Abstract
Purpose
Oxidative stress has been linked to initiation and progression of cancer and recent studies have indicated a potential translational role regarding modulation of ROS in various cancers, including acute myeloid leukemia (AML). Detailed understanding of the complex machinery regulating ROS including its producer elements in cancer is required to define potential translational therapeutic use. Based on previous studies in acute myeloid leukemia (AML) models, we considered NADPH oxidase (NOX) family members, specifically NOX4 as a potential target in AML.
Methods
Pharmacologic inhibition and genetic inactivation of NOX4 in murine and human models of AML were used to understand its functional role. For genetic inactivation, CRISPR-Cas9 technology was used in human AML cell lines in vitro and genetically engineered knockout mice for Nox4 were used for deletion of Nox4 in hematopoietic cells via Mx1-Cre recombinase activation.
Results
Pharmacologic NOX inhibitors and CRISPR-Cas9-mediated inactivation of NOX4 and p22-phox (an essential NOX component) decreased proliferative capacity and cell competition in FLT3-ITD-positive human AML cells. In contrast, conditional deletion of Nox4 enhanced the myeloproliferative phenotype of an FLT3-ITD induced knock-in mouse model. Finally, Nox4 inactivation in normal hematopoietic stem and progenitor cells (HSPCs) caused a minor reduction in HSC numbers and reconstitution capacity.
Conclusion
The role of NOX4 in myeloid malignancies appears highly context-dependent and its inactivation results in either enhancing or inhibitory effects. Therefore, targeting NOX4 in FLT3-ITD positive myeloid malignancies requires additional pre-clinical assessment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-022-03986-3.
Keywords: NADPH oxidases (NOX), Nox4, Acute myeloid leukemia (AML), FLT3-ITD, Reactive oxygen species (ROS), Oxidative stress, CRISPR-Cas9
Introduction
Acute myeloid leukemia (AML) is a heterogeneous disease of the hematopoietic system characterized by abnormal proliferation and accumulation of immature cells. Clonal evolution and acquisition of additional mutations drive leukemic transformation of myeloid progenitors. Leukemic cells arising from the malignant clone have lost the ability for differentiation and are characterized by reduced apoptosis (Chan and Majeti 2013). With improved understanding of the underlying biology, novel treatment options have emerged for patients with AML (Kantarjian et al. 2021). Of note, development of novel targeted treatment options requires the careful assessment of the molecular target and putative functional differences regarding the cellular context or underlying genetic background (Cucchi et al. 2021).
NADPH oxidases (NOXs) are transmembrane proteins that generate reactive oxygen species (ROS) as their sole function (Altenhöfer et al. 2012). NOX4 and NOX2 are expressed in hematopoietic cancers and contribute to cellular transformation by increasing ROS-levels and genomic instability (Naughton et al. 2009; Reddy et al. 2011; Stanicka et al. 2015). Recently, our group observed that genetic inactivation of Nox4 attenuated FLT3-ITD-driven leukemic transformation. Consistently, pharmacologic targeting of NOX4 decreased proliferative capacity of FLT3-ITD transformed cells (Jayavelu et al. 2016). In contrast, recent studies found tumor-promoting effects of Nox4 deficiency (Helfinger et al. 2021). Therefore, we sought to validate and confirm the therapeutic potential of NOX4 as a therapeutic target in AML.
Materials and methods
Cell lines, cell proliferation and apoptosis
Murine 32D-FLT3-ITD were provided by Prof. J. Duyster, Freiburg, Germany and Dr. R. Grundler, Munich, Germany). Leukemia cell lines were purchased from DSMZ (Braunschweig, Germany). Cells were cultured according to standard protocols and tested negative for mycoplasma. Cell viability was measured using Cell Titer-Blue reagent (Promega, Madison, WI, USA) according to the manufacturers’ instructions. For proliferation assays, the number of cells was counted with a hemocytometer. Apoptosis was measured by flow cytometry using Annexin V/SYTOX Blue staining.
Use of pharmacologic inhibitors
The following inhibitors were used for in vitro studies: ROS inhibitors: GKT137831 (Selleckchem, Houston, TX, USA) and GSK2795039 (Hycultec GmbH, Beutelsbach, Germany).
