Abstract
Vitamin D3 (VD3), an essential nutrient for animals, has been demonstrated to stimulate the uptake of certain amino acids. However, the role of VD3 in the intestine, the primary site for digestion and absorption of nutrients, remains poorly characterized. Here, the grass carp (Ctenopharyngodon idella) was studied to assess the influence of different doses of VD3 (15.2, 364.3, 782.5, 1,167.9, 1,573.8, and 1,980.1 IU/kg) on growth performance, intestinal morphology, digestive absorption, amino acid transport, and potential signaling molecule levels in a feeding experiment. As a result, dietary VD3 improved growth performance, intestinal structure, and digestive and brush border enzyme activities. Additionally, most intestinal free amino acids and their transporters were upregulated after VD3 intake, except for Ala, Lys, Asp, Leu, solute carrier (SLC) 7A7, SLC1A5, and SLC1A3 mRNA in different segments, Leu and SLC6A14 mRNA in the proximal intestine, and SLC7A5 mRNA in the mid and distal intestine. In the crucial target of rapamycin (TOR) signal pathway of amino acid transport, the gene and protein expression of TOR, S6 kinase 1, and activating transcription factor 4 were elevated, whereas 4E-binding protein 1 was decreased, further suggesting an advanced amino acid absorption capacity in the fish due to VD3 supplementation. Based on percentage weight gain, feed efficiency, and trypsin activity, the VD3 requirements of on-growing grass carp were estimated to be 968.33, 1,005.00, and 1,166.67 IU/kg, respectively. Our findings provide novel recommendations for VD3 supplementation to promote digestion and absorption capacities of fish, contributing to the overall productivity of aquaculture.
Keywords: Vitamin D3, Intestinal segment, Intestinal absorption, Amino acid transporter, Peptide transporter, TOR signaling
1. Introduction
Vitamin D3 (VD3) is of importance for animals (Mora et al., 2008). It is obtained directly from the diet by all vertebrates, including fish (Dusso et al., 2005), and is transported and absorbed by the intestines (Haddad et al., 1993). Fish accumulate VD3 throughout their lifetime, and appropriate levels are maintained through diet (Graff et al., 2002). VD3 deficiency reduces specific growth rate (SGR) and feed conversion ratio in juvenile Siberian sturgeon, Acipenser baerii (Wang et al., 2017), juvenile black carp, Mylopharyngodon piceus (Wu et al., 2020), and orange-spotted grouper, Epinephelus coioides (He et al., 2021). Growth performance and feed utilization in fish are associated with intestinal digestion and absorption functions (Zhang et al., 2019), especially in the case of stomachless fish (Le et al., 2019). However, the influence of VD3 on the intestine, the primary site of digestion and absorption, and the related underlying mechanism remain poorly understood. Chen et al. (2017) reported that, in the primary human trophoblast cells, VD3 facilitated the absorption of certain amino acids (AAs) by accelerating the activity of associated amino acid transporters (AATs), indicating that VD3 may influence the absorption capacity on the intestine. Moreover, different intestinal sections of fish exhibit varied digestive abilities (Yuan et al., 2020). Therefore, the specific effects of VD3 are likely to vary throughout the intestine and need to be further studied.
The digestion and absorption capacities of fish are reflected by the activities of digestive enzymes (e.g., trypsin and chymotrypsin) and intestinal brush border enzymes (e.g., alkaline phosphatase [AKP] and Na+-K+-ATPase) (García-Gasca et al., 2006). To date, just one study has explored the relationship between VD3 and digestive enzymes in the animal gut; the study showed that VD3 improved the activity of chymotrypsin in laying hens (Korének et al., 2000). However, no report has focused on the influence of VD3 on brush border enzymes in animal intestines. Butyrate and manganese have been proved to elevate the brush border enzyme activities (e.g. trypsin, chymotrypsin, lipase, and amylase) and the levels of creatine kinas (CK) and γ-glutamyl transpeptidase (γ-GT), respectively, in the intestines of grass carp (Tang et al., 2016; Tian et al., 2017). VD3 can increase butyrate and manganese levels in cells (Claro da Silva et al., 2016; Tanaka et al., 1990). Furthermore, these findings suggest that VD3 affects the digestive and brush border enzymes activities in the animal intestine, and this requires further explanation.
Digestive enzymes are close-connected with nutrient digestion (Mourad and Saadé, 2011). Dietary protein, a fundamental nutrient, is decomposed into AAs and small peptides in the intestines (Coomer et al., 1993). The absorption and transportation of AAs and small peptides occur via AATs and small peptide transporters, respectively (Daniel, 2004; Ogihara et al., 1999). The AATs can be classified into four types based on their transport mechanisms and substrate specificity: neutral and cationic AATs (such as solute carrier [SLC]7A7), cationic AATs (such as SLC7A1), neutral AATs (such as SLC7A5), and anionic AATs (such as SLC1A2) (Perland and Fredriksson, 2017). SLC15A1 is a peptide transporter (PepT1) that is highly expressed in animal intestines (Christensen, 1990). Chen et al. (2017) demonstrated that VD3 increases the activity of SLC38A2 in the primary human trophoblast cells. However, to our knowledge, no studies have explored the influence of VD3 on the absorption capacity of AAs and small peptides in animal intestines. In rat skeletal muscle cells, GSK3 enhanced SLC38A2 mRNA expression (Stretton et al., 2019), and VD3 increased GSK3 contents in a C2C12 cell line (Salles et al., 2013). Moreover, low levels of insulin decreased SLC7A1 expression in umbilical cord veins endothelial cells (González et al., 2011). VD3 stimulated pancreatic insulin secretion (Zeitz et al., 2003). Therefore, VD3 potentially influences the absorptive capacity of AAs and relevant transporters in the intestines, which warrants a detailed study.
AAs and peptides are regulated by the target of rapamycin (TOR) signaling pathway (Benner et al., 2011). The mammalian TOR (mTOR) signaling regulates AATs by controlling activating transcription factor 4 (ATF4) mRNA, which is negatively regulated by 4E-binding protein 1 (4E-BP1) (Park et al., 2017). Studies investigating the relationship between VD3 and the TOR signaling pathway during intestinal assimilation of animals are limited. In cardiac muscle cells, the expression of mTOR and ribosomal protein S6 kinase 1 (S6K1) can be activated by protein kinase C (PKC) (Moschella et al., 2007), the expression of which is mediated by VD3 in chondrocytes (Sylvia et al., 1996). Additionally, in the C2C12 muscle cell line, VD3 can activate c-Jun N-terminal kinase (JNK) expression (Buitrago et al., 2006), which inhibits ATF4 mRNA expression in MC3T3-E1 cells (Matsuguchi et al., 2009). These studies indicate that VD3 presumably accommodates AAs and peptide transporters in the animal intestine through the TOR signaling pathway, which requires further exploration.
Grass carp (Ctenopharyngodon idella), originally distributed in China, has now been introduced in over 100 countries and is considered one of the most important freshwater aquaculture species (Wu et al., 2012). Currently, no research has focused on the VD3 requirements of grass carp. Furthermore, the reported nutritional requirements of fish are based on different indicators (Fang et al., 2020). Hence, it is of worthiness to estimate the VD3 requirements of on-growing grass carp based on the various indicators.
In this study, we hypothesize that VD3 supplementation increases the growth performance and intestinal digestion and absorption capacities of grass carp (C. idella) through a mechanism related to the TOR signaling pathway. To test this hypothesis, we aimed to investigate the effects of VD3 on intestinal structural integrity, digestive enzyme (trypsin, chymotrypsin, and amylase), and brush border enzyme (AKP, Na+/K+-ATPase, γ-GT, and CK) activities in different segments of the grass carp intestine. To the best of our knowledge, this is the first investigation on VD3 as it pertains to AATs, PepT1 transport, and associated signaling molecules (TOR/S6K1, 4E-BP1, and ATF4) in each intestinal segment of on-growing grass carp. This study offers insight into the VD3 requirements of on-growing grass carp according to various indicators and can be used to guide the production of ideal commercial feeds, improving animal health and yield in grass carp aquaculture operations.
2. Materials and methods
The current study was approved by the procedures and guidelines of the University of Sichuan Agricultural Animal Care Advisory Committee (No. PXR-2019114009).
2.1. Experimental diets
The formula and approximate components of the experimental diets are displayed in Table 1. Protein sources were mainly composed with soybean protein concentrate, gelatin, and casein, with linseed and soy oil chosen as lipid sources. Dietary protein content reached 300 g/kg feed, which has been demonstrated to be adequate by the National Research Council (NRC, 2011). Different levels of VD3 (500,000 IU/g) was provided: 0 (un-supplemented control), 400, 800, 1,200, 1,600, and 2,000 IU/kg feed, and corn starch was used to adjust dietary weight. Following analysis approaches of Takeuchi et al. (1984), the final amounts of VD3 in each treatment were determined as 15.2 (un-supplemented control), 364.3, 782.5, 1,167.9, 1,573.8, and 1,980.1 IU/kg feed. The ingredients were mixed, pressed into pellets, and stored at −20 °C.
Table 1.
Composition and nutrient levels of basal diet (dry matter basis, g/kg).
| Item | Content |
|---|---|
| Ingredients | |
| Casein | 135.0 |
| Gelatin | 44.7 |
| Soybean protein concentrate | 220.0 |
| α-starch | 240.0 |
| Corn starch | 209.1 |
| linseed oil | 17.7 |
| Soy oil | 14.5 |
| Cellulose | 50.0 |
| Ca(H2PO4)2 | 14.8 |
| Vitamin premix 1 | 10.0 |
| Mineral premix 2 | 20.0 |
| Vitamin D3 (VD3) premix 3 | 10.0 |
| Choline chloride (50%) | 10.0 |
| Ethoxyquin (30%) | 0.5 |
| DL-Met | 3.7 |
| Total | 1,000.0 |
| Nutrients content4 | |
| Crude protein | 300.0 |
| Crude fat | 37.8 |
| n-3 PUFAs | 10.4 |
| n-6 PUFAs | 9.6 |
| n-3: n-6 PUFAs | 10.8 |
| Available phosphorus | 4.0 |
| VD3, μg/kg | 3.8 |
PUFAs = polyunsaturated fatty acids.
One kilogram of vitamin premix contained the following (g/kg): DL-α-tocopherol acetate (50%), 12.58; menadione (22.9%), 0.83; cyanocobalamin (1%), 0.94; D-biotin (2%), 0.75; folic acid (95%), 0.42; thiamine nitrate (98%), 0.09; ascorbyl acetate (95%), 4.31; niacin (99%), 4.04; meso-inositol (98%), 19.39; calcium-D-pantothenate (98%), 3.85; riboflavin (80%), 0.73; pyridoxine hydrochloride (98%), 0.62; retinyl acetate (500,000 IU/g), 2.10. All ingredients were diluted with maize starch to 1 kg.
One kilogram of mineral premix contained the following (g/kg): FeSO4.H2O (30.0% Fe), 12.2500; MgSO4.H2O (15.0% Mg), 200.0000; ZnSO4.H 2O (34.5% Zn), 8.2460; MnSO4.H2O (31.8% Mn), 2.6590; CuSO4.5H2O (25.0% Cu), 0.9560; Na2SeO3 (44.7% Se), 0.0168; KI (76.9% I), 0.0650g. All ingredients were diluted with maize starch to 1 kg.
VD3 premix: premix was added to obtain graded level of VD3 and the amount of maize starch was reduced to compensate.
The contents of crude protein and crude lipid were measured values on the air-dried matter basis. Supplement of available P, n-3 PUFAs, and n-6 PUFAs were calculated according to NRC (2011).
2.2. Experimental design, procedure and sample collection
This feeding trial was conducted in an authorized experimental base (Dayi County, Sichuan province, China). Experimental fish were acquired from a local farm (Chengdu, China). The fish selected for experiments were visibly healthy, with no parasites found under microscopic examination. Fish were acclimatized for 4 weeks before the experiment. Before the experiment, fish were provided with a basal diet (un-supplemented) for 2 weeks to reduce pre-accumulated levels of VD3. At the beginning of the feeding experiment, 540 fish were weighed (257.24 ± 0.63 g, mean ± SD) and randomly divided into 18 culture cages (140 cm × 140 cm × 140 cm), with 30 fish in each cage. Six different doses of VD3 treatments were performed with 3 replicates. All cages were located in outdoor freshwater ponds, and microporous aeration was used throughout the trial. The dissolved oxygen concentration in each cage was >6 mg/L. Water temperature and pH were 28.0 ± 2.1 °C and 7.0 ± 0.2, respectively. All fish were subject to natural light conditions, with approximately 11 h light and 13 h darkness. Fish were fed to satiation four times daily for 10 weeks. A 100 cm2 disk was equipped at the bottom of each cage to collected the feed residue. This was then dried and weighed to calculate feed intake (FI).
Toward the end of the 70-day feeding experiment, the fish were weighed and counted to determine growth performance. Twelve fish were randomly selected from each treatment and euthanized in a benzocaine bath (50.0 mg/L). The intestines were immediately removed, their length and weight were recorded, and tissues were preserved at −80 °C (Shao et al., 2021). On the basis of intestinal anatomical structure of grass carp (Ni and Wang, 1999), intestines were divided into proximal intestine (PI), mid intestine (MI), and distal intestine (DI) segments. The PI segment was determined as the length from the sphincter to the first turn, and the DI was defined as the last turn to the anus.
