Abstract
Purpose
Oral corticosteroids (OCS) are commonly used in patients with severe asthma, but they are associated with several adverse events. We estimated the prevalence of patients with OCS-dependent asthma in a large nationwide registry for severe asthma and delineated their clinical characteristics.
Methods
This cross-sectional study analyzed enrollment data of the patients recruited in the Korean Severe Asthma Registry (KoSAR) from 2010 to 2019. The clinical characteristics of patients were compared according to OCS dependency, which was defined as maintenance OCS treatment lasting at least 6 months during the 12 months prior to enrollment.
Results
Among the 562 patients with severe asthma, 121 (21.5%) patients were defined as having OCS-dependent asthma. Compared with the OCS-independent group, the OCS-dependent group was older at symptom onset and had a higher prevalence of anxiety, worse lung function, and used more medication than the control group. Despite the higher doses of daily ICS and 6-month cumulative OCS, the OCS-dependent group reported greater consumption of relievers and a higher prevalence of unscheduled emergency room visits and repeated OCS bursts. Although anti-interleukin-5 was more commonly prescribed for patients with OCS-dependent asthma, only a limited proportion of patients with severe asthma received biologics.
Conclusions
One-fifth of patients with severe asthma had OCS-dependency, which was associated with a greater disease burden compared to those with OCS-independent asthma. Active intervention including initiation of biologics and regular assessment of OCS-induced morbidities is warranted to reduce the use of OCS and its potential adverse effects.
Keywords: Asthma, prevalence, steroids, adverse reactions, severity, biologics
INTRODUCTION
Severe asthma is defined based on the asthma control status and treatment level. Despite differences in the existing diagnostic criteria for severe asthma, high-intensity anti-inflammatory treatment is a common condition required for the diagnosis.1 Compared to those with mild-to-moderate asthma, patients with severe asthma use higher doses of inhaled corticosteroids (ICS) as a controller, as well as more frequent use of short-term oral corticosteroids (OCS) due to acute exacerbations.2 In some patients with severe asthma, maintenance of OCS is needed to avoid symptom aggravation. A recent meta-analysis reported that the proportion of long-term OCS use ranged from 20% to 60% in patients with severe or uncontrolled asthma.2
Despite the substantial therapeutic effect and widespread use of OCS in respiratory diseases, OCS carry the potential risk of adverse effects.3,4 Among asthmatic patients, increases in the prevalence of OCS-related morbidities, including psychiatric disorders, diabetes, gastrointestinal disorder, osteoporosis, cardiovascular disorders, and infection along with increases in the exposed dose of OCS have been reported.5,6,7 In addition, analysis of large-sized claims data of asthmatics shows that chronic use (6 months or more in one year before index date) of corticosteroids was significantly associated with all-cause mortality.8 As such, OCS-induced complications lead to additional medical and economic burdens on severe asthma management.9
Asthmatics who need maintenance OCS for symptom control are classified as having “OCS-dependent asthma.” Recently, biologic agents such as anti-interleukin (IL)-5, anti-IL-5Rα, and anti-IL-4Rα monoclonal antibodies have become available in clinics. These agents show an OCS-sparing effect in randomized clinical trials of people with severe asthma.10,11,12,13 As therapeutic options other than OCS are now available for patients with severe asthma, especially individuals with eosinophilic inflammation, an active intervention strategy for reducing OCS use is needed.14,15 At the same time, a systemic assessment of OCS-related adverse reactions is required. In this regard, a precise estimation of the prevalence and disease burden of OCS-dependent asthma needs to be preceded. Therefore, we aimed to identify and characterize patients with OCS-dependent asthma compared to those with OCS-independent asthma by analyzing data from a large-sized nationwide registry for severe asthma.
MATERIALS AND METHODS
Study design and subjects
We used the Korean Severe Asthma Registry (KoSAR) for this study, which is the representative severe asthma registry in Korea established by the Working Group on Severe Asthma, the Korean Academy of Asthma, Allergy, and Clinical Immunology (KAAACI) in 2010.16,17,18 Currently, 25 university hospitals are participating in the KoSAR. The registry is composed of adult patients (≥ 18 years) with severe asthma who have been treated by asthma specialists for at least one year. The goal of the KoSAR is to understand the epidemiology, clinical characteristics, and management of severe asthma by obtaining real-world data in a prospective and observational manner.16,19
The diagnosis of severe asthma for enrollment was made when patients either 1) do not consistently reach a well-controlled state despite Global Initiative for Asthma (GINA) treatment step 4 or 5 or 2) have well-controlled asthma after GINA treatment step 4 or 5 but have a history of more than one unscheduled visit or 3 administrations of systemic corticosteroids in a given year, have ever had a near-fatal asthma attack, or have worsening symptoms when the OCS or ICS dose is reduced to 25%. Patients with severe bronchiectasis, a tuberculosis-destroyed lung, interstitial lung disease, severe congestive heart failure, or a disease other than asthma requiring the maintenance of OCS were excluded. For the present study, we analyzed enrollment data of the patients who had registered KoSAR between 2010 and 2019.
