Abstract
Background
Allergic sensitization is linked to allergy development, with early sensitization often associated with worse outcomes. We aimed to identify if distinct allergic sensitization trajectories existed within a diverse and multi-ethnic Asian cohort.
Methods
We administered modified ISAAC questionnaires in the first 8 years and conducted skin prick testing at ages 18 months, 3, 5 and 8 years in the Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort. We used latent class analysis to derive allergic sensitization trajectories, and adjusted odds ratios (AOR) to evaluate predictive risk factors and associations with allergic comorbidities.
Results
Among 997 children, three trajectories were identified: early food and mite sensitization (16.2%), late mite sensitization (24.2%) and no/low sensitization (59.6%). Early food and mite sensitization was associated with early eczema by 6 months [AOR (95%CI) 4.67 (1.78–12.28)], increased risk of wheeze by 3–8 years (ARR 1.72–1.99) and eczema in the first 8 years of life (ARR 1.87–2.41). Late mite sensitization was associated with female sex [AOR 0.58 (0.35–0.96)], cesarean section [AOR 0.54 (0.30–0.98)], early eczema by 6 months [AOR 3.40 (1.38–8.42)], and increased risk of eczema by 18 months [ARR 1.47 (1.03–2.08)] and 8 years [ARR 1.35 (1.05–1.73)].
Conclusion
Early onset of eczema and early allergic sensitization were strongly associated. Early sensitization, especially to house dust mites, was associated with increased risks of developing wheeze and eczema, pointing to the importance of developing preventive perinatal interventions and effective therapeutics for sensitized toddlers.
Keywords: Allergic sensitization trajectories, Latent class analysis, Eczema, Wheeze, House dust mite
Abbreviations: AOR, Adjusted odds ratio; ARR, Adjusted relative risk; EFMS, Early food and mite sensitization; GUSTO, Growing Up in Singapore Towards healthy Outcomes; ISAAC, International Study of Asthma and Allergies in Childhood; LCA, Latent class analysis; LMS, Late mite sensitization; SPT, Skin prick testing; Th2, T-helper 2
Introduction
Globally, the prevalence of allergic diseases in children is approximately 30–35%.1 Allergic sensitization is associated with a prominent T-helper 2 (Th2) immune response,2,3 which drives the development of allergic diseases.4,5 Evidence of association between early allergic sensitization and allergic outcomes has been presented by a number of studies.4 The Isle of Wight birth cohort showed that allergic sensitization was associated with higher risks of asthma, rhinitis and eczema development at age 4 years.6 In Chinese schoolchildren from Hong Kong, Malaysia and China, indoor allergic sensitization was associated with higher risks of asthma and rhinitis development.7 However, these studies did not examine the longitudinal patterns of allergic sensitization trajectories.
The use of latent class analysis (LCA), an unsupervised data driven statistical method, allows identification of allergic sensitization trajectories which are not predefined and not directly discernible from complex data.8 We define the term “trajectories” as longitudinal changes in sensitization to different number and types of allergens from infancy to early childhood. This method utilises maximum likelihood estimation to produce homogenous subgroups from a diverse population.9 Allergic sensitization trajectories can identify the number and type of allergic sensitization over different time periods and further elucidate the relationships between atopy and allergic diseases.
Increasing evidence has highlighted the importance of allergic sensitization trajectories in predicting allergy development. The WHEALS birth cohort derived four trajectories of allergic sensitization using LCA and found that only children belonging to “highly-sensitized” trajectory had increased risk of asthma by four years old.10 Similarly, the MAAS cohort used a nested two-stage LCA and found that children with mite sensitization to seven components of mite allergens had the highest risk of developing asthma with comorbid rhinitis and eczema.11 However, current studies that have evaluated allergic sensitization trajectories are mainly from European countries where allergen exposure and sensitization profiles differ from the tropical climate in Singapore. For example, more than 80% of Singaporean Chinese adults (the ethnicity of highest proportion in Singapore) had dust mite sensitization12 as compared to 10% in a Swedish adult population13 due to the perennial hot and humid weather in Singapore.14 Asian countries also tend to have higher shellfish and lower peanut and pollen sensitization rates.14,15
We hypothesized that there is an underlying heterogeneity in the trajectories of allergic sensitization development, which are in turn associated with different allergic diseases and predictors. In this study, we examined whether distinct allergic sensitization trajectories existed within our ethnically diverse population-based cohort using the results from repeated skin prick testing (SPT) over 8 years in the Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort. We also determined if the trajectories were associated with different predictors and differential risk for the clinical outcomes of eczema, wheeze with use of nebulizer and rhinitis.
Methods
Study design and definition of allergic outcomes
The GUSTO study is a prospective population-based cohort study involving 1247 healthy pregnant mothers and their offspring. Healthy pregnant women aged 18 and above who were attending their first-trimester antenatal dating ultrasound scan clinics at two major public maternity units in Singapore, National University Hospital and KK Women's and Children's Hospital from June 2009 to September 2010, were invited to participate. They had to be Singaporeans or permanent residents, belong to any of the major ethnic groups (Chinese, Malay or Indian) in Singapore, and intend to give birth at either hospital, donate their birth tissues and reside in Singapore for at least five years. Detailed methodology was described by Soh et al.16 The modified International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire was used to evaluate offspring allergic symptoms at ages 3, 6, 9, 12, 15, 18 months and 2, 3, 4, 5, 6, 7 and 8 years. Questions used are listed in Supplementary Text 1. The conduct of this study was based on the guidelines in the Declaration of Helsinki. Informed written consent was obtained from all participants.
