Abstract
The present study investigated the individual and combined effects of xylo-oligosaccharides (XOS) and gamma-irradiated astragalus polysaccharides (IAPS) on the immune response, antioxidant capacity and intestinal microbiota composition of broiler chickens. A total of 240 newly hatched Ross 308 chicks were randomly allocated into 5 dietary treatments including the basal diet (control), or the basal diet supplemented with 50 mg/kg chlortetracycline (CTC), 100 mg/kg XOS (XOS), 600 mg/kg IAPS (IAPS), and 100 mg/kg XOS + 600 mg/kg IAPS (XOS + IAPS) respectively. The results showed that birds in the control group had lower the thymus index and serum lysozyme activity than those in the other 4 groups (P < 0.05). Moreover, there was an interaction between XOS and IAPS treatments on increasing the serum lysozyme activity (P < 0.05). Birds in the CTC and XOS + IAPS groups had lower serum malondialdehyde concentration and higher serum total antioxidant capacity activity and mucosal interleukin 2 mRNA expression of jejunum than those in the control group (P < 0.05). In addition, birds in the control groups had lower duodenal and jejunal IgA-producing cells number than these in other 4 groups (P < 0.05). As compared with the CTC group, dietary individual XOS or IAPS administration increased duodenal IgA-producing cells number (P < 0.05). Meanwhile, there was an interaction between XOS and IAPS treatments on increasing duodenal and jejunal IgA-Producing cells numbers (P < 0.05). Dietary CTC administration increased the proportion of Bacteroides, and decreased the proportion of Negativibacillus (P < 0.05). However, dietary XOS + IAPS administration increased Firmicutes to Bacteroidetes ratio, the proportion of Ruminococcaceae, as well as decreased the proportion of Barnesiella and Negativibacillus (P < 0.05). In conclusion, the XOS and IAPS combination could improve intestinal mucosal immunity and barrier function of broilers by enhancing cytokine gene expression, IgA-producing cell production and modulates cecal microbiota, and the combination effect of XOS and IAPS is better than that of individual effect of CTC, XOS, or IAPS in the current study.
Key words: broiler, xylo-oligosaccharides, gamma-irradiated astragalus polysaccharides, immune response, microbiota
INTRODUCTION
The intestinal tract of poultry is a unique organ inhabited by numerous commensal microbes, which can perform digestion and uptake of nutrients while staying tolerant to commensal bacteria and self-antigens, as well as inducing immune responses against harmful microorganisms (Mason et al., 2008). The intestinal mucosal immune system and commensal microbes colonized in the intestine plays an important role in this complex physiological activity. The intestinal mucosal immune system is a self-defense mechanism of the body, which is composed of the gut-associated lymphoid tissue (GALT), including the Peyer's patches (PP), isolated lymphoid follicles (ILF) and mesenteric lymph node (MLN), and a large population of scattered immune cells (Ahluwalia et al., 2017). The GALT is the inductive and effector sites of the mucosal immune defense. The macromolecular antigens can enter GALT through M cells to activate the intestinal mucosa immune response, biomarker of which is the production of immunoglobulin (Ig) A by B cells present in the GALT (Suzuki and Fagarasan, 2008; Sanz and Palma, 2009; Million et al., 2018). The IgA is transported across the epithelium and forms secretory IgA (SIgA), which covers the gut lumen to protect the body from pathogen.
Microbes and host have cohabited and coevolved for a few million years in a well-balanced mutualism. The host provides the commensal microbes with plant polysaccharides and host-derived glycans, and in return the commensal microbes provide energy for the host by fermenting these non-digestible carbohydrates (Comstock, 2009). More importantly, the germ-free models suggest that the gut microbiota exert an important role in shaping both the gut mucosal immune system and the systemic immune system (Million et al., 2018). The development of ILF depends on stimulation by the intestine bacteria, and partial native bacteria transported into PP and MLN through M cells that can react with immune competent cells to develop complex immune responses (including the production of cytokines, chemokines, and SIgA) to ensure pathogen elimination without causing tissue damage in physiological conditions (Suzuki and Fagarasan, 2008; Sanz and Palma, 2009). Numerous studies have shown that the intestinal probiotics (such as Ruminococcaceae and Lachnospiraceae) are relative to gut health of broilers, and the higher abundance of these beneficial bacteria can reduce the number of harmful bacteria in the intestine and enhance the immune response (Lan et al., 2020; Dai et al., 2021). Chicks are susceptible to external stimulation, which always result in impaired intestinal barrier function and poor growth performance (Li et al., 2019a,b). After antibiotic growth promoters (AGPs) are banned in many countries, numerous studies began to focus on natural antibiotic alternatives with non-toxic side effects. Prebiotics and plant polysaccharides have attracted much attention because they can improve the intestinal barrier function, enhance the body's immunity and regulate the intestinal microbial community of chickens (Guo et al., 2004; Li et al., 2009; Min et al., 2016).
Xylo-oligosaccharides (XOS) is a prebiotic. It can't be disintegrated by digestive enzymes but can be utilized by hindgut probiotics. Dietary supplementation with 100 mg/kg XOS administration improved growth performance, increased the proportion of probiotics (such as Bifidobacterium and Lactobacillus) and the concentration of short-chain fatty acids (SCFAs) in the cecum of broilers (Pourabedin et al., 2015; Ribeiro et al., 2018; Singh et al., 2021). These intestine probiotics and SCFAs play important roles in promoting intestinal development and regulating immunity function (Azad et al., 2020). In addition to indirectly affecting intestinal microbes, prebiotics can also directly interact with immune cells to regulate immune response (Wu et al., 2017). Gamma-irradiated astragalus polysaccharides (IAPS) is a physical modification product of native astragalus polysaccharides (APS). Gamma irradiation modification with a proper dose can significantly reduce the molecular weight of native APS, which makes IAPS have higher immunomodulatory activity than native APS (Ren et al., 2018; Li et al., 2019a). Dietary supplementation with 600 or 900 mg/kg IAPS significantly improved the growth performance and immunity of immunosuppressed broiler (Li et al., 2019a,b). These results suggest that IAPS can be used as an immune enhancer in broiler production.
In a previous study, we found that 100 mg/kg XOS combined with 600 mg/kg IAPS showed better effects in improving broiler growth performance and promoting intestinal development than XOS or IAPS individual in the previous study (Wang et al., 2020). It is well known that changes of the intestinal microbial community and body immunity exert vital role in the healthy growth of chicks. Therefore, it is necessary to explore whether the growth-promoting effect induced by combination use of XOS and IAPS is mediated through the changes of intestinal mucosal immune barrier and microflora. Thus, the aim of this study was to evaluate the effects of dietary XOS, IAPS, and their combination administration on the immune response, antioxidant capacity intestinal microbiota composition and concentrations of SCFAs of broilers.
MATERIALS AND METHODS
Preparation of XOS, IAPS, and Chlortetracycline
Natural APS was purchased from Tianjin Sainuo Pharmaceutical Co., Ltd. (Tianjin, China), and the detected polysaccharides content was 87.64%. The APS was irradiated using a BFT-IV cobalt-60 source irradiator in XiYue Irradiation Technology Co., Ltd. (Nanjing, China) to obtain IAPS according to Ren et al. (2018) and Li et al. (2019a). XOS with a purity of 35% were purchased from Jiangsu Kangwei Biological Co., Ltd. (Nanjing, China). The chlortetracycline (CTC) with a purity of 15% was provided by Nanjing Furunde Animal Pharmaceutical Co., Ltd. (Nanjing, China).
Birds and Experimental Design
The experimental was approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University. A total of 240 one-day-old Ross 308 chicks with similar body weight (44.00 ± 0.45 g) were selected and divided into 5 groups, and each of which contained 6 replicate pens per group with 8 chicks per pen. The birds of control group were fed with a basal diet. The birds of CTC, XOS, and IAPS groups were fed with basal diets supplementation with 50 mg/kg chlortetracycline, 100 mg/kg XOS, and 600 mg/kg IAPS, respectively. The birds of XOS + IAPS group were fed with a basal diet supplementation with 100 mg/kg XOS and 600 mg/kg IAPS. The basal diet was formulated to meet or exceed the nutrient requirements of the NRC (1994) and was devoid of antibiotics (Table 1). All birds were free access to feed and water in a temperature-controlled room at Nanjing Kangxin Poultry Industry Company (Nanjing, China) during the experimental duration. The temperature of room was maintained at 33°C for the first 4 d and then reduced by 3°C per week to a final temperature of 24°C. The experiment period lasted for 21 d.
