Abstract
Background
A recent study has identified the role of CHRNA5-A3-B4 gene cluster variants rs16969968 and rs578776 of nicotinic acetylcholine receptors (nAChRs) on smoking status in Bengali ethnicity. The aim of the current study was to investigate whether these rs16969968-rs578776-rs11072768 single nucleotide polymorphisms (SNPs) of CHRNA5-A3-B4 gene cluster were associated with nicotine dependence (ND) and related phenotypes.
Methods
The Fagerstrom Test for Nicotine Dependence (FTND) and Cigarette Dependence Scale (CDS-12) were used to assess the degree of ND, and genotyping was done using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method on a cohort of 129 male smokers participating in a structured questionnaire-based survey.
Results
Smokers with AA genotype of CHRNA5 rs16969968 SNP were at significantly increased risk of developing ND compared to its wild type variant with odds ratio (ORs) of 1.20 (FTND: 95% CI 0.25–5.37, p = 0.253) and 2.48 (CDS-12: 95% CI 0.46–13.26, p = 0.081), respectively. Conversely, smokers with AA genotype of CHRNA3 rs578776 variant had a strong protective effect against ND development (ORs = 0.27, 95% CI 0.09–0.80, p = 0.076). There was no such link reported in CHRNB4 rs11072768 variant carriers. Similarly, G-A/G-A diplotype of rs16969968_rs578776 variants was discovered to be a protective factor against ND. Moreover, demographic features such as age, occupation and dwelling status were found to be significantly associated with ND.
Conclusion
Taken together, CHRNA5-A3-B4 gene cluster variants rs16969968 and rs578776 as well as specific demographic characteristics regulate ND and related smoking phenotypes in Bangladeshi male smokers. Further studies with large sample sizes are required to substantially validate the significance.
Keywords: Bangladeshi smokers, CHRNA5-A3-B4 gene cluster, Nicotine dependence, rs16969968, rs578776, rs11072768
Bangladeshi smokers; CHRNA5-A3-B4 gene cluster; Nicotine dependence; rs16969968; rs578776; rs11072768.
1. Introduction
Smoking is one of the leading causes of premature death worldwide. Study conducted on more than 200,000 people has reported that about 67 percent of smokers perished from smoking-related illness and the relative risk for adverse health effects increases with intensity of smoking [1]. In 2015, global estimated death due to smoking was 6.4 million and it has been predicted that by 2030 it will be approximately 6.8 million which is appalling [2, 3]. Besides, in Bangladesh, the death rate due to smoking has been reported to be 19% by World Health Organization (WHO) [4]. Global Adult Tobacco Survey (GATS) conducted by WHO in Bangladesh has reported that approximately 35.3% adults are currently smoking among which 46% are men and 25.2% are women and among the non-smokers 39% adults are exposed to smoking [5]. Even though different smoking cessation treatments are available, many smokers failed to act upon their intentions to quit smoking and diversity in quitting occurs due to nicotine dependence (ND). The majority of dependents are not able to stop smoking, even with professional assistance [6] and in spite of the known health benefits. Various restorative factors [7, 8, 9] are contributing to relapse after cessation among which genetic factors play an important role and can be attributable to a 50% risk for failed attempts to quit smoking [4, 5]. Recently, Genome-wide association studies (GWAS) have highlighted some new genetic variants such as CHRNA5-A3-B4 gene cluster associated with nicotine dependence (ND), which can also be candidates for the prediction of the success of the treatment of this dependence [10].
ND is a complex behavioral condition that has a slow onset and develops due to prolonged smoking which can be characterized by tolerance, cravings, withdrawal symptoms during periods of abstinence, and loss of control over the amount despite the harmful consequences. Nicotine is the main chemical constituent and psychoactive ingredient of cigarettes responsible for ND development which gives a temporary pleasing effect in the brain after inhalation and absorption, and dissipation of its transient effect impulsivity to smoke arises again. It exerts its psychopharmacological activity by binding with functionally diverse neuronal nicotinic acetylcholine receptors (nAChRs), which exists as homo or hetero pentameric and ligand gated ion channel [11, 12, 13, 14]. Among its 12 different subtypes, α5, α3, β4 receptor subunits are often co-expressed and co-regulated on chromosome 15q25, and encoded by CHRNA5-A3-B4 gene cluster [10]. Polymorphism in this gene cluster alters the receptor composition as well as binding of nicotine to this receptor which leads to changes in functional and behavioral outcomes. Additionally, variation in this gene cluster has been found associated with ND and various smoking related phenotypes [12, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32] as well as lung cancer [33, 34, 35] which makes this a plausible candidate to develop better assessment methods and produce more personalized therapy for cessation. The most well-known hereditary influence related to ND is a missense mutation at rs16969968 (G>A) on the nAChRs CHRNA5, resulting in an amino acid alteration from aspartate to asparagine at the 398th position [36, 37, 38, 39]. CHRNA3 rs578776 (G>A) is a 3'-UTR variant and the minor allele genotype (AA) was found to be a protective factor against heaviness of smoking as well as ND [15, 27, 36, 38, 39, 40, 41, 42] and CHRNB4 rs11072768 (T>G) is an intron variant significantly associated with cigarette per day (CPD) in Chinese males and lung cancer indirectly through smoking behavior [12, 43, 44, 45].
In accordance with those previous studies and as association differs in different races and ethnic groups, our previous work established a strong association between this gene cluster variants and smoking status (SS) [21]. Therefore, the current study was aimed to further explore the hypothesis that occurrences of these three SNPs (rs16969968-rs578776-rs11072768) in CHRNA5-A3-B4 gene cluster control several ND traits in Bangladeshi male smokers.
2. Materials and methods
2.1. Study design and population
A total of 129 smokers defined by WHO [46] were enrolled from our previously published study [21] but was limited only to male population due to unavailability and privacy concerns of female smokers. A prior power analysis (β = 0.2) determined that the power of the analysis of variance (ANOVA) was ≥0.8 at a 95% confidence interval (CI), indicating that the sample size (n = 129) of the study was sufficient. Each participant signed a written consent form before being enrolled and completed self-reported measures for his smoking history and behavior. Study protocol was approved by the ethical committees and the study was conducted according to the declaration of Helsinki and its further amendments [47].
2.2. Phenotype analysis
Primary data was collected by a questionnaire-based survey method where Fagerstrom Test for Nicotine Dependence (FTND) and Cigarette Dependence Scale (CDS-12), two scales were used for the measurement of degree of ND. The FTND scale measures the degree of ND from 2 (low) to 8 (high), relates with the withdrawal symptoms, ability to stop smoking, physiological dependence and tolerance, heavily weighted for cigarettes per day (CPD) and time from waking until the first cigarette (TFC) but fails to differentiate very low dependent smokers [48, 49, 50]. Whereas, the 12-items CDS scale is brief, self-administered, slightly better predictor of withdrawal symptoms and self-efficacy after cessation, has test-retest ability and suited for both high and low dependent smokers ranging from 12 (lowest) to 60 (highest) but has no ‘gold standard’ for measuring ND. Since CDS-12 scale is briefer and more sensitive than FTND scale but has no ‘gold standard’ for ND both the scales were used in order to measure the ND more precisely. Here, we assessed ND with some smoking-related phenotypes-smoking initiation (SI), CPD, TFC in association with these two scores i.e., FTND, CDS-12.
SI was assessed by comparing smokers of ≤16 years to smokers of >16 years of age as previous study reported no observable genetic effect in subjects who began daily nicotine use after the age of 16 [51]. CPD was used as a measurement of tobacco consumption where heavy smokers who smoked 20 or more cigarettes per day were compared with light smokers who smoked less than 20 cigarettes per day [52]. TFC was applied for measuring the craving phenotype and the ability to quit as well. ND was evaluated using FTND and CDS-12 scores. Participants were divided into two groups based on their scores: low dependence (<4 scores for FTND, ≤40 scores for CDS-12) and high dependence (≥4 scores for FTND, >40 scores for CDS-12).
