Abstract
Cancer, which is the uncontrolled growth of cells, is the second leading cause of death after heart disease. Targeting drugs, especially to specific genes and proteins involved in growth and survival of cancer cells, is the prime need of research world-wide. Indole moiety, which is a combination of aromatic-heterocyclic compounds, is a constructive scaffold for the development of novel leads. Owing to its bioavailability, high unique chemical properties and significant pharmacological behaviours, indole is considered as the most inquisitive scaffold for anticancer drug research. This is illustrated by the fact that the U.S. Food and Drug Administration (FDA) has recently approved several indole-based anticancer agents such as panobinostat, alectinib, sunitinib, osimertinib, anlotinib and nintedanib for clinical use. Furthermore, hundreds of studies on the synthesis and activity of the indole ring have been published in the last three years. Taking into account the facts stated above, we have presented the most recent advances in medicinal chemistry of indole derivatives, encompassing hot articles published between 2018 and 2021 in anticancer drug research. The recent advances made towards the synthesis of promising indole-based anticancer compounds that may act via various targets such as topoisomerase, tubulin, apoptosis, aromatase, kinases, etc., have been discussed. This review also summarizes some of the recent efficient green chemical synthesis for indole rings using various catalysts for the period during 2018–2021. The review also covers the synthesis, structure‒activity relationship, and mechanism by which these leads have demonstrated improved and promising anticancer activity. Indole molecules under clinical and preclinical stages are classified into groups based on their cancer targets and presented in tabular form, along with their mechanism of action. The goal of this review article is to point the way for medicinal chemists to design and develop effective indole-based anticancer agents.
Key words: Indole, Synthesis, Anticancer, Structure‒activity relationship, Topoisomerase, Apoptosis, Aromatase inhibitors, Tubulin inhibitors
Graphical abstract
Recent progress (2018–2021) in the synthesis, structure‒activity relationship and mechanism of promising indole-based anticancer compounds that may act via multiple targets has been discussed with detailed illustrations.
1. Introduction
Heterocyclic compounds are considered as one of the imperative sources of bioactive compounds, which could be synthesized in lab or can be obtained from natural sources1, 2, 3. These heterocyclic compounds owing to the presence of one or more heteroatoms such as N, O, S, having H-bond donating and acceptors capability, can easily bind to various therapeutic receptors thereby giving numerous biological effects4. Enormous developments are still continuing to investigate the synthesis of newer, safer and prominent biologically active heterocyclics.
Among these heterocyclic compounds, nitrogen heterocycles especially indole is considered as one of the most important nitrogen heterocyclic cores that have gained considerable interest in last decade due to its multiple bioactivities5 (Supporting Information Fig. S1). It is evidenced by the fact that recently, U.S. Food and Drug Administration (FDA) databases have disclosed the importance of nitrogen-containing heterocycles in drug-designing. Among various nitrogen-containing heterocycles, indole chemistry ranked in the 9th position in 2015 among the top 25 drugs approved by FDA6,7.
Generally, indole is the combination of aromatic-heterocyclic compounds in which a benzene ring is fused in 2, 3 positions of the pyrrole ring. Chemically indoles are non-basic in nature. The heterocyclic pyrrole ring part of indole is more dominated by the electrophilic substitution reactions compared to its benzene counterpart due to more electron availability at pyrrole ring. The five membered electron-rich pyrrole ring and benzene portion has resulted indole as the most exotic hetero-annulated heterocyclic nucleus in medicinal chemistry8. The tireless efforts have been shown by the researchers to strategic manipulating various substituents around the basic scaffold of indole in order to discover potential bioactive compounds9,10.
2. Synthesis
Indole was first synthetically prepared by Adolf von Baeyer11 in 1866; he oxidised indigo to get isatin, reduced the isatin to dioxindole and oxindole by means of zinc dust, and further reduced oxindole to indole by passing its vapours over hot zinc oxide. From that day, various other strategies have been developed for the synthesis of the indole nucleus (Supporting Information Fig. S2).
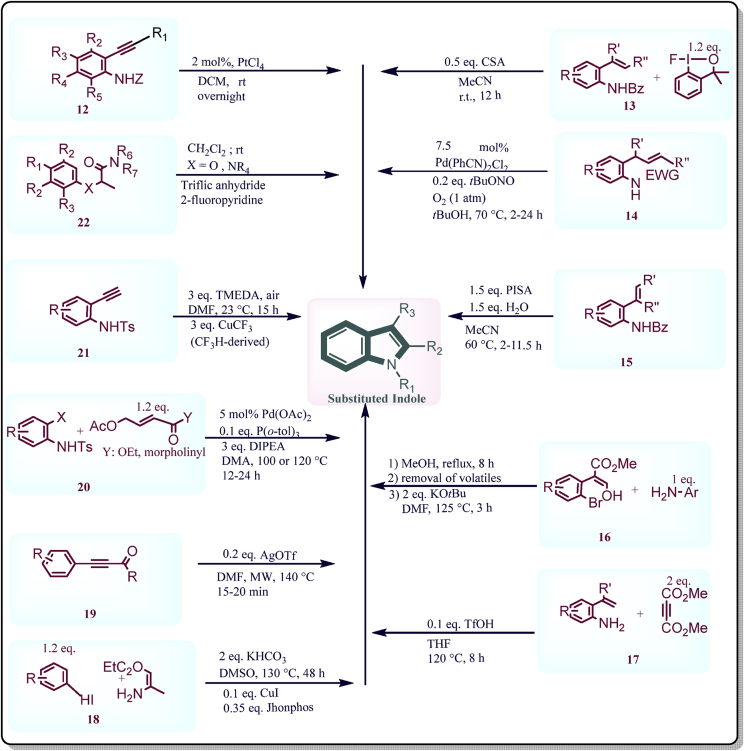
The more recent catalytic approaches for the efficient synthesis of indole ring have been illustrated in Fig. 112, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22. The catalyst PtCl4 was explored by Chaisan and co-workers12 in 2018 to promote the hydroamination of 2-alkylanylaniline for the preparation of 2-substituted indoles. The 1%–2% (mol/mol) catalyst loading was essential for the excellent synthesis of large varieties of substituted indoles at room temperature. Although, the method required reflux conditions for the substrates containing halogen or other electron withdrawing groups at some positions to obtain moderate to high yield. Authors affirmed that this approach is simple, clean and might function as an appreciable synthetic tool to bring together various N-heterocyclic scaffolds (Fig. 1). Andries-Ulmer et al.13 in 2018 reported a hypervalent F-iodane catalyst which allows a regio divergent formation of indoles and tryptophans from styrenes through a common spirocyclic intermediate i.e., F-cyclopropane. The amazing reactivity of fluoro iodanes revealed the extraordinary chemistry of hypervalent iodane in the future (Fig. 1). Ning and others14 in the same year reported Pd-tBuONO co-catalysed aerobic cycloisomerisation of o-allylanilines using molecular oxygen as terminal oxidant. The substituted functionalized indoles were obtained in moderate to high yields and method was found to be highly accessible and practical. Molecular oxygen which was used as a terminal oxidant is undeniably a clean terminal oxidant where the harmful transition metal redox cocatalysts generally cannot be prohibited in such aerobic methods. The avoiding hazardous oxidant, high boiling point solvents such as DMF and DMSO and heavy-metal cocatalysts were some of the benefits of this method which can be applied to other pharmaceutical synthesis (Fig. 1). Xia et al.15 in 2018 reported an easily available and bench-stable water-soluble hypervalent iodine (III) reagent PISA (phenyliodonio) sulfamate which efficiently synthesize numerous indoles through C‒H amination of 2-alkenylanilines including an aryl migration/intramolecular cyclization cascade with brilliant regioselectivity. A series of indoles can be synthesised in aqueous CH3CN with PISA. Also, in this reaction, PISA acts both as Brønsted acid and oxidizing agent whereas, water improves the efficacy of the reaction by acting as a catalyst (Fig. 1).
Figure 1.
Synthetic diagram for synthesis of indole derivatives by new green chemical methods.
In 2018, N-substituted indole-3-carboxylates were prepared in good yields with the help of t-BuOK/DMF system under transition metal free conditions reported by Bugaenko and others16. These conditions are mainly suitable for the production of halogenated indoles without any traces of dehalogenation products. The method makes the use of t-BuOK/DMF system to obtain the corresponding indoles in high yields via cyclization of 3-amino 2-(2-bromphenyl) acrylate. The method avoids the use of expensive catalysts and best for the fast production of various libraries of compound containing indoles (Fig. 1). In 2018, Ni and associates17 reported the preparation of 2-substituted indoles and quinolines from 2-vinylanilines and alkynoates without using any oxidant. The reaction is proceeded by means of tandem Michael addition in which C–C bond cleavage occur and 2-vinylanilines and alkynoates are cyclized to give products without the use of oxidising agent. This technique not only present a valuable approach to prepare C2-substituted indoles in high yields via cleaving simultaneously C=C and CC bonds under metal-free conditions but also affords an easy method for synthesising C2-substituted quinolines in moderate yields through Pd-catalysed CC bond cleavage. This approach indicates better functional group compatibility for the production of anticipated products in good to excellent yields (Fig. 1). For the synthesis of multi-substituted indoles in high yields from easily accessible aryl iodides and enamines at 130 °C in DMSO, Li and others18 in 2018 suggested a one-pot tandem copper-catalysed Ullman-type C–N bond formation/intramolecular cross-dehydrogenative coupling system. This kind of sequential one-pot process was reported to be practical and modular assembly for synthesizing wide varieties of heterocycles where structures of products and good functionality tolerance are considered (Fig. 1). In 2018, Rode and others19 reported a microwave irradiated reaction of β-(2-aminophenyl)-α,β-ynones catalysed by AgOTf to give 3-unsubstituted 2-acylindoles in the presence of 20% (mol/mol) AgOTf in good yields. The authors also investigated the applicability of Cu(OTf)2 as a catalyst but reaction proceeded similarly leading to the formation of products but with reduced efficiency. This transformation represents the first example of 5-endo-dig cyclization of 2-alkynylanilines bearing an acyl group linked to the triple bond (Fig. 1). Chen and others20 in 2018 reported the usage of a Pd(OAc)2/P (o-tol)3/DIPEA protocol which permits a practical cascade Tsuji-Trost reaction/Heck coupling of N-Ts o-bromoanilines with 4-acetoxy-2-butenonic acid derivatives to offer numerous substituted indole/azaindole-3-acetic acid derivatives. The method turned out to be successful in synthesizing diverse substituted indole/azaindole-three-acetic acid derivatives and almotriptan, a drug for the acute remedy of migraine. The process can be efficiently completed from easily obtainable substrates: substituted N-Ts o-haloanilines and allyl ester/amide. So, the method offered an opportunity for the synthesis of an indole-3-acetic acid scaffold treasured in medicinal chemistry (Fig. 1).
The reliance of prefunctionalised indoles having activating groups at particular positions was not convenient. So novel methods were reported by Ye et al.21 to synthesize 2-(trifluoromethyl) indoles using easily available 2-alkynylanilines and fluoroform-derived CuCF3 reagents. The control of regioselectivity (2-CF3 vs 3-CF3) was mostly problematic with substrate which does not have a blocking group and thus limit the reaction scope. But this method constructed the indole cores with no ambiguity of the CF3 position. CF3 source is inexpensive and obtained from the industrial by-product fluoroform. The same protocol can also be useful for synthesizing 3-formyl-2-(trifluoromethyl) indoles, an intermediate for many trifluoro methylated drug molecules (Fig. 1). Keteniminium salts (KI) are the reactive intermediates which are versatile in nature. An effective route was reported by Tanriver et al.22 in 2019 for the synthesis of 3-amino-indoles and other heterocyclic system from readily available acetamides containing aniline and a phenoxy group respectively. Mostly a keteniminium salt intermediate is needed for the reaction to occur. Structural and energetic studies, nature of the reactions, and the reactivity differences in electrocyclization reactions of keteniminium salts, resulted in the synthesis of various heterocyclic systems those were studied computationally (Fig. 1).
Due to the versatile nature of indole, it has gained immense popularity among the researchers to synthesize several compounds which encompass indole as a main nucleus and determined their various biological activities23. However, it is worth noting that no recent update report on indole scaffold as anticancer activity demonstrating structure‒activity relationship (SAR) has been published. As a result, the primary goal of this review is to provide current information (2018–2021) on recent advances in efficient novel green methods for the synthesis of indole cores, as well as the SAR of indole anticancer activity. The effects on anticancer activity of changing the ring substitution on indole to reveal the active pharmacophore have also been illustrated in the present review for quick understanding of medicinal chemists.
3. Indole as anticancer drugs
Cancer is considered as a global health risk nowadays. It is multifaceted lethal disease wherein a group of atypical body cells starts dividing uncontrollably instead of normal cell division. Globally, it is the second leading cause of death after cardiovascular diseases accounted for 8.8 million deaths in 2015. But recent study speculated it to be the primary cause of death in the near future. The deaths are predicted to increase 13.1 million by 203024. The developments of novel compounds that are both potent and tumour-selective are the main challenges for the researchers. Inspite of the crucial advances in technology and chemotherapy research, the non-selectivity to cancerous tissue and multiple-drug resistance by cancer cells continues to be a grave concern for the medicinal chemists. Moreover, many of the cytotoxic agents used in health facilities are highly expensive for patients in contrast to traditional drugs. Therefore, developing new anti-cancer agents with unique mechanism of action, high efficacy, low toxicity, low cost and short therapy duration profile has been a great challenge for pharmaceutical re-searchers25,26.
In last few decades, heterocyclic compounds which may be obtained naturally or synthetically, have been developed as a potential scaffold for the development of many anticancer drugs. Heterocyclic compounds owing to the presence of heteroatoms such as oxygen, nitrogen and sulphur, except at least one carbon atom in the ring, they typically can be employed as hydrogen bond donors and acceptors. Thus, they can bind suitably to pharmacological targets and receptors via intermolecular H-bonds more effectively giving pharmacological effects. Also, they are able to alter the liposolubility and hence the aqueous solubility of drug molecules to achieve the remarkable pharmacotherapeutic properties2,3. Motivated by the anticancer clinical drugs such as vincristine and vinblastine containing indole alkaloids, many researches have also been made in the areas of indole alkaloids from natural sources in the last three years27,28. The approval and use of these indole containing drugs have further inspired the medicinal chemists to design and synthesize various other molecules incorporating indole moiety, thereby creating a large number of structurally diverse leads with distinctive mechanism of anticancer action. These leads exert the anticancer action by distinctive mechanism such as inhibiting Bcl-2, Mcl-1 proteins (induce apoptosis), inhibiting PIM proteins (hinder cellular process and signal transduction in cells), inhibiting DNA topoisomerase, inhibiting aromatase (inhibit replication and transcription), modulating epigenetic mechanisms [inhibit histone deacetylase (HDAC)] and inhibiting cellular mitosis (tubulin inhibitors)29,30. The following section focus on summarizing the recent advances (2018–2021) made towards synthesis of indole core in the design of anti-cancer compounds that may act via various targets such as topoisomerase, tubulin, aromatase, kinases, etc. The review also discusses the SAR of potential lead accountable for anticancer activity31.
