Abstract
Background
MRI is invaluable for the pre-mortem diagnosis of sporadic Creutzfeldt-Jakob disease (sCJD), demonstrating characteristic diffusion abnormalities. Previous work showed these changes were often not reported (low sensitivity), leading to eventual diagnosis at a more advanced state. Here, we reviewed the situation a decade later, on the presumption of improved access and awareness over time.
Methods
We reviewed initial MRI scans of 102 consecutive suspected sCJD patients recruited to the National Prion Monitoring Cohort study between 2015 and 2019, assessing for characteristic signal changes in the striatum, thalamus and cortical ribbon. We compared our findings to formal reports from referring centres. Requesting indications were studied to assess if they were suggestive of CJD. Patients were examined and their MRC Prion Disease Rating Scale scores recorded.
Results
We identified characteristic MRI abnormalities in 101 cases (99% sensitivity), whilst referring centres reported changes in 70 cases (69% sensitivity), which was a significant improvement in reporting sensitivity from 2012. Reporting sensitivity was associated with signal change in the cerebral cortex, and with the number of regions involved, but not significantly affected by clinical information on request forms, or referring centres being regional neuroscience/non-neuroscience centres. Similar to a previous study, patients with missed abnormalities on initial reporting possessed lower MRC Scale scores when referred to the NPC than those correctly identified.
Conclusions
Whilst local MRI reporting of sCJD has improved with time, characteristic abnormalities remain significantly under detected on initial scans. Sensitivity is better when the cerebral cortex and multiple regions are involved. We re-emphasize the utility of MRI and encourage further efforts to improve awareness and sensitivity in the assessment of patients with rapidly progressive dementia.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-022-11087-x.
Keywords: Creutzfeldt-Jakob disease, CJD, MRI, Prion
Introduction
Creutzfeldt-Jakob disease (CJD) is a rapidly progressive and universally fatal neurodegenerative disease, caused by the propagation of assemblies of misfolded prion protein [1]. Sporadic CJD (sCJD) remains the most common human prion disease, contributing 85% of CJD patients. Although a definitive diagnosis of sCJD relies on histopathological confirmation, clinical assessment and investigations can make near certain pre-mortem diagnoses [2, 3]. Of the investigations included in the current diagnostic criteria, diffusion-weighted MRI (DWI) is considered an essential investigation given its ready availability, short acquisition time, and high sensitivity (up to 98%) and specificity (nearly 100%) [4–8]. Characteristic features of sCJD on DWI and fluid attenuated inversion recovery (FLAIR) sequences include high intensity signal changes in cortical, striatal and thalamic regions [9, 10].
Early identification of sCJD offers opportunities for improved care, avoidance of unnecessary tests and empirical treatments, planning for end of life, and inclusion into clinical trials [6]. PRN100, a monoclonal antibody treatment recently offered to a small number of patients with sCJD, exemplifies one such innovative opportunity which relies on early patient diagnoses. Other experimental compounds are also being developed [11].
Unfortunately, diagnostic changes of sCJD on MRI are often missed, as evidenced by our paper published in 2012, where we found an overall 47% sensitivity on initial radiology reporting [12]. Subsequent efforts by the National Prion Clinic (NPC) and others to improve awareness of CJD-specific MRI abnormalities has included delivery of lectures to hospital specialists and presentation of our findings at national conferences. The inclusion of DWI to dementia protocols has also become well established. We aimed to re-explore whether there has been any change in the sensitivity of detecting sCJD on MRI, hypothesising that increased awareness amongst clinicians, improved access to MRI and scan protocols, and improved scan quality since our previous study, might have improved rates of diagnosis.
Materials and methods
Patients
We retrospectively studied MRI scans from patients referred to the NPC with suspected sCJD between 2/4/2015 and 5/12/2019 who were enrolled into the National Prion Monitoring Cohort study [13]. This study was approved by the Scotland A Research Ethics Committee. Informed consent was obtained from participants or, when appropriate, relatives and carers. Patients who did not fulfil the clinical diagnostic criteria for sCJD or did not possess MRI imaging and reports that were transferred and archived at University College London Hospital were excluded [2].