2.3. Genotype analysis
Genomic DNA was extracted from all 129 smokers’ blood samples and genotyped for CHRNA5 rs16969968, CHRNA3 rs578776, CHRNB4 rs11072768 variants using Polymerase Chain Reaction -Restriction Fragment Length Polymorphism (PCR-RFLP) method [53]. Details of the genotype analysis can be found in our previously published study [21].
2.4. In-silico gene expression analysis
To explore the tissue specific expression of CHRNA5-A3-B4 gene cluster and its associated SNPs of interest, an in-silico gene expression analysis was performed using Genotype-Tissue Expression (GTEx) portal [54]. Furthermore, to correlate the expression of these three genes with two major histological subtypes of lung cancer i.e., lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) as well as with smoking status, UALCAN, an interactive web tool based on TCGA dataset [55], was used.
2.5. Statistical analysis
Chi-square test (χ2) was applied to measure the association of smokers’ demographic features with several smoking phenotypes. To perform multiple regression analyses for assessing odds ratios (ORs) and 95% confidence interval (CI), SPSS software package, version 17.0 (SPSS, Inc., Chicago, IL) was used and were adjusted to age, occupation and dwelling status. In all the analyses, p value less than 0.05 was considered as statistically significant. In addition, data, if required, were corrected for multiple comparisons using Bonferroni method in order to prevent false positive results.
3. Results
3.1. Association between demographic characteristics of smokers and smoking phenotypes
Age of the smokers was found to be a statistically significant factor in influencing many smoking-related phenotypes, such as age of SI, CPD, TFC, FTND and CDS-12, whereas occupation and dwelling status were important contributing factors primarily for phenotypes related to the intensity of nicotine dependence. Individual body weight and parental tobacco exposure didn’t seem to impact any ND traits. Detailed results of the correlation analysis between demographic characteristics and smoking phenotypes are shown in Table 1.
Table 1.
Correlation between demographic characteristics of smokers and different smoking phenotypes.
| Characteristics | SI age |
CPD |
TFC |
FTND |
CDS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤16 years | >16 years | p-value | ≤20 | >20 | p-value | ≤30 | >30 | p-value | <4 | ≥4 | p-value | <40 | ≥40 | p-value | |
| Age (years) | |||||||||||||||
| <25 | 4 | 27 | 0.024∗ | 31 | 0 | 0.022∗ | 6 | 25 | 0.0005∗ | 22 | 9 | 0.029∗ | 22 | 9 | 0.027∗ |
| 25–50 | 15 | 38 | 56 | 14 | 37 | 32 | 31 | 39 | 33 | 37 | |||||
| >50 | 8 | 8 | 15 | 7 | 16 | 7 | 9 | 13 | 8 | 14 | |||||
| Weight (kg) | |||||||||||||||
| <60 | 6 | 24 | 0.516 | 34 | 11 | 0.256 | 24 | 21 | 0.603 | 27 | 18 | 0.327 | 18 | 27 | 0.098 |
| 60–80 | 18 | 44 | 61 | 9 | 32 | 38 | 44 | 26 | 39 | 31 | |||||
| >80 | 3 | 5 | 7 | 1 | 3 | 5 | 7 | 1 | 6 | 2 | |||||
| Occupation | |||||||||||||||
| Student | 11 | 42 | 0.377 | 53 | 1 | 0.0001∗ | 16 | 38 | 0.0004∗ | 34 | 20 | 0.026∗ | 34 | 20 | 0.038∗ |
| Service | 3 | 9 | 16 | 3 | 8 | 11 | 11 | 8 | 11 | 8 | |||||
| Business | 5 | 10 | 15 | 5 | 13 | 8 | 7 | 13 | 6 | 14 | |||||
| Others | 8 | 12 | 18 | 12 | 22 | 7 | 10 | 20 | 12 | 18 | |||||
| Dwelling status | |||||||||||||||
| Urban | 15 | 46 | 0.497 | 57 | 3 | 0.0005∗ | 22 | 38 | 0.014∗ | 40 | 20 | 0.0004∗ | 39 | 21 | 0.003∗ |
| Rural | 12 | 27 | 45 | 18 | 37 | 26 | 22 | 41 | 24 | 39 | |||||
| PTE | |||||||||||||||
| Yes | 15 | 37 | 0.665 | 53 | 9 | 0.447 | 30 | 32 | 0.925 | 32 | 30 | 0.787 | 31 | 31 | 0.785 |
| No | 12 | 36 | 49 | 12 | 29 | 32 | 30 | 31 | 32 | 29 | |||||
PTE, Parental Tobacco Exposure; SI, Smoking initiation; CPD, Cigarette per day; TFC, Time from waking until the First Cigarette; FTND, Fagerstrom Test for Nicotine Dependence; CDS-12, Cigarette Dependence Scale -12 (∗) marks the values which are statistically significant where p < 0.05 indicates statistical significance.
3.2. Correlation of CHRNA5-A3-B4 gene cluster with smoking phenotypes
To examine the influence of these investigated SNPs on the age of daily smoking initiation, we reported a significant association of rs578776 AA genotype with late onset of smoking (OR = 3.49, 95% CI = 0.96–10.54, p = 0.016). However, no such link was discovered for the other two SNPs (Table 2).
Table 2.
Association of CHRNA5-A3-B4 gene cluster SNPs with different smoking phenotypes.
| Characteristics | SI age |
CPD |
TFC |
FTND |
CDS |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤16 years | >16 years | Adjusted OR (95%CI) | p-value | ≤20 | >20 | Adjusted OR (95%CI) | p-value | ≤30 | >30 | Adjusted OR (95%CI) | p-value | <4 | ≥4 | Adjusted OR (95% CI) | p-value | <40 | ≥40 | Adjusted OR (95% CI) | p-value | |
| rs16969968 G>A | ||||||||||||||||||||
| GG | 21 | 50 | Ref | 73 | 16 | Ref | 45 | 44 | ref | 43 | 46 | Ref | 46 | 43 | ref | |||||
| GA | 5 | 18 | 0.65 (0.21–1.99) | 0.153 | 22 | 5 | 1.03 (0.33–3.08) | 0.30 | 11 | 16 | 0.59 (0.27–1.53) | 0.121 | 16 | 11 | 0.62 (0.27–1.42) | 0.104 | 15 | 12 | 0.81 (0.35–1.95) | 0.237 |
| AA | 1 | 5 | 0.45 (0.05–4.30) | 0.168 | 7 | 0 | 0.29 (0.02–5.13) | 0.132 | 3 | 4 | 0.69 (0.16–3.34) | 0.228 | 3 | 4 | 1.20 (0.25–5.37) | 0.253 | 2 | 5 | 2.48 (0.46–13.26) | 0.081 |
| rs578776 G>A | ||||||||||||||||||||
| GG | 6 | 29 | Ref | 37 | 5 | Ref | 20 | 22 | ref | 17 | 25 | Ref | 19 | 23 | ref | |||||
| GA | 12 | 32 | 1.76 (0.57–5.13) | 0.096 | 43 | 13 | 2.16 (0.74–6.64) | 0.05 | 30 | 25 | 1.25 (0.54–2.77) | 0.159 | 27 | 29 | 0.70 (0.33–1.58) | 0.147 | 28 | 28 | 0.74 (0.34–1.76) | 0.201 |
| AA | 8 | 11 | 3.49 (0.96–10.54) | 0.016∗ | 18 | 3 | 1.18 (0.26–5.44) | 0.23 | 9 | 13 | 0.75 (0.27–1.96) | 0.198 | 15 | 6 | 0.27 (0.09–0.80) | 0.076∗ | 13 | 8 | 0.48 (0.17–1.40) | 0.08 |
| rs11072768 T>G | ||||||||||||||||||||
| TT | 11 | 29 | Ref | 42 | 11 | Ref | 26 | 28 | ref | 29 | 24 | Ref | 28 | 25 | ref | |||||
| TG | 13 | 28 | 1.20 (0.47–3.10) | 0.223 | 41 | 6 | 0.57 (0.18–1.69) | 0.092 | 23 | 23 | 1.09 (0.48–2.40) | 0.280 | 24 | 23 | 1.19 (0.50–2.41) | 0.221 | 25 | 22 | 0.94 (0.44–2.20) | 0.301 |
| GG | 2 | 14 | 0.38 (0.07–1.91) | 0.081 | 16 | 4 | 0.92 (0.27–3.31) | 0.32 | 9 | 11 | 0.85 (0.32–2.43) | 0.275 | 8 | 12 | 1.78 (0.63–4.96) | 0.09 | 9 | 11 | 1.33 (0.45–3.9) | 0.192 |
PTE, Parental Tobacco Exposure; SI, Smoking initiation; CPD, Cigarette per day; TFC, Time from waking until the First Cigarette; FTND, Fagerstrom Test for Nicotine; Dependence; CDS-12, Cigarette Dependence Scale -12 (∗) marks the values which are statistically significant after correcting for multiple testing.
