Abstract
An 80-year-old woman with myelofibrosis sought evaluation for progressive dyspnea. Her past medical history included essential thrombocytosis, which transformed to myelofibrosis. Inspiratory computed tomography of chest showed diffuse mosaic attenuation with lymphadenopathy. Flexible bronchoscopy with lymph node and pulmonary parenchymal cryo biopsy revealed nodular deposits of extramedullary hematopoiesis in lung parenchyma and moderate to severe vascular medial and intimal thickening of pulmonary vasculature consistent with pulmonary parenchymal extramedullary hematopoiesis associated with pulmonary hypertension (a rare compensatory mechanism in myeloproliferative disorders). In this report, we explore the manifestations, pathogenesis, treatment, and prognosis of pulmonary extramedullary hematopoiesis reported in the literature.
Keywords: Extramedullary hematopoiesis, Myelofibrosis, Mosaic attenuation
Abbreviations: BAL, bronchoalveolar lavage; CT, computed tomography; DLCO, diffusion capacity of lung for carbon monoxide; EBUS, endobronchial ultrasound; EMH, extramedullary hematopoiesis; FEV1, forced expiratory volume in 1; FNAB, fine-needle aspiration biopsy; FVC, forced vital capacity; LPM, liters per minute; MPO, myeloperoxidase; NSIP, nonspecific interstitial pneumonia; PEMH, pulmonary extramedullary hematopoiesis; PH, pulmonary hypertension; PFT, Pulmonary function testing; RES, reticuloendothelial system; TBLC, transbronchial lung cryobiopsy; TBNA, transbronchial needle aspiration; TLC, total lung capacity
1. Introduction
Diffuse mosaic attenuation of lung (area of variable attenuation) on the computed tomography (CT) is a common pattern. The mosaic pattern of attenuation is nonspecific and can reflect the diseases of alveoli, small airways, pulmonary interstitium, and/or pulmonary vasculature. We report a case of rare etiology of diffuse mosaic attenuation on CT chest in a patient with myelofibrosis.
2. Case vignette
An 80-year-old woman with myelofibrosis sought evaluation for progressive dyspnea and hypoxemia. Her past medical history included essential thrombocytosis (BCR-ABL negative) diagnosed in 2011, which transformed to myelofibrosis diagnosed in 2013, JAK-2 negative, del (13q) in 69% of cells, diabetes mellitus type-2, and hypertension. Treatment of essential thrombocytosis and myelofibrosis included hydroxyurea (2012), anagrelide (2012–2013), ruxolitinib (2016), and fedratinib (01/2020 to time of evaluation).
She reported worsening dyspnea with exertion and prolonged speech, despite consistent use of supplemental oxygen at 2 L per minute (LPM)She also endorsed fatigue that was historically associated with anemia for which she received periodic transfusions. She denied cough, hemoptysis, recent weight change, easy bruising, fever, chills, night sweats, chest pain, palpitations, orthopnea, or lower extremity edema. She was a former smoker with a 5 pack-year history and quit over 50 years ago. She denied any environmental or occupational exposures or international travel.
She was afebrile, had a regular heart rate of 79 beats per minute, a blood pressure 170/73 mmHg, a respiratory rate of 16 breaths per minute, and was saturating 86% on room air and 100% on 2 LPM. She was in no apparent distress and was able to converse without difficulty. Cardiac examination revealed normal S1 and S2, without 3rd or 4th heart sounds, murmurs, rubs, and jugular venous distension. Lung auscultation demonstrated bibasilar crackles without wheezing. There was no evidence of pedal edema, clubbing, cyanosis, ecchymosis, lymphadenopathy, hepatomegaly, or splenomegaly.
