Abstract
Introduction
Cancer and its treatment using various chemotherapeutic agents can have many adverse side effects. These side effects often result in significant changes in haematological and biochemical composition of blood. As a result, the regular monitoring of serum biochemical and haematological changes plays an important role in management of disease. The present study aimed to determine the relationship between haematological and biochemical changes in neutropenic cancer patients following chemotherapy. Specifically we evaluated the association between neutrophil count and serum proteins and electrolytes.
Methods
For this purpose we analysed retrospectively collected laboratory results from two independent patient cohorts. Each cohort was divided into a control group consisting of patients with normal haematological parameters and a study group which included patients with reduced neutrophil counts. Neutropenic patients (study group) were cancer patients on chemotherapy.
Results and conclusion
Blood samples of cancer patients in study group showed reduction in haemoglobin, neutrophils and platelets. Neutropenic group showed a significant reduction in serum albumin, total protein, calcium, and potassium. Our results show that patients with severe neutropenia had pronounced changes in serum protein and electrolytes and increased incidence of abnormal serum protein and electrolyte level. The changes in the neutrophil counts showed a positive correlation with the changes in serum protein and electrolyte levels. A similar trend was seen in both the patient cohorts: the discovery set (176 patients) and the validation set (200 patients). Taken together our results suggest that chemotherapy-induced neutropenia is associated with dysregulation in haemoglobin, platelets, serum proteins and electrolytes.
Keywords: Chemotherapy, Serum albumin, Serum total protein, Serum calcium, Serum potassium, Neutropenia
Chemotherapy; Serum Albumin; Serum total Protein; Serum Calcium; Serum Potassium; Neutropenia.
1. Introduction
Cancer treatment includes chemotherapy, radiation therapy, biological therapy, surgery or a combination of two or more of these therapeutic options. All of these treatment options are aimed to eradicate the cancer cells that exist in the patient's body. Chemotherapy targets and destroys all rapidly dividing cells including cancerous and normal cells. As a result chemotherapy affects the body's normal organs and tissues. The undesired consequence of damage to healthy cells is a complication of treatment, or a side effect that delays the treatment and reduces the dose [1]. Blood has four main components: plasma, erythrocytes (red blood cells), leucocytes (white blood cells), and thrombocytes (platelets) [2]. Blood cells are short-lived and need to be replenished regularly. Haematopoietic stem cells in bone marrow divide rapidly to give rise to new blood cells and are therefore adversely affected by chemotherapy. Chemotherapy suppresses the growth and maturation of stem cells in the bone marrow and reduces the production of blood cells. Cancer chemotherapy results in the reduction in blood cell production and reduced blood cell counts in peripheral circulation. Reduction in all types of blood cells results in pancytopenia [3]. Chemotherapy-induced reduction in white blood cells particularly neutrophils results in a condition known as neutropenia. Neutropenia compromises the body's immune responses against infections and can lead to serious life threatening systemic infections and sepsis. Chemotherapy-induced neutropenia is considered to be an oncologic emergency and need to be treated immediately.
Chemotherapy can also affect the hepatic and renal function of cancer patients which manifests itself as alteration of serum proteins (serum albumin, total protein) and serum urea and creatinine respectively. Albumin, the most abundant serum protein is produced by liver cells up to 9–12 g/day and it represents about 50% of total proteins of the body and the normal serum range is 3.5–5 g/L [4]. It maintains normal plasma colloid osmotic pressure, boosts immunity, and has anti-cancer properties [5, 6]. The absorption, synthesis, and decomposition of proteins and albumin in the body can be evaluated by measuring total serum protein and albumin [7]. Serum albumin levels are used to assess the patient's liver function, nutritional status, and renal function when analysed along with blood urea nitrogen (BUN) and creatinine. Hypoalbuminemia occurs due to malnutrition and significant nutrient consumption by cancer cells [5]. Cancer patients with low levels of serum albumin have severe chemotherapy-induced toxicities [8, 9]. The cancer patients on chemotherapy who had serum albumin more than 3.40 g/dl showed better overall survival. Low serum albumin is a risk factor for higher chemotherapy-induced toxicity and an independent prognostic factor that can predict the impact of chemotherapy on overall survival [10, 11]. Hypoalbuminemia before treatment was identified to be one of the five independent risk factors for developing aspiration pneumonia in cancer patients on chemotherapy and radiotherapy, which deteriorated more than 20% in 191 patients post chemo- and radio-therapy [12]. A study conducted on the nutritional status of gastric cancer patients on chemotherapy suggested hypoalbuminemia as a sensitive predictor of chemotherapy toxicities including neutropenia [13]. The success of treatment, length of hospital stay, and prognosis can be predicted by the nutritional status of patients. Serum albumin is also an indicator of the nutritional status of cancer patients on chemotherapy [14, 15, 16].
Several chemotherapy drugs cause renal dysfunction due to which kidneys are unable to excrete nitrogenous waste and creatinine that result in imbalance in fluid and electrolyte homeostasis [17, 18, 19]. Glomerular filtration rate (GFR) is commonly used to evaluate kidney function [18]. GFR is usually estimated by serum creatinine, age, weight, and gender of the person [19]. Although variation in serum creatinine could be due to an altered rate of endogenous production regulated by creatinine kinase in cancer patients [18]. But high serum creatinine levels in cancer patients on chemotherapy have been reported in different studies. Several studies have shown that chemotherapeutic drugs have nephrotoxic effects such as bleomycin [20] Cisplatin [21], and Intravesical Bacilli Calmette-Guerin (IVBCG) therapy, commonly used to treat for non-muscle invasive bladder cancer (NMIBC) is also considered to be nephrotoxic drug [22]. A study conducted on patients having testicular cancer showed elevated serum creatinine during bleomycin treatment and was considered a significant risk factor for developing pulmonary toxicities [20]. Another study suggested acute renal failure showing high blood urea and creatinine with hypokalaemia after pemetrexed treatment [23]. Electrolyte disorders or imbalances are also frequently present in cancer patients [24, 25]. In most cases, these disorders are due to combination of physiologic or metabolic changes (Hallmarks of cancer) that are commonly seen in cancer patients and chemotherapeutic regimens used for treatment [26]. These chemotherapy-induced electrolyte disorders can often be associated with other adverse conditions and result in poor response to treatment and other life threatening complications [27].