2.3. Histological analysis
Four percent paraformaldehyde was used to fix the different intestinal segments for subsequent histologic assessment (Song et al., 2021). After serial dehydration in gradually increasing concentrations of ethanol, the tissues were then xylene equilibration and paraffin embedded (Tsertou et al., 2020). The samples were dissected into 4-μm slides, dyed with hematoxylin and eosin (H&E), and observed for morphological structures using a Nikon TS100 light microscope (Tokyo, Japan).
2.4. Biochemistry assay
The PI, MI, and DI segments were homogenized in ice-cold 0.9% sterile physiological saline (1:10, wt/vol), incubated on ice for 30 min, and centrifuged at 6,000 × g at 4 °C for 20 min to collect the supernatant (Heidarieh et al., 2013). A bicinchoninic acid (BCA) assay kit was used to determine the protein concentration of the supernatant (Beyotime, Nanjing, China) according to the manufacturer's instructions. Activities of digestive enzymes trypsin, chymotrypsin, and amylase were determined following the process described by Ma et al. (2019), Korének et al. (2000), and Eshaghzadeh et al. (2015), respectively. AKP, Na+/K+-ATPase, γ-GT, and CK activities were measured according to Zhou et al. (2012), Bessey et al. (1946), Bauermeister et al. (1983), and Tanzer and Gilvarg (1959), respectively.
2.5. Free amino acid (FAA) analysis
For each sample, 400 mg tissue was homogenized in a 10% sulfosalicylic acid solution and centrifuged at 6,000 × g at 4 °C for 10 min. The collected supernatants were percolated through 0.22-μm filters for FAA measurements. The FAA content was analyzed using an automated AA analyzer (L-8080; Hitachi, Tokyo, Japan).
2.6. Real-time quantitative polymerase chain reaction (PCR)
Total intestinal RNA extraction, reverse transcription, and quantitative real-time PCR were performed following standard procedure by Zhao et al. (2020). Briefly, intestinal RNA was extracted using RNAiso Plus (TaKaRa, Dalian, China), and treated with DNase I. The purity and quantity of RNA were identified via agarose gel (1.5%) electrophoresis and spectrophotometry (A260/280 nm ratio) analysis, respectively. Successively, RNA was reverse transcribed into complementary DNA using the PrimeScript RT Reagent Kit (TaKaRa, Dalian, China). Oligonucleotide primers were adopted for quantitative real-time PCR (Table 2). The amplification efficiency of all genes was calculated based on the standard curve of a 10-step dilution sequence, which confirmed that the primer amplification efficiency was about 100%. β-actin was used as the reference gene for normalization.
Table 2.
Real-time PCR primer sequences.
| Gene | Forward sequences of primers (5′ to 3′) | Reverse sequences of primers (5′ to 3′) | Temperature, °C | GenBank number |
|---|---|---|---|---|
| SLC7A7 | GATAGCCATCACTTTCTCCAAC | GTCCCATACTTCACATAGGCA | 58.0 | JX013494 |
| SLC7A6 | GCAGAGTTGGGCACGACAATCAC | GGAAGAGCGGCTGAACCAAGTAG | 58.0 | KC206055 |
| SLC1A2a | ATCCATGGTGCCATATTCCTG | CAACGGAAAGTTACAGGCAGAG | 59.6 | KY200977 |
| SLC1A3 | GGAATGCTTTCGTCATCCTCAC | CAGAGCGGCCATACCTGTAATT | 61.0 | KY200978 |
| SLC1A5 | ACACTTTCTTGCTGGGATTGGT | GGATGGTGATGACCTGGACAAA | 62.0 | KU559898 |
| SLC6A14 | TCATTGCGTACCCTGATGCTCT | ACTTCAGAACTTTGGGATAGGCA | 62.5 | KX823959 |
| SLC6A19b | ACCACTGGAAAGGCTGTTTATGT | ACCGCATCTTGCTCACAGTTATT | 62.0 | KX823960 |
| SLC7A1 | CGACATCACTGAACCCTCCAAC | TGCACAGGCTGAACAGGACACT | 62.0 | KY200979 |
| SLC7A5 | CAACATGAGCCGACCAGGAGAC | CCAGCGACAACCCGACTGAACC | 62.0 | KY200980 |
| SLC7A8 | TGGTGAGAAGCTGTTGGGAGTGA | GCAAGTGAAGAGTAGGGCTGGAA | 59.6 | GU474428 |
| SLC7A9 | TTCTACAGTCTTCTGCCGTTGC | AGAGCTGGAGAAGGCGTGTAAC | 61.5 | KX823958 |
| SLC38A2 | AGAAGAGTCCTGCAAACCCAA | CACAAACATTCCCAGAAACGA | 61.7 | KY200981 |
| SLC6A6 | CTCCGCAAGAACAAGACACTC | CCCACACAGCGAGCAGAC | 62.5 | KX682391 |
| SLC15A1 | TGCTCTTGTTGTGTTCATCG | CTCTCTCTTGGGGTATTGCTT | 62.0 | KC782748 |
| atp1a1a.1 | TGCCATTGTAGCCGTAAC | GGTGCCCAAAGGTAGAGG | 60.3 | JX854442 |
| atp1a1a.4 | GAGGTCGTTGCTGGTGAT | CAGTGAGGGAAGAGTTGTC | 55.9 | KM112094 |
| CK | CTCCTCGTTCACCCAGAC | CAGCATCAAGGGATACGC | 61.4 | JX854444 |
| TOR | TCCCACTTTCCACCAACT | ACACCTCCACCTTCTCCA | 61.4 | JX854449 |
| S6K1 | TGGAGGAGGTAATGGACG | ACATAAAGCAGCCTGACG | 59.4 | EF373673.1 |
| 4E-BP1 | GCTGGCTGAGTTTGTGGTTG | CGAGTCGTGCTAAAAAGGGTC | 60.3 | KT757305.1 |
| ATF4 | CAACATTAGCAGCGGCAA | GTGAGCGGGTGAACGGT | 56.3 | AY437846.1 |
| β-actin | GGCTGTGCTGTCCCTGTA | GGGCATAACCCTCGTAGAT | 61.4 | M25013 |
SLC = solute carrier; atp1a1a.1 = Na+/K+-ATPase alpha-subunit isoform 1; atp1a1a.4 = Na+/K+-ATPase alpha-subunit isoform 4; CK = creatine kinase; TOR = target of rapamycin; S6K1 = ribosomal protein S6 kinase 1; 4E-BP1 = eIF4E-binding protein 1; ATF4 = activating transcription factor.
2.7. Western blot analysis
The separation of tissue protein homogenate, antibodies, and the western blotting procedure were performed as previously reported (Huang et al., 2021). In brief, protein samples of equal mass (40 μg per row) were extracted, separated on sodium dodecyl sulfate (SDS)-glycine polyacrylamide gel, and then transferred to the polyvinylidene-fluoride membrane. The membrane was blocked with blocking solution (0.5%) for 2 h at room temperature, and then incubated with primary antibody overnight at 4 °C. Primary antibodies against β-actin (1:3,000), anti-total TOR (1:1,000), p-TORSer2448 (1:1,000), and p-ATFSer219(1:1,000) were purchased from Affinity BioReagents (Golden, Colorado, USA); antibodies against p-S6K1Thr389 (1:1,000) and p-4E-BP1Thr 37/46 (1:1,000) were obtained from Affinity Technology (Shanghai, China). We washed the membranes, incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 h, and visualized the immune complexes under ChemiDoc imaging system (Bio-Rad, USA).
2.8. Statistical analyses
Calculations for growth performance parameters are listed in Table 3. Data were presented as means ± SD, as indicated in the table legends. Statistical tests were performed using SPSS Statistics v.25 (IBM Corp., Armon, NY, USA). Samples were analyzed using one-way analysis of variance and Duncan's multiple range test to evaluate statistical differences among treatments at the level of P < 0.05. We used a nonparametric Kruskal–Wallis test for normalization if the data did not display normal distribution. Regression and correlation analyses were performed using the broken-line model and Pearson's correlation, respectively.
Table 3.
Index formula for growth performance of on-growing grass carp (Ctenopharyngodon idella).
| Item | Formulas |
|---|---|
| PWG | PWG (%) = 100 × [FBW (g/fish) – IBW (g/fish)]/IBW (g) |
| SGR | SGR (%/d) = 100 × [ln FBW (g/fish) – ln IBW (g/fish)]/d |
| FE | FE (%) = 100 × [FBW (g/fish) – IBW (g/fish)]/FI (g/fish) |
| ISI | ISI (%) = 100 × Wet intestine weight (g)/Wet body weight (g) |
| ILI | ILI (%) = 100 × Wet intestine length (cm)/Wet body length (cm) |
PWG = percentage of weight gain; FBW = final body weight; IBW = initial body weight; SGR = specific growth rate; FE = feed efficiency; FI = feed intake; ISI = intestinal somatic index; ILI = intestinal length index.
3. Results
3.1. Growth performance and histopathology of on-growing grass carp
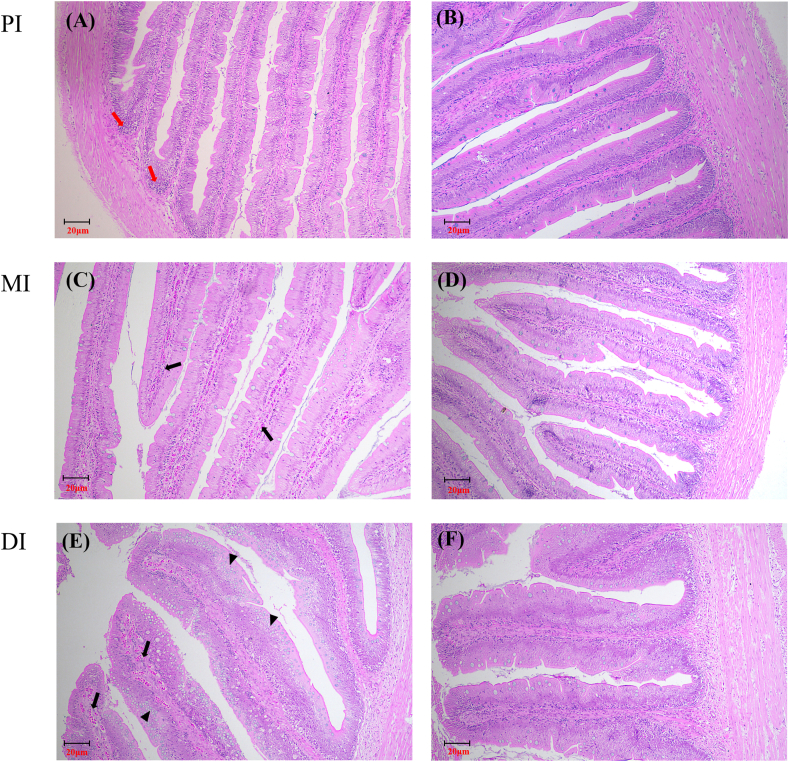
During and at end of the 70 days feeding trial, the survival rates of all experimental grass carp were 100%. As displayed in Table 4, final body weight (FBW), percentage weight gain (PWG), SGR, FI, feed efficiency (FE), intestinal length (IL), and intestinal weight (IW) increased significantly (P < 0.05) compared with those of the control for VD3 supplementation up to 1,167.9 IU/kg, and decreased significantly (P < 0.05) at higher concentrations. Intestinal somatic index (ISI) and intestinal length index (ILI) increased minimally for dietary VD3 levels up to 1,167.9 IU/kg but decreased slightly at higher levels. The folds height in the three intestinal segments increased with VD3 levels up to 1,167.9 IU/kg (P < 0.05), above which they decreased in all segments. The histopathological analysis results (Fig. 1) revealed that VD3 deficiency was associated with inflammatory cell infiltration in the PI, blood capillary hyperemia in the MI, and blood capillary hyperemia and goblet cell hyperplasia in the DI.
Table 4.
