Abstract
Elucidating the pharmaceutical mechanisms behind traditional Chinese medicine (TCM) is the key to promote its modernization process. In China, soaking TCM in liquor has a history of thousands of years, and many TCMs have to be processed into liquor before they can be used to treat diseases. Chinese liquor (Baijiu) contains more than 2,000 trace components, the interaction mechanism between TCM and Baijiu still remains unclear, making TCM a "mystery". The TCM industry commonly employs chromatographic and spectrographic technology to investigate the redox activity of TCM substances. However, only investigating the redox differences in specific active substances cannot provide a complete understanding of the redox activity of TCM substances. Thus, we employed the electrochemical approach to study the overall redox activity of substances in TCM in situ. The key result is that the redox substances in Baijiu function as a mediator for the redox reaction of Polygonum multiflorum extract. The redox efficiency of the extract is enhanced because of the faster electron transferability of the redox mediator in Baijiu.
Keywords: Traditional Chinese medicine, Polygonum multiflorum, Redox activity, Redox mediation
Traditional Chinese medicine; Polygonum multiflorum; Redox activity; Redox mediation.
1. Introduction
Traditional Chinese medicine (TCM) culture has a history of thousands of years and is a crucial cultural heritage of humankind [1, 2]. For example, artemisinin is a Chinese medicine developed on the basis of modern approaches and theories that can efficiently reduce the mortality of malaria, representing a crucial example of the modernization process of TCM [3, 4]. The modernization of TCM is an inevitable trend with the development of pharmaceutical science, and understanding the pharmaceutical mechanisms behind TCM is the key to promoting its modernization process [5].
TCM can be prepared using four approaches: cutting the pure plant into slices after natural drying [6], calcining plant tissues directly or indirectly with fire [7], extracting active ingredients from plant tissues using water [8, 9], and extracting active ingredients from plant tissues using liquids other than water [10]. The active ingredients in numerous plant tissues have very low solubility in water. Finding a suitable solvent that can dissolve these active ingredients is essential for improving the extracting efficacy of TCM [11]. Thus, the typical Chinese distilled liquor (also called Chinese Baijiu), a readily available organic solvent in ancient times, naturally became the best solvent for extracting the active ingredients of TCM [12]. In China, soaking TCM in liquor has a long history [13]; liquor was employed to soak TCM as early as the Spring and Autumn Warring States Period [14]. Many TCMs have to be processed into liquor before they can be employed for treating diseases. For example, regarding reinforcing drugs such as Rehmannia glutinosa, Cornus, and Ligustrum lucidum, soaking TCMs in liquor is critical for accomplishing the desired pharmaceutical effect [15, 16].
This study investigates the correlation between the extraction process and redox activity of active substances in TCM. Polygonum multiflorum, which is highly valued for its extensive medicinal properties, was chosen as the TCM model [17, 18]. P. multiflorum is the root tuber of polygonum, and its primary active components include polyphenols, polysaccharides, and flavonoids [19]. As a commonly used medicine, it has many functions, such as scavenging free radicals, antivirus, and antitumor effects [20]. The redox activity of substances allows molecules in blood and organelles to transform energy or exchange biological information [21]. Therefore, investigating the redox activity of medicinal substances will offer a theoretical basis for TCM application [22]. Currently, the TCM industry commonly employs chromatographic and spectrographic technology to examine the redox activity of medicine substances [23, 24]. However, investigating only the redox differences in specific active substances cannot provide a complete understanding of the redox activity of substances in TCM. Thus, this study employs electrochemical approaches to investigate the overall redox activity of substances in TCM in situ and further investigates the source of the pharmaceutical effect of P. multiflorum. Further, the extraction process of P. multiflorum has been optimized based on the electrochemical redox behavior. Studies have confirmed that when ethanol is used as a solvent, although it can extract effective ingredients, the overall redox activity of P. multiflorum is lower than that when a liquor with the same ethanol concentration is used as the solvent. The substances contained in the liquor promote the redox reaction in P. multiflorum, showing a positive synergistic effect and providing a theoretical basis for optimizing the process of extracting active substances from TCM using liquor.
2. Experimental section
2.1. Material
The P. multiflorum was purchased from Shennong Pharmaceutical Co., Ltd. (Shaoyang, China), and the identities were authenticated in accordance with the Chinese Pharmacopoeia by Ying Su. Our research complies with the laws of China. Ethanol, tetra-n-butylammonium perchlorate, dimethyl sulfoxide, and 2,2-Diphenyl-1-picrylhydrazyl were purchased from Jinshan Chemical Reagent Co., LTD, Chengdu, China. The reagents were used without further purification. The standards (emodin, cas No. 518-82-1; quercetin, cas No.117-39-5; lawsone, cas No.83-72-7) were purchased from Aladdin Biochemical Technology Co., LTD, Shanghai, China.
2.2. Ultrasonic-assisted extraction from P. multiflorum and A. sinensis
The pretreated dried plant samples were crushed, weighed at 2.0 g, and placed in a distillation flask. The sample was placed in an ultrasonic cleaner with a power of 158 W for 40 min, and at the solid/liquid ratio of 40 mL/g. Then the sample was extracted with ethanol of a desired concentration. The extraction process was optimized by single-factor experiments: ethanol concentration (30%, 40%, 50%, 60%, and 70%), temperature (50 °C, 60 °C, 70 °C, 80 °C, 90 °C, and 95 °C), and extraction time (1h, 3h, and 5h). The extracted solution was treated by the vacuum freezing & drying technology, and the dry matter was collected for follow-up experiments.