Definitions and outcomes
Patients with severe asthma were classified into either “OCS-dependent group” or “OCS-independent group” based on their medication history. OCS dependency was determined at enrollment when the maintenance OCS treatment lasted more than 6 months during the previous year. The demographic data (age, sex, and body mass index [BMI]), family history of allergic diseases (asthma, allergic rhinitis, and atopic dermatitis), history of asthma (age of symptom onset, age of diagnosis, duration of treatment), and comorbidities were collected from the KoSAR database. The analyzed asthma medications included ICS, long-acting beta-agonists (LABA), leukotriene receptor antagonists (LTRA), long-acting muscarinic antagonists (LAMA), theophylline, short-acting beta-agonists (SABA), and biologics such as anti-immunoglobulin E (IgE) and anti-IL-5. We also collected the results of lung function tests, complete blood count, serum total IgE, fractional exhaled nitric oxide (FeNO), and induced sputum analysis. Atopy was defined when a skin prick test or serum-specific IgE showed a positive result for at least one inhalant allergen. The clinicians were asked to assess whether the participants had asthma/chronic obstructive pulmonary disease (COPD) overlap (ACO) based on smoking history and presence of fixed airway obstruction. The control state of asthma was assessed based on the classification of asthma control level by GINA and the Asthma Control Test (ACT) score. Quality of life (QoL) was evaluated using the QoL Questionnaire in Adult Korean Asthmatics (QLQAKA).20 The number of OCS bursts and unscheduled healthcare use during the 12 months before enrollment was also evaluated. An OCS burst was defined as the short-term use of OCS lasting at least 3 consecutive days. This study was approved by the Institutional Review Board of each hospital and informed patient consent was obtained from study participants (IRB number: AMC 2017-1382).
Statistical analysis
All data are presented as counts and percentages for categorical variables and mean with standard deviation (SD) for continuous variables. The comparisons between groups according to OCS dependence were conducted using the χ2 test or Fisher’s exact test for categorical variables and independent t-test or Mann–Whitney U test for continuous variables, as appropriate. All statistical analyses were carried out using SPSS 24.0 (IBM Corp., Armonk, NY, USA). Two-sided P values < 0.05 were considered statistically significant.
RESULTS
Baseline characteristics and comorbidities according to OCS dependence
A total of 562 patients with severe asthma were included in this study. Among them, 121 (21.5%) patients were defined as having OCS-dependent severe asthma, while 441 (78.5%) had OCS-independent severe asthma. The baseline characteristics and comorbidities of the patients are summarized in Table 1. The mean age, proportion of male patients, BMI, and smoking status were comparable between the OCS-dependent group and the OCS-independent group. However, the OCS-dependent group showed a significantly older age at symptom onset (44.0 ± 14.7 years vs. 40.2 ± 17.2 years, P = 0.042) and a larger proportion of patients with adult-onset asthma (96.5% [111/115] vs. 89.6% [369/412], P = 0.021) compared to the OCS-independent group. Family history of allergic disease was significantly less prevalent in the OCS-dependent group (41.3% [50/121] vs. 52.4% [231/441], P = 0.031), while the prevalence of atopy was not significantly different between the 2 groups (43.2% [102/236] vs. 36.9% [24/65], P = 0.362). Approximately one-third of patients were diagnosed with ACO regardless of OCS dependency.
Table 1. Baseline characteristics and comorbidities in patients with severe asthma.
| Characteristics | OCS-independent (n = 441) | OCS-dependent (n = 121) | P value | |
|---|---|---|---|---|
| Age (yr) | 54.5 ± 14.5 | 57.7 ± 12.3 | 0.077 | |
| Male (%) | 191 (43.4) | 62 (51.2) | 0.125 | |
| BMI (kg/m2) (n = 556) | 24.2 ± 4.0 | 24.6 ± 3.6 | 0.105 | |
| History of asthma | ||||
| Age at symptom onset (yr) (n = 527) | 40.1 ± 17.1 | 44.0 ± 14.7 | 0.042 | |
| Adult-onset asthma (n = 527) | 369 (89.6) | 111 (96.5) | 0.021 | |
| Age of asthma diagnosis (yr) (n = 521) | 43.2 ± 16.6 | 46.2 ± 14.4 | 0.095 | |
| Duration of treatment (yr) (n = 558) | 9.8 ± 11.1 | 10.3 ± 9.0 | 0.057 | |
| Smoking status (n = 552) | 0.053 | |||
| Never smoker | 249 (57.5) | 56 (47.1) | ||
| Ex-smoker | 141 (32.6) | 53 (44.5) | ||
| Current smoker | 43 (9.9) | 10 (8.4) | ||
| Family history of allergic diseases | 231 (52.4) | 50 (41.3) | 0.031 | |
| Atopy (n = 301) | 102 (43.2) | 24 (36.9) | 0.362 | |
| Asthma/COPD overlap (n = 527) | 0.129 | |||
| Asthma only, more likely | 293/417 (70.3) | 69/110 (62.7) | ||
| Asthma/COPD overlap | 124/417 (29.7) | 41/100 (37.3) | ||
| Comorbidities | ||||
| Allergic rhinitis (n = 561) | 288 (65.5) | 69 (57.0) | 0.080 | |
| Chronic sinusitis (n = 396) | 105 (32.2) | 31 (44.3) | 0.054 | |
| Hypertension (n = 559) | 134 (30.5) | 42 (35.0) | 0.350 | |
| GERD (n = 559) | 92 (21.0) | 29 (24.2) | 0.449 | |
| Osteoporosis (n = 558) | 44 (10.0) | 19 (15.8) | 0.076 | |
| AERD (n = 515) | 42 (10.4) | 10 (9.0) | 0.667 | |
| Atopic dermatitis (n = 560) | 48 (10.9) | 8 (6.7) | 0.170 | |
| Allergic conjunctivitis (n = 558) | 47 (10.7) | 9 (7.6) | 0.311 | |
| Diabetes mellitus (n = 511) | 40 (10.1) | 10 (8.8) | 0.705 | |
| Depressive disorder (n = 560) | 25 (5.7) | 9 (7.5) | 0.460 | |
| Heart failure (n = 559) | 12 (2.7) | 7 (5.9) | 0.148 | |
| Arrhythmia (n = 558) | 11 (2.5) | 2 (1.7) | 0.744 | |
| Sleep apnea (n = 558) | 7 (1.6) | 5 (4.2) | 0.144 | |
| Anxiety disorder (n = 558) | 5 (1.1) | 7 (5.8) | 0.005 | |
Data are presented as mean ± standard deviation or counts (%).
