Abstract
The global burden of chronic liver disease (CLD) is substantial. Due to the limited indication of and accessibility to antiviral therapy in viral hepatitis and lack of effective pharmacological treatment in nonalcoholic fatty liver disease, the beneficial effects of antidiabetics and non–antidiabetics in clinical practice have been continuously investigated in patients with CLD. In this narrative review, we focused on non-antidiabetic drugs, including ursodeoxycholic acid, silymarin, dimethyl4,4’-dimethoxy-5,6,5’,6’-dimethylenedixoybiphenyl-2,2’-dicarboxylate, L-ornithine L-aspartate, branched chain amino acids, statin, probiotics, vitamin E, and aspirin, and summarized their beneficial effects in CLD. Based on the antioxidant, anti-inflammatory properties, and regulatory functions in glucose or lipid metabolism, several non–antidiabetic drugs have shown beneficial effects in improving liver histology, aminotransferase level, and metabolic parameters and reducing risks of hepatocellular carcinoma and mortality, without significant safety concerns, in patients with CLD. Although the effect as the centerpiece management in patients with CLD is not robust, the use of these non-antidiabetic drugs might be potentially beneficial as an adjuvant or combined treatment strategy.
Keywords: Nonalcoholic fatty liver disease, Viral hepatitis, Cirrhosis, Non-antidiabetic drugs, Treatment
INTRODUCTION
Over the last decade, chronic liver disease (CLD) has become a major cause of morbidity and mortality worldwide, accounting for 2 million deaths globally [1]. The most common etiologies of CLD are nonalcoholic fatty liver disease (NAFLD), followed by hepatitis B virus (HBV), hepatitis C virus (HCV), and alcoholic liver disease [2]. Since cirrhosis and hepatocellular carcinoma (HCC) as major complications of CLD contribute to liver-related morbidity and mortality, effective management of patients with CLD is crucial to reduce the forthcoming disease burden and health expenditures [3].
Although the prevalence of NAFLD is increasing globally and emerging as a major cause of advanced liver diseases, effective and evidence-based pharmacotherapy is still lacking [4]. In viral hepatitis, potent antiviral therapy using nucleot(s)ide analogues and direct-acting antivirals (DAAs) is the mainstay treatment; however, only selected patients are candidates for antiviral therapy, and the supply of antiviral drugs is often limited in under-developed countries [5,6]. In addition, research has shown the combined fatty load in patients with viral hepatitis to have an unfavorable influence on long-term outcomes [7]. Therefore, varying drugs, including antidiabetics, antioxidants, lipid-lowering drugs, probiotics, and anti-platelets, which might have potential beneficial effects, have been continuously investigated in patients with CLD [8-15]. In in vitro studies, diverse therapeutic mechanisms of these drugs, including hepatoprotective, antioxidative, anti-inflammatory, and anti-lipogenic properties, have been suggested [16-27].
Indeed, several clinical studies have determined that these drugs induce histological and biochemical improvements and thus improve long-term outcomes in patients with CLD [8-15]. In addition, several drugs have been found to affect metabolic parameters, including the anthropometric index, insulin resistance, and lipid profiles [28-30]. In contrast, other studies have suggested that these drugs have no beneficial therapeutic effect in patients with CLD [8,12,31-34]. In this review, we summarized the therapeutic mechanisms and beneficial effects of non–antidiabetic drugs in patients with CLD.
URSODEOXYCHOLIC ACID (UDCA)
Mechanism of action
UDCA is a hydrophilic stereoisomer of chenodeoxycholic acid whose efficacy has been proven in primary biliary cholangitis (PBC), making it the recommended first-line treatment in affected patients [35,36]. Experimental models have shown that UDCA increases the secretion of bile acids and other anionic molecules, such as glutathione conjugates or bilirubin glucuronides, which abrogates cholestasis resulting from hydrophobic bile acids, cytokines, or sex hormones [16,17]. One of the mechanisms involved in the increased secretion of bile acids is the upregulation of hepatobiliary transporter genes such as bile salt export pump and multidrug-resistance proteins 2 and 3 (Fig. 1) [37].
Figure 1.
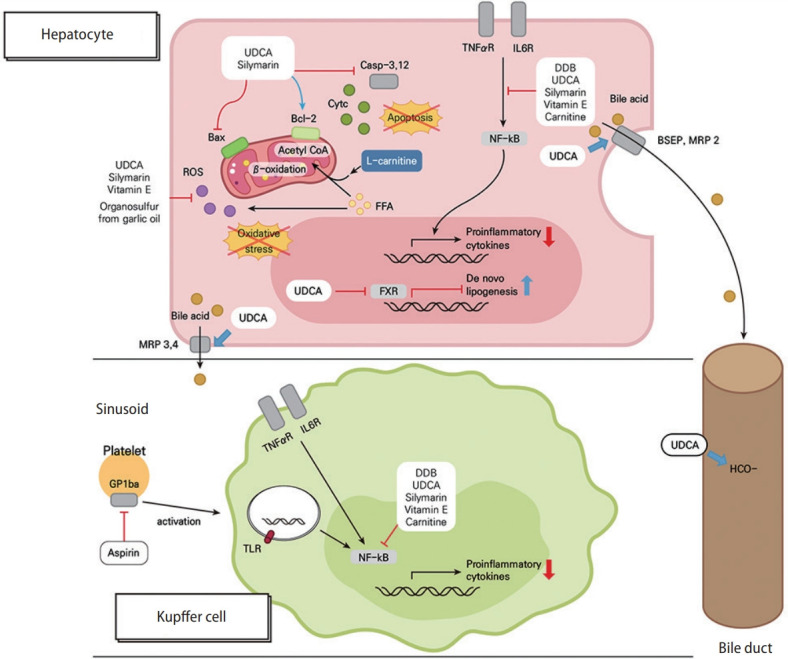
Mechanisms for protective effects by UDCA, silymarin, DDB and its combination with other supplements, vitamin E, and aspirin in chronic liver diseases. UDCA and silymarin negatively regulate the mitochondrial apoptotic pathway by inhibiting Bax translocation, reinforcing Bcl-2 activity, and blocking the activations of caspase-3 and -12, which prevents the apoptosis of hepatocytes in chronic liver diseases. Moreover, UDCA, silymarin, vitamin E, and organosulfur (from garlic oil) relieve ROS-mediated oxidative stress in hepatocytes. Cytosolic FFA contributes to the intracellular ROS pool, and carnitine shuttles FFA-derived acyl-coenzyme as into the mitochondria, making them to undergo β-oxidation. TNF-α/IL-6 receptor signaling and TLR signaling upregulates the expression of pro-inflammatory cytokines via NF-κB activation, while UDCA, silymarin, DDB, vitamin E, and carnitine block this pathway in both hepatocytes and Kupffer cells. UDCA increases the secretion of bile acids via the upregulation of the hepatobiliary transporter genes, such BSEP, MRP2, and MRP3, and also enhances biliary bicarbonate excretion. Interestingly, UDCA induces neutral lipid accumulation in hepatocytes by exerting FXR-antagonistic effects. Aspirin attenuates intrahepatic inflammation by blocking platelet-derived, GPIbα-mediated Kupffer cell activation. UDCA, ursodeoxycholic acid; MRP, multidrug resistance protein; ROS, reactive oxygen species; Bcl-2, B-cell lymphoma 2; FFA, free fatty acid; FXR, farnesoid X receptor; TNF, tumor necrosis factor; IL-6; interleukin-6; DDB, dimethyl-4,4’-dimethoxy-5,6,5’,6’-dimethylenedixoybiphenyl-2,2’-dicarboxylate; NF-κB, nuclear factor kappa-B; BSEP, bile salt export pump; GP1bα, glycoprotein 1bα; TLR, toll-like receptor.
UDCA alleviates intracellular oxidative stress via various mechanisms. Nuclear factor erythroid 2-related factor 2 (NRF2) is a critical stress sensor and a key transcription factor for detoxification, and UDCA enhances NRF2-mediated hepatocellular antioxidative processes in the rat liver [38]. UDCA was also shown to normalize excessive myeloperoxidase activity and reactive oxygen species (ROS) production in stressed rat livers by enhancing the intracellular levels of a reduced form of glutathione [39]. In liver-derived cell lines, the intracellular ROS levels were shown to be increased by palmitate treatment but reduced by co-treatment of palmitate and UDCA [40].
UDCA also protects hepatocytes from undergoing apoptosis [41]. There are multiple signaling pathways and mechanisms associated with the observed anti-apoptotic role of UDCA, such as the lowering of endoplasmic reticulum stress, enhancement of mitochondrial function and integrity, and accentuation of survival signaling among the nuclear factor kappa-B (NF-κB), phosphatidylinositol 3-kinase (PI3K), and mitogen-activated protein kinase pathways [16]. Specifically, UDCA negatively regulates the mitochondrial apoptotic pathway by inhibiting Bax translocation and reinforcing B-cell lymphoma 2 (Bcl-2) activity (Fig. 1) [42]. In stressed primary rat hepatocytes, UDCA was shown to regulate the E2F-1/p53/Bax pathway to block apoptosis [42,43]. UDCA was also proven to target the miR-34a/SIRT1/p53 pro-apoptotic pathway in free fatty acid (FFA)-treated primary rat hepatocytes and the rat liver, reducing hepatocyte apoptosis [44]. Finally, these anti-apoptotic roles of UDCA block caspase-3 activation [42].
Regarding steatosis in NAFLD livers, conflicting results have been offered. One recent report showed that UDCA induces neutral lipid accumulation in the liver in NAFLD patients by exerting farnesoid X receptor (FXR)-antagonistic effects [45]. On the contrary, another previous report showed that hepatic steatosis was decreased by UDCA in NAFLD rats, which was attributable to autophagy induction by activation of the adenosine monophosphate-activated protein kinase (AMPK) signaling pathway [46].
Importantly, UDCA also has immune-modulatory and anti-inflammatory effects in the liver. In patients with PBC, UDCA may alleviate auto-antigen–mediated liver injury by substantially reducing major histocompatibility complex class I expression in the liver [16,47]. In UDCA-fed, aged mice, inflammatory cytokines such as tumor necrosis factor (TNF)-α, C-C motif ligand 2, and interleukin-6 (IL-6) were significantly downregulated in liver and/or white adipose tissues relative to in the tissues of control mice [48]. These observed anti-inflammatory effects may occur as a consequence of the glucocorticoid receptor agonist activity of UDCA and resultant suppression of NF-κB-dependent inflammatory gene transcription in both parenchymal cells and non-parenchymal cells [49,50]. Moreover, UDCA impairs chemotaxis of liver-infiltrating T-cells by downregulation of intrahepatic interferon (IFN)-γ and C-X3-C motif chemokine ligand 1 expression [51]. Interestingly, systemic administration of UDCA attenuates experimental auto-immune arthritis by suppressing T helper 17 cell differentiation through the upregulation of small heterodimer partner interacting leucine zipper protein and by inducing the activation of AMPK and p38 in mouse CD4+ T-cells [52].
Clinically beneficial effects
NAFLD
In the past few decades, many clinical trials have been conducted to reveal whether UDCA has hepatoprotective effects in NAFLD as well as PBC. To date, however, such trials offer conflicting results because of different inclusion and exclusion criteria, treatment doses and durations, and combinations with various drugs in each study (Table 1).
Table 1.
Summary of clinical studies in UDCA
| Study | Etiology | Inclusion criteria | Intervention period | Arms (n) | Age (years) | Outcomes | Study design |
|---|---|---|---|---|---|---|---|
| Lindor et al. [8] (2004) | NAFLD | Biopsy-proven NASH with ALT >1.5×ULN | 2 years | UDCA 13–15 mg/kg/day (80) | 45.4±12.0 | · Changes in AST (mean, -21.7 U/L vs. -20.7 U/L; P=0.37) and ALT (mean, -32.7 U/L vs. -31.6 U/L; P=0.60) | RCT |
| Placebo (86) | 48.5±11.6 | · Changes in steatosis (mean, -0.6 vs. -0.3; P=0.41), inflammation (mean, 0.0 vs. -0.1; P=0.43), and fibrosis (mean, 0.0 vs. 0.0; P=0.50) stages | |||||
| Leuschner et al. [58] (2010) | NAFLD | Biopsy-proven NASH with ALT >1.5×ULN | 18 months | UDCA 23–28 mg/kg/day (94) | 41.45 (18–71) | · Difference in modified Brunt score (mean, -0.98 vs. -0.97; P=0.881) | RCT |
| Placebo (91) | 45.02 (18–73) | · Difference in NAS (mean, -1.22 vs. -1.03; P=0.355) | |||||
| Ratziu et al. [59] (2011) | NAFLD | Biopsy-proven NASH with ALT >50 IU/L | 12 months | UDCA 28–35 mg/kg/day (62) | 49.8±10.2 | · Changes in ALT (-28.3% vs. -1.6%; P<0.001) | RCT |
| Placebo (61) | 49.6±12.6 | · Proportion of ALT normalization (24.5% vs. 4.8%; P<0.003) | |||||
| · Changes in FibroTest values (median, -10.5% vs. +9.6%; P<0.006) | |||||||
| Traussnigg et al. [63] (2019) | NAFLD | ALT >0.8×ULN | 12 weeks | Nor-UDCA 1,500 mg/day (67) | 48.9±12.8 | · Changes in ALT (mean, -17.2 U/L vs. -7.0 U/L vs. +5.3 U/L;P<0.0001) | RCT |
| Nor-UDCA 500 mg/day (67) | 44.9±11.6 | · Proportion of ALT < 0.8×ULN (17.5% vs. 14.8% vs. 5.2%) | |||||
| Placebo (64) | 48.8±11.4 | · Reduction of hepatic fat fraction by MRS (-23.5% vs. +0.9% vs. -1.0%) | |||||
| Fabbri et al. [64] (2000) | CHC | Non-responders to IFN-α with ALT >1.5×ULN | 14 months | IFN-α + UDCA 600 mg/day followed by UDCA 600 mg/ day (53) | 52.6±1.8 | · Proportion of ALT normalization (38% vs. 12%; P<0.01) | RCT |
| IFN-α followed by placebo (50) | 45.8±1.8 | · Proportion of ALT relapse after withdrawal of IFN-α (55% vs. 100%; P<0.01) | |||||
| Boucher et al. [65] (2000) | CHC | Responders to IFN-α | 12 months | UDCA 10 mg/kg/day (54) | 41±15 | · Proportion of biochemical SR (30% vs. 46%; P=NS) | RCT |
| Placebo (53) | 43±15 | · Proportion of virological SR (22% vs. 32%; P=NS) | |||||
| · Post-treatment Knodell score (mean, 6.6 vs. 5.6; P=NS) | |||||||
| Omata et al. [66] (2007) | CHC | ALT >61 U/L | 24 weeks | UDCA 150 mg/day (195) | 58±12.2 | · Changes in ALT (-15.3% vs. -29.2% vs. -36.2%; P<0.001) | RCT |
| UDCA 600 mg/day (198) | 57.7±12.0 | · Changes in AST (-13.6% vs. -25.0% vs. -29.8%; P<0.001) | |||||
| UDCA 900 mg/day (193) | 59.8±10.1 | · Changes in GGT (-22.4% vs. -41.0% vs. -50.0%; P<0.001) |
Variables are expressed as median (interquartile range) or mean±standard deviation.
UDCA, ursodeoxycholic acid; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; ALT, alanine aminotransferase; ULN, upper limit of normal; AST, aspartate aminotransferase; RCT, randomized controlled trial; NAS, NAFLD activity score; nor-UDCA, norursodeoxycholic acid; MRS, magnetic resonance spectroscopy; CHC, chronic hepatitis C; IFN, interferon; SR, sustained response; NS, not significant.
A non-randomized, 1-year prospective study in 40 patients showed that 13–15 mg/kg/day of UDCA produced significant improvements in liver enzymes and hepatic steatosis compared to clofibrate [53]. In a large randomized controlled trial (RCT) with 166 patients, Lindor et al. [8] showed that treatment with 13–15 mg/kg/day of UDCA over 2 years reduced alanine aminotransferase (ALT) levels to the same extent as placebo (mean change, -32.7 vs. -31.6 U/L; P=0.60). However, there was no histologic benefit of UDCA treatment, although the proportion of subjects with improved steatosis was higher than that achieved with placebo, without statistical significance (46% vs. 37%; P=0.41). High rates of ALT and steatosis improvement in the placebo group and a high dropout rate, particularly in the UDCA group (30%), might be issues warranting careful interpretation. In a subsequent randomized trial, Dufour et al. [54] did not show that UDCA had any significant benefit over placebo in promoting biochemical and histologic improvements either, although patients in the UDCA group experienced continuous decreases in aminotransferase levels over 2 years. Instead, UDCA and vitamin E combination therapy improved aminotransferase levels, liver histology, and metabolic profile in patients with nonalcoholic steatohepatitis (NASH) [54,55].
Based on the benefits of high-dose UDCA (HD-UDCA) in other CLDs [56,57], two RCTs with HD-UDCA therapy were conducted. Leuschner et al. assigned 185 patients with biopsyproven NASH to either HD-UDCA (23–28 mg/kg/day) or placebo treatment for 18 months [58]. HD-UDCA failed to improve overall histology over placebo, the primary endpoint, although lobular inflammation (P for NAFLD activity score [NAS]=0.005) and γ-glutamyl transferase (GGT) improved significantly (mean change, -52.53 vs. -16.84 U/L; P<0.0001). On the other hand, Ratziu et al. [59] conducted another randomized trial in which a total of 126 patients with biopsyproven NASH were randomized to receive HD-UDCA (28–35 mg/kg/day) or placebo for 1 year. The reductions in ALT level (-28.3% vs. -1.6% from baseline; P<0.001) and FibroTest measure (median change, -10.5% vs. +9.6%; P<0.006), which was used as a surrogate marker for fibrosis, was significantly greater in the HD-UDCA group than the placebo group. In addition, patients treated with HD-UDCA experienced significant reductions in serum glucose level and improved insulin resistance compared to patients in the placebo group. Different UDCA doses, treatment durations, and NAS at baseline might explain the discrepant results between the two studies.
Recently, norursodeoxycholic acid (nor-UDCA), a synthetic side chain–shortened homologue of UDCA [60], was evaluated for the treatment of NAFLD based on promising results in pre-clinical studies [61,62]. In a randomized controlled, phase II clinical trial involving 198 patients, 1,500 mg/day of nor-UDCA significantly reduced serum ALT levels within 12 weeks (mean change, -17.2 vs. +5.3 U/L; P<0.0001), and nor-UDCA was found to be safe and well tolerated [63]. In addition, hepatic fat fraction measured by magnetic resonance spectroscopy was remarkably reduced in nor-UDCA-treated patients (-23.5% vs. -1.0%).
Taken together, the results of various clinical trials suggest that conventional doses of UDCA (13–15 mg/kg/day) in monotherapy have little therapeutic effect in NASH, but HD-UDCA (28–35 mg/kg/day) may be beneficial in driving biochemical improvements in NASH patients with low severity. In addition, a conventional dose of UDCA may help to improve NASH when administered in combination with vitamin E. Further studies to evaluate the beneficial effects of nor-UDCA in patients with NAFLD or NASH are warranted.
Viral hepatitis
The role of UDCA in viral hepatitis has not been studied extensively. There are a few studies evaluating the effect of UDCA in patients with chronic hepatitis C (CHC) with or without IFN treatment (Table 1). Fabbri et al. [64] conducted an RCT involving 103 patients who had not responded to IFN therapy. Patients were randomized to receive UDCA (600 mg/day) in addition to IFN or to continue on IFN-α alone. After stopping α-IFN, patients who received UDCA continued to receive UDCA for an additional 6 months. UDCA improved the response rate to α-IFN (ALT normalization, 38% vs. 12%; P<0.01) and reduced the severity of relapse. In another RCT involving 107 biochemical responders to IFN, patients were randomized to receive either UDCA (10 mg/kg/day) or placebo for 12 months [65]. Continuation of UDCA therapy after withdrawal of IFN therapy did not significantly improve the maintenance of response to IFN or liver histology parameters in IFN responders. The most recent large-scale randomized trial was conducted in 596 CHC patients with detectable HCV RNA [66]. Patients were assigned randomly to receive 150, 600, or 900 mg/day of UDCA for 24 weeks, and it was found that 600 mg/day of UDCA was the optimal dose to decrease aspartate aminotransferase (AST) (-25.0% vs. -13.6% from baseline; P<0.001) and ALT (-29.2% vs. -15.3% from baseline; P<0.001) levels compared to 150 mg/day, while the GGT concentration was significantly lower in the 900-mg/day group than the 600-mg/day group (-50.5% vs. -41.0% from baseline; P<0.001), which may indicate an improvement in cholestasis due to biliary injury in CHC. However, the serum HCV RNA did not change in any group.
Accordingly, UDCA may lead to an improvement in serum aminotransferase activities in CHC without effects on viral clearance. Since all studies were conducted in the IFN era, which carries a low treatment response rate, further considerations and studies are needed on the role of UDCA in CHC at this time of using DAAs.
Safety
UDCA is widely used in the treatment of patients with PBC and has shown an excellent safety profile [67]. In viral hepatitis or NAFLD patients as well, no safety issues have been raised in various RCTs, even in studies with long-term administration of HD-UDCA. The most commonly reported adverse event of UDCA therapy is diarrhea [8,58,59,66], although abdominal discomfort, fatigue, rash, and pruritus were also reported [8,58,59]. The rate of clinical adverse events was similar for UDCA and placebo when the conventional dose was administered, whereas diarrhea occurred more frequently with HDUDCA than placebo [8,58,59].
SILYMARIN
Mechanism of action
The bioactive extract of milk thistle, silymarin, has been documented to have several pharmacological features, including antioxidant and anti-inflammatory properties, in preclinical studies (Fig. 1) [68-73].
Silymarin uses scavengers, allowing for the elimination of free radicals; inhibits ROS producing-enzymes, preventing free radical formation; promotes protective molecule synthesis; and activates antioxidant enzymes [18]. In experimental studies, silybin, the most prevalent and biologically active flavonolignan isomer of silymarin, potently scavenges ROS such as hydroxyl and peroxyl anions and hypochlorous acid [72,74-76]. Also, silybin inhibits superoxide anion radicals and nitric oxide in isolated Kupffer cells [68]. Silymarin enhances hepatic glutathione generation by elevating cysteine availability and helps the liver to maintain glutathione by stabilizing membrane permeability through the inhibition of lipid peroxidation [69]. In an NAFLD mouse model, silymarin restored nicotinamide adenine dinucleotide+ (NAD+) homeostasis, sirtuin 1 activity, and the AMPK-α pathway to improve poly-(ADP-ribose)-polymerase function, which are important regulatory pathways linked to oxidative stress [77]. The antioxidant property of silymarin prevents or reduces hepatic inflammation by reducing oxidative stress in various liver diseases [78].
Silymarin also exerts an anti-inflammatory effect. There is increasing evidence that silymarin inhibits inflammatory mediators, such as NF-κB, which is activated in most CLD, and inflammatory metabolites [79]. In isolated rat Kupffer cells, silymarin selectively inhibits leukotriene B4 formation, but weakly inhibited prostaglandin E2 formation, which may account for its anti-inflammatory action [68]. The anti-inflammatory property of silymarin may help to prevent or improve hepatic fibrogenesis given that chronic inflammation has been a common underlying mechanism in progressive liver fibrosis [80].
Silymarin also has anti-fibrotic activity, inhibiting the conversion of hepatic stellate cells (HSCs) into myofibroblasts through the inhibition of fibrogenic pathways, including cytoskeletal formation, pro-fibrogenic collagen, and electron transfer chains. Animal and in vitro models demonstrate that silymarin down-regulates tumor growth factor (TGF)-ß1 mRNA and inhibits NF-κB to improve hepatic fibrosis [79,81,82]. In an in vitro study, silybin inhibited the growth factor-induced production of pro-collagen in activated human HSC dose-dependently, slowing down the progression of early fibrosis [81].
Insulin resistance is a well-known key mechanism in the pathogenesis of NAFLD. In a rat NAFLD model, silybin decreased insulin resistance by reducing visceral obesity, enhancing lipolysis, and inhibiting gluconeogenesis [83]. Silymarin can also restore a pathway of insulin receptor substrate-1/PI3K/Akt, which can reduce NAFLD-induced insulin resistance and steatosis, as well as activate the FXR, which in turn can decrease hepatic inflammation [26,84].
Clinically beneficial effects
NAFLD
The clinical applications of silymarin in NAFLD have been identified in several RCTs to date (Table 2) [9,28,31,85-87]. Among them, two studies investigated the efficacy of silymarin in patients with histologically confirmed NAFLD [9,31]. In an RCT with 49 patients treated with 2,100 mg/day of silymarin and 50 patients treated with placebo, the proportion of patients who showed improvements in fibrosis (≥1 stage) was significantly higher in the silymarin group compared to the placebo group (22.4% vs. 6.0%; P=0.023), while the proportion of patients who had improvements in NAS (≥30%) was statistically comparable between the silymarin and placebo groups (32.7% vs. 26.0%; P=0.467) [31]. Another RCT also showed no statistically significant difference in the improvement of NAS (≥2 points) between the silymarin and placebo groups (15– 19% vs. 12%; P=0.79) [9]; however, a retrospective cohort study demonstrated that patients with higher levels of oxidative stress markers had statistically significant improvements in NAS after silymarin treatment (variation, -70%; P=0.001), while those with lower levels of oxidative stress markers did not (variation, -29%; P=0.057), suggesting the effect of silymarin based on its antioxidant properties [88]. It is expected that further studies with selected subgroups of patients using relevant biomarkers representing the severity of oxidative stress or inflammation may reveal the clinically beneficial effect of silymarin in the histologic improvement of NAFLD more clearly.
Table 2.