Serum electrolytes include sodium, potassium, chloride calcium, and magnesium. Electrolyte imbalance is commonly seen in cancer patients due to malignancies or adverse effects of antineoplastic drugs such as diarrhoea, vomiting, and renal disorders [24, 25]. Thus, evaluation of these disorders in patients and understanding their correlation with other haematological abnormalities such as neutropenia is important in the overall care of the cancer patients. This study aimed to determine the effects of chemotherapy on serum electrolytes and proteins in cancer patients and to establish an association with chemotherapy-induced neutropenia.
2. Methods
2.1. Study design
A retrospective cohort study was conducted from February 2018 to March 2021 to analyse the haematological and biochemical profile changes in blood samples obtained from cancer patients on chemotherapy treatments, collected at the Haematology unit of University hospital, Galway (UHG). We analysed retrospectively collected laboratory results from two independent patient cohorts - the discovery set (total 176 patients- 46% male and 42% female, 12% was unspecified) and the validation set (total 200 patients- 56.5% male and 43.5% female). Each cohort was further divided into a control group consisting of patients with normal haematological parameters and a study group which included patients with abnormal neutrophil counts.
2.2. Patients and biochemistry reports
Following approved ethical guidelines from the local ethics review committee complete blood count and biochemical assessment results were obtained from the Haematology Laboratory at UHG. The data set received in 2018–2019 (discovery data set) was considered as discovery data set while the data set received in 2020–2021 (validation data set) was used to validate the results. The samples were analysed to determine changes in biochemical parameters such as serum Urea, Creatinine, Albumin, Total Protein, and serum electrolytes (Sodium, Potassium, Chloride, Phosphate, and Calcium) and haematological parameters or blood cell counts. Using the Advia 212i system, blood counts were done and biochemistry measurements were performed on serum by the Cobas 8000 modular analyser series from Roche Diagnostics. The reports of these tests were compiled for each patient and analysed for normal and abnormal absolute neutrophil counts (ANC). Each patient data obtained was further classified based on ANC into normal and moderate (0.5–1.0 × 109/L ANC) or severe (<0.5 × 109/L ANC) neutropenia. The patient data was then analysed to determine changes in biochemical parameters such as serum Urea, Creatinine, Albumin, Total Protein, and serum electrolytes (Sodium, Potassium, Chloride, Phosphate, and Calcium) which were correlated with changes in neutrophil counts.
3. Ethics statement
The analysis of patient blood biochemistry reports in this study was approved by the Clinical Research Ethics Committee, Merlin Park Hospital, Galway at the meeting held on 13th December 2017 (Ref: C.A 1888 Management of Chemotherapy-induced Neutropenia in Cancer Patients).
3.1. Statistical analysis
The Data analysis was done using Microsoft Excel Windows 10. Results were expressed as mean ± SD. Significance was determined using a paired T-test. For correlation analysis Pearson's coefficient was calculated using excel. Differences were considered to be significant at P < 0.05.
3.2. Statistical power calculation
For power calculations we used web-based algorithm (powerandsamplesize.com) and selected parameters to calculate sample size needed to compare whether the means of two groups are different (2 Means: 2-Sample, 2-Sided Equality). We used the s-standard deviation and d-expected difference between two means values from the published similar study (Wang, et al., Changes of serum albumin level and SIR in NSCLC. Journal of Cancer Research and Therapeutics - October–December 2014 - Volume 10 - Issue 4. DOI: 10.4103/0973–1482.137953). On the basis of above assumptions, the sample size of 30 per group is deemed sufficient to have 90% power to detect differences between the treatments at p < 0.001. The required sample size according to the statistical power calculation was 60 for each discovery and validation set and the study cohort included in this study was 176 in discovery set and 200 in validation set.
4. Results
Two Patient groups were included in this study, non-cancer patients attending Haematology/Out Patients Department (OPD), considered as control group, having normal haematological parameters and cancer patients post-chemotherapy having neutropenia (study group). Neutropenic patients were identified on basis of neutrophil count and defined as patients having neutrophils less than <2.0 ×109/L. First, each biochemical parameter in both the groups (control group versus neutropenic group) was compared. The percentage of patients in both groups having out of normal range values were analysed. It was observed that the percentage of patients showing abnormal levels of calcium, sodium, potassium, chloride, albumin, and total protein was higher in neutropenic group as compared to control group (Table 1). Phosphate and urea did not show much difference in both groups. The percentage of neutropenic male patients showing abnormal creatinine levels was higher than in patients in the control group having abnormal levels whereas the percentage of the neutropenic females having abnormal creatinine levels was lower than creatinine levels observed in patients control group (Table 1) in the discovery set. Similar haematological and biochemical parameters were observed for validation set as shown in Table 2.
Table 1.
Biochemistry profile of blood samples in discovery set
| Parameters | Total numbers |
Out of Normal of range n (%) |
||
|---|---|---|---|---|
| OPD patients | Neutropenic patients | OPD patients | Neutropenic patients | |
| Sodium (Na+) | 84 | 84 | 8 (9.52) | 12 (14.3) |
| Mild | - | 34 | - | 4 (11.8) |
| Moderate | - | 20 | - | 3 (15.0) |
| Severe | - | 30 | - | 5 (16.7) |
| Potassium (K+) | 80 | 82 | 6 (7.5) | 12 (14.6) |
| Mild | - | 32 | - | 2 (6.3) |
| Moderate | - | 20 | - | 0 |
| Severe | - | 30 | - | 10 (33.3) |
| Chloride (Cl-) | 83 | 84 | 10 (12.05) | 18 (21.4) |
| Mild | - | 34 | - | 5 (14.7) |
| Moderate | - | 20 | - | 6 (30) |
| Severe | - | 30 | - | 7 (23.3) |
| Calcium (Ca2+) | 76 | 84 | 1 (1.32) | 26 (31) |
| Mild | - | 34 | - | 5 (14.7) |
| Moderate | - | 20 | - | 4 (20) |
| Severe | - | 30 | - | 17 (56.7) |
| Phosphate | 74 | 83 | 48 (64.86) | 55 (66.3) |
| Mild | - | 33 | - | 19 (57.6) |
| Moderate | - | 20 | - | 13 (65) |
| Severe | - | 30 | - | 23 (76.7) |
| Albumin | 81 | 84 | 5 (6.17) | 39 (46.4) |
| Mild | - | 34 | - | 6 (17.6) |
| Moderate | - | 20 | - | 10 (50) |
| Severe | - | 30 | - | 23 (76.7) |
| Total Protein | 81 | 84 | 8 (9.88) | 36 (42.9) |
| Mild | - | 34 | - | 8 (23.5) |
| Moderate | - | 20 | - | 7 (35) |
| Severe | - | 30 | - | 21 (70) |
| Blood Urea | 83 | 84 | 18 (21.69) | 19 (22.6) |
| Mild | - | 34 | - | 9 (26.5) |
| Moderate | - | 20 | - | 4 (20) |
| Severe | - | 30 | - | 6 (20) |
| Creatinine Female | 35 | 31 | 7 (20) | 4 (12.9) |
| Mild | - | 13 | - | 1 (7.7) |
| Moderate | - | 8 | - | 1 (12.5) |
| Severe | - | 10 | - | 2 (20) |
| Creatinine Male | 40 | 40 | 3 (7.5) | 17 (42.5) |
| Mild | - | 15 | - | 8 (53.3) |
| Moderate | - | 10 | - | 3 (30) |
| Severe | - | 15 | - | 6 (40) |
For all the parameters patient numbers (males, females and unidentified) were pooled. As the normal range of creatinine for males and females is different they are shown separately. Patients with unidentified sex were excluded. All the patients were divided in to three groups, mild, moderate and severe on basis of neutrophil count. Mild 1.0-2.0 x 109/L; Moderate 1.0-0.5 × 109/L; and severe <0.5 × 109/L. a no. of cases for which data is available.
