Abstract
Gastric cancer is one of the first malignant cancers in the world and a large number of people die every year due to this disease. Many genetic and epigenetic risk factors have been identified that play a major role in gastric cancer. HOTAIR is an effective epigenetic agent known as long noncoding RNA (lncRNA). HOTAIR has been described to have biological functions in biochemical and cellular processes through interactions with many factors, leading to genomic stability, proliferation, survival, invasion, migration, metastasis, and drug resistance. In the present article, we reviewed the prognostic value of the molecular mechanisms underlying the HOTAIR regulation and its function in the development of Gastric Cancer, whereas elucidation of HOTAIR–protein and HOTAIR–DNA interactions can be helpful in the identification of cancer processes, leading to the development of potential therapeutic strategies.
Keywords: Expression, Gastric cancer, HOTAIR regulation, Regulatory pathways
Introduction
The long non-coding RNA (lncRNA) is one of the most important types of non-coding RNA discovered and has important functions in the cells. The difference between lncRNAs and other non-coding RNAs is in their length (more than 200 nucleotides).1,2
HOX transcript antisense RNA)HOTAIR (as an oncogenic lncRNA is implicated in the pathogenesis and progress of many types of malignancies.3,4 HOTAIR is located in an intergenic area between HOXC11 and HOXC12 on chromosome 12q13.13 that is implicated in epigenetic regulation of transcription in the HOXC gene cluster.5 HOTAIR has been defined to function as the regulator of various genes implicating in epithelial–mesenchymal transition, cell proliferation, migration, and metastasis.6,7
HOTAIR is capable of participating in the regulation of chromatin states and dynamics via recruiting polycomb repressive complex 2 (PRC2) occupancy. Suppression of HOTAIR leads to reduction of H3K27 trimethylation and suppression of tumor suppressor genes via the enhancer of zeste homolog 2 (EZH2).8,9
HOTAIR is capable of recruiting PRC2 complex and LSD1/CoREST/REST complexes for targeting specific genes, which results in H3K27 trimethylation and H3K4 demethylation, leading to epigenetic suppression of metastasis suppressors; while HOTAIR down-regulation has been revealed to be associated with decreased inventive phenotypes of cancer. These results indicate that IncRNAs are involved in epigenetic reprogramming that leads to cancer development; thus, they can serve as key targets for the development of novel approaches for cancer diagnosis and therapy.8,10 HOTAIR seems to be a favorable predictive marker for the biological behavior of cancer and a therapeutic strategy for targeted therapy. Therefore, the aim of this review was to assess the genetic variants in HOTAIR and their correlation with gastrointestinal cancers susceptibility, especially Gastric Cancer (GC).
Genetic variants in lncRNA HOTAIR
The functional single nucleotide polymorphisms (SNPs) are considered as genetic risk factors for gastric carcinogenesis.11,12 Some studies point out that HOTAIR SNPs are associated with the risk of many kinds of malignancies (Table 1). HOTAIR SNPs seem to be potentially capable of altering HOTAIR expression in the cell.
Table 1.
Data was obtained from LincSNP 3.0: a database for functional variant of LncRNA at http://bio-bigdata.hrbmu.edu.cn/lincsnp/index.jsp.
| HOTAIR Variant | Regulation | LncRNA Target | Cancers | Functional role | Pubmed ID |
|---|---|---|---|---|---|
| rs920778 | Up regulation | HER2 | gastric | Associated with HOTAIR up regulation, leading to GC susceptibility | 25640751 |
| rs12826786 | Up regulation | – | gastric | No considerable role in susceptibility to GC | 28342055 |
| rs12826786 | Up regulation | – | gastric cardia adenocarcinoma | may be capable of increasing HOTAIR levels | 25476857 |
| rs920778 | Up regulation | – | esophageal squamous cell carcinoma (ESCC) | conferring ESCC susceptibility | 24788237 |
| rs4759314 | Up regulation | – | pancreatic | PVT1 rs1561927 polymorphism the G allele was highly seen in ductal adenocarcinoma and pancreatic neuroendocrine tumor, HOTAIR rs4759314 G allele in pancreatic neuroendocrine tumor, serve as markers in cancer prediction | 30475759 |
| rs4759314 and rs200349340 | Up regulation | pancreatic | minor alleles of rs4759314 and rs200349340 were associated with increased expression of HOTAIR | 30867650 | |
| rs920778 | NA | – | hepatocellular carcinoma | maker for predicting Cancer | 29141248 |
| rs1899663 | Up regulation | – | lung | associated with higher risk of cancer | 30154526 |
| rs4759314 | – | – | lung | rs4759314 and rs12826786 are linked to cancer susceptibility in Chinese Han population | 30464618 |
| rs7958904 | – | – | lung | Platinum chemotherapy response | 26729200 |
| rs920778 | Up regulation | – | lung cancer | rs920778, rs1899663, and rs4759314 are associated with higher risk | 30154526 |
| rs1899663 | – | – | lung | Correlation with platinum chemotherapy response | 26729200 |
| rs12826786 | – | – | breast | genetic susceptibility to cancer and poor prognosis | 26577852 |
| rs920778 | – | – | breast | increased the risk of cancer | 25586347 |
| rs920778 | – | – | breast | increased the risk of cancer | 26547792 |
| rs12826786 | – | – | prostate | Risk of cancer and progression-free survival | 29436234 |
| rs12826786 | – | – | prostate | HOTAIR variants are related to prostate cancer and hyperplasia in an Iranian population | 28259691 |
| rs920778 | – | – | prostate | rs920778 and rs12826786 was not linked to cancer susceptibility | 29436234 |
| rs1899663 | – | – | prostate | rs1899663 T allele increased risk of disease | 28259691 |
| rs920778 | Up regulation | – | ovarian | rs920778 polymorphism was associated with susceptibility to cancer and prognosis | 27690631 |
| rs2366152 | Down regulation | miR-22 | cervical | Involved in pathogenesis of CaCx, miR-22 was capable of decreasing rs2366152C- HOTAIR lncRNA in CaCx cell, acts as a marker for singling out CaCx | 27683269 |
| rs920778 | Up regulation | – | cervical | is able to regulate HOTAIR expression, is associated with susceptibility to cancer | 27467165 |
| rs7958904 | Up regulation | cervical | Linked to cell proliferation and risk of cervical cancer; rs7958904 CC genotype showed increased HOTAIR level than those with GG genotype | 28600545 | |
| rs920778 | Up regulation | – | papillary thyroid carcinoma (PTC) | rs920778 SNP was capable of regulating HOTAIR expression, served as functional genetic variant in PTC | 27549736 |
| rs4759314 | Up regulation | HOXC | urothelial cell carcinoma | Patients are at risk disease after controlling for age and tobacco use and poor overall survival | 30982978 |
An intronic SNP rs4759314 locating on the promoter site of HOTAIR has been suggested as a marker for the prediction of GC in the Chinese populations. AG/GG genotypes of the rs4759314 were suggested to be a risk factor for GC. SNP rs4759314 is capable of influencing intronic promoter site, which results in allele-specific effects on HOTAIR and HOXC11, leading to GC susceptibility, while the effect of AG genotype was found to be markedly higher in comparison with the AA genotype.13 Rs7958904 CC genotype has been attributed to cervical cancer, compared to the GG/GC genotypes.14
TT genotype of SNP rs920778 has been found to be involved in regulating gene expression of HOTAIR and may be linked to cancer susceptibility (Table 1). Previous studies have revealed that SNP rs920778, which is located on the HOTAIR intronic enhancer site in esophageal cancer, was capable of altering this enhancer in esophageal cancer cell lines and normal esophageal tissues, which leads to increased HOTAIR levels among T allele carriers, and might be considered as a mechanism involving in esophageal squamous cell carcinoma susceptibility. These results provide functional genetic variants involving in HOTAIR regulation, thereby partly indicating the basis of esophageal squamous cell carcinoma.15 HOTAIR rs920778 T allele has been demonstrated to contribute to an increase in the risk of Gastric Cancer (GC) in comparison with the C allele. The rs920778C > T SNP has been confirmed to be capable of altering intronic enhancer site, resulting in elevation of HOTAIR RNA level among T allele carriers in GC cell lines and tissues, indicating functional genetic variants implicating in the regulation of HOTAIR in GC, which might serve as a potential factor for GC susceptibility in Chinese population.16
Overexpression of HOTAIR has been illustrated to lead to an advanced stage, poor prognosis and poor overall survival rate in patients with gastric cardia adenocarcinoma. The mutated T allele of rs12826786 led to genotype-specific change of HOTAIR expression, contributing to TNM stage and development of cardia adenocarcinoma in a population of north China.17 But some other studies have ruled out the association of 2826786 and rs920778 polymorphisms with gastric cancer.18,19
Molecular function of HOTAIR in GC
Estrogen signaling, estrogen receptors (ERs) and ER coregulators and HOTTAIR
So far, the role of estrogen signaling and its association with many cancers such as GC has not been investigated, therefore the role of HOTAIR in estrogen-mediated transcriptional regulation needs to be revealed in GC patients. It has been revealed that ERs are capable of forming a complex with the endothelial nitric oxide synthase (eNOS), associating with chromatin in an estrogen-dependent manner in prostate cancer.
