Abstract
Background
Despite the worldwide increase in life expectancy and the elderly population, very little is known about the characteristics of anaphylaxis in older adults.
Methods
A retrospective scan was made of the files of patients who presented at the Allergy Unit of our clinic between October 2011 and October 2021. The study included 971 patients aged ≥18 years who met the criteria for diagnosis of anaphylaxis. The patients were separated into 2 groups of adults (18–64 years) and older adults (≥65 years).
Results
The adult group included 887 (91.3%) patients and the older adult group, 84 (8.7%) patients. Comorbid diseases were seen more frequently in the older adults than in the adult group (p < 0.001). Drugs were seen to be the most common trigger of anaphylaxis in both groups, and this was more common in the older adult group (p = 0.039). Food was a more common trigger of anaphylaxis in the adult group than in the older adult group (p = 0.017). In both groups, the skin was the organ most affected, and was less affected in the older adults than in the adults (p = 0.020). Cardiovascular symptoms were seen significantly more and respiratory symptoms significantly less in the older adult group (p < 0.001, p = 0.002, respectively). Admission to the hospital and the intensive care unit was more frequent in the older adult group and rates of adrenalin administration were higher compared to the adult group (p < 0.001 for all).
Conclusion
Anaphylaxis in the older adults is generally caused by drugs. Older adults were found to have more cardiovascular symptoms and more frequent adrenalin injections, hospitalizations and intensive care unit admissions.
Keywords: Anaphylaxis, Older adults, Drug hypersensitivity, Adrenaline, Hospitalization
Introduction
Life expectancy is increasing, and thereby the number of older adults worldwide. Despite this increase in life expectancy, very little is known about the characteristics of anaphylaxis in older adults.1,2 The triggers of anaphylaxis show variations in different age groups.2,3 The most common triggers worldwide are drugs, insect venoms, and food.4 Although these triggers are universal, differences are seen depending on nutritional habits, exposure, and geographical differences associated with residence in a rural or urban area.5
Elderly patients are at greater risk of severe and even mortal reactions.6 This risk is further increased by comorbidities and the simultaneous use of drugs (angiotensin converting enzyme [ACE] inhibitors, beta blockers).7 Recommendations for the emergency treatment of anaphylaxis are similar for all age groups. Adrenaline is the first treatment of choice in anaphylaxis and recommended by several international guidelines. Nevertheless, its use remains suboptimal.8
The aim of this study was to obtain data related to triggers, symptoms, and treatment to be able to better detect and treat anaphylaxis in older adult patients.
Materials and methods
A retrospective scan was made of the files of patients who presented at the Allergy Unit of our clinic between October 2011 and October 2021. The study included patients aged ≥18 years who met the criteria for diagnosis of anaphylaxis. The study was approved by the local ethics committee of university (decision no. 2021/3443-2022/3623).
Data collection
Patient data were obtained from the electronic medical records and the patient files of the Allergy Unit in respect of age, gender, comorbidities, atopy history, drugs used at the same time (ACE inhibitors, beta blockers, non-steroidal anti-inflammatory drugs [NSAID]), anaphylaxis triggers, clinical symptoms, treatments applied, and history of admission to hospital and/or intensive care unit (ICU).
Statistical analysis
Data obtained in the study were analyzed statistically using SPSS for Windows v. 22.0 software. Continuous variables were presented as mean ± standard deviation (SD) or median (min-max) values, and categorical variables as number (n) and percentage (%). The Chi-squared test was used to compare categorical variables. Univariate and multivariate logistic regression analyses were used to identify risk factors for anaphylaxis in the older adults. All variables with p values < 0.1 in univariate analyses were entered into forward stepwise multivariate logistic regression analyses. A value of p < 0.05 was considered statistically significant.