Table 1.
Ingredient composition and calculated nutrient levels of the basal diet.
| 1 to 21 d | |
|---|---|
| Ingredient (%) | |
| Corn | 57.61 |
| Soybean meal | 31.00 |
| Corn gluten meal (CP, 60%) | 3.29 |
| Soybean oil | 3.11 |
| Dicalcium phosphate | 2.00 |
| Limestone | 1.20 |
| Salt | 0.30 |
| L-Lysine HCl | 0.34 |
| DL-Methionine | 0.15 |
| Premix1 | 1.00 |
| Calculated nutrient levels (%) | |
| Metabolizable energy (MJ/kg) | 12.56 |
| Crude protein | 21.00 |
| Calcium | 1.00 |
| Total phosphorus | 0.70 |
| Non-phytate phosphorus | 0.46 |
| Lysine | 1.20 |
| Methionine | 0.50 |
| Methionine + cysteine | 0.85 |
Premix provided per kilogram of diet: vitamin A, 12,000 IU; vitaminD3, 2,500 IU; vitamin E, 20 IU; menadione sodium bisulfate, 1.3 mg; thiamin, 2.2 mg; riboflavin, 8 mg; nicotinamide, 40 mg; calcium pantothenate, 10 mg; pyridoxine·HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; vitamin B12 (cobalamin), 0.013 mg; choline chloride, 400 mg; Fe (from ferrous sulfate), 80 mg; Cu (from copper sulfate), 8 mg; Mn (from manganese sulfate), 110 mg; Zn (from zinc sulfate), 60 mg; I (from calcium iodate), 1.1 mg; Se (from sodium selenite), 0.3 mg.
Sample Collection
At the age of 21 d, two birds per replicate close to the average BW were chosen and weighed. Blood samples were collected from the jugular vein in non-heparinized centrifuge tubes, and then these tubes were centrifuged at 3,000 × g for 15 min at 4°C to separate serum. The separated serum was stored at −20°C until analysis. After electrically stunning (50 V, alternating current, 400 Hz for 5 s each bird), the birds were then killed by exsanguination of the left carotid artery. Thereafter, carcasses were dissected, thymus, spleen, and bursa were respectively collected and weighed after removal of all adherent fat to calculate the lymphatic organ index. The lymphoid organ index was calculated according to the following formula: lymphoid organ index (g/kg·BW) = the weight of lymphoid organ (g)/body weight (kg). Then, the duodenum (from ventriculus to pancreo-biliary ducts), jejunum (from pancreo-biliary ducts to yolk stalk), and ileum (from yolk stalk to ileocecal junction) were separated, and about 1 cm middle portion of the small intestine were excised and fixed in 4% paraformaldehyde. Afterward, the mucosa of jejunum was scraped with sterilized slides, put into RNAase-free tube, frozen in liquid nitrogen and stored at −80°C for total RNA extraction. Finally, the digesta of 2 ceca were ligated with sterilized cotton thread, removed, and collected in the sterile freezing tubes, and stored at −80°C for DNA extraction.
Serum Analysis
The concentrations of serum interleukin 1β (IL-1β), interleukin 2 (IL-2), and tumor necrosis factor-α (TNF-α) were measured using the corresponding ELISA kits in accordance with the manufacturer's instructions. In addition, serum samples were analyzed for lysozyme activity with lysozyme assay kit (Turbidimetric method), total superoxide dismutase (T-SOD) activity with T-SOD assay kit (Hydroxylamine method), glutathione peroxidase (GSH-PX) activity with GSH-PX assay kit (Colorimetric method), malondialdehyde (MDA) concentration with MDA assay kit (TBA method), and total antioxidant capacity (T-AOC) with a T-AOC assay kit (Colorimetric method). The abovementioned kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Total RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
The total RNA was extracted from the jejunal mucosa using a Trizol reagent (Takara Biotechnology Co. Ltd., Dalian, China). The NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE) was used to determine the purity and quality of total RNA, the OD260/OD280 ratio of which at 1.8 to 2.0 was used for subsequent PCR reactions. A PrimeScript RT Master Mix kit (Takara Biotechnology Co. Ltd.) was used to reverse transcription of RNA into cDNA which were stored at −20°C.
The real-time quantitative PCR (RT-qPCR) was performed with the QuanStudio6 ABI PRISM 7500 Detection System (Applied Biosystems, Carlsbad, CA) using a SYBR Premix Ex Taq kit (Takara Biotechnology Co. Ltd). The reaction volume and reaction conditions were determined according to the method of Wang et al (2020). Primer sequences are exhibited in Table 2 and were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The mRNA expression level of β-actin was used to normalize the cycle threshold (Ct) values. The expression of target gene relative to β-actin was calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 2.
Primer sequences, amplicon sizes and GenBank numbers.
| Genes1 | Primer sequence (5′-3′) | Amplicon size (bp) | GenBank number |
|---|---|---|---|
| IL-1β | F: GTACCGAGTACAACCCCTGC | 112 | NM 204524.1 |
| R: AGCAACGGGACGGTAATGAA | |||
| IL-2 | F: GCTAATGACTACAGCTTATGGAGCA | 138 | NM 204153.1 |
| R: TGGGTCTCAGTTGGTGTGTAGAG | |||
| LITAF | F: AGACCAGATGGGAAGGGAATGAA | 219 | NM 204267.1 |
| R: GAAGAGGCCACCACACGACAG | |||
| IFN-γ | F: ATGTAGCTGACGGTGGACCT | 193 | NM 205149.1 |
| R: ACGCCATCAGGAAGGTTGTT | |||
| β-actin | F: ATCCGGACCCTCCATTGTC | 120 | NM 205518.1 |
| R: AGCCATGCCAATCTCGTCTT |
Abbreviations: IFN-γ, interferon γ; IL-1β, interleukin 1β; IL-2, interleukin 2; LITAF, lipopolysaccharide induced TNF factor.
Measurement of IgA-Producing Cells Using Immunohistochemistry
According to standard histology procedures, small intestine tissues were dehydrated in ethanol, equilibrated in xylene, and embedded in paraffin wax. About 5 μm of cross-sections were prepared and performed by immunohistochemical analysis. The histological sections were stained according to the method of Wang et al. (2009). The mouse anti-chicken IgA monoclonal antibody (Cat no. 8330–01; Southern Biotechnology Inc., Birmingham, AL) was diluted to 10 μg/mL with PBS (0.01 M, pH 7.4). The number of IgA-producing cells in the intestinal lamina propria was counted under a light microscope with a final magnification of 200 × (Olympus BX51, Olympus Optical Co. Ltd., Tokyo, Japan). The numbers of IgA-producing cells of 5 villus lamina propria from one intestinal cross-section per bird were counted for statistical analysis. The results were expressed as the number of cells per square millimeter.
DNA Extraction, PCR Amplification and Illumina Novaseq 6000 Sequencing
Microbial DNA was extracted using the HiPure Stool DNA Kits (Magen, Guangzhou, China) according to manufacturer's protocols. The DNA quality was determined by using Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific). DNA samples with 260/280 absorbance ratios between 1.8 and 2.0 were used for library construction and sequencing. The V3–V4 region of the microbial 16S rRNA gene was amplified by PCR (94°C for 2 min, followed by 30 cycles at 98°C for 10 s, 62°C for 30 s, and 68°C for 30 s and a final extension at 68°C for 5 min) using primers 341F/806R (341F: 5’-CCTACGGGNGGCWGCAG-3’, 806R: 5’-GGACTACHVGGGTATCTAAT-3’). PCR reactions were performed in triplicate 50 μL mixture containing 5 μL of 10 × KOD Buffer, 5 μL of 2 mM dNTPs, 3 μL of 25 mM MgSO4, 1.5 μL of each primer (10 μM), 1 μL of KOD Polymerase, 100 ng of template DNA, and double distilled water filled to 50 μL. The PCR reagents kits were purchased from Toyobo (Tokyo, Japan). Then amplicons were extracted from 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA and quantified using ABI StepOnePlus Real-Time PCR System (Life Technologies, Foster City, CA). Purified amplicons were pooled in equimolar and paired-end sequenced (PE250) on an Illumina platform according to the standard protocols.
