Abstract
Concussion diagnosis is complicated by a lack of objective measures. Ubiquitin carboxyl-terminal esterase L1 (UCHL1) is a biomarker that has been shown to increase following traumatic brain injury but has not been investigated in concussed athletes on the sideline of athletic events. Therefore, this study was conducted to determine if UCHL1 can be used to aid in sideline concussion diagnosis. Blood was taken via standard venipuncture from a recreationally active control group, a group of rugby players prior to match play (pre-match), rugby players following match-play (match-control), and rugby players after suffering a sport-related concussion (SRC). UCHL1 was not significantly different among groups (p > 0.05) and was unable to distinguish between SRC and controls (AUROC < 0.400, p > 0.05). However, when sex-matched data were used, it was found that the female match-control group had a significantly higher serum UCHL1 concentration than the pre-match group (p = 0.041). Differences were also found in serum UCHL1 concentrations between male and female athletes in the match-control group (p = 0.007). This study does not provide evidence supporting the use of UCHL1 in sideline concussion diagnosis when blood is collected soon after concussion but does show differences in serum UCHL1 accumulation between males and females.
Subject terms: Biomarkers, Diagnostic markers, Brain injuries
Introduction
Sport-related concussion (SRC) is defined as a traumatic brain injury induced by biomechanical forces in which any neurological deficits eventually resolve and there is no structural damage to the brain that could be detected with structural neuroimaging1. Diagnosis of SRC is extremely complex due to confounding symptoms and lack of clinically objective tests2. Furthermore, in a self-efficacy survey of 94 high school and collegiate certified athletic trainers (ATCs), self-efficacy in their clinical assessment of SRC, using common tools such as balance testing, neurocognitive testing, vestibular and oculomotor screening, the Sport Concussion Assessment Tool 3rd edition (SCAT3), etc., was reported to be moderate3. However, high self-efficacy in concussion diagnosis is important to ensure the safety of concussed athletes3. Including a validated objective blood-based biomarker in the diagnostic criteria of SRC would likely enhance the accuracy of SRC diagnosis and improve self-efficacy among ATCs and other medical practitioners.
Currently, there is no clinically validated biomarker that can be used to aid in SRC diagnosis on the sideline of athletic events4. Ubiquitin carboxyl-terminal esterase L1 (UCHL1) is an enzyme primarily found in neurons and is involved with tagging cytosolic proteins for degradation5. This enzyme has emerged as a strong biomarker candidate for assessing severe traumatic brain injury (TBI)6–8. Research has also shown that UCHL1 is elevated in moderate and mild TBI patients, and can be used to distinguish mild TBI patients requiring computed tomography (CT) imaging9–13. In 2018, UCHL1 was approved by the United States of America Food and Drug Administration (FDA) to be used in the assessment of mild TBI in lieu of head CT imaging. Recently, the company Abbott Laboratories (Chicago, IL USA) has developed the FDA approved Abbott's i-STAT™ Alinity™ handheld device which can produce UCHL1 results within 15 min after a plasma sample has been inserted. This technology has been shown to have good sensitivity in predicting acute traumatic intracranial injury14. All these advancements make the investigation of UCHL1 as a biomarker that may aid in SRC diagnosis quite lucrative.
In a clinical investigation, Papa et al. found that UCHL1 was highest at enrollment15. This study also showed that UCHL1 was able to distinguish TBI patients (most of whom were mild TBI patients) compared to controls at enrollment as well as 4, 8, 12, and 16 h post-injury15. A more recent study by Papa et al. found that UCHL1 is detectable less than one hour after mild TBI12. Based on these studies it is plausible that UCHL1 could be used to detect SRC on the sideline of an athletic event. A few studies have investigated UCHL1 in athletes who experienced an SRC and found that UCHL1 levels are elevated16–18.
Asken et al. compared UCHL1 levels in SRC subjects to baseline and controls with blood drawn 10 h after SRC (median 4.1 h post SRC)16. From this study it was found that UCHL1 levels were higher in SRC subjects compared to baseline and controls but not statistically different16. However, when data analysis was limited to blood samples obtained less than four hours after injury UCHL1 levels were significantly higher compared to baseline16.
Meier et al. compared blood draws from preseason and football player controls to football players experiencing SRC18. In this study UCHL1 was found to be significantly higher in SRC subjects at 6 h (median blood collection time point of 2 h post SRC) compared to preseason and football player controls18. In a similar study by Meier et al., blood was collected at preinjury baseline, within 6 h post SRC (median 3.8 h. post SRC) and 24 to 48 h post SRC17. These data were then compared to matched uninjured football players and non-contact-sport athletes finding that UCHL1 was elevated within 6 h post SRC compared to baseline and both control groups17.
Past studies on SRC and mild TBI all point to UCHL1 being a potential biomarker for use in sideline diagnosis based on how rapidly UCHL1 seems to accumulate in the blood. However, there are no studies to date that focus on collecting blood samples on the sideline of athletic events soon after concussion. Therefore, the purpose of this study was to investigate serum UCHL1 by attending rugby tournaments and collecting blood pitchside from concussed and non-concussed rugby players. We hypothesized that UCHL1 would be able to distinguish concussed athletes pitchside.
