Abstract
Scientists are indeed fascinated by the pharmacologically important chemicals found in medicinal plants. Citrus fruits possess several curing agents for the prevention of diseases. Therefore, experiments were carried out to test the antibacterial activity of methanolic extracts of peels from various locally available citrus fruits such as citron (Citrus medica), satkora (Citrus macroptera) and adajamir (Citrus assamensis) against Bacillus spp. and E. coli using the disc diffusion method. Different physicochemical characteristics of fruit juice were also determined. Methanolic extract of satkora peel had the highest antibacterial activity of 2.2 and 2.6 cm while adajamir had the lowest antibacterial activity of 1.7 and 2.1 cm as ZOI against Bacillus spp. and E. coli, respectively. In the case of pH and total soluble solids (TSS), the three citrus varieties showed a small variation where satkora showed the highest total phenolic content (TPC), vitamin C content, and antioxidant activity than the others. Citrus fruits can be exploited as antibacterial and antioxidant ingredients in food and nutraceuticals, according to the findings of this study.
Keywords: Citrus fruits, Methanolic extract, Antimicrobial analysis, Zone of inhibition (ZOI), Physicochemical analysis
Citrus fruits; Methanolic extract; Antimicrobial analysis; Zone of inhibition (ZOI); physicochemical analysis.
1. Introduction
Citrus fruits are characterized through a leathery rind and white pith enclosing the juicy segments. Citric acid is present in large quantities in the juice, which gives characteristic harsh flavor. Sugar, organic acid, lipids, polysaccharide, vitamins, minerals, flavonoids, carotenoids and volatile chemicals are all commonly found in citrus fruit. Vitamin C and flavonoids are abundant in them. The quantity of phytochemicals in fruit is determined by its species, variety and growing method. A variety of flavanones and flavones are present among the flavonoids (Khan et al., 2022).
Citrus fruits' non-edible components such as peels and seeds may also be quite beneficial if used properly. Peel waste is very perishable and seasonal, posing a difficulty for processing companies and pollution control authorities. Appropriate procedures are used to convert them into value-added goods (Kumar et al., 2011). Citrus peels are used for a variety of purposes, including fish feed, activated carbon, and conventional paper raw materials (Arias and Ramón-Laca, 2005). Citrus fruit products are recognized to possess antibacterial and antifungal activity (Viuda-Martos et al., 2008). Citrus fruits' peels contain an ample quantity of flavanones and polymethoxylated flavones, which are uncommon in other plants. These chemicals are not only vital for human health and the environment, but they also have a wide range of economic uses in the food and pharmaceutical sectors.
Citrus peel oils also have potent antioxidant and antibacterial properties (Raspo et al., 2020). Antioxidant compounds derived from natural sources, including fruits, rice, olive seeds, beans, spices, and vegetables, have been studied (Hossain et al., 2020b; Sarkar et al., 2020a, Sarkar et al., 2020b). Many of these bioactive components are present in many plants, including pulses, wood, bark, stem, leaf, fruit, root, herb, and seed (Roy et al., 2021; Sarkar et al., 2020a, Sarkar et al., 2020b; Zzaman et al., 2021).
Antibiotics are chemicals generated by microorganisms that destroy or inhibit the development of other microorganisms. Antibiotics are commonly used to treat some types of bacteria which infect organisms; however, if the medication is not administered appropriately, some of the adverse effects might be fatal. Antibiotics obtained from a wide range of microorganisms are nowadays used to treat a range of human diseases; therefore, measures are necessary to limit antibiotic use, develop innovative drugs, both synthetic and natural, and sustain human health for a long time. Plants have long been a valuable source of natural products. According to the World Health Organization (WHO), a medicinal plant is any plant that contains chemicals that can be utilized for therapeutic purposes or that are precursors of chemo-pharmaceuticals semi-synthetic novel medicines (Salih and Abass, 2003). For thousands of years, plant oils and extracts have been utilized for a wide range of therapeutic and medicinal reasons (Jones, 1996). There are several uses based on plant oils and extracts' antibacterial properties, such as food preservation, medicines, complementary and alternative medicine and natural therapeutics (Hammer et al., 1999; Lis-Balchin and Deans, 1997). Malaria, burns, diarrhea, stomach problems, gonorrhea, and other infectious diseases are treated by using plants. In comparison to traditional pharmaceuticals, these plants may be obtained with ease and are less expensive.
