Summary
The Xenopus embryo provides an advantageous model system where genes can be readily transplanted as DNA or mRNA or depleted with antisense techniques. Here, we present a protocol to culture and image the cell biological properties of explanted Xenopus cap cells in tissue culture. We illustrate how this protocol can be applied to visualize lysosomes, macropinocytosis, focal adhesions, Wnt signaling, and cell migration.
For complete details on the use and execution of this protocol, please refer to Tejeda-Muñoz et al. (2022).
Subject areas: Cell Biology, Cell culture, Developmental biology, Microscopy, Model Organisms, Signal Transduction
Graphical abstract
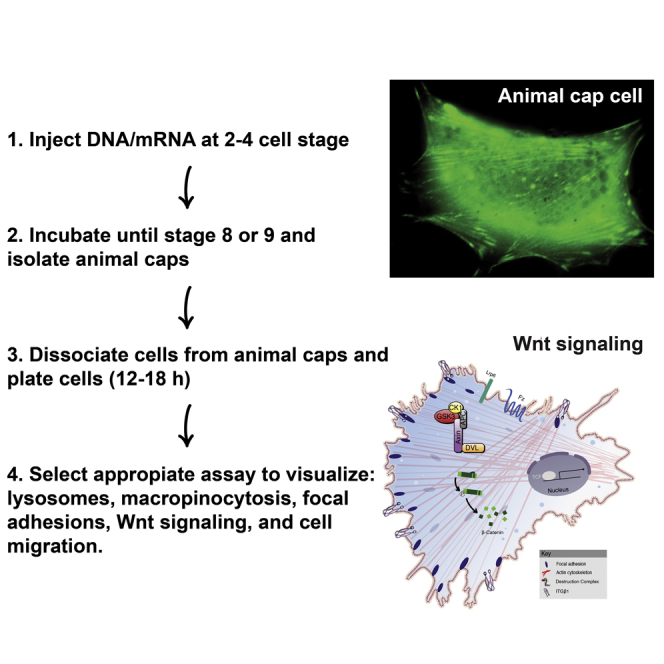
Highlights
-
•
Quick, inexpensive protocol for culturing frog embryo animal cap cells
-
•
Simple assay to visualize cell biological properties of living embryonic cells
-
•
Studying animal cap cells to reveal lysosomal function and cell adhesion
-
•
Animal cap cells for Wnt signaling and cell motility studies
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The Xenopus embryo provides an advantageous model system where genes can be readily transplanted as DNA or mRNA or depleted with antisense techniques. Here, we present a protocol to culture and image the cell biological properties of explanted Xenopus cap cells in tissue culture. We illustrate how this protocol can be applied to visualize lysosomes, macropinocytosis, focal adhesions, Wnt signaling, and cell migration.
Before you begin
Xenopus laevis animal cap cells are located in the animal (pigmented) pole of the blastula or early gastrula stage embryo (Ariizumi et al., 2009). By microinjecting the embryo during the early cleavage stages one can overexpress or deplete gene products with great ease, making these cells a favorite system for researchers (Asashima et al., 2009; Blum et al., 2015; Moriyama and De Robertis, 2018; Sosa et al., 2019). Only a few Xenopus permanent cell lines exist (Smith and Tata, 1991) and animal cap cells provide a system closer to the in vivo situation. The protocol below describes the specific steps for the visualization of living ectodermal cells. Cells cultured in L-15 medium (without serum) diluted to 50% with water survived for up to three days, provided they were attached to fibronectin-coated coverslips. Here we characterize the method and provide simple assays to visualize (1) lysosomes with lysosome-associated membrane protein 1 (LAMP1-RFP), (2) the multivesicular body marker CD63-RFP, (3) activated lysosomal Cathepsin D with the SiR-Lysosome reagent, (Marciniszyn et al., 1976), (4) macropinocytosis with added TMR-Dextran 70 kDa (Commisso et al., 2014; Albrecht et al., 2020; Tejeda-Muñoz et al., 2019; Redelman-Sidi et al., 2018), (5) focal adhesions labeled by microinjection with Tes-GFP DNA injection and, (6) F-actin during cell migration using LifeAct DNA expression (Tejeda-Muñoz et al., 2022). These assays have proven useful in the analysis of Wnt signaling (Tejeda-Muñoz et al., 2022). The protocol can in principle be adapted for any number of cell biological assays for which there are appropriate fluorescent protein tracers or gene depletion reagents.
Institutional permissions
These experiments require vertebrate animals in order to prepare embryos; all experiments reported here have been approved by the UCLA Animal Research Committee in accordance to the Public Health Service Policy on Humane Care and Used of Laboratory Animals (ARC-1995-129).
Preparation of explants
Timing: 1 day
Preparation of animal cap single cells from Xenopus provides a very useful assay system (Sive et al., 2000). Embryos were prepared by in vitro fertilization with a Xenopus laevis testis suspension and cultured as previously described (Moriyama and De Robertis, 2018). Earlier work had used Xenopus gastrula mesodermal cells cultured in simple saline solutions and demonstrated a requirement of fibronectin or laminin for cell attachment (Nakatsuji, 1986; Smith et al., 1990). This section describes the preparation of ectodermal cells from Xenopus for use in culture using Leibovitz L-15 cell culture medium (Figure 1A). The cells can be treated with growth factors as well as drugs for studies of migration and cell-cell contact inhibition.
