Abstract
Osteoarthritis (OA), in which M1 macrophage polarization in the synovium exacerbates disease progression, is a major cause of cartilage degeneration and functional disabilities. Therapeutic strategies of OA designed to interfere with the polarization of macrophages have rarely been reported. Here, we report that SHP099, as an allosteric inhibitor of src-homology 2-containing protein tyrosine phosphatase 2 (SHP2), attenuated osteoarthritis progression by inhibiting M1 macrophage polarization. We demonstrated that M1 macrophage polarization was accompanied by the overexpression of SHP2 in the synovial tissues of OA patients and OA model mice. Compared to wild-type (WT) mice, myeloid lineage conditional Shp2 knockout (cKO) mice showed decreased M1 macrophage polarization and attenuated severity of synovitis, an elevated expression of cartilage phenotype protein collagen II (COL2), and a decreased expression of cartilage degradation markers collagen X (COL10) and matrix metalloproteinase 3 (MMP3) in OA cartilage. Further mechanistic analysis showed thatSHP099 inhibited lipopolysaccharide (LPS)-induced Toll-like receptor (TLR) signaling mediated by nuclear factor kappa B (NF-κB) and PI3K–AKT signaling. Moreover, intra-articular injection of SHP099 also significantly attenuated OA progression, including joint synovitis and cartilage damage. These results indicated that allosteric inhibition of SHP2 might be a promising therapeutic strategy for the treatment of OA.
KEY WORDS: SHP2, SHP099, Osteoarthritis, Synovitis, Toll-like receptor signaling, Macrophage, Cartilage degradation, M1 macrophage polarization
Graphical abstract
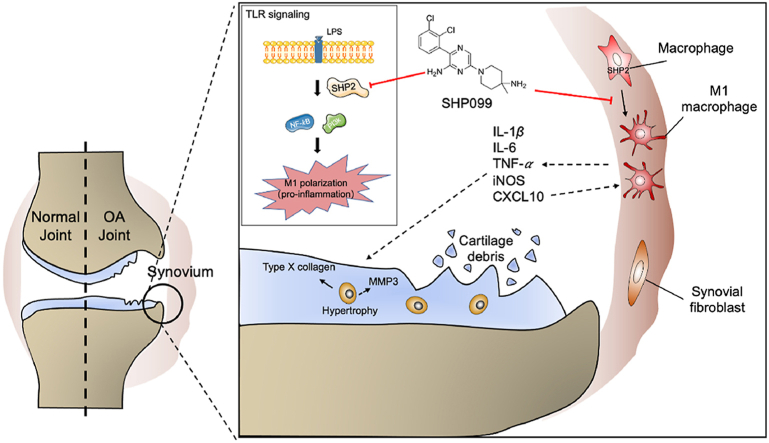
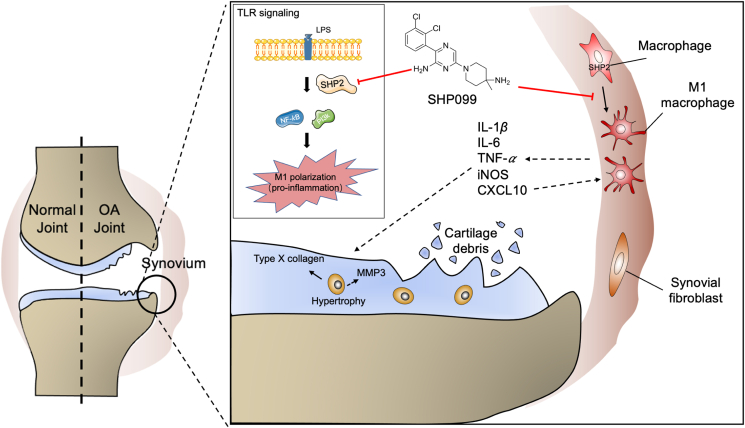
SHP2 positively regulates TLR signaling in M1 macrophage-induced osteoarthritis synovitis, indicating that targeting macrophagic SHP2 is a promising strategy for osteoarthritis.
1. Introduction
Osteoarthritis (OA) is a senile disease with a high incidence that affects 10% of men and 18% of women over the age of 60 years old1. OA is characterized by cartilage degeneration, subchondral bone remodelling and synovial inflammation (synovitis)2. Synovitis in OA is related to severe pain and joint dysfunction and accelerates cartilage loss3. Histological changes in synovitis include synovial lining hyperplasia, macrophage and lymphocyte infiltration and new blood vessel formation4.
Macrophages determine inflammatory marker expression during synovitis5 and are divided into two types, namely, M1 and M2 macrophages. M1 macrophages can release inflammatory factors, such as Interleukin (IL)-1 and IL-6, while M2 macrophages can release protective cytokines, such as IL-106. The promotion of M1 macrophage polarization can aggravate OA progression7. However, conditional macrophage deletion has no significant impact on the development of OA8. Intervention for macrophage M1 polarization to attenuate the severity of OA is awaited to be investigated.
Src-homology 2-containing protein tyrosine phosphatase 2 (SHP2) is a widely expressed non-receptor protein tyrosine phosphatase9. SHP2 plays an essential role in organism development and physiological and pathological reactions in response to growth and stimulatory factors10,11. It inhibited NOD-like receptor protein 3 (NLRP3) inflammasome activation in macrophages12. However, a recent study demonstrated that SHP2 exacerbated inflammation by inhibiting macrophage responsiveness to IL-1013. Our previous research revealed that SHP2 exacerbated the imbalance between cartilage catabolism and anabolism by regulating the DOK1–UPP1–uridine axis and promoted the progression of osteoarthritis14, but it is still unclear whether SHP2 affects immune cells in the joint microenvironment, especially macrophages, to regulate the progression of OA. SHP099 is a potent (IC50 = 71 nmol/L) and selective allosteric inhibitor of SHP2, which concurrently binds to the interface of the protein tyrosine phosphatase domains, N-terminal SH2 and C-terminal SH2, thus inhibiting SHP2 activity through an allosteric mechanism15. The research on SHP099 focuses on its ability in inhibiting cancer cell growth and enhancing anti-tumor immunity16. Exploring the role of SHP2 in macrophage polarization during the progression of OA and the potential application of its allosteric inhibitor would provide a new strategy for treating OA.
In this study, we found that SHP2 was highly expressed in OA synovial macrophages. Conditional Shp2 knockout (cKO) mice exhibited decreased M1 macrophage polarization and less cartilage degeneration in the destabilization of medial meniscus (DMM) model, a surgically induced OA mouse model17. SHP099, a SHP2 allosteric inhibitor, attenuated lipopolysaccharide (LPS)-induced M1 macrophage inflammation in bone marrow-derived macrophages (BMDMs) and RAW264.7 cells and inhibited M1 polarization of macrophages by suppressing LPS-induced Toll-like receptor (TLR) signaling, which is mediated by nuclear factor kappa B (NF-κB) and PI3K–AKT signaling. Furthermore, intra-articular injection of SHP099 significantly attenuated OA progression, including joint synovitis and cartilage damage. These findings indicated that SHP2 might be a therapeutic target for the treatment of OA.
2. Materials and methods
2.1. Clinical specimen
Human OA synovial tissues were obtained from patients who had undergone total knee replacement surgery. Normal synovial tissues were obtained from donors who underwent lower limb amputation due to traffic accidents and had no history of arthritic diseases. This study was approved by the Ethical Committee of the Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School (2020-156-01). The privacy rights of human subjects were always be observed and the informed consent was obtained for experimentation with human subjects.
