Abstract
Ubiquitination has important functions in osteoarthritis (OA), yet the mechanism remains unclear. Here, we identify the regulator of G protein signaling 12 (RGS12) in macrophages, which promotes the association between ubiquitin and IκB during inflammation. We also find that RGS12 promotes the degradation of IκB through enhancing the ubiquitination whereas the process can be inhibited by MG132. Moreover, the increased ubiquitination further inhibits the expression of MTAP, which can indirectly activate the phosphorylation of IκB. Finally, due to the degradation of IκB, the NF-κB translocates into the nucleus and further promotes the gene expression of cytokines such as IL1β, IL6, and TNFα during inflammation. Importantly, RGS12 deficiency prevents ubiquitination and inflammation in surgically or chemically induced OA. We conclude that the lack of RGS12 in macrophages interferes with the ubiquitination and degradation of IκB, thereby preventing inflammation and cartilage damage. Our results provide evidence for the relevance of RGS12 in promoting inflammation and regulating immune signaling.
Keywords: IκB, NF-κB, Osteoarthritis, RGS12, Ubiquitination
Introduction
Osteoarthritis (OA) is one of the most common bone-related diseases in the world. The pathogenesis of OA results from a complex interaction between cellular and inflammatory factors.1,2 OA is morphologically characterized by a chronic breakdown of cartilage and episodic synovitis.3 A number of reports showed that synovial inflammation can be found in both early and late stages in OA patients.4, 5, 6 Abundant proinflammatory cytokines are the main reason for inflammation and cartilage erosion found in the synovium of OA patients.7,8 The infiltration and accumulation of macrophages in the synovial tissues are considered as a hallmark of synovitis.7 Macrophages, as essential effectors of the innate immune system, play a critical role in inflammation and host defense.9 Activated macrophages can secrete proinflammatory cytokines which are involved in inflammatory bone diseases such as OA.10
Ubiquitination exerts a critical role in regulating the process of OA.11 Ubiquitination is a process that ubiquitin molecules modify the targets to control protein stability, activity, and function through an enzymatic reaction cascade.12 Ubiquitin itself is a highly conserved 76 amino acid polypeptide. The covalent attachment of ubiquitin to cellular proteins occurs at lysine (K) residues.13 Ubiquitin contains seven K residues that allow different poly-ubiquitin chains to be created including K48 and K63 chains. It has been proved that these chains play important roles in signal transduction pathways, such as the NF-κB pathway.14 A large number of ubiquitin E3 ligases and deubiquitylating enzymes have recently been linked to inflammation responses. Several reports indicated that ubiquitylation controls proximal signaling induced by inflammatory cytokines, for example, tumor necrosis factor (TNF), which increases endothelial permeability and promotes tissue damage.15 Moreover, Marta Radwan et al11 reported that inhibition of the 26S proteasome and lysine-48 linked ubiquitination can inhibit OA.
The ubiquitin-proteasome system (UPS) plays a critical role in the regulation of IκB degradation.16 IκB binds and isolates NF-κB dimers to further inhibit the nuclear translocation of NF-κB and inflammatory signaling.17 As most proteins are degraded via the UPS, IκB is modified by the attachment of the small polypeptide ubiquitin. Finally, additional ubiquitin polypeptides can be covalently attached to the original ubiquitin to regulate the client protein for proteasomal degradation.18
Regulators of G-protein signaling (RGS) proteins regulate GTPase activating protein function as negative modulators of G-protein-coupled receptors.19 Among them, RGS12 was reported to play pivotal roles in the inflammation process.20,21 RGS12 promotes macrophage activation and the release of inflammatory factors in rheumatoid arthritis as an NF-κB regulator.20 As the largest RGS protein, RGS12 involves in several signaling pathways such as receptor tyrosine kinases (RTKs), mitogen-activated protein kinases (MAPKs), G protein-coupled receptors (GPCRs), and Ras GTPases.22,23 Moreover, RGS12 regulates post-modifications of multiple proteins under physiological and pathological conditions.24 However, whether RGS12 regulates inflammatory diseases like OA through controlling the ubiquitination of component(s) in NF-κB is still unclear.
The aim of this study was to investigate the relationship of macrophage RGS12 and ubiquitination in OA mouse models and to identify novel targets of ubiquitination, all of which may have potential therapeutic relevance in OA and other inflammatory diseases.
Materials and methods
Animal
To generate macrophage lineage RGS12 conditional knock out (cKO) mice, RGS12 fl/fl mice were crossed with mice expressing Cre recombinase under the control of the lysozyme 2 (Lyz2 or LysM)-promotor (LysM-Cre+). The LysM-Cre+ (wildtype, WT) and the LysM-Cre+; RGS12 fl/fl (cKO) mice were littermates derived from the breeding of heterozygous animals. The animals were maintained under specific pathogen-free conditions. All animal studies were performed in accordance with institutional guidelines and with approval by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania.
Cell culture
The macrophage cell line RAW 264.7 was obtained from the American Type Culture Collection (TIB-71). Cells were maintained in a complete growth medium (DMEM added with 2 mM glutamine, 100 units/mL penicillin, 100 mg/mL streptomycin, and 10% FBS).
Bone marrow macrophages (BMMs) from 8-week old WT (LysM-Cre+) or RGS12 cKO (LysM-Cre+; RGS12 fl/fl) mice were isolated from the femur and cultured for 6 days in BMM media supplemented with 10% FBS, 5000 U/mL M-CSF, and 1% penicillin and streptomycin as previously described.25
Anterior cruciate ligament transection (ACLT)
The OA model was created by transection of anterior cruciate ligaments on the right knee joints of 8-week-old female mice.26,27 Briefly, the medial side of the joint was opened, and the patella was dislocated laterally to expose the femoral condyles after dissecting the soft tissue. The anterior cruciate ligament was transected, and the transection was confirmed by an anterior drawer test as previously described.26,27 The knee joints were harvested 4 weeks after ACLT surgery, and the OA pathogenesis was analyzed by histological staining.
Collagenase-induced osteoarthritis (CIOA)
CIOA was induced as previously described.28 Briefly, mice received an intra-articular injection of one unit of collagenase type VII (C0773, Sigma–Aldrich, US) with saline on days 0 and 2 to induce joint instability in the right knee. The knee joints were harvested and processed for histological examination 4 weeks after CIOA.
Histopathology analysis
To test the damage of cartilage, the sections from the knee joints of 8-week old LysM-Cre+ and RGS12 cKO mice with ACLT or CIOA were stained with Safranin-O/light green.
The positive safranin O area was detected and analyzed using Image J software (NIH, US) as previously described.29 The pathologic changes of knee joints were analyzed according to OARSI score.30 Briefly, the following 0–4 subjective scoring system was used in evaluating OA severity: (0) no apparent changes; (1) loss of superficial zone in articular cartilage; (2) defects limited above tidemark; (3) defects extending to calcified cartilage; (4) exposure of subchondral bone.