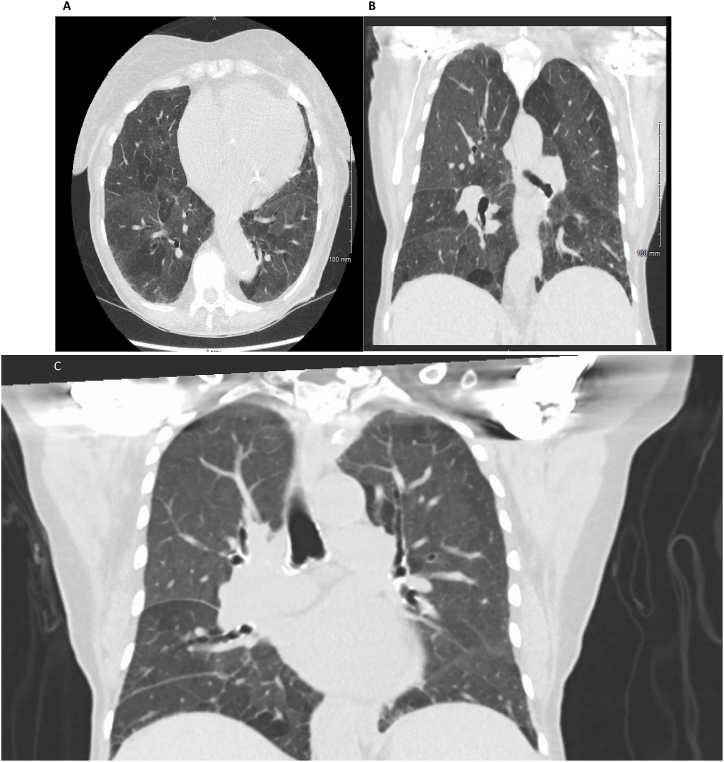
Complete blood count revealed a hemoglobin of 9.2g/dl, a white blood cell count of 10.8x10e3/μL with a differential of 55% neutrophils, 12% lymphocytes, 9% monocytes, 3% eosinophils, 1% basophils, 5% bands, 5% metamyelocytes, 9% myelocytes, and 1% promyelocyte, and a platelet count of 139x10e3/μL. Peripheral blood smear showed 2+ polychromasia, 1+ basophilic stippling, 2+ tear drop cells, 1+ microcytes, 1+ ovalocytes, 2+ anisocytosis, and 1 nucleated red blood cell. Chest x-ray was performed showing nonspecific interstitial markings. Inspiratory and expiratory computed tomography (CT) chest showed diffuse mosaic attenuation, enlarged mediastinal and hilar lymphadenopathy (Fig. 1A and B& C), enlarged pulmonary trunk (3.4 cm), and enlarged central pulmonary arteries. Pulmonary function testing (PFT) revealed a forced vital capacity (FVC) of 1.88 L (80% predicted), forced expiratory volume in 1 second (FEV1) of 1.5 L (87% predicted),FVC/FEV1 of 80%, total lung capacity (TLC) of 3.71 L (86% predicted), and diffusion capacity (DLCO) of 55% predicted. Transthoracic echocardiogram showed normal left ventricle size with normal systolic (EF of 62%) and diastolic function. There was normal right ventricle size and systolic function. Estimated pulmonary artery systolic pressure was 39 mm Hg.
Fig. 1.
CT chest showing diffuse mosaic attenuation in the (a) axial and (b) coronal views.
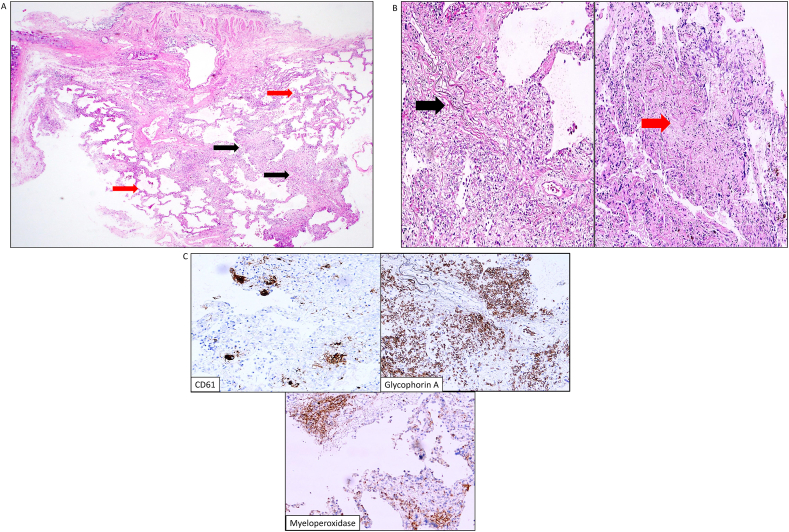
Given her dyspnea, PFT results, and new imaging findings, the differential diagnosis remained broad. The patient noticed mild improvement in dyspnea with prednisone and doxycycline but got worse when they were discontinued. The potential etiologies were atypical infection, small airway disease, central thromboembolic pulmonary hypertension, drug induced lung disease secondary to fedratinib, hypersensitivity pneumonitis, extramedullary hematopoiesis, and vasculitis. To narrow down the diagnosis, bronchoscopy with bronchoalveolar lavage (BAL), transbronchial lung cryobiopsy (TBLC) of the right lower lobe, and endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration (TBNA) of a right paratracheal lymph node was performed. Bacterial, fungal, and mycobacterial cultures were negative. Transbronchial lung cryobiopsy revealed expansile, vaguely nodular deposits of extramedullary hematopoiesis in a background of diffuse, homogeneous interstitial thickening (nonspecific interstitial pneumonia (NSIP) pattern) (Fig. 2A) and medium sized blood vessels with moderate to severe vascular medial and intimal thickening with disrupted/reduplicated elastica (Fig. 2B). Immunohistochemistry showed trilinear hematopoiesis with cluster of differentiation 61 (CD61) positive megakaryocytes, focal aggregates of glycophorin A positive erythroid precursors, and rare myeloperoxidase (MPO) positive myeloid precursors (Fig. 2C). Mediastinal lymph node transbronchial needle aspiration showed numerous atypical polylobated megakaryocytes, highlighted by CD61 immunostain, and erythroid and myeloid precursors at various stages of maturation, highlighted by E-cadherin and MPO, respectively, without any blasts in the background of small lymphocytes consistent with pulmonary extramedullary hematopoiesis (PEMH).
