Abstract
Asthma, chronic obstructive pulmonary disease, and idiopathic pulmonary fibrosis are representative chronic respiratory diseases (CRDs). Although they differ in terms of disease presentation, they are all thought to arise from unresolved inflammation. Neutrophils are not only the first responders to acute inflammation, but they also help resolve the inflammation. Notably, emerging clinical studies show that CRDs are associated with systemic and local elevation of neutrophils. Moreover, murine studies suggest that airway-infiltrating neutrophils not only help initiate airway inflammation but also prolong the inflammation. Given this background, this review describes neutrophil-mediated immune responses in CRDs and summarizes the completed, ongoing, and potential clinical trials that test the therapeutic value of targeting neutrophils in CRDs. The review also clarifies the importance of understanding how neutrophils interact with other immune cells and how these interactions contribute to chronic inflammation in specific CRDs. This information may help identify future therapeutic strategies for CRDs.
Keywords: Neutrophils, asthma, chronic obstructive pulmonary diseases, idiopathic pulmonary fibrosis, lung, inflammation
INTRODUCTION
Chronic respiratory disease (CRD) is an umbrella term for chronic diseases affecting the lungs and airways. The most common include asthma, chronic obstructive pulmonary disease (COPD), and idiopathic pulmonary fibrosis (IPF). Asthma and COPD are both accompanied by airway obstruction and prolonged airway inflammation.1 Common asthma symptoms are coughing, shortness of breath, wheezing, and chest tightness.2 Approximately 4% of adults globally have asthma, which is increasing annually.3 COPD includes chronic bronchitis and emphysema and is marked by breathing difficulties.4,5 It is the third leading cause of death worldwide (3.23 million deaths annually).6 Allergens such as pollen, house dust mites (HDMs), cockroaches, mold, and animal dander trigger asthma and aggravate COPD symptoms. These airborne allergens irritate the airway epithelium, causing it to secrete excessive mucus and chemokines.7 However, COPD is not caused by either allergies or asthma. Instead, long-term exposure to environmental hazards such as air pollution and cigarette smoke are the main causes of COPD.4,5 IPF is another progressive respiratory disease with a poor prognosis.8 It affects approximately 3 million people worldwide.9 Its cause is unknown, but risk factors include smoking, lung injury, a family history of the disease, abnormal acid reflux, environmental exposure, and chronic viral infections.10,11 Significantly, despite their different pathologies, asthma, COPD, and IPF are all initiated by airway inflammation that fails to resolve. While the immunological mechanisms that initiate and maintain these CRDs remain fully unraveled, their importance in CRD pathogenesis means that a greater understanding of them may lead to therapeutics that will help control and prevent CRDs.
Neutrophils are the most abundant cell type in human blood and critical responders in the first line of defense against pathogens.12 In the context of pulmonary infection, neutrophils are recruited to the site of inflammation and initiate inflammation by secreting various pro-inflammatory cytokines and chemokines.13,14 Besides, neutrophils can promote damaged tissue repairing by clearing nuclear debris resulting from lung injuries.15 Due to their short life span, the activities of neutrophils were long thought to be restricted to countering acute infections and clearing pathogens. However, emerging evidence suggests that neutrophils have broader activities; in particular, they may play an important role in initiating and contributing to the unabating inflammation that is the hallmark of many chronic diseases. Specifically, there is evidence of continuous recruitment of neutrophils to sites of inflammation, where they add to the chronic inflammatory responses by releasing proteases, forming neutrophil extracellular traps (NETs), and activating other immune cells.16
Since CRDs such as severe asthma, COPD, and IPF are all associated with continuously high neutrophil levels,17,18 and there is evidence that suppressing these cells or their functions ameliorates CRD symptoms and lung damage, it is of interest to re-evaluate the hitherto overlooked role of neutrophils in these diseases. Here, we will discuss the diverse roles of neutrophils in CRDs and elucidate the mechanisms by which neutrophils crosstalk with other immune cells. The research in these areas may help us identify strategies to counteract the adverse effects of neutrophils in CRDs.
NEUTROPHILS IN CHRONIC RESPIRATORY DISEASES
Neutrophils in asthma
Asthma is one of the most prevalent CRDs. It is associated with airway obstruction and excessive mucus production and is generally considered an allergic disease caused by type-2 immune responses. Specifically, inhaled allergens disrupt the airway epithelium, which increases the production of alarmins, stimulating T helper (Th) 2 cells, type 2 innate lymphoid cells (ILC2s), eosinophils, and alveolar macrophages.19 The local and systemic augmentation of eosinophils is considered a key risk factor for asthma exacerbation.20 However, 10%–33% of asthmatics have normal ranges of serum immunoglobulin E (IgE) and a non-allergic phenotype that is less responsive to standard therapy21,22 and is marked by increased neutrophilic inflammation. Triggers for this non-allergic asthma phenotype include environmental factors, including ozone, air pollutants, and viral infection.23,24,25 In addition, many clinical studies have shown that increased neutrophil numbers are associated with greater asthma severity and steroid resistance.18,26
Neutrophils secrete many different molecules, some of which have been found to contribute directly to airway inflammation and asthma exacerbation (Fig. 1). They include NETs, which are extracellular structures composed of chromatin backbone and granule proteins with antimicrobial activity.27 In healthy settings, NETs neutralize harmful pathogens in the lungs. However, when the NET formation is dysregulated and therefore excessive or continuous, it promotes tissue damage and inflammation.28 An example of this pathogenic role of NET in asthma is the study by He et al.29 They showed that when a murine model of asthma is challenged with intratracheal < 2.5-µm particulate matter (PM2.5), it increases asthma severity. Specifically, it elevates airway inflammation, mucus secretion, and neutrophil infiltration. Mechanistic analyses then showed that the lung-recruited neutrophils release NETs, which increases the local expression and activity of the oxidoreductase NQO1. This, in turn, upregulates the expression of the mucin gene MUC5AC in airway epithelial cells, thereby aggravating mucus hypersecretion.29 Similarly, Shin et al. 30 reported recently that NET-producing neutrophils contribute to air pollutant-induced asthma exacerbation. They observed that intratracheal instillation of diesel exhaust particle (DEP), as an asthma exacerbator, generates a novel neutrophil subset in the airway that expresses SiglecF. These cells produce more NETs than conventional neutrophils. Moreover, they produce more cysteinyl leukotrienes, which are known to exacerbate asthma. SiglecF+ neutrophils are upregulated in an asthma exacerbation model (i.e., HDM-induced asthma followed by DEP challenge), and the neutralization of their NET or cysteinyl leukotriene production blocks HDM+DEP-induced airway hyperresponsiveness (AHR).30
Fig. 1. Contributions of neutrophils to chronic respiratory diseases.
Environmental factors such as allergens and air pollutants induce pulmonary inflammation. In asthma, airborne antigens induce the infiltration of neutrophils, which release MMPs and inflammatory cytokines that recruit and activate other immune cells. In particular, neutrophils express TGF-β, which induces morphological changes and EMT of human bronchial epithelial cells. Neutrophils also form NETs, which promote mucus production. In COPD, neutrophils stimulate macrophage recruitment by secreting chemokines. They also activate macrophages by releasing neutrophil elastase. In IPF, neutrophil-derived NETs induce the transition of pulmonary fibroblasts into myofibroblasts. These cells mediate pulmonary fibrosis. TGF-β from the pulmonary neutrophils also induces alternative macrophage (M2) polarization in IPF, which in turn promotes fibrosis.
MMP, matrix metalloproteinase; TGF-β, transforming growth factor-β; EMT, epithelial-mesenchymal transition; COPD, chronic obstructive pulmonary disease; IPF, idiopathic pulmonary fibrosis; NET, neutrophil extracellular trap.