Liquid chromatography-tandem mass spectrometry analysis
The LC-MS experiment was performed as described in the previous study31 to compare the protein profiles in BMMs from 8-week old WT and RGS12 cKO mice. A stringent set of criteria including a low peptide and protein false discovery rate (FDR) of <0.05 was used for protein identification. Heat map visualizations were performed using the R Package cluster. The database for annotation, visualization, and integrated discovery (DAVID) was utilized to perform gene ontology (GO) enrichment analysis.
Quantitative real-time qPCR analysis
RNAs from synovial tissues or BMMs from 8-week old WT and RGS12 cKO mice were extracted using Trizol reagent (Life Technologies, USA) according to the manufacturer's instructions. Then, 1 μg of RNA was reversely transcribed into cDNA using the Reverse Transcription Kit (TAKARA, Japan). Real-time PCR was performed with the reaction mixture containing primers, the cDNA template, and SYBR Green PCR Master Mix (Bimake, USA). The sequences of real-time PCR primers were shown in Table S1.
Plasmid construction and transfection
RGS12 cDNA fragment (NM_173402.2) was cloned and inserted into the p3xFLAG-Myc-CMV-26 backbone (pCMV-RGS12) as previously described.20 Control (siCtrl, sc-37007) and MTAP siRNAs (siMTAP, sc-60007) were purchased from Santa Cruz. RAW264.7 cells or BMMs were seeded on 6-well plates at 3 × 105 cells/well. Cells were transfected with pCMV, pCMV-RGS12 plasmids, siCtrl or siMTAP by using the Lipofectamine 3000 Transfection Reagent (L3000001, ThermoFisher) for 24–48 h.
Immunoprecipitation (IP)
RAW264.7 cells were firstly lysed in NP-40 buffer supplemented with protein inhibitor cocktail (PIC) and phenylmethylsulfonyl fluoride (Sigma–Aldrich, US). The major methods were performed as described.32,33 Briefly, the lysates of equal amounts of protein were incubated at room temperature with primary antibodies mouse IgG (1:500, sc-2025, Santa Cruz) and anti-ubiquitin (Ub) (1:500, sc-166553, Santa Cruz) for 1 h and then with protein A/G beads overnight, after which the beads were washed with PBST. Bound proteins were solubilized in loading buffer for Western blot analysis.
Western blot
Synovium or macrophages (BMMs or RAW264.7 cells) were homogenized with RIPA (radioimmunoprecipitation assay) buffer containing PIC (Protease Inhibitor Cocktails, Sigma–Aldrich, US) on ice. Nuclear and cytoplasmic proteins were extracted using commercial reagents (2900, Millipore, US) according to the manufacturer's protocol. Equal amounts of protein (30 μg) were denatured in SDS and separated in 10% SDS-PAGE gels. Proteins were transferred to NC membranes in transfer buffer containing 20% methanol for 90 min. The membranes were blocked with 5% skim milk, incubated with primary antibody overnight at 4 °C and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:2,000, Jackson ImmunoResearch, PA) at room temperature for 1 h. β-Actin (1:2000, sc-47778, Santa Cruz) was used as the internal control. The following primary antibodies were used: anti-RGS12 (1:1,000, GW21317, Sigma–Aldrich), anti-ubiquitin (Ub) (1:500, sc-166553, Santa Cruz), anti-Interleukin-1 (1:500, sc-12742, Santa Cruz), anti-Interleukin-6 (1:500, sc-57315, Santa Cruz), anti-TNFα (tumor necrosis factor alpha) (1:1000, 60291-1-Ig, Proteintech), anti-MTAP (methylthioadenosine phosphorylase) (1:500, sc-100782, Santa Cruz), anti-IκBα (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha) (1:1,000, 4814, Cell Signaling Technology), and anti-Phospho IκBα (1:1,000, 2859, Cell Signaling Technology).
Immunofluorescence (IF)
The BMMs or RAW264.7 cells were fixed in 4% paraformaldehyde for 20 min at room temperature, incubated with NF-κB (p65) (1:200, 8242, Cell Signaling Technology) and α-Tubulin (1:200, 66031-1-Ig, Proteintech, US) followed by incubation with the donkey anti-rabbit IgG (H + L) Alexa Fluor 594 (1:500, A-21207, ThermoFisher) and the goat anti-mouse IgG (H + L) Alexa Fluor 488 (1:500, A28175, ThermoFisher). All images were visualized on a Leica microscope and acquired at the same exposure time with Image J software.
Statistical analysis
All data are expressed as the mean ± S.E.M. Statistical significance was determined by unpaired two-tailed Student's t-test. An analysis of variance test was first performed to compare the mean values between groups, and the Student–Newman–Keuls test was used to compare the mean values between two conditions with GraphPad software 7.0 (San Diego, CA, US). P-values less than 0.05 were considered significant.
Results
Ablation of RGS12 in macrophages does not affect the cartilage development
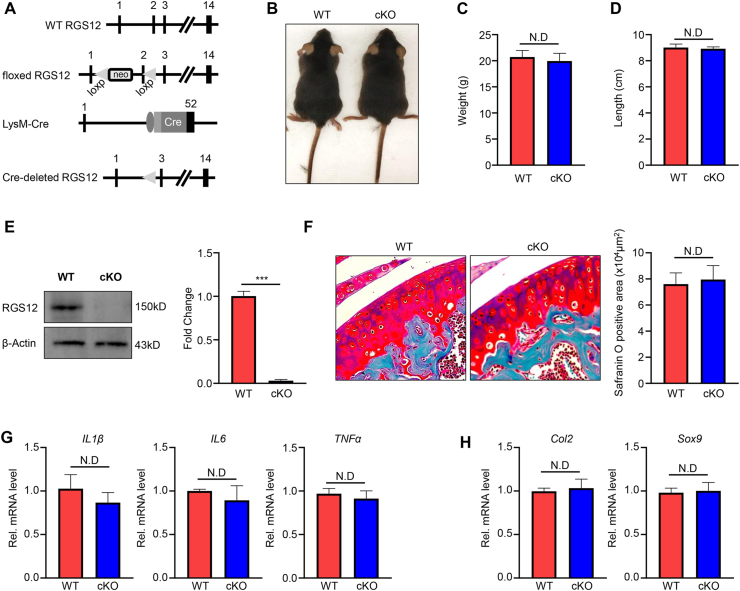
The joint inflammatory environment is critical for the initiation and aggregation of cartilage lesion.34 Macrophage accumulation in proliferative synovial tissue is the common feature of OA.35 RGS12 plays an important role in regulating the cartilage homeostasis24 and the functions of macrophages.20,24 To identify whether RGS12 cKO in macrophages affects the cartilage formation, macrophage-specific RGS12 cKO mice were generated by crossing RGS12 floxed mice with LysM-Cre transgenic mice, which is predominantly active in macrophages.36 In this setting, the Cre recombinase removed exon 2 of the floxed RGS12 gene (Fig. 1A) in LysM+ cells. The macrophage lineage RGS12 cKO mice were viable and fertile. The animals showed no difference in body weight and length at 8 weeks between LysM-Cre+ (wild-type, WT) and LysM-Cre+; RGS12 fl/fl (cKO) mice (Fig. 1B–D). Loss of RGS12 protein from BMMs was confirmed by immunoblotting from the RGS12 cKO mice (Fig. 1E). Nevertheless, loss of RGS12 in macrophages did not affect the articular cartilage development when assessed at the age of 8 weeks (Fig. 1F). The mRNA level of pro-inflammatory factors such as IL1β, IL6, and TNFα exhibited a mild decrease but no significance in synovial tissues (Fig. 1G). Moreover, the mRNA level of cartilage markers Col2 and Sox9 did not show any significant changes in synovial tissues (Fig. 1H). Thus, the loss of RGS12 in macrophages does not affect cartilage development.