Genetic inactivation by CRISPR/Cas9
Genetic inactivation by CRISPR/Cas9 was performed as previously described (Perner et al. 2021; Schnoeder et al. 2022) unless otherwise stated. Guide RNAs were designed using the Broad GPP tool (Doench, Nat Biotechnology 2014). For cloning of sgRNA sequences, the improved-scaffold-pU6-sgRNA-EF1Alpha-PURO-T2A-RFP (ipUSEPR) vector system (Uckelmann et al. 2018), with puromycin resistance and RFP selection marker was used. Genetic inactivation by CRISPR/Cas9 was performed as published before (Jayavelu et al. 2020). sgRNA sequences are provided in the Supplementary Materials. For negative selection competition assays, transduced cells were mixed with non-transduced cells at 9:1 RFP−/RFP+ ratio for applying selection pressure. The percentage of RFP+ was monitored by flow cytometry.
Genomic NOX4 knockout validation
PCR on genomic DNA was used to confirm NOX4 knockout in human AML cell lines. Primer pairs are listed in the Supplementary Materials.
Protein extraction and immunoblotting
Western Blotting was performed according to standard protocols as previously published (Heidel et al. 2006; Schnöder et al. 2015). Cell lines and whole bone marrow cells were lysed as described previously. Two different antibodies against NOX4 were provided by J. M. Doroshow (Bethesda, MD, USA) (Meitzler et al. 2017) and A. Shah (King’s College London, London, UK) (Anilkumar et al. 2008). All antibodies are indicated in the Supplementary Materials.
Animal experiments
All mice were kept under pathogen-free conditions in the accredited Animal Research facility of the University Hospital Jena. The animal experiments were approved by the Landesverwaltungsamt Thüringen (animal protocol number 02-035/16). Experimental mice were generated by crossing Nox4flox/flox mice (Schröder et al. 2012) with Mx1-Cre transgenic mice (B6.Cg-Tg(Mx1-Cre)1Cgn/J: Jackson Laboratory, Bar Harbor, USA) and Flt3ITD/ITD knock-in mice (Lee et al. 2007). Mx1-Cre-recombinase was activated by intraperitoneal injections of 100 µg low-molecular-weight poly-I-poly-C (LMW-pIpC, GE-Healthcare) on alternating days as indicated.
Competitive bone marrow (BM) transplantation and analysis of steady state hematopoiesis
Competitive bone marrow transplantation and analysis of steady state hematopoiesis were performed as previously published (Mohr et al. 2018). Nox4flox/flox Mx1-Cre + mice and Nox4flox/flox Mx1-Cre- or Nox4wt/wt Mx1-Cre + control mice were used as donors.
Excision control regarding Nox4 was performed on genomic DNA isolated from WBM cells. PCR primers included Nox4-forward, CCAAGCTTCCGATTCCCATTCTC and Nox4-reverse, GTCCTC-CAATCATGAAAGTGAAGC). An alternative forward primer was used to detect the 509 bp unexcised loxP-flanked allele (Nox4-forward-alt: AGAATGAAAAGCTAGGCGTCCTTGG).
Flow cytometry and antibody staining
Immunophenotyping of normal and leukemic cell compartments and of leukemic PB and BM was performed as described before (Heidel et al. 2012, 2013). Antibodies are provided in Supplementary Methods. Flow cytometry was performed on a FACS Canto II cytometer (Becton Dickinson, Heidelberg, Germany).