3.1. Indole as tubulin polymerization inhibitors
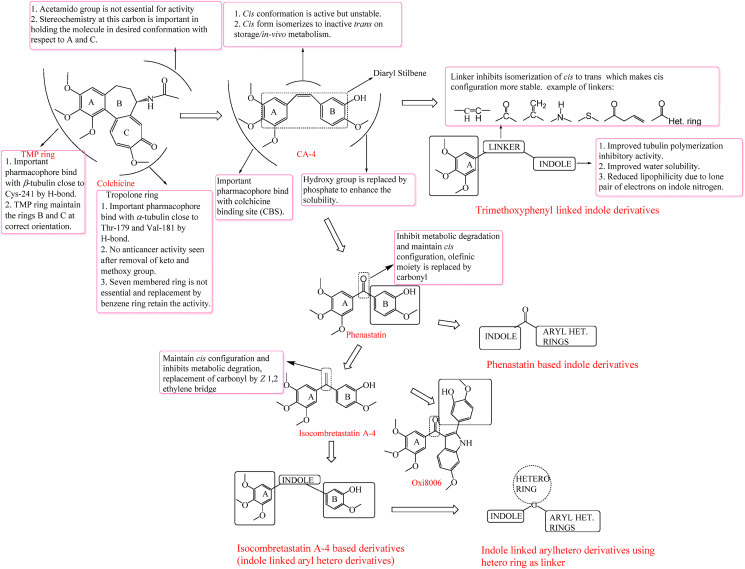
Microtubules are cytoskeletal components made up of long chains of tubulins, which are tiny subunits of proteins. Microtubules are important cellular targets for cancer chemotherapy. They are involved in a variety of vital cellular activities, including cell motility, cell division, cell shape control, and intracellular transport. Microtubules polymerize into spindle fibres, which are involved in mitotic cell division. Since microtubules play an important role in the cell division, so they are considered as an important target in the development of chemotherapeutic agents. The indole moiety is thought to hinder tubulin polymerization by dissolving mitotic spindles and disrupting microtubules, resulting in cell cycle arrest32. Microtubule-active drug generally bind on tubulin to one of the three main binding sites, the paclitaxel site, the Vinca domain and colchicine's binding site. Drugs that bond to the colchicine binding site are undergoing intensive investigation as anticancer agents. Various recent literature reports disclosed the tubulin polymerization inhibitory property of indole scaffold33. Combretastatin A-4 (CA-4) is a natural tubulin binding natural product isolated from Cape Bush willow tree, Combretum caffrum that binds to β-tubulin at colchicine binding site and inhibits the tubulin polymerization process and disrupt the formation and function of normal mitotic spindle. Moreover, it also has antiangiogenic and antivascular activity34,35. SAR studies by Pettit and co-workers36 have demonstrated CA-4 has the following pharmacophores crucial for tubulin polymerization inhibitory activity: 1) Ring A is substituted with 3,4,5-trimethoxyphenyl (TMP). 2) Ring B is substituted with 3-hydroxy-4-methoxyphenyl. 3) The two phenyl rings are separated by cis double bond.
CA-4 can exist geometric configurations―cis and trans stilbene. The cis structure has been demonstrated to be significant for anticancer action through binding to the CBS. Active cis conformation of CA-4 is easily isomerized into the more thermodynamically stable but much less active trans isomer, which has little or no biological activity36.
Despite the fact that CA-4 has been a clinical success, it has certain drawbacks, including a short biological half-life, limited aqueous solubility (due to ring B), and susceptible cis-trans isomerization (leading to conversion from cis to inactive trans form) associated with CA-4 limit their therapeutic potential37. To inhibit metabolic degradation and to maintain the cis configuration of the combretastatin, replacement of the olefinic moiety by the bioisoster carbonyl group and Z 1,2-ethylene bridge have led to the development of phenastatin38 and isoCA-439 respectively which were found to be more stable and potent as compared to CA-4 due to their inability to isomerize into an inactive trans form. The solubility problem of CA-4 has also been tackled by the formation of highly water-soluble prodrugs such as phosphates on the hydroxyl groups that led to the development of CA-4P (fosbretabulin) which is in phase II clinical trials for multi-drug combination anti-tumour therapy (Fig. 2)40.
Figure 2.
Design and development of indole based combretastatin and isocombretastatin derivatives.
3.1.1. Trimethoxyphenyl (TMP) linked indole derivatives
The trimethoxyphenyl (TMP) moiety of combretastatin has been shown to be essential for binding to the colchicine binding site and thereby suppressing tumour cell proliferation. On the other hand, the deleterious isomerization issue of natural CA-4 has been solved by linking TMP with various linkers and indole bridging to limit the cis configuration. As a result of this method, a number of combretastatin analogues with good stability and bioactivity have been developed recently.
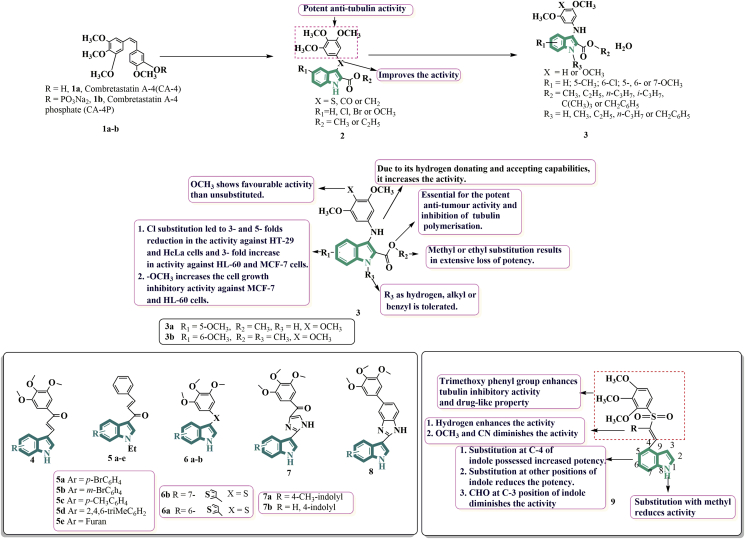
Tubulin polymerization inhibition (TPI) and cytotoxicity against human cancer cell lines are reduced when the B ring of combretastatin A-4 is replaced with an N-methyl-5-indolyl, but cyano, methoxycarbonyl, formyl, and hydroxyiminomethyl substitutions at the 3rd position of indole restore potent TPI and cytotoxicity against sensitive human cancer cell lines. Substituted combretastatin displayed higher potencies than the isomeric isocombretastatin and the highest potencies were achieved by hydroxyimidomethyl and cyano groups, with TPI values in the sub micromolar range and cytotoxicities in the nanomolar and sub nanomolar range. The 3,4,5-trimethoxyphenyl ring yielded more powerful derivatives than a 2,3,4-trimethoxyphenyl ring in every case41.
Prompted by these results and synthesizing more potent combretastatin 1a and isocombretastatin 1b, Romagnoli et al. 42 in 2020 decided to investigate the possibility of replacing the sulphur, carbonyl, or methylene 2 bridging units between the 3-position of the indole nucleus and the trimethoxy phenyl moieties with a biosteric anilinic nitrogen (NH) moiety 3 (Fig. 3) because of its hydrogen bond accepting and donating capabilities. Out of all the derivatives synthesized, compounds 3a and 3b were found to be highly potent as new anti-proliferative agents which targets tubulin at colchicine site compared to that of reference compound CA-4.
Figure 3.
Chemical structures and SAR of trimethoxy phenyl-based indole derivatives 1–9.
Several researches have been taken place in recent years to replace B ring of combretastatin with indole ring as well as replacing methylene bridge with various linkers to get rid of cis–trans isomerisation. To make keto stilbene derivatives of CA-4, Ducki et al.44 used a chalcone scaffold. They created several compounds that outperformed CA-4 in vitro antiproliferative properties at nanomolar concentrations against human chronic myelogenous leukaemia K562 cell lines by using the aryl substitution pattern of CA-4 in their chalcones. Based on the above results, Cong and colleagues45 synthesized indole-chalcone based anti-microtubule medicines in 2018, based on the fact that tubulin is the direct cellular target of chalcones, which is responsible for anti-cancer activity. The report stated that 4 (Fig. 3) possessed excellent activity at nanomolar concentration against NCI-60 human cancer cell lines with GI50 < 4 nmol/L. Further indole-chalcone derivatives were synthesized by Badria and others46 in 2019. They carried out the synthesis of five novel chalcones derived from N-ethyl-3-acetylindole 5a‒e by using different substituents. Evaluation studies revealed that the derivatives are sensitive to the triple negative breast cancer cells (MDA-MB-231) than the ER positive breast cancer cell lines (MCF-7) with IC50 values ranging from 13 to 19 μmol/L.
Regina and colleagues47 reported an indole-thiophene complex in 2018. They came up with new 3-aryl-thio and 3-aroyl-1H-indole derivatives with a heterocyclic ring at position 5, 6, or 7 of the indole moieties. A series of about 20 derivatives was synthesized and evaluated for the cytotoxic activities. Compounds 6a and 6b inhibited HT29, HepG2, HCT116, T98G cell lines and have IC50 values in the nanomolar range. Compounds 6a and 6b were effective at 20 and 100 nmol/L respectively showing that 6a is 3-fold more potent than 6b. The replacement of sulphur atom with carbonyl group also showed good activity.
In 2019, Wang and colleagues48 investigated the indole-imidazole hybrid as a powerful tubulin inhibitor, resulting in two potent compounds 7a and 7b with IC50 values ranging from 1.6 to 3.7 nmol/L, which were around 2–3 times more potent than the reference drug ABI-231. SAR studies suggested that indolyl rotations remarkably affect the cytotoxicity 30 mg/kg intraperitoneal injection caused a decrease in the tumour cell growth by 83.8% and also reduced the tumour weight by 62.8%. As a result, the hybrid could be a useful option for further studies. With an average IC50 value of 50 nmol/L and strong in vitro inhibition of tubulin polymerization (IC50 = 2.52 μmol/L), benzimidazole-indole derivative 8 showed potent inhibitory effects on cancer cell proliferation49.
In separate study published in 2019, Li and colleagues43 synthesized two series consisting of 22 novel indole-vinyl sulfone derivatives and tested them for tubulin inhibitory efficacy. Among all the derivatives of both the series, 9 (Fig. 3) was found to exhibit most potent activity against a panel of cancer cell lines. Further mechanistic research displayed that compound 9 led to cell cycle arrest at G2/M phase induced cell apoptosis and disrupted microtubule networks in K5662 cells with dose-dependent manner. Molecular modelling studies exposed that 9 interacted with tubulin at colchicine-binding site. The calculated IC50 value was found to be 3.09 μmol/L. The SAR studies revealed that the substitutions led to decrease in the activity except substitution at C-4 position. Among all the derivatives, vinyl sulfone derivative with R being H was the most potent. Substitution of aldehyde group at C-3 position of indole led to significant reduction in the activity. Besides, reducing indole to 2-H indole or substituting methyl to NH of indole also led to slight decrease in the activity.
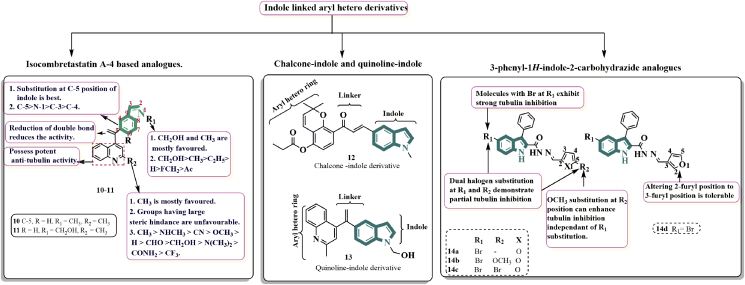
3.1.2. Indole linked arylhetero derivatives
Replacement of ethylene bridge of CA-4 leads to the formation of isoCA-4, it was found to be more stable and potent as compared to CA-4 due to its inability to isomerize into an inactive trans form.
Khelifi et al. and others50 designed and synthesised a novel series of isocombretastatin A-4 (isoCA-4) analogues considering capability of CA-4 to inhibit polymerisation process through binding with colchicine site on tubulin and blocking the formation of mitotic spindle. They created a variety of new isocombretastatin A-4 (isoCA-4) analogues by substituting quinoline and indole for the 3,4,5-trimethoxyphenyl and isovanillin moieties in isoCA-4.
Among all the derivatives, 10 and 11 exhibited the most potent activities against five cancer cell lines with IC50 values ranging from 2 to 11 μmol/L as compared to combretastatin A-4 (CA-4). The SAR studies revealed that the olefins substitution at C-3 of indole exhibited lesser activities (IC50 > 1 μmol/L). The methyl substitution at N-1 position of indole significantly enhanced the activities for about 60-fold as compared to the non-substituted. The 60 fold enhanced activity was observed for methyl substitution at N-1 position of indole than non-substitution. Furthermore, the hydroxymethyl substituent at N-1 position of indole was most effective than other groups such as ethyl, fluoride, methyl and acetyl in enhancing the anticancer activity. Furthermore, mechanism investigation revealed compound act by inhibiting the microtubule polymerisation by binding to colchicine site of tubulin, disrupted microtubule networks, arrested cell cycle at G-2/M phase, induce apoptosis and depolarised mitochondria of K562 cell lines51 (Fig. 4).
Figure 4.
Chemical structure and SAR of indole linked aryl hetero derivatives 10–14.
The N-containing heterocyclic skeleton is one of the most appealing structures in bioactive molecules with significant pharmacological importance52, particularly in terms of anti-cancer activity. Indole, indoline, and tetrahydroquinoline compounds, for example, have been used to develop novel tubulin inhibitors with significant anti-cancer efficacy that bind to the colchicine binding site. With IC50 values ranging from 0.22 to 1.80 μmol/L, the chalcone-indole derivative 12 effectively suppressed cancer cell proliferation53. Compound 12 efficiently prevented tubulin polymerization by causing cell cycle arrest in the G2/M phase. Quinoline-indole derivative 13 as an anti-tubulin drug targeting the colchicine binding site inhibited tested cancer cell lines with IC50 values ranging from 2 to 11 nmol/L, as well as considerable in vitro tubulin polymerization inhibition (IC50 = 2.09 μmol/L)51 (Fig. 4).
By using structure-based virtual screening and similarity investigations, Saruengkhanphasit and colleagues54 discovered eight novel 3-phenyl-1H-indole-2-carbohydrazide derivatives with anti-proliferative properties. The most potent candidate was furan-3-ylmethylene-3-phenyl-1H-indole-2-carbohydrazide 14a, which was evaluated experimentally for tubulin polymerization inhibition. Molecule 14a, like other tubulin inhibitors, was able to induce G2/M phase arrest in the A549 cell line. Small furan ring substitutions, halogenation at R1 position, and changes in furyl connectivity were the focus of synthetic modifications of 14a. Derivatives 14b‒d exhibited the most potent tubulin inhibitory effects and were comparable to 14a (Fig. 4).
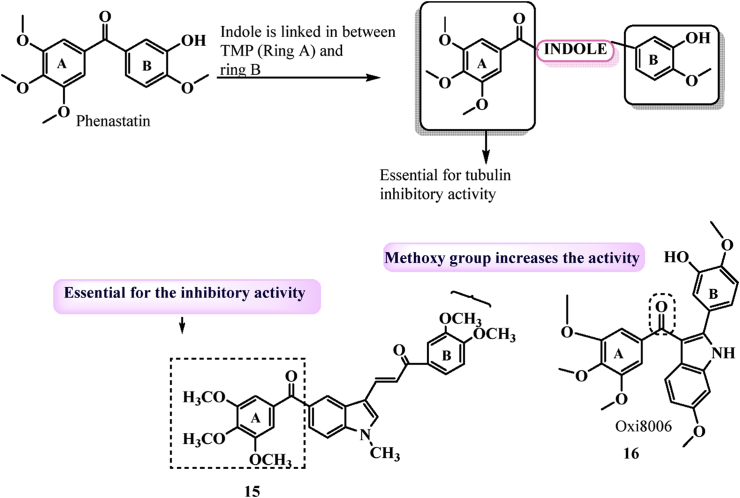
3.1.3. Phenastatin based indole derivatives
As discussed earlier, by replacing the olefinic moiety with a bioisoster carbonyl group and a Z 1,2-ethylene bridge to inhibit metabolic degradation and maintain the combretastatin's cis configuration, phenastatin and isoCA-4 were developed, which were found to be more stable and potent than CA-4 due to their inability to isomerize into an inactive trans form. In 2020, Kode and colleagues55 synthesised a library of indole linked chalcones based on phenastatin and tested them for anticancer efficacy and tubulin polymerization inhibitors. Of all, analogue 15 (Fig. 5) with 1-methyl, 2-3-methoxy substituents in the aromatic ring was important against human oral cancer cell line SCC-29B with GI50 = 0.96 μmol/L. Molecular docking studies revealed that 15 interacts and binds at colchicine binding site of tubulin. The inclusion of the trimethoxy ring and carbonyl group, which are required for tubulin action, as well as methoxy substituents at R2 and R3, increased cytotoxic activity, according to SAR analyses. Addition of indole ring in phenastatin leads to formation of Oxi8006 16 with potently inhibits tubulin assembly (IC50 = 1.1 μmol/L) and cell growth.
Figure 5.
Phenastatin based indole derivatives 15 and 16.
3.1.4. Indole linked arylhetero derivatives using hetero-ring as linker
To solve the problem of natural CA-4 isomerization, many heterocyclic bridging CA-4 analogues have been developed to limit the cis configuration and give excellent bioactivity56.