Hormonal regulation of HOTAIR was found to be associated with the formation of these complexes, but not in normal endothelial cells. HOTAIR has also been demonstrated to be involved in the regulation of estrogen receptor transcriptional targets and is capable of regulating estrogen-dependent and independent transcription of many genes, including pS2, hTERT and PSA, in prostate cancer, however, it is not capable of participating in the regulation of androgen receptor-activated pathways.20
Dysregulation of ERs has been defined to be implicated in the progression of many cancers.21 ERs and ER-coregulators (e.g., chromatin-remodeling enzymes; mixed-lineage leukemia (MLL) family of histone methyl-transferases (MLL1, MLL3) and CBP/p300) have been defined to be capable of targeting HOTAIR promoter in an E2-dependent fashion.22,23
HOTAIR exhibited direct interactions with PRC2 and histone demethylase LSD1, in turn, requiting target gene loci and inhibiting their transcriptional levels through H3K27 trimethylation (for PRC2) and H3K4 demethylation (for LSD1), which suggests its role as a modulator of chromatin scaffold.24,25 CBP/p300 is also involved in estradiol-induced HOTAIR expression, where it led to H3K4met3 promotion and histone acetylation and HOTAIR transcription.26
Down-regulation of HOTAIR was found to be linked to the suppression of invasive phenotypes of cancer, in cells with extensive PRC2 activity.10 ERβ plays a crucial role in RCC development through increasing HOTAIR and altering the ceRNA network. ERβ-modulated HOTAIR was capable of affecting many genes (EZH2, VEGFA, ZEB1, ADAM9, VIM, CCND2, and ZEB2) by antagonizing different miRNAs (i.e., miR-200c, miR-138, miR-204, miR-217); thus, HOTAIR has been suggested as a marker for the detection of progression of overall survival in RCC.4
ERα36 over-expression was indicated to be extensively involved in lymph node metastasis in GC. High expression of ERα36 protein can be observed in GC cell lines, tissues, plasma membrane and cytoplasm cells.27 A study suggested that positive ERα and negative ERβ expression are related to poor survival in patients with GC.28 Increased level of ERβ5 mRNA has been reported to be linked to pTNM stage, and lymph node metastasis.29 Positive estrogen receptor (ER) mRNA is increasingly recognized to be a crucial aspect for regional lymph nodes metastases and growth of gastric carcinoma, suggesting its potential for prognosis and endocrine therapy.30 Contradictory results addressed protective role of ERβ in GC against the invasive phenotype of GC.31 ERα, ERβ, PR, and AR expression levels were revealed to contribute to advanced tumor grade and intestinal type, while ERα and AR were linked to the early TNM stage, which indicates that their clinicopathological and prognostic values can be limited.32 Deregulated ERs have been shown to exhibit potential benefits for diagnosis, prognosis, and targeted therapy in GC. To the best of our knowledge, no study has been conducted on the role of ERs-HOTAIR axis and estradiol-induced HOTAIR expression in GC. The significance of this approach will be revealed in future studies investigating the function of HOTAIR in both physiological and pathological processes in molecular, cellular, and tissue levels, as well as from elucidation of ERs-HOTAIR associations, and interactions of ER isoforms in GC, which provides a systematic understanding of ERs for providing diagnostic, prognostic, and targeted therapy in ER-associated gastric cancer.
A study indicated that transfection of siRNA against siH3 led to the transcriptional gene silencing of heparinase in GC as well as prostate and bladder cancer, resulting in the decreased invasion, metastasis, and angiogenesis of this cancer, but transcriptional gene silencing was not found to be implicated in changes of epigenetic markers including H3K9me2, H3K27me3, or AcH3. Therefore, this evidence showed the potential benefit of TSS-targeted small RNAs for the suppression of malignant phenotypes of cancer.33
Proto-oncogenic MYC family
C-MYC as an oncoprotein is capable of up-regulating HOTAIR at the transcriptional level and its promoter activity via interacting with E-box element, leading to negative regulation of miRNA-130a and progression of gallbladder cancer cells, suggesting that C-MYC plays a major role in gallbladder cancer cells.34 C-MYC has been found to function as a regulator of many protein-coding and non-coding genes such as miRNAs.35 The interaction of IGF2BP1 with lncRNA GHET1 in GC was found to be associated with overexpression of both mRNA and protein levels of MYC.36
Overexpression of lncRNA CCAT1-L transcript variant has been identified in colorectal cancer, and MYC was capable of increasing the level of CCAT1-S variant in GC and colon cancer via binding to the E-box element.37,38 MYC-induced HOTAIR has been suggested to play a crucial role in cell cycle progression and metastasis.39 The intricate interaction between MYC and other lncRNAs such as HOTAIR has not yet been identified in GC and other types of cancer, and further investigation is required in this field.
Transforming growth factor-β1 (TGF-β) in the tumor microenvironment
It has been suggested that HOTAIR is involved in tumor progression by stemness acquisition. Another study indicated that HOTAIR was capable of affecting the TGF-β/Smad signaling pathway and can increase proliferation, and migration in the TGF-β2-induced EMT in human lens epithelial cells, while HOTAIR suppression was associated with the reduction of the TGF-β-induced cell progression.40 TGF-β was found to be capable of assembling a receptor complex involving SMAD2, SMAD3 and SMAD4 activation, leading to transcription.41 CDK5 has been found to be involved in CAF-induced EMT, where CAFs can mediate EMT and HOTAIR via CDK5 signaling. A novel epigenetic molecular mechanism has been described, in which TGF-β1/CDK5/HOTAIR axis is involved in modulating EMT and the progression of tumor cells in cancer-associated fibroblasts.42,43 Increased linc-POU3F3 could enhance regulatory T (Treg) distribution, leading to the promotion of cell proliferation by SMAD2 and SMAD3 phosphorylation and activating TGF-β signaling pathway in GC.44 Current data provide further insights into the role of HOTAIR in cancers, however, the role of TGF-β/HOTAIR axis in GC cell lines and tissues is unknown or is still poorly understood.
Tumor necrosis factor-α (TNF-α)
TNF-α is another extrinsic factor in the tumor microenvironment that is involved in up-regulating HOTAIR and inducing EMT in breast cancer.45,46 TNF-α production plays a key role in the development, progression and poor prognosis of many kinds of cancers such as GC. On the other hand, disruption of TNF-α or TNFR1 receptor genes has been clarified to be linked to the inhibition of induced malignancies in the mouse GC.47,48 TNF-α/TNFR1 signaling has been demonstrated to be capable of increasing GC progression via inducing Noxo1 and Gna14 48. HOTAIR has been illustrated to be implicated in IκBα degradation, leading to NF-κB activation and expression of its targets such as cytokine, and pro-inflammatory genes,49 which demonstrates its role in inflammatory and immune response. Studies on the role of TNF-α/HOTAIR signaling in cancer are very limited; therefore, more evidence is needed for further interpretation that is not possible right now.
Nuclear factor kappa B (NF-κB)
A study demonstrated an NF-KB-responsive element in the upstream of the HOTAIR promoter. NF-κB-driven expression of HOTAIR at the transcriptional level, triggered NF-κB, IL-6 and CHK1-p53-p21 signaling, consequently leading to cellular senescence and chemotherapy resistance in ovarian and other cancers, suggesting the involvement of RNF-κB-HOTAIR in exerting a positive-feedback loop cascade in DNA damage response.50 The potential interaction of NF-κB activation with HOTAIR has been demonstrated because of DNA damage response. As indicated, HOTAIR up-regulation could trigger EMT in many types of cancers such as ovarian and colon cancers.51,52 However, the role of NF-κB-HOTAIR axis and its network requires further investigation in cancers such as GC.
