Abstract
Background/Aims
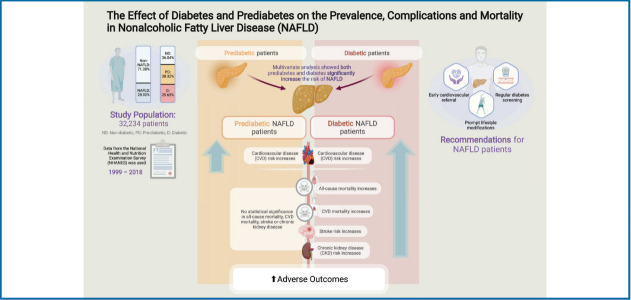
Nonalcoholic fatty liver disease (NAFLD) is closely associated with diabetes. The cumulative impact of both diseases synergistically increases risk of adverse events. However, present population analysis is predominantly conducted with reference to non-NAFLD individuals and has not yet examined the impact of prediabetes. Hence, we sought to conduct a retrospective analysis on the impact of diabetic status in NAFLD patients, referencing non-diabetic NAFLD individuals.
Methods
Data from the National Health and Nutrition Examination Survey 1999–2018 was used. Hepatic steatosis was defined with United States Fatty Liver Index (US-FLI) and FLI at a cut-off of 30 and 60 respectively, in absence of substantial alcohol use. A multivariate generalized linear model was used for risk ratios of binary outcomes while survival analysis was conducted with Cox regression and Fine Gray model for competing risk.
Results
Of 32,234 patients, 28.92% were identified to have NAFLD. 36.04%, 38.32% and 25.63% were non-diabetic, prediabetic and diabetic respectively. Diabetic NAFLD significantly increased risk of cardiovascular disease (CVD), stroke, chronic kidney disease, all-cause and CVD mortality compared to non-diabetic NAFLD. However, prediabetic NAFLD only significantly increased the risk of CVD and did not result in a higher risk of mortality.
Conclusions
Given the increased risk of adverse outcomes, this study highlights the importance of regular diabetes screening in NAFLD and adoption of prompt lifestyle modifications to reduce disease progression. Facing high cardiovascular burden, prediabetic and diabetic NAFLD individuals can benefit from early cardiovascular referrals to reduce risk of CVD events and mortality.
Keywords: Diabetes mellitus, Non-alcoholic fatty liver Disease, Prediabetic state
Graphical Abstract
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the fastest growing cause of chronic liver disease and is estimated to affect 25–33% of the global population [1-3]. The spectrum of NAFLD ranges from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH), with the latter being characterized by the presence of lobar inflammation, ballooning, and fibrosis [4]. The presence of NAFLD is associated with an increased risk of systemic complications including the development of cardiovascular disease (CVD) [5,6], hepatocellular carcinoma [7], extrahepatic malignancy, and even depression [8]. In particular, the development and progression of NAFLD is closely associated with diabetes which is driven by insulin resistance and alterations in lipoprotein metabolism [9].
A recent meta-analysis estimated that up to half of individuals with NAFLD [10] have diabetes and the cumulative impact of both diseases synergistically increases the risk of both hepatic and extrahepatic events [9]. Pooled analysis of prospective studies has also shown a 2.19 increase in hazard ratio in the development of diabetes among patients with NAFLD, which supports a bi-directional relationship between both disease pathways [11]. Current consensus by the American Diabetic Association recommends that patients with type 2 diabetes with either elevation in liver enzymes or fatty liver on ultrasound imaging should be screened for NASH [12] or fibrosis. Several observational studies have also found that the presence of diabetes in NAFLD increases the risk of all cause and cardiovascular mortality compared to non-NAFLD diabetics [13,14].
While the awareness of diabetes and NAFLD is well established, prediabetes is a lesser-known entity in NAFLD. Prediabetes, a state of dysfunction albeit to a lesser degree of insulin sensitivity and impairment of β-cell function, has been found to be associated with NAFLD and its accompanying metabolic complications [15]. However, current literature mainly focuses on the prevalence and risk factors of NAFLD in prediabetes patients [16,17] with limited studies on clinical outcomes. Additionally, studies on the outcomes of prediabetes and diabetes with NAFLD are often conducted with reference to patients without NAFLD. Hence, we sought to examine the prevalence, outcomes, and impact of prediabetes and diabetes with reference to NAFLD without diabetes, using patients recruited in the United States National Health and Nutrition Examination Survey (NHANES) between 1999–2018.
MATERIALS AND METHODS
This study analyses patients recruited between 1999–2018 of NHANES. Briefly, the NHANES study was a cross-sectional survey platform that adopted a stratified, multistage, clustered probability sampling design which studies individuals representative of the general non-institutionalised population. Longitudinal outcomes of mortality are supplemented with data from the national death index. The NHANES study also involved a structured interview conducted in patients’ home, with subsequent standardised health examination conducted at a mobile examination centre for physical examinations and laboratory tests. The original survey was approved by the National Centre for Health Statistics Research Ethics Review Board. As the data used in the analysis is publicly available and de-identified, Institutional Review Board for the present analysis was not required.
