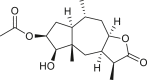
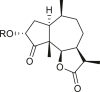
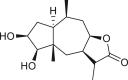
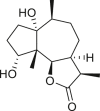
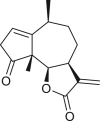
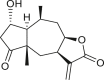
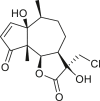
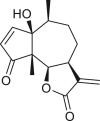
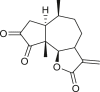
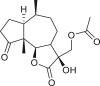
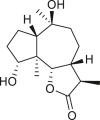
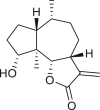
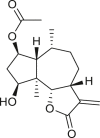
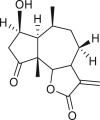
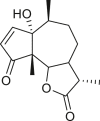
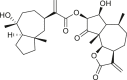
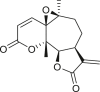
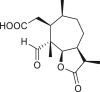
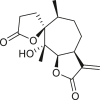
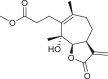
Abstract
Sesquiterpenes are bitter secondary metabolites characteristic to the genus Ambrosia (Asteraceae) and constitute one of the most diverse classes of terpenoids. These compounds exhibit broad-spectrum bioactivities, such as antiproliferative, cytotoxic, antimicrobial, anti-inflammatory, molluscicidal, schistomicidal, larvicidal, and antiprotozoal activities. This review compiles and discusses the chemistry and pharmacology of sesquiterpenes of the Ambrosia species covering the period between 1950 and 2021. The review identified 158 sesquiterpenes previously isolated from 23 different Ambrosia species collected from across the American, African, and Asian continents. These compounds have guaiane, pseudoguaiane, seco-pseudoguaiane, daucane, germacrane, eudesmane, oplopane, clavane, and aromadendrane carbon skeletons. Most sesquiterpene compounds predominantly harbor the pseudoguaiane skeleton, whereas the eudesmanes have the most varied substituents. Antiproliferative and antiprotozoal activities are the most promising bioactivities of sesquiterpenes in Ambrosia and could lead to new pathways toward drug discovery.
Keywords: Ambrosia, Antiproliferative activity, Antiprotozoal activity, Biological activities, Sesquiterpenes
Ambrosia, Antiproliferative activity, Antiprotozoal activity, Biological activities, Sesquiterpenes.
1. Introduction
The genus Ambrosia is part of the Ambrosiinae subtribe and Heliantheae tribe in the Asteraceae family. It contains approximately 50 species, with ragweed or bursage as the commonly known member. Ambrosia is a naturally occurring species in the new world, mainly in North America (León de la Luz and Rebman, 2019). It is distributed mostly in the southwestern United States and the nearby North Mexico with an apparent center of origin and diversity in the Sonoran Desert, but a few species can be found in Central America and South America (Payne, 1966).
SLs are major biochemical compounds characteristic of the Asteraceae family (Frederick, 1982) and have been detected in several angiosperm families, including Amaranthaceae, Acanthacea, Apiaceae, Magnoliaceae, Lamiaceae, Euphorbiaceae, Lauraceae, Orchidaceae, and Polygonaceae (Picman, 1986). Notably, SLs have also been reported in liverworts (Marchantiophyta) (Knoche et al., 1969). They are colorless, bitter, relatively stable, lipophilic secondary metabolites of the terpenoids with 15-carbon atom skeleton and a lactone ring (Fischer et al., 1979). To date, the presence of sesquiterpenes has been reported in 23 species from the Ambrosia genus. The predominant sesquiterpenes isolated are of pseudoguaiane type, the second most common carbon skeleton type in eudesmane compounds. Some species or collections of a single species from different geographical locations can produce more than one sesquiterpene type. The concurrent presence of all the nine sesquiterpene skeletons in a single species has not yet been reported.
SLs exert various bioactivities. The most prominent representative of this group is artemisinin, a compound isolated from Artemisia annua, that has been used in the treatment of malaria (Klayman, 1985). SLs of the genus Ambrosia species have mainly been studied in vitro for their antiproliferative, cytotoxic, antimicrobial and anti-inflammatory activities.
2. Botany and taxonomy
Several Ambrosia species have accidentally been introduced to Eurasia. Of these, A. artemisiifolia, commonly known as ragweed, is the most successful and widespread and has been recorded almost throughout Europe (Gerber et al., 2011). Aside from A. artemisiifolia, A. trifida also radiated to Europe; however, the two species no longer occur in the Sonoran Desert to date (Payne, 1964; Iamonico, 2016). Eleven species, namely, A. microcephala, A. tenuifolia, A. peruviana, A. arborescens, A. artemisioides, A. pannosa, A. parviflora, A. plystachya, A. camphorata, A. psilostachya, and A. humi, are endemic to South America or only rarely occur elsewhere. Contrarily, A. velutina and A. hispida occur mostly in the Caribbean, whereas the five cosmopolitan species, namely, A. artemisiifolia, A. confertiflora, A. tenuifolia, A. tomentosa, and A. trifida, are present in all continents, except Antarctica.