Note: Animal caps can be dissected from manually dechorionated blastula-stage embryos with eyebrow hair knives; however, this is much slower than cutting the tissue with sharp Dumont #5 forceps.
-
1.
Manually dechorionate the frog embryos at blastula stages 8 or 9 (staged according to Nieuwkoop and Faber, 1967) with forceps and dissect the animal cap region in 1 × MMR solution plastic Petri dishes covered with 2% agarose in H2O.
Note: The animal cap cells normally develop into epidermis.
-
2.Transfer the animal cap explants into fresh 2% agarose (a thin coat of agarose in H2O) plates in 1 × MMR saline.
-
a.Dissociate them manually by gently pipetting up and down 3–4 times. Avoid contact of cells with air. Use a pulled glass Pasteur pipette with a fire-blunted opening of about 1 mm.
-
a.
-
3.
Discard the more adherent, pigmented epithelial cells. Troubleshooting 1.
Note: After the pipetting process, the outer pigmented epithelial cells remain attached to each other.
-
4.
Plate the dissociated animal cap cells with a glass Pasteur pipette in L-15 medium (without serum) diluted to 50% with water in a 12-well culture dish containing circular fibronectin-coated coverslips for immunofluorescence.
-
5.For filming, plate the cells on fibronectin-coated glass-bottom chambers. Place cells from approximately 6 to 8 animal caps into each well. Troubleshooting 2.
-
a.Sterilize the coverslips before adding them to the wells by autoclaving or immersing them in 70% ethanol under a sterile tissue culture hood. Allow the ethanol to evaporate and then place coverslips into individual wells of a 12-well culture plate.
-
b.Add fibronectin to the same wells containing the coverslips used for cell adhesion and spreading (Nakatsuji, 1986). Use 10 μg/mL fibronectin for 30 min at 37°C (Sigma F4759).
-
c.Wash coverslips with phosphate buffered saline (PBS, Gibco) 3 times and then add a volume of 1 mL of 1:1 L-15:H2O medium to each 12-well and add cells.
-
a.
-
6.
Incubate the cap cells at 15°C–20°C for 12–18 h. Individual ectodermal cells divide (Figure 1A) and remain attached while sibling embryos reach late neurula or tailbud stages.
-
7.
Proceed with the respective protocol (movies or immunofluorescence) and treat the cells with chemicals or growth factors according to the chosen assay.
Note: Animal cap dissociation can be accelerated using Ca++ Mg++-free MMR or a mixture of 1:10 MMR with or without divalent cations instead of mechanical disruption.
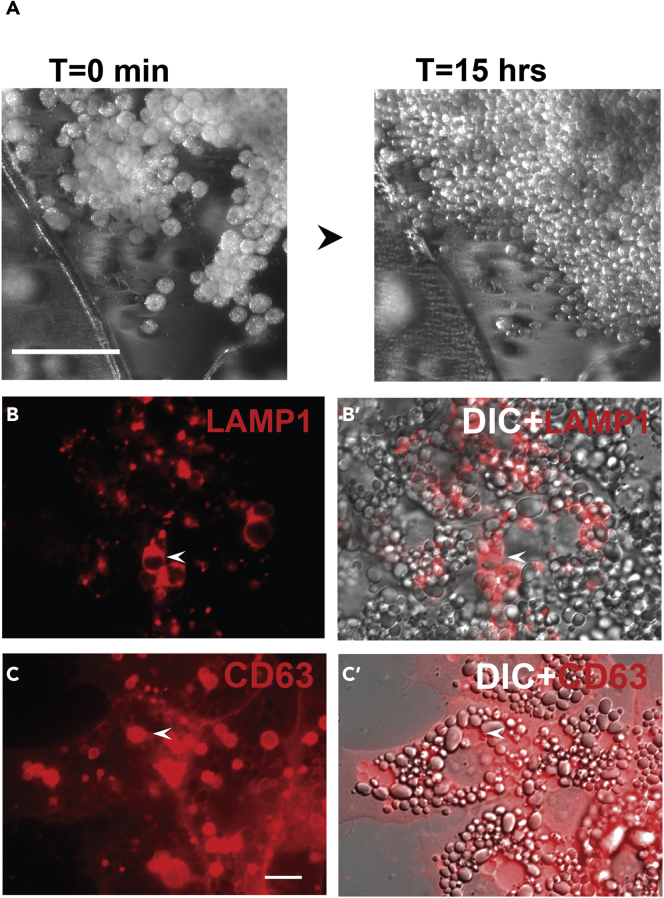
Figure 1.
Dissociation of animal caps for lysosomal studies
(A) Images from a movie of animal cap excised at blastula stage 8.5 plated in fibronectin and filmed for 15 h; note that cells divide in these conditions. Scale bar, 500 μm.