HOTAIR has been found to contribute to 5FU resistance and consequent development of colorectal cancer via recruiting EZH2, inhibiting miR-218 and activating NF-κB signaling, and facilitated foundation for the aberrant activation of VOPP1 as the target of miR-218 in colorectal cancer.53 VOPP1 could play a key role in regulating NF-κB transcription, leading to tumor resistance to apoptosis. NF-κB translocation was associated with cell-cycle progression and the loss of E2F-1 as a transcriptional factor, which resulted in TS enzyme, a target of 5FU therapeutic strategy;53, 54, 55 thus, HOTAIR has been introduced as a therapeutic target in colorectal cancer, when its suppression could lead to chemosensitivity in 5FU-based chemotherapy. HOTAIR causes chemoresistance of cells in GC,56 colorectal cancer,53 bladder cancer,57 etc. However, the role of HOTAIR in the development of tumorigenesis and chemoresistance in many cancers such as gastrointestinal cancers is poorly understood.
A growing body of evidence indicates that the NF-κB signaling cascade is involved in inflammation-induced oncogenesis, e.g., gastrointestinal cancers.58,59 NF-κB system has been defined to be associated with the inflammation and activation of inflammatory cells, which can lead to cancer development and progression of cancer in different cells. A number of tumor-suppressor or oncogenic proteins have been found to be aberrantly involved in gastric tumorigenesis and progression via regulating NF-κB activity. NF-κB is involved in regulating many cellular processes and implicating in the regulation of many properties linked to malignant phenotypes, which suggests its potential for the development of targeted therapy and diagnostic tools in patients suffering from GC cancer, and other cancers. In addition, suppression of NF-κB was effectively linked to increased cell death in cancer cells, leading to the promotion of therapeutic strategies.
CpG island methylation and HOTAIR regulation
An intergenic region or regulatory element has been also found to be located in 1-kb downstream of HOTAIR (the 3’ end of the HOTAIR, between HOTAIR and HOXC12), namely CpG island, which increased DNA methylation in this region and was associated with the up-regulation of HOTAIR; therefore, CpG island has been proposed to be involved in the prevention of repressive heterochromatin from spreading to the HOXC12 6. CpG island methylation could be biologically linked to HOTAIR regulation, but its role in cancer is poorly understood; therefore, further clarifications are needed to increase our understanding regarding the interaction between HOTAIR and CpG Island in cancer patients. CpG Island in the HOTAIR-N-HOXC11 region provided an opportunity for evaluating the regulation of a sense–antisense transcript, where regulation of antisense transcripts has been suggested to contribute to cancer development.60
Type 1 collagen (Col-1) and OPN
Unfortunately, few investigations explained the mechanisms underlying the function of HOTAIR and their correlation with Col-1, even available studies warrant further clarification of ECM-mediated regulation of HOTAIR in cancer and its association with cancer microenvironment. ECM-mediated up-regulation of HOTAIR-N has been demonstrated in Claudin-low breast cancer cells, where H3K4me3 and BRD4 binding to a HOTAIR-N promoter are associated with HOTAIR expression, leading to invasive growth in cancer cells, while siRNA HOTAIR led to inhibition of invasive growth.61 Further investigations are required to clarify the interaction of ECM, lncRNA, and epigenetic coding with hallmarks of cancer.
Osteopontin (OPN) is also implicated in the up-regulation of HOTAIR via reduction of the inhibitory effect of interferon regulatory factor-1 (IRF1), leading to malignant phenotypes of cancer cells, e.g., invasive and metastatic behavior; while IRF1 was capable of targeting HOTAIR promoter, which leads to HOTAIR suppression.62 OPN up-regulation demonstrated a high aggressiveness phenotype of GC and colorectal cancer,63 and was associated with poor overall survival in patients suffering from GC, suggesting its prognostic value for Asian patients and patients with surgical resection.64,65 Additionally, OPN was associated with the development of GC from H. Pylori.66 The role of host-derived OPN and tumor-derived OPN needs further investigation. Few studies evaluated functional overview of the mechanisms underlying the HOTAIR/OPN axis regulation in cancers, and, to the best of our knowledge, no data are available for GC in this regard.
Poly r(C)-binding protein (PCBP) and HOTARI
It has been suggested that PCBP1 is highly decreased in HOTAIR expressing cells, where the direct interaction of HOTAIR with poly r(C)-binding protein (PCBP)-1 was revealed. The findings demonstrate an oncogenic role for HOTAIR in GC via inhibition of PCBP1, leading to metastasis of GC cells.67 Replenishment of PCBP1 or miR-3978 was capable of attenuating legumain protein expression level and increasing docetaxel chemosensitivity. Replenishment of miR-3978 was found to be involved in inducing PCBP1 protein, suggesting its potential for repressing the negative regulator of PCBP1 in peritoneal GC metastasis.68
PCBP1 could function as a tumor suppressor and its repression was found to be associated with the progression and development of many cancers, such as GC, metastatic GC, liver, colon cancer, lung, cervical, and breast cancer, as well as thyroid carcinoma cells.67, 68, 69, 70, 71, 72, 73 PCBP1, as a transcription regulator, plays a crucial role in regulating cancer-related genes via multiple mechanisms,74,75 such as phosphatase of regenerating liver 3 (PRL-3) suppression in colon cancer cell,70 inactivation of TGF-β pathway,74, 75, 76, 77 stabilizing tumor suppressor mRNA (e.g., POLH, p63),78,79 promoting apoptosis pathways,80, 81, 82 and protecting chromosomal integrity.75,83 In this regard, it can be concluded that PCBP1 could participate in multiple mechanisms underlying the regulation of genes involved in tumorigenesis, however, HOTAIR/PCBP1 axis needs further investigations to clarify the underlying mechanisms that remained elusive.
HOTAIR interaction with miRNAs in GC
A growing body of evidence indicates that IncRNAs are capable of interacting with miRNAs, leading to epigenetic regulation of many genes implicating in transcriptional and post-transcriptional processes, etc. Inverse expression levels between miRNA and lncRNA may occur with or without direct binding, and even miRNAs are capable of binding to miRNA recognition elements (MRE) in lncRNAs acting as competitive endogenous RNAs (ceRNAs), leading to regulation of diverse cellular processes involving in cancer development (Table 2).
Table 2.
MiRNA targeting of HOTAIR through direct and indirect interactions and HOTAIR involving in ceRNA regulatory network.
| HOTAIR involved in direct interaction | ||||
|---|---|---|---|---|
| miRNA | mRNA targets of miRNA | HOTAIR role | Cancer type | PMID |
| miR-1 | FOXC1-activated driver of malignancy, | Hepatocellular carcinoma | 27895772 | |
| miR-130a | a c-Myc activated driver of tumorogenesis | Gallbladder cancer | 24953832 | |
| miR-148a | human leukocyte antigen-G (HLA-G) | Oncogenesis: promoted proliferation, migration and invasion | Cervical cancer | 27574106 |
| miR-152 | HLA-G | Oncogenesis: tumor escape mechanisms | Gastric cancer | 26187665 |
| miR-34a | Oncogenesis | Prostate cancer | 23936419 | |
| miR-101 | COX-2 | Increased invasion and migration of cells | nasopharyngeal carcinoma | 30314699 |
| miR-217 | ZEB1 | Oncogenesis: osteosarcoma development | osteosarcoma | 30367466 |
| HOTAIR involved in indirect interaction | ||||
| miR-7 | SETDB1 | Oncogenesis: invasive phenotype of Cancer cells, | Breast cancer stem cells | 25070049 |
| miR34a | H3K27me3 | Oncogenesis: EMT and metastasis | Gastric cancer | 26136075 |
| miR-125a-5p | CASP2 | Oncogenesis: inhibition of apoptosis, cancer cell survival | Colon cancer | 26962687 |
| HOTAIR involved in ceRNA regulatory network | ||||
| miR-326 | FGF1 | malignant behaviors of cells | Glioma | 26183397 |
| miR-331–3p | HER2 | Oncogenesis | Gastric cancer | 24775712 |
| miR-141 | SKA2 | Oncogenesis: proliferation, migration and invasion | Glioma | 27121316 |
HOTAIR up-regulation was found to be linked to invasive phenotypes of gastric carcinoma cells, whereas HOTAIR loss led to suppression of both invasive phenotypes and cell viability. HOTAIR may play a key role in ceRNA regulatory mechanism via sponging miR-331–3p for modulating the suppression of epidermal growth factor receptor type 2 (HER2), leading to induction of post-transcriptional regulation. Therefore, the positive association of HOTAIR with HER2 leads to the development of GC.88 EZH2 may function as an oncogenic regulator, thereby regulating the progression of GC.89 HOTAIR has been defined to be a target for both miR-331–3 P and miR-124, whereas MiR-331–3p or miR-124 overexpression is capable of suppressing cell proliferation in GC, and expression level of miR-331–3p/miR-124 has been reported to be inversely linked to HOTAIR level in advanced GC, which is in line with consequences of HOTAIR knockdown in GC cells (BGC-823). The current evidence of miR-331–3p/miR-124 and HOTAIR interactions highlight the role of the post-transcriptional mechanism in the development of GC. These results proposed a protecting effect for ceRNA that can be capable of sequestering miRNAs.88
Another study illustrated another example of HOTAIR interaction with miRNA and provided more direct evidence that HOTAIR can be implicated in the tumor escape mechanism in GC, showing its role as a ceRNA via suppression of miR-152, resulting in up-regulation of human leukocyte Antigen-G (HLA-G) level. It is noteworthy that the negative association of HOTAIR with miR-152 has been confirmed via bioinformatics analysis and in-vitro assessment.90 Based on this data and the regulatory role of miR-152 on the HLA-G expression, it has been hypothesized that HOTAIR may be involved in increasing HLA-G expression by suppressing miR-152 expression, leading to GC progression. This new biological function of HOTAIR suggests a key avenue for potentiating immunotherapy to increase the prognosis and survival of patients. However, the role of HOTAIR in tumor immune escape has been not sufficiently established.