A rapid and significant diversification of ragweeds may be facilitated by their predisposition to polyploidization and human-mediated dispersion. The interspecific hybridization and intraspecific morphological diversity caused by the rapid diversification led to systematic controversies in the genus (Payne et al., 1972). The complication due to species variability in this genus is augmented by a combination of the following factors: growing as habitat pioneers, migration and mixing of seed stocks by agriculture and other human activities, seed dispersal by glacial fronts, seed longevity beyond 50 years (Payne, 1976), polyploidy and disploidy (Payne, 1964; Raven et al., 1968), hybridization (Wagner and Beals, 1958), and inbreeding due to self-pollination and self-fertilization incompatibilities (Payne, 1963).
Numerous Ambrosia species are dioecious desert shrubs, such as A. ambrosioides and A. bryantii, or shrubs in arid areas, including A. arborescens, A. camphorata, and A. dumosa, with annual occurrence in A. acanthicarpa and perennial occurrence in A. canescens, A. confertiflora, and A. grayi. Contrarily, more specialized and derived species are herbs that often occur as secondary plants in ruderal habitats (e.g., A. chamissonis) or disturbed habitats (Payne, 1964).
3. Sesquiterpenes and sesquiterpene lactones of the genus Ambrosia
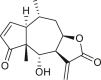
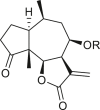
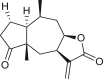
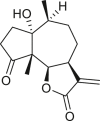
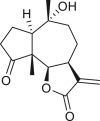
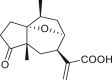
SLs are structurally diverse; the genus Ambrosia is overrepresented by pseudoguaianolides, eudesmanolides, and seco-pseudoguaianolides based on their skeletal structures (Jakupovic et al., 1988; Silva et al., 1992; An et al., 2019). Most SLs harbors a γ-lactone ring (the suffix “olide” refers to the lactone group) containing an exocyclic methylene group conjugated with a carbonyl group, and their biological activities have been attributed to this moiety. The exomethylene group on lactone ring can be substituted either with a methyl group, or a functionalized methylene group. In some cases, the exocyclic methylene is reduced, and the double bond is endocyclic, as observed in ambromaritolide (41) and 11-hydroxyambromaritolide (42) (Jakupovic et al., 1987).
In Asteraceae, sesquiterpenes are mainly synthesized in the smooth endoplasmic reticula of CGTs and then secreted into the extracellular and subcuticular space (Amrehn et al., 2014). The evolutionary and ecological significance of sesquiterpene lactones may be to deter herbivory, pathogenic bacteria, and fungi, as well as other competitors owing to their allelopathic effects (Picman, 1986a; Vidotto et al., 2013). Biosynthetically, sesquiterpene lactones are a type of naturally occurring terpenoids and are formed from a common C5 precursor, IPP, and its isomer, DMAPP, which is derived from the plastid MEP and the cytoplasmic MVA-independent pathways (Bick and Lange, 2003). Pyrophosphate esters of trans,trans-farnesol, cis,trans-farnesol, and nerolidol are formed from three isoprene units via modification and/or cyclization processes (Herz, 1986). γ-lactone can be biosynthesized via lactonization at either the C-6 or C-8 position; its configuration could be cis or trans, and the proton in its C-7 position is usually α (alpha) (Figure 1) (Koji et al., 1974; Zhang et al., 2016; Adekenov, 2017; Li et al., 2020). Numerous secondary structural modifications that affect the methyl groups are often caused by the functional groups of alcohols, carboxylic acids, and unsaturation level, which might be reduced and oxidized (epoxides, hydroxyl moieties). In addition, the hydroxyl groups are frequently esterified (Jean, 1999).
Figure 1.
The proposed biosynthetic pathways of the major structural classes of sesquiterpene lactones found in the genus Ambrosia.
An extensive literature review identified 158 sesquiterpenes of different types, including germacranes, oplopane, eudesmanes, guaianes, pseudoguaianes, seco-pseudoguaianes, daucanes (carotanes), aromadendrane, and clovane from 23 Ambrosia species (Figure 2). Sesquiterpenes are characterized by the presence of a β-oxygenated functional group at the C-6 terminal that might be present as a free hydroxyl group or involved in the formation of the C-6 or C-12 γ-lactones. Ambrosia is the only genus in the Asteraceae family that harbors an oxygen functional group at the C-8 position predominantly in an α-oriented manner, and if present, the ester functional groups are usually linked to other positions. The sesquiterpenes identified from our extensive literature search are listed alphabetically using their minor or semi-systematic names and the species in which the compounds were detected. Psilostachyin C (62) is the most common compound in the Ambrosia and was detected in 11 taxa, followed by pseudoguaianolide damsin (8) in nine taxa.
Figure 2.
Sesquiterpene carbon skeletal structures.
