Abstract
The objective of this research was to look at how the pesticide lambda-cyhalothrin (LCT) affected the liver, kidney, testis, and ovary of albino mice; and on morphological and skeletal features of the newborn of treated females. The study also aimed to test the ameliorative effects of L-carnitine (LC) against (LCT). Five sets of mice were created, Group 1 acted as the control, while Group 2 received a high dose of LCT, Group 3 received a high dose of LCT + LC, Group 4 received a low dose of LCT that was a residue of in khat (Qat), and Group 5 received a low dose of LCT + LC. The findings revealed that the treated groups' body weights were reduced significantly, whereas the absolute and relative weights of the liver in all groups were statistically decreased insignificantly. There were histopathological changes in the tissues in groups 2 and 4. While the tissues of the ovary and testis showed recovery in groups 3 and 5. When compared to the control group, the values of the seminiferous tubules parameters were statistically significant in the 3 and 5 groups. The newborn had a high dose of pesticides and showed some malformations in the skeleton. However, in group 3 the skeletal malformation was minimized and in-group 5 the skeleton malformations had completely disappeared. It could be concluded that LCT is highly harmful to mouse tissues and caused neonatal malformations, whereas LC has a marked protective effect against LCT.
Keywords: Insecticides, Pyrethroid Lambda-cyhalothrin, Antioxidant L-carnitine, Khat, Mice histological study
1. Introduction
Pesticides are substances used for eliminating or deterring pests that can cause harm to humans, animals, or the environment (Boudh and Singh, 2019, Butu et al., 2020, Warra and Prasad, 2020). Pesticides used on a large scale on crops and forests may contribute to toxic compounds being found in the environment. These compounds can find their way into water bodies; thus casting an adverse impact on the aquatic biota, animals, and humans (Fetoui et al., 2010, Velmurugan et al., 2007). Through soil moisture, these molecules enter crop and forest plants leaving the consumers vulnerable to chronic exposure. Pyrethroids are neurotoxic insecticides widely used to control agricultural and domestic insect pests. Chemically, they are similar to pyrethrins, but they are more toxic to insects and mammals, and they persist longer in the environment (Inyang et al., 2016, WHO, 2015, WHO, 2005).
Lambda-cyhalothrin is a pyrethroid insecticide that is effective on a variety of arthropods that are harmful to human and animal health, also to agricultural production (El-Demerdash, 2007, WHO, 1990). LCT has been shown in mice to have negative effects on tissues, chromosomal abnormalities, and the production of micronuclei in the bone marrow (Çelik et al., 2003, Fetoui et al., 2010).
L-carnitine an innate component of mammalian tissue synthesized from lysine and methionine essential amino acids. It is derived from dietary sources (75%) and endogenous biosynthesis (25%) mainly in the liver and kidney, is a necessary factor for the utilization of long-chain fatty acids to produce energy in mitochondria (Elkomy et al., 2020, Jin et al., 2019, Sallam et al., 2020). It has been shown to function as an antioxidant that scavenges reactive oxygen species (ROS); so could prevent mitochondrial oxidative stress-induced by mitochondrial damage and apoptosis in different cell types, and also elicits stabilizing effect on damaged cell membranes (Dokmeci et al., 2006, Edres et al., 2018, Ghonem et al., 2018, Sallam et al., 2020, Sarica et al., 2007).
L-carnitine presents in high levels in the epididymis, and plays a vital function in the metabolism, as well as in the sperm formation and maturation (Sallam et al., 2020). It has a protective role against cardiotoxicity which might be related to its antioxidant, and antiapoptotic effects (Aboubakr et al., 2020). With its profile of activities, this molecule may have ameliorative action on adverse effects of some pesticides.
Khat (Catha edulis) is a Celastraceae flowering shrub grown in parts of the Middle East and East Africa. In some countries, khat is classified as a drug of abuse, because it contains cathinone, a compound that elicits a feeling of euphoria comparable with that elicited by amphetamines (Kalix, 1992). Harmful effects of khat have been observed in various systems of the body (Al-Habori, 2005). No data might be available in the literature related to residues of (LCT) pesticides in Khat and their effects on the tissues and offspring of the experimental animals. This study aimed to see how the pesticide lambda-cyhalothrin affected histological changes and induced abnormalities in the offspring of exposed mothers, and how the medicine L-carnitine modified these changes and abnormalities.
2. Materials and methods
2.1. The insecticide: lambda-cyhalothrin
Lambda-cyhalothrin is a liquid chemical, it is an alpha-cyano-3-phenoxybenzyl-3-(2-chloro-3,3,3- trifluoroprop-1-enyl)-2, 2-dimethyl-cyclopropane-carboxylate (WHO, 1990) with molecular formula: C23H19CIF3NO3. It is available with different trade names for example, ‘Karate’, ‘Matador’, ‘Icon’ etc. Commercial lambda-cyhalothrin was purchased from the local market in Sana‘a, Yemen.
2.2. L-carnitine
L-carnitine is a liquid chemical, it is an amino acid derivative with the name β-hydroxy-γ- trimethyl-aminobutyrate (Baumgartner and Jacobs, 1997), LC was obtained from MEPACO company (Inshas Elraml, Egypt).
2.3. Khat (Catha edulis)
The khat leaves were supplied by a farm located in Al-Ahjur, Sana’a. The leaves of khat were spread by the pesticide lambda-cyhalothrin. The residues of the pesticide in the leaves were analyzed using a khat extraction, and we treated the mice using a similar concentration. The extraction process of the khat leaves was done and read by GC–MS in the laboratory of the Department of Plant Protection, Ministry of Agriculture. 10 gm of Qat leaves were macerated for 2 or 3 min in 50 ml methanol then filtered and filled up with 100 ml methanol. 5 ml from this extraction was taken and diluted with 15 ml with H2O, 2 ml concentrated NaCl solution was added, then completed the extraction by adding 3x5 ml Tetra Butyl Methyl Ether (TBME), finally this extraction was injected on GC–MS for reading the result (Steinhauer et al., 2002).
2.4. Approval on ethical grounds
The animal experiments were executed in line with the National Institute of Health's guide for the care and use of laboratory animals (Nih, 1978) and were authorized by the local ethics of animal experiments.
2.5. Experimental animals
Thirty male and thirty female mice weighing from (25–30 gm) each were used. They were made available from the Biology Department, Science College, Sana'a University's house of animals. During the trial, the animals were kept in completely sanitary surroundings and fed dry food pellets and water ad libitum. Animals under treatment were orally fed on variable doses of insecticide LCT and antioxidant (LC) every day for six weeks and their body weight and liver weight were recorded.
The mice were randomly assigned to 5 groups of 12 animals each. The control group was the first group and obtained only a routine diet and water, whereas the groups of experimental TI treated with 10 mg/kg b.w of LCT, TII treated with 10 mg/kg b.w (LCT) + 600 mg/kg b.w LC, TIII treated with 0.326 mg/kg b.w (LCT) equivalent to residual traces in ‘Qat’ extract and TIV Group treated with 0.326 mg/kg b.w (LCT) + 600 mg/kg b.w LC. At the end of the experiments, the mice were euthanized under light diethyl ether anesthesia.