Summary of clinical studies in silymarin
| Study | Etiology | Inclusion criteria | Intervention period | Arms (n) | Age (years) | Outcomes | Study design |
|---|---|---|---|---|---|---|---|
| Navarro et al. [9] (2019) | NAFLD | NAS ≥4 without cirrhosis | 48–50 weeks | Silymarin 1,260 mg/day (26) | 47.3 (10.8) | · Improvement ≥2 in NAS (19% vs. 15% vs. 12%; P=0.79) | RCT |
| Silymarin 2,100 mg/day (27) | 48.2 (11.4) | · Normalized ALT level (8% vs. 25% vs. 5%; P=0.08) | |||||
| Placebo (25) | 49.5 (10.9) | · Improved fibrosis stage (12% vs. 26% vs. 28%; P=0.30) | |||||
| Wha Kheong et al. [31] (2017) | NAFLD | NAS ≥4 | 48 weeks | Silymarin 2,100 mg/day (49) | 49.6±12.7 | · Decrease ≥30% in NAS (32.7% vs. 26.0%; P=0.467) | RCT |
| Placebo (50) | 50.1±10.2 | · Reductions in fibrosis ≥1 stage (22.4% vs. 6.0%; P=0.023) | |||||
| · Change in triglyceride level (mean, -0.2 vs. 0.04 mmol/L; P=0.017) | |||||||
| Anushiravani et al. [87] (2019) | NAFLD | Grade ≥2 steatosis in ultrasonography | 3 months | LSM (30) | 47.0±9.1 | · Changes in AST (mean, -0.9 vs. -8.3 U/mL; P<0.001) and ALT (mean, -0.6 vs. -9.3 U/mL; P<0.001) levels | RCT |
| LSM + silymarin 140 mg/day (30) | · Changes in WC (mean, -1.2 vs. -0.3 cm; P<0.001) | ||||||
| Solhi et al. [85] (2014) | NAFLD | AST and ALT >1.2×ULN | 8 weeks | LSM + silymarin 210 mg/day (33) | 43.6±8.3 | · Changes in AST (mean, 62.8 to 30.5 vs. 70.4 to 36.2; P=0.038) and ALT (mean, 91.3 to 38.4 vs. 84.6 to 52.3; P=0.026) levels (U/L) | RCT |
| LSM + placebo (31) | 9.4±10.5 | ||||||
| Hajiaghamohammadi et al. [28] (2012) | NAFLD | 8 weeks | Pioglitazone 15 mg/day (22) | 33.4±6.6 | · Changes in AST (mean, 55.0 to 37.59 vs. 54.86 to 42.5 vs. 56 to 37.77; P=0.003) and ALT (mean, 77.45 to 52.27 vs. 78.36 to 60.95 vs. 78.73 to 53.05; P<0.005) levels (U/L) | RCT | |
| Metformin 500 mg/day (22) | 32.5±6.5 | ||||||
| Silymarin 140 mg/day (22) | 33.5±6.3 | · Significant reductions in mean levels of fasting glucose, triglyceride, total cholesterol, and insulin and HOMA-IR in all groups (P<0.01) | |||||
| Hashemi et al. [86] (2009) | NAFLD | AST and ALT >1.2×ULN or biopsy-proven NASH | 6 months | Silymarin 280 mg/day (50) | 39.28±11.11 | · ALT (52% vs. 18%; P=0.001) and AST (62% vs. 20%; P=0.0001) level normalization | RCT |
| Placebo (50) | 39.0±10.70 | ||||||
| Sorrentino et al. [90] (2015) | NAFLD | WC > 94 cm in men or >80 cm in women, triglyceride >150 mg/dL, and fasting glucose >100 mg/dL | 90 days | LSM + silymarin 250 mg/day + vitamin E 60 IU/day (43) | 56.63±12.79 | · Change in hepatic steatosis index (mean, -1.85 vs. -0.19; P=0.0134) | Prospective cohort study |
| LSM (35) | 55.40±13.63 | · Changes in BMI (mean, -0.71 vs. -0.004 kg/m2; P=0.022) and WC (mean, -4.81 vs. -1.78 cm; P=0.028) | |||||
| Stiuso et al. [88] (2014) | NAFLD | Biopsy-proven NASH | 12 months | Low levels of TBARS: Silymarin 188 mg/day + phosphatidyl choline 388 mg/day + vitamin E acetate 50% 178.56 mg/day (11) | 40.8±10.3 | · Proportion of change in NAS (-29%; NS), portal inflammation (-25%; NS), and fibrosis (-50%; P=0.01) | Retrospective cohort study |
| · Proportion of change in AST (-33%; P=0.05), ALT (-14%; NS), and insulin (-8%; NS) levels and HOMA-IR (-11%; NS) | |||||||
| High levels of TBARS: silymarin 188 mg/day + phosphatidyl choline 388 mg/day + vitamin E acetate 50% 178.56 mg/day (19) | · Proportion of change in NAS (-70%, P=0.001), portal inflammation (-58%; P=0.001), and fibrosis (-60%; P=0.001) | ||||||
| · Proportion of change in AST (-42%; P=0.01), ALT (-31%; P=0.05), and insulin (-40%; P=0.001) levels and HOMA-IR (-42%; P=0.001) | |||||||
| Fried et al. [91] (2012) | HCV | ALT ≥65 U/L who were unsuccessfully treated with IFN | 24 weeks | Silymarin 1,260 mg/day (50) | 54.0 (52.0–57.0) | · Proportion of ALT ≤45 U/L (4.0% vs. 3.8% vs. 1.9%; P=0.80), at least 50% ALT decline and ALT <65 U/L at week 24 (2.0% vs. 3.8% vs. 3.8%; P=0.83) | RCT |
| Silymarin 2,100 mg/day (52) | 54.0 (48.0–58.0) | ||||||
| Placebo (52) | 56.0 (51.5–59.5) | · Changes in ALT (mean, -14.4 vs. -11.3 vs. -4.3 U/L; P=0.75) and HCV RNA (mean, -0.03 vs. 0.04 vs. 0.07 log10 IU/L; P=0.54) levels | |||||
| Yakoot and Salem [92] (2012) | HCV | Genotype 4, IFN-naïve or relapsers/nonresponders to IFN or combined therapy | 6 months | Silymarin 420 mg/day (29) | 48±12 | · ETR (3.4% vs. 13.3%; P=0.12) | RCT |
| Spirulina 1,500 mg/day (30) | 47±12 | · 3 months virological response (0% vs. 3.3%; P=0.22) | |||||
| · Reduction in ALT level (mean, -6.8 vs. -23.7 IU/L; P=0.006) | |||||||
| Tanamly et al. [94] (2004) | HCV | Presence of HCV RNA | 12 months | Silymarin 373.5 mg/day (68) | 44.1 | · All physical and mental health variables (SF-36 questionnaire) were improved in both groups. | RCT |
| Multivitamin (71) | · Persistent HCV RNA (97.1% vs. 95.8%; P=0.684) and ALT >35 IU/L (13.0% vs. 14.3%; NS) | ||||||
| Ferenci et al. [95] (2008) | HCV | Nonresponders to full-dose PegIFN/RBV | 14 days | Silymarin iv 5 (3), 10 (3), 15 (5), 20 (9) mg/kg + 24 weeks PegIFNα-2a 180 µg/week + ribavirin 1–1.2 g/day from day 8 | 52.7±12.8 | · Dose-dependent HCV RNA decrease (log drop after 14 days: mean, 1.63 [5 mg/kg], 4.16 [10 mg/kg], 3.69 [15 mg/kg], and 4.85 [20 mg/kg]; P<0.001) | Prospective cohort study |
| · Undetectable HCV RNA in 7 patients on 15 or 20 mg/kg of silymarin at week 12 |
Variables are expressed as median (interquartile range) or mean±standard deviation.
NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; ALT, alanine aminotransferase; RCT, randomized controlled trial; LSM, lifestyle modification; AST, aspartate aminotransferase; WC, waist circumference; ULN, upper limit of normal; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; NASH, nonalcoholic steatohepatitis; BMI, body mass index; TBARS, thiobarbituric acid-reactive species; NS, not significant; HCV, hepatitis C virus; IFN, interferon; ETR, end-treatment response; PegIFN/RBV, pegylated interferon and ribavirin.
The significant association between silymarin treatment and the improvement in aminotransferase levels in patients with NAFLD has been well reported in several RCTs [28,85-87]. Levels of AST (mean difference, -8.3 vs. -0.9 U/mL; P<0.001) and ALT (mean difference, -9.3 vs. -0.6 U/mL; P<0.001) significantly improved in the silymarin group compared to the placebo group [87]. In an RCT comparing pioglitazone, metformin, and silymarin, changes in AST (mean, -17.41 vs. -12.36 vs. -18.23; P=0.003) and ALT (mean, -25.18 vs. -17.41 vs. -25.68; P<0.005) levels after treatment were significantly different between the treatment groups [28]. Finally, a recent meta-analysis involving 622 patients with NAFLD revealed that silymarin was more efficacious than placebo in reducing ALT (mean difference, -14.86; 95% confidence interval [CI], -19.37 to -10.36; I2=39%; P<0.001) and AST (mean difference, -7.11; 95% CI, -14.16 to -0.05; I2=88%; P<0.05) levels [89].
In addition, significant improvements in metabolic parameters, including levels of triglyceride, fasting glucose, and total cholesterol, as well as values of the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), waist circumference, and body mass index (BMI) following treatment with silymarin have been reported in previous RCTs and a prospective cohort study, suggesting the potential of silymarin for the treatment of metabolic syndrome [28,31,87,90].
Overall, silymarin treatment in NAFLD seems beneficial in improving hepatic necro-inflammation, as reflected by the improvement of AST and ALT levels, and metabolic parameters. Therefore, silymarin may be a potential future therapy for patients with NASH, presumably in combination with other agents, but still requires large RCTs for solid validation.
Viral hepatitis
The beneficial effect of silymarin in improving aminotransferase in CHC patients remains controversial despite several RCTs and prospective studies (Table 2) [91-95]. In an RCT with CHC patients in whom IFN therapy was unsuccessful, the proportion of patients who had ALT normalization was statistically comparable between the silymarin and placebo groups (3.8–4.0% vs. 1.9%; P=0.80) [91]. Another RCT with CHC patients who were treatment-naïve or relapsers/non-responders to IFN or combined therapy showed a significant improvement in ALT level in a spirulina group compared to the silymarin group (mean,-23.7 vs. -6.8 IU/L; P=0.006) [92]. In contrast, a prospective study reported significant reductions in levels of ALT (mean, 108.7–70.3 IU/L; P<0.001) and AST (mean, 99.4–59.7 IU/L; P=0.004) after silymarin treatment [93]. Two prospective studies demonstrated significant reductions in HCV RNA levels after silymarin treatment; however, silymarin had no effect in the suppression of HCV RNA in all RCTs [91-95].
Safety
In clinical trials, silymarin has been used for up to 48 weeks at 2,100 mg/day [9,31]. Overall, silymarin is well tolerated in CLD patients with no or only a very low incidence of serious adverse events [9,28,31,85-88,90-95]. Systematic reviews demonstrated a 4% incidence of adverse events and no treatment-related serious adverse events or deaths [89,96].
DIMETHYL-4,4’-DIMETHOXY-5,6,5’,6’-DIMETHYLENEDIXOYBIPHENYL-2,2’-DICARBOXYLATE (DDB) AND ITS COMBINATION WITH OTHER SUPPLEMENTS
Mechanism of action
DDB is a synthetic compound derived from schisandrin C, an active metabolite from Schizandrae sinensis Fructus. It has been widely used in practice to lower ALT levels in chronic hepatitis for nearly 50 years [97]. The protective roles of DDB were reported in experimental models of liver injury using carbon tetrachloride (CCl4), D-galactosamine, thioacetamide, and prednisolone [19]. It is known to reduce the membranal lipid peroxidation and ALT release from damaged hepatocytes (Fig. 1) [19,98]. DDB has also shown additional inhibitory effects on lipopolysaccharides (LPS)-inducible NF-κB activation and subsequent TNF-α production [99], It is related to the inhibition of either IkBα degradation or signaling of caspases-3, -8, and -9 [99,100].
Carnitine orotate complex or diallyl sulfide from garlic oil has been used in combination with DDB to add clinical benefit with different mechanisms of action. A DDB-carnitine orotate complex prevented FFA-induced lipotoxicity by adding carnitine (Fig. 1). In a physiologic state, carnitine shuttles long-chain fatty acids into mitochondria and facilitates mitochondrial β-oxidation by acting as a coenzyme for palmitoyltransferase 1A [101]. In an in vitro study, carnitine facilitated effective mitochondrial β-oxidation, thereby reducing both intracellular fat deposits and alternative fat peroxidation, which ultimately leads to decreased production of ROS and oxidative stress [102]. Carnitine treatment in diabetic rats fed a methionine choline-deficient diet led to decreased serum ALT levels and improved lobular inflammation in vivo [102]. Meanwhile, diallyl sulfide, one of the organosulfur compounds from garlic oil, is known to inhibit the action of CYP450 2E1 [103,104]. It modulates the production of toxic or reactive intermediate during phase II detoxification (Fig. 1) [105]. Garlic oil enhanced the protective effect of DDB in the improvement of serum ALT level and was linked to decreasing numbers of Kupffer cells and dead hepatocytes in CCl4-treated rats [106]. Meanwhile, in case of alcohol-induced hepatotoxicity, it was only blocked by adding garlic oil to DDB (~40%) and not by DDB only. Furthermore, Park et al. [107] reported that garlic oil combined with DDB was protective of glutathione deficiency-induced liver injury as evidenced by improved ALT and triglyceride levels. Garlic oil combined with DDB showed a synergetic benefit according to a comparison of its histologic activity to that of treatment with only DDB or garlic oil.
Clinically beneficial effects
CLD
Apart from the widespread experimental studies with long histories, well-designed, RCTs are limited, involving only a small number of participants and short duration of treatment (Table 3). In the design of a double-blind, active-controlled trial, participants with CLDs (NASH, 69%; alcoholic hepatitis, 20%; chronic hepatitis, 11%) were treated with either DDB (750 mg/day) or UDCA (300 mg/day) for 24 weeks [10], and DDB led to a significantly higher rate of ALT normalization compared to UDCA (80.0% vs. 34.8%, P<0.001).
Table 3.
Summary of clinical studies in DDB
| Study | Etiology | Inclusion criteria | Intervention period | Arms (n) | Age (years) | Outcomes | Study design |
|---|---|---|---|---|---|---|---|
| Lee et al. [10] (2014) | CLD | (1) Persistent ALT ≥1.5×ULN more than once during the previous 6 months; (2) ALT ≥1.5×ULN at enrollment | 24 weeks | DDB 750 mg/day (67) | 51 (20–72) | ALT normalization (≤40 IU/L) (80.0% vs. 34.8%; P<0.001) | RCT; double blind, active- controlled |
| Kang et al. [108] (2001) | CLD | (1) Biopsy-proven chronic hepatitis or (2) abnormal AST/ALT for >6 months; no evidence of viral hepatitis B or C | 8 weeks | High-dose group: DDB 150 mg + carnitine/orotate complex 900 mg/day (33) | 49.03±9.74 | ALT normalization (88.5% vs. 54.6% vs.44.4%; P=0.0027) | RCT; double blind, phase II |
| Low-dose group: DDB 150 mg + carnitine/orotate complex 600 mg/day (30) | 41.58±11.73 | ||||||
| DDB 150 mg/day (32) | 44.00±11.55 | ||||||
| Park et al. [109] (2001) | CLD | (1) Biopsy- proven chronic hepatitis or (2) ALT elevation (≥1.5×ULN) more than twice during the previous 6 months; no evidence of viral hepatitis B or C | 8 weeks | High-dose group: DDB 150 mg + carnitine/orotate complex 900 mg/day (53) | 44.57±11.49 | ALT normalization (81.13% vs. 67.35% vs. 64.54%; P=0.0407) | RCT; double blind, phase III |
| Low-dose group: DDB 100 mg + carnitine/orotate complex 600 mg/day (48) | 43.24±13.01 | ||||||
| DDB 100 mg/day (52) | 45.63±13.75 | ||||||
| Kim et al. [110] (2014) | CLD | (1) Abnormal ALT or AST in previous 6 months, (2) sonographical findings of chronic hepatitis or fatty liver, or (3) history of being treated for chronic hepatitis for >30 days | 12 weeks | DDB + garlic oil 960 mg/day (100) | 44 (20–79) | ALT normalization (≤40 IU/L) (89% vs. 18.6% vs. 22.9%; P<0.001) | RCT;double blind, placebo- andactive- controlled, phaseIV |
| Silymarin 1,018 mg/day (102) | 49 (20–75) | ||||||
| Placebo (35) | 44 (24–77) | ||||||
| Hong et al. [111] (2014) | NAFLD | Combined with impaired fasting glucose metabolism | 12 weeks | Metformin 750 mg/day and DDB-carnitine orotate complex 900 mg/day (26) | 51.5±9.4 | Decrement of ALT level (mean, 51.5 vs. 16.7; P=0.001) | RCT; double blind, placebo- controlled |
| Metformin 750 mg/day and placebo (26) | 52.0±9.6 | ||||||
| Bae et al. [112] (2015) | NAFLD | Combined with type 2 diabetes | 12 weeks | DDB-carnitine orotate complex 824 mg/day (39) | 50.6±9.3 | ALT normalization (<30 IU/L in men or <19 IU/L in women) (89.7% vs. 17.9%; P<0.001) | RCT; double blind, placebo- controlled |
| Placebo (39) | 52.0±9.4 | ||||||
| Jun et al. [113] (2013) | HBV | (1) ALT ≥80 IU/L, (2) ALT < ULN×10, (3) treatment- naïve, and (4) HBV >105 copies/ mL in case of HBeAg-positive result or HBV >104 copies/ mL in case of HBeAg-negative result | 12 months | Entecavir 0.5 mg/day and DDB- carnitine orotate 2,472 mg/ day complex (67) | 43.0 ± 9.8 | ALT normalization (<40 U/L) (100% vs. 85.7%; P=0.0019) | RCT |
| Entecavir 0.5 mg/day (63) | 44.9±10.0 |
Variables are expressed as median (interquartile range) or mean±standard deviation.
DDB, dimethyl-4,4’-dimethoxy-5,6,5’,6’-dimethylenedixoybiphenyl-2,2’-dicarboxylate; CLD, chronic liver disease; ALT, alanine aminotransferase; ULN, upper limit of normal; RCT, randomized controlled trial; AST, aspartate aminotransferase; NAFLD, nonalcoholic fatty liver disease; HBV, hepatitis virus; HBeAg, hepatitis B e antigen.
Supplementing DDB with carnitine orotate complex was evaluated in two RCTs. In a phase II RCT for CLDs other than viral hepatitis, 8 weeks of supplementing DDB (150 mg) with carnitine orotate complex (900 mg) led to a significantly higher rate of ALT normalization (88.5%) compared to combined carnitine orotate complex (600 mg) and DDB (150 mg) (54.5%) treatment or treatment with DDB (150 mg) only (44.4%) (P=0.003) [108]. In a phase III trial of participants with CLDs other than viral hepatitis [109], adding a high dose of carnitine orotate complex (900 mg) to DDB (150 mg) led to a higher rate of ALT normalization (81.1%) than adding a low dose of carnitine orotate complex (600 mg) to DDB (100 mg) (67.4%) or treating with DDB (100 mg) alone (64.5%) (P=0.041) (Table 3).
The addition of garlic oil to DDB was recently evaluated in a double-blind RCT with 12 weeks of intervention (Table 3) [110]. Kim et al. [110] evaluated the beneficial effect on ALT normalization, improvement of quality of life, and safety of the DDB-garlic oil complex in patients with CLDs other than viral hepatitis. The rate of ALT normalization was 89% in the DDB–garlic oil complex group, which was significantly higher than that of the silymarin group (18.6%) or placebo group (22.9%) (P<0.001). The level of serum malondialdehyde, a lipid peroxidation marker, was decreased in the DDB-garlic oil complex group (-1.4 pmol/mg), but increased in both the silymarincontrol and placebo groups (P<0.001). Although the score for total CLD questionnaire score was significantly improved within the DDB-garlic oil complex group after treatment, the score improvement was statistically similar among the three treatment groups.
In summary, DDB and its combination with other supplements seems to significantly decrease the serum level of ALT in patients with CLDs other than viral hepatitis, when compared to silymarin or placebo.
NAFLD
Given the benefit of carnitine on the reduction in intracellular FFA level and the improvement of insulin resistance, the effect of a DDB-carnitine orotate complex was evaluated in participants with either impaired fasting glucose metabolism or type 2 diabetes (Table 3). In a double-blind RCT, either metformin and placebo or metformin and a DDB-carnitine orotate complex was given for 12 weeks to participants with both impaired fasting glucose metabolism and NAFLD [112]. Even with the small number of patients in each group, the reduction in ALT from baseline was significantly greater in the metformin and DDB-carnitine orotate complex group (mean reduction, 51.5±33.2 IU/L) than in the metformin and placebo group (mean reduction, 16.7±31.3 IU/L) among the patients with impaired fasting glucose metabolism and NAFLD (P=0.001). The change in 8-hydroxy-2’-deoxyguanosine, an oxidative stress marker, was 0.7±3.2 μg/g in the metformin and DDB-carnitine orotate complex group and -1.2±2.9 μg/g in the metformin and placebo group (P=0.034), suggesting a benefit of the DDB-carnitine orotate complex in decreasing oxidative stress. Furthermore, the fold change (2−ΔΔCt) in mitochondrial copy number was significantly greater in the metformin and DDB-carnitine orotate complex group than that of the metformin and placebo group (1.16±0.38 vs. 0.95±0.45, P<0.05), suggesting the occurrence of less mitochondrial damage in the DDB-carnitine orotate complex group. Nevertheless, the additional effects of the DDB-carnitine orotate complex on the changes of fasting plasma glucose, C-peptide, insulin, HbA1c, and HOMA-IR were not significant.
In another multicenter, double-blind, placebo-controlled RCT, Bae et al. [112] evaluated the effect of adding a DDB-carnitine orotate complex to anti-diabetic treatment on ALT normalization in patients with type 2 diabetes and NAFLD. At 12 weeks of treatment, the rate of ALT normalization was significantly higher in the DDB-carnitine orotate complex treatment group than the placebo group (89.7% vs. 17.9%, P<0.001). The liver attenuation index according to non-contrast computed tomography was also significantly increased in the DDB-carnitine orotate complex group compared to the placebo group (6.21±8.96 vs. 0.74±8.05 Hounsfield units, P=0.008), indicating that the DDB-carnitine orotate complex has a beneficial effect in terms of the improvement of steatosis among patients with both type 2 diabetes and NAFLD.
In summary, a DDB-carnitine orotate complex is effective in ALT normalization in NAFLD, but its beneficial effect on the improvement of insulin resistance in NAFLD combined with either impaired fasting glucose metabolism or type 2 diabetes should be further investigated to gather solid evidence. Additionally, there is an unmet need for further data supporting the effects of DDB-containing drugs on the improvement of liver histology.
Viral hepatitis
The beneficial effect of a DDB-carnitine orotate complex in ALT normalization was evaluated in treatment-naïve chronic hepatitis B (CHB) patients with concomitant use of entecavir (Table 3) [113]. Despite there being no effect on virologic response, the DDB-carnitine orotate complex combined with entecavir led to a higher rate of ALT normalization (100%) compared to entecavir treatment only (85.7%) after 12 months (P=0.002). This might indicate that a DDB-carnitine orotate complex can synergistically stabilize hepatic necroinflammation during antiviral therapy for CHB.
Safety
Except for a mild degree of skin urticaria [10], no severe adverse events related to DDB have been reported. Adverse events supposed to have a causal relationship with DDB and carnitine orotate complex use include abdominal discomfort, indigestion, headache, and nausea [109,113]. Rates of adverse events did not differ among the control, low-dose, and high-dose groups using DDB and carnitine orotate complex [109]. The adverse events possibly related to DDB-garlic oil treatment were diarrhea, dry mouth, epigastric soreness, rash, and so on [111]. The rates of adverse events were not different compared to those of the placebo control group or active control (silymarin) group, and no serious adverse events occurred during the 12 weeks of the study period [110].
L-ORNITHINE L-ASPARTATE (LOLA)
Mechanism of action
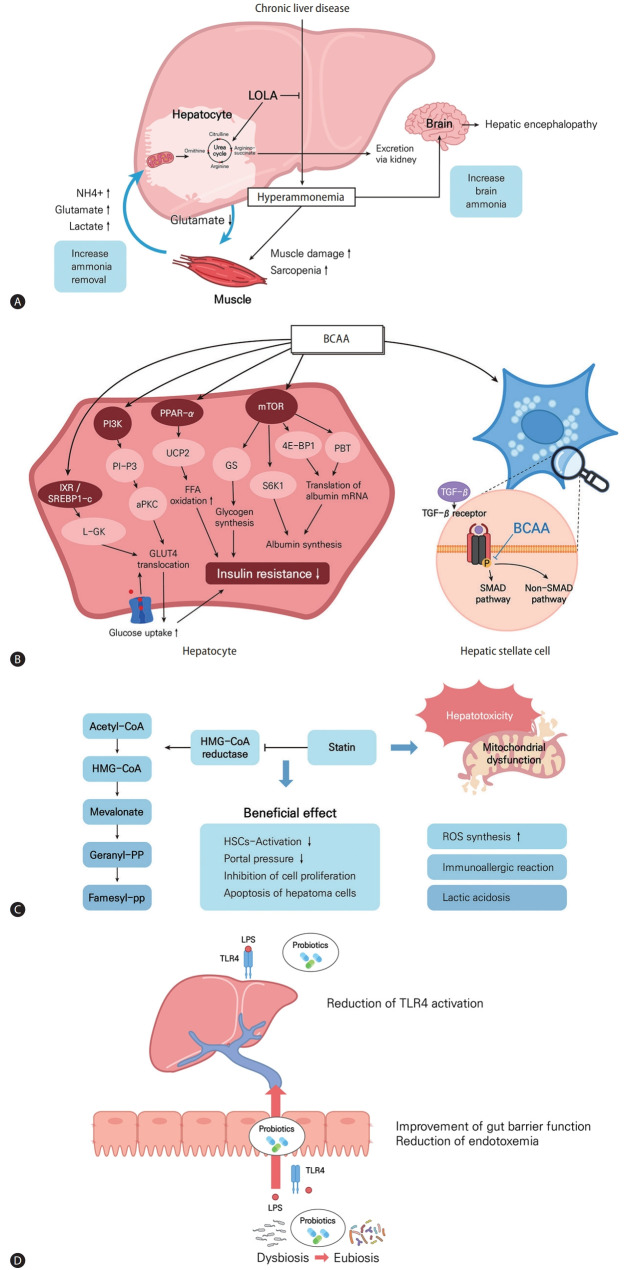
LOLA is a mixture of endogenous amino acids that promotes ammonia removal in patients with liver cirrhosis [114,115]. Ammonia is produced as a result of nitrogen metabolism in muscle and other peripheral tissues [114]. In the liver, ammonia is converted into urea, which is excreted in urine [116]. Impaired liver function can result in an elevated ammonia concentration [117]. In experimental models, the expression levels of genes encoding urea-cycle enzymes, as well as the amounts of those enzymes, are reduced, suppressing ureagenesis and inducing hyperammonemia in a pre-cirrhotic state [21]. Hyperammonemia may trigger fibrosis progression in patients with NASH.