3.1. Guaianes
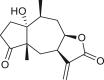
Only four guaiane-type compounds were isolated from the genus Ambrosia (Table 1). The isolated guaianes had a 1,5-trans junction with the exception of 4β-hydroxy-1α,5α,7α,9αH-guaia-10(14),11(13)-dien-12-acid 9-O-β-D-glucoside (4), which was isolated from A. artemisiifolia (Ding et al., 2015). In addition, all isolated guaiane compounds were trans-6,12 lactones, with two having a C-8 oxygen functional group, an ester in cumambrin A (1) and a β-alcohol in cumambrin B (2) (Romo et al., 1968). The other two glycosides (3, 4) have both been reported to have a β-D-glucopyranose moiety at the C-9 position (Ding et al., 2015). The 4β-hydroxy-1α,5α,7α,9αH-guaia-10(14),11(13)-dien-12-acid 9-O-β-D-glucoside (4) has an open-ring system, instead of a lactone ring, which contains a carboxyl group at the C-12 terminal. Moreover, the occurrence of a C-3 or C-4 (1, 2) and C-4 or 5 1β,7β,9β,10β,13αH-guaia-4(5)-en-12,6β-olide-9-O-β-D-glucoside (3) double bonds on the cyclopentane ring is a common feature; however, only compound 4 has a different structural moiety.
Table 1.
Guaianes isolated from the genus Ambrosia.
| Structure | Name | Taxa | Ref. |
|---|---|---|---|
 |
cumambrin A (1) R = Ac |
A. peruviana | (Romo et al., 1968) |
| cumambrin B (2) R = H |
A. acanthicarpa | (Geissman et al., 1969) | |
| A. artemisiifolia | (An et al., 2019) | ||
| A. peruviana | (Romo et al., 1968) | ||
 |
1β,7β,9β,10β,13αH-guaia-4(5)-en-12,6β-olide-9-O-β-D-glucoside (3) R = Glc |
A. artemisiifolia | (Ding et al., 2015) |
 |
4β-hydroxy-1α,5α,7α,9αH-guaia-10(14),11(13)-dien-12-acid 9-O-β-D-glucoside (4) R = Glc |
A. artemisiifolia | (Ding et al., 2015) |
3.2. Pseudoguaianes
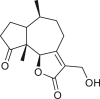
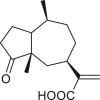
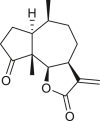
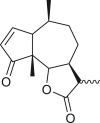
Pseudoguaianes have the highest number of derivatives, with 57 compounds (Table 2). Their main difference from guaianes is that they have a methyl group at the C-5 instead of the C-4 position. Pseudoguaianes are typically C-6 or C-12-olides with a C-11 or C-13 dihydro moiety or exocyclic C-11 and C-13 double. In this review, the lactone ring was only absent in compounds 16, 19, 30, and 31, whereas compounds 10, 17, 18, 22–24, 27, 32, 36, 60, and 61 containedc the C-8/C-12 olide ring. The following pseudoguaiane chemical features have been reported: C13-nor in 41 (Jakupovic et al., 1987); C15-nor in 30 (Taglialatela-Scafati et al., 2012); double bond at C-7/C-11 in 41–49; diol in 17, 27, 29, 33, 34, 37, 43, 45, and 52;epoxide in 19, 31, and 39; C-4 ketone in 5–11, 13–16, 18–21, 25, 26, 28, 30–32, 35–50, 55, 56, and 59; C-3/C-4 esters in 22–24, 26, 28, 60, and 61. The others contain the ester group at C-13 as observed in 50 (Abdel Salam et al., 1984) or at C-2 as detected in 54 (Jimenez-Usuga et al., 2016) and also chlorohydrin as observed in compound 37 (Mahmoud et al., 1999). Dimers are present only in this group of Ambrosia SLs and were observed in compounds 46, 47, 57, and 58.
Table 2.
Pseudoguaianes isolated from the genus Ambrosia.
3.3. Seco-pseudoguaianes
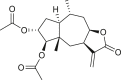
A total of 18 seco-pseudoguaianes (seco-ambrosanolides) have been reported in the genus Ambrosia (Table 3). These compounds exhibit similarity to the pseudoguaiane groupfor example, they are almost exclusively C-6 or C-12 olides, with only tomentosin (74) having a C-8 or C-12 lactone ring, and show a methyl group at the C-5 instead of the C-4 terminal (Seaman and Mabry, 1979a). Aside from the C-5 methyl group terminal, seco-pseudoguaianes possess an α- or β-hydroxyl group, for example, in metabolites 63, 64, 70, 71–73, 76, and 79, except in 10α-hydroxypsilostachyin C compound (75), where it is located at the C-10 position (Borges-del-Castillo et al., 1983). Notably, the lactone ring between the C-4 and C-1 positions or between the C-4 and C-5 positions was absent in six seco-pseudoguaiane compounds (i.e., 65, 69, 71, 74, 76, and 79). The ring A opening was at the C-3 or C-4 position in 4-oxo-3,4-seco-ambrosan-6,12-olide-3-oic acid (65), resulting in an unusual structure (Stefanović et al., 1987). The remaining seco-ambrosanolides are dilactone compounds that are more oxygenated than the other skeletal types. The complete lack of oxygen functional or hydroxyl groups at the C-8 position is a common feature of seco-pseudoguaianes, unlike the other type of sesquiterpenes isolated from this genus.