2.6. Specimen preparation
The liver, kidney, testes, and ovaries were removed and then washed with saline to remove the bloodstain. The tissues were fixed by using 10% neutral formalin, dehydrated with different grades of alcohol. The dehydrated tissues were then cleared in xylene, infiltrated, and embedded with paraffin wax. Blocks were cut at a thickness of 5 mm and stained with hematoxylin and eosin before being examined under a light microscope (Velmurugan et al., 2007). Sections of testes were used to measure the seminiferous tubule parameters (diameter, area, and perimeter) by using ocular and stage micrometers under a light microscope.
The newborns were sacrificed by euthanasia method of exposure to 80% CO₂ sec in a sealed chamber then the skin, viscera, and adipose tissue of them were removed, and they were placed in acetone for at least 2 days to remove the fat and keep the specimen firm, then they were stained for 3 days in freshly prepared staining solution which was composed of 0.3 % alcian blue in 70 % ethanol (1 vol), 0.1 % alizarin red in 95 % ethanol (1 vol), acetic acid (1 vol), and 70 % ethanol (17 vol) with the amount of 10 ml per a specimen was used. After staining the specimens were washed in distilled water, cleared in 1 % aqueous solution of KOH for 12–48 h, then in a mixture of 20 %, 50 %, 80 % glycerin + 1 % aqueous KOH solutions as follows: 1 vol 20 % glycerin + 4 volumes 1 % KOH, 1 vol 50 % glycerin + 1 vol 1 % KOH, and 4 volumes 80 % glycerin + 1 vol 1 % KOH for 2–3 days in each solution (McLeod, 1980).
2.7. Statistical analysis
For the statistical analysis of body weights, data were tested by one-way analysis of variance (ANOVA) (Ogueji and Auta, 2007). The differences in the relative liver weights were compared for statistical significance using Duncan’s multiple range test (Duncan, 1955). All seminiferous tubule parameters were analyzed by using the 2 sample t-test to assess the significance of changes between control and experimental groups (Elbetieha et al., 2001). Data were presented as mean ± SE (standard error). The p values (Probability) <0.05 were considered significant.
3. Results
3.1. The mortality rate
During the 6 weeks experimental period, the number of dead mice (including males and females) and the percentage of mortalities are illustrated in (Table 1, Table 2).
Table 1.
The number of dead male mice and the mortality rate in the control and treatment groups throughout the experiment.
| Male Groups | NO. of animals | weeks |
Total mortality | % of mortality | |||||
|---|---|---|---|---|---|---|---|---|---|
| W1 | W2 | W3 | W4 | W5 | W6 | ||||
| Control | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TI | 6 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 33 |
| TII | 6 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 33 |
| TIII | 6 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 33 |
| TIV | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Control (Group 1), TI (Group 2), TII (Group 3), TIII (Group 4), and TIV (Group 5).
Table 2.
The number of dead female mice and the mortality rate in the control and treatment groups throughout the experiment.
| Female Groups | NO.of animals | weeks |
Total mortality | % of mortality | |||||
|---|---|---|---|---|---|---|---|---|---|
| W1 | W2 | W3 | W4 | W5 | W6 | ||||
| Control | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TI | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 16 |
| TII | 6 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 16 |
| TIII | 6 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 16 |
| TIV | 6 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 16 |
Control (Group 1), TI (Group 2), TII (Group 3), TIII (Group 4), and TIV (Group 5).
In the control group, there was no case of death, therefore no percentage of mortality was recorded.
In contrast, Table 1 in TI group of males only, group TII and group TIII, two cases of death were recorded. The percentage of male mortality was 33% in each of these three groups. But there were no cases of death in group TIV and hence the rate of mortality was zero.
There was one occurrence of death in each of the female mouse groups TI, TII, TIII, and TIV (Table 2), resulting in a mortality rate of 16%.
3.2. Body and liver weights
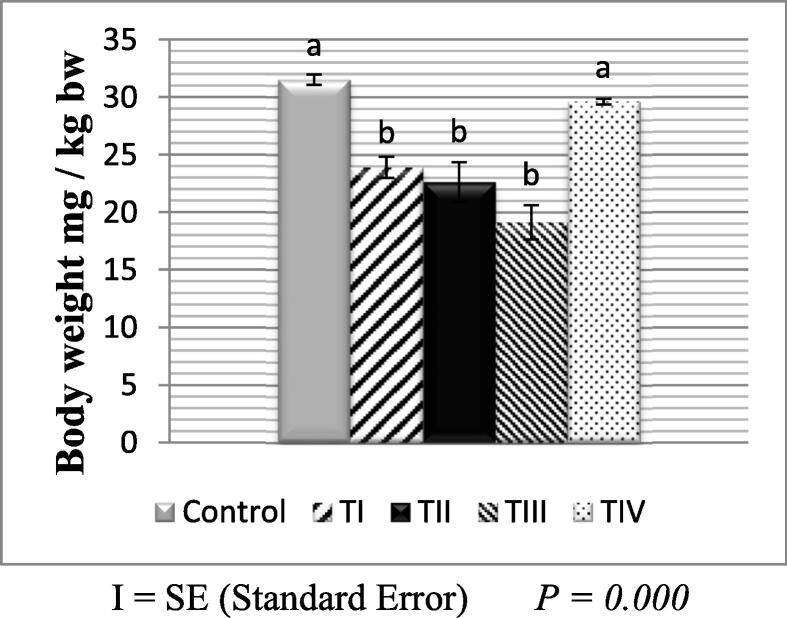
The body weight of the control group was statistically significant when compared with the other treated groups, which included both males and females (p < 0.05). In the group of control, the body weight gradually increased. While in the groups of males (TI, TII, and TIII) the body weight was dropped compared to the group of control. A statistically significant difference existed. When compared to the control group, the bodyweight difference in group TIV was statistically insignificant.
