Abstract
Purpose
The molecular links between metabolism and inflammation that drive different inflammatory phenotypes in asthma are poorly understood. We aimed to identify the metabolic signatures and underlying molecular pathways of different inflammatory asthma phenotypes.
Methods
In the discovery set (n = 119), untargeted ultra-high-performance liquid chromatography-mass spectrometry (UHPLC-MS) was applied to characterize the induced sputum metabolic profiles of asthmatic patients with different inflammatory phenotypes using orthogonal partial least-squares discriminant analysis (OPLS-DA), and pathway topology enrichment analysis. In the validation set (n = 114), differential metabolites were selected to perform targeted quantification. Correlations between targeted metabolites and clinical indices in asthmatic patients were analyzed. Logistic and negative binomial regression models were established to assess the association between metabolites and severe asthma exacerbations.
Results
Seventy-seven differential metabolites were identified in the discovery set. Pathway topology analysis uncovered that histidine metabolism, glycerophospholipid metabolism, nicotinate and nicotinamide metabolism, linoleic acid metabolism as well as phenylalanine, tyrosine and tryptophan biosynthesis were involved in the pathogenesis of different asthma phenotypes. In the validation set, 24 targeted quantification metabolites were significantly expressed between asthma inflammatory phenotypes. Finally, adenosine 5′-monophosphate (adjusted relative risk [adj RR] = 1.000; 95% confidence interval [CI] = 1.000–1.000; P = 0.050), allantoin (adj RR = 1.000; 95% CI = 1.000–1.000; P = 0.043) and nicotinamide (adj RR = 1.001; 95% CI = 1.000–1.002; P = 0.021) were demonstrated to predict severe asthma exacerbation rates.
Conclusions
Different inflammatory asthma phenotypes have specific metabolic profiles in induced sputum. The potential metabolic signatures may identify therapeutic targets in different inflammatory asthma phenotypes.
Keywords: Asthma, sputum, metabolomics, phenotype, biomarkers
INTRODUCTION
Asthma is a serious global health problem of chronic inflammatory airway disease characterized by airway hyperresponsiveness and reversible airway obstruction resulting in recurrent episodes of wheezing, shortness of breath, chest tightness, and cough.1 Asthma is a heterogeneous disease with different phenotypes and endotypes, within which the inflammatory phenotypes of asthma (eosinophilic asthma [EA], neutrophilic asthma [NA], and paucigranulocytic asthma [PGA]) are widely recognized by differential proportions of induced sputum granulocytes.2 Although some guidelines recommend tailored management approaches for patients with different inflammatory phenotypes,1,3,4 treatment strategy for specific inflammatory phenotypes, especially NA, is lacking and represents an unmet medical need in asthma.5,6 Meanwhile, molecular mechanisms driving different inflammatory phenotypes of asthma are poorly understood and are likely to be heterogeneous. Therefore, it is important to identify the specific biomarkers and understand molecular mechanisms for different inflammatory phenotypes of asthma, which may lead to more personalized medicine.7
Metabolomics is the systematic identification and quantitative scientific study of chemical processes involving metabolites, small molecule substrates, intermediates, and products of cell metabolism in a given organism or biological sample.8,9 Metabolomics represents an integrated pathophysiological profile encompassing genetic and environmental interactions, which could prove instrumental in elucidating the biological mechanisms of asthma. There is growing evidence that metabolomic profiles in exhaled breath condensate (EBC), urine, and blood could distinguish asthma and asthma phenotypes.10 Our previous study has also shown for the first time that metabolite profiles in induced sputum better separate asthma phenotypes.11 Although some studies have demonstrated that inflammatory asthma phenotypes can be discriminated by an electronic nose breath analyzer,12 there are no specific metabolic biomarkers and molecular pathways identified in terms of different inflammatory asthma phenotypes in the study. Therefore, it can be more justified to use induced sputum to obtain a close molecular mechanism of different inflammatory phenotypes of asthma.
The primary aim of this study was to identify the metabolic signatures and underlying molecular pathways of different inflammatory asthma phenotypes. The secondary aim was to explore if these signatures are related to asthma control and exacerbation risk. We applied ultra-high performance liquid chromatography–mass spectrometry (UHPLC-MS) to characterize the induced sputum metabolic profiles from healthy controls and asthmatic patients classified according to different inflammatory phenotypes as EA, NA, and PGA in the discovery set. Following multivariate modeling, the concentrations of selected metabolites were confirmed with targeted mass spectrometry assays in the validation set.
MATERIALS AND METHODS
Study design and subjects
This study included 2 parts: 1) discovery set: a cross-sectional study to identify the metabolic signatures and underlying molecular pathways of different inflammatory asthma phenotypes; 2) validation set: a prospective cohort study to explore the association between those identified signatures identified in the discovery set and asthma exacerbations in the 12-month followed-up period. This was a real-world study based on the Australasian Severe Asthma Network (ASAN),13 in which patients with asthma were consecutively recruited from the asthma clinic of the West China Hospital, Sichuan University in China and we did not exclude the asthmatic patients with chronic obstructive pulmonary disease (COPD), namely asthma-COPD overlap (ACO). Asthmatic participants in the discovery set (n = 119) of the cross-sectional study and in the validation set (n = 114) of the prospective cohort study were recruited from the Asthma Clinic of West China Hospital, Sichuan University. In addition, 20 healthy volunteers were recruited by advertisement between March 2014 and April 2019. All participants were aged ≥ 18 years with stable asthma (no respiratory tract infection and no exacerbation or systemic corticosteroid use in the previous 4 weeks). Asthma was diagnosed according to American Thoracic Society (ATS) guidelines14 and Global Initiative for Asthma (GINA).1 Subjects were excluded if: 1) they were smoking or ever smoked, pregnant or breastfeeding; and 2) they had cognitive impairment or current solid organ malignancy. Asthma inflammatory phenotype was assigned based on sputum cell counts. Subjects with a sputum proportion of < 61% neutrophils and < 3% eosinophils were classified as PGA; those with ≥ 61% neutrophils and < 3% eosinophils were classified as NA, those with < 61% neutrophils and ≥ 3% eosinophils were classified as EA.2 This study was reviewed and approved by the Institutional Review Board of West China Hospital, Sichuan University (Chengdu, China) (No.2014-30), and all subjects provided written informed consent.
Clinical assessment
Data on demographics and clinical characteristics were collected using the standardized case report form and detailed as shown in the Supplementary Data S1. Asthma control questionnaire (ACQ) score15 and Asthma Quality of Life Questionnaire (AQLQ) score,16 were analyzed in all participants. In the validation set, severe asthma exacerbations were recorded during the 12-month follow-up, which were defined as worsening asthma symptoms that led to one of the following: 1) use of systemic corticosteroid for more than 3 days; and 2) a hospitalization or emergency department (ED) visit because of asthma, requiring systemic corticosteroids according to the ATS/European Respiratory Society (ERS) guidelines.17
Sputum induction, processing, and airway inflammatory cytokine assay
In this study, all the included patients underwent sputum induction between 8:00 a.m. and 10:00 a.m. on the day of the enrollment, and all the sputum samples were processed by well-trained researchers within 2 hours according to the standard protocols established by the ASAN as described in our previous published studies.18,19 The inflammatory phenotype was then determined based on the induced sputum cell counts. Sputum plugs were collected for subsequent metabolism analysis with sputum supernatants for airway inflammatory cytokine assay. Additional details are described in Supplementary Data S1. In the validation set, sputum supernatant cytokines, regarded as airway inflammatory biomarkers, including interleukin (IL)-1β, IL-5, IL-8, IL-13, IL-17A, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α, were detected using a Luminex-based MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel Kit (EMD Millipore Corporation, Billerica, MA, USA) with the MILLIPLEX Analyst 5.1 software. Spiking experiments on cytokines in sputum supernatants showed that recovery ranged from 70% to 130% in all detectable analyses.20,21
UHPLC-MS method for metabolomics
In the discovery set, sputum plugs were collected, and an aliquot of 200 μL of sputum plug was quick-frozen immediately by liquid nitrogen and stored at −80°C for subsequent metabolism analysis. The sputum samples collected were thawed on ice, and metabolites were extracted with acetonitrile and methanol using the previously described method.22,23 Sputum supernatant samples were analyzed using a UHPLC system (1290; Agilent Technologies, Santa Clara, CA, USA) with a UPLC BEH Amide (2.1 mm × 100 mm, 1.7 μm) coupled to TripleTOF 6550+6600 (Q-TOF, AB Sciex Pte. Ltd., Singapore) via a previously described method with modifications.24 The detailed sputum preparation, spectrometry method, and metabolite identification are described in Supplementary Data S1.