Growth performance and intestinal growth of on-growing grass carp (Ctenopharyngodon idella) fed diets containing graded levels of VD3 for 10 weeks.
| Item | VD3 level, IU/kg diet |
P-value | |||||
|---|---|---|---|---|---|---|---|
| 15.2 (control) | 364.3 | 782.5 | 1,167.9 | 1,573.8 | 1,980.1 | ||
| IBW1, g/fish | 257.00 ± 0.67 | 257.11 ± 0.38 | 258.00 ± 0.67 | 257.11 ± 0.77 | 257.33 ± 0.67 | 256.89 ± 0.38 | 0.328 |
| FBW1, g/fish | 854.99 ± 14.42a | 965.39 ± 15.01c | 1,051.49 ± 13.21d | 1,129.67 ± 14.73e | 973.48 ± 10.55c | 883.08 ± 10.87b | <0.001 |
| PWG1, % | 232.68 ± 5.76a | 275.48 ± 5.86c | 306.85 ± 5.28d | 339.37 ± 7.03e | 276.02 ± 3.33c | 242.57 ± 4.45b | <0.001 |
| SGR1, %/d | 1.72 ± 0.02a | 1.89 ± 0.02c | 2.01 ± 0.01d | 2.11 ± 0.02e | 1.90 ± 0.02c | 1.76 ± 0.02b | <0.001 |
| FI1, g/fish | 927.47 ± 1.11a | 1,051.64 ± 1.66c | 1,084.60 ± 1.93d | 1,143.64 ± 0.91e | 1,051.06 ± 2.69c | 954.36 ± 1.36b | <0.001 |
| FE1, % | 0.64 ± 0.02a | 0.67 ± 0.01bc | 0.73 ± 0.01d | 0.76 ± 0.01e | 0.68 ± 0.01c | 0.66 ± 0.01ab | <0.001 |
| IL2, cm | 61.36 ± 2.48a | 64.92 ± 2.52b | 69.85 ± 4.95c | 68.83 ± 4.89c | 65.00 ± 3.07b | 62.92 ± 2.85ab | <0.001 |
| ISI2, % | 1.68 ± 0.07a | 1.76 ± 0.10abc | 1.79 ± 0.05bc | 1.82 ± 0.07c | 1.76 ± 0.17abc | 1.73 ± 0.11ab | 0.02 |
| IW2, g | 14.28 ± 0.91a | 16.83 ± 0.81b | 18.52 ± 1.16c | 20.12 ± 1.91d | 17.13 ± 1.35b | 15.1 ± 1.27a | <0.001 |
| ILI2, % | 175.78 ± 6.87a | 179.74 ± 7.64ab | 186.43 ± 13.46bc | 187.99 ± 10.54c | 181.23 ± 7.50abc | 179.02 ± 7.21ab | 0.015 |
| Folds height | |||||||
| PI3, μm | 1,274.17 ± 191.08a | 1,520.17 ± 192.56b | 1,734.50 ± 190.88bc | 1,914.50 ± 254.33c | 1,641.50 ± 195.55b | 1,573.83 ± 192.98b | <0.001 |
| MI3, μm | 1,001.17 ± 115.53a | 1,220.67 ± 177.77b | 1,280.83 ± 176.36bc | 1,445.33 ± 200.79c | 1,320.17 ± 153.27bc | 1,201.67 ± 202.87ab | 0.004 |
| DI3, μm | 976.83 ± 140.05a | 1,017.67 ± 130.43a | 1,141.00 ± 133.27ab | 1,214.67 ± 161.81b | 1,049.67 ± 140.06ab | 1,004.67 ± 128.87a | 0.046 |
IBW = initial body weight; FBW = final body weight; PWG = percentage of weight gain; SGR = specific growth rate; FI = feed intake; FE = feed efficiency; IL = intestinal length; ISI = intestinal somatic index; IW = intestinal weight; ILI = intestinal length index; PI = proximal intestine; MI = mid intestine; DI = distal intestine.
a-e Mean values within a row with different superscript letters indicate significant difference (one-way ANDOVA and Duncan's multiple-range tests, P < 0.05).
Values are means ± SD for 3 replicate groups, with 30 fish in each group.
Values are means ± SD (n = 3).
Values are means ± SD (n = 6).
Fig. 1.
The hematoxylin and eosin (H&E) histology of PI, MI, and DI (100× ) in on-growing grass carp fed diets containing different levels of VD3 (IU/kg). (A), (C), and (E), 15.2 IU/kg diet (Un-supplement control); (B), (D), and (F), 1,167.9 IU/kg diet. Red arrowhead, black arrowhead, and triangle showed, respectively, the inflammatory cell infiltration, the blood capillary hyperemia, and the goblet cell hyperplasia. VD3 = vitamin D3; PI = proximal intestine; MI = mid intestine; DI = distal intestine.
3.2. Digestive and absorption enzyme activities, and Na+/K+-ATPase alpha-subunit isoform 1 (atp1a1a1), atp1a1a4, and creatine kinase (CK) mRNA levels
All intestinal digestion enzyme activities under different VD3 levels are displayed in Table 5. Peak levels of trypsin and amylase activities were observed with dietary VD3 supplementation levels of 782.5 and 1,167.9 IU/kg, respectively, and activities plateaued at higher values (P > 0.05). Chymotrypsin activity increased slightly along with an enhancement in dietary VD3, up to 1,167.9 IU/kg, and declined slightly at higher levels. The levels of brush border enzymes are presented in Table 6. The AKP activities in the PI, MI, and DI slightly increased with the supplementation of dietary VD3 levels up to 1,167.9, 1,573.8 and 1,167.9 IU/kg, respectively, and they were lower in all intestinal segments at higher VD3 levels. Na+/K+-ATPase activities were increased by the supplementation of dietary VD3, with the highest levels at VD3 supplementation level of 1,167.9 IU/kg diet, and at higher levels maintained constant level in the PI and MI (P > 0.05), while decreasing slightly in the DI. The activities of γ-GT reached the highest values at dietary VD3 levels of 1,167.9, 1,167.9, and 782.5 IU/kg in PI, MI, and DI segments, respectively. At higher levels, the activities decreased slightly in the PI and significantly in the MI and DI (P < 0.05). Dietary VD3 supplementation elevated CK activities within all intestinal segments, and peak values were obtained in the 1,167.9 IU/kg treatment, decreasing minimally at higher levels.
Table 5.
Digestive enzymes activities in the intestine of the on-growing grass carp (Ctenopharyngodon idella) fed diets containing graded levels of vitamin D3 for 10 weeks (U/g tissue)1.
| Item | VD3 level, IU/kg diet |
P-value | |||||
|---|---|---|---|---|---|---|---|
| 15.2 (control) | 364.3 | 782.5 | 1,167.9 | 1,573.8 | 1,980.1 | ||
| Trypsin | 0.82 ± 0.08a | 0.98 ± 0.09b | 1.25 ± 0.11c | 1.22 ± 0.13c | 1.20 ± 0.09c | 1.02 ± 0.13c | <0.001 |
| Chymotrypsin | 9.07 ± 0.77a | 9.13 ± 0.74a | 10.61 ± 0.82b | 12.02 ± 1.32c | 11.99 ± 1.09b | 11.39 ± 0.83b | <0.001 |
| Amylase | 1,009.12 ± 86.67a | 1,180.49 ± 66.10bc | 1,245.55 ± 75.92c | 1,293.35 ± 139.44c | 1,164.97 ± 127.77bc | 1,091.18 ± 102.44ab | 0.001 |
a-c Mean values within a row with different superscript letters indicate significant difference (one-way ANOVA and Duncan's multiple-range tests, P < 0.05).
Values are means ± SD (n = 6).
Table 6.
Brush border enzymes of the PI, MI, and DI in on-growing grass carp (Ctenopharyngodon idella) fed diets containing graded levels of VD3 for 10 weeks1.
| Item | VD3 level, IU/kg diet |
P-value | |||||
|---|---|---|---|---|---|---|---|
| 15.2 (control) | 364.3 | 782.5 | 1,167.9 | 1,573.8 | 1,980.1 | ||
| PI | |||||||
| AKP | 69.11 ± 7.09a | 75.45 ± 4.26abc | 78.7796 ± 7.23bc | 80.96 ± 7.47c | 74.21 ± 1.99abc | 72.61 ± 4.19ab | 0.017 |
| Na+/K+-ATPase | 190.96 ± 11.63a | 207.50 ± 8.49b | 220.49 ± 11.41bc | 231.28 ± 11.95c | 209.23 ± 18.51b | 207.97 ± 10.02b | <0.001 |
| γ-GT | 23.58 ± 2.46a | 26.91 ± 2.52bc | 27.00 ± 2.55bc | 29.05 ± 2.71c | 26.04 ± 2.70abc | 23.79 ± 2.19ab | 0.006 |
| CK | 91.77 ± 5.73a | 107.47 ± 11.34a | 110.69 ± 10.55ab | 128.18 ± 13.52b | 115.62 ± 12.56ab | 106.60 ± 10.60a | 0.037 |
| MI | |||||||
| AKP | 63.53 ± 5.38a | 66.14 ± 6.32ab | 72.97 ± 6.91bc | 73.19 ± 6.30bc | 74.77 ± 7.53c | 69.52 ± 4.36abc | 0.024 |
| Na+/K+-ATPase | 123.13 ± 12.41a | 131.46 ± 12.10ab | 139.54 ± 15.55bc | 149.07 ± 11.76c | 129.74 ± 11.60ab | 126.31 ± 8.73ab | 0.01 |
| γ-GT | 20.98 ± 2.24a | 24.16 ± 1.76b | 26.93 ± 1.68c | 27.85 ± 2.34c | 23.72 ± 2.13b | 21.79 ± 1.68ab | 0.002 |
| CK | 100.34 ± 7.24a | 105.69 ± 11.04ab | 120.42 ± 9.86c | 122.83 ± 6.83c | 113.91 ± 10.33bc | 112.67 ± 9.34bc | <0.001 |
| DI | |||||||
| AKP | 58.41 ± 6.70a | 60.01 ± 6.88a | 64.80 ± 5.16bc | 70.65 ± 6.54c | 63.28 ± 6.69bc | 58.95 ± 6.00a | 0.02 |
| Na+/K+-ATPase | 106.30 ± 7.60a | 111.11 ± 9.26a | 119.60 ± 5.66ab | 127.78 ± 18.02b | 116.06 ± 9.52ab | 107.00 ± 11.36a | 0.015 |
| γ-GT | 18.21 ± 0.77a | 19.47 ± 0.79ab | 21.38 ± 2.78c | 21.26 ± 1.40c | 20.35 ± 2.22ab | 18.94 ± 1.56a | 0.019 |
| CK | 50.29 ± 5.66a | 56.38 ± 6.59abc | 58.53 ± 4.64bc | 60.21 ± 4.98c | 55.21 ± 3.87abc | 53.08 ± 5.55ab | 0.034 |
PI = proximal intestine; MI = mid intestine; DI = distal intestine; AKP = alkaline phosphatase, mmol of nitrophenol released/g tissue; Na+/K+-ATPase, μmol of phosphorus released/g tissue; γ-GT = γ-glutamyl transpeptidase, mmol of 5-amino-2-nitrobenzoate released/g tissue; CK = creatine kinase, μmol of phosphorus released/g tissue.
a-c Mean values within a row with different superscript letters indicate significant difference (one-way ANOVA and Duncan's multiple-range tests, P < 0.05).
Values are means ± SD (n = 6).
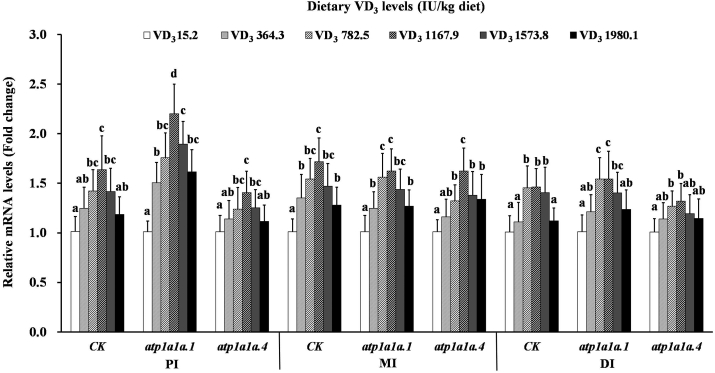
Intestinal CK, atp1a1a1, and atp1a1a4 mRNA levels under different VD3 treatments are presented in Fig. 2. Following dietary VD3 supplementation of up to 1,167.9 IU/kg, the CK mRNA was upregulated minimally in the PI and DI but significantly in the MI (P < 0.05) and then was downregulated slightly in all segments. atp1a1a1 levels increased significantly in the PI and MI under VD3 supplementation up to 1,167.9 IU/kg (P < 0.05) but increased only minimally in the DI; it level was decreased at higher supplement levels. The mRNA levels of atp1a1a4 increased slightly with VD3 supplementation up to 1,167.9 IU/kg in the three intestinal segments and decreased slightly in the PI; however, atp1a1a4 mRNA levels exhibited a stabilizing trend in the MI and DI above 1,167.9 IU/kg treatment.
Fig. 2.
Relative expression of CK, atp1a1a.1, and atp1a1a.4 in the PI, MI, and DI of on-growing grass carp (Ctenopharyngodon idella) fed diets containing graded levels of VD3 for 10 weeks. Mean values ± SD. n = 6 for each bar. Different letters above the bars indicate significant difference (ANOVA and Duncan's multiple-range test, P < 0.05). VD3 = vitamin D3; PI = proximal intestine; MI = mid intestine; DI = distal intestine; CK = creatine kinase.
3.3. FAA profile of intestinal segments
As displayed in Table 7, with dietary VD3 supplementation of 1,167.9 IU/kg, the Met, Gly, Trp, Thr, Ile, Phe, Gly, Pro, Ser, Tyr, Tau, His, Arg, His, and Glu contents increased in the PI, but were lower at higher treatment levels. Levels of Val and Cys elevated with an increase in VD3 levels up to 782.5 IU/kg and then decreased at higher levels. However, VD3 had no effect on Leu in the PI (P > 0.05). In the MI, the contents of Met, Trp, Thr, Ile, Leu, Val, Phe, Ser, Tyr, Tau, Arg, His, and Glu increased with dietary VD3 levels up to 1,167.9 IU/kg and decreased slightly at higher levels (P > 0.05). The contents of Gly and Cys minimally increased with increasing VD3 levels up to 1,573.8 IU/kg and then decreased minimally at higher levels (P > 0.05). The content of Pro was higher in VD3 supplement groups than that in control groups and stayed relatively constant. In the DI, levels of Met, Thr, Ile, Leu, Val, Phe, Pro, Ser, Tyr, Cys, Tau, Arg, His, and Glu increased with the supplementation of VD3 up to 1,167.9 IU/kg, and decreased slightly above this level (P > 0.05). Tyr and Gly contents enhanced with the VD3 levels increased up to 1,573.8 IU/kg, decreasing slightly at higher levels. Notably, VD3 supplementation did not affect Ala, Lys, or Asp contents in any intestinal segment (P > 0.05).