Definition
The definition of NAFLD was adapted based on the American Association for the Study of Liver Disease (AASLD) guidelines for NAFLD. We defined NAFLD as the presence of steatosis is the absence of substantial alcohol use (≥2 drinks a day in men, ≥3 drinks a day in women). The presence of steatosis in NAFLD was quantified with either Fatty Liver Index (FLI) or United States FLI (US-FLI) with a cut-off of ≥60 [18] and ≥30 [19], respectively. Diabetes was defined as glycohemoglobin (HbA1c) ≥6.5%, fasting plasma glucose ≥7 mmol/L, self-reported diabetes or the use of anti-diabetic medications. Prediabetes was defined as HbA1c between 5.7–6.5% or fasting plasma glucose between 5.6–7 mmol/L [20]. Non-invasive tests (NITs) for fibrosis include Aspartate Aminotransferase to Platelet Ratio Index (APRI), fibrosis-4 (FIB-4) index and NAFLD Fibrosis Score. These tests have accuracies of area under curve 0.74, 0.80 and 0.75–0.82, respectively in the diagnosis of advanced fibrosis [21]. Lean patients were defined as having a body mass index (BMI) of <23 kg/m2 for Asians and a BMI <25 kg/m2 for other races. Patients were considered overweight when they had BMI between 23–27.5 kg/m2 for Asians and 25–30 kg/m2 for other races. Obese patients were defined as BMI >27.5 kg/m2 for Asians and BMI >30 kg/m2 for other races [22]. Hypertension was defined as a systolic or diastolic blood pressure ≥130/85 or the use of anti-hypertensive medications. Chronic kidney disease (CKD) was defined as the presence of kidney damage or an estimated glomerular filtration rate of less than 60 mL/min/1.73 m2 under the modification of diet in renal disease (MDRD) equation [23].
Statistical analysis
All statistical analysis was conducted in STATA (ver. 16.1; Statacorp, Chicaco, IL, USA) and Rstudio (ver. 4.0.3; PBC, Boston, MA, USA). Continuous variables were examined with Wilcoxon ranked sum test and Kruskal-Wallis analysis of variance while binary variables were examined with chi-square test and fisher exact where appropriate. A multivariate generalized linear model with a log link and robust variance estimator was use to examine the risk of binary events including risk of NAFLD, CVD, CKD, stroke [24]. A risk ratio (RR) was used to compare risk between both groups. The RR is a better approximation of events in common events and provides better interpretability compared to odds ratios [25]. Survival analysis was conducted with Cox proportional model for all-cause mortality and a Fine Gray sub-distribution hazard ratio (SHR) was calculated for cardiovascular mortality to account for competing risk. The multivariate model included variables that are common confounders of both all-cause and cardiovascular mortality in NAFLD including age, gender, race, BMI, previous myocardial infraction, and CKD.
RESULTS
Baseline characteristics and associated factors
A total of 32,234 patients were included in the analysis and 13,112 (28.92%) individuals were found to have NAFLD. A total of 20,139 individuals were non-NAFLD and non-diabetic. Of the 13,112 individuals with NAFLD, 12,932 had glycaemic measures quantified and of these, 4,661 (36.04%; 95% confidence interval [CI], 35.22% to 36.87%) were non-diabetic, while 4,956 (38.32%; 95% CI, 37.49% to 39.16%) and 3,315 (25.63%; 95% CI, 24.89% to 26.39%) were NAFLD patients with prediabetes and diabetes respectively (Fig. 1). A comparison between baseline characteristics of diabetic NAFLD, prediabetic NAFLD and non-diabetic NAFLD is summarized in Table 1. After adjusting for confounders including age, gender, race, and BMI, results from a generalized linear regression with robust estimator found older age (RR, 1.02; 95% CI, 1.02 to 1.03; P<0.01) and higher BMI (RR, 1.02; 95% CI, 1.02 to 1.03; P<0.01) to be associated risk factors of prediabetes in NAFLD. Hispanics (RR, 1.10; 95% CI, 1.04 to 1.15; P<0.01) and African Americans (RR, 1.07; 95% CI, 1.03 to 1.12; P<0.01) were also associated with an increased risk of prediabetes in NAFLD. Conversely, Caucasians (RR, 0.81; 95% CI, 0.78 to 0.84; P<0.001) and female gender (RR, 0.84; 95% CI, 0.82 to 0.86; P<0.01) were associated with reduced risks of prediabetes in NAFLD compared to non-diabetic NAFLD patients. Similarly, older age (RR, 1.04; 95% CI, 1.02 to 1.04; P<0.01), and higher BMI (RR, 1.04; 95% CI, 1.04 to 1.05; P<0.01) were found to be associated risk factors for diabetes in NAFLD. Female gender (RR, 0.82; 95% CI, 0.79 to 0.85; P<0.01) was associated with lower risk of diabetes in NAFLD. Comparisons of NITs for fibrosis can be found in Figure 2. The NITs were generally found to be the highest in diabetic individuals, although the presence of prediabetes also significantly increased NITs for hepatic steatosis and fibrosis compared to non-diabetic NAFLD. A multivariate generalized linear model with robust variance estimator found that both prediabetes (RR, 1.27; 95% CI, 1.22 to 1.32; P<0.01) and diabetes (RR, 1.38; 95% CI, 1.30 to 1.50; P<0.01) significantly increased the associated risk of NAFLD respectively.
Figure 1.
