Abstract
Cutibacterium acnes (previously known as Propionibacterium) infections are reportedly increasing in patients with implanted foreign material. Though it is a rare cause of bacterial endocarditis, patients with implanted prosthetic valves and devices have potential increased risk. Cutibacterium species are an ubiquitous environmental surface contaminant and typically difficult to culture, in case of high suspicion for infective endocarditis extended duration incubation of blood or any tissue sample and 16S RNA sequencing of any tissue sample is helpful for a microbiological identification. We report a case of a 50 year old male with culture negative prosthetic valve endocarditis in which the pathogen was identified by molecular testing 16S RNA gene sequencing.
Highlights
-
•
Prosthetic valve endocarditis.
-
•
Cutibacterium acnes.
-
•
16S gene sequencing.
-
•
Management challange for prostetic valve endocarditis.
Introduction
Cutibacterium acnes (previously known as Propionibacterium, C. acnes) infections are reportedly increasing in patients with implanted foreign material [1]. Though it is a rare cause of bacterial endocarditis, patients with implanted prosthetic valves and devices have potential increased risk [2], [3], [4]. We report a case of a 50 year old male with culture negative prosthetic valve endocarditis in which the pathogen was identified by molecular testing 16S RNA gene sequencing.
Case
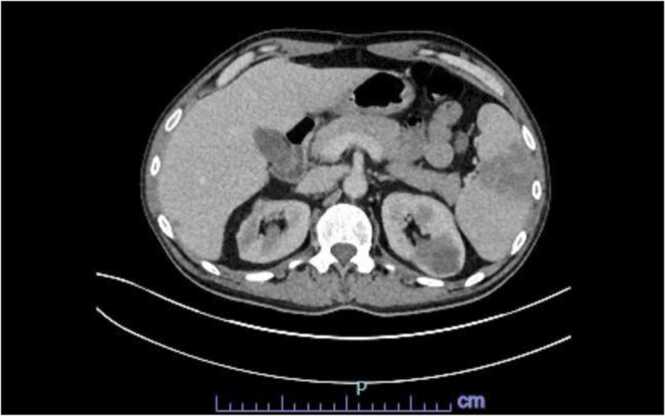
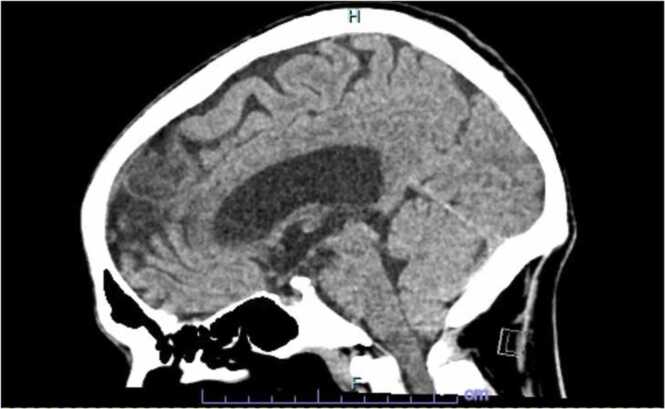
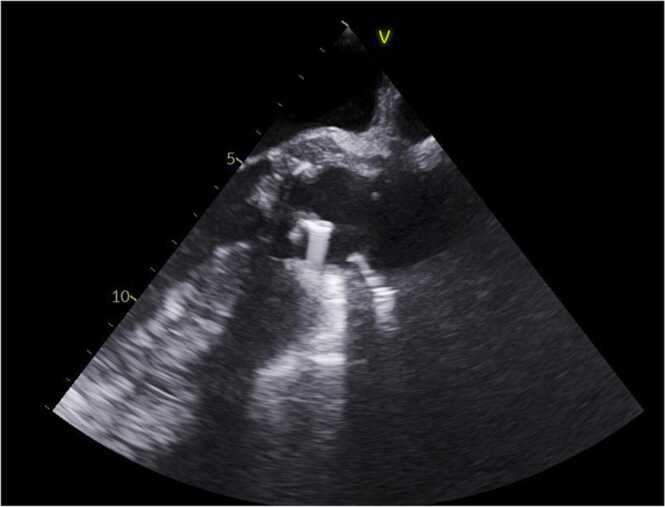
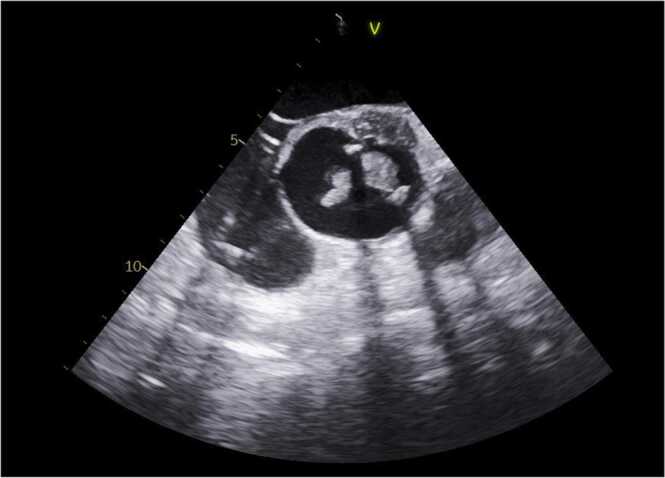
This is a 50 year old male with pertinent history of a congenital bicuspid aortic valve with ascending aortic aneurysmal dilatation status post aortic valve replacement in 2013 complicated by complete heart block, requiring pacemaker placement during that same admission. He presented to our hospital with a constellation of symptoms for the better part of 6–8 weeks including intermittent low grade fevers, night sweats (described as drenching), worsening dyspnea on exertion, weight loss of about 10–15 pounds in the set time frame, chronic fatigue and poor appetite. He had been worked up as an outpatient without an etiological diagnosis. He presented to our hospital with complaints of abdominal pain, worse over his left upper quadrant and associated with nausea and vomiting. His dentition was poor and