OCS, oral corticosteroids; BMI, body mass index; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; AERD, aspirin-exacerbated respiratory disease.
Among the co-existing diseases, the prevalence of anxiety disorder was significantly higher in patients with OCS-dependent asthma (5.8% [7/120] vs. 1.1% [5/438], P = 0.005) (Table 1). Allergic diseases including allergic rhinitis, atopic dermatitis, and atopic conjunctivitis were more prevalent in the OCS-independent group. The proportion of potential OCS complications (e.g., hypertension, osteoporosis, and diabetes mellitus) was not significantly different between the 2 groups at enrollment, although osteoporosis was more common in the OCS-dependent group without statistical significance (15.8% [19/120] vs. 10.0% [44/438], P = 0.076).
Clinical characteristics of asthma according to OCS dependence
Patients in the OCS-dependent group reported significantly lower values of forced expiratory volume in 1 second (FEV1; % predicted) (62.2% ± 21.8% vs. 67.7% ± 19.2%, P = 0.014) and forced vital capacity (FVC; % predicted) (75.5% ± 16.8% vs. 80.04% ± 16.5%, P = 0.009) compared to those in the OCS-independent group, while the FEV1/FVC ratio was similar between the 2 groups (0.66 ± 0.14 vs. 0.67 ± 0.15, P = 0.428) (Table 2). In terms of laboratory tests, the OCS-dependent group had a notably higher total white blood cell count than the OCS-independent group (8,860 ± 2,590 /μL vs. 8,220 ± 2,760 /μL, P = 0.004). However, the levels of T2 inflammatory markers, including blood eosinophils (379.6 ± 479.1 /μL vs. 401.3 ± 497.4 /μL, P = 0.236), total IgE (328.1 ± 408.0 IU/L vs. 504.1 ± 699.7 IU/L, P = 0.170), sputum eosinophils (11.9% ± 19.5% vs. 13.4% ± 19.2%, P = 0.974), and FeNO (47.6 ± 32.3 ppb vs. 42.3 ± 29.7 ppb, P = 0.532) were comparable between the 2 groups, regardless of maintenance OCS use. In line with these findings, the proportion of patients presenting with the T2 phenotype was similar between the groups. Among the participants, 78 had data regarding blood eosinophils after one year of treatment. Although the mean eosinophil level was lower in the patients who were OCS-dependent at enrollment, this was not statistically significant (210.0 ± 198.8 /μL vs. 330.3 ± 434.1 /μL, P = 0.487).
Table 2. Comparison of the clinical characteristics of asthma according to OCS dependence.
| Characteristics | OCS-independent (n = 441) | OCS-dependent (n = 121) | P value | |
|---|---|---|---|---|
| Lung function | ||||
| FEV1 (% predicted) (n = 553) | 67.7 ± 19.2 | 62.2 ± 21.8 | 0.014 | |
| FEV1 (L) (n = 558) | 1.8 ± 0.7 | 1.6 ± 0.7 | 0.008 | |
| FVC (% predicted) (n = 553) | 80.04 ± 16.5 | 75.5 ± 16.8 | 0.009 | |
| FVC (L) (n = 558) | 2.8 ± 0.9 | 2.5 ± 0.8 | 0.008 | |
| FEV1/FVC ratio (n = 556) | 0.67 ± 0.15 | 0.66 ± 0.14 | 0.428 | |
| Laboratory tests | ||||
| WBC (/μL) (n = 521) | 8,220 ± 2,760 | 8,860 ± 2,590 | 0.004 | |
| Blood eosinophils (/μL) (n = 518) | 401.3 ± 497.4 | 379.6 ± 479.1 | 0.236 | |
| Total IgE (IU/L) (n = 161) | 504.1 ± 699.7 | 328.1 ± 408.0 | 0.170 | |
| Sputum neutrophils (%) (n = 148) | 50.4 ± 31.6 | 41.0 ± 33.2 | 0.133 | |
| Sputum eosinophils (%) (n = 144) | 13.4 ± 19.2 | 11.9 ± 19.5 | 0.974 | |
| FeNO (ppb) (n = 136) | 42.3 ± 29.7 | 47.6 ± 32.3 | 0.532 | |
| Phenotype of asthma | ||||
| Type 2 inflammation* | 315 (71.4) | 87 (71.9) | 0.919 | |
| Blood eosinophils ≥ 150 cells/μL | 261 (64.6) | 68 (59.6) | 0.332 | |
| FeNO ≥ 20 ppb | 88 (75.2) | 16 (84.2) | 0.562 | |
| Sputum eosinophil ≥ 2% | 68 (63.0) | 24 (66.7) | 0.689 | |
| Presence of atopy | 102 (43.2) | 24 (36.9) | 0.362 | |
| Level of asthma control (n = 512) | 0.090 | |||
| Uncontrolled | 130 (32.4) | 36 (32.4) | ||
| Partly controlled | 169 (42.1) | 57 (51.4) | ||
| Controlled | 102 (25.4) | 18 (16.2) | ||
| ACT score (n = 550) | 18.1 ± 5.1 | 17.2 ± 5.7 | 0.218 | |
| QLQAKA score (n = 550) | 61.1 ± 14.9 | 59.5 ± 15.0 | 0.296 | |
Data are presented as mean ± standard deviation or counts (%).