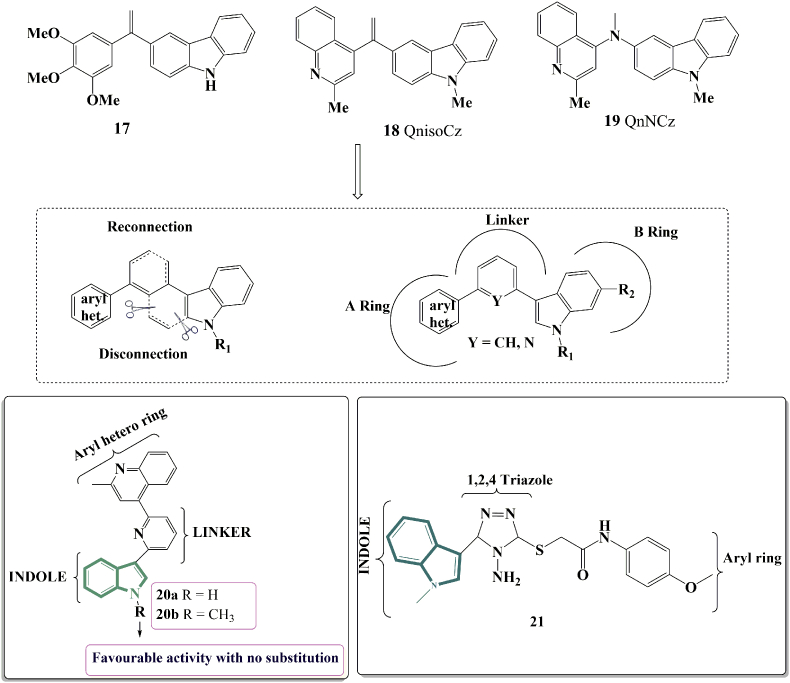
Recently, it has been seen that classical ring B of CA-4 or isoCA-4 can be substituted effectively by a carbazolyl group (compound 17, Fig. 6)57. Following that, it was found that the combination of a quinolinyl ring as ring A and a carbazolyl group as ring B had the highest efficacy. The quinalinyl-iso-carbazolyl (QnisoCz) 18 compound was found to be more active than isoCA-4 against A569, U87-MG and HUVEC cells58. Also, QnisoCz 18 was 67 times more cytotoxic than CA-4 against lung adenocarcinoma epithelial cells (A549). A similar replacement as in compound 18 was also achieved with a N (Me) linker in compound 1959. In order to continue studying SARs in the isoCA-4 series, and inspired by the promising results obtained with quinolines and quinazolines, the current study was undertaken to investigate the effect of novel bridge structure variations on the antiproliferative activities of the resulting CA-4 analogues. A series of novel cyclic bridging analogues (CBAs) of CA-4 with a phenyl or pyridine linker were developed, produced, and analysed precisely. The indole nucleus was investigated as a B-ring analogue using a disconnection and reconnection technique using carbazole derivatives (17–19).
Figure 6.
Design and structures of cyclic bridge analogues 17–21.
In the year 2021, Pecnard and colleagues60 published their findings on the synthesis of a series of cyclic bridging analogues of isocombretastatin A-4 using phenyl or pyridine linkers. They developed a series of derivatives from which 20 displayed a potent anti-proliferative activity having IC50 value 5.6 nmol/L. Further, N-methylation of the indole ring of 20a resulted in 20b which was obtained in high yields. But the antiproliferative activity of 20a was found to be very high against various cancer cell lines. Also, it was more lipophilic than the reference compounds CA-4 and also isoCA-4. The best mixture related in their structure was discovered by SAR analyses (quinaldine-pyridyl-indole). It was discovered that a quinaldine nucleus (A ring) coupled to a pyridine (linker) attached to an indole ring (B ring) was very effective. It lacks meta-OH group in the B-ring which makes it highly active in the H-29 cells which is not present in isoCA-4 and CA-4 (Fig. 6). Yang and collegues61 designed and synthesized 36 novel indole-based1,2,4-triazole derivatives through the molecular hybrid strategy. The bioassay results revealed that 21 displayed excellent antiproliferative efficacies in the nanomolar range against HeLa cells. Importantly, the compound exhibited no obvious cytotoxic activity (IC50 > 100 μmol/L) toward HEK-293, a normal human embryonic kidney cell line. More results demonstrated that the promising compound effectively inhibited tubulin polymerization with an IC50 value of 8.3 μmol/L, and molecular docking studies revealed that 21 well occupied the colchicine-site in tubulin (Fig. 6).
3.1.5. Miscellaneous indole derivatives
Chang and colleagues62 described that, by binding to the colchicine binding site, the 7-aroyl-amino-2,3-dihydroindole-1-sulfonamide effectively reduced tubulin polymerization (IC50 = 1.1 μmol/L). With IC50 values ranging from 8.6 to 10.8 nmol/L, compound significantly reduced the development of KB, MKN45, H460, HT29, and TSGH cells (Supporting Information Fig. S3).
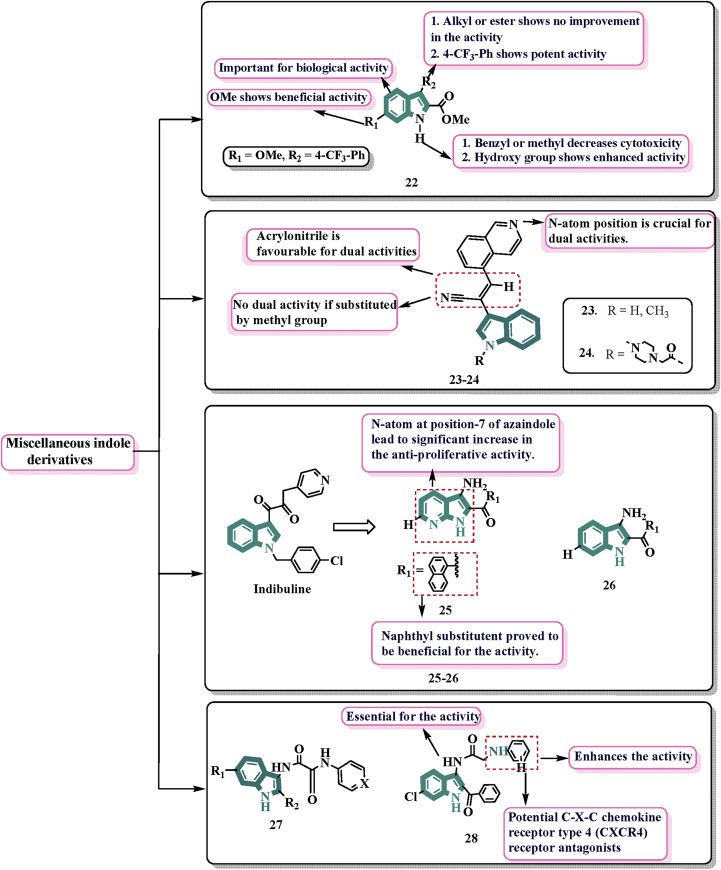
In 2019, Cury and colleagues63 described the synthetic and mechanistic study of 21 novel 3-substituted and 3,6-disubstituted-2-carbalkoxy indoles. Among them, 22 (Fig. 7) showed good cytotoxicity against CEM cells at the nanomolar scale with EC50 = 0.20 μmol/L. It decreased β-tubulins protein in leukaemia cells in a time-dependent manner. The methoxy group at the N-atom of indole showed good activity whereas substitution by methyl or benzyl diminishes the activity. It enhances microtubule depolymerization and cell cycle arrest at G2/M signifying that the structural alteration in the indole nucleus did not affect its well-known molecular target in cancerous cells. The 22 demonstrated selective cytotoxicity to leukaemia cells with no apparent cytotoxicity for normal T-lymphocytes, even at 100 mmol/L. See and others in 2018 reported the discovery of indole iso-quinoline hybrid 23 (Fig. 7) which was found to have broad-spectrum efficacy against 30 cancer cell lines and GI50 = 1.5 μmol/L64. Further in 2019, they carried out some modifications on the indole nitrogen and studied various prodrugs based on the modifications. A series of 12 derivatives was prepared and evaluated for anti-cancer activity. Compound 24 when administered orally 80 mg/kg twice a day showed 76% inhibition of tumour development in a mouse model of paclitaxel-resistant colon cancer65.
Figure 7.
Chemical structures and SAR of miscellaneous derivatives 22–28.
The powerful anti-tumour action was also aided by hybridization of this ring with other moieties. Only a few papers have claimed that attaching a heterocyclic ring to the parent scaffold increases the activity of colchicine binding medicines66. Hybridization of indole ring with other heterocyclic moieties also proved to be beneficial for anticancer activity. Few articles published have stated that there is an increase in the activity of colchicine binding drugs via attachment of a heterocyclic ring to the parent scaffold67.
Indibuline (indolyl-3-glyoxamide) is presently in phase I clinical trials for cancer chemotherapy due to its potent anticancer activity with minimal neurotoxicity. Based on its structure, Diao et al.68 in 2019 developed an efficient method for one-pot synthesis of substituted 3-amino-1H-7-azaindole derivatives 25 (Fig. 7) and 3-amino-1H-indole derivatives with general structure 26 (Fig. 7) tested for their anti-proliferative activities against four cancer cell lines. Among both, derivative 25 was found to be highly potent which indicated that the common 3-amino-1H-7-azaindole is very favourable scaffold for anti-cancer agents and displayed 13-, 5-, and 1.4-fold increase in activity compared to 5-fluorouracil in inhibiting HeLa, HepG2, and MCF-7 cell proliferation with IC50 values of 3.7, 8.0, and 19.9 μmol/L respectively. The SAR investigation suggested that the nitrogen atom at the 7-position of the azaindole ring was crucial for the anti-proliferative activity of the molecule. N atom of azaindole was found crucial for the activity.
They recently reported the synthesis of two new indole-based oxalamide 27 and aminoacetamide derivatives 28 to further investigate the actions of indole (Fig. 7). All the synthesised compounds were evaluated for in-vitro anti-proliferative activities against human cervical cancer cells. None of the indole based oxalamide derivatives with general structure 27 were found to be potent. The cytotoxicity varies depending on the substitution at position 3 of the indole ring. The SAR revealed that within the series of oxalamide derivatives, replacing phenyl ring with pyridine ring slightly improved the anti-proliferative activities, but none of the compounds were found to be enough active for further investigations. Meanwhile in the series of amino-acetamide derivatives, activities depend on the substitution pattern on phenyl ring in amino-acetamide portion at position-3 of indole. Derivatives possessing amino-acetamide moiety at position-3 of indole ring possessed strong anti-proliferative activities. Among all, 28 was found to be most potent with inhibition activity (46%) where colchicine exhibited 77% inhibition effect at the tested concentration and IC50 values 11.99 ± 1.62 μmol/L against HCT116 and 14.43 ± 2.1 μmol/L against PC-3. Also, 28 made stable interaction in the colchicine-binding of tubulin. These results encouraged further investigation on 28 to develop into new anti-cancer agents69.
In the year 2019, Sigalapalli and colleagues70 identified benzyl/phenethyl thiazolidinone indole hybrids as effective cytotoxic agents and tested their efficacy against a variety of cancer cell lines. Among the 44 derivatives synthesized, one of them (Supporting Information Fig. S4) was revealed to be highly active.
3.2. Indole as topoisomerase inhibitors
DNA topoisomerases (I and II) are nuclear enzymes those were taken into consideration as essential targets for anticancer drug designing because of their important role in DNA replication and recombination as well as repair and transcription71. These enzymes introduce transient breaks in the DNA molecule by cleaving one strand (type I topoisomerases) or both strands (type II topoisomerases, also called Topo II), thereby relieving the topological tension, relaxing the DNA double helices and thus play an important role of DNA replication in the cell cycle. Both Human DNA topoisomerase I and II are considered as important targets for anticancer drug design and indole molecule such as indolocarbazole (BMS-250749) is developed as potential Topo I inhibitor which has been under clinical trial72.
TOP1, TOP1MT, TOP2α, TOP2β, TOP3α, and TOP3β are six topoisomerases that pack and unpack the 2 m of DNA that must be contained in the nucleus, whose diameter (6 μm) is 3 million times smaller. While all six human topoisomerases remove DNA negative supercoiling (underwinding), only TOP2α and TOP2β cleave both DNA strands, allowing them to resolve DNA knots and interwoven DNA circles (decatenation). Only TOP3β serves as an RNA topoisomerase, whereas TOP3α resolves hemicatenate and double-holiday junctions73. Topoisomerase inhibitors are incredibly selective and clearly qualify as “targeted medicines”. TOP1 inhibitors have no effect on TOP enzymes, and TOP2 inhibitors have no effect on TOP1 enzymes. Furthermore, bacterial topoisomerase inhibitors (gyrase and Topo IV) are inert against host cell topoisomerases (TOP2 and TOP1), which explains their antibacterial activity without affecting the host genome. In addition to interfacial inhibition, the therapeutic mode of action of topoisomerase inhibitors provides a new paradigm for pharmacological activity: drug action is driven by enzyme poisoning rather than catalytic inhibition74. Lima et al.75 in 2017 revealed anti-proliferative activity of 4-thiazolidinones indole derivatives via inhibition of topoisomerase 11α against eight human cancer cell lines (Supporting Information Fig. S5).
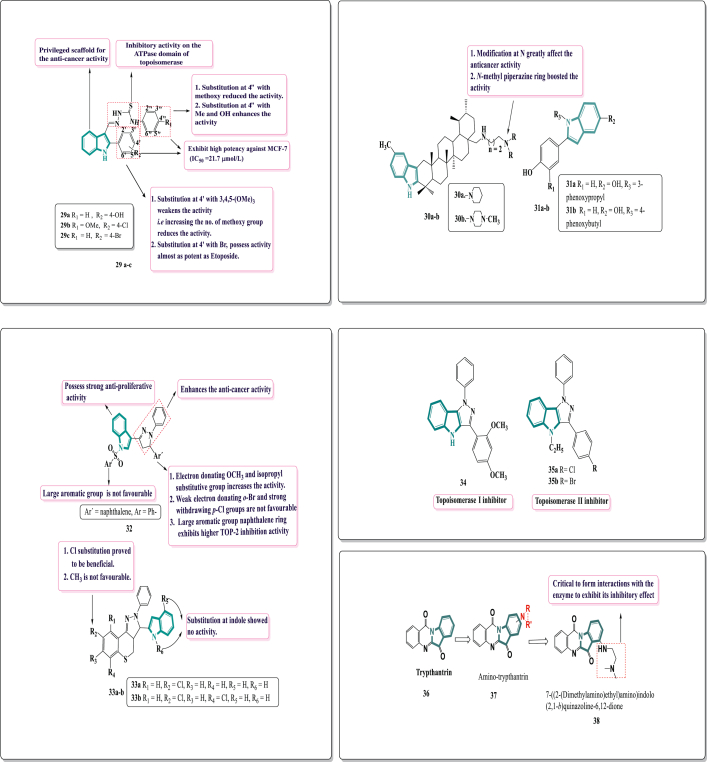
After all the successful synthesis of above derivatives, Bakherad et al.76, in 2019 designed the novel-indole thiosemicarbazone derivatives as antiproliferative agents by using the idea of multi-target compounds having synergistic impact to design effective cytotoxic agents. They synthesized a series of compounds having phenyl substituted aryl group and 4-methoxyphenyl. The phenyl substitution was discovered to be very influential for cytotoxic activity. The SAR revealed that compounds consisting of phenyl group attached to thiosemicarbazone moieties and 4-hydroxyphenyl group at C-2 of indole ring exhibited high potency against MCF-7 (IC50 = 21.76 μmol/L) than etoposide (IC50 = 33.45 μmol/L). Replacement of phenyl attached to thiosemicarbazone by 4-methoxyphenyl weakens the activity (IC50 > 100 μmol/L). Substitution by 3,4,5-(OMe)3 at C-2 position of indole exhibited reduced activity. From all the synthesised compounds of this series, 29a‒c (Fig. 8) showed good activity but 29b possessed the highest potency against A-549 and HEP-G2and also induced apoptosis in A-549 cells. The molecular docking study of the 29b (Fig. 8) suggested suitable H-bonding and hydrophobic interactions in the tubulin and ATPase domain of topoisomerase IIα binding sites with IC50 = 11.77 and 11.91 μmol/L against A-549 and Hep-G2 respectively76. Encouraged by the promising results of combining indole and thiosemicarbazone scaffolds, Bakherad and co-workers77 concentrated on designing more novel multitarget indole anti-tumour agents. They substituted thiosemicarbazone moiety with a methyl group which resulted in improved cytotoxic activity against four human tumours cell lines.
Figure 8.
Chemical structure and SAR of indole-based topoisomerase inhibitors 29–38.