Sequencing Data Processing
Raw data containing low quality reads were further filtered using FASTP (version 0.18.0) (Chen et al., 2018). Then paired end clean reads were merged as raw tags using FLSAH (version 1.2.11) with a minimum overlap of 10 bp and mismatch error rates of 2%. Noisy sequences of raw tags were filtered under specific filtering conditions to obtain the high quality clean tags (Bokulich et al., 2013). The clean tags were clustered into operational taxonomic units (OTUs) of ≥ 97% similarity using UPARSE (version 9.2.64) pipeline (Edgar, 2013). All chimeric tags were removed using UCHIME algorithm and finally obtained effective tags for further analysis (Edgar et al., 2011). The representative OTU sequences were classified into organisms by a naive Bayesian model using RDP classifier (version 2.2) based on SILVA database (version 132) with the confidence threshold value of 0.8. The abundance statistics for each taxonomy was visualized using Krona (version 2.6). The stacked bar plot of the community composition was visualized in R project ggplot2 package (version 2.2.1). Chao1, Shannon, Simpson index were calculated in QIIME (version 1.9.1). Principal coordinates analysis (PCoA) of Unweighted distances were generated in R project Vegan package (version 2.5.3) and plotted in R project ggplot2 package (version 2.2.1).
Cecal SCFAs Concentrations
According to the method of Liu et al. (2021), the digesta sample was processed and the supernatant was obtained. Then the supernatant was analyzed on a capillary column gas chromatograph (GC-14B; Shimadzu, Kyoto, Japan) equipped with a capillary column (Agilent HP-5MS; 30 m × 0.32 mm × 0.25 μm, Agilent Technologies Inc., Santa Clara, CA). The concentrations of acetate, propionate, and butyrate were calculated and expressed as mmol per g of wet cecal digesta.
Statistical Analysis
All data were analyzed using 1-way ANOVA followed by Duncan's multiple range tests using SPSS 20.0 statistical software (SPSS Inc., Chicago, IL). Besides, data excluded CTC group were evaluated by 2-way ANOVA using the general linear model procedure of SPSS 20.0, and the model included the main effects of XOS and IAPS, as well as the interaction between XOS and IAPS. The indexes were analyzed by the mean of 2 chickens per cage as a replicate. All results were presented by mean values and the standard error of the mean (SEM). Differences were considered significant at P < 0.05.
RESULTS
MDA Concentration and Antioxidant Enzyme Activity
As shown in Table 3, the one-way ANOVA analysis results showed that birds in the CTC, IAPS, and XOS + IAPS groups had lower MDA concentrations than those in the control and XOS groups (P < 0.05). As compared with the control group, dietary XOS + IAPS administration increased serum T-AOC activity of broilers than that in the control group (P < 0.05), to a level equal to that of the CTC (P > 0.05). In addition, the main effect analysis showed that IAPS treatment increased serum T-SOD activity and decreased serum MDA concentration (P < 0.05). There was no interaction between XOS and IAPS treatments on serum antioxidative indices of broilers (P > 0.05).
Table 3.
Serum antioxidative ability of broilers fed diets supplementation with xylo-oligosaccharides (XOS), 60Co γ-ray irradiated astragalus polysaccharides (IAPS), and their combination.
| Treatments1 | MDA2 (nmol/mL) | T-AOC2 (U/mL) | T-SOD2 (U/mL) | GSH-PX2 (U/mL) |
|---|---|---|---|---|
| Control | 3.90a | 7.34b | 511.15 | 2,195.45 |
| CTC | 2.17b | 13.41a | 537.14 | 2,418.18 |
| XOS | 3.75a | 11.31ab | 531.17 | 2,240.91 |
| IAPS | 2.22b | 10.83ab | 616.51 | 2,013.64 |
| XOS + IAPS | 2.38b | 12.07a | 592.26 | 2,213.64 |
| SEM | 0.16 | 0.66 | 17.12 | 88.41 |
| P value | <0.01 | 0.04 | 0.25 | 0.74 |
| Main effects | ||||
| XOS | ||||
| - | 3.06 | 9.08 | 563.83 | 2,104.55 |
| + | 3.07 | 11.69 | 561.72 | 2,227.27 |
| IAPS | ||||
| - | 3.82y | 9.32 | 521.16y | 2,218.18 |
| + | 2.30x | 11.45 | 604.39x | 2,113.64 |
| P values | ||||
| XOS | 0.97 | 0.07 | 0.95 | 0.56 |
| IAPS | <0.01 | 0.14 | 0.03 | 0.62 |
| XOS × IAPS | 0.34 | 0.33 | 0.54 | 0.71 |
Superscripts for means belong to 1-way ANOVA analyses among 5 treatment groups.
superscripts for means belong to 2 × 2 factorial arrangement (XOS and IAPS). Means with no common superscripts within the column of each classification are significantly different (P < 0.05).
Control, birds fed with basal diet; CTC, XOS, and IAPS, birds fed with basal diets supplementation with 50 mg/kg chlortetracycline, 100 mg/kg XOS, and 600 mg/kg IAPS, respectively; XOS + IAPS, birds fed with a basal diet supplementation with 100 mg/kg XOS and 600 mg/kg IAPS.
MDA, malondialdehyde; T-AOC, total antioxidant capacity; T-SOD, total superoxide dismutase; GSH-PX, glutathione peroxidase.
Immune Organ Index
As shown in Table 4, the one-way ANOVA analysis results showed that there was no significant difference in spleen and bursa indexes of birds among 5 groups (P > 0.05). However, birds in the control group had lower thymus index than those in the other 4 groups (P < 0.05). The main effect analysis results showed birds fed dietary supplementation with IAPS had higher thymus index than those fed diets without IAPS (P < 0.05). There was no interaction between XOS and IAPS treatments on immune organ index (P > 0.05).
Table 4.
Immune organ index of broilers fed diets supplementation with xylo-oligosaccharides (XOS), 60Co γ-ray irradiated astragalus polysaccharides (IAPS), and their combination.
| Treatments1 | Thymus index (g/kg·BW) | Spleen index (g/kg·BW) | Bursa index (g/kg·BW) |
|---|---|---|---|
| Control | 3.23b | 2.61 | 0.96 |
| CTC | 4.72a | 2.71 | 0.93 |
| XOS | 4.32a | 2.56 | 1.03 |
| IAPS | 4.58a | 2.88 | 1.06 |
| XOS + IAPS | 4.56a | 2.73 | 1.11 |
| SEM | 0.17 | 0.07 | 0.03 |
| P value | 0.02 | 0.71 | 0.28 |
| Main effects | |||
| XOS | |||
| - | 3.91 | 2.75 | 1.01 |
| + | 4.44 | 2.64 | 1.07 |
| IAPS | |||
| - | 3.77y | 2.58 | 0.99 |
| + | 4.57x | 2.81 | 1.08 |
| P values | |||
| XOS | 0.12 | 0.56 | 0.33 |
| IAPS | 0.03 | 0.22 | 0.17 |
| XOS × IAPS | 0.11 | 0.77 | 0.90 |
Superscripts for means belong to one-way ANOVA analyses among 5 treatment groups.
superscripts for means belong to 2 × 2 factorial arrangement (XOS and IAPS). Means with no common superscripts within the column of each classification are significantly different (P < 0.05).
Control, birds fed with basal diet; CTC, XOS, and IAPS, birds fed with basal diets supplementation with 50 mg/kg chlortetracycline, 100 mg/kg XOS, and 600 mg/kg IAPS, respectively; XOS + IAPS, birds fed with a basal diet supplementation with 100 mg/kg XOS and 600 mg/kg IAPS.
Serum Lysozyme Activity and Cytokines Concentration
As shown in Table 5, the one-way ANOVA analysis results showed that birds in the CTC group had higher serum lysozyme activity than those in the control group (P < 0.05). Meanwhile, birds in the XOS, IAPS, and XOS + IAPS groups showed higher serum lysozyme activity than those in the control and CTC groups (P < 0.05). In addition, the main effect analysis showed that the birds fed XOS supplementation diets showed higher serum lysozyme activity than those fed diets without XOS (P < 0.05), and the birds fed IAPS supplementation diets showed higher serum lysozyme activity and TNF-α concentration than those fed diets without IAPS (P < 0.05). Moreover, there was an interaction between XOS and IAPS treatments on increasing the serum lysozyme activity (P < 0.05).