Methods
The Appalachian State University (Boone, NC, USA) (IRB #s 20-0033, 19-0232, and 17-0345) and Rutgers University (New Brunswick, NJ, USA) (IRB# Pro20140000411) Institutional Review Board (IRB) for the Protection of Human Subjects approved this study. All data was collected in accordance with guidelines and regulations of both the Appalachian State University and Rutgers University IRBs. Prior to participation, written informed consent was obtained from all subjects. All subjects acknowledged that they cannot be identified via the paper and subjects were fully anonymized following data collection.
Study population and study design
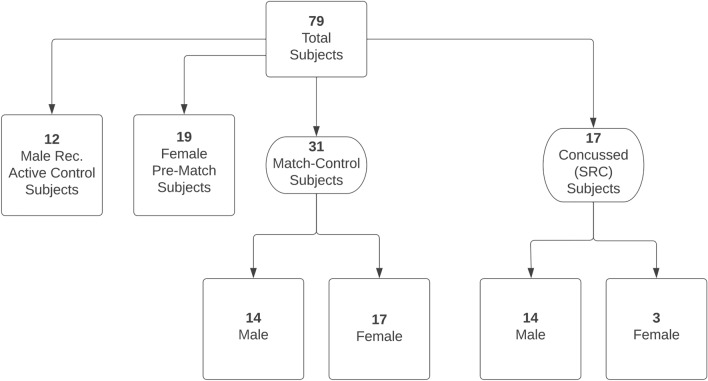
This study was an observational cross-sectional study with the primary objective of determining if UCHL1 can distinguish among concussed and non-concussed subjects at the sideline of athletic events. Rugby players were separated into pre-match (n = 19), match-control (n = 31) and SRC groups (n = 17) (Fig. 1). Prior to rugby matches, athletes were asked to donate a blood sample for the study. Athletes included in the pre-match group were in-season but had gone seven days without rugby play prior to blood draw. The athletes donating blood were asked to return to the research tent following their match to participate in the match-control group and increase the number of paired samples. There were only females rugby players in the pre-match group because none of the male rugby players agreed to donate a blood sample pre-match despite repeated recruitment. Since no male rugby players volunteered to donate blood pre-match, we included a separate pool of male Caucasians (n = 12) who were of similar age to the majority of match-control male athletes, recreationally active, and not participating in any contact sport to serve as a male control group. These recreationally active controls had not exercised eight hours prior to blood draw. Match-control and SRC athletes were in-season and had not played a match in the seven days prior to participation in the study. Match-control subjects included females (n = 17) and males (n = 14) who were recruited by asking rugby players to participate in the study following their rugby match. None of the rugby athletes participating in the match-control group reported injury of any kind. Subjects in the SRC group included females (n = 3) and males (n = 14) recruited by asking athletes who were diagnosed with concussion by on-site ATCs to participate in the study. None of the SRC subjects experienced loss of consciousness and all were able to give consent. Although ATCs have reported moderate self-efficacy in diagnosing concussion3, they are the best tool we currently have for diagnosing concussion and is the reason their diagnosis was relied upon for this study. Subjects in all groups were asked about their concussion history (number of past concussions), years of rugby play, age, body mass, and all subjects reported they had never been diagnosed with any central nervous system dysfunction such as epilepsy, cerebral palsy, or multiple sclerosis. Demographic characteristics of each group can be found in Table 1.
Figure 1.
Schematic explanation of subject groups. Rec. recreationally, SRC sport related concussion.
Table 1.
Descriptive statistics for each group with separated male and female data. Subjects were all Caucasian.
| Group | Sex (n) | Age (yrs.) | Mass (kg) | Concussion Hx (n) | Years of play (yrs.) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| Rec. active ctrl (n = 12) | 12 | 0 | 20.3 (1.5) | N/A | 68.4 (6.6) | N/A | 0 (0) | N/A | 0 (0) | N/A |
| Pre-match (n = 19) | 0 | 19 | N/A | 20.2 (4.6) | N/A | 73.3 (15.0) | N/A | 0 (0, 1.5) | N/A | 1.0 (1.0, 2.0) |
| Match-ctrl (n = 31) | 14 | 17 | 37.5 (24.3, 49.5) | 20.5 (2.9) | 93.6 (14.0) | 72.6 (14.4) | 2.0 (1.0, 2.8) | 0 (0, 1.0) | 11.5 (5.8, 26) | 1.0 (1.0, 3.0) |
| SRC (n = 17) | 14 | 3 | 24.5 (22.0, 27.5) | 18.3 (0.5) | 88.5 (9.4) | 63.6 (6.7) | 0.5 (0, 1.8) | 0 (0) | 7.0 (2.5, 9.5) | 1.0 (1.0, 1.5) |
All data reported as mean (SD) except when the SD is greater than 1/3 of the mean. In these cases data are reported as median (Q1, Q3). Rec. Active Ctrl = Recreationally active control group, Match-Ctrl = Match-Control group, SRC = Sport-related Concussion group, Hx = history, SD = standard deviation, Q1 = 1st quartile, Q3 = 3rd quartile.