Li and colleagues78 in 2020 worked on the novel indole derivatives of ursolic acid containing different N-(aminoalkyl)carboxamide side chains. The synthesised compounds were then evaluated for their in vitro cytotoxic activities for two human hepatocarcinoma cell lines (SMMC-7721 and HepG2) and normal hepatocyte cell line (LO2) by utilising the protocol of MTT assay. It was found that compound 30a and 30b (Fig. 8) were the most potent among all the derivatives. 30a showed interesting activities against SMMC-7721 cells and with IC50 values of 0.89 ± 0.11 μmol/L. Whereas 30b possessed significant topoisomerase IIα inhibitory activity with IC50 values 0.56 ± 0.08 and 0.91 ± 0.13 μmol/L against SMMC-7721 and HepG2 cells respectively. Actually, anti-cancer activities were mainly affected by N-containing moieties at the side chain. SAR evaluation indicated that N-methylpiperazine moiety 30b possessed the brilliant anti-tumour activities and could be very helpful as a lead for the discovery of novel anti-tumour agents.
Zidar and others79 in 2020 reported the synthesis and topoisomerase II inhibitory activity of 3-methyl-2-phenyl-1H-indoles. A series of analogues was designed and evaluated against HeLa, A2780 and MSTO-211H cells. Compounds 31a and 31b (Fig. 8) were found to be highly potent. 31a have GI50 values of 4.4, 2.2 and 2.4 μmol/L whereas 31b having GI50 values 4.0, 2.0 and 2.9 μmol/L against HeLa, A2780 and MSTO-211H cell lines respectively. SAR evaluation showed that presence of –OH was R1 is not favourable for the activity whereas no substituted is favourable. Improved activity was observed by the addition of phenoxy group to the propyl side chain of R3 (31a). As the length of the alkyl linker was increased from propylene to butylene, rise in the activity was observed (31b). Appropriate side chain length is essential for the activity. Hydroxyl (‒OH) at R2 showed high potency than unsubstituted.
Pyrazoline is emerging as an interesting moiety in the field of anti-tumour agents. Several analogs have been synthesised and evaluated for cytotoxicity80. Shu and others81 in 2019, synthesised and evaluated a series of novel indole pyrazoline hybrid derivatives as potential topoisomerase-1 inhibitors. Among all the derivatives, 32 (Fig. 8) with large aromatic group naphthalene ring at Ar' was found to exhibit strong TOP-1 inhibitor which was measured by TOP-1 mediated relaxation assay with CPT (camptothecin) as a positive control. However, unfavourable effects can be observed by large aromatic substitution at Ar.
Pyrazoline analogs have various biological activities and have the capability of hydrogen bonding. Thiochroman have also shown favourable cytotoxic activities82. Songand and others83 in 2020 reported novel pyrazoline derivatives having indole nucleus. Utilising MTT assay, the derivatives were then evaluated against four human cancer cell lines MCF-7, HeLa, MGC-803 and Bel-740H. They synthesised nearly 13 derivatives out of which 33a and 33b (Fig. 8) were found to be highly potent as topoisomerase inhibitors. The IC50 values of 33a and 33b were found to be 15.43 and 20.53 μmol/L respectively. SAR evaluation indicated that chlorine substitution was favourable whereas methoxy group was not found to be beneficial for increasing the cytotoxicity.
Various 1,4-dihydropyrazolo [4,3-b]indoles were synthesized by Kaur and team84, all of the target compounds were evaluated for cytotoxicity against the cancer cell lines A549 (lung), HCT-116 (colon), MDA-MB-231 (breast), and MCF-7 (breast). Although all the tested compounds exhibited low μmol/L IC50s compound 34, 35a and 35b emerged as broad-spectrum antiproliferative compound (IC50 range 0.58–2.41 μmol/L) comparable to standard drugs. To examine Topo I inhibitory ability, compounds 34, 35a, and 35b were investigated in a Topo I induced DNA relaxation assay. The results of the Topo I inhibition experiment revealed that these drugs had inhibitory effect against Topo I. In addition, topoisomerase binding experiments demonstrated that 34 inhibited Topo I, whereas 35a and 35b inhibited Topo II more than the conventional medication (Fig. 8). Recently Kwon and associates85 reported the importance of benzo containing tryptanthrin derivatives. These benzo [b]tryptanthrin derivatives inhibits the DNA non-intercalative Topo I/II enzyme and shows cytotoxic activity. Tryptanthrin 36 is a natural indoloquinazoline alkaloid which has been widely explored as anti-bacterial, anti-malarial and anti-cancer agents. On the basis of structure of trypthanthrin, Catanzaro and others in 2020 reported the synthesis of newTopo II inhibitor, 38 which was developed from trypthantrin 36 via amino-trypthantrin 37 (amination of tryptanthrin) resulting in an effective anti-proliferative activity. Compound 38 (Fig. 8) was developed with the aim of improving water-solubility (as trypthantrin has low water solubility) and improving drug-interaction by ionic and H-bonding. Among the 12 compounds synthesized, 38 (Fig. 8) was found to be the most potent and inhibited Topo II with an IC50 of 26.6 ± 4.7 μmol/L which was about 2.5 times better activity than standard drug etoposide. Moreover, 38 was also found to be five times more cytotoxic than tryptanthrin (IC50 72 h: 44.36 μmol/L) indicating the role of indole nucleus in enhancing cytotoxic ability of the tryptanthrin. The N,N-dimethyl alkylamine is found to be crucial for interacting with enzyme to exhibit inhibitory activity. In addition, the high-water solubility and favourable pharmacokinetics and ADME properties make 38 as potential lead for further research86.
3.3. Indole as potent anti-cancer and apoptosis inducers
Apoptosis which is also called as “cellular suicide” is a form of programmed cell death in which cancerous and infected cells are eliminated by the body. Cells normally try to repair the damaged DNA. But sometimes, damage is beyond repair and then apoptosis comes into play and removes the damage DNA. When cell with damaged DNA failed to undergo apoptosis, it may lead to cancer87. There must be balance between the mitosis (proliferation) and apoptosis (cell death). The apoptotic pathway can be inhibited by Bcl-2 (including Mcl-1, Bcl-x and Bcl-2) require either Bax or Bak, two pro-apoptotic Bcl-2 family members. Tumour suppressor gene P53 induces apoptosis by indirectly antagonizing anti-apoptotic Bcl-2 family members. Activating P53 and antagonising Bcl-2 are two ways used to enhance cancer cell death. MDM2 is a key antagonist of the P53 tumour suppressor. New drugs have been created that bind to MDM2, displacing P53 and activating the P53 pathway, resulting in cell cycle arrest and apoptosis88. A cascade of initiator and effector caspases is activated progressively by another protein family known as IAPs throughout the apoptosis process. Except caspases several interconnected kinase-driven signalling pathways are also involved. For the reason that many of these signalling cascades negatively alter apoptosis in most cancer cells, inhibition of kinases is reasonable approach for anticancer remedy. Multikinase inhibitors are renowned apoptotic inducers. For therapeutic applications, inducing apoptosis is favourable than induction of necrosis because apoptosis does not damage the host by using causing inflammatory reactions89. An understanding of the process of apoptosis is therefore important in developing efficient chemotherapeutic agents. There are some commercially available anticancer agents which are derivatives of indole. Attachment of heterocyclic rings to indole increases the anticancer activity90. In their previous study, El Sharief and co-workers91 observed that morpholino-isatin substitution at position-3 of indole enhanced the anticancer activity. Therefore, these observations helped them to resume their previous work. Morpholine is believed to be very important anticancer pharmacophore which are known to be modulating the pharmacokinetic property of whole molecule. They designed and synthesised morpholino-isatin nucleus and then substituted it to the 3-position of indole along with the different bioactive pharmacophores. Then they combined this pharmacophore with other active heterocycles and then finally designed spiro analogues of this scaffold with the ambition to get more active derivatives. Synthesis of some novel spiroindole derivatives containing sulfonamide moiety was attained via chalcone intermediates92.
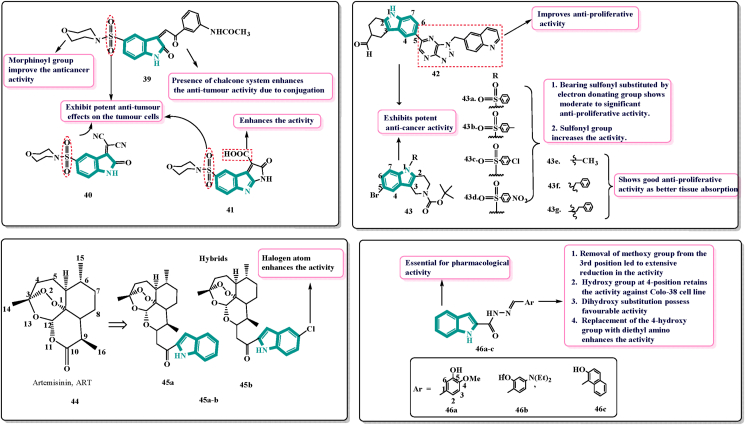
In 2019, El Sharief and associates93 designed, synthesised certain new indole derivatives as effective anticancer drugs and apoptosis inducers, and studied their biological activity. The authors reported an expeditious strategy material to construct 2-oxoindoles derivatives, fused pyrrole [2,3k-b] indoles and spiro-indole derivatives. Fused derivatives showed potent anti-cancer activity over spiro derivatives. Compounds 39, 40 and 41 (Fig. 9) were more effective than lapatinib against EGFR. Furthermore, 39–41 demonstrated greater potency against EGFR than lapatinib, with IC50 values ranging from 0.019 to 0.026 μmol/L compared to lapatinib's 0.028 μmol/L. The binding mechanism, amino acid interactions, and free binding energy of these powerful derivatives were investigated using molecular docking. Cell cycle was arrested at G2/M phase by compounds 39 and 40, at G0‒G1 phase by compounds 41. The cytotoxicity of arylidine of 2-oxoindoles derivative 40 was outstanding, and it had broad-spectrum action against all cell lines. Through the carbonyl group of its indole scaffold, it displayed the same amino acid interaction as erlotinib. Among all the chemicals evaluated in the study, substance 41 dihydropyrrolo [2,3-b] indole-3-carboxylic acid is the most cytotoxic. On Hep G-2 and HCT-116, the molecule outperformed the reference medication, while on MCF-7, it came close. Finally, the presence of the chalcone system in 39 and the carboxyl group in 41 increased anti-tumour efficacies, resulting in results that were comparable to or better than those of the reference medication lapatinib. Furthermore, all promising compounds induced apoptosis in pre-G1 phase cells94.
Figure 9.
Chemical structures and SAR of indole-based apoptosis inducers 39–46.
Feng and others95 in 2019 designed novel indole analogues encouraged by their previous research. In 2012, they designed and synthesised a new compound 42 (Fig. 9) bearing active skeleton 2,3,4,5-tetrahydro-1H-pyrido-[4,3-b] indole which showed high anti-proliferative activity. They synthesised novel derivatives 43 (Fig. 9) based on combination principles utilising 42 as the lead molecule in an ongoing quest to develop novel anti-tumour medicines. The substitution of sulfonamide group was basically for structurally similar amino sulfonic esters and has revealed good anticancer activity in-vivo and in-vitro as stated by the biological isostere principles. Also, it was expected that having an alkyl or arylated alkyl in position N-5 will help in absorption by various tissues and improves the anticancer activity. The introduction of sulfonyl, alkyl, or aralkyl enhanced the activity of 2,3,4,5-tetrahydro-1H-pyrido-[4,3-b] indole and expectantly, it was rapidly and efficiently absorbed by the improved polarity and lipid solubility. All derivatives possessed slight to brilliant anti-tumour activity with IC50 values between 0 and 100 μmol/L against tumour cells.
The HeLa, A549, HepG2, and MCF-7 cells were used to test all of the synthesised compounds. The anti-proliferative effect of compound 43a‒43b (Fig. 9) with sulfonyl replaced by an electron donating group was moderate to substantial. The best compound was 43c, which had IC50 values of 13.71, 9.42, 15.06, and 14.77 μmol/L. As a result, it was hypothesised that adding a sulfonyl group boosts activity. The anti-proliferative effect of compound 43e‒43g with alkyl, phenyl, and arylated alkyl groups was good. As a result, the findings revealed that these series of derivatives displayed selectivity for A549 cancer cell lines and had the potential to be effective lung cancer therapies. The authors’ compounds showed a good inhibitory impact on cancer cells, and they might be used as a lead compound for the development of new anticancer inhibitors. This work was useful for building a chemical library as well as for future research projects96.
Artemisinin (ART, 44, Fig. 9), a Chinese traditional drug obtained from the sweet wormwood plant, Artemesia annua, has been extensively used in the treatment of malaria and has shown excellent safety for decades. It is a sesquiterpene lactone whose structure consists of endoperoxide, acetal, lactone, seven chiral carbons and the tetracyclic system which is responsible for its poor solubility both in oil and water97. Till 1993, none of the researchers found that artemisinin had the potential for anticancer activity. Afterwards, artemisinin and its derivatives gained more attention for searching anticancer agents. Researchers found that beyond the antimalarial effects, artemisinin has also been effective as anticancer agents98.
Hu et al.99 in 2019 worked on the artemisinin indole and artemisinin-imidazole hybrids and accessed on multi-drug resistance in MCF-7/ADR cells. The structural modifications were done at C-10 position of artemisinin. Most of the new compounds showed higher anti-cancer activities than artemisinin. Among all, 45a and 45b (Fig. 9) were found possessing higher potency with IC50 = 6.78 and 5.25 μmol/L (10-fold) against MCF-7, respectively. Reversal activities of artemisinin-indole derivatives were better than artemisinin-imidazole derivatives as revealed by SAR studies. Electron withdrawing group at position-5 of the indole enhanced the activity when compared to unsubstituted derivative100.
To further explore the cytotoxic activities of indole derivatives, Demurtas and co-workers103 in 2019, synthesised a series of indole derivatives combined with hydrazone moiety and evaluated for anti-oxidant, photoprotective as well as anti-proliferative activity. Hydrazono-indole derivatives have reported to induce apoptosis100 and to interact with tubulin101. They have reported anti-proliferative activity of benzofuranhydrazones in 2018102. Therefore, encouraged by those outcomes, they reported a small library of hydrazono-indole derivatives. All the derivatives were evaluated on human melanoma Colo 38 cell line as well as human erythroleukemic K562 cells to reveal their anti-proliferative activity. The compound 46a (Fig. 9) was found to possess highest cytotoxic activity of this series effective on both the Colo38 and K562 cells at nano molar concentrations [IC50 = 0.59 ± 0.03 and 0.067 ± 0.001 μmol/L] respectively. The removal of 4-methoxy group from 46a led to extensive reduction in the activity. The shift of 3-hydroxy group to the 4th position retains the anti-proliferative activity against Colo38 cell lines. Presence of 4-diethylamino 46b or naphthyl ring 46c showed enhanced activity. The compound 46b (Fig. 9) showed an inhibitory activity profile very similar to 46c (Fig. 9) on both the cell lines (IC50 values between 0.54 and 0.83 μmol/L). The SAR data obtained above suggested that 46a, 46b and 46c exhibited anti-proliferative activity at sub-micromolar concentrations on human erythroleukemia K-562 and melanoma Colo-38 cells and confirmed the importance of phenol and naphthol substitution.
3.4. Indole as aromatase inhibitors
Aromatase is a C4P (cytochrome P-450) hemoprotein, a monooxygenase enzyme responsible for the conversion of androgens to estrogens by demethylation and aromatisation of the steroidal ring. It also catalyses the conversion of androstenedione and testosterone to estrone and estradiol. Increased level of these hormones fuels the growth of various types of cancer especially breast cancer. Aromatase inhibitors functions by inhibiting the aromatase activity through interaction with the substrate binding site of aromatase. By stopping of aromatase enzyme, it lowers estrogen level in the body which is responsible for breast cancer. On the basis of their structure, they were normally divided in steroidal and non-steroidal anastrozole104. Indole derivatives endowed with encouraging aromatase inhibiting potential.