Furthermore, the CPD value was used to assess the intensity of nicotine consumption, and our study found no significant connection between the three SNPs studied and the amount of tobacco consumed (Table 2).
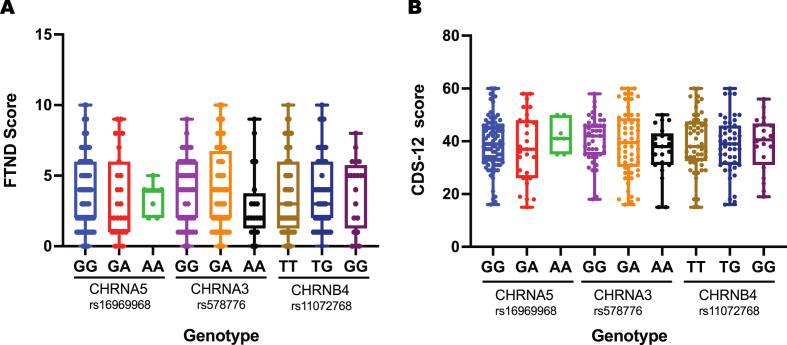
FTND, CDS scores, and TFC phenotype were recorded afterwards to study the influence of these selected polymorphisms on the degree of ND in smokers. According to the FTND and CDS-12 scores, rs16969968 AA genotype contributed to the development of ND. Conversely, given its high linkage disequilibrium (D′ = 1.0) and low correlation (r2 = 0.2478) with rs16969968 in Bengali ethnicity (Supplementary Figure 1), the rs578776 AA genotype showed a protective function against ND. In addition, the TFC phenotype was found to be unrelated to the SNPs studied (Table 2). Figure 1 shows the FTND and CDS-12 scores distribution among smokers of different CHRNA5-A3-B4 genotypes.
Figure 1.
Distribution of nicotine dependence test scores among smokers of different CHRNA5-A3-B4 genotypes A) FTND score, B) CDS-12 score.
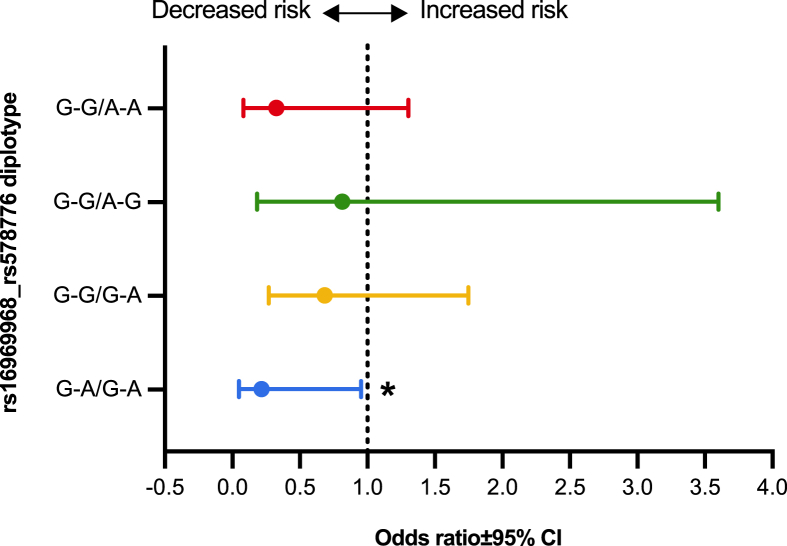
To further dig into the collective role of rs16969968 and rs578776 SNPs, different diplotypes, matched pair of haplotypes on homologous chromosomes [56], of these two polymorphisms having at least <5.0% frequency were correlated with their FTND scores. The diplotype analysis revealed that individuals having two low-risk G alleles for rs16969968 along with two low-risk A alleles for rs578776 were associated with significantly reduced risk of ND (OR = 0.2167, 95% CI = 0.05–0.95, p = 0.043) (Figure 2).
Figure 2.
Association of different rs16969968_rs578776 diplotypes with FTND scores. ∗p < 0.05.
3.3. Correlation between CHRNA5-A3-B4 gene expression and lung cancer risk
Our in-silico mRNA expression analysis of these three genes in different regions of brain and lungs revealed that the median expression of both CHRNA5 and CHRNA3 genes is highest in the cerebellum region, whereas basal ganglia express the highest level of CHRNB4 gene. Compared to the cerebellum, CHRNA5 and CHRNB4 genes are relatively lowly expressed in lung tissues, approximately by 3-fold and 4-fold, respectively and the expression of CHRNA3 was found negligible (only 1.2% relative to cerebellum) (Supplementary Figure 2 and Supplementary Table 1).