OCS, oral corticosteroids; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; WBC, white blood cell; FeNO, fractional exhaled nitric oxide; ACT, Asthma Control Test; QLQAKA, Quality of Life Questionnaire in Adult Korean Asthmatics.
*Type 2 inflammation was defined when the patients were either eosinophilic (blood eosinophils ≥ 150 cells/μL, FeNO ≥ 20 ppb, or sputum eosinophil ≥ 2%) or allergic (presence of atopy).
At the enrollment visit, asthma control status was similar between the groups, along with a comparable ACT score (17.2 ± 5.7 vs. 18.1 ± 5.1, P = 0.218). The QoL as evaluated by QLQAKA was also similar between the 2 groups (59.5 ± 15.0 vs. 61.0 ± 14.9, P = 0.296) (Table 2).
Use of asthma medications according to OCS dependence
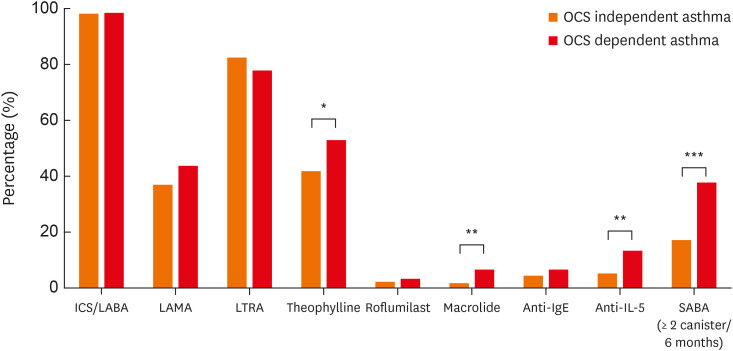
Regarding controller medications for asthma, 98.2% of the total patients were using ICS-LABA (Fig. 1). Additional inhaled LAMA was prescribed in 38.4% as an add-on therapy. The most common oral controller was LTRA (81.3%), followed by theophylline (44.1%), macrolide (2.7%), and roflumilast (2.5%). When comparing the use of asthma medications according to OCS dependence, theophylline (52.9% vs. 41.7%, P = 0.028) and macrolides (6.6% vs. 1.6%, P = 0.006) were significantly more commonly used in the OCS-dependent group than in the OCS-independent group. Among the study patients, only a small portion received anti-IL-5 (6.9%) and anti-IgE (5.0%). Anti-IL-5 was more commonly prescribed for patients with OCS-dependent asthma (13.2% vs. 5.1%, P = 0.002), while no significant difference was noted for the prescription of anti-IgE (6.6% vs. 4.5%, P = 0.325). The proportion of patients who had used 2 or more canisters of SABA as a rescue medication during the previous 6 months was significantly larger in the OCS-dependent group than in the OCS-independent group (37.7% vs. 17.0%, P < 0.001).
Fig. 1. Use of asthma medications according to OCS dependence.
ICS, inhaled corticosteroids; LABA, long-acting beta-agonists; LAMA, long-acting muscarinic antagonists; LTRA, leukotriene receptor antagonists; OCS, oral corticosteroids; SABA, short-acting beta-agonists.
*P < 0.05, **P < 0.01, and ***P < 0.001.
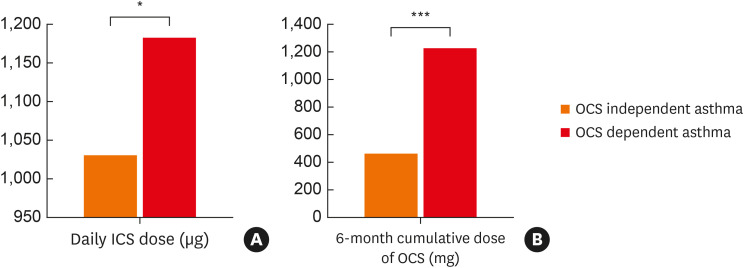
The daily dose of ICS (budesonide equivalent dose) was significantly higher in the OCS-dependent group compared with the OCS-independent group (1,181 ± 664 μg vs. 1,030 ± 726 μg, P = 0.039) (Fig. 2A). Moreover, the cumulative prednisolone-equivalent dose of OCS during the previous 6 months was notably higher in the OCS-dependent group as well (1,222 ± 839 mg vs. 461 ± 460 mg, P < 0.001) (Fig. 2B). The mean daily OCS dose was 6.8 mg in OCS dependent group. Among 66 OCS-dependent patients who had follow-up data on medication 6 months after enrollment, 32 (48.5%) patients were still on maintenance OCS.
