To the Editor:
Neural tube defects (NTDs) are among the most common human birth defects affecting about 1/1000 live births worldwide.1 The etiology of NTDs is attributed to complex genetic and environmental risk factors. Over 200 genes have been identified to cause NTDs in animal models, suggesting the involvement of distinct molecular basis in NTD etiology, including the Sonic hedgehog (Shh) signaling. Recently, several negative regulators of Shh pathway null mutants displayed severe NTDs, indicating abnormal activation of Shh signaling is commonly associated with NTDs.2 Tubby-like protein 3 (Tulp3) is a typical repressor of Shh signaling and knock out Tulp3 causes severe cranial and caudal NTDs in mice.3 This strongly implied that TULP3 is essential for embryonic neural tube development and that variants in TULP3 might contribute to human NTDs, which hasn't been demonstrated thus far.
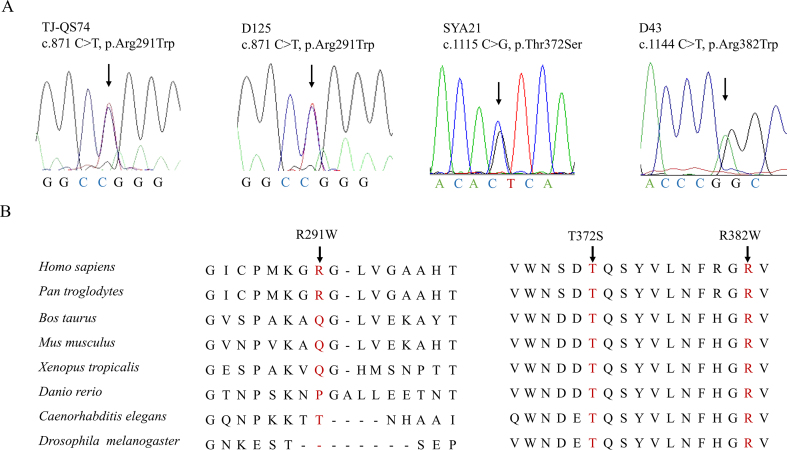
To clarify this, we conducted next generation sequencing in a Chinese NTDs cohort including 352 patients and 224 matched healthy controls to screen TULP3 variants. We identified three case-specific rare variants: c.871C > T (p. Arg291Trp), c.1115C > G (p. Thr372Ser) and c.1144C > T (p. Arg382Trp) in TULP3, which only occurred in NTDs patients and were totally absent in our controls. The clinical phenotypes of variants carriers were listed in Table S1. All these three variants were heterozygous and verified by Sanger sequencing (Fig. S1A). They were all located in the C-terminal tubby domain of TULP3 (Fig. 1A). Sequences alignment implied that variant p. Thr372Ser and p. Arg382Trp were highly conserved among spices, even in invertebrates, while variant p. Arg291Trp wasn't conserved (Fig. S1B). In silico predictions based on SIFT, PolyPhen2, PROVEAN and Mutation Taster uniformly suggested that variant p. Arg382Trp was probably deleterious to TULP3 function. This variant had a frequency of 1.6e-05 in the gnomAD database (Table S2). Its distribution in our NTDs group was significantly different from that in the general population (1/352 vs. 1.6e-05, P = 0.007, Fisher's exact test).