Allergic sensitization
SPT was conducted at ages 18 months, 3, 5 and 8 years to major relevant allergens in Singapore, which included cow's milk, egg, peanut and house dust mites, Dermatophagoides pteronyssinus, Dermatophagoides farina (Greer Laboratories, Lenoir, NC, USA) and Blomia tropicalis. SPT was defined as positive at a timepoint if any of the allergens tested positive at the timepoint with an average wheal size of at least 3 mm.
Statistical methods
The study aimed to find out subgroups of children with distinct allergic sensitization profiles over 8 years of follow-up based on SPT to the major relevant allergens. An exploratory, unsupervised LCA was performed by using the doLCA program in the LCA Stata plugin17 to derive the allergic sensitization trajectories based on SPT at ages 18 months, 3, 5, and 8 years.18 LCA was employed because it is often used to identify subgroups of people based on their individual characteristics.19 Both latent variable (ie, children subgroups) and observed variables (ie, allergic sensitization) are categorical in this context. LCA assumes local independence, which means that observed variables are independent conditional on a particular latent class. Participants who had completed at least one SPT were included into the analysis. We fitted models from one through six classes. Each model was repeatedly estimated 100 times with a prespecified seed, to find the optimal seed or starting values of parameters that maximized the likelihood function. This step also helped to check model identification and avoid suboptimal local maxima. The final number of latent classes were chosen based on the Akaike information criterion and Bayesian information criterion, as well as the identification and interpretability of the model. Posterior probabilities of class membership were generated for each child. The child was subsequently assigned to the class with the highest posterior probability. Variables were summarized by frequency (percentage) and mean (standard deviation) as appropriate. Chi-square, Fisher's exact, independent two-sample t and Wilcoxon rank-sum tests were used to compare demographic variables. Associations between predictors and allergic sensitization trajectories were evaluated by multivariable multinomial logistic regression. Associations between allergic sensitization trajectories and allergic diseases were assessed by multivariable Poisson regression with robust error variance, adjusted for confounders. The Benjamini-Hochberg procedure with a prespecified false discovery rate was applied to reduce type I errors associated with multiple comparisons. All statistical analyses were conducted in Stata/SE 16.1 for Windows (StataCorp LLC, College Station, TX USA) assuming two-sided tests with a 5% significance level.
Results
The study population comprised 997 mother-child dyads after excluding subjects without data on SPT. The majority of the mothers were of Chinese ethnicity [565 (56.7%)] and had post-secondary and higher education [692 (70.3%)]; 52.5% of participants were boys (Table 1). There were no differences in demographic characteristics such as child's sex and ethnicity between included and excluded participants while mean maternal age was higher for included participants (Supplementary Table 1).
Table 1.
Characteristics of participants according to allergic sensitization trajectory.
| Total (n = 997) | No/low sensitization (n = 594) | Early food and mite sensitization (n = 162) | P | Late mite sensitization (n = 241) | P | |
|---|---|---|---|---|---|---|
| Demographics, n (%) | ||||||
| Sex | 0.008a | 0.042a | ||||
| Male | 523 (52.5) | 289 (48.7) | 98 (60.5) | 136 (56.4) | ||
