype bar was 3D‐printed using a polymer with the intent to increase the effectiveness of a workflow using s‐CAM. To fulfil this requirement, high accuracy and dimensional stability of a‐CAM products are crucial. Based on a recent systematic review, the accuracy of 3D‐printed dental models varies widely and can reach a maximum mean error of 579 μm (Etemad‐Shahidi et al., 2020). The precision of 3D‐printed polymer models is significantly influenced by the digital workflow applied (Muhlemann et al., 2018), the settings of manufacturing parameters (e.g. build direction) (Park et al., 2019) and the type of post‐processing and storage (Etemad‐Shahidi et al., 2020). It is evident that accuracy of a‐CAM products has to achieve the level of s‐CAM to be successfully integrated in the digital fabrication workflow.
Further clinical studies on production time applying a digital workflow for the fabrication of implant prostheses should (a) focus on the use of a‐CAM technologies, (b) specify the applied a‐CAM technology and the parameter settings (e.g. layer thickness) and (c) document the prosthetic and auxiliary components in detail. In addition, the time for post‐processing of the a‐CAM product (debedding, sintering, cleaning, curing, surface treatments) needs to be recorded. Finally, effectiveness should be reported by the number, type and time of chairside adjustments needed for each prosthesis rather than providing mean time for chairside adjustments.
5. CONCLUSIONS
The scientific evidence obtained through the present systematic review provides no data on production time, effectiveness and costs in the digital workflow comparing s‐CAM and a‐CAM. Post‐processing of CAM products may substantially contribute to the overall production time. Monolithic CAD‐CAM implant crowns may be preferred because the need for chairside/laboratory adjustments was lower compared to veneered implant crowns.
CONFLICT OF INTEREST
The authors report no conflict of interest.
AUTHOR CONTRIBUTION
Sven Mühlemann: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (lead); Methodology (lead); Validation (lead); Writing‐original draft (lead); Writing‐review & editing (lead). Jenni Hjerppe: Conceptualization (supporting); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Writing‐original draft (supporting); Writing‐review & editing (supporting). Christoph H. F. Hämmerle: Conceptualization (supporting); Investigation (supporting); Methodology (supporting); Resources (lead); Supervision (supporting). Daniel S. Thoma: Conceptualization (equal); Data curation (supporting); Formal analysis (supporting); Investigation (equal); Methodology (supporting); Writing‐original draft (equal); Writing‐review & editing (equal).
Supporting information
Table S1
ACKNOWLEDGEMENTS
Open Access Funding provided by Universitat Zurich.
Mühlemann, S. , Hjerppe, J. , Hämmerle, C. H. F. , & Thoma, D. S. (2021). Production time, effectiveness and costs of additive and subtractive computer‐aided manufacturing (CAM) of implant prostheses: A systematic review. Clinical Oral Implants Research, 32, 289–302. 10.1111/clr.13801
DATA AVAILABILITY STATEMENT
Data can be provided upon request.
REFERENCES
- Atzeni, E. , & Salmi, A. (2012). Economics of additive manufacturing for end‐usable metal parts. The International Journal of Advanced Manufacturing Technology, 62, 1147–1155. 10.1007/s00170-011-3878-1 [DOI] [Google Scholar]
- Bosch, G. , Ender, A. , & Mehl, A. (2014). A 3‐dimensional accuracy analysis of chairside CAD/CAM milling processes. Journal of Prosthetic Dentistry, 112, 1425–1431. 10.1016/j.prosdent.2014.05.012 [DOI] [PubMed] [Google Scholar]
- de Oliveira, N. R. C. , Pigozzo, M. N. , Sesma, N. , & Lagana, D. C. (2020). Clinical efficiency and patient preference of digital and conventional workflow for single implant crowns using immediate and regular digital impression: A meta‐analysis. Clinical Oral Implants Research, 31, 669–686. 10.1111/clr.13604 [DOI] [PubMed] [Google Scholar]
- Di Fiore, A. , Vigolo, P. , Graiff, L. , & Stellini, E. (2018). Digital vs conventional workflow for screw‐retained single‐implant crowns: A comparison of key considerations. International Journal of Prosthodontics, 31, 577–579. 10.11607/ijp.5938 [DOI] [PubMed] [Google Scholar]