Results
Patient population
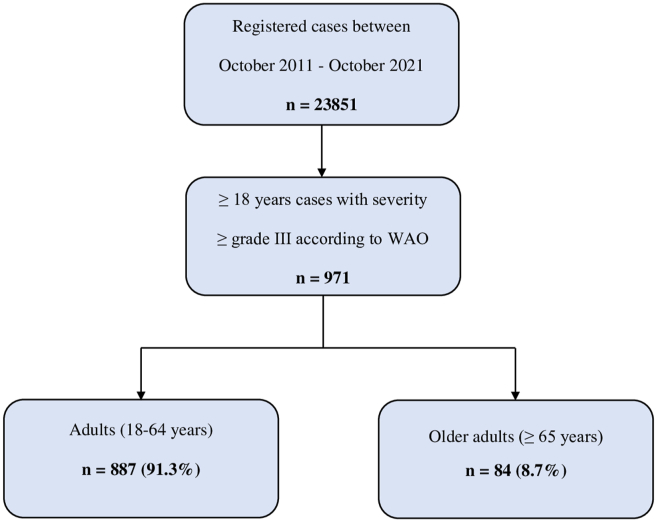
In the defined 10-year period, of the 23 851 patients who presented at the Allergy Unit of our clinic, 971 met the World Allergy Organization (WAO) diagnostic criteria for anaphylaxis (≥ grade III).3 The patients were separated into 2 groups of adults (18-64 years) and older adults (≥65 years) (Fig. 1). The adult group included 887 (91.3%) patients with a median age of 38 years (range, 18–64 years) and the older adult group included 84 (8.7%) patients with a median age of 68 years (range, 65–87 years). There was a greater number of female patients in both groups (62.1% and 56%, respectively) (Table 1).
Fig. 1.
Flow chart of the patients included in the study. WAO, World Allergy Organization
Table 1.
Clinical characteristics of anaphylaxis patients.
| Adults (18–64 years) n (%) | Older adults (≥65 years) n (%) | pa | |
|---|---|---|---|
| Total | 887 (91.3) | 84 (8.7) | |
| Age, years, median (min-max) | 38 (18–64) | 68 (65–87) | |
| Sex | |||
| Female | 551 (62.1) | 47 (56) | 0.267 |
| Male | 336 (37.9) | 37 (44) | 0.267 |
| Atopy | 240 (27) | 12 (14.3) | 0.010 |
| Comorbidities | 401 (45.2) | 76 (90.4) | < 0.001 |
| Asthma and other respiratory diseases | 161 (18.1) | 29 (34.5) | < 0.001 |
| Cardiovascular diseases | 141 (15.9) | 64 (73.6) | < 0.001 |
| Diabetes mellitus | 75 (8.5) | 20 (23.8) | < 0.001 |
| Neuropsychiatric diseases | 51 (5.7) | 6 (7.1) | 0.604 |
| Thyroid diseases | 38 (4.3) | 3 (3.6) | 0.756 |
| Rheumatological diseases | 27 (3.0) | 2 (2.4) | 0.733 |
| Malignant diseases | 18 (2) | 4 (4.7) | 0.108 |
| Gastrointestinal-liver diseases | 14 (1.6) | 3 (3.6) | 0.682 |
| Concurrent medications | 240 (27) | 55 (65.5) | < 0.001 |
| ACE inhibitors | 39 (4.4) | 22 (26.2) | < 0.001 |
| Beta blockers | 69 (7.8) | 27 (32.1) | < 0.001 |
| NSAIDs | 172 (19.4) | 25 (29.8) | 0.024 |
ACE, angiotensin converting enzyme; NSAIDs, nonsteroidal anti-inflammatory drugs.
Chi Square test (data were shown as number and percentages)
Comorbid diseases
Comorbid diseases were seen more often in the older adults than in the adult group (90.4%, 45.2%, respectively) (p < 0.001). The most frequently seen comorbidities in both groups were asthma and other respiratory diseases, cardiovascular diseases, and diabetes mellitus, and the rates were higher in the older adult group (p < 0.001 for all). Atopic diseases were more common in the adults than in the older adult group (27%, 14.3%, respectively) (p = 0.010) (Table 1).
Concurrent medications
The use of ACE inhibitors, beta blockers, and NSAIDs was more common in the older adult group than in the adult group (p < 0.001, p < 0.001, p = 0.024) (Table 1).