(B–C′) Animal cap cells serve as a model for lysosomal studies using LAMP1-RFP or CD63-RFP as markers; the DIC visible light channel shows yolk platelets present in the embryonic cells. See also Methods video S1. Scale bar, 10 μm.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Digitonin | Sigma | Cat#300410 |
| Sir-Lysosome | Cytoskeleton Inc | Cat#CYSC012 |
| Tetramethylrhodamine Dextran (TMR-Dx) 70,000 kDa | Thermo Fisher Scientific | Cat# D1818 |
| Lithium chloride (LiCl) | Sigma | Cat#L4408 |
| 10-cm dish | Thermo Fisher Scientific | Cat#174903 |
| 8-well glass-bottom chamber slides | ibidi | Cat#80827 |
| Circular coverslips | ibidi | Cat#10815 |
| Culture chambers #0 cover glass | E&G | Cat#GBD00003-200 |
| Leibovitz L-15 cell culture medium | Thermo Fisher Scientific | Cat#11415064 |
| Glutamine | Thermo Fisher Scientific | Cat#25030081 |
| Bovine Serum Albumin (BSA) | Thermo Fisher Scientific | Cat#9048468 |
| Pen-Strep antibiotics | Thermo Fisher Scientific | Cat#15140122 |
| Triton X-100 | Thermo Fisher Scientific | Cat#HFH10 |
| Paraformaldehyde (PFA) | Sigma | Cat#P6148 |
| Fibronectin | Sigma | Cat# F4759 |
| PBS for cell culture applications | Gibco | Cat#10-010-023 |
| PBS for immunostaining washes | Thermo Fisher Scientific | Cat#BP3994 |
| Mounting Medium with DAPI | Abcam | Cat# ab104135 |
| Protease Inhibitors | Roche | Cat#04693132001 |
| Phosphatase inhibitors | Calbiochem | Cat#524629 |
| mMESSAGE mMACHINE™ SP6 Transcription Kit | Thermo Fisher Scientific | Cat#AM1340 |
| Experimental models: Organisms/strains | ||
| Xenopus laevis (wild-type) | Xenopus I | N/A |
| Recombinant DNA | ||
| LifeAct | IMSR | RRID: IMSR_EM:12427 |
| pCS2-mGFP | Addgene | RRID: Addgene_14757 |
| CD63-RFP | Addgene | RRID: Addgene_62964 |
| xWnt8myc | Addgene | RRID: Addgene_16863 |
| Software and algorithms | ||
| ImageJ | NIH | http://imagej.nih.gov/ij/ |
| Axiovision 4.8 | ZEISS | http://Zeiss.com |
| Zen 2.3 imaging software | ZEISS | http://Zeiss.com |
| Other | ||
| IM 300 microinjection pump | Narishige International USA, Inc | N/A |
| Axio Observer Z1 Inverted Microscope with Apotome | ZEISS | N/A |
Materials and equipment
Preparation of macropinocytosis and lysosomal reagents prior to use
| Reagent | Traces | [Working] | Contents | No. of Exp. / Kit | λabs (nm) | λem (nm) | Probe |
|---|---|---|---|---|---|---|---|
| TMR-Dextran 70 kDa | Macropinocytosis | 1 mg/mL | 25 mg | 25 coverslips | 555 | 580 | Intensiometric |
| SiR-Lysosome (1 mM) | Cathepsin D | 1 μM | 50 nmol probe 1 μmol verapamil |
200 coverslips | 652 | 674 | Intensiometric |
Note: For all of the following reagents, limit light exposure throughout use.
SiR-Lysosome:
-
•
For 1 mM stock concentration: dissolve a SiR-Lysosome vial in 50 μL anhydrous dimethyl sulfoxide (DMSO).
-
•
Compound is unaffected by freeze-thawing. Do not aliquot as this will increase decay. Store SiR-Lysosome at −20°C until use, product is stable for at least three months.
TMR-dextran 70 kDa:
-
•
For 1 mM stock concentration: Dissolve 25 mg TMR-dextran vial in 360 μL anhydrous DMSO.
-
•
Compound is affected by repeated freeze-thaw cycles. For long-term storage, store aliquots of TMR-dextran 70 kDa at −20°C for several weeks.
1 × MMR solution (Marc’s Modified Ringers):
-
•
100 mM NaCl, 2 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES pH 7.4.
Microinjection and microscopy
Use M8 Wild dissecting microscopes for microinjection of Wnt pathway signaling components, multivesicular body (MVB) markers, focal adhesion markers, and mRNA or DNA encoding fluorescence markers in Xenopus embryos at the 2–4 cell stage. Microinjected DNA should not exceed 20 pg/cell. The Narishige microinjectors used were powered by an in-house air pressure line using Singer micromanipulators (Moriyama and De Robertis, 2018).
Live imaging was performed using a Zeiss Observer.Z.1 inverted microscope equipped with Apotome.2, an automated scanning stage, and Zeiss PEECON stage top incubation for Temperature/CO2 for cell culture. However, L-15 medium has the great advantage that it does not require CO2, and Xenopus cells develop at room temperature. Software image analyses can be achieved across an array of platforms including Imaris or the publicly available and free resource Image J.
Step-by-step method details
This protocol is divided into three main sections that each offers a unique readout for culturing animal cap cells by monitoring lysosomal activity and macropinocytosis, cell migration and focal adhesions, as well as drug treatments that mimic Wnt signaling.