Table 2.
Biochemistry profile of blood samples in the validation set.
| Parameters | Total numbers |
Out of Normal of range n (%) |
||
|---|---|---|---|---|
| OPD patients | Neutropenic patients | OPD patients | Neutropenic patients | |
| Sodium (Na+) | 92 | 99 | 5(5.43) | 30(30.3) |
| Mild | - | 13 | - | 4(30.8) |
| Moderate | - | 23 | - | 8(34.8) |
| Severe | - | 63 | - | 18(28.6) |
| Potassium (K+) | 86 | 99 | 2(2.33) | 22(22.2) |
| Mild | - | 13 | - | 1(7.7) |
| Moderate | - | 23 | - | 2(8.7) |
| Severe | - | 63 | - | 19(30.2) |
| Chloride (Cl-) | 92 | 98 | 13(14.13) | 24(24.5) |
| Mild | - | 13 | - | 3(23.1) |
| Moderate | - | 23 | - | 3(13.0) |
| Severe | - | 62 | - | 18(29.0) |
| Calcium (Ca2+) | 80 | 99 | 6(7.50) | 22(22.2) |
| Mild | - | 13 | - | 2(15.4) |
| Moderate | - | 23 | - | 4(17.4) |
| Severe | - | 63 | - | 16(25.4) |
| Phosphate | 77 | 99 | 48(62.34) | 64(64.6) |
| Mild | - | 13 | - | 7(53.8) |
| Moderate | - | 23 | - | 17(73.9) |
| Severe | - | 63 | - | 40(63.5) |
| Albumin | 84 | 99 | 7(8.33) | 46(46.5) |
| Mild | - | 13 | - | 4(30.8) |
| Moderate | - | 23 | - | 10(43.5) |
| Severe | - | 63 | - | 32(50.8) |
| Total Protein | 83 | 99 | 4(4.82) | 57(57.6) |
| Mild | - | 13 | - | 8(61.5) |
| Moderate | - | 23 | - | 13(56.5) |
| Severe | - | 63 | - | 36(57.1) |
| Blood Urea | 92 | 99 | 25(27.17) | 20(20.2) |
| Mild | - | 13 | - | 3(23.1) |
| Moderate | - | 23 | - | 2(8.7) |
| Severe | - | 63 | - | 15(23.8) |
| Creatinine Female | 37 | 42 | 7(18.92) | 12(28.6) |
| Mild | - | 6 | - | 3(50.0) |
| Moderate | - | 15 | - | 6(40.0) |
| Severe | - | 21 | - | 3(14.3) |
| Creatinine Male | 54 | 57 | 12(22.22) | 11(19.3) |
| Mild | - | 7 | - | Nil |
| Moderate | - | 8 | - | 1(12.5) |
| Severe | - | 42 | - | 10(23.8) |
For all the parameters patient numbers (males, females and unidentified) were pooled. As the normal range of creatinine for males and females is different they are shown separately. Patients with unidentified sex were excluded. All the patients were divided in to three groups, mild, moderate and severe on basis of neutrophil count. Mild 1.0-2.0 x 109/L; Moderate 1.0-0.5 × 109/L; and severe <0.5 × 109/L. a no. of cases for which data is available.
Further we sub-divided the neutropenic group into three subgroups based on their neutrophil count (Mild 1.0–2.0 × 109/L; Moderate 1.0–0.5 × 109/L; and severe <0.5 × 109/L) to assess if changes in above mentioned biochemical parameters correlate with the severity of neutropenia (Table 1). It was observed that a higher percentage of patients in the severely neutropenic patient group showed abnormal levels of calcium, albumin, potassium and total protein whereas the percentage of severely neutropenic patients having abnormal sodium and chloride was not very high as compared to control group. Blood urea and creatinine levels for female patients did not show much variation in both groups while the higher percentage of male patients with severe neutropenia showed abnormal creatinine levels than in patients in control group (Table 1). Similar trend was observed in the validation set except for creatinine in male patients which did not show much difference when compared to creatinine level of male patients in control group (Table 2).
4.1. Loss of cellular components of blood in cancer patients on chemotherapy
In this study, blood cells data of cancer patients on chemotherapy was analysed and compared with control group patients’ data to confirm the effects of chemotherapy on the cellular component of blood. A highly significant reduction was noticed in haemoglobin (male and female), neutrophils and platelets of cancer patients on chemotherapy (Figure 1A-D) suggestive of pancytopenia. Similar results were observed in the validation set (Figure 1E-H). The blood samples from cancer patients on chemotherapy showing reduced levels of neutrophils will be referred to as neutropenic group in the paper.
Figure 1.
Reduced haemoglobin, neutrophils and platelets in cancer patients post-chemotherapy. Comparison of serum haemoglobin in (A) female patients control group (n = 40) and Cancer patients post-chemotherapy (n = 41); (B) male patients control group (n = 42) and Cancer patients post-chemotherapy (n = 32) from discovery set is shown. Comparison of (C) Neutrophils and (D) Platelets in control group (n = 90) and Cancer patients post-chemotherapy (n = 86) from discovery set is shown. Comparison of serum haemoglobin in (E) female patients control group (n = 45) and Cancer patients post-chemotherapy (n = 42); (F) male patients control group (n = 55) and Cancer patients post-chemotherapy (n = 58) from validation set is shown. Comparison of (G) Neutrophils and (H) Platelets in control group (n = 100) and Cancer patients post-chemotherapy (n = 100) from validation set is shown.