MiR-152 over-expression has been found to be associated with repression of HLA-G expression by targeting its 3′-UTR sites, which lead to increasing NK cell-mediated cytolysis, while miR-152 was not able to effect invasive phenotypes of JEG-3 cells, supporting its role in enhancing the immune system.91 Previous findings support the suppressive role of miR-152 in cell proliferation, migration/invasion, and motility via inhibiting tetraspanin membrane protein CD151. CD151 was capable of accelerating GC by inducing invasion and metastasis in GC.92 The role of CD151 in promoting cell motility and metastasis has been reported in vivo.93,94 Decreased expression levels of miR-148a and miR-152 have been reported to be linked to the progression and development of gastrointestinal cancers.95
In another study on cervical cancer, it was indicated that HOTAIR acts in the up-regulation of HLA-G expression by negative regulation of miR-148a through direct interaction and leading to in vivo proliferation, migration, and invasion, while siRNA HOTAIR reversed these phenotypes, and promoted apoptosis, suggesting the potential of HOTAIR-miR-148a-HLG-A axis in the development of a novel therapeutic strategy in cervical cancer.96 However, an in-depth understanding of the mechanism underlying HOTAIR interaction with miRNAs is required, especially in GC cancer. In GC, miR-148a, a member of the miR-148/152 family, exhibited a low expression level in GC tissues and cell lines, which was capable of regulating multiple targets and pathways implicating in malignant phenotypes, e.g., cell growth, proliferation, invasion, and metastasis in GC. Emerging evidence has demonstrated the existence of lncRNA MeG3-miR-148a interaction network that enables miRNAs suppression and inhibits MeG3 via modulation of the Dnamethyl transferase 1 (DnMT1) in GC, while its overexpression led to DnMT1 suppression, contributing to MeG3 expression.97,98 Thus, an in-depth understanding of the interaction between HOTAIR and miRNAs, especially the miR-148/152 family may serve as a novel therapeutic strategy in GC.
HOTAIR plays a key role in increasing GC cell EMT and metastasis via binding to PRC2 and epigenetic suppression of miR34a through H3K27me3 modification, which subsequently could lead to the promotion of C-Met, HGF/C-Met/Snail pathway components, and in turn, increased Snail level in GC.87
LncRNA networks have complex regulatory mechanisms, contributing to diverse biological functions; thus, these complex regulatory mechanisms require further clarification for choosing the appropriate therapeutic targets and exploring novel potential therapeutic strategies.
Clinicopathological characteristics and prognostic value of HOTAIR in GC
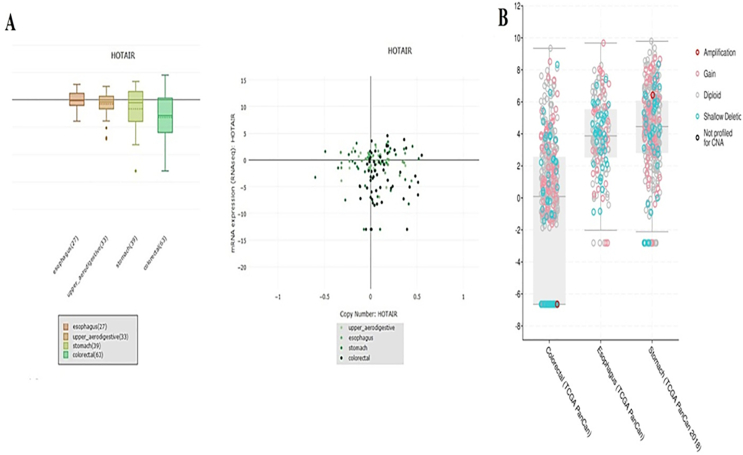
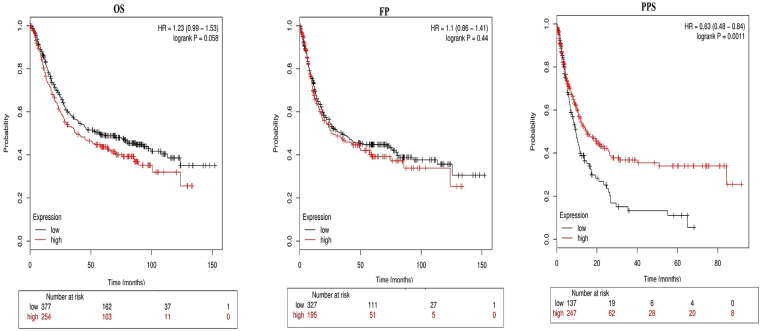
HOTAIR RNA expression levels in gastrointestinal cancer cell lines were adapted from Cancer Cell Line Encyclopedia (CCLE) database (https://portals.broadinstitute.org/ccle), (Fig. 1A). Furthermore, HOTAIR expression RNA (Seq V2; log2), copy number alterations, was indicated in Figure 1B (https://www.cbioportal.org). Furthermore, Kaplan–Meier Plotter was applied to generate overall survival (OS), first progression (FP), and post progression survival (PPS) plots (Fig. 2).
Figure 1.
Factors involving in transcriptional up-regulation of HOTAIR. HOTAIR RNA expression levels in cancer cell lines were adapted from Cancer Cell Line Encyclopedia (CCLE) (A). HOTAIR expression RNA (Seq V2; log2), copy number alterations, was indicated in (B).
Figure 2.
Kaplan–Meier Plotter was applied to generate overall survival (OS), first progression (FP), post progression survival (PPS) plots in gastric cancer.
A meta-analysis indicated the significant association of HOTAIR up-regulation with worse OS in GC,99 and lncRNA HOTAIR could play an oncogenic role and led to malignant transformation of normal cells. HOTAIR expression has a key role in lymph node metastasis, venous invasion, and poor prognosis (poor overall survival) in the diffuse type of GC, and overexpression was capable of not only increasing anchorage-independent cell growth in cell line but also promoting metastases to the liver in vivo.100 HOTAIR expression was found to be linked to lymph node metastasis, TNM stage, and poor overall survival in GC patients. Suppression of HOTAIR was capable of not only decreasing invasive phenotypes of GC cells but also reducing MMP1 and MMP3 expression levels in vitro. Findings demonstrated a reversed EMT process by HOTAIR.86 These findings help develop potentially effective therapeutic and diagnostic approaches for GC patients.
A study demonstrated that patients with up-regulated levels of HOTAIR showed a poor survival rate as compared to patients with lower levels of HOTAIR, suggesting the predictive value of HOTAIR for prognosis. In addition, HOTAIR expression was capable of promoting tumor growth and metastasis in vivo.101 HOTAIR lncRNA up-regulation in patients with GC was found to be associated with tumor size, differentiation, lymph-vascular invasion, deeper invasion depth, lymph node metastasis, distant metastasis, and TNM stage, as well as overall survival and progression-free survival, indicating that it can be applied to potentially develop a prognostic biomarker.102,103 HOTAIR was revealed to be linked to tumor size and lymphatic metastasis in GC, whereas it showed a potential role in the progression of GC.104
Overexpression of HOTAIR was linked to peritoneal metastasis and poor prognosis in patients suffering from GC, suggesting that this marker might serve as a prognostic factor for peritoneal dissemination as well as a diagnostic marker for GC patients.105 Up-regulation of HOTAIR in advanced gastric adenocarcinoma cases has been suggested as an independent poor prognostic indicator, and its expression was associated with poor survival in patients with poorly differentiated and advanced stage (TNM stages IIIA and IIIB),.56
It should be noted that evidence was highlighted based on HOTAIR as the predictor of overall survival, and its association with TNM stage and lymph node metastasis was reported.84 A considerable association was found between HOTAIR up-regulation and disease-free survival, where CDH1 or epithelial cadherin loss as a suppressor was negatively linked to HOTAIR in GC 87. A significant correlation of HOTAIR with lymphovascular invasion, lymph node metastasis, advanced TNM stage, and poor overall survival has been indicated in GC.85
Ample evidence indicates that HOTAIR may potentially be a diagnostic and prognostic marker for high-risk GC patients, whereas its key role in GC development and progression has been revealed in both in vitro and in vivo studies. Thereby, this lncRNA may be added to the pool of candidate targets to aid and provide more effective regimens for targeted therapy in GC.