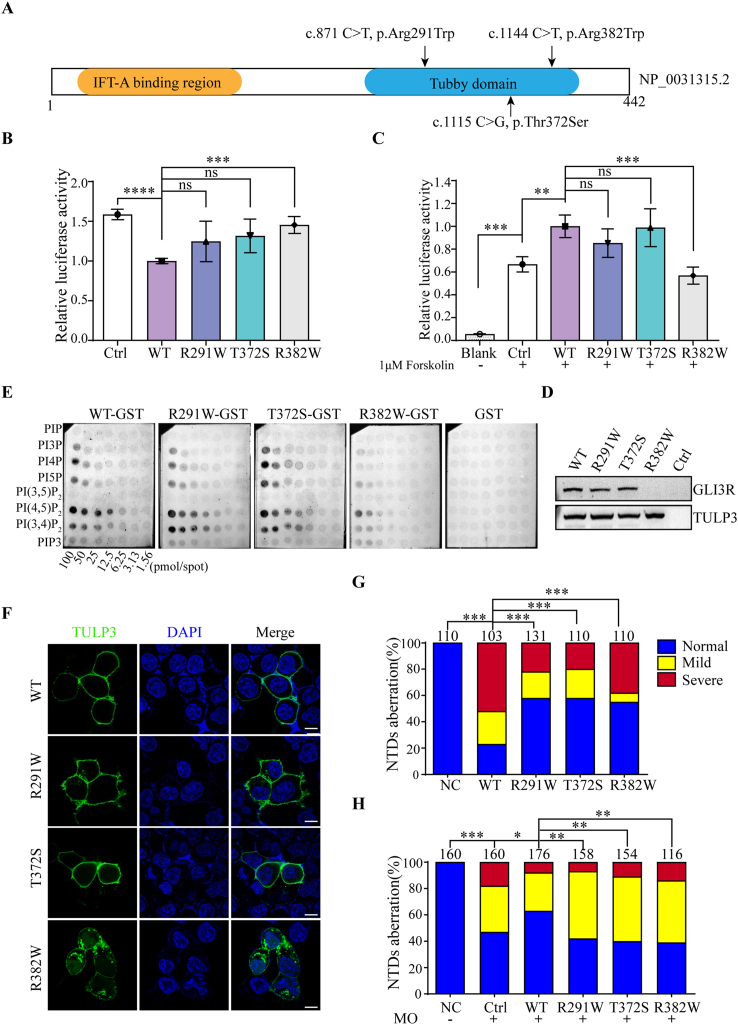
Figure 1.
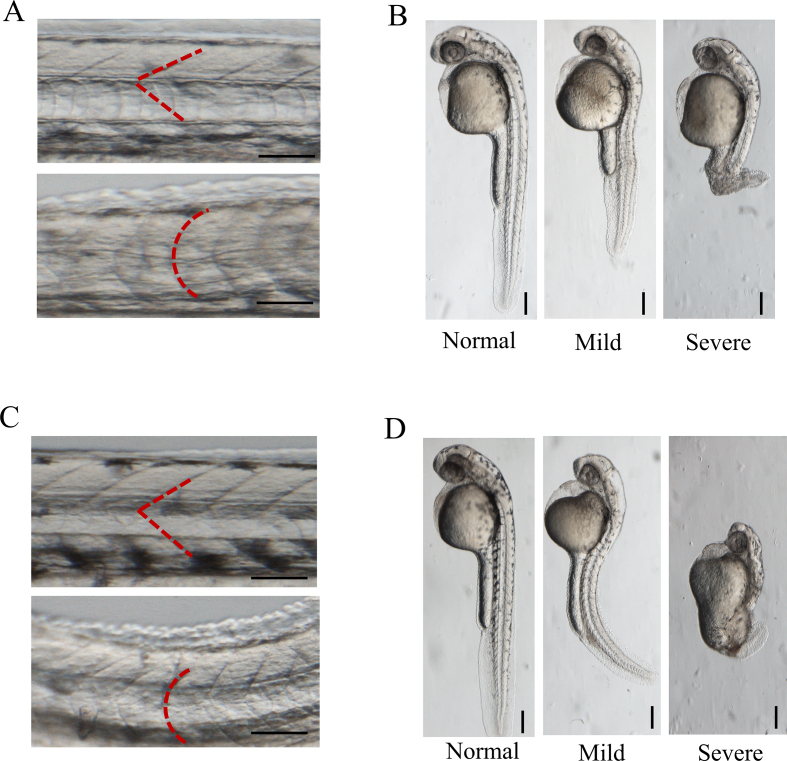
Functional analysis of rare TULP3 variants identified from human neural tube defects patients. (A) Linear structure diagram of human TULP3 protein. The N-terminal IFT-A binding domain and C-terminal tubby domain are shown in orange and blue, respectively. Distributions of three variants in TULP3 protein were marked with arrows above the diagram. (B) Variant p. Arg382Trp significantly lost its suppression of Shh signaling. HEK293T cells were co-transfected with GLI-responsive luciferase reporter and empty vector (Ctrl), wild-type or mutant pCMV6-Myc-TULP3 plasmids. Twenty-four hours later, the cells were harvested and lysed for the luciferase assay. (C) Variant p. Arg382Trp significantly decreased cAMP levels. A cAMP-responsive luciferase reporter was co-transfected with empty vector (Ctrl), wild-type or mutant pCMV6-Myc-TULP3 plasmids into HEK293T cells. At 24 h post-transfection, cells were treated with 1 μM forskolin or not as indicated for another 1 h. (D) Variant p. Arg382Trp reduced the formation of GLI3R. Empty vector, wild-type or mutant pCMV6-Myc-TULP3 plasmids were transfected into HEK293T cells. Endogenous GLI3R was detected by Western blotting. All experiments were performed for three independent times, and representative results are shown as the mean ± SD. The statistical analysis used an unpaired two-tailed t-test. Asterisks above horizontal lines indicated a statistically significant difference (ns, not significant, P > 0.05, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001). (E) Variant p. Arg382Trp decreased its affinity to PtdIns(4,5)P2 and dissociated from the membrane in PIP array assay. Membranes with PtdInsPs dots in gradient concentrations were used to test the affinity of the GST-TULP3 fusion protein towards PtdInsPs. GST protein was used as a negative control. The darker dots