4.2. Serum albumin and total protein was significantly reduced in neutropenic patients
Next we analysed the serum protein profile (albumin and total protein) in control and neutropenic group. We observed a statistically significant decrease in serum albumin and total protein in the neutropenic group (mean value serum albumin 36.30 g/L and total protein 61.30 g/L) as compared to the control group (mean value serum albumin 42.91 g/L and total protein 68.39 g/L) (Figure 2A-B). Similarly, serum albumin and total protein were also statistically reduced in validation set (Figure 2C-D). These results show a significant reduction of serum albumin and total protein in patients having chemotherapy-induced neutropenia.
Figure 2.
Reduced serum albumin and total protein in neutropenic patients. Comparison of (A) serum albumin in control group (n = 81) and neutropenic group (n = 84); (B) total protein in control group (n = 81) and neutropenic group (n = 84) from discovery set is shown. Comparison of (C) serum albumin in control group (n = 84) and neutropenic group (n = 100); (D) total protein in control group (n = 84) and neutropenic group (n = 100) from validation set is shown.
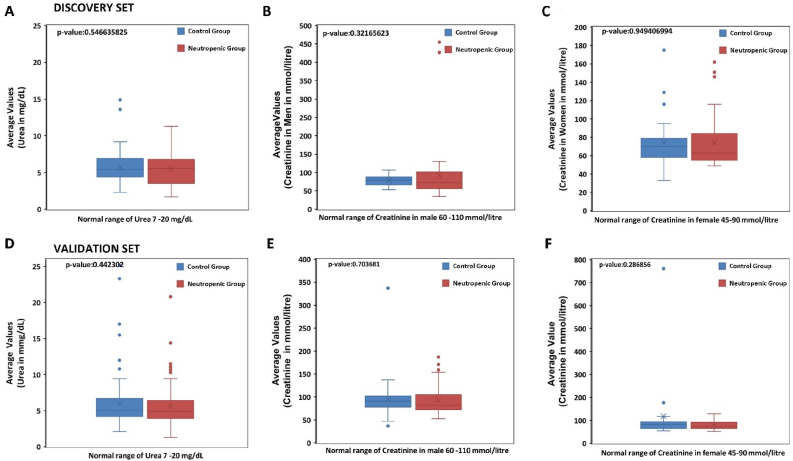
4.3. No change in serum urea and creatinine in neutropenic patients
Changes in serum urea level was noticed in neutropenic group when compared to control group but the reduction was not significant (Figure 3A), similarly serum creatinine levels in neutropenic male and female patients were slightly raised as compared to control group (Figure 3B-C) but was not statistically significant. In agreement with these results validation set did not show any statistically significant change in serum urea and creatinine levels of neutropenic patients (Figure 3D-F).
Figure 3.
Altered serum urea and creatinine in neutropenic patients. Comparison of (A) serum urea in control group (n = 83) and neutropenic group (n = 84); (B) serum Creatinine in male patients control group (n = 40) and neutropenic group (n = 40); (C) serum creatine in female control group (n = 35) and neutropenic group (n = 31) from discovery set is shown. Comparison of (A) serum urea in control group (n = 92) and neutropenic group (n = 99); (B) serum Creatinine in male patients control group (n = 54) and neutropenic group (n = 57); (C) serum creatine in female control group (n = 37) and neutropenic group (n = 42) from validation set is shown.
4.4. Hypokalemia and hypocalcemia in neutropenic patients
Major serum electrolytes include sodium, potassium, phosphate, calcium, magnesium, and chloride. The regulation of electrolytes is primarily done by kidneys. The physiological balance of serum electrolytes is essential for the body to function properly. Electrolyte imbalance, whether acute or chronic, due to the renal or non-renal cause has various adverse effects on the body. It is important to monitor the electrolytes of the cancer patient on chemotherapy [33, 34]. We found that neutropenic group showed a statistically significant decrease in serum potassium and calcium level (mean value serum potassium 4.01 mMol/L and calcium 2.25 mMol/L) than control group (mean value serum potassium 4.34 mMol/L and calcium 2.37 mMol/L) (Figure 4A-B). There was no significant change in serum phosphate, chloride and sodium levels in neutropenic group (Figure 4C-E). In keeping with these results validation set also showed significant decrease in serum potassium and calcium levels while no change in serum phosphate, chloride and sodium levels (Figure 4F-J) was observed.
Figure 4.
Altered serum electrolytes in neutropenic patients. Comparison of (A) serum potassium in control group (n = 80) and neutropenic group (n = 82) (B) serum calcium in control group (n = 76) and neutropenic group (n = 84); (C) serum phosphate in control group (n = 74) and neutropenic group (n = 83); (D) serum chloride in control group (n = 83) and neutropenic group (n = 84) and (E) serum sodium in control group (n = 84) and neutropenic group (n = 84) from discovery set is shown. Comparison of (F) serum potassium in control group (n = 86) and neutropenic group (n = 99); (G) serum calcium in control group (n = 80) and neutropenic group (n = 99); (H) serum phosphate in control group (n = 77) and neutropenic group (n = 99); (I) serum chloride in control group (n = 92) and neutropenic group (n = 98) and (J) serum sodium in control group (n = 92) and neutropenic group (n = 99) from validation set is shown.
4.5. Serum protein and electrolytes in severely neutropenic patients
Since we did not find any significant change in the serum level of phosphate, chloride, sodium, urea and creatinine between control group and neutropenic group, we decided to perform in-depth analysis by sub-dividing neutropenic group into three categories (Mild 1.0–2.0 × 109/L; Moderate 1.0–0.5 × 109/L; and severe <0.5 × 109/L) on the basis of neutrophil counts. Serum albumin, total protein, urea, creatinine, and serum electrolyte levels were analyzed (Table 1) in three categories of neutropenia. In line with our results on serum albumin and total protein (Figure 2), we found robust drop in serum albumin and total protein in patients having severe neutropenia as compared to control group (Figure 5A-B). Further, the reduction in serum albumin and total protein in patients having severe neutropenia was more pronounced than observed for all the patients in neutropenic group. Similarly, serum albumin and total protein were also robustly reduced in severe neutropenic group of validation set (Figure 5C-D).
Figure 5.
Reduced serum albumin and total protein in severe neutropenic patients. Comparison of (A) serum albumin in control group (n = 81) and severe neutropenic group (n = 30) and (B) total Protein in control group (n = 81) and severe neutropenic group (n = 30) from discovery set is shown. Comparison of (C) serum albumin in control group (n = 84) and severe neutropenic group (n = 63) and (D) total Protein in control group (n = 84) and severe neutropenic group (n = 63) from validation set is shown.