Therapeutic approach
Knockdown of HOTAIR has been found to be associated with apoptosis, inhibition of growth of GC xenograft, and peritoneal metastasis, as well as disruption of GC cells [85, 105]. Okugawa et al. (2014) [105] provided evidence that siRNA HOTAIR could suppress cell proliferation and migration/invasion in GC cells, but it was capable of increasing the anoikis rate in transfected cells; on the other hand, suppression of both xenograft tumor growth and peritoneal metastasis was observed when HOTAIR siRNA-transfected cells were injected into nude mice. An increasing body of evidence highlights that HOTAIR may potentially be a useful diagnostic and prognostic marker for peritoneal metastasis, whereas its biological and pathological association with GC has been demonstrated.
Another study has also shown the high level of HOTAIR in lymphovascular invasion and lymph node metastasis in GC, while its Knockdown by siRNA caused suppression of viability, motility, and proliferation in GC cells and led to reduction of cell invasiveness and migration, as well as induction of apoptosis; thus, ample evidence support the potential role of HOTAIR in suppressing apoptosis and increasing invasive phenotypes of GC.85
A study applied shRNA and MKN 74 and KATO III cell lines for HOTAIR modulation, in which HOTAIR loss in KATO III cell lines by shRNA led to reduction of peritoneal dissemination.100
HOTAIR exhibited the highest differential expression pattern in GC compared to other lncRNAs including UCA1, LINC00589, DKFZP434K028, LINC00698, FAM225A, HOXA11-AS, and FIRRE. Suppression of HOTAIR expression by siRNA led to repression of proliferation and invasion; its suppression was also capable of not only enhancing chemosensitivity but also inducing apoptosis in GC cells.101 Another study indicated that siRNA HOTTIP suppressed cell proliferation, invasion/migration, and promoted cell apoptosis.102 As indicated in the above parts, the sh-HOTAIR group showed a lower frequency of lung metastases in vitro and displayed the EMT inhibition of GC cells by regulating Met and Snail, whereas increased levels of HOTAIR were partially linked to the suppression of mi34a overexpression in GC.87
HOTAIR expression has been illustrated to influence the survival of patients with advanced gastric adenocarcinoma, whereas drug resistance was observed in patients receiving fluorouracil and platinum chemotherapy. Nevertheless, HOTAIR overexpression is linked to a poor prognosis of GC.56
HOTAIR siRNA1 was associated with the loss of HOTAIR expression, leading to the reduction of viability of GC cells, and improvement of the chemosensitivity to5-fluorouracil (5-FU) and Cisplatin in GC cells BGC-823 and MKN-45.101
Therapeutic interventions
To date, no clinical trials have been performed on recombinant protein and regulatory agents of HOTAIR as an anticancer drug. However, high expression of HOTAIR has been used as a predictor of poor overall survival in patients with GC [86, 100]. Findings have also shown that inhibition of HOTAIR can reduce the invasion of GC cells by regulating effective transcription factors such as E-cadherin, vimentin, and N-cadherin.86 Other studies have shown that HOTAIR increases cisplatin resistance in GC cells by binding and inhibiting miR-126. In addition, by affecting kinases, HOTAIR enhances the expression of vascular endothelial growth factor A (VEGFA) and PI3–K.106 Other findings suggest that targeting HOTAIR in gastric cancer cells may be effective in increasing therapeutic efficacy and improving survival in patients with GC.90 In general, the evidence gathered suggests that HOTAIR plays an important role in gastric cancer, as well as resistance to treatment. Providing intervention and therapeutic solutions to evaluate the effectiveness of this biomolecule as a drug is highly required.
Conclusion
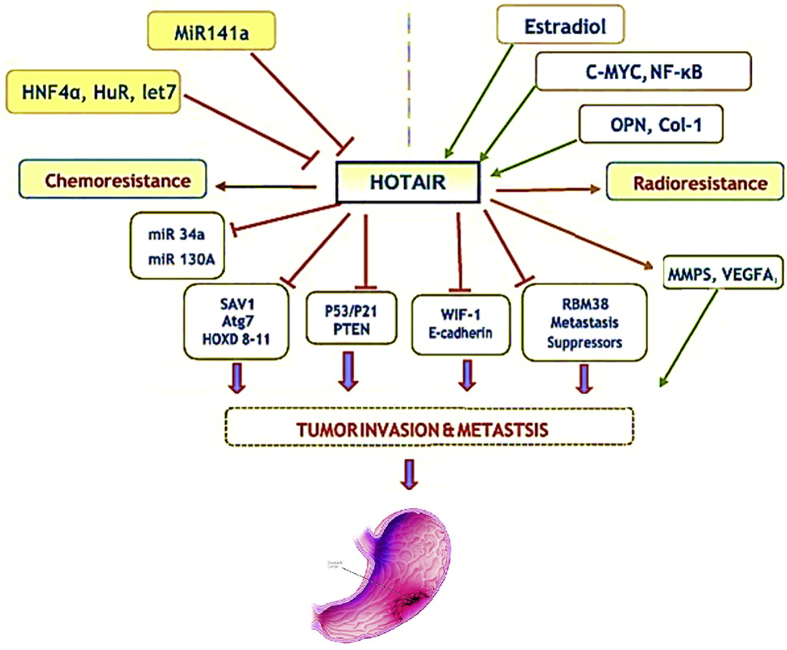
Functional polymorphisms of HOTAIR have been described to contribute to the susceptibility of many kinds of malignancies, thus, genetic variations can be considered for risk assessment, early detection, and targeted therapy in GC. HOTAIR is implicated in biological functions and biochemical and cellular processes via interactions with many factors, leading to genomic stability, proliferation, survival, invasion, migration, metastasis, and drug resistance. Furthermore, up-regulation of HOTAIR contributes to malignant phenotypes of GC, e.g., metastases, tumor progression, and EMT process cancer (Fig. 3). However, further studies are required to clarify the underlying role of HOTAIR in GC, and to describe the complicated mechanism of action of HOTAIR in different cancers.
Figure 3.
Model of action proposed for HOTAIR and cancer.
Transcriptional regulation of HOTAIR is associated with many mechanisms and various factors, including multiple estrogen response elements (ERE 1, 2, 3 and 4), Proto-oncogenic MYC family, nuclear factor kappa B (NF-κB), HREs, activator protein 1 (AP1), CpG-islands, HREs, specificity protein 1 (Sp1), proteins of the SRC family, as well as miRNAs. Other factors including post-synthetic methylation, MiR-141, Argonaute2 (Ago2), IRF1, siRNA, and some functional SNPs are involved in the down-regulation of HOTAIR.
Author contributions
WK, GY, SZ, XL, AZ, PY, JZ, YS, RY, and HJ participated in draft and design the study, editing, revise and writing of review article. All authors read and approved the final manuscript.
Conflict of interests
The authors declare that they have no competing interests.