Triggers of anaphylaxis
Drugs were seen to be the most common trigger of anaphylaxis in both groups, and this was more common in the older adult compared to the adult group (73.8%, 62.4%, respectively) (p = 0.039). The drug most often causing anaphylaxis was determined to be antibiotics in the older adult group (41.9%) and NSAIDs in the adult group (44.7%). The second most common cause of anaphylaxis in both groups was hymenoptera venom allergy (HVA) (adults: 25.5%, older adults: 20.2%) (p = 0.289). Older adult males were seen to be more predisposed to HVA-related anaphylaxis (males: 35.1%, females: 8.5%, p = 0.003) and older adult females to drug-related anaphylaxis (females: 80.8%, males: 64.8%, p = 0.098) (data not shown). Food was a more common trigger of anaphylaxis in the adult group than in the older adult group (8.6%, 1.2%, respectively) (p = 0.017) (Table 2).
Table 2.
Comparison of anaphylaxis triggers in the adults and older adults.
| Adults (18–64 years) n (%) | Older adults (≥65 years) n (%) | pa | |
|---|---|---|---|
| Total | 887 (91.3) | 84 (8.7) | |
| Drug | 554 (62.4) | 62 (73.8) | 0.039 |
| NSAIDs | 248 (44.7) | 24 (38.7) | 0.905 |
| Antibiotics | 214 (38.6) | 26 (41.9) | 0.166 |
| Beta lactams | 173 (81) | 20 (77) | 0.635 |
| Non-beta lactams | 41 (19) | 6 (23) | 0.635 |
| Proton pump inhibitors | 23 (4.2) | 2 (3.2) | 0.907 |
| Contrast agents | 17 (3.0) | 1 (1.6) | 0.637 |
| Local anesthetics | 12 (2.1) | 1 (1.6) | 0.901 |
| Insect venom | 226 (25.5) | 17 (20.2) | 0.289 |
| Vespula spp. | 80 (35.4) | 7 (41.2) | 0.833 |
| Apis mellifera | 62 (27.4) | 5 (29.4) | 0.720 |
| Apis mellifera + Vespula spp. | 63 (27.9) | 4 (23.5) | 0.419 |
| Unknown | 21 (9.3) | 1 (5.9) | 0.488 |
| Food | 76 (8.6) | 1 (1.2) | 0.017 |
| Egg whole | 13 | 0 | – |
| Walnut | 12 | 0 | – |
| Peanut | 10 | 1 | 0.958 |
| Peach | 10 | 0 | – |
| Almond | 8 | 0 | – |
| Latex | 12 (1.4) | 1 (1.2) | 0.901 |
| Idiopathic | 19 (2.1) | 3 (3.6) | 0.400 |
NSAID, nonsteroidal anti-inflammatory drugs.
Chi Square test (data were shown as number and percentages)
Clinical manifestations of anaphylaxis
In both groups, the skin was the organ most affected, and was less affected in the older adults than in the adults (85.7%, 76.2%, respectively) (p = 0.020). A severe reaction (grade V)3 was seen more often in the older adults with no skin symptoms compared to the adults with no skin symptoms (40%, 15.7%, respectively) (p = 0.010) (data not shown). Cardiovascular symptoms were seen significantly more in the older adult group than in the adult group (66.6%, 44.5%, respectively) (p < 0.001). Loss of consciousness was more frequent in the older adult group than in the adult group (40.5%, 23.7%, respectively (p = 0.001) (data not shown). Cardiac arrest developed in 24 cases (adults 2.6%, older adults 4.8%) (p = 0.151), and 2 of these were determined as Kounis syndrome related to HVA (data not shown). Respiratory symptoms were seen less often in the older adult group than in the adult group (64.3%, 79%, respectively) (p = 0.002). Laryngeal edema was seen to be present more in the older adults than in the adult group (23.8%, 13.7%, respectively) (p = 0.013) (data not shown). Similar rates of gastrointestinal symptoms were seen in the older adult and adult groups (14%, 13.1%, respectively) (p = 0.852) (Table 3).
Table 3.