2.3. Scavenging of DPPH radical by extracts of P. multiflorum
The DPPH was dissolved in ethanol to a final concentration of 2.2 mmol/L. The extract solution was prepared in ethanol with the desired concentration. The scavenging reaction was started by addition of extract solution into the DPPH solution. The reaction mixture was kept at room temperature for 20 min and the absorbance was measured at 325 and 525 nm with an ultra-violet and visible spectrophotometer (UV-2600, Shimadzu Corporation, Japan).
2.4. Electrochemistry
Electrochemistry was performed with an electrochemical workstation (CHI 660C, CHI Instrument, Shanghai, China). A typical three-electrode electrochemical system was used in this work. A glassy carbon electrode with a radius of 1.5 mm was used as the working electrode, platinum wire as the counter electrode, and Ag/AgNO3 electrode (saturated with AgNO3) as the reference electrode. Before the electrochemical test, the glassy carbon electrode was polished with 1 μm, 0.5 μm, and 0.3 μm alumina slurry to make its surface clean, and further cleaned with a large amount of sulfuric acid, ethanol and water, respectively. All electrodes were purchased from CHI instrument. Tetra-n-butylammonium perchlorate was used as the supporting electrolyte and dimethyl sulfoxide was used as the solvent for electrochemical analysis.
2.5. UPLC-MS analysis
Constituent analysis of the liquor extract and alcohol extract performed by UPLC-MS. Chromatographic analysis was performed with an UPLC system (Agilent1290UPLC), equipped with a Waters BEH C18 LC column (2.1∗100 mm∗1.7 um). The binary mobile phases included solution A (0.1% formic acid-water solution) and solution B (0.1% formic acid-acetonitrile solution). Flow velocity: 0.3 mL/min. Injection volume: 5 μL. A high-resolution mass spectrometry detection system (Agilent QTOF6550) was used, which equipped with an electrospray ion (ESI) source. Sheath gas temp 350 °C, Sheath gas flow 12 L/min.
3. Results and discussion
3.1. Redox picture of P. multiflorum extract revealed by electrochemistry
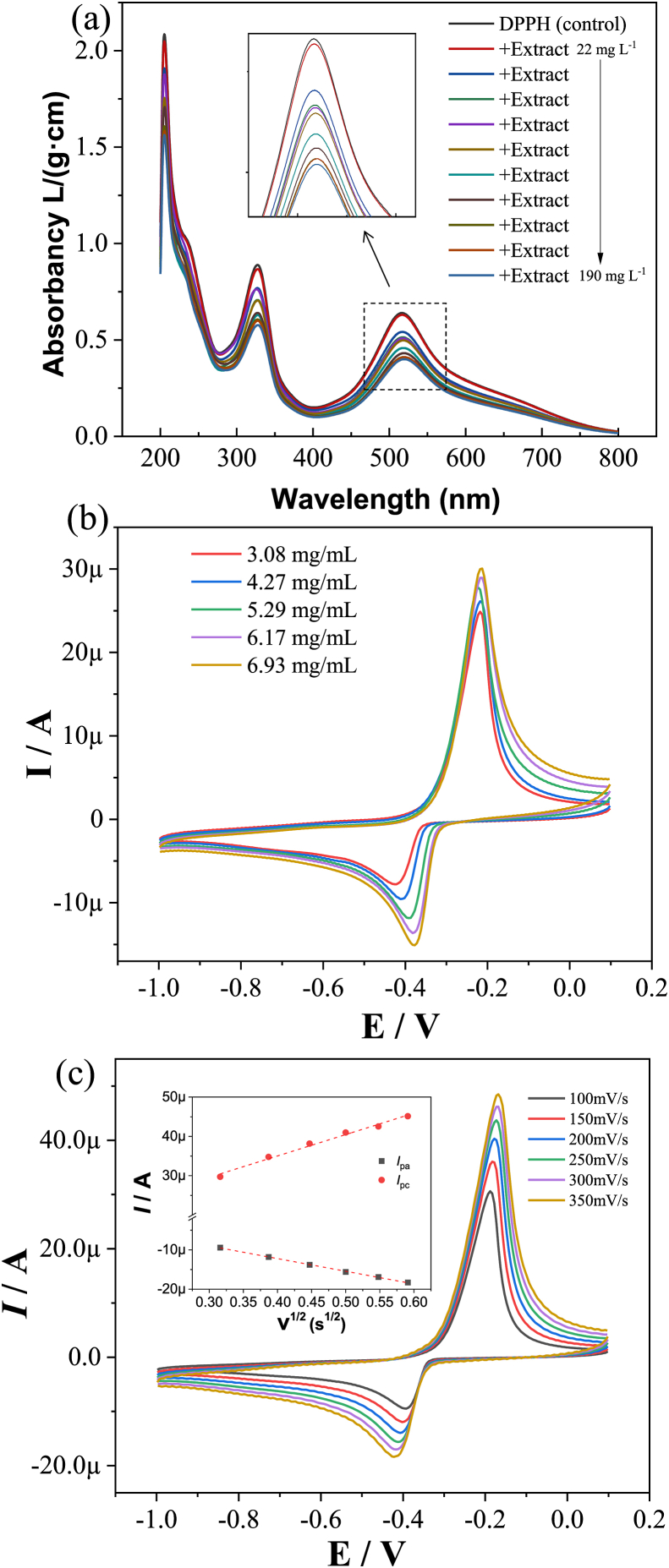
We employed the stable free radical diphenylpicrylhydrazyl (DPPH) to indirectly feedback the redox properties of P. multiflorum extract: DPPH can maintain a stable free radical state in an organic solution, and its solution is purple [25]. When there is a free radical scavenger, the electron of DPPH will be captured, making its color lighter. The degree of fading is quantitatively related to the number of accepted electrons. As shown in Figure 1a, the intensity of the two absorption peaks (325 nm and 525 nm) of DPPH decreases with the increase in the concentration of P. multiflorum extract. It indicates that the single electron of DPPH is captured by the extract of P. multiflorum, which confirms the free radical scavenging ability of the extract [26]. Although P. multiflorum has a complex composition, it is considered to contain the main components: flavonoids, anthraquinones and stilbene glycosides [27]. All these substances contain active phenolic hydroxyl groups which can be oxidized into ketones, thus the P. multiflorum extract possesses the free radical scavenging ability. The DPPH-based spectroscopy method can prove the antioxidant capacity of the alcohol extract of P. multiflorum without complicated procedures [28]. However, this method is an indirect method and fails to provide further information on the overall redox properties of the extract.