| Female | 474 (47.5) | 305 (51.4) | 64 (39.5) | 105 (43.6) | ||
| Ethnicity | 0.002a | 0.001a | ||||
| Chinese | 565 (56.7) | 317 (53.4) | 100 (61.7) | 148 (61.7) | ||
| Malay | 248 (24.9) | 139 (23.4) | 45 (27.8) | 64 (26.7) | ||
| Indian | 183 (18.4) | 138 (23.2) | 17 (10.5) | 28 (11.7) | ||
| Maternal education level | 0.244 | 0.119 | ||||
| Secondary school education or less | 293 (29.7) | 187 (31.9) | 43 (27.0) | 63 (26.4) | ||
| Post-secondary and higher | 692 (70.3) | 400 (68.1) | 116 (73.0) | 176 (73.6) | ||
| Family history of allergy | 482 (54.1) | 265 (49.9) | 84 (59.2) | 0.050a | 133 (61.0) | 0.006a |
| Maternal plasma Vitamin D3 | 0.476 | 0.264 | ||||
| Deficient | 117 (13.7) | 74 (14.6) | 19 (13.8) | 24 (11.6) | ||
| Insufficient | 230 (27.0) | 145 (28.6) | 33 (23.9) | 52 (25.1) | ||
| Sufficient | 505 (59.3) | 288 (56.8) | 86 (62.3) | 131 (63.3) | ||
| Maternal age in yearsb | 31 (5) | 31 (5) | 32 (5) | 0.334 | 32 (5) | 0.119 |
| Parity | 0.920 | 0.671 | ||||
| 0 | 448 (44.9) | 270 (45.5) | 76 (46.9) | 102 (42.3) | ||
| 1 | 334 (33.5) | 195 (32.8) | 53 (32.7) | 86 (35.7) | ||
| ≥2 | 215 (21.6) | 129 (21.7) | 33 (20.4) | 53 (22.0) | ||
| Gestational age in weeksb | 38.8 (1.5) | 38.8 (1.5) | 38.8 (1.6) | 0.742 | 38.8 (1.3) | 0.450 |
| Environmental factors, n (%) | ||||||
| Tobacco exposure during pregnancy | 355 (37.5) | 205 (36.6) | 57 (36.8) | 0.970 | 93 (39.9) | 0.381 |
| Mode of delivery | 0.213 | 0.505 | ||||
| Vaginal delivery | 693 (69.6) | 405 (68.2) | 118 (73.3) | 170 (70.5) | ||
| Cesarean section | 303 (30.4) | 189 (31.8) | 43 (26.7) | 71 (29.5) | ||
| Breastfeeding | 0.001a | 0.095 | ||||
| Mainly breastfeeding | 116 (12.3) | 72 (12.8) | 13 (8.8) | 31 (13.3) | ||
| Mixed feeding | 425 (44.9) | 227 (40.3) | 86 (58.1) | 112 (47.9) | ||
| Mainly formula feeding | 405 (42.8) | 265 (47.0) | 49 (33.1) | 91 (38.9) | ||
| Eczema in early life by 6 months | 76 (8.8) | 29 (5.7) | 27 (19.6) | <0.001a | 20 (9.2) | 0.082 |
| Cat ownership by 1 year | 31 (3.9) | 19 (4.2) | 5 (3.9) | 0.874 | 7 (3.5) | 0.682 |
| Dog ownership by 1 year | 59 (7.5) | 40 (8.8) | 2 (1.5) | 0.003a | 17 (8.5) | 0.889 |
| Childcare attendance by 1 year | 70 (12.8) | 30 (9.7) | 19 (20.7) | 0.005a | 21 (14.6) | 0.123 |
p value that was significant after using the Benjamini-Hochberg procedure with a proposed false discovery rate of 5%.
Data presented as mean (SD)
Identification of allergic sensitization trajectories
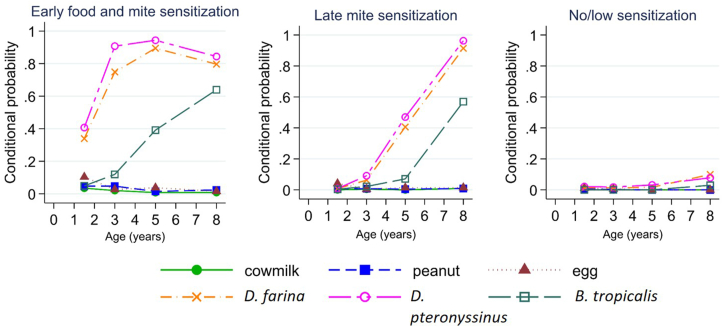
LCA classified the children into three allergic sensitization trajectories (Fig. 1 and Supplementary Figure 1). Conditional probability is the probability of an allergic sensitization at a time point conditional on a particular trajectory. Depending on the time of onset, we labelled these as early food and mite sensitization (EFMS) (16.2%), late mite sensitization (LMS) (24.2%), and no/low sensitization (59.6%, reference).
Fig. 1.
Probability of allergic sensitization conditional on latent class membership
Early food and mite sensitization (EFMS)
Compared to children from the no/low sensitization trajectory, those in the EFMS trajectory were more likely to be boys, have a family history of allergy, mixed breastfeeding and formula feeding, eczema in early life by 6 months and to have attended childcare during infancy (Table 1). They were also less likely to be of Indian ethnicity and to have owned a dog during infancy (Table 1). Multivariable analysis adjusting for confounders showed that children with EFMS had higher odds of having early eczema by 6 months (Table 2).
Table 2.
Association between characteristics of participants and allergic sensitization trajectory by multivariate multinomial logistic regression (n = 396).