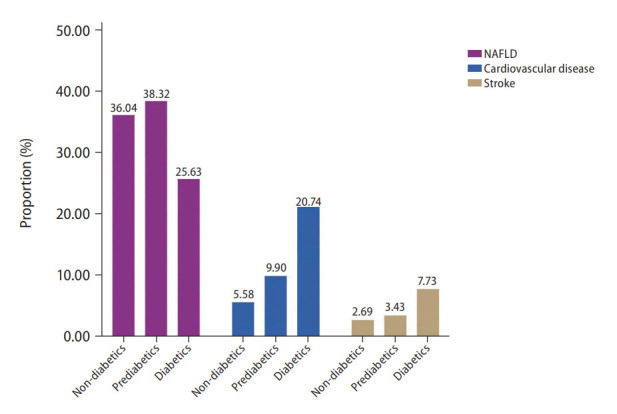
Proportion of nonalcoholic fatty liver disease (NAFLD), cardiovascular disease, and stroke.
Table 1.
Baseline demographics of diabetes, prediabetes and non-diabetes with NAFLD
| Diabetes (n=3,315) | Pre-diabetes (n=4,956) | No diabetes (n=4,661) | P-value | ||
|---|---|---|---|---|---|
| Age (years) | 62.00 (IQR, 53.00 to 71.00) | 56.00 (IQR, 43.00 to 68.00) | 44.00 (IQR, 32.00 to 60.00) | <0.01* | |
| Gender, male | 0.44 (95% CI, 0.43 to 0.46) | 0.46 (95% CI, 0.45 to 0.47) | 0.44 (95% CI, 0.43 to 0.46) | 0.14 | |
| Platelet (1,000 cells/μL) | 242.00 (IQR, 202.00 to 292.00) | 250.00 (IQR, 210.00 to 295.00) | 256.00 (IQR, 217.00 to 301.00) | <0.01* | |
| Glycohemoglobin (%) | 6.90 (IQR, 6.30 to 8.00) | 5.80 (IQR, 5.60 to 6.00) | 5.30 (IQR, 5.10 to 5.50) | <0.01* | |
| Fasting glucose (mmol/L) | 7.88 (IQR, 6.88 to 9.99) | 5.88 (IQR, 5.66 to 6.22) | 5.22 (IQR, 4.94 to 5.38) | 0.01* | |
| Total bilirubin (umol/L) | 10.26 (IQR, 6.84 to 13.68) | 10.26 (IQR, 6.84 to 13.68) | 10.26 (IQR, 6.84 to 13.68) | 0.17 | |
| AST (IU/L) | 22.00 (IQR, 19.00 to 28.00) | 23.00 (IQR, 19.00 to 28.00) | 22.00 (IQR, 19.00 to 26.00) | <0.01* | |
| ALT (IU/L) | 22.00 (IQR, 16.00 to 29.00) | 21.00 (IQR, 17.00 to 29.00) | 19.00 (IQR, 15.00 to 26.00) | <0.01* | |
| GGT (IU/L) | 25.00 (IQR, 17.00 to 39.00) | 22.00 (IQR, 16.00 to 33.00) | 17.00 (IQR, 13.00 to 26.00) | <0.01* | |
| LDL (mg/dL) | 103.00 (IQR, 79.00 to 130.00) | 118.00 (IQR, 95.00 to 142.00) | 109.00 (IQR, 88.00 to 133.00) | <0.01* | |
| HDL (mg/dL) | 46.00 (IQR, 39.00 to 56.00) | 50.00 (IQR, 41.00 to 60.00) | 53.00 (IQR, 44.00 to 65.00) | <0.01* | |
| Total cholesterol (mg/dL) | 186.00 (IQR, 158.00 to 217.00) | 197.00 (IQR, 172.00 to 226.00) | 189.00 (IQR, 163.00 to 217.00) | <0.01* | |
| Triglycerides (mg/dL) | 153.00 (IQR, 104.00 to 229.00) | 124.00 (IQR, 85.00 to 184.00) | 103.00 (IQR, 70.00 to 158.00) | <0.01* | |
| Waist circumference (cm) | 111.40 (IQR, 103.50 to 121.10) | 108.20 (IQR, 101.40 to 117.10) | 107.20 (IQR, 101.20 to 115.00) | <0.01* | |
| Body mass index (kg/m2) | 32.88 (IQR, 29.40 to 37.60) | 32.40 (IQR, 29.21 to 36.56) | 32.32 (IQR, 29.40 to 36.20) | <0.01* | |
| Weight (kg) | 90.30 (IQR, 78.10 to 105.10) | 90.50 (IQR, 79.60 to 104.20) | 91.50 (IQR, 81.20 to 103.50) | 0.03* | |
| Hypertension | 0.83 (95% CI, 0.82 to 0.85) | 0.65 (95% CI, 0.64 to 0.67) | 0.48 (95% CI, 0.46 to 0.49) | <0.01* | |
| Ethnicity | <0.01* | ||||
| Mexican American | 0.20 (95% CI, 0.19 to 0.22) | 0.18 (95% CI, 0.17 to 0.19) | 0.19 (95% CI, 0.18 to 0.20) | ||
| Hispanic | 0.09 (95% CI, 0.08 to 0.10) | 0.09 (95% CI, 0.08 to 0.09) | 0.07 (95% CI, 0.07 to 0.08) | ||
| White | 0.38 (95% CI, 0.37 to 0.40) | 0.43 (95% CI, 0.41 to 0.44) | 0.49 (95% CI, 0.48 to 0.51) | ||
| Black | 0.25 (95% CI, 0.23 to0.26) | 0.23 (95% CI, 0.21 to 0.24) | 0.18 (95% CI, 0.17 to 0.20) | ||
| Other race | 0.08 (95% CI, 0.07 to 0.09) | 0.08 (95% CI, 0.08 to 0.09) | 0.06 (95% CI, 0.06 to 0.07) | ||
| Overweight | 0.87 (95% CI, 0.85 to 0.89) | 0.91 (95% CI, 0.90 to 0.93) | 0.93 (95% CI, 0.92 to 0.94) | <0.01* | |
| Obese | 0.72 (95% CI, 0.71 to 0.74) | 0.71 (95% CI, 0.69 to 0.72) | 0.71 (95% CI, 0.70 to 0.72) | 0.21 | |
NAFLD, nonalcoholic fatty liver disease; IQR, interquartile range; CI, confidence interval; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyl transferase; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
P-value ≤0.05 denotes statistical significance.