Figure 1.
(a) DPPH radical scavenging assay with the increase in the concentration of Polygonum multiflorum extract. (b) Cyclic voltammetry of Polygonum multiflorum extract in different concentrations: 3.08 mg/L, 4.27 mg/L, 5.29 mg/L, 6.17 mg/L, and 6.93 mg/L. Scan rate, 100 mV/s. (c) Cyclic voltammetry of Polygonum multiflorum extract in different scan rates: 100 mV/s, 150 mV/s, 200 mV/s, 250 mV/s, 300 mV/s, and 350 mV/s. Extract concentration, 6.93 mg/L.
Therefore, we employ the electrochemical method to directly explore the redox properties of the extract. The electrochemical method is highly sensitive and can detect the redox ability of the extract without damaging its molecular structure [29]. As shown in Figure 1b, both the oxidation and reduction current of the cyclic voltammetry spectrum have increased along with increasing extract concentration, suggesting that the extract compound exchanges electrons with the surface of the carbon electrode, which confirms the redox ability of the extract [30]. As shown in Figure 1c, both the oxidation current and the reduction current have increased with the increase of the scanning speed. The current value is proportional to the square root of the scan rate, indicating that the electron transfer from the redox active ingredient to the electrode surface is controlled by the diffusion process (the diffusion process and electron transfer process are two consecutive steps in the same process, and the diffusion process has slower speed than the electron transfer process), which confirms the good solubility of the active ingredient in the solvent. At the same time, the above results indicate that electrochemistry is an advantageous technique and can be employed to directly test the redox "picture" of medicine extracts [31].
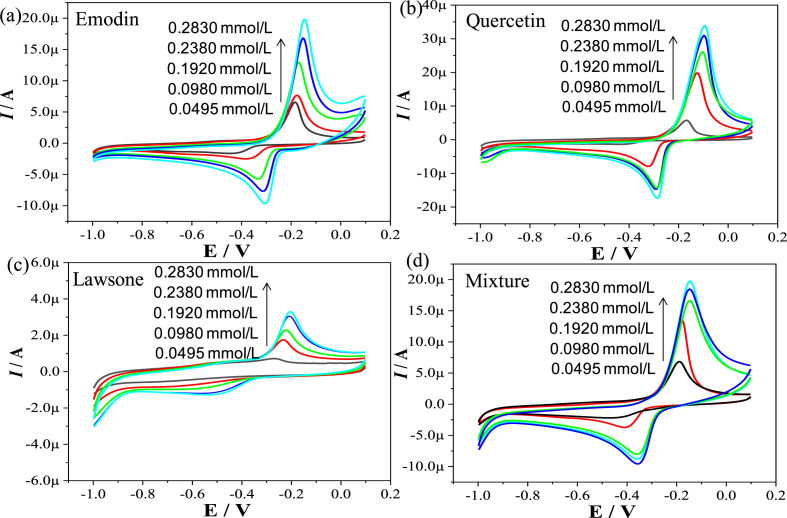
Next step, we employed the electrochemical method to investigate the redox signal characteristics of each component in the extract. As revealed by liquid chromatography, emodin, quercetin, and lawsone have a relatively high content in P. multiflorum extract [32]. Therefore, we selected the standards of the above three substances to study the redox characteristics of P. multiflorum extract. As shown in Figures 2a, 2b, and 2c, the cyclic voltammetry peaks of all three substances present a semi-reversible redox process, and the current value of the oxidation peak exceeds that of the reduction peak, indicating that these ingredients tend to be oxidized. In other words, when these substances exert a pharmaceutical effect, they are preferentially oxidized, thereby preventing the oxidization of the substances in the blood [33]. The separation potential between anodic peak and cathodic peak (ΔEp) of these three substances are 0.169 V (emodin), 0.194 V (quercetin), and 0.304 V (lawsone), respectively. As seen, the cyclic voltammetry spectrum of lawsone has the largest value of ΔEp, indicating the most irreversible nature of lawsone [34]. In other words, lawsone tends to be maintained in an oxidizable state, that is, lawsone has the strongest ability to maintain oxidability. In a study for measuring the antioxidant activity of dietary polyphenols, lawsone, catechin and quercetin showed significantly different abilities in inhibition the oxidation of low-density lipoprotein [35]. Antioxidant activity of flavonoids is considered to be related to the positioning of phenolic hydroxyl groups as well as the various types of substitution. And the feature of B-ring with an ortho-dihydroxy group induces effective ROS (reactive oxygen species) scavenging ability [36]. The structural formulas of the above-mentioned compounds in the oxidation state (the state of ketone) are shown in Figure 3. As seen, the ketone state of lawsone has an active B-ring and the smallest electron delocalization area, suggesting that the redox reversibility of these ingredients is correlated to the position of the active groups (phenolic hydroxyl groups or ketones),which is consistent with the previous report [37].
Figure 2.
Cyclic voltammetry of emodin (a), quercetin (b), and lawsone (c) in different concentrations (0.0495 mmol/L, 0.0980 mmol/L, 0.1920 mmol/L, 0.2380 mmol/L, and 0.2830 mmol/L). Scan rate, 100 mV/s. (d) Cyclic voltammetry of the mixture of emodin, quercetin, and lawsone, in a fixed-ratio (1:1:1). Scan rate, 100 mV/s.