Funding
This study was supported by Zhejiang Provincial Natural Science Foundation of China (No. Q17H030001, LQ18H070006).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Mattick J.S. Non-coding RNAs: the architects of eukaryotic complexity. EMBO Rep. 2001;2(11):986–991. doi: 10.1093/embo-reports/kve230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawaji H., Hayashizaki Y. Exploration of small RNAs. PLoS Genet. 2008;4(1):e22. doi: 10.1371/journal.pgen.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasgupta P., Kulkarni P., Majid S., et al. MicroRNA-203 inhibits long noncoding RNA HOTAIR and regulates tumorigenesis through epithelial-to-mesenchymal transition pathway in renal cell carcinoma. Mol Canc Therapeut. 2018;17(5):1061–1069. doi: 10.1158/1535-7163.MCT-17-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding J., Yeh C.R., Sun Y., et al. Estrogen receptor β promotes renal cell carcinoma progression via regulating LncRNA HOTAIR-miR-138/200c/204/217 associated CeRNA network. Oncogene. 2018;37(37):5037–5053. doi: 10.1038/s41388-018-0175-6. [DOI] [PubMed] [Google Scholar]
- 5.Woo C.J., Kingston R.E. HOTAIR lifts noncoding RNAs to new levels. Cell. 2007;129(7):1257–1259. doi: 10.1016/j.cell.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Lu L., Zhu G., Zhang C., et al. Association of large noncoding RNA HOTAIR expression and its downstream intergenic CpG island methylation with survival in breast cancer. Breast Cancer Res Treat. 2012;136(3):875–883. doi: 10.1007/s10549-012-2314-z. [DOI] [PubMed] [Google Scholar]
- 7.Cheng D., Deng J., Zhang B., et al. LncRNA HOTAIR epigenetically suppresses miR-122 expression in hepatocellular carcinoma via DNA methylation. EBioMedicine. 2018;36:159–170. doi: 10.1016/j.ebiom.2018.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai M.C., Manor O., Wan Y., et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329(5992):689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhan A., Mandal S.S. Long noncoding RNAs: emerging stars in gene regulation, epigenetics and human disease. ChemMedChem. 2014;9(9):1932–1956. doi: 10.1002/cmdc.201300534. [DOI] [PubMed] [Google Scholar]
- 10.Gupta R.A., Shah N., Wang K.C., et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resende C., Thiel A., Machado J.C., Ristimäki A. Gastric cancer: basic aspects. Helicobacter. 2011;16(Sp 1):38–44. doi: 10.1111/j.1523-5378.2011.00879.x. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig J.A., Weinstein J.N. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5(11):845–856. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 13.Du M., Wang W., Jin H., et al. The association analysis of lncRNA HOTAIR genetic variants and gastric cancer risk in a Chinese population. Oncotarget. 2015;6(31):31255–31262. doi: 10.18632/oncotarget.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin H., Lu X., Ni J., et al. HOTAIR rs7958904 polymorphism is associated with increased cervical cancer risk in a Chinese population. Sci Rep. 2017;7(1):3144. doi: 10.1038/s41598-017-03174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X., Zhou L., Fu G., et al. The identification of an ESCC susceptibility SNP rs920778 that regulates the expression of lncRNA HOTAIR via a novel intronic enhancer. Carcinogenesis. 2014;35(9):2062–2067. doi: 10.1093/carcin/bgu103. [DOI] [PubMed] [Google Scholar]
- 16.Pan W., Liu L., Wei J., et al. A functional lncRNA HOTAIR genetic variant contributes to gastric cancer susceptibility. Mol Carcinog. 2016;55(1):90–96. doi: 10.1002/mc.22261. [DOI] [PubMed] [Google Scholar]
- 17.Guo W., Dong Z., Bai Y., et al. Associations between polymorphisms of HOTAIR and risk of gastric cardia adenocarcinoma in a population of north China. Tumour Biol. 2015;36(4):2845–2854. doi: 10.1007/s13277-014-2912-y. [DOI] [PubMed] [Google Scholar]
- 18.Bayram S., Ülger Y., Sümbül A.T., et al. A functional HOTAIR rs920778 polymorphism does not contributes to gastric cancer in a Turkish population: a case-control study. Fam Cancer. 2015;14(4):561–567. doi: 10.1007/s10689-015-9813-0. [DOI] [PubMed] [Google Scholar]
- 19.Ülger Y., Dadaş E., Yalinbaş Kaya B., Sümbül A.T., Genç A., Bayram S. The analysis of lncRNA HOTAIR rs12826786 C>T polymorphism and gastric cancer susceptibility in a Turkish population: lack of any association in a hospital-based case-control study. Ir J Med Sci. 2017;186(4):859–865. doi: 10.1007/s11845-017-1596-x. [DOI] [PubMed] [Google Scholar]
- 20.Aiello A., Bacci L., Re A., et al. MALAT1 and HOTAIR long non-coding RNAs play opposite role in estrogen-mediated transcriptional regulation in prostate cancer cells. Sci Rep. 2016;6:38414. doi: 10.1038/srep38414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ur Rahman M.S., Cao J. Estrogen receptors in gastric cancer: advances and perspectives. World J Gastroenterol. 2016;22(8):2475–2482. doi: 10.3748/wjg.v22.i8.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson S., Gustafsson J.A. Estrogen receptor action. Crit Rev Eukaryot Gene Expr. 2002;12(4):237–257. doi: 10.1615/critreveukaryotgeneexpr.v12.i4.10. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson S., Gustafsson J.A. Biological role of estrogen and estrogen receptors. Crit Rev Biochem Mol Biol. 2002;37(1):1–28. doi: 10.1080/10409230290771438. [DOI] [PubMed] [Google Scholar]
- 24.Zhao W., Geng D., Li S., Chen Z., Sun M. LncRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer Med. 2018;7(3):842–855. doi: 10.1002/cam4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Pawłowska E., Szczepanska J., Blasiak J. The long noncoding RNA HOTAIR in breast cancer: does autophagy play a role? Int J Mol Sci. 2017;18(11):e2317. doi: 10.3390/ijms18112317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhan A., Hussain I., Ansari K.I., Kasiri S., Bashyal A., Mandal S.S. Antisense transcript long noncoding RNA (lncRNA) HOTAIR is transcriptionally induced by estradiol. J Mol Biol. 2013;425(19):3707–3722. doi: 10.1016/j.jmb.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng H., Huang X., Fan J., et al. A variant of estrogen receptor-alpha, ER-alpha 36 is expressed in human gastric cancer and is highly correlated with lymph node metastasis. Oncol Rep. 2010;24(1):171–176. [PMC free article] [PubMed] [Google Scholar]
- 28.Xu C.Y., Guo J.L., Jiang Z.N., et al. Prognostic role of estrogen receptor alpha and estrogen receptor beta in gastric cancer. Ann Surg Oncol. 2010;17(9):2503–2509. doi: 10.1245/s10434-010-1031-2. [DOI] [PubMed] [Google Scholar]
- 29.Guo J.L., Xu C.Y., Jiang Z.N., et al. Estrogen receptor beta variants mRNA expressions in gastric cancer tissues and association with clinicopathologic parameters. Hepatogastroenterol. 2010;57(104):1584–1588. [PubMed] [Google Scholar]
- 30.Zhao X.H., Gu S.Z., Liu S.X., Pan B.R. Expression of estrogen receptor and estrogen receptor messenger RNA in gastric carcinoma tissues. World J Gastroenterol. 2003;9(4):665–669. doi: 10.3748/wjg.v9.i4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryu W.S., Kim J.H., Jang Y.J., et al. Expression of estrogen receptors in gastric cancer and their clinical significance. J Surg Oncol. 2012;106(4):456–461. doi: 10.1002/jso.23097. [DOI] [PubMed] [Google Scholar]
- 32.Gan L., He J., Zhang X., et al. Expression profile and prognostic role of sex hormone receptors in gastric cancer. BMC Cancer. 2012;12:566. doi: 10.1186/1471-2407-12-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang G., Zheng L., Pu J., et al. Small RNAs targeting transcription start site induce heparanase silencing through interference with transcription initiation in human cancer cells. PLoS One. 2012;7(2):e31379. doi: 10.1371/journal.pone.0031379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma M.Z., Li C.X., Zhang Y., et al. Long non-coding RNA HOTAIR, a c-Myc activated driver of malignancy, negatively regulates miRNA-130a in gallbladder cancer. Mol Cancer. 2014;13:156. doi: 10.1186/1476-4598-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao J., Zhao X., Tao J. c-MYC-miRNA circuitry: a central regulator of aggressive B-cell malignancies. Cell Cycle. 2014;13(2):191–198. doi: 10.4161/cc.27646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang F., Xue X., Zheng L., et al. Long non-coding RNA GHET1 promotes gastric carcinoma cell proliferation by increasing c-Myc mRNA stability. FEBS J. 2014;281(3):802–813. doi: 10.1111/febs.12625. [DOI] [PubMed] [Google Scholar]