MRI evaluation and reporting
The earliest MRI scan of each patient was assessed by one of three research team members (one senior neuroradiologist and two clinical research fellows, specifically trained for the purposes of the study) at the National Hospital for Neurology and Neurosurgery (NHNN) and NPC. The research team were aware of a probable sCJD diagnosis. DWI b1000 and FLAIR sequences were assessed for three types of high intensity signal change, which are characteristic of CJD: in the striatum (sufficient even if only one of caudate or putamen were affected), thalamus, or at least two cortical regions (temporal, parietal, occipital), being aware of regions that tend to give non-specific signal changes [3, 14]. It should be emphasised that thalamic signal changes alone are not included in the current sCJD diagnostic criteria. Apparent diffusion coefficient (ADC) maps were available for all patients, which were additionally used for MRI evaluation. Ambiguous findings were reviewed by all three members of the research team and a consensus agreed.
External reports from referring centres were reviewed to assess whether they included CJD as a potential diagnosis. Clinical indications within the request section of radiological reports were also reviewed to determine if they were suggestive of a diagnosis of CJD, searching for key words such as “CJD”, “prion”, “dementia”, and “cognitive decline”. The Medical Research Council Prion Disease Rating Scale (MRC Scale, a functionally-oriented 20-point outcome measure designed for patients with CJD) scores at initial assessment were recorded [13].
Statistical analysis
Comparison of groups were made using McNemar’s test (for paired comparisons), χ2 test, or logistic regression (STATA 16.0). A p-value < 0.05 was deemed significant.
Results
We obtained MRI scans and neuroradiological reports of 106 consecutive patients (56 males, 50 females). DWI sequences were available for 104 of these patients, including four patients who were later diagnosed with inherited prion disease (2 E200K, 2 P102L) following PRNP sequencing, whose clinical picture at the time of referral was indistinguishable from sCJD. Characteristics of the remaining 102 suspected sCJD cases (98% with DWI and 2% solely possessing FLAIR), including ultimate diagnosis, are summarised in Table 1, noting that “probable sCJD” is known to have an extremely high correlation with post-mortem diagnosis. We identified CJD-associated MRI changes in 101 of the 102 sCJD cases (99% sensitivity), with the remaining scan having subtle cortical ribboning uncorrelatable with its ADC map, which was deemed insufficient to meet diagnostic criteria and was subsequently excluded from our further analysis. In contrast, referring centres reported changes in 70 cases (69% sensitivity, p < 0.0001 using McNemar’s test). Of the 4 patients who were ultimately diagnosed with inherited prion disease, 3 possessed scans that had been reported to have features of prion disease by both the NPC and referring centres.
Table 1.
Characteristics of the 102 suspected sCJD cases when referred to the NPC
| N (% of recorded) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Final diagnosis | Definite sCJD | Probable sCJD | |||||||||
| 20 | 82 | ||||||||||
| Gender | Male | Female | |||||||||
| 56 | 46 | ||||||||||
| Average age (range) | 66 (33–86) | ||||||||||
| Average MRC scale score (range) | 11 (0–20) | ||||||||||
| Codon 129 genotype | MM | MV | VV | Not tested | |||||||
| 28 (35) | 31 (39) | 21 (26) | 22 | ||||||||
| EEG findings | PSWCs | General slowing | Non-specific abnormalities | Normal | Unrecorded | ||||||
| 9 | 50 | 29 | 10 | 32 | |||||||
| RT-QuIC | Positive | Negative | Uninterpretable | Insufficient CSF | No CSF | ||||||
| 84 (90) | 9 (10) | 1 | 2 | 6 | |||||||
| Region of referring centre | Greater London (28), South East England (12), South West England (12), East England (10), East Midlands (3), West Midlands (6), Yorkshire and the Humber (7), North East England (6), North West England (9), Scotland (4), Wales (2), Northern Ireland (3) | ||||||||||
|
Number of cases with MRI changes (% sensitivity) |
Year | Total (n) | NPC review | Referring centre | |||||||
| 2012 | 91 | 83 (91%) | 43 (47%)* | ||||||||
| 2020 | 102 | 101 (99%)** | 70 (69%) | ||||||||
A comparison of the number of cases with CJD-associated MRI changes reported by the NPC and referring centres in 2012 and 2020 is also included
PSWC periodic sharp wave complexes
*p < 0.01 compares the referring centre's opinion of CJD-associated changes in 2020 with 2012 using χ2 with Yates’ correction and 1 degree of freedom
**p < 0.0001 compares the referring centre’s opinion of CJD-associated changes in 2020 with the NPC opinion in 2020 using McNemar’s test
Of the sCJD cases that were reported as showing features of prion disease by referring centres, 46 had MRI requests that were suggestive of sCJD with 14 specifically mentioning CJD, whilst 19 requests were not suggestive of sCJD and 5 requests were unavailable to us. For the cases in which a differential diagnosis of sCJD was not reported, 18 cases had suggestive requests with 7 specifically mentioning CJD, whilst 9 requests were not suggestive, and 4 requests were unavailable. Suggestive clinical information within the request was not found to have a significant impact on the sensitivity of reporting by referring centres (p = 0.7 using logistic regression).