Citrus macroptera is a citrus fruit known as satkora in the region, and it is used for a variety of things, including cooking and as an odorant in the fragrance industry. Local communities in Assam, India, exploit semi-wild species mostly for medicinal purposes (Shahriar et al., 2018). Citrus assamensis, commonly known as adajamir in Bangladesh, is the most prominent and costly citrus fruit cultivated in the larger Sylhet region. Citrus medica, or the citron, is one of the original citrus fruits, from which all other citrus varieties descended through natural hybrid speciation or artificial hybridization. Asian cuisine and traditional remedies, as well as religious rites and offerings, make extensive use of this species. The aim of this study was to assess the antimicrobial activity of peels and to evaluate the physicochemical properties and antioxidant activity of juices prepared from different citrus fruits.
2. Materials and methods
2.1. Sample collection
Citrus fruits were collected from a garden at Haripur, Sylhet, Bangladesh. The fruits were citron (Citrus medica), satkora (Citrus macroptera), adajamir (Citrus assamensis) (Figure 1).
Figure 1.
Adajamir (Citrus assamensis), citron (Citrus medica), and satkora (Citrus macroptera) samples.
2.2. Preparation of peels
Peels were removed from fruits and rinsed in sterile distilled water after being washed in tap water. After that, it was dried for 24 h at 60 °C. The dried peels were ground into a fine powder in a clean household blender and kept at room temperature in a research laboratory (Pandey et al., 2011).
2.3. Preparation of juice
The flesh of three different fruits was collected. After that juice was prepared using a juicer from the flesh. Then juices were preserved at freeze temperature (−20 °C) for further physiochemical and antioxidant analysis.
2.4. Preparation of peel extracts
10 g of each sample were weighed into a disinfected, clean conical flask, and 50 ml of extraction solvent (methanol) were added in a 1:5 ratio (g/ml) and left to extract for 2–3 days at room temperature by covering the flask with foil paper. Thereafter, Whatman number 1 filter paper was used to remove the impurities and supernatant was preserved.
2.5. Concentration of the peel extract
The filtrates were concentrated by oven drier at 60 °C. The concentrated filtrate was then mixed with the sterile DMSO (Dimethyl sulfoxide) in 1:2 proportion. These were kept at a temperature of 4 °C in the refrigerator for antibacterial sensitivity testing (Pandey et al., 2011).
2.6. Preparation of culture medium
Nutrient agar media was freshly prepared. For 1000 ml solution 28 g nutrient agar was needed. It was autoclaved at 121 °C, 15 psi for 20 min before use.
2.7. Microorganisms used
The test organisms were collected from the Dept. of Genetic Engineering and Biotechnology, Shahjalal University of Science and Technology (SUST) to investigate the potential activity of the extracts. They included gram-positive bacteria Bacillus spp. and gram-negative Escherichia coli. The organisms used, are all human pathogenic organisms of clinical origin.
2.7.1. Inoculum preparation
Each bacterium was initially subcultured in a nutrient agar medium for 24 h at 37 °C. To achieve consistency, a standardized inoculum of each bacterium was distributed on a nutrient agar plate using a sterilized cotton bar.
2.7.2. Inoculation of test plates
After dipping a sterile cotton swab into the solution, surplus fluid was collected by pressing and spinning the swab against the wall. The swab was used to streak the whole dry surface of the nutrient agar Plates 2–3 times. As a result, the inoculum is evenly distributed throughout the whole surface. The aseptic condition was maintained in the process (Pandey et al., 2011).
2.8. Antibacterial activity
The antibacterial activity of different citrus fruits extracts was estimated by the disc diffusion method of Bonev et al. (2008). Blank discs were soaked into the sample in a Petri plate for about 2 h. Then the discs were ready to use. Discs were placed into the inoculated nutrient agar plates. The plates were left for 1 h to take place diffusion before being incubated at 37 °C for 24 h. A standard ruler was used to measure the zone of inhibition (ZOI). The size of ZOI was calculated in millimeters and then converted to centimeters. Ciprofloxacin (5 μg/ml) was used as an antibiotic for the positive control test. Additionally, a negative control (dH2O) was also used in the experiment.