2.2. Animals
All animal experiments were authorized by the Animal Care and Use Committee of Nanjing Drum Tower Hospital, the affiliated hospital of Nanjing University (2019AE01120). We have complied with all rules and regulations. Shp2f/f mice were crossed with LyzCre mice on the C57BL/6 background for more than two generations to generate LyzCre; Shp2f/f mice as previously described18, Shp2 was conditionally knocked out in macrophages of LyzCre; Shp2f/f mice, genotype identification was performed, and SHP2 protein was knocked out in BMDMs of LyzCre; Shp2f/f mice (Supporting Information Fig. S1A and S1B). OA was induced in 8-week-old male cKO and wild-type (WT) mice by DMM surgery as previously described19. After exposure to a 3-mm longitudinal incision over the distal patella to the proximal tibial plateau, the medial meniscotibial ligament (MMTL) was sheared to make the medial meniscus unstable. At 6 weeks after surgery, mice were euthanized for collection of joint tissues. Twenty-four C57BL/6 mice were purchased from the Animal Center of Nanjing Medical University (Nanjing, China). After one week of adaptive feeding, the mice were randomly divided into sham, SHP099, DMM or DMM + SHP099 groups. DMM surgery was performed in the DMM and DMM + SHP099 groups, and two weeks later, intra-articular injection of 10 μL of 20 μmol/L SHP099 (MCE) was performed twice a week in the SHP099 and DMM+SHP099 groups, while the remaining groups were injected with 10 μL phosphate-buffered saline (PBS).
2.3. Cell culture condition and transfection
RAW264.7 cells were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Science (Shanghai, China). BMDMs were obtained and cultured as described previously20,21. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco) with 10% Fetal Bovine Serum (FBS, Gibco) and 1% penicillin and streptomycin (Gibco) at 37 °C with 5% CO2. Lentivirus was purchased from GeneChem (Shanghai, China), and cells were transfected according to the manufacturer's requirements.
To evaluate the role of SHP2 in M1 macrophage polarization, 100 ng/mL LPS (Sigma–Aldrich) was used to induce M1 macrophage polarization, and 20 μmol/L SHP099 was used to inactivate the SHP2 protein. The M1 macrophage surface marker CD80 (Biolegend) was analysed using a Flow Cytometer (BD Accuri C6 Plus).
2.4. Western blotting
Total protein was extracted using RIPA Lysis Buffer (Solarbio) with 1 mmol/L phenylmethanesulfonyl fluoride (Solarbio) and 1 mmol/L phosphatase inhibitor cocktail (Bimake). Nuclear and cytoplasmic proteins were extracted using a Nucleoplasmic Protein Extraction Kit (Solarbio), and protein concentrations were measured by the BCA Assay Kit (Thermo Scientific). A 10% (w/v) SDS-polyacrylamide gel was used to separate proteins (EpiZyme), which were transferred onto polyvinylidene fluoride membranes (Bio-Rad). After blocking with 5% (w/v) milk (Bio-Rad) for 1 h at 37 °C, the membrane was incubated with primary antibodies overnight at 4 °C. A horseradish peroxidase-conjugated goat anti-rabbit/mouse IgG (Biosharp) was used as a secondary antibody. All images were obtained using a Western Blotting Imaging System (Tanon).
2.5. Quantitative real-time polymerase chain reaction (qPCR)
TRIzol reagent (Thermo Fisher Scientific) was used to extract RNA from cells. qPCR was conducted in a 20 μL system using the SYBR Green q-PCR Kit (Vazyme) on a Light Cycler (Roche) with the primers presented in Supporting Information Table S1.
2.6. Histological analysis
Human synovium was fixed with 4% paraformaldehyde (PFA) for 48 h. The total knee joints of mice were decalcified with 10% EDTA at a 7.4 pH for 21 days. They were then embedded in paraffin and cut into 3-micrometer-thick sections for haematoxylin and eosin (H&E) staining and Safranin O/Fast green staining. The synovitis score was evaluated for synovial lining cell thickness (0–3) and synovial stroma density (0–3)22. The Osteoarthritis Research Society International (OARSI) score23 was evaluated for cartilage degradation. Each section of the histology specimen was evaluated by double-blinded, independent observers.
2.7. Immunohistochemistry and immunofluorescence
After being dewaxed and rehydrated with gradient alcohol, the sections were rinsed with 3% hydrogen peroxide to inactivate endogenous peroxidases. After antigen retrieval using 0.1% Pepsin (Sigma), the sections were blocked with goat serum (Gibco) for 1 h at 37 °C and incubated with primary antibodies overnight at 4 °C. Immunohistochemistry sections were incubated with horseradish peroxidase-conjugated secondary antibody (Biosharp) for 1 h at 37 °C. The positive cells were visualized using 3,3-diaminobenzidine (Typng). Images were received by Optical Microscope (Zeiss). Immunofluorescence sections were incubated with FITC- or TRITC-conjugated secondary antibodies for 1 h at 37 °C, and then the nuclei were stained with 4,6-diamidino-2-phenylindole (Abcam) for 4 min. Images were obtained on a Fluorescence Microscope (Zeiss, Germany). For cytological immunofluorescence, the cells were fixed in 4% PFA. After blocking with 5% bovine serum albumin (BSA) for 1 h, the primary antibody was incubated overnight, and the following procedures were the same as those for immunofluorescence.
2.8. Antibodies
The following antibodies were used: mouse anti-F4/80 (Santa Cruz), rabbit anti-iNOS (Cell Signaling Technology), mouse anti-CD80 (Invitrogen Antibodies), rabbit anti-CD206 (Abcam), rabbit anti-SHP2 (Cell Signaling Technology), rabbit anti-COL2 (Abcam), rabbit anti-COL10 (Abcam), rabbit anti-MMP3 (Proteintech), rabbit anti-COX2 (Cell Signaling Technology), rabbit anti-GAPDH (Cell Signaling Technology), rabbit anti-p-P65 (Cell Signaling Technology), rabbit anti-P65 (Cell Signaling Technology), rabbit anti-β-actin (Cell Signaling Technology), rabbit anti-histone H3 (Cell Signaling Technology), rabbit anti-p-AKT (Cell Signaling Technology), rabbit anti-AKT (Cell Signaling Technology), and rabbit anti-p-IKKα/β (Cell Signaling Technology).
2.9. Enzyme-linked immunosorbent assay (ELISA)
The tumor necrosis factor (TNF)-α and IL-6 levels in the supernatant of cultured RAW264.7 cells and BMDMs were investigated using ELISA Kits (R&D Systems) according to the manufacturer's instructions. The absorbance at 450 nm was detected by a Microplate Reader (Thermo Scientific).
2.10. RNA sequence (RNA-seq)
Total RNA was extracted from RAW264.7 cells after LPS treatment with or without SHP099 (n = 3) for one day. They were submitted to GeneChem company (Shanghai, China) to acquire the FPKM values of all genes. Pearson's correlation analysis was performed, and heatmaps were generated. In our studies, differentially expressed genes (DEGs) were defined as fold changes > 1.4 and P < 0.05. Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was performed to evaluate the biological function of DEGs.