Figure 2.
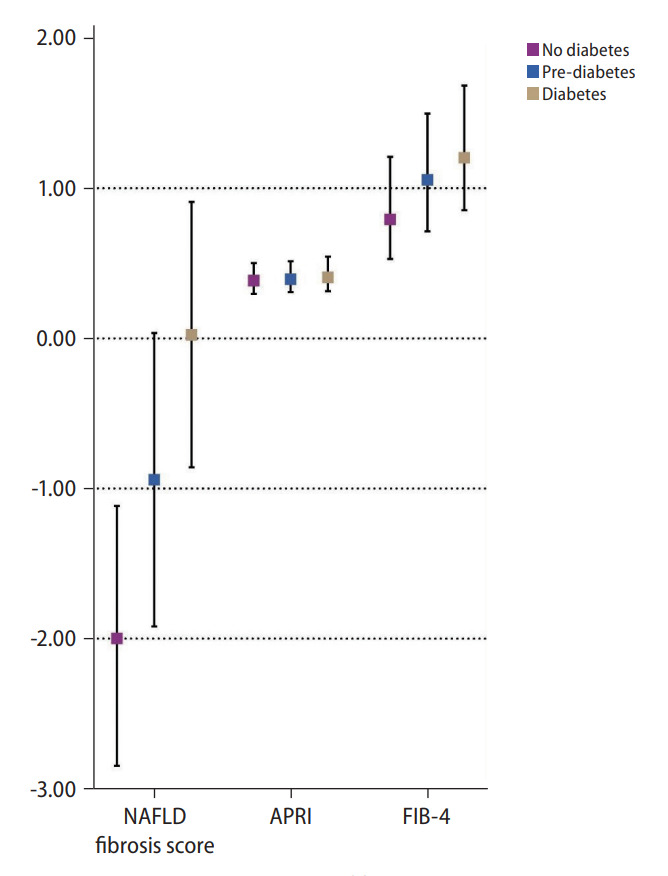
Non-invasive measures of fibrosis in non-diabetic, prediabetic and diabetic nonalcoholic fatty liver disease (NAFLD). APRI, Aspartate Aminotransferase to Platelet Ratio Index; FIB-4, fibrosis-4.
Diabetic and prediabetic NAFLD vs. non-diabetic NAFLD
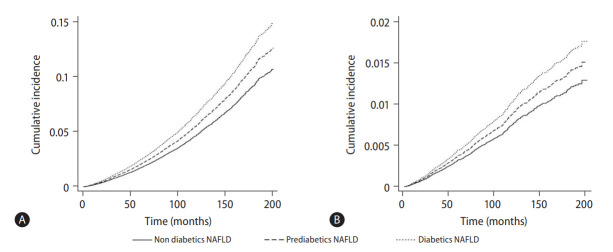
A multivariate generalized linear model was conducted to examine the risk of CVD, stroke, and CKD in NAFLD patients with prediabetes and diabetes (Fig. 3, Table 2). Both the presence of prediabetic NAFLD (RR, 1.20; 95% CI, 1.03 to 1.41; P=0.02) and diabetes NAFLD (RR, 1.90; 95% CI, 1.64 to 2.21; P<0.01) were associated with a significant increase in risk of CVD compared to non-diabetic NAFLD in an adjusted model. However, only diabetic NAFLD (RR, 1.66; 95% CI, 1.29 to 2.15; P<0.01) and not prediabetic NAFLD (RR, 0.89; 95% CI, 0.69 to 1.66; P=0.41) was associated with an increased risk of stroke compared to non-diabetic NAFLD in an adjusted multivariate analysis. Similarly, the risk of CKD was only significantly higher in diabetic NAFLD (RR, 1.47; 95% CI, 1.10 to 1.97; P=0.01) and not in prediabetic NAFLD (RR, 0.93; 95% CI, 0.81 to 1.09; P=0.41), compared to non-diabetic NAFLD. Survival analysis was conducted to examine the overall mortality and CVD mortality between diabetic NAFLD, prediabetic NAFLD, and non-diabetic NAFLD (Table 2). In a multivariate cox proportional model adjusted for age, gender, race, BMI, previous myocardial infraction, and CKD, diabetic NAFLD (HR, 1.60; 95% CI, 1.38 to 1.85; P<0.01; Fig. 4A) but not prediabetic NAFLD (HR, 1.14; 95% CI, 0.99 to 1.34; P=0.06; Fig. 4A) increased all-cause mortality compared to non-diabetic NAFLD. A competing risk analysis was conducted with a Fine Grey model for the SHR of CVD mortality with NAFLD. There was a significant increased risk of CVD mortality in diabetic NAFLD (SHR, 1.41; 95% CI, 1.02 to 1.94; P=0.04; Fig. 4B) but not in prediabetic NAFLD (SHR, 1.11; 95% CI, 0.82 to 1.52; P=0.49; Fig. 4B), compared to non-diabetic NAFLD.