Table 3.
Seco-pseudoguaianes isolated from the genus Ambrosia.
3.4. Daucanes (carotanes)
The four daucane compounds detected in the genus Ambrosia are listed in Table 4. These compounds contain at least two oxygenation sites that include an esterified hydroxyl group at the C-1 position. The C-1 position in lasidiol anisate (82) and 2,3-dihydro-2,3-dihydroxylasidiol 1-anisate (83) are substituted with aromatic acid 1α-anisoyloxy derivatives (Taglialatela-Scafati et al., 2012), whereas the position is occupied by simple aliphatic acids (angeloyl and 2-methylbutyric acids) in 1α-angeloyloxycarotol (80) and 1α-(2′-methylbutyroyloxy)-carotol (81) (Tiansheng et al., 1993). An alternative oxygen functional group, β-alcohol, is a common feature at the C-6 position of daucanes. These compounds contain a double bond at the C-2 or C-3 position, except in 2,3-dihydro-2,3-dihydroxylasidiol 1-anisate (83), which is a diol bearing two α-oriented hydroxyl groups.
Table 4.
Daucanes (carotanes) isolated from the genus Ambrosia.
| Structure | Name | Taxa | Ref. |
|---|---|---|---|
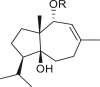 |
1α-angeloyloxycarotol (80) R = Ang |
A. trifida | (Tiansheng et al., 1993) |
| 1α-(2′-methylbutyroyloxy)-carotol (81) R = 2-methylbutyrate |
A. trifida | (Tiansheng et al., 1993) | |
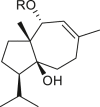 |
lasidiol anisate (82) R = p-methoxybenzoyl |
A. artemisiifolia | (Taglialatela-Scafati et al., 2012) |
 |
2,3-dihydro-2,3-dihydroxylasidiol 1-anisate (83) R = p-methoxybenzoyl |
A. artemisiifolia | (Taglialatela-Scafati et al., 2012) |
3.5. Germacranes
The 18 germacrane compounds detected in the genus Ambrosia are listed in Table 5. The olefinic group at the C-1 or C-10, C-3 or C-4, and C-4 or C-5 positions is always trans. The olefinic group in isoartemisiifolinic acid (99) and diacetyl isoartemisiifolinic acid (100) compounds from A. artemisiifolia was located at the C-4 or C-15 positions, whereas the exomethylene groups were observed at the C-10 or C-14 and C-4 or C-15 positions in 5-oxo-8α-hydroxy-1-epiartemorin (96) (Taglialatela-Scafati et al., 2012). Germacranes of Ambrosia species are almost exclusively C-6 or C-12 and C-8 or C-12 olides, with a typical exocyclic C-11 or C-13 double bond, except for dihydroparthenolide (87), which has a C-11 or C-13 dihydro moiety (Bianchi et al., 1968). The artemisiifolin derivatives, including 84–86, 91, 92, and 97–100, harbors a C-8 or C-12 olide ring and an oxygen functional group at the C-6 position. A primary alcohol functional group was only detected in compounds 85 and 97, whereas an acetoxy at the C-15 site is exclusively present in diacetyl-artemisiifolinic acid (98) (Taglialatela-Scafati et al., 2012). Almost all germacranolides have a C-15 methyl group, with only three members having an α-oxygen functional group at the C-8 position, whereas isabelin (86) is a dilactone derivative (Yoshioka and Mabry, 1969).
Table 5.
Germacranes isolated from the genus Ambrosia.
