Abstract
The genus Burkholderia comprises 19 species, including Burkholderia vietnamiensis which is the only known N2-fixing species of this bacterial genus. The first isolates of B. vietnamiensis were recovered from the rhizosphere of rice plants grown in a phytotron, but its existence in natural environments and its geographic distribution were not reported. In the present study, most N2-fixing isolates recovered from the environment of field-grown maize and coffee plants cultivated in widely separated regions of Mexico were phenotypically identified as B. cepacia using the API 20NE system. Nevertheless, a number of these isolates recovered from inside of maize roots, as well as from the rhizosphere and rhizoplane of maize and coffee plants, showed similar or identical features to those of B. vietnamiensis TVV75T. These features include nitrogenase activity with 10 different carbon sources, identical or very similar nifHDK hybridization patterns, very similar protein electrophoregrams, identical amplified 16S rDNA restriction (ARDRA) profiles, and levels of DNA-DNA reassociation higher than 70% with total DNA from strain TVV75T. Although the ability to fix N2 is not reported to be a common feature among the known species of the genus Burkholderia, the results obtained show that many diazotrophic Burkholderia isolates analyzed showed phenotypic and genotypic features different from those of the known N2-fixing species B. vietnamiensis as well as from those of B. kururiensis, a bacterium identified in the present study as a diazotrophic species. DNA-DNA reassociation assays confirmed the existence of N2-fixing Burkholderia species different from B. vietnamiensis. In addition, this study shows the wide geographic distribution and substantial capability of N2-fixing Burkholderia spp. for colonizing diverse host plants in distantly separated environments.
There exist many examples of the wide geographic distribution of Rhizobium species in symbiotic association with legumes (see reference 23). Commonly, this association is referred to as being restricted to legume plants with the exception of the genus Parasponia (41). Recently, Rhizobium leguminosarum bv. trifolii and Azorhizobium caulinodans have also been found in natural endophytic association with field-grown rice (12, 45). Similarly, Gluconacetobacter (formerly Acetobacter) diazotrophicus was considered in early studies as an endophyte associated only with sugarcane and with two other sucrose-accumulating plants (11). However, in the last few years G. diazotrophicus has been found in endophytic association with multiple host plants such as Coffea arabica (19), Eleusine coracana (22), and Ananas comosus (36), all of which were cultivated in very distant geographical regions. A similar picture has been described for Azoarcus species. The first Azoarcus species, Azoarcus indigens and Azoarcus communis, were described in association with Kallar grass cultivated in Pakistan (30). Interestingly, one of the strains used in this study was isolated in France in 1982 from a refinery oil sludge and identified as A. communis. Recently, Azoarcus indigens has also been isolated from field-grown rice cultivated in Nepal (12). Azoarcus tolulyticus has been recovered from a variety of environments and regions (13, 48). These data suggest that Azoarcus spp. are widely distributed. Apparently, many bacterial species are able to flourish in very different and distant habitats. Staley (34) pointed out that the bacterial biogeography data tend to support the hypothesis that many bacteria are cosmopolitan in their distribution. However, the geographic and environmental distribution of many bacterial species remain unknown. For instance, Burkholderia vietnamiensis was discovered in association with roots of rice plants grown in a Vietnamese soil (15). To date, this species has not been isolated from any other plant.
The genus Burkholderia comprises 19 species, which includes soil and rhizosphere bacteria as well as plant and human pathogens (1, 35, 39, 42, 47). In addition, the GenBank database contains the 16S rRNA sequence (accession number AJ238360) of a N2-fixing bacterium, “B. brasilensis”, which has not been officially described. B. vietnamiensis is the only N2-fixing species of this bacterial genus validly described (15).
Over the last few years there has been an increasing interest in B. cepacia, the type species of the genus, because of its wide distribution in natural and clinical environments (18, 31, 39). B. cepacia is recognized for its abilities to promote maize growth (5), to enhance crop yields (8, 35), and to suppress many soilborne plant pathogens (5, 17, 25), as well as to degrade diverse pesticides (9, 26). Similarly, B. vietnamiensis has attracted interest because of its abilities to promote rice plant growth and to enhance grain yield (37, 38). However, both B. cepacia, particularly the named genomovar III and B. vietnamiensis have been cultured from patients with cystic fibrosis (39). Recently, it has been described that B. cepacia genomovar III is a common plant-associated bacterium (3).
The determination of the extent and distribution of microbial diversity on the planet will help in understanding the role of specific microbial taxa in their natural habitats (34). In addition, studies on the diversity of plant-associated bacteria may contribute to the discovery of new beneficial plant-microbe interactions.
In this study, we report on the association of N2-fixing Burkholderia species with field-grown maize and coffee plants cultivated in widely separated geographical regions of Mexico. Our results show that B. vietnamiensis is widely distributed and reveal the existence of new N2-fixing Burkholderia spp. We also report on the ability of B. kururiensis to fix N2, a feature hitherto unknown in this species.
MATERIALS AND METHODS
Plant samples.
From four to eight complete maize and coffee plants grown under field conditions were collected in different geographical regions of Mexico. The origins of maize and coffee plants analyzed are summarized in Table 1.
TABLE 1.
Representative nitrogen-fixing Burkholderia strains associated with maize and coffee plants