- Etemad‐Shahidi, Y. , Qallandar, O. B. , Evenden, J. , Alifui‐Segbaya, F. , & Ahmed, K. E. (2020). Accuracy of 3‐dimensionally printed full‐arch dental models: A systematic review. Journal of Clinical Medicine, 9, 10.3390/jcm9103357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadi, Y. , Bahrami, G. , & Isidor, F. (2018). Effect of software version on the accuracy of an intraoral scanning device. International Journal of Prosthodontics, 31, 375–376. 10.11607/ijp.5781 [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T. , & Green, S. (2011). Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). London: The Cochrane Collaboration. Retrieved from http://handbook.cochrane.org [Google Scholar]
- ISO/ASTM 52900‐15 . (2009). Standard Terminology for Additive Manufacturing – General Principles – Terminology. ASTM International. [Google Scholar]
- Jerman, E. , Wiedenmann, F. , Eichberger, M. , Reichert, A. , & Stawarczyk, B. (2020). Effect of high‐speed sintering on the flexural strength of hydrothermal and thermo‐mechanically aged zirconia materials. Dental Materials, 36, 1144–1150. 10.1016/j.dental.2020.05.013 [DOI] [PubMed] [Google Scholar]
- Joda, T. , & Bragger, U. (2014). Complete digital workflow for the production of implant‐supported single‐unit monolithic crowns. Clinical Oral Implants Research, 25, 1304–1306. 10.1111/clr.12270 [DOI] [PubMed] [Google Scholar]
- Joda, T. , & Bragger, U. (2015). Time‐efficiency analysis comparing digital and conventional workflows for implant crowns: A prospective clinical crossover trial. International Journal of Oral and Maxillofacial Implants, 30, 1047–1053. 10.11607/jomi.3963 [DOI] [PubMed] [Google Scholar]
- Joda, T. , & Bragger, U. (2016). Time‐efficiency analysis of the treatment with monolithic implant crowns in a digital workflow: A randomized controlled trial. Clinical Oral Implants Research, 27, 1401–1406. 10.1111/clr.12753 [DOI] [PubMed] [Google Scholar]
- Kaleli, N. , & Ural, C. (2020). Digital evaluation of laser scanning speed effects on the intaglio surface adaptation of laser‐sintered metal frameworks. Journal of Prosthetic Dentistry, 123, 874 e871–874 e877. 10.1016/j.prosdent.2019.12.020 [DOI] [PubMed] [Google Scholar]
- Kaleli, N. , Ural, C. , Ozkoylu, G. , & Duran, I. (2019). Effect of layer thickness on the marginal and internal adaptation of laser‐sintered metal frameworks. Journal of Prosthetic Dentistry, 121, 922–928. 10.1016/j.prosdent.2018.08.018 [DOI] [PubMed] [Google Scholar]
- Kessler, A. , Hickel, R. , & Reymus, M. (2020). 3D printing in dentistry‐state of the art. Operative Dentistry, 45, 30–40. 10.2341/18-229-L [DOI] [PubMed] [Google Scholar]
- Koch, G. K. , Gallucci, G. O. , & Lee, S. J. (2016). Accuracy in the digital workflow: From data acquisition to the digitally milled cast. Journal of Prosthetic Dentistry, 115, 749–754. 10.1016/j.prosdent.2015.12.004 [DOI] [PubMed] [Google Scholar]
- Liberati, A. , Altman, D. G. , Tetzlaff, J. , Mulrow, C. , Gotzsche, P. C. , Ioannidis, J. P. , Clarke, M. , Devereaux, P. J. , Kleijnen, J. , & Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: Explanation and elaboration. Journal of Clinical Epidemiology, 62, e1–34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- Mangano, F. , Mangano, C. , Margiani, B. , & Admakin, O. (2019). Combining intraoral and face scans for the design and fabrication of computer‐assisted design/Computer‐Assisted Manufacturing (CAD/CAM) Polyether‐Ether‐Ketone (PEEK) implant‐supported bars for maxillary overdentures. Scanning, 2019, 1–14. 10.1155/2019/4274715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano, F. , Margiani, B. , & Admakin, O. (2019). A Novel Full‐Digital Protocol (SCAN‐PLAN‐MAKE‐DONE((R))) for the design and fabrication of implant‐supported monolithic translucent zirconia crowns cemented on customized hybrid abutments: A retrospective clinical study on 25 patients. International Journal of Environmental Research and Public Health, 16, 1–20. 10.3390/ijerph16030317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano, F. , & Veronesi, G. (2018). Digital versus analog procedures for the prosthetic restoration of single implants: A randomized controlled trial with 1 year of follow‐up. BioMed Research International, 2018, 1–20. 10.1155/2018/5325032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello, C. C. , Lemos, C. A. A. , de Luna Gomes, J. M. , Verri, F. R. , & Pellizzer, E. P. (2019). CAD/CAM vs conventional technique for fabrication of implant‐supported frameworks: A systematic review and meta‐analysis of in vitro studies. International Journal of Prosthodontics, 32, 182–192. 10.11607/ijp.5616 [DOI] [PubMed] [Google Scholar]