| Structure | Name | Taxa | Ref. |
|---|---|---|---|
 |
chamissonin (84) | A. acanthicarpa | (Geissman and Levy, 1967) |
| (Geissman et al., 1969) | |||
| A. chamissonis | (Geissman et al., 1966) | ||
| (Lh́omme et al., 1969) | |||
| (Payne et al., 1973) | |||
| (Geissman et al., 1973) | |||
| A. dumosa | (Seaman and Mabry, 1979a) | ||
 |
artemisiifolin (85) | A. artemisiifolia | (Porter et al., 1970) |
 |
isabelin (86) | A. artemisiifolia | (Porter et al., 1970) |
| (Molinaro et al., 2016) | |||
| (Molinaro et al., 2018) | |||
| A. psilostachya | (Yoshioka et al., 1968) | ||
| (Yoshioka and Mabry, 1969) | |||
| (Potter and Mabry, 1972) | |||
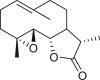 |
dihydroparthenolide (87) | A. artemisiifolia | (Bianchi et al., 1968) |
| (Silva et al., 1992) | |||
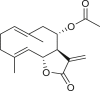 |
tulipinolide (88) | A. camphorata | (Seaman and Mabry, 1979b) |
| A. dumosa | (Seaman and Mabry, 1979a) | ||
 |
costunolide (89) | A. camphorata | (Seaman and Mabry, 1979b) |
| A. chamissonin | (Geissman et al., 1973) | ||
| A. chamissonis | (Payne et al., 1973) | ||
 |
chamissanthin (90) | A. chamissonis | (Payne et al., 1973) |
| (Geissman et al., 1973) | |||
| A. dumosa | (Seaman and Mabry, 1979a) | ||
 |
chamisselin (91) | A. chamissonis | (Payne et al., 1973) |
| (Geissman et al., 1973) | |||
| A. dumosa | (Seaman and Mabry, 1979a) | ||
 |
chamissarin (92) | A. chamissonis | (Payne et al., 1973) |
| (Geissman et al., 1973) | |||
| A. dumosa | (Seaman and Mabry, 1979a) | ||
 |
tamaulipin-A (93) | A. confertiflora | (Fischer et al., 1968) |
| (Yoshioka et al., 1970) | |||
 |
tamaulipin-B (94) | A. confertiflora | (Fischer and Mabry, 1967) |
| (Yoshioka et al., 1970) | |||
| A. dumosa | (Seaman and Mabry, 1979a) | ||
 |
parthenolide (95) | A. dumosa | (Geissman and Matsueda, 1968) |
| (Seaman and Mabry, 1979a) | |||
| A. confertiflora | (Yoshioka et al., 1970) | ||
 |
5-oxo-8α-hydroxy-1-epiartemorin (96) | A. artemisiifolia | (Taglialatela-Scafati et al., 2012) |
 |
artemisiifolinic acid (97) | A. artemisiifolia | (Taglialatela-Scafati et al., 2012) |
 |
diacetyl-artemisiifolinic acid (98) | A. artemisiifolia | (Taglialatela-Scafati et al., 2012) |
 |
isoartemisiifolinic acid (99) R1 = H R2 = H |
A. artemisiifolia | (Taglialatela-Scafati et al., 2012) |
| diacetyl-isoartemisiifolinic acid (100) R1 = Ac R2 = Ac |
A. artemisiifolia | (Taglialatela-Scafati et al., 2012) | |
 |
1,10-epoxyparthenolide (101) | A. confertiflora | (Coronado-Aceves et al., 2016) |
3.6. Eudesmanes
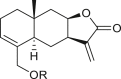
A total of 46 eudesmane compounds identified are listed in Table 6. Eudesmanes are characterized by the presence of a trans fused decalin (decahydronaphthalene) moiety. This group of SLs is the most diverse in terms of their substituents, which are predominantly non-lactonized compounds. Several representatives of this group contain a free α- or β-oriented hydroxyl group, with only 12-hydroxy-4(5),11(13)-eudesmadien-15-al (125) harboring an aldehyde functional group at the C-4 position (De Leo et al., 2010). Three compounds, namely, 144, 145, and 146, harbor a C-6 or C-12 olide ring, whereas 13 compounds are C-8 or C-12 olides. Ilicic acid (130) contains a carboxy group at the C-12 terminal (Herz et al., 1966), whereas it was located at C-13 in costic acid (141) (Seaman and Mabry, 1979b). In contrast, a hydroxymethyl group at the C-12 terminal instead of γ-lactone was reported in 12-hydroxy-4(5),11(13)-eudesmadien-15-al (125) (De Leo et al., 2010). An epoxy group can be found in different positions, including C-4 or C-5, with α orientation as observed in 102 and 103, at C-1 or C-4 with β orientation as observed in 121 and 122 (Jakupovic et al., 1988), and at C-3 or C-4 with an α orientation as observed in 146 (Yoshioka et al., 1970). The hydroperoxy group-containing compounds were only isolated from A. arborescens and were located in an α orientation at C-3 in 108, or in a β orientation at C-3 in 117 and 119 and at C-4 in 118 (Jakupovic et al., 1988). Numerous eudesmane SLs carry a cinnamoyl part attached to the C-1 (111), C-4 (106, 107), or C-6 (113, 116–121) position. The 3″-hydroxy-3″-methylglutaryl moieties at the C-1 or C-9 positions were observed in compounds 132–135 (An et al., 2019). Some glycosides have also been reported, such as the β-D-glucopyranose attached to the C-6 terminal in compounds 114, 131–135, 137, and 139 or to the C-1 terminal in compounds 128 and 129, which is the most common sugar substituent. The β-D-apiofuranose moieties have also been reported in 127 and 138. In addition, the 1α,6β,9β-trihydroxy-5,10-bis-epi-eudesm-3-ene-1-O-α-L-arabinopyranosyl-6-O-β-D-glucopyranoside (136) harbors the β-D-arabinopyranose rather than the glucopyranose moiety (An et al., 2019).
Table 6.