Table 5.
Serum lysozyme activity and cytokines concentration of broilers fed diets supplementation with xylo-oligosaccharides (XOS), 60Co γ-ray irradiated astragalus polysaccharides (IAPS), and their combination.
| Treatments1 | Lysozyme (U/mL) | IL-1β2 (ng/L) | IL-22 (ng/L) | TNF-α2 (ng/L) |
|---|---|---|---|---|
| Control | 524.88c | 40.45 | 32.61 | 51.15 |
| CTC | 863.43b | 43.59 | 37.74 | 75.39 |
| XOS | 1,353.23a | 35.38 | 30.16 | 65.13 |
| IAPS | 1,425.47a | 38.22 | 33.63 | 82.31 |
| XOS + IAPS | 1,430.35a | 43.94 | 36.13 | 83.1 |
| SEM | 78.22 | 1.4 | 1.74 | 4.09 |
| P value | <0.01 | 0.26 | 0.71 | 0.06 |
| Main effects | ||||
| XOS | ||||
| - | 975.13y | 39.34 | 33.12 | 66.73 |
| + | 1,391.79x | 39.66 | 33.14 | 72.38 |
| IAPS | ||||
| - | 939.06y | 37.92 | 31.38 | 56.40y |
| + | 1,427.86x | 41.08 | 34.88 | 82.70x |
| P values | ||||
| XOS | <0.01 | 0.91 | 1.00 | 0.40 |
| IAPS | <0.01 | 0.26 | 0.39 | <0.01 |
| XOS × IAPS | <0.01 | 0.06 | 0.55 | 0.47 |
Superscripts for means belong to one-way ANOVA analyses among 5 treatment groups.
Superscripts for means belong to 2 × 2 factorial arrangement (XOS and IAPS). Means with no common superscripts within the column of each classification are significantly different (P < 0.05).
Control, birds fed with basal diet; CTC, XOS, and IAPS, birds fed with basal diets supplementation with 50 mg/kg chlortetracycline, 100 mg/kg XOS, and 600 mg/kg IAPS, respectively; XOS + IAPS, birds fed with a basal diet supplementation with 100 mg/kg XOS and 600 mg/kg IAPS.
IL-1β, interleukin 1β; IL-2, interleukin 2; TNF-α, tumor necrosis factor α.
Relative mRNA Expression of IL-1β, IL-2, IFN-γ, and LITAF in Jejunum
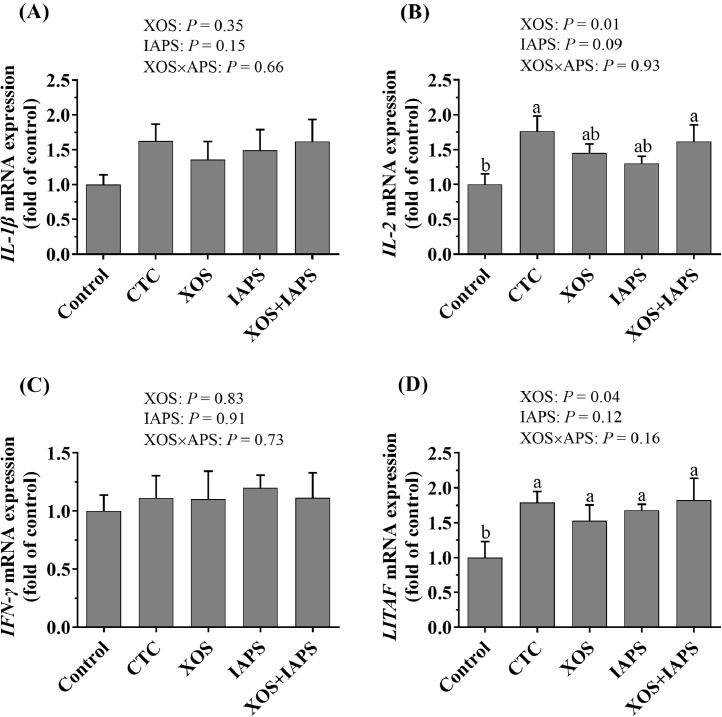
As shown in Figure 1, the one-way ANOVA analysis results showed that birds in the CTC and XOS + IAPS groups had higher IL-2 mRNA expression of the jejunum than those in the control group (P < 0.05). Moreover, the birds in the control group had lower LITAF mRNA expression of the jejunum than those in the other 4 groups (P < 0.05). In addition, the main effect analysis showed that XOS treatment significantly increased IL-2 and LITAF mRNA expression of the jejunum (P < 0.05).
Figure 1.
The relative mRNA expression of IL-1β (A), IL-2 (B), IFN-γ (C), and LITAF (D) in jejunal mucosa of broilers fed diets supplementation with xylo-oligosaccharides (XOS), 60Co γ-ray irradiated astragalus polysaccharides (IAPS), and their combination. The data are represented as the mean value ± SEM of each treatment. Means without a common letter significantly differ (P < 0.05). a-b Superscripts for means belong to 1-way ANOVA analyses among 5 treatment groups; P values means the significant from 2 × 2 factorial analysis (XOS and IAPS). Control, birds fed with basal diet; CTC, XOS, and IAPS, birds fed with basal diets supplementation with 50 mg/kg chlortetracycline, 100 mg/kg XOS, and 600 mg/kg IAPS, respectively; XOS + IAPS, birds fed with a basal diet supplementation with 100 mg/kg XOS and 600 mg/kg IAPS. Abbreviation: CTC, chlortetracycline.
The Numbers of IgA-Producing Cells in Intestine
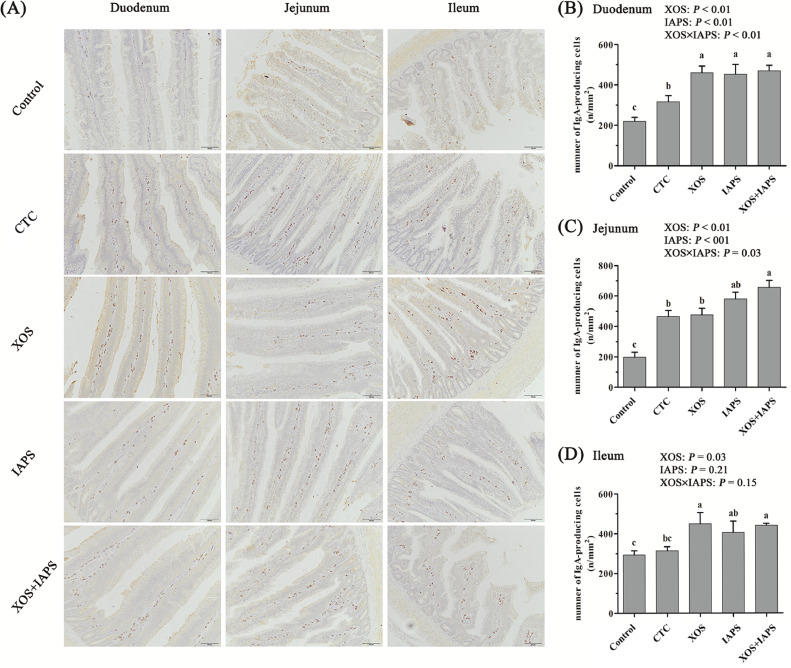
As exhibited in Figure 2, the one-way ANOVA analysis results showed that birds in the CTC group had higher IgA-producing cells numbers of duodenum and jejunum than those in the control group (P < 0.05). Birds in the XOS, IAPS, and XOS + IAPS groups showed higher IgA-producing cells number of duodenum and ileum than those in the control and CTC groups (P < 0.05). Moreover, birds in the XOS + IAPS group had higher IgA-producing cells numbers of jejunum than those in the control, CTC and XOS groups (P < 0.05). The main effect analysis results showed that both XOS and IAPS treatments significantly increased IgA-producing cells numbers of duodenum and jejunum (P < 0.05). Moreover, there was an interaction between XOS and IAPS treatments on increasing IgA-Producing cells numbers of duodenum and jejunum (P < 0.05).
Figure 2.