Other potentially pathogenic neutrophil-derived molecules are transforming growth factor (TGF)-β. Haddad et al. 31 showed that the blood neutrophils from severe asthmatic patients induced changes in morphology and epithelial-mesenchymal transition (EMT) of human bronchial epithelial cells more than healthy control neutrophils. They also found that the asthmatic neutrophils increased TGF-β productions and neutralizing TGF-β reduced EMT-related gene expressions from primary epithelial cells. In addition, increased TGF-β production could be associated with poor lung function.31 Torrego et al.32 demonstrated that the allergen challenge decreased forced expiratory volume in one second (FEV1) and augmented TGF-β secreting neutrophils in the bronchial mucosa. These two data suggested that the TGF-β-producing neutrophils destroy airway epithelium and worsen asthma phenotype.
Neutrophil-derived proteases could damage the airway and worsen asthma symptoms. One is neutrophil elastase (NE), which is stored in neutrophil azurophilic granules and is released when neutrophils are activated by exposure to cigarette smoke or inflammation.33 Suzuki et al.34 showed that when healthy rodents inhale human NE, they display increased immune cell infiltration, epithelial cell damage, and airway constriction. Moreover, patients with neutrophilic asthma exhibit high systemic expression of the elastase gene ELA2. 35 In addition, mice with OVA-induced asthma exhibit attenuated AHR, airway immune cell recruitment, and type 2 inflammation when an NE inhibitor is administered prior to the OVA challenge.36 Another neutrophil-derived protease is matrix metallopeptidase (MMP)-9. It is secreted when neutrophils physically encounter allergens in the presence of IgE.37 Moreover, patients who have severe asthma despite receiving high-dose glucocorticoids display higher MMP9 levels in their bronchoalveolar lavage fluid (BALF) than healthy controls or steroid-untreated patients with moderate or mild asthma. Notably, the BALF MMP9 levels in the cohort correlate with BALF neutrophil counts. Moreover, in vitro analyses showed that steroids do not reduce MMP production by BALF neutrophils.38 These findings suggest that further studies on neutrophils may reveal mechanisms that can be targeted to control non-allergic and severe asthma.
Neutrophils in COPD
COPD is characterized by irreversible airflow obstruction with a persistent cough, shortness of breath, and a decline in lung function. Since it has a deleterious effect on patient quality of life and is a leading cause of death, it is increasingly posing a significant social and economic burden.4 Smoking is a major risk factor for COPD, followed by air pollutants and occupational chemicals.39,40 Exposure to these agents induces local and systemic inflammation and subsequent pulmonary symptoms.41,42 Although bronchodilators can alleviate COPD symptoms,43 they cannot arrest or ameliorate the underlying disease. Therefore, the development of new therapeutic targets is urgently needed for COPD.
Compared to other CRDs, COPD is associated with consistently higher neutrophilic inflammation (Fig. 1). Indeed, neutrophils are the most abundant cells in the sputum or BALF of patients with both stable and exacerbated COPD.17,44,45 Moreover, neutrophil numbers in sputum correlate with the severity of airflow obstruction in COPD.46 Like in asthma, NE plays an important pathogenic role in COPD. First, by proteolyzing lung tissues, NE is a major cause of alveolar enlargement and destruction.47,48,49 Secondly, NE stimulates airway epithelial cells to express MUC5AC,50 thereby inducing hyperplasia of secretory cells and mucin production.51 Thirdly, NE activates macrophages, releasing active MMPs that induce lung destruction and emphysema. The macrophages also produce proinflammatory cytokines that trigger inflammation.52 Notably, neutrophils also secrete MMPs in COPD.53 In particular, neutrophils in the induced sputum of COPD patients display elevated levels of MMP8 and MMP9 that are associated with COPD exacerbations.54,55 Moreover, smoking (a major risk factor for COPD) increases the expression of MMP9 and MMP12 in lung neutrophils in mice.56,57 Smoking also has the same effect on neutrophils in the induced sputum of COPD patients compared to healthy non-smokers or smokers.58 The role of NETs in COPD is currently unclear: while some studies showed that high NET levels in the sputum of patients with COPD are associated with more frequent exacerbations,59,60 another observed that patients with COPD exhibit reduced NET formation. The latter study also suggested that this low NET formation is associated with higher frequencies of exacerbations due to defective bacterial clearance.61
Neutrophils in IPF
IPF is a progressive respiratory disease with a poor prognosis.8 Unlike the other CRDs, the contribution of neutrophils to the pathogenesis of IPF and their clinical significance remains controversial. Nonetheless, several clinical investigations suggest neutrophil-mediated inflammation plays an essential role in the onset, progression, and acute exacerbation of IPF, as follows (Fig. 1). First, the lungs and sputum of IPF patients have higher numbers of neutrophils than the specimens from healthy controls.62,63 Secondly, compared to healthy controls, IPF patients have significantly higher levels of interleukin (IL)-8 in BALF. IL-8 is a potent chemokine that attracts neutrophils to sites of inflammation. Significantly, the numbers of neutrophils in the BALF from IPF patients also correlate with BALF IL-8 levels.64 Thirdly, compared to patients with other fibrotic interstitial pneumonia, IPF patients contain elevated levels of the calcium-binding S100 protein in their serum and BALF. S100A9 is mainly produced by neutrophils and macrophages. Notably, the serum and BALF levels of S100A9 in the IPF patients correlated positively with the neutrophil counts in BALF.65 Finally, patients with acute exacerbation of IPF demonstrate abnormally high serum NE levels.66
Multiple lines of evidence also suggest that neutrophil-secreted factors contribute to the fibrotic microenvironment in IPF, which is characterized by the formation of myofibroblasts that deposit collagen in the lung interstitial spaces. First, neutrophil-derived NETs induce fibroblasts to differentiate into myofibroblasts and are critical for developing bleomycin-induced lung fibrosis, an animal model of IPF.67,68 Secondly, a murine study showed that NE in the lung is upregulated during the onset of IPF and contributes to the pathogenesis of IPF by remodeling the extracellular matrix (ECM).69 Thirdly, neutrophil MMPs also remodel the ECM in the early stage of IPF and lung injury, thereby promoting disease exacerbation.70,71,72 Fourthly, several studies suggest that neutrophil-derived leukotrienes (LTs) stimulate the infiltration of other immune cells into the lung, thereby boosting IPF progression. Specifically, patients with IPF have significantly higher levels of LTC4 and LTB4 in BALF compared to non-asthmatic controls.73,74 Similarly, mice with bleomycin-induced fibrosis have higher LTB4 levels in BALF than vehicle-treated control mice.75 Moreover, LTC4 transgenic mice develop more severe IPF than wild-type mice.76 Interestingly, a recent study showed that senescent lung fibroblasts could also secrete LTs, which induces naïve fibroblasts to synthesize collagen, thereby promoting lung fibrosis.77 Finally, neutrophil-derived cytokines may contribute to fibrosis in different ways depending on the stage of pulmonary fibrosis. Thus, in the early inflammatory phase, the infiltrating neutrophils may injure the lung by secreting proinflammatory cytokines such as IL-1β, tumor necrosis factor (TNF)α, IL-6, and IL-8.78 Conversely, neutrophils can secrete profibrotic mediators during the chronic fibrotic stage.78 Regarding this, TGF-β shifts the neutrophil phenotype to N2-type, increasing its profibrotic functions.79 This is significant because TGF-β is a potent pro-fibrotic mediator produced by various immune cells (especially macrophages) and strongly promotes pulmonary fibrosis.80
CROSSTALK BETWEEN NEUTROPHILS AND IMMUNE CELLS IN THE AIRWAYS
As described above, neutrophils contribute to CRDs in many ways, particularly by secreting molecules that drive the pathogenic activities of other immune cells. Therefore, it is important to understand the interactions between neutrophils and other immune cells in the lungs since this will allow us to identify the cellular/molecular mechanisms that drive CRDs. This information may point to molecules that can be targeted for therapy. Here, we will discuss how neutrophils interact with other innate immune cells.