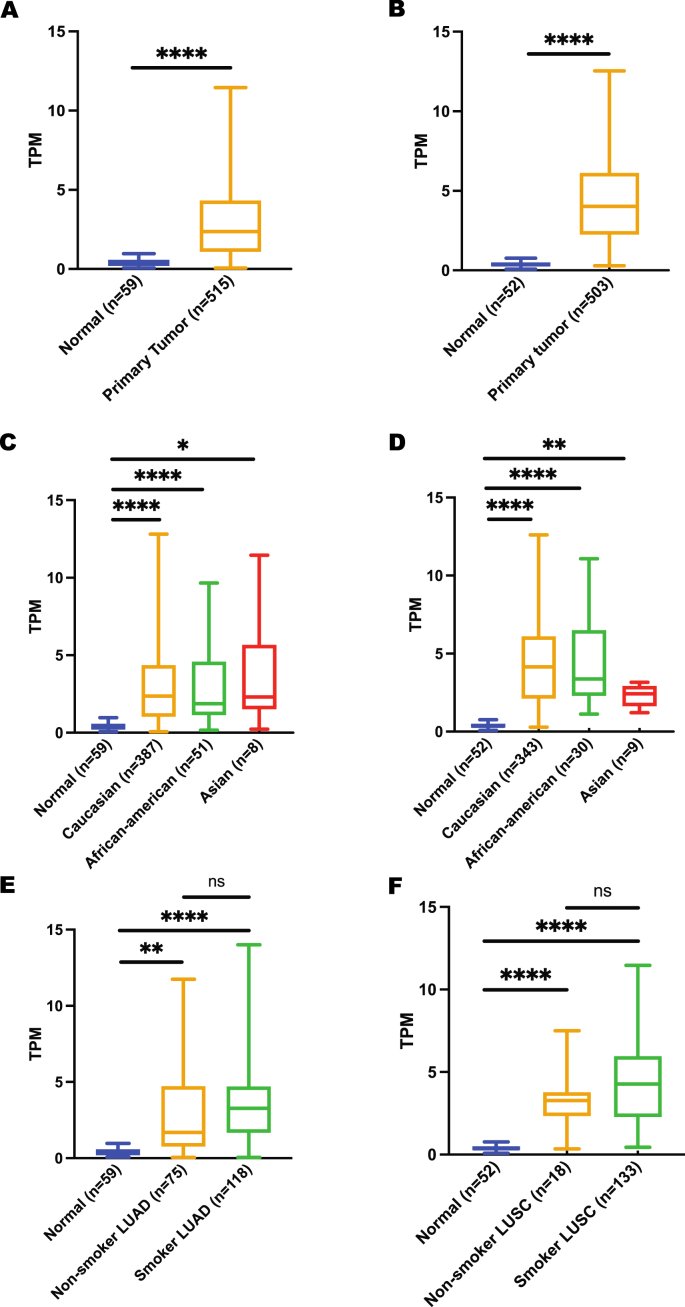
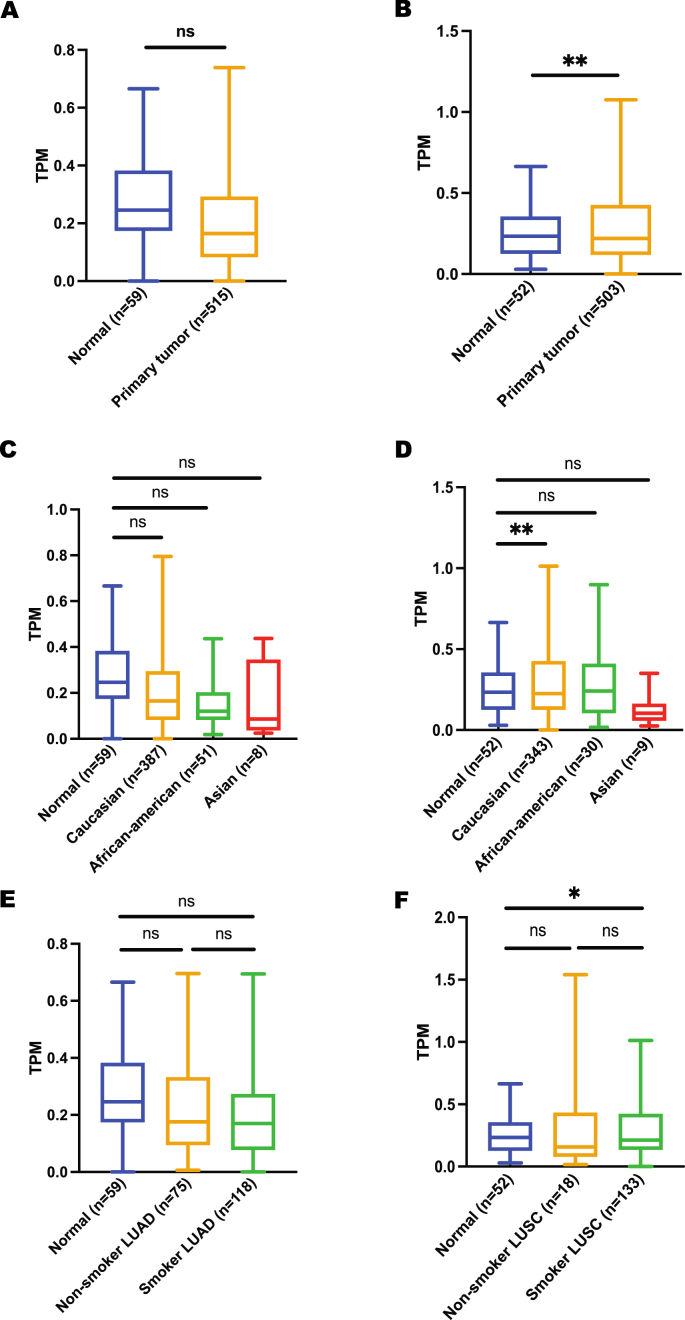
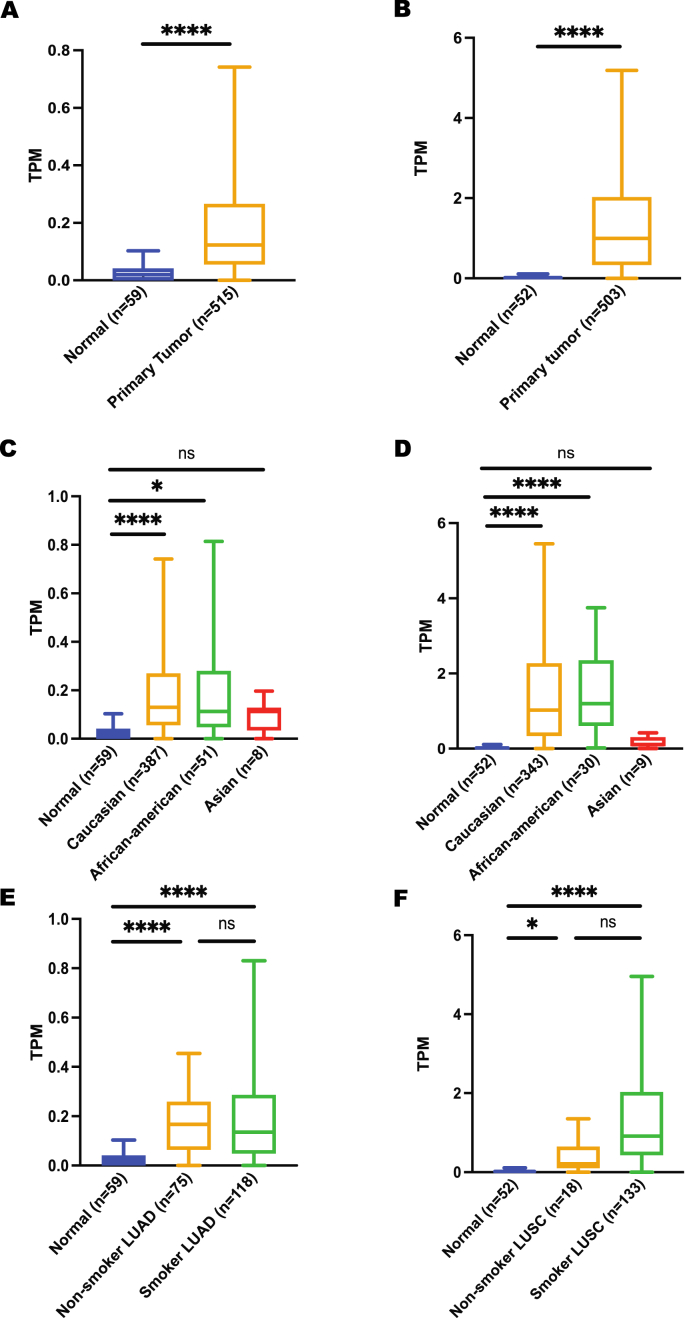
To correlate these expression data with lung cancer risk, expression between normal and lung cancer was obtained from the UALCAN database. CHRNA5 gene was found to be significantly upregulated in lung cancer patients irrespective of their cancer subtypes, ethnicities and smoking habits (Figure 3), suggesting a link between high CHRNA5 expression and lung cancer risk. In contrast to CHRNA5 overexpression in lung primary tumors, expression of CHRNA3 gene showed no significant difference between normal and lung cancer patients except for Caucasian ethnicity and LUSC subtypes (Figure 4), which could possibly be justified by the low expression level of this gene in lungs compared to major brain regions. In case of CHRNB4 gene expression, a trend similar to CHRNA5 expression was observed. However, it is important to highlight the fact that CHRNB4 expression exhibits insignificant results for Asian lung cancer patients (Figure 5). More importantly, despite the existence of some sort of relationship between normal and lung cancer patients’ gene expression for CHRNA5-A3-B4 gene cluster in terms of their smoking habits, none of these genes showed any significant difference when a comparison was made between smoker and non-smoker lung cancer patients.
Figure 3.
Expression analysis of CHRNA5 gene using UALCAN A) expression in LUAD, B) expression in LUSC, C) expression in LUAD based on patient’s ethnicity, D) expression in LUSC based on patient’s ethnicity, E) expression in LUAD based on patient’s smoking habit, F) expression in LUSC based on patient’s smoking habit. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001, ns p > 0.05.
Figure 4.
Expression analysis of CHRNA3 gene using UALCAN A) expression in LUAD, B) expression in LUSC, C) expression in LUAD based on patient’s ethnicity, D) expression in LUSC based on patient’s ethnicity, E) expression in LUAD based on patient’s smoking habit, F) expression in LUSC based on patient’s smoking habit. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001, ns p > 0.05.
Figure 5.