LOLA promotes ammonia removal by increasing the synthesis of urea (Fig. 2A). Hypermethylation of urea cycle-related genes and reduced quantities and activities of urea-cycle enzymes have been noted in patients with NASH [20]. These changes increase the plasma ammonia concentration and result in ammonia accumulation in the liver tissue of patients with NASH. LOLA reduces the serum concentrations of liver enzymes and triglycerides in patients with NASH. The underlying mechanisms of this effect may be enhanced by ammonia removal, increased antioxidant activity, attenuated lipid peroxidation by glutamine and GHS, and improved hepatic microcirculation by L-arginine-derived nitric oxide.
Figure 2.
Mechanisms and effects of LOLA, BCAA, statins, and probiotics in chronic liver diseases. (A) In patients with chronic liver disease, hepatic ammonia removal is decreased and muscle ammonia removal is increased. LOLA acts to prevent hyperammonemia by increasing the synthesis of urea. (B) BCAA treatment acts on hepatocytes to decrease insulin resistance and affects albumin synthesis and acts on stellate cells to inhibit fibrosis by regulating TGF-β pathways. (C) Statins inhibit HMG-CoA reductase and induce pleiotropic effects by the deactivation of hepatic stellate cells, reduction of portal pressure, and inhibition of cell proliferation and induction of hepatoma cells. The liver toxicity of statins can be mediated by mitochondrial dysfunction, ROS synthesis, immno-allergic reactions, and lactic acidosis. (D) Therapeutic effects of probiotics modulating the gut microbiota and the gut-liver axis to improve liver diseases. LOLA, L-ornithine L-aspartate; BCAA, branched chain amino acid; PI3K, phosphatidylinositol 3-kinase; PPAR, peroxisome proliferator-activated receptor; mTOR, mammalian target of rapamycin; L-GK, liver type glucokinase; UCP2, uncoupling protein 2; GS, glycogen synthase; 4E-BP1, 4E-binding protein 1; GLUT, glucose transporter; TGF, tumor growth factor; BCAA, branched chain amino acid; SMAD, suppressor of mothers against decapentaplegic; HMG-CoA, hydroxymethylglutaryl coenzyme A; HSC, hepatic stellate cell; ROS, reactive oxygen species; LPS, lipopolysaccharides; TLR, toll-like receptor.
Clinically beneficial effects
NAFLD
Few studies have evaluated LOLA as a treatment for NASH. A total of 463 patients with fatty liver, 29% of whom had NAFLD, were treated with LOLA during 1–3 months [96]. It was determined that LOLA reduced serum AST, ALT, and GGT levels by up to 70% in patients with CLD (AST, from mean of 48.1±53.7 to 25.7±16.1 U/L; ALT, from mean of 52.6±44.7 to 39.2±36.5 U/L; GGT, from mean of 155.4±236.7 to 60.9±56.3 U/L) [96]. Moreover, beneficial treatment outcomes were more pronounced in patients with fatty liver than those with cirrhosis [118]. In a multicenter open-label, multidose RCT [119], the efficacy of LOLA was assessed in 72 patients with NASH. Patients were prescribed high-dose (6 g bid; n=38) or low-dose (3 g bid; n=34) LOLA for 12 weeks. After 6 and 12 weeks of treatment, the serum levels of AST (baseline: mean, 82.28±29.92; 6 weeks: mean, 66.64±29.17; 12 weeks: mean, 61.86±26.69 U/L), ALT (baseline: mean, 106.95±39.90; 6 weeks: mean, 84.10±38.98; 12 weeks: mean, 65.80±26.73 U/L), and GGT (baseline: mean, 114.29±44.72; 6 weeks: mean, 90.10±34.96; 12 weeks: mean, 70.87±23.57 U/L) were significantly lowered in both groups than at baseline. Additionally, LOLA resulted in a significant dose-related reduction in the levels of ALT (low-dose vs. high dose group: 6-week mean, 22.85±26.88 vs. 35.92±32.28 U/L; P<0,0001; 12-week mean, 41.15±24.07 vs. 50.19±28.08 U/L; P<0.0001) [119]. In another study with 78 patients with NASH, LOLA improved hepatic microcirculation as evaluated by polyhepatography (a modified technique for the non-invasive estimation of intrahepatic blood flow) in the presence of stage 0–1 fibrosis [120]. However, the gathered data were limited due to the small sample size and use of serum transaminase as the main outcome. Thus, further studies of the effect of LOLA in patients with NAFLD are needed.
Hepatic encephalopathy (HE)
HE is a severe neuropsychiatric complication of cirrhosis [121], characterized by deficits in attention, visuospatial construction, and impaired motor speed. Hyperammonemia is consistently reported in such patients. Treatment strategies principally seek to lower the levels of circulating ammonia. In a systematic review and meta-analysis, LOLA appeared to improve the mental state (pooled risk ratio [RR], 1.36; 95% CI, 1.10–1.69; P=0.005) and lower the ammonia level (mean difference, -17.50 μmol/L; P=0.0008) of patients with overt HE or minimal HE [115]. A recent double-blind, randomized, placebo-controlled trial showed that combining intravenous LOLA with lactulose and rifaximin significantly improved the HE grade (92.5% vs. 66%; P<0.001), shortened the recovery time (mean, 2.70±0.46 vs. 3.00±0.87 days; P=0.03), and reduced the 28-day mortality rate (16.4% vs. 41.8%; P=0.001) compared to only lactulose and rifaximin use [24]. In addition, the LOLA group showed significantly higher reductions in levels of blood ammonia (mean, 51.69±10.835 vs. 37.52±12.41 μmol/L; P<0.001) and inflammatory markers such as IL-6 (mean, 36.43±27.51 vs. 26.93±20.55 pg/mL; P=0.025) and TNF-α (mean, 10.83±5.12 vs. 8.77±5.56; P=0.027), compared to the placebo group [24].
Safety
The rate of adverse events was low (4.4–4.8%) and most were gastrointestinal-related [119].
BRANCHED CHAIN AMINO ACIDS (BCAAs)
Mechanism of action
BCAAs are some of the essential amino acids and consist of leucine, valine, and isoleucine. In basic research, BCAAs have been studied in relation to metabolism, liver fibrosis, and immunity (Fig. 2B). First, with respect to metabolism, BCAAs were reported to have major effects on 1) protein/albumin synthesis and 2) glucose/lipid metabolism and insulin resistance. Among the BCAAs, leucine, in particular, increases albumin production [22]. Specifically, leucine activates the mTOR pathway and increases the transcription of albumin mRNA by increasing 4E-binding protein downstream of the mTOR pathway [122-124]. In addition, in HepG2 cells, a mechanism by which BCAA-stimulated polypyrimidine tract-binding protein binds to albumin mRNA and increases albumin translation has been reported [125]. The mechanisms by which BCAAs affect glucose metabolism have also been variously reported, such as the PI3KAkt pathway [126,127], induced glucose transporter (GLUT)-4 and GLUT-1 translocation, and increased glycogen synthase activity [128]. BCAAs improve insulin resistance through liver, skeletal muscle, and adipose tissue, respectively, and PI3K, peroxisome proliferator-activated receptor (PPAR)-α, and Akt pathways are involved [126,128-130]. Recently, it has been reported that BCAA administration inhibits the lipogenesis-related genes FAS and ACC through the proliferation of intestinal flora, thereby inhibiting lipogenesis [131]. Also, BCAA administration can help improve hepatic fibrosis, and the main mechanism is inhibition of the TGF-β signaling pathway of HSCs [132-135]. Lastly, among BCAAs, valine especially can help to restore immune function by regulating the maturation and function of monocyte-derived dendritic cells in cirrhotic patients [136].
Clinically beneficial effects
Viral hepatitis
In viral hepatitis, BCAA administration improved HOMA-IR in insulin-resistant CHC patients (mean after treatment, 4.5 in the BCAA group vs. 5.3 in the control group; P=0.047) [29]. Also, 2-year BCAA administration in obese CHC patients was effective in preventing HCC and improving IFN signaling promoted by malnutrition (event-free survival for HCC: hazard ratio [HR], 0.3; 95% CI, 0.12–0.78, P=0.008) (Table 4) [137].
Table 4.
Summary of clinical studies in BCAA
| Study | Etiology | Inclusion criteria | Intervention period | Arms (n) | Age (years) | Outcome | Study design |
|---|---|---|---|---|---|---|---|
| Muto et al. [11] (2005) | Mixed (mainly HCV) | Decompensated cirrhosis | 2 years | BCAA (314) | 62±8 | Higher/longer event-free survival in BCAA group (death, varix rupture, HCC, hepatic failure) (HR, 0.67; 95% CI, 0.49–0.93, P=0.015) | RCT |
| Control (308) | 61±9 | ||||||
| Marchesini et al. [144] (2003) | Mixed (mainly viral) | Advanced cirrhosis (CTP B or C) | 1 year | BCAA (59) | 59±1 | Event (death, varix rupture, HCC, hepatic failure) (15.5% vs. 32.1% vs. 27.1%; P=0.037) | RCT |
| Lactoalbumin (56) | 60±1 | ||||||
| Maltodextrins (59) | 59±1 | ||||||
| Ichikawa et al. [145] (2010) | Mixed (mainly viral) | Liver cirrhosis | 8 weeks | BCAA (12) | 66.2±8.2 | Change in ESS (mean, -5.5 vs. 1.2; P<0.05) | RCT |
| Control (9) | 67.4±9.8 | ||||||
| Yamamoto et al. [154] (2005) | Mixed (mainly viral) | Liver cirrhosis | 1 hour | BCAA (16) | 63±8 | Change in cerebral blood flow (PET) (mean, 0.81 vs. 0.75; P<0.05) | RCT |
| Control (13) | 62±9 | ||||||
| Kawamura et al. [138] (2009) | Mixed (mainly HCV) | Liver cirrhosis with CTP class A | 1 year | BCAA (27) | 62.7±10.08 | Cirrhosis-related complications (HCC, ascites, varix, HE) (14.8% vs. 30.4%; P=0.043) | RCT |
| Control (23) | 62.3±7.3 | ||||||
| Les et al. [32] (2011) | Mixed (mainly HCV, alcohol) | Liver cirrhosis with episode of HE within 2 months | 56 weeks | BCAA (21) | 64.1±10.4 | Recurrence of HE (47% vs. 34%; P=0.274) | RCT |
| Control, maltodextrin (27) | 62.5±10.4 | ||||||
| Nakaya et al. [139] (2007) | HCV | Liver cirrhosis | 3 months | BCAA (19) | 67±9 | Change in albumin level (mean, 3.2 vs. 3.0; P<0.05) | RCT |
| Control (19) | 67±8 | ||||||
| Takeshita et al. [29] (2012) | HCV | HCV with insulin resistance | 24 weeks | BCAA (14) | 58.6±2.9 | HOMA-IR after treatment (mean, 4.5 vs. 5.3; P=0.047) | RCT |
| Control (13) | 64.2±3.0 | ||||||
| Koreeda et al. [149] (2011) | Mixed (mainly HCV) | Liver cirrhosis | 6 months | BCAA (17) | 68±10 | Change in Rmax (mean, 0.23 to 0.25; P=0.059) | Prospective cohort study |
| Park et al. [142] (2020) | Mixed (mainly alcohol) | Liver cirrhosis with CTP score of 8–10 points | 6 months | BCAA (63) | 60±10 | Higher/longer event-free survival in BCAA group (death, varix rupture, HCC, hepatic failure) (HR, 0.38; 95% CI, 0.22–0.68, P<0.001) | Prospective cohort study |
| Control (61) | 58±11 | ||||||
| Park et al. [143] (2017) | Mixed (mainly HBV, alcohol) | Liver cirrhosis with CTP score of 8–10 points | 6 months | BCAA (166) | 59±11 | Cirrhotic complication-free survival (median, 19.3 vs. 19.2 months; P=0.973) | Retrospective cohort study |
| Control (141) | 60±9 | ||||||
| Hanai et al. [277] (2015) | Mixed (mainly HCV) | Liver cirrhosis patients who were not transplantation candidates | 1 year | BCAA (94) | 64 (28–91) | Higher/longer overall survival in BCAA group (log-rank test P=0.02) | Retrospective cohort study |
| Control (36) | 66 (33–84) | ||||||
| Hanai et al. [148] (2020) | Mixed (mainly HCV, alcohol) | Liver cirrhosis | NA | BCAA (87) | 69 (59–74) | Lower mortality in BCAA group (HR, 0.57; 95% CI, 0.33–0.99, P=0.046) | Retrospective cohort study |
| Control (436) | 66 (55–74) |
Variables are expressed as median (interquartile range) or mean±standard deviation.
BCAA, branched chain amino acid; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; RCT, randomized controlled trial; CTP, Child-Turcotte-Pugh; ESS, Epworth Sleepiness Scale; PET, positron emission tomography; HE, hepatic encephalopathy; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; HBV, hepatitis B virus.
Liver cirrhosis
In patients with liver cirrhosis, the effect of BCAA on the prognosis of cirrhotic patients has been verified through various RCTs (Table 4). BCAA administration for >6–12 months has been commonly reported to reduce clinical decompensation (14.8% vs. 30.4%; P=0.043) [138]. In particular, BCAA was effective in reducing the occurrence of varix rupture, the rate of hepatic failure, and incidence of de novo HE [138-143]. BCAA was also found to be helpful for improving aspects of the quality of life (i.e., physical functioning improved from 67%±4% to 73%±3%; P=0.023) [144]. such as sleep disturbance (change in Epworth Sleepiness Scale: BCAA group, -5.5 vs. control group, 1.2; P<0.05) [145]. and nutritional status or sarcopenia [139,146,147]. As for the method of BCAA administration, both taking BCAAs as drugs and eating BCAA-rich foods during late-night meals were effective [139,148,149]. On the other hand, BCAA administration did not lead to improvements in measures of liver function itself, such as Model for End-stage Liver Disease (MELD) or Child-Turcotte-Pugh (CTP) scores, in many studies [138,140,142-144]. Also, whether or not BCAAs can prolong overall survival (OS) differs between studies. Recently, two meta-analyses showed that BCAA supplements had no effect on survival [150,151].
Although BCAA administration was effective in improving liver function and reducing the risk of decompensation in patients with liver cirrhosis, further prospective studies are still required to discern whether BCAA is effective for patients with non-cirrhosis.
HE
BCAAs have been reported to be effective in both minimal HE and overt HE (Table 4) [32,151]. According to meta-analysis results, BCAA administration improved HE compared to the control group (RR, 1.71; 95% CI, 1.17–2.51) [150]. However, in patients with a previous history of HE, BCAA administration had no significant effect on the recurrence of HE (BCAA group, 47% vs. control group, 34%; P=0.274) [32]. This phenomenon can be explained by that facts that BCAAs reduce ammonia levels [152,153] and increase cerebral blood flow in cirrhotic patients [154], respectively.
To date, BCAA administration has shown a beneficial effect in patients with HE. However, additional research is needed to determine whether BCAA administration will be effective for decompensation in conditions other than HE, such as variceal bleeding, ascites, and jaundice.
Safety
Adverse events related to BCAA administration were not reported in most studies, including RCTs. In a Cochrane review of 5 studies [11,144,155-157], BCAA did not increase the risk of serious adverse events, but they were associated with nausea and diarrhea, although not to a significant degree (RR, 3.39; 95% CI, 0.7–16.46) [151].
STATIN
Mechanism of action
Statins are inhibitors of hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase and are used as lipid-lowering agents by >200 million patients worldwide [158]. The major pharmacologic effect of statin is decreased production of cholesterol precursors and cholesterol biosynthesis, resulting in the prevention of atherosclerosis to reduce cardiovascular and cerebrovascular events and mortality [23]. In addition to their lipid-lowering effect, statins are well known to have other beneficial effects, causing improvements in endothelial function and displaying anti-inflammatory, immunomodulatory, and anti-thrombotic effects (Fig. 2C) [159]. Statins interfere with the activation of small GTPases like RhoA and Ras proteins, which can modulate endothelial nitric oxide synthaseand nitric oxide activity [160]. Statins activate PPAR-α and -β oxidation, resulting in the reduction of intrahepatic inflammation [161]. Statins can also induce the protection of liver sinusoidal endothelial cells (LSECs) and mediate paracrine endothelial-HSC deactivation through the induction of transcription factor Kruppel-like factor 2 [162]. Deactivation of HSCs and stabilization of LSECs lead to the alleviation of hepatic fibrosis and portal pressure [163]. Statins also showed anti-tumor effects in HCC by reducing cell proliferation and tumor cell adhesion [164].
Clinically beneficial effects
NAFLD
Several reports have suggested beneficial effects of statins in NAFLD and NASH (Table 5). Nelson et al. [12] conducted an RCT that investigated the therapeutic effects of simvastatin in biopsy-proven NASH patients. This study included a small number of patients, and there was no significant difference in necroinflammatory activity (mean, 1.4±0.5 vs. 1.0±1.4; P>0.05) or fibrosis stage (1.50±0.9 vs. 1.0±1.4; P>0.05) between the simvastatin group and placebo group. However, two cross-sectional studies reported that statin treatment had protective effects against NASH progression. One study enrolled 108 statin users and 1,094 controls who received liver biopsy for suspected NASH [165]. In this study, statin treatment was associated with a reduced risk of steatosis (odds ratio [OR], 0.09; 95% CI, 0.01–0.32, P=0.004), NASH (OR, 0.25; 95% CI, 0.13–0.47, P<0.001), and F2–F4 fibrosis (OR, 0.42 95% CI, 0.20–0.80; P=0.017) after matching [165]. The other study enrolled 346 diabetes patients with biopsy-proven NAFLD and reported that statin treatment reduced the risk of NASH (OR, 0.57; 95% CI, 0.32–1.01, P=0.055) and F2–F4 fibrosis (OR, 0.47; 95% CI, 0.26–0.84, P=0.011) [166]. In addition, another retrospective cohort study reported that statin treatment significantly reduced the amount of hepatic steatosis from a mean rate of 20.4% at baseline to 11.1% at follow-up (P=0.001), whereas the control group did not experience such a change [167].
Table 5.
Summary of clinical studies in statin
| Study | Etiology | Inclusion criteria | Intervention period | Arms (n) | Age (years) | Outcomes | Study design |
|---|---|---|---|---|---|---|---|
| Nelson et al. [12] (2009) | NAFLD | Biopsy-proven NASH | 12 months | Simvastatin 40 mg/day (10) | 52.6±8.6 | · Necroinflammatory activity (mean, 1.4 vs. 1.0; P>0.05) | RCT |
| Control (6) | 52.5±13.0 | · Fibrosis stage (mean, 1.50 vs. 1.0; P>0.05) | |||||
| Dongiovanni et al. [165] (2015) | NAFLD | Patients who underwent liver biopsy for suspected NASH | ≥6 months | Statin (107) | 53±10 | · Lower presence of steatosis (OR, 0.09; 95% CI, 0.01–0.32; P=0.004), NASH (OR, 0.25; 95% CI, 0.13–0.47; P<0.001), F2–F4 fibrosis (OR, 0.42; 95% CI, 0.20–0.80; P=0.017)* in statin group | Cross-sectional study |
| Control (1,094) | 41±16 | ||||||
| Nascimbeni et al. [166] (2016) | NAFLD | Biopsy-proven NAFLD with type 2 DM | NA | Statin (154) | 55 (48–61) | · Lower presence of NASH (OR, 0.57; 95% CI, 0.32–1.01; P=0.055) and significant fibrosis (OR, 0.47; 95% CI, 0.26–0.84; P<0.011) in statin group | Cross-sectional study |
| Control (192) | 52 (42–58) | ||||||
| Ekstedt et al. [167] (2007) | NAFLD | Biopsy-proven NAFLD with elevated ALT and/or AST >41 U/L and/ or elevated ALP >106 U/L | 10.3–16.3 years | Statin (17) | 48.7±9.1 | · Significant reduction of liver steatosis in statin group (20.4% to 11.1%, P=0.001) | Retrospective cohort study |
| Control (51) | 46.3±11.8 | ||||||
| Rallidis et al. [168] (2004) | NAFLD | Biopsy-proven NASH and abnormal liver enzyme | 6 months | Pravastatin 20 mg/day (5) | 40±8 | · Improvement in the grade of inflammation (75%) and steatosis (25%) | Prospective cohort study |
| Hyogo et al. [169] (2008) | NAFLD | Biopsy-proven NASH with lipidemia | 24 months | Atorvastatin 10 mg/day (31) | 52.5 (27–68) | · Significant improvement of steatosis grade (1.6 to 0.8; P<0.001) and NAS (4.1 to 2.9; P<0.001) | Prospective cohort study |
| Hyogo et al. [172] (2011) | NAFLD | Biopsy-proven NASH and hyperlipidemia | 12 months | Pitavastatin 2 mg/day (20) | 50.6 (25–75) | · Change of NAS (6.7 to 6.3) and fibrosis stage (2.3 to 2.1) | Prospective cohort study |
| Nakahara et al. [170] (2012) | NAFLD | Biopsy-proven NASH and hyperlipidemia | 24 months | Rosuvastatin 2.5 mg/day (19) | 46.3 (20–65) | · Change of NAS (3.89 to 3.44) and fibrosis stage (2.33 to 2.00) | Prospective cohort study |
| Kargiotis et al. [171] (2015) | NAFLD | Biopsy-proven NASH and metabolic syndrome and lipidemia | 12 months | Rosuvastatin 10 mg/day (20) | 40.5±5.6 | · Complete resolution of NASH (95%) | Prospective cohort study |
| Lee et al. [278] (2021) | NAFLD | Age ≥20 years who participated the NHIS physical health examination | 72 months | NAFLD (164,856) | 41.4±12.4 | · Reduced risk of NAFLD development in statin group (adjusted OR 0.66; 95% CI, 0.65–0.67) | Retrospective cohort study |
| Control (824,280) | 41.4±12.4 | ||||||
| Zou et al. [175] (2022) | NAFLD | Diabetes or obesity and ICD- 10 codes (K76.0 and K758.1) | 1.92 years | Statin (73,385) | 58.0±12.4 | · Lower risk of HCC development in statin group (HR, 0.47; 95% CI, 0.36–0.60; P<0.001) | Retrospective cohort study |
| Control (199,046) | 50.0±14.9 | ||||||
| Avins et al. [176] (2008) | Mixed | Patients with evidence of liver disease showing elevated AST or ALT levels or diagnosis of liver disease | 28.8 months (12.1–58.2) | Lovastatin (13,491) | 53.9±11.4 | · Lower incidence of liver function test abnormalities in statin group (incident RR 0.28; 95% CI, 0.12–0.55; P=NA) | Retrospective cohort study |
| Control (79,615) | 47.5±13.6 | ||||||
| Hsiang et al. [177] (2015) | HBV | 1.6 years | Statin (1,176) | 58.7±12.4 | · Lower HCC development in statin group (subHR 0.68; 95% CI, 0.48–0.97; P=0.033) | Retrospective cohort study | |
| 4.9 years | Control (52,337) | 37.6±14.1 | |||||
| Huang et al. [178] (2016) | HBV | 4.71±3.21 | Statin (22,544) | 52.87±11.51 | · Lower incidence of cirrhosis (RR, 0.433; 95% CI, 0.344–0.515; P<0.001) and decompensated cirrhosis (RR, 0.468; 95% CI, 0.344–0.637; P<0.001) in stain group | Retrospective cohort study | |
| 4.57±3.20 years | Control (215,802) | 39.73±13.14 | |||||
| Butt et al. [273] (2015) | HCV | Received HCV treatment ≥14 days | >24 months | Statin (3,347) | 53 (49–56) | · Higher SVR rate (OR, 1.44; 95% CI, 1.29–1.61; P<0.0001) in statin group | Retrospective cohort study |
| Control (3,901) | 52 (48–56) | · Cirrhosis development (17.3% vs. 25.2%; P<0.001) | |||||
| · HCC incidence (1.2% vs. 2.6%, P<0.01) | |||||||
| Simon et al. [179] (2015) | HCV | Previous non- response to standard interferon therapies and advanced hepatic fibrosis on liver biopsy | 3.5 years | Statin (29) | 54.2±7.2 | · Lower risk of fibrosis progression in statin group (unadjusted HR, 0.32; 95% CI, 0.10–0.97; P=0.048; adjusted HR, 0.31; 95% CI, 0.10–0.97; P=0.044) | Retrospective cohort study |
| Control (514) | |||||||
| Yang et. el. [180] (2015) | HCV | 2,874,031.7 personyears | Statin (29,204) | Only distribution available | · Incidence rate of cirrhosis (445.5/100,000 person-years vs. 1311.2/100,000 personyears) | Retrospective cohort study | |
| Control (197,652) | |||||||
| Mohanty et al. [181] (2016) | HCV | 2.6 years | Statin (1,323) | 56 (52–60) | · Lower risk of decompensation (HR, 0.22; 95% CI, 0.17–0.28) and death (HR, 0.39; 95% CI, 0.34–0.44) before matching in statin group | Retrospective cohort study | |
| 1.9 years | Control (12,522) | 54 (50–58) | · Lower risk of decompensation (HR, 0.55; 95% CI, 0.39–0.77 and death (HR, 0.56; 95% CI, 0.46–0.69) after matching in statin group | ||||
| Abraldes et al. [182] (2009) | Mixed (mainly alcohol) | Liver cirrhosis patients with severe portal HTN defined as HVPG of ≥12 mmHg | 30±4 days | Simvastatin (28) | 58±10 | · Change in HVPG (mean, -8.3% vs. -1.6%; P=0.041) | RCT |
| Control (27) | 56±10 | ||||||
| Pollo-Flores et al. [183] (2015) | Mixed (mainly HCV) | Cirrhosis with portal HTN | 3 months | Simvastatin (14) | 56.5 (IQR, 8.7) | · Decreased HVPG of at least 20% from baseline or to ≤12 mmHg) (55% vs. 0%; P=0.03) | RCT |
| Control (20) | 58.5 (IQR, 13.5) | ||||||
| Abraldes et al. [184] (2016) | Mixed (mainly alcohol) | Diagnosis of liver cirrhosis, and variceal bleeding within the previous 5–10 days, and plan to start standard prophylactic treatment for variceal bleeding | 371 days | Simvastatin (69) | 57.42±11.31 | · Higher survival (HR, 0.387; 95% CI, 0.152–0.986; P=0.030) in statin group | RCT |
| 382 days | Control (78) | 57.62±10.59 | · Lower rebleeding risk (HR, 0.858; 95% CI, 0.455–1.620, P=0.583) in statin group | ||||
| Kumar et al. [185] (2014) | Mixed (mainly HCV) | Biopsy-proven liver cirrhosis on statin therapy at biopsy and for ≥3 months after biopsy | 36 months | Statin (81) | 59.79±10.91 | · Lower mortality (HR, 0.53; 95% CI, 0.334–0.856; P=0.01) in statin group | Retrospective cohort study |
| 30 months | Control (162) | 59.64±10.60 | · Lowe risk of decompensation (HR, 0.58, 0.34–0.98; P=0.04) in statin group |
Variables are expressed as median (interquartile range) or mean±standard deviation.