| Early food and mite sensitization |
Late mite sensitization |
|||
|---|---|---|---|---|
| AOR (95% CI) | P | AOR (95% CI) | P | |
| Sex | ||||
| Male | Ref | – | Ref | – |
| Female | 0.72 (0.40–1.32) | 0.294 | 0.58 (0.35–0.96) | 0.033a |
| Ethnicity | 0.532 | 0.121 | ||
| Chinese | Ref | – | Ref | – |
| Malay | 1.02 (0.41–2.49) | 0.971 | 1.26 (0.63–2.52) | 0.521 |
| Indian | 0.54 (0.18–1.63) | 0.273 | 0.45 (0.18–1.09) | 0.078 |
| Maternal education level | ||||
| Secondary school education or less | Ref | – | Ref | – |
| Post-secondary and higher | 1.22 (0.53–2.78) | 0.642 | 1.61 (0.85–3.03) | 0.142 |
| Family history of allergy | 1.27 (0.68–2.37) | 0.458 | 1.08 (0.65–1.80) | 0.775 |
| Maternal plasma Vitamin D3 | 0.730 | 0.530 | ||
| Deficient | Ref | – | Ref | – |
| Insufficient | 0.93 (0.30–2.89) | 0.905 | 0.82 (0.34–1.99) | 0.658 |
| Sufficient | 1.24 (0.42–3.62) | 0.697 | 1.16 (0.50–2.66) | 0.735 |
| Maternal age in years | 1.03 (0.96–1.10) | 0.453 | 0.97 (0.92–1.03) | 0.365 |
| Parity | 0.981 | 0.444 | ||
| 0 | Ref | – | Ref | – |
| 1 | 0.97 (0.47–1.97) | 0.924 | 1.35 (0.74–2.46) | 0.326 |
| ≥2 | 0.91 (0.35–2.36) | 0.844 | 1.55 (0.74–3.25) | 0.240 |
| Gestational age in weeks | 1.01 (0.81–1.27) | 0.915 | 1.09 (0.89–1.33) | 0.401 |
| Tobacco exposure during pregnancy | 0.85 (0.39–1.85) | 0.688 | 1.23 (0.68–2.22) | 0.490 |
| Mode of delivery | ||||
| Vaginal delivery | Ref | – | Ref | – |
| Cesarean section | 0.72 (0.37–1.42) | 0.348 | 0.54 (0.30–0.98) | 0.042a |
| Breastfeeding | 0.026 | 0.360 | ||
| Mainly breastfeeding | Ref | – | Ref | – |
| Mixed feeding | 2.43 (0.96–6.16) | 0.061 | 1.47 (0.71–3.04) | 0.305 |
| Mainly formula feeding | 1.03 (0.36–2.98) | 0.952 | 1.01 (0.46–2.21) | 0.988 |
| Eczema in early life by 6 months | 4.67 (1.78–12.28) | 0.002a | 3.40 (1.38–8.42) | 0.008a |
| Cat ownership by 1 year | 1.51 (0.43–5.35) | 0.525 | 0.85 (0.27–2.61) | 0.771 |
| Dog ownership by 1 year | 0.13 (0.02–1.08) | 0.059 | 0.97 (0.39–2.41) | 0.945 |
| Childcare attendance by 1 year | 2.12 (0.92–4.90) | 0.078 | 2.01 (0.97–4.15) | 0.059 |
AOR, adjusted odds ratio; CI, confidence interval.
No/low sensitization trajectory was the baseline group for odds.
p value that was significant
In multivariable analysis, compared to children in the no/low sensitization trajectory, children with EFMS had higher odds of wheeze development by ages 3, 5, and 8 years and eczema development in the first 8 years of life (Table 3 and Supplementary Table 2). No association was observed between EFMS and rhinitis development in the first 8 years of life.
Table 3.
Association between allergic sensitization trajectory and allergic comorbidity by multivariate Poisson regression with robust error variance.
| Early food and mite sensitization |
Late mite sensitization |
|||
|---|---|---|---|---|
| ARR (95% CI) | P | ARR (95% CI) | P | |
| Wheezing by 18 months | 1.32 (0.80–2.18) | 0.271 | 1.14 (0.72–1.79) | 0.580 |
| Wheezing by 3 years | 1.72 (1.26–2.35) | 0.001a | 1.20 (0.86–1.68) | 0.285 |
| Wheezing by 5 years | 1.81 (1.41–2.33) | <0.001a | 1.21 (0.91–1.60) | 0.181 |
| Wheezing by 8 years | 1.99 (1.56–2.53) | <0.001a | 1.31 (1.00–1.71) | 0.050 |
| Eczema by 18 months | 2.41 (1.74–3.34) | <0.001a | 1.47 (1.03–2.08) | 0.033a |
| Eczema by 3 years | 2.08 (1.57–2.74) | <0.001a | 1.34 (0.99–1.82) | 0.058 |
| Eczema by 5 years | 1.90 (1.46–2.46) | <0.001a | 1.31 (0.99–1.73) | 0.058 |
| Eczema by 8 years | 1.87 (1.47–2.37) | <0.001a | 1.35 (1.05–1.73) | 0.019a |
| Rhinitis by 18 months | 0.97 (0.81–1.17) | 0.788 | 0.89 (0.74–1.06) | 0.192 |
| Rhinitis by 3 years | 1.00 (0.86–1.16) | 0.974 | 0.96 (0.83–1.11) | 0.545 |
| Rhinitis by 5 years | 0.98 (0.84–1.13) | 0.743 | 0.95 (0.84–1.09) | 0.498 |
| Rhinitis by 8 years | 0.99 (0.86–1.15) | 0.906 | 0.93 (0.82–1.06) | 0.290 |
ARR, adjusted relative risk; CI, confidence interval.
No/low sensitization trajectory was the reference group for relative risk.