Figure 3.
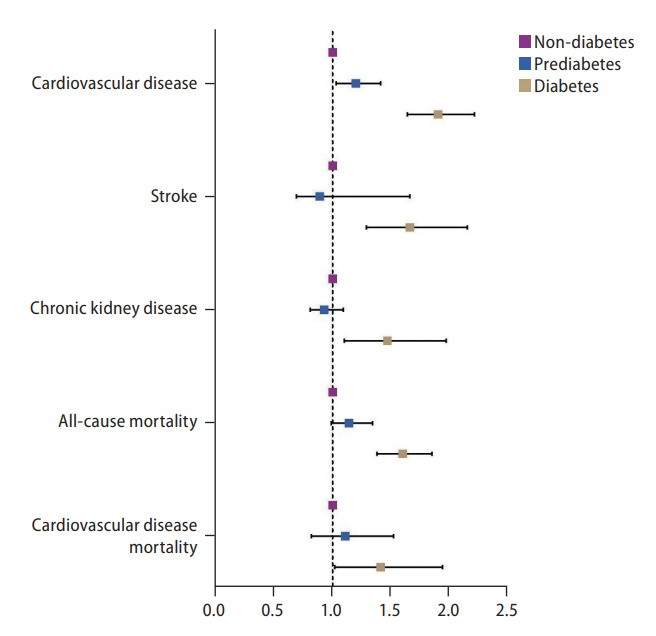
Forest plot of outcomes in non-diabetic, pre-diabetic, and diabetic nonalcoholic fatty liver disease.
Table 2.
Multivariate analysis of outcomes in pre-diabetic and diabetic NAFLD
| Relative to non-diabetic NAFLD |
Relative to non-diabetic non-NAFLD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Diabetes |
Pre-diabetes |
Diabetes |
Pre-diabetes |
|||||
| Effect size | P-value | Effect size | P-value | Effect size | P-value | Effect size | P-value | |
| CVD | RR, 1.90 (95% CI, 1.64 to 2.21) | <0.01* | RR, 1.20 (95% CI, 1.03 to 1.41) | 0.02* | RR, 1.85 (95% CI, 1.63 to 2.10) | <0.01* | RR, 1.18 (95% CI, 1.03 to 1.35) | 0.02* |
| Stroke | RR, 1.66 (95% CI, 1.29 to 2.15) | <0.01* | RR, 0.89 (95% CI, 0.69 to 1.66) | 0.41 | RR, 1.35 (95% CI, 1.26 to 1.43) | <0.01* | RR, 0.98 (95% CI, 0.91 to 1.05) | 0.61 |
| CKD | RR, 1.47 (95% CI, 1.10 to 1.97) | 0.01* | RR, 0.93 (95% CI, 0.81 to 1.09) | 0.41 | RR, 1.82 (95% CI, 1.60 to 2.08) | <0.01* | RR, 1.17 (95% CI, 1.01 to 1.36) | 0.04* |
| CVD mortality | SHR, 1.41 (95% CI, 1.02 to 1.94) | 0.04* | SHR, 1.11 (95% CI, 0.82 to 1.52) | 0.49 | SHR, 1.30 (95% CI, 0.99 to 1.71) | 0.06 | SHR, 1.00 (95% CI, 0.77 to 1.31) | 0.99 |
| Overall mortality | HR, 1.60 (95% CI, 1.38 to 1.85) | <0.01* | HR, 1.14 (95% CI, 0.99 to 1.34) | 0.06 | HR, 1.35 (95% CI, 1.19 to 1.52) | <0.01* | HR, 0.98 (95% CI, 0.87 to 1.10) | 0.98 |
NAFLD, nonalcoholic fatty liver disease; CVD, cardiovascular disease; RR, risk ratio; CI, confidence interval; CKD, chronic kidney disease; SHR, sub-distribution hazard ratio; HR, hazard ratio.
P-value ≤0.05 denotes statistical significance.
Figure 4.
(A) Overall mortality in non-diabetic, prediabetic, and diabetic nonalcoholic fatty liver disease (NAFLD). (B) Cardiovascular mortality in non-diabetic, prediabetic and diabetic NAFLD.