| 16S rDNA genotype | Taxon | Strain | Sourcea | Cultivar | Plant | Location codeb |
|---|---|---|---|---|---|---|
| 1 | B. vietnamiensis | MMi-324 | Rhizosphere | VS-535 | Maize | 1 |
| 1 | B. vietnamiensis | MMi-334 | Rhizoplane | VS-535 | Maize | 1 |
| 1 | B. vietnamiensis | MMi-353 | Rhizoplane | VS-535 | Maize | 1 |
| 1 | B. vietnamiensis | MMi-1486 | Rhizosphere | VS-535 | Maize | 1 |
| 1 | B. vietnamiensis | MMi-1537 | Roots* | VS-535 | Maize | 1 |
| 1 | B. vietnamiensis | MMi-1547 | Rhizoplane | VS-535 | Maize | 1 |
| 1 | B. vietnamiensis | MMi-1556 | Roots* | VS-535 | Maize | 1 |
| 1 | B. vietnamiensis | MMi-1577 | Roots* | VS-535 | Maize | 1 |
| 1 | B. vietnamiensis | MMi-1776 | Rhizosphere | VS-535 | Maize | 1 |
| 1 | B. vietnamiensis | CCE-101 | Rhizoplane | Caturra | Coffee | 5 |
| 1 | B. vietnamiensis | CCE-115 | Roots† | Caturra | Coffee | 5 |
| 1 | B. vietnamiensis | CCE-201 | Rhizoplane | Caturra | Coffee | 5 |
| 1 | B. vietnamiensis | CCE-211 | Roots† | Caturra | Coffee | 5 |
| 1 | B. vietnamiensis | CCE-303 | Rhizoplane | Caturra | Coffee | 5 |
| 1 | B. vietnamiensis | CCE-312 | Roots† | Caturra | Coffee | 5 |
| 2 | B. vietnamiensis | MMi-302 | Rhizosphere | VS-535 | Maize | 1 |
| 2 | B. vietnamiensis | MMi-313 | Rhizosphere | VS-535 | Maize | 1 |
| 2 | B. vietnamiensis | MMi-344 | Roots* | VS-535 | Maize | 1 |
| 5 | Burkholderia sp. | CCE-414 | Roots† | Caturra | Coffee | 5 |
| 6 | Burkholderia sp. | CCE-421 | Rhizosphere | Caturra | Coffee | 5 |
| 7 | Burkholderia sp. | MTl-441 | Rhizosphere | Landrace | Maize | 3 |
| 8 | Burkholderia sp. | CAC-124 | Rhizosphere | Arabiga | Coffee | 7 |
| 13 | Burkholderia sp. | CAC-98 | Rhizosphere | Arabiga | Coffee | 7 |
| 13 | Burkholderia sp. | CAC-142 | Rhizosphere | Arabiga | Coffee | 7 |
| 13 | Burkholderia sp. | CAC-369 | Rhizosphere | Arabiga | Coffee | 7 |
| 13 | Burkholderia sp. | CAC-382 | Rhizoplane | Arabiga | Coffee | 7 |
| 14 | Burkholderia sp. | CGC-321 | Rhizosphere | Garnica | Coffee | 7 |
| 15 | Burkholderia sp. | MTl-641 | Rhizosphere | Landrace | Maize | 3 |
| 15 | Burkholderia sp. | CGC-72 | Rhizoplane | Garnica | Coffee | 7 |
| 15 | Burkholderia sp. | CAC-112 | Rhizoplane | Arabiga | Coffee | 7 |
| 15 | Burkholderia sp. | CGC-316 | Rhizosphere | Garnica | Coffee | 7 |
| 16 | Burkholderia sp. | MOc-235 | Rhizosphere | Landrace | Maize | 2 |
| 16 | Burkholderia sp. | MOc-255 | Rhizosphere | Landrace | Maize | 2 |
| 16 | Burkholderia sp. | MOc-725 | Rhizoplane | Landrace | Maize | 2 |
| 17 | Burkholderia sp. | MMi-786 | Rhizoplane | VS-535 | Maize | 1 |
| 17 | Burkholderia sp. | MTo-41 | Rhizosphere | Landrace | Maize | 4 |
| 17 | Burkholderia sp. | MTo-431 | Rhizosphere | Landrace | Maize | 4 |
| 17 | Burkholderia sp. | MTo-432 | Rhizoplane | Landrace | Maize | 4 |
| 17 | Burkholderia sp. | MTo-452 | Rhizoplane | Landrace | Maize | 4 |
| 18 | Burkholderia sp. | CBN-516 | Rhizosphere | Bourbon | Coffee | 6 |
| 18 | Burkholderia sp. | CBN-523 | Rhizoplane | Bourbon | Coffee | 6 |
| 18 | Burkholderia sp. | CBN-721 | Rhizoplane | Bourbon | Coffee | 6 |
| 18 | Burkholderia sp. | CBN-724 | Rhizoplane | Bourbon | Coffee | 6 |
| 19 | Burkholderia sp. | MTo-16 | Rhizosphere | Landrace | Maize | 4 |
| 19 | Burkholderia sp. | MTo-293 | Stem | Landrace | Maize | 4 |
| 20 | Burkholderia sp. | MMi-493 | Rhizosphere | VS-535 | Maize | 1 |
| 21 | Burkholderia sp. | CCE-401 | Rhizoplane | Caturra | Coffee | 5 |
| 21 | Burkholderia sp. | CBN-15 | Rhizoplane | Bourbon | Coffee | 6 |
| 21 | Burkholderia sp. | CBN-23 | Rhizoplane | Bourbon | Coffee | 6 |
| 21 | Burkholderia sp. | CBN-25 | Rhizosphere | Bourbon | Coffee | 6 |
| 21 | Burkholderia sp. | CAC-92 | Rhizosphere | Arabiga | Coffee | 7 |
∗, Surface-sterilized roots; †, unwashed roots.
Location codes: 1, Miacatlán, Morelos State; 2, Ocotepec, Morelos State; 3, Tlayacapan, Morelos State; 4, Totontepec, Oaxaca State; 5, El Eden, Chiapas State; 6, La Neblina, Querétaro State; 7, Coatepec, Veracruz State (all in Mexico).
Media and cultural conditions.
A nitrogen-free semisolid medium (BAz) was used as an enrichment culture and for the enumeration of N2-fixing Burkholderia species. BAz medium had the following composition (in grams/liter): azelaic acid, 2.0; K2HPO4, 0.4; KH2PO4, 0.4; MgSO4 · 7H2O, 0.2; CaCl2, 0.02; Na2MoO4 · H2O, 0.002; FeCl3, 0.01; bromothymol blue, 0.075; and agar, 2.3. The medium was adjusted with KOH to pH 5.7. Vials containing 5 ml of BAz medium were autoclaved at 121°C for 20 min, and filter-sterilized cycloheximide (200 μg/tube) was then added. PCAT medium is considered selective for B. cepacia (6). Because azelaic acid is used as a carbon source for most of the known Burkholderia species and tryptamine is used by B. cepacia but not by many other Burkholderia species (15), the PCAT medium was modified. Tryptamine was omitted as a nitrogen source to avoid the overgrowth of B. cepacia and to allow the growth of N2-fixing Burkholderia species. In this modified medium (PCATm) chlorothalanil was omitted as well, and bromothymol blue (75 mg/liter) was added. A BAc medium (0.2% azelaic acid, 0.02% l-citrulline, 0.04% K2HPO4, 0.04% KH2PO4, and 0.02%, MgSO4 · 7H2O) was also used for isolation and culturing of Burkholderia species. The pH was adjusted to 5.7, and the medium was sterilized at 121°C for 20 min prior to the addition of filter-sterilized (pore size, 0.22 μm) citrulline as the sole nitrogen source. In addition to N-free BAz medium used as an enrichment culture and for acetylene reduction activity (ARA) assays, we also tested a modified BAz medium, one lacking azelaic acid but supplemented with a single carbon source (0.5% fructose, glucose, sucrose, mannitol, glycerol, malate, succinate or 0.2% azelate, benzoate, or propionate) or with three carbon sources (0.2% malic acid, glucose, and 0.1% mannitol). This medium was named BMGM. Burkholderia spp. isolates were grown in BSE medium (0.5% succinate, 0.04% K2HPO4, 0.04% KH2PO4, 0.02% MgSO4 · 7H2O, 0.05% yeast extract; pH 6.5) for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) assays, and BDN medium (0.02% peptone, 0.01% yeast extract, 0.04% K2HPO4, 0.04% KH2PO4, 0.02% MgSO4 · 7H2O; pH 6.5) for total DNA isolation.
Isolation and enumeration.