Melatonin, a natural indolic hormone, has been shown to inhibit aromatase, which prevents free radical-induced carcinogenesis and blocks local oestrogen synthesis in breast tissue. However, melatonin's therapeutic potential is limited by a number of factors. In this study, the aromatase inhibitory potential of 2-methyl indole hydrazones was investigated and compared to melatonin using two in vitro models: a cell-free assay using a fluorescence substrate and a cell-based assay in which cell proliferation was measured in ER + human breast cancer cells (MCF-7 BUS) in the absence of oestrogen and the presence of testosterone. Molecular modelling studies are also looking into the aromatase inhibitory impact. Monochloro substituted indole hydrazones were shown to have the strongest aromatase inhibitory effect among all investigated derivatives, and were more potent than melatonin in biological activity studies. This conclusion is supported by molecular modelling. These findings could aid in the development and manufacture of new melatonin analogues with stronger aromatase inhibitory potencies105.
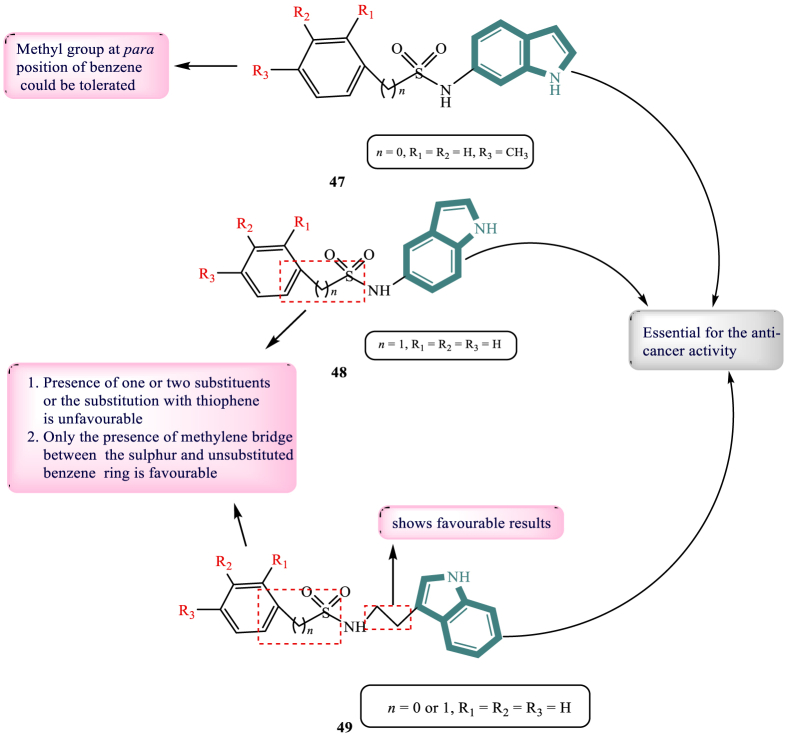
Fantacuzzi and others106 in 2019 synthesised and evaluated indole aryl sulphonamide as aromatase inhibitors. They synthesised 30 derivatives, out of which, three derivatives showed IC50 values in the range of micromolar. SAR studies revealed that only the presence of a methyl in p-position in benzene could be tolerated. The presence of methylene bridge between the sulphur and unsubstituted benzene ring was favourable for 48 (129.3 and 75.2%, Fig. 10). The compound 48, unsubstituted on aryl moiety with a linker of 2 carbon atoms bonded to C-3 of indole, showed a percentage of inhibition equal to 109.79. The evaluation of ability to inhibit the aromatase enzyme revealed that three compounds 47–49 (Fig. 10) showed IC50 value in the range of sub-micromolar (0.49, 0.16, and 0.20 μmol/L) respectively. The benzene ring and sulphonamide moiety both contributed to the activity in general. The aliphatic hydrocarbon chains that connect the sulphonamide moiety to the indole ring in compound 48 contributed to the action.
Figure 10.
Chemical structures and SAR of aryl sulphonamide-based indole derivatives 47–49.
3.5. Indole as kinase inhibitors
Protein kinase (tyrosin and serine-thronine kinases) driven phosphorylation is one of the essential mechanisms that activate intracellular signalling pathways through gene expression responsible for cell proliferation, growth and apoptosis. These protein kinases are frequently recognized as oncogenic and can be crucial for the survival and spread of cancer cells. Therefore, an intensive search for new kinase inhibitors has been continuing for the last two decades. Lots of research has been continuing in the area of kinase inhibitors. Various potential anticancer leads as kinase inhibitors are obtained which are under clinical trial and illustrated in Supporting Information Table 1.
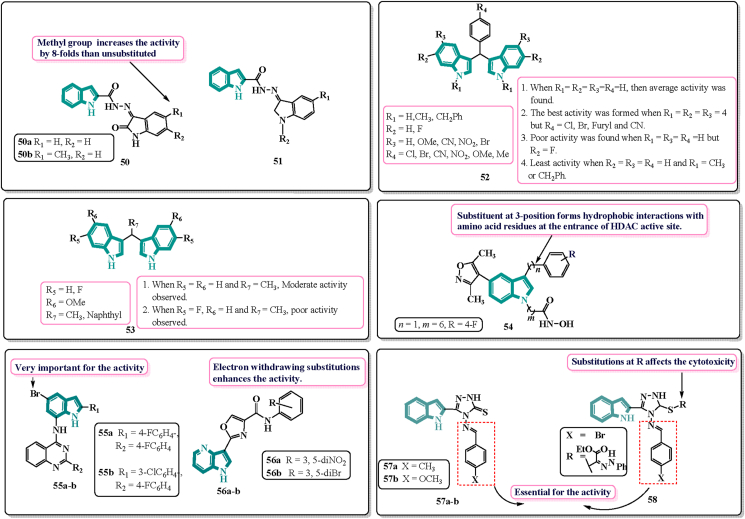
Among a number of kinases, cyclin-dependent kinaes (CDKs) are required for cell cycle progression107. They are potential cancer targets. Warhi and associates108 worked on the valuable strategy for a hybrid pharmacophore approach to synthesise Oxindole-Indole conjugates as anti-cancer CDK inhibitors. They developed two series 50 and 51 (Fig. 11). Out of which, 50a and 50b showed favourable CDK-4 inhibitory activity with IC50 = 1.82 and 1.2 μmol/L respectively. SAR studies revealed that substitution at C-5 of oxindole with methyl 50b remarkably increases the activity by 8-fold as compared to 50a suggesting that an electron donating group is favourable for the activity.
Figure 11.
Chemical structures and SAR of indole-based kinase inhibitors 50–58.
PIM kinases, a type of serine/threonine kinase, function as oncoproteins that aid cell survival and proliferation. The family of PIM kinase consists of three isoforms, PIM-1, PIM-2, and PIM-3. PIM-1 is overexpressed in many types of cancer including prostate109. The indole derivatives i.e., bisindolemethanes were developed by Bahuguna et al.110. in 2019. They synthesised a series of bisindolemethane derivatives derived from 3-substituted indole as the basic skeleton and investigated their anti-cancer activity under in vitro conditions against two different cell lines i.e., HeLa and A549 cell lines. They selected cyano-substituted turbomycin B (CN-TBM) to apprehend the mechanism of most cancer cell death. The targets were assumed with the help of Pharm Mapper and Id Target. Out of four targets assumed, Pim-1 becomes recognized as the direct target of CN-TBM. The SAR was described on the basis of two structures, 52 and 53 (Fig. 11). In 52, electron donating group at R4 displayed excellent cytotoxicity only if R1, R2 and R3 do not have any substituent, but R1 has a benzyl, then activity decreases considerably. In 53, it was found that if R5 and R6 do not have any substituent and R7 consists of methyl substitution, anti-cancer activity was found to be moderate. Among all of the bisindole moieties, molecules with substitution with cyano at R4 disclosed better anti-cancer activity only when there is no substitution at R1, R2 and R3. The cause is probably because of extended conjugation or flow of electrons towards the nitrogen atom of cyano group110.
HDAC by removing acetyl from N-acetyl lysine of histones, helps in wrapping DNA more tightly during DNA replication thereby playing essential role in cell proliferation and cell death. So, HDAC is considered as valuable target for design of anti-cancer agents having HDAC inhibitory activity. Now researchers have paid much attention for developing HDAC inhibitors with dual targeting capability due to its limited efficacy, drug resistance and toxicities111. Various studies have demonstrated that HDAC and BRD4 take part in the regulation of some protein kinase signalling pathways like PI3K-AKT.
Cheng et al.112 in 2019 worked on the synthesis of novel derivatives as potential HDAC/BRD4 dual inhibitors and anti-leukemic agents. They created a series of indole derivatives that integrate the inhibitory actions of BRD4 and HDAC into a single molecule using a structure-based design strategy. Among all the 15 synthesised derivatives, 54 (Fig. 11) was found to be the most potent inhibitor against HDAC3 with IC50 value of 5 nmol/L and BRD4 with inhibition rate of 88% at the concentration of 10 μmol/L.
Epidermal growth factor receptor (EGFR) is a protein on the surface of cells which has been associated with various cellular processes such as cell proliferation, angiogenesis and inhibition of apoptosis. Over expression of EGFR has been found in various types cancers leading to uncontrolled cell multiplications. EGFR inhibitors acts by blocking the activity of EGFR and thereby inhibiting all cellular processes113. Further EGFR inhibitors were synthesised by Mphahele and others114 in 2018. They worked on the synthesis of indole-aminoquinazolines via the amination of 2-aryl-4-chloroquiazolines with 7-amino-2-aryl-bromoindoles. The derivatives synthesised then evaluated for the anti-tumour activity against A549, CaCo-2, C3A, MCF-7 and HeLa. Derivatives 55a and 55b (Fig. 11) were found the most active one. The addition of a 4-fluorophenyl group at the C-2 position of the quinazoline and indole moieties increased the cytotoxicity, according to SAR studies. 55a and 55b showed potent activity towards EGFR (epidermal growth factor) when compared to the reference drug gefitinib (LC50 = 38.9 nmol/L).
Another EGFR targeting derivatives were prepared by Swammy and others115 in 2019. They designed a series of 11 amide derivatives of azaindole-oxazoles and evaluated for their anti-cancer activity against 3 cancer cell lines. Among all, 56a and 56b (Fig. 11) possessed magnificent activity in MCF-7 cell lines with IC50 values 0.034 and 0.036 μmol/L. Both the derivatives bind firmly to EGFRs which is strongly correlated with the anti-tumour activity.
VEGFR-2 is a receptor tyrosine kinase which is responsible for the stimulation of various signaling pathways116. Indole framework has already gained much attention due to anti-proliferative activities in drug discovery. Also, 1,2,4-triazole nucleus has been researched for various bioactivities. Al-Hussain and others117 synthesised indolyl-1,2,4-triazole hybrids and evaluated for their anti-cancer activities. Derivatives 57a, 57b and 58 (Fig. 11) were found to be highly active possessing VEGFR-2 inhibitory activity than the reference drug sunitinib. The derivatives were evaluated in-vitro against 2 human renal cancer cell lines and were found to be five-fold more potent than sunitinib with IC50 values 0.034, 0.002 and 0.046 μmol/L respectively. Also, they showed better safety profile than that of sunitinib. SAR studies indicated 4-methylbenzylidene boosted up the activity against VEGFR-2 kinase. p-Chlorophenacyl moiety at S-atom reduced the activity whereas bromobenzylidene derivative was more potent. Kamel et al.118 described the synthesis of bio isosteres of marine indole alkaloids, as well as anti-proliferative activity against the HCT-116 cell line (Supporting Information Fig. S6).
3.6. Miscellaneous targets of indole derivatives
3.6.1. IDO1/TDO dual inhibitors
Kynurenine (Kyn) pathway which converts tryptophan to kynurenine, an immunosuppressive metabolite, is a significant metabolic pathway of tryptophan (Trp). In many cancer types, two key enzymes of the Kyn pathway, indoleamine-2,3-dioxygenase (IDO) and tryptophan-2,3-dioxygenase (TDO or TDO2), are overexpressed, which are directly or indirectly involved in the origin of numerous types of cancer119.
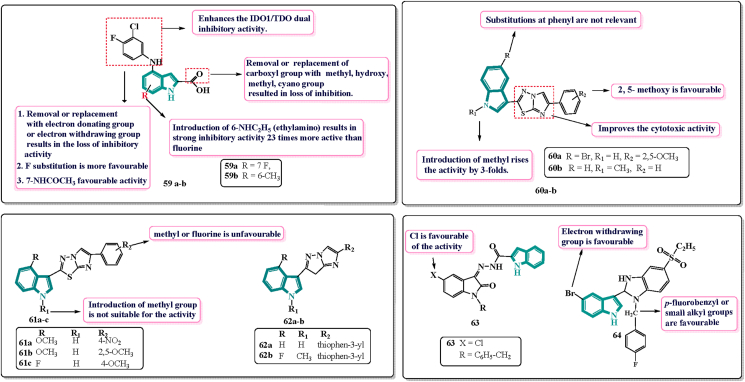
Cui and colleagues120 in the year 2020 synthesised and evaluated indole-2-carboxylic acid derivatives as IDO1/TDO dual inhibitors. They performed a random screening of their in-house compound library which featured an indole scaffold. The indole 2-carboxylic acid 59a and 59b (Fig. 12) were found to be dual inhibitors with a novel scaffold with IC50 = 99.2 and IC50 = 22.5 μmol/L against TDO. By scrutinizing the hits 59a and 59b, they speculated that the indole aryl amine frame as a unique structural feature for this class of inhibitors. The SAR is shown in below.
Figure 12.
Chemical structures and SAR of indole drugs acting on miscellaneous targets 59–64.
3.6.2. Against pancreatic cancer cells
The indole scaffold has been developed as one of the important scaffolds because of its exceptional capability to mimic peptide derivatives and reversibly bind proteins121.
Furthermore, the imidazo [2,1-b][1,3,4] thiadiazole ring has been emerged as an interesting scaffold for the design and synthesis of derivatives having various pharmacological properties122. Based on these discoveries, as well as on the concept of the “one compound-multi target”, Li petri and others123 in 2019 designed and synthesised a series of hybrid structures, 3-(6-phenylimidazo [2,1-b][1,3,4] thiadiazole-2-yl)-1H-indoles to get active derivatives with different therapeutic activities. They synthesised about 10 derivatives out of which two derivatives were able to inhibit both migration and proliferation of SUIT-2 cells at concentration lesser than 10 μmol/L. Seven out of ten compounds were submitted to National Cancer Institute (NCI) for the screening to evaluate their antitumor activity on a panel of 60 human cancer cell lines. Compound 60a (Fig. 12) was found to be active against both SUIT-2 and Panc-1 cells having IC50 values of 8.4 and 9.8 μmol/L respectively. Another derivative 60b (Fig. 12) displayed strong anti-tumour activities against SUIT-2 cells having IC50 value of 5.16 μmol/L. The SAR studies revealed that 2,5-methoxy is favourable for the cytotoxic activity. Introduction of methyl group at N-atom of indole enhanced the activity by 3-fold. Overall, the results of cytotoxicity and cell migration obtained suggested its role as an interesting hit compound to create a library of new derivatives.
Same authors then synthesised a new series of imidazo [2,1-b][1,3,4] thiadiazole derivatives and evaluated against SUIT-2, CAPAN-1 and Panc-1. Compounds 61a and 61b (Fig. 12) were of great importance. They were found active against SUIT-2, CAPAN-1 and Panc-1 with IC50 value about 5 μmol/L whereas 61b showed activity only against Panc-1 cells. Derivative 61c (Fig. 12) was able to inhibit the cell proliferation in SUIT-2 and CAPAN-1 cells having IC50 value of 11.8 and 10.49 μmol/L respectively. SAR studies revealed that the N-methylation of indole is not favourable for the activity whereas unsubstituted one was favourable. Presence of methoxy or fluorine atom at phenyl ring is not favourable. From overall results, 61a turned out to be an interesting derivative which may help to synthesise a new library of derivatives124.
Further in 2020, same authors reported a novel series of 18 imidazothiadiazole derivatives. Out of 18 designed analogs, 62a and 62b (Fig. 12) possessed outstanding cytotoxicity with IC50 values ranging from 0.85 to 4.86 μmol/L. In-vitro evaluation studies on PDAC cells, SUIT-2, Panc-1 were carried out by sulforhodamine assay. The SAR studies suggested that thiophene moiety is crucial for the activity and the replacement with phenyl ring diminishes the activity. Also, both the hybrids were found active against gemcitabine resistant Panc-1R cells and higher activity than 5-fluorouracil (GI50 = 4.5 μmol/L) and gemcitabine (GI50 > 10 μmol/L)124.
Recently Reem and colleagues125 reported novel Isatin-Indole conjugates and evaluated for their in-vitro anti-tumour activity against three human cancer cell lines. 63 (Fig. 12) was found to be the most potent and had IC50 value of 1.17 μmol/L which was found to be seven-fold greater than the reference drug sunitinib (IC50 = 8.11 μmol/L).