Cultured animal cap cells for lysosomal studies
Timing: 1 day
This assay provides a powerful tool to study and visualize lysosome movements utilizing animal caps as an in vivo model in Xenopus using lysosome associated membrane protein 1 (LAMP-1-RFP) or the multivesicular body maker CD63-RFP after microinjection of DNA plasmids (Figures 1B–1C′). Animal cap cells also provide a good model to study how Wnt signaling stabilizes lysosome accumulation (Albrecht et al., 2021) using the CD-63-RFP multivesicular body marker co-injected with xWnt8 mRNA (Figure 2). SiR-Lysosome is a very useful reagent that specifically labels active lysosomes (Tejeda-Muñoz and De Robertis, 2022). It contains a fluorescent photostable silicon rhodamine (SiR) dye linked to a pepstatin A peptide (Marciniszyn et al., 1976). SiR-Lysosome binds to the activated and cleaved form of lysosomal Cathepsin D actin as a reporter of lysosomal activity. Its far-red emission (imaged in standard Cy5 filter sets) decreases phototoxicity and autofluorescence with compatibility for GFP- and mCherry-tagged proteins. By simply adding SiR-Lysosome to the culture medium, lysosomes containing active Cathepsin D can be visualized and their activation is increased by the Wnt mimic lithium chloride (LiCl) lysosomes (Albrecht et al., 2020; Tejeda-Muñoz and De Robertis, 2022). (Figures 3A–3B′).
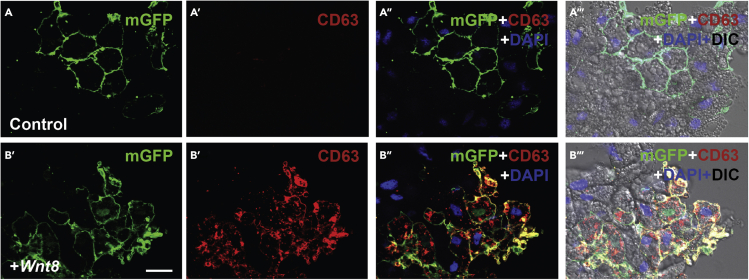
Figure 2.
Xenopus animal cap cells showing that microinjection of 2 pg xWnt8 mRNA at the 2–4 cell stage greatly stabilizes CD63 lysosomes
(A–A‴) Control animal cap injected with DNA encoding membrane GFP and the MVB marker CD63; uninjected cells serve as an internal control.
(B–B‴). Co-injection of xWnt8 mRNA results in a striking stabilization of CD63, a marker of the intraluminal vesicles presents in multivesicular bodies and lysosomes. Canonical Wnt is a potent regulator of lysosome function. The images on the right panels show the merged red, green, blue and visible light channels. Scale bar, 10 μm.
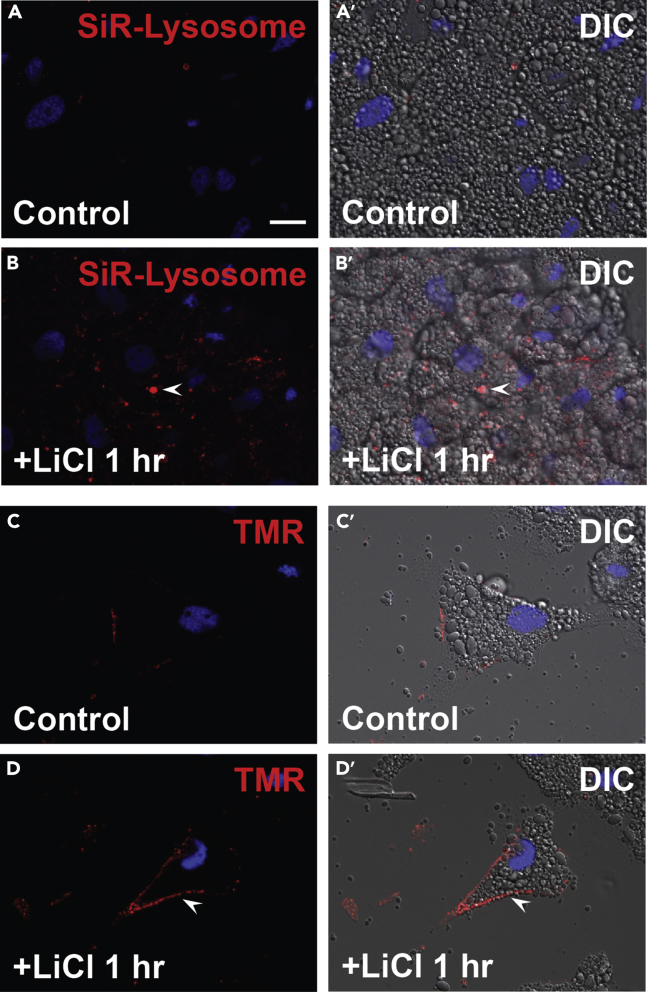
Figure 3.
Xenopus animal cells display lysosomes and macropinocytosis activation after LiCl treatment, which mimics Wnt signaling by inhibiting GSK3 inhibition
(A and A′) Control cells treated with SiR-Lysosome.
(B and B′) LiCl treatment increases active lysosomes. Mimicking Wnt signaling with LiCl shows that lysosomes become more active.
(C and C′) Untreated cells show low uptake of the macropinocytosis marker TMR-Dextran 70 kDa.
(D and D′) LiCl treatment increases macropinocytosis (arrowhead). Scale bar, 10 μm.
Dilute SiR-Lysosome (1 mM stock) to a working concentration of 1 μM in culture medium (0.5 μL of the reagent per 500 μL L-15 medium 50% diluted with water).