Interactions between neutrophils and macrophages
In the lungs, macrophages act as the foremost immune barrier against foreign particles or infection.81 When they encounter cigarette smoke, pollutants, or microorganisms, they become activated and secrete neutrophil chemoattractants, including CXCL1, CXCL2, IL-1α, and CCL2.82 They also promote neutrophil infiltration by releasing LTB4.83 , 84 Moreover, they secrete various cytokines such as IL-1, IL-6, and IL-13 that regulate neutrophil functions. In addition, they produce granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF), which can increase the lifespan of neutrophils in the inflammatory site (Fig. 2).85,86
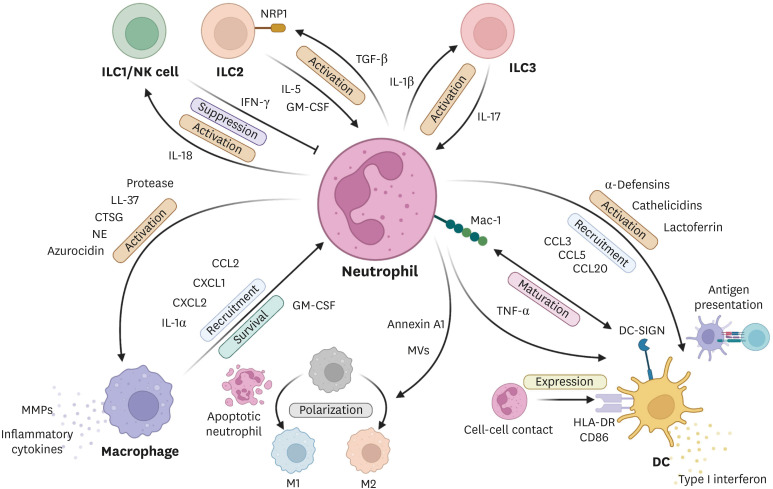
Fig. 2. Interactions between neutrophils and other innate immune cells.
Neutrophils directly activate macrophages by secreting granule components: proteases, LL-37, CTSG, NE, and azurocidin. Neutrophils can also induce M2 and M1 macrophage polarization by releasing annexin A1-bearing MVs and apoptotic neutrophil components, respectively. Conversely, activated macrophages can recruit neutrophils by releasing chemokines and IL-1α. Macrophages can also increase local neutrophil survival by secreting GM-CSF. DCs and neutrophils can bind to each other via Mac-1 and DC-SIGN. This interaction promotes the maturation of both neutrophils and DCs. Cell-cell contact with neutrophils also increases DC expression of HLA-DR and CD86 and, therefore, DC antigen presentation. DC migration is also activated and increased by neutrophil-derived chemokines and granule proteins. Neutrophils are negatively regulated by the IFN-γ from activated ILC1 and NK cells. Conversely, neutrophils stimulate ILC1/NK cells by releasing IL-18. Neutrophils reciprocally activate both ILC2s and ILC3s. Thus, neutrophils activate NRP-1-expressing ILC2s by releasing TGF-β, which, in turn, activates neutrophils by secreting IL-5 and GM-CSF. Meanwhile, neutrophils activate ILC3s by producing IL-1β while ILC3s stimulate neutrophils by releasing IL-17. IL-5, GM-CSF, and IL-17 also act to extend neutrophil life span.
CTSG, cathepsin G; NE, neutrophil elastase; MV, microvesicle; IL, interleukin; GM-CSF, granulocyte macrophage-colony stimulating factor; DC, dendritic cell; Mac-1, macrophage antigen-1; ILC, innate lymphoid cell; NK, natural killer cell; NRP-1, neuropilin-1; TGF-β, transforming growth factor-β; IFN, interferon.
In turn, the recruited neutrophils become activated and secrete interferon (IFN)-γ and macrophage inflammatory protein (MIP)-1α and MIP-1β, which recruit circulating monocytes to the site of inflammation.87 Neutrophils also produce granule proteins such as cathelicidin-related antimicrobial peptides, LL-37, azurocidin, cathepsin G (CTSG), and proteinases88,89 that increase the inflammatory responses of macrophages.90 The NE from neutrophils also causes macrophages to produce MMPs such as MMP2, MMP9, and MMP14. In addition, NE acts synergistically with lipopolysaccharide to induce macrophages to produce the inflammatory cytokines IL-8, IL-1β, and TNF-α.52 Neutrophils also shape the microbicidal capability and polarization of macrophages. Thus, when neutrophils undergo apoptosis, they release azurocidin and lactoferrin, which are taken up by macrophages. This enhances both the microbicidal activity and the M1 polarization of macrophages.91 Similarly, Soehnlein et al.92 showed that neutrophil granule proteins trigger the release by macrophages of TNF-α and IFN-γ, which then act in an autocrine loop to promote macrophage phagocytosis. Notably, there are reports that neutrophils can induce the opposite polarization of macrophages towards the M2 phenotype.93 This may be mediated by neutrophil microvesicles bearing annexin A1 and phosphatidylserine.94,95 Moreover, when M2 macrophages are co-cultured with NETs, their secretion of proinflammatory cytokines rises; by contrast, NET-treated M1 macrophages undergo cell death.96
Thus, resident macrophages recruit neutrophils to the site of inflammation, after which the neutrophils recruit new macrophages. The two cell types then regulate each other’s functions. In particular, neutrophil-secreted granule proteins and NETs promote macrophage efferocytosis, pathogen phagocytosis, and the resolution of inflammation. This close reciprocal relationship warrants further investigation into macrophage-neutrophil interactions in chronic inflammation.
Interactions between neutrophils and dendritic cells
Dendritic cells (DCs) are professional antigen-presenting cells that can initiate T-cell and B-cell responses. Numerous studies have shown that neutrophil-DC interactions can modulate adaptive immune responses. Thus, when DCs are co-cultured with human neutrophils, their expression of CD86 and HLA-DR is upregulated via cell contact-dependent mechanisms and promotes antigen-specific T-cell responses.97 Moreover, neutrophils that express macrophage antigen-1 (Mac-1) can bind to the C-type lectin receptor DC-SIGN on DCs, thereby generating mature DCs that trigger strong T-cell proliferation. This DC-maturing effect of neutrophils is also mediated by their production of TNF-α.98 Moreover, neutrophils shape DC migration from sites of infection to the lymph nodes, where they activate T cells. Specifically, when neutrophils in Mycobacterium tuberculosis (Mt)-infected mice are depleted, DCs are more likely to be infected rather than acquiring Mt antigens by ingesting infected neutrophils. This downregulates DC migration to the mediastinal lymph nodes and delays the activation and proliferation of antigen-specific CD4+ T cells.99
Neutrophils also promote DC migration to inflamed sites by releasing the chemokines CCL3, CCL5, and CCL20.100 This may be mediated by the effect of environmental agents on neutrophils. For example, when peripheral blood neutrophils from smokers with or without COPD and healthy non-smokers were cultured with lipopolysaccharide or organic dust, they all produced the DC chemokine CCL3. However, while monoclonal antibody (mAb)-mediated neutralization of the TNF-α produced by the neutrophils blocked neutrophil production of CCL3 in healthy smokers and non-smokers, it was less effective in smokers with COPD. This suggests that while inflammatory responses or foreign molecules can activate neutrophils to recruit DCs into the lungs, the COPD environment may hamper this DC-recruiting function of neutrophils.101 Neutrophils may also promote DC recruitment by releasing proteases such as CTSG and NE that process the inactive precursors of DC chemoattractants (e.g., chemerin) into their highly active forms.102 In addition, neutrophils can activate DCs. One mechanism involves the secretion of the granule proteins α-defensins, cathelicidins, and lactoferrin, which bind to their corresponding receptors on the DC surface.103 Another mechanism involves NETs: a study with an in vivo model of emphysema showed that cigarette smoke promotes NET formation and that these NETs activate plasmacytoid DCs (pDCs) to produce IFN-α, a type I IFN. This, in turn, promotes the maturation and antigen presentation of the pDCs, which then strongly activate Th1 and Th17 differentiation.104 Notably, IFN-α from pDCs also stimulates neutrophils to form NETs.105 Thus, neutrophils and DCs may reciprocally augment each other’s functions, potentially creating vicious circles. There is also evidence that, conversely, DCs promote neutrophil recruitment: Kim et al.106 showed with a murine model of emphysema that DCs and monocytes recruited to the lungs promote emphysema by secreting serum amyloid A, which has chemotactic activity and induces neutrophil recruitment.