Indoles and benzimidazole are gaining attention because of their anti-tumour and anti-estrogenic effects. Karadayi and associates126 in 2020 reported novel indole-benzimidazole derivatives. They synthesised a set of indole-benzimidazoles having benzene sulfonyl structure and evaluated to assess their anti-proliferative activity. The derivative 64 (Fig. 12) with p-fluorophenyl at R1 position and Br at R2 was found to be the most potent of all. The IC50 value of 64 equals to 32.2, 22.3 and 9.95 μmol/L against MCF-7, MDA-MB-231, HEPG2 respectively.
4. Clinical advancement and off-target challenges of indole anticancer drugs
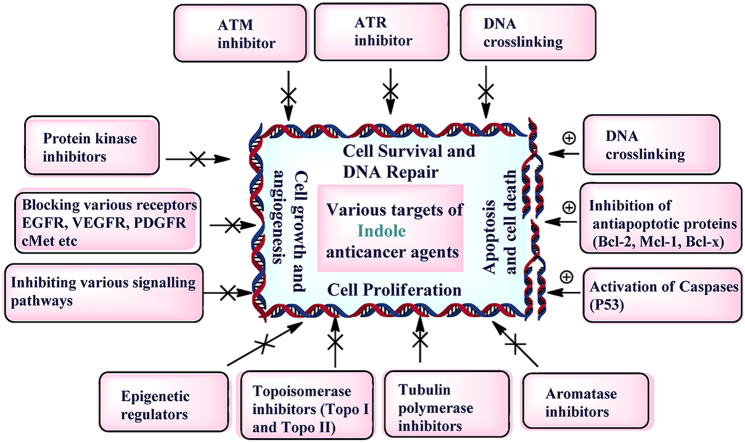
A vast data available in the literature mentioned various FDA approved indole drugs effective against various kinds of cancers. Most of the indole containing drugs under clinical trials are protein kinase inhibitors. These kinases after activation regulate various cellular processes of cell proliferation and growth via various downstream regulatory pathways. Hence, blocking this signalling pathway by inhibitors provides a promising approach for treating many types of cancers and resulted in the development of various drugs in clinical and preclinical trials (Fig. 13). Indole derivatives under advanced stages of development are mentioned in Supporting Information Table S1 showing the molecular targets along with short description of mechanism of action.
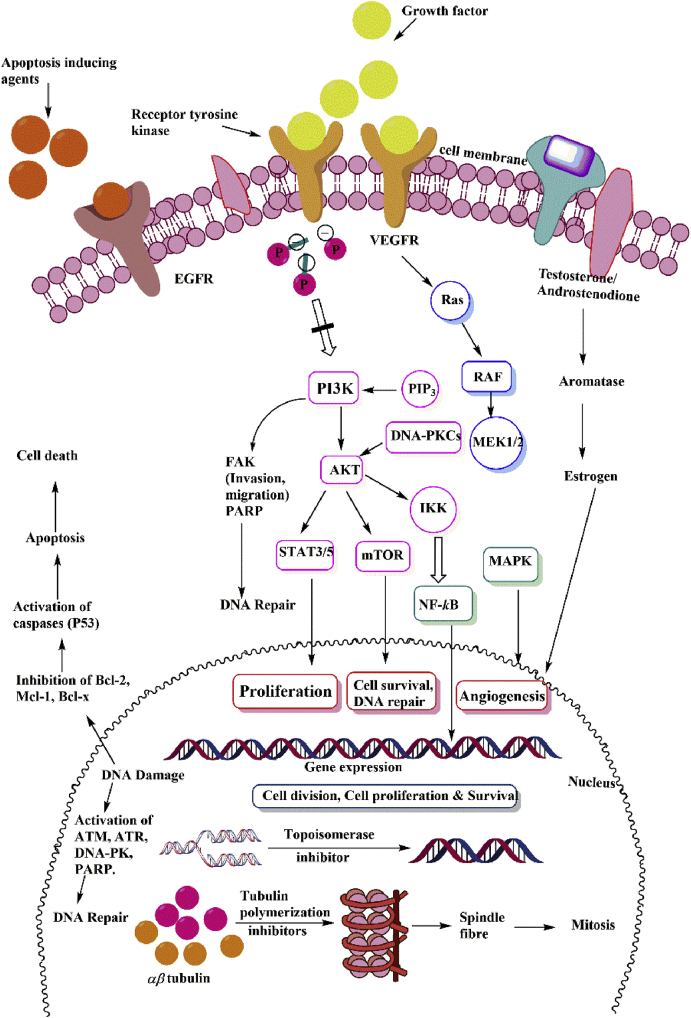
Figure 13.
Mechanistic pathways depicting the anticancer mechanism of indole drugs under clinical trial. Protein kinases enzymes driven by tyrosine and serine-threonine kinases phosphorylate protein and modulate various cellular functions by activating various signalling pathways. Ligand (growth factors) binding induces the activation of PI3K through the generation of PIP3 in the membrane. It induces the central signalling chain resulting in cell growth, proliferation and angiogenesis. PI3-Kinase related protein kinases-ATM, ATR, and DNA-PKc act via its downstream targets to induce cell cycle arrest and promote DNA repair. All protein kinase inhibitors drugs act as anticancer agents by blocking tyrosine kinase, EGFR, VEGFR, ATM, ATR, etc., thereby inhibiting cell proliferation and DNA repair. Apoptosis inducers promote apoptosis by a number of methods, including DNA cross-linking and antiapoptotic protein inhibition (Bcl-2, Mcl-1, Bcl-x) and activation of caspases (P53). Apoptosis inducers enhance apoptotic cell death by acting on numerous apoptosis-related proteins. Tubulin inhibitors act by destabilizing microtubule assembly by binding to tubulin binding sites during spindle formation thereby inhibiting cell proliferation and growth. Inhibiting aromatase enzymes and epigenetic regulators (HDAC and EZH2) are other mechanism by which indole derivatives are acting as anticancer agents.
Comparable to many other human diseases, cancer today has plethora of potential molecular targets for treatment research. Traditional drug development which mostly based on the “one molecule, one target, one protein” concept sometimes ignores drug-protein interactions. Many complicated diseases are linked to a multitude of target proteins which often overlooked during drug development. Furthermore, because of the poly-pharmacological features of indole nucleus derived from off-targets (drug binds with other enzymes ion channels and/or receptors than those that it is meant to) resulted in an unpredictable and unavoidable activity that may result in unwanted side-effects. The various off-target challenges of indole anticancer drugs under advanced stage of development have been discussed (Supporting Information).
5. Conclusions
Currently pharmaceutical industries are spending 30% of their total expenditure on R & D on developing kinase inhibitors especially for anticancer research. The 75 drugs have already been approved by FDA and 100 kinase inhibitors are in the late stage of development and likely to be approved. Only 10% of kinases have been investigated comprehensively. That mean, there is lot of scope in the area of kinase inhibitors. Moreover, aromatase inhibitors have been proved to provide the effective anticancer agents. These aromatase inhibitors seem likely to soon become the standard adjuvant therapy, either alone or in sequence with other agents. The identification of novel human topoisomerase inhibitors was prompted by the discovery of several new bacterial topoisomerase inhibitors and regulatory proteins. Hence, topoisomerase remain as important enzymatic targets of anticancer agents. The research in the field of apoptosis has extended to incorporate research in about all areas of cell biology from cancer to cardiovascular diseases. Understanding of the molecular mechanism of apoptosis has gained rising curiosity in anticancer drug discovery. Indole moiety has a unique pharmacophore which help it to bind with various receptors and enzymes and also has good bioavailability and distinctive chemical properties. These properties make indole-based compounds as a powerful probe for medicinal chemists working in the area of drug research127. It is also discussed that indole moiety has the capability to enhance the potency, overcoming drug-resistance, reducing side-effects of the overall molecule. Considerable researches in this area are evidenced by the facts that numbers of indole-based molecules are approved recently or are in pre-clinical and clinical stages. But still new research is required to get better understanding about the origin of cancer and drug resistance in cancer patients along with advancement in multi-drug therapy by indole-based molecules can be expected in the near future.
Acknowledgments
The author expresses gratitude to the Managing Committee of Shivalik College of Pharmacy for their constant encouragement and support.
Author contributions
Ashima Dhiman collected the data and wrote the initial draft of the manuscript. Rupam Sharma revised and wrote the final manuscript. Rajesh K. Singh designed the study, checked and reviewed the manuscript. All of the authors have read and approved the final draft.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2022.03.021.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ali I., Lone M.N., Al-Othman Z.A., Al-Warthan A., Sanagi M.M. Heterocyclic scaffolds: centrality in anticancer drug development. Curr Drug Targets. 2015;16:711–734. doi: 10.2174/1389450116666150309115922. [DOI] [PubMed] [Google Scholar]
- 2.Gholap S.S. Pyrrole: an emerging scaffold for construction of valuable therapeutic agents. Eur J Med Chem. 2016;110:13–31. doi: 10.1016/j.ejmech.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad S., Alam O., Naim M.J., Shaquiquzzaman M., MumtajAlam M., Iqbal M. Pyrrole: an insight into recent pharmacological advances with structure activity relationship. Eur J Med Chem. 2018;157:527–561. doi: 10.1016/j.ejmech.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Tantawy M.A., Nafie M.S., Elmegeed G.A., Ali I.A.I. Auspicious role of the steroidal heterocyclic derivatives as a platform for anti-cancer drugs. Bioorg Chem. 2017;73:128–146. doi: 10.1016/j.bioorg.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Sharma V., Kumar P., Pathak D. Biological importance of the indole nucleus in recent years: a comprehensive review. J Heterocycl Chem. 2010;47:491–502. [Google Scholar]
- 6.Vikatu Smith D.T., Njardarson J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals: miniperspective. J Med Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 7.Patil S.A., Patil R. Medicinal applications of (benz)imidazole- and indole-based macrocycles. Chem Biol Drug Des. 2017;89:639–649. doi: 10.1111/cbdd.12802. [DOI] [PubMed] [Google Scholar]
- 8.Kumar D., Kumar N.M., Tantak M.P., Ogura M., Kusaka E., Ito T. Synthesis and identification of ⍺-cyano bis(indolyl)chalcones as novel anticancer agents. Bioorg Med Chem Lett. 2014;24:5170–5174. doi: 10.1016/j.bmcl.2014.09.085. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y., Duan K., Zhu L., Liu Z., Zhang C., Yang L., et al. Synthesis and cytotoxic activity of novel hexahydropyrrolo[2,3-b]indole imidazolium salts. Bioorg Med Chem Lett. 2016;26:460–465. doi: 10.1016/j.bmcl.2015.11.092. [DOI] [PubMed] [Google Scholar]
- 10.Fortes M.P., da Silva P.B.N., da Silva T.G., Kaufman T.S., Militão G.C.G., Silveira C.C. Synthesis and preliminary evaluation of 3-thiocyanato-1H-indoles as potential anticancer agents. Eur J Med Chem. 2016;118:21–26. doi: 10.1016/j.ejmech.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 11.Baeyer A. Ueber die reduction aromatischer verbindungen mittelst zinkstaub [On the reduction of aromatic compounds by means of zinc dust]. Annalen der Chemie und Pharmacie. Eur J Org Chem. 1866;140:295–296. [Google Scholar]
- 12.Chaisan N., Kaewsri W., Thongsornkleeba C., Tummatorna J., Ruchirawat S. PtCl4-catalyzed cyclization of N-acetyl-2-alkynylanilines: a mild and efficient synthesis of N-acetyl-2-substituted indoles. Tetrahedron Lett. 2018;59:675–680. [Google Scholar]
- 13.Andries-Ulmer A., Brunner C., Rehbein J., Gulder T. Fluorine as a traceless directing group for the regio divergent synthesis of indoles and tryptophans. J Am Chem Soc. 2018;140:13034–13041. doi: 10.1021/jacs.8b08350. [DOI] [PubMed] [Google Scholar]
- 14.Ning X.S., Wang M.M., Qu J.P., Kang Y.B. Synthesis of functionalized indoles via palladium-catalyzed aero biccyclo isomerization of o-allylanilines using organic redox cocatalyst. J Org Chem. 2018;21:13523–13529. doi: 10.1021/acs.joc.8b01999. [DOI] [PubMed] [Google Scholar]
- 15.Xia H.D., Zhang Y.D., Wang Y.H., Zhang C. Water soluble hypervalent Iodine having I‒N bond. A reagent for the synthesis of Indoles. Org Lett. 2018;20:4052–4056. doi: 10.1021/acs.orglett.8b01615. [DOI] [PubMed] [Google Scholar]
- 16.Bugaenko D.I., Dubrovina A.A., Yurovskaya M.A., Karchava A.V. Synthesis of indoles via electron-catalyzed intramolecular C–N bond formation. Org Lett. 2018;20:7358–7362. doi: 10.1021/acs.orglett.8b02784. [DOI] [PubMed] [Google Scholar]
- 17.Ni J., Jiang Y., An Z., Yan R. Cleavage of C–C bonds for the synthesis of C-2 substituted quinolines and indoles by catalyst-controlled tandem annulation of 2-vinylanilines and alkynoates. Org Lett. 2018;20:1534–1537. doi: 10.1021/acs.orglett.8b00260. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Peng J., Chen X., Mo B., Li X., Sun P., Chen C. Copper-catalyzed synthesis of multi substituted indoles through tandem Ullman-type C‒N formation and cross-dehydrogenative coupling reactions. J Org Chem. 2018;83:5288–5294. doi: 10.1021/acs.joc.8b00353. [DOI] [PubMed] [Google Scholar]
- 19.Rode N.D., Abdalghani I., Arcadi A., Aschi M., Chiarini M., Marinelli F. Synthesis of 2-acylindoles via Ag- and Cu-catalyzed anti-Michael hydroamination of β-(2-aminophenyl)-α,β-ynones: experimental results and DFT calculations. J Org Chem. 2018;83:6354–6362. doi: 10.1021/acs.joc.8b00508. [DOI] [PubMed] [Google Scholar]
- 20.Chen D., Chen Y., Ma Z., Zou L., Li J., Liu Y. One-pot synthesis of indole-3-acetic acid derivatives through the cascade Tsuji–Trost reaction and Heck coupling. J Org Chem. 2018;83:6805–6814. doi: 10.1021/acs.joc.8b01056. [DOI] [PubMed] [Google Scholar]
- 21.Ye Y., Cheung K.P.S., He L., Tsui G.C. Synthesis of 2-(trifluoromethyl)indoles via domino trifluoromethylation/cyclization of 2-alkynylanilines. Org Lett. 2018;20:1676–1679. doi: 10.1021/acs.orglett.8b00509. [DOI] [PubMed] [Google Scholar]
- 22.Tanriver G., Dagoneau D., Karadeniz U., Kolleth A., Lumbroso A., Sulzer-Mossé S., et al. Keteniminium salts: reactivity and propensity toward electrocyclization reactions. J Org Chem. 2020;85:449–463. doi: 10.1021/acs.joc.9b02466. [DOI] [PubMed] [Google Scholar]
- 23.Kumari A., Singh R.K. Morpholine as ubiquitous pharmacophore in medicinal chemistry: deep insight into the structure‒activity relationship (SAR) Bioorg Chem. 2020;96:103578. doi: 10.1016/j.bioorg.2020.103578. [DOI] [PubMed] [Google Scholar]
- 24.Harding M.C., Sloan C.D., Merril R.M., Harding T.M., Thacker B.J., Thacker E.L. Transitions from heart disease to cancer as the leading cause of death in US states, 1999–2016. Prev Chronic Dis. 2018;133:AMP67. doi: 10.5888/pcd15.180151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rashid H., Xu Y., Muhammad Y., Wang L., Jiang J. Research advances on anticancer activities of marine and its derivatives: an updated overview. Eur J Med Chem. 2019;161:205–238. doi: 10.1016/j.ejmech.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Singh R.K., Kumar S., Prasad D.N., Bhardwaj T.R. Therapeutic journey of nitrogen mustard as alkylating anticancer agents: historic to future perspectives. Eur J Med Chem. 2018;151:401–433. doi: 10.1016/j.ejmech.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Rosales P.F., Marinbo F.F., Gower A., Chiarellp M., Canci B., Roesch-Ely M., et al. Bio-guided search of active indole alkaloids from Tabernaemontana catharinensis: antitumour activity, toxicity in-silico and molecular modelling studies. Bioorg Chem. 2019;85:66–74. doi: 10.1016/j.bioorg.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Pajaniradje S., Mohan kumar K., Radhakrishanan R., Sufi S.A., Subramaniam S., Anaikutti P., et al. Indole curcumin reverses multidrug resistance by reducing the expression of ABCD1 and COX2 in induced multidrug resistant human lung cancer cells. Lett Drug Des Discov. 2020;17:1146–1154. [Google Scholar]
- 29.Wan Y., Li Y., Yan C., Yan M., Tang Z. Indole: a privileged scaffold for the design of anti-cancer agents. Eur J Med Chem. 2019;183:111691. doi: 10.1016/j.ejmech.2019.111691. [DOI] [PubMed] [Google Scholar]
- 30.Han Y., Dong W., Guo Q., Li X., Huang L. The importance of indole and azaindole scaffold in the development of anti-tumour agents. Eur J Med Chem. 2020;203:112506. doi: 10.1016/j.ejmech.2020.112506. [DOI] [PubMed] [Google Scholar]