Optional: Addition of the calcium channel blocker, verapamil (working concentration of 1 μM), to SiR-Lysosome L-15 solution can increase signal intensity. This reagent is included with the commercial kit indicated in the Table above. Final concentrations of DMSO should not exceed 2% for treatment of cells, and a DMSO alone control should always be carried out.
For visualizing and studying lysosomes by microinjecting MVB markers in frog embryos by dissociating and culturing animal caps:
-
1.
Fertilize Xenopus laevis embryos in vitro using excised testes and culture them in 0.1 × MMR solution. Then inject frog embryos with DNAs or mRNAs (in our examples 20 pg per blastomere of LAMP1-RFP or CD63-RFP DNAs) (Figures 1B–1C′), as well as CD63-RFP and membrane GFP DNAs ± Wnt8 mRNA) (Figure 2).
Note: Perform the microinjection into the animal pole at 2–4 cell stage and culture them until midblastula stage 8.5 before excision (Nieuwkoop and Faber, 1967).
-
2.
Dissect Animal caps at blastula stage 8.5–9 in 1 × MMR solution.
-
3.
Dissociate the cap manually by pipetting the caps 3–5 times with a glass Pasteur pipette in 1 × MMR solution.
-
4.
Plate the cells in L-15 medium diluted to 50% with H20 (Tejeda-Muñoz et al., 2022) on fibronectin-coated coverslips in a 12-well plate (6–8 caps per condition).
-
5.
Incubate the cells for 12–18 h between 15°C–20°C in normal atmosphere.
-
6.
Wash with PBS 3 times to remove dead cells (Thermo Fisher Scientific).
-
7.
Fix cells for 15 min using 4% Formaldehyde diluted in PBS (4% PFA) at 20°C with limited light exposure (0.5 mL per well).
-
8.
Wash with PBS 3 times to remove PFA (0.5 mL per well).
-
9.
Apply mounting medium with DAPI onto a glass microscope slide using 10 μL per coverslip. Avoid air bubbles in the pipette tip and place the coverslip carefully onto the mounting medium.
-
10.
Microscopic examination of RFP and GFP and image acquisition was performed using a Carl Zeiss Axio Observer Z1 Inverted Microscope with Apotome and Nomarski differential interference contrast (DIC) and fluorescent filters for DAPI, FITC, and rhodamine. Images were acquired using 63× objectives and the Zeiss Zen software.
-
11.
For image acquisition Fiji software is recommended. This can be downloaded at http://fiji.sc.
For live-cell analyses (as seen in Methods video S1): plate the dissociated animal cap cells (6–8 caps per condition) in a glass-bottom chamber (#0 cover glass, Cell E&G: GBD00003-200) for 12–18 h.
Movie showing that the lysosomal marker LAMP1-RFP can be visualized in frog embryo cells. Lysosomes are very dynamic and quite large. Scale bar, 10 μm.
After step 6 of the preparation of explants section described above:
-
12.
Insert cell chamber for live imaging with conditions in the inverted microscope at 20°C (without CO2).
-
13.
Image cells for 20 min or more with an image acquisition rate of 1 frame per 30 s. Images were collected with a Zeiss Observer.Z.1 inverted microscope with Apotome, DIC, and Colibri LED with green fluorescence filters using a 63× oil Plan-APOchromat objective. The microscope, including the fluorescence filters, was controlled by Axiovision 4.8 software. Both vertical and inverted microscopes were fully motorized.
-
14.
Video editing was performed using the Adobe Premiere Pro CC 2021 software.
-
15.
Cell viability was maintained for 72 h.
For SiR-Lysosome assay (Figures 3A–3B′), after step 6 of preparation of explants:
-
a.
Incubate cells with SiR-Lysosome in 50% L-15 culture medium for 60 min at room temperature (500 μL per well). Troubleshooting 3.
-
b.
Repeat steps 6–11 animal cap cell cultures as described in the cultured animal cap cells for lysosomal studies section.
For macropinocytosis with lysosomal tracers (Figures 3C–3D′), following step 6 of preparation of explants:
-
c.
Dilute TMR-Dextran 70 kDa (1 mg/mL) in 50% L-15 culture medium and incubate cells for 60 min at room temperature, then continue with steps 6–11 in cultured animal cap cells for lysosomal studies section. Troubleshooting 4.
Animal cap cells for migration and focal adhesions
Timing: 1 day
This section provides a way of monitoring changes in cell-cell adhesion, as well as cell migration, that can be triggered by activation of the Wnt pathway (Bachir et al., 2017; Nusse and Clevers, 2017) driving a cell growth program that can become inappropriately activated in cancer. Knowing how Wnt, focal adhesions, and cell migration cooperate will improve our understanding of embryonic development and tumorigenesis. In this improved protocol animal cap cells are able to adhere to fibronectin and display focal adhesions and actin stress fibers.
For live-cell analyses of focal adhesions:
Follow steps 1–6 in the preparation of explants section using frog embryos microinjected with 20 pg of DNA/cell at the 2–4 cell stage encoding the focal adhesion marker Tes-GFP or the F-actin tracer LifeAct (Figures 4A–4C). After 12–18 h following cell plating onto glass-bottom chambers:
-
16.