Figure 1.
Ablation of RGS12 in macrophages does not affect the cartilage development in mice. (A) Generation of RGS12 cKO mice. RGS12 genomic locus, the floxed RGS12 targeting allele, the LysM-Cre transgene, and the recombinant RGS12 cKO allele. Exons are indicated by black boxes and numbered. Neo, neomycin resistance gene; loxP, loxP sites; Cre, Cre recombinase coding sequence. (B) Two-month-old female RGS12 cKO (LysM-Cre+; RGS12 fl/fl) mice show no growth retardation in comparison to WT (LysM-Cre+) mice (n = 10). (C) Measurement of body weight at 8 weeks shows no significant change (n = 10). ND: no difference (P > 0.05). (D) Measurement of body length at 8 weeks shows no significant change (n = 10). ND: no difference (P > 0.05). (E) BMMs derived from WT and RGS12 cKO were immunoblotted with an antibody against mouse RGS12. β-Actin was used as a loading control. ∗∗∗P < 0.001, vs. WT group, n = 5. (F) Sagittal sections of the knee joint of WT and RGS12 cKO mice at the age of 8 weeks (n = 10). Note that the Safranin O positive area showed no significant change. ND: no difference (P > 0.05). (G) Gene expression levels of IL1β, IL6, and TNFα in the cartilage tissue from WT and RGS12 KO mice were measured by RT-qPCR. Data are reported as means ± SEM (n = 5). ND: no difference (P > 0.05). (H) Gene expression levels of Col2 and Sox9 in the cartilage tissue from WT and RGS12 KO mice. Data are reported as means ± SEM (n = 5). ND: no difference (P > 0.05).
The loss of RGS12 in macrophages attenuates the cartilage destruction and inflammation in OA mouse models
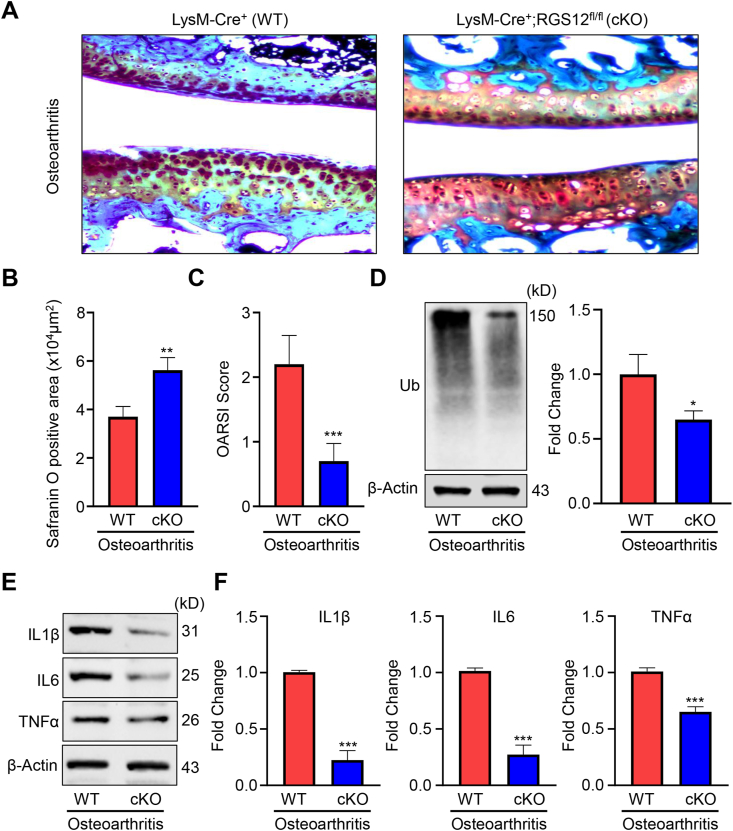
To further explore the effect of RGS12 in OA, the surgical OA model was created in 8-week old WT and RGS12 cKO mice through the anterior cruciate ligament transection (ACLT), and then mice were harvested at 4 weeks following the ACLT. Histological analysis of OA was performed by safranin O staining of the cartilage (Fig. 2A). The ACLT in WT group resulted in cartilage destruction, massive proteoglycan loss, and apparent hypocellularity compared to the RGS12 cKO group (Fig. 2A). Quantitative analysis was performed by evaluating the positive safranin area and the Osteoarthritis Research Society International (OARSI) score. The WT (ACLT) group showed a less positive safranin area and higher OARSI score compared to RGS12 cKO (ACLT) group (Fig. 2B, C). To further confirm the effect of RGS12 in OA, we also conducted the collagenase induced osteoarthritis mouse models (CIOA) and found the loss of RGS12 in macrophages (RGS12 cKO) attenuates the cartilage destruction by indicating the more positive safranin area and decreased OARSI score (Fig. S1A, B). Recently, the UPS was proved to be involved in regulating inflammatory diseases such as OA.11 To identify whether RGS12 is involved in the regulation of ubiquitination, we extracted the synovial tissues from the OA or CIOA models of WT and RGS12 cKO mice and performed immunoblotting. As shown in Figure 2D and S1C, RGS12 cKO resulted in a significant reduction of ubiquitin protein levels in synovial tissues (Fig. 2D, S1C). Moreover, the expression levels of the proinflammatory factors including IL1β, IL6, and TNFα were decreased in RGS12 cKO synovial tissues during OA (Fig. 2E, F, S1).
Figure 2.