Thus, while there is clear evidence that neutrophils can regulate DCs and adaptive immune responses, there is also some evidence of the converse relationship. Further investigations into neutrophil-DC relationships in the context of chronic inflammation may prove fruitful in identifying therapeutic targets.
Interactions between neutrophils and ILCs
ILCs are innate immune cells ubiquitously distributed throughout various tissues but are enriched at the mucosal and barrier surfaces.107 ILCs were first identified in 2010 and have now been categorized as ILC1, ILC2, and ILC3 based on their hallmark cytokines and transcription factors.
The ILC1 group, characterized by high production of IFN-γ and TNF-α, includes natural killer (NK) cells. There is evidence that neutrophils and NK cells can shape each other’s functions during bacterial infection. Thus, in Legionella pneumophila infection, neutrophils release IL-18, which is essential for the MyD88-mediated production of IFN-γ by NK cells and the clearance of the bacteria.108 However, in Mt infection, the IFN-γ produced by NK cells suppresses the influx of neutrophils into the lungs, thereby inhibiting host defense against the bacterium.109 Also, inflammatory cytokines from ILC1s could induce myeloid cells (including neutrophils) to produce MMPs, disrupting the epithelial barrier and rendering it vulnerable to the ingress of infectious agents and their products.
The ILC2 group produces GM-CSF (which induces neutrophil differentiation) and the type 2 cytokines IL-4, IL-5, IL-13, and IL-9.110 Several lines of evidence suggest that ILC2s can regulate neutrophils. First, ILC2s may be elicited soon after severe traumatic injuries since such injuries are associated with a rapid elevation in circulating IL-4, IL-5, and IL-13 levels. Xu et al.111 showed recently that patients with multiple injury trauma also quickly exhibit high serum levels of IL-33, an alarmin released after barrier integrity has been breached and is a known stimulator of ILC2s. The authors then showed with a mouse model of hemorrhagic shock and tissue trauma that post-traumatic IL-33 induces ILC2s to produce IL-5 and that this, in turn, causes neutrophils to produce more IL-5. This ultimately results in early lung injury, a common consequence of polytrauma.111 Secondly, when a cigarette smoke-induced COPD model is conducted in mice that lack ILC2s, the neutrophil numbers in the lung are lower. This effect results in significantly greater collagen deposition in the airways compared to wild-type mice.112 Thirdly, ILC2s in the bone marrow help hematopoietic and progenitor cells to recover from 5-fluorouracil-induced stress by producing GM-CSF, which induces the differentiation of myeloid cells (including neutrophils). This role of ILC2s is mediated by IL-33 produced by the stressed progenitor cells.113 Finally, Zhang et al.114 showed that lung ILC2s differ from ILC2s in other tissues by expressing neuropilin-1 (NRP-1), a transmembrane glycoprotein that is upregulated by TGF-β. The authors then observed that knocking out NRP-1 in the bleomycin-induced model of lung fibrosis suppresses IL-5 and IL-13 production by lung ILC2s and protects the mice from lung fibrosis. Importantly, they observed that NRP-1 knockout reduces neutrophil recruitment to the lung. Since neutrophils are potent producers of TGF-β, these findings suggest that 1) ILC2s can induce neutrophil recruitment, and 2) conversely, neutrophils can promote profibrotic ILC2 functions in the lungs.114 Interestingly, a recent study by Patel et al.115 asked why neutrophil depletion worsens airway damage in an HDM-induced model of allergic inflammation. They then found that the upregulation of G-CSF, induced by neutropenia, directly increases ILC2 production of IL-5 and IL-13.115 The latter study shows how specific mechanisms that regulate either ILC2s or neutrophils can also shape functions of other cells.
The group 3 ILCs also express GM-CSF along with the Th17-associated cytokines IL-17A, IL-17F, and IL-22.110 Several studies showed that ILC3s regulate neutrophils and vice versa. First, ILC3s are the most abundant lung ILC subset in COPD, and in COPD patients with emphysema, their numbers in the lung correlate significantly with lung neutrophil frequencies.116,117 Notably, both ILC3 and neutrophil frequencies in the lung also correlate with decreased lung function.106 These correlations between ILC3 and neutrophil numbers reflect the fact that IL-17 produced by ILC3s is a key driver of neutrophil recruitment to the lung in COPD.116,117 Moreover, ILC3s can induce neutrophil migration and survival by secreting the neutrophil chemoattractant CXCL8 and GM-CSF, respectively.118 Also, analyses of the murine model of emphysema showed that neutrophil-derived IL-1 causes ILC3s to proliferate and produce IL-17A and that the IL-17A from ILC3s, in turn, induces the neutrophils to produce MMP12, which generates emphysema.106 Thus, ILC3s and neutrophils can cross-regulate each other in CRDs.
Given that ILCs are tissue-resident cells and there is considerable crosstalk and cross-regulation between ILCs and neutrophils, it is possible that novel therapeutics could prevent the onset of adaptive immune activation in CRDs by altering pathogenic ILC3-neutrophil interactions and thereby reprogramming the local cytokine milieu.
TARGETING NEUTROPHILS IN CHRONIC RESPIRATORY DISEASES
Since neutrophils participate in the initiation and aggravation of chronic lung inflammation, many preclinical studies have examined the therapeutic possibilities of targeting neutrophils. Neutrophilic proteases are key mediators of tissue damage and inflammation in CRDs, therefore, it has been suggested that proteases from neutrophils may be a suitable target for asthma and COPD. A potential neutrophil protease inhibitor may be α1-antitrypsin (A1AT), which is a protease inhibitor that is endogenously synthesized in the liver and released into the bloodstream.119 Although A1AT inhibits various proteases, its primary substrate is NE.120 Clinical studies suggest that A1AT deficiency is associated with asthma-like phenotypes and that A1AT-deficient patients are more susceptible to asthma.121,122,123 Therefore, augmenting A1AT production may be a potent therapy for relieving neutrophil-mediated lung inflammation. Indeed, when patients with severe congenital A1AT deficiency are infused with A1AT, their emphysema improves (as measured by computed tomography).124 Other possibilities are Alvelestat (AZD9886) and Sivelestat, which are chemical inhibitors of NE. However, their clinical effects on COPD remain to be investigated.125,126
Other targets are the MMPs, which also play a key role in the tissue damage in CRDs. They also have endogenous inhibitors, namely, the tissue inhibitors of metalloproteinases (TIMPs).127 However, clinical trials targeting TIMPs have not yet been conducted. Another possibility is AZD1236, which selectively inhibits MMP9 and MMP12. A short-term trial indicated that it is safe in patients with COPD but may lack therapeutic efficacy.128 Yet another candidate is FP-025, an inhibitor of MMP-12. It is currently being tested for efficacy in asthma and COPD by a phase 2 trial (NCT03858686).
Neutrophil chemokines or chemokine receptors may also be candidate therapeutic targets for CRDs. One is CXCR2, which is critical for the influx of neutrophils into inflamed tissues.85 A study with a murine model of asthma showed that the CXCR2 antagonist ladarixin reduced acute and chronic neutrophil influx and attenuated AHR.129 Moreover, administration of ladarixin decreased bleomycin-induced lung fibrosis and significantly increased survival rates in mice with cigarette smoke-induced exacerbation of influenza A infection.129 Several clinical trials have also shown that CXCR2 antagonists have potential in CRDs. Thus, the CXCR1/2 antagonist MK-7123 effectively improves lung function in patients with COPD, and this is associated with reduced neutrophil counts in sputum and lower plasma MMP9 levels.130 Similarly, oral treatment with the CXCR2 antagonist AZD5069 reduces blood neutrophil counts in COPD patients without serious adverse effects.131 A phase 2 clinical trial (NCT00688467) evaluating the efficacy of MK-7123 in mild asthma has been conducted. However, the results have not yet been published. Finally, there are two phase-2 trials on the ability of BIIL 284 to treat COPD (NCT02249338 and NCT02249247). It is an LTB4 receptor antagonist that blocks neutrophil infiltration. The results of these studies have also not been published to date.