The root was shaken gently to remove the loosely attached soil, and the adhering soil was rinsed in 9.0 ml of 10 mM MgSO4 · 7H2O (Mgsol). The resulting rinse solution containing the rhizosphere bacteria was serially diluted with Mgsol. The root was subsequently washed with Mgsol containing 0.01% (vol/vol) Tween 20, followed by two rinses with Mgsol, and then immersed in 9.9 ml of Mgsol and vortexed for 3 min. The resulting suspension, which was considered to contain bacteria from the rhizoplane, was serially diluted with Mgsol. The vortexed root was immersed for 5 min under agitation in full-strength bleach solution containing Tween 20 and rinsed three times in sterile H2O. The roots were rolled on to Luria-Bertani agar plates to verify root surface sterilization and then were macerated in a blender in a known volume of Mgsol, and the suspension was serially diluted. The aerial parts of maize plants were surface sterilized for 10 min and treated as described above for the root samples. The macerates were serially diluted with Mgsol and used to calculate the most probable number (MPN) and for recovering the Burkholderia endophytic community. Vials containing N-free semisolid BAz medium were inoculated with 100-μl aliquots from diluted samples and incubated for 5 to 7 days at 29°C. Thereafter, cultures were replicated once more under the same conditions. Vials with a white or yellowish pellicle at a depth of 1 to 4 mm below of surface were streaked onto PCATm and BAc medium plates and incubated at 29°C. After 4 to 5 days, predominant colonies with different morphology were individually inoculated in vials containing N-free BAz medium, incubated at 29°C for 4 days and assayed for ARA as described previously (24). When ARA was not detected in N-free BAz medium, the isolates were inoculated in N-free semisolid BMGM medium, incubated for 3 days before the ARA assays were carried out. All the acetylene-reducing colonies were further verified for culture purity, and then the colony morphology was recorded and their distribution was used to calculate the MPN of N2-fixing bacteria, using the McCrady tables. Three replicates per 10-fold dilution were made from each sample. Three N2-fixing isolates from each colony morphology type were chosen from the highest dilutions of each sample. These isolates were maintained in 20% glycerol at −80°C prior to analysis.
Phenotypic characterization.
The isolates were presumptively identified with the API 20NE system (bioMérieux). The results were interpreted by using the API analytical profile index, which provided the percentage of identification. In addition, representative isolates were evaluated for their ability to reduce acetylene using glucose, fructose, sucrose, mannitol, glycerol, malate, succinate, azelate, benzoate, or propionate, as the single carbon sources. Acetylene was injected after the cultures were incubated at 29°C for 72 h, but when azelate, benzoate, and propionate were tested the incubation was for 4 days.
SDS-PAGE.
Cultures were grown in BSE medium with reciprocal shaking (200 rpm) for 15 h at 29°C, and 1.0-ml samples were harvested by centrifugation at 12,300 × g for 10 min. The pellet were resuspended in 70 μl of 0.125 M Tris-HCl, 4% SDS, 20% glycerol, and 10% mercaptoethanol at pH 6.8. Aliquots of 10 μl were used for SDS-PAGE performed as described by Laemmli (20).
Total DNA isolation and amplified DNA restriction analysis (ARDRA).
Cultures were grown in BDN medium for 24 h and centrifuged at 12,300 × g for total DNA preparation as described previously (2). The 16S ribosomal DNA (rDNA) genes from N2-fixing Burkholderia isolates were PCR amplified with the primers fD1 and rD1 (44), using Taq polymerase (Boehringer-Roche). The PCR conditions consisted of an initial denaturing cycle (94°C, 3 min), 35 amplification cycles (94°C, 1 min; 55°C, 1 min; 72°C, 2 min), and then a final elongation cycle (72°C, 5 min). The PCR amplified 16S rRNA gene fragments (ca. 1.5 kb) were restricted with 5 U each of AluI, DdeI, HaeIII, HhaI, HinfI, MspI, and RsaI. The lengths of the restriction fragments of the different 16S rRNA genes were determined by electrophoresis in 3% agarose gels, and the restriction patterns from each isolate were compared. Each isolate was assigned to a 16S rDNA genotype, defined by the combination of the restriction patterns obtained with the seven restriction endonucleases. Similarities among the 16S rRNA gene sequences were estimated from the proportion of shared restriction fragments by the method of Nei and Li (28). A dendrogram was constructed from the resulting distance matrix using the unweighted pair group method with averages (UPGMA) (32).
nifHDK hybridizing patterns and DNA-DNA relatedness analysis.
Total DNA was digested with EcoRI, and restriction fragments were electrophoresed, blotted, and hybridized as described previously (7). Total EcoRI DNA digests from Burkholderia isolates were hybridized with pCQ12, which contains a 4.1-kb segment of the nifHDK region of R. etli CFN 42 (29). DNA-DNA homology was based on relative levels of hybridization to 32P-labeled DNA from B. vietnamiensis TVV75T. DNA-DNA hybridization was for 12 h at 65°C, and the nylon filters were washed once in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at room temperature for 10 min, once in 1× SSC at 55°C for 5 min, and once in 0.1× SSC at 65°C for 5 min. The percentage of total homologous reassociation was calculated for each strain tested as described previously (19).
RESULTS
Isolation and enumeration.
The inoculation of N-free semisolid BAz medium with samples from rhizosphere soil of maize and coffee plants as well as with maize plant tissues, followed by subsequent streaking on the PCATm and BAc culture media, allowed the recovery of more than 200 N2-fixing isolates. Although many N2-fixing isolates obtained from each sample of rhizosphere, rhizoplane, or plant tissues were recovered and analyzed, we included only one representative isolate recovered from each sample in Table 1.
Bacterial growth in N-free semisolid BAz medium resulted in the formation of surface pellicles with different characteristics and acetylene reduction activities. In this medium some cultures formed pellicles at a depth of 1 to 2 mm below the surface, while others formed pellicles 4 mm below the surface. The pellicles were white, whitish, or yellowish and dense and fine or thick and diffuse. In general, pH changes were not observed in N-free BAz medium, but the growth of some isolates resulted in a slightly raised pH as indicated by a green-blue color in the medium.
Predominant colonies on PCATm medium were yellowish, round, smooth, flat or convex with entire margins and a diameter of from ≤1.0 to 1.5 mm. Many but not all of these isolates reduced acetylene in N-free semisolid BAz medium. However, more N2-fixing isolates were detected when tested in the N-free semisolid BMGM medium. Many N2-fixing isolates formed white colonies, while others formed whitish or yellowish colonies on BAc agar. However, all of the colonies were round and smooth, with entire margins varying in diameter from 1 to 2 mm. White colonies were flat or slightly convex, and these isolates turned the medium from green to deep blue, while isolates with whitish or yellowish colonies were convex and turned the medium a light blue color. However, several N2-fixing isolates were not able to grow on BAc medium plates. MPN values for colonies with the features described varied from 4 × 106 CFU/g of rhizosphere soil to 4 × 105 CFU/g of fresh tissue of maize.
Phenotypic characterization.
Biochemical tests based on the use of API 20NE showed that a majority of the N2-fixing isolates recovered on PCATm agar plates belonged to the species B. cepacia (81.1 to 99.6% confidence limits based on the API analytical profile index), but some diazotrophic isolates were identified as Pseudomonas aureofaciens with confidence limits from 63.5 to 89.7%. Similarly, N2-fixing isolates growing on BAc medium plates were identified as B. cepacia and P. aureofaciens. However, several isolates recovered from PCATm agar plates but incapable of growing on BAc medium were identified as belonging mainly to Enterobacter cloacae and Klebsiella pneumoniae subsp. pneumoniae (data not shown). Frequently, strains of these species were isolated from within the roots and stems of maize plants.
The ability to fix N2 varied among the different diazotrophs isolated from the maize and coffee plants (Table 2). All of these isolates were capable of N2 fixation with fructose, mannitol, malate, and succinate as single carbon sources, but this ability was variable with other carbon substrates. B. vietnamiensis TVV75T and several isolates recovered from maize and coffee plants showed ARA with all of the carbon sources tested except glucose. However, the isolates CCE-312 and CCE-101 were capable of reducing acetylene when glucose was used as a single carbon source. This inability to reduce acetylene when glucose was the carbon source was also observed with the collection of B. vietnamiensis strains TVV69, TVV72, and TVV115 (data not shown) recovered from the rhizosphere of rice (15). Inexplicably, isolates corresponding to the 16S rDNA genotypes 16, 17, 18, and 19 showed an inconsistent ARA even among replicates from the same assay. Frequently, one or two of three replicates did not exhibit ARA. The carbon source was eliminated as a possible cause of this inconsistency because these isolates were able to grow with glucose, fructose, sucrose, mannitol, succinate, and other carbon sources when NH4NO3 is supplied as nitrogen source (data not shown). Also, contamination was discarded as a possible cause of inconsistency. Interestingly, B. kururiensis KP23T was capable of fixing N2 when grown on several carbon sources (Table 2).