Figure 3.
The redox reaction of emodin, quercetin, and lawsone respectively. The structural transformation between the phenolic hydroxyl state (reduction state) and ketone state (oxidation state).
Moreover, it can be seen that the oxidation peak potentials of the cyclic voltammetry peaks of the above-mentioned substances are -0.203 V, -0.148 V, and -0.098 V, respectively. Lawone has the lowest oxidation peak potential, indicating that when these substances exert the pharmaceutical effect, lawsone is preferentially oxidized [38]. All above results illustrate the importance of lawsone's antioxidant capacity in the P. multiflorum extract. Figure 2d shows the cyclic voltammogram of a mixture of these substances in a fixed-ratio (1:1:1). The voltammogram is similar to that of the three substances on the whole. As the peak potentials of these substances are relatively close to each other, thus the smaller peaks (0.2830 mmol/L of emodin contributed a current of 18 μA; 0.2830 mmol/L of lawsone contributed a current of 3 μA) are covered by the larger peak (0.2830 mmol/L of emodin contributed a current of 35 μA). The oxidation potential of the superimposed peak is -0.112 V, which is intermediate between the oxidation potential of these substances, indicating that the redox ability of these active ingredients when they are mixed is the sum of the abilities of the three substances when they exist alone, reflecting the collaborative contribution characteristic of redox ability.
3.2. Correlation between the redox activity and its extraction technology
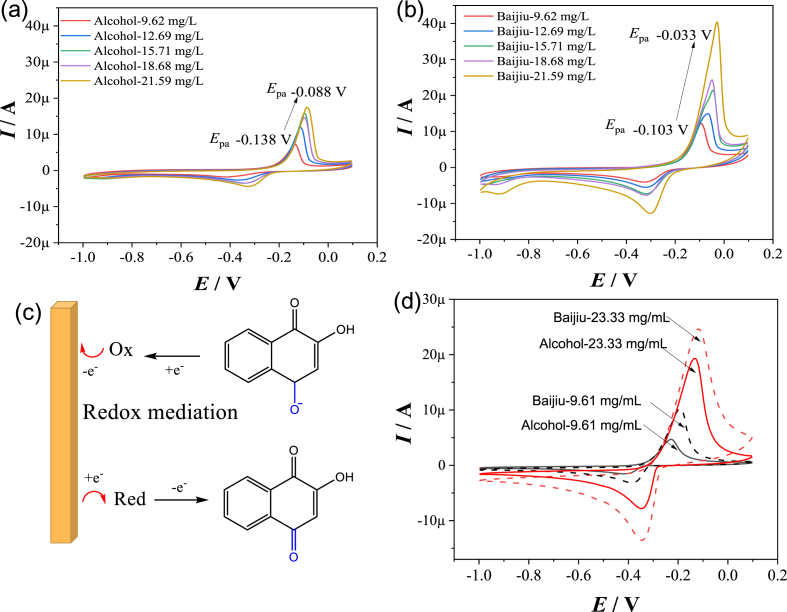
We next compared the difference between alcohol extraction (60% ethanol) and liquor extraction (Baijiu in 60% ethanol). As shown in Figures 4a and 4b, under the same concentration of extract, the Baijiu group has higher peak current than the alcohol group. Under the extract concentration of 9.62 mg/L, the oxidation current values of the Baijiu group and the alcohol group are 11.3 μA and 4.8 μA, respectively. Under the extract concentration of 21.59 mg/L, the oxidation current values of the Baijiu group and the alcohol group are 41.2 μA and 16.3 μA, respectively. As seen, in the presence of liquor, the extract showed an enhanced redox activity. The enhancement phenomenon of redox activity can be caused by more than one reason [39]. One reason for the enhancement phenomenon is that there is an increase in the amount of substances capable of exerting redox effects in Baijiu extracts. Constituent analysis of the liquor extract and alcohol extract was performed by UPLC-MS. The relative concentration of the constituents, which showed significant changes, were shown in Table 1. As seen, some constituents showed an increase (>120%) in relative concentration: tracheloside (125.7%), mayurone (120.5%), while some constituents showed a decrease (<90%) in relative concentration: 4-ethylresorcin (84.3%), D-(+)-Cellobios (89.6%). Although the aroma substances in Baijiu account for only 1%–2% of the total volume [40], they may have enhanced the extraction efficiency for redox species. It is reasonable as the molecular polarity of redox species in the extract is similar with the aroma substances in Baijiu, such as organic acids, esters [41].
Figure 4.
(a) Cyclic voltammetry of Polygonum multiflorum in different concentrations, extracted with 60% ethanol. (b) Cyclic voltammetry of Polygonum multiflorum in different concentrations, extracted with liquor (Baijiu in 60% ethanol). (c) Schematic representation of the redox mediation mechanism: The redox substances in Baijiu act as the redox mediator. (d) Cyclic voltammetry of 60% ethanol extracted Angelica sinensis (solid line) and liquor (Baijiu in 60% ethanol) extracted Angelica sinensis (dotted line).
Table 1.