Fig. 2. A daily dose of ICS (A) and a 6-month cumulative dose of OCS (B).
The budesonide and prednisolone equivalent dose was used for ICS and OCS, respectively.
ICS, inhaled corticosteroids; OCS, oral corticosteroids.
*P < 0.05, ***P < 0.001.
Asthma exacerbations and healthcare use according to OCS dependence
The percentage of patients who had visited healthcare facilities at least once due to asthma exacerbations in the previous year from enrollment was not significantly different between the 2 groups (38.3% vs. 44.0%, P = 0.264) (Table 3). In terms of unexpected healthcare use, emergency room visits were significantly more common in the OCS-dependent group than in the OCS-independent group (24.8% vs. 16.9%, P = 0.049). The frequencies of unscheduled visits to an outpatient clinic (24.0% vs. 29.0%, P = 0.278), hospitalizations (26.4% vs. 22.2%, P = 0.333), and admission to an intensive care unit (1.7% vs. 0.2%, P = 0.119) were not significantly different between the 2 groups. The proportion of patients who had consumed 3 or more OCS bursts for exacerbated asthma during a given year was significantly higher in the OCS-dependent group than in the OCS-independent group (37.9% vs. 24.1%, P = 0.030).
Table 3. Comparison of asthma exacerbations in the previous year from enrollment according to OCS dependence.
| Variables | OCS-independent (n = 441) | OCS-dependent (n = 121) | P value | |
|---|---|---|---|---|
| Unscheduled healthcare visit (n = 556) | ||||
| At least one visit | 192 (44.0) | 46 (38.3) | 0.264 | |
| Outpatient clinic visit | 126 (29.0) | 29 (24.0) | 0.278 | |
| Emergency room visit | 74 (16.9) | 30 (24.8) | 0.049 | |
| Hospitalization | 97 (22.2) | 32 (26.4) | 0.333 | |
| ICU admission | 1 (0.2) | 2 (1.7) | 0.119 | |
| Number of OCS bursts (n = 352) | 0.030 | |||
| Less than three | 223 (75.9) | 36 (62.1) | ||
| Three or more | 71 (24.1) | 22 (37.9) | ||
Data are presented as counts (%).
OCS; oral corticosteroids, ICU; intensive care unit.
DISCUSSION
In the present study, we identified and characterized patients with severe asthma with OCS dependency from the KoSAR registry and compared them to those without OCS dependency. Patients with OCS-dependent asthma comprised 21% of severe asthmatics and had a higher proportion of adult-onset asthma and a higher prevalence of anxiety disorders compared to those with OCS-independent asthma. In addition, the OCS-dependent group had poorer lung function and used more medication as controllers. Despite the higher doses of daily ICS and 6-month cumulative OCS, patients in the OCS-dependent group had significantly higher proportions of those who consumed relievers, made unscheduled emergency room visits, and had repeated OCS bursts.
We noted that one-fifth of the patients with severe asthma were defined as having OCS-dependent asthma. Prescription of regular OCS aims to avoid exacerbations in patients whose symptoms worsen when reducing OCS. The proportion of chronic OCS users among severe asthmatics varied across studies, ranging from 17% to 63%.21,22,23,24 The wide range could be attributed to the heterogeneity in the study populations, ethnicities, diagnostic criteria of OCS-dependence, and different referral systems in each country. The studies comprehensively assessing characteristics of severe asthma were summarized in Table 4.4,22 The higher prevalence of adult-onset asthma in the OCS-dependent group is in line with a previous study from the Belgian Severe Asthma Registry, in which the maintenance use of OCS was associated with late-onset (≥ 40 years of age) asthma. In the Belgian registry, OCS maintenance was also related to a lower prevalence of atopy.22 Considering the lower prevalence of family history of allergic disease and fewer self-reported allergic diseases, there may be significantly fewer patients with atopy in the OCS-dependent group. However, it was difficult to precisely evaluate this issue because only 53.4% (301/562) of participants underwent a skin prick test or a serum-specific IgE test. The substantial proportion of OCS-dependent patients and shared phenotypes in this population suggest the presence of specific pathophysiology leading to OCS reliance.