Results
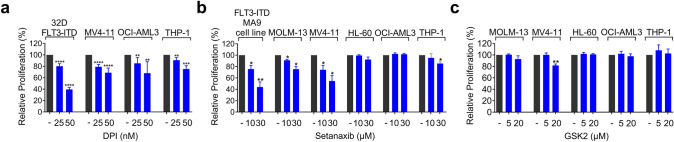
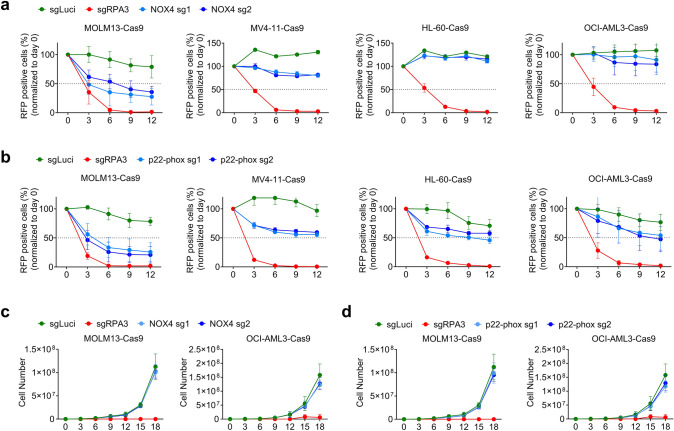
First, we sought to define the potential of pharmacological NOX-inhibition in more detail using murine and human cell models of AML. Treatment with the rather unselective NOX inhibitor DPI reduced proliferative capacity of leukemia cell lines (Fig. 1a). However, as DPI is not suitable for in vivo treatment and known to inhibit some other enzymes like eNOS, and xanthine oxidase (Altenhöfer et al. 2015), we decided to test the more selective NOX4/1 inhibitor setanaxib and the NOX2 inhibitor GSK2795039. While murine and human FLT3-ITD-positive AML cell lines showed higher sensitivity to setanaxib (Fig. 1b), only MV4-11 cells were inhibited in growth by GSK2795039 (Fig. 1c). Given the potential off-target effects of NOX-inhibitors (Augsburger et al. 2019), we aimed to validate the sensitivity of FLT3-ITD cell lines by genetic inactivation of NOX-enzymes. Human AML cell lines with stable expression of Cas9 were generated as described before (Jayavelu et al. 2020; Perner et al. 2021; Schnoeder et al. 2022). To assess the functional importance of NOX4 (NOX4) and of the essential NOX-component p22-phox (CYBA), we used a CRISPR–Cas9-mediated gene-editing and negative-selection strategy. The genetic deletion was confirmed by Western blotting, and gPCR (Supplementary Fig. S1 and S2). Cells expressing different NOX4 single guide RNAs (sgRNAs) were (partially) outcompeted by non-transduced cells in FLT3-ITD positive cell lines (MOLM13, MV4-11), while non-FLT3-mutated cell lines (OCI-AML3, HL-60) appeared less sensitive (Fig. 2a). In contrast, at least partial dropout was observed in all cell lines with p22-phox targeting sgRNAs (Fig. 2b). The more pronounced phenotype of p22-phox targeting compared to NOX4 targeting may reflect the essential function of p22-phox for several NOX-enzymes (NOX1-4). However, we observed rather subtle effects of NOX4 and p22-phox deletion on leukemia cell proliferation or apoptosis compared to cell competition and negative selection assays (Fig. 2c, d and Supplementary Fig. S3). Taken together, these in vitro results suggest that NOX4 is relevant under conditions of leukemia cell competition in FLT3-mutated cell lines.
Fig. 1.
Effect of NOX inhibitors on the growth of AML cells. a, b The proliferation/viability of the leukemic cells was measured by Cell Titer Blue assay. Upon 72 h of treatment, the fluorescent signals, which are directly proportional to the number of viable cells, were measured and normalized to the fluorescent signal of the DMSO control group. Three independent experiments (technical triplicates) were conducted. a DPI treatment (25 nM and 50 nM) b Setanaxib treatment (10 µM and 30 µM) and c GSK2795039 treatment (5 µM and 20 µM) (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by two-tailed t-test)
Fig. 2.