Univariate and multivariate analysis for metabolomic profiling
After the high-throughput screening of the metabolic features using the above rules, a total of 772 (473 from positive ion mode and 299 from negative ion mode) features were extracted from the UHPLC-MS data. For each metabolite peak reproducibly detected in whole samples, the unpaired two-tailed Wilcoxon rank-sum test (a nonparametric univariate method) was carried out to examine the significance of each metabolite among different groups and adjusted by false discovery rate (FDR) correction (Benjamini–Hochberg method). The merged UHPLC-MS data were log-transformed for subsequent analyses. The filtered and normalized data matrix was exported for multivariate statistical analysis using principal component analysis (PCA) and orthogonal partial least-squares discriminant analysis (OPLS-DA) with SIMCA 16.1 software package (Umetrics, Umea, Sweden). The permutation tests (n = 200) proceeded to validate the model further. By combining the univariate and multivariate statistical analyses, based on variable importance in the projection (VIP) threshold of 1 from the 10-fold cross-validated OPLS-DA model, fold change < 0.83 or > 1.2, FDR < 0.05, numbers of different metabolites among different groups were obtained.
Pathway analysis
Metabolomics Pathway Analysis (MetPA) was performed by the online tool MetaboAnalyst with the hypergeometric test for over-representation analysis and relative betweenness centrality for pathway topology analysis.25
Targeted quantification in the validation set
After evaluations from 3 aspects: 1) reference standard availability, 2) acceptable chromatography peak, and 3) quality control criteria (the recovery and relative standard deviation [RSD] of standards mixture quality control sample were in 80%-120% and less than 20%, respectively). Only 39 metabolites could be available and simultaneously quantified in the sputum samples in multiple reaction monitoring modes by liquid chromatography-tandem mass spectrometry using an EXION LC System (SCIEX) coupled with SCIEX 6500 QTRAP + Triple Quadrupole mass spectrometry (SCIEX). Details were described in Supplementary Data S1.
Statistics analysis
Continuous variables are expressed as the mean ± standard deviation or median (quartiles [Q]1, [Q]3) depending on their distribution. Categorical variables are summarized as numbers and percentages. For continuous variables, the differences among different inflammatory asthma phenotypes and healthy controls were analyzed using the Student’s t-test or analysis of variance if they were normally distributed; otherwise, Wilcoxon rank-sum test was used to assess them. Categorical data were compared using the χ2 test or Fisher’s exact test. Correlations between targeted metabolites and clinical indices in asthmatic patients in the validation set were analyzed using Spearman’s correlation coefficients and adjusted by FDR correction (Benjamini–Hochberg method). Between-group comparisons of targeted metabolites were examined by receiver operating characteristic (ROC) curves and the area under the curve (AUC) was calculated to determine their capability to discriminate different groups in the validation set. Logistic and negative binomial regression models were established to assess the association between metabolites and severe asthma exacerbations during follow-up in the validation set. These analyses were conducted using SPSS version 25.0 (IBM Corp, Armonk, NY, USA). A 2-tailed P value of < 0.05 was considered statistically significant in all statistical analyses.
RESULTS
Demographics and clinical characteristics
The characteristics of the participants are summarized in Table 1 and Supplementary Tables S1 and S2. There were 20 healthy control subjects and 119 asthmatic patients (40 patients with EA, 36 patients with NA, and 43 patients with PGA) recruited in the discovery set and 114 asthmatic patients (35 patients with EA, 30 patients with NA, and 49 patients with PGA) recruited in the validation set. Detail demographics and clinical characteristics are described in Supplementary Data S1.
Table 1. Clinical characteristics grouped by airway inflammatory phenotypes in the validation set.
| Variables | EA (n = 35) | NA (n = 30) | PGA (n = 49) | χ2/F/Z | P value | |
|---|---|---|---|---|---|---|
| Sex (female) | 24 (68.6) | 19 (63.3) | 24 (49) | 3.584 | 0.167 | |
| Age (yr) | 45.37 ± 10.58 | 51.94 ± 13.34b | 44.10 ± 12.08d | 4.220 | 0.017 | |
| BMI (kg/m2) | 23.68 ± 2.86 | 23.28 ± 3.78 | 24.63 ± 4.13 | 1.404 | 0.250 | |
| Family history | 10 (28.6) | 14 (46.7) | 15 (30.6) | 2.844 | 0.241 | |
| Age onset (yr) | 34.00 (8.00, 42.00) | 39.00 (22.50, 50.75) | 33.00 (16.50, 39.50) | 3.050 | 0.218 | |
| Asthma duration (yr) | 11.62 (3.31, 35.26) | 10.92 (4.61, 22.22) | 10.21 (4.06, 20.42) | 0.661 | 0.719 | |
| Severe asthma | 3 (8.6) | 5 (16.7) | 5 (10.2) | 1.170 | 0.557 | |
| BDP (ug) | 200 (80, 400) | 400 (400, 1,000) | 400 (0, 800) | 12.516 | 0.002 | |
| LTRA | 15 (42.9) | 10 (33.3) | 18 (36.7) | 0.659 | 0.719 | |
| Theophylline | 3 (8.6) | 10 (33.3) | 11 (22.4) | 6.060 | 0.048 | |
| Proportions of patients experiencing exacerbation in the previous 12 months | 14 (40) | 11 (36.7) | 15 (30.6) | 0.835 | 0.659 | |
| Atopic comorbidity | ||||||
| Allergic rhinitis | 23 (65.7) | 9 (31) | 25 (51) | 7.642 | 0.022 | |
| Eczema | 7 (20) | 8 (26.7) | 8 (16.3) | 1.236 | 0.539 | |
| SPT | 16 (45.7) | 12 (40) | 23 (46.9) | 7.790 | 0.100 | |
| ACQ | 0.50 (0.17, 1.83) | 0.83 (0.33, 1.33) | 0.33 (0.00, 1.17) | 4.533 | 0.103 | |
| AQLQ | 5.63 (4.87, 6.42) | 5.90 (5.28, 6.42) | 6.13 (5.58, 6.59) | 3.471 | 0.176 | |
| Pre-bronchodilator | r | |||||
| FEV1 (L) | 2.02 ± 0.61 | 1.74 ± 0.57 | 2.45 ± 0.98b,c | 8.370 | < 0.001 | |
| FEV1 (%pred) | 69.69 ± 19.41 | 63.73 ± 20.05 | 77.39 ± 20.64c | 4.477 | 0.013 | |
| FVC (L) | 3.19 ± 0.73 | 2.83 ± 0.63 | 3.46 ± 1.10c | 4.730 | 0.011 | |
| FVC (%) | 91.77 ± 17.75 | 85.07 ± 12.451 | 91.88 ± 17.02 | 1.930 | 0.150 | |
| FEV1/FVC (%) | 63.00 ± 11.02 | 61.57 ± 15.43 | 70.07 ± 12.90b,c | 4.972 | 0.009 | |