- 31.Lu H., Zhu G., Tang T., Ma Z., Chen Q., Chen Z. Anticancer molecule discovery via C2-substituent promoted oxidative coupling of indole and enolate. iScience. 2019;22:214–228. doi: 10.1016/j.isci.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prinz H. Recent advances in the field of tubulin polymerization inhibitors. Expert Rev Anticancer Ther. 2014;2:695–708. doi: 10.1586/14737140.2.6.695. [DOI] [PubMed] [Google Scholar]
- 33.Patil A., Patil S.A., Beaman K.D., Patil S.A. Indole molecules as inhibitors of tubulin polymerization: potential new anticancer agents, an update (2013‒2015) Future Med Chem. 2016;8:1291–1316. doi: 10.4155/fmc-2016-0047. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Perez M.J., Priego E.M., Bueno O., Martins M.S., Canela M.D., Liekens S. Blocking blood flow to solid tumours by destabilizing tubulin: an approach to targeting tumour growth. J Med Chem. 2016;59:8685–8711. doi: 10.1021/acs.jmedchem.6b00463. [DOI] [PubMed] [Google Scholar]
- 35.Greene L.M., Meegan M.J., Zisterer D.M. Combretastatin: more than just vascular targeting agents. J Pharmacol Exp Therapeut. 2015;355:212–227. doi: 10.1124/jpet.115.226225. [DOI] [PubMed] [Google Scholar]
- 36.Pettit G.R., Rhodes M.R., Herald D.L., Hamel E., Schmidt J.M., Pettit R.K. Antineoplastic agents 445 synthesis and evaluation of structural modification of (Z) and (E) combretastatin A-4. J Med Chem. 2005;48:4087–4099. doi: 10.1021/jm0205797. [DOI] [PubMed] [Google Scholar]
- 37.Gaspari R., Prota A.E., Bargsten K., Cavalli A., Steinmetz M.O. Structural basis of cis and trans combretastatin binding to tubulin. Inside Chem. 2017;2:102–113. [Google Scholar]
- 38.Le Broc-Ryckewaert D., Pommery N., Pommery J., Ghinet A., Frace A., Wiart J.F., et al. In-vitro metabolism of phenastatin: potential pharmacological consequences. Drug Metabol Lett. 2011;5:209–215. doi: 10.2174/187231211796904973. [DOI] [PubMed] [Google Scholar]
- 39.Messaoudi S., Treguier B., Hamez A., Provot O., Peyrat J.F., De Losada J.R., et al. Isocombretastatin A versus combretastatin A: the forgotten isoCA-4 isomer as a highly promising cytotoxic and antitubulin agent. J Med Chem. 2009;52:4538–4542. doi: 10.1021/jm900321u. [DOI] [PubMed] [Google Scholar]
- 40.Seddigi Z.S., Malik M.S., Saraswati A.P., Ahmed S.A., Babalghith A.O., Lamfon H.A., et al. Recent advances in combretastatin based derivatives and prodrugs as anti-mitotic agents. Med Chem Comm. 2017;8:1592–1603. doi: 10.1039/c7md00227k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarez R., Gajate C., Pubela P., Mollinedo F., Medarde M., Pelaeza R. Substitution at the indole 3 position yields highly potent indolecombretastatins against human tumor cells. Eur J Med Chem. 2018;158:167e183. doi: 10.1016/j.ejmech.2018.08.078. [DOI] [PubMed] [Google Scholar]
- 42.Romagnoli R., Prencipe F., Oliva P., Salvador M.K., Brancale A., Ferla S., et al. Design, synthesis and biological evaluation of 2-alkoxycarbonyl-3-anilinoindoles as a new class of potent inhibitors of tubulin polymerization. Bioorg Chem. 2020;97 doi: 10.1016/j.bioorg.2020.103665. [DOI] [PubMed] [Google Scholar]
- 43.Li W., Sun H., Xu F., Shuai W., Liu J., Xu S., et al. Synthesis, molecular properties prediction and biological evaluation of indole vinyl sulfone derivatives as novel tubulin polymerization inhibitors targeting the colchicine binding site. Bioorg Chem. 2019;85:49–59. doi: 10.1016/j.bioorg.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 44.Ducki S., Rennison D., Woo M., Kendall A., Chabert J.F., McGown A.T., Lawrence N.J. Combretastatin-like chalcones as inhibitors of microtubule polymerization. Part 1: synthesis and biological evaluation of antivascular activity. Bioorg Med Chem. 2009;17:7698–7710. doi: 10.1016/j.bmc.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 45.Cong H., Zhao X., Castle B.T., Pomeroy E.J., Zhou B., Lee J., et al. An indole–chalcone inhibits multidrug-resistant cancer cell growth by targeting microtubules. Mol Pharm. 2018;15:3892–3900. doi: 10.1021/acs.molpharmaceut.8b00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badria F.F., Soliman S.M., Atef S., Islam M.S., Al-Majid A.M., Dege N., et al. Anticancer indole-based chalcones: a structural and theoretical analysis. Molecules. 2019;24:3728. doi: 10.3390/molecules24203728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.La Regina G., Bai R., Coluccia A., Naccarato V., Famiglini V., Nalli M., et al. New 6- and 7-heterocyclyl-1H-indole derivatives as potent tubulin assembly and cancer cell growth inhibitors. Eur J Med Chem. 2018;152:283–297. doi: 10.1016/j.ejmech.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 48.Wang Q., Arnst K.E., Wang Y., Kumar G., Ma D., White S.W., et al. Structure-guided design, synthesis, and biological evaluation of (2-(1H-indol-3-yl)-1H-imidazole-4-yl) (3,4,5-trimethoxyphenyl) methanone (ABI-231) analogues targeting the colchicine binding site in tubulin. J Med Chem. 2019;62:6734–6750. doi: 10.1021/acs.jmedchem.9b00706. [DOI] [PubMed] [Google Scholar]
- 49.Ren Y., Wang Y., Li G., Zhang Z., Ma L., Cheng B., et al. Discovery of novel benzimidazole and indazole analogues as tubulin polymerization inhibitors with potent anticancer activities. J Med Chem. 2021;64:4498–4515. doi: 10.1021/acs.jmedchem.0c01837. [DOI] [PubMed] [Google Scholar]
- 50.Khelifi I., Naret T., Renko D., Hamze A., Bernadat G., Bignon J., et al. Design, synthesis and anticancer properties of isoCombretaQuinolines as potent tubulin assembly inhibitors. Eur J Med Chem. 2017;127:1025–1034. doi: 10.1016/j.ejmech.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 51.Li W., Shuai W., Sun H., Xu F., Bi Y., Xu J., et al. Design, synthesis and biological evaluation of quinoline-indole derivatives as anti-tubulin agents targeting the colchicine binding site. Eur J Med Chem. 2019;163:428–442. doi: 10.1016/j.ejmech.2018.11.070. [DOI] [PubMed] [Google Scholar]
- 52.Akhtar J., Khan A.A., Ali Z., Haider R., Yar M.S. Structure‒activity relationship (SAR) study and design strategies of nitrogen containing heterocyclic moieties for their anticancer activities. Eur J Med Chem. 2017;125:143–189. doi: 10.1016/j.ejmech.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 53.Wang G., Li C., He L., Lei K., Wang F., Pu Y., et al. Design, synthesis and biological evaluation of a series of pyrano chalcone derivatives containing indole moiety as novel anti-tubulin agents. Bioorg Med Chem. 2014;22:2060–2079. doi: 10.1016/j.bmc.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 54.Rungroj S., Chutikarn B., Narittira O., Kriengsak L., Worawat N., Jisnuson S., et al. Identification of new 3-phenyl-1H-indole-2-carbohydrazide derivatives and their structure‒activity relationships as potent tubulin inhibitors and anticancer agents: a combined in silico, in vitro and synthetic study. Bioorg Chem. 2021;110:104795. doi: 10.1016/j.bioorg.2021.104795. [DOI] [PubMed] [Google Scholar]
- 55.Kode J., Kovvuri J., Nagaraju B., Jadhav S., Barkume Madan, Sen S., et al. Synthesis, biological evaluation, and molecular docking analysis of phenstatin based indole linked chalcones as anticancer agents and tubulin polymerization inhibitors. Bioorg Chem. 2021;105:104447. doi: 10.1016/j.bioorg.2020.104447. [DOI] [PubMed] [Google Scholar]
- 56.Lu Y., Chen J., Xiao M., Li W., Miller D.D. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm Res. 2012;29:2943–2971. doi: 10.1007/s11095-012-0828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bzeih T., Naret T., Hachem A., Jaber N., Khalaf A., Bignon J., et al. A general synthesis of arylindoles and (1-arylvinyl) carbazoles via a one-pot reaction from N-tosylhydrazones and 2-nitro-haloarenes and their potential application to colon cancer. Chem Commun (Camb) 2016;52:13027–13030. doi: 10.1039/c6cc07666a. [DOI] [PubMed] [Google Scholar]
- 58.Naret T., Khelifi I., Provot O., Bignon J., Levaique H., Dubois J., et al. 1,1-Diheterocyclic ethylenes derived from quinaldine and carbazole as new tubulin-polymerization inhibitors: synthesis, metabolism, and biological evaluation. J Med Chem. 2019;62:1902–1916. doi: 10.1021/acs.jmedchem.8b01386. [DOI] [PubMed] [Google Scholar]
- 59.Khelifi I., Naret T., Hamze A., Bignon J., Levaique H., Garcia Alvarez M.C., et al. N-bis-heteroaryl methylamines: potent anti-mitotic and highly cytotoxic agents. Eur J Med Chem. 2019;168:176–188. doi: 10.1016/j.ejmech.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 60.Pecnard S., Provot O., Levaique H., Bignon J., Askenatzis L., Saller F., et al. Cyclic bridged analogs of isoCA-4: design, synthesis and biological evaluation. Eur J Med Chem. 2021;209:112873. doi: 10.1016/j.ejmech.2020.112873. [DOI] [PubMed] [Google Scholar]
- 61.Yang F., Jian X.E., Chen L., Ma Y.F., Liu Y.X., You W.W., et al. Discovery of new indole-based 1,2,4-triazole derivatives as potent tubulin polymerization inhibitors with anticancer activity. New J Chem. 2021;45:21869–21880. [Google Scholar]
- 62.Chang J.Y., Hsieh H.P., Chang C.Y., Hsu K.S., Chiang Y.F., Chen C.M., et al. 7-Aroyl-aminoindoline-1-sulfonamides as a novel class of potent antitubulin agents. J Med Chem. 2006;49:6656–6659. doi: 10.1021/jm061076u. [DOI] [PubMed] [Google Scholar]
- 63.Cury N.M., Capitão R.M., Almeida R.D.C.B., Artico L.L., Corrêa J.R., Simão Dos Santos E.F., et al. Synthesis and evaluation of 2-carboxy indole derivatives as potent and selective anti-leukemic agents. Eur J Med Chem. 2019;181:111570. doi: 10.1016/j.ejmech.2019.111570. [DOI] [PubMed] [Google Scholar]
- 64.See C.S., Kitagawa M., Liao P.J., Lee K.H., Wong J., Lee S.H., et al. Discovery of the cancer cell selective dual acting anti-cancer agent (Z)-2-(1H-indol-3-yl)-3-(isoquinolin-5-yl) acrylonitrile (A131) Eur J Med Chem. 2018;156:344–367. doi: 10.1016/j.ejmech.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 65.See C.S., Kitagawa M., Liao P.J., Lee K.H., Wong J., Lee S.H., et al. Prodrugs of the cancer cell selective anti-cancer agent (Z)-2-(1H-indol-3-yl)-3-(isoquinolin-5-yl) acrylonitrile (A131) are orally efficacious in a mouse model of resistant colon cancer. Bioorg Med Chem Lett. 2018;29:216–219. doi: 10.1016/j.bmcl.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 66.Wu X., Wang Q., Li W. Recent advances in heterocyclic tubulin inhibitors targeting the colchicine binding site. Anticancer Agents Med Chem. 2016;16:1325–1338. doi: 10.2174/1871520616666160219161921. [DOI] [PubMed] [Google Scholar]
- 67.Carta D., Ferlin M.G. An overview on 2-arylquinolin-4(1H)-ones and related structures as tubulin polymerisation inhibitors. Curr Top Med Chem. 2014;14:2322–2345. doi: 10.2174/1568026614666141127120421. [DOI] [PubMed] [Google Scholar]
- 68.Diao P.C., Hu M.J., Yang H.K., You W.W., Zhao P.L. Facile one-pot synthesis, anti-proliferative evaluation and structure-activity relationships of 3-amino-1H-indoles and 3-amino-1H-7-azaindoles. Bioorg Chem. 2019;85:102914. doi: 10.1016/j.bioorg.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 69.Diao P.C., Jian X.E., Chen P., Huang C., Yin J., Chun Huang J., et al. Design, synthesis and biological evaluation of novel indole-based oxalamide and aminoacetamide derivatives as tubulin polymerization inhibitors. Bioorg Med Chem Lett. 2019;30:126816. doi: 10.1016/j.bmcl.2019.126816. [DOI] [PubMed] [Google Scholar]
- 70.Sigalapalli D.K., Pooladanda V., Singh P., Kadagathur M., Guggilapu S.D., Uppu J.L., et al. Discovery of certain benzyl/phenethyl thiazolidinone-indole hybrids as potential anti-proliferative agents: synthesis, molecular modelling and tubulin polymerization inhibition study. Bioorg Chem. 2019;92:103188. doi: 10.1016/j.bioorg.2019.103188. [DOI] [PubMed] [Google Scholar]
- 71.Aher S.B., Muskawar P.N., Thenmozhi K., Bhagat P.R. Recent developments of metal N-heterocyclic carbenes as anticancer agents. Eur J Med Chem. 2014;81:408–419. doi: 10.1016/j.ejmech.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 72.Hevener K., Verstak T.A., Lutat K.E., Riggsbee D.L., Mooney J.W. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm Sin B. 2018;8:844–861. doi: 10.1016/j.apsb.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomas A., Pommier Y. Targeting topoisomerase I in the era of precision medicine. Clin Cancer Res. 2019;25:6581–6589. doi: 10.1158/1078-0432.CCR-19-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pommier Y. Drugging topoisomerases: lessons and challenges. ACS Chem Biol. 2013;8:82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Oliveira J.F., Lima T.S., Vendramini-Costa D.B., de Lacerda Pedrosa S.C.B., Lafayette E.A., da Silva R.M.F., et al. Thiosemicarbazones and 4-thiazolidinones indole-based derivatives: synthesis, evaluation of antiproliferative activity, cell death mechanisms and topoisomerase inhibition assay. Eur J Med Chem. 2017;136:305–314. doi: 10.1016/j.ejmech.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 76.Bakherad Z., Safavi M., Fassihi A., Sadeghi-Aliabadi H., Bakherad M., Rastegar H., et al. Design and synthesis of novel cytotoxic indole-thiosemicarbazone derivatives: biological evaluation and docking study. Chem Biodivers. 2019;16 doi: 10.1002/cbdv.201800470. [DOI] [PubMed] [Google Scholar]
- 77.Bakherad Z., Safavi M., Fassih A., Sadeghi-Aliabadi H., Bakherad M., Rastegar H., et al. Anti-cancer, anti-oxidant and molecular docking studies of thiosemicarbazone indole-based derivatives. Res Chem Intermed. 2019;45:2827–2854. [Google Scholar]