Our data were compared to the findings reported in 2012 (Table 1), showing a significant improvement in referring centre identification of CJD-associated MRI signal changes (p < 0.01 using χ2 test).
Of the 101 sCJD cases, we found that 67% of cases had restricted diffusion in the cortex, 80% had restricted diffusion in the basal ganglia and 51% had restriction of the thalamus. No MRI scans displayed thalamic signal change alone. We reviewed whether sensitivity of reporting by referring centres varied by the location of signal change (Supplementary Table 1). We fitted logistic regression models to test whether the regions of signal change (cortex, striatum, thalamus), or a simple sum of the number of sites affected (sum of sites), determined sensitivity of reporting. Both signal change within the cortex (OR 2.50, CI 1.01–6.18, p = 0.048) and sum of sites (OR 2.61, 1.28–5.33, p = 0.0083) positively predicted a report of CJD.
We also compared reporting sensitivity between referring centres that were known to be regional neuroscience centres against non-neuroscience centres. 62 patients were referred to the NPC by regional neuroscience centres, of which 45 cases were correctly reported on initial MRI scan whilst 17 possessed changes that were missed. 39 patients were referred by non-neuroscience centres, of which 25 cases were correctly reported and 14 cases were missed diagnoses. No significant difference in reporting sensitivity could subsequently be found between regional neuroscience and non-neuroscience referring centres (p = 0.22 using logistic regression).
Where CJD-associated MRI abnormalities were detected by the referring hospital, patients had a mean MRC score of 11.5/20 (range 0–20) on initial assessment by the NPC whilst patients with missed MRI abnormalities possessed a lower mean score of 9.7/20 (range 0–19, p = 0.07 using logistic regression), similar to our previous report [12]. Additionally, days since symptom onset did not predict an accurate test report (p = 0.44 using logistic regression).
Discussion
In this study, we found MRI changes had not been identified by referring centres in 30% of patients with sCJD, despite well-defined imaging diagnostic criteria that have a sensitivity greater than other, more invasive diagnostic tests (Fig. 1) [3, 14]. Although identification of sCJD-related diffusion abnormalities has improved since our study in 2012, these findings suggest that for a considerable number of patients, diagnosis, support and stratification for future clinical trials may be delayed [12].