Table 7.
Free amino acid contents of the PI, MI, and DI in on-growing grass carp (Ctenopharyngodon idella) fed diets containing graded levels of VD3 for 10 weeks (mg/100g tissue) 1.
| Item | VD3 level, IU/kg diet |
P-value | |||||
|---|---|---|---|---|---|---|---|
| 15.2 (control) | 364.3 | 782.5 | 1,167.9 | 1,573.8 | 1,980.1 | ||
| PI | |||||||
| Neutral amino acids | |||||||
| Met | 0.83 ± 0.06a | 0.89 ± 0.09a | 1.11 ± 0.13b | 1.12 ± 0.13b | 1.03 ± 0.15ab | 0.84 ± 0.10a | 0.023 |
| Trp | 0.69 ± 0.06a | 0.75 ± 0.10ab | 0.89 ± 0.04bc | 0.99 ± 0.10c | 0.78 ± 0.10ab | 0.75 ± 0.05ab | 0.006 |
| Thr | 1.21 ± 0.08a | 1.40 ± 0.15ab | 1.52 ± 0.15b | 1.57 ± 0.20ab | 1.30 ± 0.10a | 1.22 ± 0.05a | 0.050 |
| Ile | 1.10 ± 0.10a | 1.30 ± 0.08abc | 1.37 ± 0.13bc | 1.47 ± 0.21c | 1.32 ± 0.13abc | 1.15 ± 0.09ab | 0.039 |
| Ala | 1.72 ± 0.11 | 1.73 ± 0.11 | 1.80 ± 0.11 | 1.84 ± 0.08 | 1.79 ± 0.15 | 1.72 ± 0.09 | 0.766 |
| Leu | 2.11 ± 0.16 | 2.29 ± 0.16 | 2.32 ± 0.15 | 2.62 ± 0.17 | 2.44 ± 0.35 | 2.36 ± 0.17 | 0.980 |
| Val | 1.20 ± 0.10a | 1.41 ± 0.12ab | 1.60 ± 0.08b | 1.58 ± 0.15b | 1.36 ± 0.12ab | 1.29 ± 0.20a | 0.018 |
| Phe | 1.35 ± 0.10a | 1.42 ± 0.08ab | 1.56 ± 0.03b | 1.81 ± 0.15c | 1.58 ± 0.17b | 1.41 ± 0.13ab | 0.002 |
| Gly | 1.10 ± 0.12a | 1.24 ± 0.08a | 1.32 ± 0.17ab | 1.52 ± 0.16b | 1.21 ± 0.16a | 1.12 ± 0.11a | 0.028 |
| Pro | 1.13 ± 0.09a | 1.38 ± 0.10bc | 1.44 ± 0.11c | 1.46 ± 0.08c | 1.30 ± 0.05bc | 1.28 ± 0.07b | 0.004 |
| Ser | 1.27 ± 0.09a | 1.29 ± 0.11ab | 1.55 ± 0.09bc | 1.68 ± 0.26c | 1.54 ± 0.08c | 1.33 ± 0.10ab | 0.016 |
| Tyr | 1.52 ± 0.16a | 1.73 ± 0.23ab | 1.87 ± 0.19bc | 2.16 ± 0.18c | 1.83 ± 0.19ab | 1.65 ± 0.10ab | 0.014 |
| Cys | 0.21 ± 0.02a | 0.26 ± 0.03ab | 0.36 ± 0.04c | 0.32 ± 0.04bc | 0.31 ± 0.06bc | 0.29 ± 0.02bc | 0.010 |
| Tau | 1.33 ± 0.14a | 1.50 ± 0.20ab | 1.67 ± 0.16bc | 1.84 ± 0.09c | 1.54 ± 0.16ab | 1.39 ± 0.12ab | 0.016 |
| Basic amino acids | |||||||
| Lys | 1.25 ± 0.09 | 1.30 ± 0.09 | 1.41 ± 0.07 | 1.35 ± 0.11 | 1.30 ± 0.13 | 1.21 ± 0.12 | 0.336 |
| Arg | 1.49 ± 0.08a | 1.62 ± 0.10a | 1.81 ± 0.17ab | 2.16 ± 0.25c | 1.87 ± 0.18bc | 1.52 ± 0.12a | 0.002 |
| His | 0.45 ± 0.07a | 0.62 ± 0.11b | 0.66 ± 0.05bc | 0.77 ± 0.06c | 0.76 ± 0.05c | 0.63 ± 0.02b | 0.001 |
| Acidic amino acids | |||||||
| Asp | 1.99 ± 0.14 | 2.10 ± 0.15 | 2.10 ± 0.13 | 2.00 ± 0.19 | 1.96 ± 0.22 | 1.95 ± 0.07 | 0.776 |
| Glu | 2.12 ± 0.24a | 2.32 ± 0.14abc | 2.56 ± 0.17cd | 2.82 ± 0.22d | 2.50 ± 0.25bcd | 2.15 ± 0.09ab | 0.007 |
| MI | |||||||
| Neutral amino acids | |||||||
| Met | 0.75 ± 0.09a | 0.97 ± 0.15ab | 1.22 ± 0.14bc | 1.37 ± 0.18c | 1.23 ± 0.10bc | 0.98 ± 0.18ab | 0.003 |
| Trp | 0.56 ± 0.07a | 0.66 ± 0.08ab | 0.79 ± 0.05bc | 0.89 ± 0.09c | 0.85 ± 0.11c | 0.67 ± 0.05ab | 0.001 |
| Thr | 1.31 ± 0.21a | 1.46 ± 0.17a | 1.65 ± 0.12ab | 1.84 ± 0.27b | 1.41 ± 0.10a | 1.32 ± 0.10a | 0.018 |
| Ile | 1.08 ± 0.14a | 1.18 ± 0.15abc | 1.40 ± 0.09cd | 1.50 ± 0.10d | 1.33 ± 0.12bcd | 1.13 ± 0.13ab | 0.009 |
| Ala | 1.84 ± 0.13 | 1.91 ± 0.24 | 1.94 ± 0.28 | 1.84 ± 0.16 | 1.77 ± 0.11 | 1.65 ± 0.04 | 0.455 |
| Leu | 2.03 ± 0.16a | 2.16 ± 0.10a | 2.33 ± 0.29ab | 2.65 ± 0.26b | 2.40 ± 0.20ab | 2.18 ± 0.17a | 0.042 |
| Val | 1.33 ± 0.16a | 1.49 ± 0.10ab | 1.64 ± 0.10b | 1.68 ± 0.15b | 1.47 ± 0.15ab | 1.36 ± 0.09a | 0.029 |
| Phe | 1.39 ± 0.11a | 1.60 ± 0.20abc | 1.68 ± 0.13bc | 1.75 ± 0.12c | 1.44 ± 0.15ab | 1.44 ± 0.10ab | 0.037 |
| Gly | 1.043 ± 0.15a | 1.11 ± 0.10a | 1.27 ± 0.14ab | 1.48 ± 0.20b | 1.50 ± 0.11b | 1.13 ± 0.11a | 0.005 |
| Pro | 1.15 ± 0.10a | 1.39 ± 0.10b | 1.47 ± 0.13b | 1.59 ± 0.09b | 1.41 ± 0.17b | 1.36 ± 0.13b | 0.019 |
| Ser | 1.17 ± 0.12a | 1.40 ± 0.17ab | 1.49 ± 0.13b | 1.61 ± 0.16bc | 1.57 ± 0.11c | 1.36 ± 0.08ab | 0.003 |
| Tyr | 1.51 ± 0.20a | 1.86 ± 0.19b | 1.99 ± 0.13b | 2.07 ± 0.14b | 2.01 ± 0.20b | 1.80 ± 0.13ab | 0.016 |
| Cys | 0.22 ± 0.02a | 0.27 ± 0.02a | 0.34 ± 0.04b | 0.37 ± 0.05bc | 0.42 ± 0.04c | 0.36 ± 0.04bc | <0.001 |
| Tau | 1.40 ± 0.15a | 1.48 ± 0.08ab | 1.64 ± 0.11bc | 1.81 ± 0.11c | 1.77 ± 0.11c | 1.42 ± 0.11a | 0.002 |
| Basic amino acids | |||||||
| Lys | 1.47 ± 0.15 | 1.50 ± 0.13 | 1.57 ± 0.08 | 1.45 ± 0.10 | 1.41 ± 0.11 | 1.41 ± 0.10 | 0.573 |
| Arg | 1.483 ± 0.12a | 1.62 ± 0.15ab | 1.75 ± 0.14b | 2.16 ± 0.29b | 1.85 ± 0.24b | 1.68 ± 0.10ab | 0.016 |
| His | 0.45 ± 0.06a | 0.54 ± 0.09ab | 0.67 ± 0.07bc | 0.74 ± 0.06d | 0.73 ± 0.06cd | 0.68 ± 0.04b | <0.001 |
| Acidic amino acids | |||||||
| Asp | 1.88 ± 0.17 | 2.05 ± 0.10 | 2.20 ± 0.10 | 2.05 ± 0.29 | 1.93 ± 0.22 | 1.83 ± 0.18 | 0.261 |
| Glu | 2.07 ± 0.25a | 2.22 ± 0.21ab | 2.58 ± 0.13bc | 2.63 ± 0.26c | 2.29 ± 0.23abc | 2.20 ± 0.13ab | 0.036 |
| DI | |||||||
| Neutral amino acids | |||||||
| Met | 0.74 ± 0.11a | 0.83 ± 0.09a | 1.02 ± 0.14b | 1.24 ± 0.08c | 1.15 ± 0.10bc | 1.04 ± 0.10bc | 0.001 |
| Trp | 0.57 ± 0.05a | 0.63 ± 0.09a | 0.82 ± 0.04bc | 0.91 ± 0.07c | 1.02 ± 0.10bc | 0.87 ± 0.05ab | 0.001 |
| Thr | 1.32 ± 0.19a | 1.40 ± 0.12ab | 1.63 ± 0.11b | 1.65 ± 0.14b | 1.52 ± 0.09ab | 1.35 ± 0.11a | 0.033 |
| Ile | 1.20 ± 0.19a | 1.21 ± 0.14a | 1.32 ± 0.10a | 1.58 ± 0.10b | 1.39 ± 0.12ab | 1.37 ± 0.09ab | 0.029 |
| Ala | 1.70 ± 0.18 | 1.85 ± 0.22 | 1.82 ± 0.18 | 1.87 ± 0.23 | 1.75 ± 0.10 | 1.67 ± 0.05 | 0.662 |
| Leu | 2.10 ± 0.23a | 2.42 ± 0.25abc | 2.64 ± 0.27bc | 2.81 ± 0.22c | 2.54 ± 0.19abc | 2.33 ± 0.27ab | 0.043 |
| Val | 1.49 ± 0.17a | 1.58 ± 0.17a | 2.05 ± 0.29b | 2.14 ± 0.27b | 1.77 ± 0.29ab | 1.57 ± 0.23a | 0.026 |
| Phe | 1.42 ± 0.16a | 1.47 ± 0.08a | 1.80 ± 0.19bc | 1.96 ± 0.27c | 1.51 ± 0.13ab | 1.45 ± 0.10a | 0.008 |
| Gly | 1.12 ± 0.14a | 1.14 ± 0.08a | 1.33 ± 0.13ab | 1.54 ± 0.19b | 1.57 ± 0.11b | 1.47 ± 0.11b | 0.003 |
| Pro | 1.15 ± 0.09a | 1.37 ± 0.15ab | 1.51 ± 0.19b | 1.63 ± 0.12b | 1.45 ± 0.17b | 1.39 ± 0.13ab | 0.031 |
| Ser | 1.37 ± 0.10a | 1.64 ± 0.18b | 1.68 ± 0.16b | 1.94 ± 0.16c | 1.65 ± 0.15b | 1.56 ± 0.07ab | 0.009 |
| Tyr | 1.57 ± 0.25a | 1.76 ± 0.20ab | 2.02 ± 0.15bc | 2.16 ± 0.26c | 1.70 ± 0.23ab | 1.61 ± 0.11a | 0.024 |
| Cys | 0.24 ± 0.03a | 0.30 ± 0.04ab | 0.37 ± 0.05bc | 0.44 ± 0.06c | 0.43 ± 0.05c | 0.34 ± 0.03b | 0.001 |
| Tau | 1.40 ± 0.18a | 1.50 ± 0.14abc | 1.66 ± 0.20abc | 1.78 ± 0.12c | 1.74 ± 0.15bc | 1.46 ± 0.11ab | 0.045 |
| Basic amino acids | |||||||
| Lys | 1.44 ± 0.17 | 1.49 ± 0.13 | 1.55 ± 0.12 | 1.56 ± 0.11 | 1.56 ± 0.17 | 1.49 ± 0.08 | 0.842 |
| Arg | 1.45 ± 0.15a | 1.62 ± 0.12ab | 1.82 ± 0.21bc | 2.16 ± 0.30b | 1.87 ± 0.14bc | 1.68 ± 0.16ab | 0.011 |
| His | 0.41 ± 0.04a | 0.53 ± 0.07b | 0.61 ± 0.04bc | 0.75 ± 0.06d | 0.67 ± 0.05cd | 0.60 ± 0.09bc | <0.001 |
| Acidic amino acids | |||||||
| Asp | 1.89 ± 0.14 | 2.08 ± 0.13 | 2.29 ± 0.21 | 2.25 ± 0.19 | 2.10 ± 0.33 | 1.97 ± 0.20 | 0.223 |
| Glu | 2.15 ± 0.22a | 2.56 ± 0.36ab | 2.76 ± 0.31ab | 3.11 ± 0.45b | 2.72 ± 0.39ab | 2.34 ± 0.16a | 0.048 |
PI = proximal intestine; MI = mid intestine; DI = distal intestine.
a-d Mean values within a row with different superscript letters indicate significant difference (one-way ANOVA and Duncan's multiple-range tests, P < 0.05).