Expression analysis of CHRNB4 gene using UALCAN A) expression in LUAD, B) expression in LUSC, C) expression in LUAD based on patient’s ethnicity, D) expression in LUSC based on patient’s ethnicity, E) expression in LUAD based on patient’s smoking habit, F) expression in LUSC based on patient’s smoking habit. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001, ns p > 0.05.
Our analysis of CHRNA5 rs16969968 polymorphism also reported a significantly low expression for AA genotype compared to GG genotype (p = 5.00 × 10-09) in lung tissue (Supplementary Figure 3). No lung tissue specific data was found for CHRNA3 rs578776 and CHRNB4 rs11072768 polymorphisms in the GTEx portal.
4. Discussion
Numerous lines of evidence including ours have indicated a compelling link between CHRNA5-A3-B4 gene cluster variants (rs16969968-rs578776-rs11072768) and SS in Bangladeshi male smokers. In the present study, which primarily focused on the transition from regular smoking to addiction, we performed analyses to explore their relationship with ND by utilizing two independent ND measurement scales (FTND and CDS-12) covering a broad spectrum of smoking related phenotypes including smoking initiation age, smoking quantity and craving as well as dependence.
Individuals carrying AA genotype of CHRNA5 rs16969968 variant had 1.20 (FTND) and 2.48 (CDS-12) higher odds of developing ND, if compared with noncarriers, replicating several previously reported studies [18, 26, 26]. However, our findings failed to reach sufficient statistical significance considerably owing to the small study population or probably a true finding in reference to few previous similar reports [57, 58, 59]. Association of rs16969968 AA genotype with ND was well documented by in vitro functional studies [18, 60]. A change from aspartic acid (D398) to asparagine (N398) in rs16969968 variant initiates a series of event including the reduction of receptor’s function, concomitant prolongation of receptor desensitization and reduction of calcium ions permeability [60, 61], which ultimately generates an increased demand for nicotine in particular brain areas leading to development of ND [27, 36, 62]. In addition, conservation of D398 amino acid across divergent species [18] and a drastic reduction of sensitivity to nicotine induced behaviors and seizures in CHRNA5 mutant mouse model [63] might also reason the high ND in CHRNA5 rs16969968 AA genotype carriers. The CHRNA3 rs578776 variant AA genotype was found to be protective against ND and late-onset smoking which corroborates several previous studies [18, 64]. A study conducted on the brain reward system reported that rs578776 protective A-allele carriers show normal intrinsic reward sensitivity to intrinsically pleasant activity in smokers whereas G-allele carriers demonstrate increased sensitivity to nicotine induced stimulation [65]. Moreover, Hong and colleagues identified rs578776 at-risk G-allele mediated overactivation of a specific brain circuit in dorsal anterior cingulate cortex and left anterior thalamus regions using fMRI technology [66], which might be sensitive to nicotine exposure suggesting a plausible link between rs578776 at-risk G-allele and ND. Being an intron variant, CHRNB4 rs11072768 polymorphism has been found to be associated with smoking cessation, CPD [12, 43] as well as lung cancer [45]. Molecular study performed on B4 subunit null mice reported an increased tolerance development after chronically treated with nicotine [67], which might lead to neuroadaptation and subsequently to ND. Surprisingly, our analysis could not identify any association of rs11072768 with any of the investigated smoking-related phenotypes.
Analysis of different diplotypes of rs16969968_rs578776 also detected that smoker with common allele homozygous for rs16969968 and minor allele homozygous for rs578776 demonstrated an overall protection against ND, showing an independent role of each SNP. This finding was also potentially backed by the low correlation (r2 = 0.2478) value reported in BEB ethnicity (current study) and differences between rs578776 related dorsal anterior cingulate cortex (dACC)-thalamus circuit and rs16969968 related dACC-ventral striatum circuit [66].
ND features may also be modified by many social environment factors, according to social cognitive theory [68]. Age, occupation, and residence status were all found to be strongly correlated with ND-related traits in this study. On the other hand, parental smoking exposure had no effect on future ND development, which is in direct opposition to the social cognitive paradigm [68]. This discrepancy could possibly be due to other confounding factors which might be ruled out by large scale population study.
Our study also investigated the mRNA expression pattern of CHRNA5-A3-B4 gene cluster in normal and lung cancer patients using an in-silico approach. We reported overexpression of CHRNA5 and CHRNB4 in lung cancer patients compared to normal volunteers which is consistent with several previously published results [33, 34, 45, 69]. However, CHRNB4 expression was found to be nonsignificant in case of Asian ethnicity which in a way corroborates our findings regarding rs11072768 and ND. No such association was found for CHRNA3 gene. In case of smoker lung cancer patients, expression of all three genes was significantly higher than that of normal individuals. Interestingly, there was no significant difference in expression of CHRNA5-A3-B4 gene region revealed between non-smokers (n = 18) and smokers (n = 133) lung cancer patients, hence, requiring a large-scale investigation. SNP specific expression analysis using GTEx portal showed low expression of rs16969968 AA genotype in normal lung tissues and no data was found for other two SNPs. Altogether, based on the findings of our preliminary in-silico analysis, a comprehensive study comprising a substantial number of lung cancer patients with varying smoking status is required to establish any link among CHRNA5-A3-B4 gene cluster, ND and lung cancer risk.
In conclusion, our findings showed that rs16969968 and rs578776 SNPs of CHRNA5-A3-B4 gene cluster on chromosome 15q25 and certain demographic features influence ND in Bangladeshi male smokers, though our study was limited by small sample size and lack of any biochemical biomarker e.g. serum/urine cotinine (COT), trans-3'-hydroxy cotinine (3HC) levels measurement. This study replicating the gene to phenotype association with those self-reported phenotypes and genetic measurements allowed a further step towards understanding the relationship between the variants and ND in the Bangladeshi population. Further study with a large sample size should be performed to substantially validate these findings.