- 37.He X., Tan X., Wang X., et al. C-Myc-activated long noncoding RNA CCAT1 promotes colon cancer cell proliferation and invasion. Tumour Biol. 2014;35(12):12181–12188. doi: 10.1007/s13277-014-2526-4. [DOI] [PubMed] [Google Scholar]
- 38.Yang F., Xue X., Bi J., et al. Long noncoding RNA CCAT1, which could be activated by c-Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol. 2013;139(3):437–445. doi: 10.1007/s00432-012-1324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swier L.J.Y.M., Dzikiewicz-Krawczyk A., Winkle M., van den Berg A., Kluiver J. Intricate crosstalk between MYC and non-coding RNAs regulates hallmarks of cancer. Mol Oncol. 2019;13(1):26–45. doi: 10.1002/1878-0261.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z., Zhu H., Liu Y., Quan F., Zhang X., Yu L. LncRNA HOTAIR mediates TGF-β2-induced cell growth and epithelial-mesenchymal transition in human lens epithelial cells. Acta Biochim Biophys Sin. 2018;50(10):1028–1037. doi: 10.1093/abbs/gmy101. [DOI] [PubMed] [Google Scholar]
- 41.Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 42.Ren Y., Jia H.H., Xu Y.Q., et al. Paracrine and epigenetic control of CAF-induced metastasis: the role of HOTAIR stimulated by TGF-ß1 secretion. Mol Cancer. 2018;17(1):5. doi: 10.1186/s12943-018-0758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Botti G., Scognamiglio G., Aquino G., Liguori G., Cantile M. LncRNA HOTAIR in tumor microenvironment: what role? Int J Mol Sci. 2019;20(9):2279. doi: 10.3390/ijms20092279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiong G., Yang L., Chen Y., Fan Z. Linc-POU3F3 promotes cell proliferation in gastric cancer via increasing T-reg distribution. Am J Transl Res. 2015;7(11):2262–2269. [PMC free article] [PubMed] [Google Scholar]
- 45.Zhuang Y., Nguyen H.T., Burow M.E., et al. Elevated expression of long intergenic non-coding RNA HOTAIR in a basal-like variant of MCF-7 breast cancer cells. Mol Carcinog. 2015;54(12):1656–1667. doi: 10.1002/mc.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antoon J.W., Lai R., Struckhoff A.P., et al. Altered death receptor signaling promotes epithelial-to-mesenchymal transition and acquired chemoresistance. Sci Rep. 2012;2:539. doi: 10.1038/srep00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25(3):409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 48.Oshima H., Ishikawa T., Yoshida G.J., et al. TNF-α/TNFR1 signaling promotes gastric tumorigenesis through induction of Noxo1 and Gna14 in tumor cells. Oncogene. 2014;33(29):3820–3829. doi: 10.1038/onc.2013.356. [DOI] [PubMed] [Google Scholar]
- 49.Obaid M., Udden S.M.N., Deb P., Shihabeddin N., Zaki M.H., Mandal S.S. LncRNA HOTAIR regulates lipopolysaccharide-induced cytokine expression and inflammatory response in macrophages. Sci Rep. 2018;8(1):15670. doi: 10.1038/s41598-018-33722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Özeş A.R., Miller D.F., Özeş O.N., et al. NF-κB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene. 2016;35(41):5350–5361. doi: 10.1038/onc.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Z.H., Wang X.L., Tang H.M., et al. Long non-coding RNA HOTAIR is a powerful predictor of metastasis and poor prognosis and is associated with epithelial-mesenchymal transition in colon cancer. Oncol Rep. 2014;32(1):395–402. doi: 10.3892/or.2014.3186. [DOI] [PubMed] [Google Scholar]
- 52.Qiu J.J., Lin Y.Y., Ye L.C., et al. Overexpression of long non-coding RNA HOTAIR predicts poor patient prognosis and promotes tumor metastasis in epithelial ovarian cancer. Gynecol Oncol. 2014;134(1):121–128. doi: 10.1016/j.ygyno.2014.03.556. [DOI] [PubMed] [Google Scholar]
- 53.Li P., Zhang X., Wang L., et al. lncRNA HOTAIR contributes to 5FU resistance through suppressing miR-218 and activating NF-κB/TS signaling in colorectal cancer. Mol Ther Nucleic Acids. 2017;8:356–369. doi: 10.1016/j.omtn.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagaraju G.P., Alese O.B., Landry J., Diaz R., El-Rayes B.F. HSP90 inhibition downregulates thymidylate synthase and sensitizes colorectal cancer cell lines to the effect of 5FU-based chemotherapy. Oncotarget. 2014;5(20):9980–9991. doi: 10.18632/oncotarget.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson P.M., Danenberg P.V., Johnston P.G., Lenz H.J., Ladner R.D. Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat Rev Clin Oncol. 2014;11(5):282–298. doi: 10.1038/nrclinonc.2014.51. [DOI] [PubMed] [Google Scholar]
- 56.Zhao W., Dong S., Duan B., et al. HOTAIR is a predictive and prognostic biomarker for patients with advanced gastric adenocarcinoma receiving fluorouracil and platinum combination chemotherapy. Am J Transl Res. 2015;7(7):1295–1302. [PMC free article] [PubMed] [Google Scholar]
- 57.Shang C., Guo Y., Zhang H., Xue Y.X. Long noncoding RNA HOTAIR is a prognostic biomarker and inhibits chemosensitivity to doxorubicin in bladder transitional cell carcinoma. Canc Chemother Pharmacol. 2016;77(3):507–513. doi: 10.1007/s00280-016-2964-3. [DOI] [PubMed] [Google Scholar]
- 58.Gambhir S., Vyas D., Hollis M., Aekka A., Vyas A. Nuclear factor kappa B role in inflammation associated gastrointestinal malignancies. World J Gastroenterol. 2015;21(11):3174–3183. doi: 10.3748/wjg.v21.i11.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sokolova O., Naumann M. NF-κB signaling in gastric cancer. Toxins. 2017;9(4):119. doi: 10.3390/toxins9040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maruyama R., Shipitsin M., Choudhury S., et al. Altered antisense-to-sense transcript ratios in breast cancer. Proc Natl Acad Sci U S A. 2012;109(8):2820–2824. doi: 10.1073/pnas.1010559107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M., Li X., Zhuang Y., Flemington E.K., Lin Z., Shan B. Induction of a novel isoform of the lncRNA HOTAIR in Claudin-low breast cancer cells attached to extracellular matrix. Mol Oncol. 2017;11(12):1698–1710. doi: 10.1002/1878-0261.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang G., Zhang S., Gao F., et al. Osteopontin enhances the expression of HOTAIR in cancer cells via IRF1. Biochim Biophys Acta. 2014;1839(9):837–848. doi: 10.1016/j.bbagrm.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 63.Higashiyama M., Ito T., Tanaka E., Shimada Y. Prognostic significance of osteopontin expression in human gastric carcinoma. Ann Surg Oncol. 2007;14(12):3419–3427. doi: 10.1245/s10434-007-9564-8. [DOI] [PubMed] [Google Scholar]
- 64.Gu X., Gao X.S., Ma M., et al. Prognostic significance of osteopontin expression in gastric cancer: a meta-analysis. Oncotarget. 2016;7(43):69666–69673. doi: 10.18632/oncotarget.11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ng L., Wan T.M., Lam C.S., et al. Post-operative plasma osteopontin predicts distant metastasis in human colorectal cancer. PLoS One. 2015;10(5):e0126219. doi: 10.1371/journal.pone.0126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee S.H., Park J.W., Go D.M., et al. Ablation of osteopontin suppresses N-methyl-N-nitrosourea and Helicobacter pylori-induced gastric cancer development in mice. Carcinogenesis. 2015;36(12):1550–1560. doi: 10.1093/carcin/bgv144. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Z.Z., Shen Z.Y., Shen Y.Y., et al. HOTAIR long noncoding RNA promotes gastric cancer metastasis through suppression of poly r(C)-Binding protein (PCBP) 1. Mol Canc Therapeut. 2015;14(5):1162–1170. doi: 10.1158/1535-7163.MCT-14-0695. [DOI] [PubMed] [Google Scholar]
- 68.Ji F.J., Wu Y.Y., An Z., et al. Expression of both poly r(C) binding protein 1 (PCBP1) and miRNA-3978 is suppressed in peritoneal gastric cancer metastasis. Sci Rep. 2017;7(1):15488. doi: 10.1038/s41598-017-15448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang T., Huang X.H., Dong L., et al. PCBP-1 regulates alternative splicing of the CD44 gene and inhibits invasion in human hepatoma cell line HepG2 cells. Mol Cancer. 2010;9:72. doi: 10.1186/1476-4598-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H., Vardy L.A., Tan C.P., et al. PCBP1 suppresses the translation of metastasis-associated PRL-3 phosphatase. Cancer Cell. 2010;18(1):52–62. doi: 10.1016/j.ccr.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 71.Pillai M.R., Chacko P., Kesari L.A., Jayaprakash P.G., Jayaram H.N., Antony A.C. Expression of folate receptors and heterogeneous nuclear ribonucleoprotein E1 in women with human papillomavirus mediated transformation of cervical tissue to cancer. J Clin Pathol. 2003;56(8):569–574. doi: 10.1136/jcp.56.8.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thakur S., Nakamura T., Calin G., et al. Regulation of BRCA1 transcription by specific single-stranded DNA binding factors. Mol Cell Biol. 2003;23(11):3774–3787. doi: 10.1128/MCB.23.11.3774-3787.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang M., Wang X., Tan J., Zhao M., Lian L., Zhang W. Poly r(C) binding protein (PCBP) 1 is a negative regulator of thyroid carcinoma. Am J Transl Res. 2016;8(8):3567–3573. [PMC free article] [PubMed] [Google Scholar]