Comparison of clinical features of anaphylaxis in the adults and older adults.
| Adults (18–64 years) n (%) | Older adults (≥65 years) n (%) | pa | |
|---|---|---|---|
| Total | 887 (91.3) | 84 (8.7) | |
| Symptoms | |||
| Mucocutaneous | 760 (85.7) | 64 (76.2) | 0.020 |
| Respiratory | 702 (79) | 54 (64.3) | 0.002 |
| Cardiovascular | 395 (44.5) | 56 (66.6) | <0.001 |
| Gastrointestinal | 124 (14) | 11 (13.1) | 0.852 |
| Severity | |||
| Grade III | 372 (41.9) | 40 (47.6) | 0.314 |
| Grade IV | 424 (47.8) | 27 (32.1) | 0.006 |
| Grade V | 91 (10.3) | 17 (20.3) | 0.005 |
| Biphasic reaction | 33 (3.7) | 1 (1.2) | 0.228 |
| Hospitalization | 108 (12.2) | 31 (36.9) | <0.001 |
| Treatment in the ICU | 71 (8) | 13 (15.5) | <0.001 |
ICU, intensive care unit.
Chi Square test (data were shown as number and percentages)
Anaphylaxis severity grading
The WAO systemic allergic reaction grading system was used for grading the severity of anaphylaxis. Grade III-V reactions are accepted as anaphylaxis.3 Grade III and V reactions were more frequent in older adults vs adults and that Grade IV reactions were more frequent in adults vs. older adults (p = 0.314, p = 0.005, p = 0.006, respectively) (Table 3).
Hospitalization and intensive care unit admission
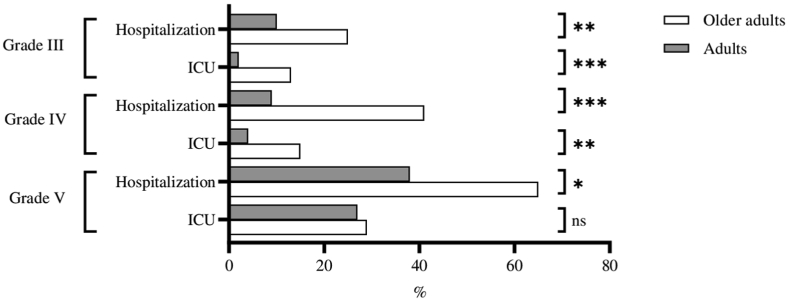
Hospitalization because of anaphylaxis was more frequent in the older adult group than in the adult group (36.9%, 12.2%, respectively) (p < 0.001). Rates of admission to the ICU were higher for older adults than for adults (15.5%, 8%, respectively) (p < 0.001) (Table 3). The rates of hospitalization and ICU admission were higher in the older adult group irrespective of the grade of severity (grade III: p = 0.005, p < 0.001, grade IV: p < 0.001, p = 0.007, grade V: p = 0.045, p = 0.513) (Fig. 2).
Fig. 2.
Rates of hospitalization and intensive care unit admissions by severity of anaphylaxis. ICU, intensive care unit. ∗∗∗p < 0.001; ∗∗p < 0.01; ∗p < 0.05; ns, not significant
Anaphylaxis treatment
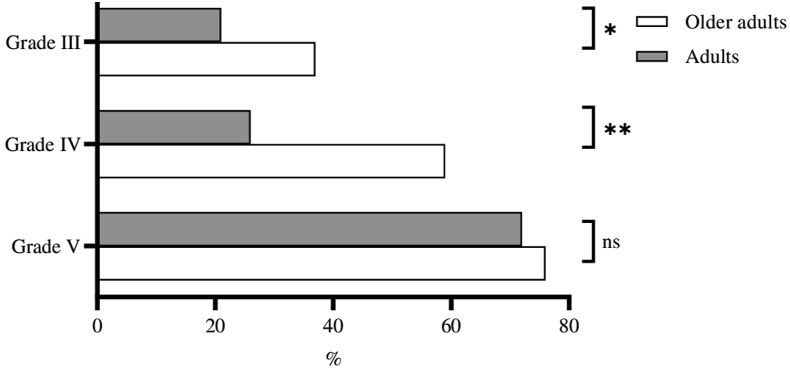
The rate of adrenalin administration was higher in the older adult group (53.1%, 28.4%, respectively) (p < 0.001) (Table 4). At all the severity grades, the rate of adrenalin administration was higher in the older adult group (grade III: p = 0.038, grade IV: p < 0.001, grade V: p = 0.736) (Fig. 3).