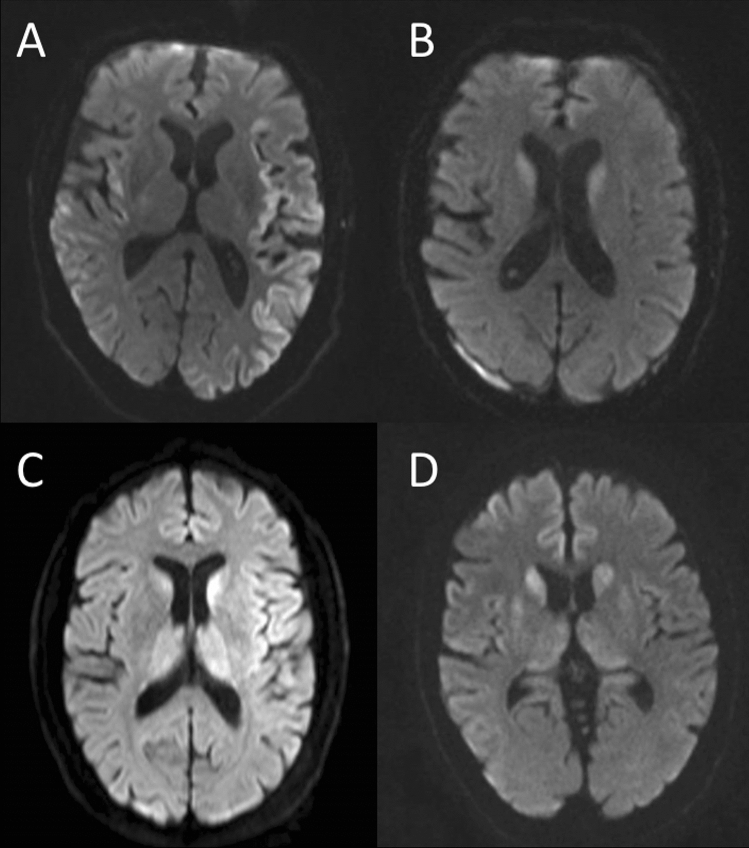
Fig. 1.
Initial MRI scans from four separate cases, not reported by referring centres as being suspicious for CJD. Image A displays significant cortical involvement which was noted by the radiologist, with differentials of seizure or hypoxia listed. Image B shows high intensity change in the striatum, which was not documented on the report. Images C and D demonstrate high intensity changes in all three regions that are characteristically affected in CJD (cortex, thalamus and striatum). These changes were missed in the report for Image C, whilst the report for image D mentioned abnormal signal in the basal ganglia only, and suggested differentials including hypoglycaemia, hypoxia, mitochondrial and metabolic causes
Several possible reasons may explain the difference in reporting of sCJD by referring centres when compared to our reporting. Firstly, we were aware of the probable diagnosis of sCJD prior to reviewing the imaging, whereas referring centre radiologists were not provided with an imaging request suggestive of sCJD in 26% of the unreported cases. Although our results indicate the clinical information on the imaging request form does not significantly influence reporting sensitivity, our analysis did not take into account the grade of signal hyperintensity in the reported and missed groups, and it may have been that a relevant clinical indication improves reporting sensitivity in cases with subtle MRI findings [15]. As a separate consideration, any addenda to the initial MRI report following multidisciplinary meetings, may not have been updated on the actual MRI report available to us, contributing to an underestimated referring centre reporting sensitivity.
Our analysis looking at the impact of location of signal change on the sensitivity of reporting revealed that this did play a significant role on missed diagnoses, with referring centres generally picking up CJD more reliably if the cerebral cortex was involved (in isolation) and additionally as the number of involved sites increased. In those cases where a diagnosis of sCJD was missed, it may have been that high intensity signal changes were dismissed as artefact, particularly in poor-quality scans. However, DWI sequences were available in virtually all included cases, which when combined with the ADC map, should aid a radiologist to discriminate between real and artefactual signal change [9].
Similar to our study undertaken in 2012, we found that patients with MRI findings that are missed on initial scan, are referred to the NPC at a more advanced stage of disease, as demonstrated by a lower MRC Scale score on initial assessment [12], although on this occasion the finding did not achieve statistical significance. This emphasises the importance of prompt identification of CJD on MRI to allow an earlier window for specialist clinical care and recruitment into clinical trials.
The improved sensitivity of reporting is encouraging. This may, in part, be driven by an increased awareness of CJD over the years and increased utility of DWI sequences, performed in 98% of cases here compared to 79% in the previous study, potentially indicating radiologists were better provided with scan modalities to identify signal changes [5, 6, 9, 14]. The use of DWI sequences with higher b-values (e.g. b = 3000 s/mm2), correlating to the applied diffusion weighting, is also encouraged to further enhance sensitivity to CJD-associated changes [16]. Moreover, computed aided diagnosis (CAD) systems are being studied to facilitate early, accurate diagnoses of neurological disease on MR imaging, which if developed and validated for CJD, could significantly improve imaging interpretation [17].