Blood collection and processing
One four milliliter blood sample from subjects in all groups was obtained from a prominent vein in the antecubital space by standard venipuncture. Blood collection was performed in a laboratory setting for the recreationally active control group and occurred in a research tent pitchside for the pre-match, match-control and SRC groups. Our goal was to collect blood less than 60 min from the end of the match in the match-control subjects and less than 60 min from the impact that caused concussion in the SRC group. Blood was drawn into a silicone-coated serum tube and allowed to clot at ambient temperature for at least 30 min but no more than 60 min. After the ambient temperature incubation period the blood was centrifuged using a StatSpin Express 3 portable centrifuge (Beckman Coulter, Inc., Brea, CA USA) for five minutes at 5600 revolutions per minute (2685 relative centrifugal force) to separate the serum from red blood cells. Serum was then removed using a pipettor and placed in a cryotube which was placed in a cryobox and then stored in a −80 °C portable freezer (model ULT25NEU, STIRLING ULTRACOLD A division of Global Cooling, Inc., Athens, OH USA) until the sample could be placed in a permanent −80 °C freezer. The samples remained frozen at −80 °C until biomarker analysis.
Biomarker analysis
Serum UCHL1 concentration was measured using human UCHL1 ELISA-96-well plate assay kits (OKEH01247, Aviva Systems Biology Corporation, San Diego, CA USA). The detection range of the assays were 78–5,000 pg mL−1, with a limit of detection (LOD) less than 34 pg mL−1. A linear regression analysis was used to formulate a standard curve for each assay. The average intra-assay coefficient of variation was 16.3% and the inter-assay coefficient of variation was 6.66%.
Analysis of all standards and samples were performed in duplicate. All values found to be below the detection range of the assay were reported to be one-half the LOD. In addition, all values found to be below the blank value were reported as undetectable. Absorbance for all five assays were read at 450 nm using an Eon spectrophotometer (BioTek Instruments, Inc., Winooski, VT USA).
Statistical analyses
G*power analysis (G*Power 3.1.9.6)19 using previously published data16,20 determined that 12 subjects in each group were needed to obtain a power of 0.80 and effect size of 0.5.
A Kruskal–Wallis non-parametric test was used to compare medians of UCHL1, age, body mass, concussion history, and years of rugby play among groups due to unequal sample sizes and a lack of normal distribution as examined using a Shapiro–Wilk normality test. Partial eta squared (η2) was used to calculate effect size. The area under the receiver operating characteristic curve (AUROC) was calculated to determine the diagnostic accuracy of UCHL1 to distinguish between groups. Since Asken et al. found differences in resting UCHL1 concentration between males and females21 we decided to perform a separate statistical analysis comparing male data only and female data only among the protocols (i.e. pre-match females vs. match-control females, pre-match females vs. SRC females, match-control females vs. SRC females, recreationally active males vs. match-control males, recreationally active males vs. SRC males, and match-control males vs. SRC males). We also performed a separate statistical analysis comparing male and female data within the match-control and SRC groups (i.e. match-control females vs. match-control males and SRC females vs. SRC males). Since the Shapiro–wilk normality test also showed a lack of normality for the male and female match-control and SRC data, a two-sided Mann–Whitney U test was used to compare medians between sexes and a Cohen’s d (d) test was used to calculate effect size. A sensitivity analysis was performed to determine if age affects UCHL1 levels since age has been shown to affect the appearance of certain brain injury biomarkers, specifically neurofilament light (NfL)22, and because there was a statistical difference between the pre-match group and both the match-control and SRC groups. The sensitivity analysis removed data from subjects aged 40 years or older based upon outliers considered as a Z score less than − 1.5 or greater than 1.5 (a conservative Z score for determining outliers was used as using the typical Z score of 3 and − 3 would have only removed two subjects), which ended up being a total of 9 subjects. We used this same sensitivity analysis method to detect outliers in the time of blood draw in the match-control and SRC groups since there was a broad range in blood collection times. A sensitivity analysis was also conducted for serum UCHL1 concentration for each group. However, we used the more common Z score of − 3 and 3 as a cutoff value for UCHL1 concentration outliers. Statistical significance was determined at p < 0.05. All statistical analyses were generated using Statistical Package for the Social Sciences (SPSS) version 27 (SPSS Inc., Chicago, IL USA).
Ethics approval and consent to participate
The Appalachian State University (Boone, NC, USA) (IRB #s 20-0033, 19-0232, and 17-0345) and Rutgers University (New Brunswick, NJ, USA) (IRB# Pro20140000411) Institutional Review Board (IRB) for the Protection of Human Subjects approved this study.
Results
The study sample consisted of 79 total participants. We recruited 19 individuals pre-match, all of which were female. Post-match, 31 individuals (14 male and 17 female) consented to giving their blood, among which 5 female individuals also provided their blood pre-match. The recreational active male control group consisted of 12 individuals. Finally, blood sampling for UCHL1 was available for 17 athletes (14 male and 3 female) with SRC. Blood was collected an average of 42.3 min, (median = 16.0 min, Q1 = 10.0 min, Q3 = 45.0 min) post-match in the match-control group and blood was collected an average of 102.3 min (median = 37.0 min, Q1 = 10.0, Q3 = 156.0 min) post-concussion in the SRC group.