TABLE 2.
ARA by representative N2-fixing Burkholderia isolates and strains of related species
| 16S rDNA genotype | Taxon | Reference strain | ARA witha:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fru | Glu | Scr | Man | Gly | Suc | Mal | Aze | Ben | Pro | |||
| 1 | B. vietnamiensis | TVV75T | 88 | − | + | 38 | + | 88 | + | 78 | + | 90 |
| 1 | B. vietnamiensis | MMi-324 | 34 | − | − | 24 | + | 84 | + | 22 | + | 24 |
| 1 | B. vietnamiensis | MMi-1537 | 30 | − | + | 26 | + | 118 | + | 28 | + | 28 |
| 1 | B. vietnamiensis | MMi-1547 | 24 | − | + | 30 | + | 100 | + | 22 | + | 22 |
| 1 | B. vietnamiensis | CCE-201 | 26 | − | + | 22 | + | 172 | + | 29 | + | 26 |
| 1 | B. vietnamiensis | CCE-312 | 40 | 50 | + | 36 | + | 123 | + | 48 | + | 130 |
| 1 | B. vietnamiensis | CCE-101 | 50 | 34 | + | 32 | + | 156 | + | 10 | + | 24 |
| 1 | B. vietnamiensis | SXo-702 | 42 | 40 | + | 32 | + | 128 | + | 24 | + | 22 |
| 2 | B. vietnamiensis | MMi-302 | 60 | − | − | 42 | + | 82 | + | 33 | + | 52 |
| 2 | B. vietnamiensis | MMi-344 | 78 | 36 | + | 66 | + | 94 | + | 70 | + | 72 |
| 3 | B. cepacia | ATCC 29352 | − | − | − | − | − | − | − | − | − | − |
| 5 | Burkholderia sp. | CCE-414 | 86 | − | + | 22 | + | 104 | + | 74 | + | 44 |
| 6 | Burkholderia sp. | CCE-421 | 28 | − | − | <10 | + | 114 | + | 46 | − | <10 |
| 8 | Burkholderia sp. | CAC-124 | 79 | 48 | + | 41 | + | 48 | + | 34 | + | 36 |
| 11 | B. caribensis | MWAP64T | − | − | − | − | − | − | − | − | − | − |
| 12 | B. graminis | C4D1MT | − | − | − | − | − | − | − | − | − | − |
| 13 | Burkholderia sp. | CAC-382 | 28 | 62 | + | 34 | + | 218 | + | 58 | − | 26 |
| 13 | Burkholderia sp. | CAC-98 | 173 | 254 | − | 94 | + | 236 | + | 220 | + | 130 |
| 14 | Burkholderia sp. | CGC-321 | 112∗ | 48 | + | 36 | + | 436 | + | 56 | − | 26 |
| 15 | Burkholderia sp. | MTl-641 | 72∗ | 345 | + | 86 | + | 152 | + | 206 | + | 206 |
| 15 | Burkholderia sp. | CGC-72 | 165 | 256 | ± | 142 | + | 346 | + | 180 | + | 142 |
| 16 | Burkholderia sp. | MOc-235 | 48∗ | − | + | 62∗ | + | 68∗ | ± | 32 | − | 28 |
| 16 | Burkholderia sp. | MOc-725 | 82 | − | + | 48 | + | 198 | + | 54 | + | 104 |
| 16 | Burkholderia sp. | SMi-583 | 112 | − | + | 49 | + | 194 | + | 64 | − | 42 |
| 17 | Burkholderia sp. | MMi-786 | 40 | − | + | <10∗ | − | 216 | ± | − | − | − |
| 17 | Burkholderia sp. | MTo-431 | 64 | − | + | 42∗ | − | 156∗ | + | − | − | − |
| 18 | Burkholderia sp. | SXo-252 | 76 | − | + | <10∗ | − | 164 | + | <10∗ | ± | − |
| 18 | Burkholderia sp. | CBN-516 | 60∗ | 72∗ | − | 16∗ | − | 312 | + | 16∗ | ± | − |
| 18 | Burkholderia sp. | CBN-721 | 86∗ | 66∗ | − | <10 | − | 184 | + | <10∗ | ± | − |
| 19 | Burkholderia sp. | MTo-293 | 80 | 96 | ± | 38 | − | 166 | + | 40 | − | − |
| 19 | Burkholderia sp. | MTo-16 | 152 | 40 | ± | 58 | + | 196 | + | 66 | − | 208 |
| 20 | Burkholderia sp. | MMi-493 | 52 | 28∗ | − | 46 | + | 220 | + | 44 | − | 62 |
| 21 | Burkholderia sp. | CCE-401 | 34 | 34 | + | 20 | − | 158 | + | 20 | − | − |
| 21 | Burkholderia sp. | CBN-23 | 34 | 15 | + | 24 | − | 270 | + | 44 | − | − |
| 21 | Burkholderia sp. | CAC-92 | 24 | 60 | + | 22 | − | 80 | + | 38 | − | − |
| 22 | B. kururiensis | KP23T | 69 | 160 | + | 46 | + | 132 | + | 68 | − | − |
| 22 | “B. brasilensis” | M-130 | 30 | 78 | ± | 26 | + | 74 | ± | <10∗ | − | − |
Values represent nanomoles of C2H4/h/culture and the means of two replicate cultures. When inconsistent ARA was observed, up to six replicates were done in independent experiments. +, Positive activity; −, negative activity; ± or ∗, inconsistent activity. Fru, fructose; Glu, glucose; Scr, sucrose; Man, mannitol; Gly, glycerol; Mal, malate; Suc, succinate; Aze, azelate; Ben, benzoate; Pro, propionate.
nifHDK hybridizing patterns.
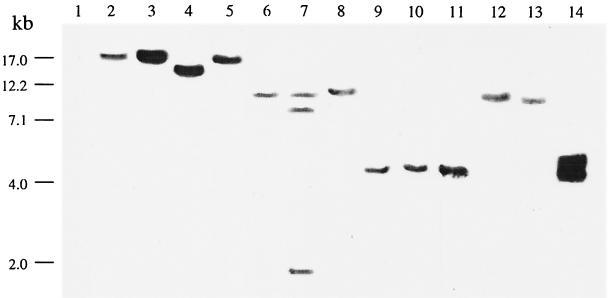
The nifHDK patterns of representative Burkholderia isolates are shown in Fig. 1. Several isolates showed an nifHDK hybridization pattern identical (e.g., MMi-1486) or very similar (e.g., CCE-101) to that of the B. vietnamiensis type strain. In addition, other groups of N2-fixing Burkholderia isolates showed a hybridization pattern of the nifHDK genes different from that of the B. vietnamiensis type strain. A band hybridizing to the nifHDK genes of R. etli was observed in total EcoRI DNA fingerprints from B. kururiensis KP23T. The nifHDK hybridization pattern of B. kururiensis was different from that of B. vietnamiensis but was very similar to that of strain CAC-92 recovered from a coffee plant. These results confirmed the ability of the Burkholderia isolates to fix N2, even by isolates (e.g., MMi-786 and SXo-252) which showed an inconsistent ARA.