2.11. Statistical analysis
All results were observed by different researchers. Differences between two groups were analysed by paired or unpaired Student's t test (parametric or nonparametric test), while the results of three or four groups were analysed by one-way analysis of variance (ANOVA, parametric test). The nonparametric data (such as OARSI scores and synovitis scores) were analysed using the Kruskal–Wallis test with multiple comparisons. All graphics were made by Prism 8 (GraphPad Software Inc.). The results are presented as the mean ± standard error of mean (SEM), and P < 0.05 were considered as statistically significant (∗P < 0.05, ∗∗P < 0.01), ns represents no significance.
3. Results
3.1. M1 macrophages are significantly increased in the synovium from OA patients and DMM mice
To explore the role of synovial macrophages in OA, we examined the synovitis score and macrophage polarization in the synovium of OA patients. Consistent with a previous study7, the OA synovium presented a higher synovitis score, which was accompanied by increased cell infiltration and synovial thickness (Supporting Information Fig. S2A and S2B). In OA synovium, F4/80 (macrophage marker)-positive cells were significantly increased, which was accompanied by an elevated proportion of inducible nitric oxide synthase (iNOS) (M1 macrophage marker)- and CD80 (M1 macrophage marker)-positive cells (Fig. S2C and S2D). However, the proportion of CD206 (M2 macrophage marker)-positive cells was lower than that of iNOS-positive cells, with no significant difference observed between the normal and OA groups (Supporting Information Fig. S3). We also investigated synovitis and macrophage polarization in a DMM-induced OA mouse model. The cell infiltration and tissue proliferation of the synovium of DMM mice were observed, and their synovitis scores were significantly elevated from 2 weeks to 8 weeks after DMM surgery (Fig. S2E and S2F). Similar to OA patients’ synovium, the accumulation of macrophages and the percentage of M1 macrophages were significantly increased in 6-week DMM mice (Fig. S2G and S2H).
3.2. SHP2 is located in the accumulated macrophages and upregulated in the synovium from OA patients and DMM mice
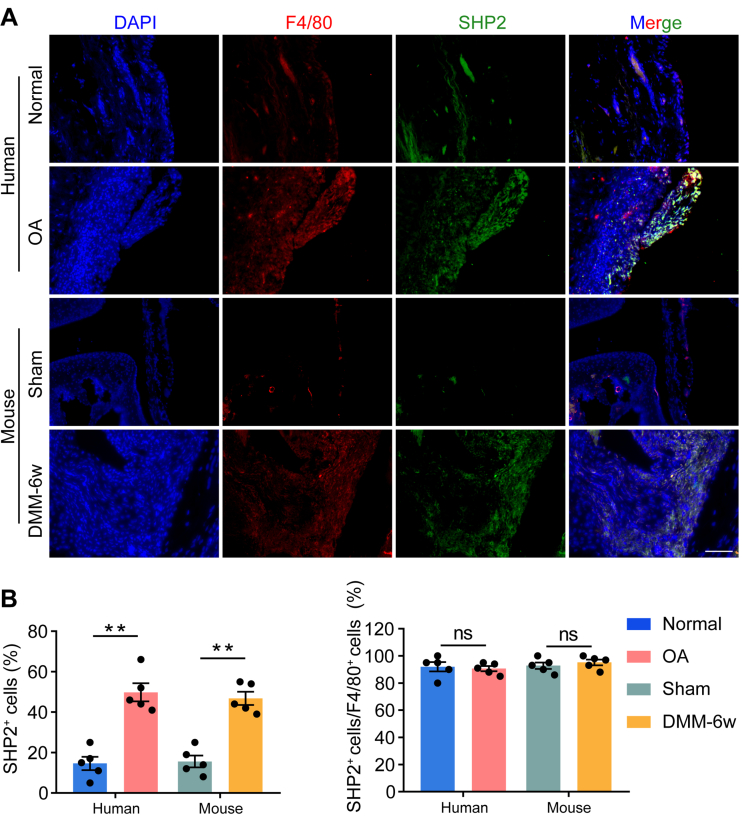
To determine whether SHP2 participated in the synovitis of OA, SHP2 expression and its colocalization with a macrophage marker (F4/80) were examined. Compared to that in normal subjects, SHP2 expression in the synovium was significantly elevated in OA patients. In addition, SHP2 was mostly located in the accumulated macrophages. Similar results were observed in the synovium of DMM mice (Fig. 1A and B).
Figure 1.
SHP2 expression and located cells in the synovium of OA patents and DMM mice. (A) Representative images of immunofluorescence of src-homology 2-containing protein tyrosine phosphatase 2 (SHP2), F4/80 and their colocalization in normal synovium and osteoarthritis (OA) synovium from human, and the representative immunofluorescence images of SHP2, F4/80 and their colocalization in the synovium of sham and 6-week destabilization of medial meniscus (DMM) mice. Scale bar: 100 μm. (B) Quantification of the proportion of SHP2-positive cells in synovial cells and F4/80-positive cells. Two-way analysis of variance (ANOVA) with Tukey's multiple comparison test. All data are shown as the mean ± SEM, n = 5; ∗∗P < 0.01, ns represents no significance.
3.3. SHP2 deletion in macrophages attenuates OA by inhibiting M1 macrophage polarization
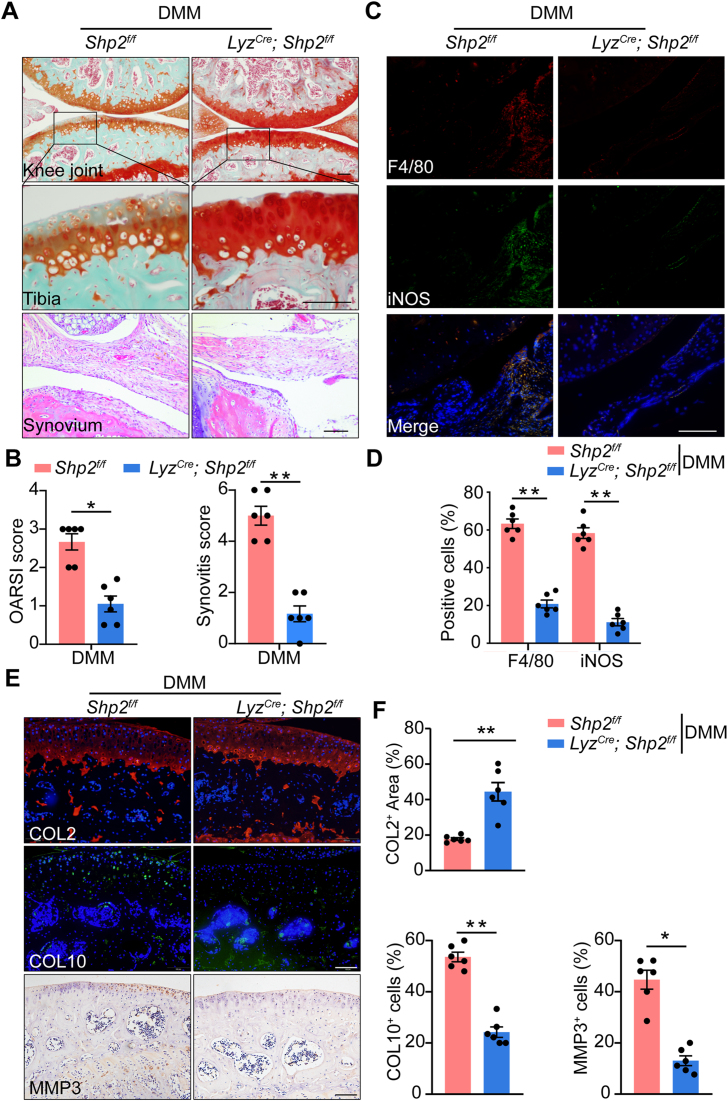
Compared to Shp2f/f mice, the cartilage degeneration of Shp2f/f; LyzCre mice was significantly attenuated during the OA process. The cell infiltration of synovium from the cKO mice was decreased (Fig. 2A). The synovitis and OARSI scores of Shp2f/f; LyzCre mice were lower (Fig. 2B). Moreover, decreased macrophage accumulation was shown in the cKO mice, which was accompanied by a lower rate of M1 macrophage markers, including iNOS and CD80 ((Fig. 2C and D, Supporting Information Fig. S4A and S4B). Compared to Shp2f/f mice, collagen II (COL2) was elevated in the cartilage of Shp2f/f; LyzCre mice at 6 weeks after DMM surgery, while the hypertrophic cartilage marker collagen X (COL10) was decreased. Furthermore, the expression of the cartilage degradation enzyme matrix metalloproteinases 3 (MMP3) was significantly reduced when Shp2 was conditional deleted, which may explain the cartilage protection effect in Shp2f/f; LyzCre mice (Fig. 2E and F).