Following any desired incubation with growth factor or drugs, wash 3 times with PBS.
-
17.
Proceed with the movie generation by mounting a cell chamber for live imaging in the inverted microscope. Movie condition generation as previously described in the section about culturing animal cap cells for lysosomal studies.
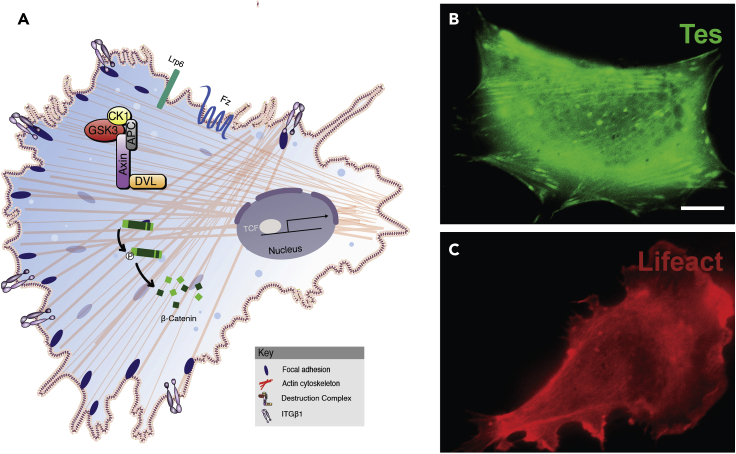
Figure 4.
Focal adhesions in animal cap cells
(A) Diagram showing actin cytoskeletal cables, membrane integrins, and focal adhesions in the leading edge of a migratory cell, as well as the β-catenin destruction complex that regulates canonical Wnt signaling.
(B) Xenopus animal cap cells showing focal adhesions in the leading edge and actin cables marked by TES-GFP. Focal adhesions are critical for cell adhesion and migration.
(C) A DNA plasmid encoding the F-actin tracer LifeAct (Gonagen) (in red) was microinjected into the animal pole at the 4 cell-stage (20 pg/injection). Scale bar, 10 μm. See also Methods video S2.
Drug treatment
Timing: 1 day
The activation of the canonical Wnt pathway is a driving force in many human cancers, especially colorectal, hepatocellular, and mammary carcinomas (MacDonald et al., 2009; Nusse and Clevers, 2017; Galluzzi et al., 2019; Tejeda-Muñoz et al., 2022, Tejeda-Muñoz and Robles-Flores, 2015). Wnt causes the stabilization and nuclear transport of newly-synthesized transcriptional regulator β-catenin, and the generally accepted view in the field is that the canonical effects of Wnt growth factors are caused by the transcription of β-catenin target genes. Recent studies indicate that Wnt is also a regulator of many other cellular physiological activities, such as macropinocytosis, endosome trafficking (Colozza et al., 2020), protein stability, lysosomal activity (Holland et al., 2020; Lawrence and Zoncu, 2019; Taelman et al., 2010; Tejeda-Muñoz et al., 2019), and cell adhesion (Tejeda-Muñoz et al., 2022).
For drug treatment assay of cultured animal cap cells:
After 12–18 h following cell plating onto glass-bottom chambers (steps 1–6 in the preparation of explants section):
-
18.
Wash 3 times in 50% L-15 medium.
-
19.
Proceed with the movie generation by mounting a cell chamber for live imaging in the inverted microscope at room temperature and imaging as described in the section on cultured animal cap cells for lysosomal studies under the live-cell analyses protocol.
-
20.
Add drug treatments: GSK3 inhibitors (40 mM LiCl) or controls (40 mM NaCl or DMSO). Troubleshooting 5.
-
21.
Start recording immediately.
-
22.
Generate a movie in the same cells using the conditions used to film before drug treatment.
Note: The use of antibodies for focal adhesions such as Tes, Zyxin, Vinculin can also be performed in cells plated on coverslips. In this case, following steps 1–6 in preparation of explants:
-
23.
Wash 3 times with PBS (0.5 mL per well) to remove dead cells.
-
24.
Fix cells for 15 min in 4% PFA diluted in PBS (0.5 mL per well).
-
25.
Wash with PBS 3 times to remove PFA (0.5 mL per well).
-
26.
Permeabilize cells with digitonin (6.5 μg/mL) for 10 min in PBS (0.5 mL per well).
-
27.
Wash 3 times with PBS to remove detergent (0.5 mL per well).
-
28.
Block cells for 1 h in blocking solution containing 5% bovine serum albumin in PBS to reduce background from nonspecific binding.
-
29.
Incubate the samples with the primary antibody for 24 h at 4°C.
-
30.
Wash 3 times with PBS (0.5 mL per well).
-
31.
Incubate with secondary antibodies for 1 h at 20°C (0.5 mL per well) (ab150117 goat anti-mouse and ab150084 goat anti-rabbit are recommended).
-
32.
Wash 3 times with PBS (0.5 mL per well).
-
33.
Mount cells in mounting media with DAPI.
-
34.
Dry slides in a dark area at 20°C for 16 h prior to imaging.