Constituent analysis of the liquor extract and alcohol extract performed by UPLC-MS.
| No | CAS | Name | Retention time/min | Molecular weight | Molecular formula | Relative concentration (liquor/alcohol) |
|---|---|---|---|---|---|---|
| 1 | 33464-71-0 | Tracheloside | 29.858 | 550.2057 | C27H34O12 | 125.7% |
| 2 | 4677-90-1 | Mayurone | 28.816 | 204.1523 | C14H20O | 120.5% |
| 3 | 550-10-7 | Hydrocotarnine | 26.535 | 221.1056 | C12H15NO3 | 118.6% |
| 4 | 24399-19-7 | Theaspirone | 23.348 | 208.1471 | C13H20O2 | 112.5% |
| 5 | 63644-71-3 | γ-Methoxyisoeugenol hoxy-1-propenyl)-phenol | 23.835 | 194.0952 | C11H14O3 | 111.2% |
| 6 | 2896-60-8 | 4-Ethylresorcinol | 16.023 | 138.0687 | C8H10O2 | 84.3% |
| 7 | 528-50-7 | D-(+)-Cellobiose | 2.546 | 342.1167 | C12H22O11 | 89.6% |
| 8 | 60125-23-7 | 2-Propenal | 17.438 | 148.0532 | C9H8O2 | 97.4% |
| 9 | 483-64-7 | Damascenine | 25.687 | 195.0902 | C10H13NO3 | 97.5% |
| 10 | 64274-28-8 | Aucubigenin | 16.018 | 184.0742 | C9H12O4 | 97.7% |
Another reason for the enhancement phenomenon is that the redox substances in Baijiu (e.g. aldehydes and ketones) act as the redox mediator [42], and the redox efficiency of the extract is improved due to the faster electron transfer ability of redox mediator in Baijiu. Aldehydes and ketones in Baijiu are chemicals with electrochemical activity, which will exchange electrons with oxidants or reductants in P. multiflorum extract, and then diffuse to the electrode surface and exchange electrons there. The mediation process is repeated, and the mediator functions as an electron shuttle between the oxidants/reductants and electrode, thus the redox efficiency of the extract is improved. A redox mediation [43] mechanism is illustrated in Figure 4c. Further, cyclic voltammetry of another TCM model (Angelica sinensis) was shown in Figure 4d. As seen, under the same concentration of extract, the Baijiu group has higher peak current than the alcohol group, indicating a similar phenomenon with P. multiflorum. A comparative study [13] on the pharmacodynamic difference of TCM accompanied with Chinese Baijiu and water (without Baijiu) has demonstrated the function of Baijiu in activating anti-inflammation, analgesia and anti-mammary gland hyperplasia, which also suggests a mediation effect.
At the same time, it should be noted that the Baijiu group has higher oxidation potential than the alcohol group. Under the extract concentration of 9.62 mg/L, the oxidation potential values of the Baijiu group and the alcohol group are -0.103 V and -0.138 V, respectively. Under the extract concentration of 21.59 mg/L, the oxidation potential values of the liquor group and the alcohol group are -0.033 V and -0.088 V, respectively. It suggests that under the mediation effect of redox substances in Baijiu, the oxidation process of the extract is disturbed by redox polarization, which increases the oxidation potential and is thus unfavorable for its preferential oxidation (then unfavorable for its pharmaceutical effect). Although redox mediation increases the amount of antioxidants, polarization reduces the antioxidant ability. In view of the overall redox efficacy, the mediation effect and the polarization effect are contradictory. Therefore, how to regulate these two effects in the extracting process is the key to optimizing the redox efficacy.
In addition, literatures reported that flavonoids act as highly active antioxidants by way of metal chelation [36]. Thus the concentrations of the metals in Baijiu were determined by ICP-MS/OES analysis [44]. It is found that eight metals (Al, Ba, Ca, Cu, Fe, Mg, K, and Na) have a relatively high content. The results suggest that the ortho-dihydroxyl group in flavonoids may takes part in metal chelation via these ions (Al3+, Ba2+, Ca2+, Cu2+, Fe3+, Mg2+) thus enhancing the redox efficiency of the extract. However, flavonoids can chelate metals by diversified possible route which depends on flavonoids structure, the pH of the reaction and the type of metal ion, emphasizing the need for further research.
3.3. Optimized extraction process based on the redox activity
We further optimized the P. multiflorum extraction process based on the redox activity of the extract. As shown in Figure 5a, the higher the temperature in the extraction process, the greater the current of the oxidation peak and the reduction peak of the extract, indicating an increase in the amount of extracted substances capable of redox reactions. Moreover, the higher the temperature in the extraction process, the greater the ΔEp between the oxidation peak and the reduction peak, indicating the poor reversibility of the redox reaction. It can be seen that higher temperature in the extraction process increases the total amount of extracted active substances, but some substances lose their redox activity at the same time. Figure 5b shows the free radical scavenging ability of the extract at different temperatures. It can be seen that under the extraction temperature of 90 °C, the absorption peak has the smallest intensity, suggesting that this temperature is beneficial for the extract to exert its free radical scavenging ability. It should be noted that the extract will undergo molecular decomposition, isomerization, and chemical bond breakage at high temperatures, which will change the antioxidant capacity of the extract [45]. At 95 °C, the amount of extracted substances capable of redox reactions is the highest. However, in view of the redox reversibility, a temperature lower than 95 °C is advantageous. Moreover, the absorption spectrum suggests that the free radical scavenging ability of the extract is relatively strong at 90 °C. Therefore, from an overall point of view, the extraction temperature of 90 °C is conducive to the redox activity of P. multiflorum extract.
Figure 5.
Cyclic voltammetry (a) and DPPH radical scavenging assay (b) of the extract of Polygonum multiflorum, extracted in the condition of different temperatures. Cyclic voltammetry (c) and DPPH radical scavenging assay (d) of the extract of Polygonum multiflorum, extracted in the condition of different alcohol concentrations. Cyclic voltammetry (e) and DPPH radical scavenging assay (f) of the extract of Polygonum multiflorum, extracted in the condition of different times.