Figure 2.
Conditional Shp2 deletion in macrophages attenuated the severity of osteoarthritis. (A) Representative images of Safranin O/Fast Green of the cartilage and synovium from Shp2f/f and Shp2f/f; LyzCre mice. Scale bar: 100 μm. (B) Quantification of the Osteoarthritis Research Society International (OARSI) score and Synovitis score of Shpf/f (n = 6) and Shp2f/f; LyzCre (n = 6) mice. Unpaired Student's t-test (non-parametric test), ∗∗P < 0.01. (C) Representative images of immunofluorescence of F4/80 and iNOS in the synovium from Shpf/f and Shp2f/f; LyzCre mice. Scale bar: 100 μm. (D) Quantification of the proportion of F4/80- and iNOS- positive cells in the synovium from Shp2f/f (n = 6) and Shp2f/f; LyzCre (n = 6) mice. Unpaired Student’ t-test (parametric test), ∗∗P < 0.01. (E) Representative images of immunofluorescence of collage II (COL2), collagen X (COL10), and matrix metalloproteinase 3 (MMP3). Scale bar: 100 μm. (F) Quantification of the proportion of the positive area of COL2, the proportion of cells expressing COL10 and MMP3 in the cartilage of Shp2f/f and Shp2f/f; LyzCre mice (n = 6). Unpaired Student's t-test (parametric test); ∗P < 0.05, ∗∗P < 0.01. All Data are shown as the mean ± SEM.
3.4. SHP099 treatment inhibits LPS-induced M1 macrophage polarization
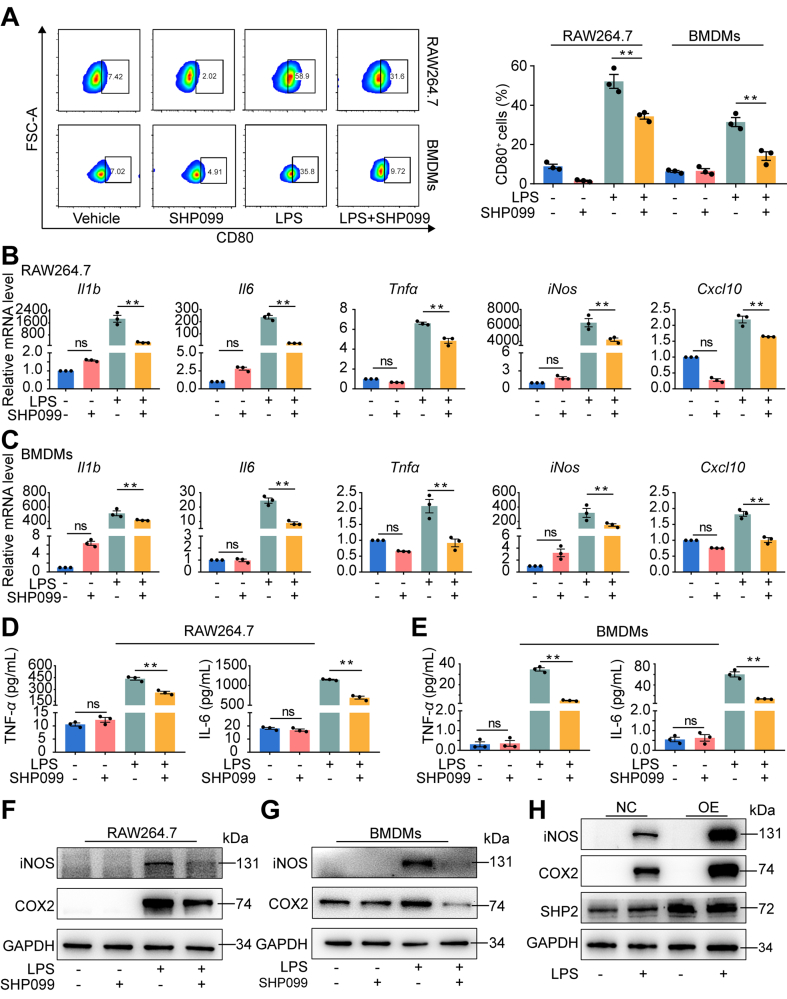
To determine the purity of RAW264.7 cells and BMDMs, F4/80 (macrophage surface marker) of RAW264.7 cells and BMDMs was identified using flow cytometry (Supporting Information Fig. S5A). After stimulation with LPS for 3 h, the Shp2 mRNA level was significantly elevated in RAW264.7 cells (Fig. S5B). BMDMs from LyzCre; Shp2f/f mice attenuated LPS-induced IL-6 and TNF-α release (Supporting Information Fig. S6A and S6B) and M1-related mRNA expression (Fig. S6C). To determine the impact of allosteric SHP2 inhibitor on macrophage polarization in vitro, we applied SHP099, an SHP2 allosteric inhibitor, to RAW264.7 cells and BMDMs stimulated with LPS for 24 h. The assessment of the expression level of M1 macrophage markers by flow cytometry revealed that the proportion of CD80+ cells decreased remarkably after SHP099 treatment (Fig. 3A). Similarly, inhibition of SHP2 in RAW264.7 cells and BMDMs significantly downregulated M1 macrophage-expressed inflammatory genes [Il1b, Il6, Tnfa, CXC chemokineligand-10 (Cxcl10) and iNos] (Fig. 3B and C). ELISA results showed that SHP099 significantly decreased the secretion of LPS-induced inflammatory cytokines (TNF-α and IL-6) (Fig. 3D and E). Furthermore, M1 macrophage-related proinflammatory proteins [iNOS and cyclooxygenase 2 (COX2)] were decreased after SHP099 treatment (Fig. 3F and G). Furthermore, SHP2 overexpression enhanced the expression of iNOS and COX2 (Fig. 3H). To avoid the off-target effect of SHP099, we also found that another SHP2 allosteric inhibitor, SHP836, inhibited M1 macrophage-related inflammatory protein and gene expression (Supporting Information Fig. S7A and S7B).
Figure 3.