Effect of genetic inactivation of NOXs on cell competition and proliferation of human AML cells. a, b CRISPR-Cas9 cell competition assay in MOLM13, OCI-AML3, MV4-11 and HL-60 cells after (a) NOX4 or (b) p22-phox deletion using 2 different sgRNAs compared to knockout of RPA3 (positive control) or a sgRNA (sgLuci) targeting luciferase (negative control) through 12 days (x-axis) after starting the competition assay. sgRNA targeted transduced (RFP+) cells were mixed with their non-transduced counterparts (RFP−) at a 1:10 ratio. c, d Proliferation was assessed by cell counting with trypan blue exclusion in (c) NOX4 or (d) p22-phox knockout in MOLM13 and OCI-AML3 cells over 18 days (x-axis). No significant difference was found between sgLuci and NOX4 or p22-phox targeted groups using two-tailed t-test
ROS balance and NOX activity in cells in vivo are also regulated by cell-extrinsic factors and neighboring niche cells (Tarafdar and Pula 2018). Also, recent publications provide evidence for the functional relevance of Nox2 in normal hematopoiesis and for leukemogenesis in mice (Adane et al. 2019). Therefore, we sought to confirm the above effects of Nox4 on FLT3-mutated myeloid disease in vivo. In brief, conditional Nox4fl/fl mice (Schröder et al. 2012) were crossed with an Mx1-Cre-recombinase (B6.Cg-Tg(Mx1-Cre)1Cgn/J, received from The Jackson Laboratory, Bar Harbor, USA) and a conventional Flt3-ITD knock-in mouse strain (Lee et al. 2007).
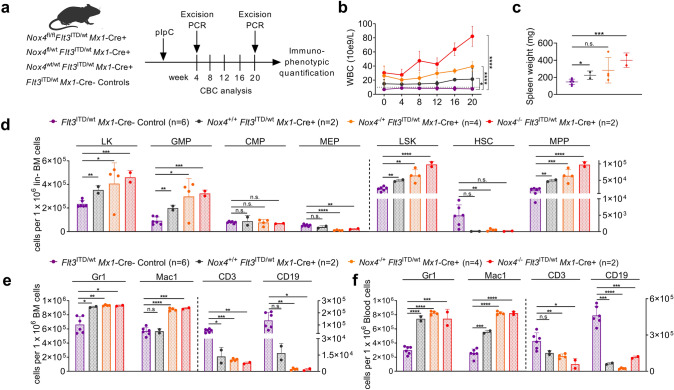
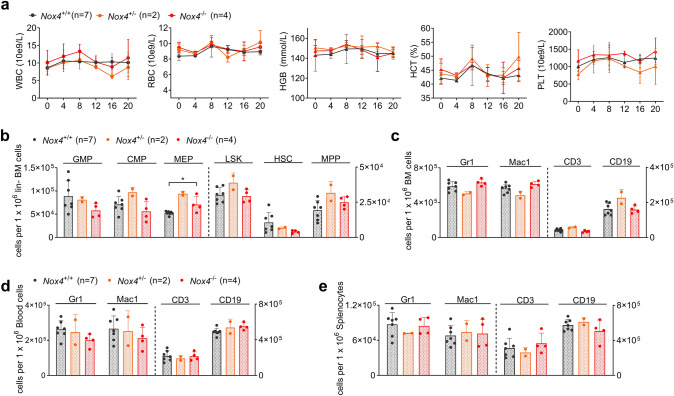
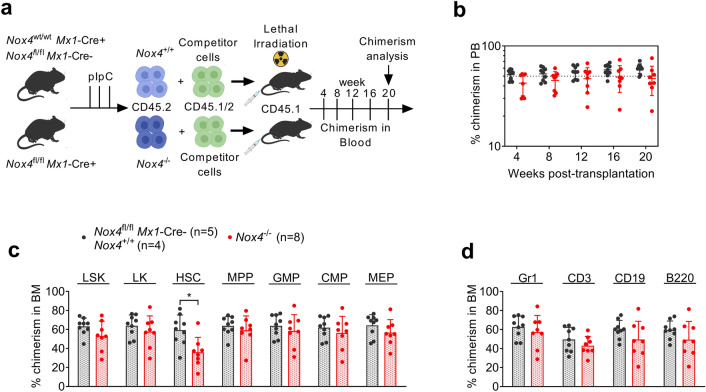
This model allows functional assessment of disease development in vivo and its dependency on Nox4 without being dependent on bone marrow transplantation and irradiation. Mx1-Cre recombinase was induced by administration of pIpC in Nox4fl/fl Flt3ITD/wt Mx1-Cre + , Nox4fl/wt Flt3ITD/wt Mx1-Cre + , or Nox4wt/wt Flt3ITD/wt Mx1-Cre + mice and Mx1-Cre- littermate controls (Fig. 3a). Excision of the Nox4 gene was confirmed by PCR in PB cells at week 4 (Supplementary Fig. S4b, c). As expected, Nox4wt/wt Flt3ITD/wt Mx1-Cre + mice showed elevated white blood count (WBC) compared to Flt3ITD/wt Mx1-Cre- controls. Unexpectedly, Nox4 deletion in Flt3ITD/wt Mx1-Cre + background induced a further increase in leukocytosis in a gene-dose-dependent manner (Fig. 3b). Consistent with this finding, splenomegaly was more pronounced in Nox4 deficient animals, when investigated at week 20 (Fig. 3c). Immunophenotypic analysis of stem and progenitor cell compartments in the BM revealed that Nox4 deletion resulted in increased cell numbers of LKs, LSKs, MPPs, and GMPs in Flt3ITD/wt Mx1-Cre + mice. Conversely, the number of MEPs was decreased in Nox4 deficient mice, indicating a lineage bias depending on the presence of Nox4. As described before (Lee et al. 2007; Li et al. 2008), HSC numbers appeared reduced in FLT3-mutated animals when compared to wildtype controls (Fig. 3d). Immunophenotypic analysis of mature cell compartments in BM, PB, and spleen revealed an expanded myeloid compartment with increased Mac1 expression in Nox4 knockout mice and