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; RCT, randomized controlled trial; OR, odds ratio; CI, confidence interval; DM, diabetes mellitus; NA, not available; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; NAS, NAFLD activity score; NHIS, National Health Interview Survey; ICD-10, International Classification of Diseases; HCC, hepatocellular carcinoma; HR, hazard ratio; RR, risk ratio; HBV, hepatitis B virus; HCV, hepatitis C virus; SVR, sustained viral response; HTN, hypertension; HVPG, hepatic venous pressure gradient; IQR, interquartile range.
These data are after matching.
Several prospective cohort studies have reported that statin treatments using pravastatin, atorvastatin, and rosuvastatin have beneficial effects in improving the histologic grade of NAFLD and NASH [168-171]. On the other hand, pitavastatin did not significantly change NAS or fibrosis stage [172]. In a large population-based study, statin treatment reduced the risk of NAFLD development (adjusted OR, 0.66; 95% CI, 0.65–0.67) and fibrosis development (adjusted OR, 0.43; 95% CI, 0.42–0.44). In a study using data from the National Health Information database of South Korea, statin treatment decreased not only the risk of NAFLD occurrence but also the development of fibrosis attributed to NAFLD, regardless of diabetes mellitus [173]. A meta-analysis by Fatima et al. [174] reported that statin treatment reduced the risk of NAFLD development (OR, 0.69; 95% CI, 0.57–0.84; I2=36%; P=0.0002); ALT and GGT levels; and histologic grades with steatosis, inflammation, and fibrosis. There was also a report that statin treatment reduced the HCC risk in patients with NAFLD (HR, 0.47; 95% CI, 0.36–0.60) [175].
Collectively, there is increasing evidence that statin treatment might have protective effects on NAFLD/NASH development and a beneficial effect of histologic improvement for NAFLD/NASH. Therefore, statins can be used in NAFLD and NASH, and they are considered first-line treatments to lower low-density lipoprotein (LDL) cholesterol and prevent atherosclerotic cerebrovascular disease. However, well-organized RCTs are required to establish a therapeutic effect of statin in NAFLD/NASH patients with histologic confirmation, and long-term, large-scale prospective cohort studies are also needed to identify whether statin treatments are associated with liver-related outcomes in patients with NAFLD/NASH.
Viral hepatitis
Three studies with CHB patients reported another beneficial effect of statin use. According to a large-scale retrospective cohort study including patients with CLD, lovastatin use was associated with lower incidence rates of liver-test abnormalities (incident RR [IRR], 0.28; 95% CI, 0.12–0.55), moderate liver injury (IRR, 0.56; 95% CI, 0.47–0.65), severe liver injury (IRR, 0.50; 95% CI, 0.29–0.81), and the occurrence of liver cirrhosis and liver failure (IRR, 0.29; 95% CI, 0.21–0.38) [176]. Another study by Hsiang et al. [177] reported that statin treatment is associated with a lower risk of HCC development (weighted sub-HR, 0.68; 95% CI, 0.48–0.97; P=0.033). Another nationwide cohort study reported that statin use significantly lowered the incidence of cirrhosis (RR, 0.433; 95% CI, 0.344–0.515; P<0.001) and decompensated cirrhosis (RR, 0.468; 95% CI, 0.344–0.637; P<0.001) compared to that of a non-statin group [178].
Statins might be associated with a sustained virologic response (SVR) in patients with HCV. Patients treated with statins showed higher SVRs (OR, 1.44; 95% CI, 1.29–1.61; P<0.0001) and decreased progression of liver fibrosis and incidence of HCC among CHC patients who received pegylated IFN-based HCV treatment for ≥14 days [173]. Other retrospective studies also showed that statin treatment is associated with the decreased risk of fibrosis and cirrhosis progression in patients with HCV (Table 5) [179-181].
Despite the positive results in patients with HBV or HCV, the clinical relevance of statins might be limited mainly due to the weakness of a retrospective study design. Therefore, it is premature to routinely prescribe statins for clinical benefit before well-designed prospective studies are available.
Liver cirrhosis
Two RCTs showed that simvastatin significantly reduced the hepatic venous pressure gradient (HVPG) and improved hepatic perfusion in patients with cirrhosis [182,183]. In another RCT, simvastatin failed to reduce the risk of rebleeding (HR, 0.858; 95% CI, 0.455–1.620; P=0.583) but prolonged survival (HR, 0.387; 95% CI, 0.152–0.986, P=0.030) in cirrhotic patients with variceal bleeding [184]. A retrospective study reported that statin use was associated with lower risks of mortality and decompensation (Table 5) [185].
Statins have potential beneficial effects in cirrhosis patients, but their long-term beneficial effects are limited. In addition, safety is also a concern, especially in patients with cirrhosis. Further prospective long-term follow-up data are required to confirm the beneficial effect of statins in patients with cirrhosis.
Safety
There are several concerns surrounding the adverse effects of statins, which include myopathy, a risk of type 2 diabetes development, deterioration of neurological and neurocognitive conditions, renal toxicity, and hepatotoxicity [186]. Aminotransferase elevation was found in up to 2% of patients in early clinical trials [187]. The mechanism of statin-induced hepatotoxicity is not clearly defined: mitochondrial dysfunction by oxidative stress, increase of ROS synthesis, immune-allergic reactions, and lactic acidosis may be potential mechanisms (Fig. 2C) [188]. However, most cases of aminotransferase elevation are mild and transient. Although the increase in liver enzyme levels depends on the statin dose, the incidence of AST or ALT level >3 times of the upper normal range is 0–1.8%, and clinically significant acute liver injury or fulminant hepatic failure is very rare [189]. In a previous study of patients with cirrhosis, two cases of severe rhabdomyolysis (2.8%) developed in the simvastatin group [184]. As even a small dose of statin than the usual dose can lead to adverse events, including rhabdomyolysis, in patients with decompensated cirrhosis, the administration of statins to patients with decompensated cirrhosis or acute liver failure should be avoided. Further studies for determining the safety dose of statin are required in patients with advanced liver disease.
PROBIOTICS
Mechanism of action
The gut microbiota has been considered a novel environmental factor involved in the pathophysiology of liver diseases [190]. A growing body of evidence points towards the suggestion that intestinal dysbiosis contributes to impaired barrier function of the intestinal mucosa [191]. Enhanced intestinal permeability allows bacterial metabolites such as LPS to reach the liver [192,193]. LPS trigger inflammation and insulin resistance by activating toll-like receptor (TLR)-4 and initiating pro-inflammatory cascades [194]. Overall, intestinal dysbiosis, bacterial translocation, and TLR-4 activation lead to increased hepatic fat accumulation, prompting the development of NAFLD and progression to NASH [195].
Modulation of the gut microbiota would represent an attractive target for therapeutic interventions in NAFLD subjects. In vivo studies have demonstrated the therapeutic effects of probiotics on NAFLD (Fig. 2D) [25]. In high-fat diet-fed murine models, probiotics induce Bifidobacterium abundance and a more beneficial composition of gut microbiota [196]. Ingestion of Lactobacillus also ameliorates the progression of NAFLD in murine models with a Western diet [197].
In terms of the gut-liver-brain axis, conditions of altered communication between the gut microbiota and the brain, such as dysbiosis, leaky gut, metabolic endotoxemia, and brain changes, may induce the development of HE in patients with liver cirrhosis [190,198]. Conversely, probiotics can reduce ammonia absorption by decreasing the urease activity of gut bacteria in the intestinal lumen and the intestinal pH [199].
Clinically beneficial effects
NAFLD
Probiotics are live bacteria that intend to improve the “good” gut microbiota by competitive colonization and acidification of the intestinal lumen [200]. Lactobacillus and Bifidobacterium are the most commonly used species in probiotics. Seven representative RCTs have investigated the therapeutic effect of probiotics in patients with NAFLD (Table 6) [201-207]. Although heterogeneities exist in terms of the dosing and type of probiotics and the treatment period in these RCTs, probiotic intervention could be related to diminishing liver steatosis assessed by imaging modalities compared to placebo [202,204-207]. In addition, improvements in several biochemical markers, including ALT, AST, and GGT, and metabolic profiles such as total and LDL cholesterols were also observed in probiotic groups [201-207]. Unfortunately, these studies included small numbers of patients, and few of them examined the effect of probiotics on histologic markers of NASH.
Table 6.
Summary of clinical studies in probiotics
| Study | Etiology | Inclusion criteria | Treatment | Intervention period | Arms (n) | Age (years) | Outcomes | Study design |
|---|---|---|---|---|---|---|---|---|
| Wong et al. [202] (2013) | NAFLD | Biopsy-proven NASH | Mixture of L. plantarum, L. delbrueckii, L. acidophilus, L. rhamnosus, and B. bifidum | 6 months | Probiotics (10) | 42±9 | · Decreased liver fat contents in probiotics (22.6% to 14.9%; P=0.034) | RCT |
| Usual care (10) | 55±9 | · Decreased AST level in probiotics (mean, 83.3 to 46.1; P=0.008) | ||||||
| Shavakhi et al. [205] (2013) | NAFLD | Biopsy-proven NASH on metformin | Lactobacillus, Bifidobacterium, Streptococcus | 6 months | Probiotics + metformin (31) | 41.5±12.7 | · Decreased ALT and AST levels (mean, 133.7 to 45.2 vs. 123.1 to 44.2; P<0.001) | RCT |
| Placebo + metformin (32) | 55±9 | · Reduction in grade of hepatic steatosis measured by US | ||||||
| Nabavi et al. [203] (2014) | NAFLD | NAFLD on US | L. acidophilus and B. lactis | 8 weeks | Probiotic yoghurt (36) | 42.74±8.72 | · Lower AST, ALT, total cholesterol, and LDL-cholesterol levels after treatment in probiotic yogurt groups than control (P<0.05) | RCT |
| No probiotics (15) | 44.05±8.14 | |||||||
| Abdel Monem [201] (2017) | NAFLD | Biopsy-proven NASH | L. acidophilus | 1 month | Probiotics (15) | 44.20±5.51 | · Decreased ALT level in probiotics (mean, 83.3 to 46.1; P<0.001) | RCT |
| No probiotics (15) | 44.33±5.62 | · Decreased AST level in probiotics (mean, 57.1 to 38.2; P=0.03) | ||||||
| Manzhalii et al. [210] (2017) | NAFLD | NASH fed a low- fat/low-calorie diet | L. casei, L. rhamnosus, L. bulgaris, B. longum, S. thermophilus and oligofructose | 12 weeks | Probiotic cocktail (38) | 44.3±1.5 | · Greater reduction in AST and ALT levels in synbiotics than placebo (P<0.05) | RCT |
| No probiotics (37) | 43.5±1.3 | · Greater reduction in the fibrosis score by TE in synbiotics than placebo (P<0.05) | ||||||
| Kobyliak et al. [204] (2018) | NAFLD | Diabetes | Bifidobacterium, Lactobacillus, Lactococcus, Propionibacterium | 8 weeks | Probiotics (30) | 53.4±9.55 | · Decreased AST and GGT levels in probiotics (P<0.05) | RCT |
| Placebo (28) | 57.3±10.5 | · Decreased fatty liver index in probiotics (84.33 to 78.73; P<0.001) | ||||||
| Ahn et al. [207] (2019) | NAFLD | Obesity and liver fat >5% on proton density fat fraction | Lactobacillus, Pediococcus, Bifidobacterium | 12 weeks | Probiotics (30) | 41.7±12.49 | · Decreased liver fat contents (mean 16.3% to 14.1%; P=0.032) | RCT |
| Placebo (35) | 44.71±13.31 | · Greater reduction in the triglyceride level in probiotics than placebo (P=0.003) | ||||||
| Duseja et al. [206] (2019) | NAFLD | Biopsy-proven NAFLD | Lactobacillus, Bifidobacterium, Streptococcus | 1 year | Oral multistrain probiotic (19) | 38±10 | · Greater reduction in ALT level in probiotics than placebo (P=0.046) | RCT |
| Placebo (20) | 33±6 | · Greater reduction in the NAS and hepatic fibrosis in probiotics than placebo (P<0.05) | ||||||
| Malaguarnera et al. [30] (2012) | NAFLD | Abnormal serum aminotransferase levels | B. longum and oligofructose | 24 weeks | Synbiotics + LSM (33) | 46.9±5.4 | · Lower AST and LDL-cholesterol levels after treatment in synbiotics than placebo (P<0.05) | RCT |
| Placebo + LSM (33) | 46.7±5.7 | · Greater reduction in HOMA-IR and NASH activity score in synbiotics than placebo (P<0.05) | ||||||
| Eslamparast et al. [13] (2014) | NAFLD | NAFLD on US with ALT >60 IU/L for 6 months | Combination of L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. longum, L. bulgaricus and oligofructose | 28 weeks | Synbiotic capsule (26) | 46.35±8.8 | · Lower AST, ALT and GGT levels after treatment in synbiotics than placebo (P<0.001) | RCT |
| Placebo (26) | 45.69±9.5 | · Greater reduction in the fibrosis score by TE (mean, 9.36 to 6.38 vs. 7.92 to 7.16; P<0.001) | ||||||
| Asgharian et al. [219] (2016) | NAFLD | Combination of L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. longum, L. bulgaricus and oligofructose | 8 weeks | Synbiotics (40) | 46.57±1.7 | · Decreased grade of steatosis on US in synbiotics (P<0.005) | RCT | |
| Placebo (40) | 47.78±1.7 | |||||||
| Mofidi et al. [208] (2017) | NAFLD | BMI ≤25 | Combination of L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. longum, L. bulgaricus and oligofructose | 28 weeks | Synbiotics (21) | 40.09±11.44 | · Greater reduction in AST and fasting glucose levels in synbiotics than placebo (P<0.05) | RCT |
| Placebo (21) | 44.61±10.12 | · Greater reduction in the fibrosis score and steatosis by TE in synbiotics than placebo (P<0.001) | ||||||
| Sayari et al. [211] (2018) | NAFLD | Taking sitagliptin | Lactobacillus, Bifidobacterium, Streptococcus and fructooligosaccharide | 16 weeks | Synbiotics + sitagliptin (70) | 42.48±11.41 | · Greater reduction of glucose, AST, total cholesterol, and LDL-cholesterol levels in synbiotics than placebo (P<0.05) | RCT |
| Placebo +sitagliptin (68) | 43.42±11.65 | |||||||
| Scorletti et al. [33] (2020) | NAFLD | NAFLD diagnosed by histologic confirmation or imaging evidence of liver fat | Bifidobacterium and fructooligosaccharide | 10–14 months | Synbiotic agents (55) | 50.2±12.4 | · No significant difference in MRS-based liver fat reduction between groups | RCT |
| Placebo (49) | 51.6±13.1 |
Variables are expressed as mean±standard deviation.
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; AST, aspartate aminotransferase; RCT, randomized controlled trial; ALT, alanine aminotransferase; US, ultrasonography; LDL, low-density lipoprotein; TE, transient elastography; GGT, γ-glutamyl transferase; NAS, NAFLD activity score; LSM, lifestyle modification; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; BMI, body mass index; MRS, magnetic resonance spectroscopy.
Synbiotics are a combination of advantageous gut bacteria (probiotics) and non-digestible fibers that help good bacteria to grow (prebiotics). Most RCTs evaluating the effect of synbiotics in NAFLD demonstrated significant reductions in liver enzymes and steatosis as measured by ultrasound [13,30,208-211]. Interestingly, an European RCT including 75 patients with NASH fed a low-fat/low-calorie diet reported that end-of-study liver stiffness as measured by transient elastography was significantly lower in patients treated with 12 weeks of synbiotics compared to the control group (mean, 5.2±0.2 vs. 5.9±0.2 kPa; P<0.05), with significant differences in the serum cholesterol (mean, 5.4±0.2 vs. 6.0±0.2 mmol/L; P<0.05) and BMI (mean, 21.1±0.6 vs. 23.9±0.6 kg/m2; P<0.05) [210]. Synbiotic supplementation was also associated with a greater reduction in fibrosis among Iranian lean NAFLD subjects who underwent lifestyle modification (mean change±standard error, -1.71±0.25 vs. -0.71±0.18 kPa; P<0.001) [208]. A recent U.K. phase II RCT showed that the administration of synbiotics without lifestyle intervention altered the fecal microbiome, with increased proportions of Bifidobacterium and Faecalibacterium and reductions in Oscillibacter and Alistipes, compared with baseline [33]. However, such did not significantly improve magnetic resonance imaging–based liver fat content or indirect markers of liver fibrosis. Finally, a recent meta-analysis involving 28 clinical trials enrolling 1,555 patients with NAFLD revealed that syn-/probiotic therapy had beneficial effects on BMI (mean difference, -1.46; 95% CI, -2.44 to -0.48; I2=97%; P<0.001), ALT (mean difference, -13.40; 95% CI, -17.03 to -9.77; I2=94%; P<0.001), AST (mean difference, -13.54; 95% CI, -17.86 to -9.22; I2=96%; P<0.001), HOMA-IR (mean difference, -0.42; 95% CI, -0.73 to -0.12; I2=79%; P=0.007), and total cholesterol (mean difference, -15.38; 95% CI, -26.50 to -4.25; I2=93%; P=0.007) levels [212].
Collectively, probiotic supplementation can be used as a complementary approach for managing patients with NAFLD, especially in combination with lifestyle interventions. However, the identification of appropriate bacterial strains and proper duration of treatment as well as potential interactions with other targeted agents require further investigation.
Liver cirrhosis
Data exist on the beneficial role of probiotics in treating patients with liver cirrhosis, especially HE. The effect of probiotics in secondary prophylaxis was evaluated in an RCT involving 130 Indian patients who had recovered from HE [213]. The probability of hospitalization for HE was significantly lower in patients treated with 6 months of probiotics compared to others (19.7% vs. 42.2%; HR, 0.45; 95% CI, 0.23–0.87; P=0.02). Significant improvements in CTP score and MELD scores from baseline were observed only in the probiotic group (median [interquartile range]: 8.81 [7.98–9.64] to 7.19 [6.63–7.75], P<0.001 for CTP score; 17.00 [13.60–20.40] to 13.25 [11.88–14.62], P<0.26 for MELD score). Another Indian RCT including 160 patients with minimal HE indicated that probiotics could effectively prevent overt HE (1.2% vs. 19%; HR for control vs. probiotic group, 2.1; 95% CI, 1.31–6.53; P<0.05) [214]. A recent meta-analysis including 14 trials compared probiotics to placebo/no treatment in patients with HE demonstrated that the probiotics group had a significant lower prevalence of incomplete resolution of HE (RR, 0.67; 95% CI, 0.56–0.79) and development of overt HE (RR, 0.29; 95% CI, 0.16–0.51) [215]. The plasma ammonia level was also lower for probiotics-treated patients (mean difference, -8.29 μmol/L; 95% CI, -13.17 to -3.41). However, no difference in mortality was observed (RR, 0.58; 95% CI, 0.23–1.44). Overall, all these studies hold the promise that manipulation of intestinal microbiota may be helpful for the management of HE.
Safety
Probiotics and synbiotics are inexpensive nutritional supplements that are widely available worldwide. Although most clinical trials focused on the beneficial effect of probiotics rather than their safety [216], probiotics may be safe in immunocompetent adults based on a history of safe use of probiotics in foods. However, probiotics have been associated with a higher risk of bacterial or fungal infection in neonates, infants, and critically ill patients [217,218]. In terms of patients with liver disease, all studies reviewed here reported no significant safety issues with the clinical use of supplementation with probiotic microorganisms in this setting.
VITAMIN E
Mechanism of action
Vitamin E (tocopheraol) shows antioxidant activity by scavenging ROS and nitrogen species [219]. Vitamin E also increases the action of antioxidative enzymes like superoxide dismutase, catalase, and glutathione peroxidase (Fig. 1) [220,221]. In addition to its antioxidative effect, vitamin E has anti-fibrotic, anti-inflammatory, and anti-apoptotic effects [222-224]. Vitamin E supplementation inhibits the activation and proliferation of HSCs in acute damage by CCl4 [224]. Vitamin E shows an anti-inflammatory effect through inhibiting cyclo-oxygenase-2 and 5-lipoxygenase–mediated eicosanoids and suppressing NF-κB and Janus kinase (JAK)-signal transducer and activator of transcription protein (STAT) 6 or JAK/STAT3 pathways [222]. Vitamin E also inhibits apoptosis by decreasing the proapoptotic proteins [223].
Clinically beneficial effects
NAFLD
In NAFLD, oxidative stress plays a crucial role in the progression to NASH [225]. Therefore, vitamin E, an antioxidant, has recently been studied a lot in NAFLD. These studies varied in the duration (3 months to 2 years) and dose (100–800 IU/mL) of vitamin E used and are summarized in Table 7. Vitamin E monotherapy or in combination with other agents significantly improved liver enzymes [34,87,226-231]. In an RCT comparing pioglitazone, vitamin E, and placebo, ALT was significantly decreased in the vitamin E treatment group compared to placebo (mean, -37.0 vs. -20.1; P=0.001) [14]. Vitamin E treatment significantly improved the hepatic steatosis assessed by ultrasound compared to placebo (34.9% vs. 18.2%; P=0.038) [226]. Pervez et al. [230] reported that vitamin E supplementation for 12 weeks significantly reduced the fatty liver index (FLI) score compared to placebo (mean change, -12.82 vs. -3.86; P<0.001). Aller et al. [232] showed that treatment with a combination of vitamin E and silymarin for 3 months significantly decreased the FLI (from 86.2 to 76.9; P<0.05). Vitamin E treatment also improved parameters of the fibrotic burden, as reflected by non-invasive fibrosis surrogates, such as the ratio of AST to platelets score (mean, 0.55 to 0.4; P<0.001) [34] and NAFLD fibrosis score (mean, -1.6 to -2.1; P<0.05) [232]. Vitamin E treatment also decreased inflammatory cytokines (IL-6 change: mean, -3.42 vs. -1.56 pg/mL; P<0.001 and TNF-α change: mean, -3.26 vs. -1.15 pg/mL; P=0.001) [230] or chemokines (CCL-2/monocyte chemo-attractant protein 1: from mean 289 to 131; P<0.05) [227] and improved insulin resistance (HOMA-IR change: mean, -0.52 vs. -0.13; P<0.001) [230]. In addition, vitamin E treatment with other agents improved the serum level of adipokine, increasing adiponectin (mean change, +3,808 in UDCA/vitamin E vs. -1,626 in UDCA/placebo vs. -687 ng/mL in placebo/placebo; P<0.03) [55] and decreasing leptin (mean change, -0.48 vs. 2.54; P<0.05) concentrations [231].
Table 7.