Fig. 2.
Histology from right lower lobe transbronchial lung cryobiopsy. (a). Low-power view reveals central lung parenchyma with overlying bronchial mucosa. There are expansile, vaguely nodular deposits of extramedullary hematopoiesis (black arrow) in a background of diffuse, homogeneous interstitial thickening (red arrow). H&E (4X). (b). Medium sized blood vessels showing moderate (black arrow) to severe (red arrow) vascular medial and intimal thickening with disrupted elastica. H&E (10x). (c). Immunohistochemistry highlights trilinear hematopoiesis. CD61, glycophorin A, and myeloperoxidase reveal frequent presence of megakaryocytes, erythroid, and myeloid precursors, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Because our patient demonstrated progression of her myelofibrosis with evidence of PEMH, she elected to proceed with enrollment in a clinical trial utilizing CPI-0610 for potential treatment. Since initiation of therapy, she has reported stable symptoms of dyspnea and has required 2 L of supplemental oxygen while resting and 4 L while ambulating. Right heart catheterization revealed mean pulmonary artery pressure of 28 mm Hg, pulmonary artery wedge pressure of 4 mm Hg, and pulmonary vascular resistance of PVR 4.3–4.6 wood units suggestive of pulmonary artery hypertension. Ventilation perfusion scan ruled out chronic thromboembolic PH. She is scheduled for pulmonary vasodilatory therapy.
3. Discussion
Extramedullary hematopoiesis (EMH) is the production of hematopoietic stem and progenitor cells outside the bone marrow [1]. During human embryonic and fetal development, hematopoiesis first occurs in extramedullary anatomical sites like the yolk sac, chorion, and dorsal aorta. The hematopoiesis then migrates to the fetal liver and spleen, and finally colonizes the bone marrow just before birth transitioning to definitive phase hematopoiesis [1]. After birth, EMH is considered abnormal but may occur if the bone marrow becomes uninhabitable due to fibrosis, lymphoma, leukemia, thalassemia, essential thrombocytosis, or hemolytic disorders [[2], [3]]. Most commonly, it occurs in the liver, spleen, lymph nodes, and para-spinal region. Rarely, other organs may also be involved, including the pleura, lungs, pericardium, mediastinum, central nervous system, prostate, gastrointestinal track, retroperitoneum, ovaries, and breasts [2].
Pulmonary extramedullary hematopoiesis is a rare manifestation, with many reported cases related to myelofibrosis and other myeloproliferative neoplasms. There are several hypotheses, including the splenic filtration theory, in which displaced hematopoietic stem cells in the vasculature are filtered and accumulate in the spleen, with splenectomy allowing the cells to accumulate in other sites. It does not explain the development of EMH in organs and tissues that do not filter blood. The compensatory phenomenon theory attempts to explain EMH as a response to myelofibrosis (as in this case). It too does not explain all cases, as EMH can arise in the absence of marrow fibrosis and anemia. The myelostimulatory theory suggests the possibility of up regulation of hematopoiesis in the bone marrow and extra medullary sites by apparent secretion of yet unknown factor(s). Somewhat related, the redirected differentiation theory suggests that circulating factors (e.g., cytokines such as interleukins-12 and 13) induce adult stem cell populations to differentiate into hematopoietic lineage cells. None of the explanations are definitive and remain postulations without direct experimental evidence linking them to EMH.