Values are means ± SD (n = 6).
3.4. Intestinal AATs and PepT1 mRNA levels
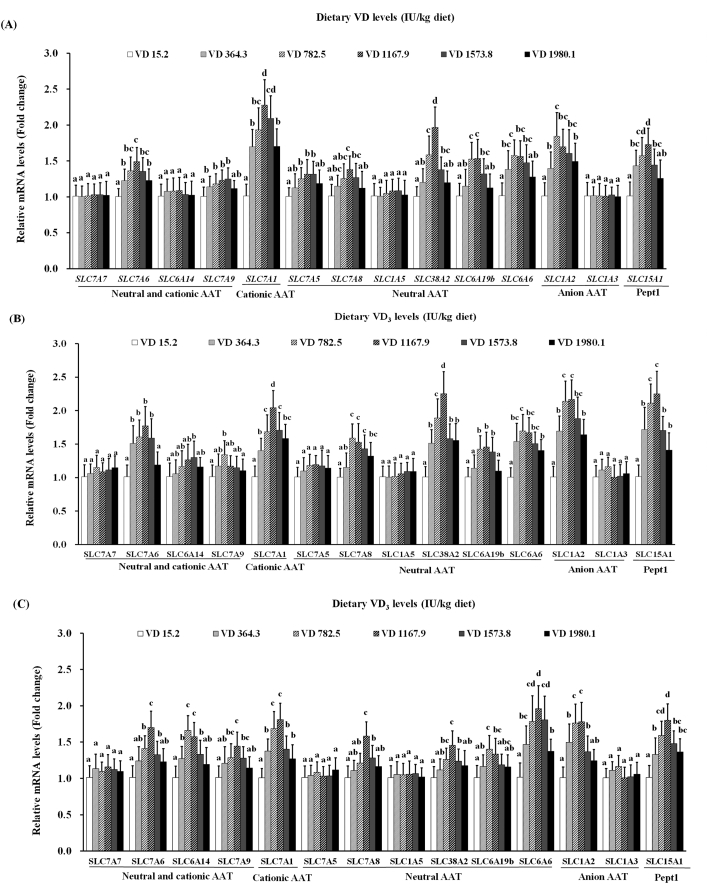
As illustrated in Fig. 3, VD3 supplementation up to 1,167.9 IU/kg corresponded to increased SLC7A6, SLC7A1, SLC7A5, SLC7A8, SLC38A2, SLC6A19b, and SLC15A1 mRNA levels in the PI; at higher treatment levels, all were downregulated. SLC7A9, SLC6A6, and SLC1A2 mRNA was upregulated with VD3 supplementation of up to 1,573.8, 782.5, and 782.5 IU/kg diet, respectively, and under higher VD3 concentrations, they declined minimally. No significant differences were observed in SLC6A14 levels in the PI (P > 0.05). In the MI, as dietary VD3 levels increased from 15.2 to 1,167.9 IU/kg, SLC7A6, SLC7A1, SLC38A2, SLC6A19b, SLC1A2, and SLC15A1 mRNA expression increased, and then their expression decreased at higher VD3 concentrations. The mRNA levels of SLC7A9, SLC7A8, and SLC6A6 all increased with VD3 supplementation up to 782.5 IU/kg, and were downregulated at higher levels. The peak level of SLC6A14 was found at VD3 supplementation of 1,573.8 IU/kg. Notably, VD3 had no marked effect on SLC7A5 in the MI (P > 0.05). In the DI, with the supplementation of VD3 up to 1,167.9 IU/kg, SLC7A6, SLC7A9, SLC7A1, SLC7A8, SLC38A2, SLC6A6, SLC1A2, and SLC15A1 mRNA levels were upregulated and then downregulated at higher levels. Following VD3 supplementation up to 782.5 IU/kg, SLC6A14, and SLC6A19b mRNA levels in the DI increased and then decreased slightly at higher levels of dietary VD3. The mRNA levels of SLC7A5 were not affected by VD3 in the DI (P > 0.05). Notably, SLC7A7, SLC1A5, and SLC1A3 mRNA levels exhibited no marked changes in on-growing grass carp (P > 0.05).
Fig. 3.
Relative expression of amino acid transporters in the PI (A), MI (B), and DI (C) of the on-growing grass carp (Ctenopharyngodon idella) fed diets containing graded levels of VD3 for 10 weeks. Mean values ± SD. n = 6 for each bar. Different letters above the bars indicate significant difference (ANOVA and Duncan's multiple-range test, P < 0.05). PI = proximal intestine; MI = mid intestine; DI = distal intestine.
3.5. Intestinal mRNA and protein phosphorylation levels of TOR, 4E-BP1, S6K1, and ATF4 of on-growing grass carp
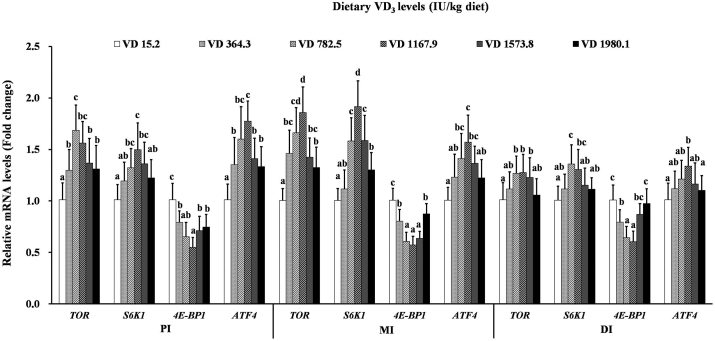
As shown in Fig. 4, TOR mRNA was upregulated at VD3 supplementation levels of 782.5, 1,167.9, and 1,167.9 IU/kg diet in the PI, MI, and DI, respectively, and downregulated at higher levels. In addition, S6K1 mRNA was upregulated slightly with VD3 supplementation of 1,167.9, 1,167.9, and 782.5 IU/kg within the PI, MI, and DI, respectively, and downregulated at higher levels. Furthermore, the mRNA levels of 4E-BP1 decreased significantly with dietary VD3 supplementation of 1,167.9 IU/kg (P < 0.05) and increased slightly in the MI and DI (P < 0.05); however, levels remained steady in the PI (P > 0.05). ATF4 mRNA was upregulated with VD3 supplementation up to 1,167.9 IU/kg diet and slightly downregulated in the MI and DI at higher levels; however, a stabilizing trend was observed in the PI (P > 0.05).
Fig. 4.
Relative expression of TOR, S6K1, 4E-BP1, and ATF4 in the PI, MI, and DI of the on-growing grass carp (Ctenopharyngodon idella) fed diets containing graded levels of VD3 for 10 weeks. Mean values ± SD. n = 6 for each bar. Different letters above the bars indicate significant difference (ANOVA and Duncan's multiple-range test, P < 0.05). TOR = total target of rapamycin; S6K1 = S6 kinase 1; 4E-BP1 = eukaryotic translation initiation factor 4E-binding protein 1; ATF4 = activating transcription factor 4; PI = proximal intestine; MI = mid intestine; DI = distal intestine.
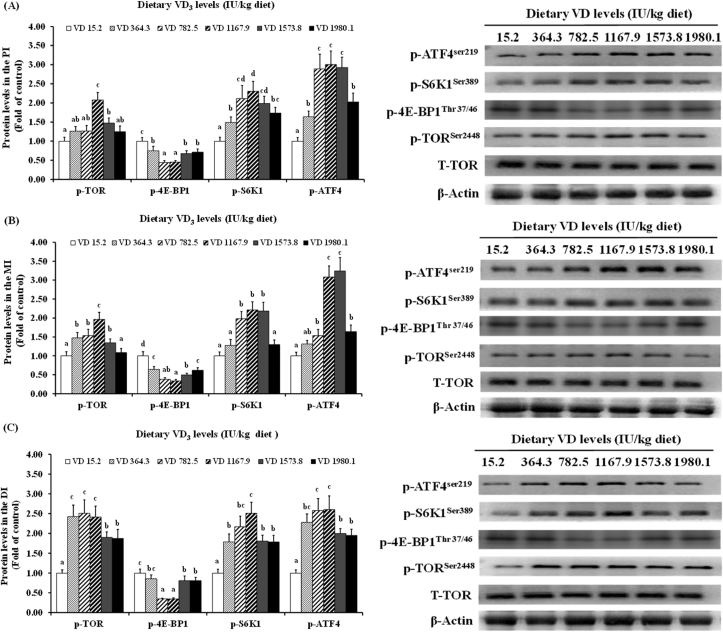
Protein levels of signaling molecules in the three intestinal segments of on-growing grass carp with varying VD3 supplementation are illustrated in Fig. 5. No significant differences were detected in protein levels of T-TOR within the PI, MI, or DI (P > 0.05) under different VD3 levels. The p-TOR protein levels increased with VD3 supplementation up to 1,167.9, 1,167.9, and 782.5IU/kg diets in the PI, MI, and DI, respectively, and were lower under higher levels in the PI and MI, while remaining stable in the DI (P > 0.05). Protein levels of p-4E-BP1 decreased with VD3 supplementation up to 1,167.9 IU/kg and plateaued in the PI and DI at higher levels (P > 0.05), while increasing minimally in the MI. Increasing levels of p-S6K1 were observed in all segments, with increasing VD3 supplementation up to 1,167.9 IU/kg diet. Levels decreased slightly in the PI and significantly in the MI at higher levels (P < 0.05), while plateauing in the DI. The p-ATF4 protein levels reached the highest level when VD3 levels were 1,167.9 IU/kg, 1,573.8, and 1,167.9 IU/kg in the PI, MI, and DI, respectively, and then stabilized in the DI (P > 0.05); however, the levels decreased significantly in the PI and MI segments (P < 0.05).
Fig. 5.
Western blot analysis of protein expression of genes in the PI (A), MI (B), and DI (C) of the on-growing grass carp (Ctenopharyngodon idella) fed diets with graded levels of VD3 (IU/kg) for 10 weeks. Mean values ± SD. n = 6 for each bar. Different letters above the bars indicate significant difference (ANOVA and Duncan's multiple-range test, P < 0.05). PI = proximal intestine; MI = mid intestine; DI = distal intestine.
4. Discussion
It has been demonstrated that VD3 increases the uptake of certain AAs by facilitating the activities of AATs, such as SLC38A2 in cultured cells (Chen et al., 2017). However, the function of VD3 in the intestine, the primary position for nutrients digestion and absorption, is not fully understood. In the current study, for the first time, to our knowledge, we explored the role and mechanisms of VD3 in the intestinal absorption of nutrients by analyzing its effects on digestion and brush border enzyme activities, AAs and AATs levels in different intestinal segments in fish, as well as the key TOR signaling pathway.
4.1. VD3 deficiency decreased growth performance and disrupted intestinal morphology of fish
Reductions in FBW, PWG, and SGR were observed in fish under VD3 deficiency (control group, 15.2 IU/kg), which was consistent with observations of Wuchang bream, Megalobrama amblycephala (Hong et al., 2015) and Atlantic salmon, Salmo salar L (Graff et al., 2002). Fish growth performance is associated with FI and FE (Zhang et al., 2013); thus, our findings revealed that VD3 deficiency was associated with poor growth. Additionally, fish growth, reflected by the IL, ISI, IW, and ILI indices, is closely related to intestinal development (Wu et al., 2011). Our results demonstrated that dietary VD3 deficiency decreased IL, ISI, IW, and ILI, suggesting VD3 deficiency impairs growth and development of fish intestines.
In fish, intestinal growth is correlated with structural integrity (Zhang et al., 2013). Pathological changes in intestinal structure reflected in the form of inflammatory cell infiltration, goblet cell hyperplasia (Erben et al., 2014), and blood capillary hyperemia (Wu et al., 2016). In the current study, histopathological examinations revealed that dietary VD3 deficiency caused pathological changes in the three intestinal segments (Fig. 1), indicating damage to fish intestinal structure integrity. Intestinal health affects the intestinal function, including digestion and absorption capacity (Liu, 2015). In addition, an increase in intestinal villus height promotes the contact area between the tract and chyme and, in turn, enhances the digestion and absorption of nutrients (Wang et al., 2020). Accordingly, we assessed the influence of VD3 on digestion and absorption capacities in fish intestines.