Table 4. Studies comparing characteristics of severe asthma according to OCS dependence.
| Study | Year | Number of severe asthmatics | Definition of mOCS and proportion | Daily OCS dose | Characteristics of patients with mOCS (vs. non-mOCS-users) |
|---|---|---|---|---|---|
| KoSAR (Korea) | 2022 | 562 | OCS treatment for more than 6 months in a year: 21.5% | 6.8 mg (prednisolone equivalent) | • Demographics: ↑Adult-onset asthma |
| • Lung function: ↓FEV1, FVC | |||||
| • Inflammatory markers: Blood eosinophils, sputum eosinophils, FeNO (−) | |||||
| • Comorbidities: ↑anxiety disorder | |||||
| Sweeny et al.4 (UK) | 2016 | 770 | Requirement of mOCS: 57.4% | 15 mg (prednisolone equivalent) | • Demographics: ↑Male, age at diagnosis of asthma (−), ↑BMI |
| • Lung function: ↓FEV1, FEV1/FVC ratio | |||||
| • Comorbidities: ↑Corticosteroid-induced morbidities | |||||
| Graff et al.22 (Belgium) | 2020 | 982 | Daily use of OCS: 21.0% | 8 mg (prednisone equivalent) | • Demographics: ↑Male, ↑late-onset asthma |
| • Lung function: FEV1, FVC (−) | |||||
| • Inflammatory markers: blood eosinophils, sputum eosinophils (−), ↑FeNO | |||||
| • Comorbidities: ↑Emphysema, bronchiectasis, GERD, EGPA |
OCS, oral corticosteroids; mOCS, maintenance oral corticosteroids; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; BMI, body mass index; FeNO, fractional exhaled nitric oxide; GERD, gastroesophageal reflux disease; EGPA, Eosinophilic granulomatosis with polyangiitis.
Despite the well-known adverse effects of chronic OCS use, only anxiety showed a significantly higher prevalence in the OCS-dependent group compared with the OCS-independent group. In patients with asthma, the presence of anxiety was related to worsening asthma symptoms and poor asthma control.25,26 Consequently, patients with an anxiety disorder could be more prone to using higher doses of OCS to relieve their symptoms. On the other hand, chronic OCS use might have played a role, at least partly, in the development of anxiety disorders, as corticosteroids are reportedly associated with a higher risk of a mood or anxiety disorder.27 A recent qualitative study in severe asthmatics described the psychologic side effects of OCS, such as mood swings and anxiety derived from OCS reliance.28 In our data, the prevalence of hypertension, osteoporosis, and diabetes mellitus were similar between the OCS-dependent group and the OCS-independent group.
In contrast, a study from the British Thoracic Society (BTS) Difficult Asthma Registry reported that corticosteroid-dependent severe asthma was associated with higher frequencies of complications such as diabetes, hypertension, obesity, and sleep disorders. One possible reason for this discrepancy is differences in the OCS daily dose, which was higher in the BTS registry than in the KoSAR (15 mg vs. 6.8 mg).4 Despite a similar prevalence between the groups at the time of investigation, a higher incidence of comorbidities is expected in patients treated with prolonged OCS. These findings also highlight the need for a regular assessment of potential OCS-induced morbidities, as their risk increases according to the increase in cumulative OCS dose and duration of exposure.29 Unlike anxiety disorder, asymptomatic metabolomic or cardiovascular disorders are hard for patients to recognize, especially in the early phase; thus, timely screening before progression is required.30
In our analysis, the OCS-dependent group had a significantly worse lung function than the OCS-independent group, while there were no significant differences in the levels and total scores of symptom control. These findings imply that, in the management of OCS-dependent severe asthma, OCS is prescribed to maintain symptom control to a level similar to that in OCS-independent asthma. Nonetheless, the decline of lung function could not be readily prevented by the chronic use of OCS. Another notable finding was the comparable levels of eosinophilic markers in severe asthmatics regardless of chronic OCS use. Persistent eosinophilic inflammation despite OCS maintenance has been reported in the Belgian severe asthma registry and participants in clinical trials of mepolizumab.22,31 This warrants the necessity of highly efficient T2-targeting therapies, including mepolizumab, reslizumab, dupilumab, and benralizumab, to reduce OCS consumption. At the same time, worse lung function and less OCS-responsive inflammatory markers suggest the presence of pathologic mechanisms that could not be attenuated by OCS. These might be the involvement of airway fibrosis, inherent eosinophil characteristics, or other host factors affecting corticosteroid metabolism.32
In terms of asthma medication, patients in the OCS-dependent group reported greater ICS doses and more theophylline, macrolide, and SABA use. Despite the OCS maintenance, the OCS-dependent group still experienced more frequent unscheduled emergency room visits and more repeated use of OCS bursts compared with the OCS-independent group. In addition to the maintenance use of OCS, intermittent use of OCS bursts for exacerbations was also reported to induce various OCS-related adverse reactions.6 Moreover, overuse of SABA was associated with an increased risk of asthma exacerbation and mortality.33 Collectively, these findings suggest the substantial economic and disease burden stemming from medications and medication-associated adverse effects in OCS-dependent asthma. Frequent exacerbations also impose an additional burden on these patients.28,34 However, compared with the patients with severe asthma in other countries,24 fewer patients were using biologics (anti-IgE and anti-IL-5) in our study. Although more patients in the OCS-dependent group received anti-IL-5 compared with the OCS-independent group, the majority of patients still relied on chronic OCS use. We speculate that the high medication costs and lack of reimbursement by the National Health Insurance Service in Korea imposed a significant barrier to the use of biologics in patients with severe asthma.35
When further examining the cumulative dose of corticosteroids, the mean OCS dose consumed in the OCS-dependent group during 6 months was 1,222 mg, which was more than twice the dose consumed in the OCS-independent group (461 mg). Considering that a cumulative yearly dose of 1,000 mg has been suggested as a threshold for referral given its association with corticosteroid-induced morbidity, special efforts are needed to reduce OCS use and prevent adverse events.32 The potential risk from corticosteroid-associated side-effects could be underestimated in clinical practice,5 because it is not reflected in ACT scores or spirometry results. This is in accordance with our results showing a similar degree of asthma control status in the OCS-dependent group and the OCS-independent group. Despite the similar control status at specific times, the potential risks of the 2 groups may vary greatly. Therefore, identification of high-risk patients and screening for corticosteroid-induced side effects in clinical practice are required. Initiating biologic agents also needs to be actively considered for reducing not only acute exacerbations but also OCS use. Moreover, further study is warranted to assess the impact of chronic OCS use on clinical course and disease burden of asthma in a longitudinal manner.