The number of small intestinal IgA-producing cells of broilers fed diets supplementation with xylo-oligosaccharides (XOS), 60Co γ-ray irradiated astragalus polysaccharides (IAPS), and their combination. The data are represented as the mean value ± SEM of each treatment. Means without a common letter significantly differ (P < 0.05). a-c Superscripts for means belong to 1-way ANOVA analyses among 5 treatment groups; P values means the significant from 2 × 2 factorial analysis (XOS and IAPS). Control, birds fed with basal diet; CTC, XOS, and IAPS, birds fed with basal diets supplementation with 50 mg/kg chlortetracycline, 100 mg/kg XOS, and 600 mg/kg IAPS, respectively; XOS + IAPS, birds fed with a basal diet supplementation with 100 mg/kg XOS and 600 mg/kg IAPS. Abbreviation: CTC, chlortetracycline.
The Quality Analysis of Sequencing Data
After filtering of raw tags and removing of chimeric tags, the number of tags obtained by clustering according to 100% similarity is 63810. The estimate of Good's coverage reached greater than 99.42% for all cecal samples. The Venn diagram (Figure 3A) showed a total of 580 OTU are shared among the 5 treatment groups. In addition, the unique OUT numbers corresponding to control, CTC, XOS, IAPS. and XOS + IAPS groups were 133, 143, 127, 103, and 125, respectively.
Figure 3.
OUT analysis and alpha diversity index analysis among 5 groups. Venn diagram based on OTU (A), Chao1 index (B), Shannon index (C), and Simpson index (D). Means without a common letter significantly differ (P < 0.05). a, b Superscripts for means belong to one-way ANOVA analyses among 5 treatment groups; P values means the significant from 2 × 2 factorial analysis (XOS and IAPS). Control, birds fed with basal diet; CTC, XOS, and IAPS, birds fed with basal diets supplementation with 50 mg/kg chlortetracycline, 100 mg/kg XOS, and 600 mg/kg IAPS, respectively; XOS + IAPS, birds fed with a basal diet supplementation with 100 mg/kg XOS and 600 mg/kg IAPS. Abbreviations: CTC, chlortetracycline; IAPS, irradiated astragalus polysaccharides; XOS, xylo-oligosaccharides.
Microbial Diversity
The bacterial α diversity indices among 5 groups are presented in Figure 3. The one-way ANOVA analysis results showed that dietary IAPS and XOS + IAPS supplementation significantly decreased Chao1 index compared with the control and CTC groups (P < 0.05; Figure 3B). The main effect analysis results showed that only IAPS treatment decreased Chao1 index. There was no interaction between XOS and IAPS treatments on α diversity (P > 0.05). In addition, as shown in Figure 4, no distinguishable clustering of the samples was evident based on the dietary treatments because of high interindividual variation. The results demonstrated that the core set of microbiota in cecum of broilers had not been changed by dietary treatments.
Figure 4.
Principal coordinate analysis (PCoA) plot of cecal microbiota composition based on unweight UniFrace on 21-d broilers. Control, birds fed with basal diet; CTC, XOS, and IAPS, birds fed with basal diets supplementation with 50 mg/kg chlortetracycline, 100 mg/kg XOS, and 600 mg/kg IAPS, respectively; XOS + IAPS, birds fed with a basal diet supplementation with 100 mg/kg XOS and 600 mg/kg IAPS. Abbreviations: CTC, chlortetracycline; IAPS, irradiated astragalus polysaccharides; XOS, xylo-oligosaccharides.
Microbial Community Compositions
As shown in Figure 5, the relative abundance of OTUs was analyzed at different ranking levels at phylum, family, and genus. At the phylum level, Firmicutes and Bacteroidetes were the most dominant phyla, followed by Proteobacteria, Epsilonbacteraeota and Cyanobacteria (Figure 5A). The one-way ANOVA analysis results showed birds in the IAPS and XOS + IAPS groups had higher the percentage of Firmicutes than those in the CTC group (P < 0.05; Figure 5B). In addition, birds in the XOS + IAPS group showed lower the percentage of Bacteroidetes than those in the control, CTC, and XOS groups (P < 0.05; Figure 5C). The main effect analysis results showed that IAPS treatment significantly increased the percentage of Firmicutes, while decreased the percentage of Bacteroidetes (P < 0.05; Figures 5B and 5C).
Figure 5.
Compositions of cecal microbiota and differential species identified of broilers at phylum, family, and genus level on d 21. (A, D, H) were the bacterial community compositions at phylum, family, and genus level, respectively; (B, C, E, F, G, I, J, K) were significantly differential bacteria at phylum family and genus level, respectively. Means without a common letter significantly differ (P < 0.05). a-c Superscripts for means belong to 1-way ANOVA analyses among 5 treatment groups; P values means the significant from 2 × 2 factorial analysis (XOS and IAPS). Control, birds fed with basal diet; CTC, XOS, and IAPS, birds fed with basal diets supplementation with 50 mg/kg chlortetracycline, 100 mg/kg XOS, and 600 mg/kg IAPS, respectively; XOS + IAPS, birds fed with a basal diet supplementation with 100 mg/kg XOS and 600 mg/kg IAPS. Abbreviations: CTC, chlortetracycline; IAPS, irradiated astragalus polysaccharides; XOS, xylo-oligosaccharides.
At the family level, the one-way ANOVA analysis results showed that the birds from IAPS group showed higher the percentage of Ruminococcaceae than those in the CTC group (P < 0.05; Figure 5E). Birds in the XOS + IAPS group had higher the percentage of Ruminococcaceae and lower the percentage of Barnesiellaceae than those in the control, CTC, and XOS groups (P < 0.05; Figure 5E and 5G). In addition, birds in the CTC group showed higher the percentage Bacteroidaceae than those in the other 4 groups (P < 0.05; Figure 5F). The main effect analysis results showed that IAPS treatment increased the percentage of Ruminococcaceae, while decreased the percentage of Bacteroidaceae and Barnesiellaceae (P < 0.05; Figures 5E–5G).
At the genus level, the one-way ANOVA analysis results showed that birds in the IAPS and XOS + IAPS groups had lower the percentage of Barnesiella than that in the control, CTC, or XOS groups (P < 0.05; Figure 5J). In addition, the CTC group significantly increased the percentage of Bacteroides than that in the control, IAPS, and XOS + IAPS groups (P < 0.05; Figure 5I). Birds in the control group had higher the percentage of Negativibacillus than those in the other 4 groups (P < 0.05). The main effect analysis results showed that XOS treatment significantly increased the percentage of Bacteroides and decreased the percentage of Negativibacillus (P < 0.05; Figure 5K). IAPS treatment significantly decreased the percentage of Bacteroides, Barnesiella, and Negativibacillus (P < 0.05; Figures 5I–5K). Taking together, there was no interaction between XOS and IAPS treatments on microbial community compositions in cecum of broilers (P > 0.05).
The Concentrations of Short-Chain Fatty Acids in Cecal Digesta
As shown in Table 6, dietary CTC, XOS, IAPS, or XOS + IAPS administration had no significant effects on the concentration of cecal acetate, propionate, and butyrate among 5 groups (P > 0.05).
Table 6.
The cecal short-chain fatty acids concentrations of broilers fed diets supplementation with xylo-oligosaccharides (XOS), 60Co γ-ray irradiated astragalus polysaccharides (IAPS), and their combination.
| Treatments1 | Acetate (mmol/g) | Propionate (mmol/g) | Butyrate (mmol/g) |
|---|---|---|---|
| Control | 8.58 | 2.08 | 2.12 |
| CTC | 10.48 | 1.91 | 2.27 |
| XOS | 10.10 | 2.34 | 2.30 |
| IAPS | 10.01 | 2.33 | 2.16 |
| XOS + IAPS | 9.34 | 1.84 | 2.19 |
| SEM | 0.46 | 0.21 | 0.13 |
| P value | 0.75 | 0.91 | 0.99 |
| Main effects | |||
| XOS | |||
| - | 9.29 | 2.21 | 2.14 |
| + | 9.72 | 2.09 | 2.24 |
| IAPS | |||
| - | 9.34 | 2.21 | 2.21 |
| + | 9.68 | 2.09 | 2.18 |
| P values | |||
| XOS | 0.70 | 0.83 | 0.74 |
| IAPS | 0.76 | 0.81 | 0.93 |
| XOS × IAPS | 0.32 | 0.48 | 0.82 |
Control, birds fed with basal diet; CTC, XOS, and IAPS, birds fed with basal diets supplementation with 50 mg/kg chlortetracycline, 100 mg/kg XOS, and 600 mg/kg IAPS, respectively; XOS + IAPS, birds fed with a basal diet supplementation with 100 mg/kg XOS and 600 mg/kg IAPS.