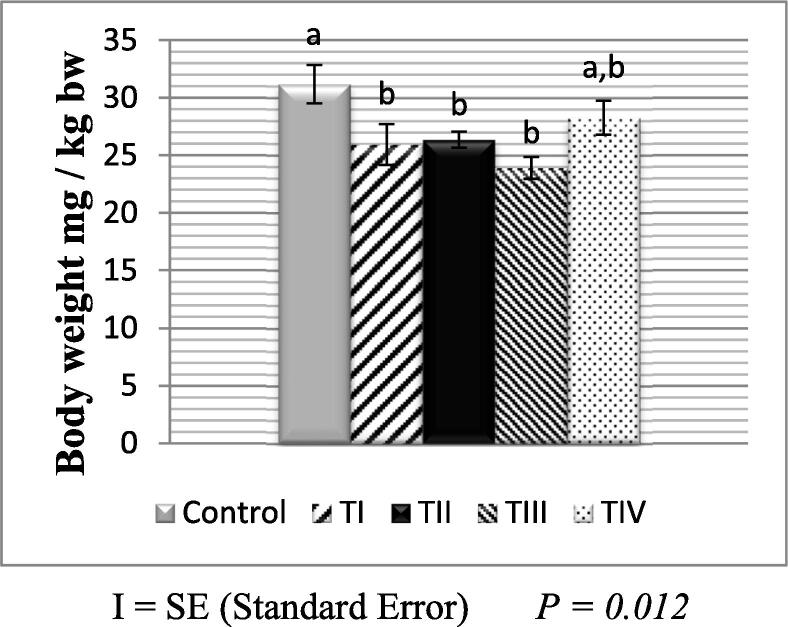
Female body weights in the three groups (TI, TII, and TIII) were decreased gradually and then increased during pregnancy. This decrease was significant in comparison with the control group. While in the group TIV did not decrease, and the difference was statistically insignificant.
At the conclusion of the experiment, the mean male body weight was as in (Fig. 1) illustrates that the maximum mean value was (29.57 ± 0.24gm) in TIV compared with the control group. Fig. 2 shows that the maximum mean value of body weight in females was also (28.26 ± 1.48gm) in TIV compared to the control group.
Fig. 1.
Mean body weight in the control and treated groups of male albino mice.
Fig. 2.
Mean body weight in the control and treated groups of female albino mice.
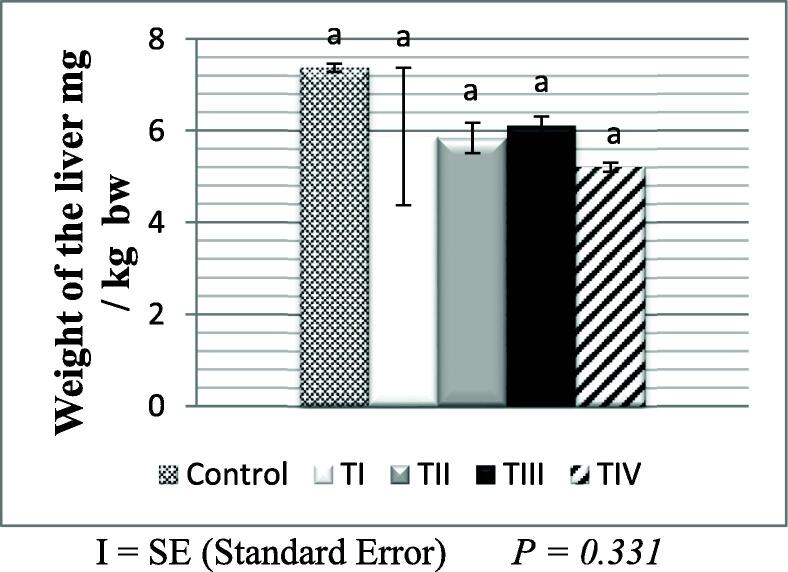
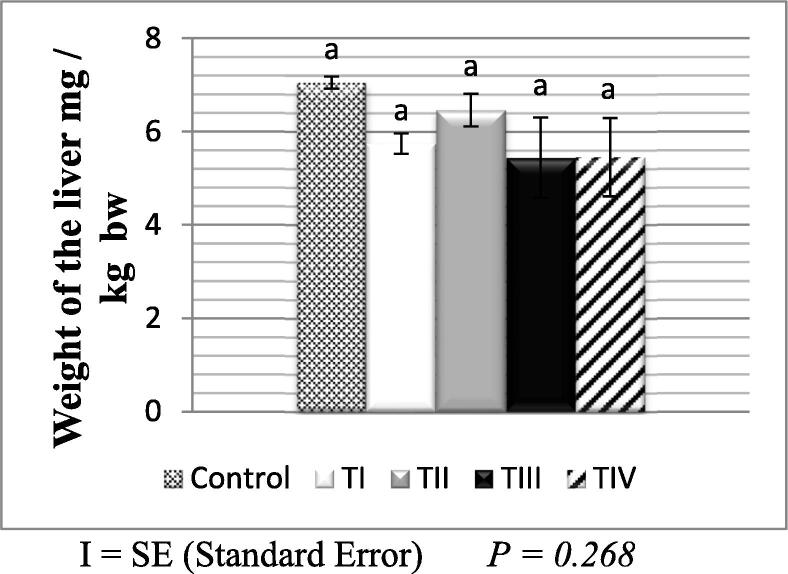
In comparison to the control group, the absolute and relative weights of male and female livers in all treated groups were statistically insignificant (P > 0.05) as shown in (Fig. 3, Fig. 4).
Fig. 3.
Relative liver weight of the control and treated groups of male albino mice.
Fig. 4.
Relative liver weight of the control and treated groups of female albino mic.
3.3. Histological changes
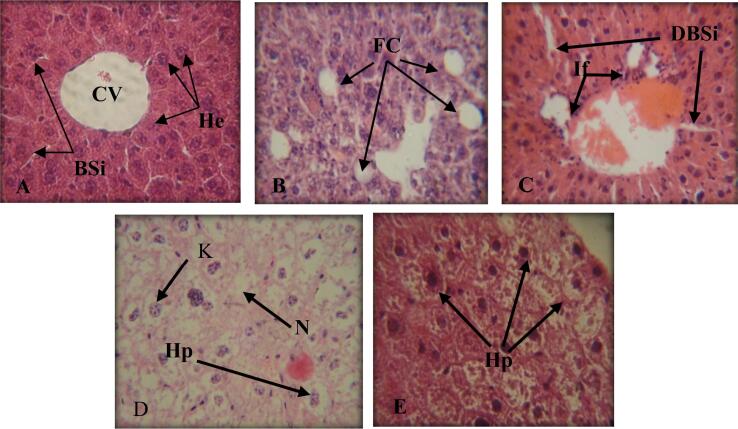
The liver section of the control group showed normal structure (Fig. 5A), in contrast to the control group, the treated mice's liver sections showed histological abnormalities. In all treated groups, the histopathological alterations included: congestion in the central vein of the hepatic lobule, a wide area of fatty changes, dilated blood vessels, the presence of necrotic cells, karyorrhexis, and hypertrophy (Fig. 5B,C,D, and E).
Fig. 5.
Sections in the livers of albino mice. A: (Control Group) showing normal central vein (CV), normal hepatocytes (He), normal blood sinusoids (BSi). 800X.B: (TI Group) showing fatty change (FC) 800X. C: (TII Group) showing neutrophils infiltration (If), dilated blood sinusoids (DBSi). 400X D: (TIII Group) showing hypertrophy (Hp), necrosis (N), karyorrhexis (K). 800X. E: (TIV Group) showing hypertrophy (Hp). 800X.