Declarations
Author contribution statement
Nusrat Islam Chaity: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mohd Nazmul Hasan Apu: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are thankful to the volunteers, their families, nurses and physicians who helped us to the successful completion of the study. The authors would also like to thank the Department of Clinical Pharmacy and Pharmacology, University of Dhaka, Bangladesh to provide lab facilities and other opportunities to carry out the research work.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Paquette D. 2015. The terrifying rate at which smokers die from smoking - The Washington Post.https://www.washingtonpost.com/news/wonk/wp/2015/02/26/the-terrifying-rate-at-which-smokers-die-from-smoking/ (accessed June 13, 2021) [Google Scholar]
- 2.Ezzati M., Lopez A.D. Regional, disease specific patterns of smoking-attributable mortality in 2000. Tobac. Control. 2004;13:388–395. doi: 10.1136/tc.2003.005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamruzzaman Md., Hossain A., Kabir E. Smoker’s characteristics, general health and their perception of smoking in the social environment: a study of smokers in Rajshahi City, Bangladesh. Z Gesundh Wiss. 2021:1–12. doi: 10.1007/s10389-020-01413-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasan M.K. Dhaka Tribune; 2018. WHO: Tobacco Responsible for 1 in 5 Deaths in Bangladesh.https://www.dhakatribune.com/health/2018/06/01/tobacco-1-in-5-deaths-bangladesh [Google Scholar]
- 5.WHO, Global Adult Tobacco Survey 2017. https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/global-adult-tobacco-survey
- 6.Clinical L. Practice guideline treating tobacco use and dependence 2008 update panel and staff, A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public health service report. Am. J. Prev. Med. 2008;35:158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aloise-Young P.A., Graham J.W., Hansen W.B. Peer influence on smoking initiation during early adolescence: a comparison of group members and group outsiders. J. Appl. Psychol. 1994;79:281–287. doi: 10.1037/0021-9010.79.2.281. [DOI] [PubMed] [Google Scholar]
- 8.Buller D.B., Borland R., Woodall W.G., Hall J.R., Burris-Woodall P., Voeks J.H. Understanding factors that influence smoking uptake. Tobac. Control. 2003;12(Suppl 4):IV16–25. doi: 10.1136/tc.12.suppl_4.iv16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitoria P.D., Salgueiro M.F., Silva S.A., de Vries H. Social influence, intention to smoke, and adolescent smoking behaviour longitudinal relations. Br. J. Health Psychol. 2011;16:779–798. doi: 10.1111/j.2044-8287.2010.02014.x. [DOI] [PubMed] [Google Scholar]
- 10.Lassi G., Taylor A.E., Timpson N.J., Kenny P.J., Mather R.J., Eisen T., Munafò M.R. The CHRNA5–A3–B4 gene cluster and smoking: from discovery to therapeutics. Trends Neurosci. 2016;39:851–861. doi: 10.1016/j.tins.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaimarri A., Moretti M., Riganti L., Zanardi A., Clementi F., Gotti C. Regulation of neuronal nicotinic receptor traffic and expression. Brain Res. Rev. 2007;55:134–143. doi: 10.1016/j.brainresrev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Li M.D., Yoon D., Lee J.-Y., Han B.-G., Niu T., Payne T.J., Ma J.Z., Park T. Associations of variants in CHRNA5/A3/B4 gene cluster with smoking behaviors in a Korean population. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012183. e12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picciotto M.R., Caldarone B.J., King S.L., Zachariou V. Nicotinic receptors in the brain: links between molecular biology and behavior. Neuropsychopharmacology. 2000;22:451–465. doi: 10.1016/S0893-133X(99)00146-3. [DOI] [PubMed] [Google Scholar]
- 14.Watkins S.S., Koob G.F., Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob. Res. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- 15.Baker T.B., Weiss R.B., Bolt D., von Niederhausern A., Fiore M.C., Dunn D.M., Piper M.E., Matsunami N., Smith S.S., Coon H., McMahon W.M., Scholand M.B., Singh N., Hoidal J.R., Kim S.-Y., Leppert M.F., Cannon D.S. Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob. Res. 2009;11:785–796. doi: 10.1093/ntr/ntp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berrettini W., Yuan X., Tozzi F., Song K., Francks C., Chilcoat H., Waterworth D., Muglia P., Mooser V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol. Psychiatr. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bierut L.J., Madden P.A.F., Breslau N., Johnson E.O., Hatsukami D., Pomerleau O.F., Swan G.E., Rutter J., Bertelsen S., Fox L., Fugman D., Goate A.M., Hinrichs A.L., Konvicka K., Martin N.G., Montgomery G.W., Saccone N.L., Saccone S.F., Wang J.C., Chase G.A., Rice J.P., Ballinger D.G. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum. Mol. Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bierut L.J., Stitzel J.A., Wang J.C., Hinrichs A.L., Grucza R.A., Xuei X., Saccone N.L., Saccone S.F., Bertelsen S., Fox L., Horton W.J., Breslau N., Budde J., Cloninger C.R., Dick D.M., Foroud T., Hatsukami D., Hesselbrock V., Johnson E.O., Kramer J., Kuperman S., Madden P.A.F., Mayo K., Nurnberger J., Pomerleau O., Porjesz B., Reyes O., Schuckit M., Swan G., Tischfield J.A., Edenberg H.J., Rice J.P., Goate A.M. Variants in nicotinic receptors and risk for nicotine dependence. Am. J. Psychiatr. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breitling L.P., Dahmen N., Mittelstraß K., Illig T., Rujescu D., Raum E., Winterer G., Brenner H. Smoking cessation and variations in nicotinic acetylcholine receptor subunits α-5, α-3, and β-4 genes. Biol. Psychiatr. 2009;65:691–695. doi: 10.1016/j.biopsych.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Caporaso N., Gu F., Chatterjee N., Sheng-Chih J., Yu K., Yeager M., Chen C., Jacobs K., Wheeler W., Landi M.T., Ziegler R.G., Hunter D.J., Chanock S., Hankinson S., Kraft P., Bergen A.W. Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaity N.I., Sultana T.N., Hasan M.M., Shrabonee I.I., Nahid N.A., Islam M.S., Apu M.N.H. Nicotinic acetylcholine gene cluster CHRNA5-A3-B4 variants influence smoking status in a Bangladeshi population. Pharmacol. Rep. 2021;73:574–582. doi: 10.1007/s43440-021-00243-1. [DOI] [PubMed] [Google Scholar]
- 22.Doyle G.A., Wang M.-J., Chou A.D., Oleynick J.U., Arnold S.E., Buono R.J., Ferraro T.N., Berrettini W.H. Vitro and ex vivo analysis of CHRNA3 and CHRNA5 haplotype expression. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freathy R.M., Ring S.M., Shields B., Galobardes B., Knight B., Weedon M.N., Smith G.D., Frayling T.M., Hattersley A.T. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum. Mol. Genet. 2009;18:2922–2927. doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lessov-Schlaggar C.N., Pergadia M.L., Khroyan T.V., Swan G.E. Genetics of nicotine dependence and pharmacotherapy. Biochem. Pharmacol. 2008;75:178–195. doi: 10.1016/j.bcp.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M.D., Burmeister M. New insights into the genetics of addiction. Nat. Rev. Genet. 2009;10:225–231. doi: 10.1038/nrg2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saccone N.L., Saccone S.F., Hinrichs A.L., Stitzel J.A., Duan W., Pergadia M.L., Agrawal A., Breslau N., Grucza R.A., Hatsukami D., Johnson E.O., Madden P.A.F., Swan G.E., Wang J.C., Goate A.M., Rice J.P., Bierut L.J. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009;150B:453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saccone N.L., Wang J.C., Breslau N., Johnson E.O., Hatsukami D., Saccone S.F., Grucza R.A., Sun L., Duan W., Budde J., Culverhouse R.C., Fox L., Hinrichs A.L., Steinbach J.H., Wu M., Rice J.P., Goate A.M., Bierut L.J. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 2009;69:6848–6856. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saccone S.F., Hinrichs A.L., Saccone N.L., Chase G.A., Konvicka K., Madden P.A.F., Breslau N., Johnson E.O., Hatsukami D., Pomerleau O., Swan G.E., Goate A.M., Rutter J., Bertelsen S., Fox L., Fugman D., Martin N.G., Montgomery G.W., Wang J.C., Ballinger D.G., Rice J.P., Bierut L.J. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum. Mol. Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlaepfer I.R., Hoft N.R., Collins A.C., Corley R.P., Hewitt J.K., Hopfer C.J., Lessem J.M., McQueen M.B., Rhee S.H., Ehringer M.A. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol. Psychiatr. 2008;63:1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorgeirsson T.E., Stefansson K. Genetics of smoking behavior and its consequences: the role of nicotinic acetylcholine receptors. Biol. Psychiatr. 2008;64:919–921. doi: 10.1016/j.biopsych.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorgeirsson T.E., Stefansson K. Commentary: gene-environment interactions and smoking-related cancers. Int. J. Epidemiol. 2010;39:577–579. doi: 10.1093/ije/dyp385. [DOI] [PubMed] [Google Scholar]