SHP099 inhibited macrophage M1 polarization and inflammatory cytokines secretion during LPS-induced inflammation for 24 h. (A) Flow cytometry for detection and quantitative analysis of CD80+ cells after the treatment of lipopolysaccharide (LPS) with or without SHP099 for 24 h in RAW264.7 cells and bone marrow-derived macrophages (BMDMs) (n = 3). One-way ANOVA with Tukey's multiple comparison test; ∗∗P < 0.01. (B) qPCR analysis of mRNA of M1-related genes, interleukin-1β (Il1b), Il6, tumor necrosis factor α (Tnfa), inducible nitric oxide synthase (iNos) and CXC chemokineligand-10 (Cxcl10) in RAW264.7 cells after LPS stimulation with or without SHP099 treatment for 24 h (n = 3). One-way ANOVA with Tukey's multiple comparison test; ∗∗P < 0.01, ns represents no significance. (C) qPCR analysis of mRNA of M1-related genes, Il1b, Il6, Tnfa, iNos and Cxcl10 in BMDMs after LPS stimulation with or without SHP099 treatment for 24 h (n = 3). One-way ANOVA with Tukey's multiple comparison test; ∗∗P < 0.01, ns represents no significance. (D) Enzyme-linked immunosorbent assay (ELISA) results of TNF-α and IL-6 secretion of RAW264.7 cells after LPS stimulation with or without SHP099 treatment for 24 h (n = 3). One-way ANOVA with Tukey's multiple comparison test; ∗∗P < 0.01, ns represents no significance. (E) ELISA results of TNF-α and IL-6 secretion of BMDMs cells after LPS stimulation with or without SHP099 treatment for 24 h. One-way ANOVA with Tukey's multiple comparison test. ∗∗P < 0.01. (F) Western blot analysis of iNOS and cyclooxygenase 2 (COX2) in RAW264.7 after LPS stimulation with or without SHP099 treatment for 48 h. (G) Western blot analysis of iNOS and COX2 in BMDMs after LPS stimulation with or without SHP099 treatment for 48 h. (H) Western blot analysis of iNOS and COX2 in RAW264.7 after LPS stimulation with over-expression (OE) of SHP2 or not (NC) for 48 h. All data are shown as the mean ± SEM.
3.5. SHP099 downregulates the TLR pathway by inhibiting MyD88-dependent transduction
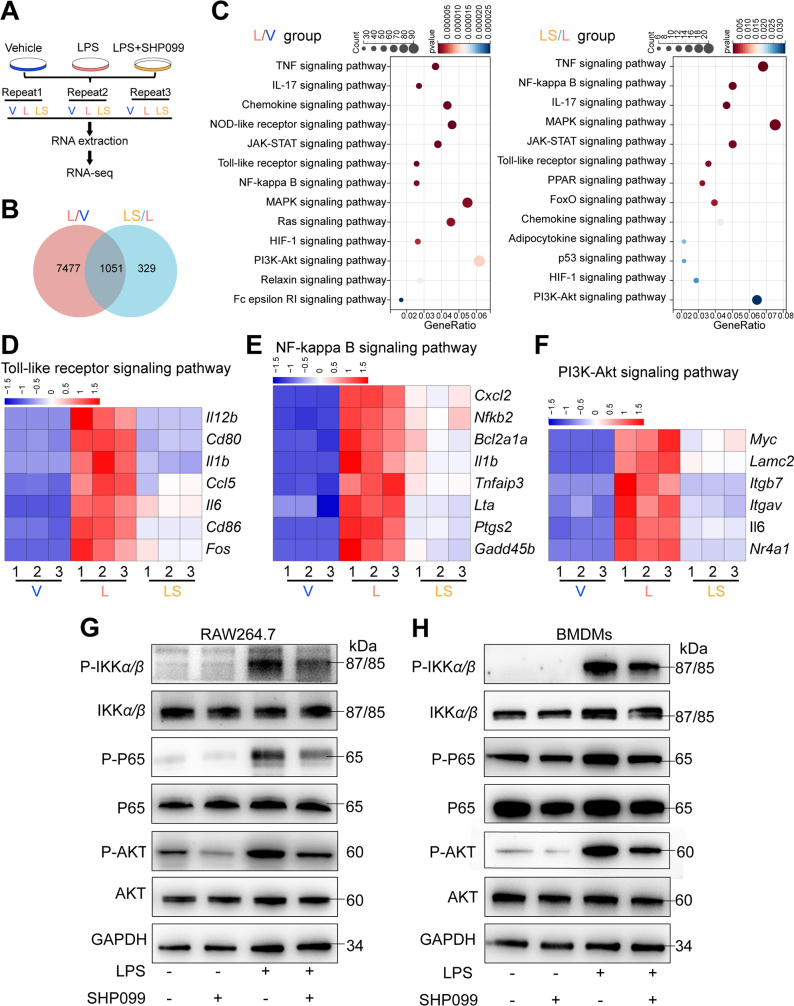
To identify the underlying mechanism by which SHP099 modulates M1 macrophage polarization, we performed RNA sequencing (RNA-seq) analysis on RAW264.7 cells stimulated by LPS with or without SHP099 treatment (Fig. 4A). A total of 8528 DEGs were identified between the vehicle (V) and LPS (L) groups, and 1380 DEGs were identified between the LPS (L) group and LPS+SHP099 (LS) group (Fig. 4B). Pathway enrichment analysis identified significant enrichment of DEGs, such as TLR signaling, NF-κB signaling and PI3K–AKT signaling (Fig. 4C). LPS is a classic stimulator of TLR signaling, and the myeloid differentiation primary response protein 88 (MyD88)-dependent pathway is a key pathway involved in TLR signaling and IL-1 receptor family signaling, which includes the NF-κB24 and PI3K–AKT pathways25. SHP099 treatment significantly downregulated TLR and NF-κB signaling, with several related genes stimulated by LPS, such as Cxcl2, Nfkb2, Bcl2a1a, and Il1b (Fig. 4D and E). Moreover, the expression of many PI3K–AKT-related genes was also decreased in the SHP099 group (Fig. 4F). LPS stimulation significantly elevated the protein levels of p-IKKα/β, p-P65, and p-AKT, which decreased after SHP099 treatment in RAW264.7 cells and BMDMs (Fig. 4G and H). Moreover, the translocation of P65 into the nucleus was observed after 3 or 6 h of LPS stimulation and was inhibited by SHP099 in RAW264.7 cells (Supporting Information Fig. S8A). Immunofluorescence revealed that SHP099 partially inhibited the translocation of P65 induced by LPS in RAW264.7 cells (Fig. S8B and S8C). These results indicate that SHP099 attenuates the TLR pathway and its downstream signaling pathways, including the NF-κB and PI3K–AKT pathways.
Figure 4.
SHP099 inhibited Toll-like signaling (TLR) pathway, including its downstream pathways, including nuclear factor kappaB (NF-κB) pathway, and PI3K–AKT pathway. (A) Schematic diagram for RNA-sequence analysis. (B) Venn diagram. (C) Encyclopedia of Genes and Genomes (KEGG) analysis of the biological function of differentially expressed genes (DEGs). (D) DEGs in the TLR signaling pathway. (E) DEGs in the NF-κB signaling pathway. (F) DEGs in the PI3K–AKT signaling pathway. (G) Western blot analysis of p-IKKα/β, IKKα/β, p-P65, P65, p-AKT, AKT, GAPDH after LPS stimulation with or without SHP099 treatment in RAW264.7 cells. (H) Western blot analysis of p-IKKα/β, IKKα/β, p-P65, P65, p-AKT, AKT, GAPDH after LPS stimulation with or without SHP099 treatment in BMDMs.