PEMH patients may present as asymptomatic or with varying findings, including hemoptysis, acute or progressive dyspnea, chest pain, and various nonspecific symptoms like fatigue, fever, night sweats, or pruritis [1,4,5]. While rare, life-threatening complications such as thrombotic events, hemorrhagic pleural effusion, alveolar hemorrhage, cardiac tamponade, or acute spinal cord compression attributed to mediastinal or epidural EMH are also reported [1,[4], [5], [6]]. Radiographic findings in PEMH vary widely and include ground glass opacities, pulmonary nodules/masses, interstitial infiltrates, fibrosis, consolidation, pleural effusion, and septal thickening [7,8]. Given these variable imaging findings, assessing for a broad differential diagnosis, including drug induced lung injury based on prior treatments, volume overload, infection, and possible leukemic transformation, is imperative.
Pulmonary hypertension (PH) has been reported in 55–60% of patients with PEMH [16]. It has been attributed to various mechanisms. Chronic thromboembolism with occlusion of pulmonary vasculature due to thrombocytosis (from increased platelet activation and platelet derived growth factor secretion), high cardiac output states - either drug-induced (resulting in pulmonary venoocclusive disease or arterial hypertension) or as a result of aberrant JAK/Src-STAT3 signaling pathway (resulting in inflammation, hyperproliferative state and angiogenesis), or direct pulmonary vascular remodeling by myeloid cells, have all been postulated as underlying mechanisms. In our patient, there was significant interstitial fibrosis in the NSIP pattern, which could have played a part in the pulmonary hypertensive changes noticed in the cryobiopsy specimen. The prognosis is reported to be poor in patients with myeloproliferative disorders and concurrent pulmonary hypertension. In a case series of twenty-six patients by Dingli et–al., it was reported that the patients with pulmonary hypertension ascribed to myelofibrosis or various myeloproliferative diseases had a median survival of 18 months [9]. Although data are limited, it may be reasonable to consider screening patients with myelofibrosis and dyspnea for pulmonary hypertension to prognosticate disease course.
The diagnosis of PEMH can be established with fine needle aspiration or tissue biopsy of the lung or cytologic analysis of pleural fluid. Fine-needle aspiration biopsy (FNAB) may be adequate for a diagnosis of EMH in many cases, with reduced risk of complications including hemorrhage. FNAB diagnosis relies upon cellularity consisting of dispersed trilinear hematopoietic elements. Lung biopsies (surgical open lung biopsies or transbronchial cryobiopsies, as in this case) have the advantage of highlighting EMH, as well as parenchymal and vascular changes, which may influence disease course and treatment. In this case, the biopsy revealed extensive EMH on a background of diffuse interstitial fibrosis (NSIP pattern) with pulmonary hypertensive changes. This case exemplifies the ability of a transbronchial cryobiopsy to provide adequate tissue for diagnosis and evaluation of parenchymal/vascular changes, while offsetting some of the risks associated with a surgical lung biopsy.
Noninvasive diagnosis of PEMH relies on whole-body bone marrow scintigraphy with use of radiotracers, such as technetium Tc-99M sulfur colloid, which after injection is phagocytized by the reticuloendothelial system (RES). Because RES and erythropoietic tissues coexist in the bone marrow, the injected colloid distribution serves as a surrogate marker of erythropoietic activity in extramedullary sites. Bone marrow scintigraphy is about 90% accurate for diagnosing EMH [11]. False negatives may occur if there is minimal medullary stroma present or if there is low cellular activity.
Treatment options for PEMH include blood transfusion, medical therapy, external beam radiation therapy, and surgical management. Given the rarity of this process, there are no clear guidelines. Only case reports are available for each treatment modality. Medical therapy with hydroxyurea has been shown to improve clinical symptoms. While its mechanism is unknown, hydroxyurea has been shown to stimulate fetal hemoglobin synthesis in thalassemia patients and induce a cytoreductive effect, which may contribute to a reduction of EMH in general and for those patients with PEMH specifically [12]. There are also cases of successful treatment of PEMH with the JAK1/2 inhibitor ruxolitinib (RUX) [13,14]. Preliminary clinical data from a phase two study using CPI-0610, a small molecule inhibitor of bromodomain and extra-terminal (BET) proteins, as monotherapy or as add-on to ruxolitinib for patients with myelofibrosis showed spleen volume reduction, symptom alleviation, hemoglobin and platelet count improvement, reduction in transfusion dependence, suppression of proinflammatory cytokines, and improvement in bone marrow fibrosis [15]. In contrast, case reports using steroids for treatment have shown poor outcomes [7].