| Post-bronchodilator | ||||||
| FEV1 (L) | 2.29 ± 0.61 | 1.89 ± 0.64b | 2.61 ± 0.88c | 8.236 | < 0.001 | |
| FEV1 (%) | 73.79 ± 18.85 | 68.44 ± 19.37b | 84.30 ± 17.05c | 6.749 | 0.002 | |
| FVC (L) | 3.39 ± 0.70 | 3.01 ± 0.67 | 3.56 ± 0.98c | 3.811 | 0.025 | |
| FVC (%) | 99.16 ± 15.99 | 89.36 ± 10.56a | 95.99 ± 13.62d | 3.995 | 0.021 | |
| FEV1/FVC (%) | 67.71 ± 10.54 | 62.71 ± 15.46 | 73.42 ± 13.04 | 9.963 | 0.007 | |
| BDR | ||||||
| Δ FEV1 (L) | 0.29 (0.16, 0.46) | 0.17 (0.05, 0.25)a | 0.15 (0.04, 0.32)a | 10.118 | 0.006 | |
| Δ FEV1 (%) | 18.06 (7.30, 29.06) | 10.68 (3.47, 18.25)b | 6.80 (1.27, 17.08)a | 9.364 | 0.009 | |
| FeNO (ppb) | 53.50 (38.50, 79.75) | 26.50 (17.75, 49.25)b | 29.50 (14.25, 49.75)b | 14.470 | 0.001 | |
| IgE (IU/mL) | 119.71 (46.80, 378.21) | 88.76 (26.16, 391.25) | 82.83 (4547, 347.16) | 0.871 | 0.647 | |
| Sputum cytology | ||||||
| Total cell | 0.30 (0.4, 0.42) | 0.68 (0.42, 1.23) | 0.26 (0.13, 0.54) | 24.495 | < 0.001 | |
| Eosinophil (%) | 14.63 (7.44, 38.75) | 0.00 (0.00, 0.25)a | 0.00 (0.00, 0.38)a | 78.829 | < 0.001 | |
| Eosinophil (×104/mL) | 4.24 (1.50, 10.66) | 0.00 (0.00, 0.16) | 0.00 (0.00, 0.08) | 74.370 | < 0.001 | |
| Neutrophil (%) | 26.00 (13.00, 43.00) | 90.25 (78.50, 98.31)a | 17.75 (6.75, 35.25)b,c | 67.844 | < 0.001 | |
| Neutrophil (×104/mL) | 5.55 (2.97, 12.83) | 57.33 (32.13, 107.30) | 3.80 (1.32, 11.19) | 57.524 | < 0.001 | |
| Lymphocyte (%) | 1.00 (0.50, 1.50) | 0.50 (0.25, 0.75)b | 0.75 (0.25, 2.00)d | 6.245 | 0.044 | |
| Lymphocyte (×104/mL) | 0.24 (0.05, 0.41) | 0.30 (0.10, 0.53) | 0.25 (0.06, 0.65) | 0.510 | 0.775 | |
| Macrophage (%) | 48.13 (28.75, 66.63) | 8.63 (1.38, 19.31)a | 80.50 (63.75, 92.00)a,c | 76.359 | < 0.001 | |
| Macrophage (×104/mL) | 11.04 (8.01, 19.08) | 5.85 (1.05, 12.57) | 21.60 (8.32, 39.80) | 23.856 | < 0.001 | |
| Sputum cytokines | ||||||
| TNF-α (pg/mL) | 6.13 (2.67, 13.12) | 32.58 (5.53, 58.27)a | 12.24 (5.07, 26.46)a | 13.398 | < 0.001 | |
| IFN-γ (pg/mL) | 2.01 (1.80, 2.77) | 2.18 (1.90, 2.44) | 2.01 (1.66, 2.631) | 0.631 | 0.729 | |
| IL-1β (pg/mL) | 8.28 (4.44, 18.38) | 64.30 (21.35, 286.80)a | 18.14 (5.63, 57.70)b,c | 20.533 | < 0.001 | |
| IL-4 (pg/mL) | 43.75 (21.99, 67.96) | 52.19 (35.78, 59.16) | 43.75 (10.30, 65.42) | 1.543 | 0.462 | |
| IL-5 (pg/mL) | 2.49 (1.40, 7.21) | 0.83 (0.72, 1.26)a | 1.23 (0.87, 1.85)a,c | 25.185 | < 0.001 | |
| IL-6 (pg/mL) | 10.92 (3.74, 19.94) | 22.76 (2.76, 46.12) | 19.57 (7.26, 46.62) | 4.320 | 0.115 | |
| IL-8 (pg/mL) | 953.15 (426.06, 1,517.75) | 1,307.50 (701.92, 3,757.75)a | 1,209.0 (732.36, 1,976.50)b | 9.165 | 0.010 | |
| IL-13 (pg/mL) | 3.33 (1.95, 8.69) | 3.39 (2.14, 4.50) | 3.62 (2.44, 5.64) | 1.687 | 0.430 | |
| IL-17A (pg/mL) | 2.08 (1.58, 3.18) | 3.56 (2.11, 5.08)a | 2.66 (1.72, 4.18) | 8.764 | 0.013 | |
| MDC (pg/mL) | 41.09 (20.57, 75.15) | 38.58 (24.70, 64.29) | 46.01 (26.46, 75.14) | 2.487 | 0.288 | |
| Exacerbation in the 12-month follow-up | ||||||
| Withdraw | 0 (0) | 3 (10) | 3 (6.1) | 3.367 | 0.186 | |
| Severe exacerbation, n (%) | 6 (17.1) | 10 (37) | 3 (6.5)c | 10.935 | 0.004 | |
| Severe exacerbation times, n (frequency/person/year) | 0.29 ± 0.75 | 0.70 ± 1.33 | 0.09 ± 0.35c | 10.965 | 0.004 | |
| Emergency visit, n (%) | 3 (8.6) | 2 (7.4) | 2 (4.3) | 0.636 | 0.728 | |
| Emergency visit times, n (frequency/person/year) | 0.11 ± 0.40 | 0.07 ± 0.27 | 0.09 ± 0.41 | 0.552 | 0.759 | |
| Hospitalization, n (%) | 5 (14.3) | 5 (18.5) | 3 (6.5) | 2.560 | 0.278 | |
| Hospitalization times, n (frequency/person/year) | 0.26 ± 0.74 | 0.26 ± 0.66 | 0.09 ± 0.35 | 2.513 | 0.285 | |
Values are presented as mean ± standard deviation, number (%), or median (quartile 1, quartile 3).
EA, eosinophilic asthma; NA, neutrophilic asthma; PGA, paucigranulocytic asthma; BMI, body mass index; BDP, beclomethasone dipropionate; LTRA, leukotriene receptor antagonist; SPT, skin prick test; ACQ, asthma control questionnaire; AQLQ, asthma quality of life questionnaire; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; BDR, bronchodilator response; FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; TNF, tumor necrosis factor; IFN, interferon; IL, interleukin; MDC, macrophage-derived chemokines.
Versus EA, aP < 0.01, bP < 0.05; Versus NA, cP < 0.01, dP < 0.05.
In the validation set, sputum supernatant inflammatory cytokines were assessed. Among the asthma phenotypes, patients with EA had the highest level of IL-5 and the lowest levels of TNF-α, IL-1β, and IL-8 (all P < 0.05). However, patients with NA had the highest IL-1β and the lowest IL-5 levels (all P < 0.05) (Table 1). In the 12-month follow-up period, 3 (10%) patients with NA and 3 (6.1%) with PGA were lost to follow-up. Finally, there were 108 patients with asthma (EA, n = 35; NA, n = 27; PGA, n = 46) were included in the analysis of asthma exacerbations. As a result, there were significant differences in the proportion of patients experiencing severe asthma exacerbation (EA vs. NA vs. PGA: 17.1% vs. 37% vs. 6.5%, P =0.004) and the frequency of severe asthma exacerbation times (EA vs. NA vs. PGA: 0.29 ± 0.75 vs. 0.70 ± 1.33 vs. 0.09 ± 0.35, P = 0.004) among the phenotypic groups (Table 1).
Untargeted metabolic profiles discriminate between different inflammatory asthma phenotypes and healthy subjects in the discovery set
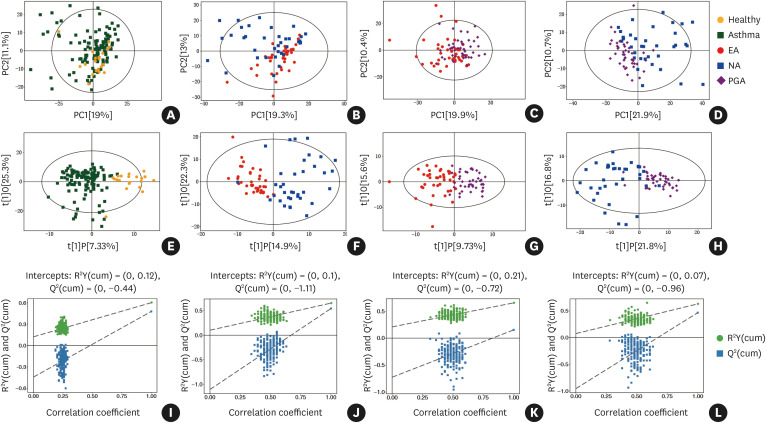
The score plot of OPLS-DA analysis showed a distinct separation between different asthma inflammatory phenotypes. Furthermore, the asthma group was also clearly separated from the healthy controls (Fig. 1). The parameters for the classification models from the software are shown in Supplementary Table S3, suggesting all models had very good fitness and predictive capability. Furthermore, a leave-one-out cross-validation (LOOCV) was used to estimate the robustness and predictive ability of each model; thus, a 200 permutation test was applied. The low value of the Q2 intercept in each model indicated the robustness of these models and thus showed a low risk of over-fitting in all comparisons.