CONCLUSIONS
This review explored the role of neutrophils in various inflammatory lung diseases. The evidence to date suggests that neutrophils not only mediate acute lung inflammation caused by acute infection but also play essential roles in chronic inflammatory diseases such as asthma, COPD, and IPF. These roles involve neutrophil-derived proteases, cytokines, and NET formation, which induce airway epithelial cell remodeling and hyperplasia. Moreover, neutrophils can exacerbate CRD severity by interacting with many different immune cells (here, we mainly dealt with innate immune cells, namely, macrophages, DCs, and ILCs). Thus, these interactions and cross-communication may be novel therapeutic targets, especially because neutrophils contribute to the etiology of chronic inflammatory settings via similar mechanisms; this suggests that the neutrophil-targeting treatment strategies that work in one disease may also be similarly beneficial in other chronic diseases.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (2022R1A2C3007730 and SRC 2017R1A5A1014560) and the Cooperative Research Program of Basic Medical Science and Clinical Science from Seoul National University College of Medicine (800-2021288).
Footnotes
Disclosure: There are no financial or other issues that might lead to a conflict of interest.
References
- 1.Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64:728–735. doi: 10.1136/thx.2008.108027. [DOI] [PubMed] [Google Scholar]
- 2.Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56:219–233. doi: 10.1007/s12016-018-8712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asher MI, García-Marcos L, Pearce NE, Strachan DP. Trends in worldwide asthma prevalence. Eur Respir J. 2020;56:56. doi: 10.1183/13993003.02094-2020. [DOI] [PubMed] [Google Scholar]
- 4.Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med. 2019;381:1257–1266. doi: 10.1056/NEJMra1900500. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ, Burney PG, Silverman EK, Celli BR, Vestbo J, Wedzicha JA, et al. Chronic obstructive pulmonary disease. Nat Rev Dis Primers. 2015;1:15076. doi: 10.1038/nrdp.2015.76. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) The top 10 causes of death. Geneva: WHO; 2020. [cited 2020 May 17]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. [Google Scholar]
- 7.Lee YG, Lee PH, Choi SM, An MH, Jang AS. Effects of air pollutants on airway diseases. Int J Environ Res Public Health. 2021;18:9905. doi: 10.3390/ijerph18189905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 9.Boehringer Ingelheim. IPF overview. Ingelheim am Rhein: Boehringer Ingelheim; c2022. [cited 2022 May 17]. Available from: https://www.boehringer-ingelheim.com/respiratory/ipf/facts-about-ipf. [Google Scholar]
- 10.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 11.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. 2016;16:378–391. doi: 10.1038/nri.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balamayooran G, Batra S, Fessler MB, Happel KI, Jeyaseelan S. Mechanisms of neutrophil accumulation in the lungs against bacteria. Am J Respir Cell Mol Biol. 2010;43:5–16. doi: 10.1165/rcmb.2009-0047TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun. 2009;77:568–575. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oved JH, Paris AJ, Gollomp K, Dai N, Rubey K, Wang P, et al. Neutrophils promote clearance of nuclear debris following acid-induced lung injury. Blood. 2021;137:392–397. doi: 10.1182/blood.2020005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrero-Cervera A, Soehnlein O, Kenne E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. 2022;19:177–191. doi: 10.1038/s41423-021-00832-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartoli ML, Di Franco A, Vagaggini B, Bacci E, Cianchetti S, Dente FL, et al. Biological markers in induced sputum of patients with different phenotypes of chronic airway obstruction. Respiration. 2009;77:265–272. doi: 10.1159/000176385. [DOI] [PubMed] [Google Scholar]
- 18.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133:1557–63.e5. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ham J, Shin JW, Ko BC, Kim HY. Targeting the epithelium-derived innate cytokines: from bench to bedside. Immune Netw. 2022;22:e11. doi: 10.4110/in.2022.22.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price DB, Rigazio A, Campbell JD, Bleecker ER, Corrigan CJ, Thomas M, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med. 2015;3:849–858. doi: 10.1016/S2213-2600(15)00367-7. [DOI] [PubMed] [Google Scholar]
- 21.Peters SP. Asthma phenotypes: nonallergic (intrinsic) asthma. J Allergy Clin Immunol Pract. 2014;2:650–652. doi: 10.1016/j.jaip.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Baos S, Calzada D, Cremades-Jimeno L, Sastre J, Picado C, Quiralte J, et al. Nonallergic asthma and its severity: biomarkers for its discrimination in peripheral samples. Front Immunol. 2018;9:1416. doi: 10.3389/fimmu.2018.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatri SB, Holguin FC, Ryan PB, Mannino D, Erzurum SC, Teague WG. Association of ambient ozone exposure with airway inflammation and allergy in adults with asthma. J Asthma. 2009;46:777–785. [PMC free article] [PubMed] [Google Scholar]
- 24.Brauer M, Hoek G, Smit HA, de Jongste JC, Gerritsen J, Postma DS, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J. 2007;29:879–888. doi: 10.1183/09031936.00083406. [DOI] [PubMed] [Google Scholar]
- 25.Oliver BG, Robinson P, Peters M, Black J. Viral infections and asthma: an inflammatory interface? Eur Respir J. 2014;44:1666–1681. doi: 10.1183/09031936.00047714. [DOI] [PubMed] [Google Scholar]
- 26.Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57:875–879. doi: 10.1136/thorax.57.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 28.Keir HR, Chalmers JD. Neutrophil extracellular traps in chronic lung disease: implications for pathogenesis and therapy. Eur Respir Rev. 2022;31:210241. doi: 10.1183/16000617.0241-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He X, Zhang L, Xiong A, Ran Q, Wang J, Wu D, et al. PM2.5 aggravates NQO1-induced mucus hyper-secretion through release of neutrophil extracellular traps in an asthma model. Ecotoxicol Environ Saf. 2021;218:112272. doi: 10.1016/j.ecoenv.2021.112272. [DOI] [PubMed] [Google Scholar]
- 30.Shin JW, Kim J, Ham S, Choi SM, Lee CH, Lee JC, et al. A unique population of neutrophils generated by air pollutant-induced lung damage exacerbates airway inflammation. J Allergy Clin Immunol. 2022;149:1253–1269.e8. doi: 10.1016/j.jaci.2021.09.031. [DOI] [PubMed] [Google Scholar]
- 31.Haddad A, Gaudet M, Plesa M, Allakhverdi Z, Mogas AK, Audusseau S, et al. Neutrophils from severe asthmatic patients induce epithelial to mesenchymal transition in healthy bronchial epithelial cells. Respir Res. 2019;20:234. doi: 10.1186/s12931-019-1186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torrego A, Hew M, Oates T, Sukkar M, Fan Chung K. Expression and activation of TGF-beta isoforms in acute allergen-induced remodelling in asthma. Thorax. 2007;62:307–313. doi: 10.1136/thx.2006.063487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy EA, Danna-Lopes D, Sarfati I, Rao SK, Cohen JR. Nicotine-stimulated elastase activity release by neutrophils in patients with abdominal aortic aneurysms. Ann Vasc Surg. 1998;12:41–45. doi: 10.1007/s100169900113. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, Wang W, Lin JT, Shirato K, Mitsuhashi H, Inoue H. Aerosolized human neutrophil elastase induces airway constriction and hyperresponsiveness with protection by intravenous pretreatment with half-length secretory leukoprotease inhibitor. Am J Respir Crit Care Med. 1996;153:1405–1411. doi: 10.1164/ajrccm.153.4.8616573. [DOI] [PubMed] [Google Scholar]