Table 4.
Comparison of anaphylaxis treatment in the adults and older adults.
| Adults (18–64 years) n (%) | Older adults (≥65 years) n (%) | pa | |
|---|---|---|---|
| Total | 887 (91.3) | 84 (8.7) | |
| Treatment | |||
| Antihistamines (H1 and/or H2) | 836 (94.2) | 77 (91.7) | 0.340 |
| Steroids | 810 (91.3) | 74 (88.1) | 0.323 |
| Epinephrine | 252 (28.4) | 43 (53.1) | <0.001 |
| Epinephrine autoinjector prescription | 330 (37.2) | 24 (28.6) | 0.116 |
| Immunotherapyb | 96 (10.8) | 2 (2.4) | 0.014 |
Chi Square test (data were shown as number and percentages).
Venom immunotherapy
Fig. 3.
Adrenaline administration rates according to the severity of anaphylaxis. ∗∗p < 0.001; ∗p < 0.05; ns, not significant
Risk factors for anaphylaxis in the older adults
In the univariate logistic regression analysis, the risk factors for anaphylaxis in the older adults were determined to be comorbidities (Odds ratio [OR], 11.514; 95% confidence interval [CI], 5.491–24.142, p < 0.001), use of ACE inhibitors (OR, 7.715; 95% CI, 4.308–13.818, p < 0.001), and use of beta blockers (OR, 5.616; 95% CI, 3.340–9.442, p < 0.001) (Table 5).
Table 5.
Anaphylaxis risk factors in the older adults.
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p | OR | 95% CI | p |
| Sex (female vs. male) | 0.775 | 0.493–1.217 | 0.268 | |||
| Comorbidity (yes vs. no) | 11.514 | 5.491–24.142 | <0.001 | 7.527 | 3.487–16.249 | <0.001 |
| ACE inhibitor use (yes vs. no) | 7.715 | 4.308–13.818 | <0.001 | 3.405 | 1.849–6.272 | <0.001 |
| Beta-blocker use (yes vs. no) | 5.616 | 3.340–9.442 | <0.001 | 2.375 | 1.364–4.135 | 0.002 |
OR, odds ratio; CI, confidence interval
In the multivariate logistic regression analysis, the risk factors for anaphylaxis in the older adults were determined to be comorbidities (OR, 7.527; 95% CI, 3.487–16.249, p < 0.001), use of ACE inhibitors (OR, 3.405; 95% CI, 1.849–6.272, p < 0.001), and use of beta blockers (OR, 2.375; 95% CI, 1.364–4.135, p = 0.002) (Table 5).
Discussion
This large-scale report presents the triggers, clinical characteristics, and treatment of anaphylaxis, based on a 10-year observation period. Drugs were determined to be the most common cause of anaphylaxis in both adults and older adults. Hospitalization and admission to ICU was more frequent and the rate of adrenalin administration was higher in older adults than in adults.
With aging, there is a reduction in natural and acquired immunity to antigens (Immunosenescence) which results in suppression of type-1 hypersensitivity reactions.1,9,10 Consequently, as seen in the current study, anaphylaxis is seen less in older adults than in adults. Apart from some small changes in diagnosis and treatment, anaphylaxis in older adults is the same as in adults.2,11
Current data have shown age-related differences in respect of the triggers and symptoms of anaphylaxis.2,3 The most common agents of anaphylaxis are foods in children and adolescents, and drugs and venoms in adults and older adults.12,13 In addition, idiopathic anaphylaxis is seen more frequently in adults and older adults than in children.14,15 In the current study, drugs were determined to be the most common trigger of anaphylaxis in both groups. Moreover, anaphylaxis of drug origin was seen more frequently in the older adults than in the adults (73.8% vs. 62.4%). The drug group most often leading to anaphylaxis was determined to be NSAIDs in adults, and beta-lactam antibiotics in older adults, who are probably the population group who use the most drugs. As in the current study, previous studies have shown that multiple comorbidities and multiple drug use increase the probability of drug-induced anaphylaxis.16,17 It has also been shown that a high frequency of drug-related anaphylaxis may be related to potentially inappropriate drug prescriptions associated with multiple comorbidities in older adults.18
In the current study, food as a trigger of anaphylaxis was determined to be a less frequent cause in older adults than in adults (1.2% vs. 8.6%). However, there was a higher rate of older adult patients for whom no trigger could be determined (3.6% vs. 2.1%). The current study results demonstrated that the frequency of anaphylaxis related to food decreased together with age, and the frequency of idiopathic and drug-related anaphylaxis increased.