It is important, however, to acknowledge that in certain patients, particularly those with encephalopathies who require extensive sedation for scanning to be undertaken, MRI may not be the most appropriate investigation and less invasive tests such as CSF analysis may alternatively be required. We also acknowledge that our review was not blinded to suspected diagnosis, meaning we had a very high prior suspicion of CJD related scan abnormalities, which may bias sensitivity. The balance between sensitivity and specificity in overall accuracy of MRI reporting necessitates further investigation. We could not reliably investigate this aspect because CJD mimic conditions are often identified prior to recruitment into our Cohort study.
Conclusion
MRI is undoubtedly an investigation of great utility for the diagnosis of CJD, particularly due to its ready accessibility, however the characteristic high intensity signal changes are still often not correctly linked to CJD on initial scan reports. Encouragingly, there has been significant progress in radiological detection since 2012, although further efforts are still required to improve these figures. It is subsequently anticipated that, with further increased alertness of sCJD and its diagnostic criteria, improvements in MRI accessibility, scan quality and potentially the use of CAD, there could be significant positive strides toward earlier identification and effective management of sCJD patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
AJ, DS and HH reviewed scans. AJ and DS undertook the data analysis. AN, THM, HO, SM and PR were involved in patient recruitment. SM and JC developed the idea for the study and funding. All authors contributed to review of the manuscript.
Abbreviations
- MRI
Magnetic resonance imaging
- sCJD
Sporadic Creutzfeldt-Jakob disease
- NPC
National prion clinic
- DWI
Diffusion weighted imaging
- FLAIR
Fluid attenuated inversion recovery
- NHNN
National hospital for neurology and neurosurgery
- MRC Scale
Medical research council prion disease rating scale
- ADC
Apparent diffusion coefficient
- CAD
Computer aided diagnosis
Funding
The study was funded by the National Institute for Health Research’s Biomedical Research Centre at UCLH NHS Foundation Trust (Project Number 541735). Danielle Sequeira is supported by the UCLH BRC grant. Tze How Mok is supported by a Fellowship award from Alzheimer’s Society. Akin Nihat is supported by a Medical Research Council Clinical Research Training Fellowship. Both Tze How Mok and Akin Nihat are also supported by CJD Support Network UK Research Support Grants. John Collinge and Simon Mead are NIHR Senior Investigators.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
We studied MRI scans from patients referred to the NPC with suspected sCJD between 2/4/2015 and 5/12/2019 who were enrolled into the National Prion Monitoring Cohort study [13]. This study was approved by the Scotland A Research Ethics Committee, and was performed in accordance with the Declaration of Helsinki. Informed consent had been obtained from participants or, when appropriate, relatives and carers.
Footnotes
Aaron Jesuthasan and Danielle Sequeira contributed equally to this work.
References
- 1.Collinge J. Mammalian prions and their wider relevance in neurodegenerative diseases. Nature. 2016;539(7628):217–226. doi: 10.1038/nature20415. [DOI] [PubMed] [Google Scholar]
- 2.Hermann P, Appleby B, Brandel JP, Caughey B, Collins S, Geschwind MD, Green A, Haïk S, Kovacs GG, Ladogana A, Llorens F, Mead S, Nishida N, Pal S, Parchi P, Pocchiari M, Satoh K, Zanusso G, Zerr I. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol. 2021;20(3):235–246. doi: 10.1016/S1474-4422(20)30477-4.Erratum.In:LancetNeurol.2021Apr;20(4):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diagnostic Criteria|H|Prion Disease|CDC. 2019. https://www.cdc.gov/prions/cjd/diagnostic-criteria.html. Accessed 28 Dec 2020.