Diabetic and prediabetic NAFLD vs. non-diabetic non-NAFLD
A sensitivity analysis was also conducted to examine the relative effect of prediabetic NAFLD and diabetic NAFLD with non-diabetic non-NAFLD (Table 2). Both prediabetic NAFLD (RR, 1.18; 95% CI, 1.03 to 1.35; P=0.02; RR, 1.17; 95% CI, 1.01 to 1.36; P=0.04) and diabetic NAFLD (RR, 1.85; 95% CI, 1.63 to 2.10; P<0.01; RR, 1.82; 95% CI, 1.60 to 2.08; P<0.01) were at an increased risk of CVD events and CKD, respectively. Stroke however was only associated with an increased risk in diabetic NAFLD (RR, 1.35; 95% CI, 1.26 to 1.43; P<0.01), but not in prediabetic NAFLD (RR, 0.98; 95% CI, 0.91 to 1.05; P=0.61) when compared to non-diabetic non-NAFLD. In survival analysis of overall mortality, there was no significant increased risk of overall mortality in prediabetic NAFLD compared to non-diabetic non-NAFLD (HR, 0.98; 95% CI, 0.87 to 1.10; P=0.98). However, diabetic NAFLD significantly increased the risk of overall mortality compared to non-diabetic non-NAFLD (HR, 1.35; 95% CI, 1.19 to 1.52; P<0.01). There was no increase in CVD mortality in prediabetic NAFLD (SHR, 1.00; 95% CI, 0.77 to 1.31; P=0.99) compared to non-diabetic non-NAFLD. However, diabetic NAFLD (SHR, 1.30; 95% CI, 0.99 to 1.71; P=0.06) resulted in a borderline non-significant increase in CVD mortality compared to non-diabetic non-NAFLD.
DISCUSSION
Evidence from meta-analyses have shown that NAFLD increases the risk of CKD, CVD, stroke, cardiovascular and all-cause mortality compared to the general population [13,14]. While insulin resistance is a core pathway in the pathogenesis of NAFLD [9], current large scale population studies [13,14] were conducted with reference to non-NAFLD diabetic patients, and were less focused on the effect relative to NAFLD without diabetes or have not accounted for competing risk [26] mortality. Moreover, the implications of prediabetes, a sign of metabolic perturbation, have not been examined among NAFLD patients. The present analysis of 23,987 patients adds to the literature by examining the synergistic effect of prediabetes and diabetes respectively in NAFLD compared to controls of non-diabetic NAFLD. In a population level analysis of NAFLD, 38.32% and 25.63% of NAFLD individuals had prediabetes and diabetes respectively which was associated with an increase in end organ complications in NAFLD.
Prediabetes is an intermediate state of hyperglycaemia with raised glycaemic parameters below the diabetic threshold [27]. Pathogenically, it differs from diabetes in its smaller extent of insulin resistance [28] and its potential for reversibility with lifestyle modification [29]. Additionally, it is a condition that is easily detectable in clinical settings. In our study, prediabetic NAFLD was found to increase only CVD but was not associated with a higher risk of stroke, CKD, overall mortality and CVD mortality. Importantly, only diabetics with NAFLD was associated with an increased risk of cardiovascular mortality compared to non-diabetic NAFLD in a competing risk analysis. Conventional wisdom suggests CVD disease to be the leading cause of death in NAFLD [30] and a previous meta-analysis conducted by Mantovani et al. [31] found a higher rate of fatal and non-fatal myocardial infarction among NAFLD patients with diabetes. CVD is similarly a major burden in diabetics with synergistic effect from both diseases, where patients with diabetes may experience adverse CVD events at an earlier age [32]. Previous studies have found up to 2.5 times increase in risk of CVD mortality in diabetic patients as compared to non-diabetic patients [33]. Considering the aggregate burden of diabetes and NAFLD, individuals with prediabetic and diabetic NAFLD may benefit from early referrals for cardiovascular risk assessment.
Despite the significant burden of NAFLD, current guidelines by the AASLD have not yet emphasised the need for routine assessment of glycaemic assessment in NAFLD individuals [34] without diabetes. NAFLD patients without diabetes at presentation may benefit from frequent monitoring of the Hba1c test. Prompt treatment with lifestyle modifications should be initiated to prevent progression from prediabetes to diabetes, which can significantly reduce the morbidity and mortality of these individuals. Lifestyle modifications are an essential aspect of both conditions and can potentially be synergistically employed with wearable technologies or electronic health applications. NAFLD patients with diabetes on the other hand may benefit from the use of glucagon-like peptide-1 receptor agonists (GLP1-RA [35]) or sodium-glucose co-transporter-2 inhibitors (SGLT2i [36]) in addition to metformin, with both agents showing significant reduction in fibrosis, steatosis and added benefits of cardiovascular protection in diabetics [37].
Limitations
The current analysis uses a population database from the NHANES study from 1999 to 2018. There are, however, several limitations to the results. Firstly, the quantification of alcohol intake is subject to recall bias. Next, FLI and US-FLI are NITs that only offer a gauge measure of steatosis but were deemed suitable measures in the setting of population studies. Glycaemic control is a flexible measure that can change with time, and the analysis only captured a snapshot of control at the point of inclusion. Despite adjusting for age, a younger age may also serve to be a confounder of the analysis since events of mortality may have yet to occur in younger individuals.
Conclusion
The current analysis reinforces the importance of glycaemic control in NAFLD. Individuals identified with NAFLD will benefit from frequent monitoring and prompt lifestyle changes should be initiated early in the course of disease to prevent the progression into type 2 diabetes which can significantly increase mortality and morbidity of the disease. Individuals with prediabetic and diabetic NAFLD might also benefit from early cardiovascular risk assessment. Pharmacological agents similarly should be targeted to improve glycaemic control, fibrosis and provide cardiovascular protection.
Acknowledgments
All authors have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted. No writing assistance was obtained in the preparation of the manuscript. The manuscript, including related data, figures and tables has not been previously published and that the manuscript is not under consideration elsewhere.