DISCUSSION
Broilers are usually susceptible to oxidative stress because of improper feeding and management, which causes a large amount of oxygen free radicals (ROS) to accumulate in the body (Zhao et al., 2021). Excessive ROS could cause adverse effects such as protein and lipid peroxidation, disrupting nucleic acid (e.g., DNA) functions, which could cause oxidative damage to tissues and cells. Thus, it is essential to maintain the balance of the redox state of broilers by improving the antioxidant capacity. The body's antioxidant capacity is generally characterized by the activities of T-AOC, T-SOD, and GSH-PX in serum. MDA, an end product from lipid peroxidation, is used as a biomarker to measure the level of oxidative stress (Mousavi et al., 2018). In the current study, we found that dietary IAPS administration had a tendency to improve the T-AOC activity in serum, and significantly decreased the serum MDA concentration. Similarly, Wu (2018) reported that dietary APS administration enhanced the antioxidant capacity of broilers by increasing the serum activities of T-SOD and GSH-PX and decreasing the serum MDA concentration. Although there was no interaction between XOS and IAPS, we found that dietary XOS + IAPS administration significantly improved the T-AOC activity and decreased the MDA concentration in serum, suggesting that the combination of XOS and IAPS could play a better antioxidant effect.
During the early stage after hatch, broiler chicks exhibit weak adaptability to environment and poor resistance to diseases, therefore, it is very important to improve the immunity of chick so as to prevent infectious diseases (Wu, 2018). Thymus, spleen, and bursa are important part of the poultry immune system which is responsible for the production, differentiation and proliferation of immune cells (Allan et al., 2020). After antigen stimulation, immune cells can secrete cytokines such as IL-2 and TNF-α (Ding et al., 2018). These cytokines play important roles in activating the innate host defense system and regulating the adaptive immune response (Wang et al., 2014). Lysozyme produced by monocytes and neutrophils is an important part of the innate host defense system. It can destroy the cell walls of bacteria and dissolve pathogenic bacteria. Ding et al. (2018) indicated that XOS supplementation improved immunity by increasing plasma IL-2 and TNF-α concentration of laying hens. In addition, Li et al. (2019a) reported that 600 mg/kg IAPS significantly increased the thymus index and T lymphocytes proliferation of immunosuppressed broilers treated with cyclophosphamide. Similar to abovementioned previous studies, we found that dietary XOS and IAPS significantly increased the thymus index and the serum lysozyme activity of broilers. Moreover, there was interaction between XOS and IAPS treatments on increasing serum lysozyme activity. These results suggested that XOS and IAPS had better immune enhancement effects than CTC, and the combined of XOS and IAPS could exert the best immune promotion effect.
The immune barrier composed of the intestinal mucosal immune system can identify and kill foreign antigens by releasing cytokines, activating immune cells, and producing sIgA (Ahluwalia et al., 2017; Million et al., 2018). A better intestinal immune barrier function is important for the healthy growth of broilers by protecting the body from pathogenic bacteria. In the current study, we found that dietary CTC, XOS, IAPS, and XOS + IAPS administration significantly increased LITAF mRNA expression of jejunum, indicating that dietary CTC, XOS, APS, and XOS + IAPS supplementation activated the intestinal mucosal immune system and enhance the intestinal immune barrier function. Li et al. (2019b) reported that both 300 and 600 mg/kg IAPS could upregulate IL-2 mRNA expression of jejunum, thereby enhancing the intestinal immune barrier function of immunosuppressive broilers. Our present study found that XOS + IAPS increased the jejunal IL-2 mRNA expression, and the effect of which is better than individual supplementation of XOS and IAPS, suggesting that the combination of XOS and IAPS could better strengthen the intestinal immune barrier function of broilers. Therefore, we concluded that the XOS + IAPS could more effectively activate the intestinal mucosal immune system, thereby enhancing the immunity of broilers.
It is well known that biomarker of the intestinal mucosa immune response is the production of secretory immunoglobulin A (sIgA). The IgA-producing cells distributed in the intestinal lamina propria are responsible for producing sIgA, which is the most prominent antibody present in mucosal surfaces and protects intestinal mucosa against the invasion of enteric toxins and pathogenic microorganisms (Zhai et al., 2011; Min et al., 2016). The current study showed that dietary XOS or IAPS administration could strengthen intestinal mucosal immune barrier by increasing the number of IgA-producing cells of small intestinal, and the effect of which was better than CTC. Similar to our results, Min et al. (2016) reported that dietary prebiotics (XOS and mannanoligosaccharide) administration significantly increased small intestinal mucosa sIgA content. In addition, Li et al. (2019b) also indicated that IAPS were beneficial for regulating the intestinal immune response by increasing the number of IgA-producing cells in duodenum. What stands out is that there was interaction between XOS and IAPS treatments on increasing the number of IgA-Producing cells. As compared with dietary XOS and IAPS administration, dietary XOS + IAPS administration showed a better effect on increasing the number of cells in jejunum. Those results further confirmed that XOS and IAPS enhanced the intestinal immune barrier function of broilers, and the combination of XOS and IAPS can exert better efficacy.
Up to now, several studies had found that complex gut microbiota and their metabolites could affect the immune function and intestinal barrier function, and be easily altered by various stimuli such as probiotics, organic acid and feed enzymes (Jacobson et al., 2018; Shu et al., 2020; Dai et al., 2021). In the current study, neither dietary CTC nor XOS administration affected diversity of cecal microbial communities, while they altered relative abundance of certain taxa such as Bacteroides and Negativibacillus genus. Pourabedin et al. (2015) indicated that 2 g/kg XOS treatment increased the proportion of cecal Lactobacillus genus of broiler chickens. These results were inconsistent with the current study, which might be a result of differences in the experimental duration and XOS supplementation dosage. Unexpectedly, we found that the proportion of Lactobacillus and Bifidobacteria genus were less than 1% and not different among all 5 treatments, which might indicate that 100 mg/kg XOS treatment was not enough to achieve the purpose of increasing probiotics such as Lactobacillus. Interestingly, we found that dietary CTC administration increased the proportion of Bacteroides genus and decreased the proportion of Negativibacillus genus, and dietary XOS also had the same trend. Negativibacillus genus is a kind of bacillus with a Gram-negative cell wall structure. It has been clearly demonstrated that the increased Negativibacillus proportion caused a distal gut dysbiosis after calves infected with Escherichia coli O157: H7 (Larzabal, et al., 2020). In addition, Negativibacillus genus was higher in cecal contents of mice with obesity-related disorders (Wang et al., 2019). Thereby, we speculated that the decrease in abundance of Negativibacillus was beneficial to intestinal health. As for Bacteroides genus, numerous studies had indicated that as a member of intestinal commensal communities, it plays an important role in fermenting carbohydrates to produce SCFAs, participating in the metabolism of polysaccharides, mediating colonization resistance to intestinal invasive pathogens, and regulating immunity (inducing the secretion of sIgA; Jacobson et al., 2018; Comstock, 2019; Yang et al., 2020). We thus thought that the changes of Bacteroides by the CTC supplementation might exert an important role in improving immunity and increasing the intestine IgA-producing cells of broilers in the present study.