3.6. Inhibition of the PI3K and NF-κB pathways inhibits M1 macrophage polarization
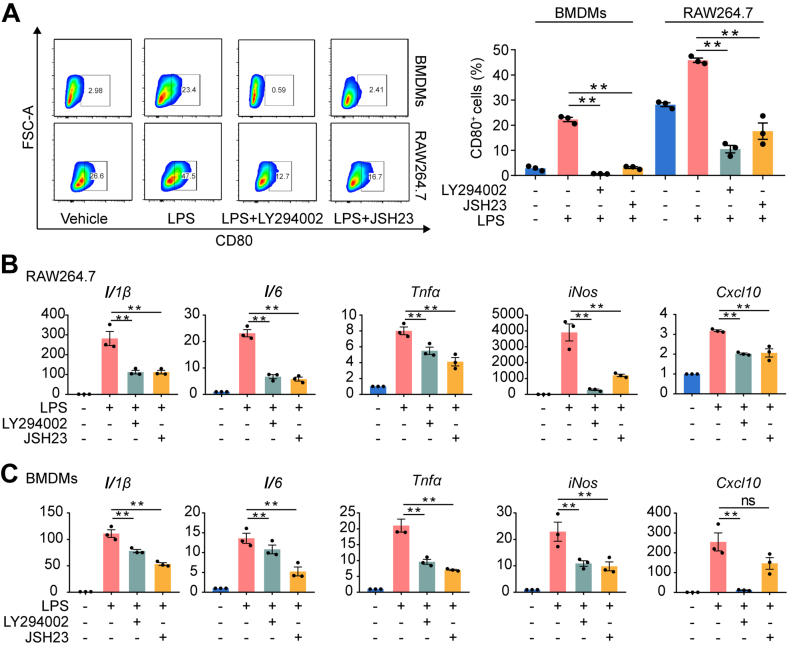
To investigate whether the PI3K and NF-κB pathways mediate SHP099-induced macrophage polarization, PI3K (LY294002) and NF-κB (JSH23) inhibitors were used in vitro. The rate of CD80+ cells was significantly decreased after LY294002 and JSH23 treatment in RAW264.7 cells and BMDMs (Fig. 5A). The mRNA expression of M1 macrophage markers (iNos, Il1b, Il6, Tnfa and Cxcl10) was also decreased after LY294002 and JSH23 treatment in RAW264.7 cells and BMDMs (Fig. 5B and C), and the inhibition of the M1 phenotype induced by SHP099 was not promoted in RAW264.7 cells in which the PI3K–AKT and NF-κB pathways were blocked (Supporting Information Fig. S9).
Figure 5.
Inhibition of NF-κB pathway and PI3K pathway inhibited macrophage M1 polarization. (A) Flow cytometry for detection and quantitative analysis of CD80-positive cells after the treatment of LPS with or without treatment of LY294002 and JSH23 for 24 h in RAW264.7 cells and BMDMs (n = 3). One-way ANOVA with Tukey's multiple comparison test, ∗∗P < 0.01. (B) qPCR analysis of mRNA of M1-related genes, Il1b, Il6, Tnfa, iNos and Cxcl10 in RAW264.6 cells after LPS stimulation with or without LY294002 and JSH23 treatment for 24 h (n = 3). One-way ANOVA with Tukey's multiple comparison test, ∗∗P < 0.01. (C) qPCR analysis of mRNA of M1-related genes, Il1b, Il6, Tnfa, iNos and Cxcl10 in BMDMs after LPS stimulation with or without LY294002 and JSH23 treatment for 24 h (n = 3). One-way ANOVA with Tukey's multiple comparison test, ∗∗P < 0.01. All data are shown as the mean ± SEM.
3.7. Intra-articular injection of SHP099 alleviates OA by inhibiting M1 macrophage polarization
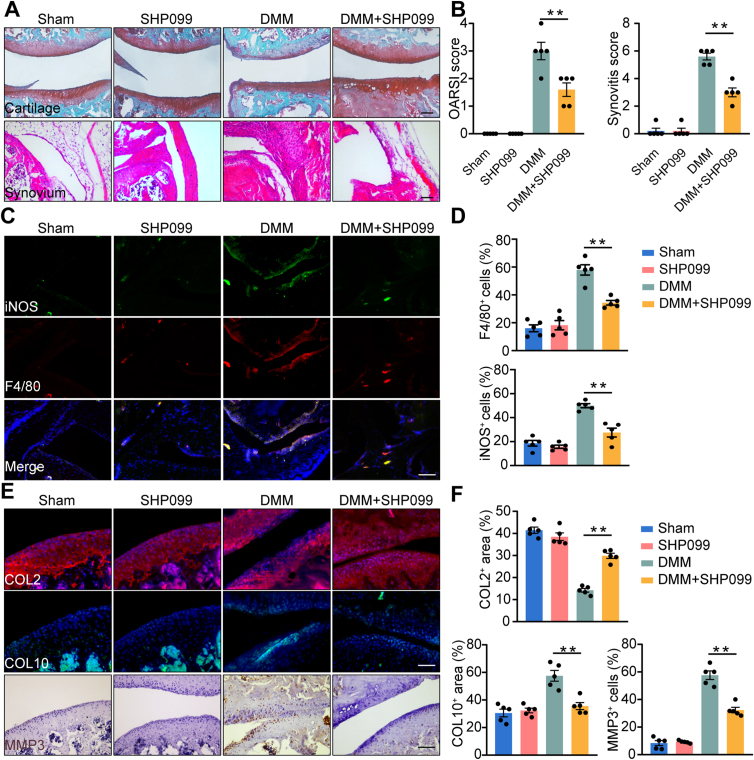
To examine whether SHP099 could inhibit M1 macrophage polarization and alleviate OA in vivo, intra-articular injection of SHP099 was performed in a mouse DMM model. After intra-articular injection of SHP099, the abundance of cartilage extracellular matrix increased compared to that in the DMM group. Cell infiltration of the synovium was remarkably decreased after SHP099 administration (Fig. 6A). The OARSI score and synovitis score decreased significantly (Fig. 6B). Consistent with the synovitis score, there was a substantial reduction in the proportion of F4/80-, iNOS-, and CD80-positive cells in the synovium following SHP099 injection (Fig. 6C and D, Supporting Information Fig. S10A and S10B). Furthermore, the expression of COL2 was elevated in the cartilage of the SHP099 group after DMM surgery, while COL10 was decreased. Furthermore, the expression of MMP3 was significantly reduced (Fig. 6E and F), indicating that the SHP2 allosteric inhibitor could be a potential therapeutic drug for OA.
Figure 6.
Intra-articular injection of SHP099 attenuated DMM-induced OA progression in mice model. (A) Representative images of Safranin O/Fast Green of the cartilage and H&E staining of synovium from mice divided into four groups, including Sham group, intra-articular injection of SHP099 group, DMM group and DMM with injection of SHP099 group. Scale bar: 100 μm. (B) Quantification of synovitis score and OARSI score of four groups (n = 5). One-way ANOVA with Tukey's multiple comparison test, ∗∗P < 0.01. (C) Representative images of immunofluorescence of F4/80 and iNOS in the synovium from mice in four groups. Scale bar: 100 μm. (D) Quantification of the proportion of F4/80- and iNOS- positive cells in the synovium from mice in four groups (n = 5). One-way ANOVA with Tukey's multiple comparison test, ∗∗P < 0.01. (E) Representative images of immunofluorescence of COL2, COL10 and MMP3. Scale bar: 100 μm. (F) Quantification of the proportion of the positive area of COL2, the proportion of cells expressing COL10 and the proportion of cells expressing MMP3 in the cartilage of mice divided into four groups (n = 5). One-way ANOVA with Tukey's multiple comparison test, ∗∗P < 0.01. All data are shown as the mean ± SEM.