Expected outcomes
The loss of normal cell polarity and adhesion caused by Wnt signaling activation is a fundamental step for tumor progression and metastasis. The crosstalk between Wnt signaling and focal adhesions is responsible for regulating adhesion, migration, and Wnt pathway component activities. Therefore, understanding how cell adhesion is affected in Wnt-driven cancers will be a useful tool in understanding the cellular dynamics during cell division, spreading, and adhesion, as well as in understanding how they can act as a tumor suppressor. For example Methods video S2, shows how mimicking Wnt activation using the GSK3 inhibitor LiCl treatment triggered increased cell motility. This illustrates that animal cap cells are a good model system to study cell movement, Wnt signaling, and cancer in embryo-derived cells.
Mimicking Wnt signaling through GSK3 inhibition with LiCl strongly increased cell motility; note the actin-driven ruffling membrane motility at the leading edge of the cell in the direction of movement.
Limitations
Problems with reagents such as SiR-lysosome or TMR-Dextran can occur as a result from incubation times that are too short, resulting in low fluorescence intensity. Possible contamination of the animal cap cells can also happen if sterile conditions are not kept, giving nonspecific artificial staining or high background. Staining between individual cells within a whole in vivo system during drug testing can be variable, requiring examination of multiple cells. The troubleshooting guidelines outlined below should be incorporated into the use of this protocol to help mitigate some of these risks. Environmental factors such as growth medium and drug treatment will require further optimization in independent lab settings. For this reason, the protocols included here describe methods with transparency and maximal details to increase the validity of data analyses.
Troubleshooting
Problem 1
Pigmented cells present in the animal cap.
Potential solution
Avoid plating pigmented cells from the animal cap external layer as they quench fluorescence.
Problem 2
Bacterial contamination of the animal cap cell cultures.
Potential solution
Cleaning all the areas and materials with ethanol and using L-15 medium in sterile conditions to avoid cell contamination. Additionally, gentamicin (50 mg/mL) solution can be added to the culture medium.
Problem 3
Low signal with lysosomal markers.
Potential solution
Low fluorescence can result from high cell density on coverslips.
Problem 4
Nonspecific artificial staining or high background in fixed cell imaging.
Potential solution
Permeabilization with digitonin, or low concentrations of Triton X-100 0.15% (both diluted in PBS) can help reduce background fluorescence in immunostaining experiments. This procedure should be performed prior to blocking, with cells incubated with 0.5 mL of either 6.5 μg/mL digitonin or 0.15% Triton X-100 with 0.5 mL per coverslip in an individual well of a 12-well dish for 10 min. Following this, detergent should be removed with three washes of PBS (0.5 mL per well for each wash).
Problem 5
Individual cells within a preparation system can show variability.
Potential solution
Performing multiple independent experiments and quantitating the results in Fiji software.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, N Tejeda-Muñoz (ntejedamunoz@mednet.ucla.edu).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
We are grateful to P. Sheladiya for comments on the manuscript and M. F. Domowicz for help with the illustrations. Funding bodies were UC Cancer Research Coordinating Committee (grant C21CR2039); National Institutes of Health grant P20CA016042 to the University of California, Los Angeles Jonsson Comprehensive Cancer Center; and the UCLA Norman Sprague Endowment for Molecular Oncology.
Author contributions
Conceptualization, N.T.M.; Investigation, N.T.M., J.M., and E.M.D.R.; Writing – Original Draft, N.T.M.; Writing – Review & Editing, N.T.M., J. M., and E.M.D.R.; Funding Acquisition, E.M.D.R.; Supervision, N.T.M.
Declaration of interests
Authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2022.101455.
Data and code availability
No data or code was generated in this study.
References
- Albrecht L.V., Tejeda-Munoz N., Bui M.H., Cicchetto A.C., Di Biagio D., Colozza G., Schmid E., Piccolo S., Christofk H.R., De Robertis E.M. GSK3 inhibits macropinocytosis and lysosomal activity through the Wnt destruction complex machinery. Cell Rep. 2020;32:107973. doi: 10.1016/j.celrep.2020.107973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht L.V., Tejeda-Muñoz N., De Robertis E.M. Cell biology of canonical Wnt signaling. Annu. Rev. Cell Dev. Biol. 2021;37:369–389. doi: 10.1146/annurev-cellbio-120319-023657. [DOI] [PubMed] [Google Scholar]
- Ariizumi T., Takahashi S., Chan T., Ito Y., Michiue T., Asashima M. Isolation and differentiation of Xenopus animal cap cells. Curr Prot Stem Cell Biol. 2009;9:ID.5.1–ID.5.31. doi: 10.1002/9780470151808.sc01d05s9. [DOI] [PubMed] [Google Scholar]
- Asashima M., Ito Y., Chan T., Michiue T., Nakanishi M., Suzuki K., Hitachi K., Okabayashi K., Kondow A., Ariizumi T. In vitro organogenesis from undifferentiated cells in Xenopus. Dev. Dynam. 2009;238:1309–1320. doi: 10.1002/dvdy.21979. [DOI] [PubMed] [Google Scholar]