- 32.Wang J.C., Cruchaga C., Saccone N.L., Bertelsen S., Liu P., Budde J.P., Duan W., Fox L., Grucza R.A., Kern J., Mayo K., Reyes O., Rice J., Saccone S.F., Spiegel N., Steinbach J.H., Stitzel J.A., Anderson M.W., You M., Stevens V.L., Bierut L.J., Goate A.M. COGEND collaborators and GELCC collaborators, Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum. Mol. Genet. 2009;18:3125–3135. doi: 10.1093/hmg/ddp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amos C.I., Wu X., Broderick P., Gorlov I.P., Gu J., Eisen T., Dong Q., Zhang Q., Gu X., Vijayakrishnan J., Sullivan K., Matakidou A., Wang Y., Mills G., Doheny K., Tsai Y.-Y., Chen W.V., Shete S., Spitz M.R., Houlston R.S. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat. Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hung R.J., McKay J.D., Gaborieau V., Boffetta P., Hashibe M., Zaridze D., Mukeria A., Szeszenia-Dabrowska N., Lissowska J., Rudnai P., Fabianova E., Mates D., Bencko V., Foretova L., Janout V., Chen C., Goodman G., Field J.K., Liloglou T., Xinarianos G., Cassidy A., McLaughlin J., Liu G., Narod S., Krokan H.E., Skorpen F., Elvestad M.B., Hveem K., Vatten L., Linseisen J., Clavel-Chapelon F., Vineis P., Bueno-de-Mesquita H.B., Lund E., Martinez C., Bingham S., Rasmuson T., Hainaut P., Riboli E., Ahrens W., Benhamou S., Lagiou P., Trichopoulos D., Holcátová I., Merletti F., Kjaerheim K., Agudo A., Macfarlane G., Talamini R., Simonato L., Lowry R., Conway D.I., Znaor A., Healy C., Zelenika D., Boland A., Delepine M., Foglio M., Lechner D., Matsuda F., Blanche H., Gut I., Heath S., Lathrop M., Brennan P. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 35.Liu P., Vikis H.G., Wang D., Lu Y., Wang Y., Schwartz A.G., Pinney S.M., Yang P., de Andrade M., Petersen G.M., Wiest J.S., Fain P.R., Gazdar A., Gaba C., Rothschild H., Mandal D., Coons T., Lee J., Kupert E., Seminara D., Minna J., Bailey-Wilson J.E., Wu X., Spitz M.R., Eisen T., Houlston R.S., Amos C.I., Anderson M.W., You M. Familial aggregation of common sequence variants on 15q24-25.1 in lung cancer. J. Natl. Cancer Inst. 2008;100:1326–1330. doi: 10.1093/jnci/djn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X., Chen J., Williamson V.S., An S.-S., Hettema J.M., Aggen S.H., Neale M.C., Kendler K.S. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009;150B:926–933. doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong L.E., Yang X., Wonodi I., Hodgkinson C.A., Goldman D., Stine O.C., Stein E.S., Thaker G.K. A CHRNA5 allele related to nicotine addiction and schizophrenia. Gene Brain Behav. 2011;10:530–535. doi: 10.1111/j.1601-183X.2011.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kendler K.S., Chen X., Dick D., Maes H., Gillespie N., Neale M.C., Riley B. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nat. Neurosci. 2012;15:181–189. doi: 10.1038/nn.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu A.Z.X., Renner C.C., Hatsukami D.K., Benowitz N.L., Tyndale R.F. CHRNA5-A3-B4 genetic variants alter nicotine intake and interact with tobacco use to influence body weight in Alaska Native tobacco users. Addiction. 2013;108:1818–1828. doi: 10.1111/add.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartz S.M., Lin P., Edenberg H.J., Xuei X., Rochberg N., Saccone S., Berrettini W., Nelson E., Nurnberger J., Bierut L.J., Rice J.P. NIMH Genetics Initiative Bipolar Disorder Consortium, Genetic association of bipolar disorder with the β(3) nicotinic receptor subunit gene. Psychiatr. Genet. 2011;21:77–84. doi: 10.1097/YPG.0b013e32834135eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Improgo Ma.R.D., Scofield M.D., Tapper A.R., Gardner P.D. The nicotinic acetylcholine receptor CHRNA5/A3/B4 gene cluster: dual role in nicotine addiction and lung cancer. Prog. Neurobiol. 2010;92:212–226. doi: 10.1016/j.pneurobio.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saccone N.L., Culverhouse R.C., Schwantes-An T.-H., Cannon D.S., Chen X., Cichon S., Giegling I., Han S., Han Y., Keskitalo-Vuokko K., Kong X., Landi M.T., Ma J.Z., Short S.E., Stephens S.H., Stevens V.L., Sun L., Wang Y., Wenzlaff A.S., Aggen S.H., Breslau N., Broderick P., Chatterjee N., Chen J., Heath A.C., Heliövaara M., Hoft N.R., Hunter D.J., Jensen M.K., Martin N.G., Montgomery G.W., Niu T., Payne T.J., Peltonen L., Pergadia M.L., Rice J.P., Sherva R., Spitz M.R., Sun J., Wang J.C., Weiss R.B., Wheeler W., Witt S.H., Yang B.-Z., Caporaso N.E., Ehringer M.A., Eisen T., Gapstur S.M., Gelernter J., Houlston R., Kaprio J., Kendler K.S., Kraft P., Leppert M.F., Li M.D., Madden P.A.F., Nöthen M.M., Pillai S., Rietschel M., Rujescu D., Schwartz A., Amos C.I., Bierut L.J. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamidovic A., Kasberger J.L., Young T.R., Goodloe R.J., Redline S., Buxbaum S.G., Benowitz N.L., Bergen A.W., Butler K.R., Franceschini N., Gharib S.A., Hitsman B., Levy D., Meng Y., Papanicolaou G.J., Preis S.R., Spring B., Styn M.A., Tong E.K., White W.B., Wiggins K.L., Jorgenson E. Genetic variability of smoking persistence in African Americans. Cancer Prev. Res. 2011;4:729–734. doi: 10.1158/1940-6207.CAPR-10-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J.Z., Tozzi F., Waterworth D.M., Pillai S.G., Muglia P., Middleton L., Berrettini W., Knouff C.W., Yuan X., Waeber G., Vollenweider P., Preisig M., Wareham N.J., Zhao J.H., Loos R.J.F., Barroso I., Khaw K.-T., Grundy S., Barter P., Mahley R., Kesaniemi A., McPherson R., Vincent J.B., Strauss J., Kennedy J.L., Farmer A., McGuffin P., Day R., Matthews K., Bakke P., Gulsvik A., Lucae S., Ising M., Brueckl T., Horstmann S., Wichmann H.-E., Rawal R., Dahmen N., Lamina C., Polasek O., Zgaga L., Huffman J., Campbell S., Kooner J., Chambers J.C., Burnett M.S., Devaney J.M., Pichard A.D., Kent K.M., Satler L., Lindsay J.M., Waksman R., Epstein S., Wilson J.F., Wild S.H., Campbell H., Vitart V., Reilly M.P., Li M., Qu L., Wilensky R., Matthai W., Hakonarson H.H., Rader D.J., Franke A., Wittig M., Schäfer A., Uda M., Terracciano A., Xiao X., Busonero F., Scheet P., Schlessinger D., St Clair D., Rujescu D., Abecasis G.R., Grabe H.J., Teumer A., Völzke H., Petersmann A., John U., Rudan I., Hayward C., Wright A.F., Kolcic I., Wright B.J., Thompson J.R., Balmforth A.J., Hall A.S., Samani N.J., Anderson C.A., Ahmad T., Mathew C.G., Parkes M., Satsangi J., Caulfield M., Munroe P.B., Farrall M., Dominiczak A., Worthington J., Thomson W., Eyre S., Barton A., Wellcome Trust Case Control Consortium. Mooser V., Francks C., Marchini J. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat. Genet. 2010;42:436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y., Jiang M., Li Q., Liang W., He Q., Chen W., He J. Chromosome 15q25 (CHRNA3-CHRNB4) variation indirectly impacts lung cancer risk in Chinese males. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization, editor. Guidelines for Controlling and Monitoring the Tobacco Epidemic. World Health Organization; Geneva: 1998. [Google Scholar]