As shown in Figure 5c, as the ethanol concentration during the extraction process increases, the peak current first increases and then decreases. Under 60% ethanol concentration, the peak current is the highest, indicating that the amount of extracted substances capable of redox reaction is more desirable under this concentration. On the one hand, the dissolution of polyphenols is related to the polarity of the extraction solvent. As the ethanol concentration increases, there is a smaller polarity gap between the solvent and the solute, and the extracted amount is higher. On the other hand, polyphenols are combined with substances such as proteins and polysaccharides in plant tissues through the force of hydrogen bonds [46]. Under excessive ethanol concentration, a large amount of alcohol-soluble substances are eluted, resulting in greater polarity difference between the polyphenol and the solvent, so the amount of the extracted polyphenol decreases. Figure 5d shows the free radical scavenging ability of the extract under different ethanol concentrations. Under an ethanol concentration of 60%, the absorption peak has the smallest intensity, which implies that the condition is the optimal ethanol concentration for the extract to exert its free radical scavenging ability. Seen from the two aspects of extraction amount and free radical scavenging ability, an ethanol concentration of 60% is ideal for optimization the extraction process. Figure 5e shows the change in extraction amount under different extraction times. It can be seen that the extraction amount under the condition of 1h is significantly higher than that under the condition of 3h and 5h. Polyphenols diffuse rapidly into the solvent within 1h, showing the strongest redox capacity. As the extraction time increases, parts of the polyphenols are decomposed, with the redox capacity decreased. As shown in Figure 5f, under the extraction condition of 1h, the extract also exhibits significant free radical scavenging ability. Using the electrochemical redox method and optical absorption spectroscopy, the optimal extraction process of P. multiflorum can be determined: the extraction temperature of 90 °C, the extraction ethanol concentration of 60%, and the extraction time of 1 h.
4. Conclusions
In conclusion, the liquor extraction process shows a correlation with the redox efficacy of active substances in P. multiflorum. On the one hand, the redox substances in the liquor function as redox mediators to enhance the redox efficiency of the extract. On the other hand, the extract oxidation process is disturbed by redox polarization, which is unconducive to the pharmaceutical effect of the extract. Thus, the results indicate that the process of extracting active substances from TCM using liquor may be optimized by regulating the mediation and polarization effect. Additionally, the results confirm that electrochemical technology is an efficient tool to directly test the oxidation activity of plant extracts and can be used for elucidating the pharmaceutical mechanisms behind TCM and promoting its modernization process.
Declarations
Author contribution statement
Ying Su: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Zihao Wang: Performed the experiments; Analyzed and interpreted the data.
Yougui Yu: Conceived and designed the experiments; Wrote the paper.
Qing Zheng: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Natural Science Foundation of Hunan Province (2020JJ5516), the Scientific Research Fund of Hunan Provincial Education Department (20A458), Graduate Education Teaching Foundation (2021JGSY002), and the Innovation Foundation For Postgraduate of Shaoyang University (CX2021SY067).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Yin J., Wu M., Lin R., Li X., Ding H., Han L., Yang W., Song X., Li W., Qu H., Yu H., Li Z. Application and development trends of gas chromatography–ion mobility spectrometry for traditional Chinese medicine, clinical, food and environmental analysis. Microchem. J. 2021;168:106527. [Google Scholar]
- 2.Liu Y., Cheng Y. Combined development of traditional Chinese medicine and interventional medicine. J. Intervent. Med. 2021;4:136–138. doi: 10.1016/j.jimed.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J., Xu C., Wong Y.K., Li Y., Liao F., Jiang T., Tu Y. Artemisinin, the magic drug discovered from traditional Chinese medicine. Engineering. 2019;5:32–39. [Google Scholar]
- 4.Pacios-Michelena A., Kasaragod V.B., Schindelin H. Artemisinins and their impact on inhibitory neurotransmission. Curr. Opin. Pharmacol. 2021;59:19–25. doi: 10.1016/j.coph.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Zhang B., Yang S., Guo D.-a. The quest for the modernization and internationalization of traditional chinese medicine. Engineering. 2019;5:1–2. [Google Scholar]
- 6.Li X., Wu L., Wu R., Sun M., Fu K., Kuang T., Wang Z. Comparison of medicinal preparations of Ayurveda in India and five traditional medicines in China. J. Ethnopharmacol. 2022;284 doi: 10.1016/j.jep.2021.114775. [DOI] [PubMed] [Google Scholar]