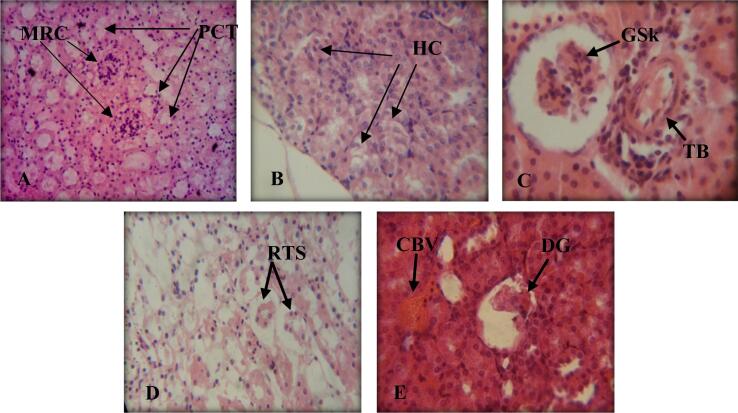
Concerning the kidney section of control female mice showing the general histological structure is present in (Fig. 6A). Sections in the kidney of albino mice treated first with doses of LCT alone and later with LCT together with LC, showed both histological alterations such as hydropic change, extensive glomerular shrinkage, thickened blood vessels, and degenerated glomerulus with dilated renal tubules. on the other hand, recorded amyloid cast within the renal tubular epithelium and occurrence of congested blood vessels (Fig. 6B,C, D, and E).
Fig. 6.
Cross-sections in kidneys of albino mice. (H&E) A: (Control Group) showing normal malpighian renal corpuscles(MRC), normal proximal convoluted tubules (PCT). 400X B: (TI Group) showing hydropic change (HC). 800X C: (TII Group) showing glomerular shrinkage (GSk), thickened blood vessel (TBV). 800X D: (TIII Group) showing renal tubular shrinkage (RTS). 400X E: (TIV Group) showing degenerated glomerulus (DG), congested blood vessel (CBV). 800X.
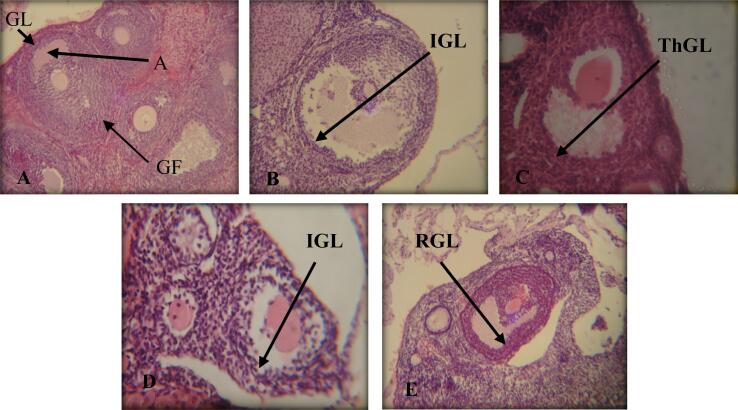
The control group's ovary has a variety of ovarian follicles: primary follicles, secondary, and Graafian follicles. After the Graafian follicle ruptures, the corpus luteum forms in the ovary as a temporary endocrine organ (Fig. 7A). Meanwhile, the ovary of the mice group orally administered lambda-cyhalothrin showed in groups TI and TIII irregular follicular cells around the follicular cysts, also a portion of the Graafian follicles was lined with thin layers of granulosa cells (Fig. 7B,D).
Fig. 7.
Longitudinal-sections in ovaries of albino mice (H&E) A: (Control Group) showing graafian follicle (GF) with its antrum (A) and granulosa layer (GL). 400X B: (TI Group) showing irregular granulosa layer (IGL) 800X C: (TII Group) showing thick granulosa layer (ThGL). 800X D: (TIII Group) showing irregular granulosa layer (IGL). 800X E: (TII Group) showing regular granulosa layer (RGL). 400X.
In TII and TIV Groups, no histopathological alterations were seen. Regular follicular cells were present around the follicular cytes, and the Graafian follicles were lined with thick granulosa layers, as in the control group (Fig. 7C,E).
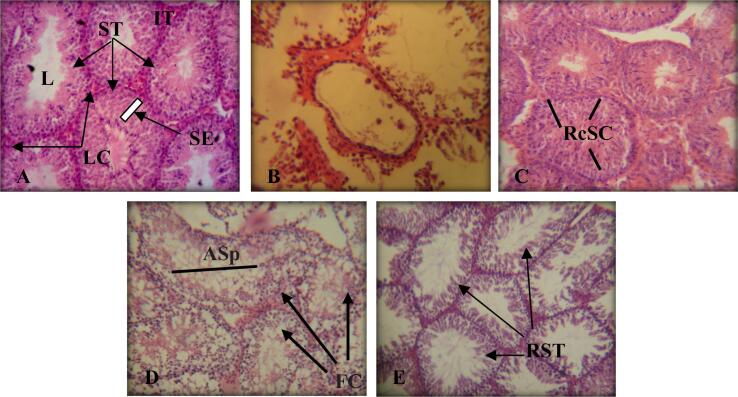
Control males' testes have convoluted seminiferous tubules and Leydig (interstitial cells). The spermatogenic cells are structured in the following order, beginning at the basement membrane and ending at seminiferous tubule, the spermatogenic cells are organized in the following order: Spermatogonia, primary spermatocytes, secondary spermatocytes, spermatids, and spermatozoa. Sertoli cells are simple columnar cells present between the spermatogenic cells (Fig. 8A). While in groups TI and TIII degenerated spermatogenic cells were noticed in seminiferous tubules which were lined by very few spermatogenic cells or by cell debris with a complete absence of mature spermatozoa in the tubular lumen (Fig. 8B, D). In groups TII and TIV there was a recovery of the seminiferous tubules (Fig. 8C,E).
Fig. 8.
Cross-sections in a testes of albino mice (H&E) A: (Control Group) showing normal seminiferous tubules (ST), lumen (L), interstitial tissue (IT), seminiferous epithelium (SE), leydig cells (LC). 400X B: (TI Group) showing spermatogenic arrest in the tubules which are lined with very few spermatogenic cells or with cell debris with complete absence of mature spermatozoa in tubular lumen. 800X C: (TII Group) showing recovered seminiferous cells (RcSC). 400X D: (TIII Group) showing fatty change in seminiferous tubules (FC), complete absence of mature spermatozoa in tubular lumen (ASp). 400X E: (TIVGroup) showing regular seminiferous tubules (RST). 400X.
The parameters of seminiferous tubules (diameter, area, and perimeter) were measured in all groups, and a summary of these results is presented in (Table 3). Compared with the testis of the control group, all values of parameters in TI, and TIII groups (which were treated with lambda-cyhalothrin alone with high and low doses), were statistically insignificant.
Table 3.