Fig. 1. Untargeted metabolic profiles discriminate different inflammatory phenotypes of asthma and healthy subjects in the discovery set. (A-D) Score plot of PCA model obtained retrospectively from different inflammatory asthma phenotypes, and healthy subjects; (E-H) Score plot of OPLS-DA model obtained retrospectively from different inflammatory asthma phenotypes and healthy subjects; The labels t[1] and t[2] along the axes represent the scores (the first 2 partial least-squares components) of the model, which are sufficient to build a satisfactory classification model. (I-L) Permutation test of the OPLS-DA model was obtained retrospectively from different inflammatory asthma phenotypes and healthy subjects; 200 permutations were performed, and the resulting R2 and Q2 values were plotted.
PCA, principal component analysis; OPLS-DA, orthogonal partial least-squares discriminant analysis; EA, eosinophilic asthma; NA, neutrophilic asthma; PGA, paucigranulocytic asthma.
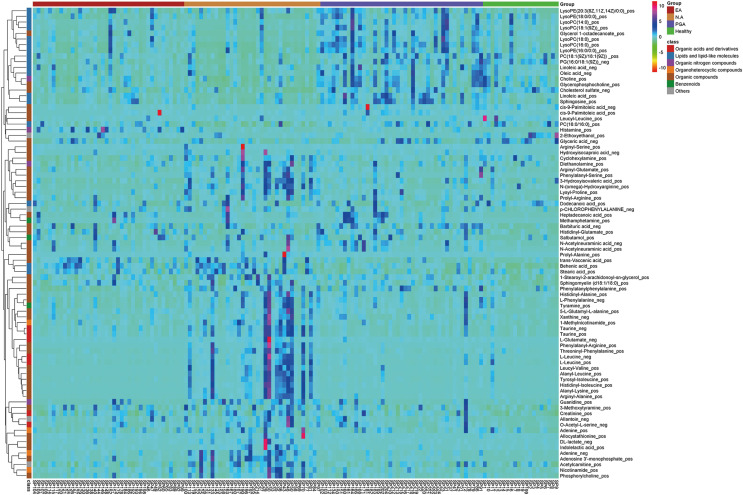
By combining the univariate and multivariate statistical analyses, based on a VIP threshold of 1 from the 10-fold cross-validated OPLS-DA model, fold change < 0.83 or > 1.2 and FDR < 0.05, a total of 77 differential metabolites were identified between different asthma phenotypes and healthy subjects as shown in the heatmap in Fig. 2.
Fig. 2. Heatmap of identified differential metabolites in sputum samples in different inflammatory phenotypes of asthma and healthy subjects in the discovery set.
Pathway topology analysis in the discovery set
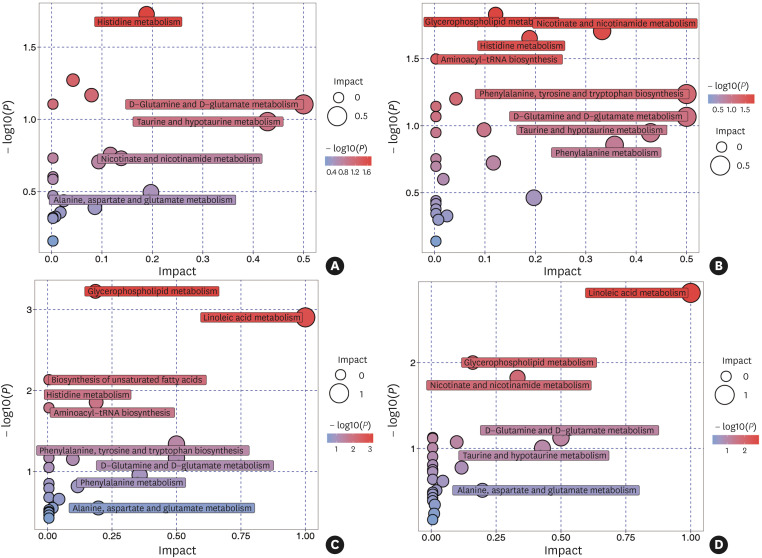
To analyze the most relevant pathways after identifying differential metabolites, the metabolic pathway topology enrichment analysis was applied with the impact value to evaluate the importance of the pathways underlying the pathophysiology mechanism in different asthma phenotypes and healthy subjects (Fig. 3). As a result, histidine metabolism (P = 0.019; impact value = 0.189) was identified as different between asthmatics and healthy controls. Differential metabolites between EA and NA were involved in glycerophospholipid metabolism (P = 0.015; impact value = 0.121), nicotinate and nicotinamide metabolism (P = 0.020; impact value = 0.332) and histidine metabolism (P = 0.022; impact value = 0.189). Differential metabolites between EA and PGA were involved in glycerophospholipid metabolism (P = 0.001; impact value = 0.186), linoleic acid metabolism (P = 0.001; impact value = 1.000), histidine metabolism (P = 0.014; impact value = 0.189) and phenylalanine, tyrosine and tryptophan biosynthesis (P = 0.046; impact value = 0.500). Differential metabolites between NA and PGA were involved in linoleic acid metabolism (P = 0.002; impact value = 1.000), glycerophospholipid metabolism (P = 0.010; impact value = 0.160) and nicotinate and nicotinamide metabolism (P = 0.015; impact value = 0.332).
Fig. 3. MetaboAnalyst pathway Impact based on selected and more representative metabolites responsible for the class separation in sputum samples in different inflammatory phenotypes of asthma and healthy subjects. (A) Asthma and healthy; (B) EA and NA; (C) EA and PGA; (D) NA and PGA. Circles represent metabolic pathways potentially involved in class separation. EA, eosinophilic asthma; NA, neutrophilic asthma; PGA, paucigranulocytic asthma.
Targeted quantification of identified metabolites between different inflammatory asthma phenotypes and healthy subjects in the validation set
To validate and determine the expression levels of 39 differentially expressed metabolites, which were selected to perform targeted quantification in patients in the validation set. Among these metabolites, 24 were significantly expressed between asthma inflammatory phenotypes (Table 2), with 9 reaching a significant P value of 0.001 or less. The expression of histamine, the histidine metabolism-associated metabolite, was highest, whereas concentrations of 5-L-glutamyl-L-alanine, nicotinamide, dihydrothymine, L-leucine, L-phenylalanine, alanyl-leucine, phenylalanyl-serine, phenylalanylphenylalanine were the lowest in patients with EA. These results were consistent with those of pathway topology analysis, indicating that histidine metabolism may play an important role in EA. In patients with NA, concentrations of adenosine 5′-monophosphate, glyceric acid, taurine, dihydrothymine, L-leucine, tyramine, L-glutamate, alanyl-leucine, phenylalanyl-serine, and threoninyl-phenylalanine were highest, which involved in purine metabolism, glyoxylate, and dicarboxylate metabolism, taurine, and hypotaurine metabolism, and aminoacyl-tRNA biosynthesis. In contrast, the concentration of glycerophospholipid metabolism-associated metabolites, glycerophosphocholine, was the lowest. However, concentrations of glycerophosphocholine were highest in patients with PGA. In addition, biosynthesis of unsaturated fatty acids associated metabolites, heptadecanoic acid, and oleic acid was also highest, but concentrations of dodecanoic acid were lowest in patients with PGA (Table 2).
Table 2. Targeted Metabolites grouped by airway inflammatory phenotypes in the validation set.