- 78.Li A.L., Hao Y., Wang W.Y., Liu Q.S., Sun Y., Gu W. Design, synthesis, and anticancer evaluation of novel indole derivatives of ursolic acid as potential topoisomerase II inhibitors. Int J Mol Sci. 2020;21:2876. doi: 10.3390/ijms21082876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zidar N., Secci D., Tomašič T., Mašič L.P., Kikelj D., Passarella D., et al. Synthesis, antiproliferative effect, and topoisomerase II inhibitory activity of 3-methyl-2-phenyl-1H-indoles. ACS Med Chem Lett. 2020;11:691–697. doi: 10.1021/acsmedchemlett.9b00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tok F., İrem Abas B., Çevik Ö., Koçyiğit-Kaymakçıoğlu B. Design, synthesis and biological evaluation of some new 2-pyrazoline derivatives as potential anticancer agents. Bioorg Chem. 2020;102:104063. doi: 10.1016/j.bioorg.2020.104063. [DOI] [PubMed] [Google Scholar]
- 81.Shu B., Yu Q., Hu D.X., Che T., Zhang S.S., Li D. Synthesis and biological evaluation of novel indole-pyrazoline hybrid derivatives as potential topoisomerase 1 inhibitors. Bioorg Med Chem Lett. 2020;30:126925. doi: 10.1016/j.bmcl.2019.126925. [DOI] [PubMed] [Google Scholar]
- 82.Song Y.L., Dong Y.F., Yang T., Zhang C.C., Su L.M., Huang X., et al. Synthesis and pharmacological evaluation of novel bisin-dolylalkanes analogues. Bioorg Med Chem. 2013;21:7624–7627. doi: 10.1016/j.bmc.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 83.Song Y., Feng S., Feng J., Dong J., Yang K., Liu Z., et al. Synthesis and biological evaluation of novel pyrazoline derivatives containing indole skeleton as anti-cancer agents targeting topoisomerase II. Eur J Med Chem. 2020;200:112459. doi: 10.1016/j.ejmech.2020.112459. [DOI] [PubMed] [Google Scholar]
- 84.Kaur M., Mehta V., Abdullah Wani A., Arora S., Bharatam P.V., Sharon A., et al. Synthesis of 1,4-dihydropyrazolo[4,3-b]indoles via intramolecular C(sp2)‒N bond formation involving nitrene insertion, DFT study and their anticancer assessment. Bioorg Chem. 2021;114:105114. doi: 10.1016/j.bioorg.2021.105114. [DOI] [PubMed] [Google Scholar]
- 85.Jun K.Y., Park S.E., Liang J.L., Jahng Y., Kwon Y. Benzo[b]tryptanthrin inhibits MDR1, topoisomerase activity, and reverses adriamycin resistance in breast cancer cells. ChemMedChem. 2015;10:827–835. doi: 10.1002/cmdc.201500068. [DOI] [PubMed] [Google Scholar]
- 86.Catanzaro E., Betari N., Arencibia J.M., Montanari S., Sissi C., De Simone A., et al. Targeting topoisomerase II with trypthantrin derivatives: discovery of 7-((2-(dimethylamino) ethyl) amino) indolo[2,1-b] quinazoline-6,12-dione as an antiproliferative agent and to treat cancer. Eur J Med Chem. 2020;202:112504. doi: 10.1016/j.ejmech.2020.112504. [DOI] [PubMed] [Google Scholar]
- 87.Wang C., Engelke L., Bickel D., Hamacher A., Frank M., Proksch P., et al. The tetrahydroxanthone-dimer phomoxanthone A is a strong inducer of apoptosis in cisplatin-resistant solid cancer cells. Bioorg Med Chem. 2019;27:115044. doi: 10.1016/j.bmc.2019.115044. [DOI] [PubMed] [Google Scholar]
- 88.Singh R., Letai A., Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20:175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Binju M., Amaya-Padilla M.A., Wan G., Gunosewoyo H., SuryoRahmanto Y., Yu Y. Therapeutic inducers of apoptosis in ovarian cancer. Cancers (Basel) 2019;11:1786. doi: 10.3390/cancers11111786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pandeya S.N., Smitha S., Jyoti M., Sridhar S.K. Iological activities of isatin and its derivatives. Acta Pharm. 2005;55:27–46. [PubMed] [Google Scholar]
- 91.Ammar Y.A., Sh El-Sharief A.M., Belal A., Abbas S.Y., Mohamed Y.A., Mehany A.B.M., et al. Design, synthesis, antiproliferative activity, molecular docking and cell cycle analysis of some novel (morpholinosulfonyl) isatins with potential EGFR inhibitory activity. Eur J Med Chem. 2018;156:918–932. doi: 10.1016/j.ejmech.2018.06.061. [DOI] [PubMed] [Google Scholar]
- 92.Arshad F., Khan M.F., Akhtar W., Alam M.M., Nainwal L.M., Kaushik S.K., et al. Revealing quinquennial anticancer journey of morpholine: a SAR based review. Eur J Med Chem. 2019;167:324–356. doi: 10.1016/j.ejmech.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 93.El-Sharief A.M.S., Ammar Y.A., Belal A., El-Sharief M.A.M.S., Mohamed Y.A., Mehany A.B.M., et al. Design, synthesis, molecular docking and biological activity evaluation of some novel indole derivatives as potent anticancer active agents and apoptosis inducers. Bioorg Chem. 2019;85:399–412. doi: 10.1016/j.bioorg.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 94.Ye L., Tian Y., Li Z., Zhang J., Wu S. Design and synthesis of some novel 2,3,4,5-tetra hydro-1H-pyrido[4,3-b]indoles as potential c-Met inhibitors. Helv Chim Acta. 2012;95:320–326. [Google Scholar]
- 95.Yu Feng, Teng X., Gu J., Yu B., Luo Y., Ye L. Novel anti-cancer agents: design, synthesis, biological activity, molecular docking, and MD simulations of 2,3,4,5-tetrahydro-1H-pyrido-[4,3-b]indole derivatives. Med Chem Res. 2019;28:133–142. [Google Scholar]
- 96.Kumar M.S., Yadav T.T., Khair R.R., Peters G.J., Yergeri M.C. Combination therapies of Artemisinin and its derivatives as viable approach for future cancer treatment. Curr Pharmaceut Des. 2019;25:3323–3338. doi: 10.2174/1381612825666190902155957. [DOI] [PubMed] [Google Scholar]
- 97.La Pensée L., Sabbani S., Sharma R., Bhamra I., Shore E., Chadwick A.E., et al. Artemisinin-polypyrrole conjugates: synthesis, DNA binding studies and preliminary antiproliferative evaluation. ChemMedChem. 2013;8:709–718. doi: 10.1002/cmdc.201200536. [DOI] [PubMed] [Google Scholar]
- 98.Wong Y.K., Xu C., Kalesh K.A., He Y., Lin Q., Wong W.S.F., et al. Artemisinin as an anticancer drug: recent advances in target profiling and mechanisms of action. Med Res Rev. 2017;37:1492–1517. doi: 10.1002/med.21446. [DOI] [PubMed] [Google Scholar]
- 99.Hu Y., Li N., Zhang J., Wang Y., Chen L., Sun J. Artemisinin-indole and artemisinin-imidazole hybrids: synthesis, cytotoxic evaluation and reversal effects on multidrug resistance in MCF-7/ADR cells. Bioorg Med Chem Lett. 2019;29:1138–1142. doi: 10.1016/j.bmcl.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 100.Eldehna W.M., Abo-Ashour M.F., Ibrahim H.S., Al-Ansary G.H., Ghabbour H.A., Elaasser M.M., et al. Novel [(3-indolylmethylene)hydrazono]indolin-2-ones as apoptotic anti-proliferative agents: design, synthesis and in vitro biological evaluation. J Enzym Inhib Med Chem. 2018;33:686–700. doi: 10.1080/14756366.2017.1421181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tantak M.P., Klingler L., Arun V., Kumar A., Sadana R., Kumar D. Design and synthesis of bis(indolyl)ketohydrazide-hydrazones: identification of potent and selective novel tubulin inhibitors. Eur J Med Chem. 2017;136:184–194. doi: 10.1016/j.ejmech.2017.04.078. [DOI] [PubMed] [Google Scholar]
- 102.Baldisserotto A., Demurtas M., Lampronti I., Moi D., Balboni G., Vertuani S., et al. Benzofuran hydrazones as potential scaffold in the development of multifunctional drugs: synthesis and evaluation of antioxidant, photoprotective and antiproliferative activity. Eur J Med Chem. 2018;156:118–125. doi: 10.1016/j.ejmech.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 103.Demurtas M., Baldisserotto A., Lampronti I., Moi D., Balboni G., Pacifico S., et al. Indole derivatives as multifunctional drugs: synthesis and evaluation of antioxidant, photoprotective and antiproliferative activity of indole hydrazones. Bioorg Chem. 2019;85:568–576. doi: 10.1016/j.bioorg.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 104.Saha T., Makar S., Swetha R., Gutti G., Singh S.K. Estrogensignaling: an emanating therapeutic target for breast cancer treatment. Eur J Med Chem. 2019;177:116–143. doi: 10.1016/j.ejmech.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 105.Ozcan-Sezer S., Ince E., Akdemir A., Ceylan Ö.Ö., Suzen S., Gurer-Orhan H. Aromatase inhibition by 2-methyl indole hydrazone derivatives evaluated via molecular docking and in vitro activity studies. Xenobiotica. 2019;49:549–556. doi: 10.1080/00498254.2018.1482029. [DOI] [PubMed] [Google Scholar]
- 106.Fantacuzzi M., De Filippis B., Gallorini M., Ammazzalorso A., Giampietro L., Maccallini C., et al. Synthesis, biological evaluation, and docking study of indole aryl sulfonamides as aromatase inhibitors. Eur J Med Chem. 2020;185:111815. doi: 10.1016/j.ejmech.2019.111815. [DOI] [PubMed] [Google Scholar]
- 107.Bhatia R., Singh R.K. In: Protein kinases promising targets for anticancer drug research. Singh R.K., editor. IntechOpen; London: 2021. Introductory Chapter: protein kinases as promising targets for drug design against cancer; pp. 1–14. [Google Scholar]
- 108.Al-Warhi T., El Kerdawy A.M., Aljaeed N., Ismael O.E., Ayyad R.R., Eldehna W.M., et al. Synthesis, biological evaluation and in silico studies of certain oxindole-indole conjugates as anticancer CDK inhibitors. Molecules. 2020;25:2031. doi: 10.3390/molecules25092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang C., Qie Y., Yang T., Wang L., Du E., Liu Y., et al. Kinase PIM1 promotes prostate cancer cell growth via c-Myc-RPS7-driven ribosomal stress. Carcinogenesis. 2019;40:52–60. doi: 10.1093/carcin/bgy126. [DOI] [PubMed] [Google Scholar]
- 110.Bahuguna A., Singh A., Kumar P., Dhasmana D., Krishnan V., Garg N. Bisindolemethane derivatives as highly potent anticancer agents: synthesis, medicinal activity evaluation, cell-based compound discovery, and computational target predictions. Comput Biol Med. 2020;116:103574. doi: 10.1016/j.compbiomed.2019.103574. [DOI] [PubMed] [Google Scholar]
- 111.Amemiya S., Yamaguchi T., Hashimoto Y., Noguchi-Yachide T. Synthesis and evaluation of novel dual BRD4/HDAC inhibitors. Bioorg Med Chem. 2017;25:3677–3684. doi: 10.1016/j.bmc.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 112.Cheng G., Wang Z., Yang J., Bao Y., Xu Q., Zhao L., et al. Design, synthesis and biological evaluation of novel indole derivatives as potential HDAC/BRD4 dual inhibitors and anti-leukemia agents. Bioorg Chem. 2019;84:410–417. doi: 10.1016/j.bioorg.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 113.Ayati A., Moghimi S., Salarinejad S., Safavi M., Pouramiri B., Foroumadi A. A review on progression of epidermal growth factor receptor (EGFR) inhibitors as an efficient approach in cancer targeted therapy. Bioorg Chem. 2020;99:103811. doi: 10.1016/j.bioorg.2020.103811. [DOI] [PubMed] [Google Scholar]
- 114.Mphahele M.J., Mmonwa M.M., Aro A., McGaw L.J., Choong Y.S. Synthesis, biological evaluation and molecular docking of novel indole-amnoquinazoline hybrids for anticancer properties. Int J Mol Sci. 2018;19:2232. doi: 10.3390/ijms19082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Swamy P.V., Kumar V.K., Raju R.R., Reddy R.V., Chatterjee A., Kiran G., et al. Amide derivatives of 4-azaindole: design, synthesis, and EGFR targeting anticancer agents. Synth Commun. 2020;50:71–84. [Google Scholar]
- 116.Zhang Y., Zhang M., Wang Y., Fan Y., Chen X., Yang Y., et al. Protein‒ligand interaction-guided discovery of novel VEGFR-2 inhibitors. J Biomol Struct Dyn. 2020;38:2559–2574. doi: 10.1080/07391102.2019.1635915. [DOI] [PubMed] [Google Scholar]
- 117.Al-Hussain S.A., Farghaly T.A., Zaki M.E.A., Abdulwahab H.G., Al-Qurashi N.T., Muhammad Z.A. Discovery of novel indolyl-1,2,4-triazole hybrids as potent vascular endothelial growth factor receptor-2 (VEGFR-2) inhibitors with potential anti-renal cancer activity. Bioorg Chem. 2020;105:104330. doi: 10.1016/j.bioorg.2020.104330. [DOI] [PubMed] [Google Scholar]
- 118.Kamel M.M., Abdel-Hameid M.K., El-Nassan H.B., El-Khouly E.A. Synthesis and cytotoxicity evaluation of novel indole derivatives as potential anti-cancer agents. Med Chem. 2019;15:873–882. doi: 10.2174/1573406415666190408125514. [DOI] [PubMed] [Google Scholar]
- 119.Badawy A.A. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. 2017;15 doi: 10.1177/1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cui G., Lai F., Wang X., Chen X., Xu B. Design, synthesis and biological evaluation of indole-2-carboxylic acid derivatives as Ido1/TDO dual inhibitors. Eur J Med Chem. 2020;188:111985. doi: 10.1016/j.ejmech.2019.111985. [DOI] [PubMed] [Google Scholar]
- 121.de Sá Alves F.R., Barreiro E.J., Fraga C.A. From nature to drug discovery: the indole scaffold as a ‘privileged structure’. Mini Rev Med Chem. 2009;9:782–793. doi: 10.2174/138955709788452649. [DOI] [PubMed] [Google Scholar]
- 122.Romagnoli R., Baraldi P.G., Prencipe F., Balzarini J., Liekens S., Estévez F. Design, synthesis and antiproliferative activity of novel heterobivalent hybrids based on imidazo[2,1-b][1,3,4]thiadiazole and imidazo[2,1-b][1,3]thiazole scaffolds. Eur J Med Chem. 2015;101:205–217. doi: 10.1016/j.ejmech.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 123.Li Petri G., Cascioferro S., El Hassouni B., Carbone D., Parrino B., Cirrincione G., et al. Biological evaluation of the antiproliferative and anti-migratory activity of a series of 3-(6-phenylimidazo[2,1-b][1,3,4]thiadiazol-2-yl)-1H-indole derivatives against pancreatic cancer cells. Anticancer Res. 2019;39:3615–3620. doi: 10.21873/anticanres.13509. [DOI] [PubMed] [Google Scholar]
- 124.Cascioferro S., Petri G.L., Parrino B., Carbone D., Funel N., Bergonzini C., et al. Imidazo[2,1-b][1,3,4]thiadiazoles with antiproliferative activity against primary and gemcitabine-resistant pancreatic cancer cells. Eur J Med Chem. 2020;189:112088. doi: 10.1016/j.ejmech.2020.112088. [DOI] [PubMed] [Google Scholar]
- 125.Al-Wabli R.I., Almomen A.A., Almutairi M.S., Keeton A.B., Piazza G.A., Attia M.I. New isatin‒indole conjugates: synthesis, characterization, and a plausible mechanism of their in vitro antiproliferative activity. Drug Des Dev Ther. 2020;14:483–495. doi: 10.2147/DDDT.S227862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karadayi F.Z., Yaman M., Kisla M.M., Keskus A.G., Konu O., Ates-Alagoz Z. Design, synthesis and anticancer/antiestrogenic activities of novel indole-benzimidazoles. Bioorg Chem. 2020;100:103929. doi: 10.1016/j.bioorg.2020.103929. [DOI] [PubMed] [Google Scholar]
- 127.Kumari A., Singh R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorg Chem. 2019;89 doi: 10.1016/j.bioorg.2019.103021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.