- 7.Jin W., Zhou T., Li G. Recent advances of modern sample preparation techniques for traditional Chinese medicines. J. Chromatogr. A. 2019;1606 doi: 10.1016/j.chroma.2019.460377. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X., Zhang Y., Meng X., Yin P., Deng C., Chen J., Wang Z., Xu G. Effect of a traditional Chinese medicine preparation Xindi soft capsule on rat model of acute blood stasis: A urinary metabonomics study based on liquid chromatography–mass spectrometry. J. Chromatogr. B. 2008;873:151–158. doi: 10.1016/j.jchromb.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Liu J., Sun Y., Liu L., Yu C. A water-soluble polysaccharide (EFP-AW1) from the alkaline extract of the roots of a traditional Chinese medicine, Euphorbia fischeriana: Fraction and characterization. Carbohydr. Polym. 2012;88:1299–1303. [Google Scholar]
- 10.Bu L., Dai O., Zhou F., Liu F., Chen J.-F., Peng C., Xiong L. Traditional Chinese medicine formulas, extracts, and compounds promote angiogenesis. Biomed. Pharmacother. 2020;132 doi: 10.1016/j.biopha.2020.110855. [DOI] [PubMed] [Google Scholar]
- 11.Wu J., Wu X., Wu R., Wang Z., Tan N. Research for improvement on the extract efficiency of lignans in traditional Chinese medicines by hybrid ionic liquids: As a case of Suhuang antitussive capsule. Ultrason. Sonochem. 2021;73 doi: 10.1016/j.ultsonch.2021.105539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiang Z., Wang Y., Liu S. The chemical and metabolite profiles of Gualou-Xiebai-Banxia-Baijiu decoction, a classical traditional Chinese medicine formula, by using high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry and in-house software. J. Ethnopharmacol. 2022 doi: 10.1016/j.jep.2022.114994. [DOI] [PubMed] [Google Scholar]
- 13.Song J., Feng B., Zhang D., Qiu M., Ran F., Cao B., Xu H., Lin J., Xu R., Han L. Comparative study on the pharmacodynamic difference of oral administration of Xiaojin Pills accompanied with Chinese Baijiu and water. J. Ethnopharmacol. 2021;278 doi: 10.1016/j.jep.2021.114284. [DOI] [PubMed] [Google Scholar]
- 14.Lin P., Qin Z., Yao Z., Wang L., Zhang W., Yu Y., Dai Y., Zhou H., Yao X. Metabolites profile of Gualou Xiebai Baijiu decoction (a classical traditional Chinese medicine prescription) in rats by ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. B. 2018;1085:72–88. doi: 10.1016/j.jchromb.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Chen X., Cao Y.-g., Zhang Y.-h., Zeng M.-n., Ren Y.-j., Liu Y.-l., He C., Fan X.-l., Zheng X.-k., Feng W.-s. Two new ionones from the fresh roots of Rehmannia glutinosa. Phytochem. Lett. 2021;46:114–118. [Google Scholar]
- 16.Ota M., Nakazaki J., Tabuchi Y., Ono T., Makino T. Historical and pharmacological studies on rehmannia root processing– Trends in usage and comparison of the immunostimulatory effects of its products with or without steam processing and pretreatment with liquor. J. Ethnopharmacol. 2019;242 doi: 10.1016/j.jep.2019.112059. [DOI] [PubMed] [Google Scholar]
- 17.Kang Y., Lee K., Choi J., Komakech R., Min J., Ju S., Kim S.W., Youn C., Kim Y.G., Moon B.C. Maximizing seedling and root tuber production in Polygonum multiflorum for use in ethnomedicine. S. Afr. J. Bot. 2018;119:119–131. [Google Scholar]
- 18.Zhang Q., Xu Y., Lv J., Cheng M., Wu Y., Cao K., Zhang X., Mou X., Fan Q. Structure characterization of two functional polysaccharides from Polygonum multiflorum and its immunomodulatory. Int. J. Biol. Macromol. 2018;113:195–204. doi: 10.1016/j.ijbiomac.2018.02.064. [DOI] [PubMed] [Google Scholar]
- 19.Park H.-J., Zhang N., Park D.K. Topical application of Polygonum multiflorum extract induces hair growth of resting hair follicles through upregulating Shh and β-catenin expression in C57BL/6 mice. J. Ethnopharmacol. 2011;135:369–375. doi: 10.1016/j.jep.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 20.Tang W., Li S., Liu Y., Huang M.-T., Ho C.-T. Anti-inflammatory effects of trans-2,3,5,4′-tetrahydroxystilbene 2-O-β-glucopyranoside (THSG) from Polygonum multiflorum (PM) and hypoglycemic effect of cis-THSG enriched PM extract. J. Funct.Foods. 2017;34:1–6. [Google Scholar]
- 21.Ding Q., Luo L., Yu L., Huang S.-l., Wang X.-q., Zhang B. The critical role of glutathione redox homeostasis towards oxidation in ermanin-induced melanogenesis. Free Radical Biol. Med. 2021;176:392–405. doi: 10.1016/j.freeradbiomed.2021.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Selyutina O.Y., Kononova P.A., Koshman V.E., Shelepova E.A., Azad M.G., Afroz R., Dharmasivam M., Bernhardt P.V., Polyakov N.E., Richardson D.R. Ascorbate-and iron-driven redox activity of Dp44mT and emodin facilitates peroxidation of micelles and bicelles. Biochim. Biophys. Acta (BBA) - Gen. Subj. 2021 doi: 10.1016/j.bbagen.2021.130078. [DOI] [PubMed] [Google Scholar]
- 23.Shen M.-R., He Y., Shi S.-M. Development of chromatographic technologies for the quality control of Traditional Chinese Medicine in the Chinese Pharmacopoeia. J. Pharmaceut. Anal. 2021;11:155–162. doi: 10.1016/j.jpha.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao X., Yang L., Bai Y., Li Q., Zhao X., Bian L., Zheng X. Screening of bioactive components from traditional Chinese medicine by immobilized β2 adrenergic receptor coupled with high performance liquid chromatography/mass spectrometry. J. Chromatogr. B. 2019;1134–1135 doi: 10.1016/j.jchromb.2019.121782. [DOI] [PubMed] [Google Scholar]
- 25.Kandi S., Charles A.L. Statistical comparative study between the conventional DPPH spectrophotometric and dropping DPPH analytical method without spectrophotometer: Evaluation for the advancement of antioxidant activity analysis. Food Chem. 2019;287:338–345. doi: 10.1016/j.foodchem.2019.02.110. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Li Q., Xing H., Lu X., Zhao L., Qu K., Bi K. Evaluation of antioxidant activity of ten compounds in different tea samples by means of an on-line HPLC–DPPH assay. Food Res. Int. 2013;53:847–856. [Google Scholar]