The major strength of this study is that it presents the demographic and clinical characteristics of patients with OCS-dependent severe asthma using a nationwide representative multicenter registry, in which all participants with severe asthma were diagnosed and managed by asthma specialists at dedicated referral centers. However, this study has several limitations that should be acknowledged. First, as the registry did not include patients with severe asthma managed at primary or secondary centers without a referral, the findings may not represent the whole population of patients with OCS-dependent severe asthma. Secondly, the comorbidities were self-reported and not assessed based on objective diagnostic criteria. The data on comorbidities were collected once at the enrollment visit without follow-up. Therefore, we could not tell the causal relationship between OCS use and potential complications. In addition, we did not include eosinophilic granulomatosis with polyangiitis or allergic bronchopulmonary aspergillosis as comorbidities in our original case report form which might have caused long-term OCS use. Thirdly, considering that the definitions of severe asthma and OCS dependence are heterogeneous among registries and studies, the findings in this study should be interpreted with caution when compared with OCS-dependent severe asthma in different study populations.
In conclusion, approximately 20% of patients with severe asthma in the KoSAR had OCS dependency. Patients with OCS-dependent asthma reported a greater disease burden compared to those with OCS-independent asthma in terms of worse lung function, more medication use, and frequent exacerbations. Patients with OCS-dependent asthma remained eosinophilic despite chronic OCS use. Active intervention is needed for this population, including initiation of biologic agents and regular assessment of OCS-induced morbidities.
ACKNOWLEDGMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI21C0198) and the Korea National Institute of Health research project (project No.2022-ER1205-00).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 2.Bleecker ER, Menzies-Gow AN, Price DB, Bourdin A, Sweet S, Martin AL, et al. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med. 2020;201:276–293. doi: 10.1164/rccm.201904-0903SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. QJM. 2000;93:105–111. doi: 10.1093/qjmed/93.2.105. [DOI] [PubMed] [Google Scholar]
- 4.Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71:339–346. doi: 10.1136/thoraxjnl-2015-207630. [DOI] [PubMed] [Google Scholar]
- 5.Song WJ, Lee JH, Kang Y, Joung WJ, Chung KF. Future risks in patients with severe asthma. Allergy Asthma Immunol Res. 2019;11:763–778. doi: 10.4168/aair.2019.11.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141:110–116.e7. doi: 10.1016/j.jaci.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52:1800703. doi: 10.1183/13993003.00703-2018. [DOI] [PubMed] [Google Scholar]
- 8.Lee H, Ryu J, Nam E, Chung SJ, Yeo Y, Park DW, et al. Increased mortality in patients with corticosteroid-dependent asthma: a nationwide population-based study. Eur Respir J. 2019;54:1900804. doi: 10.1183/13993003.00804-2019. [DOI] [PubMed] [Google Scholar]
- 9.Barry LE, Sweeney J, O’Neill C, Price D, Heaney LG. The cost of systemic corticosteroid-induced morbidity in severe asthma: a health economic analysis. Respir Res. 2017;18:129. doi: 10.1186/s12931-017-0614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378:2475–2485. doi: 10.1056/NEJMoa1804093. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein JA, Virchow JC, Murphy K, Maspero JF, Jacobs J, Adir Y, et al. Effect of fixed-dose subcutaneous reslizumab on asthma exacerbations in patients with severe uncontrolled asthma and corticosteroid sparing in patients with oral corticosteroid-dependent asthma: results from two phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med. 2020;8:461–474. doi: 10.1016/S2213-2600(19)30372-8. [DOI] [PubMed] [Google Scholar]
- 12.Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 13.Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 14.Suehs CM, Menzies-Gow A, Price D, Bleecker ER, Canonica GW, Gurnell M, et al. Expert consensus on the tapering of oral corticosteroids for the treatment of asthma. A Delphi study. Am J Respir Crit Care Med. 2021;203:871–881. doi: 10.1164/rccm.202007-2721OC. [DOI] [PubMed] [Google Scholar]