Next we analysed the serum electrolytes such as sodium, potassium, phosphate, calcium, and chloride in control group and severe neutropenic patients. In agreement with our results on serum potassium and calcium (Figure 4A-B), we found marked decrease in serum potassium and calcium in patients having severe neutropenia as compared to patients in control group (Figure 6A-B). Furthermore, the reduction in serum potassium and calcium in patients having severe neutropenia was more noticeable than observed neutropenic group. Similarly, serum potassium and calcium were robustly reduced in severe neutropenic patients of validation set (Figure 6F-G).
Figure 6.
Altered serum electrolytes in severe neutropenic patient group. Comparison of (A) serum potassium in control group (n = 80) and severe neutropenic patient group (n = 30); (B) serum calcium in control group (n = 76) and severe neutropenic patient group (n = 30); (C) serum phosphate in control group (n = 74) and neutropenic group (n = 30); (D) serum chloride in control group (n = 83) and severe neutropenic group (n = 30) and (E) serum sodium in control group (n = 84) and severe neutropenic patient group (n = 30) from discovery set is shown. Comparison of (F) serum potassium in control group (n = 86) and severe neutropenic patient group (n = 63); (G) serum calcium in control group (n = 80) and severe neutropenic patient group (n = 63); (H) serum phosphate in control group (n = 77) and severe neutropenic group (n = 63); (I) serum chloride in control group (n = 92) and severe neutropenic group (n = 62) and (J) serum sodium in control group (n = 92) and severe neutropenic patient group (n = 63) from validation set is shown.
Unlike the results of serum phosphate and chloride for all neutropenic patients which were not significantly different then control group (Figure 4), serum phosphate and chloride level in severely neutropenic patients were markedly altered than control group. The level of serum phosphate was significantly reduced (Figure 6C) while serum chloride levels were increased (Figure 6D) in severe neutropenic patients group and serum sodium did not show any difference between in either group, severe neuropenic or control group (Figure 6E). However, validation set did not show similar changes in serum phosphate, chloride and sodium levels in severe neutropenic patients (Figure 6H-J). In agreement with results in Figure 3, serum creatinine and urea level did not show any significant change in the severely neutropenic patients as compared to control group (Figure 7).
Figure 7.
Altered serum urea and creatinine in severe neutropenic patients. Comparison of (A) serum urea in control group (n = 83) and severe neutropenic group (n = 30); (B) serum Creatinine in male patients control group (n = 40) and severe neutropenic group (n = 15); (C) serum creatine in female control group (n = 35) and severe neutropenic group (n = 10) from discovery set is shown. Comparison of (D) serum urea in control group (n = 92) and severe neutropenic group (n = 63); (E) serum Creatinine in male patients control group (n = 54) and severe neutropenic group (n = 42); (F) serum creatine in female control group (n = 37) and severe neutropenic group (n = 21) from validation set is shown.
4.6. Association between serum protein and electrolyte imbalances and severity of neutropenia
We determined the incidence of altered haemoglobin, platelets, serum albumin, total protein and serum electrolyte levels in neutropenic group. We found that percentage of patients showing out of normal ranges values for haemoglobin, platelets, serum albumin, total protein and serum electrolyte increased steadily with the severity of neutropenia in discovery set (Figure 8A) as well as validation set (Figure 8F). A small percentage of patients in control group showed out of normal range values for haemoglobin, platelets, serum albumin, total protein and serum electrolyte (Figure 8A) and validation set (Figure 8F). We found a positive correlation between serum albumin, total protein and serum electrolytes (potassium and calcium) and neutrophils in the chemotherapy-induced neutropenia patients (Figure 8B-E). Furthermore, similar association between serum protein and electrolyte imbalances and severity of neutropenia was observed in validation set (Figure 8G-J).
Figure 8.
Association of Serum proteins and electrolytes with neutrophil count. (A) The percentage of patients (control group, mild, moderate and severe) having out of normal range values for the indicated parameters from discovery set is shown. (B-D) The dot plot of neutrophil counts in the neutropenic patient group and (B) serum albumin, (C) serum total protein, (D) serum potassium ans (E) serum calcium from discovery set is shown. The best fit line and R2 value is shown. (F) The percentage of patients (control group, mild, moderate and severe) having out of normal range values for the indicated parameters from validation set is shown. (G-J) The dot plot of neutrophil counts in the neutropenic patient group and (G) serum albumin, (H) serum total protein, (I) serum potassium ans (J) serum calcium from validation set is shown. The best fit line and R2 value is shown.
5. Discussion
This study was conducted to evaluate the effects of chemotherapy on haematological and biochemical components of blood of cancer patients. Chemotherapy is one the most successful treatment option for cancer patients but has various adverse side effects. Vomiting, diarrhoea and anorexia being the most common side effects of almost all chemotherapeutic agents, but a certain chemotherapeutic agents can be hepatotoxic and nephrotoxic as well. In this study, we report that neutropenic cancer patients experienced pancytopenia along with significant reduction in serum albumin, total protein and electrolyte (potassium and calcium) imbalance. Pancytopenia refers to a deficiency in all three major types of blood cells namely, erythrocytes (red blood cells), leucocytes (white blood cells), and thrombocytes (platelets). There are a number of different conditions that can cause pancytopenia, including bone marrow diseases, some cancers, and some infections; chemotherapy treatment can also cause pancytopenia. Since our study group included cancer patients on chemotherapy with reduced neutrophils counts the most likely reason for pancytopenia observed in this study is chemotherapy treatment.