Sex, ethnicity, maternal education level and family history of allergy were adjusted in the multivariate Poisson regression with robust error variance for each allergic comorbidity.
p value that was significant
Late mite sensitization (LMS)
Compared to children from the no/low sensitization trajectory, those in the LMS trajectory were less likely to be girls or to be of Indian ethnicity and more likely to have a family history of allergy (Table 1). Multivariable analysis showed that female sex and cesarean section were associated with lower odds of LMS and children with LMS had higher odds having eczema in early life by 6 months (Table 2).
In multivariable analysis, compared to children in the no/low sensitization trajectory, children with LMS had higher odds of eczema by 18 months and 8 years (Table 3 and Supplementary Table 2). No association was observed between LMS and wheeze and rhinitis development in the first 8 years of life.
Discussion
In this study, LCA of data collected over multiple timepoints in the first 8 years of life generated three trajectories of allergic sensitization, EFMS, LMS and no/low sensitization. These trajectories were associated with different clinical outcomes. EFMS was associated with wheeze development by 3–8 years and eczema development in the first 8 years. LMS was associated with eczema by 18 months and 8 years only (Fig. 2). Notably, there was low food allergen sensitization and high house dust mite sensitization in this cohort study conducted in Singapore with a year-round tropical climate.
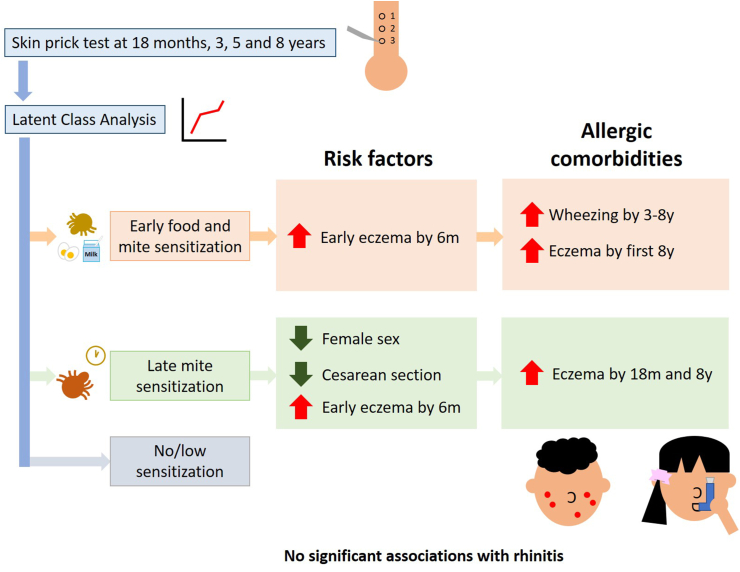
Fig. 2.
Three allergic sensitization trajectories using latent class analysis were identified. The trajectories identified were early food and mite sensitization (EFMS), late mite sensitization (LMS) and no/low sensitization. Early onset of eczema were associated with higher odds of development of EFMS and LMS. Female sex and cesarean section were also associated with lower odds of LMS. EFMS was associated with wheeze development by 3–8 years and eczema development in the first 8 years. LMS was associated with eczema by 18 months and 8 years only. None of the trajectories were associated with rhinitis
We found EFMS to be associated with higher odds of wheeze and eczema development, highlighting the association between early allergic sensitization and the development of wheeze and eczema. Our findings are supported by other cohorts; the CAPS cohort in Australia observed that early mixed food and inhalant trajectory was associated with the highest odds of development of eczema and asthma at 8 years as compared to other trajectories.20 The PARIS birth cohort in France used k-means clustering and found that early and persistent sensitization was associated with highest odds of isolated atopic dermatitis development in childhood.21 The MAS cohort in Germany and the PASTURE cohort on five European countries used LCA and found that the severe atopy trajectory featuring early food and mite sensitization was also associated with highest odds of lifetime eczema and asthma development by six years.22 Early sensitization may signify the onset of the atopic march leading to subsequent development of other allergic diseases. To reduce the risk of development of allergic diseases, it is likely to be beneficial to prevent allergic sensitization in early life.
Conversely, LMS was only associated with higher risk of eczema by 18 months and 8 years and was not associated with wheeze and rhinitis in the first 8 years of life. Longer follow up is needed to evaluate the influence of LMS on subsequent development of allergic outcomes. Besides this, none of the sensitization trajectories was associated with rhinitis in our study, contrary to findings in other studies.20,23 A possible reason could be that we did not differentiate between transient and persistent as well as allergic and non-allergic or infectious rhinitis.