The clinical findings of anaphylaxis vary according to the organ systems involved. As in several previous studies, the current study data determined that the organ most often involved was the skin.2,19,20 Moreover, despite the vast majority of anaphylaxis cases having skin symptoms, they may not be seen initially or may never be seen.3,21 Interestingly, in the current study, skin findings were seen less in older adults than in adults. This may be related to changes in skin structure in older adults. In addition, severe anaphylaxis was seen more often in older patients with no skin findings compared to adults with no skin findings (40% vs. 15.7%). The absence of skin findings together with a severe reaction may be related to a delay in the diagnosis of anaphylaxis. In the past this was attributed to the development of a severe circulation disorder without skin findings.22 In the current study group of older adults, cardiovascular symptoms were seen more than in adults. Other studies have similarly shown that loss of consciousness is seen more often in older adults.2,20 Increasing cardiovascular symptoms and loss of consciousness may be related to cardiovascular comorbidities seen more often in older patients. In the current study, respiratory symptoms were seen less but laryngeal edema was seen more often in the older adult group.
Anaphylaxis is a life-threatening, urgent clinical condition. Treatment must be applied rapidly after the diagnosis is made.23 Adrenalin is the most important drug in anaphylaxis treatment, and is life-saving. It is recommended as the first step in treatment by several guidelines.3,8 In contrast, antihistamines and corticosteroids are usually the first treatment step used by healthcare professionals. As seen in the current study data, only some anaphylaxis cases are treated with adrenalin, and this proportion varies between populations.24 However, the data obtained in the current study showed that the rate of adrenalin administration increased together with the severity of reaction and age. Although adrenalin was administered more often to older adults than adults, it was only applied to half of the older adults. This demonstrates that most patients were not treated in accordance with the guidelines. The results also show the need for more widespread training programs about anaphylaxis treatment for healthcare professionals.
Cardiovascular diseases and the drugs used (ACE inhibitors, beta blockers) are the most important factors making the treatment of anaphylaxis in older adults more difficult.25 It can be difficult to decide to administer adrenalin for anaphylaxis in older adults with cardiovascular disease. In such situations, the decision must be made taking into account the potential cardiovascular complications that may emerge if the anaphylaxis is not treated.7 However, there is no absolute contra-indication in any age group for the administration of adrenalin in the treatment of anaphylaxis.8 Polypharmacy and more frequent cardiovascular diseases in older patients may explain the low rates of adrenalin administration.
Similar to the findings of previous studies, higher rates of hospitalization and ICU admission were determined in the older adults in the current study.2,11,26 In addition, as the severity of anaphylaxis increased, there was also seen to be an increase in the rates of hospitalization and ICU admission. As comorbid diseases are more common in older adults, this could also affect the hospitalization and ICU admission rates.
Finally, several risk factors have been defined for anaphylaxis. The most important of these are age, comorbid diseases, and concurrent drug use (ACE inhibitors, beta blockers).8,20 Similarly in the current study, comorbid diseases and concurrent use of ACE inhibitors and/or beta blockers were determined to be risk factors for anaphylaxis in older adults.
The characteristics of anaphylaxis in older adults and the differences in these from those of adults were evaluated in this study. There were some limitations to this study; (1) although the sample size was sufficient, it was conducted in a single centre and did not reflect the general population, (2) Our study groups did not have homogeneous samples, since the older adult group represents less than 10% of our study population, (3) the data were limited to the patient records so there have been variable quality of information in the medical records. Despite these limitations, the study data can be considered to be of guidance for further larger scale multicentre studies.