Mean of seminiferous tubules parameters in albino mice including the control and the treated groups.
| Mean ± SE |
|||||
|---|---|---|---|---|---|
| Control | TI | TII | TIII | TIV | |
| Diameter | (0.18 ± 0.002)a | (0.17 ± 0.002)a | (0.14 ± 0.003)b | (0.18 ± 0.002)a | (0.21 ± 0.0003)c |
| Area | (0.029 ± 0.001)a | (0.027 ± 0.001)a | (0.021 ± 0.0004)b | (0.028 ± 0.001)a | (0.038 ± 0.0002)c |
| Perimeter | (0.6 ± 0.007)a | (0.53 ± 0.009)a | (0.47 ± 0.011)b | (0.62 ± 0.005)a | (0.66 ± 0.002)c |
Data expressed as (Mean ± SE). (a, b, c) Different superscripts within rows are significantly different (p < 0.05). Control (Group 1), TI (Group 2), TII (Group 3), TIII (Group 4), and TIV (Group 5).
The values were statistically significant in TII, and TIV groups (which were both treated with lambda-cyhalothrin + L-carnitine) compared with values in the control group. The minimum values were in the TII group and the maximum values were in the TIV group.
3.4. Newborn malformations
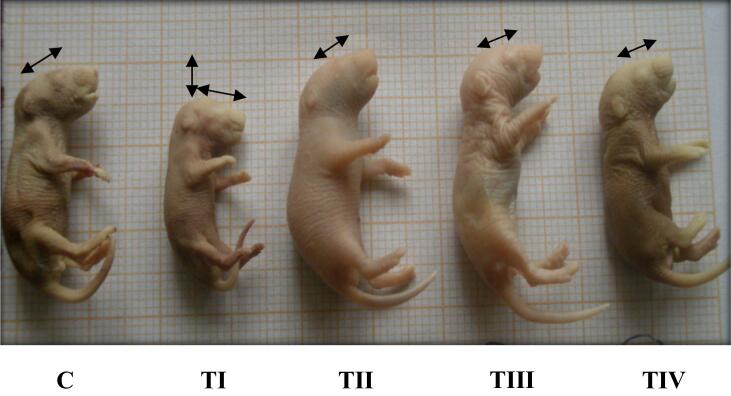
In the present investigation, the insecticide LCT caused some malformation in the newborn female mice treated with the percentage of (10 mg/kg BW) of this insecticide. This newborn had an approximately normal morphological appearance but showed a reduction in size and curvature of the head down to its bottom. These malformations did not appear when LC was used with the same dose.
The newborn of mothers who had the low dose of the insecticide did not have any malformations. Moreover, the LC had no effect when used with the same low dose of insecticide (Fig. 9).
Fig. 9.
Offspring of albino mice under experiment: control (C), treated groups (TI, TII, TIII, and TIV). (TI): Reduction in size and curvature of the head down to its bottom. (TII), (TIII), and (TIV): morphological changes were not observed.
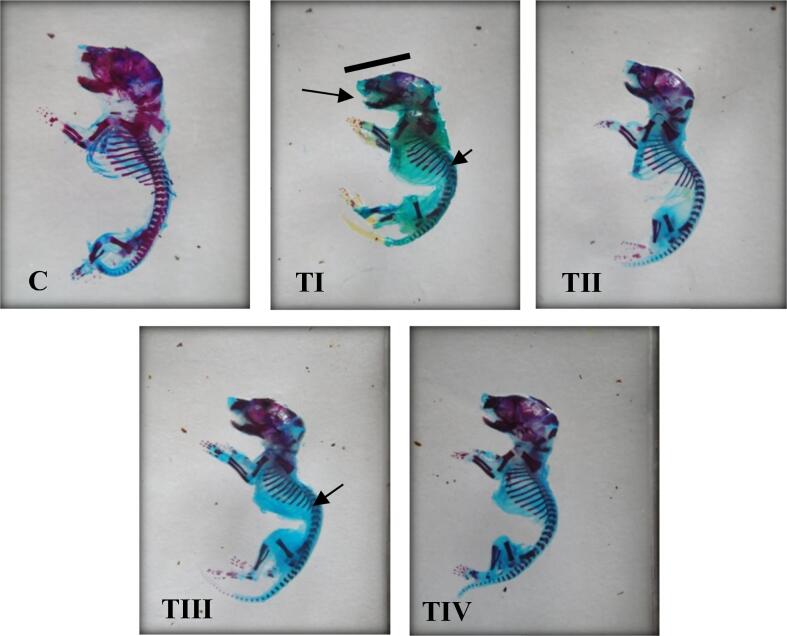
3.5. Skeletal investigation
The normal skeleton of offspring obtained from the control group is represented in (Fig.10C), the skeletons of TI group offspring showed an increase in vertebral column curvature, a curvature of the head down to its bottom, elongation of the mandible bone, little ossification in the cervical and lumbar vertebrae, and parietal and interparietal bones of the skull. Meanwhile, the offspring of group TIII were investigated after they were treated with a low dose of pesticide found in khat extraction (0.326 mg/kg b.w.) including the curvature of the head down to its bottom and the fork end of the tibia only.
Fig. 10.
Side view of newborns of albino mice stained with alizarin and alcian blue. Control (C), treated groups (TI, TII, TIII, and TIV).
In Group TII when LC was used in a high dose of lambda-cyhalothrin, the skeleton malformations were minimized. While in Group TIV, the skeleton malformations which were caused by the low dose of lambda-cyhalothrin and LC have completely disappeared (Fig. 10).
4. Discussion
Humans are exposed to a variety of environmental contaminants, including pesticides, through different routes of exposure: air, food, and water. These compounds have the potential to disrupt endocrine processes, resulting in reproductive problems, cancers, and other effects (Prater et al., 2002). Lambda-cyhalothrin is a synthetic pyrethroid insecticide that affects many organs and is commonly used in pesticides (Righi and Palermo-Neto, 2005).
The current study found that giving albino mice 10 mg/kg b.w of LCT resulted in a decrease in female body weight (Groups: TI, TII, and TIII). However, the TIV group's body weight did not drop, possibly because the LC might reduce the effect of LCT on this parameter. This might be attributable to LCT's toxicity, which reduced dietary intake and absorption through the gastrointestinal tract, as well as the effectiveness of food conversion (Ball and Chhabra, 1981). This result agrees with the findings of (Ben Abdallah et al., 2013, Fetoui et al., 2009, Yousef, 2010) who reported that LCT decreased the food intake and body weight in rabbits, rats, and female mice. On the other hand, our result disagrees that showed no statistically significant differences in body weight or weight increase between the LCT-treated mice and the control group.
Data in (Figs. 1 and 2) in comparison to the control group, the liver absolute and relative weight differences in the treated groups were statistically insignificant. Similar result reported with (Nieradko-Iwanicka and Konopelko, 2020).
Higher circulation caused by increased demands for detoxification of hazardous drugs, a proliferation of hepatic cytochrome P 450 monooxygenase, and keeping the normal liver functional capability may all cause increasing the weight of the liver (Ambali et al., 2007). But this result is inconsistent with that of (Fetoui et al., 2009) who used the same insecticide and discovered that rats' absolute and relative liver weights were lowered significantly compared to the control group.