The loss of RGS12 in macrophages attenuates the cartilage destruction and inflammation in OA mouse models. (A) Safranin O staining of the cartilage from WT and RGS12 cKO groups at 4 weeks post-surgery. (B,C) The safranin O positive area and cartilage OARIS scores were analyzed as depicted in (A). The data in the figures represent the mean ± SEM. Significant differences between WT and RGS12 cKO groups are indicated as ∗∗P < 0.01, ∗∗∗P < 0.001, vs. WT group, n = 5. (D) The ubiquitin (Ub) levels were detected by immunoblotting in synovial tissues lysis from (A). Quantitative analysis of Ub level was depicted on the right. The data are represented as means ± SEM. ∗P < 0.05, n = 3. (E) Western blot analysis of the levels of IL1β, IL6, and TNFα in the synovial tissues from (A). β-Actin was used as a loading control. (F) Quantitative analysis of the Western blots in Figure 1E. The data are represented as means ± SEM. ∗∗∗P < 0.001, n = 3.
RGS12 promotes ubiquitination and IκB degradation in macrophages
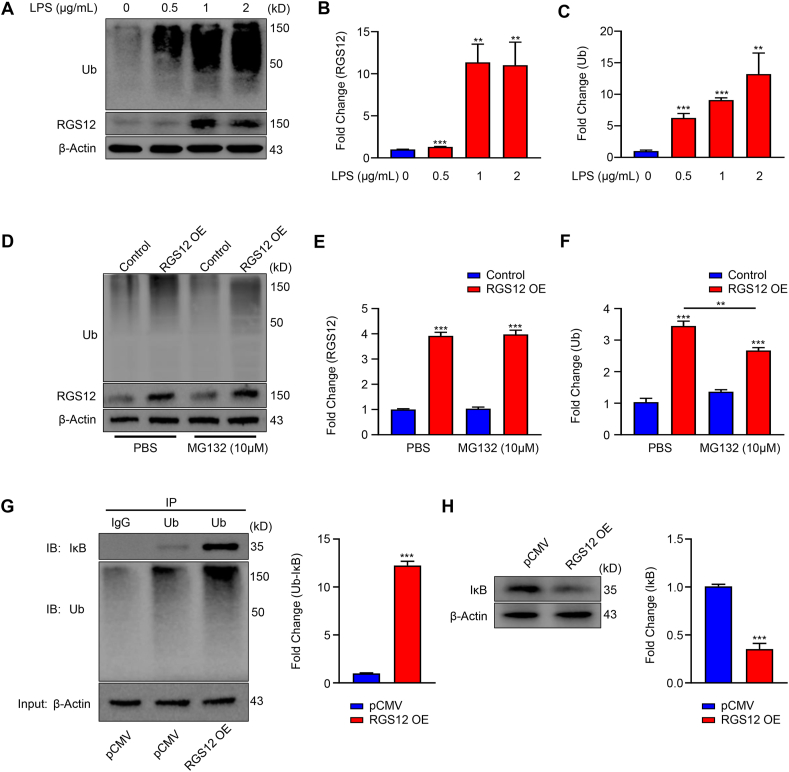
To get further insights into the potential association, we treated the RAW264.7 cells with LPS at indicated different doses (Fig. 3A). We found that LPS upregulated the expression levels of RGS12 and ubiquitin protein in a dose-dependent manner (Fig. 3B, C).
Figure 3.
RGS12 promotes ubiquitination and IκB degradation in macrophages. (A) Increased levels of ubiquitin (Ub) and RGS12 in LPS-treated macrophages. Protein expression levels of Ub and RGS12 following treatment of LPS with different doses (0, 0.5, 1, and 2 μg/mL) in RAW264.7 cells for 24 h. (B) Quantitative analysis of RGS12 level in (A). β-Actin as an internal control. Data are mean ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001 versus RGS12 fl/fl group, (n = 3). (C) Quantitative analysis of Ub level in (A). β-Actin as an internal control. Data are mean ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001 versus control group (LPS, 0 μg/mL). The representative of three experiments. (D) RAW264.7 cells were transfected with pCMV control and pCMV-RGS12 plasmids (RGS12 OE) for 24 h. Then the cells were treated with PBS and MG132 (10 μm) for 12 h. The Ub and RGS12 protein levels were detected by Western blot. (E) The RGS12 protein level was quantitatively analyzed as depicted in (D). ∗∗∗P < 0.001 versus Control group (pCMV OE), (n = 3). (F) The Ub protein level was quantitatively analyzed as depicted in (D). β-Actin as an internal control. Note that RGS12 increases the Ub levels while MG132 could inhibit this process. Data are mean ± SEM. ∗∗P < 0.01 and ∗∗∗P < 0.001, n = 3. (G) RGS12 promotes the interaction of Ub and IκB in macrophages. The RAW264.7 cells were transfected with pCMV or pCMV-RGS12 plasmids for 48 h with Lipofectamine 3000 (ThermoFisher, US). The cell lysates of macrophages were incubated with anti-Ub or control immunoglobulin G (IgG) antibody, and bound protein was examined by Western blotting with the corresponding antibodies. The level of Ub associated IκB (Ub-IκB) was analyzed in the right panel. Note that the overexpression of RGS12 can promote the combination of Ub and IκB. ∗∗∗P < 0.001 versus pCMV group, (n = 3). (H) RAW264.7 cells were transfected with pCMV control and pCMV-RGS12 plasmids (RGS12 OE) for 48 h. The IκB protein levels were detected by Western blot. Note that the overexpression of RGS12 enhances the degradation of IκB in macrophages. ∗∗∗P < 0.001 versus pCMV group, (n = 3).
To determine whether RGS12 regulates ubiquitin levels in macrophages, we transfected the RAW264.7 cells with pCMV (3 μg) and pCMV-RGS12 (3 μg) plasmids for 24 h. Then the cells were incubated with MG132 for 12 h for Western blot analysis (Fig. 3D). The results showed RGS12 overexpression (OE) significantly enhanced the ubiquitin level in macrophages which was significantly inhibited by MG132 (proteasome inhibitor) treatment (Fig. 3E, F). This suggested that MG132 could suppress RGS12-induced ubiquitination.
Ubiquitin targets IκB for degradation and then activates NF-κB and its downstream signaling pathways.16 To further explore the relationship between RGS12 and IκB, we performed the IP experiments. The results showed the ubiquitinated-IκB was significantly increased in RGS12 OE group (Fig. 3G). Moreover, the total IκB levels were decreased in the RGS12 OE group (Fig. 3H). These results indicated that RGS12 promotes ubiquitination to degrade the IκB in macrophages.