4.2. VD3 deficiency decreased fish digestion and absorption capacities
The presence of digestive enzymes reflects nutrient digestion capacity in fish (Johnston et al., 2004). Our results showed that dietary VD3 deficiency decreased trypsin, chymotrypsin, and amylase activities in the intestines of on-growing grass carp. Nutrient absorption is mainly influenced by brush border activities (Silva et al., 2010), particularly AKP, Na+/K+-ATPase, γ- GT, and CK, which are responsible for the final stage of nutrient degradation and assimilation (Jiang et al., 2014). To the best of our knowledge, this is the first study to examine the effect of VD3 on absorption enzymes of different intestinal segments in animals. Our results revealed that VD3 deficiency decreased brush border enzyme activities and downregulated CK, atp1a1a1, and atp1a1a4 mRNA levels in different intestinal segments of fish. In rats, brush border enzyme activities indicate the function of the growth status of epithelial cells (Hodin et al., 1995), which is reflected by IL, ILI, and fold height. In the present study, dietary VD3 supplementation increased these indexes. It is known that VD3 mediates the differentiation of epithelial cells to establish small intestinal villi and, in turn, promotes nutrient absorption (Christakos et al., 2020). The enterocyte absorption of dipeptides and tripeptides relies on microvillous peptidases without hydrolysis (Jose et al., 1997). γ-GT is involved in peptide transport by catalyzing the hydrolysis of γ-glutamyl peptide bonds and simultaneously provides membrane transport of amino acids (Rust 2002). CK is vital for large or demanding energy transfers with large energy demands, as it catalyzes the transfer of phosphate to creatine in an ATP-dependent manner. In our study, VD3 deficiency depressed γ-GT and CK levels, suggesting that VD3 reduces amino acid and energy transport. To support this assumption, we investigated the influence of VD3 on the absorption of AAs and small peptides in the three intestinal segments.
4.3. VD3 deficiency decreased AA and peptide absorption in fish intestines
AAs are absorbed efficiently within epithelial cells in the animal digestive tract (Sans et al., 2021). FAA contents in tissue can reflect the absorption capacity of intestinal AAs (Berge et al., 2004). AA and small peptide transport in fish intestines require AATs and SLC15A1 (Rosario et al., 2016). Enteral AAs are mainly transported by their corresponding AATs in animals (Appendix Table 1) (Bröer, 2008; Hyde et al., 2003). In this study, we investigated, for the first time, the effect of VD3 on AAs absorption in the gut.
4.3.1. VD3 deficiency decreased neutral and basic AA absorption capacity partly related to neutral AATs and neutral cationic AATs in the intestine of fish
The absorption of neutral and basic AAs in intestine depends on neutral AATs and neutral and cationic AATs (Wu, 2013). The present results demonstrated that VD3 deficiency decreased the contents of most neutral AAs (except Leu in the PI, and Ala in all three segments) and basic AAs (except Lys), as well as the regulation of most neutral and cationic AATs (except SLC6A14 in the PI, and SLC7A7 in all three segments) and of cationic AAT mRNA in different segments. Correlation analysis (Appendix Table 2) revealed that the abundance of AAT mRNA positively correlated with coincident FAA concentration. These results indicate that decreased AA levels in fish intestines may be related to a decrease in relative AAT mRNA.
Some of the notable phenomena observed with respect to the FAA and AATs merit an further investigation. First, the lack of influence of VD3 over Leu content in the PI may be related to SLC6A14, which is highly responsible for Leu transport (Anderson et al., 2008). The results also indicated that VD3 had no influence on SLC6A14 expression in the PI. Second, VD3 had no impact on the SLC7A7 levels, which could be attributed to the affected Lys content. SLC7A7 was high responsible for Lys transport in the intestine (Sperandeo et al., 2007). A study also demonstrated that knock out of SLC7A7 disrupts intestinal reabsorption with Lys (Bodoy et al., 2019). Our results showed that VD3 supplementation did not influence Lys contents, which was consistent with the observation regarding the impact of VD3 on SLC7A7 mRNA levels. Third, VD3 deficiency downregulated SLC6A14 mRNA expression in the MI and DI (rather than PI), which could be correlated with butyrate, IL-1β, and iNOS. Butyrate increased the mRNA of IL-1β in the MI and DI of young grass carp (Tian et al., 2017). iNOS can be activated by IL-1β in myeloid suppressor cells (Popovic et al., 2007), and iNOS also upregulates SLC6A14 in the carcinoma cells of the cervix (Gupta et al., 2006). Tanaka et al. (1990) demonstrated that VD3 enhanced butyrate in HT-29 cell lines. Therefore, VD3 deficiency could decrease butyrate production, and, in turn, reduce IL-1β in the MI and DI; IL-1β would also reduce the amount of iNOS that could decrease SLC6A14 expression. Fourth, VD3 reduced SLC7A5 abundance only in the PI, which may be due to zinc and TGF-β1. In the PI (not in the MI and DI) of young grass carp, zinc increased TGF-β1 expression (Song et al., 2017), and TGF-β1 promoted the expression of SLC7A5 (Zhao et al., 2017). VD3 significantly increases the zinc levels in bone (Worker and Migicovsky, 1961). Therefore, we speculated that VD3 could increase zinc contents and TGF-β1 expression, resulting in upregulated SLC7A5 expression in the PI. Fifth, VD3 deficiency had no effect on SLC1A5 expression, which could be associated with the unchanged content of Ala. Ala was identified as substrate specificity of SLC1A5. SLC1A5 displayed the high affinity with Ala and is involved in Ala transport (Scalise et al., 2015). Our result revealed that VD3 treatment did not affect the content of Ala, which supported our hypothesis.
4.3.2. VD3 deficiency decreased the acidic AA absorption capacity partly related to anionic AAT in the intestine of fish
Acidic AAs (Asp and Glu) can be adjusted by anionic AATs (SLC1a2a and SLC1A3) in the intestine (Bröer, 2008). The present study showed that VD3 deficiency caused a decline in acidic AAs (except Asp). The correlation analysis (Appendix Table 3) indicated that the expression of these AATs correlated with coincident FAA concentrations. The lack of change to Asp may be due to Ala. Ala and α-ketoglutarate can be catalyzed by aspartate aminotransferase and thus form Asp (Schindhelm et al., 2006). In the present study, VD3 did not affect Asp content, which may support our assumption.
SLC1A3 mRNA levels were not affected by dietary VD3 across the intestinal segments. This observation could potentially be related to insulin. The activity of SLC1A3 is unaffected by insulin in adipocytes (Krycer et al., 2017). VD3 stimulates pancreatic insulin secretion (Zeitz et al., 2003). Therefore, VD3 may not affect SLC1A3 mRNA abundance because of insulin, which requires further research.
4.3.3. VD3 deficiency decreased AAs absorption capacity partly related to SLC15A1 in the intestine of fish
The physiological function of intestinal peptide transport (SLC15A1) is to absorb dipeptides and tripeptides resulting from the digestion of dietary proteins (Frazier et al., 2008; Yang et al., 1999). In our study, we found that SLC15A1 mRNA abundance, as well as most FAA levels, decreased in the three intestinal segments under VD3 deficiency. The probable cause for the decreased SLC15A1 levels may be associated with intestine weight. The small intestine weights in chickens are strongly positive correlated with SLC15A1 gene expression (Li et al., 2013). In this study, we observed that VD3 increased intestine weight, although further validation of this hypothesis is required.
4.4. VD3 deficiency suppressed AA and peptide transporters partly associated with TOR, ATF4, 4E-BP1, and S6K1 signaling in on-growing grass carp intestine
mTORC1 regulates AATs by suppressing 4E-BP1, which inhibits ATF4 in HEK293T cells (Park et al., 2017), and phosphorylates S6K1, which controls the expression of AATs and PepT1 in humans (Benner et al., 2011; Hay and Sonenberg, 2004; Rosario et al., 2016). The present study stands as a first step to explore the mechanistic effects of VD3 on absorption in animal intestinal segments. The results demonstrated that VD3 deficiency downregulated S6K1, ATF4, and p-TOR mRNA levels and upregulated 4E-BP1 mRNA in all intestinal segments. Furthermore, a correlation analysis (Appendix Table 3) revealed that most AATs and SLC15A1 were correlated positively with TOR, S6K1, and ATF4 and negatively related to 4E-BP1 in different intestinal segments. Therefore, we inferred that VD3 mediates AA transport and is potentially related to TOR signaling molecules in fish intestines.
4.5. Excess levels of VD3 led to adverse effects on fish growth
Compared with the results observed at optimal VD3 supplement levels (1,167.9 IU/kg diet), excess VD3 (1,980.1IU/kg diet) led to significantly decreased growth performance and feed utilization. This agrees with results from studies on juvenile Siberian sturgeon (Wang et al., 2017), juvenile black carp (Wu et al., 2020), and orange-spotted grouper (He et al., 2021). This phenomenon could be associated with 1) reduced ability for digestion and absorption; 2) increased oxidative stress and decreased antioxidant capacity (He et al., 2021); 3) depressed immune response (Jiang et al., 2015); or 4) increased risk of skeletal deformities (Darias et al., 2010).
4.6. Comparison of VD3 requirements for on-growing grass carp based on different indices
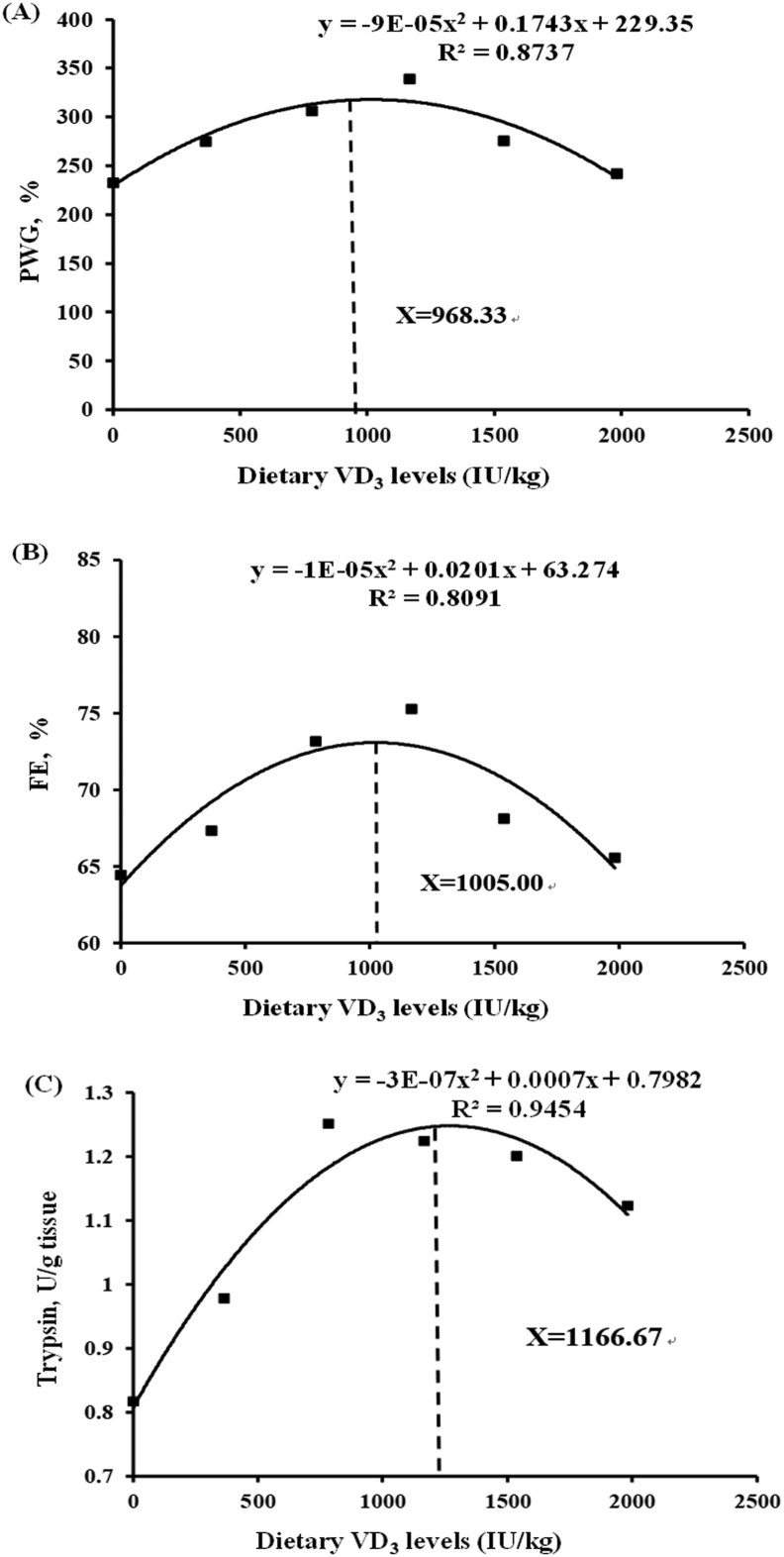
Based on PWG (Fig. 6A) and FE (Fig. 6B), the estimated optimal VD3 supplement level was 968.33 and 1,005.00 IU/kg, respectively. Alternatively, on basis of trypsin activity (Fig. 6C), VD3 requirements are estimated to be 1,166.67 IU/kg diet. A higher activity of trypsin was required compared to growth performance, indicating that greater amounts of VD3 were required for optimal digestion.
Fig. 6.
Quadratic regression analysis of PWG (%, A), FE (%, B) and trypsin activity (U/g tissue, C) for the on-growing grass carp (Ctenopharyngodon idella) fed diets with graded levels of VD3 (IU/kg) for 10 weeks.