FIG. 1.
Autoradiogram of a Southern blot of total EcoRI-digested DNA hybridized with the nifHDK probe of R. etli CFN 42. Lane 1, B. cepacia ATCC 29352 used as a negative control; lane 2, B. vietnamiensis TVV75T. Lanes 3 through 12 are examples of representative N2-fixing Burkholderia isolates; lane 3, MMi-1486; lane 4, CCE-101; lane 5, MMi-302; lane 6, CCE-414; lane 7, CCE-421; lane 8, CGC-321, lane 9, MMi-786; lane 10, SXo-252; lane 11, MTo-293; lane 12, CAC-92; lane 13, B. kururiensis KP23T; lane 14, R. etli CFN42.
Protein electrophoregrams.
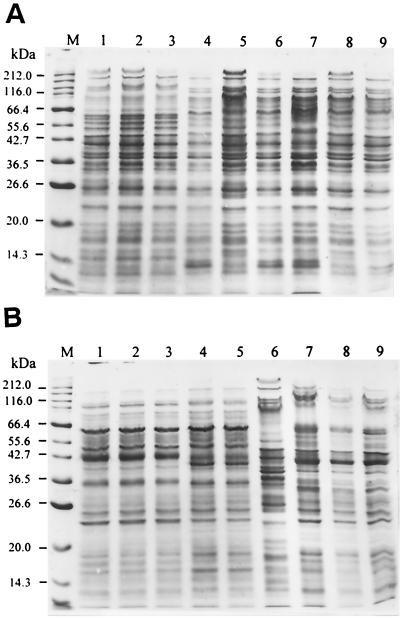
The whole-cell protein patterns of representative N2-fixing Burkholderia strains are shown in Fig. 2. Several isolates (e.g., MMi-1547 and MMi-1556) recovered from the rhizosphere, rhizoplane, and inside of maize roots, as well as isolates (e.g., CCE-101 and CCE-312) from the roots and rhizosphere of coffee plants showed protein patterns very similar to those of the B. vietnamiensis type strain (Fig. 2A). The differences were mostly observed in the 55.6- to 116-kDa region. In addition, other groups of N2-fixing isolates recovered from the maize and coffee environment showed almost identical protein electrophoregrams (Fig. 2B), but those (e.g., MOc-235 or MTl-641) were clearly different from the protein patterns of B. vietnamiensis (Fig. 2B) and from other known Burkholderia species (data not shown). Interestingly, a few N2-fixing isolates (e.g., SXo-702 and SXo-252) recovered from the sorghum plant (cv. D-65) environment in Xoxocotla, Morelos State (data not shown), exhibited very similar protein patterns to those of the isolates recovered from maize plants in Morelos State but were almost indistinguishable from the protein patterns of isolates (e.g., CCE-312 and CBN-516) from coffee plants cultivated in Chiapas State at a distance of about 1,200 km (Fig. 2A and B).
FIG. 2.
Protein electrophoregrams (SDS-PAGE) of representatives N2-fixing Burkholderia strains. (A) B. vietnamiensis isolates recovered: from maize, lane 1, MMi-324; lane 2, MMi-1547; lane 3, MMi-1556; lane 9, MMi-302; from coffee, lane 6, CCE-312; lane 7, CCE-101; lane 8, CCE-201; from sorghum, lane 4, SXo-702; from rice, lane 5, type strain TVV75T. M, protein marker. (B) N2-fixing Burkholderia spp. Lane 1, sorghum isolate SXo-252; coffee isolates, lane 2, CBN-516; lane 3, CBN-721; lane 7, CAG-98; lane 8, CGC-321; maize isolates, lane 4, MOc-235; lane 5, MOc-255; lane 9, MTl-641; rice isolate, lane 6, B. vietnamiensis TVV75T. M, protein marker.
ARDRA profiles.
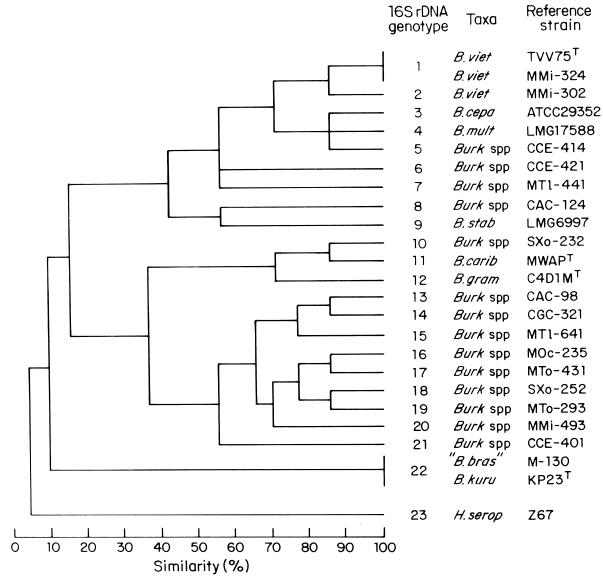
Although ARDRA has been used successfully to differentiate Rhizobium (21) and Burkholderia (31) species, coefficients of similarity to define species limits do not exist. However, in the present study, B. vietnamiensis and B. cepacia–B. multivorans, as well as B. graminis and B. caribensis, were differentiated by coefficients of 70% similarity (Fig. 3). On this basis, we used the level of 70% similarity as indicative of a separate species for the diazotrophic Burkholderia recovered from the maize and coffee plant environment. Twenty-two different 16S rDNA genotypes were identified among the strains of known Burkholderia species analyzed and the N2-fixing isolates recovered from maize and coffee plants (Fig. 3). Only the 16S rDNA genotypes 1 and 15 were recovered from both maize and coffee plants, while genotypes 2, 16, 17, and 19 were identified only among isolates recovered from maize plants, and the genotypes 5, 6, 8, 13, 14, 18, and 21 were identified only among isolates recovered from coffee plants (Table 1). One isolate (SMi-583) recovered from the surface-sterilized roots of a sorghum plant showed an ARDRA profile identical (16S rDNA genotype 16) to that of isolates (e.g., MOc-235 and MOc-725) recovered from maize plants. Interestingly, several N2-fixing isolates recovered from maize and coffee plants showed ARDRA profiles identical (16S rDNA genotype 1) or almost identical (16S rDNA genotype 2) to that of the B. vietnamiensis strains TVV75T, TVV69, and TVV72 (Fig. 3). In addition, other N2-fixing isolates corresponding to the 16S rDNA genotypes 13 to 21 were phylogenetically related to B. caribensis and B. graminis but were clearly different and largely distant (15% similarity) from the diazotrophic species B. vietnamiensis and from B. cepacia, B. multivorans, and B. stabilis (Fig. 3), all of which are included in the named “B. cepacia complex” (39). Strain KP23T of B. kururiensis and “B. brasilensis” M-130 showed the same ARDRA profiles obtained with the seven restriction enzymes used.
FIG. 3.