prior dental work (with appropriate prophylaxis) was done about 6–7 months prior to admission. Initial blood work was unremarkable. Admission CT abdomen with contrast revealed findings concerning for a large splenic infarction and prior renal infarcts (Fig. 4). Additionally, he was found to have multiple areas of cerebral hypo attenuation concerning for embolic infarcts on brain CT imaging ( Fig. 3). His workup included a trans esophageal echocardiography (TEE) which revealed a prosthetic aortic valve vegetation plus an aortic root abscess ( Fig. 1, Fig. 2). Interestingly, the patient had no prior antibiotic exposure and negative blood cultures on day 10 of incubation period x 4 on this admission which was extended from the standard 5 day period in this case. Clinical and objective data were consistent with possible IE based on Duke's criteria [5]. Given how high risk this patient was, we initiated empiric ceftriaxone and vancomycin. Thoracic and cardiovascular surgery (TCVS) was consulted for a possible redo valve replacement. On day 10 of admission permanent pacemaker and lead wires was extracted with no pathological evidence of inflammation. On day 23 of empiric antimicrobial therapy, the patient underwent Redo-sternotomy, redo- mechanical aortic valve replacement (On-x), replacement of the ascending aorta and hemi arch, right axillary artery cut down with chimney graft and permanent pacemaker placement. The prosthetic valve tissue was sent to pathology and forwarded to University of Washington for 16S RNA gene sequencing. Intra operative tissue gram stain showed no organisms and few white blood cells, culture was incubated for an extended period at our facility which remained sterile on day 10. The pathology revealed focal acute aortic valvulitis with fibrinopurulent exudate and focal dystrophic calcifications on microscopic examination of tissue block of the excised prosthetic valve tissue and 16S RNA gene sequencing eventually returned positive for Cutibacterium acnes. Though 16S RNA gene testing doesn’t provide antimicrobial susceptibility, but based on microbiological organism, he received 4 weeks of effective empiric antimicrobial therapy post-surgery and pacemaker placement. Due to complexity of his re-do surgery and aortic root abscess, he was then placed on long term oral suppressive therapy with cephalexin to cover the aforementioned organism.
Fig. 4.