2.8.1. Determination of zone of inhibition (ZOI)
Each plate was analyzed after 24 h of incubation. On the surface, there existed a circular inhibitory zone. A ruler was used to calculate the diameters of the zones to the closest full centimeter.
2.9. Physicochemical properties of different citrus fruits
2.9.1. pH
The pH was determined by preparing a buffer with a pH of 7.0 and setting the temperature to 28 °C, standardizing the glass electrode with a standard buffer solution, and then rinsing the electrode with distilled water before placing it into the sample solution (Hossain et al., 2021).
2.9.2. Total soluble solids (TSS)
A hand refractometer was used to evaluate the TSS of the sample juice, and the results were recorded as degree Brix (Sarkar et al., 2021).
2.9.3. Titrableacidity (TA)
For the determination of TA, we used the method of Ranganna (1986). The citric acid factor (citric acid factor = 0.0064 g) was determined by multiplying the volume of NaOH with the dilution factor. The result was expressed as percentages of citric acid.
2.10. Antioxidant activities of different citrus fruits
2.10.1. Determination of ascorbic acid (vitamin C)
Vitamin C was determined by the dye method used in Kumar, and Shukla (2017). To make the stock solution, 100 mg of pure dry crystalline ascorbic acid was taken and diluted to 100 ml with 4% oxalic acid. The 100 ml standard solution was made by diluting a 10 ml stock solution with 4% oxalic acid. In a conical flask, 5 ml of standard solution and 4% oxalic acid were pipetted and titrated against the dye solution. The quantity of ascorbic acid (mg) present in 100 g of sample was calculated by following equation (1).
| (1) |
where V1, V2, and Vs are the amount of dye consumed, amount of dye, and the homogenized pulp respectively.
2.10.2. Determination of DPP Hradical scavenging activity
The antioxidant activity of the extract was determined by the DPP Hradical scavenging method of Rahman et al. (2015). The absorbance of the sample solution (As) and blank (Ac) solution without the extract were measured using a UV-Vis spectrophotometer at 517 nm. The sample's capacity to scavenge the DPPH radical was evaluated using the following equation (2):
| (2) |
The EC50 value was calculated from the free radical scavenging activity evaluated by DPPH. EC50 is the concentration necessary to provide a 50% reduction on DPPH radical, is a commonly used parameter to express antioxidant capacity and compare the antioxidant activity of different substances.
2.10.3. Total phenolic contents (TPC)
Total phenolic content in the extracts was determined by the method of Slinkard, and Singleton (1977) with minor modifications. For the assay, 1.0 ml of the extract were mixed with 2.0 ml of 1 M Folin-Ciocalteu reagent and kept for 5 min. Then it was treated with 2.0 ml of 7.5% sodium carbonate (w/v). After shaking vigorously for 30 min at room temperature, the mixture was centrifuged at 2000 rpm for 10 min. The absorbance of the supernatant was measured at a wavelength of 765 nm. A gallic acid standard curve (y = 0.9028 x + 0.054, R2 = 0.9998) was used to quantify the extract's total phenolic content which was reported in milligrams of gallic acid equivalents (mg GAE) per 100 g of extract. Concentrations of standards of Gallic acid, used for the standard curve, were 0.02, 0.04, 0.06, 0.08, 0.10 mg/ml.
2.11. Statistical analysis
All tests were carried out in three replicates, and the values were reported as Mean ± Standard Deviation. We used one-way ANOVA (analysis of variance) followed by Tukey's multiple comparison test in Graph Pad Prism 8 to find out whether there was a significant difference among the samples. The significant difference was evaluated at p ≤ 0.05.