Dendrogram of genetic relationships among N2-fixing Burkholderia isolates recovered from maize, coffee, and sorghum plants and related species of Burkholderia based on ARDRA analysis. Burk, Burkholderia; B. viet, B. vietnamiensis; B. cepa, B. cepacia; B. mult, B. multivorans; B. stab, B. stabilis; B. carib, B. caribensis; B. gram, B. graminis; B. kuru, B. kururiensis; “B. bras,” “B. brasilensis”; and H. serop, Herbaspirillum seropedicae. Isolates identified with a same 16S rDNA genotype, in addition to reference strain: genotype 1, MMi-334, MMi-353, MMi-1486, MMi-1537, MMi-1547, MMi-1556, MMi-1577, MMi-1776, SXo-702, CCE-101, CCE-115, CCE-201, CCE-211, CCE-303, and CCE-312 and reference strains TVV69 and TVV72; genotype 2, MMi-313 and MMi-344; genotype 13, CAC-142, CAC-369, and CAC-382; genotype 15, CAC-112, CGC-72, and CGC-316; genotype 16, MOc-255, MOc-725, and SMi-583; genotype 17, MMi-786, MTo-41, MTo-432, and MTo-452; genotype 18, CBN-516; CBN-523, CBN-721, and CBN-724; genotype 19, MTo-16; genotype 21, CBN-15, CBN-23, CBN-25, and CAC-92.
DNA-DNA relatedness.
Twenty-five Burkholderia strains were analyzed in the DNA-DNA reassociation assays. Nine N2-fixing Burkholderia strains analyzed corresponding to the 16S rDNA genotypes 1 and 2 constituted a homogeneous group related to B. vietnamiensis TVV75T, with DNA homology values ranging from 65 to 102%. One or two N2-fixing isolates corresponding to each of the 16S rDNA genotypes 13 to 18 and genotype 21 exhibited very low DNA homology levels, ranging from 6 to 13% with the reference strain TVV75T. The type strains B. caribensis, B. graminis, B. kururiensis, and “B. brasilensis” exhibited low DNA homology (14 to 38%) with the same reference strain TVV75T, as is expected for different species.
DISCUSSION
In this study, we report the isolation of many diazotrophs from inside maize roots as well as from the rhizosphere and rhizoplane of maize and coffee plants cultivated in distant geographical regions of Mexico. The successful recovery of N2-fixing Burkholderia spp. associated with maize and coffee plants is partially attributed to the semiselective enrichment using N-free semisolid BAz medium, as well as to the subsequent isolation on PCATm agar plates and growth on BAc medium. Although some strains of the family Enterobacteriaceae were isolated from maize plants on PCATm agar plates, they cannot grow with citrulline on the BAc medium. The selectivity of the BAc medium was based on the ability of Burkholderia spp. to grow with azelaic acid and citrulline (15), substrates which are not used by bacteria associated with coffee plants such as G. diazotrophicus (19), G. johannae, and G. azotocaptans (14) nor by other common plant-associated bacteria such as Azospirillum and Rhizobium spp. (data not shown).
Phenotypic identification of the N2-fixing isolates with the API 20NE system showed that most of the isolates recovered on PCATm medium and all of the isolates which grew on BAc medium plates belonged to B. cepacia and a few belonged to P. aureofaciens. Recently, the inability to differentiate B. cepacia from P. aureofaciens or from other Burkholderia species using the API 20NE identification system has been reported (31). However, among the 51 B. cepacia isolates analyzed in that study only one was misidentified at the genus level. On this basis, the N2-fixing isolates recovered from the environment of maize and coffee plants were considered to belong to the genus Burkholderia. PCR amplification of 16S rDNA genes with selected primers described previously (4) confirmed that these N2-fixing isolates are members of the genus Burkholderia (data not shown).
A number of Burkholderia isolates recovered from the environment of maize and coffee plants showed very similar or identical features to those of type strain TVV75T of B. vietnamiensis. These features include N2-fixing ability, almost identical protein patterns, and identical ARDRA profiles. In addition, these isolates showed high levels of DNA-DNA reassociation (mean homology, 81%) with total DNA from strain TVV75T. Taking into account that bacteria with very similar protein patterns possess high genome similarity (40) and that a bacterial genomic species includes strains with 70% or greater DNA-DNA relatedness (33), these isolates were assigned to the species B. vietnamiensis.
It is worth noting that the first isolates of B. vietnamiensis, including the type strain TVV75T, were recovered from the rhizosphere of young rice plants grown on a Vietnamese soil in a phytotron (38). In the present study, the isolates of this species were recovered from the rhizosphere and rhizoplane of maize and coffee plants grown under natural field conditions, as well as endophytically from the maize plants. Moreover, this study shows the substantial capability of the bacterial species for colonizing different host plants and environments. This is further supported by the recovery of B. vietnamiensis isolates (e.g., SXo-702) from the rhizoplane of sorghum plants.
In Mexico, maize has been traditionally cultivated for thousands of years, and even today this crop is grown in many rural regions with a sustainable agriculture where fertilizer has not been used for generations. It is conceivable that the occurrence of B. vietnamiensis (4 × 106 CFU/g of rhizosphere soil and 4 × 104 CFU/g of fresh tissue of roots) might contribute to the growth of the maize plant and hence to crop production, as has been observed with field inoculation of rice with B. vietnamiensis TVV75T (37, 38).
Although the ability to fix N2 is not reported to be a common feature among the known species of the genus Burkholderia, we have found that this bacterial genus is very rich in diazotrophic species, as was demonstrated with ARA assays and confirmed with the presence of nifHDK genes. Our results confirm that diazotrophy is a common property among bacteria. However, the ability of some bacterial species to fix N2 is unknown because this feature is not routinely evaluated when a new species is described (46). This is especially true for the B. kururiensis species. In this study, both the presence of nifHDK genes in strain KP23T of B. kururiensis and the ARA assays revealed that this species is capable of fixing N2 with selected carbon sources under microaerophilic conditions. It is known that many N2-fixing bacterial species do not show such ability except under special growth conditions (46). Nevertheless, this possibility does not explain the erratic acetylene reduction activity observed with some isolates corresponding to the 16S rDNA genotypes 16, 17, 18, and 19. Further studies are required to resolve the observed discrepancies.
Interestingly, strains KP23T of B. kururiensis and M-130 of “B. brasilensis” were capable of fixing N2 in a similar manner, and both strains showed the same ARDRA profile. In addition, an analysis of the 16S rRNA sequences revealed 99.9% similarity between B. kururiensis KP23T and “B. brasilensis” M-130 (data not shown), suggesting that both strains belong to the same species. This finding emphasizes the wide geographic and environmental distribution of the bacterial species. While B. kururiensis KP23T was recovered from an aquifer polluted with trichlorethylene in Japan (47), the “B. brasilensis” M-130 strain was found to be plant associated in Brazil (V. L. D. Baldani, G. Kirchhof, V. M. Reis, E. de Oliveira, I. J. Baldani, N. Springer, W. Ludwig, A. Hartmann, and J. Döbereiner, NCBI GenBank database, accession number AJ238360, 1999).
In this study, most of the N2-fixing isolates analyzed showed phenotypic (colony morphology, N2-fixing ability, and protein patterns) and genotypic (ARDRA and nifHDK profiles) features different from those of the known N2-fixing species B. vietnamiensis, as well as from those of B. kururiensis, previously unknown to be a diazotrophic species. In addition, DNA-DNA reassociation assays confirmed the existence of N2-fixing Burkholderia species different from B. vietnamiensis. Nevertheless, a polyphasic taxonomy analysis, as recommended by Vandamme et al. (40), is required for the validation of novel N2-fixing Burkholderia species.