Summary of clinical studies in vitamin E
| Study | Etiology | Inclusion criteria | Intervention period | Arms (n) | Age (years) | Outcomes | Study design |
|---|---|---|---|---|---|---|---|
| Harrison et al. [233] (2003) | NAFLD | Histologic diagnosis of NASH | 6 months | Vitamin E 1,000 IU/day and C 1,000 mg/day (23) | 50.2 | · Improvement in fibrosis score (47.8% vs. 40.9%; P=0.005) | RCT |
| Placebo (22) | 52.5 | · No changes in inflammation (P>0.05) | |||||
| · ALT improvement (P=0.007) | |||||||
| Sanyal et al. [279] (2004) | NAFLD | Non-diabetic, non-cirrhotic | 6 months | Vitamin E 400 IU/day (10) | 46±13 | · Vitamin E: Improvement in steatosis (P=0.02), ballooning (P=0.055) and portal fibrosis (P>0.05) | RCT |
| Vitamin E 400 IU/day + pioglitazone 30 mg/day (10) | 47±12 | · Vitamin E + pioglitazone: Improvement in steatosis (P=0.002), ballooning (P=0.01), and portal fibrosis (P>0.05) | |||||
| · Comparison between the two groups: steatosis (P<0.05), ballooning (P>0.05), portal fibrosis (P>0.05) | |||||||
| Ersöz et al. [280] (2005) | NAFLD | Histologically proven NAFLD | 6 months | Vitamin E 600 IU/day and C 500 mg/day (28) | 46.3±9.4 | · ALT change (IU/L) (mean, 91.9 to 39.1, P<0.05 vs. 93.7 to 49.1, P<0.05; P>0.05) | Open-label RCT |
| UDCA 10 mg/kg/day (29) | 47.9±10.6 | ||||||
| Dufour et al. [54] (2006) | NAFLD | Histologic diagnosis of NASH | 6 months | UDCA 12–15 mg/kg/day + vitamin E 800 IU/day (15) | 46±14 | · Decrease in AST and ALT levels (IU/L) (UDCA + vitamin E vs. placebo, P<0.05; UDCA vs. placebo, P>0.05) | Double-blinded RCT |
| UDCA 12–15 mg/kg/day + placebo (18) | 47±12 | · Improvement in steatosis (UDCA + vitamin E vs. placebo, P<0.05; UDCA vs. placebo, P>0.05) | |||||
| Placebo + placebo (15) | 44±14 | · No improvement in inflammation and fibrosis (P>0.05) | |||||
| Balmer et al. [55] (2009) | NAFLD | Histologic diagnosis of NAFLD | 2 years | UDCA 12–15 mg/kg/day + vitamin E 800 IU/day (14) | 47±14 | · Adiponectin level change (ng/mL) (mean, +3,808 vs. -1,626 vs. -687; P<0.03) | Double-blinded RCT |
| UDCA 12–15 mg/kg/day + placebo (14) | 47±12 | ||||||
| Placebo + placebo (13) | 46±13 | ||||||
| Sanyal et al. [14] (2010) | NAFLD | Histologic diagnosis of NASH without diabetes | 96 weeks | Pioglitazone 30 mg/day (80) | 47.0±12.6 | · Improvement in NASH (vitamin E 43% vs. placebo 19%, P=0.001; pioglitazone 34% vs. placebo 19%, P=0.04) | Double-blinded RCT |
| Vitamin E 800 IU/day (84) | 46.6±12.1 | · Improvement in fibrosis (vitamin E 41% vs. placebo 31%, P=0.24; pioglitazone 44% vs. placebo 31%, P=0.12) | |||||
| Placebo (83) | 45.4±11.2 | · Changes in serum aminotransferase level (IU/L) (mean, vitamin E -37.0 vs. placebo -20.1, P=0.001; pioglitazone -40.8 vs. placebo -20.1, P<0.001) | |||||
| Aller et al. [232] (2015) | NAFLD | Histologic diagnosis of NAFLD | 3 months | Silymarin 1,080.6 mg/day + vitamin E 72 mg/day (18) | 47.4±11.2 | · Decrease in fatty liver index (mean, 86.2 to 76.9; P<0.05 vs. 85.2 to 77.5; P<0.05) | RCT |
| Control (18) | 43.75±3.5 | · Decrease in NFS (mean, -1.6 to -2.1; P<0.05 vs. -1.0 to -1.5; P<0.05) | |||||
| Parikh et al. [34] (2016) | NAFLD | Non-diabetic, non-cirrhotic | 52 weeks | Vitamin E 800 IU/day (100) | 40.19±2.9 | · ALT normalization (14% vs. 19%; P=0.2) | Open-label RCT |
| UDCA 600 mg/day (150) | · ALT reduction (56% vs. 63%; P=0.2) | ||||||
| Polyzos et al. [229] (2017) | NAFLD | Histologic diagnosis of NASH | 52 weeks | Vitamin E 800 IU/day + spironolactone 25 mg/day (14) | 54.9±1.8 | · NFS reduction (44% vs. 47%; P=0.69) | Open-label RCT |
| Vitamin E 800 IU/day (17) | 53.8±3.4 | · ALT reduction (IU/L) (43.5 to 40.0, P>0.05 vs. 66.0 to 42.1, P>0.05; P>0.05) | |||||
| Bril et al. [237] (2019) | NAFLD | Histologic diagnosis of NASH and type 2 diabetes | 18 months | Vitamin E 800 IU/day (36) | 60±9 | · NAFLD liver fat score reduction (P=0.028 vs. P=0.61) | Double-blinded RCT |
| Vitamin E 800 IU/day + pioglitazone 30-45 mg/day (37) | 60±6 | · Reduction of NAS (vitamin E 31% vs. placebo 19%, P=0.26; vitamin E + pioglitazone 54% vs. placebo 19%, P=0.003) | |||||
| Placebo (32) | 57±11 | · Resolution of NASH (vitamin E 33% vs. placebo 12%, P=0.04; vitamin E + pioglitazone 43% vs. placebo 12%, P=0.005) | |||||
| Fouda et al. [227] (2021) | NAFLD | Histologic diagnosis of NASH | 3 months | Vitamin E 800 IU/day (34) | 44.8±9.7 | · Fibrosis change (vitamin E 50% vs. placebo 30%, P=0.09; vitamin E + pioglitazone 52% vs. placebo 30%, P=0.07) | Single-blind RCT |
| UDCA 500 mg/day (34) | 43.4±11 | · ALT reduction (P<0.05) | |||||
| Pentoxifylline 800 mg/day (34) | 45.2±11 | · Inflammatory cytokine reduction (IL-6, CCL-2/MCP-1) (P<0.05) | |||||
| Kedarisetty et al. [228] (2021) | NAFLD | Histologic diagnosis of NASH | 1 year | Vitamin E 800 IU/day (33) | 35 (16–64) | · ALT reduction (IU/L) (mean 85.5 to 28 vs. 97 to 24; P=0.23) | Open-label RCT |
| Vitamin E 800 IU/day + pentoxifylline 1,200 mg/day (36) | 40 (20–64) | · NAS change (mean 4.3 to 3.1 vs. 5 to 2.8; P=0.45) | |||||
| · Fibrosis stage change (mean 1.7 to 1.7 vs. 2.1 to 1.0; P=0.004) | |||||||
| · Insulin change (mU/L) (mean 12.4 to 10.8 vs. 12.9 to 7.6; P=0.048) | |||||||
| · TNF-α change (pg/mL) (mean 7.14 to 3.83 vs. 7.85 to 1.59; P=0.001) | |||||||
| Groenbaek et al. [248] (2006) | HCV | Elevated ALT | 6 months | Vitamin C 500 mg/day + vitamin E 945 IU/day + selenium 200 µg/day (12) | 45 (33–53) | · Change in serum ALT (IU/L) (mean, -8 vs. -6; P=0.60) | Double-blinded RCT |
| Placebo (11) | 45 (23–55) | · Change in HCV RNA (log10 eqv/L) (mean, 0.17 vs. 0.41; P=0.24) | |||||
| Marotta et al. [246] (2007) | HCV | Cirrhosis, genotype 1, and elevated ALT | 6 months | Vitamin E 900 IU/day (25) | 62 (54–75) | · Improvement of redox status, GSH, GSSG, GSH/GSSG and MDA: vitamin E (P<0.05) and FPP (P<0.05) | RCT |
| Fermented papaya preparation 9 g/day (25) | |||||||
| Control (10) | |||||||
| Bunchorntavakul et al. [249] (2014) | HCV | Genotype 3 | 12 weeks | Vitamin E 800 IU/day (19) | 48.8±8.3 | · Decrease in serum ALT (mean, 105.1 to 96.5; P=0.260 vs. 107.5 to 120.4) | Double- blinded RCT |
| Placebo (18) | 49.5±8.6 | · ALT responder (57.8% vs. 29.4%; P=0.106) | |||||
| Malaguarnera et al. [245] (2015) | HCV | Received PegIFN- α2b + ribavirin | 12 months | Silybin 47 mg/day + vitamin E 15 mg/day + phospholipid 97 mg/day (32) | 46.4±6.9 | · Decrease in ALT level (IU/L) (mean, 170.2 to 36.9, P<0.001 vs. 161.6 to 69.2, P<0.001; P<0.001) | Double- blinded RCT |
| · Decrease in viremia (106 IU/mL) (mean, 5.32 to 1.67, P<0.05 vs. 5.4 to 3.24, P<0.001; P<0.05) | |||||||
| Placebo (32) | 45.2±6.7 | · Decrease in TGF-β (ng/mL) (mean, 54.2 to 32.8, P<0.05 vs. 51.8 to 45.2, P<0.05; P<0.05) | |||||
| · Decrease in PIIINP (ng/mL) (mean, 43.8 to 33.4, P<0.001 vs. 44.7 to 39.8, P<0.05; P<0.05) | |||||||
| · Decrease in TIMP-1 (ng/mL) (mean, 480.2 to 310.6, P<0.001 vs. 487.2 to 421.0, P<0.001; P<0.001) | |||||||
| Andreone et al. [251] (2001) | HBV | Positive HBV DNA and raised ALT (1.5×ULN) | 3 months | Vitamin E 600 IU/day (15) | 37±15 | · ALT normalization (47% vs. 6%; P=0.011) | RCT |
| Control (17) | 42±14 | · Negative HBV DNA (53% and 18%; P=0.039) | |||||
| · Complete response (47% vs. 0%; P=0.0019) |
Variables are expressed as median (interquartile range) or mean±standard deviation.
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; ALT, alanine aminotransferase; RCT, randomized controlled trial; UDCA, ursodeoxycholic acid; AST, aspartate aminotransferase; NFS, NAFLD fibrosis score; IL-6, interleukin-6; CCL2, C-C motif ligand 2; MCP, monocyte chemoattractant protein; NAS, NAFLD activity score; TNF, tumor necrosis factor; HCV, hepatitis C virus; GSH, glutathione; GSSG, glutathione disulfide, MDA, malondialdehyde; FPP, fermented papaya preparation; PegIFN, pegylated interferon; TGF, transforming growth factor; PIIINP, pro-collagen III N-terminal peptide; TIMP, tissue inhibitor of metalloproteinase; HBV, hepatitis B virus; ULN, upper limit of normal.
There have been studies showing that vitamin E treatment led to histologic improvement in NAFLD. Harrison et al. [233] reported that treatment with a combination of vitamin E (1,000 IU/day) and vitamin C (1,000 IU/day) for 6 months significantly improved the hepatic fibrosis score (P=0.002) in histologically proven NASH, while there was no significant change in inflammation score (P>0.05). Dufour et al. [54] showed that treatment with a combination of vitamin E (800 IU/day) and UDCA (12–15 mg/kg/day) for 6 months significantly improved hepatic steatosis (P<0.05), while there was no significant change in inflammation or fibrosis. In the PIVENS (Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis) trial of pioglitazone or vitamin E for the treatment of biopsy-confirmed NASH patients without diabetes, vitamin E treatment (800 IU/day) for 96 weeks, compared to placebo, significantly improved the inflammation (43% vs. 19%; P=0.001) [14]. Histologic analysis revealed that vitamin E treatment, compared to placebo, reduced the steatosis (54% vs. 31%; P=0.005), lobular inflammation (54% vs. 35%; P=0.02), and hepatocellular ballooning (50% vs. 29%; P=0.01), but fibrosis was not significantly improved (41% vs. 31%; P=0.24) [14]. Based on the PIVENS trial, guidelines for NAFLD of major societies recommend vitamin E as a treatment in non-diabetic adults with biopsy-proven NASH [4,234-236]. Recently, Bril et al. [237] also reported that vitamin E (800 IU/day) for 18 months achieved a resolution of NASH (33% vs. 12%; P=0.04) compared to placebo in adult patients with diabetes and biopsyproven NASH. In this study, the improvement of fibrosis was not statistically significant (50% vs. 30%; P=0.09), but it showed a trend toward a higher rate in vitamin E treatment [237].
A meta-analysis of histological changes after vitamin E treatment in adult NASH patients revealed significant improvements in steatosis, lobular inflammation, and hepatocellular ballooning [238,239]. However, the results of meta-analyses on fibrosis changes after vitamin E were not consistent. Said and Akhter [239] reported that vitamin E treatment did not significantly improve fibrosis (P=0.09), while Vadarlis et al. [238] reported that vitamin E treatment significantly improved fibrosis (P=0.005). Meanwhile, a network analysis of studies that assessed the effect of different pharmacological interventions on NASH demonstrated that vitamin E achieved a significant fibrosis improvement compared to placebo (OR, 1.72; 95% CI, 1.04–2.85) [240]. Therefore, vitamin E may be an effective treatment in biopsy-proven NASH by improving the steatosis and inflammation. Considering the conflicting results on fibrosis, however, further studies are needed to determine whether vitamin E would improve hepatic fibrosis.
Viral hepatitis
HCV infection is associated with systemic oxidative stress, which is characterized by increased ROS and nitrogen species and decreased antioxidants [241]. Oxidative stress in HCV patients occurs in the early phase of disease [241,242] and is associated with the progression of fibrosis and development of HCC [242,243]. Because of the association between HCV infection and oxidative stress, vitamin E has been studied in HCV patients as an antioxidant (Table 7). In a double-blinded RCT with HCV patients refractory to IFN-α therapy by von Herbay et al. [244], vitamin E treatment (800 IU/day) for 12 weeks significantly lowered serum ALT levels (mean ALT change, -22 vs. +1; P<0.001) compared to placebo. Malaguarnera et al. [245] showed that addition of a complex of antioxidants, including vitamin E (15 mg/day), silybin (47 mg/day), and phospholipids (97 mg/day), to IFN-α2b plus ribavirin led to a significantly greater reduction in viral load (mean, -3.65×106 vs. -2.16×106 IU/mL; P<0.05) and serum levels of hepatic fibrosis markers, such as TGF-β (mean, -21.4 vs. -6.6 ng/mL; P<0.05), pro-collagen III N-terminal peptide (mean, -10.4 vs. -4.9 ng/mL; P<0.05), and tissue inhibitor of metalloproteinase 1 (mean, -169.6 vs. -66.2 ng/mL; P<0.001). Marotta et al. [246] showed that vitamin E treatment in HCV-related cirrhosis patients improved parameters of the redox status, such as glutathione, glutathione disulfide (GSSG), glutathione/GSSG, and malondialdehyde (all P<0.005). However, other studies failed to reveal an improvement in serum ALT level [247-249] or viral load [248]. Furthermore, cessation of vitamin E treatment was followed by a rapid relapse of ALT elevation and viremia [244,250].
Most of the studies on the effects of vitamin E in HCV patients were conducted during a period in which IFN-α treatment was the standard treatment for HCV, and their results are also inconsistent. In recent years, as DAAs, which are very effective treatments, have become the standard therapeutic approach for HCV, further studies are needed on the role of vitamin E treatment in HCV patients in the DAA era.
Studies on the effectiveness of vitamin E treatment in HBV patients are very rare. Andreone et al. [251] reported that ALT normalization (47% vs. 6%; P=0.011) and a complete response (47% vs. 0%; P=0.0019) were achieved at a significantly higher rate following vitamin E treatment (600 IU/day) for 3 months compared to in the control group among patients with a positive HBV DNA status and heightened ALT levels.
Vitamin E treatment decreased the serum aminotransferase level and improved inflammatory cytokine, chemokine, and adipokine concentrations in NAFLD patients with its antioxidant and anti-inflammatory properties. In addition, vitamin E treatment not only led to histological improvement in hepatic steatosis, lobular inflammation, and ballooning but also showed a potential to improve hepatic fibrosis in histologically proven NASH patients. Therefore, vitamin E may be an alternative treatment option for NASH patients who have no effective treatment other than weight loss. On the other side, the usefulness of vitamin E has been reduced due to the use of effective antiviral agents in chronic viral hepatitis, and further studies are necessary on the role of vitamin E treatment.
Safety
There are concerns about the long-term safety of vitamin E treatment. Some studies reported that vitamin E treatment is associated with an increase in overall mortality [252,253], prostate cancer [254], and hemorrhagic stroke [255]. In a meta-analysis of preventive studies of antioxidant supplements, vitamin E (duration, 0.5–6.3 years; dose, 16.5–800 IU/day) was significantly associated with increased mortality (RR, 1.04; 95% CI, 1.01–1.07) [253]. In another meta-analysis of trials, high-dose vitamin E (≥400 IU/day) for >1 year led to an increased risk for all-cause mortality (P=0.022), and there was a significant relationship between vitamin E dosage and all-cause mortality (P=0.027) [252]. In a prospective study comparing the long-term effect of selenium (200 μg/day), vitamin E (400 IU/day), selenium plus vitamin E, and placebo, vitamin E treatment (follow-up period, 7–12 years) increased the risk of prostate cancer compared to placebo (HR, 1.17; 99% CI, 1.004–1.36; P=0.008) [254]. A meta-analysis of RCTs with ≥1 year of followup investigating the effect of vitamin E (50–800 IU/day) on stroke showed that vitamin E increased the risk for hemorrhagic stroke (RR, 1.22; 95% CI, 1.00–1.48; P=0.048) [255]. Therefore, long-term use of vitamin E should be avoided.
ASPIRIN
Mechanism of action
Following tissue injury, platelet activation and degranulation mediate the normal physiological tissue repair process. However, in chronic inflammation, over-activation of the platelet can cause fibrosis in various tissues [256]. In the liver, platelets could directly activate HSCs [257] or could interact with many effector cells, such as macrophages, cytotoxic T-cells, and natural killer T (NKT) cells, eventually causing hepatic fibrosis [258]. Aspirin could prevent hepatic fibrosis progression and HCC directly by blocking platelet function (Fig. 1).
Extrahepatic platelet–derived growth factor β produced by platelets may have directly activated HSCs in a biliary fibrosis model [257]. Platelet number and activation were increased in a NASH model. In the same study, a platelet receptor subunit, glycoprotein 1b alpha (GP1bα), was an important mediator in NASH. The combination of GP1bα, hyaluronic acids, cytokines, and chemokines mediates the immune response, causing progression to NASH and HCC [259]. The crosstalk between cytotoxic T-cells, NKT cells, and hepatocytes can cause liver disease progression. Hepatocyte-driven lymphotoxin β receptor and NF-κB signaling could trigger NASH to HCC progression [258]. Prolyl 4-hydroxylase subunit α2 inhibition by aspirin could also decrease collagen deposition and HCC development [260].
In a viral hepatitis model, platelets mediated cytotoxic T-cell-mediated liver damage. Aspirin treatment reduced the platelet activation, and intrahepatic cytokine release related in the inflammation process [27]. Aspirin treatment also reduces the homing and accumulation of virus specific cytotoxic Tcells as well as virus-non-specific lymphocytes in the liver [261].
Clinically beneficial effects
NAFLD
A nationwide cross-sectional study in the United States showed that regular aspirin use was associated with a lower prevalence of NAFLD itself (HR, 0.62; 95% CI, 0.51–0.74, P=0.04) (Table 8) [262]. A prospective cohort study with biopsyconfirmed NAFLD patients showed that aspirin use reduced the risk of advanced fibrosis (HR, 0.63; 95% CI, 0.43–0.85) [263]. Another cross-sectional study using nationwide health survey data from the United States showed that regular aspirin use was associated with a decreased risk of liver fibrosis in CLD patients confirmed by ultrasonography (β-coefficients measured at 0.24 standard deviations lower; 95% CI, -0.42 to -0.06; P=0.009) [256]. To date, two studies have shown the association between aspirin use and the risk of HCC development. In a pooled analysis of 10 prospective cohort studies in the United States, aspirin led to a 32% decrease in HCC development (HR, 0.68; 95% CI, 0.57–0.81) [264]. Meanwhile, in another pooled analysis of two prospective cohort studies in the United States, aspirin caused a 46% decrease in HCC development (HR, 0.54; 95% CI, 0.36–0.80) [265]. Although both pooled analysis studies did not specify the etiology of liver disease, it is assumed that NAFLD may be the major etiology of liver disease.
Table 8.
Summary of clinical studies in aspirin
| Study | Etiology | Inclusion criteria | Intervention period | Arms (n) | Age (years) | Outcomes | Study design |
|---|---|---|---|---|---|---|---|
| Simon et al. [265] (2018) | Variable | Pooled analysis of cohort | NA | Aspirin (58,855) | 64±8 | · Decreased HCC development in aspirin group (HR, 0.54; 95% CI, 0.36–0.80) | Prospective cohort study |
| Control (74,516) | 62±8 | ||||||
| Petrick et al. [264] (2015) | Variable | Pooled analysis of cohort | NA | Aspirin (477,470) | NA | · Decreased HCC development in aspirin group (HR, 0.68; 95% CI, 0.57–0.81) | Prospective cohort study |
| Control (606,663) | NA | ||||||
| Simon et al. [263] (2019) | NAFLD | Biopsy confirmed NAFLD | NA | Aspirin (151) | 59.9±8.6 | · Decreased prevalence of advanced fibrosis in aspirin group (HR, 0.63; 95% CI, 0.43–0.85) | Prospective cohort study |
| Control (210) | 48.2±13.5 | ||||||
| Shen et al. [262] (2014) | NAFLD | US-confirmed NAFLD | 1 month | NAFLD (2,889) | 54.6±0.3 | · Decreased NAFLD prevalence in aspirin group (HR, 0.62; 95% CI, 0.51–0.74) | Cross-sectional retrospective study |
| Control (8,527) | 48.7±0.5 | ||||||
| Jiang et al. [256] (2016) | CLD | US-confirmed CLD | 1 month | Aspirin (520) | 46.6±15.4 | · Decreased non-invasive fibrosis index in apsirin group, 0.24 standard deviation lower; 95% CI, -0.42 to 0.06 | Cross-sectional retrospective study |
| Control (1,336) | 43.2±14.7 | ||||||
| Hui et al. [271] (2021) | CHB | Receiving nucleos(t)ide analog | Over 90 days | Aspirin (1,744) | 62.2±10.8 | · Decreased HCC development in aspirin group (HR, 0.60; 95% CI, 0.46–0.78) | Retrospective cohort study |
| Control (33,367) | 52.5±12.5 | ||||||
| Choi et al. [267] (2021) | CHB | Over 90 days | Aspirin (7,718) | NA | · Decreased HCC development in aspirin group (OR, 0.92; 95% CI, 0.85–0.99) | Retrospective cohort study | |
| Control (24,977) | NA | ||||||
| Simon et al. [15] (2020) | Viral hepatitis | CHB, CHC monoinfection | Over 90 days | Aspirin (14,205) | 50.5±13.0 | · Decreased HCC development in aspirin group (HR, 0.69; 95% CI, 0.62–0.76) | Prospective cohort study |
| Control (36,070) | 39.6±13.5 | · Decreased liver-related death in aspirin group (HR, 0.73; 95% CI, 0.67–0.81) | |||||
| Liao et al. [270] (2020) | CHC | NA | Aspirin (1,991) | NA | · Decreased HCC development in aspirin group (HR, 0.56; 95% CI, 0.43–0.72) | Retrospective cohort study | |
| Control (1,991) | NA | ||||||
| Lee et al. [268] (2020) | CHC | Over 90 days | Aspirin (2,478) | 63.2±10.0 | · Decreased HCC development in aspirin group (HR, 0.78; 95% CI, 0.64–0.95) | Retrospective cohort study | |
| Control (4,956) | 63.2±10.0 | ||||||
| Lee et al. [269] (2019) | CHB | Over 90 days | Aspirin (2,123) | 58.9±11.8 | · Decreased HCC development in aspirin group (HR, 0.71; 95% CI, 0.58–0.86) | Retrospective cohort study | |
| Control (8,492) | 58.8±11.8 | ||||||
| Lee et al. [276] (2017) | CHB | Low-level viremia | Median 38.5 months | Aspirin (343) | 54.2±11.1 | · Decreased HCC development in aspirin group (HR, 0.26; 95% CI, 0.09–0.74) | Retrospective cohort study |
| Control (1,116) | 50.3±10.8 |
Variables are expressed as median (interquartile range) or mean±standard deviation.
NA, not applicable; HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; NAFLD, nonalcoholic fatty liver disease; US, ultrasonography; CLD, chronic liver disease; CHB, chronic hepatitis B; OR, odds ratio; CHC, chronic hepatitis C.
In NAFLD patients, aspirin use was associated with various outcomes, such as prevalence, fibrosis, and HCC development. However, there are still very few studies considering the outcomes of aspirin use in NAFLD. Since it is difficult to perform randomized controlled studies with aspirin, there is an unmet need for more cohort studies involving NAFLD patients.
Viral hepatitis
Most studies performed in viral hepatitis analyzed HCC development as primary outcome (Table 8). A recent metaanalysis of seven studies that studied CHB or CHC patients showed a 27% decrease in HCC development [266]. A prospective study using Swedish nationwide registry data including CHB or CHC patients with median 7.9 years of follow-up revealed a 31% decrease in HCC development in the aspirin group (HR, 0.69; 95% CI, 0.62–0.76). The reduction in HCC development was duration-dependent [15]. The authors also evaluated survival data and showed a 27% reduction in liver-related death in the aspirin treatment group (HR, 0.73; 95% CI, 0.67–0.81) [15]. In addition, several population-based studies have been made available, mostly enrolling the Asian population. A Korean study using nationwide reimbursement data showed an 8% decrease in HCC development (OR, 0.92; 95% CI, 0.85–0.99) [267]. Two Taiwan studies using nationwide reimbursement data revealed a 29% decrease (HR, 0.71; 95% CI, 0.58–0.86; P<0.001) and a 22% decrease (HR, 0.78; 95% CI, 0.64–0.95; P=0.011), respectively, in HCC development in CHB and CHC patients [268,269]. Another Taiwan study using nationwide reimbursement data compared aspirin using CHC patients matched 1:1 with aspirin non-using CHC patients. The result included a 44% rate of HCC reduction (HR, 0.56; 95% CI, 0.43–0.72; P<0.001) [270]. A Hong Kong study using a nationwide electronic healthcare data repository analyzed antiviral-treated CHB patients and showed a 40% decrease in HCC development (HR, 0.60; 95% CI, 0.46–0.78, P<0.001) [271].
In addition, several studies have shown the beneficial effects of aspirin use in prolonging OS in HCC patients treated with transarterial chemoembolization and curative resection [272,273]. In addition, it has been revealed that aspirin use can reduce HCC recurrence in several clinical settings [272,274,275].