3. Results and discussion
3.1. Antibacterial activity of peels
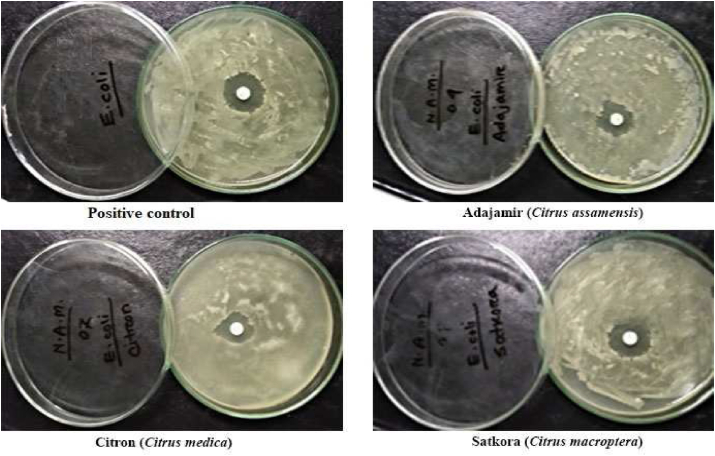
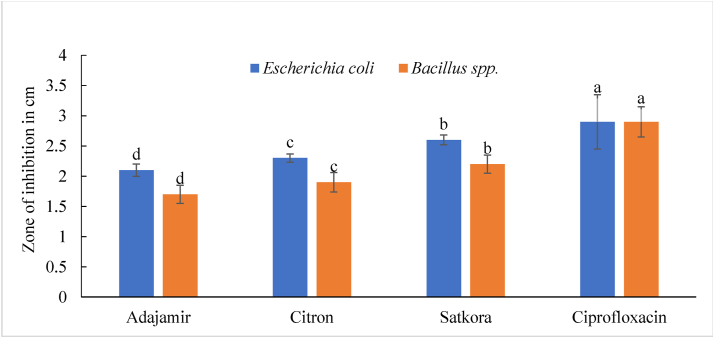
Compounds that kill or inhibit the development of bacteria locally without being very harmful to adjacent tissues are known as antibacterial active molecules (Mohapatra et al., 2018). Results showed that methanolic extract of satkora peel had the highest antibacterial activity against Bacillus spp. and E. coli, respectively. On the other hand, adajamir had the lowest antibacterial activity while citron had moderate activity against both Bacillus spp. and E. coli. Figures 2 and 3 are the graphical presentation of the zone of inhibition of adajamir, citron, satkora extracts, and blank against Bacillus spp. and E. coli respectively.
Figure 2.
Graphical representation of zone of inhibition of adajamir, citron, satkora extracts, and positive control (ciprofloxacin) against Bacillus spp.
Figure 3.
Graphical representation of zone of inhibition of antibacterial activity analysis of adajamir, citron, satkora extracts, and positive control (ciprofloxacin) against E. coli.
Satkora peel showed the highest antibacterial activity of 2.6 cm, citron showed moderate activity of 2.3 cm while adajamir showed the least 2.1 cm activity against E. coli (Figure 4). All the values were significantly different from each other (p ≤ 0.05).
Figure 4.
Antibacterial activity of adajamir (Citrus assamensis), citron (Citrus medica), satkora (Citrus macroptera) peel extracts, and ciprofloxacin against E. coli and Bacillus spp. Values with a different letter in each specific color column are significantly different (p ≤ 0.05).
Satkora showed the highest 2.2 cm, citron showed moderate 1.9 cm and adajamir showed least 1.7 cm antibacterial activity against Bacillus spp. which were significantly different from each other (p ≤ 0.05) (Figure 4). These variations in antibacterial activity might be due to the various species, variety, regions and mode of cultivation.
According to Mohapatra et al. (2018), ZOI for citron and satkora were 2.5 cm and 2.9 cm respectively against E. coli. These results are in accordance with this present study.
3.2. Physicochemical analysis of fruit juice
A solution's acidity or alkalinity is represented by the pH scale, which measures the concentration of hydrogen ions in the solution. Adajamir had the highest pH value of 2.39 while citron had moderate and satkora had the lowest pH value (Table 1). So, satkora was found more acidic than the other two varieties. The acidity of adajamir was found lower than that of citron. According to Nongalleima et al. (2017), the pH of citron and satkora were 2.42 and 2.39 respectively. These results were quite similar to this present study.
Table 1.