indicate higher affinity between PtdInsPs and GST-TULP3 fusion protein. (F) Variant p. Arg382Trp resulted in subcellular localization change of TULP3. Confocal microscopy of wild-type or mutant pCMV6-EGFP-TULP3 plasmids transfected HEK293T cells at 24 h post-transfection with 63 × oil lenses. GFP (green) and DAPI (blue) represent TULP3 and nucleus, respectively. Scale bar: 10 μm (bottom white lines). (G, H) Human TULP3 variants caused severe somite development defects in zebrafish. (G) Distribution of the three categories in each group in overexpression assay. For overexpression, embryos were injected with wild-type or mutant human TULP3 mRNAs, respectively. (H) Distribution of the three categories in each group in Tulp3-MO knock down and rescue assay. To knock down endogenous Tulp3, 8 ng of Tulp3-MO was injected into each embryo. For rescue, 100 pg of wild-type or mutant human TULP3 mRNAs and 8 ng of Tulp3-MO were co-injected into each embryo. Zebrafish embryos were clustered into three categories, namely normal, mild and severe according to the severity of defects in somite shape. The distribution of the three categories in the indicated groups was calculated. The number above each column indicated the total number of embryos counted. Scale bars are 200 μm. Statistical significance (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001) was evaluated by χ2 analysis in GraphPad Prism and shown above the horizontal lines. NC, un-injected group serves as the negative control; hpf, hour post fertilization.
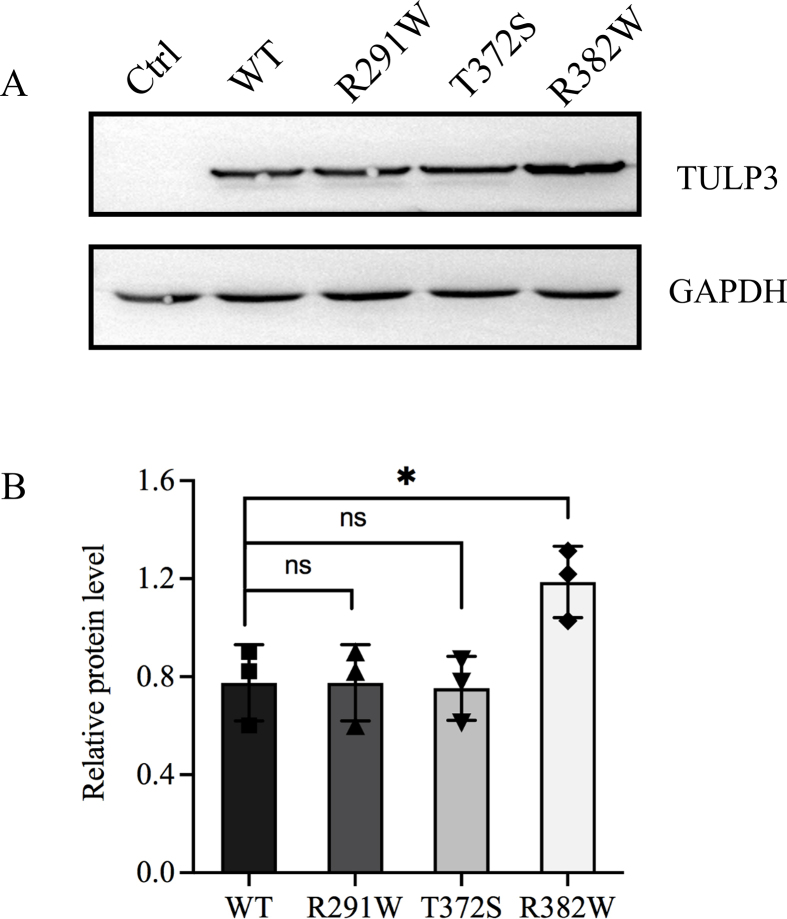
Then we performed in vitro and in vivo functional assays on these variants. Western blot analysis indicated that variant p. Arg382Trp significantly increased the protein level compared with wild-type, while the other two variants showed no difference (Fig. S2). Since TULP3 functions as a negative regulator of Shh pathway, we next determined the effect of TULP3 