Conclusion
The results of this study demonstrated that the characteristics of anaphylaxis in older adults are different from those of adults. Drugs are usually the cause of anaphylaxis in older adults. Although skin symptoms were seen most often in older adults, cardiovascular symptoms were seen more frequently than in adults. Adrenalin was administered more frequently to older adults, and they required hospitalization or ICU admission more often. The most important risk factors for anaphylaxis in older adults were determined to be comorbidities and the use of ACE inhibitors and beta blockers.
Abbreviations
ACE, angiotensin converting enzyme; NSAID, non-steroidal anti-inflammatory drugs; ICU, intensive care unit; WAO, World Allergy Organization; HVA, hymenoptera venom allergy.
Funding
The authors have no funding to declare.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Author's contributions
Concept and design: EY, ŞA, FÇ, Data collection or processing: EY, RE, FSA, MK, Analysis or Interpretation: EY, ŞA, FÇ, Literature search: RE, FSA, MK, Writing: EY, FÇ, RE, FSA, MK, Approval: EY, ŞA, FÇ.
Ethics approval
The study was approved by the Local Ethics Committee of Necmettin Erbakan University Meram Faculty of Medicine (decision no. 2021/3443-2022/3623); the study adhered to all the relevant tenets of the Declaration of Helsinki (1975).
Authors’ consent for publication
All the authors reviewed the final draft and provided consent for publication.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
Not applicable.
Footnotes
Full list of author information is available at the end of the article
Contributor Information
Eray Yıldız, Email: drerayyldz@gmail.com.
Şevket Arslan, Email: arslansevket@hotmail.com.
Fatih Çölkesen, Email: drvefa42@hotmail.com.
Recep Evcen, Email: r_evcen@hotmail.com.
Filiz Sadi Aykan, Email: filizsadi@yahoo.com.
Mehmet Kılınç, Email: mehmet915002@hotmail.com.
References
- 1.Cardona V., Guilarte M., Luengo O., Labrador-Horrillo M., Sala-Cunill A., Garriga T. Allergic diseases in the elderly. Clin Transl Allergy. 2011;1(1):11. doi: 10.1186/2045-7022-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aurich S., Dölle-Bierke S., Francuzik W., et al. Anaphylaxis in elderly patients-data from the European anaphylaxis registry. Front Immunol. 2019;10:750. doi: 10.3389/fimmu.2019.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardona V., Ansotegui I.J., Ebisawa M., et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 2020;13(10) doi: 10.1016/j.waojou.2020.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worm M., Moneret-Vautrin A., Scherer K., et al. First European data from the network of severe allergic reactions (NORA) Allergy. 2014;69(10):1397–1404. doi: 10.1111/all.12475. [DOI] [PubMed] [Google Scholar]
- 5.González-de-Olano D., Lombardo C., González-Mancebo E. The difficult management of anaphylaxis in the elderly. Curr Opin Allergy Clin Immunol. 2016;16(4):352–360. doi: 10.1097/ACI.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 6.Francuzik W., Dölle S., Worm M. Risk factors and treatment of refractory anaphylaxis - a review of case reports. Expet Rev Clin Immunol. 2018;14(4):307–314. doi: 10.1080/1744666X.2018.1450140. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman P., Simons F.E. Anaphylaxis and cardiovascular disease: therapeutic dilemmas. Clin Exp Allergy. 2015;45(8):1288–1295. doi: 10.1111/cea.12520. [DOI] [PubMed] [Google Scholar]
- 8.Muraro A., Roberts G., Worm M., et al. Anaphylaxis: guidelines from the European academy of allergy and clinical immunology. Allergy. 2014;69(8):1026–1045. doi: 10.1111/all.12437. [DOI] [PubMed] [Google Scholar]
- 9.Fülöp T., Dupuis G., Witkowski J.M., Larbi A. The role of immunosenescence in the development of age-related diseases. Rev Invest Clin. 2016;68(2):84–91. [PubMed] [Google Scholar]