The loss of RGS12 inhibits ubiquitination and promotes molecule metabolic process
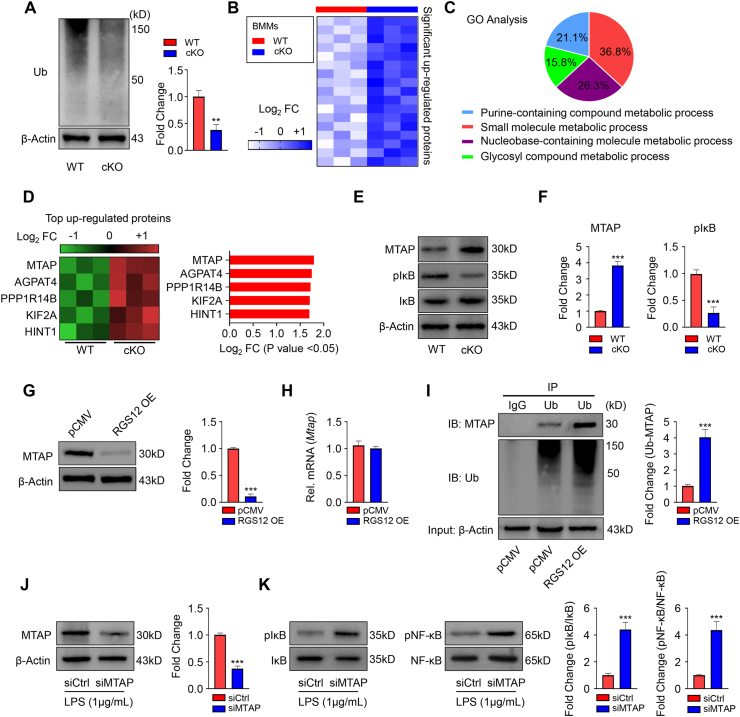
To further understand the mechanism of RGS12, we collected the BMMs from the WT and RGS12 cKO mice. We first detected the ubiquitin level and found that was significantly decreased in the RGS12 cKO BMMs in comparison to WT (Fig. 4A). We then performed the liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based quantitative proteomics strategy to profile the dynamics in the global proteins in WT and RGS12 cKO BMMs and identified the most significant up and down-regulated proteins (Fig. S2 and Table S2). Within this dataset, we identified 55 unique proteins that were significantly changed in RGS12 cKO BMMs relative to control. Among these proteins, 17 proteins were found up-regulated in RGS12 cKO BMMs (Fig. 4B and Table S2).
Figure 4.
The loss of RGS12 inhibits ubiquitination and promotes molecule metabolic process. (A) Immunoblot showed the Ub protein in WT and RGS12 cKO BMMs. Quantitative data showed the relative levels of Ub. A t test showed significant differences between the two groups, ∗∗P < 0.01 versus WT. Data are representative of three separate experiments. (B) Heatmap depicting the proteins which were up-regulated in RGS12 KO BMMs as compared to control cells. Optimized cutoff thresholds for significantly altered proteins was set at 1.3 log2-transformed ratios and P < 0.05, (n = 3). (C) Gene ontology (GO) enrichment analysis to identify biological processes corresponding to the significantly altered proteins. The percentage of significant biological processes was shown in the pie chart with various colors. (D) Heatmap and bar graph depicting the top 5 significant up-regulated proteins as depicted in (B). (E) Immunoblot showed the MTAP, pIκB, IκB, and β-Actin protein levels in WT and RGS12 cKO BMMs. (F) Quantitative data showed the MTAP and pIκB expression as depicted in (E). A t test showed significant differences between the two groups, ∗∗P < 0.001 versus WT. Data are representative of three separate experiments. (G) RAW264.7 cells were transfected with pCMV control and pCMV-RGS12 plasmids (RGS12 OE) for 48 h. The MTAP protein levels were detected by Western blot. Note that the overexpression of RGS12 promotes the degradation of MTAP in macrophages. ∗∗∗P < 0.001 versus pCMV group, (n = 3). (H) Gene expression levels of MTAP in the macrophages as described in (G). Data are reported as means ± SEM (n = 5). (I) RAW264.7 cells were transfected with pCMV or pCMV-RGS12 plasmids for 48 h with Lipofectamine 3000 (ThermoFisher, US). The cell lysates of those cells were incubated with anti-Ub or control immunoglobulin G (IgG) antibody, and bound protein was examined by Western blotting with the corresponding antibodies. The level of Ub associated MTAP (Ub-MTAP) was analyzed. Note that the overexpression of RGS12 enhanced the combination of Ub and MTAP. ∗∗∗P < 0.001 versus pCMV group, (n = 3). (J) The BMMs from WT mice were transfected with siCtrl and siMTAP for 24 h and treated with LPS (1 μg/mL) for 24 h. The MTAP protein level was determined by Western blot. Quantitative analysis of the MTAP level was shown on the right panel. The data are represented as means ± SEM. ∗∗∗P < 0.001, n = 3. (K) The levels of pIκB, IκB, pNF-κB, and NF-κB in BMM lysis were detected by immunoblotting as described in (J). Quantitative analyses were shown on the right panel. Note that reduced MTAP enhanced the expression of pNF-κB and pIκB. ∗∗∗P < 0.001, n = 3.
Proteins were considered significantly changed if they exceeded the empirically determined threshold set at P < 0.05. To analyze the biological significance of these changed proteins, we performed gene ontology (GO) analysis to identify the biological functions (Fig. 4C). The analysis revealed several biological functions related to the metabolic process that were impacted by RGS12 deletion including purine-containing compound metabolic process, small molecule metabolic process, nucleobase-containing molecule metabolic process, and glycosyl compound metabolic process (Fig. 4C). We identified the top 5 up-regulated proteins including MTAP, AGPAT4, PPP1R14B, KIF2A, and HINT1 in RGS12 cKO BMMs (Fig. 4D). MTAP can phosphate the downstream MTA to further inhibit the IκB activation.37 We found that the MTAP was increased and pIκB was decreased in the RGS12 cKO BMMs by immunoblotting assay (Fig. 4E, F). To determine the relationship between RGS12 and MTAP, we overexpressed the RGS12 in RAW264.7 cells and found RGS12 decreases the protein levels of MTAP but does not affect its transcriptional expression (Fig. 4G, H). We then performed the IP experiments to further explore whether RGS12 affects MTAP degradation (Fig. 4I). The results showed the ubiquitinated-MTAP was significantly increased in RGS12 OE group (Fig. 4I). These results indicated that RGS12 also promotes the degradation of MTAP through ubiquitination in macrophages. To further determine whether MTAP regulates the activation of NF-κB signaling under inflammatory conditions, BMMs were transfected with MTAP siRNAs and then treated with LPS for 24 h to knock down MTAP (Fig. 4J). We found that the knockdown of MTAP further promoted the expressions of pIκB and pNF-κB under inflammatory conditions (Fig. 4K).
MG132 inhibits the activation of NF-κB caused by RGS12
RGS12 is increased and promotes the activation of NF-κB in macrophages under inflammatory conditions.20 Under normal conditions, we found the loss of RGS12 in BMMs only mildly decreases the expressions of pIκB and pNF-κB, the nuclear translocation activity of NF-κB, and the expressions of proinflammatory cytokines (Fig. S3, P > 0.05). However, the loss of RGS12 in BMM significantly reduced the expression levels of Ub levels, pIκB and pNF-κB, IL1β, and IL6 after stimulating the cells with LPS for 24 h (Fig. S4).