- 4.Rudge P, Hyare H, Green A, et al. Imaging and CSF analyses effectively distinguish CJD from its mimics. J Neurol Neurosurg Psychiatry. 2018;89:461–466. doi: 10.1136/jnnp-2017-316853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kallenberg K, Schulz-Schaeffer WJ, Jastrow U, Poser S, Meissner B, Tschampa HJ, et al. Creutzfeldt-Jakob disease: comparative analysis of MR imaging sequences. Am J Neuroradiol. 2006;27(7):1459–1462. [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenmenger L, Porter M-C, Carswell CJ, Thompson A, Mead S, Rudge P, et al. Evolution of diffusion-weighted magnetic resonance imaging signal abnormality in sporadic Creutzfeldt-Jakob disease with histopathological correlation. JAMA Neurol. 2016;73(1):76–84. doi: 10.1001/jamaneurol.2015.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young GS, Geschwind MD, Fischbein NJ, Martindale JL, Henry RG, Liu S, Lu Y, Wong S, Liu H, Miller BL, Dillon WP. Diffusion-weighted and fluid-attenuated inversion recovery imaging in Creutzfeldt-Jakob disease: high sensitivity and specificity for diagnosis. Am J Neuroradiol. 2005;26(6):1551–1562. [PMC free article] [PubMed] [Google Scholar]
- 8.Shiga Y, Miyazawa K, Sato S, Fukushima R, Shibuya S, Sato Y, Konno H, Doh-ura K, Mugikura S, Tamura H, Higano S, Takahashi S, Itoyama Y. Diffusion-weighted MRI abnormalities as an early diagnostic marker for Creutzfeldt-Jakob disease. Neurology. 2004;63(3):443–449. doi: 10.1212/01.WNL.0000134555.59460.5D. [DOI] [PubMed] [Google Scholar]
- 9.Vitali P, Maccagnano E, Caverzasi E, Henry RG, Haman A, Torres-Chae C, et al. Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias. Neurology. 2011;76(20):1711–1719. doi: 10.1212/WNL.0b013e31821a4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bizzi A, Pascuzzo R, Blevins J, et al. Evaluation of a new criterion for detecting prion disease with diffusion magnetic resonance imaging. JAMA Neurol. 2020;77(9):1141–1149. doi: 10.1001/jamaneurol.2020.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mead S, Khalili-Shirazi A, Potter C, Mok T, Nihat A, Hyare H, Canning S, Schmidt C, Campbell T, Darwent L, Muirhead N, Ebsworth N, Hextall P, Wakeling M, Linehan J, Libri V, Williams B, Jaunmuktane Z, Brandner S, Rudge P, Collinge J. Prion protein monoclonal antibody (PRN100) therapy for Creutzfeldt–Jakob disease: evaluation of a first-in-human treatment programme. The Lancet Neurology. 2022;21(4):342–354. doi: 10.1016/S1474-4422(22)00082-5. [DOI] [PubMed] [Google Scholar]
- 12.Carswell C, Thompson A, Lukic A, Stevens J, Rudge P, Mead S, et al. MRI findings are often missed in the diagnosis of Creutzfeldt-Jakob disease. BMC Neurol. 2012;5(12):153. doi: 10.1186/1471-2377-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson AGB, Lowe J, Fox Z, Lukic A, Porter M-C, Ford L, et al. The Medical Research Council prion disease rating scale: a new outcome measure for prion disease therapeutic trials developed and validated using systematic observational studies. Brain J Neurol. 2013;136(Pt 4):1116–1127. doi: 10.1093/brain/awt048. [DOI] [PubMed] [Google Scholar]
- 14.Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob. Brain. 2009;132(10):2659–2668. doi: 10.1093/brain/awp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo C, Steffens T, Sim L, Caffery L. The effect of clinical information on radiology reporting: a systematic review. J Med Radiat Sci. 2021;68(1):60–74. doi: 10.1002/jmrs.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyare H, Thornton J, Stevens J, Mead S, Rudge P, Collinge J, et al. High-b-value diffusion MR imaging and basal nuclei apparent diffusion coefficient measurements in variant and sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2010;31(3):521–526. doi: 10.3174/ajnr.A1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellegrini E, Ballerini L, Hernandez MDCV, Chappell FM, González-Castro V, Anblagan D, Danso S, Muñoz-Maniega S, Job D, Pernet C, Mair G, MacGillivray TJ, Trucco E, Wardlaw JM. Machine learning of neuroimaging for assisted diagnosis of cognitive impairment and dementia: a systematic review. Alzheimers Dement. 2018;11(10):519–535. doi: 10.1016/j.dadm.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files.