Abbreviations
- AASLD
American Association for the Study of Liver Disease
- APRI
Aspartate Aminotransferase to Platelet Ratio Index
- BMI
body mass index
- CI
confidence interval
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- FIB-4
fibrosis-4
- FLI
Fatty Liver Index
- GLP1-RA
glucagon-like peptide-1 receptor agonist
- HbA1c
glycohemoglobin
- MDRD
modification of diet in renal disease
- NAFL
non-alcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NHANES
National Health and Nutrition Examination Survey
- NITs
non-invasive tests
- RR
risk ratio
- SGLT2i
sodium-glucose co-transporter-2 inhibitor
- SHR
sub-distribution hazard ratio
- US-FLI
United States Fatty Liver Index
Study Highlights
• Diabetes is a common comorbidity present with NAFLD subjects.
• Longitudinal outcomes on NAFLD subjects with diabetes were poorer compared NAFLD subjects without diabetes: increasing risks of cardiovascular mortality, cardiovascular disease, stroke and chronic kidney disease.
Footnotes
Authors’ contributions
All authors approve the final version of the manuscript, including the authorship list and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of Article: Cheng Han Ng; Conceptualisation and Design: Cheng Han Ng, Mark Muthiah; Acquisition of Data: Cheng Han Ng, Wen Hui Lim, Yip Han Chin, Kai En Chan; Analysis and Interpretation of Data: Cheng Han Ng, Wen Hui Lim, Yip Han Chin, Kai En Chan, Rebecca Wenling Zeng, Pei Chen Tsai, Jie Ning Yong, Darren Jun Hao Tan; Writing – original draft: Cheng Han Ng, Wen Hui Lim, Yip Han Chin, Kai En Chan, Rebecca Wenling Zeng, Jie Ning Yong, Pei Chen Tsai, Darren Jun Hao Tan; Writing – review & editing: Chin Meng Khoo, Lay Hoon Goh, Zheng Jye Ling, Anand Kulkarni, Lung-Yi Loey Mak, Daniel Q Huang, Mark Chan, Nicholas WS Chew, Mohammad Shadab Siddiqui, Arun J. Sanyal, Mark Muthiah
Conflicts of Interest
AJS is President of Sanyal Biotechnology and has stock options in Genfit, Akarna, Tiziana, Indalo, Durect and Galmed. He has served as a consultant to Astra Zeneca, Nitto Denko, Enyo, Ardelyx, Conatus, Nimbus, Amarin, Salix, Tobira, Takeda, Jannsen, Gilead, Terns, Birdrock, Merck, Valeant, Boehringer-Ingelheim, Lilly, Hemoshear, Zafgen, Novartis, Novo Nordisk, Pfizer, Exhalenz and Genfit. He has been an unpaid consultant to Intercept, Echosens, Immuron, Galectin, Fractyl, Syntlogic, Affimune, Chemomab, Zydus, Nordic Bioscience, Albireo, Prosciento, Surrozen and Bristol Myers Squibb. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers, Shire, Intercept, Merck, Astra Zeneca, Malinckrodt, Cumberland and Norvatis. He receives royalties from Elsevier and UptoDate. MN has been on the advisory board for 89BIO, Gilead, Intercept, Pfizer, Novo Nordisk, Blade, EchoSens, Fractyl, Terns, Siemens and Roche diagnostic; MN has received research support from Allergan, BMS, Gilead, Galmed, Galectin, Genfit, Conatus, Enanta, Madrigal, Novartis, Pfizer, Shire, Viking and Zydus; MN is a minor shareholder or has stocks in Anaetos, Rivus Pharma and Viking.
REFERENCES
- 1.Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2019;16:377–386. doi: 10.1038/s41575-019-0144-8. [DOI] [PubMed] [Google Scholar]
- 2.Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, et al. An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2021.11.038. 2021 Dec 4. doi: [DOI] [PubMed] [Google Scholar]
- 3.Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, et al. 2019 Global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2021.12.002. 2021 Dec 7. doi: [DOI] [PubMed] [Google Scholar]
- 4.Muthiah MD, Cheng Han N, Sanyal AJ. A clinical overview of non-alcoholic fatty liver disease: a guide to diagnosis, the clinical features, and complications-what the non-specialist needs to know. Diabetes Obes Metab. 2022;24 Suppl 2:3–14. doi: 10.1111/dom.14521. [DOI] [PubMed] [Google Scholar]
- 5.Tang ASP, Chan KE, Quek J, Xiao J, Tay P, Teng M, et al. Non-alcoholic fatty liver disease increases risk of carotid atherosclerosis and ischemic stroke: an updated meta-analysis with 135,602 individuals. Clin Mol Hepatol. 2022;28:483–496. doi: 10.3350/cmh.2021.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toh JZK, Pan XH, Tay PWL, Ng CH, Yong JN, Xiao J, et al. A meta-analysis on the global prevalence, risk factors and screening of coronary heart disease in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2021.09.021. 2021 Sep 22. doi: [DOI] [PubMed] [Google Scholar]
- 7.Tan DJH, Ng CH, Lin SY, Pan XH, Tay P, Lim WH, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 2022;23:521–530. doi: 10.1016/S1470-2045(22)00078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao J, Lim LKE, Ng CH, Tan DJH, Lim WH, Ho CSH, et al. Is fatty liver associated with depression? A meta-analysis and systematic review on the prevalence, risk factors, and outcomes of depression and non-alcoholic fatty liver disease. Front Med (Lausanne) 2021;8:691696. doi: 10.3389/fmed.2021.691696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18:599–612. doi: 10.1038/s41575-021-00448-y. [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. 2021;70:962–969. doi: 10.1136/gutjnl-2020-322572. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S34–S45. doi: 10.2337/dc19-S004. [DOI] [PubMed] [Google Scholar]