Because EMH tumors are extremely radiosensitive, low-dose radiation therapy has been used successfully for patients with PEMH manifesting as pleural effusions and interstitial lung disease. Based on the limited case series level evidence, the recommended dose of radiation therapy is in the 100–2000 cGy range [10]. Acute spinal cord or nerve compression attributed to EMH needs emergent surgical decompression and excision of the mass to prevent permanent injury. Mass effect from EMH has been known to cause acute gastrointestinal or bladder outlet obstruction requiring surgical debulking. Chemical pleurodesis with sclerosing agents such as talc or tetracycline can worsen the hemorrhagic pleural effusion by irritating the EMH tissue and should be avoided [7]. Neoadjuvant radiation therapy followed by chemical pleurodesis is superior as a combined modality for EMH related recurrent hemothorax.
4. Conclusions
While rare, pulmonary extramedullary hematopoiesis (PEMH) should be considered for patients with myelofibrosis, respiratory symptoms, and chest imaging abnormalities. Diagnosis of PEMH often requires lung tissue as nuclear medicine imaging modalities may be falsely negative. Transbronchial lung cryobiopsy offers the opportunity to procure sufficient tissue for diagnosis of PEMH and associated changes, which can influence disease course and management without significant risk of complications. Because patients with PEMH related pulmonary hypertension may have a worse prognosis, a thorough evaluation for pulmonary hypertension in this patient population is warranted. Treatment of PEMH focuses on medical therapy for myelofibrosis with various agents; guidelines are not available.
Statement of ethics
-
A.
Study approval statement: As per our institution policy (Medical College of Wisconsin Affiliated hospitals), ethical approval is not required for case reports.
-
B.
Consent to publish statement: Written informed consent was obtained from the patient which included publication of images.
Funding sources
The authors report no funding sources.
Author contributions
Dr. Singh, Jani contributed to writing the manuscript. Drs. Benn and Kurman, and Rao provided essential revisions. Dr. Rao contributed the pathology images.
Data availability statement
All the relevant data can be provided upon request.
Declaration of competing interest
The authors report no conflicts of interest.
References
- 1.Mack R., Zhang L., Breslin Sj P., Zhang J. The fetal-to-adult hematopoietic stem cell transition and its role in childhood hematopoietic malignancies. Stem Cell Rev. Rep. 2021 Aug 23 doi: 10.1007/s12015-021-10230-x. Epub ahead of print. PMID: 34424480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan N., Lavu S., Hanson C.A., Tefferi A. Extramedullary hematopoiesis in the absence of myeloproliferative neoplasm: mayo Clinic case series of 309 patients. Blood Cancer J. 2018 Nov 19;8(12):119. doi: 10.1038/s41408-018-0156-6. PMID: 30455416; PMCID: PMC6242913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh I., Mikita G., Green D., Risquez C., Sanders A. Pulmonary extra-medullary hematopoiesis and pulmonary hypertension from underlying polycythemia vera: a case series. Pulm. Circ. 2017 Mar 10;7(1):261–267. doi: 10.1177/2045893217702064. PMID: 28680586; PMCID: PMC5448544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgiades C.S., Neyman E.G., Francis I.R., Sneider M.B., Fishman E.K. Typical and atypical presentations of extramedullary hemopoiesis. AJR Am. J. Roentgenol. 2002 Nov;179(5):1239–1243. doi: 10.2214/ajr.179.5.1791239. PMID: 12388506. [DOI] [PubMed] [Google Scholar]
- 5.Rumi E., Passamonti F., Boveri E., De Amici M., Astori C., Braschi M., Castagnola C., Magrini U., Cazzola M., Lazzarino M. Dyspnea secondary to pulmonary hematopoiesis as presenting symptom of myelofibrosis with myeloid metaplasia. Am. J. Hematol. 2006 Feb;81(2):124–127. doi: 10.1002/ajh.20509. PMID: 16432861. [DOI] [PubMed] [Google Scholar]