Early onset eczema by six months was associated with both EFMS and LMS. Our result is supported by the Melbourne Atopy Cohort Study which also reported that early eczema by six months preceded and predicted allergic sensitization at one year.24 Recently, the “outside-in” hypothesis, which postulates that early onset of eczema increases susceptibility to transcutaneous sensitization by allergens and initiates the atopic march leading to asthma and rhinitis, has gained attention.25 Eczema with filaggrin mutations, a key gene in the skin barrier, has been associated with skin barrier integrity and degree of inhalant allergic sensitization, while such associations were not found for children with eczema who did not have filaggrin mutations.26 Allergic sensitization can in turn increase the risk of having subsequent eczema later in childhood.4 The Danish Allergy Research Centre cohort showed that, similar to our study, development of subsequent atopic dermatitis at age six years was associated with early food and inhalant sensitization and early atopic dermatitis from 0 to 18 months.27
Besides early onset of eczema, female sex was also associated with lower odds of LMS. Sex-related differences in atopy development in childhood is well-documented, where prepubertal boys tend to have higher rates of sensitization likely due to higher expression of Th2 cytokines, interleukin-5 and interleukin-13.28
In this study, we used LCA to derive allergic sensitization trajectories. LCA has been widely used in social and behavioural sciences and both open-source and commercial software are available for the implementation of the methods.19 The full information maximum likelihood approach is adopted in most software to maximize the usage of data and reduce the impact of missing data. However, in situations where subjects are to be assigned to different latent classes for subsequent analysis, there may be classification uncertainty in some individuals. Moreover, when applied to longitudinal data over multiple follow-up times, LCA directly computes item-response probabilities without assuming any time trend. This may result in large number of parameters to be estimated as well as discontinuous patterns over time.
Strengths of this study include multiple SPT performed at regular intervals, which allowed us to observe the changes in allergic sensitization over time as compared to other studies with fewer timepoints of data collection.21 The extensive data collection also allowed us to study predictors of these trajectories. A limitation of this study is parental report of doctor-diagnosed allergic outcomes. However, the very regular follow up of the participants at multiple timepoints with structured interviewer-administered questionnaires would have minimised recall bias and increased the likelihood of capturing information on a child's visit to the doctor for allergy-related issues. We also tested for a limited panel of six allergens in SPT. However, we focused on major relevant allergens in Singapore;29 hence, we were likely to have captured the most important sensitization profiles in Singaporean children. Cat and dog dander were not studied due to low ownership and sensitization rates.29 In addition, the results from this study may not be generalizable to Singapore's population as women of Malay and Indian ethnicities were overrepresented on purpose at recruitment.16
In conclusion, this study identified three distinct allergic sensitization trajectories as well as differential predictors and risks associated with clinical outcomes in a longitudinal study of Singapore children from birth to eight years using LCA. Our results showed that early sensitization, especially to house dust mites, was associated with a higher risk of developing wheeze and eczema, pointing to the importance of implementing preventive perinatal interventions and effective therapeutics for sensitized toddlers. As early development of eczema by six months was a common risk factor for both allergic sensitization trajectories, it is hence important to prevent and seek medical attention for early onset eczema.
Financial support
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health's National Medical Research Council (NMRC), Singapore - NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A∗STAR), Singapore. EH Tham is supported by the National Medical Research Council (NMRC) Transition Award grant [MOH-TA18nov-003] from NMRC, Singapore. Godfrey KM is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042) and NIHR Southampton Biomedical Research Centre (IS-BRC-1215-20004)), the European Union (Erasmus + Programme ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP) and the British Heart Foundation (RG/15/17/3174, SP/F/21/150013). All authors declare that the study sponsors had no role in the study design, collection, analysis and interpretation of data, in the writing of the report and in the decision to submit the article for publication.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author. The data is not publicly available due to privacy or ethical restrictions.
Authors’ contribution
Lau HX and Chen ZJ analysed the data and wrote the manuscript. Chan YH provided statistical advice. Tham EH, Goh AEN, Van Bever HP, Teoh OH, Karnani N, Gluckman PD, Tan KH, Yap FKP, Godfrey KM, Eriksson JG, Chong YS, Lee BW and Shek LPC contributed to the study design and provided intellectual input. Loo EXL conceptualized the study design, contributed to the analysis and wrote the manuscript. All authors critically reviewed and approved the manuscript as submitted and agreed to be accountable for all aspects of the work.
Ethics, consent and permissions
Ethics approval was obtained from the Domain Specific Review Board of Singapore National Healthcare Group (D/2009/021) and the Centralised Institutional Review Board of SingHealth (2018/2767). The conduct of this study was based on the guidelines in the Declaration of Helsinki. Informed written consent was obtained from all participants.
Agreement to publish the work
All authors have read through and approved the publication of the manuscript.
Editorial policy confirmation and agreement
All authors confirm and agree to the editorial policy.
Confirmation of unpublished work
All authors confirm that the manuscript is original, has not been published before, is not currently being considered for publication elsewhere, and has not been posted to a preprint server.
Declaration of competing interest
Godfrey KM has received reimbursement for speaking at conferences sponsored by Nestle. Godfrey KM and Chong YS are part of an academic consortium that has received research funding from Abbot Nutrition, Nestle and Danone. All other authors declare no conflict of interest.