Among the bacteria associated with the rhizosphere and roots of maize plants, B. cepacia seems to be one of the predominant species (10, 27). Because of its abilities, B. cepacia is considered as a potential agricultural agent (5, 8, 9, 17, 25, 26, 35). However, despite the undoubted economic and ecological benefits of utilizing B. cepacia in agriculture, there exist diverse opinions on the use of this bacterial species (16, 18, 43) because of its importance as an opportunistic pathogen in nosocomial infections and in patients with cystic fibrosis. Taking into account this fact as well as the wide geographic distribution and the riches of N2-fixing Burkholderia isolates associated with maize, coffee, and sorghum plants, we consider it important to assess the N2-fixing Burkholderia genotypes distantly related to B. cepacia, both for their ability to promote plant growth and for their potential as biocontrol and bioremediation agents.
ACKNOWLEDGEMENTS
We are grateful to Les Barran and Michael Dunn for constructive English corrections. We gratefully acknowledge Jacques Balandreau (Université Lyon) for supplying the strains C4D1MT of B. graminis, B. vietnamiensis TVV75T, and B. caribensis MWAP64T and Richard Goldstein (Boston University) for supplying B. vietnamiensis strains TVV69, TVV72, and TVV115, B. multivorans strain LMG 17588, and B. stabilis strain LMG 6997. We are also grateful to Hui Zhang (Tsukuba, Japan), who kindly provided B. kururiensis strain KP23T, and Rosa M. Pitard (EMBRAPA, Brazil) for supplying “B. brasilensis” strain M-130. We thank Isabel López-Lara (CIFN-UNAM) for help with SDS-PAGE and Guadalupe Paredes-Valdez, Sandra Hernández-Bustillos, and Norma Garcia-Calderón for technical assistance.
Paulina Estrada-De Los Santos is supported by Consejo Nacional de Ciencia y Tecnología (CONACyT). This research was partially funded by grant CONACyT 400343-5-33576-V.
REFERENCES
- 1.Achouak W, Christen R, Barakat M, Martel M-H, Heulin T. Burkholderia caribensis sp. nov., and exopolysaccharide-producing bacterium isolated from vertisol microaggregates in Martinique. Int J Syst Bacteriol. 1999;49:787–794. doi: 10.1099/00207713-49-2-787. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, More D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 3.Balandreau J, Viallard V, Cournoyer B, Coenye T, Laevens S, Vandamme P. Burkholderia cepacia genomovar III is a common plant-associated bacterium. Appl Environ Microbiol. 2001;67:982–985. doi: 10.1128/AEM.67.2.982-985.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauernfeind A, Schneider I, Jungwirth R, Roller C. Discrimination of Burkholderia multivorans and Burkholderia vietnamiensis from Burkholderia cepacia genomovars I, III, and IV by PCR. J Clin Microbiol. 1999;37:1335–1339. doi: 10.1128/jcm.37.5.1335-1339.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevivino A, Sarrocco S, Dalmastri C, Tabacchioni S, Cantale C, Chiarini L. Characterization of a free-living maize-rhizosphere population of Burkholderia cepacia: effect of seed treatment on disease suppression and growth promotion of maize. FEMS Microbiol Ecol. 1998;27:225–237. [Google Scholar]
- 6.Burbage D A, Sasser M. A medium selective for Pseudomonas cepacia. Phytopathology. 1982;76:706. [Google Scholar]
- 7.Caballero-Mellado J, Martínez-Romero E. Limited genetic diversity in the endophytic sugarcane bacterium Acetobacter diazotrophicus. Appl Environ Microbiol. 1994;60:1532–1537. doi: 10.1128/aem.60.5.1532-1537.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiarini L, Bevivino A, Tabacchioni S, Dalmastri C. Inoculation of Burkholderia cepacia, Pseudomonas fluorescens and Enterobacter sp. on Sorghum bicolor: root colonization and plant growth promotion of dual strain inocula. Soil Biol Biochem. 1998;30:81–87. [Google Scholar]
- 9.Daubaras D L, Danganan C E, Hübner A, Ye R W, Hendrickson W, Chakrabarty A M. Biodegradation of 2,4,5-trichlorophenoxyacetic acid by Burkholderia cepacia strain AC1100: evolutionary insight. Gene. 1996;179:1–8. doi: 10.1016/s0378-1119(96)00326-5. [DOI] [PubMed] [Google Scholar]
- 10.Di Cello F, Bevivino A, Chiarini L, Fani R, Paffetti D, Tabacchioni S, Dalmastri C. Biodiversity of a Burkholderia cepacia population isolated from the maize rhizosphere at different plant growth stages. Appl Environ Microbiol. 1997;63:4485–4493. doi: 10.1128/aem.63.11.4485-4493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Döbereiner J. History and new perspectives of diazotrophs in association with non-leguminous plants. Symbiosis. 1993;13:1–13. [Google Scholar]
- 12.Engelhard M, Hurek T, Reinhold-Hurek B. Preferential occurrence of diazotrophic endophytes, Azoarcus spp., in wild rice species and land races of Oryza sativa in comparison with modern races. Environ Microbiol. 2000;2:131–141. doi: 10.1046/j.1462-2920.2000.00078.x. [DOI] [PubMed] [Google Scholar]
- 13.Fries M R, Zhou J-Z, Chee-Sanford J, Tiedje J M. Isolation, characterization, and distribution of denitrifying toluene degraders from a variety of habitats. Appl Environ Microbiol. 1994;60:2802–2810. doi: 10.1128/aem.60.8.2802-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuentes-Ramírez, L. E., R. Bustillos-Cristales, A. Tapia-Hernández, T. Jiménez-Salgado, E. T. Wang, E. Martínez-Romero, and J. Caballero-Mellado. Novel nitrogen-fixing acetic acid bacteria, Gluconacetobacter johannae sp. nov., and Gluconacetobacter azotocaptans sp. nov., associated with coffee plants. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 15.Gillis M, Tran Van V, Bardin R, Goor M, Hebbar P, Willems A, Segers P, Kersters K, Heulin T, Fernandez M P. Polyphasic taxonomy in the genus Burkholderia leading to an emended description of the genus and proposition of Burkholderia vietnamiensis sp. nov. for N2-fixing isolates from rice in Vietnam. Int J Syst Bacteriol. 1995;45:274–289. [Google Scholar]
- 16.Govan J R W, Balandreau J, Vandamme P. Burkholderia cepacia—friend and foe. ASM News. 2000;66:124–125. [Google Scholar]
- 17.Hebbar K P, Martel M H, Heulin T. Suppression of pre- and postemergence damping-off in corn by Burkholderia cepacia. Eur J Plant Pathol. 1998;104:29–36. [Google Scholar]
- 18.Holmes A, Govan J, Goldstein R. Agricultural use of Burkholderia (Pseudomonas) cepacia: a threat to human health? Emerg Infect Dis. 1998;4:221–227. doi: 10.3201/eid0402.980209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiménez-Salgado T, Fuentes-Ramírez L E, Tapia-Hernández A, Mascarúa-Esparza M A, Martínez-Romero E, Caballero-Mellado J. Coffea arabica L., a new host plant for Acetobacter diazotrophicus, and isolation of other nitrogen-fixing acetobacteria. Appl Environ Microbiol. 1997;63:3676–3683. doi: 10.1128/aem.63.9.3676-3683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Laguerre G, Allard M-R, Revoy F, Amarger N. Rapid identification of rhizobia by restriction fragment length polymorphism analysis of PCR-amplified 16S rRNA genes. Appl Environ Microbiol. 1994;60:56–63. doi: 10.1128/aem.60.1.56-63.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loganathan P, Sunita R, Parida A K, Nair S. Isolation and characterization of two genetically distant groups of Acetobacter diazotrophicus from a new host plant Eleusine coracana L. J Appl Microbiol. 1999;87:167–172. [Google Scholar]