- 6.Scott I.C., Poynton C.H. Polycythaemia rubra vera and myelofibrosis with spinal cord compression. J. Clin. Pathol. 2008 May;61(5):681–683. doi: 10.1136/jcp.2007.053751. PMID: 18441161. [DOI] [PubMed] [Google Scholar]
- 7.Bowling M.R., Cauthen C.G., Perry C.D., Patel N.P., Bergman S., Link K.M., Sane A.C., Conforti J.F. Pulmonary extramedullary hematopoiesis. J. Thorac. Imag. 2008 May;23(2):138–141. doi: 10.1097/RTI.0b013e31815b89aa. PMID: 18520574. [DOI] [PubMed] [Google Scholar]
- 8.Marchiori E., Escuissato D.L., Irion K.L., Zanetti G., Rodrigues R.S., Meirelles G.S., Hochhegger B. Extramedullary hematopoiesis: findings on computed tomography scans of the chest in 6 patients. J. Bras. Pneumol. 2008 Oct;34(10):812–816. doi: 10.1590/s1806-37132008001000009. English, Portuguese, PMID: 19009214. [DOI] [PubMed] [Google Scholar]
- 9.Dingli D., Utz J.P., Krowka M.J., Oberg A.L., Tefferi A. Unexplained pulmonary hypertension in chronic myeloproliferative disorders. Chest. 2001 Sep;120(3):801–808. doi: 10.1378/chest.120.3.801. PMID: 11555513. [DOI] [PubMed] [Google Scholar]
- 10.Weinschenker P., Kutner J.M., Salvajoli J.V., Hanriot R.M., Ribeiro A.F., Capelozzi V.L., Del Giglio A. Whole-pulmonary low-dose radiation therapy in agnogenic myeloid metaplasia with diffuse lung involvement. Am. J. Hematol. 2002 Apr;69(4):277–280. doi: 10.1002/ajh.10075. PMID: 11921022. [DOI] [PubMed] [Google Scholar]
- 11.Yang M., Roarke M. Diffuse pulmonary extramedullary hematopoiesis in myelofibrosis diagnosed with technetium-99m sulfur colloid bone marrow scintigraphy and single photon emission computerized tomography/CT. Am. J. Hematol. 2017 Mar;92(3):323–324. doi: 10.1002/ajh.24616. Epub 2017 Jan 23. PMID: 27883206. [DOI] [PubMed] [Google Scholar]
- 12.Meo A., Cassinerio E., Castelli R., Bignamini D., Perego L., Cappellini M.D. Effect of hydroxyurea on extramedullary haematopoiesis in thalassaemia intermedia: case reports and literature review. Int J Lab Hematol. 2008 Oct;30(5):425–431. doi: 10.1111/j.1751-553X.2007.00965.x. PMID: 19046318. [DOI] [PubMed] [Google Scholar]
- 13.Song M.K., Park B.B., Uhm J.E. Understanding splenomegaly in myelofibrosis: association with molecular pathogenesis. Int. J. Mol. Sci. 2018 Mar 18;19(3):898. doi: 10.3390/ijms19030898. PMID: 29562644; PMCID: PMC5877759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison C., Kiladjian J.J., Al-Ali H.K., Gisslinger H., Waltzman R., Stalbovskaya V., McQuitty M., Hunter D.S., Levy R., Knoops L., Cervantes F., Vannucchi A.M., Barbui T., Barosi G. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl. J. Med. 2012 Mar 1;366(9):787–798. doi: 10.1056/NEJMoa1110556.PMID:22375970. [DOI] [PubMed] [Google Scholar]
- 15.Mascarenhas John, Kremyanskaya Marina, Hoffman Ronald, Bose Prithviraj, Talpaz Moshe, Harrison Claire N., Gupta Vikas, Leber Brian, Sirhan Shireen, Kabir Sujan, Adrian Senderowicz, Shao James, Mertz Jennifer, Trojer Patrick, Verstovsek Srdan. MANIFEST, a phase 2 study of CPI-0610, a bromodomain and extraterminal domain inhibitor (BETi), as monotherapy or "Add-on" to ruxolitinib, in patients with refractory or intolerant advanced myelofibrosis. Blood. 2019;134(Supplement_1):670. doi: 10.1182/blood-2019-127119. [DOI] [Google Scholar]
- 16.Yang M., Covington M.F., Nguyen B.D., Johnson G.B., Mesa R.A., Roarke M.C. 99mTc-Sulfur colloid bone marrow scintigraphy in diagnosis of diffuse pulmonary extramedullary hematopoiesis secondary to myelofibrosis. J. Nucl. Med. Technol. 2018 Dec;46(4):368–372. doi: 10.2967/jnmt.118.210534. Epub 2018 Jun 8. PMID: 29884685; PMCID: PMC6944180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the relevant data can be provided upon request.