| Variables | EA (n = 35) | NA (n = 30) | PGA (n = 49) | F/χ2 | P value | |
|---|---|---|---|---|---|---|
| Glycerophospholipid metabolism | ||||||
| Acetylcholine (nmol/L) | 0.31 (0.31, 187.96) | 23.89 (0.31, 112.99) | 0.31 (0.31, 64.59) | 2.797 | 0.247 | |
| Phosphorylcholine (nmol/L) | 189.63 (110.08, 3,201.77) | 1,851.12 (264.00, 15,225.36)b | 438.43 (198.54, 26,775.05) | 6.037 | 0.049 | |
| Glycerophosphocholine (nmol/L) | 2,553.49 (682.95, 8,122.17) | 716.74 (341.96, 2,290.81)a | 5,114.09 (1,689.96, 20,211.77)b,c | 20.165 | < 0.001 | |
| Histidine metabolism | ||||||
| L-Glutamate (nmol/L) | 1,290.79 (943.51, 1,532.14) | 2,641.40 (1,279.93, 6,459.78)a | 1,521.64 (868.38, 1,970.67)c | 13.690 | 0.001 | |
| Histamine (nmol/L) | 186.97 (49.93, 663.85) | 33.88 (9.68, 93.17)a | 118.88 (13.54, 263.37)b | 16.174 | < 0.001 | |
| Linoleic acid metabolism | ||||||
| Linoleic acid (μg/mL) | 4.73 (2.34, 8.68) | 5.25 (2.30, 10.61) | 6.99 (4.80, 14.13)a | 7.356 | 0.025 | |
| Nicotinate and nicotinamide metabolism | ||||||
| Nicotinamide (nmol/L) | 96.35 (65.83, 213.90) | 186.95 (68.17, 467.24)b | 178.73 (76.51, 328.91)b | 7.383 | 0.025 | |
| Biosynthesis of unsaturated fatty acids | ||||||
| Dodecanoic acid (μg/mL) | 0.19 (0.15, 0.28) | 0.20 (0.11, 0.31) | 0.11 (0.07, 0.22)a,d | 9.645 | 0.008 | |
| cis-9-Palmitoleic acid (μg/mL) | 3.90 (3.02, 6.34) | 4.41 (3.24, 6.19) | 3.69 (2.77, 5.41) | 1.041 | 0.594 | |
| Heptadecanoic acid (μg/mL) | 1.28 (1.01, 1.87) | 1.18 (0.93, 1.67) | 1.59 (1.22, 2.40)b,c | 9.641 | 0.008 | |
| Stearic acid (μg/mL) | 30.80 (20.19, 40.30) | 33.17 (19.88, 51.15) | 23.59 (16.50, 44.16) | 2.101 | 0.350 | |
| Oleic acid (μg/mL) | 24.43 (13.53, 44.19) | 27.60 (14.44, 50.94) | 40.75 (24.93, 88.11)a,d | 8.847 | 0.012 | |
| Aminoacyl-tRNA biosynthesis | ||||||
| L-Phenylalanine (nmol/L) | 2,232.30 (1,476.07, 3,956.09) | 4,312.25 (2,156.40, 8,653.09)a | 3,444.02 (2,327.59, 4,802.50)a | 12.856 | 0.002 | |
| L-Leucine (nmol/L) | 1,301.48 (1,042.41, 2,866.16) | 6,101.80 (2,371.16, 14,427.98)a | 2,217.51 (1,575.11, 3,415.91)a,c | 22.558 | < 0.001 | |
| Pyruvate metabolism | ||||||
| Pyruvate (nmol/L) | 9,066.60 (5,386.23, 14,086.09) | 8,976.54 (5,732.44, 13,815.05) | 7,607.23 (4,837.88, 12,730.67) | 0.327 | 0.849 | |
| Lactate (nmol/L) | 31,323.49 (11,645.66, 70,410.53) | 45,079.44 (29,664.26, 99,143.60) | 27,791.58 (13,036.10, 61,869.96) | 5.307 | 0.070 | |
| Purine metabolism | ||||||
| Adenine (nmol/L) | 158.85 (89.21, 251.09) | 130.82 (52.38, 262.55) | 198.31 (111.25, 350.34) | 3.881 | 0.144 | |
| Adenosine 5'-monophosphate (nmol/L) | 63.01 (44.71, 719.82) | 1,296.76 (92.87, 7,312.97)a | 152.13 (44.38, 664.24)c | 11.814 | 0.003 | |
| Xanthine (nmol/L) | 249.41 (160.61, 414.90) | 443.45 (247.05, 816.71)a | 266.67 (135.92, 575.51) | 7.461 | 0.024 | |
| Taurine and hypotaurine metabolism | ||||||
| Taurine (nmol/L) | 11,941.08 (8,989.49, 15,724.86) | 27,304.15 (17,812.74, 49,177.67)a | 12,408.37 (7,877.01, 19,915.33)c | 28.064 | < 0.001 | |
| Glyoxylate and dicarboxylate metabolism | ||||||
| Glyceric acid (nmol/L) | 82.31 (82.31, 452.54) | 434.50 (82.31, 641.01)b | 82.31 (82.131, 461.93)d | 6.534 | 0.038 | |
| Tyrosine metabolism | ||||||
| Tyramine (nmol/L) | 9.16 (8.51, 10.76) | 11.13 (9.36, 15.28)a | 9.52 (8.58, 11.63)d | 10.445 | 0.005 | |
| Glutathione metabolism | ||||||
| 5-L-Glutamyl-L-alanine (nmol/L) | 20.72 (2.75, 46.35) | 63.63 (14.49, 184.20)a | 37.63 (14.30, 95.90)b | 10.915 | 0.004 | |
| Peptides and analogues | ||||||
| Arginyl-Serine (nmol/L) | 8.23 (8.23, 8.23) | 8.23 (8.23, 70.81) | 8.23 (8.23, 8.23) | 1.433 | 0.488 | |
| Alanyl-Leucine (nmol/L) | 8.46 (3.83, 13.41) | 143.98 (42.70, 677.71)a | 13.48 (6.59, 25.49)b,c | 54.661 | < 0.001 | |
| Phenylalanyl-Serine (nmol/L) | 4.96 (2.50, 7.65) | 29.35 (13.33, 71.03)a | 6.48 (4.30, 12.89)b,c | 39.718 | < 0.001 | |
| Threoninyl-Phenylalanine (nmol/L) | 26.02 (6.84, 45.36) | 158.42 (90.13, 429.76)a | 36.52 (16.56, 90.06)c | 42.313 | < 0.001 | |
| Phenylalanylphenylalanine (nmol/L) | 35.54 (10.67, 85.26) | 92.04 (50.64, 190.22)a | 63.79 (42.91, 139.07)a | 12.310 | 0.002 | |
| Other organic acids and derivatives | ||||||
| Diethanolamine (nmol/L) | 1.13 (0.31, 37.48) | 14.36 (0.31, 29.96) | 5.03 (0.31, 31.76) | 0.419 | 0.811 | |
| Creatinine (nmol/L) | 364.55 (275.61, 503.29) | 418.74 (318.41, 730.73) | 368.45 (292.09, 623.45) | 3.739 | 0.154 | |
| 3-Hydroxyisovaleric acid (nmol/L) | 195.62 (59.68, 315.78) | 73.59 (42.69, 193.64) | 117.49 (64.34, 254.28) | 4.394 | 0.111 | |
| Barbituric acid (nmol/L) | 9.15 (9.15, 36.34) | 9.15 (9.15, 57.99) | 9.15 (9.15, 9.15) | 3.698 | 0.157 | |
| Allantoin (nmol/L) | 9,948.25 (552.17, 29,158.91) | 10,041.04 (5,825.87, 14,776.30) | 10,029.46 (4,741.30, 23,504.00) | 0.051 | 0.975 | |
| N-Acetylneuraminic acid (nmol/L) | 14,613.85 (7,482.63, 25,123.29) | 15,285.09 (10,212.22, 29,827.90) | 19,793.16 (14,160.44, 36,193.90)b | 7.077 | 0.029 | |
| Cyclohexylamine (nmol/L) | 8.19 (5.85, 13.77) | 8.63 (3.67, 12.36) | 12.17 (8.32, 19.33)b,d | 7.347 | 0.025 | |
| Dihydrothymine (nmol/L) | 76.08 (63.07, 100.40) | 119.85 (87.71, 172.38)a | 99.94 (65.00, 141.26)b,d | 14.549 | 0.001 | |
| Allocystathionine (nmol/L) | 2.75 (1.48, 3.98) | 4.27 (1.50, 6.53) | 2.49 (0.95, 5.86) | 3.135 | 0.209 | |
| Salbutamol (nmol/L) | 312.90 (141.64, 1,060.22) | 331.76 (127.14, 974.82) | 365.77 (106.66, 1,401.31) | 0.069 | 0.966 | |
| Indolelactic acid (nmol/L) | 2.79 (2.79, 7.67) | 6.58 (2.79, 15.06) | 4.11 (2.79, 12.14) | 3.141 | 0.208 | |
Values are presented as median (quartile 1, quartile 3).
EA, eosinophilic asthma; NA, neutrophilic asthma; PGA, paucigranulocytic asthma.
Versus EA, aP < 0.01, bP < 0.05; Versus NA, cP < 0.01, dP < 0.05.
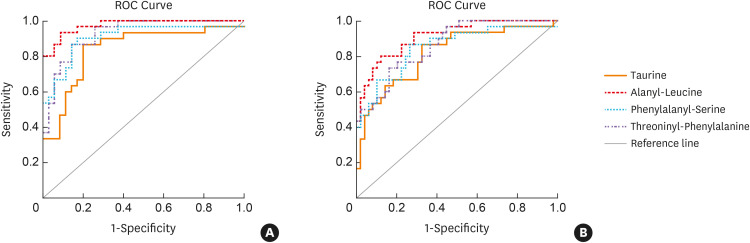
Furthermore, these differentially expressed metabolites were examined by ROC curves to discriminate different inflammatory asthma phenotypes (Supplementary Table S4). As a result, taurine, alanyl-leucine, phenylalanyl-serine, and threoninyl-phenylalanine could be used as candidate metabolite markers to discriminate between NA and EA or PGA with the AUC ranging from 0.816 to 0.975 (all P < 0.05) (Fig. 4). However, no single metabolite could satisfactorily discriminate between EA and PGA as all of the AUC < 0.7.