The current findings revealed that LCT treatment could cause liver injury, hepatic necrosis, inflammation, congestion, hemorrhage, blood vessel dilation, pyknosis, hypertrophy, karyorrhexis, and fatty changes.
The results of this study corroborated previous reports (Al-Sarar et al., 2014, Fetoui et al., 2009) who have demonstrated that exposure to LCT induced liver injury in mice and rats similar to the present findings. Basir et al. (2011) reported that the liver of LCT-treated rabbits exhibited extensive perihepatitis, bile duct hyperplasia, necrosis, hemorrhage, and congestion. Infiltrations were noted in the area of the central veins of the lobules in the portal tract and between hepatocytes (Ahmad et al., 2011, Luty et al., 2000) also found that cypermethrin produced histological lesions in rabbit livers.
In this study, the LCT + LC treated groups caused liver alterations in the mice and there was no protective impact of LC as an antioxidant. These changes included inflammation, necrosis, dilated blood vessels, and hypertrophy. This result is inconsistent that of (Yapar et al., 2007) who reported that LC has a prominent protective effect against hepatotoxicity in mice caused by acetaminophen (which is analgesic and antipyretic).
Comparing the LCT treated groups with the control group in the kidney showed the existence of hydropic change, convoluted tubule dilated, dilated and congested blood vessels, amyloid cast, inflammation, and enlarged glomerulus. These results agree with those of (Ahmad et al., 2011, Basir et al., 2011, Khaldoun Oularbi, 2014) who have revealed that LCT caused kidney injury in mice, rabbits, and rats similar to the current results. Using lambda-cyhalothrin + LC together induced kidney injury in the mice of the present study but also no protective effect of LC as an antioxidant was observed. The kidney injury included glomerular shrinkage, hydropic change, thickened blood vessels, amyloid cast, renal tubular shrinkage, degenerated glomerulus, dilated renal tubules, and fatty changes. This result does not agree with that of (Kart et al., 2006) who reported that LC decreased the oxidative stress caused by gentamicin (antibiotic) in the kidney.
Sections in the ovary of LCT-treated animals revealed some changes. These changes included irregular follicular cells around the follicular cysts, also a portion of the Graafian follicles were lined with thin layers of the granulosa layer. These results are similar to that of (Ahmad et al., 2011) who discovered that diazinon, a pesticide, was harmful to ovarian tissue and produced histological alterations in mice's ovaries.
The LCT + LC treated groups demonstrated that the ovary looked like that of the control group. There were regular follicular cells around the follicular cysts, also the Graafian follicles were lined by thick layers of the granulosa cells. These results agree those of (Azarnia et al., 2004) who showed that the antioxidant L- cysteine protected mice's ovary from damage caused by specific poisons.
The testis of the lambda-cyhalothrin-treated animals also suffered severe damage. This insecticide may cause testicular injuries like seminiferous tubules irregularity caused by blood vessel thickness in the interstitial tissue, interstitial spaces expansion, spermatogenic cell degeneration, and fatty change in the seminiferous tubule. These results are corroborated with previous reports of (Al-Sarar et al., 2014) who have demonstrated that exposure to lambda-cyhalothrin caused testis injury in mice similar to the present findings. In permethrin-exposed mice, abnormal seminiferous tubules with vacuoles or a lack of germ cells have been seen (Zhang et al., 2007).
Histopathological alterations were observed in the testis of lambda-cyhalothrin + L-carnitine treated mice (TII). The changes included seminiferous tubules dilation and interstitial spaces expansion, but the seminiferous tubules were retrieved, and the spermatogenic cells had more spermatozoa. The testis was regular in the TIV group, with recovered seminiferous tubules, spermatogenic cells, and an increase in spermatozoa productivity. These results may agree with those of (Zhai et al., 2007) who reported that L-carnitine possesses antioxidant properties which increase sperm concentration by preventing lipid peroxidation in White Leghorns.
When the values of the seminiferous tubules parameters (diameter, area, and perimeter) of the treated groups (TI, TIII) were compared to the control group, they were found to be insignificantly lower. This result agrees with (Elbetieha et al., 2001) who investigated how cypermethrin affects mice males and discovered that the perimeter of seminiferous tubules shrank but the seminiferous lumen diameter stayed the same. The numerous types of pesticides and the various amounts that were used may have contributed to this result.
While these parameters' values of the treated groups (TII, TIV) were statistically significant in comparison with the control group, in TII values being lower and TIV values being higher. This could be attributed to the combined effect of high lambda-cyhalothrin and L-carnitine doses in TII, which resulted in a decrease in seminiferous tubule parameters. L-carnitine increased seminiferous tubule parameters in TIV, which could be attributed to the low dose of lambda-cyhalothrin, which could not produce a combined effect with L-carnitine. So, the influence of L-carnitine on the seminiferous tubules by raising their parameters might be ascribed to the regrowth of numerous spermatogenic cells. This result agrees with the findings of (Topcu-Tarladacalisir et al., 2009) who cleared that the exposure of male albino rats to L-carnitine at 21 and 44 days after irradiation caused increasing the diameter of the seminiferous tubules, and an increase of their seminiferous tubules epithelium height at 21 days after irradiation.
The present study illustrated that the insecticide LCT when applied alone caused slight embryo malformations (TI and TIII groups). These were represented by less ossification of some bones of the skeleton. This result agrees with that of (El-Bayomy et al., 2002) who revealed that the insecticide Mirex caused embryo malformations in mice at the early organogenesis stage. Moreover, the present results agree with (Syed et al., 2009) who found that the Cyfluthrin insecticide inhibited the ossification of embryonic skeletons in Swiss albino mice.
The current study's findings also revealed that the antioxidant LC decreased the malformations of newborns induced by the LCT insecticide. This result agrees with (Chen et al., 2004) who discovered that using antioxidants lowered the incidence of ethanol-induced embryo abnormalities in mice.
Even though the dosage was only utilized for a short time, our data suggest that LCT had a significant influence on the liver, kidney, ovary, and testis of albino mice, and produced mild embryo malformations. The antioxidant L-carnitine can be utilized to address any changes to the testis, ovary, or embryo abnormalities induced by exposure to the insecticides.
Funding
The Researchers Supporting Project number (RSP 2021/232) of King Saud University in Riyadh, Saudi Arabia, funded this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to the Researchers Supporting Project number (RSP 2021/232) of King Saud University in Riyadh, Saudi Arabia, for funding this research.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Nawal M. Al Malahi, Email: nalmalahi@ksu.edu.sa.
Reem A. Alajmi, Email: ralajmi@ksu.edu.sa.
Afrah F. Alkhuriji, Email: aalkhuriji@ksu.edu.sa.
Jameel Al-Tamimi, Email: Jtamimi@ksu.edu.sa.
Ahmad R Alhimaidi, Email: ahimaidi@ksu.edu.sa.