Physicochemical properties of adajamir (Citrus assamensis), citron (Citrus medica), satkora (Citrus macroptera) fruit juices.
| Parameters | Samples |
||
|---|---|---|---|
| Adajamir | Citron | Satkora | |
| PH value | 2.39 ± 0.21a | 2.36 ± 0.12a | 2.31 ± 0.15a |
| TSS value (°Brix) | 5.90 ± 0.39c | 6.20 ± 0.41b | 6.52 ± 0.29a |
| TA value (%) | 3.74 ± 0.57a | 3.81 ± 1.12a | 3.93 ± 1.23a |
All the values are expressed as mean ± SD for 3 replicates (n = 3). Values with a different letter in a roware significantly different (p ≤ 0.05).
Adajamir had the lowest TSS value of 5.9° Brix while citron had moderate and satkora had the highest TSS value (Table 1). According to Nongalleima et al. (2017), the TSS value of citron and satkora were 6.5 and 7.2 °Brix respectively which were quite similar to this present study.
The total concentration of free protons and undissociated acids in a solution that may be neutralized by reacting with a strong base is referred to as titratable acidity (TA). Satkora had the highest TA value of 3.93% while citron had moderate and adajamir had the lowest TA value (Table 1). These variations might be due to the different origins, variety, and species. So, satkora was found more acidic than the other two varieties. According to Mehmood et al. (2015), the TA value of adajamir and citron were found at 3.92 and 3.87% respectively. Our present finding were found to be similar to the findings of Rekha et al. (2012).
3.3. Antioxidant activities of different citrus fruits
An abundant source of Vitamin C contains in citrus fruits and green vegetables. It's critical for strong, flexible connective tissue, and it may also serve as an antioxidant. Scurvy occurs when there is a severe nutritional deficit of ascorbic acid.
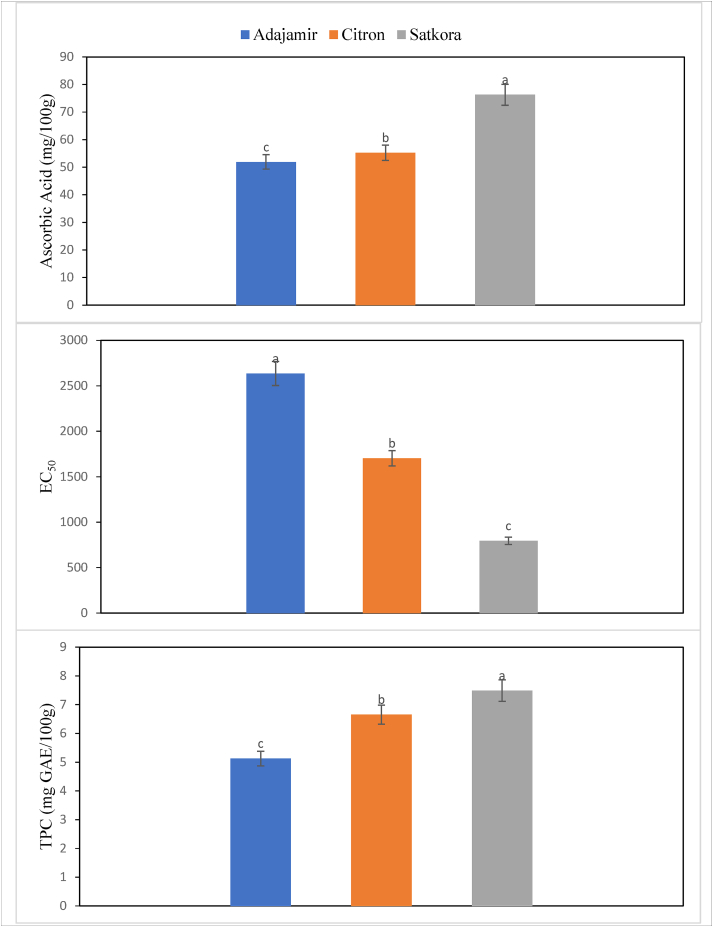
Satkora had the highest ascorbic acid content 76.26 mg/100 g while citron had moderate 55.27 mg/100 g and adajamir had the lowest 51.89 mg/100 g which were significantly different from each other (p ≤ 0.05) (Figure 5). According to Rekha et al. (2012), the ascorbic acid content of citron and satkora were 52.35 and 69.29 mg/100 g respectively which were in line with our study.
Figure 5.
Ascorbic acid content, EC50 value, and Total phenolic contents (TPC) of adajamir (Citrus assamensis), citron (Citrus medica), and satkora (Citrus macroptera) juice.