Fig. 4. The ROC curves of metabolites to discriminate between NA and EA (A)/PGA (B).
ROC, receiver operating characteristic; EA, eosinophilic asthma; NA, neutrophilic asthma; PGA, paucigranulocytic asthma.
Correlations of differential metabolites with clinical and inflammatory profiles in the validation set
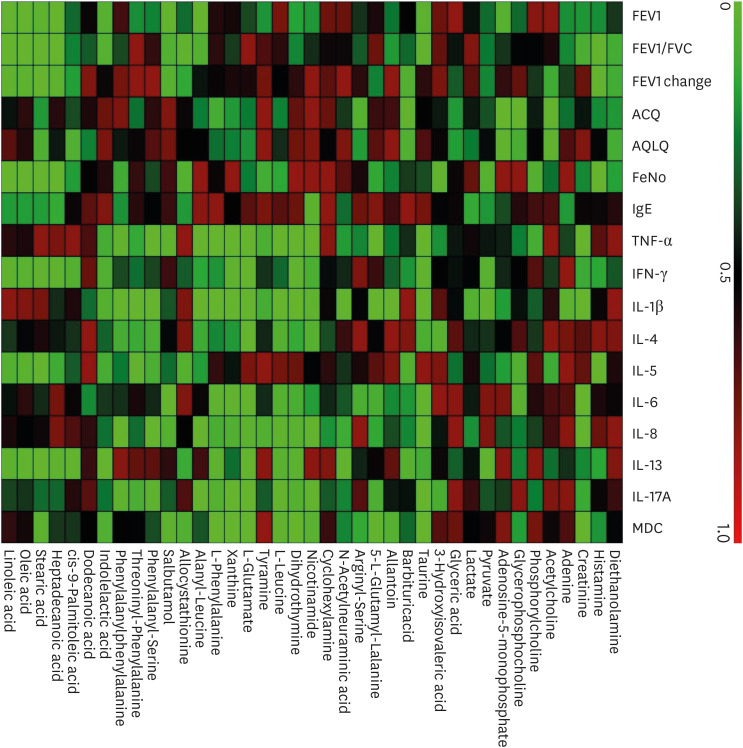
Correlation analysis showed that differentially expressed metabolites correlated to lung function, asthma control, and inflammatory profiles in all subjects with asthma (Fig. 5 and Supplementary Table S5) with the detail in the Supplementary Data S1.
Fig. 5. Heatmap of correlations of differential metabolites with clinical and inflammatory profiles in all subjects with asthma in the validation set.
FEV1, forced expiratory volume in one second; FVC, forced vital capacity; ACQ, asthma control questionnaire; AQLQ, asthma quality of life questionnaire; FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; TNF, tumor necrosis factor; IFN, interferon; IL, interleukin; MDC, macrophage-derived chemokines.
We explored the association between metabolites and severe asthma exacerbation during the 12-month follow-up period using logistic regression and negative binomial regression models in all subjects with asthma (Supplementary Table S6). As a result, adenosine 5′-monophosphate was significantly associated with severe asthma exacerbations (Table 3). Adenosine 5′-monophosphate was positively associated with the proportion of patients experiencing severe asthma exacerbations (adjusted relative risk [adj RR] = 1.000; 95% confidence interval [CI] = 1.000–1.000; P = 0.050) and the frequency of severe asthma exacerbations (RRadj = 1.000; 95% CI = 1.000–1.000; P = 0.008) after adjusting for age, gender, BMI and FEV1%. Meanwhile, allantoin and nicotinamide both positively related with the frequency of severe asthma exacerbations (RRadj = 1.000; 95% CI = 1.000–1.000; P = 0.043, and RRadj = 1.001; 95% CI = 1.000–1.002; P = 0.021, respectively). Furthermore, we performed the subgroup analysis according to EA or non-eosinophilic asthma (NEA). As a result, adenosine 5′-monophosphate was still significantly associated with severe asthma exacerbations in the NEA group, but not in the EA group. In addition, it was found that adenine, allocystahionine, and nicotinamide were significantly associated with severe asthma exacerbations in the NEA group. However, limited by sample size in the EA group, we found that only tyramine was related to the frequency of severe asthma exacerbations. The results of the subgroup analysis are described in Supplementary Data S1.
Table 3. Metabolites for predicting asthma exacerbations (adjusted model).
| Metabolites (nmol/L) | Proportions of patients experiencing severe asthma exacerbation | Severe exacerbation frequency | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Adenine | 0.997 (0.994–1.001) | 0.146 | 0.998 (0.995–1.001) | 0.123 |
| Phosphorylcholine | 1.030 (0.986–1.076) | 0.181 | 1.000 (1.000–1.000) | 0.090 |
| Adenosine 5'-monophosphate | 1.000 (1.000–1.000) | 0.050 | 1.000 (1.000–1.000) | 0.008 |
| Pyruvate | 1.000 (1.000–1.000) | 0.350 | 1.000 (1.000–1.000) | 0.179 |
| 3-Hydroxyisovaleric acid | 0.996 (0.992–1.001) | 0.108 | 0.996 (0.992–1.000) | 0.053 |
| Allantoin | 1.000 (1.000–1.000) | 0.136 | 1.000 (1.000–1.000) | 0.043 |
| N-Acetylneuraminic acid | 1.000 (1.000–1.000) | 0.125 | 1.000 (1.000–1.000) | 0.124 |
| Nicotinamide | 1.001 (0.999–1.003) | 0.248 | 1.001 (1.000–1.002) | 0.021 |
| Tyramine | 1.015 (0.955–1.080) | 0.626 | 1.041 (0.988–1.097) | 0.135 |
| Allocystathionine | 1.014 (0.929–1.106) | 0.756 | 1.068 (0.992–1.150) | 0.083 |
| Threoninyl-Phenylalanine | 0.999 (0.998–1.001) | 0.558 | 1.000 (0.999–1.002) | 0.623 |
Adjusted for age, gender, BMI, and FEV1.
OR, odds ratio; CI, confidence interval; BMI, body mass index; FEV1, forced expiratory volume in one second.
DISCUSSION
To our knowledge, this is the first study establishing the metabolic signatures and underlying molecular pathways of different inflammatory phenotypes of asthma in induced sputum samples by combing untargeted and targeted metabolomics. This study demonstrated the unique metabolic profiles between different inflammatory phenotypes of asthma and healthy subjects, with 77 differential metabolites identified. Pathway topology analysis uncovered 5 pathways that may be involved in the pathogenesis of different asthma phenotypes. Further, 24 targeted quantification metabolites were validated as significantly different between asthma inflammatory phenotypes, which were significantly correlated with clinical and inflammatory profiles. Finally, adenosine 5′-monophosphate, allantoin, and nicotinamide predicted rate ratios of severe asthma exacerbations. These findings indicated novel immunometabolic mechanisms associated with different inflammatory phenotypes of asthma, highlighting potential new therapeutic directions.
A substantial body of research has focused on metabolomic in asthma compared to healthy controls and according to asthma severity.10,26 However, few studies have evaluated the metabolome in relation to inflammatory phenotypes of asthma. A previous study demonstrated that inflammatory asthma phenotypes could be discriminated by an electronic nose breath analyzer.12 Brinkman et al.27 further demonstrated that inflammatory phenotypes of severe asthma could be identified by unbiased clustering of exhaled breath profiles using eNose technology. Although eNose technology seems suitable for the noninvasive identification of asthma inflammatory phenotypes, it is difficult to identify the individual metabolic pathway and metabolites driving the distinction between the subgroups.
The current study showed that histidine metabolism, glycerophospholipid metabolism, nicotinate and nicotinamide metabolism, linoleic acid metabolism, as well as phenylalanine, tyrosine and tryptophan biosyntheses were involved in the pathogenesis of different asthma phenotypes. In fact, previous studies supported our results as histidine metabolism was identified as an implicated pathway between asthma and healthy subjects,28 and glycerophospholipid metabolism also participated in the pathogenesis of asthma, especially EA.29,30 In addition, linoleic acid metabolism was the most significant pathway between NA and EA or PGA (all impact value = 1, P < 0.05), which indicated the important role of linoleic acid metabolism in NA. Panda et al.31 proved that linoleic acid metabolite partially leads to steroid-resistant asthma features through nuclear factor (NF)-κB. Therefore, it is speculated that the development of targeted treatment of linoleic acid metabolism has important implications for the individualized treatment of NA.