References
- Aboubakr M., Elsayd F., Soliman A., Fadl S.E., El-Shafey A., Abdelhiee E.Y. L-Carnitine and vitamin E ameliorate cardiotoxicity induced by tilmicosin in rats. Environ. Sci. Pollut. Res. 2020;27:23026–23034. doi: 10.1007/s11356-020-08919-6. [DOI] [PubMed] [Google Scholar]
- Ahmad K.R., Tahir M.Z., Khan S.Y., Tahir H.M., Raees K., Arshad M., Abbas T. Effects of diazinon on the ovarian micro-anatomical and micrometric parameters of pregnant mice. African J. Biotechnol. 2011;10:14656–14662. [Google Scholar]
- Al-Habori M. The potential adverse effects of habitual use of Catha edulis (khat) Expert Opin. Drug Saf. 2005;4:1145–1154. doi: 10.1517/14740338.4.6.1145. [DOI] [PubMed] [Google Scholar]
- Al-Sarar A.S., Abobakr Y., Bayoumi A.E., Hussein H.I., Al-Ghothemi M. Reproductive toxicity and histopathological changes induced by lambda-cyhalothrin in male mice. Environ. Toxicol. 2014;29:750–762. doi: 10.1002/tox.21802. [DOI] [PubMed] [Google Scholar]
- Ambali S., Akanbi D., Igbokwe N., Shittu M., Kawu M., Ayo J. Evaluation of subchronic chlorpyrifos poisoning on hematological and serum biochemical changes in mice and protective effect of vitamin C. J. Toxicol. Sci. 2007;32:111–120. doi: 10.2131/jts.32.111. [DOI] [PubMed] [Google Scholar]
- Azarnia M., Shakour A., Rostami P., Sanaie-Mehr A. The protective role of L-Cysteine against follicular atresia induced by lead in mouse ovary. Acta Med. Iran. 2004;42(2):83–88. [Google Scholar]
- Ball L.M., Chhabra R.S. Intestinal absorption of nutrients in rats treated with 2,3,7,8-tetrachlorodibenzo-p-Dioxln (TCDD) J. Toxicol. Environ. Heal. Part A Curr. 1981;8(4):629–638. doi: 10.1080/15287398109530097. [DOI] [PubMed] [Google Scholar]
- Basir A., Khan A., Mustafa R., Zargham Khan M., Rizvi F., Mahmood F., Yousaf A. Toxicopathological effects of lambda-cyhalothrin in female rabbits (Oryctolagus cuniculus) Hum. Exp. Toxicol. 2011;30:591–602. doi: 10.1177/0960327110376550. [DOI] [PubMed] [Google Scholar]
- Baumgartner M., Jacobs S. L-carnitine: importance for pig breeding. Lohmann Inf. 1997;22:15–19. [Google Scholar]
- Ben Abdallah F., Fetoui H., Zribi N., Fakhfakh F., Keskes L. Quercetin attenuates lambda cyhalothrin-induced reproductive toxicity in male rats. Environ. Toxicol. 2013;28:673–680. doi: 10.1002/tox.20762. [DOI] [PubMed] [Google Scholar]
- Boudh, S., Singh, J.S., 2019. Pesticide contamination: environmental problems and remediation strategies, in: Emerging and Eco-Friendly Approaches for Waste Management, Springer, pp. 245–269.
- Butu M., Stef R., Grozea I., Corneanu M., Butnariu M. Bioremediation and Biotechnology. Springer; 2020. Biopesticides: clean and viable technology for healthy environment; pp. 107–151. [Google Scholar]
- Çelik A., Mazmanci B., Çamlica Y., Aşkin A., Çömelekoǧlu Ü. Cytogenetic effects of lambda-cyhalothrin on Wistar rat bone marrow. Mutat. Res. Toxicol. Environ. Mutagen. 2003;539:91–97. [PubMed] [Google Scholar]
- Chen S., Dehart D.B., Sulik K.K. Protection from ethanol-induced limb malformations by the superoxide dismutase/catalase mimetic, EUK-134. FASEB J. 2004;18:1234–1236. doi: 10.1096/fj.03-0850fje. [DOI] [PubMed] [Google Scholar]
- Dokmeci D., Akpolat M., Aydogdu N., Uzal C., Doganay L., Turan F.N. The protective effect of L-carnitine on ionizing radiation-induced free oxygen radicals. Scand. J. Lab. Anim. Sci. 2006;33:75–83. [Google Scholar]
- Duncan D.B. Multiple range and multiple F tests. Biometrics. 1955;11(1):1–42. [Google Scholar]
- Edres H.A., Taha N.M., Mandour A.-E.-W.-A., Lebda M.A. Impact of L-carnitine on Bisphenol A-induced kidney damage in rats. Alexandria J. Vet. Sci. 2018;56(1):11–17. [Google Scholar]
- El-Bayomy A.A., Smoak I.W., Branch S. Embryotoxicity of the pesticide mirex in vitro. Teratog. Carcinog. Mutagen. 2002;22:239–249. doi: 10.1002/tcm.10016. [DOI] [PubMed] [Google Scholar]
- Elbetieha A., Da’as S.I., Khamas W., Darmani H. Evaluation of the toxic potentials of cypermethrin pesticide on some reproductive and fertility parameters in the male rats. Arch. Environ. Contam. Toxicol. 2001;41:522–528. doi: 10.1007/s002440010280. [DOI] [PubMed] [Google Scholar]
- El-Demerdash F.M. Lambda-cyhalothrin-induced changes in oxidative stress biomarkers in rabbit erythrocytes and alleviation effect of some antioxidants. Toxicol. Vitr. 2007;21:392–397. doi: 10.1016/j.tiv.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Elkomy A., Abdelhiee E.Y., Fadl S.E., Emam M.A., Gad F.A., Sallam A., Alarifi S., Abdel-Daim M.M., Aboubakr M. L-carnitine mitigates oxidative stress and disorganization of cytoskeleton intermediate filaments in cisplatin-induced Hepato-renal toxicity in rats. Front. Pharmacol. 2020;11:1–12. doi: 10.3389/fphar.2020.574441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetoui H., Garoui E.M., Zeghal N. Lambda-cyhalothrin-induced biochemical and histopathological changes in the liver of rats: ameliorative effect of ascorbic acid. Exp. Toxicol. Pathol. 2009;61:189–196. doi: 10.1016/j.etp.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Fetoui H., Makni M., Garoui E.M., Zeghal N. Toxic effects of lambda-cyhalothrin, a synthetic pyrethroid pesticide, on the rat kidney: Involvement of oxidative stress and protective role of ascorbic acid. Exp. Toxicol. Pathol. 2010;62:593–599. doi: 10.1016/j.etp.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Ghonem M.M., Lashin H.I., Hodeib A.A., Soliman N.A. L-carnitine as an adjuvant treatment in acute organophosphorus pesticides poisoning: a randomized clinical trial. Mansoura J. Forensic Med. Clin. Toxicol. 2018;26:37–52. [Google Scholar]
- Inyang I.R., Obidiozo O.Z., Izah S.C. Effects of Lambda cyhalothrin in protein and Albumin content in the kidney and liver of Parpohiocephalus obscurus. EC Pharmacol. Toxicol. 2016;2:148–153. [Google Scholar]
- Jin M., Pan T., Cheng X., Zhu T.T., Sun P., Zhou F., Ding X., Zhou Q. Effects of supplemental dietary L-carnitine and bile acids on growth performance, antioxidant and immune ability, histopathological changes and inflammatory response in juvenile black seabream (Acanthopagrus schlegelii) fed high-fat diet. Aquaculture. 2019;504:199–209. [Google Scholar]
- Kalix P. Cathinone, a natural amphetamine. Pharmacol. Toxicol. 1992;70:77–86. doi: 10.1111/j.1600-0773.1992.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Kart A., Yapar K., Karapehlivan M., Tunca R., Ogun M., Citil M. Effects of L-carnitine on kidney histopathology, plasma and tissue total sialic acid, malondialdehyde and glutathione concentrations in response to gentamicin administration in Balb/C mice. Revue De Medecine Veterinaire. 2006;157(4):179–184. [Google Scholar]
- Khaldoun Oularbi H. Biochemical and histopathological changes in the kidney and ddrenal gland of rats following repeated exposure to Lambda-Cyhalothrin. J. Xenobiotics. 2014;4:8–13. [Google Scholar]
- Luty S., Latuszynska J., Obuchowska-Przebirowska D., Tokarska M., Haratym-Maj A. Subacute toxicity of orally applied alpha-cypermethrin in Swiss mice. Ann. Agric. Environ. Med. 2000;7:33–41. [PubMed] [Google Scholar]
- McLeod M.J. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980;22:299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- Nih, 1978. Grants Peer Review, vol. 2, pp. 25–33.