Eudesmanes isolated from the genus Ambrosia.
| Structure | Name | Taxa | Ref. |
|---|---|---|---|
 |
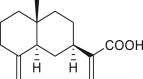
4α,15-epoxy-iso-alantolactone (102) R = H |
A. arborescens | (Jakupovic et al., 1988) |
| 3α-hydroxy-4α,15-epoxy-iso-alantolactone (103) R = OH |
|||
 |
15-hydroxy-iso-allo-alantolactone (104) R = H |
A. arborescens | (Jakupovic et al., 1988) |
| 15-acetoxy-iso-allo-alantolactone (105) R = Ac |
|||
| 15-cinnamoyloxy-iso-allo-alantolactone (106) R = Cinn |
|||
| 15-[2Z-cinnamoyloxy]-iso-allo-alantolactone (107) R = Cinn cis |
|||
 |
15-acetoxy-3α-hydroperoxy-allo-alantolactone (108) R1 = αOOH, H R2 = Ac |
A. arborescens | (Jakupovic et al., 1988) |
| 3α,15-dihydroxy-allo-alantolactone (109) R1 = αOH, H R2 = H |
|||
| 15-hydroxy-3-oxo-allo-alantolactone (110) R1 = O R2 = H |
|||
 |
1α-(E)-cinnamoyloxy-6β-hydroxy-5,10-bis-epi-eudesm-3-ene (111) R1 = Cinn R2 = H |
A. arborescens | (Jakupovic et al., 1988) |
| 1α-hydroxy-6β-anisoyloxy-5,10-bis-epi-eudesm-3-ene (112) R1 = H R2 = p-methoxybenzoyl |
|||
| 6β-cinnamoyloxy-1α-hydroxy-5,10-bis-epi-eudesm-3-ene (113) R1 = H R2 = Cinn |
|||
| 1α,6β-dihydroxy-5,10-bis-epi-eudesm-3-ene-6-O-[β-D-glucopyranoside] (114) R1 = H R2 = β-D-glucopyranosyl |
|||
| 6β-angeloyloxy-1α-hydroxy-5,10-bis-epi-eudesm-4(15)-ene (115) R1 = H R2 = Ang |
|||
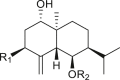 |
β-chaenocephalol cinnamate (116) R1 = H R2 = Cinn |
A. arborescens | (Jakupovic et al., 1988) |
| 6β-cinnamoyloxy-1α-hydroxy-3β-hydroperoxy-5,10-bis-epi-eudesm-4(15)-ene (117) R1 = OOH R2 = Cinn |
|||
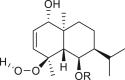 |
6β-(E)-cinnamoyloxy-1α-hydroxy-4β-hydroperoxy-5,10-bis-epi-eudesm-2-ene (118) R = Cinn |
A. arborescens | (Jakupovic et al., 1988) |
 |
6β-(E)-cinnamoyloxy-1α-hydroxy-3β-hydroperoxy-5,10-bis-epi-eudesm-4-ene (119) R1 = βOOH, H R2 = Cinn |
A. arborescens | (Jakupovic et al., 1988) |
| 6β-cinnamoyloxy-1α-hydroxy-5,10-bis-epi-eudesm-4-en-3-one (120) R1 = O R2 = Cinn |
|||
 |
6β-cinnamoyloxy-5,10-bis-epi-eudesmane-1β,4β-epoxide (121) R = Cinn |
A. arborescens | (Jakupovic et al., 1988) |
| 6β-anisoyloxy-5,10-bis-epi-eudesmane-1β,4β-epoxide (122) R = p-methoxybenzoyl |
|||
 |
eudesm-11(13)-en-4β,9β-diol (123) | A. arborescens | (De Leo et al., 2010) |
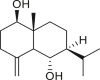 |
volenol (124) | A. arborescens | (De Leo et al., 2010) |
 |
12-hydroxy-4(5),11(13)-eudesmadien-15-al (125) | A. arborescens | (De Leo et al., 2010) |
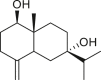 |
4(15)-eudesmene-1β,7α-diol (126) | A. arborescens | (De Leo et al., 2010) |
 |
1β,6α-dihydroxyeudesman-4(15)-ene-1-O-β-D-apiofuranosyl(1″-6′)-O-β-D-glucopyranoside (127) R = Glc (6′-1″)-Api |
A. artemisiifolia | (Huang et al., 2014) |
| 1β,6α-dihydroxyeudesman-4(15)-ene-1-O-β-D-glucopyranoside (128) R = Glc |
|||
 |
7-epi-eudesm-4(15)-ene-1α,6α-diol 1-O-β-D-glucopyranoside (129) R = Glc |
A. artemisiifolia | (Huang et al., 2014) |
 |
ilicic acid (130) | A. artemisiifolia | (Huang et al., 2014) |
| A. camphorata | (Seaman and Mabry, 1979a, Seaman and Mabry, 1979b) | ||
| A. ilicifolia | (Herz et al., 1966) | ||
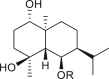 |
pumilaside A (131) R = Glc |
A. artemisiifolia | (Huang et al., 2014) |
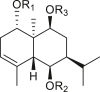 |
1α,6β,9β-trihydroxy-5,10-bis-epi-eudesm-3-ene-9-O-[(S)-3″-hydroxy-3″-methylglutaryl]-6-O-β-D-glucopyranoside (132) R1 = H R2 = Glc R3 = HMG |