4. Discussion
In this study, we demonstrated for the first time that increased SHP2 was mostly located in accumulated macrophages in the synovium of OA patients and OA model mice, thus indicating its potential role in OA. Conditional deletion of Shp2 in macrophages could decrease M1 macrophage polarization, attenuate synovitis and decrease cartilage degradation during OA progression. Moreover, SHP2 inhibition reduced LPS-induced M1 macrophage polarization via the TLR signaling pathway in vitro. Intra-articular injection of SHP099 alleviated OA progression in a mouse DMM model. These results provide a new perspective for intervention in the OA process by modulating macrophage polarization (Fig. 7).
Figure 7.
The graphic illustration of the mechanism of SHP2-mediated macrophage polarization in osteoarthritis. SHP2 aggravates M1 macrophage-induced synovitis in OA by inhibiting NF-κB and PI3K–AKT signal pathway, indicating that targeting macrophagic SHP2 is a promising strategy for OA.
Studies have shown that enhanced synovial M1 macrophage polarization is responsible for the increased severity of OA7. Consistent with previous studies7,22, we found increased synovitis score and highly infiltrated M1 macrophages in the synovium of OA patients. Compared to the collagenase-induced OA model, the DMM-induced OA mouse model was described as a low synovial-activation OA model26, but we found that in the DMM model, the synovitis scores were also increased significantly from 2 weeks after DMM surgery, thus indicating that synovitis participated in the process of OA progression from the early to late phases. Furthermore, macrophage accumulation and M1 polarization in the synovium of the DMM-induced OA mouse model were also observed. The DMM model simulates the pathological process of OA well, and these results suggested that synovitis and macrophage polarization played a vital role in the development of OA.
A recent study revealed that SHP2 could promote the invasion and survival of synovial fibroblasts during RA27, but whether SHP2 participates in macrophage function and polarization during the pathologic process of OA was not investigated. Compared to the WT mice, the cartilage degradation of the cKO mice was significantly decreased, which might be associated with the lower expression of degrading enzymes, such as MMP3. Furthermore, as we expected, macrophage accumulation in cKO mice was significantly attenuated. At the same time, the proportion of M1 macrophages was decreased, which might explain why cartilage degradation decreased. Intra-articular injection of SHP099 also has an ideal therapeutic effect. Several studies have tried to influence the OA process by interfering with the function of macrophages, such as physical therapy represented by low-intensity pulsed ultrasound28 and small molecule drugs represented by kinsenoside29 and rapamycin7. Therefore, finding a specific target modulating macrophage polarization will provide new ideas regarding potential treatments for OA, and our results showed that SHP2 is a new ideal target through which to influence the polarization of macrophages during the OA process.
To explore the underlying mechanism of SHP2 on macrophage polarization, RAW 264.7 cells, a mouse macrophage cell line that is widely used as M0 macrophages30 and BMDMs, were used. Under LPS stimulation, SHP099 treatment inhibited M1 macrophage polarization. LPS is a classic stimulator of TLRs, whose downstream signaling is regulated by MyD88-and TRIF-dependent pathways31. Recent studies revealed that the MyD88 pathway modulated the M1 polarization of macrophages32,33, but whether this process is involved in SHP2-regulated macrophage polarization is unknown. According to our RNA-seq results, SHP099 significantly attenuated LPS-induced TLR signaling, and p-P6534 and p-AKT35 were decreased after SHP099 treatment, which are key proteins in the TLR/MyD88 proinflammatory pathway. These results showed that SHP2 might be a possible modulator of the TLR/MyD88 pathway.
There are several limitations of this study. First, we did not evaluate the effect of Shp2 conditional deletion on other OA models, such as spontaneous OA, which would hamper a more comprehensive understanding of the role of SHP2 in OA. Second, we did not evaluate the periarticular osteophyte formation of mice and hence might ignore the possible role of SHP2 in bone remodelling.
5. Conclusions
To conclude, we found that SHP2 inhibition is a potential therapeutic strategy that can be used to attenuate the severity of OA by repolarizing M1 macrophages in synovial tissues. Future studies are warranted to explore the underlying mechanisms of how SHP2 allosteric inhibitors modulate the transduction of the TLR/MyD88 proinflammatory pathway.
Acknowledgments
We also thank Professor Tao Shi from the Comprehensive Cancer Centre of Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School & Clinical Cancer Institue of Nanjing University (Nanjing, China) for the help for us. This work was supported by the National Science Foundation of China (NSFC 81802196, 81572129, 81872877, 91853109, and 81772335), Key Program of NSFC (81730067, China), Special Program of Chinese Academy of Science (XDA16020805, China), Jiangsu Provincial Key Medical Center Foundation (China), Jiangsu Provincial Medical Outstanding Talent Foundation (China), and Jiangsu Provincial Key Medical Talent Foundation (China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2022.02.010.
Contributor Information
Yang Sun, Email: yangsun@nju.edu.cn.
Dongquan Shi, Email: shidongquan@nju.edu.cn.