- Nieradko-Iwanicka B., Konopelko M. Effect of Lambda cyhalothrin on Locomotor Activity, Memory, Selected Biochemical Parameters, Tumor Necrosis Factor α, and Interleukin 1ß in a Mouse Model. Int. J. Environ. Res. Public Health. 2020;17(24):1–11. doi: 10.3390/ijerph17249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogueji E.O., Auta J. Investigation of biochemical effects of acute concentrations of Lambda-Cyhalotrin on African Catfish Clarias gariepinus-Teugels. J. Fish. Int. 2007;2(1):86–90. [Google Scholar]
- Prater M.R., Gogal R.M., Jr, Blaylock B.L., Longstreth J., Holladay S.D. Single-dose topical exposure to the pyrethroid insecticide, permethrin in C57BL/6N mice: effects on thymus and spleen. Food Chem. Toxicol. 2002;40:1863–1873. doi: 10.1016/s0278-6915(02)00163-1. [DOI] [PubMed] [Google Scholar]
- Righi D.A., Palermo-Neto J. Effects of type II pyrethroid cyhalothrin on peritoneal macrophage activity in rats. Toxicol. 2005;212:98–106. doi: 10.1016/j.tox.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Sallam A.O., Rizk H.A., Emam M.A., Fadl S.E., Abdelhiee E.Y., Khater H., Elkomy A., Aboubakr M. The ameliorative effects of L-carnitine against cisplatin-induced gonadal toxicity in rats. Pak. Vet. J. 2020;41(1):147–151. [Google Scholar]
- Sarica S., Corduk M., Suicmez M., Cedden F., Yildirim M., Kilinc K. The effects of dietary L-carnitine supplementation on semen traits, reproductive parameters, and testicular histology of Japanese quail breeders. J. Appl. Poult. Res. 2007;16:178–186. [Google Scholar]
- Steinhauer, S.; Rzepka, S.; Jernberg, K.., 2002. Combined decline and magnitude of residues of cymoxanil and famoxadone in protected tomatoes (Fruiting vegetables: Combined decline and magnitude of residues of cymoxanil and famoxadone in protected tomatoes (Fruiting vegetables: solanaceae) following applications of cymoxanil/famoxadone (DPX-KX007) WG (1.3:1) – southern europe, season 2001. Dr. Specht & Partner chemische laboratorien gmbh, Hamburg, germany. DuPont-5519 (Sited in WHO. 2003, Pesticide Residues in Food. Evaluations, vol. 1).
- Syed F., Soni I., John P., Bhatnagar P. Embryotoxic and teratogenic evaluation of cyfluthrin in Swiss albino mice. Toxicol. Int. 2009;16(2):121–126. [Google Scholar]
- Topcu-Tarladacalisir Y., Kanter M., Uzal M.C. Role of l-carnitine in the prevention of seminiferous tubules damage induced by gamma radiation: a light and electron microscopic study. Arch. Toxicol. 2009;83:735–746. doi: 10.1007/s00204-008-0382-y. [DOI] [PubMed] [Google Scholar]
- Velmurugan B., Selvanayagam M., Cengiz E.I., Unlu E. Histopathology of lambda-cyhalothrin on tissues (gill, kidney, liver and intestine) of Cirrhinus mrigala. Environ. Toxicol. Pharmacol. 2007;24:286–291. doi: 10.1016/j.etap.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Warra A.A., Prasad M.N.V. Agrochemicals Detection, Treatment and Remediation. Elsevier; 2020. African perspective of chemical usage in agriculture and horticulture their impact on human health and environment; pp. 401–436. [Google Scholar]
- WHO, I., 1990. Environmental health criteria 101: Methylmercury. World Heal. Organ. Geneva, pp. 144.
- WHO, 2005. Safety of Pyrethroids for Public Health Use. World Health Organization.
- WHO, 2015. Specifications and evaluations for public health pesticides: Lambda-cyhalothrin. Geneva, Switz. World Heal. Organ.
- Yapar K., Kart A., Karapehlivan M., Atakisi O., Tunca R., Erginsoy S., Citil M. Hepatoprotective effect of L-carnitine against acute acetaminophen toxicity in mice. Exp. Toxicol. Pathol. 2007;59:121–128. doi: 10.1016/j.etp.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Yousef M.I. Vitamin E modulates reproductive toxicity of pyrethroid lambda-cyhalothrin in male rabbits. Food Chem. Toxicol. 2010;48:1152–1159. doi: 10.1016/j.fct.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Zhai W., Neuman S.L., Latour M.A., Hester P.Y. The effect of dietary L-carnitine on semen traits of white leghorns. Poult. Sci. 2007;86:2228–2235. doi: 10.1093/ps/86.10.2228. [DOI] [PubMed] [Google Scholar]
- Zhang S.Y., Ito Y., Yamanoshita O., Yanagiba Y., Kobayashi M., Taya K., Li C.M., Okamura A., Miyata M., Ueyama J., Lee C.H., Kamijima M., Nakajima T. Permethrin may disrupt testosterone biosynthesis via mitochondrial membrane damage of leydig cells in adult male mouse. Endocrinol. 2007;148(8):3941–3949. doi: 10.1210/en.2006-1497. [DOI] [PubMed] [Google Scholar]