CT abdomen demonstrated a large splenic infaction and prior renal infacts.
Fig. 3.
CT head demonstrated multiple areas of cerebral hyp attenuation concerning for embolic infacts.
Fig. 1.
TEE demonstrated prosthetic aortic valve vegetaion and aortic root abscess.
Fig. 2.
TEE demonstrtaed prosthetic aortic valve vegetation and aortic root abscess.
Discussion
Cutibacterium species are normal commensals of human skin, sebaceous glands and hair follicles; they can also be found in the conjunctiva, external ear, nasopharynx, oral cavity, and genitourinary tract [6]. In addition, C. acnes is an ubiquitous environmental surface contaminant. Therefore, it is often difficult to determine whether positive culture results for Cutibacterium species reflect contamination or true infection. Most clinical infections due to Cutibacterium species are due to C. acnes; other less common species of possible clinical significance include C. granulosum, C. avidum, and C. propionicum.
C. acnes is of relatively low virulence but is capable of adherence and biofilm formation; this characteristic plays an important role in the pathogenesis of infection due to this organism [7]. Infiltration of the glycocalyx with adherence to the implanted foreign material has been observed [8], [9]. C. acnes strains exhibiting the hemolytic phenotype appear to be more pathogenic, whereas nonhemolytic strains are more likely to represent contamination [10]. A combination of clinical, microbiological, and histopathological evidence is important in establishing a diagnosis of infection.
Blood cultures are usually incubated for 3–5 days based on individual hospital guidelines. Given advancement in microbiological techniques, prolonged incubation periods are not routinely recommended for HACEK organisms when considering IE as a diagnosis [11]. Cutibacterium species is typically difficult to culture from blood or tissue at times requiring extended incubation periods for times up to 10–14 days [3]. Moreover, cases require an appropriate clinical setting for diagnosis to aide in differentiation of colonization versus infection. In the event of culture sterility, tissue analysis for 16 S RNA gene sequencing has been found to be helpful in the identification of more indolent pathogens.
16S rDNA is used for identifying bacteria by sequencing codes for a small subunit of ribosomal RNA. Identification relies on matching the sequence from a sample against a database of all known 16S RNA sequences 0.16S RNA sequencing is particularly important in cases of bacteria with unusual phenotypic profiles; such as rare bacteria, slow-growing bacteria, bacteria incapable of growth on culture media and culture-negative infections [12]. As our case illustrates, the diagnostic value of 16S RNA gene sequencing was instrumental for guiding long-term antimicrobial therapy in this high risk patient with increased probability of IE clinically. The limitation of 16S RNA gene sequencing includes no anti-microbial susceptibility to guide definitive therapy.
In complicated cases of prosthetic valve endocarditis, patients require prolonged antimicrobial therapy and many cases may even require surgical intervention. As per the American Association of Cardiology and ESC (European Society of Cardiology) Guidelines, surgery is indicated in valve dysfunction that leads to heart failure, uncontrolled infection (defined as a paravalvular extension, abscess, or persistent bacteremia), and recurrent/high risk of embolism. Although 20–40 % of patients are not able to undergo surgery due to operative risk, our patient safely underwent redo operation. Due to the complicated nature of the surgery performed, high perioperative risk for any future redo procedures and placement of vascular graft to already inflamed ascending aorta. The decision was made for initiation of long-term suppressive therapy. There are no clear guidelines on long term suppressive antimicrobial therapy currently, but the benefits in this case outweigh the potential risks of long term antimicrobial therapy. The decision for long-term suppressive therapy should be made on an individual clinical and microbiological data, and was made possible based on organism identification from 16S RNA sequencing.
Conclusion
Routine use of extended duration incubation of cultures and potentially 16S RNA gene sequencing can help identify a microbiological organisms in patients who would have otherwise not had an etiological organism for their IE.
Funding source
No funding source.
Ethical approval
Informed consent obtained. National research council’s guidelines have been followed.
Consent
Written consent obtained from patient.