- 35.Baines KJ, Simpson JL, Wood LG, Scott RJ, Gibson PG. Systemic upregulation of neutrophil α-defensins and serine proteases in neutrophilic asthma. Thorax. 2011;66:942–947. doi: 10.1136/thx.2010.157719. [DOI] [PubMed] [Google Scholar]
- 36.Koga H, Miyahara N, Fuchimoto Y, Ikeda G, Waseda K, Ono K, et al. Inhibition of neutrophil elastase attenuates airway hyperresponsiveness and inflammation in a mouse model of secondary allergen challenge: neutrophil elastase inhibition attenuates allergic airway responses. Respir Res. 2013;14:8. doi: 10.1186/1465-9921-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ventura I, Vega A, Chacón P, Chamorro C, Aroca R, Gómez E, et al. Neutrophils from allergic asthmatic patients produce and release metalloproteinase-9 upon direct exposure to allergens. Allergy. 2014;69:898–905. doi: 10.1111/all.12414. [DOI] [PubMed] [Google Scholar]
- 38.Cundall M, Sun Y, Miranda C, Trudeau JB, Barnes S, Wenzel SE. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucocorticoids. J Allergy Clin Immunol. 2003;112:1064–1071. doi: 10.1016/j.jaci.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Mathioudakis AG, Vanfleteren LE, Lahousse L, Higham A, Allinson JP, Gotera C, et al. Current developments and future directions in COPD. Eur Respir Rev. 2020;29:200289. doi: 10.1183/16000617.0289-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salvi S. Tobacco smoking and environmental risk factors for chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:17–27. doi: 10.1016/j.ccm.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 41.He Z, Chen Y, Chen P, Wu G, Cai S. Local inflammation occurs before systemic inflammation in patients with COPD. Respirology. 2010;15:478–484. doi: 10.1111/j.1440-1843.2010.01709.x. [DOI] [PubMed] [Google Scholar]
- 42.Wouters EF. Local and systemic inflammation in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:26–33. doi: 10.1513/pats.200408-039MS. [DOI] [PubMed] [Google Scholar]
- 43.Wedzicha JA, Decramer M, Seemungal TA. The role of bronchodilator treatment in the prevention of exacerbations of COPD. Eur Respir J. 2012;40:1545–1554. doi: 10.1183/09031936.00048912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lacoste JY, Bousquet J, Chanez P, Van Vyve T, Simony-Lafontaine J, Lequeu N, et al. Eosinophilic and neutrophilic inflammation in asthma, chronic bronchitis, and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 1993;92:537–548. doi: 10.1016/0091-6749(93)90078-t. [DOI] [PubMed] [Google Scholar]
- 45.Baraldo S, Turato G, Badin C, Bazzan E, Beghé B, Zuin R, et al. Neutrophilic infiltration within the airway smooth muscle in patients with COPD. Thorax. 2004;59:308–312. doi: 10.1136/thx.2003.012146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujimoto K, Yasuo M, Urushibata K, Hanaoka M, Koizumi T, Kubo K. Airway inflammation during stable and acutely exacerbated chronic obstructive pulmonary disease. Eur Respir J. 2005;25:640–646. doi: 10.1183/09031936.05.00047504. [DOI] [PubMed] [Google Scholar]
- 47.Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol. 2003;163:2329–2335. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demkow U, van Overveld FJ. Role of elastases in the pathogenesis of chronic obstructive pulmonary disease: implications for treatment. Eur J Med Res. 2010;15(Suppl 2):27–35. doi: 10.1186/2047-783X-15-S2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benjamin JT, Plosa EJ, Sucre JM, van der Meer R, Dave S, Gutor S, et al. Neutrophilic inflammation during lung development disrupts elastin assembly and predisposes adult mice to COPD. J Clin Invest. 2021;131:e139481. doi: 10.1172/JCI139481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucey EC, Stone PJ, Breuer R, Christensen TG, Calore JD, Catanese A, et al. Effect of combined human neutrophil cathepsin G and elastase on induction of secretory cell metaplasia and emphysema in hamsters, with in vitro observations on elastolysis by these enzymes. Am Rev Respir Dis. 1985;132:362–366. doi: 10.1164/arrd.1985.132.2.362. [DOI] [PubMed] [Google Scholar]
- 51.Voynow JA, Young LR, Wang Y, Horger T, Rose MC, Fischer BM. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am J Physiol. 1999;276:L835–L843. doi: 10.1152/ajplung.1999.276.5.L835. [DOI] [PubMed] [Google Scholar]
- 52.Krotova K, Khodayari N, Oshins R, Aslanidi G, Brantly ML. Neutrophil elastase promotes macrophage cell adhesion and cytokine production through the integrin-Src kinases pathway. Sci Rep. 2020;10:15874. doi: 10.1038/s41598-020-72667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vandooren J, Goeminne P, Boon L, Ugarte-Berzal E, Rybakin V, Proost P, et al. Neutrophils and activated macrophages control mucosal immunity by proteolytic cleavage of antileukoproteinase. Front Immunol. 2018;9:1154. doi: 10.3389/fimmu.2018.01154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ilumets H, Rytilä PH, Sovijärvi AR, Tervahartiala T, Myllärniemi M, Sorsa TA, et al. Transient elevation of neutrophil proteinases in induced sputum during COPD exacerbation. Scand J Clin Lab Invest. 2008;68:618–623. doi: 10.1080/00365510801983773. [DOI] [PubMed] [Google Scholar]
- 55.Omachi TA, Eisner MD, Rames A, Markovtsova L, Blanc PD. Matrix metalloproteinase-9 predicts pulmonary status declines in α1-antitrypsin deficiency. Respir Res. 2011;12:35. doi: 10.1186/1465-9921-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vlahos R, Bozinovski S, Jones JE, Powell J, Gras J, Lilja A, et al. Differential protease, innate immunity, and NF-kappaB induction profiles during lung inflammation induced by subchronic cigarette smoke exposure in mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L931–L945. doi: 10.1152/ajplung.00201.2005. [DOI] [PubMed] [Google Scholar]
- 57.Foronjy R, Nkyimbeng T, Wallace A, Thankachen J, Okada Y, Lemaitre V, et al. Transgenic expression of matrix metalloproteinase-9 causes adult-onset emphysema in mice associated with the loss of alveolar elastin. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1149–L1157. doi: 10.1152/ajplung.00481.2007. [DOI] [PubMed] [Google Scholar]
- 58.Demedts IK, Morel-Montero A, Lebecque S, Pacheco Y, Cataldo D, Joos GF, et al. Elevated MMP-12 protein levels in induced sputum from patients with COPD. Thorax. 2006;61:196–201. doi: 10.1136/thx.2005.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grabcanovic-Musija F, Obermayer A, Stoiber W, Krautgartner WD, Steinbacher P, Winterberg N, et al. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir Res. 2015;16:59. doi: 10.1186/s12931-015-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dicker AJ, Crichton ML, Pumphrey EG, Cassidy AJ, Suarez-Cuartin G, Sibila O, et al. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:117–127. doi: 10.1016/j.jaci.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pullan J, Greenwood H, Walton GM, Stockley RA, Sapey E. Neutrophil extracellular traps (NETs) in COPD: a potential novel mechanism for host damage in acute exacerbations. Eur Respir J. 2015;46:PA5055 [Google Scholar]
- 62.Hope-Gill BD, Hilldrup S, Davies C, Newton RP, Harrison NK. A study of the cough reflex in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:995–1002. doi: 10.1164/rccm.200304-597OC. [DOI] [PubMed] [Google Scholar]
- 63.Obayashi Y, Yamadori I, Fujita J, Yoshinouchi T, Ueda N, Takahara J. The role of neutrophils in the pathogenesis of idiopathic pulmonary fibrosis. Chest. 1997;112:1338–1343. doi: 10.1378/chest.112.5.1338. [DOI] [PubMed] [Google Scholar]