variants on Shh signaling using a GLI-responsive luciferase reporter. As expected, wild-type TULP3 could significantly repress Shh activity. Variant p. Arg382Trp dramatically lost inhibition on Shh signaling, while the other two variants were not significantly different from wild-type (Fig. 1B). Based on previous study that TULP3/IFT-A regulated GPCR repressed Shh signaling by modulating cAMP signaling,4 we suspected that variants in TULP3 may disrupt the downstream signaling transduction. Subsequent measurement verified this. As shown in Figure 1C, variant p. Arg382Trp notably decreased the downstream cAMP level compared to the wild-type. In addition, we detected the process of endogenous GLI3R in wild-type or mutant TULP3-transfected HEK293T cells. Consistent with the cAMP results, variant p. Arg382Trp led to a defect in GLI3R formation, while the other two variants acted as wild-type TULP3 (Fig. 1D). These combined results may account for the hyper-activated Shh activity in variant p. Arg382Trp.
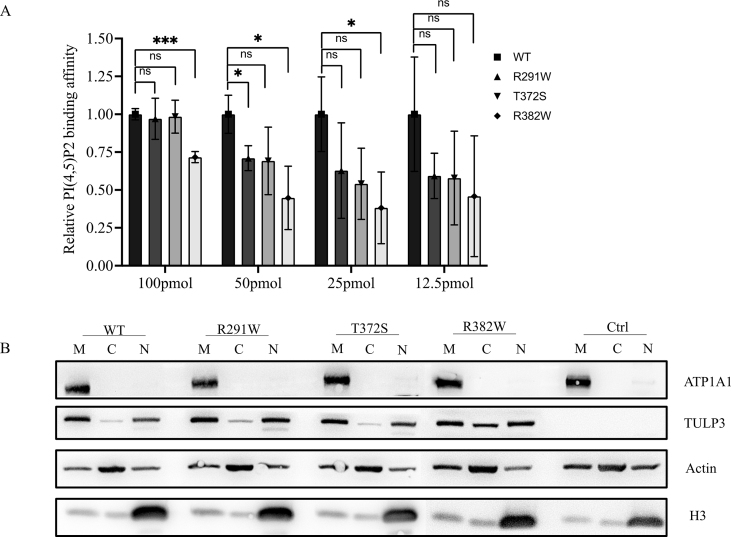
The interaction between the TULP3 tubby domain and PtdInsPs directs attachment of TULP3 to the membrane and Shh signaling regulation. Since the variant p. Arg382Trp is situated in the tubby domain, we performed a PIP array assay to evaluate the affinity between TULP3 variants and PtdInsPs. As results showed, wild-type tubby domain could bind to PtdIns(4,5)P2 well, consistent with a previous study.5 With the concentration of PtdIns(4,5)P2 decreasing from 100 pmol/spot to 25 pmol/spot, the interactions between variant p. Arg382Trp and PtdIns(4,5)P2 were significantly weakened compared with the wild-type. The other two variants attenuated interactions with PtdIns(4,5)P2 to certain degrees, while statistic differences weren't significant. At the concentration of 12.5 pmol/spot, all variants had a similar affinity to PtdIns(4,5)P2 as the wild-type TULP3 (Fig. 1E, S3A).