Collectively, in patients with viral hepatitis, aspirin seems to reduce the risk of HCC development. However, few studies have adopted liver fibrosis or OS as their primary endpoints, and most studies have enrolled population-based cohorts. Accordingly, further prospective studies based on hospital data with varying endpoints are warranted for solid validation.
Safety
The safety concern related to aspirin therapy is mainly gastrointestinal bleeding. Most studies did not report an increased risk of gastrointestinal bleeding in the aspirin group [15,269,276]. However, one study reported increased peptic ulcer bleeding in cirrhosis patients with aspirin use compared to aspirin-untreated patients [268]. Another study reported increased gastrointestinal bleeding in patients only with shortterm aspirin use [271].
CONCLUSION
In this review, we summarized the mechanisms of several non-antidiabetics and their evidence regarding a beneficial effect in patients with CLD. Although the evidence is not sufficiently solid, the different nonanti-diabetics showed beneficial effects in improving histology, aminotransferase level, metabolic parameters, and the risk of long-term outcomes in patients with CLD without significant safety concerns (Fig. 3). However, further studies are still warranted to consolidate their potential benefit in adjuvant or combination settings in the era of potent antiviral therapy.
Figure 3.
Clinically beneficial effects of non-antidiabetic drugs in chronic liver diseases. UDCA, ursodeoxycholic acid; LOLA, L-ornithine L-aspartate; DDB, dimethyl-4,4’-dimethoxy-5,6,5’,6’-dimethylenedixoybiphenyl-2,2’-dicarboxylate; HCC, hepatocellular carcinoma.
Acknowledgments
This study was supported by the Technology Innovation Program (20013712) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea), the Basic Science Research Program through a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2021R1C1C1005844 to P.S.S.), and the Soonchunhyang University Research Fund.
We would like to thank Editage (www.editage.co.kr) for English language editing.
Abbreviations
- ALT
alanine aminotransferase
- AMPK
adenosine monophosphate-activated protein kinase
- AST
aspartate aminotransferase
- BCAA
branched chain amino acid
- Bcl2
B-cell lymphoma 2
- BMI
body mass index
- CCl4
carbon tetrachloride
- CHB
chronic hepatitis B
- CHC
chronic hepatitis C
- CI
confidence interval
- CLD
chronic liver disease
- CTP
Child-Turcotte-Pugh
- DAA
direct-acting antiviral
- DDB
dimethyl-4
- FFA
free fatty acid
- FLI
fatty liver index
- FXR
farnesoid X receptor
- GGT
γ-glutamyl transferase
- GLUT
glucose transporter
- GP1bα
glycoprotein 1bα
- GSSG
glutathione disulfide
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HD-UDCA
high-dose UDCA
- HE
hepatic encephalopathy
- HMG-CoA
hydroxymethylglutaryl coenzyme A
- HOMA-IR
Homeostatic Model Assessment for Insulin Resistance
- HR
hazard ratio
- HSC
hepatic stellate cell
- HVPG
hepatic venous pressure gradient
- IFN
interferon
- IL-6
interleukin-6
- IRR
incident risk ratio
- JAK
Janus kinase
- LDL
low-density lipoprotein
- LOLA
L-ornithine L-aspartate
- LPS
lipopolysaccharides
- LSEC
liver sinusoidal endothelial cell
- MELD
Model for End-stage Liver Disease
- mTOR
FULL NAME
- NAD+
nicotinamide adenine dinucleotide+
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
nonalcoholic steatohepatitis
- NF-κB
nuclear factor kappa-B
- NKT
natural killer T
- nor-UDCA
norursodeoxycholic acid
- NRF2
nuclear factor erythroid 2-related factor 2
- OR
odds ratio
- OS
overall survival
- PBC
primary biliary cholangitis
- PI3K
phosphatidylinositol 3-kinase
- PIVENS
Pioglitazone
- PPAR
peroxisome proliferator-activated receptor
- RCT
randomized controlled trial
- ROS
reactive oxygen species
- RR
risk ratio
- STAT
signal transducer and activator of transcription protein
- SVR
sustained virologic response
- TGF
tumor growth factor
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- UDCA
ursodeoxycholic acid
Footnotes
Authors’ contributions
Conception and design: S.U. Kim and Y.J. Kim; Writing, review, and/or revision of the manuscript: H.A. Lee, Y. Chang, P.S. Sung, E.L. Yoon, H.W. Lee, J. Yoo, Y. Lee, J. An, D.S. Song, Y.Y. Cho, S.U. Kim, and Y.J. Kim.
Conflicts of Interest
Seung Up Kim has served as an advisory committee member for Gilead Sciences, GSK, Bayer, Novo Nordisk, and Eisai. He is a speaker for Gilead Sciences, GSK, Bayer, Eisai, Abbive, EchoSens, MSD, Bristol-Myers Squibb, Hanhwa, Yuhan, Samil, PharmaKing, Celltrion, and Bukwang. He has also received research grants from Abbive and Bristol-Myers Squibb.
Yoon Jun Kim has received research grant support from BTG, Boston Scientific, AstraZeneca, Gilead Sciences, Samjin, BL&H, and Bayer. He has served as an advisory committee member of Bayer, BMS, Samil, PharmaKing, Celltrion, and Bukwang. He is also a speaker for Abbvie, Ipsen, Eisai, Boston Scientific, BMS, BTG, Bayer, MSD, Gilead, Novo Nordisk Green Cross Cell, Boehringer Ingelheim, AstraZeneca, BL&H, and Gilead Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Other authors have no conflicts to disclose.
REFERENCES
- 1.Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650–2666. doi: 10.1016/j.cgh.2019.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Kang SH, Lee HW, Yoo JJ, Cho Y, Kim SU, Lee TH, et al. KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2021;27:363–401. doi: 10.3350/cmh.2021.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korean Association for the Study of the Liver (KASL) KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 2019;25:93–159. doi: 10.3350/cmh.2019.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mbaga DS, Kenmoe S, Kengne-Ndé C, Ebogo-Belobo JT, Mahamat G, Foe-Essomba JR, et al. Hepatitis B, C and D virus infections and risk of hepatocellular carcinoma in Africa: a meta-analysis including sensitivity analyses for studies comparable for confounders. PLoS One. 2022;17:e0262903. doi: 10.1371/journal.pone.0262903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, et al. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25:52–64. doi: 10.3350/cmh.2018.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 9.Navarro VJ, Belle SH, D’Amato M, Adfhal N, Brunt EM, Fried MW, et al. Silymarin in non-cirrhotics with non-alcoholic steatohepatitis: a randomized, double-blind, placebo controlled trial. PLoS One. 2019;14:e0221683. doi: 10.1371/journal.pone.0221683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SH, Cheon GJ, Kim HS, Kim YD, Kim SG, Kim YS, et al. Comparison on the efficacy and safety of biphenyl dimethyl dicarboxylate and ursodeoxycholic acid in patients with abnormal alanine aminotransferase: multicenter, double-blinded, randomized, active-controlled clinical trial. Korean J Gastroenterol. 2014;64:31–39. doi: 10.4166/kjg.2014.64.1.31. [DOI] [PubMed] [Google Scholar]
- 11.Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, et al. Effects of oral branched-chain amino acid granules on eventfree survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705–713. doi: 10.1016/s1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- 12.Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomized placebo-controlled trial. J Clin Gastroenterol. 2009;43:990–994. doi: 10.1097/MCG.0b013e31819c392e. [DOI] [PubMed] [Google Scholar]
- 13.Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebocontrolled pilot study. Am J Clin Nutr. 2014;99:535–542. doi: 10.3945/ajcn.113.068890. [DOI] [PubMed] [Google Scholar]
- 14.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon TG, Duberg AS, Aleman S, Chung RT, Chan AT, Ludvigsson JF. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N Engl J Med. 2020;382:1018–1028. doi: 10.1056/NEJMoa1912035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poupon R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: an overview of their mechanisms of action. Clin Res Hepatol Gastroenterol. 2012;36 Suppl 1:S3–S12. doi: 10.1016/S2210-7401(12)70015-3. [DOI] [PubMed] [Google Scholar]
- 17.Roma MG, Toledo FD, Boaglio AC, Basiglio CL, Crocenzi FA, Sánchez Pozzi EJ. Ursodeoxycholic acid in cholestasis: linking action mechanisms to therapeutic applications. Clin Sci (Lond) 2011;121:523–544. doi: 10.1042/CS20110184. [DOI] [PubMed] [Google Scholar]
- 18.Surai PF. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants (Basel) 2015;4:204–247. doi: 10.3390/antiox4010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu T, Liu G. Protective effects of dimethyl-4,4’-dimethoxy-5,6,5’,6’-dimethylene dioxybiphenyl-2,2’-dicarboxylate on damages of isolated rat hepatocytes induced by carbon tetrachloride and D-galactosamine. Biomed Environ Sci. 1992;5:185–194. [PubMed] [Google Scholar]
- 20.De Chiara F, Heebøll S, Marrone G, Montoliu C, Hamilton-Dutoit S, Ferrandez A, et al. Urea cycle dysregulation in non-alcoholic fatty liver disease. J Hepatol. 2018;69:905–915. doi: 10.1016/j.jhep.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 21.Thomsen KL, De Chiara F, Rombouts K, Vilstrup H, Andreola F, Mookerjee RP, et al. Ammonia: a novel target for the treatment of non-alcoholic steatohepatitis. Med Hypotheses. 2018;113:91–97. doi: 10.1016/j.mehy.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Kimball SR, Jefferson LS. New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr. 2006;83:500S–507S. doi: 10.1093/ajcn/83.2.500S. [DOI] [PubMed] [Google Scholar]
- 23.Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US preventive services task force. JAMA. 2016;316:2008–2024. doi: 10.1001/jama.2015.15629. [DOI] [PubMed] [Google Scholar]
- 24.Jain A, Sharma BC, Mahajan B, Srivastava S, Kumar A, Sachdeva S, et al. L-ornithine L-aspartate in acute treatment of severe hepatic encephalopathy: a double-blind randomized controlled trial. Hepatology. 2022;75:1194–1203. doi: 10.1002/hep.32255. [DOI] [PubMed] [Google Scholar]
- 25.Porras D, Nistal E, Martínez-Flórez S, González-Gallego J, García-Mediavilla MV, Sánchez-Campos S. Intestinal microbiota modulation in obesity-related non-alcoholic fatty liver disease. Front Physiol. 2018;9:1813. doi: 10.3389/fphys.2018.01813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Hai J, Cao M, Zhang Y, Pei S, Wang J, et al. Silibinin ameliorates steatosis and insulin resistance during non-alcoholic fatty liver disease development partly through targeting IRS-1/PI3K/Akt pathway. Int Immunopharmacol. 2013;17:714–720. doi: 10.1016/j.intimp.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, et al. Platelets mediate cytotoxic T lymphocyteinduced liver damage. Nat Med. 2005;11:1167–1169. doi: 10.1038/nm1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hajiaghamohammadi AA, Ziaee A, Oveisi S, Masroor H. Effects of metformin, pioglitazone, and silymarin treatment on nonalcoholic fatty liver disease: a randomized controlled pilot study. Hepat Mon. 2012;12:e6099. doi: 10.5812/hepatmon.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeshita Y, Takamura T, Kita Y, Ando H, Ueda T, Kato K, et al. Beneficial effect of branched-chain amino acid supplementation on glycemic control in chronic hepatitis C patients with insulin resistance: implications for type 2 diabetes. Metabolism. 2012;61:1388–1394. doi: 10.1016/j.metabol.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57:545–553. doi: 10.1007/s10620-011-1887-4. [DOI] [PubMed] [Google Scholar]
- 31.Wah Kheong C, Nik Mustapha NR, Mahadeva S. A randomized trial of silymarin for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2017;15:1940–1949.e8. doi: 10.1016/j.cgh.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Les I, Doval E, García-Martínez R, Planas M, Cárdenas G, Gómez P, et al. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol. 2011;106:1081–1088. doi: 10.1038/ajg.2011.9. [DOI] [PubMed] [Google Scholar]
- 33.Scorletti E, Afolabi PR, Miles EA, Smith DE, Almehmadi A, Alshathry A, et al. Synbiotics alter fecal microbiomes, but not liver fat or fibrosis, in a randomized trial of patients with nonalcoholic fatty liver disease. Gastroenterology. 2020;158:1597–1610.e7. doi: 10.1053/j.gastro.2020.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parikh P, Ingle M, Patel J, Bhate P, Pandey V, Sawant P. An openlabel randomized control study to compare the efficacy of vitamin E versus ursodeoxycholic acid in nondiabetic and noncirrhotic Indian NAFLD patients. Saudi J Gastroenterol. 2016;22:192–197. doi: 10.4103/1319-3767.182451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394–419. doi: 10.1002/hep.30145. [DOI] [PubMed] [Google Scholar]
- 36.Leuschner U, Fischer H, Kurtz W, Güldütuna S, Hübner K, Hellstern A, et al. Ursodeoxycholic acid in primary biliary cirrhosis: results of a controlled double-blind trial. Gastroenterology. 1989;97:1268–1274. doi: 10.1016/0016-5085(89)91698-3. [DOI] [PubMed] [Google Scholar]
- 37.Ikebuchi Y, Shimizu H, Ito K, Yoshikado T, Yamanashi Y, Takada T, et al. Ursodeoxycholic acid stimulates the formation of the bile canalicular network. Biochem Pharmacol. 2012;84:925–935. doi: 10.1016/j.bcp.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, et al. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G735–G747. doi: 10.1152/ajpgi.90321.2008. [DOI] [PubMed] [Google Scholar]
- 39.El-Sherbiny GA, Taye A, Abdel-Raheem IT. Role of ursodeoxycholic acid in prevention of hepatotoxicity caused by amoxicillin-clavulanic acid in rats. Ann Hepatol. 2009;8:134–140. [PubMed] [Google Scholar]
- 40.Yokoyama K, Tatsumi Y, Hayashi K, Goto H, Ishikawa T, Wakusawa S. Effects of ursodeoxycholic acid and insulin on palmitate-induced ROS production and down-regulation of PI3K/Akt signaling activity. Biol Pharm Bull. 2017;40:2001–2004. doi: 10.1248/bpb.b17-00423. [DOI] [PubMed] [Google Scholar]
- 41.Mueller M, Castro RE, Thorell A, Marschall HU, Auer N, Herac M, et al. Ursodeoxycholic acid: effects on hepatic unfolded protein response, apoptosis and oxidative stress in morbidly obese patients. Liver Int. 2018;38:523–531. doi: 10.1111/liv.13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amaral JD, Viana RJ, Ramalho RM, Steer CJ, Rodrigues CM. Bile acids: regulation of apoptosis by ursodeoxycholic acid. J Lipid Res. 2009;50:1721–1734. doi: 10.1194/jlr.R900011-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solá S, Castro RE, Kren BT, Steer CJ, Rodrigues CM. Modulation of nuclear steroid receptors by ursodeoxycholic acid inhibits TGF-beta1-induced E2F-1/p53-mediated apoptosis of rat hepatocytes. Biochemistry. 2004;43:8429–8438. doi: 10.1021/bi049781x. [DOI] [PubMed] [Google Scholar]
- 44.Castro RE, Ferreira DM, Afonso MB, Borralho PM, Machado MV, Cortez-Pinto H, et al. miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non-alcoholic fatty liver disease. J Hepatol. 2013;58:119–125. doi: 10.1016/j.jhep.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Mueller M, Thorell A, Claudel T, Jha P, Koefeler H, Lackner C, et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J Hepatol. 2015;62:1398–1404. doi: 10.1016/j.jhep.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu P, Zhao J, Guo Y, Yu Y, Wu X, Xiao H. Ursodeoxycholic acid alleviates nonalcoholic fatty liver disease by inhibiting apoptosis and improving autophagy via activating AMPK. Biochem Biophys Res Commun. 2020;529:834–838. doi: 10.1016/j.bbrc.2020.05.128. [DOI] [PubMed] [Google Scholar]
- 47.Calmus Y, Gane P, Rouger P, Poupon R. Hepatic expression of class I and class II major histocompatibility complex molecules in primary biliary cirrhosis: effect of ursodeoxycholic acid. Hepatology. 1990;11:12–15. doi: 10.1002/hep.1840110104. [DOI] [PubMed] [Google Scholar]
- 48.Oh AR, Bae JS, Lee J, Shin E, Oh BC, Park SC, et al. Ursodeoxycholic acid decreases age-related adiposity and inflammation in mice. BMB Rep. 2016;49:105–110. doi: 10.5483/BMBRep.2016.49.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miura T, Ouchida R, Yoshikawa N, Okamoto K, Makino Y, Nakamura T, et al. Functional modulation of the glucocorticoid receptor and suppression of NF-kappaB-dependent transcription by ursodeoxycholic acid. J Biol Chem. 2001;276:47371–47378. doi: 10.1074/jbc.M107098200. [DOI] [PubMed] [Google Scholar]
- 50.Takigawa T, Miyazaki H, Kinoshita M, Kawarabayashi N, Nishiyama K, Hatsuse K, et al. Glucocorticoid receptor-dependent immunomodulatory effect of ursodeoxycholic acid on liver lymphocytes in mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G427–G438. doi: 10.1152/ajpgi.00205.2012. [DOI] [PubMed] [Google Scholar]
- 51.Shimoyama S, Kawata K, Ohta K, Chida T, Suzuki T, Tsuneyama K, et al. Ursodeoxycholic acid impairs liver-infiltrating T-cell chemotaxis through IFN-γ and CX3CL1 production in primary biliary cholangitis. Eur J Immunol. 2021;51:1519–1530. doi: 10.1002/eji.202048589. [DOI] [PubMed] [Google Scholar]
- 52.Lee EJ, Kwon JE, Park MJ, Jung KA, Kim DS, Kim EK, et al. Ursodeoxycholic acid attenuates experimental autoimmune arthritis by targeting Th17 and inducing pAMPK and transcriptional corepressor SMILE. Immunol Lett. 2017;188:1–8. doi: 10.1016/j.imlet.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, et al. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464–1467. doi: 10.1002/hep.510230624. [DOI] [PubMed] [Google Scholar]
- 54.Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin E in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–1543. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 55.Balmer ML, Siegrist K, Zimmermann A, Dufour JF. Effects of ursodeoxycholic acid in combination with vitamin E on adipokines and apoptosis in patients with nonalcoholic steatohepatitis. Liver Int. 2009;29:1184–1188. doi: 10.1111/j.1478-3231.2009.02037.x. [DOI] [PubMed] [Google Scholar]
- 56.Colombo C, Crosignani A, Assaisso M, Battezzati PM, Podda M, Giunta A, et al. Ursodeoxycholic acid therapy in cystic fibrosisassociated liver disease: a dose-response study. Hepatology. 1992;16:924–930. doi: 10.1002/hep.1840160412. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell SA, Bansi DS, Hunt N, Von Bergmann K, Fleming KA, Chapman RW. A preliminary trial of high-dose ursodeoxycholic acid in primary sclerosing cholangitis. Gastroenterology. 2001;121:900–907. doi: 10.1053/gast.2001.27965. [DOI] [PubMed] [Google Scholar]
- 58.Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rössle M, Cordes HJ, et al. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:472–479. doi: 10.1002/hep.23727. [DOI] [PubMed] [Google Scholar]
- 59.Ratziu V, de Ledinghen V, Oberti F, Mathurin P, Wartelle-Bladou C, Renou C, et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011–1019. doi: 10.1016/j.jhep.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 60.Yoon YB, Hagey LR, Hofmann AF, Gurantz D, Michelotti EL, Steinbach JH. Effect of side-chain shortening on the physiologic properties of bile acids: hepatic transport and effect on biliary secretion of 23-nor-ursodeoxycholate in rodents. Gastroenterology. 1986;90:837–852. doi: 10.1016/0016-5085(86)90859-0. [DOI] [PubMed] [Google Scholar]
- 61.Steinacher D, Claudel T, Trauner M. Therapeutic mechanisms of bile acids and nor-ursodeoxycholic acid in non-alcoholic fatty liver disease. Dig Dis. 2017;35:282–287. doi: 10.1159/000454853. [DOI] [PubMed] [Google Scholar]
- 62.Beraza N, Ofner-Ziegenfuss L, Ehedego H, Boekschoten M, Bischoff SC, Mueller M, et al. Nor-ursodeoxycholic acid reverses hepatocyte-specific nemo-dependent steatohepatitis. Gut. 2011;60:387–396. doi: 10.1136/gut.2010.223834. [DOI] [PubMed] [Google Scholar]
- 63.Traussnigg S, Schattenberg JM, Demir M, Wiegand J, Geier A, Teuber G, et al. Norursodeoxycholic acid versus placebo in the treatment of non-alcoholic fatty liver disease: a double-blind, randomised, placebo-controlled, phase 2 dose-finding trial. Lancet Gastroenterol Hepatol. 2019;4:781–793. doi: 10.1016/S2468-1253(19)30184-0. [DOI] [PubMed] [Google Scholar]
- 64.Fabbri C, Marchetto S, Pezzoli A, Accogli E, Fusaroli P, Azzaroli F, et al. Efficacy of ursodeoxycholic acid in association with alpha-interferon for chronic hepatitis C in alpha-interferon nonresponder patients. Eur J Gastroenterol Hepatol. 2000;12:511–515. doi: 10.1097/00042737-200012050-00006. [DOI] [PubMed] [Google Scholar]
- 65.Boucher E, Guyader D, Jacquelinet S, Andre P, Mendler MH, Turlin B, et al. Interferon and ursodeoxycholic acid combined therapy in chronic viral C hepatitis: controlled randomized trial in 203 patients. Dig Liver Dis. 2000;32:29–33. doi: 10.1016/s1590-8658(00)80041-9. [DOI] [PubMed] [Google Scholar]
- 66.Omata M, Yoshida H, Toyota J, Tomita E, Nishiguchi S, Hayashi N, et al. A large-scale, multicentre, double-blind trial of ursodeoxycholic acid in patients with chronic hepatitis C. Gut. 2007;56:1747–1753. doi: 10.1136/gut.2007.120956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.European Association for the Study of the Liver EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 68.Dehmlow C, Erhard J, de Groot H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23:749–754. doi: 10.1053/jhep.1996.v23.pm0008666328. [DOI] [PubMed] [Google Scholar]
- 69.Kwon DY, Jung YS, Kim SJ, Kim YS, Choi DW, Kim YC. Alterations in sulfur amino acid metabolism in mice treated with silymarin: a novel mechanism of its action involved in enhancement of the antioxidant defense in liver. Planta Med. 2013;79:997–1002. doi: 10.1055/s-0032-1328704. [DOI] [PubMed] [Google Scholar]
- 70.Song Z, Deaciuc I, Song M, Lee DY, Liu Y, Ji X, et al. Silymarin protects against acute ethanol-induced hepatotoxicity in mice. Alcohol Clin Exp Res. 2006;30:407–413. doi: 10.1111/j.1530-0277.2006.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valenzuela A, Garrido A. Biochemical bases of the pharmacological action of the flavonoid silymarin and of its structural isomer silibinin. Biol Res. 1994;27:105–112. [PubMed] [Google Scholar]
- 72.Valenzuela A, Guerra R. Differential effect of silybin on the Fe2+-ADP and t-butyl hydroperoxide-induced microsomal lipid peroxidation. Experientia. 1986;42:139–141. doi: 10.1007/BF01952435. [DOI] [PubMed] [Google Scholar]
- 73.van Pelt JF, Verslype C, Crabbé T, Zaman Z, Fevery J. Primary human hepatocytes are protected against prolonged and repeated exposure to ethanol by silibinin-dihemisuccinate. Alcohol Alcohol. 2003;38:411–414. doi: 10.1093/alcalc/agg099. [DOI] [PubMed] [Google Scholar]
- 74.Dehmlow C, Murawski N, de Groot H. Scavenging of reactive oxygen species and inhibition of arachidonic acid metabolism by silibinin in human cells. Life Sci. 1996;58:1591–1600. doi: 10.1016/0024-3205(96)00134-8. [DOI] [PubMed] [Google Scholar]
- 75.Valenzuela A, Guerra R, Garrido A. Silybin dihemisuccinate protects rat erythrocytes against phenylhydrazine-induced lipid peroxidation and hemolysis. Planta Med. 1987;53:402–405. doi: 10.1055/s-2006-962757. [DOI] [PubMed] [Google Scholar]
- 76.Noel-Hudson MS, de Belilovsky C, Petit N, Lindenbaum A, Wepierre J. In vitro cytotoxic effects of enzymatically induced oxygen radicals in human fibroblasts: experimental procedures and protection by radical scavengers. Toxicol In Vitro. 1989;3:103–109. doi: 10.1016/0887-2333(89)90052-0. [DOI] [PubMed] [Google Scholar]
- 77.Salomone F, Barbagallo I, Godos J, Lembo V, Currenti W, Cinà D, et al. Silibinin restores NAD+ levels and induces the SIRT1/AMPK pathway in non-alcoholic fatty liver. Nutrients. 2017;9:1086. doi: 10.3390/nu9101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hahn G, Lehmann HD, Kürten M, Uebel H, Vogel G. On the pharmacology and toxicology of silymarin, an antihepatotoxic active principle from Silybum marianum (L.) Gaertn. Arzneimittelforschung. 1968;18:698–704. [PubMed] [Google Scholar]
- 79.Lieber CS, Leo MA, Cao Q, Ren C, DeCarli LM. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J Clin Gastroenterol. 2003;37:336–339. doi: 10.1097/00004836-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 80.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver diseasemeta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 81.Trappoliere M, Caligiuri A, Schmid M, Bertolani C, Failli P, Vizzutti F, et al. Silybin, a component of sylimarin, exerts antiinflammatory and anti-fibrogenic effects on human hepatic stellate cells. J Hepatol. 2009;50:1102–1111. doi: 10.1016/j.jhep.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 82.Kim M, Yang SG, Kim JM, Lee JW, Kim YS, Lee JI. Silymarin suppresses hepatic stellate cell activation in a dietary rat model of non-alcoholic steatohepatitis: analysis of isolated hepatic stellate cells. Int J Mol Med. 2012;30:473–479. doi: 10.3892/ijmm.2012.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yao J, Zhi M, Gao X, Hu P, Li C, Yang X. Effect and the probable mechanisms of silibinin in regulating insulin resistance in the liver of rats with non-alcoholic fatty liver. Braz J Med Biol Res. 2013;46:270–277. doi: 10.1590/1414-431X20122551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gu M, Zhao P, Huang J, Zhao Y, Wang Y, Li Y, et al. Silymarin ameliorates metabolic dysfunction associated with diet-induced obesity via activation of farnesyl X receptor. Front Pharmacol. 2016;7:345. doi: 10.3389/fphar.2016.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Solhi H, Ghahremani R, Kazemifar AM, Hoseini Yazdi Z. Silymarin in treatment of non-alcoholic steatohepatitis: a randomized clinical trial. Caspian J Intern Med. 2014;5:9–12. [PMC free article] [PubMed] [Google Scholar]