Acknowledgements
We thank the GUSTO study group and all clinical and home-visit staff involved. The voluntary participation of all subjects is greatly appreciated. The GUSTO study group includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit Froukje Philipp Broekman, Boon Long Quah, Chai Kiat Chng, Cheryl Shufen Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Daniel Yam Thiam Goh, Doris Ngiuk Lan Loh, Evelyn Xiu Ling Loo, Elizabeth Huiwen Tham, Fabian Kok Peng Yap, George Seow Heong Yeo, Helen Yu Chen, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna Dawn Holbrook, Joshua J. Gooley, Karen Tan Mei Ling, Keith M. Godfrey, Kenneth Yung Chiang Kwek, Kok Hian Tan, Krishnamoorthy Naiduvaje, Leher Singh, Lin Lin Su, Lourdes Mary Daniel, Lynette Pei-Chi Shek, Marielle V. Fortier, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Michael J. Meaney, Mya Thway Tint, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Peter David Gluckman, Pratibha Keshav Agarwal, Rob Martinus van Dam, Salome A. Rebello, Seang Mei Saw, Shang Chee Chong, Shirong Cai, Shu-E Soh, Sok Bee Lim, Stephen Chin-Ying Hsu, Victor Samuel Rajadurai, Walter Stunkel, Wee Meng Han, Wei Wei Pang, Yap Seng Chong, Yin Bun Cheung, Yiong Huak Chan and Yung Seng Lee.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2022.100667.
Contributor Information
Hui Xing Lau, Email: lau_hui_xing@sics.a-star.edu.sg.
Zhaojin Chen, Email: medcz@nus.edu.sg.
Yiong Huak Chan, Email: medcyh@nus.edu.sg.
Elizabeth Huiwen Tham, Email: elizabeth_tham@nuhs.edu.sg.
Anne Eng Neo Goh, Email: anne.goh.e.n@singhealth.com.sg.
Hugo Van Bever, Email: hugo_van_bever@nuhs.edu.sg.
Oon Hoe Teoh, Email: teoh.oon.hoe@singhealth.com.sg.
Neerja Karnani, Email: neerja_karnani@sics.a-star.edu.sg.
Peter D. Gluckman, Email: pd.gluckman@auckland.ac.nz.
Kok Hian Tan, Email: tan.kok.hian@singhealth.com.sg.
Fabian Kok Peng Yap, Email: fabian.yap.k.p@singhealth.com.sg.
Keith M. Godfrey, Email: kmg@mrc.soton.ac.uk.
Johan G. Eriksson, Email: obgjge@nus.edu.sg.
Yap Seng Chong, Email: obgcys@nus.edu.sg.
Bee Wah Lee, Email: paeleebw@nus.edu.sg.
Lynette Pei-Chi Shek, Email: lynette_pc_shek@nuhs.edu.sg.
Evelyn Xiu Ling Loo, Email: evelyn_loo@sics.a-star.edu.sg.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chad Z. Allergies in children. Paediatr Child Health. 2001;6(8):555–566. doi: 10.1093/pch/6.8.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenmalm M., Van Snick J., Cormont F., Salman B. Allergen-induced Th1 and Th2 cytokine secretion in relation to specific allergen sensitization and atopic symptoms in children. Clin Exp Allergy. 2001;31(10):1528–1535. doi: 10.1046/j.1365-2222.2001.01190.x. [DOI] [PubMed] [Google Scholar]
- 3.Jang Y.H., Choi J.K., Jin M., et al. House dust mite increases pro-Th2 cytokines IL-25 and IL-33 via the activation of TLR1/6 signaling. J Invest Dermatol. 2017;137(11):2354–2361. doi: 10.1016/j.jid.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 4.Alduraywish S.A., Lodge C.J., Campbell B., et al. The march from early life food sensitization to allergic disease: a systematic review and meta-analyses of birth cohort studies. Allergy. 2016;71(1):77–89. doi: 10.1111/all.12784. [DOI] [PubMed] [Google Scholar]
- 5.Miller J.D. The role of dust mites in allergy. Clin Rev Allergy Immunol. 2019;57(3):312–329. doi: 10.1007/s12016-018-8693-0. [DOI] [PubMed] [Google Scholar]
- 6.Arshad S.H., Tariq S.M., Matthews S., Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 Years: a whole population birth cohort study. Pediatrics. 2001;108(2):e33. doi: 10.1542/peds.108.2.e33. [DOI] [PubMed] [Google Scholar]
- 7.Leung R., Ho P., Lam C.K., Lai C.W. Sensitization to inhaled allergens as a risk factor for asthma and allergic diseases in Chinese population. J Allergy Clin Immunol. 1997;99(5):594–599. doi: 10.1016/s0091-6749(97)70018-6. [DOI] [PubMed] [Google Scholar]
- 8.Mori M., Krumholz H.M., Allore H.G. Using latent class Analysis to identify hidden clinical phenotypes. JAMA. 2020;324(7):700–701. doi: 10.1001/jama.2020.2278. [DOI] [PubMed] [Google Scholar]
- 9.Berlin K.S., Williams N.A., Parra G.R. An introduction to latent variable mixture modeling (part 1): overview and cross-sectional latent class and latent profile analyses. J Pediatr Psychol. 2014;39(2):174–187. doi: 10.1093/jpepsy/jst084. [DOI] [PubMed] [Google Scholar]