The DPPH free radical scavenging test was used to evaluate the free radical scavenging activity of citrus fruit juices. The DPPH free radical scavenging technique is a commonly used method for evaluating the ability of various samples to neutralize free radicals.
The highest antioxidant capacities were detected for satkora and the lowest for adajamir. Citron had moderate antioxidant capacities among the three citrus fruits (Figure 5). According to Mehmood et al. (2015), the antioxidant activity of adajamir and citron were found 2550 and 1690 mg/ml respectively by EC50.
TPC is used to ascertain the samples' phenolic content. Phenolic chemicals found in plants have redox characteristics that make them antioxidants. Phenolic compounds are found in plants.
Significant differences were observed in the total phenolic contents among different citrus fruits. The total phenolic content ranged from 5.125 to 7.488 mg GAE/100 g of fruit juices (Figure 5). High phenolic content was observed in satkora. The variation in total phenolic content among these three samples might be due to the variety, species, and mode of cultivation. Our finding were in line with the findings of Rekha et al. (2012).
4. Conclusion
The antibacterial properties of adajamir, citron and satkora peels against gram-positive bacteria Bacillus spp. and gram-negative bacteria Escherichia coli were demonstrated using an antimicrobial susceptibility assay. Among them, satkora showed the highest antibacterial activity than the others. It provides a reliable indication for producing the drug which can inhibit the growth of microorganisms. Therefore, citrus fruits can be used as a natural antibiotic instead of synthetic antibiotics. Satkora also showed the highest TPC, vitamin C and antioxidant activity compared to other samples. The juice obtained from the three locally grown citrus fruits was of good quality based on physicochemical properties. Citrus fruits can be employed as a useful source of health-promoting components, based on the findings.
Declarations
Author contribution statement
Moinul Hasan, Provakar Roy, Mahabub Alam: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mozammel Hoque, Wahidu Zzaman: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors acknowledged supplies and laboratory help from the department of Food Engineering and Tea Technology at Shahjalal University of Science and Technology, Sylhet, Bangladesh.
References
- Arias B.Á., Ramón-Laca L. Pharmacological properties of citrus and their ancient and medieval uses in the Mediterranean region. J. Ethnopharmacol. 2005;97(1):89–95. doi: 10.1016/j.jep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Bonev B., Hooper J., Parisot J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008;61(6):1295–1301. doi: 10.1093/jac/dkn090. [DOI] [PubMed] [Google Scholar]
- Hammer K.A., Carson C.F., Riley T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999;86(6):985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- Hossain M., Arafat M., Alam M., Hossain M. Effect of solvent types on the antioxidant activity and total flavonoids of some Bangladeshi legumes. Food Res. 2021;5(4):329–335. [Google Scholar]
- Hossain M., Evan M., Moazzem M., Roy M., Zzaman W. Response surface optimization for antioxidant extraction from jackfruit (artocarpus heterophyllus lam.) seed and pulp. J. Sci. Res. 2020;12(3):397–409. [Google Scholar]
- Jones F.A. Herbs–useful plants. Their role in history and today. Eur. J. Gastroenterol. Hepatol. 1996;8(12):1227–1231. doi: 10.1097/00042737-199612000-00018. [DOI] [PubMed] [Google Scholar]
- Khan W.A., Hu H., Ann Cuin T., Hao Y., Ji X., Wang J., Hu C. Untargeted metabolomics and comparative flavonoid analysis reveal the nutritional aspects of pak choi. Food Chem. 2022;383 doi: 10.1016/j.foodchem.2022.132375. [DOI] [PubMed] [Google Scholar]
- Kumar K.A., Narayani M., Subanthini A., Jayakumar M. Antimicrobial activity and phytochemical analysis of citrus fruit peels-utilization of fruit waste. Int. J. Eng. Sci. Technol. 2011;3(6):5414–5421. [Google Scholar]