- 64.Ziegenhagen MW, Zabel P, Zissel G, Schlaak M, Müller-Quernheim J. Serum level of interleukin 8 is elevated in idiopathic pulmonary fibrosis and indicates disease activity. Am J Respir Crit Care Med. 1998;157:762–768. doi: 10.1164/ajrccm.157.3.9705014. [DOI] [PubMed] [Google Scholar]
- 65.Hara A, Sakamoto N, Ishimatsu Y, Kakugawa T, Nakashima S, Hara S, et al. S100A9 in BALF is a candidate biomarker of idiopathic pulmonary fibrosis. Respir Med. 2012;106:571–580. doi: 10.1016/j.rmed.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 66.Sugino K, Nakamura Y, Muramatsu Y, Hata Y, Shibuya K, Homma S. Analysis of blood neutrophil elastase, glutathione levels and pathological findings in postoperative acute exacerbation of idiopathic pulmonary fibrosis associated with lung cancer: two case reports. Mol Clin Oncol. 2016;5:402–406. doi: 10.3892/mco.2016.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rohrbach AS, Slade DJ, Thompson PR, Mowen KA. Activation of PAD4 in NET formation. Front Immunol. 2012;3:360. doi: 10.3389/fimmu.2012.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki M, Ikari J, Anazawa R, Tanaka N, Katsumata Y, Shimada A, et al. PAD4 deficiency improves bleomycin-induced neutrophil extracellular traps and fibrosis in mouse lung. Am J Respir Cell Mol Biol. 2020;63:806–818. doi: 10.1165/rcmb.2019-0433OC. [DOI] [PubMed] [Google Scholar]
- 69.Takemasa A, Ishii Y, Fukuda T. A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. Eur Respir J. 2012;40:1475–1482. doi: 10.1183/09031936.00127011. [DOI] [PubMed] [Google Scholar]
- 70.Yamashita CM, Dolgonos L, Zemans RL, Young SK, Robertson J, Briones N, et al. Matrix metalloproteinase 3 is a mediator of pulmonary fibrosis. Am J Pathol. 2011;179:1733–1745. doi: 10.1016/j.ajpath.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.García-Prieto E, González-López A, Cabrera S, Astudillo A, Gutiérrez-Fernández A, Fanjul-Fernandez M, et al. Resistance to bleomycin-induced lung fibrosis in MMP-8 deficient mice is mediated by interleukin-10. PLoS One. 2010;5:e13242. doi: 10.1371/journal.pone.0013242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henry MT, McMahon K, Mackarel AJ, Prikk K, Sorsa T, Maisi P, et al. Matrix metalloproteinases and tissue inhibitor of metalloproteinase-1 in sarcoidosis and IPF. Eur Respir J. 2002;20:1220–1227. doi: 10.1183/09031936.02.00022302. [DOI] [PubMed] [Google Scholar]
- 73.Wilborn J, Bailie M, Coffey M, Burdick M, Strieter R, Peters-Golden M. Constitutive activation of 5-lipoxygenase in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1996;97:1827–1836. doi: 10.1172/JCI118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wardlaw AJ, Hay H, Cromwell O, Collins JV, Kay AB. Leukotrienes, LTC4 and LTB4, in bronchoalveolar lavage in bronchial asthma and other respiratory diseases. J Allergy Clin Immunol. 1989;84:19–26. doi: 10.1016/0091-6749(89)90173-5. [DOI] [PubMed] [Google Scholar]
- 75.Izumo T, Kondo M, Nagai A. Effects of a leukotriene B4 receptor antagonist on bleomycin-induced pulmonary fibrosis. Eur Respir J. 2009;34:1444–1451. doi: 10.1183/09031936.00143708. [DOI] [PubMed] [Google Scholar]
- 76.Hirata H, Arima M, Fukushima Y, Sugiyama K, Tokuhisa T, Fukuda T. Leukotriene C4 aggravates bleomycin-induced pulmonary fibrosis in mice. Respirology. 2013;18:674–681. doi: 10.1111/resp.12072. [DOI] [PubMed] [Google Scholar]
- 77.Wiley CD, Brumwell AN, Davis SS, Jackson JR, Valdovinos A, Calhoun C, et al. Secretion of leukotrienes by senescent lung fibroblasts promotes pulmonary fibrosis. JCI Insight. 2019;4:e130056. doi: 10.1172/jci.insight.130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frangogiannis N. Transforming growth factor-β in tissue fibrosis. J Exp Med. 2020;217:e20190103. doi: 10.1084/jem.20190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arora S, Dev K, Agarwal B, Das P, Syed MA. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology. 2018;223:383–396. doi: 10.1016/j.imbio.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barnes PJ. The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2009;41:631–638. doi: 10.1165/rcmb.2009-0220TR. [DOI] [PubMed] [Google Scholar]
- 83.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 84.Shim YM, Paige M, Hanna H, Kim SH, Burdick MD, Strieter RM. Role of LTB4 in the pathogenesis of elastase-induced murine pulmonary emphysema. Am J Physiol Lung Cell Mol Physiol. 2010;299:L749–L759. doi: 10.1152/ajplung.00116.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaur M, Singh D. Neutrophil chemotaxis caused by chronic obstructive pulmonary disease alveolar macrophages: the role of CXCL8 and the receptors CXCR1/CXCR2. J Pharmacol Exp Ther. 2013;347:173–180. doi: 10.1124/jpet.112.201855. [DOI] [PubMed] [Google Scholar]
- 86.Prame Kumar K, Nicholls AJ, Wong CH. Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018;371:551–565. doi: 10.1007/s00441-017-2753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Selders GS, Fetz AE, Radic MZ, Bowlin GL. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen Biomater. 2017;4:55–68. doi: 10.1093/rb/rbw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 89.Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peiró T, Patel DF, Akthar S, Gregory LG, Pyle CJ, Harker JA, et al. Neutrophils drive alveolar macrophage IL-1β release during respiratory viral infection. Thorax. 2018;73:546–556. doi: 10.1136/thoraxjnl-2017-210010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Påhlman LI, Mörgelin M, Eckert J, Johansson L, Russell W, Riesbeck K, et al. Streptococcal M protein: a multipotent and powerful inducer of inflammation. J Immunol. 2006;177:1221–1228. doi: 10.4049/jimmunol.177.2.1221. [DOI] [PubMed] [Google Scholar]
- 92.Soehnlein O, Kai-Larsen Y, Frithiof R, Sorensen OE, Kenne E, Scharffetter-Kochanek K, et al. Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages. J Clin Invest. 2008;118:3491–3502. doi: 10.1172/JCI35740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gong D, Shi W, Yi SJ, Chen H, Groffen J, Heisterkamp N. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012;13:31. doi: 10.1186/1471-2172-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eken C, Gasser O, Zenhaeusern G, Oehri I, Hess C, Schifferli JA. Polymorphonuclear neutrophil-derived ectosomes interfere with the maturation of monocyte-derived dendritic cells. J Immunol. 2008;180:817–824. doi: 10.4049/jimmunol.180.2.817. [DOI] [PubMed] [Google Scholar]
- 95.Rhys HI, Dell’Accio F, Pitzalis C, Moore A, Norling LV, Perretti M. Neutrophil microvesicles from healthy control and rheumatoid arthritis patients prevent the inflammatory activation of macrophages. EBioMedicine. 2018;29:60–69. doi: 10.1016/j.ebiom.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakazawa D, Shida H, Kusunoki Y, Miyoshi A, Nishio S, Tomaru U, et al. The responses of macrophages in interaction with neutrophils that undergo NETosis. J Autoimmun. 2016;67:19–28. doi: 10.1016/j.jaut.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 97.Megiovanni AM, Sanchez F, Robledo-Sarmiento M, Morel C, Gluckman JC, Boudaly S. Polymorphonuclear neutrophils deliver activation signals and antigenic molecules to dendritic cells: a new link between leukocytes upstream of T lymphocytes. J Leukoc Biol. 2006;79:977–988. doi: 10.1189/jlb.0905526. [DOI] [PubMed] [Google Scholar]