In this study, we found that cancer patients on chemotherapy showed a significant reduction in serum albumin and total protein levels. There could be various possible reasons for the reduction of serum albumin and total protein in cancer patients on chemotherapy. Cancer patients face issues such as indigestion and insufficient diet along with nutrient consumption by cancer cells which is one of the reasons, these patients develop hypoalbuminemia [5]. Although, albumin is not a specific marker to detect liver damage as the chemotherapy causes liver cell damage and about 20–30% albumin is produced by liver cells, therefore, it could be a possible cause of hypoalbuminemia and hypoproteinemia along with other possible reason such as malnutrition in cancer patients. Malnutrition can also occur due to the common side effects of chemotherapy such as vomiting, diarrhoea, and anorexia. The nutritional status of patients can be assessed by serum albumin and total protein [28, 29]. Depending on the type, stage, and chemotherapy regime given to the cancer patients, 28–87% of patients experience poor nutritional status leading to decreased serum albumin and total protein [30, 31]. Thus, hypoalbuminemia and hypoproteinemia found in this study could be either because of liver damage and/or poor nutritional status of the cancer patients on chemotherapy. A strong relationship is noticed between hypoalbuminemia and increasing age but does not show any correlation with gender, treatment, or BMI of the cancer patient [5]. In this study, no significant difference in male and female albumin and total protein levels was seen, therefore, male and female data was pooled together and analysed. Certain chemotherapeutic agents are solely metabolized by the liver and can deteriorate liver function in patients with pre-existing liver diseases and should be used with caution. These drugs can cause an asymptomatic rise of liver enzymes, acute hepatic failure, or fibrosis leading to end-stage liver diseases [32]. Hypoalbuminemia was an important risk factor along with proteinuria, decreased performance status, and creatinine clearance for changing chemotherapy regime [33]. Reduced serum albumin and total protein is a common finding in many malignancies and considered to be a negative prognostic factor for survival [34, 35]. Hypoalbuminemia is a predictor of the overall survival of cancer patients [36]. A study conducted to identify risk factors for early termination of chemotherapy in cancer patients within the first 21 days of treatment reported hypoalbuminemia to be one of the factors to stop treatment at an early stage [37]. Further due to lack of information such as age, type of cancer, and type of treatment it is difficult to identify the main cause of hypoalbuminemia and hypoproteinaemia.
The physiological balance of serum electrolytes is essential for the body to function properly. Electrolyte imbalance, whether acute or chronic, due to the renal or non-renal cause has various adverse effects on the body. It is important to monitor the electrolytes of the cancer patient on chemotherapy [38, 39]. We observed a decrease in serum potassium and calcium in patients having neutropenia as compared to control group patients. Usually electrolyte imbalances are asymptomatic however, sometimes they are associated with clinical manifestations that can hinder patient's management leading to serious life-threatening conditions. Furthermore, several clinical studies have shown that electrolyte imbalance correlates with a poor quality of life and performance status, reduced probability of response to anti-cancer treatment and poor outcomes and reduced survival. Here, it is difficult to pinpoint whether these changes were drug-induced or disease-induced as for most of the patients' blood biochemistry data, pre and post chemotherapy, was not available and this is main limitation of this study. Electrolyte disorders in cancer patients are dependent on several factors: physiopathology of cancer, anti-cancer treatments and clinical comorbidities. Further analysis is required using large data set to generalize the key finding of this study.
Calcium is a mineral found in different places in the body, including blood. It helps form bones and teeth, and it is required for muscles, nerves, and brain to work correctly. Most of the calcium in human body is present in bones and blood contains only a small amount. There are various causes of low levels of serum calcium, such as inadequate dietary intake, vitamin D deficiency, low or abnormal serum magnesium, and phosphate levels, and PTH deficiency [40]. Hypercalcemia is more common in many cancers while hypocalcemia occurs in patients with renal failure and osteosclerotic bone metastases. Hyopcalcemia is seen in some malignancies due to the release of osteoblastic factors or the adverse effects of some neoplastic drugs [41]. A study reported that cancer patients treated with platinum-based chemotherapy had significant hypocalcemia which was related to hypomagnesemia as magnesium is essential for PTH release [42]. Hypocalcemia in chemotherapy patients also occurs in tumour lysis syndrome [43, 44]. In hypocalcemic patients, it is essential to consider vitamin D deficiency, low magnesium, and chronic renal disease [45]. Hypocalcemia in patients can manifest itself as fatigue, irritability, anxiety, and depression. Further, the typical manifestations of hypocalcemia include muscular irritability with tetany, perioral numbness, distal paraesthesia and muscle cramps. If severe, hypocalcemia may cause bronchospasm and/or laryngospasm, seizures, and at the cardiac level, hypocalcemia may prolong QT and ST intervals in the electrocardiogram. In this study we report robust reduction of serum calcium in severe neutropenic patients suggesting that some of the cardiotoxic effects seen in cancer patient could be due to hypoclacemia.
Potassium maintains intracellular and extracellular charge difference, which is responsible for muscle and nerve excitability and for maintaining normal pH. Regulation of serum potassium is through combination of oral intake, renal elimination, and balance between intracellular and extracellular concentration [42, 46]. Antineoplastic drugs can cause hypokalemia either by its side effects of anorexia/low nutritional status, vomiting, diarrhoea or renal loss. Chemotherapeutic agents such as ifosfamide, cause potassium excretion through the proximal tubule and leads to proximal tubulopathy or Fanconi syndrome, and continue even after completion of chemotherapy regime [47]. Another study reported platinum-based chemotherapeutic agents to be responsible for inducing potassium excretion due to their adverse effects such as vomiting, diarrhoea, and nephrotoxicity [48]. Cancers such as small cell lung carcinoma (SCLC), thymus, and bronchial malignancies thyroid cancer secrete ectopic adrenocorticotropin hormone (ACTH) causing potassium wastage through kidneys by activating mineralocorticoid pathway due to increased cortisol secretion [49, 50, 51]. Studies have reported acute myeloid leukaemia (AML), specifically the M4 and M5 subtype, to cause renal potassium wasting by increasing blood lysozyme and inducing lysozymuria that ultimately results in tubular injury and excessive potassium excretion in urine. Another possible mechanism believed to cause hypokalemia is an activation of the mineralocorticoid pathway by the renin-like activity of AML blast cells [52, 53, 54]. In this study, it was found that cancer patients on chemotherapy suffering from severe neutropenia, had significantly reduced serum potassium levels. Clinical presentation depends on severity of hypokalemia. Patients with mild hypokalemia are often asymptomatic, but patients with severe hypokalemia can present with neurological and psychiatric symptoms and/or cardiac signs (bradycardia) until acute respiratory failure with cardiovascular collapse, secondary to muscle paralysis, might occur.
One of the main limitation is retrospective nature of the study and lack of sufficient data regarding cancer type, chemotherapy treatment and other demographic data. Nonetheless our results show that changes in serum proteins and electrolytes among the cancer patients increase with the severity of chemotherapy-induced neutropenia. Our results show that patients with severe neutropenia had pronounced changes in serum protein and electrolytes and increased incidence of abnormal serum protein and electrolyte level. The changes in the neutrophil counts showed a positive correlation with the changes in serum protein and electrolyte levels. Future studies are required along with data on chemotherapy regimen, cancer type and patients' demographic features.