We also observed the subcellular localization of TULP3, which may be disturbed by the impairment of interaction between TULP3 and membrane PtdIns(4,5)P2, in cultured HEK293T cells 24 h post-transfection with confocal microscopy. Surprisingly, the variant p. Arg382Trp exhibited obvious distribution in cytoplasm, while the wild-type TULP3 was entirely localized on the plasma membrane. There was no visible change for the other two variants (Fig. 1F). To obtain a better understanding of the appearance of variant p. Arg382Trp in cytoplasm, we segregated wild-type and mutant TULP3 transfected cells into membrane, cytoplasmic and nuclear fractions at 36 h after transfection for Western blot analysis. We found that wild-type TULP3 was prominently distributed in both membrane and nuclear fractions, but rarely distributed in the cytoplasm, in line with a previous study.5 Moreover, there was also a distinct retention of variant p. Arg382Trp in the cytoplasm, consistent with our confocal results (Fig. S3B).
To elucidate the underlying pathogenic effect of TULP3 variants in vivo, we performed zebrafish microinjections. In the overexpression assay, the somite development of TULP3-injected embryos was impaired mimicking the dysregulation of Shh signaling. They displayed unique U-shaped somites (Fig. S4A, lower panel) and shortened body axises (Fig. S4B), which were different from the characteristic chevron-shaped somites in uninjected group (Fig. S4A, upper panel). We found that injection of wild-type human TULP3 mRNA caused a significant deformity ratio comparing with the uninjected group, and the deformity ratios in variants-injected groups reduced to varying degrees compared with wild-type group (Fig. 1G). This suggested that these three variants were loss-of-function. In the rescue experiments, we first knocked down endogenous Tulp3 expression with zebrafish Tulp3-MO. Aberrant somite development was also found in MO-injected group (Fig. S4C, D). Then, a mixture of Tulp3-MO and wild-type or mutant human TULP3 mRNAs were injected into embryos to rescue. As shown in Figure 1H, wild-type TULP3 could partially reverse the defects due to Tulp3-MO knockdown. In contrast, these three variants did not behave well as wild-type. Taken together, we speculated that all three variants manifested as loss-of-function during zebrafish embryo development.
Our finding provided the evidence that TULP3 variant was associated with human NTDs and variant p. Arg382Trp may contribute to the etiology of human NTDs via abolishing suppression on Shh signaling.
Author contributions
L.K and X.Y designed the study. L.K conducted experiments, collected data and wrote the original draft of manuscript. Y.J performed zebrafish experiments. S.C, K.S and R.P participated in cell experiment and samples collection. X.Y and H.W revised the manuscript, supervised the project and provided funding support. All authors reviewed and approved the final version.
Conflict of interests
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2021.11.010.
Contributor Information
Xueyan Yang, Email: xueyanyang@fudan.edu.cn.
Hongyan Wang, Email: wanghy@fudan.edu.cn.
Funding
This work was supported by National Key Research and Development Program, China (No. 2016YFC1000502 to H.W. and X.Y.); the National Natural Science Foundation of China (No. 81930036, 31521003 and 31771669 to H.W.); and the Basic Research Project of “Innovation Action Plan” from Shanghai Science and Technology Commission Shanghai Municipality, China (No. 17JC1400902 to H.W.)
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
References
- 1.Copp A.J., Adzick N.S., Chitty L.S., Fletcher J.M., Holmbeck G.N., Shaw G.M. Spina bifida. Nat Rev Dis Primers. 2015;1:15007. doi: 10.1038/nrdp.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murdoch J.N., Copp A.J. The relationship between sonic Hedgehog signaling, cilia, and neural tube defects. Birth Defects Res A Clin Mol Teratol. 2010;88(8):633–652. doi: 10.1002/bdra.20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norman R.X., Ko H.W., Huang V., et al. Tubby-like protein 3 (TULP3) regulates patterning in the mouse embryo through inhibition of Hedgehog signaling. Hum Mol Genet. 2009;18(10):1740–1754. doi: 10.1093/hmg/ddp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukhopadhyay S., Wen X., Ratti N., et al. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell. 2013;152(1–2):210–223. doi: 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 5.Santagata S., Boggon T.J., Baird C.L., et al. G-protein signaling through tubby proteins. Science. 2001;292(5524):2041–2050. doi: 10.1126/science.1061233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.