conversely reduced abundance of lymphoid cells (Fig. 3e, f and Supplementary Fig. S4a). Counter-selection of partially excised clones could be excluded by PCR of peripheral blood cells at week 20 (Supplementary Fig. S4d, e). Taken together, these results suggest for the first time that Nox4 deletion may promote the myeloproliferative phenotype in a FLT3-ITD-driven mouse model. To assess for a functional impact of Nox4 inactivation on normal HSCs, we investigated steady-state hematopoiesis after conditional deletion of Nox4 (Supplementary Fig. S5). Following activation of Mx1-Cre by pIpC injections into Nox4fl/fl Mx1-Cre + , Nox4fl/wt Mx1-Cre + , Nox4wt/wt Mx1-Cre + or Cre-negative mice, we found no significant differences in peripheral blood counts (Fig. 4a) and immunophenotypic analysis of myeloid or lymphoid blood compartments in peripheral blood, BM, and spleen (Fig. 4c–e). The total number of hematopoietic stem and progenitor cells (HSPCs) was not altered by Nox4 deletion except for a subtle increase in MEP numbers (Fig. 4b). To test for the function of Nox4 deficient HSPCs, we used the most stringent assay to assess for hematopoietic stem and progenitor cell function (Purton and Scadden 2007), which is transplantation into irradiated recipient hosts (Fig. 5a). When Nox4−/− whole bone marrow cells and Nox4+/+ controls were transplanted into primary recipient hosts in a competitive manner (ratio of 1:1), we found no loss or gain of function in Nox4-deficient cells. Nox4 knockout cells (Supplementary Fig. S6a, b) competed against wildtype controls as indicated by stable PB chimerism over 16 weeks and showed a mild decrease on week 20 (Fig. 5b). Likewise, no difference was observed in chimerism of total BM or immunophenotypic abundance of immature or mature compartments at week 20 except for rather decreased numbers of Nox4−/− cells in the CD34− LSK cell population (Fig. 5c, d). This finding is in line with a previous study, highlighting a decrease in repopulation capacity of Nox4 knockout HSCs in secondary recipient hosts (Prieto-Bermejo et al. 2021). Taken together, inactivation of Nox4 does not enhance normal HSPC function in vivo but may result in decreased abundance and function of long-term HSCs.
Fig. 3.
Role of Nox4 in the context of FLT3-ITD-driven myeloproliferative disease. a Experimental protocol for assessing the impact of Nox4 deletion on steady-state hematopoiesis in Flt3ITD/wt Mx1-Cre + mice. b WBCs after conditional deletion of Nox4 through 20 weeks. c Spleen weights of Nox4 wt or knockout Flt3ITD/wt Mx1-Cre + mice. d Immunophenotypic quantification of stem- and progenitor cell abundance, specifically of L−K+, L−S+K+, HSC (CD48−CD150+L−S+K+), MPP (CD48+CD150+L-S+K+ or CD48+CD150−L−S+K+ or CD48−CD150−L−S+K+), GMP (CD34highFcgRhighL−S−K+), CMP (CD34highFcgRlowL−S−K+), and MEP (CD34lowFcgRlowL−S−K+). e, f Immunophenotypic quantification of mature myeloid (Gr-1+ or Mac1+), B-lymphoid (CD19+), and T-lymphoid (CD3+) cells in e BM and f blood. Error bars indicate the standard deviation. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by two-tailed t-test)
Fig. 4.