- Methani, M. M. , Revilla‐Leon, M. , & Zandinejad, A. (2020). The potential of additive manufacturing technologies and their processing parameters for the fabrication of all‐ceramic crowns: A review. Journal of Esthetic Restorative Dentistry, 32, 182–192. 10.1111/jerd.12535 [DOI] [PubMed] [Google Scholar]
- Michailova, M. , Elsayed, A. , Fabel, G. , Edelhoff, D. , Zylla, I. M. , & Stawarczyk, B. (2020). Comparison between novel strength‐gradient and color‐gradient multilayered zirconia using conventional and high‐speed sintering. Journal of the Mechanical Behavior of Biomedical Materials, 111, 103977. 10.1016/j.jmbbm.2020.103977 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. & Group, P . (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Med, 6, e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlemann, S. , Greter, E. A. , Park, J. M. , Hammerle, C. H. F. , & Thoma, D. S. (2018). Precision of digital implant models compared to conventional implant models for posterior single implant crowns: A within‐subject comparison. Clinical Oral Implants Research, 29, 931–936. 10.1111/clr.13349 [DOI] [PubMed] [Google Scholar]
- Muhlemann, S. , Kraus, R. D. , Hammerle, C. H. F. , & Thoma, D. S. (2018). Is the use of digital technologies for the fabrication of implant‐supported reconstructions more efficient and/or more effective than conventional techniques: A systematic review. Clinical Oral Implants Research, 29(Suppl 18), 184–195. 10.1111/clr.13300 [DOI] [PubMed] [Google Scholar]
- Muhlemann, S. , Lakha, T. , Jung, R. E. , Hammerle, C. H. F. , & Benic, G. I. (2020). Prosthetic outcomes and clinical performance of CAD‐CAM monolithic zirconia versus porcelain‐fused‐to‐metal implant crowns in the molar region: 1‐year results of a RCT. Clinical Oral Implants Research, 31, 856–864. 10.1111/clr.13631 [DOI] [PubMed] [Google Scholar]
- Padros, R. , Giner, L. , Herrero‐Climent, M. , Falcao‐Costa, C. , Rios‐Santos, J. V. , & Gil, F. J. (2020). Influence of the CAD‐CAM Systems on the Marginal Accuracy and Mechanical Properties of Dental Restorations. International Journal of Environmental Research and Public Health, 17, 4276. 10.3390/ijerph17124276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, S. , Guo, D. , Zhou, Y. , Jung, R. E. , Hammerle, C. H. F. , & Muhlemann, S. (2019). Time efficiency and quality of outcomes in a model‐free digital workflow using digital impression immediately after implant placement: A double‐blind self‐controlled clinical trial. Clinical Oral Implants Research, 30, 617–626. 10.1111/clr.13447 [DOI] [PubMed] [Google Scholar]
- Park, G. S. , Kim, S. K. , Heo, S. J. , Koak, J. Y. , & Seo, D. G. (2019). Effects of printing parameters on the fit of implant‐supported 3D printing resin prosthetics. Materials (Basel), 12, 10.3390/ma12162533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presotto, A. G. C. , Barao, V. A. R. , Bhering, C. L. B. , & Mesquita, M. F. (2019). Dimensional precision of implant‐supported frameworks fabricated by 3D printing. Journal of Prosthetic Dentistry, 122, 38–45. 10.1016/j.prosdent.2019.01.019 [DOI] [PubMed] [Google Scholar]
- Revilla‐Leon, M. , Meyer, M. J. , & Ozcan, M. (2019). Metal additive manufacturing technologies: Literature review of current status and prosthodontic applications. International Journal of Computerized Dentistry, 22, 55–67. [PubMed] [Google Scholar]
- Revilla‐Leon, M. , Meyer, M. J. , Zandinejad, A. , & Ozcan, M. (2020). Additive manufacturing technologies for processing zirconia in dental applications. International Journal of Computerized Dentistry, 23, 27–37. [PubMed] [Google Scholar]