- 13.Mallet V, Parlati L, Martinino A, Scarano Pereira JP, Jimenez CN, Sakka M, et al. Burden of liver disease progression in hospitalized patients with type 2 diabetes mellitus. J Hepatol. 2022;76:265–274. doi: 10.1016/j.jhep.2021.09.030. [DOI] [PubMed] [Google Scholar]
- 14.Wild SH, Walker JJ, Morling JR, McAllister DA, Colhoun HM, Farran B, et al. Cardiovascular disease, cancer, and mortality among people with type 2 diabetes and alcoholic or nonalcoholic fatty liver disease hospital admission. Diabetes Care. 2018;41:341–347. doi: 10.2337/dc17-1590. [DOI] [PubMed] [Google Scholar]
- 15.Taharboucht S, Guermaz R, Brouri M, Chibane A. Pre-diabetes and NAFLD; a study of an Algerian population sample. Endocrine and Metabolic Science. 2020;1:100060. [Google Scholar]
- 16.Vesa CM, Behl T, Nemeth S, Bratu OG, Diaconu CC, Moleriu RD, et al. Prediction of NAFLD occurrence in prediabetes patients. Exp Ther Med. 2020;20:190. doi: 10.3892/etm.2020.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajput R, Ahlawat P. Prevalence and predictors of non-alcoholic fatty liver disease in prediabetes. Diabetes Metab Syndr. 2019;13:2957–2960. doi: 10.1016/j.dsx.2019.07.060. [DOI] [PubMed] [Google Scholar]
- 18.Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2015;41:65–76. doi: 10.1111/apt.13012. [DOI] [PubMed] [Google Scholar]
- 19.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Standards of medical care in diabetes--2015: summary of revisions. Diabetes Care. 2015;38 Suppl:S4. doi: 10.2337/dc15-S003. [DOI] [PubMed] [Google Scholar]
- 21.Wong VW, Adams LA, de Lédinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15:461–478. doi: 10.1038/s41575-018-0014-9. [DOI] [PubMed] [Google Scholar]
- 22.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 24.Cummings P. Methods for estimating adjusted risk ratios. Stata J. 2009;9:175–196. [Google Scholar]
- 25.Cummings P. The relative merits of risk ratios and odds ratios. Arch Pediatr Adolesc Med. 2009;163:438–445. doi: 10.1001/archpediatrics.2009.31. [DOI] [PubMed] [Google Scholar]
- 26.Wu W, Xiang J, Chen X. Association between diabetes mellitus and all-cause and cardiovascular mortality among individuals with ultrasound-defined non-alcoholic fatty liver disease. Front Endocrinol (Lausanne) 2021;12:773342. doi: 10.3389/fendo.2021.773342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hostalek U. Global epidemiology of prediabetes - present and future perspectives. Clin Diabetes Endocrinol. 2019;5:5. doi: 10.1186/s40842-019-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bansal N. Prediabetes diagnosis and treatment: a review. World J Diabetes. 2015;6:296–303. doi: 10.4239/wjd.v6.i2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Przybyszewski EM, Targher G, Roden M, Corey KE. Nonalcoholic fatty liver disease and cardiovascular disease. Clin Liver Dis (Hoboken) 2021;17:19–22. doi: 10.1002/cld.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantovani A, Csermely A, Petracca G, Beatrice G, Corey KE, Simon TG, et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:903–913. doi: 10.1016/S2468-1253(21)00308-3. [DOI] [PubMed] [Google Scholar]
- 32.Almourani R, Chinnakotla B, Patel R, Kurukulasuriya LR, Sowers J. Diabetes and cardiovascular disease: an update. Curr Diab Rep. 2019;19:161. doi: 10.1007/s11892-019-1239-x. [DOI] [PubMed] [Google Scholar]
- 33.Yang JJ, Yu D, Wen W, Saito E, Rahman S, Shu XO, et al. Association of diabetes with all-cause and cause-specific mortality in Asia: a pooled analysis of more than 1 million participants. JAMA Netw Open. 2019;2:e192696. doi: 10.1001/jamanetworkopen.2019.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Targher G. Glucagon-like peptide-1 receptor agonists for treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: an updated meta-analysis of randomized controlled trials. Metabolites. 2021;11:73. doi: 10.3390/metabo11020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong C, Yaow CYL, Ng CH, Chin YH, Low YF, Lim AYL, et al. Sodium-glucose co-transporter 2 inhibitors for non-alcoholic fatty liver disease in asian patients with type 2 diabetes: a meta-analysis. Front Endocrinol (Lausanne) 2021;11:609135. doi: 10.3389/fendo.2020.609135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng CH, Lin SY, Chin YH, Lee MH, Syn N, Goh XL, et al. Antidiabetic medications for type 2 diabetics with nonalcoholic fatty liver disease: evidence from a network meta-analysis of randomized controlled trials. Endocr Pract. 2022;28:223–230. doi: 10.1016/j.eprac.2021.09.013. [DOI] [PubMed] [Google Scholar]