Role of Nox4 in normal steady-state hematopoiesis. a Peripheral blood counts; white blood count (WBC), red blood count (RBC), hematocrit (HCT) level, hemoglobin (HGB) level, and platelet count (PLT) after conditional deletion of Nox4 during normal steady-state hematopoiesis through 20 weeks. b Immunophenotypic quantification of HSPCs as indicated in Fig. 3d. c–e Immunophenotypic quantification of mature myeloid (Gr-1+ or Mac1+), B-lymphoid (CD19+), and T-lymphoid (CD3+) cells in c BM, d peripheral blood and e spleen. Error bars indicate the standard deviation. (*P < 0.05, by two-tailed t-test)
Fig. 5.
Impact of Nox4 deletion on the reconstituting capability of HSPCs. a Experimental protocol for assessing the impact of Nox4 deletion on repopulation capacity of HPSCs. Competitive repopulation assay was performed by the competition of Nox4−/− or Nox4+/+ donor cells against WT competitor cells. b Peripheral blood chimerism of primary recipient mice. P value = 0.045 for the difference on day 20. c Chimerism of immature BM compartments: L-K + , L-S + K + , HSC (CD34−/lowL−S+K+), MPP (CD34+L−S+K+), GMP (CD34highFcgRhighL−S−K+), CMP (CD34highFcgRlowL−S−K+), and MEP (CD34lowFcgRlowL−S−K+). P value = 0.011 for the difference in HSC compartment. d Chimerism of myeloid (Gr1+) and lymphoid (CD3+, CD19+, B220+) linages in BM. Two independent cohorts; Nox4−/− (n = 8), controls Nox4+/+ (n = 4) and Nox4fl/fl Mx1-Cre + (n = 5), mean ± SD. Statistical analyses were performed using a two-tailed Mann–Whitney test
Discussion
For the first time, our findings provide evidence that Nox4 deletion may enhance an FLT3-ITD induced myeloproliferative phenotype in vivo. This phenotype is in contrast to the in vitro data of previous reports: genetic and pharmacologic targeting of NOX4 decreased the growth of leukemic cells in vitro (Jayavelu et al. 2016; Naughton et al. 2009; Reddy et al. 2011), which is also consistent with our genetic targeting approach in human AML. Using transplantation models of FLT3-ITD driven cells into irradiated recipient hosts, genetic inactivation of Nox4 by RNAi had resulted in prolonged survival. Of note, these models had been performed using murine AML cell lines or primary murine cells harboring more than one oncogene (MLL-AF9 and FLT3-ITD).
As indicated above, a recent study investigated the role of Nox4 in a different cancer model and described a tumor suppressor role of Nox4. In this study, Nox4 deletion enhanced cancerogen-induced tumor formation in murine models of carcinoma and sarcoma (Helfinger et al. 2021). The phenotype observed here suggests that the role of Nox4 in leukemia is highly context dependent. Targeting Nox4 may therefore result in leukemia inhibitory or promoting effects and underlines the necessary caution in developing NOX4-modulating drugs. Moreover, suitable compounds that may specifically target NOX4 are still lacking. Setanaxib (GKT137831), a drug currently developed in advanced clinical trials, was recently proposed as a NOX1/4 specific inhibitor. However, its specificity regarding NOX enzymes has recently been questioned (Augsburger et al. 2019; Dao et al. 2020). Detailed pre-clinical genetic and pharmacologic studies of NOX4 modulation are clearly required before defining it as a bona fide target in leukemia therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the shared grant of the German Cancer Aid (Deutsche Krebshilfe, 70112607) to F.D.B. and F.H.H.