Demographic comparisons
In comparing demographic data among groups it was found that age was significantly greater in the match-control (median = 22.0 years, Q1 = 19.5 years, Q3 = 27.5 years, p = 0.010, η2 = 0.117) and SRC (median = 24.0 years, Q1 = 20.0 years, Q3 = 26.0 years, p = 0.022, η2 = 0.117) groups compared to the pre-match group (median = 19 years, Q1 = 18.5 years, Q3 = 20.0 years), but no other differences were found among groups (p > 0.05).
When comparing body mass among groups it was found that the SRC group (median = 87.3 kg, Q1 = 75.0 kg, Q3 = 90.9 kg) had a significantly greater body mass than the recreationally active control group (median = 68.0 kg, Q1 = 63.1 kg, Q3 = 72.9 kg) (p = 0.026, η2 = 0.138), but no other differences among groups (p > 0.05, η2 = 0.138) were found. Concussion history (number of past concussions) was significantly different between the match-control group (median = 1.0, Q1 = 0.0, Q3 = 2.0) and recreationally active control group (median = 0.0, Q1 = 0, Q3 = 0) (p = 0.002, η2 = 0.062), with no differences among any of the rugby groups (p > 0.05). Similarly, years of rugby play were all significantly greater in the rugby groups compared to the non-athlete group (p ≤ 0.002, η2 = 0.140), with no differences among the rugby groups (p > 0.05). Table 2 shows the comparison of demographic data among groups.
Table 2.
Descriptive statistics for combined sex data.
| Group | Age (yrs.) | Mass (kg) | Concussion Hx (n) | Years of play (yrs.) |
|---|---|---|---|---|
| Rec. active ctrl (n = 12) | 20.5 (19.5, 21.3) | 68.0 (63.1, 72.9) | 0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| Pre-match (n = 19) | 19.0 (18.5, 20.0) | 70.5 (63.6, 78.4) | 0.0 (0.0, 1.5) | 1.0 (1.0, 2.0)* |
| Match-ctrl (n = 31) | 22.0 (19.5, 27.5)+ | 81.8 (69.3, 96.1) | 1.0 (0.0, 2.0)* | 3.0 (1.0, 10.0)* |
| SRC (n = 17) | 24.0 (20.0, 26.0)+ | 87.3 (75.0, 90.9)* | 0.0 (0.0, 1.0) | 4.0 (2.0, 8.0)* |
Subjects were all Caucasian.
All data reported as median(Q1, Q3).
Rec. Active Ctrl = Recreationally active control group, Match-Ctrl = Match-Control group, SRC = Sport-related Concussion group, Hx = history, Q1 = 1st quartile, Q3 = 3rd quartile. * = statistically different compared to recreationally active control group (p < 0.05), + = statistically different compared to pre-match (p < 0.05).
Comparisons of UCHL1 concentration
Descriptive statistics of UCHL1 data used for analysis among all protocols can be seen in Table 3. There were no significant differences in serum UCHL1 concentration among all four groups (p = 0.186, η2 = 0.138). UCHL1 concentration was significantly higher in the match-control females (median = 124.3 pg mL−1, Q1 = 65.5 pg mL−1, Q3 = 261.8 pg mL−1) compared to the pre-match group (median = 17.0 pg mL−1, Q1 = 17.0 pg mL−1, Q3 = 73.6 pg mL−1) (p = 0.041, η2 = 0.080) but there were no differences in males among groups (p = 0.089, η2 = 0.303). Match-control female UCHL1 concentration (median = 124.3 pg mL−1, Q1 = 65.5 pg mL−1, Q3 = 261.8 pg mL−1) was significantly greater than match-control male UCHL1 concentration (median = 8.5 pg mL−1, Q1 = 0.0 pg mL−1, Q3 = 17.0 pg mL−1) (p = 0.002, d = 0.590), but there were no differences between the recreational active control group (median = 263.7 pg mL−1, Q1 = 0.0 pg mL−1, Q3 = 822.1 pg mL−1) and pre-match group (median = 17.0 pg mL−1, Q1 = 17.0 pg mL−1, Q3 = 73.6 pg mL−1) (p = 0.236, d = 1.148) or between female (median = 17.0 pg mL−1, Q1 = 8.5 pg mL−1, Q3 = 161.3 pg mL−1) and male (median = 17.0 pg mL−1, Q1 = 0.0 pg mL−1, Q3 = 17.0 pg mL−1) UCHL1 concentration in the SRC group (p = 0.591, d = 0.752). Descriptive statistics comparing UCHL1 concentration within sex-matched data and between male and female data can be seen in Table 3.
Table 3.