However, the regulation of microbial communities by IAPS and XOS + IAPS was different from CTC and XOS. We found that dietary IAPS or XOS + IAPS administration decreased the Chao 1 indices, but did not affect the Shannon and Simpson indices. In addition, further β diversity analysis showed that the core set of microbiota in cecum of broilers had not been changed by dietary treatments. This meant that IAPS and XOS+IAPS reduced the number of species in the cecal microbial community, while was no impact on the overall diversity of the community. It is well known that intestinal probiotics mediate colonization resistance against bacterial pathogens by producing SCFAs, activating antibacterial host immune pathways, or competing with pathogens for attachment sites of intestinal epithelial cells (Bevins and Salzman, 2011; Sassone-Corsi and Raffatellu, 2015; Jacobson et al., 2018). In the current study, the increased the abundant of Ruminococcaceae by the IAPS or XOS + IAPS supplementation might exert an important role in controlling potential pathogenic bacteria, thereby resulting in the decrease of the microbiota richness. A similar study reported that the APS treatment resulted in a beneficial modulation of the microbiota that increases in the concentrations of beneficial bacteria numbers and decreases in the concentrations of harmful bacteria numbers (Escherichia coli; Li et al., 2009). Numerous studies indicated that the higher Firmicutes to Bacteroidetes ratio was responsible for the higher nutrient digestibility and body weight (Singh et al., 2013; Dai et al., 2021). We found that birds in the XOS + IAPS group showed that had higher the proportion of Firmicutes and lower Bacteroidetes than those in the control, CTC, and XOS groups, suggesting that higher Firmicutes to Bacteroidetes ratio induced by XOS + IAPS might contribute to the nutrient utilization, thereby improving the growth performance of broilers that was had been reported in our previous study (Wang et al., 2020). In addition, the observations of our study showed that dietary XOS + IAPS administration altered Firmicutes to Bacteroidetes ratio because of increasing the proportion of Ruminococcaceae and decreasing the proportion of Barnesiella. Ruminococcaceae, belonging to phyla Firmicutes, can ferment polysaccharides to produce SCFAs, which can supply energy for intestinal epithelial cells (Hooda et al., 2012). The abundance of Ruminococcaceae had positive correlation with body weight and intestinal barrier function of chickens (Dai et al., 2020, 2021). In addition, a previous reported that the relative abundant of “lean microbiota” (Barnesiella) have negative correlation with the total body weight gain of high fat diet fed mice (Zhong et al., 2020). These indicated that the microbial changes induced by XOS + IAPS exerted an important role in improving the growth performance and intestine barrier function in the current study. Dai et al. (2021) reported that the increased abundant of Bacteroides caused by dietary CTC was an unstable factor for intestinal microecology. Although the increased abundant of Bacteroides was relative to excellent growth performance of broilers, Bacteroides as neutral bacteria could cause endogenous infection when the intestinal barrier function was disturbed. Thereby we thought that XOS + IAPS could provide a healthier intestinal microecology than CTC for broilers. Moreover, IAPS play a major role when dietary combination of XOS and IAPS. It is well known that intestinal probiotics can proliferate by fermenting non-digestible oligosaccharides and polysaccharides, which is one of the principles of prebiotic value-added beneficial bacteria (Ding et al., 2018; Comstock, 2019). Therefore, the proportion of prebiotics in the diet might be the main factor affecting its efficacy. In the current study, the reason that IAPS play a major role might be the proportion of IAPS added is much higher than that of XOS. Although dietary IAPS and XOS+IAPS administration changed the relative abundant of SCFAs-producing bacteria like Ruminococcaceae, we did not find significantly different in the SCFAs concentrations among all 5 group in the current study. Similarly, Singh et al. (2021) reported that XOS in low concentration (50 or 100 mg/kg) is not expected to produce sufficient SCFAs in cecum of broilers. Ding et al. (2018) reported that there was a dose-response effect of XOS on SCFAs production in cecum of laying hens. We thus speculated that the supplementation dosage of XOS and IAPS in this study was beneficial to enhanced the fermentation of some SCFAs-producing bacteria, but these substrates was not enough to produce a large number of SCFAs.
CONCLUSIONS
Taken together, XOS, IAPS, and XOS+IAPS as feed additives can enhance the immunity and improve the antioxidant capacity of broiler chickens, while XOS + IAPS can provide birds with better the redox environment and more complete mucosal immune barrier function as compared with XOS or IAPS. In addition, XOS+IAPS can increase the SCFA-producing bacteria which inhibits the proliferation of harmful bacteria so as to provide a healthier intestinal microecology. These results suggested that combination of XOS and IAPS in CTC-free diets had potential to improve broiler immunity and gastrointestinal health.
ACKNOWLEDGMENTS
This work was financed by the National Key Research and Development Program of China (2017YFD0500505), the National Natural Science Foundation of China (31601957), the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS [2020] 407), and Jiangsu Overseas Visiting Scholar Program for University Prominent Young & Middle-aged Teachers and Presidents (2018)
DISCLOSURES
The authors declare that there are no conflicts of interest.
REFERENCES
- Ahluwalia B., Magnusson M.K., Ohman L. Mucosal immune system of the gastrointestinal tract: maintaining balance between the good and the bad. Scand. J. Gastroenterol. 2017;52:1185–1193. doi: 10.1080/00365521.2017.1349173. [DOI] [PubMed] [Google Scholar]
- Allan H.A., Samy H.M., Ibrahim M.T., Mahmoud M.M.A. Nanoselenium effect on growth performance, carcass traits, antioxidant activity, and immune status of broilers. Environ. Sci. Pollut. Res. 2020;27:38607–38616. doi: 10.1007/s11356-020-09952-1. [DOI] [PubMed] [Google Scholar]
- Azad Md A.K., Gao J., Ma J., Li T., Tan B., Huang X., Yin J. Opportunities of prebiotics for the intestinal health of monogastric animals. Anim. Nutri. 2020;6:379–388. doi: 10.1016/j.aninu.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins C.L., Salzman N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- Bokulich N.A., Subramanian S., Faith J.J., Gevers D., Gordon J.I., Knight R., Mills D.A., Caporaso J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods. 2013;10 doi: 10.1038/nmeth.2276. 57–U11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.F., Zhou Y.Q., Chen Y.R., Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:884–890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock L.E. Importance of glycans to the Host-Bacteroides mutualism in the mammalian intestine. Cell Host Microbe. 2009;5:522–526. doi: 10.1016/j.chom.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Dai D., Qiu K., Zhang H.J., Wu S.G., Han Y.M., Wu Y.Y., Qi G.H., Wang J. Organic acids as alternatives for antibiotic growth promoters alter the intestinal structure and microbiota and improve the growth performance in broilers. Front. Microbiol. 2021;11 doi: 10.3389/fmicb.2020.618144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D., Wu S.G., Zhang H.J., Qi G.H., Wang J. Dynamic alterations in early intestinal development, microbiota and metabolome induced by in ovo feeding of L-arginine in a layer chick model. J. Anim. Sci. Biotechnol. 2020;11:19. doi: 10.1186/s40104-020-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X.M., Li D.D., Bai S.P., Wang J.P., Zeng Q.F., Su Z.W., Xuan Y., Zhang K.Y. Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens. Poult. Sci. 2018;97:874–881. doi: 10.3382/ps/pex372. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F.C., Williams B.A., Kwakkel R.P., Li H.S., Li X.P., Luo J.Y., Li W.K., Verstegen M.W.A. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on the cecal microbial ecosystem in broiler chickens. Poult. Sci. 2004;83:175–182. doi: 10.1093/ps/83.2.175. [DOI] [PubMed] [Google Scholar]