Author contributions
MBD, TMS, PCM and FHH performed research. MBD, TMS, KS, FDB and FHH provided material, analyzed data and contributed to the writing of the manuscript. FHH and FDB designed research, analyzed data and wrote the paper.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Conflict of interest
The authors declare no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adane B, Ye H, Khan N, Pei S, Minhajuddin M, Stevens BM, Jones CL, D’Alessandro A, Reisz JA, Zaberezhnyy V. The hematopoietic oxidase NOX2 regulates self-renewal of leukemic stem cells. Cell Rep. 2019;27:238–254. doi: 10.1016/j.celrep.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenhöfer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans J, Schiffers P, Ho H, Wingler K, Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci. 2012;69:2327–2343. doi: 10.1007/s00018-012-1010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenhöfer S, Radermacher KA, Kleikers PW, Wingler K, Schmidt HH. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid Redox Signal. 2015;23:406–427. doi: 10.1089/ars.2013.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol. 2008;28:1347–1354. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- Augsburger F, Filippova A, Rasti D, Seredenina T, Lam M, Maghzal G, Mahiout Z, Jansen-Dürr P, Knaus UG, Doroshow J. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol. 2019;26:101272. doi: 10.1016/j.redox.2019.101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SM, Majeti R. Role of DNMT3A, TET2, and IDH1/2 mutations in pre-leukemic stem cells in acute myeloid leukemia. Int J Hematol. 2013;98:648–657. doi: 10.1007/s12185-013-1407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchi DG, Polak TB, Ossenkoppele GJ, Groot UD, Carin A, Cloos J, Zweegman S, Janssen JJ. Two decades of targeted therapies in acute myeloid leukemia. Leukemia. 2021;35:651–660. doi: 10.1038/s41375-021-01164-x. [DOI] [PubMed] [Google Scholar]
- Dao VT-V, Elbatreek MH, Altenhöfer S, Casas AI, Pachado MP, Neullens CT, Knaus UG, Schmidt HH. Isoform-selective NADPH oxidase inhibitor panel for pharmacological target validation. Free Radical Biol Med. 2020;148:60–69. doi: 10.1016/j.freeradbiomed.2019.12.038. [DOI] [PubMed] [Google Scholar]
- Heidel F, Solem FK, Breitenbuecher F, Lipka DB, Kasper S, Thiede M, Brandts C, Serve H, Roesel J, Giles F. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006;107:293–300. doi: 10.1182/blood-2005-06-2469. [DOI] [PubMed] [Google Scholar]
- Heidel FH, Bullinger L, Feng Z, Wang Z, Neff TA, Stein L, Kalaitzidis D, Lane SW, Armstrong SA. Genetic and pharmacologic inhibition of β-catenin targets imatinib-resistant leukemia stem cells in CML. Cell Stem Cell. 2012;10:412–424. doi: 10.1016/j.stem.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidel FH, Bullinger L, Arreba-Tutusaus P, Wang Z, Gaebel J, Hirt C, Niederwieser D, Lane SW, Döhner K, Vasioukhin V. The cell fate determinant Llgl1 influences HSC fitness and prognosis in AML. J Exp Med. 2013;210:15–22. doi: 10.1084/jem.20120596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinger V, Von Gall FF, Henke N, Kunze MM, Schmid T, Rezende F, Heidler J, Wittig I, Radeke HH, Marschall V (2021) Genetic deletion of Nox4 enhances cancerogen-induced formation of solid tumors. Proc Natl Acad Sci 118 [DOI] [PMC free article] [PubMed]
- Jayavelu A, Müller J, Bauer R, Böhmer S, Lässig J, Cerny-Reiterer S, Sperr W, Valent P, Maurer B, Moriggl R. NOX4-driven ROS formation mediates PTP inactivation and cell transformation in FLT3ITD-positive AML cells. Leukemia. 2016;30:473–483. doi: 10.1038/leu.2015.234. [DOI] [PubMed] [Google Scholar]