- 27.Li Q., Tu Y., Zhu C., Luo W., Huang W., Liu W., Li Y. Cholinesterase, β-amyloid aggregation inhibitory and antioxidant capacities of Chinese medicinal plants. Ind. Crop. Prod. 2017;108:512–519. [Google Scholar]
- 28.de Menezes B.B., Frescura L.M., Duarte R., Villetti M.A., da Rosa M.B. A critical examination of the DPPH method: Mistakes and inconsistencies in stoichiometry and IC50 determination by UV–Vis spectroscopy. Anal. Chim. Acta. 2021;1157 doi: 10.1016/j.aca.2021.338398. [DOI] [PubMed] [Google Scholar]
- 29.Petroniene J., Morkvenaite-Vilkonciene I., Miksiunas R., Bironaite D., Ramanaviciene A., Rucinskas K., Janusauskas V., Ramanavicius A. Scanning electrochemical microscopy for the investigation of redox potential of human myocardium-derived mesenchymal stem cells grown at 2D and 3D conditions. Electrochim. Acta. 2020;360 [Google Scholar]
- 30.Cámara C.I., Bornancini C.A., Cabrera J.L., Ortega M.G., Yudi L.M. Quantitative analysis of boldine alkaloid in natural extracts by cyclic voltammetry at a liquid–liquid interface and validation of the method by comparison with high performance liquid chromatography. Talanta. 2010;83:623–630. doi: 10.1016/j.talanta.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Sia J., Yee H.-B., Santos J.H., Abdurrahman M.K.-A. Cyclic voltammetric analysis of antioxidant activity in cane sugars and palm sugars from Southeast Asia. Food Chem. 2010;118:840–846. [Google Scholar]
- 32.Ahn S.M., Kim H.N., Kim Y.R., Choi Y.W., Kim C.M., Shin H.K., Choi B.T. Emodin from Polygonum multiflorum ameliorates oxidative toxicity in HT22 cells and deficits in photothrombotic ischemia. J. Ethnopharmacol. 2016;188:13–20. doi: 10.1016/j.jep.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 33.Wang X., Yu J.-y., Sun Y., Wang H., Shan H., Wang S. Baicalin protects LPS-induced blood–brain barrier damage and activates Nrf2-mediated antioxidant stress pathway. Int. Immunopharm. 2021;96 doi: 10.1016/j.intimp.2021.107725. [DOI] [PubMed] [Google Scholar]
- 34.Venton B.J., DiScenza D.J. In: Patel B., editor. Elsevier; 2020. in Electrochemistry for Bioanalysis; pp. 27–50. [Google Scholar]
- 35.Sánchez-Moreno C., Jiménez-Escrig A., Saura-Calixto F. Study of low-density lipoprotein oxidizability indexes to measure the antioxidant activity of dietary polyphenols. Nutr. Res. 2000;20:941–953. [Google Scholar]
- 36.Wojtunik-Kulesza K., Oniszczuk A., Oniszczuk T., Combrzyński M., Nowakowska D., Matwijczuk A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—a non-systematic review. Nutrients. 2020;12 doi: 10.3390/nu12051401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y., Wang S., Chang W., Zhang L., Wu Z., Jin R., Xing Y. Co-monomer engineering optimized electron delocalization system in carbon-bridging modified g-C3N4 nanosheets with efficient visible-light photocatalytic performance. Appl. Catal. B Environ. 2020;274 [Google Scholar]
- 38.Nagao A., Maoka T., Ono H., Kotake-Nara E., Kobayashi M., Tomita M. A 3-hydroxy %-end group in xanthophylls is preferentially oxidized to a 3-oxo ε-end group in mammals[S] J. Lipid Res. 2015;56:449–462. doi: 10.1194/jlr.P055459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cecchet F., Marcaccio M., Margotti M., Paolucci F., Rapino S., Rudolf P. Redox mediation at 11-mercaptoundecanoic acid self-assembled monolayers on gold. J. Phys. Chem. B. 2006;110:2241–2248. doi: 10.1021/jp054290n. [DOI] [PubMed] [Google Scholar]
- 40.Hong J., Tian W., Zhao D. Research progress of trace components in sesame-aroma type of baijiu. Food Res. Int. 2020;137 doi: 10.1016/j.foodres.2020.109695. [DOI] [PubMed] [Google Scholar]
- 41.Wang L., Gao M., Liu Z., Chen S., Xu Y. Three extraction methods in combination with GC×GC-tofms for the detailed investigation of volatiles in chinese herbaceous aroma-type baijiu. Molecules. 2020;25 doi: 10.3390/molecules25194429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou L., Zhang X., Huang Y., Wang M., Chen X., Lin T., Tan Y., Zhao S. A ratiometric electrochemical biosensor via alkaline phosphatase mediated dissolution of nano-MnO2 and Ru(III) redox recycling for the determination of dimethoate. J. Pharmaceut. Biomed. Anal. 2022;207 doi: 10.1016/j.jpba.2021.114400. [DOI] [PubMed] [Google Scholar]
- 43.Wang X., Chen T., Gao C., Xie Y., Zhang A. Use of extracellular polymeric substances as natural redox mediators to enhance denitrification performance by accelerating electron transfer and carbon source metabolism. Bioresour. Technol. 2022;345 doi: 10.1016/j.biortech.2021.126522. [DOI] [PubMed] [Google Scholar]
- 44.Zheng Q., Hu Y., Xiong A., Su Y., Wang Z., Zhao K., Yu Y. Elucidating metal ion-regulated flavour formation mechanism in the aging process of Chinese distilled spirits (Baijiu) by electrochemistry, ICP-MS/OES, and UPLC-Q-Orbitrap-MS/MS. Food Funct. 2021;12:8899–8906. doi: 10.1039/d1fo01505b. [DOI] [PubMed] [Google Scholar]
- 45.Arbidar E., Sahari M.A., Mirzaei H., Kashaninejad M., Molaee M. Evaluation of the effect of gamma and microwave irradiation and high temperature on the antioxidant properties of the Avicennia marina leaf extract. Radiat. Phys. Chem. 2022 [Google Scholar]
- 46.Pan T.g., Wu Y.n., He S., Wu Z., Jin R. Food allergenic protein conjugation with plant polyphenols for allergenicity reduction. Curr. Opin. Food Sci. 2022;43:36–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.