- Hooda S., Boler B.M.V., Serao M.C.R., Brulc J.M., Staeger M.A., Boileau T.W., Dowd S.E., Fahey G.C., Swanson K.S. 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J. Nutr. 2012;142:1259–1265. doi: 10.3945/jn.112.158766. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Lam L., Rajendram M., Tamburini F., Honeycutt J., Trung P., Van Treuren W., Pruss K., Stabler S.R., Lugo K., Bouley D.M., Vilches-Moure J.G., Smith M., Sonnenburg J.L., Bhatt A.S., Huang K.C., Monack D. A gut commensal-produced metabolite mediates colonization resistance to Salmonella infection. Cell Host Microbe. 2018;24:296. doi: 10.1016/j.chom.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., Chen G., Cao G., Tang J., Li Q., Zhang B., Yang C. Effects of α-glyceryl monolaurateon on growth, immune function, volatile fatty acids, and gut microbiota in broiler chickens. Poult. Sci. 2020;100 doi: 10.1016/j.psj.2020.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larzabal M., Silva W.M.D., Multani A., Vagnoni L.E., Moore D.P., Marin M.S., Riviere N.A., Delgado F.O., Vilte D.A., Victorica M.R., Ma T., Guan L.L., Talia P., Cataldi A., Cobo E.R. Early immune innate hallmarks and microbiome changes across the gut during Escherichia coli O157: H7 infection in cattle. Sci. Rep. 2020;10:21535. doi: 10.1038/s41598-020-78752-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Ren L.N., Zhu X.D., Li J.L., Zhang L., Wang X.F., Gao F., Zhou G.H. Immunomodulatory effect of gamma-irradiated Astragalus polysaccharides on immunosuppressed broilers. Anim. Sci. J. 2019;90:117–127. doi: 10.1111/asj.13133. [DOI] [PubMed] [Google Scholar]
- Li S., Wang X.F., Ren L.N., Li J.L., Zhu X.D., Xing T., Zhang L., Gao F., Zhou G.H. Protective affects gamma-irradiated Astragalus polysaccharides on intestinal development and mucosal immune function of immunosuppressed broilers. Poult. Sci. 2019;98:6400–6410. doi: 10.3382/ps/pez478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.P., Zhao X.J., Wang J.Y. Synergy of Astragalus polysaccharides and probiotics (Lactobacillus and Bacillus cereus) on immunity and intestinal microbiota in chicks. Poult. Sci. 2009;88:519–525. doi: 10.3382/ps.2008-00365. [DOI] [PubMed] [Google Scholar]
- Liu Y.S., Li S., Wang X.F., Xing T., Li J.L., Zhu X.D., Zhang L., Gao F. Microbiota populations and short-chain fatty acids production in cecum of immunosuppressed broilers consuming diets containing γ-irradiated Astragalus polysaccharides. Poult. Sci. 2021;100:273–282. doi: 10.1016/j.psj.2020.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mason K.L., Huffnagle G.B., Noverr M.C., Kao J.Y. Overview of gut immunology. Adv. Exp. Med. Biol. 2008;635:1–14. doi: 10.1007/978-0-387-09550-9_1. [DOI] [PubMed] [Google Scholar]
- Million M., Tomas J., Wagner C., Lelouard H., Raoult D., Gorvel J. New insights in gut microbiota and mucosal immunity of the small intestine. Hum. Microbiome J. 2018;7:23–32. [Google Scholar]
- Min Y.N., Yang H.L., Xu Y.X., Gao Y.P. Effects of dietary supplementation of synbiotics on growth performance, intestinal morphology, sIgA content and antioxidant capacities of broilers. J. Anim. Physiol. Anim. Nutr. 2016;100:1073–1080. doi: 10.1111/jpn.12479. [DOI] [PubMed] [Google Scholar]
- Mousavi S.N., Faghihi A., Motaghinejad M., Shiasi M., Imanparast F., Amiri H.L., Shidfar F. Zinc and selenium co-supplementation reduces some lipid peroxidation and angiogenesis markers in a rat model of NAFLD-fed high fat diet. Biol. Trace Elem. Res. 2018;181:288–295. doi: 10.1007/s12011-017-1059-2. [DOI] [PubMed] [Google Scholar]
- Pourabedin M., Guan L., Zhao X. Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome. 2015;3:15. doi: 10.1186/s40168-015-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Wang X., Li S., Li J., Zhu X., Zhang L., Gao F., Zhou G. Effect of gamma irradiation on structure, physicochemical and immunomodulatory properties of Astragalus polysaccharides. Int. J. Biol. Macromol. 2018;120:641–649. doi: 10.1016/j.ijbiomac.2018.08.138. [DOI] [PubMed] [Google Scholar]
- Ribeiro T., Cardoso V., Ferreira L.M.A., Lordelo M.M.S., Coelho E., Moreira A.S.P, Domingues M.R.M., Coimbra M.A., Bedford M.R., Fontes C.M.G.A. Xylo-oligosaccharides display a prebiotic activity when used to supplement wheat or corn-based diets for broilers. Poult. Sci. 2018;97:4330–4341. doi: 10.3382/ps/pey336. [DOI] [PubMed] [Google Scholar]
- Sanz Y., Palma G.D. Gut microbiota and probiotics in modulation of epithelium and gut-associated lymphoid tissue function. Int. Rev. Immunol. 2009;28:397–413. doi: 10.3109/08830180903215613. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi M., Raffatellu M. No vacancy: how beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J. Immunol. 2015;194:4081–4087. doi: 10.4049/jimmunol.1403169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu G., Kong F., Xu D., Yin L., He C., Lin J., Fu H., Wang K., Tian Y., Zhao X. Bamboo leaf flavone changed the community of cecum microbiota and improved the immune function in broilers. Sci. Rep. 2020;10:12324. doi: 10.1038/s41598-020-69010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Mishra B., Bedford M.R., Jha R. Effects of supplemental xylanase and xylooligosaccharides on production performance and gut health variables of broiler chickens. J. Anim. Sci. Biotechnol. 2021;12:98. doi: 10.1186/s40104-021-00617-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Karimi A., Devendra K., Waldroup P.W., Cho K.K., Kwon Y.M. Influence of penicillin on microbial diversity of the cecal microbiota in broiler chickens. Poult. Sci. 2013;92:272–276. doi: 10.3382/ps.2012-02603. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Fagarasan S. How host-bacterial interactions lead to IgA synthesis in the gut. Trends Immunol. 2008;29:523–531. doi: 10.1016/j.it.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Wang D., Ma W., She R., Sun Q., Liu Y., Hu Y., Liu L., Yang Y., Peng K. Effects of swine gut antimicrobial peptides on the intestinal mucosal immunity in specific-pathogen-free chickens. Poult. Sci. 2009;88:967–974. doi: 10.3382/ps.2008-00533. [DOI] [PubMed] [Google Scholar]
- Wang Q., Wang X.F., Xing T., Li J.L., Zhu X.D., Zhang L., Gao F. The combined impact of xylo-oligosaccharides and gamma-irradiated Astragalus polysaccharides on growth performance and intestinal mucosal barrier function of broilers. Poult. Sci. 2020;100 doi: 10.1016/j.psj.2020.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Pan Y., Wang L., Zhou H., Song G., Wang Y., Liu J., Li A. Optimal dietary ferulic acid for suppressing the obesity-related disorders in leptin-deficient obese C57BL/6J-ob/ob mice. J. Agric. Food Chem. 2019;67:4250–4258. doi: 10.1021/acs.jafc.8b06760. [DOI] [PubMed] [Google Scholar]
- Wang X., Shen J., Li S., Zhi L., Yang X., Yao J., et al. Sulfated astragalus polysaccharide regulates the inflammatory reaction in LPS-infected broiler chicks. Int. J. Biol. Macromol. 2014;69:146–150. doi: 10.1016/j.ijbiomac.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Wu R.Y., Maattanen P., Napper S., Scruten E., Li B., Koike Y., Johnson-Henry K.C., Pierro A., Rossi L., Botts S.R., Surette M.G., Shermen P.M. Non-digestible oligosaccharides directly regulate host kinome to modulate host inflammatory responses without alterations in the gut microbiota. Mcrobiome. 2017;5:135. doi: 10.1186/s40168-017-0357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018;97:3489–3493. doi: 10.3382/ps/pey220. [DOI] [PubMed] [Google Scholar]
- Yang C., Mogno I., Contijoch E.J., Borgerding J.N., Aggarwala V., Li Z., Siu S., Grasset E.K., Helmus D.S., Dubinsky M.C., Mehandru S., Cerutti A., Faith J.J. Fecal IgA levels are determined by strain-level differences in bacteroides ovatus and are modifiable by gut microbiota manipulation. Cell Host Microbe. 2020;27:467. doi: 10.1016/j.chom.2020.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L., Li Y., Wang W., Wang Y., Hu S. Effect of oral administration of ginseng stem-and-leaf saponins (GSLS) on the immune responses to Newcastle disease vaccine in chickens. Vaccine. 2011;29:5007–5014. doi: 10.1016/j.vaccine.2011.04.097. [DOI] [PubMed] [Google Scholar]
- Zhao W., Li J.L., Xing T., Zhang L., Gao F. Effects of guanidinoacetic acid and complex antioxidant supplementation on growth performance, meat quality, and antioxidant function of broiler chickens. J. Sci. Food Agric. 2021;101:3961–3968. doi: 10.1002/jsfa.11036. [DOI] [PubMed] [Google Scholar]
- Zhong H., Abdullah L.Deng, Zhao M., Tang J., Liu T., Zhang H., Feng F. Probiotic-fermented blueberry juice prevents obesity and hyperglycemia in high fat diet-fed mice in association with modulating the gut microbiota. Food Funct. 2020;11:9192–9207. doi: 10.1039/d0fo00334d. [DOI] [PubMed] [Google Scholar]