- Revilla‐Leon, M. , & Ozcan, M. (2019). Additive manufacturing technologies used for processing polymers: current status and potential application in prosthetic dentistry. Journal of Prosthodontics, 28, 146–158. 10.1111/jopr.12801 [DOI] [PubMed] [Google Scholar]
- Revilla‐Leon, M. , Sadeghpour, M. , & Ozcan, M. (2020). An update on applications of 3D printing technologies used for processing polymers used in implant dentistry. Odontology, 108, 331–338. 10.1007/s10266-019-00441-7 [DOI] [PubMed] [Google Scholar]
- Sames, W. J. , List, F. A. , Pannala, S. , Dehoff, R. R. , & Babu, S. S. (2016). The metallurgy and processing science of metal additive manufacturing. International Materials Reviews, 61, 315–360. 10.1080/09506608.2015.1116649 [DOI] [Google Scholar]
- Shim, J. S. , Kim, J. E. , Jeong, S. H. , Choi, Y. J. , & Ryu, J. J. (2020). Printing accuracy, mechanical properties, surface characteristics, and microbial adhesion of 3D‐printed resins with various printing orientations. Journal of Prosthetic Dentistry, 124, 468–475. 10.1016/j.prosdent.2019.05.034 [DOI] [PubMed] [Google Scholar]
- Shim, J. S. , Lee, J. S. , Lee, J. Y. , Choi, Y. J. , Shin, S. W. , & Ryu, J. J. (2015). Effect of software version and parameter settings on the marginal and internal adaptation of crowns fabricated with the CAD/CAM system. Journal of Applied Oral Science, 23, 515–522. 10.1590/1678-775720150081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, N. R. , Witek, L. , Coelho, P. G. , Thompson, V. P. , Rekow, E. D. , & Smay, J. (2011). Additive CAD/CAM process for dental prostheses. Journal of Prosthodontics, 20, 93–96. 10.1111/j.1532-849X.2010.00623.x [DOI] [PubMed] [Google Scholar]
- Strub, J. R. , Rekow, E. D. , & Witkowski, S. (2006). Computer‐aided design and fabrication of dental restorations: Current systems and future possibilities. Journal of the American Dental Association, 137, 1289–1296. 10.14219/jada.archive.2006.0389 [DOI] [PubMed] [Google Scholar]
- Sun, J. , & Zhang, F. Q. (2012). The application of rapid prototyping in prosthodontics. Journal of Prosthodontics, 21, 641–644. 10.1111/j.1532-849X.2012.00888.x [DOI] [PubMed] [Google Scholar]
- Torabi, K. , Farjood, E. , & Hamedani, S. (2015). Rapid prototyping technologies and their applications in prosthodontics, a review of literature. Journal of Dentistry (Shiraz), 16, 1–9. [PMC free article] [PubMed] [Google Scholar]
- Williams, F. C. , Hammer, D. A. , Wentland, T. R. , & Kim, R. Y. (2020). Immediate teeth in fibulas: Planning and digital workflow with point‐of‐care 3D printing. Journal of Oral and Maxillofacial Surgery, 78, 1320–1327. 10.1016/j.joms.2020.04.006 [DOI] [PubMed] [Google Scholar]
- Yara, A. , Ogura, H. , Shinya, A. , Tomita, S. , Miyazaki, T. , Sugai, Y. , & Sakamoto, Y. (2005). Durability of diamond burs for the fabrication of ceramic crowns using dental CAD/CAM. Dental Materials Journal, 24, 134–139. 10.4012/dmj.24.134 [DOI] [PubMed] [Google Scholar]
- Zeltner, M. , Sailer, I. , Muhlemann, S. , Ozcan, M. , Hammerle, C. H. , & Benic, G. I. (2017). Randomized controlled within‐subject evaluation of digital and conventional workflows for the fabrication of lithium disilicate single crowns. Part III: Marginal and internal fit. Journal of Prosthetic Dentistry, 117, 354–362. 10.1016/j.prosdent.2016.04.028 [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Qiu, D. , Gibson, M. A. , Zheng, Y. , Fraser, H. L. , StJohn, D. H. , & Easton, M. A. (2019). Additive manufacturing of ultrafine‐grained high‐strength titanium alloys. Nature, 576, 91–95. 10.1038/s41586-019-1783-1 [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Sun, S. , Qiu, D. , Gibson, M. A. , Dargusch, M. S. , Brandt, M. , Qian, M. , & Easton, M. (2018). Metal alloys for fusion‐based additive manufacturing. Advanced Engineering Materials, 20, 1700952. 10.1002/adem.201700952 [DOI] [Google Scholar]
- Zhang, Y. , Tian, J. , Wei, D. , Di, P. , & Lin, Y. (2019). Quantitative clinical adjustment analysis of posterior single implant crown in a chairside digital workflow: A randomized controlled trial. Clinical Oral Implants Research, 30, 1059–1066. 10.1111/clr.13519 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Data can be provided upon request.