- Jayavelu AK, Schnoder TM, Perner F, Herzog C, Meiler A, Krishnamoorthy G, Huber N, Mohr J, Edelmann-Stephan B, Austin R, et al. Splicing factor YBX1 mediates persistence of JAK2-mutated neoplasms. Nature. 2020;588:157–163. doi: 10.1038/s41586-020-2968-3. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Kadia T, DiNardo C, Daver N, Borthakur G, Jabbour E, Garcia-Manero G, Konopleva M, Ravandi F. Acute myeloid leukemia: current progress and future directions. Blood Cancer J. 2021;11:1–25. doi: 10.1038/s41408-021-00425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Tothova Z, Levine RL, Anderson K, Buza-Vidas N, Cullen DE, McDowell EP, Adelsperger J, Fröhling S, Huntly BJ. FLT3 mutations confer enhanced proliferation and survival properties to multipotent progenitors in a murine model of chronic myelomonocytic leukemia. Cancer Cell. 2007;12:367–380. doi: 10.1016/j.ccr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Piloto O, Nguyen HB, Greenberg K, Takamiya K, Racke F, Huso D, Small D. Knock-in of an internal tandem duplication mutation into murine FLT3 confers myeloproliferative disease in a mouse model. Blood J Am Soc Hematol. 2008;111:3849–3858. doi: 10.1182/blood-2007-08-109942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzler JL, Makhlouf HR, Antony S, Wu Y, Butcher D, Jiang G, Juhasz A, Lu J, Dahan I, Jansen-Dürr P. Decoding NADPH oxidase 4 expression in human tumors. Redox Biol. 2017;13:182–195. doi: 10.1016/j.redox.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr J, Dash BP, Schnoeder TM, Wolleschak D, Herzog C, Tubio Santamaria N, Weinert S, Godavarthy S, Zanetti C, Naumann M. The cell fate determinant Scribble is required for maintenance of hematopoietic stem cell function. Leukemia. 2018;32:1211–1221. doi: 10.1038/s41375-018-0025-0. [DOI] [PubMed] [Google Scholar]
- Naughton R, Quiney C, Turner S, Cotter T. Bcr-Abl-mediated redox regulation of the PI3K/AKT pathway. Leukemia. 2009;23:1432–1440. doi: 10.1038/leu.2009.49. [DOI] [PubMed] [Google Scholar]
- Perner F, Schnoeder TM, Xiong Y, Jayavelu AK, Mashamba N, Santamaria NT, Huber N, Todorova K, Hatton C, Perner B (2021) YBX1 mediates translation of oncogenic transcripts to control cell competition in AML. Leukemia 1–12 [DOI] [PMC free article] [PubMed]
- Prieto-Bermejo R, Romo-González M, Pérez-Fernández A, García-Tuñón I, Sánchez-Martín M, Hernández-Hernández Á. Cyba-deficient mice display an increase in hematopoietic stem cells and an overproduction of immunoglobulins. Haematologica. 2021;106:142. doi: 10.3324/haematol.2019.233064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Fernandes MS, Salgia R, Levine RL, Griffin JD, Sattler M. NADPH oxidases regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. Leukemia. 2011;25:281–289. doi: 10.1038/leu.2010.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnöder T, Arreba-Tutusaus P, Griehl I, Bullinger L, Buschbeck M, Lane S, Döhner K, Plass C, Lipka D, Heidel F. Epo-induced erythroid maturation is dependent on Plcγ1 signaling. Cell Death Differ. 2015;22:974–985. doi: 10.1038/cdd.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoeder TM, Schwarzer A, Jayavelu AK, Hsu C-J, Kirkpatrick J, Döhner K, Perner F, Eifert T, Huber N, Arreba-Tutusaus P. PLCG1 is required for AML1-ETO leukemia stem cell self-renewal. Blood J Am Soc Hematol. 2022;139:1080–1097. doi: 10.1182/blood.2021012778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- Stanicka J, Russell EG, Woolley JF, Cotter TG. NADPH oxidase-generated hydrogen peroxide induces DNA damage in mutant FLT3-expressing leukemia cells. J Biol Chem. 2015;290:9348–9361. doi: 10.1074/jbc.M113.510495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarafdar A, Pula G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int J Mol Sci. 2018;19:3824. doi: 10.3390/ijms19123824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckelmann HJ, Kim SM, Antonissen NJ, Krivtsov AV, Hatton C, McGeehan GM, Levine RL, Vassiliou GS, Armstrong SA. MLL-Menin inhibition reverses pre-leukemic progenitor self-renewal induced by NPM1 mutations and prevents AML development. Blood. 2018;132:546. doi: 10.1182/blood-2018-99-110851. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.