6. Conclusion
The use of chemotherapy drugs has a crucial role in treatment of cancer patients but is usually associated with numerous undesired effects which could manifest as a life-threatening conditions. Cancer patients on chemotherapy with reduced neutrophils counts showed a reduction in haemoglobin, platelets, serum albumin, total protein, calcium, and potassium. Serum proteins and electrolytes showed a positive correlation with neutrophils counts among cancer patients on chemotherapy. Clinicians and oncologists should be aware of the side effects of chemotherapy reported in the manuscript, especially escalating changes in serum protein and electrolytes and increased incidence of abnormal serum protein and electrolyte level in patients with increasing severity of chemotherapy-induced neutropenia, in order include preventive measures and appropriate interventions to mitigate them and achieve better outcomes for their patients.
Declarations
Author contribution statement
Benazir Abbasi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Amjad Hayat: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mark Lyons: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ananya Gupta, Sanjeev Gupta: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Benazir Abbasi was supported by Higher Education Commission, Pakistan [SMBBMU/P&D/ HRD/2017/24].
Ananya Gupta was supported by Enterprise Ireland [CF-2018-1041].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We acknowledge the Haematology Department, University Hospital Galway, for providing the patients' data.
Contributor Information
Ananya Gupta, Email: Ananya.Gupta@nuigalway.ie.
Sanjeev Gupta, Email: Sanjeev.Gupta@nuigalway.ie.
References
- 1.Kasi P.M., Grothey A. Chemotherapy-induced neutropenia as a prognostic and predictive marker of outcomes in solid-tumor patients. Drugs. 2018;78(7) doi: 10.1007/s40265-018-0909-3. [DOI] [PubMed] [Google Scholar]
- 2.George J.N., Aster R.H. American Society of Hematology. Education Program; 2009. Drug-induced thrombocytopenia: pathogenesis, evaluation, and management. Hematology/the Education Program of the American Society of Hematology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weycker D., et al. Risk and consequences of chemotherapy-induced thrombocytopenia in US clinical practice. BMC Cancer. 2019;19(1) doi: 10.1186/s12885-019-5354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardi M., Maggioli C., Zaccherini G. Human albumin in the management of complications of liver cirrhosis. Crit. Care. 2012 doi: 10.1186/cc11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin W., Zheng G., Yang K., Song H., Liang Y. Analysis of prognostic factors of patients with malignant peritoneal mesothelioma. World J. Surg. Oncol. 2018;16(1):1–8. doi: 10.1186/s12957-018-1350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gleeson M.W., Dickson R.C. Albumin gains immune boosting credibility. Clin. Transl. Gastroenterol. 2015;6:86. doi: 10.1038/ctg.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Don B.R., Kaysen G. Poor nutritional status and inflammation: serum albumin: relationship to inflammation and nutrition. Semin. Dial. Nov. 2004;17(6):432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang X., Han H., Duan Q., Khan U., Hu Y., Yao X. Changes of serum albumin level and systemic inflammatory response in inoperable non-small cell lung cancer patients after chemotherapy. J. Cancer Res. Therapeut. 2014;10(4):1019–1023. doi: 10.4103/0973-1482.137953. [DOI] [PubMed] [Google Scholar]
- 9.Arrieta O., et al. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: a prospective study. BMC Cancer. 2010;10(22):1–7. doi: 10.1186/1471-2407-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda S., et al. Serum albumin level as a potential marker for deciding chemotherapy or best supportive care in elderly, advanced non-small cell lung cancer patients with poor performance status. BMC Cancer. 2017;17(1):1–8. doi: 10.1186/s12885-017-3814-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakano Y., et al. Prognostic significance of the postoperative level and recovery rate of serum albumin in patients with curatively resected pancreatic ductal adenocarcinoma. Mol. Clin. Oncol. 2019;11(3) doi: 10.3892/mco.2019.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai S., et al. Risk factors for aspiration pneumonia after definitive chemoradiotherapy or bio-radiotherapy for locally advanced head and neck cancer: a monocentric case control study. BMC Cancer. 2017;17(1):1–11. doi: 10.1186/s12885-017-3052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo S.H., et al. Association of nutritional status-related indices and chemotherapy-induced adverse events in gastric cancer patients. BMC Cancer. 2016;16(1):1–9. doi: 10.1186/s12885-016-2934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz-Tovar J., Martín-Pérez E., Fernández-Contreras M.E., Reguero-Callejas M.E., Gamallo-Amat C. Vol. 102. 2010. Impact of Preoperative Levels of Hemoglobin and Albumin on the Survival of Pancreatic Carcinoma; pp. 631–636. [DOI] [PubMed] [Google Scholar]
- 15.McMillan D.C., Watson W.S., O’Gorman P., Preston T., Scott H.R., McArdle C.S. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr. Cancer. 2001;39(2) doi: 10.1207/S15327914nc392_8. [DOI] [PubMed] [Google Scholar]
- 16.Li P., et al. Perioperative changes of serum albumin are a predictor of postoperative pulmonary complications in lung cancer patients: a retrospective cohort study. J. Thorac. Dis. 2018;10(10) doi: 10.21037/jtd.2018.09.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah S.R., et al. Acute kidney injury recognition and management: a review of the literature and current evidence. Global J. Health Sci. 2015;8(5):120–124. doi: 10.5539/gjhs.v8n5p120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson L.L., et al. Association between renal function and chemotherapy-related toxicity in older adults with cancer. J. Geriatr. Oncol. 2017;8(2):96–101. doi: 10.1016/j.jgo.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cockcroft D.W., Gault M.H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 20.Kawai K., Hinotsu S., Tomobe M., Akaza H. Serum creatinine level during chemotherapy for testicular cancer as a possible predictor of bleomycin-induced pulmonary toxicity. Jpn. J. Clin. Oncol. 1998;28(9):546–550. doi: 10.1093/jjco/28.9.546. [DOI] [PubMed] [Google Scholar]
- 21.Jakob S.M., Arnold W., Marti H.P. Progressive renal failure after cisplatin therapy. Nephrol. Dial. Transplant. 1996;11(2):370–373. doi: 10.1093/oxfordjournals.ndt.a027273. [DOI] [PubMed] [Google Scholar]