- 74.Chaudhury A., Chander P., Howe P.H. Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: focus on hnRNP E1's multifunctional regulatory roles. RNA. 2010;16(8):1449–1462. doi: 10.1261/rna.2254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo J., Jia R. Splicing factor poly(rC)-binding protein 1 is a novel and distinctive tumor suppressor. J Cell Physiol. 2018;234(1):33–41. doi: 10.1002/jcp.26873. [DOI] [PubMed] [Google Scholar]
- 76.Hussey G.S., Chaudhury A., Dawson A.E., et al. Identification of an mRNP complex regulating tumorigenesis at the translational elongation step. Mol Cell. 2011;41(4):419–431. doi: 10.1016/j.molcel.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grelet S., Link L.A., Howley B., et al. Addendum: a regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat Cell Biol. 2017;19(12):1443. doi: 10.1038/ncb3647. [DOI] [PubMed] [Google Scholar]
- 78.Ren C., Cho S.J., Jung Y.S., Chen X. DNA polymerase η is regulated by poly(rC)-binding protein 1 via mRNA stability. Biochem J. 2014;464(3):377–386. doi: 10.1042/BJ20141164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho S.J., Jung Y.S., Chen X. Poly (C)-binding protein 1 regulates p63 expression through mRNA stability. PLoS One. 2013;8(8):e71724. doi: 10.1371/journal.pone.0071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang X., Hua L., Yan D., et al. Overexpression of PCBP2 contributes to poor prognosis and enhanced cell growth in human hepatocellular carcinoma. Oncol Rep. 2016;36(6):3456–3464. doi: 10.3892/or.2016.5167. [DOI] [PubMed] [Google Scholar]
- 81.Yang X., Hao Y., Ding Z., Pater A. BAG-1 promotes apoptosis induced by N-(4-hydroxyphenyl)retinamide in human cervical carcinoma cells. Exp Cell Res. 2000;256(2):491–499. doi: 10.1006/excr.2000.4829. [DOI] [PubMed] [Google Scholar]
- 82.Pickering B.M., Mitchell S.A., Evans J.R., Willis A.E. Polypyrimidine tract binding protein and poly r(C) binding protein 1 interact with the BAG-1 IRES and stimulate its activity in vitro and in vivo. Nucleic Acids Res. 2003;31(2):639–646. doi: 10.1093/nar/gkg146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Link L.A., Howley B.V., Hussey G.S., Howe P.H. PCBP1/HNRNP E1 protects chromosomal integrity by translational regulation of CDC27. Mol Cancer Res. 2016;14(7):634–646. doi: 10.1158/1541-7786.MCR-16-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen W.M., Chen W.D., Jiang X.M., et al. HOX transcript antisense intergenic RNA represses E-cadherin expression by binding to EZH2 in gastric cancer. World J Gastroenterol. 2017;23(33):6100–6110. doi: 10.3748/wjg.v23.i33.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee N.K., Lee J.H., Park C.H., et al. Long non-coding RNA HOTAIR promotes carcinogenesis and invasion of gastric adenocarcinoma. Biochem Biophys Res Commun. 2014;451(2):171–178. doi: 10.1016/j.bbrc.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 86.Xu Z.Y., Yu Q.M., Du Y.A., et al. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9(6):587–597. doi: 10.7150/ijbs.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y.W., Sun M., Xia R., et al. LincHOTAIR epigenetically silences miR34a by binding to PRC2 to promote the epithelial-to-mesenchymal transition in human gastric cancer. Cell Death Dis. 2015;6(7):e1802. doi: 10.1038/cddis.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu X.H., Sun M., Nie F.Q., et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsukawa Y., Semba S., Kato H., Ito A., Yanagihara K., Yokozaki H. Expression of the enhancer of zeste homolog 2 is correlated with poor prognosis in human gastric cancer. Cancer Sci. 2006;97(6):484–491. doi: 10.1111/j.1349-7006.2006.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song B., Guan Z., Liu F., Sun D., Wang K., Qu H. Long non-coding RNA HOTAIR promotes HLA-G expression via inhibiting miR-152 in gastric cancer cells. Biochem Biophys Res Commun. 2015;464(3):807–813. doi: 10.1016/j.bbrc.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 91.Zhu X.M., Han T., Wang X.H., et al. Overexpression of miR-152 leads to reduced expression of human leukocyte antigen-G and increased natural killer cell mediated cytolysis in JEG-3 cells. Am J Obstet Gynecol. 2010;202(6):e592. doi: 10.1016/j.ajog.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 92.Zhai R., Kan X., Wang B., et al. miR-152 suppresses gastric cancer cell proliferation and motility by targeting CD151. Tumour Biol. 2014;35(11):11367–11373. doi: 10.1007/s13277-014-2471-2. [DOI] [PubMed] [Google Scholar]
- 93.Rubinstein E. The complexity of tetraspanins. Biochem Soc Trans. 2011;39(2):501–505. doi: 10.1042/BST0390501. [DOI] [PubMed] [Google Scholar]
- 94.Fei Y., Wang J., Liu W., et al. CD151 promotes cancer cell metastasis via integrins α3β1 and α6β1 in vitro. Mol Med Rep. 2012;6(6):1226–1230. doi: 10.3892/mmr.2012.1095. [DOI] [PubMed] [Google Scholar]
- 95.Chen Y., Song Y., Wang Z., et al. Altered expression of MiR-148a and MiR-152 in gastrointestinal cancers and its clinical significance. J Gastrointest Surg. 2010;14(7):1170–1179. doi: 10.1007/s11605-010-1202-2. [DOI] [PubMed] [Google Scholar]
- 96.Sun J., Chu H., Ji J., et al. Long non-coding RNA HOTAIR modulates HLA-G expression by absorbing miR-148a in human cervical cancer. Int J Oncol. 2016;49(3):943–952. doi: 10.3892/ijo.2016.3589. [DOI] [PubMed] [Google Scholar]
- 97.Yan J., Guo X., Xia J., et al. MiR-148a regulates MEG3 in gastric cancer by targeting DNA methyltransferase 1. Med Oncol. 2014;31(3):879. doi: 10.1007/s12032-014-0879-6. [DOI] [PubMed] [Google Scholar]
- 98.Xia J., Guo X., Yan J., Deng K. The role of miR-148a in gastric cancer. J Cancer Res Clin Oncol. 2014;140(9):1451–1456. doi: 10.1007/s00432-014-1649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao S., Zhao Z.Y., Wu R., Zhang Y., Zhang Z.Y. Prognostic value of long noncoding RNAs in gastric cancer: a meta-analysis. Onco Targets Ther. 2018;11:4877–4891. doi: 10.2147/OTT.S169823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Endo H., Shiroki T., Nakagawa T., et al. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One. 2013;8(10):e77070. doi: 10.1371/journal.pone.0077070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feng X., Huang S. Effect and mechanism of long noncoding RNAs HOTAIR on occurrence and development of gastric cancer. J Cell Biochem. 2017 doi: 10.1002/jcb.26594. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 102.Ye H., Liu K., Qian K. Overexpression of long noncoding RNA HOTTIP promotes tumor invasion and predicts poor prognosis in gastric cancer. OncoTargets Ther. 2016;9:2081–2088. doi: 10.2147/OTT.S95414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li H., Li J., Zhang B., Zeng H. Long-chain non-coding RNA HOTAIR expression in tissue samples correlates with gastric cancer survival. Int J Clin Exp Med. 2018;11(2):856–862. [Google Scholar]
- 104.Li C.Y., Liang G.Y., Yao W.Z., et al. Integrated analysis of long non-coding RNA competing interactions reveals the potential role in progression of human gastric cancer. Int J Oncol. 2016;48(5):1965–1976. doi: 10.3892/ijo.2016.3407. [DOI] [PubMed] [Google Scholar]
- 105.Okugawa Y., Toiyama Y., Hur K., et al. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis. 2014;35(12):2731–2739. doi: 10.1093/carcin/bgu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yan J., Dang Y., Liu S., Zhang Y., Zhang G. LncRNA HOTAIR promotes cisplatin resistance in gastric cancer by targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumor Biol. 2016;37:16345–16355. doi: 10.1007/s13277-016-5448-5. [DOI] [PubMed] [Google Scholar]