- 10.Havstad S., Johnson C.C., Kim H., et al. Atopic phenotypes identified with latent class analyses at age 2 years. J Allergy Clin Immunol. 2014;134(3):722–727. doi: 10.1016/j.jaci.2014.01.022. e722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Custovic A., Sonntag H.-J., Buchan I.E., Belgrave D., Simpson A., Prosperi M.C. Evolution pathways of IgE responses to grass and mite allergens throughout childhood. J Allergy Clin Immunol. 2015;136(6):1645–1652. doi: 10.1016/j.jaci.2015.03.041. e1648. [DOI] [PubMed] [Google Scholar]
- 12.Andiappan A.K., Puan K.J., Lee B., et al. Allergic airway diseases in a tropical urban environment are driven by dominant mono-specific sensitization against house dust mites. Allergy. 2014;69(4):501–509. doi: 10.1111/all.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Björstad Å., Cardell L.-O., Hahn-Pedersen J., Svärd M. A cost-minimisation analysis comparing sublingual immunotherapy to subcutaneous immunotherapy for the treatment of house dust mite allergy in a Swedish setting. Clin Drug Invest. 2017;37(6):541–549. doi: 10.1007/s40261-017-0516-1. [DOI] [PubMed] [Google Scholar]
- 14.Tham E.H., Lee A.J., Bever H.V. Aeroallergen sensitization and allergic disease phenotypes in Asia. Asian Pac J Allergy Immunol. 2016;34(3):181–189. doi: 10.12932/AP0770. [DOI] [PubMed] [Google Scholar]
- 15.Lee A.J., Thalayasingam M., Lee B.W. Food allergy in Asia: how does it compare? Asia Pac Allergy. 2013;3(1):3–14. doi: 10.5415/apallergy.2013.3.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soh S.E., Tint M.T., Gluckman P.D., Godfrey K.M., Rifkin-Graboi A., Chan Y.H., et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014 Oct;43(5):1401–1409. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 17.LCA Stata Plugin (Version 1.2) [Software]. methodology.psu.Edu. 2015. [Google Scholar]
- 18.Lanza S., Dziak J., Huang L., Wagner A., Collins L. 2015. LCA Stata Plugin Users' Guide (Version 1.2). methodology.psu.Edu. [Google Scholar]
- 19.Collins L.M., Lanza S. vol. 718. John Wiley & Sons; New Jersey (USA): 2009. (Latent Class and Latent Transition Analysis: With Applications in the Social, Behavioral, and Health Sciences). [Google Scholar]
- 20.Garden F., Simpson J., Marks G., Investigators C. Atopy phenotypes in the Childhood Asthma Prevention Study (CAPS) cohort and the relationship with allergic disease: clinical mechanisms in allergic disease. Clin Exp Allergy. 2013;43(6):633–641. doi: 10.1111/cea.12095. [DOI] [PubMed] [Google Scholar]
- 21.Gabet S., Rancière F., Just J., et al. Asthma and allergic rhinitis risk depends on house dust mite specific IgE levels in PARIS birth cohort children. World Allergy Organ J. 2019;12(9) doi: 10.1016/j.waojou.2019.100057. 100057-100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hose A.J., Depner M., Illi S., et al. Latent class analysis reveals clinically relevant atopy phenotypes in 2 birth cohorts. J Allergy Clin Immunol. 2017;139(6):1935–1945. doi: 10.1016/j.jaci.2016.08.046. e1912. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt F., Hose A.J., Mueller-Rompa S., et al. Development of atopic sensitization in Finnish and Estonian children: a latent class analysis in a multicenter cohort. J Allergy Clin Immunol. 2019;143(5):1904–1913. doi: 10.1016/j.jaci.2018.12.1014. e1909. [DOI] [PubMed] [Google Scholar]
- 24.Lowe A.J., Abramson M.J., Hosking C.S., et al. The temporal sequence of allergic sensitization and onset of infantile eczema. Clin Exp Allergy. 2007;37(4):536–542. doi: 10.1111/j.1365-2222.2007.02691.x. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg N.B., Silverberg J.I. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis. Cutis. 2015;96(6):359–361. [PubMed] [Google Scholar]
- 26.Nemoto-Hasebe I., Akiyama M., Nomura T., Sandilands A., McLean W.I., Shimizu H. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009;129(3):682–689. doi: 10.1038/jid.2008.280. [DOI] [PubMed] [Google Scholar]
- 27.Kjaer H.F., Eller E., Andersen K.E., Høst A., Bindslev-Jensen C. The association between early sensitization patterns and subsequent allergic disease. The DARC birth cohort study. Pediatr Allergy Immunol. 2009 Dec;20(8):726–734. doi: 10.1111/j.1399-3038.2009.00862.x. [DOI] [PubMed] [Google Scholar]
- 28.Uekert S.J., Akan G., Evans M.D., et al. Sex-related differences in immune development and the expression of atopy in early childhood. J Allergy Clin Immunol. 2006 Dec;118(6):1375–1381. doi: 10.1016/j.jaci.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Khoo J., Shek L.P., Khor E.S., Wang D.Y., Lee B.W. Pattern of sensitization to common environmental allergens amongst atopic Singapore children in the first 3 years of life. Asian Pac J Allergy Immunol. 2001 Dec;19(4):225–229. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data is not publicly available due to privacy or ethical restrictions.