Serum ubiquitin carboxyl-terminal esterase L1 (UCHL1) concentration among groups and between sexes.
| Group | Male serum UCHL1 concentration (pg mL−1) | Female serum UCHL1 concentration (pg mL−1) | Sex combined serum UCHL1 concentration (pg mL−1) |
|---|---|---|---|
| Rec. active control group (n = 12 males) | 263.7 (0.0, 822.1) | N/A | 263.7 (0.0, 822.1) |
| Pre-match group (n = 19 females) | N/A | 17.0 (17.0, 73.6)+ | 17.0 (17.0, 73.6) |
| Match-control group (n = 14 males and 17 females) | 8.5 (0.0, 17.0) | 124.3 (65.5, 261.8)* | 54.0 (0.0, 197.0) |
| SRC group (n = 14 males and 3 females) | 17.0 (0.0, 17.0) | 17.0 (8.5, 161.3) | 17.0 (0.0, 17.0) |
All subjects were Caucasian. Values are presented as median (Q1, Q3). Rec. = Recreationally, SRC = Sport-related Concussion group, Q1 = 1st quartile, Q3 = 3rd quartile. * = Significantly different compared to male match-control group (p = 0.002), + = Significantly different compared to female match-control group (p = 0.041).
Diagnostic accuracy
The AUROC of UCHL1 comparing all groups can be found in Table 4. All comparisons show that UCHL1 did not distinguish between groups in this study (AUROC < 0.400, p > 0.05). We also compared sex-matched groups (Table 5) and found significance between the pre-match and female match-control group (AUROC = 0.745, p = 0.012). However, comparison of all other sex-matched groups showed that UCHL1 did not distinguish between groups (AUROC < 0.600, p > 0.05).
Table 4.
Area under the receiver operating characteristic curve (AUROC) comparing the different groups using serum.
| Group comparison | AUROC (95% CI) for UCHL1 |
|---|---|
| Pre-match vs. rec. active control | 0.371 (0.124–0.617), p = 0.232 |
| Match-control vs. rec. active control | 0.378 (0.154–0.601), p = 0.218 |
| Match-control vs. pre-match | 0.319 (0.140–0.499), p = 0.070 |
| SRC vs. rec. active control | 0.309 (0.088–0.530), p = 0.084 |
| SRC vs. pre-match | 0.373 (0.186–0.560), p = 0.194 |
| SRC vs. match-control | 0.363 (0.202–0.524), p = 0.121 |
Ubiquitin carboxyl-terminal esterase L1 (UCHL1) concentration. Values are presented as AUROC (95% confidence interval), p-value (p).
Rec. = recreationally; SRC = sport-related concussion.
Table 5.
Area under the receiver operating characteristic curve (AUROC) comparing sex-matched groups using serum.
| Group comparison | AUROC (95% CI) for UCHL1 |
|---|---|
| Pre-match vs. female match-control | 0.745 (0.574–0.915), p = 0.012* |
| Pre-match vs. female SRC | 0.474 (0.041–0.907), p = 0.886 |
| Females SRC vs. female match-control | 0.371 (0.000–0.758), p = 0.477 |
| Rec. active control vs. male match-control | 0.291 (0.092–0.489), p = 0.060 |
| Rec. active control vs. male SRC | 0.314 (0.110–0.518), p = 0.094 |
| Male SRC vs. male match-control | 0.521 (0.308–0.735), p = 0.844 |
Ubiquitin carboxyl-terminal esterase L1 (UCHL1) concentration. Values are presented as AUROC (95% confidence interval), p-value (p), Rec. = recreationally, SRC = Sport-related concussion, * = significantly different (p = 0.012).
Sensitivity analysis
Since age was shown to be a confounding variable in other brain-based biomarkers22 we removed nine subjects over the age of 40 and re-ran the statistics. Removal of these data did not affect statistical analysis so these subjects were left in the final data pool. A sensitivity analysis was also conducted on the time of blood collection in the match-control and SRC groups resulting in the removal of one subject from the match-control group and two subjects from the SRC group. Removal of these data points did not alter the statistical outcome so these subjects were left in the final data pool. The sensitivity analysis conducted on serum UCHL1 concentrations found one data point in the match-control female group (UCHL1 concentration = 2805.5 pg mL−1) to be an outlier. Upon further analysis of this subject’s demographic data and rugby match notes, we could not find a logical reason to disregard this subject’s data from analysis. However, it is important to note that when this data point was removed from analysis, the statistical difference between the pre-match and female match-control group is no longer statistically significant with a p-value of 0.069 compared to 0.041 when the data point is left in the data set. However, removal of this data point did not change the lack of significance among the sex combined groups nor did it affect significance, or lack thereof, for the AUROC of UCHL1.
Discussion
This investigation sought to determine if serum UCHL1 concentration from blood collected pitchside could distinguish among concussed rugby players and rugby players who played in a match but did not suffer a concussion, rugby players prior to match play, or recreationally active non-contact sport athletes. The data showed no significant differences in serum UCHL1 concentration among the four experimental groups and AUROC showed that UCHL1 concentration did not distinguish among groups. A large reason no differences were found among groups was due to the high variability of UCHL1 concentration among our subjects. High variability in serum UCHL1 concentration has been previously reported20 and may make it difficult to use UCHL1 as a biomarker of concussive injury.