Notably, it has been found that differential metabolites correlated to clinical and inflammatory profiles. Adenosine-5′-monophosphate was related to poor asthma control and severe asthma exacerbations. The discovery that adenosine-5′-monophosphate levels are increased in the bronchoalveolar lavage fluid of patients with asthma and increase further after allergen challenge raises the possibility that the adenosine generated in asthmatic airways itself contributes to the pathogenesis of asthma.32,33 It is possible that the recruitment and activation of inflammatory cells and subsequent smooth muscle contraction occurring during asthma exacerbation leads to an increase in oxygen and energy demand, thus increasing the level of adenosine in the airway in asthma.34 We also demonstrated for the first time that allantoin and nicotinamide were involved in severe asthma exacerbation. The roles of these metabolites in asthma pathogenesis and clinical outcomes remain unknown, and further studies are required on their involvement in asthma.
As for the inflammation markers, histamine was significantly related to fractional exhaled nitric oxide (FeNO) and IL-5. Histamine has been shown to play a key role in the pathogenesis of a variety of allergic diseases, including allergic asthma.35 Except for the partial expression of histamine by immune cells such as basophil,36 mast cells are the main source of histamine expression and express a large number of different receptors on their cell surface, such as FcεR1, FcγRI, complement receptors (C3AR and C5AR), and ligand receptors such as neurotrophic factor, substance P, vasoactive intestinal peptide, and adenosine phosphate.37,38,39,40 These ligands and allergens can activate mast cells to release pro-inflammatory mediators including histamine. Furthermore, histamine has been shown to promote TH cell differentiation and release type 2 inflammatory factors such as IL-5. Consistently, antihistmine has been demonstrated to significantly reduce the risk of emergency visits and hospitalization, especially in asthmatic patients with other allergic diseases, such as allergic rhinitis.41,42 Therefore, antihistamine therapy may be considered an additive treatment for EA, because it is often associated with allergic conditions such as allergic rhinitis, which was also found in the current study.
Various amino acids and dipeptides, such as taurine and alanyl-leucine, were significantly correlated with TNF-α, IL-1β, IL-8, and IL-17, indicating these differential metabolites may play a vital role in the development of NA. For example, taurine, a β-amino acid that is not integrated into proteins, is highly expressed in the intracellular chambers of most tissues. Taurine has been reported to be increased in bronchoalveolar lavage fluid in asthmatic patients.43,44 It has been shown that plasma taurine is formed with arachidonic acid in asthmatic patients.45 Animal studies have also demonstrated that branching-chain amino acids valine, leucine, and isoleucine mediated asthma-related airway inflammation through lipid oxidation pathways.46 Notably, this study also found several amino acid dipeptides that had not been reported in previous studies, and their differential expression was closely related to NA. Because induced sputum samples are biological specimens of open airways, they do not simply reflect the changes in human metabolism but also may reflect the changes in the local airway microenvironment, including local microbiota and the human host. In fact, recent studies have shown a significant correlation between neutrophilic asthma and airway microbiology.47 Therefore, this study illustrated important differences in these amino acid dipeptides that may reflect the metabolic characteristics of airway microorganisms in NA. The analysis combined with microbiology may provide further scientific evidence.
This study has several limitations to be addressed. Firstly, external validation in a new cohort was lacking. However, the differential metabolites identified in the discovery set were validated by exploring the relationships of differential metabolites with clinical and inflammation profiles of asthma in a second independent cohort population, which strengthens the results. Secondly, due to the unavailability of reference standards, some of the identified metabolites in the discovery set are undetected, which somewhat limits exploring the potential metabolic signature. Thirdly, this was a real-world study based on the ASAN, in which patients with ACO were not excluded. However, most patients with ACO were in non-eosinophilic asthma, which has been proved to be featured by incompletely reversible airflow obstruction.19,48,49 Thus, the population reflects different inflammatory phenotypes in asthma.
In conclusion, different inflammatory asthma phenotypes have specific metabolic profiles with 77 differential metabolic signatures and 5 underlying molecular pathways. The metabolic pathways identified involve histidine metabolism, glycerophospholipid metabolism, nicotinate and nicotinamide metabolism, linoleic acid metabolism as well as phenylalanine, tyrosine and tryptophan biosynthesis. Differential metabolites identified correlate with clinical and inflammatory profiles of asthma, which may serve as potential therapeutic targets in different inflammatory asthma phenotypes.
ACKNOWLEDGMENTS
The authors are grateful to Ms. Michelle Gleeson (Hunter Medical Research Institute, University of Newcastle, Australia), Ms. Dan Wang (West China Hospital, Sichuan University, China), and Ms. Zhi Lin (West China Hospital, Sichuan University, China) for their blood and sputum processing, and to all patients who volunteered for this study.
This study was supported by the National Natural Science Foundation of China (81900026, 81920108002, and 81870027), China Postdoctoral Science Foundation (2019M653437), Post-Doctor Research Project, West China Hospital, Sichuan University (2019HXBH066; 2021HXBH013), the National Key Development Plan for Precision Medicine Research (2017YFC091004), and 1.3.5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (2018HXFH016).
Footnotes
Disclosure: There are no financial or other issues that might lead to a conflict of interest.
SUPPLEMENTARY MATERIALS
Online data supplement
Clinical characteristics of asthma
Clinical characteristics grouped by airway inflammatory phenotypes in the discovery set
Models information
The AUC for metabolites to discriminate asthma and different inflammatory phenotypes
Correlations of differential metabolites with clinical and inflammatory profiles in the validation set
Metabolites predict asthma exacerbations (unadjusted model)
Metabolites predict asthma exacerbations in EA (unadjusted model)
Metabolites predict asthma exacerbations in EA (adjusted model)
Metabolites predict asthma exacerbations in NEA (unadjusted model)
Metabolites predict asthma exacerbations in NEA (adjusted model)
References
- 1.Global Initiative for Asthma. Global strategy for asthma management and prevention [Internet] place unknown: Global Initiative for Asthma; 2021. [cited 2021 Jul]. Available from: www.ginasthma.org. [Google Scholar]
- 2.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 3.British Thoracic Society; Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax. 2014;69(Suppl 1):1–192. [PubMed] [Google Scholar]
- 4.Lougheed MD, Lemiere C, Ducharme FM, Licskai C, Dell SD, Rowe BH, et al. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can Respir J. 2012;19:127–164. doi: 10.1155/2012/635624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corren J, Lemanske RF, Jr, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 6.Ortega HG, Yancey SW, Mayer B, Gunsoy NB, Keene ON, Bleecker ER, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4:549–556. doi: 10.1016/S2213-2600(16)30031-5. [DOI] [PubMed] [Google Scholar]
- 7.Levy BD, Noel PJ, Freemer MM, Cloutier MM, Georas SN, Jarjour NN, et al. Future research directions in asthma. An NHLBI Working Group Report. Am J Respir Crit Care Med. 2015;192:1366–1372. doi: 10.1164/rccm.201505-0963WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Idle JR, Gonzalez FJ. Metabolomics. Cell Metab. 2007;6:348–351. doi: 10.1016/j.cmet.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn WB, Broadhurst DI, Atherton HJ, Goodacre R, Griffin JL. Systems level studies of mammalian metabolomes: the roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem Soc Rev. 2011;40:387–426. doi: 10.1039/b906712b. [DOI] [PubMed] [Google Scholar]
- 10.Kelly RS, Dahlin A, McGeachie MJ, Qiu W, Sordillo J, Wan ES, et al. Asthma metabolomics and the potential for integrative omics in research and the clinic. Chest. 2017;151:262–277. doi: 10.1016/j.chest.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Zheng J, Zhang HP, Zhang X, Wang L, Wood L, et al. Obesity-associated metabolic signatures correlate to clinical and inflammatory profiles of asthma: a pilot study. Allergy Asthma Immunol Res. 2018;10:628–647. doi: 10.4168/aair.2018.10.6.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plaza V, Crespo A, Giner J, Merino JL, Ramos-Barbón D, Mateus EF, et al. Inflammatory asthma phenotype discrimination using an electronic nose breath analyzer. J Investig Allergol Clin Immunol. 2015;25:431–437. [PubMed] [Google Scholar]