A. artemisiifolia | (An et al., 2019) |
| 1α,6β,9β-trihydroxy-5,10-bis-epi-eudesm-3-ene-1-O-[(S)-3″-hydroxy-3″-methylglutaryl]-6-O-β-D-glucopyranoside (133) R1 = HMG R2 = Glc R3 = H |
|||
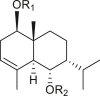 |
1β,6α-dihydroxy-7-epi-eudesm-3-ene-1-O-[(S)-3″-hdroxy-3″-methylglutaryl]-6-O-β-D-glucopyranoside (134) R1 = HMG R2 = Glc |
A. artemisiifolia | (An et al., 2019) |
 |
1α,6β,9β-trihydroxy-5,10-bis-epi-eudesm-3-ene-9-O-[(S)-3″-hydroxy-3″-methylglutaryl]-6-O-(6′-O-acetyl)-β-D-glucopyranoside (135) R1 = H R2 = AcGlc R3 = HMG |
A. artemisiifolia | (An et al., 2019) |
| 1α,6β,9β-trihydroxy-5,10-bis-epi-eudesm-3-ene-1-O-α-L-arabinopyranosyl-6-O-β-D-glucopyranoside (136) R1 = Ara R2 = Glc R3 = H |
|||
| 1α,6β,9β-trihydroxy-5,10-bis-epi-eudesm-3-ene-6-O-β-D-glucopyranoside (137) R1 = H R2 = Glc R3 = H |
|||
 |
1β,6α-dihydroxy-7-epi-eudesm-3-ene-6-O-β-D-apiofuranosyl-(1 6)-β-D-glucopyranoside (138) R = GlcApi |
A. artemisiifolia | (An et al., 2019) |
 |
1β,6α-dihydroxyeudesman-4(15)-ene-1-O-β-D-glucopyranoside (139) R = Glc |
A. artemisiifolia | (An et al., 2019) |
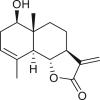
 |
isoalantolactone (140) | A. artemisiifolia | (Taglialatela-Scafati et al., 2012) |
| A. camphorata | (Seaman and Mabry, 1979a, Seaman and Mabry, 1979b) | ||
 |
costic acid (141) | A. artemisiifolia | (Taglialatela-Scafati et al., 2012) |
| A. camphorata | (Seaman and Mabry, 1979a, Seaman and Mabry, 1979b) | ||
 |
ivasperin (142) | A. psilostachya | (Vichnewski et al., 1976) |
 |
granilin (143) | A. artemisiifolia | (Vichnewski et al., 1976) |
 |
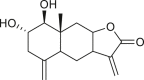
reynosin (144) | A. confertiflora | (Yoshioka et al., 1970) |
| (Coronado-Aceves et al., 2016) | |||
 |
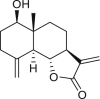
santamarine (145) | A. confertiflora | (Yoshioka et al., 1970) |
| (Coronado-Aceves et al., 2016) | |||
 |
α-epoxysantamarine (146) | A. confertiflora | (Yoshioka et al., 1970) |
 |
isotelekin (147) | A. confertiflora | (Yoshioka et al., 1970) |
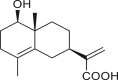 |
1β-hydroxyeudesma-4,11(13)-dien-12-oic acid (148) | A. artemisiifolia | (Ohmoto et al., 1987) |
| (Huang et al., 2014) | |||
 |
1β,6α-dihydroxyeudesm-4(15)-ene (149) | A. artemisiifolia | (Ohmoto et al., 1987) |
 |
6α-hydroxyeudesm-4(15)-ene-9β-O-anisate (150) R = p-methoxybenzoyl |
A. artemisiifolia | (Ohmoto et al., 1987) |
3.7. Oplopanes
All the six oplopane compounds were isolated from Ambrosia arborescens collected in Peru and Chile (Table 7) (Jakupovic et al., 1988). The presence of an ester at the C-1 position is a common feature. Compounds 151 and 153 have an angelate group, 1α-cinnamoyloxy-7-oxo-iso-anhydrooplopanone (154) contains a cinnamate group, whereas 1α-anisoyloxy-7-oxo-iso-anhydrooplopanone (155) is a 1α-anisoyloxy (p-methoxybenzoyl) derivative. Ester group is absent in 1-oxo-iso-anhydrooplopanone (152) and 1α-anisoyloxy-7-oxo-iso-anhydrooplopanone (156), which instead harbor a ketone in this position. Notably, no oplopane compounds have a γ-lactone ring. Diketone derivatives and the occurrence of a C-8 or C-9 double bond in the six-membered ring are a common feature in 152–156 oplopane compounds, except in 1α-angeloyloxyanhydrooplopanone (151), which show this feature as an exocyclic double bond at the C-8 or C-10 position.