Previous research has reported that athletes experiencing SRC did not have significantly higher UCHL1 levels compared to baseline in serum collected 10 h post-concussion16. However, when data analysis was limited to blood samples obtained less than four hours after injury, UCHL1 levels were significantly higher compared to baseline16. Our study collected blood samples a median of 37 min after concussion and may not have allowed for significant UCHL1 accumulation, thereby causing our results to not be significant among groups. Although not our primary hypothesis, we postulated that serum UCHL1 concentration may be higher in the match-control group compared to the pre-match and recreationally active group due to subconcussive injury. Subconcussive head injury may occur due to acceleration of the body, torso, or head and may occur during contact sport, such as rugby, despite lack of clinical concussion symptoms23. After analysis, it was found that the match-control subjects in our study did not have a significantly greater serum UCHL1 concentration than the recreationally active control group or the pre-match group. This finding is in agreement with a previous study in football players, in which serum UCHL1 concentration was not found to correlate with the number of sub-concussive head hits24. This study also agrees with our findings in that UCHL1 levels were unable to distinguish among post-game football players, emergency room patients who experienced mild traumatic brain injury, and healthy controls24. Although these studies agree with our findings, suggesting UCHL1 to be an unreliable biomarker of brain injury, other sports-related studies have shown more promise for UCHL1 as a biomarker of brain injury. For example, serum UCHL1 levels were reported to be significantly greater between football players experiencing high-acceleration head impacts compared to those not experiencing high-acceleration head impacts25. In our study, we did not separate rugby players based on the number or magnitude of head impacts so it is possible that our match-control group consisted of players experiencing a low magnitude and quantity of hits, and therefore a lack of damage to neurons which would have resulted in elevated serum UCHL1 concentrations. Two other studies investigating concussed football players found that serum UCHL1 concentration was significantly elevated post-concussion compared to baseline with a fair ability to distinguish between SRC and contact athlete controls or non-athlete controls17,18. Blood in these two studies was collected 6 h (median of 3.8 h17 and median of 2 h18) post-concussion and may be the critical difference in their findings compared to ours. In our study blood draws were taken on average 102.29 min (1 h and 42 min) with a median time of 37 min post-SRC. Furthermore, in trauma patients experiencing mild to moderate TBI, although the presence of UCHL1 was found in serum one-hour post injury, it was not until 8-h post-injury that UCHL1 levels were found to peak15. Due to the timing of our blood draws, we may not have seen large increases in serum UCHL1 concentration due to the kinetics of UCHL1 appearing in the blood. In a one-compartment kinetic model developed by Aziz et al. (2021), it was determined that UCHL1 levels likely reach a maximum appearance in the blood at 8 h following concussion, while gradually increasing up to that point26. Brophy et al. investigated the kinetics of UCHL1 levels in severe traumatic brain injury serum obtained 6 h after injury up to 7 days post-injury and found maximum UCHL1 concentration in serum appeared approximately 9 h after severe traumatic brain injury6. Our findings seem to support both of these kinetic models predicting that more time may need to elapse before UCHL1 is able to accumulate in the blood and indicate brain injury. Kinetics of UCHL1 appearance in the blood may point to difficulty in using UCHL1 levels to diagnose concussion at the sideline of athletic events when diagnosis must be made almost immediately post-injury to protect the athlete. Based on our results, and those of other studies on concussed athletes, UCHL1concentration does not appear to be a viable a biomarker in diagnosing SRC at the sideline of athletic events as it has shown to be in mild or moderate traumatic brain injury patients10. Although, similar results to our findings have also been shown when examining blood concentrations of UCHL1 in adult and pediatric trauma patients.
A study investigating adult and pediatric trauma patients between 4 and 180 h post injury did not find significant differences in serum UCHL1 concentration between concussion patients and head trauma control or body trauma control patients27. Another study investigating brain injury in children under the age of 15 years found that serum UCHL1 concentration in blood collected within 24 h of head injury was significantly greater in subjects with moderate and severe TBI compared to healthy controls but no difference in UCHL1 concentration was found between mild TBI subjects and healthy controls28. In a study investigating plasma UCHL1 concentration in blood samples collected between 2 and 6 h post injury in children with mild head injury, it was found that plasma UCHL1 levels were below the detection limit of the assay (0.78 ng/mL) for all subjects29. Based on these studies it may be possible that concussion does not necessarily cause enough neurotrauma to significantly increase UCHL1 levels, and UCHL1 concentration may be better at indicating more severe types of brain injury than typically seen during athletic events.
Very little research has compared UCHL1 levels in male and female athletes. Asken et al. (2018) found that male athletes tend to have higher median serum baseline UCHL1 levels than female athletes21. Our study agrees with this finding as values for the pre-match group (solely composed of females) had a lower, although not statistically significant, UCHL1 concentration than the recreationally active control group (solely composed of males). However, the lack of statistical significance may be due to the male group being composed of recreationally active individuals not participating in a contact sport whereas the female group was composed of in-season rugby players who may have had elevated UCHL1 levels. In addition to this difference in sexes, we found statistically significant sex differences within the match-control group. In this group the data was inverse to that of the baseline data, with females having higher UCHL1 levels than males. Females may have had higher UCHL1 levels post-match compared to males due to differences in neck size and strength between females and males. Research has shown that females are at greater risk for sustaining concussion than males, possibly due to female athletes having smaller and weaker neck muscles30. Females have also shown significantly greater head-neck segment peak angular acceleration and displacement than males when subjected to external loads to simulate real-life sport situations31. Because of this, the subconcussive impacts experienced during a rugby match may cause more neuronal damage in female athletes compared to male athletes, and therefore greater serum UCHL1 concentration. However, since neck girth or strength were not investigated in the current study, this hypothesis is purely speculative.