- 98.van Gisbergen KP, Sanchez-Hernandez M, Geijtenbeek TB, van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J Exp Med. 2005;201:1281–1292. doi: 10.1084/jem.20041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blomgran R, Ernst JD. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J Immunol. 2011;186:7110–7119. doi: 10.4049/jimmunol.1100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Charmoy M, Brunner-Agten S, Aebischer D, Auderset F, Launois P, Milon G, et al. Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS Pathog. 2010;6:e1000755. doi: 10.1371/journal.ppat.1000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Blidberg K, Palmberg L, Dahlén B, Lantz AS, Larsson K. Chemokine release by neutrophils in chronic obstructive pulmonary disease. Innate Immun. 2012;18:503–510. doi: 10.1177/1753425911423270. [DOI] [PubMed] [Google Scholar]
- 102.Wittamer V, Bondue B, Guillabert A, Vassart G, Parmentier M, Communi D. Neutrophil-mediated maturation of chemerin: a link between innate and adaptive immunity. J Immunol. 2005;175:487–493. doi: 10.4049/jimmunol.175.1.487. [DOI] [PubMed] [Google Scholar]
- 103.Yang D, de la Rosa G, Tewary P, Oppenheim JJ. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009;30:531–537. doi: 10.1016/j.it.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qiu SL, Zhang H, Tang QY, Bai J, He ZY, Zhang JQ, et al. Neutrophil extracellular traps induced by cigarette smoke activate plasmacytoid dendritic cells. Thorax. 2017;72:1084–1093. doi: 10.1136/thoraxjnl-2016-209887. [DOI] [PubMed] [Google Scholar]
- 105.Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F, et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med. 2013;19:65–73. doi: 10.1038/nm.3042. [DOI] [PubMed] [Google Scholar]
- 106.Kim J, Shin JW, Lee HJ, Kim JH, Choi SM, Lee CH, et al. Serum amyloid A promotes emphysema by triggering the reciprocal activation of neutrophils and ILC3s. Clin Transl Med. 2021;11:e637. doi: 10.1002/ctm2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tait Wojno ED, Artis D. Emerging concepts and future challenges in innate lymphoid cell biology. J Exp Med. 2016;213:2229–2248. doi: 10.1084/jem.20160525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spörri R, Joller N, Hilbi H, Oxenius A. A novel role for neutrophils as critical activators of NK cells. J Immunol. 2008;181:7121–7130. doi: 10.4049/jimmunol.181.10.7121. [DOI] [PubMed] [Google Scholar]
- 109.Feng CG, Kaviratne M, Rothfuchs AG, Cheever A, Hieny S, Young HA, et al. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis . J Immunol. 2006;177:7086–7093. doi: 10.4049/jimmunol.177.10.7086. [DOI] [PubMed] [Google Scholar]
- 110.Kim J, Ryu S, Kim HY. Innate lymphoid cells in tissue homeostasis and disease pathogenesis. Mol Cells. 2021;44:301–309. doi: 10.14348/molcells.2021.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu J, Guardado J, Hoffman R, Xu H, Namas R, Vodovotz Y, et al. IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translation study from a human cohort to a mouse trauma model. PLoS Med. 2017;14:e1002365. doi: 10.1371/journal.pmed.1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Donovan C, Starkey MR, Kim RY, Rana BM, Barlow JL, Jones B, et al. Roles for T/B lymphocytes and ILC2s in experimental chronic obstructive pulmonary disease. J Leukoc Biol. 2019;105:143–150. doi: 10.1002/JLB.3AB0518-178R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sudo T, Motomura Y, Okuzaki D, Hasegawa T, Yokota T, Kikuta J, et al. Group 2 innate lymphoid cells support hematopoietic recovery under stress conditions. J Exp Med. 2021;218:e20200817. doi: 10.1084/jem.20200817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang J, Qiu J, Zhou W, Cao J, Hu X, Mi W, et al. Neuropilin-1 mediates lung tissue-specific control of ILC2 function in type 2 immunity. Nat Immunol. 2022;23:237–250. doi: 10.1038/s41590-021-01097-8. [DOI] [PubMed] [Google Scholar]
- 115.Patel DF, Peiró T, Bruno N, Vuononvirta J, Akthar S, Puttur F, et al. Neutrophils restrain allergic airway inflammation by limiting ILC2 function and monocyte-dendritic cell antigen presentation. Sci Immunol. 2019;4:eaax7006. doi: 10.1126/sciimmunol.aax7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.De Grove KC, Provoost S, Verhamme FM, Bracke KR, Joos GF, Maes T, et al. Characterization and quantification of innate lymphoid cell subsets in human lung. PLoS One. 2016;11:e0145961. doi: 10.1371/journal.pone.0145961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ardain A, Porterfield JZ, Kløverpris HN, Leslie A. Type 3 ILCs in lung disease. Front Immunol. 2019;10:92. doi: 10.3389/fimmu.2019.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Croxatto D, Micheletti A, Montaldo E, Orecchia P, Loiacono F, Canegallo F, et al. Group 3 innate lymphoid cells regulate neutrophil migration and function in human decidua. Mucosal Immunol. 2016;9:1372–1383. doi: 10.1038/mi.2016.10. [DOI] [PubMed] [Google Scholar]
- 119.Gettins PG. Serpin structure, mechanism, and function. Chem Rev. 2002;102:4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 120.Stoller JK, Aboussouan LS. A review of α1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185:246–259. doi: 10.1164/rccm.201108-1428CI. [DOI] [PubMed] [Google Scholar]
- 121.Eden E, Strange C, Holladay B, Xie L. Asthma and allergy in alpha-1 antitrypsin deficiency. Respir Med. 2006;100:1384–1391. doi: 10.1016/j.rmed.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 122.Eden E. Asthma and COPD in alpha-1 antitrypsin deficiency. Evidence for the Dutch hypothesis. COPD. 2010;7:366–374. doi: 10.3109/15412555.2010.510159. [DOI] [PubMed] [Google Scholar]
- 123.Siri D, Farah H, Hogarth DK. Distinguishing alpha1-antitrypsin deficiency from asthma. Ann Allergy Asthma Immunol. 2013;111:458–464. doi: 10.1016/j.anai.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 124.Dirksen A, Piitulainen E, Parr DG, Deng C, Wencker M, Shaker SB, et al. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J. 2009;33:1345–1353. doi: 10.1183/09031936.00159408. [DOI] [PubMed] [Google Scholar]
- 125.Gunawardena KA, Gullstrand H, Perrett J. Pharmacokinetics and safety of AZD9668, an oral neutrophil elastase inhibitor, in healthy volunteers and patients with COPD. Int J Clin Pharmacol Ther. 2013;51:288–304. doi: 10.5414/CP201674. [DOI] [PubMed] [Google Scholar]
- 126.Lucas SD, Costa E, Guedes RC, Moreira R. Targeting COPD: advances on low-molecular-weight inhibitors of human neutrophil elastase. Med Res Rev. 2013;33(Suppl 1):E73–101. doi: 10.1002/med.20247. [DOI] [PubMed] [Google Scholar]
- 127.Murphy G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011;12:233. doi: 10.1186/gb-2011-12-11-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dahl R, Titlestad I, Lindqvist A, Wielders P, Wray H, Wang M, et al. Effects of an oral MMP-9 and -12 inhibitor, AZD1236, on biomarkers in moderate/severe COPD: a randomised controlled trial. Pulm Pharmacol Ther. 2012;25:169–177. doi: 10.1016/j.pupt.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 129.Mattos MS, Ferrero MR, Kraemer L, Lopes GA, Reis DC, Cassali GD, et al. CXCR1 and CXCR2 inhibition by ladarixin improves neutrophil-dependent airway inflammation in mice. Front Immunol. 2020;11:566953. doi: 10.3389/fimmu.2020.566953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rennard SI, Dale DC, Donohue JF, Kanniess F, Magnussen H, Sutherland ER, et al. CXCR2 antagonist MK-7123. A phase 2 Proof-of-concept trial for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:1001–1011. doi: 10.1164/rccm.201405-0992OC. [DOI] [PubMed] [Google Scholar]
- 131.Kirsten AM, Förster K, Radeczky E, Linnhoff A, Balint B, Watz H, et al. The safety and tolerability of oral AZD5069, a selective CXCR2 antagonist, in patients with moderate-to-severe COPD. Pulm Pharmacol Ther. 2015;31:36–41. doi: 10.1016/j.pupt.2015.02.001. [DOI] [PubMed] [Google Scholar]