- 10.Colonna-Romano G., Bulati M., Aquino A., et al. B cell immunosenescence in the elderly and in centenarians. Rejuvenation Res. 2008;11(2):433–439. doi: 10.1089/rej.2008.0664. [DOI] [PubMed] [Google Scholar]
- 11.Campbell R.L., Hagan J.B., Li J.T., et al. Anaphylaxis in emergency department patients 50 or 65 years or older. Ann Allergy Asthma Immunol. 2011;106(5):401–406. doi: 10.1016/j.anai.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Grabenhenrich L.B., Dölle S., Moneret-Vautrin A., et al. Anaphylaxis in children and adolescents: the European anaphylaxis registry. J Allergy Clin Immunol. 2016;137(4):1128–11237.e1. doi: 10.1016/j.jaci.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Yildiz E., Çölkesen F., Arslan Ş., et al. Allergic diseases in the elderly population: a single-center experience. Turk J Med Sci. 2021;51(5):2631–2640. doi: 10.3906/sag-2104-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orhan F., Canitez Y., Bakirtas A., et al. Anaphylaxis in Turkish children: a multi-centre, retrospective, case study. Clin Exp Allergy. 2011;41(12):1767–1776. doi: 10.1111/j.1365-2222.2011.03859.x. [DOI] [PubMed] [Google Scholar]
- 15.Oropeza A.R., Bindslev-Jensen C., Broesby-Olsen S., et al. Patterns of anaphylaxis after diagnostic workup: a follow-up study of 226 patients with suspected anaphylaxis. Allergy. 2017;72(12):1944–1952. doi: 10.1111/all.13207. [DOI] [PubMed] [Google Scholar]
- 16.O'Mahony D., Gudmundsson A., Soiza R.L., et al. Prevention of adverse drug reactions in hospitalized older patients with multi-morbidity and polypharmacy: the SENATOR∗ randomized controlled clinical trial. Age Ageing. 2020;49(4):605–614. doi: 10.1093/ageing/afaa072. [DOI] [PubMed] [Google Scholar]
- 17.Davies E.A., O'Mahony M.S. Adverse drug reactions in special populations – the elderly. Br J Clin Pharmacol. 2015;80(4):796–807. doi: 10.1111/bcp.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedna K., Hakkarainen K.M., Gyllensten H., Jönsson A.K., Petzold M., Hägg S. Potentially inappropriate prescribing and adverse drug reactions in the elderly: a population-based study. Eur J Clin Pharmacol. 2015;71(12):1525–1533. doi: 10.1007/s00228-015-1950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown S.G. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114(2):371–376. doi: 10.1016/j.jaci.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Worm M., Edenharter G., Ruëff F., et al. Symptom profile and risk factors of anaphylaxis in Central Europe. Allergy. 2012;67(5):691–698. doi: 10.1111/j.1398-9995.2012.02795.x. [DOI] [PubMed] [Google Scholar]
- 21.Gelincik A., Demirtürk M., Yılmaz E., et al. Anaphylaxis in a tertiary adult allergy clinic: a retrospective review of 516 patients. Ann Allergy Asthma Immunol. 2013;110(2):96–100. doi: 10.1016/j.anai.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Sampson H.A., Mendelson L., Rosen J.P. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327(6):380–384. doi: 10.1056/NEJM199208063270603. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman P., Nicklas R.A., Oppenheimer J., et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126(3):477–480. doi: 10.1016/j.jaci.2010.06.022. e1-42. [DOI] [PubMed] [Google Scholar]
- 24.Grabenhenrich L.B., Dölle S., Ruëff F., et al. Epinephrine in severe allergic reactions: the European anaphylaxis register. J Allergy Clin Immunol Pract. 2018;6(6):1898. doi: 10.1016/j.jaip.2018.02.026. 906.e1. [DOI] [PubMed] [Google Scholar]
- 25.Mueller U.R. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7(4):337–341. doi: 10.1097/ACI.0b013e328259c328. [DOI] [PubMed] [Google Scholar]
- 26.Mulla Z.D., Simon M.R. Hospitalizations for anaphylaxis in Florida: epidemiologic analysis of a population-based dataset. Int Arch Allergy Immunol. 2007;144(2):128–136. doi: 10.1159/000103224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.