- 23.Martínez-Romero E, Caballero-Mellado J. Rhizobium phylogenies and bacterial genetic diversity. Crit Rev Plant Sci. 1996;15:113–140. [Google Scholar]
- 24.Mascarúa-Esparza M A, Villa-González R, Caballero-Mellado J. Acetylene reduction and indoleacetic acid production by Azospirillum isolates from cactaceous plants. Plant Soil. 1988;106:91–95. [Google Scholar]
- 25.McLoughlin T J, Quinn J P, Bettermann A, Booklands R. Pseudomonas cepacia suppression of sunflower wilt fungus and role of antifungal compounds in controlling the disease. Appl Environ Microbiol. 1992;58:1760–1763. doi: 10.1128/aem.58.5.1760-1763.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller J G, Devereux R, Santavy D L, Lantz S E, Willis S G, Pritchard P H. Phylogenetic and physiological compartment of PAH-degrading bacteria from geographically diverse soils. Antonie Leeuwenhoek. 1997;71:329–343. doi: 10.1023/a:1000277008064. [DOI] [PubMed] [Google Scholar]
- 27.Nacamulli C, Bevivino A, Dalmastri C, Tabacchioni S, Chiarini L. Perturbation of maize rhizosphere following seed bacterization with Burkholderia cepacia MC17. FEMS Microb Ecol. 1997;23:183–193. [Google Scholar]
- 28.Nei M, Li W H. Mathematical model for studying genetic variations in terms of restriction endonucleases. Proc Natl Acad Sci USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinto C, de la Vega H, Flores M, Leemans J, Cevallos M A, Pardo M A, Azpiroz R, Girard M L, Calva E, Palacios R. Nitrogenase reductase: a functional multigene family in Rhizobium phaseoli. Proc Natl Acad Sci USA. 1985;82:1170–1174. doi: 10.1073/pnas.82.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reinhold-Hurek B, Hurek T, Gillis M, Hoste B, Vancanneyt M, Kersters K, De Ley J. Azoarcus gen. nov., nitrogen-fixing Proteobacteria associated with roots of Kallar grass (Leptochloa fusca (L) Kunth), and description of two species, Azoarcus indigens sp. nov. and Azoarcus communis sp. nov. Int J Syst Bacteriol. 1993;43:574–584. [Google Scholar]
- 31.Segonds C, Heulin T, Marty N, Chabanon G. Differentiation of Burkholderia species by PCR-restriction fragment length polymorphism analysis of the 16S rRNA gene and application to cystic fibrosis isolates. J Clin Microbiol. 1999;37:2201–2208. doi: 10.1128/jcm.37.7.2201-2208.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sneath P H A, Sokal R R. Numerical taxonomy. W. H. San Francisco, Calif: Freeman & Co.; 1973. [Google Scholar]
- 33.Stackebrandt E, Goebel B M. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44:846–849. [Google Scholar]
- 34.Staley J T. Bacterial biodiversity: a time for place. ASM News. 1999;65:681–687. [Google Scholar]
- 35.Tabacchioni S, Bevivino A, Chiarini L, Visca P, Del Gallo M. Characteristics of two rhizosphere isolates of Pseudomonas cepacia and their potential plant-growth-promoting activity. Microb Rel. 1993;2:161–168. [Google Scholar]
- 36.Tapia-Hernández A, Bustillos-Cristales M R, Jiménez-Salgado T, Caballero-Mellado J, Fuentes-Ramirez L E. Natural endophytic occurrence of Acetobacter diazotrophicus in pineapple plants. Microb Ecol. 2000;39:49–55. doi: 10.1007/s002489900190. [DOI] [PubMed] [Google Scholar]
- 37.Trân Van V, Berge O, Ngo Ke S, Balandreau J, Heulin T. Repeated beneficial effects of rice inoculation with a strain of Burkholderia vietnamiensis on early and late yield component in low fertility sulphate acid soils of Vietnam. Plant Soil. 2000;218:273–284. [Google Scholar]
- 38.Trân Van V, Mavingui P, Berge O, Balandreau J, Heulin T. Promotion de croissance du riz inoculé par une bactérie fixatrice d'azote, Burkholderia vietnamiensis, isolée d'un sol sulfaté acide du Viêt-nam. Agronomie. 1994;14:697–707. [Google Scholar]
- 39.Vandamme P, Holmes B, Vancanneyt M, Coenye T, Hoste B, Coopman R, Revets H, Lauwers S, Gillis M, Kersters K, Govan J R W. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int J Syst Bacteriol. 1997;47:1188–1200. doi: 10.1099/00207713-47-4-1188. [DOI] [PubMed] [Google Scholar]
- 40.Vandamme P, Pot B, Gillis M, de Vos P, Kersters K, Swings J. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Rev. 1996;60:407–438. doi: 10.1128/mr.60.2.407-438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Rhijn P, Vanderleyden J. The Rhizobium-plant symbiosis. Microbiol Rev. 1995;59:124–142. doi: 10.1128/mr.59.1.124-142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viallard V, Poirier I, Cournoyer B, Haurat J, Wiebkin S, Ophel-Keller K, Balandreau J. Burkholderia graminis sp. nov., a rhizospheric Burkholderia species, and reassessment of [Pseudomonas] phenazinium, [Pseudomonas] pyrrocinia, and [Pseudomonas] glathei as Burkholderia. Int J Syst Bacteriol. 1998;48:549–563. doi: 10.1099/00207713-48-2-549. [DOI] [PubMed] [Google Scholar]
- 43.Vidaver A K, Doyle M P, Gerone P J, Gonzalez C F, Hall P, Hunter-Cevera J C, Loria R, Newsome R L, Shore S H, Wilkins T. Burkholderia cepacia-friend or foe? ASM News. 1999;65:587. [Google Scholar]
- 44.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanni Y G, Rizk R Y, Corich V, Squartini A, Ninke K, Philip-Hollingsworth S, Orgambide G, de Bruijn F, Stoltzfus J, Buckley D, Scmidt T M, Mateos P F, Ladha J K, Dazzo F B. Natural endophytic association between Rhizobium leguminosarum bv. trifolii and rice roots and assessment of its potential to promote rice growth. Plant Soil. 1997;194:99–114. [Google Scholar]
- 46.Young J P W. Phylogenetic classification of nitrogen-fixing organisms. In: Stacey G, Burris R H, Evans G, editors. Biological nitrogen fixation. New York, N.Y: Chapman & Hall; 1992. pp. 43–86. [Google Scholar]
- 47.Zhang H, Hanada S, Shigematsu T, Shibuya K, Kamagata Y, Kanagawa T, Kurane R. Burkholderia kururiensis sp. nov., a trichloroethylene (TCE)-degrading bacterium isolated from an aquifer polluted with TCE. Int J Syst Evol Microbiol. 2000;50:743–749. doi: 10.1099/00207713-50-2-743. [DOI] [PubMed] [Google Scholar]
- 48.Zhou J, Fries M R, Chee-Sanford J C, Tiedje J M. Phylogenetic analyses of a new group of denitrifiers capable of anaerobic growth on toluene and description of Azoarcus tolulyticus sp. nov. Int J Syst Bacteriol. 1995;45:500–506. doi: 10.1099/00207713-45-3-500. [DOI] [PubMed] [Google Scholar]