- 86.Hashemi SJ, Hajiani E, Heydari SE. A placebo-controlled trial of silymarin in patients with nonalcoholic fatty liver disease. Hepat Mon. 2009;9:265–270. [Google Scholar]
- 87.Anushiravani A, Haddadi N, Pourfarmanbar M, Mohammadkarimi V. Treatment options for nonalcoholic fatty liver disease: a double-blinded randomized placebo-controlled trial. Eur J Gastroenterol Hepatol. 2019;31:613–617. doi: 10.1097/MEG.0000000000001369. [DOI] [PubMed] [Google Scholar]
- 88.Stiuso P, Scognamiglio I, Murolo M, Ferranti P, De Simone C, Rizzo MR, et al. Serum oxidative stress markers and lipidomic profile to detect NASH patients responsive to an antioxidant treatment: a pilot study. Oxid Med Cell Longev. 2014;2014:169216. doi: 10.1155/2014/169216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kalopitas G, Antza C, Doundoulakis I, Siargkas A, Kouroumalis E, Germanidis G, et al. Impact of silymarin in individuals with nonalcoholic fatty liver disease: a systematic review and metaanalysis. Nutrition. 2021;83:111092. doi: 10.1016/j.nut.2020.111092. [DOI] [PubMed] [Google Scholar]
- 90.Sorrentino G, Crispino P, Coppola D, De Stefano G. Efficacy of lifestyle changes in subjects with non-alcoholic liver steatosis and metabolic syndrome may be improved with an antioxidant nutraceutical: a controlled clinical study. Drugs R D. 2015;15:21–25. doi: 10.1007/s40268-015-0084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fried MW, Navarro VJ, Afdhal N, Belle SH, Wahed AS, Hawke RL, et al. Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: a randomized controlled trial. JAMA. 2012;308:274–282. doi: 10.1001/jama.2012.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yakoot M, Salem A. Spirulina platensis versus silymarin in the treatment of chronic hepatitis C virus infection. A pilot randomized, comparative clinical trial. BMC Gastroenterol. 2012;12:32. doi: 10.1186/1471-230X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kalantari H, Shahshahan Z, Hejazi SM, Ghafghazi T, Sebghatolahi V. Effects of silybum marianum on patients with chronic hepatitis C. J Res Med Sci. 2011;16:287–290. [PMC free article] [PubMed] [Google Scholar]
- 94.Tanamly MD, Tadros F, Labeeb S, Makld H, Shehata M, Mikhail N, et al. Randomised double-blinded trial evaluating silymarin for chronic hepatitis C in an Egyptian village: study description and 12-month results. Dig Liver Dis. 2004;36:752–759. doi: 10.1016/j.dld.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 95.Ferenci P, Scherzer TM, Kerschner H, Rutter K, Beinhardt S, Hofer H, et al. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology. 2008;135:1561–1567. doi: 10.1053/j.gastro.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 96.Saller R, Brignoli R, Melzer J, Meier R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch Komplementmed. 2008;15:9–20. doi: 10.1159/000113648. [DOI] [PubMed] [Google Scholar]
- 97.Li XY. Bioactivity of neolignans from fructus Schizandrae. Mem Inst Oswaldo Cruz. 1991;86 Suppl 2:31–37. doi: 10.1590/s0074-02761991000600010. [DOI] [PubMed] [Google Scholar]
- 98.El-Beshbishy HA. The effect of dimethyl dimethoxy biphenyl dicarboxylate (DDB) against tamoxifen-induced liver injury in rats: DDB use is curative or protective. J Biochem Mol Biol. 2005;38:300–306. doi: 10.5483/bmbrep.2005.38.3.300. [DOI] [PubMed] [Google Scholar]
- 99.Kim SG, Kim HJ, Choi SH, Ryu JY. Inhibition of lipopolysaccharide-induced I-kappaB degradation and tumor necrosis factoralpha expression by dimethyl-4,4’-dimethoxy-5,6,5’,6’-dimethylene dioxybiphenyl-2,2’-dicarboxylate (DDB): minor role in hepatic detoxifying enzyme expression. Liver. 2000;20:319–329. doi: 10.1034/j.1600-0676.2000.020004319.x. [DOI] [PubMed] [Google Scholar]
- 100.El-Bahy AA, Kassem LA, Heikal OA, Mahran LG. Antiapoptotic effect of DDB against hepatic ischemia-reperfusion injury. J Toxicol Sci. 2011;36:145–154. doi: 10.2131/jts.36.145. [DOI] [PubMed] [Google Scholar]
- 101.Rebouche CJ. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann N Y Acad Sci. 2004;1033:30–41. doi: 10.1196/annals.1320.003. [DOI] [PubMed] [Google Scholar]
- 102.Jun DW, Cho WK, Jun JH, Kwon HJ, Jang KS, Kim HJ, et al. Prevention of free fatty acid-induced hepatic lipotoxicity by carnitine via reversal of mitochondrial dysfunction. Liver Int. 2011;31:1315–1324. doi: 10.1111/j.1478-3231.2011.02602.x. [DOI] [PubMed] [Google Scholar]
- 103.Brady JF, Li DC, Ishizaki H, Yang CS. Effect of diallyl sulfide on rat liver microsomal nitrosamine metabolism and other monooxygenase activities. Cancer Res. 1988;48:5937–5940. [PubMed] [Google Scholar]
- 104.Hayes MA, Rushmore TH, Goldberg MT. Inhibition of hepatocarcinogenic responses to 1,2-dimethylhydrazine by diallyl sulfide, a component of garlic oil. Carcinogenesis. 1987;8:1155–1157. doi: 10.1093/carcin/8.8.1155. [DOI] [PubMed] [Google Scholar]
- 105.Kim SG, Kwak JY, Lee JW, Novak RF, Park SS, Kim ND. Malotilate, a hepatoprotectant, suppresses CYP2E1 expression in rats. Biochem Biophys Res Commun. 1994;200:1414–1420. doi: 10.1006/bbrc.1994.1608. [DOI] [PubMed] [Google Scholar]
- 106.Kim SG, Nam SY, Chung HC, Hong SY, Jung KH. Enhanced effectiveness of dimethyl-4,4’-dimethoxy-5,6,5’,6’-dimethylene dioxybiphenyl-2,2’-dicarboxylate in combination with garlic oil against experimental hepatic injury in rats and mice. J Pharm Pharmacol. 1995;47:678–682. doi: 10.1111/j.2042-7158.1995.tb05859.x. [DOI] [PubMed] [Google Scholar]
- 107.Park EY, Ki SH, Ko MS, Kim CW, Lee MH, Lee YS, et al. Garlic oil and DDB, comprised in a pharmaceutical composition for the treatment of patients with viral hepatitis, prevents acute liver injuries potentiated by glutathione deficiency in rats. Chem Biol Interact. 2005;155:82–96. doi: 10.1016/j.cbi.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 108.Kang JS, Hong JH, Park MS, Lee MH, Lee JB, Lee JW. A randomized controlled, double-blind evaluation of the short-term(8 weeks) efficacy and safety of godex in Korean chronic hepatitis patients. J Korean Soc Clin Pharmacol Ther. 2001;9:81–96. [Google Scholar]
- 109.Park MS, Kang JS, Chon CY, Paik SW, Rim KS, Kwak MJ, et al. Oral godex capsule for chronic liver disease: a double-blind, randomized, multicenter controlled trial. J Korean Soc Clin Pharmacol Ther. 2001;9:151–162. [Google Scholar]
- 110.Kim HJ, Lee JS, Lee HW, Kim MY, Nam SW, Sohn JH, et al. The PERFECT Study (PEnnel Real liFe Efficacy Clinical Trial), a double-blind, randomized, multicenter trial examining the efficacy of biphenyl dimethyl dicarboxylate combined with garlic oil in patients with transaminase elevated chronic liver disease. Korean J Med. 2014;86:179–189. [Google Scholar]
- 111.Hong ES, Kim EK, Kang SM, Khang AR, Choi SH, Park KS, et al. Effect of carnitine-orotate complex on glucose metabolism and fatty liver: a double-blind, placebo-controlled study. J Gastroenterol Hepatol. 2014;29:1449–1457. doi: 10.1111/jgh.12536. [DOI] [PubMed] [Google Scholar]
- 112.Bae JC, Lee WY, Yoon KH, Park JY, Son HS, Han KA, et al. Improvement of nonalcoholic fatty liver disease with carnitineorotate complex in type 2 diabetes (CORONA): a randomized controlled trial. Diabetes Care. 2015;38:1245–1252. doi: 10.2337/dc14-2852. [DOI] [PubMed] [Google Scholar]
- 113.Jun DW, Kim BI, Cho YK, Kim HJ, Kwon YO, Park SY, et al. Efficacy and safety of entecavir plus carnitine complex (GODEX®) compared to entecavir monotherapy in patient with ALT elevated chronic hepatitis B: randomized, multicenter openlabel trials. The GOAL study. Clin Mol Hepatol. 2013;19:165–172. doi: 10.3350/cmh.2013.19.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grüngreiff K, Baumann JL. Efficacy of L-ornitin L-aspartate granules in the treatment of chronic liver disease. Med Welt. 2001;52:219–226. [Google Scholar]
- 115.Butterworth RF, Kircheis G, Hilger N, McPhail MJW. Efficacy of l-ornithine l-aspartate for the treatment of hepatic encephalopathy and hyperammonemia in cirrhosis: systematic review and meta-analysis of randomized controlled trials. J Clin Exp Hepatol. 2018;8:301–313. doi: 10.1016/j.jceh.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gutiérrez-de-Juan V, López de Davalillo S, Fernández-Ramos D, Barbier-Torres L, Zubiete-Franco I, Fernández-Tussy P, et al. A morphological method for ammonia detection in liver. PLoS One. 2017;12:e0173914. doi: 10.1371/journal.pone.0173914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675–1682. doi: 10.1002/hep.23500. [DOI] [PubMed] [Google Scholar]
- 118.Butterworth RF, Canbay A. Hepatoprotection by L-ornithine L-aspartate in non-alcoholic fatty liver disease. Dig Dis. 2019;37:63–68. doi: 10.1159/000491429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tian LY, Lu LG, Tang CW, Xie Y, Luo HS, Tan SY, et al. Aspartateornithine granules in the treatment of nonalcoholic steatohepatitis: a multiple-dose parallel controlled clinical trial. Zhonghua Gan Zang Bing Za Zhi. 2013;21:528–532. doi: 10.3760/cma.j.issn.1007-3418.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 120.Ermolova T, Ermolov S. Correction of intrahepatic microcirculation disorders by L-ornithine-L-aspartate at the chronic liver diseases patients. J Hepatol. 2018;68:S585–S586. [Google Scholar]
- 121.Rudler M, Weiss N, Bouzbib C, Thabut D. Diagnosis and management of hepatic encephalopathy. Clin Liver Dis. 2021;25:393–417. doi: 10.1016/j.cld.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 122.Okuno M, Moriwaki H, Kato M, Muto Y, Kojima S. Changes in the ratio of branched-chain to aromatic amino acids affect the secretion of albumin in cultured rat hepatocytes. Biochem Biophys Res Commun. 1995;214:1045–1050. doi: 10.1006/bbrc.1995.2391. [DOI] [PubMed] [Google Scholar]
- 123.Ijichi C, Matsumura T, Tsuji T, Eto Y. Branched-chain amino acids promote albumin synthesis in rat primary hepatocytes through the mTOR signal transduction system. Biochem Biophys Res Commun. 2003;303:59–64. doi: 10.1016/s0006-291x(03)00295-x. [DOI] [PubMed] [Google Scholar]
- 124.Montoya A, Gómez-Lechón MJ, Castell JV. Influence of branched-chain amino acid composition of culture media on the synthesis of plasma proteins by serum-free cultured rat hepatocytes. In Vitro Cell Dev Biol. 1989;25:358–364. doi: 10.1007/BF02624599. [DOI] [PubMed] [Google Scholar]
- 125.Kuwahata M, Yoshimura T, Sawai Y, Amano S, Tomoe Y, Segawa H, et al. Localization of polypyrimidine-tract-binding protein is involved in the regulation of albumin synthesis by branchedchain amino acids in HepG2 cells. J Nutr Biochem. 2008;19:438–447. doi: 10.1016/j.jnutbio.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 126.Nishitani S, Matsumura T, Fujitani S, Sonaka I, Miura Y, Yagasaki K. Leucine promotes glucose uptake in skeletal muscles of rats. Biochem Biophys Res Commun. 2002;299:693–696. doi: 10.1016/s0006-291x(02)02717-1. [DOI] [PubMed] [Google Scholar]
- 127.Doi M, Yamaoka I, Fukunaga T, Nakayama M. Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes. Biochem Biophys Res Commun. 2003;312:1111–1117. doi: 10.1016/j.bbrc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 128.Nishitani S, Takehana K, Fujitani S, Sonaka I. Branched-chain amino acids improve glucose metabolism in rats with liver cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1292–G1300. doi: 10.1152/ajpgi.00510.2003. [DOI] [PubMed] [Google Scholar]
- 129.Hinault C, Mothe-Satney I, Gautier N, Lawrence JC, Jr, Van Obberghen E. Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/db mice. FASEB J. 2004;18:1894–1896. doi: 10.1096/fj.03-1409fje. [DOI] [PubMed] [Google Scholar]
- 130.Higuchi N, Kato M, Miyazaki M, Tanaka M, Kohjima M, Ito T, et al. Potential role of branched-chain amino acids in glucose metabolism through the accelerated induction of the glucosesensing apparatus in the liver. J Cell Biochem. 2011;112:30–38. doi: 10.1002/jcb.22688. [DOI] [PubMed] [Google Scholar]
- 131.Iwao M, Gotoh K, Arakawa M, Endo M, Honda K, Seike M, et al. Supplementation of branched-chain amino acids decreases fat accumulation in the liver through intestinal microbiota-mediated production of acetic acid. Sci Rep. 2020;10:18768. doi: 10.1038/s41598-020-75542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee HL, Lee J, Cha JH, Cho S, Sung PS, Hur W, et al. Anti-fibrotic effects of branched-chain amino acids on hepatic stellate cells. Korean J Intern Med. 2022;37:53–62. doi: 10.3904/kjim.2020.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takegoshi K, Honda M, Okada H, Takabatake R, MatsuzawaNagata N, Campbell JS, et al. Branched-chain amino acids prevent hepatic fibrosis and development of hepatocellular carcinoma in a non-alcoholic steatohepatitis mouse model. Oncotarget. 2017;8:18191–18205. doi: 10.18632/oncotarget.15304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khedr NF, Khedr EG. Branched chain amino acids supplementation modulates TGF-β1/Smad signaling pathway and interleukins in CCl4-induced liver fibrosis. Fundam Clin Pharmacol. 2017;31:534–545. doi: 10.1111/fcp.12297. [DOI] [PubMed] [Google Scholar]
- 135.Cha JH, Bae SH, Kim HL, Park NR, Choi ES, Jung ES, et al. Branched-chain amino acids ameliorate fibrosis and suppress tumor growth in a rat model of hepatocellular carcinoma with liver cirrhosis. PLoS One. 2013;8:e77899. doi: 10.1371/journal.pone.0077899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kakazu E, Kanno N, Ueno Y, Shimosegawa T. Extracellular branched-chain amino acids, especially valine, regulate maturation and function of monocyte-derived dendritic cells. J Immunol. 2007;179:7137–7146. doi: 10.4049/jimmunol.179.10.7137. [DOI] [PubMed] [Google Scholar]
- 137.Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, et al. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol Res. 2006;35:204–214. doi: 10.1016/j.hepres.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 138.Kawamura E, Habu D, Morikawa H, Enomoto M, Kawabe J, Tamori A, et al. A randomized pilot trial of oral branchedchain amino acids in early cirrhosis: validation using prognostic markers for pre-liver transplant status. Liver Transpl. 2009;15:790–797. doi: 10.1002/lt.21758. [DOI] [PubMed] [Google Scholar]
- 139.Nakaya Y, Okita K, Suzuki K, Moriwaki H, Kato A, Miwa Y, et al. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition. 2007;23:113–120. doi: 10.1016/j.nut.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 140.Koya S, Kawaguchi T, Hashida R, Goto E, Matsuse H, Saito H, et al. Effects of in-hospital exercise on liver function, physical ability, and muscle mass during treatment of hepatoma in patients with chronic liver disease. Hepatol Res. 2017;47:E22–E34. doi: 10.1111/hepr.12718. [DOI] [PubMed] [Google Scholar]
- 141.Habu D, Nishiguchi S, Nakatani S, Lee C, Enomoto M, Tamori A, et al. Comparison of the effect of BCAA granules on between decompensated and compensated cirrhosis. Hepatogastroenterology. 2009;56:1719–1723. [PubMed] [Google Scholar]
- 142.Park JG, Tak WY, Park SY, Kweon YO, Chung WJ, Jang BK, et al. Effects of branched-chain amino acid (BCAA) supplementation on the progression of advanced liver disease: a Korean nationwide, multicenter, prospective, observational, cohort study. Nutrients. 2020;12:1429. doi: 10.3390/nu12051429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park JG, Tak WY, Park SY, Kweon YO, Jang SY, Lee YR, et al. Effects of branched-chain amino acids (BCAAs) on the progression of advanced liver disease: a Korean nationwide, multicenter, retrospective, observational, cohort study. Medicine (Baltimore) 2017;96:e6580. doi: 10.1097/MD.0000000000006580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, et al. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792–1801. doi: 10.1016/s0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 145.Ichikawa T, Naota T, Miyaaki H, Miuma S, Isomoto H, Takeshima F, et al. Effect of an oral branched chain amino acid-enriched snack in cirrhotic patients with sleep disturbance. Hepatol Res. 2010;40:971–978. doi: 10.1111/j.1872-034X.2010.00701.x. [DOI] [PubMed] [Google Scholar]
- 146.Ishikawa T. Early administration of branched-chain amino acid granules. World J Gastroenterol. 2012;18:4486–4490. doi: 10.3748/wjg.v18.i33.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ismaiel A, Bucsa C, Farcas A, Leucuta DC, Popa SL, Dumitrascu DL. Effects of branched-chain amino acids on parameters evaluating sarcopenia in liver cirrhosis: systematic review and meta-analysis. Front Nutr. 2022;9:749969. doi: 10.3389/fnut.2022.749969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hanai T, Shiraki M, Imai K, Suetsugu A, Takai K, Shimizu M. Late evening snack with branched-chain amino acids supplementation improves survival in patients with cirrhosis. J Clin Med. 2020;9:1013. doi: 10.3390/jcm9041013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koreeda C, Seki T, Okazaki K, Ha-Kawa SK, Sawada S. Effects of late evening snack including branched-chain amino acid on the function of hepatic parenchymal cells in patients with liver cirrhosis. Hepatol Res. 2011;41:417–422. doi: 10.1111/j.1872-034X.2011.00795.x. [DOI] [PubMed] [Google Scholar]
- 150.Gluud LL, Dam G, Borre M, Les I, Cordoba J, Marchesini G, et al. Oral branched-chain amino acids have a beneficial effect on manifestations of hepatic encephalopathy in a systematic review with meta-analyses of randomized controlled trials. J Nutr. 2013;143:1263–1268. doi: 10.3945/jn.113.174375. [DOI] [PubMed] [Google Scholar]
- 151.Gluud LL, Dam G, Les I, Marchesini G, Borre M, Aagaard NK, et al. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;5:CD001939. doi: 10.1002/14651858.CD001939.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dam G, Aamann L, Vistrup H, Gluud LL. The role of branched chain amino acids in the treatment of hepatic encephalopathy. J Clin Exp Hepatol. 2018;8:448–451. doi: 10.1016/j.jceh.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dam G, Ott P, Aagaard NK, Vilstrup H. Branched-chain amino acids and muscle ammonia detoxification in cirrhosis. Metab Brain Dis. 2013;28:217–220. doi: 10.1007/s11011-013-9377-3. [DOI] [PubMed] [Google Scholar]
- 154.Yamamoto M, Iwasa M, Matsumura K, Nakagawa Y, Fujita N, Kobayashi Y, et al. Improvement of regional cerebral blood flow after oral intake of branched-chain amino acids in patients with cirrhosis. World J Gastroenterol. 2005;11:6792–6799. doi: 10.3748/wjg.v11.i43.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Plauth M, Egberts EH, Hamster W, Török M, Müller PH, Brand O, et al. Long-term treatment of latent portosystemic encephalopathy with branched-chain amino acids. A double-blind placebo-controlled crossover study. J Hepatol. 1993;17:308–314. doi: 10.1016/s0168-8278(05)80210-7. [DOI] [PubMed] [Google Scholar]
- 156.Hayashi S, Aoyagi Y, Fujiwara K, Oka H, Oda T. A randomized controlled trial of branched-chain amino acid (BCAA)-enriched elemental diet (ED-H) for hepatic encephalopathy. J Gastroenterol Hepatol. 1991;6:191. [Google Scholar]
- 157.Horst D, Grace ND, Conn HO, Schiff E, Schenker S, Viteri A, et al. Comparison of dietary protein with an oral, branched chainenriched amino acid supplement in chronic portal-systemic encephalopathy: a randomized controlled trial. Hepatology. 1984;4:279–287. doi: 10.1002/hep.1840040218. [DOI] [PubMed] [Google Scholar]
- 158.Blaha MJ, Martin SS. How do statins work?: changing paradigms with implications for statin allocation. J Am Coll Cardiol. 2013;62:2392–2394. doi: 10.1016/j.jacc.2013.08.1626. [DOI] [PubMed] [Google Scholar]
- 159.Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Statins: pros and cons. Med Clin (Barc) 2018;150:398–402. doi: 10.1016/j.medcli.2017.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schierwagen R, Uschner FE, Magdaleno F, Klein S, Trebicka J. Rationale for the use of statins in liver disease. Am J Physiol Gastrointest Liver Physiol. 2017;312:G407–G412. doi: 10.1152/ajpgi.00441.2016. [DOI] [PubMed] [Google Scholar]
- 161.Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161:161–172. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marrone G, Russo L, Rosado E, Hide D, García-Cardeña G, García-Pagán JC, et al. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. J Hepatol. 2013;58:98–103. doi: 10.1016/j.jhep.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 163.Vargas JI, Arrese M, Shah VH, Arab JP. Use of statins in patients with chronic liver disease and cirrhosis: current views and prospects. Curr Gastroenterol Rep. 2017;19:43. doi: 10.1007/s11894-017-0584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Relja B, Meder F, Wang M, Blaheta R, Henrich D, Marzi I, et al. Simvastatin modulates the adhesion and growth of hepatocellular carcinoma cells via decrease of integrin expression and ROCK. Int J Oncol. 2011;38:879–885. doi: 10.3892/ijo.2010.892. [DOI] [PubMed] [Google Scholar]
- 165.Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63:705–712. doi: 10.1016/j.jhep.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 166.Nascimbeni F, Aron-Wisnewsky J, Pais R, Tordjman J, Poitou C, Charlotte F, et al. Statins, antidiabetic medications and liver histology in patients with diabetes with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000075. doi: 10.1136/bmjgast-2015-000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ekstedt M, Franzén LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological followup study. J Hepatol. 2007;47:135–141. doi: 10.1016/j.jhep.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 168.Rallidis LS, Drakoulis CK, Parasi AS. Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis. 2004;174:193–196. doi: 10.1016/j.atherosclerosis.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 169.Hyogo H, Tazuma S, Arihiro K, Iwamoto K, Nabeshima Y, Inoue M, et al. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57:1711–1718. doi: 10.1016/j.metabol.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 170.Nakahara T, Hyogo H, Kimura Y, Ishitobi T, Arihiro K, Aikata H, et al. Efficacy of rosuvastatin for the treatment of non-alcoholic steatohepatitis with dyslipidemia: an open-label, pilot study. Hepatol Res. 2012;42:1065–1072. doi: 10.1111/j.1872-034X.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 171.Kargiotis K, Athyros VG, Giouleme O, Katsiki N, Katsiki E, Anagnostis P, et al. Resolution of non-alcoholic steatohepatitis by rosuvastatin monotherapy in patients with metabolic syndrome. World J Gastroenterol. 2015;21:7860–7868. doi: 10.3748/wjg.v21.i25.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hyogo H, Ikegami T, Tokushige K, Hashimoto E, Inui K, Matsuzaki Y, et al. Efficacy of pitavastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia: an open-label, pilot study. Hepatol Res. 2011;41:1057–1065. doi: 10.1111/j.1872-034X.2011.00849.x. [DOI] [PubMed] [Google Scholar]
- 173.Butt AA, Yan P, Bonilla H, Abou-Samra AB, Shaikh OS, Simon TG, et al. Effect of addition of statins to antiviral therapy in hepatitis C virus-infected persons: results from ERCHIVES. Hepatology. 2015;62:365–374. doi: 10.1002/hep.27835. [DOI] [PubMed] [Google Scholar]
- 174.Fatima K, Moeed A, Waqar E, Atif AR, Kamran A, Rizvi H, et al. Efficacy of statins in treatment and development of nonalcoholic fatty liver disease and steatohepatitis: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2022;46:101816. doi: 10.1016/j.clinre.2021.101816. [DOI] [PubMed] [Google Scholar]
- 175.Zou B, Odden MC, Nguyen MH. Statin use and reduced hepatocellular carcinoma risk in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2022 Feb 11; doi: 10.1016/j.cgh.2022.01.057. doi: 10.1016/j.cgh.2022.01.057. [DOI] [PubMed] [Google Scholar]
- 176.Avins AL, Manos MM, Ackerson L, Zhao W, Murphy R, Levin TR, et al. Hepatic effects of lovastatin exposure in patients with liver disease: a retrospective cohort study. Drug Saf. 2008;31:325–334. doi: 10.2165/00002018-200831040-00006. [DOI] [PubMed] [Google Scholar]