- 15.Chaves Loureiro C, Branco Ferreira M, Ferreira J, Lima R, Marques J, Sokolova A, et al. Reducing oral corticosteroids in severe asthma (ROSA Project): a nationwide Portuguese consensus. Pulmonology. 2021;27:313–327. doi: 10.1016/j.pulmoe.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Kim MH, Kim SH, Park SY, Ban GY, Kim JH, Jung JW, et al. Characteristics of adult severe refractory asthma in Korea analyzed from the Severe Asthma Registry. Allergy Asthma Immunol Res. 2019;11:43–54. doi: 10.4168/aair.2019.11.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim BK, Park SY, Ban GY, Kim MA, Lee JH, An J, et al. Evaluation and management of difficult-to-treat and severe asthma: an expert opinion from the Korean Academy of Asthma, Allergy and Clinical Immunology, the Working Group on Severe Asthma. Allergy Asthma Immunol Res. 2020;12:910–933. doi: 10.4168/aair.2020.12.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SH, Lee H, Park SY, Park SY, Song WJ, Kim JH, et al. The Korean Severe Asthma Registry (KoSAR): real world research in severe asthma. Korean J Intern Med. 2022;37:249–260. doi: 10.3904/kjim.2021.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H, Kim SH, Kim BK, Lee Y, Lee HY, Ban GY, et al. Characteristics of specialist-diagnosed asthma-COPD overlap in severe asthma: observations from the Korean Severe Asthma Registry (KoSAR) Allergy. 2021;76:223–232. doi: 10.1111/all.14483. [DOI] [PubMed] [Google Scholar]
- 20.Kwon HS, Lee SH, Yang MS, Lee SM, Kim SH, Kim DI, et al. Correlation between the Korean version of Asthma Control Test and health-related quality of life in adult asthmatics. J Korean Med Sci. 2008;23:621–627. doi: 10.3346/jkms.2008.23.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taube C, Bramlage P, Hofer A, Anderson D. Prevalence of oral corticosteroid use in the German severe asthma population. ERJ Open Res. 2019;5:00092-2019. doi: 10.1183/23120541.00092-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graff S, Vanwynsberghe S, Brusselle G, Hanon S, Sohy C, Dupont LJ, et al. Chronic oral corticosteroids use and persistent eosinophilia in severe asthmatics from the Belgian severe asthma registry. Respir Res. 2020;21:214. doi: 10.1186/s12931-020-01460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson DJ, Busby J, Pfeffer PE, Menzies-Gow A, Brown T, Gore R, et al. Characterisation of patients with severe asthma in the UK Severe Asthma Registry in the biologic era. Thorax. 2021;76:220–227. doi: 10.1136/thoraxjnl-2020-215168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang E, Wechsler ME, Tran TN, Heaney LG, Jones RC, Menzies-Gow AN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry. Chest. 2020;157:790–804. doi: 10.1016/j.chest.2019.10.053. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Jiang Q, Ji Y, Cao C. Anxiety and depression may associate with poorer control and quality of life in adults with asthma. Allergy. 2020;75:1759–1762. doi: 10.1111/all.14189. [DOI] [PubMed] [Google Scholar]
- 26.Lacwik P, Szydłowska D, Kupczyk M, Pałczyński C, Kuna P. High levels of anxiety during the COVID-19 pandemic as a risk factor of clinical worsening in patients with severe asthma. J Allergy Clin Immunol Pract. 2021;9:1381–1383. doi: 10.1016/j.jaip.2020.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savas M, Vinkers CH, Rosmalen JG, Hartman CA, Wester VL, van den Akker EL, et al. Systemic and local corticosteroid use is associated with reduced executive cognition, and mood and anxiety disorders. Neuroendocrinology. 2020;110:282–291. doi: 10.1159/000501617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song WJ, Won HK, Lee SY, Park HK, Cho YS, Chung KF, et al. Patients' experiences of asthma exacerbation and management: a qualitative study of severe asthma. ERJ Open Res. 2021;7:00528-2020. doi: 10.1183/23120541.00528-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalal AA, Duh MS, Gozalo L, Robitaille MN, Albers F, Yancey S, et al. Dose-response relationship between long-term systemic corticosteroid use and related complications in patients with severe asthma. J Manag Care Spec Pharm. 2016;22:833–847. doi: 10.18553/jmcp.2016.22.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2020 executive summary. Endocr Pract. 2020;26:107–139. doi: 10.4158/CS-2019-0472. [DOI] [PubMed] [Google Scholar]
- 31.Prazma CM, Wenzel S, Barnes N, Douglass JA, Hartley BF, Ortega H. Characterisation of an OCS-dependent severe asthma population treated with mepolizumab. Thorax. 2014;69:1141–1142. doi: 10.1136/thoraxjnl-2014-205581. [DOI] [PubMed] [Google Scholar]
- 32.Bourdin A, Adcock I, Berger P, Bonniaud P, Chanson P, Chenivesse C, et al. How can we minimise the use of regular oral corticosteroids in asthma? Eur Respir Rev. 2020;29:190085. doi: 10.1183/16000617.0085-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55:1901872. doi: 10.1183/13993003.01872-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark VL, Gibson PG, McDonald VM. The patients’ experience of severe asthma add-on pharmacotherapies: a qualitative descriptive study. J Asthma Allergy. 2021;14:245–258. doi: 10.2147/JAA.S296147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SH, Moon JY, Lee JH, Ban GY, Kim S, Kim MA, et al. Perceptions of Severe asthma and asthma-COPD overlap syndrome among specialists: a questionnaire survey. Allergy Asthma Immunol Res. 2018;10:225–235. doi: 10.4168/aair.2018.10.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]