- 47.World Medical Association World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 48.Etter J.F., Duc T.V., Perneger T.V. Validity of the Fagerström test for nicotine dependence and of the Heaviness of Smoking Index among relatively light smokers. Addiction. 1999;94:269–281. doi: 10.1046/j.1360-0443.1999.94226910.x. [DOI] [PubMed] [Google Scholar]
- 49.Heatherton T.F., Kozlowski L.T., Frecker R.C., Fagerström K.O. The fagerström test for nicotine dependence: a revision of the fagerström tolerance questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 50.John U., Meyer C., Hapke U., Rumpf H.-J., Schumann A., Adam C., Alte D., Lüdemann J. The Fagerström test for nicotine dependence in two adult population samples-potential influence of lifetime amount of tobacco smoked on the degree of dependence. Drug Alcohol Depend. 2003;71:1–6. doi: 10.1016/s0376-8716(03)00038-3. [DOI] [PubMed] [Google Scholar]
- 51.Weiss R.B., Baker T.B., Cannon D.S., von Niederhausern A., Dunn D.M., Matsunami N., Singh N.A., Baird L., Coon H., McMahon W.M., Piper M.E., Fiore M.C., Scholand M.B., Connett J.E., Kanner R.E., Gahring L.C., Rogers S.W., Hoidal J.R., Leppert M.F. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Broms U., Wedenoja J., Largeau M.R., Korhonen T., Pitkäniemi J., Keskitalo-Vuokko K., Häppölä A., Heikkilä K.H., Heikkilä K., Ripatti S., Sarin A.-P., Salminen O., Paunio T., Pergadia M.L., Madden P.A.F., Kaprio J., Loukola A. Analysis of detailed phenotype profiles reveals CHRNA5-CHRNA3-CHRNB4 gene cluster association with several nicotine dependence traits. Nicotine Tob. Res. 2012;14:720–733. doi: 10.1093/ntr/ntr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bin Sayeed M.S., Hasan Apu M.N., Munir M.T., Ahmed M.U., Islam M.S., Haq M.M., Ahsan C.H., Rashid M.A., Shin J.G., Hasnat A. Prevalence of CYP2C19 alleles, pharmacokinetic and pharmacodynamic variation of clopidogrel and prasugrel in Bangladeshi population. Clin. Exp. Pharmacol. Physiol. 2015;42:451–457. doi: 10.1111/1440-1681.12390. [DOI] [PubMed] [Google Scholar]
- 54.Lonsdale J., Thomas J., Salvatore M., Phillips R., Lo E., Shad S., Hasz R., Walters G., Garcia F., Young N., Foster B., Moser M., Karasik E., Gillard B., Ramsey K., Sullivan S., Bridge J., Magazine H., Syron J., Fleming J., Siminoff L., Traino H., Mosavel M., Barker L., Jewell S., Rohrer D., Maxim D., Filkins D., Harbach P., Cortadillo E., Berghuis B., Turner L., Hudson E., Feenstra K., Sobin L., Robb J., Branton P., Korzeniewski G., Shive C., Tabor D., Qi L., Groch K., Nampally S., Buia S., Zimmerman A., Smith A., Burges R., Robinson K., Valentino K., Bradbury D., Cosentino M., Diaz-Mayoral N., Kennedy M., Engel T., Williams P., Erickson K., Ardlie K., Winckler W., Getz G., DeLuca D., MacArthur D., Kellis M., Thomson A., Young T., Gelfand E., Donovan M., Meng Y., Grant G., Mash D., Marcus Y., Basile M., Liu J., Zhu J., Tu Z., Cox N.J., Nicolae D.L., Gamazon E.R., Im H.K., Konkashbaev A., Pritchard J., Stevens M., Flutre T., Wen X., Dermitzakis E.T., Lappalainen T., Guigo R., Monlong J., Sammeth M., Koller D., Battle A., Mostafavi S., McCarthy M., Rivas M., Maller J., Rusyn I., Nobel A., Wright F., Shabalin A., Feolo M., Sharopova N., Sturcke A., Paschal J., Anderson J.M., Wilder E.L., Derr L.K., Green E.D., Struewing J.P., Temple G., Volpi S., Boyer J.T., Thomson E.J., Guyer M.S., Ng C., Abdallah A., Colantuoni D., Insel T.R., Koester S.E., Little A.R., Bender P.K., Lehner T., Yao Y., Compton C.C., Vaught J.B., Sawyer S., Lockhart N.C., Demchok J., Moore H.F. The genotype-tissue expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chandrashekar D.S., Bashel B., Balasubramanya S.A.H., Creighton C.J., Ponce-Rodriguez I., Chakravarthi B.V.S.K., Varambally S. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Q., Cui Y., Wu R. A multilocus likelihood approach to joint modeling of linkage, parental diplotype and gene order in a full-sib family. BMC Genet. 2004;5:20. doi: 10.1186/1471-2156-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amos C.I., Gorlov I.P., Dong Q., Wu X., Zhang H., Lu E.Y., Scheet P., Greisinger A.J., Mills G.B., Spitz M.R. Nicotinic acetylcholine receptor region on chromosome 15q25 and lung cancer risk among African Americans: a case-control study. J. Natl. Cancer Inst. 2010;102:1199–1205. doi: 10.1093/jnci/djq232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Etter J.-F., Hoda J.-C., Perroud N., Munafò M., Buresi C., Duret C., Neidhart E., Malafosse A., Bertrand D. Association of genes coding for the alpha-4, alpha-5, beta-2 and beta-3 subunits of nicotinic receptors with cigarette smoking and nicotine dependence. Addict. Behav. 2009;34:772–775. doi: 10.1016/j.addbeh.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 59.Verde Z., Santiago C., González-Moro J.M.R., Ramos P. de L., Martín S.L., Bandrés F., Lucia A., Gómez-Gallego F. ‘Smoking genes’: a genetic association study. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuryatov A., Berrettini W., Lindstrom J. Acetylcholine receptor (AChR) α5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (α4β2)₂α5 AChR function. Mol. Pharmacol. 2011;79:119–125. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tammimäki A., Herder P., Li P., Esch C., Laughlin J.R., Akk G., Stitzel J.A. Impact of human D398N single nucleotide polymorphism on intracellular calcium response mediated by α3β4α5 nicotinic acetylcholine receptors. Neuropharmacology. 2012;63:1002–1011. doi: 10.1016/j.neuropharm.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherva R., Wilhelmsen K., Pomerleau C.S., Chasse S.A., Rice J.P., Snedecor S.M., Bierut L.J., Neuman R.J., Pomerleau O.F. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction. 2008;103:1544–1552. doi: 10.1111/j.1360-0443.2008.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salas R., Orr-Urtreger A., Broide R.S., Beaudet A., Paylor R., De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol. Pharmacol. 2003;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- 64.Stevens V.L., Bierut L.J., Talbot J.T., Wang J.C., Sun J., Hinrichs A.L., Thun M.J., Goate A., Calle E.E. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol. Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robinson J.D., Versace F., Lam C.Y., Minnix J.A., Engelmann J.M., Cui Y., Karam-Hage M., Shete S.S., Tomlinson G.E., Chen T.T.-L., Wetter D.W., Green C.E., Cinciripini P.M. The CHRNA3 rs578776 variant is associated with an intrinsic reward sensitivity deficit in smokers. Front. Psychiatr. 2013;4:114. doi: 10.3389/fpsyt.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hong L.E., Hodgkinson C.A., Yang Y., Sampath H., Ross T.J., Buchholz B., Salmeron B.J., Srivastava V., Thaker G.K., Goldman D., Stein E.A. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13509–13514. doi: 10.1073/pnas.1004745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meyers E.E., Loetz E.C., Marks M.J. Differential expression of the beta4 neuronal nicotinic receptor subunit affects tolerance development and nicotinic binding sites following chronic nicotine treatment. Pharmacol. Biochem. Behav. 2015;130:1–8. doi: 10.1016/j.pbb.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bandura A. Prentice-Hall, Inc; Englewood Cliffs, NJ, US: 1986. Social Foundations of Thought and Action: A Social Cognitive Theory. [Google Scholar]
- 69.Thorgeirsson T.E., Geller F., Sulem P., Rafnar T., Wiste A., Magnusson K.P., Manolescu A., Thorleifsson G., Stefansson H., Ingason A., Stacey S.N., Bergthorsson J.T., Thorlacius S., Gudmundsson J., Jonsson T., Jakobsdottir M., Saemundsdottir J., Olafsdottir O., Gudmundsson L.J., Bjornsdottir G., Kristjansson K., Skuladottir H., Isaksson H.J., Gudbjartsson T., Jones G.T., Mueller T., Gottsäter A., Flex A., Aben K.K.H., de Vegt F., Mulders P.F.A., Isla D., Vidal M.J., Asin L., Saez B., Murillo L., Blondal T., Kolbeinsson H., Stefansson J.G., Hansdottir I., Runarsdottir V., Pola R., Lindblad B., van Rij A.M., Dieplinger B., Haltmayer M., Mayordomo J.I., Kiemeney L.A., Matthiasson S.E., Oskarsson H., Tyrfingsson T., Gudbjartsson D.F., Gulcher J.R., Jonsson S., Thorsteinsdottir U., Kong A., Stefansson K. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.