- 177.Hsiang JC, Wong GL, Tse YK, Wong VW, Yip TC, Chan HL. Statin and the risk of hepatocellular carcinoma and death in a hospital-based hepatitis B-infected population: a propensity score landmark analysis. J Hepatol. 2015;63:1190–1197. doi: 10.1016/j.jhep.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 178.Huang YW, Lee CL, Yang SS, Fu SC, Chen YY, Wang TC, et al. Statins reduce the risk of cirrhosis and its decompensation in chronic hepatitis B patients: a nationwide cohort study. Am J Gastroenterol. 2016;111:976–985. doi: 10.1038/ajg.2016.179. [DOI] [PubMed] [Google Scholar]
- 179.Simon TG, King LY, Zheng H, Chung RT. Statin use is associated with a reduced risk of fibrosis progression in chronic hepatitis C. J Hepatol. 2015;62:18–23. doi: 10.1016/j.jhep.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang YH, Chen WC, Tsan YT, Chen MJ, Shih WT, Tsai YH, et al. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J Hepatol. 2015;63:1111–1117. doi: 10.1016/j.jhep.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 181.Mohanty A, Tate JP, Garcia-Tsao G. Statins are associated with a decreased risk of decompensation and death in veterans with hepatitis C-related compensated cirrhosis. Gastroenterology. 2016;150:430–40. doi: 10.1053/j.gastro.2015.10.007. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Abraldes JG, Albillos A, Bañares R, Turnes J, González R, GarcíaPagán JC, et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651–1658. doi: 10.1053/j.gastro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 183.Pollo-Flores P, Soldan M, Santos UC, Kunz DG, Mattos DE, da Silva AC, et al. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: a randomized controlled trial. Dig Liver Dis. 2015;47:957–963. doi: 10.1016/j.dld.2015.07.156. [DOI] [PubMed] [Google Scholar]
- 184.Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology. 2016;150:1160–1170.e3. doi: 10.1053/j.gastro.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 185.Kumar S, Grace ND, Qamar AA. Statin use in patients with cirrhosis: a retrospective cohort study. Dig Dis Sci. 2014;59:1958–1965. doi: 10.1007/s10620-014-3179-2. [DOI] [PubMed] [Google Scholar]
- 186.Ward NC, Watts GF, Eckel RH. Response by Ward et al to letter regarding article, “Statin Toxicity: Mechanistic Insights and Clinical Implications”. Circ Res. 2019;124:e121–e122. doi: 10.1161/CIRCRESAHA.119.315233. [DOI] [PubMed] [Google Scholar]
- 187.Bhardwaj SS, Chalasani N. Lipid-lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis. 2007;11:597–vii. doi: 10.1016/j.cld.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Karahalil B, Hare E, Koç G, Uslu İ, Şentürk K, Özkan Y. Hepatotoxicity associated with statins. Arh Hig Rada Toksikol. 2017;68:254–260. doi: 10.1515/aiht-2017-68-2994. [DOI] [PubMed] [Google Scholar]
- 189.Björnsson ES. Hepatotoxicity of statins and other lipid-lowering agents. Liver Int. 2017;37:173–178. doi: 10.1111/liv.13308. [DOI] [PubMed] [Google Scholar]
- 190.Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65:2035–2044. doi: 10.1136/gutjnl-2016-312729. [DOI] [PubMed] [Google Scholar]
- 191.Fukui H. Leaky gut and gut-liver axis in liver cirrhosis: clinical studies update. Gut Liver. 2021;15:666–676. doi: 10.5009/gnl20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 193.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182:375–387. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément K. Nonalcoholic fatty liver disease: modulating gut microbiota to improve severity? Gastroenterology. 2020;158:1881–1898. doi: 10.1053/j.gastro.2020.01.049. [DOI] [PubMed] [Google Scholar]
- 196.Moya-Pérez A, Neef A, Sanz Y. Bifidobacterium pseudocatenulatum CECT 7765 reduces obesity-associated inflammation by restoring the lymphocyte-macrophage balance and gut microbiota structure in high-fat diet-fed mice. PLoS One. 2015;10:e0126976. doi: 10.1371/journal.pone.0126976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lee NY, Shin MJ, Youn GS, Yoon SJ, Choi YR, Kim HS, et al. Lactobacillus attenuates progression of nonalcoholic fatty liver disease by lowering cholesterol and steatosis. Clin Mol Hepatol. 2021;27:110–124. doi: 10.3350/cmh.2020.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rocco A, Sgamato C, Compare D, Coccoli P, Nardone OM, Nardone G. Gut microbes and hepatic encephalopathy: from the old concepts to new perspectives. Front Cell Dev Biol. 2021;9:748253. doi: 10.3389/fcell.2021.748253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rai R, Saraswat VA, Dhiman RK. Gut microbiota: its role in hepatic encephalopathy. J Clin Exp Hepatol. 2015;5(Suppl 1):S29–S36. doi: 10.1016/j.jceh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Anand G, Zarrinpar A, Loomba R. Targeting dysbiosis for the treatment of liver disease. Semin Liver Dis. 2016;36:37–47. doi: 10.1055/s-0035-1571276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Abdel Monem SM. Probiotic therapy in patients with nonalcoholic steatohepatitis in Zagazig University Hospitals. Euroasian J Hepatogastroenterol. 2017;7:101–106. doi: 10.5005/jp-journals-10018-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wong VW, Won GL, Chim AM, Chu WC, Yeung DK, Li KC, et al. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. 2013;12:256–262. [PubMed] [Google Scholar]
- 203.Nabavi S, Rafraf M, Somi MH, Homayouni-Rad A, Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci. 2014;97:7386–7393. doi: 10.3168/jds.2014-8500. [DOI] [PubMed] [Google Scholar]
- 204.Kobyliak N, Abenavoli L, Mykhalchyshyn G, Kononenko L, Boccuto L, Kyriienko D, et al. A multi-strain probiotic reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD patients: evidence from a randomized clinical trial. J Gastrointestin Liver Dis. 2018;27:41–49. doi: 10.15403/jgld.2014.1121.271.kby. [DOI] [PubMed] [Google Scholar]
- 205.Shavakhi A, Minakari M, Firouzian H, Assali R, Hekmatdoost A, Ferns G. Effect of a probiotic and metformin on liver aminotransferases in non-alcoholic steatohepatitis: a double blind randomized clinical trial. Int J Prev Med. 2013;4:531–537. [PMC free article] [PubMed] [Google Scholar]
- 206.Duseja A, Acharya SK, Mehta M, Chhabra S, Rana S, et al. High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): a randomised, double-blind, proof of concept study. BMJ Open Gastroenterol. 2019;6:e000315. doi: 10.1136/bmjgast-2019-000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ahn SB, Jun DW, Kang BK, Lim JH, Lim S, Chung MJ. Randomized, double-blind, placebo-controlled study of a multispecies probiotic mixture in nonalcoholic fatty liver disease. Sci Rep. 2019;9:5688. doi: 10.1038/s41598-019-42059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mofidi F, Poustchi H, Yari Z, Nourinayyer B, Merat S, Sharafkhah M, et al. Synbiotic supplementation in lean patients with nonalcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr. 2017;117:662–668. doi: 10.1017/S0007114517000204. [DOI] [PubMed] [Google Scholar]
- 209.Asgharian A, Mohammadi V, Gholi Z, Esmaillzadeh A, Feizi A, Askari G. The effect of synbiotic supplementation on body composition and lipid profile in patients with nafld: a randomized, double blind, placebo-controlled clinical trial study. Iran Red Crescent Med J. 2017;19:e42902. [Google Scholar]
- 210.Manzhalii E, Virchenko O, Falalyeyeva T, Beregova T, Stremmel W. Treatment efficacy of a probiotic preparation for nonalcoholic steatohepatitis: a pilot trial. J Dig Dis. 2017;18:698–703. doi: 10.1111/1751-2980.12561. [DOI] [PubMed] [Google Scholar]
- 211.Sayari S, Neishaboori H, Jameshorani M. Combined effects of synbiotic and sitagliptin versus sitagliptin alone in patients with nonalcoholic fatty liver disease. Clin Mol Hepatol. 2018;24:331–338. doi: 10.3350/cmh.2018.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xiao MW, Lin SX, Shen ZH, Luo WW, Wang XY. Systematic review with meta-analysis: the effects of probiotics in nonalcoholic fatty liver disease. Gastroenterol Res Pract. 2019;2019:1484598. doi: 10.1155/2019/1484598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147:1327–37.e3. doi: 10.1053/j.gastro.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 214.Lunia MK, Sharma BC, Sharma P, Sachdeva S, Srivastava S. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2014;12:1003–1008.e1. doi: 10.1016/j.cgh.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 215.Dalal R, McGee RG, Riordan SM, Webster AC. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;2:CD008716. doi: 10.1002/14651858.CD008716.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bafeta A, Koh M, Riveros C, Ravaud P. Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota: a systematic review. Ann Intern Med. 2018;169:240–247. doi: 10.7326/M18-0343. [DOI] [PubMed] [Google Scholar]
- 217.Didari T, Solki S, Mozaffari S, Nikfar S, Abdollahi M. A systematic review of the safety of probiotics. Expert Opin Drug Saf. 2014;13:227–239. doi: 10.1517/14740338.2014.872627. [DOI] [PubMed] [Google Scholar]
- 218.Goldenberg JZ, Lytvyn L, Steurich J, Parkin P, Mahant S, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2015;(12):CD004827. doi: 10.1002/14651858.CD004827.pub4. [DOI] [PubMed] [Google Scholar]
- 219.Zingg JM. Vitamin E: an overview of major research directions. Mol Aspects Med. 2007;28:400–422. doi: 10.1016/j.mam.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 220.Ahsan H, Ahad A, Iqbal J, Siddiqui WA. Pharmacological potential of tocotrienols: a review. Nutr Metab (Lond) 2014;11:52. doi: 10.1186/1743-7075-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tabei SM, Fakher S, Djalali M, Javanbakht MH, Zarei M, Derakhshanian H, et al. Effect of vitamins A, E, C and omega-3 fatty acids supplementation on the level of catalase and superoxide dismutase activities in streptozotocin-induced diabetic rats. Bratisl Lek Listy. 2015;116:115–118. doi: 10.4149/bll_2015_022. [DOI] [PubMed] [Google Scholar]
- 222.Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jin X, Song L, Liu X, Chen M, Li Z, Cheng L, et al. Protective efficacy of vitamins C and E on p,p’-DDT-induced cytotoxicity via the ROS-mediated mitochondrial pathway and NF-κB/FasL pathway. PLoS One. 2014;9:e113257. doi: 10.1371/journal.pone.0113257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shen XH, Cheng WF, Li XH, Sun JQ, Li F, Ma L, et al. Effects of dietary supplementation with vitamin E and selenium on rat hepatic stellate cell apoptosis. World J Gastroenterol. 2005;11:4957–4961. doi: 10.3748/wjg.v11.i32.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, et al. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2018;2018:9547613. doi: 10.1155/2018/9547613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Magosso E, Ansari MA, Gopalan Y, Shuaib IL, Wong JW, Khan NA, et al. Tocotrienols for normalisation of hepatic echogenic response in nonalcoholic fatty liver: a randomised placebocontrolled clinical trial. Nutr J. 2013;12:166. doi: 10.1186/1475-2891-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fouda A, Abdelaziz AE, Hussien M, Ali AA, Abdelkawy KS, Elbarbry F. A randomized controlled trial comparing the effects of vitamin E, ursodeoxycholic acid and pentoxifylline on Egyptian non-alcoholic steatohepatitis patients. Eur Rev Med Pharmacol Sci. 2021;25:7449–7459. doi: 10.26355/eurrev_202112_27442. [DOI] [PubMed] [Google Scholar]
- 228.Kedarisetty CK, Bhardwaj A, Kumar G, Rastogi A, Bihari C, Kumar M, et al. Efficacy of combining pentoxiphylline and vitamin E versus vitamin E alone in non-alcoholic steatohepatitis-a randomized pilot study. Indian J Gastroenterol. 2021;40:41–49. doi: 10.1007/s12664-020-01131-x. [DOI] [PubMed] [Google Scholar]
- 229.Polyzos SA, Kountouras J, Mantzoros CS, Polymerou V, Katsinelos P. Effects of combined low-dose spironolactone plus vitamin E vs vitamin E monotherapy on insulin resistance, non-invasive indices of steatosis and fibrosis, and adipokine levels in non-alcoholic fatty liver disease: a randomized controlled trial. Diabetes Obes Metab. 2017;19:1805–1809. doi: 10.1111/dom.12989. [DOI] [PubMed] [Google Scholar]
- 230.Pervez MA, Khan DA, Slehria AUR, Ijaz A. Delta-tocotrienol supplementation improves biochemical markers of hepatocellular injury and steatosis in patients with nonalcoholic fatty liver disease: A randomized, placebo-controlled trial. Complement Ther Med. 2020;52:102494. doi: 10.1016/j.ctim.2020.102494. [DOI] [PubMed] [Google Scholar]
- 231.Ekhlasi G, Kolahdouz Mohammadi R, Agah S, Zarrati M, Hosseini AF, Arabshahi SS, et al. Do symbiotic and vitamin E supplementation have favorite effects in nonalcoholic fatty liver disease? A randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2016;21:106. doi: 10.4103/1735-1995.193178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Aller R, Izaola O, Gómez S, Tafur C, González G, Berroa E, et al. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur Rev Med Pharmacol Sci. 2015;19:3118–3124. [PubMed] [Google Scholar]
- 233.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 234.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 235.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASDEASO clinical practice guidelines for the management of nonalcoholic fatty liver disease. Diabetologia. 2016;59:1121–1140. doi: 10.1007/s00125-016-3902-y. [DOI] [PubMed] [Google Scholar]
- 236.Chitturi S, Wong VW, Chan WK, Wong GL, Wong SK, Sollano J, et al. The Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017-part 2: management and special groups. J Gastroenterol Hepatol. 2018;33:86–98. doi: 10.1111/jgh.13856. [DOI] [PubMed] [Google Scholar]
- 237.Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J, et al. Role of vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2019;42:1481–1488. doi: 10.2337/dc19-0167. [DOI] [PubMed] [Google Scholar]
- 238.Vadarlis A, Antza C, Bakaloudi DR, Doundoulakis I, Kalopitas G, Samara M, et al. Systematic review with meta-analysis: the effect of vitamin E supplementation in adult patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2021;36:311–319. doi: 10.1111/jgh.15221. [DOI] [PubMed] [Google Scholar]
- 239.Said A, Akhter A. Meta-analysis of randomized controlled trials of pharmacologic agents in non-alcoholic steatohepatitis. Ann Hepatol. 2017;16:538–547. doi: 10.5604/01.3001.0010.0284. [DOI] [PubMed] [Google Scholar]
- 240.Majzoub AM, Nayfeh T, Barnard A, Munaganuru N, Dave S, Singh S, et al. Systematic review with network meta-analysis: comparative efficacy of pharmacologic therapies for fibrosis improvement and resolution of NASH. Aliment Pharmacol Ther. 2021;54:880–889. doi: 10.1111/apt.16583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Choi J, Ou JH. Mechanisms of liver injury. III. Oxidative stress in the pathogenesis of hepatitis C virus. Am J Physiol Gastrointest Liver Physiol. 2006;290:G847–G851. doi: 10.1152/ajpgi.00522.2005. [DOI] [PubMed] [Google Scholar]
- 242.Jain SK, Pemberton PW, Smith A, McMahon RF, Burrows PC, Aboutwerat A, et al. Oxidative stress in chronic hepatitis C: not just a feature of late stage disease. J Hepatol. 2002;36:805–811. doi: 10.1016/s0168-8278(02)00060-0. [DOI] [PubMed] [Google Scholar]
- 243.Chuma M, Hige S, Nakanishi M, Ogawa K, Natsuizaka M, Yamamoto Y, et al. 8-Hydroxy-2’-deoxy-guanosine is a risk factor for development of hepatocellular carcinoma in patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2008;23:1431–1436. doi: 10.1111/j.1440-1746.2008.05502.x. [DOI] [PubMed] [Google Scholar]
- 244.von Herbay A, Stahl W, Niederau C, Sies H. Vitamin E improves the aminotransferase status of patients suffering from viral hepatitis C: a randomized, double-blind, placebo-controlled study. Free Radic Res. 1997;27:599–605. doi: 10.3109/10715769709097863. [DOI] [PubMed] [Google Scholar]
- 245.Malaguarnera M, Motta M, Vacante M, Malaguarnera G, Caraci F, Nunnari G, et al. Silybin-vitamin E-phospholipids complex reduces liver fibrosis in patients with chronic hepatitis C treated with pegylated interferon α and ribavirin. Am J Transl Res. 2015;7:2510–2518. [PMC free article] [PubMed] [Google Scholar]
- 246.Marotta F, Yoshida C, Barreto R, Naito Y, Packer L. Oxidativeinflammatory damage in cirrhosis: effect of vitamin E and a fermented papaya preparation. J Gastroenterol Hepatol. 2007;22:697–703. doi: 10.1111/j.1440-1746.2007.04937.x. [DOI] [PubMed] [Google Scholar]
- 247.Idéo G, Bellobuono A, Tempini S, Mondazzi L, Airoldi A, Benetti G, et al. Antioxidant drugs combined with alpha-interferon in chronic hepatitis C not responsive to alpha-interferon alone: a randomized, multicentre study. Eur J Gastroenterol Hepatol. 1999;11:1203–1207. doi: 10.1097/00042737-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 248.Groenbaek K, Friis H, Hansen M, Ring-Larsen H, Krarup HB. The effect of antioxidant supplementation on hepatitis C viral load, transaminases and oxidative status: a randomized trial among chronic hepatitis C virus-infected patients. Eur J Gastroenterol Hepatol. 2006;18:985–989. doi: 10.1097/01.meg.0000231746.76136.4a. [DOI] [PubMed] [Google Scholar]
- 249.Bunchorntavakul C, Wootthananont T, Atsawarungruangkit A. Effects of vitamin E on chronic hepatitis C genotype 3: a randomized, double-blind, placebo-controlled study. J Med Assoc Thai. 2014;97 Suppl 11:S31–S40. [PubMed] [Google Scholar]
- 250.Look MP, Gerard A, Rao GS, Sudhop T, Fischer HP, Sauerbruch T, et al. Interferon/antioxidant combination therapy for chronic hepatitis C--a controlled pilot trial. Antiviral Res. 1999;43:113–122. doi: 10.1016/s0166-3542(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 251.Andreone P, Fiorino S, Cursaro C, Gramenzi A, Margotti M, Di Giammarino L, et al. Vitamin E as treatment for chronic hepatitis B: results of a randomized controlled pilot trial. Antiviral Res. 2001;49:75–81. doi: 10.1016/s0166-3542(00)00141-8. [DOI] [PubMed] [Google Scholar]
- 252.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 253.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 254.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Schürks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Jiang ZG, Feldbrügge L, Tapper EB, Popov Y, Ghaziani T, Afdhal N, et al. Aspirin use is associated with lower indices of liver fibrosis among adults in the United States. Aliment Pharmacol Ther. 2016;43:734–743. doi: 10.1111/apt.13515. [DOI] [PubMed] [Google Scholar]
- 257.Yoshida S, Ikenaga N, Liu SB, Peng ZW, Chung J, Sverdlov DY, et al. Extrahepatic platelet-derived growth factor-β, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology. 2014;147:1378–1392. doi: 10.1053/j.gastro.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 258.Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 259.Malehmir M, Pfister D, Gallage S, Szydlowska M, Inverso D, Kotsiliti E, et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019;25:641–655. doi: 10.1038/s41591-019-0379-5. [DOI] [PubMed] [Google Scholar]
- 260.Wang T, Fu X, Jin T, Zhang L, Liu B, Wu Y, et al. Aspirin targets P4HA2 through inhibiting NF-κB and LMCD1-AS1/let-7g to inhibit tumour growth and collagen deposition in hepatocellular carcinoma. EBioMedicine. 2019;45:168–180. doi: 10.1016/j.ebiom.2019.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Iannacone M, Sitia G, Narvaiza I, Ruggeri ZM, Guidotti LG. Antiplatelet drug therapy moderates immune-mediated liver disease and inhibits viral clearance in mice infected with a replication-deficient adenovirus. Clin Vaccine Immunol. 2007;14:1532–1535. doi: 10.1128/CVI.00298-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Shen H, Shahzad G, Jawairia M, Bostick RM, Mustacchia P. Association between aspirin use and the prevalence of nonalcoholic fatty liver disease: a cross-sectional study from the third national health and nutrition examination survey. Aliment Pharmacol Ther. 2014;40:1066–1073. doi: 10.1111/apt.12944. [DOI] [PubMed] [Google Scholar]
- 263.Simon TG, Henson J, Osganian S, Masia R, Chan AT, Chung RT, et al. Daily aspirin use associated with reduced risk for fibrosis progression in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:2776–2784. doi: 10.1016/j.cgh.2019.04.061. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Petrick JL, Sahasrabuddhe VV, Chan AT, Alavanja MC, BeaneFreeman LE, Buring JE, et al. NSAID use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the liver cancer pooling project. Cancer Prev Res (Phila) 2015;8:1156–1162. doi: 10.1158/1940-6207.CAPR-15-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Simon TG, Ma Y, Ludvigsson JF, Chong DQ, Giovannucci EL, Fuchs CS, et al. Association between aspirin use and risk of hepatocellular carcinoma. JAMA Oncol. 2018;4:1683–1690. doi: 10.1001/jamaoncol.2018.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Li X, Wu S, Yu Y. Aspirin use and the incidence of hepatocellular carcinoma in patients with hepatitis B virus or hepatitis C virus infection: a meta-analysis of cohort studies. Front Med (Lausanne) 2021;7:569759. doi: 10.3389/fmed.2020.569759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Choi WM, Kim HJ, Jo AJ, Choi SH, Han S, Ko MJ, et al. Association of aspirin and statin use with the risk of liver cancer in chronic hepatitis B: a nationwide population-based study. Liver Int. 2021;41:2777–2785. doi: 10.1111/liv.15011. [DOI] [PubMed] [Google Scholar]
- 268.Lee TY, Hsu YC, Tseng HC, Lin JT, Wu MS, Wu CY. Association of daily aspirin therapy with hepatocellular carcinoma risk in patients with chronic hepatitis c virus infection. Clin Gastroenterol Hepatol. 2020;18:2784–2792. doi: 10.1016/j.cgh.2020.04.036. e7. [DOI] [PubMed] [Google Scholar]
- 269.Lee TY, Hsu YC, Tseng HC, Yu SH, Lin JT, Wu MS, et al. Association of daily aspirin therapy with risk of hepatocellular carcinoma in patients with chronic hepatitis B. JAMA Intern Med. 2019;179:633–640. doi: 10.1001/jamainternmed.2018.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Liao YH, Hsu RJ, Wang TH, Wu CT, Huang SY, Hsu CY, et al. Aspirin decreases hepatocellular carcinoma risk in hepatitis C virus carriers: a nationwide cohort study. BMC Gastroenterol. 2020;20:6. doi: 10.1186/s12876-020-1158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hui VW, Yip TC, Wong VW, Tse YK, Chan HL, Lui GC, et al. Aspirin reduces the incidence of hepatocellular carcinoma in patients with chronic hepatitis b receiving oral nucleos(t)ide analog. Clin Transl Gastroenterol. 2021;12:e00324. doi: 10.14309/ctg.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lee PC, Yeh CM, Hu YW, Chen CC, Liu CJ, Su CW, et al. Antiplatelet therapy is associated with a better prognosis for patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Ann Surg Oncol. 2016;23(Suppl 5):874–883. doi: 10.1245/s10434-016-5520-9. [DOI] [PubMed] [Google Scholar]
- 273.Li JH, Wang Y, Xie XY, Yin X, Zhang L, Chen RX, et al. Aspirin in combination with TACE in treatment of unresectable HCC: a matched-pairs analysis. Am J Cancer Res. 2016;6:2109–2116. [PMC free article] [PubMed] [Google Scholar]
- 274.Young SH, Chau GY, Lee IC, Yeh YC, Chao Y, Huo TI, et al. Aspirin is associated with low recurrent risk in hepatitis B virus-related hepatocellular carcinoma patients after curative resection. J Formos Med Assoc. 2020;119(1 Pt 2):218–229. doi: 10.1016/j.jfma.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 275.Tan RZH, Lockart I, Abdel Shaheed C, Danta M. Systematic review with meta-analysis: the effects of non-steroidal antiinflammatory drugs and anti-platelet therapy on the incidence and recurrence of hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;54:356–367. doi: 10.1111/apt.16515. [DOI] [PubMed] [Google Scholar]
- 276.Lee M, Chung GE, Lee JH, Oh S, Nam JY, Chang Y, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017;66:1556–1569. doi: 10.1002/hep.29318. [DOI] [PubMed] [Google Scholar]
- 277.Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193–199. doi: 10.1016/j.nut.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 278.Lee JI, Lee HW, Lee KS, Lee HS, Park JY. Effects of statin use on the development and progression of nonalcoholic fatty liver disease: a nationwide nested case-control study. Am J Gastroenterol. 2021;116:116–124. doi: 10.14309/ajg.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 279.Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–1115. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 280.Ersöz G, Günşar F, Karasu Z, Akay S, Batur Y, Akarca US. Management of fatty liver disease with vitamin E and C compared to ursodeoxycholic acid treatment. Turk J Gastroenterol. 2005;16:124–128. [PubMed] [Google Scholar]