Because of these sex differences, we decided to compare sex-match data among the different groups in our study. After doing this, we found that the female match-control group had significantly higher serum UCHL1 concentration than the pre-match group and the AUROC was also significant for distinguishing between the female match-control group and pre-match group. However, there were still no significant differences among the male matched data groups. These comparisons seem to support the hypothesis that subconcussive hits may result in more neurotrauma in female athletes compared to their male counterparts.
There are several limitations in our study. Our sample population was small and lacked matching in age, sex, years of play, and concussion history. Our data also lacked paired data from pre- to post-match and pre-match to post-SRC. Paired data would have been beneficial in determining if UCHL1 levels could distinguish match-play and concussion within individuals. Another limitation is that we only obtained blood samples at one time point and the time points had large variation from 5 to 303 min post-match and 6 min to 480 min post-concussion. It would have been ideal to collect initial blood samples at the same time post-match and post-concussion, along with analyzing how UCHL1 accumulated in the serum at later time points. This would also have made our data more comparable to other studies. Relying on on-site ATCs not involved in the study to diagnose concussion is also a limitation, as ATCs have reported moderate efficacy in diagnosing concussion. This is a necessary limitation as current SRC diagnosis largely relies on athlete assessment by ATCs. A lack of concussion assessment on the match-control subjects is also a limitation since it is possible that some of the subjects in the match-control group were concussed even though they reported not having any symptoms of concussion.
To the best of our knowledge this is the first study focused on analyzing serum UCHL1 concentration from blood samples obtained soon after SRC. No difference in serum UCHL1 concentration was found in any of the combined sex groups, suggesting that UCHL1 levels may not be a viable biomarker for sideline SRC diagnosis at early timepoints. However, as indicated by other studies, UCHL1 levels may be useful in diagnosing SRC at later time points, specifically 8 h or more after concussion. Interestingly, sex differences in UCHL1 concentration were found, indicating the need for sex-matched groups in future studies investigating UCHL1 levels. In order for biomarkers to be used in sideline concussion diagnosis future research must focus on collecting blood within one-hour post injury along with later time points. As seen with past studies investigating UCHL1 concentration, blood collection at later time points such as 4–8 h post-injury may be useful in distinguishing concussed from non-concussed subjects but, as indicated in our study, blood collected soon after injury may not provide the same results.
Acknowledgements
We thank undergraduate research assistants Chandler Bartol, Melina Gonzalez, Ashlyn Loring, Elisia Holderfield, and Elise Pigue for their help with subject recruitment and blood processing during portions of this study.
Author contributions
J.O.H.—involved in data analysis and manuscript writing. J.E.M.—involved in experimental design, data collection, and data analysis. S.D.B., I.C.S.—involved in data collection and data analysis. J.S.B.—involved in experimental design. A.K., J.M.S.—involved in experimental design and data collection. M.J.R.—involved in experimental design, data collection, data analysis, and manuscript writing. All authors read and approved the final manuscript.
Funding
The Beaver College of Health Sciences Summer Scholarly Support Program, Appalachian State University, Boone, NC funded this study but did not play any role in the design of the study, data collection, data analysis, interpretation of the data, or in writing the manuscript.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McCrory P, et al. Consenus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br. J. Sports Med. 2017;51:838–847. doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 2.Harmon KG, et al. American medical society for sports medicine position statement on concussion in sport. Clin. J. Sport Med. 2019;29:87–100. doi: 10.1097/JSM.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 3.Savage JL, Covassin T. The self-efficacy of certified athletic trainers in assessing and managing sport-related concussions. J. Athl. Train. 2018;53:983–989. doi: 10.4085/1062-6050-394-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huibregtse ME, Bazarian JJ, Shultz SR, Kawata K. The biological significance and clinical utility of emerging blood biomarkers for traumatic brain injury. Neurosci. Biobehav. Rev. 2021;130:433–447. doi: 10.1016/j.neubiorev.2021.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong B, Leznik E. The role of ubiquitin C-terminal hydrolase L1 in neurodegenerative disorders. Drug News Perspect. 2007;20:365–370. doi: 10.1358/dnp.2007.20.6.1138160. [DOI] [PubMed] [Google Scholar]