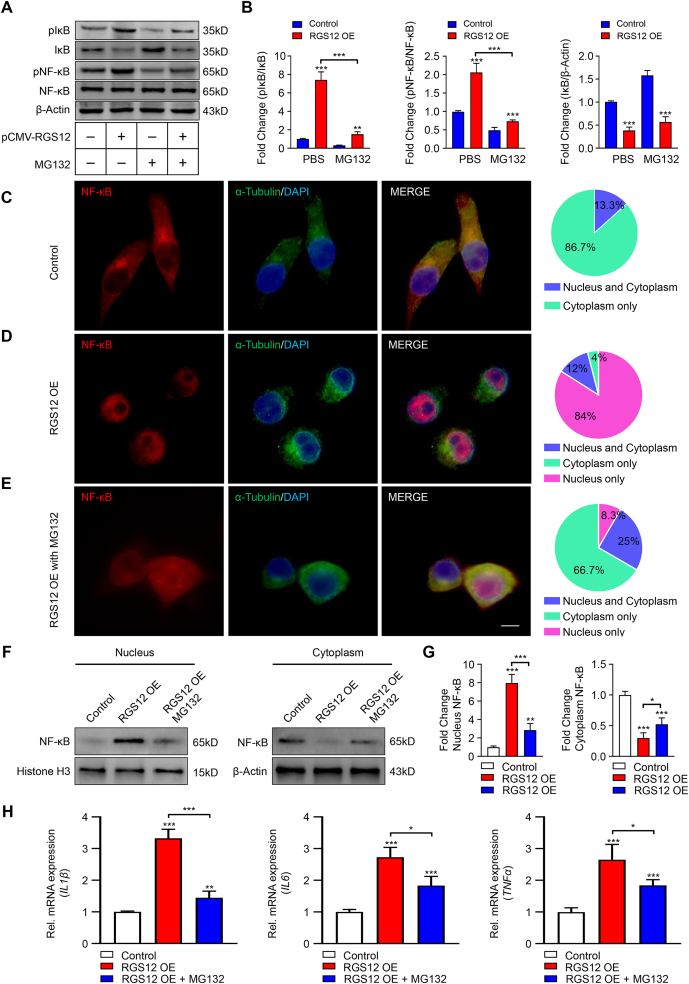
To determine whether MG132 inhibits the RGS12-induced activation of IκB and NF-κB, we transfected the BMMs with pCMV or pCMV-RGS12 for 24 h and treated the cells with MG132 for 12 h (Fig. 5A). The results showed MG132 could inhibit pIκB/pNF-κB expression and IκB degradation (Fig. 5A, B). IκB degradation promotes the nuclear translocation of NF-κB (p65). In comparison to control, the NF-κB translocates into the nucleus in RGS12 OE macrophages (Fig. 5C–E). However, MG132 inhibits the nuclear translocation of NF-κB (p65) caused by the RGS12 OE (Fig. 5C–E). We further confirmed the nuclear translocation of NF-κB in control, RGS12 OE, and RGS12 OE with MG132 conditions by Western blot analysis (Fig. 5F, G). Consistently, RGS12 OE promotes the nuclear translocation of NF-κB (p65) whereas MG132 can inhibit NF-κB translocation caused by RGS12 OE (Fig. 5F, G). Moreover, by analyzing the downstream genes of NF-κB such as IL1β, IL6, and TNFα, we found that MG132 inhibits IL1β, IL6 and TNFα mRNA expression caused by RGS12 OE in macrophages (Fig. 5H). These results suggest that MG132 blocks the activation of NF-κB (p65) signaling pathways induced by RGS12.
Figure 5.
MG132 inhibits the activation of NF-κB caused by RGS12. (A) BMMs were transfected with pCMV-RGS12 or pCMV control for 48 h and treated with MG132 for 12 h. The protein levels of pIκB, IκB, pNF-κB, NF-κB, and β-Actin were measured by immunoblotting assay. (B) The ratio of pIκB/IκB, pNF-κB/NF-κB, and IκB/β-Actin in (A) were calculated. β-Actin as an internal control. Note that the MG132 could inhibit the pIκB and pNF-κB activation induced by RGS12 in macrophages. Data are mean ± SEM. ∗∗∗P < 0.001 and ∗∗P < 0.01 versus WT group, (n = 3). (C, D) The RAW264.7 cells were transfected with pCMV (Control) (C) and pCMV-RGS12 (RGS12 OE) (D) for 24 h. Immunofluorescence showing that the nuclear translocation of NF-κB (p65) in control and RGS12 OE group (Red, NF-κB (p65); Green, α-Tubulin; Blue, DAPI; bar = 5 μm). Parts of the whole table showed the location of NF-κB (Blue, nucleus and cytoplasm, green, cytoplasm only and purple, nucleus only). (E) The RAW264.7 cells were transfected with pCMV-RGS12 (RGS12 OE) and added MG132 for 24 h. Immunofluorescence showing that the nuclear translocation of NF-κB (p65) in RGS12 OE with MG132 group (Red, NF-κB (p65); Green, α-Tubulin; Blue, DAPI; bar = 5 μm). The pie chart table showed the location of NF-κB (Blue: nucleus and cytoplasm, green: cytoplasm only and purple: nucleus only). (F, G) The RAW264.7 cells were transfected with pCMV (Control) or pCMV-RGS12 (RGS12 OE) with or without MG132 for 24 h. Immunoblots showing the expression of NF-κB in the nucleus and cytoplasm. Data are mean ± SEM. ∗∗∗P < 0.001, ∗∗P < 0.01, and ∗P < 0.05, (n = 3). (H) Real-time PCR analysis for the expression of TNFα, IL6, and IL1β in Control, RGS12 OE and RGS12 OE + MG132 groups as depicted in (F). Data are presented as the mean ± SEM. ∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001 versus the control group (n = 5).
Discussion
The pathogenesis of OA is associated with mechanical, inflammatory and metabolic factors, which finally leads to structural destruction.3 New insights indicated that synovial macrophages have a large impact on the pathogenesis of OA.7 O'Brien et al38 reported that more macrophages are showed in the early stages of synovial OA than in the late stages. They also discovered that MSCs and macrophages are spatially closer to each other in normal joints than in OA cases.38 Our study also found that ablation of RGS12 in macrophages can protect the cartilage destruction in OA by inhibiting the expression of proinflammatory factors such as IL1β, IL6, and TNFα. These findings suggest that RGS12 regulation in macrophages plays important role in OA pathogenesis and RGS12 may be a promising target for future therapeutic advances.