- 13.Wang G, Wang F, Gibson PG, Guo M, Zhang WJ, Gao P, et al. Severe and uncontrolled asthma in China: a cross-sectional survey from the Australasian Severe Asthma Network. J Thorac Dis. 2017;9:1333–1344. doi: 10.21037/jtd.2017.04.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 15.Jia CE, Zhang HP, Lv Y, Liang R, Jiang YQ, Powell H, et al. The asthma control test and asthma control questionnaire for assessing asthma control: systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131:695–703. doi: 10.1016/j.jaci.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Xu KF, Luo XC, Chen Y, Zhang YJ, Li Y, Hu B, et al. The use of Juniper’s asthma quality of life questionnaire in Chinese asthmatics. Zhonghua Nei Ke Za Zhi. 2003;42:760–763. [PubMed] [Google Scholar]
- 17.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Baines KJ, Fu JJ, Wood LG, Simpson JL, McDonald VM, et al. Sputum mast cell subtypes relate to eosinophilia and corticosteroid response in asthma. Eur Respir J. 2016;47:1123–1133. doi: 10.1183/13993003.01098-2015. [DOI] [PubMed] [Google Scholar]
- 19.Deng K, Zhang X, Liu Y, Zhang L, Wang G, Feng M, et al. Heterogeneity of paucigranulocytic asthma: a prospective cohort study with hierarchical cluster analysis. J Allergy Clin Immunol Pract. 2021;9:2344–2355. doi: 10.1016/j.jaip.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Manise M, Holtappels G, Van Crombruggen K, Schleich F, Bachert C, Louis R. Sputum IgE and cytokines in asthma: relationship with sputum cellular profile. PLoS One. 2013;8:e58388. doi: 10.1371/journal.pone.0058388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedersen F, Holz O, Lauer G, Quintini G, Kiwull-Schöne H, Kirsten AM, et al. Multi-analyte profiling of inflammatory mediators in COPD sputum--the effects of processing. Cytokine. 2015;71:401–404. doi: 10.1016/j.cyto.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Dunn WB, Broadhurst D, Begley P, Zelena E, Francis-McIntyre S, Anderson N, et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc. 2011;6:1060–1083. doi: 10.1038/nprot.2011.335. [DOI] [PubMed] [Google Scholar]
- 23.Contrepois K, Wu S, Moneghetti KJ, Hornburg D, Ahadi S, Tsai MS, et al. Molecular choreography of acute exercise. Cell. 2020;181:1112–1130.e16. doi: 10.1016/j.cell.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Zhang T, Shen X, Liu J, Zhao D, Sun Y, et al. Serum metabolomics for early diagnosis of esophageal squamous cell carcinoma by UHPLC-QTOF/MS. Metabolomics. 2016;12:116. [Google Scholar]
- 25.Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46:W486–94. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turi KN, Romick-Rosendale L, Ryckman KK, Hartert TV. A review of metabolomics approaches and their application in identifying causal pathways of childhood asthma. J Allergy Clin Immunol. 2018;141:1191–1201. doi: 10.1016/j.jaci.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinkman P, Wagener AH, Hekking PP, Bansal AT, Maitland-van der Zee AH, Wang Y, et al. Identification and prospective stability of electronic nose (eNose)-derived inflammatory phenotypes in patients with severe asthma. J Allergy Clin Immunol. 2019;143:1811–1820.e7. doi: 10.1016/j.jaci.2018.10.058. [DOI] [PubMed] [Google Scholar]
- 28.Motta A, Paris D, D’Amato M, Melck D, Calabrese C, Vitale C, et al. NMR metabolomic analysis of exhaled breath condensate of asthmatic patients at two different temperatures. J Proteome Res. 2014;13:6107–6120. doi: 10.1021/pr5010407. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Tang K, Lu Y, Tian Z, Huang Z, Wang M, et al. Revealing the role of glycerophospholipid metabolism in asthma through plasma lipidomics. Clin Chim Acta. 2021;513:34–42. doi: 10.1016/j.cca.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 30.Pang Z, Wang G, Wang C, Zhang W, Liu J, Wang F. Serum metabolomics analysis of asthma in different inflammatory phenotypes: a cross-sectional study in Northeast China. BioMed Res Int. 2018;2018:2860521. doi: 10.1155/2018/2860521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panda L, Gheware A, Rehman R, Yadav MK, Jayaraj BS, Madhunapantula SV, et al. Linoleic acid metabolite leads to steroid resistant asthma features partially through NF-κB. Sci Rep. 2017;7:9565. doi: 10.1038/s41598-017-09869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis. 1993;148:91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- 33.Mann JS, Holgate ST, Renwick AG, Cushley MJ. Airway effects of purine nucleosides and nucleotides and release with bronchial provocation in asthma. J Appl Physiol (1985) 1986;61:1667–1676. doi: 10.1152/jappl.1986.61.5.1667. [DOI] [PubMed] [Google Scholar]
- 34.van den Berge M, Polosa R, Kerstjens HA, Postma DS. The role of endogenous and exogenous AMP in asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2004;114:737–746. doi: 10.1016/j.jaci.2004.05.071. [DOI] [PubMed] [Google Scholar]
- 35.Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, et al. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Front Immunol. 2018;9:1873. doi: 10.3389/fimmu.2018.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saluja R, Ketelaar ME, Hawro T, Church MK, Maurer M, Nawijn MC. The role of the IL-33/IL-1RL1 axis in mast cell and basophil activation in allergic disorders. Mol Immunol. 2015;63:80–85. doi: 10.1016/j.molimm.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Metz M, Siebenhaar F, Maurer M. Mast cell functions in the innate skin immune system. Immunobiology. 2008;213:251–260. doi: 10.1016/j.imbio.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Saito H. Mast cell research. Chem Immunol Allergy. 2014;100:165–171. doi: 10.1159/000358733. [DOI] [PubMed] [Google Scholar]
- 39.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 40.Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 2006;349:1322–1328. doi: 10.1016/j.bbrc.2006.08.177. [DOI] [PubMed] [Google Scholar]
- 41.Adams RJ, Fuhlbrigge AL, Finkelstein JA, Weiss ST. Intranasal steroids and the risk of emergency department visits for asthma. J Allergy Clin Immunol. 2002;109:636–642. doi: 10.1067/mai.2002.123237. [DOI] [PubMed] [Google Scholar]
- 42.Corren J, Manning BE, Thompson SF, Hennessy S, Strom BL. Rhinitis therapy and the prevention of hospital care for asthma: a case-control study. J Allergy Clin Immunol. 2004;113:415–419. doi: 10.1016/j.jaci.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 43.Santangelo F, Cortijo J, Morcillo E. Taurine and the lung: which role in asthma? Adv Exp Med Biol. 2003;526:403–410. [PubMed] [Google Scholar]
- 44.Hofford JM, Milakofsky L, Pell S, Fish JE, Peters SP, Pollice M, et al. Levels of amino acids and related compounds in bronchoalveolar lavage fluids of asthmatic patients. Am J Respir Crit Care Med. 1997;155:432–435. doi: 10.1164/ajrccm.155.2.9032174. [DOI] [PubMed] [Google Scholar]
- 45.Comhair SA, McDunn J, Bennett C, Fettig J, Erzurum SC, Kalhan SC. Metabolomic endotype of asthma. J Immunol. 2015;195:643–650. doi: 10.4049/jimmunol.1500736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bazzano M, Laghi L, Zhu C, Magi GE, Serri E, Spaterna A, et al. Metabolomics of tracheal wash samples and exhaled breath condensates in healthy horses and horses affected by equine asthma. J Breath Res. 2018;12:046015. doi: 10.1088/1752-7163/aade13. [DOI] [PubMed] [Google Scholar]
- 47.Taylor SL, Leong LE, Choo JM, Wesselingh S, Yang IA, Upham JW, et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J Allergy Clin Immunol. 2018;141:94–103.e15. doi: 10.1016/j.jaci.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 48.Gibson PG, Foster PS. Neutrophilic asthma: welcome back! Eur Respir J. 2019;54:1901846. doi: 10.1183/13993003.01846-2019. [DOI] [PubMed] [Google Scholar]
- 49.Shaw DE, Berry MA, Hargadon B, McKenna S, Shelley MJ, Green RH, et al. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest. 2007;132:1871–1875. doi: 10.1378/chest.07-1047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online data supplement
Clinical characteristics of asthma
Clinical characteristics grouped by airway inflammatory phenotypes in the discovery set
Models information
The AUC for metabolites to discriminate asthma and different inflammatory phenotypes
Correlations of differential metabolites with clinical and inflammatory profiles in the validation set
Metabolites predict asthma exacerbations (unadjusted model)
Metabolites predict asthma exacerbations in EA (unadjusted model)
Metabolites predict asthma exacerbations in EA (adjusted model)
Metabolites predict asthma exacerbations in NEA (unadjusted model)
Metabolites predict asthma exacerbations in NEA (adjusted model)