- 6.Brophy GM, et al. Biokinetic analysis of ubiquitin C-terminal hydrolase-L1 (UCH-L1) in severe traumatic brain injury patient biofluids. J. Neurotrauma. 2011;28:860–870. doi: 10.1089/neu.2010.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mondello S, et al. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: A case contral study. Crit. Care. 2011;15:R156. doi: 10.1186/cc10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mondello S, et al. Glial neuronal ratio: A novel index for differentiating injury type in patients with severe traumatic brain injury. J. Neurotrauma. 2012;29:1096–1104. doi: 10.1089/neu.2011.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz-Arrastia R, et al. Acute biomarkers of traumatic brain injury: Relationships between plasma levels of ubiquitin c-terminal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma. 2014;31:19–25. doi: 10.1089/neu.2013.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welch RD, et al. Ability of serum glial fibrillary acidic protein, ubiquitin c-terminal hydrolas-L1, and S100B to differentiate normal and abnormal head computed tomography findings in patients with suspected mild or moderate traumatic brain injury. J. Neurotrauma. 2016;33:203–214. doi: 10.1089/neu.2015.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papa L, et al. Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma control and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J. Trauma Acute Care Surg. 2012;72:1335–1344. doi: 10.1097/TA.0b013e3182491e3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papa L, et al. Neuronal biomarker ubiquitin c-terminal hydrolase detects traumatic intracranial lesions on computed tomography in children and youth with mild traumatic brain injury. J. Neurotrauma. 2017;34:2132–2140. doi: 10.1089/neu.2016.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bazarian JJ, et al. Serum GFAP and UCH-L1 for prediction of absence of intracreanial injuries on head CT (ALERT-TBI): A multicentre observational study. Lancet. Neurol. 2018;17:782–789. doi: 10.1016/S1474-4422(18)30231-X. [DOI] [PubMed] [Google Scholar]
- 14.Bazarian JJ, et al. Accuracy of a rapid glial fibrillary acidic protein/ubiquitin carboxyl-terminal hydrolase L1 test for the prediction of intracranial injuries on head computed tomography after mild traumatic brain injury. Acad. Emerg. Med. 2021;28:1308–1317. doi: 10.1111/acem.14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papa L, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 2016;73:551–560. doi: 10.1001/jamaneurol.2016.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asken BM, et al. Concussion BASICS III: Serum biomarker changes following sport-related concussion. Neurology. 2018;91:e2133–e2143. doi: 10.1212/WNL.0000000000006617. [DOI] [PubMed] [Google Scholar]
- 17.Meier TB, et al. A prospective study of acute blood-based biomarkers for sport-related concussion. Ann. Neurol. 2020;87:907–920. doi: 10.1002/ana.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier TB, et al. Prospective assessment of acute blood markers of brain injury in sport-related concussion. J. Neurotrauma. 2017;34:3134–3142. doi: 10.1089/neu.2017.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 20.Asken BM, et al. Concussion biomarkers assessed in collegiate student-athletes (BASICS) I. Neurology. 2018;91:e2109–e2122. doi: 10.1212/WNL.0000000000006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asken BM, et al. Concussion BASICS II: Baseline serum biomarkers, head impact exposure, and clinical measures. Neurology. 2018;91:e2123–32132. doi: 10.1212/WNL.0000000000006616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalil M, et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018;14:577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 23.Bailes JE, Petraglia AL, Omalu BI, Nauman E, Talavage T. Role of subconcussion in repetitive mild traumatic brain injury. J. Neurosurg. 2013;119:1235–1245. doi: 10.3171/2013.7.JNS121822. [DOI] [PubMed] [Google Scholar]
- 24.Puvenna V, et al. Significance of ubiquitin carboxy-terminal hydrolase l1 elevations in athletes after sub-concussive head hits. PLoS ONE. 2014;9:e96296. doi: 10.1371/journal.pone.0096296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph, J. R. et al. Elevated markers of brain injury as a result of clinically asymptomatic high-acceleration head impacts in high-school football athletes. J. Neurosurg. 1–7 (2018) (Epub ahead of print). [DOI] [PubMed]
- 26.Azizi, S. et al. A kinetic model for blood biomarker levels after mild traumatic brain injury. Front. Neurol. (2021) (ecollection). [DOI] [PMC free article] [PubMed]
- 27.Papa L, et al. Evaluating glial and neuronal blood biomarkers GFAP and UCH-L1 as gradients of brain injury in concussive, subconcussive and non-concussive trauma: A prospective cohort study. BMJ Paediatr. Open. 2019;3:1–13. doi: 10.1136/bmjpo-2019-000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger RP, Hayes RL, Richichi R, Beers SR, Wang KKW. Serum concentrations of ubiquitin C-terminal hydrolase-L1 and αII-spectrin breakdown product 145 kDa correlate with outcome after pediatric TBI. J. Neurotrauma. 2012;29:162–167. doi: 10.1089/neu.2011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tylicka, M. & Matuszczak, E. BDN and IL-8, but not UCHL-1 and IL-11, are markers of brain injury in children caused by mild head trauma. [DOI] [PMC free article] [PubMed]
- 30.Zuckerman SL, et al. Epidemiology of sports-related concussion in NCAA athletes from 2009–2010 to 2013–2014. Am. J. Sports Med. 2015;43:2654–2662. doi: 10.1177/0363546515599634. [DOI] [PubMed] [Google Scholar]
- 31.Tierney RT, et al. Gender differences in head-neck segment dynamic stabilization during head acceleration. Med. Sci. Sports Exerc. 2005;37:272–279. doi: 10.1249/01.MSS.0000152734.47516.AA. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.