5. Conclusions
To the best of our knowledge, the present study is the first to identify the effects of VD3 on growth performance, absorption capacity, AATs, and PepT1 in different intestinal segments through a mechanism associated to the TOR signaling pathway. TOR signaling, supported by dietary VD3, caused the increase of AAT, PepT1, and brush border enzyme mRNA levels. These conclusions were supported by the findings that VD3 deficiency compromised the integrity of intestinal structure; decreased trypsin, chymotrypsin, amylase, CK, γ-GT, Na+/K+-ATPase, and AKP activities; downregulated mRNA expression of atp1a1a1, atp1a1a4, and CK; depressed the levels of most neutral, basic, and acidic AAs, in partial correlation with the downregulation of most corresponding AATs, and in relation to the TOR signaling pathway (TOR/[4E-BP1/S6K1 and ATF4]). According to the results of quadratic regression analysis of PWG (Fig. 6A), FE (Fig. 6B), and the activity of trypsin (Fig. 6C), the dietary VD3 requirement of on-growing grass carp (257.24 ± 0.40 g) was estimated to be 968.33, 1,005.00, and 1,166.67 IU/kg, respectively. These findings provide new insights into the development of VD3 supplements to improve productivity in the aquaculture of grass carp.
Author contributions
Yao Zhang: Manuscript writing, Formal analysis; Chao-Nan Li: Investigation; Wei-Dan Jiang: Data curation; Pei Wu, Yang Liu: Methodology; Sheng-Yao Kuang, Ling Tang, Hua-Wei Li: Resources; Xiao-Wan Jin, Hong-Mei Ren: Management; Xiao-Qiu Zhou, Lin Feng: Conceptualization, Supervision. Lin Feng had primary responsibility for the final content of the manuscript. All authors carefully read and approved the final revision of the manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This research was financially supported by National Key R&D Program of China (2019YFD0900200, 2018YFD0900400), National Natural Science Foundation of China for Outstanding Youth Science Foundation (31922086), Young Top-Notch Talent Support Program, China Agriculture Research System of MOF and MARA (CARS-45) and Sichuan Science and Technology Program (2019YFN0036). The authors would like to express their sincere thanks to the personnel of these teams for their kind assistance.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2022.05.004.
Contributor Information
Xiao-Qiu Zhou, Email: zhouxq@sicau.edu.cn.
Lin Feng, Email: fenglin@sicau.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Anderson C.M., Ganapathy V., Thwaites D.T. Human solute carrier SLC6A14 is the beta-alanine SLC7A5carrier. J Physiol. 2008;586:4061–4067. doi: 10.1113/jphysiol.2008.154500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauermeister A., Lewendon A., Ramage P., Nimmo I.A. Distribution and some properties of the glutathione stransferase and c-glutamyl transpeptidase activities of rainbow trout. Comp Biochem Physiol C. 1983;74:89–93. doi: 10.1016/0742-8413(83)90155-x. [DOI] [PubMed] [Google Scholar]
- Benner J., Daniel H., Spanier B. A glutathione peroxidase, intracellular peptidases and the TOR complexes regulate peptide transporter PEPT-1 in C. elegans. PLoS One. 2011;6(9):e25624. doi: 10.1371/journal.pone.0025624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge G.E., Goodman M., Espe M., Lied E. Intestinal absorption of amino acids in fish: kinetics and interaction of the in vitro uptake of L-methionine in Atlantic salmon (Salmo salar L.) Aquaculture. 2004;229:265–273. [Google Scholar]
- Bessey O.A., Lowry O.H., Brock M.J. A method for rapid determination of alkaline phosphatase with five cubic millimeters of serum. J Biol Chem. 1946;164:321–329. [PubMed] [Google Scholar]
- Bodoy S., Sotillo F., Guarch E.M., Sperandeo M.P., Ormazabal A., Zorzano A., Sebastio G., Artuch R., Palacín M. Inducible Slc7a7 knockout mouse model recapitulates lysinuric protein intolerance disease. Int J Mol Sci. 2019;20(21):5294. doi: 10.3390/ijms20215294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer S. Apical transporters for neutral amino acids: physiology and pathophysiology. Physiology. 2008;23:95–103. doi: 10.1152/physiol.00045.2007. [DOI] [PubMed] [Google Scholar]
- Buitrago C.G., Ronda A.C., Boland A.R.D., Boland R. MAP kinases p38 and JNK are activated by the steroid hormone 1α,25(OH)2-vitamin D3 in the C2C12 muscle cell line. J Cell Biochem. 2006;97:698–708. doi: 10.1002/jcb.20639. [DOI] [PubMed] [Google Scholar]
- Chen Y.Y., Powell T.L., Jansson T. 1,25-Dihydroxy vitamin D3 stimulates system A amino acid transport in primary human trophoblast cells. Mol Cell Endocrinol. 2017;442:90–97. doi: 10.1016/j.mce.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S., Li S., Cruz J.D.L., Shroyer N.F., Criss Z.K., Verzi M.P., et al. Vitamin D and the intestine: review and update. J Steroid Biochem Mol Biol. 2020;196:105501. doi: 10.1016/j.jsbmb.2019.105501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H.N. Role of amino acid transport and counter transport in nutrition and metabolism. Physiol Rev. 1990;7:43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Claro da Silva T., Hiller C., Gai Z., Kullak-Ublick G.A. Vitamin D3 transactivates the zinc and manganese transporter SLC30A10 via the Vitamin D receptor. J Steroid Biochem Mol Biol. 2016;163:77–87. doi: 10.1016/j.jsbmb.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Coomer J.C., Amos H.E., Froetschel M.A., Ragland K.K., Williams C.C. Effects of supplemental protein source on ruminal fermentation, protein degradation, and amino acid absorption in steers and on growth and feed efficiency in steers and heifers. J Anim Sci. 1993;71:3078–3086. doi: 10.2527/1993.71113078x. [DOI] [PubMed] [Google Scholar]
- Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol. 2004;66:361–384. doi: 10.1146/annurev.physiol.66.032102.144149. [DOI] [PubMed] [Google Scholar]
- Darias M.J., Mazurais D., Koumoundouros G., Glynatsi N., Christodoulopoulou S., Huelvan C., et al. Dietary vitamin D3 affects digestive system ontogenesis and ossification in european sea bass (dicentrachus labrax, linnaeus, 1758) Aquaculture. 2010;298(3–4):300–307. [Google Scholar]
- Dusso A.S., Brown A.J., Slatopolsky E. Vitamin D. Am J Physiol Ren Physiol. 2005;289:F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- Erben U., Loddenkemper C., Doerfel K., Spieckermann S., Haller D., Heimesaat M.M., et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol. 2014;7:4557–4576. [PMC free article] [PubMed] [Google Scholar]
- Eshaghzadeh H., Hoseinifar S.H., Vahabzadeh H., Ringø E. The effects of dietary inulin on growth performances, survival and digestive enzyme activities of common carp (Cyprinus carpio) fry. Aquacult Nutr. 2015;21:242–247. [Google Scholar]
- Fang C.C., Feng L., Jiang W.D., Wu P., Liu Y., Kuang S.Y., et al. Effects of dietary methionine on growth performance, muscle nutritive deposition, muscle fibre growth and type I collagen synthesis of on-growing grass carp (Ctenopharyngodon idella) Br J Nutr. 2020:1–36. doi: 10.1017/S0007114520002998. [DOI] [PubMed] [Google Scholar]
- Frazier S., Ajiboye K., Olds A., Wyatt T., Luetkemeier E.S., Wong E.A. Functional characterization of the chicken peptide transporter 1 (pept1, slc15a1) gene. Anim Biotechnol. 2008;19:201–210. doi: 10.1080/10495390802240206. [DOI] [PubMed] [Google Scholar]
- García-Gasca A., Galaviz M.A., Gutiérrez J.N., García-Ortega A. Development of the digestive tract, trypsin activity and gene expression in eggs and larvae of the bullseye puffer fish sphoeroides annulatus. Aquaculture. 2006;251(2–4):366–376. [Google Scholar]
- González M., Gallardo V., Rodríguez N., Salomón C., Westermeier F., Guzmán- Gutiérrez E., et al. Insulin-stimulated L-arginine transport requires SLC7A1 gene expression and is associated with human umbilical vein relaxation. J Cell Physiol. 2011;226(11):2916–2924. doi: 10.1002/jcp.22635. [DOI] [PubMed] [Google Scholar]
- Graff I.E., Høie S., Totland G.K., Lie Ø. Three different levels of dietary vitamin D3 fed to first-feeding fry of Atlantic salmon (Salmo salar L.): effect on growth, mortality, calcium content and bone formation. Aquacult Nutr. 2002;8:103–111. [Google Scholar]
- Gupta N., Prasad P.D., Ghamande S., Moore-Martin P., Herdman A.V., Martindale R.G., et al. Up-regulation of the amino acid transporter ATB(0,+) (SLC6A14) in carcinoma of the cervix. Gynecol Oncol. 2006;100:8–13. doi: 10.1016/j.ygyno.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Haddad J.G., Matsuoka L.Y., Hollis B.W., Hu Y.Z., Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 1993;91:2552–2555. doi: 10.1172/JCI116492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N., Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- He S., Ding M., Ray G.W., Yang Q., Tan B., Dong X., et al. Effect of dietary vitamin D levels on growth, serum biochemical parameters, lipid metabolism enzyme activities, fatty acid synthase and hepatic lipase mRNA expression for orange-spotted grouper (Epinephelus coioides) in growth mid-stage. Aquacult Nutr. 2021;27(3):655–665. [Google Scholar]
- Heidarieh M., Mirvaghefi A.R., Akbari M., Sheikhzadeh N., Kamyabi-Moghaddam Z., Askari H., et al. Evaluations of Hilyses™, fermented Saccharomyces cerevisiae, on rainbow trout (Oncorhynchus mykiss) growth performance, enzymatic activities and gastrointestinal structure. Aquacult Nutr. 2013;19:343–348. [Google Scholar]
- Hodin R.A., Chamberlain S.M., Meng S. Pattern of rat intestinal brush-border enzyme gene expression changes with epithelial growth state. Am J Physiol Cell Physiol. 1995;269(2 Pt 1):385–391. doi: 10.1152/ajpcell.1995.269.2.C385. [DOI] [PubMed] [Google Scholar]
- Huang D., Guo Y., Li X., Pan M., Liu J., Zhang W., et al. Vitamin D3/VDR inhibits inflammation through NF-κB pathway accompanied by resisting apoptosis and inducing autophagy in abalone Haliotis discus hannai. Cell Biol Toxicol. 2021 doi: 10.1007/s10565-021-09647-4. [DOI] [PubMed] [Google Scholar]
- Hyde R., Taylor P.M., Hundal H.S. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem J. 2003;373(1):1–18. doi: 10.1042/bj20030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T.T., Feng L., Liu Y., Jiang W.D., Jiang J., Li S.H., et al. Effects of exogenous xylanase supplementation in plant protein-enriched diets on growth performance, intestinal enzyme activities and microflora of juvenile Jian carp (Cyprinus carpio var. Jian) Aquacult Nutr. 2014;20:632–645. [Google Scholar]
- Jiang J., Shi D., Zhou X.Q., Yin L., Feng L., Jiang W.D., et al. Vitamin D inhibits lipopolysaccharide-induced inflammatory response potentially through the Toll-like receptor 4 signalling pathway in the intestine and enterocytes of juvenile Jian carp (Cyprinus carpio var. Jian) Br J Nutr. 2015;114:1560–1568. doi: 10.1017/S0007114515003256. [DOI] [PubMed] [Google Scholar]
- Johnston D.J., Ritar A.J., Thomas C.W. Digestive enzyme profiles reveal digestive capacity and potential energy sources in fed and starved spiny lobster (Jasus edwardsii) phyllosoma larvae. Comp Biochem Physiol B Biochem Mol Biol. 2004;138:137–144. doi: 10.1016/j.cbpc.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Jose L.Z.I., Chantal L.C., Armande P. Partial substitution of di- and tripeptides for native proteins in sea bass diet improves Dicentrarchus labrax larval development. J Nutr. 1997;127:608–614. doi: 10.1093/jn/127.4.608. [DOI] [PubMed] [Google Scholar]
- Korének M., Vaško L., Koréneková B., Jacková A., Skalická M., Siklenka P., et al. Effect of vitamin D3 on chymotrypsin activity in the droppings of laying hens after an exposure to cadmium. Bull Vet Inst Pulawy. 2000;44:227–233. [Google Scholar]
- Krycer J.R., Fazakerley D.J., Cater R.J., Thomas K.C., Naghiloo S., Burchfield J.G., et al. The amino acid transporter, SLC1A3, is plasma membrane-localised in adipocytes and its activity is insensitive to insulin. FEBS Lett. 2017;59:322–330. doi: 10.1002/1873-3468.12549. [DOI] [PubMed] [Google Scholar]
- Le H.T.M.D., Shao X., Krogdahl Å., Kortner T.M., Lein I., Kousoulaki K., et al. Intestinal function of the stomachless fish, ballan wrasse (Labrus bergylta) Front Mar Sci. 2019;6 [页码] [Google Scholar]