- Bachir A.I., Horwitz A.R., Nelson W.J., Bianchini J.M. Actin-based adhesion modules mediate cell interactions with the extracellular matrix and neighboring cells. Cold Spring Harbor Perspect. Biol. 2017;9:a023234. doi: 10.1101/cshperspect.a023234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M., De Robertis E.M., Wallingford J.B., Niehrs C. Morpholinos: antisense and sensibility. Dev. Cell. 2015;35:145–149. doi: 10.1016/j.devcel.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Colozza G., Jami-Alahmadi Y., Dsouza A., Tejeda-Muñoz N., Albrecht L.V., Sosa E.A., Wohlschlegel J.A., De Robertis E.M. Wnt-inducible LRP6-APEX2 interacting proteins identify escrt machinery and trk-fused gene as components of the Wnt Signaling Pathway. Sci. Rep. 2020;10:21555. doi: 10.1038/s41598-020-78019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commisso C., Flinn R.J., Bar-Sagi D. Determining the macropinocytic index of cells through a quantitative image-based assay. Nat. Protoc. 2014;9:182–192. doi: 10.1038/nprot.2014.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland L., Nielsen I., Maeda K., Jäättelä M. SnapShot: lysosomal functions. Cell. 2020;181:748–748.e1. doi: 10.1016/j.cell.2020.03.043. [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Spranger S., Fuchs E., Lopez-Soto A. WNT signaling in cancer immunosurveillance. Trends Cell Biol. 2019;29:44–65. doi: 10.1016/j.tcb.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R.E., Zoncu R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 2019;21:133–142. doi: 10.1038/s41556-018-0244-7. [DOI] [PubMed] [Google Scholar]
- MacDonald B.T., Tamai K., He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniszyn J., Jr., Hartsuck J.A., Tang J. Mode of inhibition of acid proteases by pepstatin. J. Biol. Chem. 1976;251:7088–7094. doi: 10.1016/s0021-9258(17)32945-9. [DOI] [PubMed] [Google Scholar]
- Moriyama Y., De Robertis E.M. Embryonic regeneration by relocalization of the Spemann organizer during twinning in Xenopus. Proc. Natl. Acad. Sci. U.S.A. 2018;115:E4815–E4822. doi: 10.1073/pnas.1802749115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji N. Presumptive mesoderm cells from xenopus laevis gastrulae attach to and migrate on substrata coated with fibronectin or laminin. J. Cell Sci. 1986;86:109–118. doi: 10.1242/jcs.86.1.109. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P.D., Faber J. North-Holland Publishing Co; 1967. Normal Table of Xenopus laevis (Daudin) [Google Scholar]
- Nusse R., Clevers H. Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Redelman-Sidi G., Binyamin A., Gaeta I., Palm W., Thompson C., Romesser P., Lowe S., Bagul M., Doench J., Root D., et al. The canonical Wnt pathway drives macropinocytosis in cancer. Cancer Res. 2018;78:4658–4670. doi: 10.1158/0008-5472.can-17-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive H.L., Grainger R., Harland R.M., editors. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Smith J.C., Symes K., Hynes R.O., DeSimone D. Mesoderm induction and the control of gastrulation in xenopus laevis: the roles of fibronectin and Integrins. Development. 1990;108:229–238. doi: 10.1242/dev.108.2.229. [DOI] [PubMed] [Google Scholar]
- Smith J.C., Tata J.R. Chapter 32 Xenopus cell lines. Methods Cell Biol. 1991;36:635–654. doi: 10.1016/s0091-679x(08)60300-3. [DOI] [PubMed] [Google Scholar]
- Sosa E.A., Moriyama Y., Ding Y., Tejeda-Muñoz N., Colozza G., De Robertis E.M. Transcriptome analysis of regeneration during xenopus laevis experimental twinning. Int. J. Dev. Biol. 2019;63:301–309. doi: 10.1387/ijdb.190006ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taelman V.F., Dobrowolski R., Plouhinec J.-L., Fuentealba L.C., Vorwald P.P., Gumper I., Sabatini D.D., De Robertis E.M. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda-Muñoz N., Robles-Flores M. Glycogen synthase kinase 3 in Wnt signaling pathway and cancer. IUBMB Life. 2015;67:914–922. doi: 10.1002/iub.1454. [DOI] [PubMed] [Google Scholar]
- Tejeda-Muñoz N., De Robertis E.M. Lysosomes are required for early dorsal signaling in the Xenopus embryo. Proc. Natl. Acad. Sci. U.S.A. 2022;119 doi: 10.1073/pnas.2201008119. e2201008119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda-Muñoz N., Albrecht L., Bui M., Robertis E. Wnt canonical pathway activates macropinocytosis and lysosomal degradation of extracellular proteins. Proc. Natl. Acad. Sci. U.S.A. 2019;116:10402–10411. doi: 10.1073/pnas.1903506116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda-Muñoz N., Mei K.-C., Sheladiya P., Monka J. Targeting Membrane Trafficking as a Strategy for Cancer Treatment. Vaccines. 2022;10:790. doi: 10.3390/vaccines10050790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda-Muñoz N., Morselli M., Moriyama Y., Sheladiya P., Matteo P., De Robertis E.M. Canonical Wnt signaling induces focal adhesion and integrin beta-1 endocytosis. iScience. 2022;25:104123. doi: 10.1016/j.isci.2022.104123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie showing that the lysosomal marker LAMP1-RFP can be visualized in frog embryo cells. Lysosomes are very dynamic and quite large. Scale bar, 10 μm.
Mimicking Wnt signaling through GSK3 inhibition with LiCl strongly increased cell motility; note the actin-driven ruffling membrane motility at the leading edge of the cell in the direction of movement.
Data Availability Statement
No data or code was generated in this study.