The ubiquitin-proteasome pathway plays a pivotal role in the pathways of NF-κB.16 Ubiquitin is first activated by a ubiquitin-activating enzyme (E1) and then transferred to a ubiquitin-conjugating enzyme (E2 or Ubc). Finally, in the presence of a ubiquitin-protein ligase (E3), ubiquitin is attached to a target protein to promote protein degradation. Upon stimulation, IκB is phosphorylated and subsequently ubiquitinated and degraded by 26S proteasome, thus allowing NF-κB to translocate to the nucleus, where it regulates the expression of a plethora of genes.39 Interestingly, it has been shown that IL-1-induced NF-κB activation is dependent on Ubc13, which is highly specific in synthesizing K63-linked polyubiquitin.40 In our study, we found RGS12 could affect the LPS induced ubiquitination and IκB degradation. Forced expression of RGS12 in macrophages can promote ubiquitination and NF-κB activation. Moreover, we found that MG132 could specifically inhibit the increase of ubiquitin caused by RGS12 in the macrophages. MG132 was reported to protect the cartilage from cytokine-mediated resorption and degradation in DMM-induced OA mice.11 Inhibition of the lysine-48 linked ubiquitination is a proved protection against murine OA. Similarly, our study showed that MG132 could inhibit the NF-κB nuclear translocation caused by RGS12, and then further inhibit the expressions of inflammatory cytokines such as IL1β, IL6, and TNFα.
Interestingly, we also found that the loss of RGS12 in macrophages depicted the decreased ubiquitination while increasing the proteins related to molecular metabolism such as MTAP. Moreover, the forced expression of RGS12 drives the degradation of MTAP in macrophages. Supportively, Georgi Kirovski reported that the downregulation of MTAP can promote IL-8 and MMP1 expression and activate the NF-κB signaling.37 Similarly, we found the knockdown of MTAP can promote the NF-κB and pIκB expressions in BMMs under inflammatory conditions. These results demonstrated that RGS12 promotes NF-κB expression and activation through the degradation of MTAP. As the largest RGS protein, RGS12 contains PDZ, PTB, RGS, RBD1/2, and GoLoco motif that may act as a scaffold to associate with ubiquitin-activating enzyme or ubiquitin-protein ligase. Thus, our future study will focus on studying the potential ubiquitin-activating enzyme(s) that are regulated by RGS12.
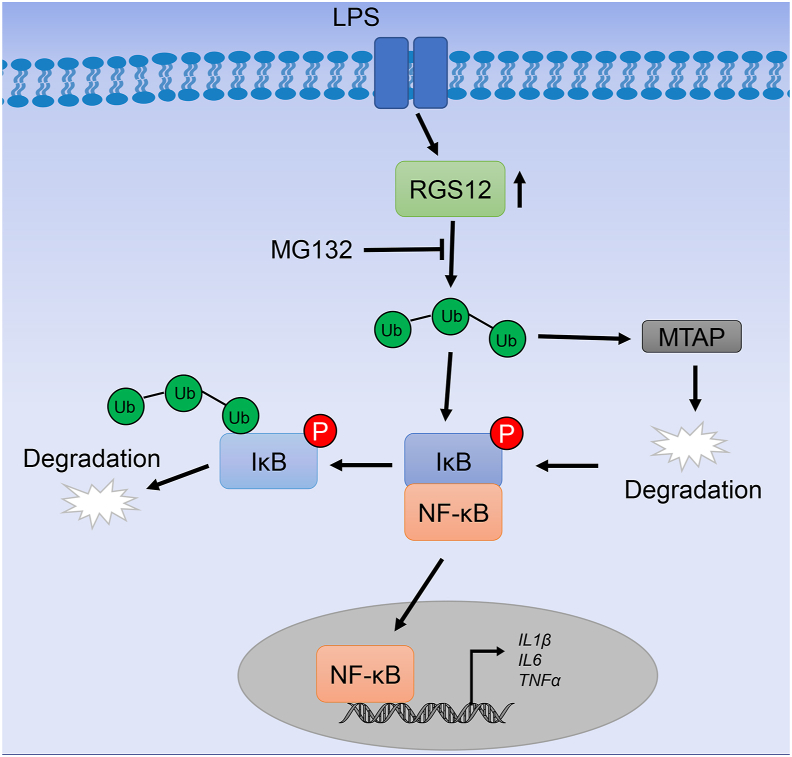
In summary, the findings of this study provide new evidence that RGS12 in macrophages acts as a ubiquitination enhancer to promote OA. During inflammation, RGS12 promotes ubiquitination and IκB degradation which further activates the nuclear translocation of NF-κB. However, deficiency of RGS12 in macrophages inhibits ubiquitination and activates MTAP to inhibit the degradation of IκB and finally inhibits the OA (Fig. 6). Therefore, this study provides the first evidence that RGS12 may become a potential target for the treatment of OA.
Figure 6.
Proposed model of RGS12 regulation of inflammation via enhancing the ubiquitination. Under inflammatory conditions such as OA, RGS12 promotes the degradation of IκB through enhancing the ubiquitination whereas the process can be inhibited by MG132. Moreover, the increased ubiquitination further degrades the MTAP protein, which can indirectly activate the phosphorylation of IκB. Finally, due to the degradation of IκB, the NF-κB translocates into the nucleus and further promotes the gene expression of cytokines such as IL1β, IL6, and TNFα during inflammation.
Author contributions
Shuying Yang and Gongsheng Yuan: conceptualization, methodology, writing-reviewing and editing. Gongsheng Yuan and Shuting Yang: validation and investigation.
Conflict of interests
The authors have no conflicts to declare.