- Li X.G., Chen X.L., Wang X.Q. Changes in relative organ weights and intestinal transporter gene expression in embryos from White Plymouth Rock and WENS Yellow Feather Chickens. Comp Biochem Physiol Mol Integr Physiol. 2013;164:368–375. doi: 10.1016/j.cbpa.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Liu Y. Fatty acids, inflammation and intestinal health in pigs. J Anim Sci Biotechnol. 2015;6:41. doi: 10.1186/s40104-015-0040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Hassan M., Allais L., He T., Leterme S., Ellis A., et al. Comparison of partial replacement of fishmeal with soybean meal and EnzoMeal on growth performance of Asian seabass Lates calcarifer. Comp Biochem Physiol C. 2019;216:29–37. doi: 10.1016/j.cbpc.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Matsuguchi T., Chiba N., Bandow K., Kakimoto K., Masuda A., Ohnishi T. JNK activity is essential for ATF4 expression and late-stage osteoblast differentiation. J Bone Miner Res. 2009;24:398–410. doi: 10.1359/jbmr.081107. [DOI] [PubMed] [Google Scholar]
- Mora J.R., Iwata M., Andrian U.H. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschella P.C., Rao V.U., McDermott P.J., Kuppuswamy D. Regulation of mTOR and S6K1 activation by the nPKC isoforms, PKCepsilon and PKCdelta, in adult cardiac muscle cells. J Mol Cell Cardiol. 2007;43:754–766. doi: 10.1016/j.yjmcc.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad F.H., Saadé N.E. Neural regulation of intestinal nutrient absorption. Prog Neurobiol. 2011;95(2):149–162. doi: 10.1016/j.pneurobio.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Ni D.S., Wang J.G. 1st ed. Science Press; Beijing: 1999. Biology and diseases of grass carp; pp. 29–33. (in Chinese) [Google Scholar]
- National Research Council . National Academies Press; Washington, DC: 2011. Nutrient requirements of fish and shrimp. [Google Scholar]
- Ogihara H., Suzuki T., Nagamachi Y., Inui K., Takata K. Peptide transporter in the rat small intestine: ultrastructural localization and the effect of starvation and administration of amino acids. Histochem J. 1999;31:169–174. doi: 10.1023/a:1003515413550. [DOI] [PubMed] [Google Scholar]
- Park Y., Reyna-Neyra A., Philippe L., Thoreen C.C. mTORC1 balances cellular amino acid supply with demand for protein synthesis through post-transcriptional control of ATF4. Cell Rep. 2017;19:1083–1090. doi: 10.1016/j.celrep.2017.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perland E., Fredriksson R. Classification systems of secondary active transporters. Trends Pharmacol Sci. 2017;38:305–315. doi: 10.1016/j.tips.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Popovic P.J., Zeh H.J., Ochoa J.B. Arginine and immunity. J Nutr. 2007;137 doi: 10.1093/jn/137.6.1681S. 1681s-6s. [DOI] [PubMed] [Google Scholar]
- Rosario F.J., Dimasuay K.G., Kanai Y., Powell T.L., Jansson T. Regulation of amino acid transporter trafficking by mTORC1 in primary human trophoblast cells is mediated by the ubiquitin ligase Nedd4-2. Clin Sci (Lond) 2016;130:499–512. doi: 10.1042/CS20150554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust M.B. In: Nutritional physiology in fish nutrition. Halver J.E., Hardy R.W., editors. Academic Press; California, USA: 2002. pp. 367–452. [Google Scholar]
- Salles J., Chanet A., Giraudet C., Patrac V., Pierre P., Jourdan M., et al. 1,25(OH)2-vitamin D3 enhances the stimulating effect of leucine and insulin on protein synthesis rate through Akt/PKB and mTOR mediated pathways in murine C2C12 skeletal myotubes. Mol Nutr Food Res. 2013;57:2137–2146. doi: 10.1002/mnfr.201300074. [DOI] [PubMed] [Google Scholar]
- Sans M.D., Crozier S.J., Vogel N.L., D'Alecy L.G., Williams J.A. Dietary protein and amino acid deficiency inhibit pancreatic digestive enzyme mRNA translation by multiple mechanisms. Cell Mol Gastroenter. 2021;11:99–115. doi: 10.1016/j.jcmgh.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalise M., Pochini L., Pingitore P., Hedfalk K., Indiveri C. Cysteine is not a substrate but a specific modulator of human ASCT2 (SLC1A5) transporter. FEBS Lett. 2015;589(23):3617–3623. doi: 10.1016/j.febslet.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Schindhelm R.K., Diamant M., Dekker J.M., Tushuizen M.E., Teerlink T., Heine R.J. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev. 2006;22:437–443. doi: 10.1002/dmrr.666. [DOI] [PubMed] [Google Scholar]
- Shao X.Y., Wu P., Feng L., Jiang W.D., Liu Y., Kuang S.Y., et al. Growth performance, digestive and absorptive capacity of on-growing grass carp (Ctenopharyngodon idellus) fed with graded level of dietary fibre from soybean hulls. Aquacult Nutr. 2021;27:198–216. [Google Scholar]
- Silva F.C.P., Nicoli J.R., Zambonino-Infante J.L., Gall M.M.L., Kaushik S., Gatesoupe F.J. Influence of partial substitution of dietary fish meal on the activity of digestive enzymes in the intestinal brush border membrane of gilthead sea bream (Sparus aurata) and goldfish (Carassius auratus) Aquaculture. 2010;306:233–237. [Google Scholar]
- Song Z.X., Jiang W.D., Liu Y., Wu P., Jiang J., Zhou X.Q., et al. Dietary zinc deficiency reduced growth performance, intestinal immune and physical barrier functions related to NF-κB, TOR, Nrf2, JNK and MLCK signaling pathway of young grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2017;66:497–523. doi: 10.1016/j.fsi.2017.05.048. [DOI] [PubMed] [Google Scholar]
- Song M., Zhang F., Chen L., Yang Q., Su H., Yang X., et al. Dietary chenodeoxycholic acid improves growth performance and intestinal health by altering serum metabolic profiles and gut bacteria in weaned piglets. Anim Nutr. 2021;7(2):365–375. doi: 10.1016/j.aninu.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandeo M.P., Annunziata P., Bozzato A., Piccolo P., Maiuri L., D'Armiento M., et al. Slc7A7 disruption causes fetal growth retardation by downregulating Igf1 in the mouse model of lysinuric protein intolerance. Am J Physiol Cell Physiol. 2007;293(1) doi: 10.1152/ajpcell.00583.2006. [DOI] [PubMed] [Google Scholar]
- Stretton C., Lipina C., Hyde R., Cwiklinski E., Hoffmann T.M., Taylor P.M., et al. CDK7 is a component of the integrated stress response regulating SNAT2 (SLC38A2)/System A adaptation in response to cellular amino acid deprivation. BBA-Mol Cell Res. 2019;1866:978–991. doi: 10.1016/j.bbamcr.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia V.L., Schwartz Z., Ellis E.B., Helm S.H., Gomez R., Dean D.D., et al. Nongenomic regulation of protein kinase C isoforms by the vitamin D metabolites 1α,25-(OH)2D3 and 24R,25-(OH)2D3. J Cell Physiol. 1996;167:380–393. doi: 10.1002/(SICI)1097-4652(199606)167:3<380::AID-JCP2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Okano T., Teraoka S., Murakami Y., Kobayashi T. High-performance liquid chromatographic determination of vitamin D in foods, feeds and pharmaceuticals by successive use of reversed-phase and straight-phase columns. J Nutr Sci Vitaminol. 1984;30:11–25. doi: 10.3177/jnsv.30.11. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Bush K.K., Eguchi T., Ikekawa N., Taguchi T., Kobayashi Y., et al. Effects of 1,25-dihydroxyvitamin D3 and its analogs on butyrate-induced differentiation of HT-29 human colonic carcinoma cells and on the reversal of the differentiated phenotype. Arch Biochem Biophys. 1990;276:415–423. doi: 10.1016/0003-9861(90)90740-p. [DOI] [PubMed] [Google Scholar]
- Tang R.J., Feng L., Jiang W.D., Liu Y., Kuang S.Y., Jiang J., et al. Growth, digestive and absorptive abilities and antioxidative capacity in the hepatopancreas and intestine of young grass carp (Ctenopharyngodon idellus) fed graded levels of dietary manganese. Aquacult Res. 2016;47:1917–1931. [Google Scholar]
- Tanzer M.L., Gilvarg C. Creatine and creatine kinase measurement. J Biol Chem. 1959;234:3201–3204. [PubMed] [Google Scholar]
- Tian L., Zhou X.Q., Jiang W.D., Liu Y., Wu P., Jiang J., et al. Sodium butyrate improved intestinal immune function associated with NF-κB and p38MAPK signalling pathways in young grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2017;66:548–563. doi: 10.1016/j.fsi.2017.05.049. [DOI] [PubMed] [Google Scholar]
- Tsertou M.I., Chatzifotis S., Fontanillas R., Cotou E., Fountoulaki E., Antonopoulou E., et al. The effect of dietary vitamin D3, minerals (Ca, P) and plant-protein sources in the development of systemic granulomatosis in meagre (Argyrosomus regius, Asso, 1801) Aquaculture. 2020;521:735052. [Google Scholar]
- Wang L., Xu H., Wang Y., Wang C., Li J., Zhao Z., et al. Effects of the supplementation of vitamin D3 on the growth and vitamin D metabolites in juvenile Siberian sturgeon (Acipenser baerii) Fish Physiol Biochem. 2017;43:901–909. doi: 10.1007/s10695-017-0344-5. [DOI] [PubMed] [Google Scholar]
- Wang J., Ni X., Wen B., Zhou Y., Liu L., Zeng Y., et al. Bacillus strains improve growth performance via enhancing digestive function and anti-disease ability in young and weaning rex rabbits. Appl Microbiol Biotechnol. 2020;104:4493–4504. doi: 10.1007/s00253-020-10536-9. [DOI] [PubMed] [Google Scholar]
- Worker N.A., Migicovsky B.B. Effect of vitamin D on the utilization of zinc, cadmium and mercury in the chick. J Nutr. 1961;75:222–224. doi: 10.1093/jn/75.2.222. [DOI] [PubMed] [Google Scholar]
- Wu G. CRC Press; 2013. Amino acids: biochemistry and nutrition. [Google Scholar]
- Wu P., Feng L., Kuang S.Y., Liu Y., Jiang J., Hu K., et al. Effect of dietary choline on growth, intestinal enzyme activities and relative expressions of target of rapamycin and eIF4E-binding protein 2 gene in muscle, hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian) Aquaculture. 2011;317:107–116. [Google Scholar]
- Wu S., Wang G., Angert E.R., Wang W., Li W., Zou H. Composition, diversity, and origin of the bacterial community in grass carp intestine. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Jiang W.D., Jiang J., Zhao J., Liu Y., Zhang Y.A., et al. Dietary choline deficiency and excess induced intestinal inflammation and alteration of intestinal tight junction protein transcription potentially by modulating NF-κB, STAT and p38 MAPK signaling molecules in juvenile Jian carp. Fish Shellfish Immunol. 2016;58:462–473. doi: 10.1016/j.fsi.2016.09.055. [DOI] [PubMed] [Google Scholar]
- Wu C., Lu B., Wang Y., Jin C., Zhang Y., Ye J. Effects of dietary vitamin D3 on growth performance, antioxidant capacities and innate immune responses in juvenile black carp (Mylopharyngodon piceus) Fish Physiol Biochem. 2020;46(6):2243–2256. doi: 10.1007/s10695-020-00876-8. [DOI] [PubMed] [Google Scholar]
- Yang C.Y., Dantzig A.H., Pidgeon C. Intestinal peptide transport systems and oral drug availability. Pharm Res (N Y) 1999;16:1331–1343. doi: 10.1023/a:1018982505021. [DOI] [PubMed] [Google Scholar]
- Yuan Z.H., Feng L., Jiang W.D., Wu P., Liu Y., Jiang J., et al. Choline deficiency decreased the growth performances and damaged the amino acid absorption capacity in juvenile grass carp (Ctenopharyngodon idella) Aquaculture. 2020;518:734829. [Google Scholar]
- Zeitz U., Weber K., Soegiarto D.W., Wolf E., Balling R., Erben R.G. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. Faseb J. 2003;17:509–511. doi: 10.1096/fj.02-0424fje. [DOI] [PubMed] [Google Scholar]
- Zhang J., Yin Y., Shu X.G., Li T., Li F., Tan B., et al. Oral administration of MSG increases expression of glutamate receptors and transporters in the gastrointestinal tract of young piglets. Amino Acids. 2013;45:1169–1177. doi: 10.1007/s00726-013-1573-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y.L., Duan X.D., Jiang W.D., Feng L., Wu P., Liu Y., et al. Soybean glycinin decreased growth performance, impaired intestinal health, and amino acid absorption capacity of juvenile grass carp (Ctenopharyngodon idella) Fish Physiol Biochem. 2019;45:1589–1602. doi: 10.1007/s10695-019-00648-z. [DOI] [PubMed] [Google Scholar]
- Zhao T., Li D., Liu X., Liu Z., Wu D., Zhou S., et al. Effect of SLC7A5 on the proliferation of tumor cells and its relationship with transforming growth factor-β1 signal pathway. J Cent South Univ. 2017;42(5):485–492. doi: 10.11817/j.issn.1672-7347.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhang T.R., Li Q., Feng L., Liu Y., Jiang W.D., et al. Effect of dietary L-glutamate levels on growth, digestive and absorptive capability, and intestinal physical barrier function in Jian carp (Cyprinus carpio var. Jian) Anim Nutr. 2020;6(2):198–209. doi: 10.1016/j.aninu.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Wang L., Wang H., Xie F., Wang T. Effect of dietary vitamin C on the growth performance and innate immunity of juvenile cobia (Rachycentron canadum) Fish Shellfish Immunol. 2012;32(6):969–975. doi: 10.1016/j.fsi.2012.01.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.