Author contributions
Dongquan Shi and Yang Sun conceived and supervised the study. Ziying Sun, Qianqian Liu performed the cell line experiments and animal experiments and analyzed the data. Zhongyang Lv, Jiawei Li, and Xingquan Xu performed bioinformatics analysis. Heng Sun, Maochun Wang, Kuoyang Sun and Tianshu Shi collected clinical samples. Zizheng Liu, Guihua Tan, Wenqiang Yan, Rui Wu, Yannick Xiaofan Yang, Shiro Ikegawa. Dongdong Sun, Haibo Cheng and Yuxian Shen gave methodological support and conceptual advice. Ziying Sun and Qianqian Liu wrote the manuscript. All authors discussed the results and commented on the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Appendix A. Supporrting information
The following is the Supplementary data to this article:
References
- 1.Woolf A.D., Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 2.Wei Y., Bai L. Recent advances in the understanding of molecular mechanisms of cartilage degeneration, synovitis and subchondral bone changes in osteoarthritis. Connect Tissue Res. 2016;57:245–261. doi: 10.1080/03008207.2016.1177036. [DOI] [PubMed] [Google Scholar]
- 3.Baker K., Grainger A., Niu J., Clancy M., Guermazi A., Crema M., et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann Rheum Dis. 2010;69:1779–1783. doi: 10.1136/ard.2009.121426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scanzello C.R., Goldring S.R. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haraden C.A., Huebner J.L., Hsueh M.F., Li Y.J., Kraus V.B. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res Ther. 2019;21:146. doi: 10.1186/s13075-019-1923-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H., Lin C., Zeng C., Wang Z., Wang H., Lu J., et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann Rheum Dis. 2018;77:1524–1534. doi: 10.1136/annrheumdis-2018-213450. [DOI] [PubMed] [Google Scholar]
- 8.Wu C.L., McNeill J., Goon K., Little D., Kimmerling K., Huebner J., Kraus V., et al. Conditional macrophage depletion increases inflammation and does not inhibit the development of osteoarthritis in obese macrophage fas-induced apoptosis-transgenic mice. Arthritis Rheumatol. 2017;69:1772–1783. doi: 10.1002/art.40161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng G.S., Hui C.C., Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein—tyrosine kinases. Science. 1993;259:1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- 10.Tajan M., de Rocca Serra A., Valet P., Edouard T., Yart A. SHP2 sails from physiology to pathology. Eur J Med Genet. 2015;58:509–525. doi: 10.1016/j.ejmg.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J., Zhang F., Niu R. Functions of SHP2 in cancer. J Cell Mol Med. 2015;19:2075–2083. doi: 10.1111/jcmm.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo W., Liu W., Chen Z., Gu Y., Peng S., Shen L., et al. Tyrosine phosphatase SHP2 negatively regulates NLRP3 inflammasome activation via ANT1-dependent mitochondrial homeostasis. Nat Commun. 2017;8:2168. doi: 10.1038/s41467-017-02351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao P., Zhang H., Zhang Y., Zheng M., Liu R., Zhao Y., et al. Phosphatase Shp2 exacerbates intestinal inflammation by disrupting macrophage responsiveness to interleukin-10. J Exp Med. 2019;216:337–349. doi: 10.1084/jem.20181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q., Zhai L., Han M., Shi D., Sun Z., Peng S., et al. SH2 domain-containing phosphatase 2 inhibition attenuates osteoarthritis by maintaining homeostasis of cartilage metabolism via the docking protein 1/uridine phosphorylase 1/uridine cascade. Arthritis Rheumatol. 2022;74:462–474. doi: 10.1002/art.41988. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y.N., LaMarche M.J., Chan H.M., Fekkes P., Garcia-Fortanet J., Acker M.G., et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature. 2016;535:148–152. doi: 10.1038/nature18621. [DOI] [PubMed] [Google Scholar]
- 16.Zhao M., Guo W., Wu Y., Yang C., Zhong L., Deng G., et al. SHP2 inhibition triggers anti-tumor immunity and synergizes with PD-1 blockade. Acta Pharm Sin B. 2019;9:304–315. doi: 10.1016/j.apsb.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasson S.S., Blanchet T.J., Morris E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Tao B., Jin W., Xu J., Liang Z., Yao J., Zhang Y., et al. Myeloid-specific disruption of tyrosine phosphatase Shp2 promotes alternative activation of macrophages and predisposes mice to pulmonary fibrosis. J Immunol. 2014;193:2801–2811. doi: 10.4049/jimmunol.1303463. [DOI] [PubMed] [Google Scholar]
- 19.Kuyinu E.L., Narayanan G., Nair L.S., Laurencin C.T. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res. 2016;11:19. doi: 10.1186/s13018-016-0346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z., Jiang Y., Zhou Z., Huang J., Chen S., Zhou W., et al. Scavenger receptor A1 attenuates aortic dissection via promoting efferocytosis in macrophages. Biochem Pharmacol. 2019;168:392–403. doi: 10.1016/j.bcp.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 21.Li H., Ghazanfari R., Zacharaki D., Lim H.C., Scheding S. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann N Y Acad Sci. 2016;1370:109–118. doi: 10.1111/nyas.13102. [DOI] [PubMed] [Google Scholar]
- 22.Krenn V., Morawietz L., Burmester G.R., Kinne R.W., Mueller-Ladner U., Muller B., et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–364. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H., Wang H., Zeng C., Yan B., Ouyang J., Liu X., et al. mTORC1 activation downregulates FGFR3 and PTH/PTHrP receptor in articular chondrocytes to initiate osteoarthritis. Osteoarthritis Cartilage. 2017;25:952–963. doi: 10.1016/j.joca.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Zhou M., Xu W., Wang J., Yan J., Shi Y., Zhang C., et al. Boosting mTOR-dependent autophagy via upstream TLR4–MyD88–MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine. 2018;35:345–360. doi: 10.1016/j.ebiom.2018.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan J., Li J., Zhang L., Sun Y., Jiang J., Huang Y., et al. Nrf2 protects against acute lung injury and inflammation by modulating TLR4 and Akt signaling. Free Radic Biol Med. 2018;121:78–85. doi: 10.1016/j.freeradbiomed.2018.04.557. [DOI] [PubMed] [Google Scholar]
- 26.Schelbergen R.F., Geven E.J., van den Bosch M.H., Eriksson H., Leanderson T., Vogl T., et al. Prophylactic treatment with S100A9 inhibitor paquinimod reduces pathology in experimental collagenase-induced osteoarthritis. Ann Rheum Dis. 2015;74:2254–2258. doi: 10.1136/annrheumdis-2014-206517. [DOI] [PubMed] [Google Scholar]
- 27.Ganesan R., Rasool M. Interleukin 17 regulates SHP-2 and IL-17RA/STAT-3 dependent Cyr61, IL-23 and GM-CSF expression and RANKL mediated osteoclastogenesis by fibroblast-like synoviocytes in rheumatoid arthritis. Mol Immunol. 2017;91:134–144. doi: 10.1016/j.molimm.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Uddin S.M.Z., Komatsu D.E. Therapeutic potential low-intensity pulsed ultrasound for osteoarthritis: pre-clinical and clinical perspectives. Ultrasound Med Biol. 2020;46:909–920. doi: 10.1016/j.ultrasmedbio.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Zhou F., Mei J., Han X., Li H., Yang S., Wang M., et al. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF-κB/MAPK signaling and protecting chondrocytes. Acta Pharm Sin B. 2019;9:973–985. doi: 10.1016/j.apsb.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B.C., Li Z., Xu W., Xiang C.H., Ma Y.F. Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Am J Transl Res. 2018;10:265–273. [PMC free article] [PubMed] [Google Scholar]
- 31.Kagan J.C., Su T., Horng T., Chow A., Akira S., Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J.T., Wu S.X., Zhang H., Kuang F. Inhibition of MyD88 signaling skews microglia/macrophage polarization and attenuates neuronal apoptosis in the hippocampus after status epilepticus in mice. Neurotherapeutics. 2018;15:1093–1111. doi: 10.1007/s13311-018-0653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arora H., Wilcox S.M., Johnson L.A., Munro L., Eyford B.A., Pfeifer C.G., et al. The ATP-binding cassette gene ABCF1 functions as an E2 ubiquitin-conjugating enzyme controlling macrophage polarization to dampen lethal septic shock. Immunity. 2019;50 doi: 10.1016/j.immuni.2019.01.014. 418-31.e6. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y.P., Ke L.F., Lu J.P., Wang J.C., Zhu W.F., Chen F.F., et al. Prevalence and clinical significance of oncogenic CD79B and MYD88 mutations in primary testicular diffuse large B-cell lymphoma: a retrospective study in China. OncoTargets Ther. 2019;12:10165–10175. doi: 10.2147/OTT.S222189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong J., Qiu X., Yu Q., Chen H., Yan C. A novel polysaccharide from Acorus tatarinowii protects against LPS-induced neuroinflammation and neurotoxicity by inhibiting TLR4-mediated MyD88/NF-κB and PI3K/Akt signaling pathways. Int J Biol Macromol. 2020;163:464–475. doi: 10.1016/j.ijbiomac.2020.06.266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.