Funding
This work was supported by grants from the National Institute on Aging (NIA) (No. AG048388); National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (No. AR066101) to S Yang. This work was also supported by grant from the Penn Center for Musculoskeletal Disorders (PCMD), NIH/NIAMS P30-AR069619.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2021.08.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 2.Barnett R. Osteoarthritis. Lancet. 2018;391(10134):1985. doi: 10.1016/S0140-6736(18)31064-X. [DOI] [PubMed] [Google Scholar]
- 3.Sokolove J., Lepus C.M. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iagnocco A., Filippucci E., Ossandon A., et al. High resolution ultrasonography in detection of bone erosions in patients with hand osteoarthritis. J Rheumatol. 2005;32(12):2381–2383. [PubMed] [Google Scholar]
- 5.Loeuille D., Chary-Valckenaere I., Champigneulle J., et al. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 2005;52(11):3492–3501. doi: 10.1002/art.21373. [DOI] [PubMed] [Google Scholar]
- 6.Sellam J., Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6(11):625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y., Jiang W., Yong H., et al. Macrophages in osteoarthritis: pathophysiology and therapeutics. Am J Transl Res. 2020;12(1):261–268. [PMC free article] [PubMed] [Google Scholar]
- 8.Takano S., Uchida K., Miyagi M., et al. Synovial macrophage-derived IL-1β regulates the calcitonin receptor in osteoarthritic mice. Clin Exp Immunol. 2016;183(1):143–149. doi: 10.1111/cei.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirayama D., Iida T., Nakase H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int J Mol Sci. 2017;19(1):92. doi: 10.3390/ijms19010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radwan M., Wilkinson D.J., Hui W., et al. Protection against murine osteoarthritis by inhibition of the 26S proteasome and lysine-48 linked ubiquitination. Ann Rheum Dis. 2015;74(8):1580–1587. doi: 10.1136/annrheumdis-2013-204962. [DOI] [PubMed] [Google Scholar]
- 12.Ebner P., Versteeg G.A., Ikeda F. Ubiquitin enzymes in the regulation of immune responses. Crit Rev Biochem Mol Biol. 2017;52(4):425–460. doi: 10.1080/10409238.2017.1325829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClellan A.J., Laugesen S.H., Ellgaard L. Cellular functions and molecular mechanisms of non-lysine ubiquitination. Open Biol. 2019;9(9):190147. doi: 10.1098/rsob.190147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suryadinata R., Roesley S.N., Yang G., Sarčević B. Mechanisms of generating polyubiquitin chains of different topology. Cells. 2014;3(3):674–689. doi: 10.3390/cells3030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y., Kang J., Zhang L., et al. Ubiquitination regulation of inflammatory responses through NF-kappaB pathway. Am J Transl Res. 2018;10(3):881–891. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z.J. Ubiquitin signalling in the NF-κB pathway. Nat Cell Biol. 2005;7(8):758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oeckinghaus A., Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1(4):a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myung J., Kim K.B., Crews C.M. The ubiquitin-proteasome pathway and proteasome inhibitors. Med Res Rev. 2001;21(4):245–273. doi: 10.1002/med.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roman D.L., Traynor J.R. Regulators of G protein signaling (RGS) proteins as drug targets: modulating G-protein-coupled receptor (GPCR) signal transduction. J Med Chem. 2011;54(21):7433–7440. doi: 10.1021/jm101572n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan G., Yang S., Ng A., et al. RGS12 is a novel critical NF-κB activator in inflammatory arthritis. iScience. 2020;23(6):101172. doi: 10.1016/j.isci.2020.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan G., Yang S., Gautam M., Luo W., Yang S. Macrophage regulator of G-protein signaling 12 contributes to inflammatory pain hypersensitivity. Ann Transl Med. 2021;9(6):448. doi: 10.21037/atm-20-5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snow B.E., Antonio L., Suggs S., Gutstein H.B., Siderovski D.P. Molecular cloning and expression analysis of rat Rgs12 and Rgs14. Biochem Biophys Res Commun. 1997;233(3):770–777. doi: 10.1006/bbrc.1997.6537. [DOI] [PubMed] [Google Scholar]
- 23.Schroer A.B., Mohamed J.S., Willard M.D., Setola V., Oestreich E., Siderovski D.P. A role for regulator of G protein signaling-12 (RGS12) in the balance between myoblast proliferation and differentiation. PLoS One. 2019;14(8):e0216167. doi: 10.1371/journal.pone.0216167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan G., Yang S., Liu M., Yang S. RGS12 is required for the maintenance of mitochondrial function during skeletal development. Cell Discov. 2020;6:59. doi: 10.1038/s41421-020-00190-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weischenfeldt J., Porse B. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot5080. pdb prot5080. [DOI] [PubMed] [Google Scholar]
- 26.Tawonsawatruk T., Sriwatananukulkit O., Himakhun W., Hemstapat W. Comparison of pain behaviour and osteoarthritis progression between anterior cruciate ligament transection and osteochondral injury in rat models. Bone Joint Res. 2018;7(3):244–251. doi: 10.1302/2046-3758.73.BJR-2017-0121.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glasson S.S., Blanchet T.J., Morris E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9):1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Cook A.D., Pobjoy J., Steidl S., et al. Granulocyte-macrophage colony-stimulating factor is a key mediator in experimental osteoarthritis pain and disease development. Arthritis Res Ther. 2012;14(5):R199. doi: 10.1186/ar4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang H., Zhang M., Wang X., et al. TNF accelerates death of mandibular condyle chondrocytes in rats with biomechanical stimulation-induced temporomandibular joint disease. PLoS One. 2015;10(11):e0141774. doi: 10.1371/journal.pone.0141774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamekura S., Hoshi K., Shimoaka T., et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005;13(7):632–641. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Ng A.Y.H., Li Z., Jones M.M., et al. Regulator of G protein signaling 12 enhances osteoclastogenesis by suppressing Nrf2-dependent antioxidant proteins to promote the generation of reactive oxygen species. Elife. 2019;8:42951. doi: 10.7554/eLife.42951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan G., Xu L., Cai T., et al. Clock mutant promotes osteoarthritis by inhibiting the acetylation of NFκB. Osteoarthritis Cartilage. 2019;27(6):922–931. doi: 10.1016/j.joca.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Yuan G., Hua B., Cai T., et al. Clock mediates liver senescence by controlling ER stress. Aging (Albany NY) 2017;9(12):2647–2665. doi: 10.18632/aging.101353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bondeson J., Blom A.B., Wainwright S., Hughes C., Caterson B., van den Berg W.B. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62(3):647–657. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H., Lin C., Zeng C., et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann Rheum Dis. 2018;77(10):1524–1534. doi: 10.1136/annrheumdis-2018-213450. [DOI] [PubMed] [Google Scholar]
- 36.Shi J., Hua L., Harmer D., Li P., Ren G. Cre driver mice targeting macrophages. Methods Mol Biol. 2018;1784:263–275. doi: 10.1007/978-1-4939-7837-3_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirovski G., Stevens A.P., Czech B., et al. Down-regulation of methylthioadenosine phosphorylase (MTAP) induces progression of hepatocellular carcinoma via accumulation of 5′-deoxy-5′-methylthioadenosine (MTA) Am J Pathol. 2011;178(3):1145–1152. doi: 10.1016/j.ajpath.2010.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Brien K., Tailor P., Leonard C., et al. Enumeration and localization of mesenchymal progenitor cells and macrophages in synovium from normal individuals and patients with pre-osteoarthritis or clinically diagnosed osteoarthritis. Int J Mol Sci. 2017;18(4):774. doi: 10.3390/ijms18040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins P.E., Mitxitorena I., Carmody R.J. The ubiquitination of NF-κB subunits in the control of transcription. Cells. 2016;5(2):23. doi: 10.3390/cells5020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J., Chen Z.J. Regulation of NF-κB by ubiquitination. Curr Opin Immunol. 2013;25(1):4–12. doi: 10.1016/j.coi.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.