CRediT authorship contribution statement
Premalkumar M Patel, MD: Study design, Literature search, Writing, Editing. Nicholas S Camp: Literature search, Writing, Editing. Cynthia I Rivera, MD FACP: Editing, Suggestion. Claudio Tuda, MD FACP, Editing, Review. Isabel Gomez, MD, Review.
Conflict of interest
We have no conflicts of interest to disclose.
References
- 1.Banzon J.M., Rehm S.J., Gordon S.M., Hussain S.T., Pettersson G.B., Shrestha N.K. Propionibacterium acnes endocarditis: a case series. Clin Microbiol Infect. 2017;23(6):396–399. doi: 10.1016/j.cmi.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 2.Mook W.R., Klement M.R., Green C.L., Hazen K.C., Garrigues G.E. The incidence of Propionibacterium acnes in open shoulder surgery: a controlled diagnostic study. journals.lww.com [Internet]. [cited 2022 Jan 5]; Available from: 〈https://journals.lww.com/jbjsjournal/Fulltext/2015/06170/The_Incidence_of_Propionibacterium_acnes_in_Open.1.aspx〉. [DOI] [PubMed]
- 3.Achermann Y., Goldstein E.J., Coenye T., Shirtliff M.E. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev. 2014;27:419–440. doi: 10.1128/CMR.00092-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindell F., Söderquist B., Sundman K., Olaison L., Källman J. Prosthetic valve endocarditis caused by Propionibacterium species: a national registry-based study of 51 Swedish cases. Eur J Clin Microbiol Infect Dis. 2018;37(4):765–771. doi: 10.1007/s10096-017-3172-8. Epub 2018 Jan 29. PMID: 29380224; PMCID: PMC5978902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kupferwasser L.I., Darius H., Müller A.M., Martin C., Mohr-Kahaly S., Erbel R., Meyer J. Diagnosis of culture-negative endocarditis: the role of the Duke criteria and the impact of transesophageal echocardiography. Am Heart J. 2001;142(1):146–152. doi: 10.1067/mhj.2001.115586. [DOI] [PubMed] [Google Scholar]
- 6.Brook I., Frazier E.H. Infections caused by Propionibacterium species. Rev Infect Dis. 1991;13(5):819–822. doi: 10.1093/clinids/13.5.819. [DOI] [PubMed] [Google Scholar]
- 7.Bayston R., Nuradeen B., Ashraf W., Freeman B.J.C. Antibiotics for the eradication of Propionibacterium acnes biofilms in surgical infection. J Antimicrob Chemother. 2007;60(6):1298–1301. doi: 10.1093/jac/dkm408. 〈https://pubmed.ncbi.nlm.nih.gov/17959732/〉 Available from. [DOI] [PubMed] [Google Scholar]
- 8.Tunney M.M., Patrick S., Curran M.D., Ramage G., Hanna D., Nixon J.R., et al. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol. 1999;37(10):3281–3290. doi: 10.1128/jcm.37.10.3281-3290.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramage G., Tunney M.M., Patrick S., Gorman S.P., Nixon J.R. Formation of Propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials. 2003;24(19):3221–3227. doi: 10.1016/s0142-9612(03)00173-x. 〈https://pubmed.ncbi.nlm.nih.gov/12763449/〉 Available from. [DOI] [PubMed] [Google Scholar]
- 10.Nodzo S.R., Hohman D.W., Crane J.K., Duquin T.R. Hemolysis as a clinical marker for propionibacterium acnes orthopedic infection. Am J Orthop. 2014;43(5):E93–E97. [PubMed] [Google Scholar]
- 11.Baron E.J., Scott J.D., Tompkins L.S. Prolonged incubation and extensive subculturing do not increase recovery of clinically significant microorganisms from standard automated blood cultures. Clin Infect Dis. 2005;41(11):1677–1680. doi: 10.1086/497595. [DOI] [PubMed] [Google Scholar]
- 12.Woo P.C.Y., Lau S.K.P., Teng J.L.L., Tse H., Yuen K.Y. Then and now: use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin Microbiol Infect. 2008;14(10):908–934. doi: 10.1111/j.1469-0691.2008.02070.x. [DOI] [PubMed] [Google Scholar]