- Kumar S., Shukla R. Different pre-treatments and storage stability of dehydrated pineapple slices. Int. J. Agric. Sci. Res. 2017;7(2):413–424. [Google Scholar]
- Lis-Balchin M., Deans S. Bioactivity of selected plant essential oils against Listeria monocytogenes. J. Appl. Microbiol. 1997;82(6):759–762. doi: 10.1046/j.1365-2672.1997.00153.x. [DOI] [PubMed] [Google Scholar]
- Mehmood B., Dar K.K., Ali S., Awan U.A., Nayyer A.Q., Ghous T., Andleeb S. In vitro assessment of antioxidant, antibacterial and phytochemical analysis of peel of Citrus sinensis. Pak. J. Pharm. Sci. 2015;28(1) [PubMed] [Google Scholar]
- Mohapatra S., Ranjan S., Dasgupta N., Kumar R., Thomas S. Elsevier; 2018. Applications of Targeted Nano Drugs and Delivery Systems: Nanoscience and Nanotechnology in Drug Delivery. [Google Scholar]
- Nongalleima K., Ajungla T., Singh C.B., Chingakham C., Singh B. Phytochemical, total phenolic, total flavonoid and total flavonols content estimation in Citrus macroptera Montruz. J. Med. Plants Stud. 2017;5(3):114–211. [Google Scholar]
- Pandey A., Kaushik A., Tiwari S. Evaluation of antimicrobial activity and phytochemical analysis of Citrus limon. J. Pharmaceut. Biomed. Sci. 2011;13(17):1–7. [Google Scholar]
- Rahman M.M., Islam M.B., Biswas M., Alam A.K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes. 2015;8(1):1–9. doi: 10.1186/s13104-015-1618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganna S. second ed. Tata McGraw-Hill Education; 1986. Handbook of Analysis and Quality Control for Fruit and Vegetable Products. [Google Scholar]
- Raspo M.A., Vignola M.B., Andreatta A.E., Juliani H.R. Antioxidant and antimicrobial activities of citrus essential oils from Argentina and the United States. Food Biosci. 2020;36 [Google Scholar]
- Rekha C., Poornima G., Manasa M., Abhipsa V., Devi J.P., Kumar H.T.V., Kekuda T.R.P. Ascorbic acid, total phenol content and antioxidant activity of fresh juices of four ripe and unripe citrus fruits. Chem. Sci. Trans. 2012;1(2):303–310. [Google Scholar]
- Roy M., Imran M.Z.H., Alam M., Rahman M. Effect of boiling and roasting on physicochemical and antioxidant properties of dark red kidney bean (Phaseolus vulgaris) Food Res. 2021;5(3):438–445. [Google Scholar]
- Salih H., Abass A. Study of the fruit peels of Citrus sinesis and Punica granatum. J. Babylon Uni. 2003;(9):243–342. 3. [Google Scholar]
- Sarkar A., Ahmed T., Alam M., Rahman S., Pramanik S.K. Influences of osmotic dehydration on drying behavior and product quality of coconut (cocos nucifera) Asian Food Sci. J. 2020;15(3):21–30. [Google Scholar]
- Sarkar A., Rahman M.M., Sarkar J.K., Alam M., Rashid M.H. Growth and quality parameters of tea (Camellia sinensis) mediated by arbuscular mycorrhizal fungi. Nova Biotechnol. Chim. 2020;19(2):232–239. [Google Scholar]
- Sarkar A., Rahman S., Roy M., Alam M., Hossain M.A., Ahmed T. Impact of blanching pretreatment on physicochemical properties, and drying characteristics of cabbage (Brassica oleracea) Food Res. 2021;5(2):393–400. [Google Scholar]
- Shahriar M., Bhuiyan M., Rana M. Screening of antibacterial, thrombolytic, membrane stabilizing, anti-inflammatory and antitumor activity of Citrus assamensis leaf extracts. J. Sci. Res. 2018;10(2):195–210. [Google Scholar]
- Slinkard K., Singleton V.L. Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Vitic. 1977;28(1):49–55. [Google Scholar]
- Viuda-Martos M., Ruiz-Navajas Y., Fernández-López J., Pérez-Álvarez J. Antifungal activity of lemon (Citrus lemon L.), Mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control. 2008;19(12):1130–1138. [Google Scholar]
- Zzaman W., Biswas R., Hossain M.A. Application of immersion pre-treatments and drying temperatures to improve the comprehensive quality of pineapple (Ananas comosus) slices. Heliyon. 2021;7(1) doi: 10.1016/j.heliyon.2020.e05882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.