- 22.Mohammed A., Arastu Z. Emerging concepts and spectrum of renal injury following Intravesical BCG for non-muscle invasive bladder cancer. BMC Urol. 2017;17(1):114. doi: 10.1186/s12894-017-0304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vootukuru V., Liew Y.P., Nally J.V. Pemetrexed-induced acute renal failure, nephrogenic diabetes insipidus, and renal tubular acidosis in a patient with non-small cell lung cancer. Med. Oncol. 2006 doi: 10.1385/MO:23:3:419. [DOI] [PubMed] [Google Scholar]
- 24.Berardi R., Torniai M., Lenci E., Pecci F., Morgese F., Rinaldi S. Electrolyte disorders in cancer patients: a systematic review. J. Cancer Metastasis Treat. 2019 [Google Scholar]
- 25.Shirali A.C. Chapter 5 : electrolyte and acid – base disorders in malignancy. Onco Nephrol. Curric. 2016:1–7. https://www.asn-online.org/education/distancelearning/curricula/onco/Chapter5.pdf [Online]. Available: [Google Scholar]
- 26.Miyake M., et al. Long-term changes in renal function, blood electrolyte levels, and nutritional indices after radical cystectomy and ileal conduit in patients with bladder cancer. Urol. J. 2019;16(2) doi: 10.22037/uj.v0i0.4531. [DOI] [PubMed] [Google Scholar]
- 27.Verzicco I., et al. Electrolyte disorders induced by antineoplastic drugs. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.M R, F E, M Z M, G J, J MV Consequences of radiotherapy on nutritional status, dietary intake, serum zinc and copper levels in patients with gastrointestinal tract and head and neck cancer. Saudi Med. J. 2007 [PubMed] [Google Scholar]
- 29.Bozzetti F., et al. Impact of cancer, type, site, stage and treatment on the nutritional status of patients. Ann. Surg. 1982 doi: 10.1097/00000658-198208000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross P.J., et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br. J. Cancer. 2004 doi: 10.1038/sj.bjc.6601781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudakwashe Nyarota D.T.Z. Albumin and total protein in cancer patients at radiotherapy clinic, Zimbabwe. Saudi J. Med. Pharm. Sci. 2017 [Google Scholar]
- 32.Grigorian Alla, Christopher B., O’Brien Hepatotoxicity secondary to chemotherapy. J. Clin. Transl. Hepatol. 2014 doi: 10.14218/JCTH.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lheureux S., et al. Evaluation of current practice: management of chemotherapy-related toxicities. Anti Cancer Drugs. 2011 doi: 10.1097/CAD.0b013e328349d7f1. [DOI] [PubMed] [Google Scholar]
- 34.Rouia Dawsar A.R.A., Ismael K., Nawfal Nada A.M.N., Al-Shawi N., Hussain Saad A.R. The effect of radiotherapy on oxidative stress, biochemical and hematological parameters in women with breast cancer. Al-Mustansiriyah J. Pharm. Sci. 2014 [Google Scholar]
- 35.Jin Y., Zhao L., Peng F. Prognostic impact of serum albumin levels on the recurrence of stage I non-small cell lung cancer. Clinics. 2013 doi: 10.6061/clinics/2013(05)17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta D., Lis C.G. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr. J. 2010 doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hyman D.M., et al. Predictors of early treatment discontinuation in patients enrolled on phase i oncology trials. Oncotarget. 2015 doi: 10.18632/oncotarget.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duong C.D., Loh J.Y. Laboratory monitoring in oncology. J. Oncol. Pharm. Pract. 2006 doi: 10.1177/1078155206072982. [DOI] [PubMed] [Google Scholar]
- 39.Briggs J.P. Overview of Renal Function and Structure. Prim. kidney Dis. 1998 [Google Scholar]
- 40.Riccardi D., Brown E.M. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am. J. Physiol. Ren. Physiol. 2010 doi: 10.1152/ajprenal.00608.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diniotis MD B., Sternberg E., Shakuntala NP S., Chiha MD M., Khosla MD P. Hypocalcemia in Malignancy - Unexpected but common. Cureus. 2015 doi: 10.7759/cureus.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oronsky B., et al. Electrolyte disorders with platinum-based chemotherapy: mechanisms, manifestations and management. Cancer Chemother. Pharmacol. 2017 doi: 10.1007/s00280-017-3392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Humphreys B.D., Soiffer R.J., Magee C.C. Renal failure associated with cancer and its treatment: an update. J. Am. Soc. Nephrol. 2005;16(1):151–161. doi: 10.1681/ASN.2004100843. [DOI] [PubMed] [Google Scholar]
- 44.Aslam H.M., Zhi C., Wallach S.L. Tumor lysis syndrome: A rare complication of chemotherapy for metastatic breast cancer. Cureus. 2019 doi: 10.7759/cureus.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper M.S., Gittoes N.J.L. Diagnosis and management of hypocalcaemia. BMJ. 2008 doi: 10.1136/bmj.39582.589433.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rose B.D., Post T.W. Introduction to disorders of potassium balance. Clin. Physiol. Acid Base Electrolyte Disord. 2001:715–756. McGraw-Hill, New York. [Google Scholar]
- 47.Skinner R., Cotterill S.J., Stevens M.C.G. Risk factors for nephrotoxicity after ifosfamide treatment in children: a UKCCSG Late Effects Group study. Br. J. Cancer. 2000 doi: 10.1054/bjoc.2000.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.du Bois A., et al. Comparison of the emetogenic potential between cisplatin and carboplatin in combination with alkylating agents. Acta Oncol. (Madr.) 1994 doi: 10.3109/02841869409083931. [DOI] [PubMed] [Google Scholar]
- 49.Alexandraki K.I., Grossman A.B. The ectopic ACTH syndrome. Rev. Endocr. Metab. Disord. 2010 doi: 10.1007/s11154-010-9139-z. [DOI] [PubMed] [Google Scholar]
- 50.Martínez-Valles M.A., Palafox-Cazarez A., Paredes-Avina J.A. Severe hypokalemia, metabolic alkalosis and hypertension in a 54 year old male with ectopic ACTH syndrome: a case report. Cases J. 2009;2(7) doi: 10.4076/1757-1626-2-6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferreira T.L., Da Silva T.N., Canário D., Delerue M.F. Hypertension and severe hypokalaemia associated with ectopic ACTH production. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-223406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perazella M.A., Eisen R.N., Frederick W.G., Brown E. Renal failure and severe hypokalemia associated with acute myelomonocytic leukemia. Am. J. Kidney Dis. 1993 doi: 10.1016/s0272-6386(12)70154-3. [DOI] [PubMed] [Google Scholar]
- 53.Muggia F.M., Heinemann H.O., Farhangi M., Osserman E.F. Lysozymuria and renal tubular dysfunction in monocytic and myelomonocytic leukemia. Am. J. Med. 1969 doi: 10.1016/0002-9343(69)90219-8. [DOI] [PubMed] [Google Scholar]
- 54.Wulf G.G., et al. Paraneoplastic hypokalemia in acute myeloid leukemia: a case of renin activity in AML blast cells. Ann. Hematol. 1996 doi: 10.1007/s002770050215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.