Table 7.
Oplopanes isolated from the genus Ambrosia.
| Structure | Name | Taxa | Ref. |
|---|---|---|---|
 |
1α-angeloyloxyanhydrooplopanone (151) R = Ang |
A. arborescens | (Jakupovic et al., 1988) |
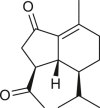 |
1-oxo-iso-anhydrooplopanone (152) | A. arborescens | (Jakupovic et al., 1988) |
 |
1α-angeloyloxy-7-oxo-iso-anhydrooplopanone (153) R = Ang |
A. arborescens | (Jakupovic et al., 1988) |
| 1α-cinnamoyloxy-7-oxo-iso-anhydrooplopanone (154) R = Cinn |
|||
| 1α-anisoyloxy-7-oxo-iso-anhydrooplopanone (155) R = p-methoxybenzoyl |
|||
| 1α-hydroxy-7-oxo-iso-anhydrooplopanone (156) R = H |
A. arborescens | (De Leo et al., 2010) |
3.8. Clovanes
Clovandiol (157) is the only representative of this group in the Ambrosia genus (Table 8) (Delgado et al., 1988). It is a diol with β-hydroxyl and α-alcohol functional groups at the C-2 and C-9 terminals, respectively. This clovane derivative shares a common feature with oplopane, aromadendrane, and daucane sesquiterpenes, with both lacking the γ-lactone ring.
Table 8.
Clovanes isolated from the genus Ambrosia.
| Structure | Name | Taxa | Ref. |
|---|---|---|---|
 |
clovandiol (157) | A. confertiflora | (Delgado et al., 1988) |
3.9. Aromadendranes
Only one aromadendrane compound, aromadendrane-4β,10α-diol (158), has been isolated so far from a Jamaican A. peruviana sample (Table 9) (Goldsby and Burke, 1987). It has no UV-absorbing chromophore group due to the absence of the lactone ring; conversely, it shows a cis-oriented cyclopropane ring in this position. As a diol, it harbors β-hydroxyl and α-hydroxyl at the C-4 and C-10 terminals, respectively.
Table 9.
Aromadendranes isolated from the genus Ambrosia.
| Structure | Name | Taxa | Ref. |
|---|---|---|---|
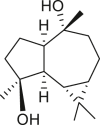 |
aromadendrane-4β,10α-diol (158) | A. peruviana | (Goldsby and Burke, 1987) |
| (Silva et al., 1992) |
4. Occurrence of Ambrosia sesquiterpenes
A. artemisiifolia is the most widely studied species in the genus, and most reports have isolated the guaiane, pseudoguaiane, seco-pseudoguaiane, eudesmane, germacrene, and daucane sesquiterpene backbones in samples from different geographical origins. Daucane sesquiterpenes were isolated from a collection of two different species, A. artemisiifolia and A. trifida, obtained from Italy and the USA, respectively. Two eudesmane compounds, 129 and 131, were isolated from A. artemisiifolia, and their presence might be an important chemotaxonomic marker for differentiating this species from other Ambrosia members. In two different collections of A. arborescens from Peru and Ecuador oplopane derivatives were isolated, which have exclusively been identified in this species. Collections of A. arborescens from southern Peru and northern Chile yielded two types of eudesmane, namely, γ-lactone ring containing 8,12-olides and the 5,10-bis-epi-eudesmanes (Jakupovic et al., 1988), whereas only one type of eudesmane was identified in samples from Ecuador (De Leo et al., 2010). However, analysis of other sample collections from northern Chile revealed no lactones (Silva et al., 1992).
Interestingly, several studies on A. maritima that exclusively occurs in Africa have only isolated the pseudoguaiane-type sesquiterpenes from this taxon, whereas the aromadendrane-type sesquiterpene (158) was isolated from A. peruviana in a Jamaican collection, which represented the first report of a sesquiterpene diol from Ambrosia species (Goldsby and Burke, 1987). The structure of this compound (158) is related to that isolated from a soft coral taxon, Sinularia mayi (Beechan et al., 1978). The relationship between sesquiterpenes derived from terrestrial and marine organisms has previously been determined (Goldsby and Burke, 1987). Notably, the carbon skeleton of the aforementioned secondary metabolite, aromadendrane-4β,10α-diol (158) with its methyl group at the C-4 position rather than at the ring junction in the C-5 position is structurally more similar to the guaiane-type sesquiterpenes detected in the genus Artemisia than the pseudoguianes of Ambrosia species. This observation further demonstrates the relationship between the genus Ambrosia and Artemisia.
Phytochemical studies have not confirmed the presence of SLs in some species, such as A. eriocentra (Herz et al., 1973), A. grayi, A. linearis, and A. tomentosa (Heywood et al., 1977). This has either been attributed to the fact that some of these species are relatively primitive within the genus and have not developed the biosynthetic pathway for sesquiterpene or the absence of lactones in some cases due to mutations, which completely inactivated the biogenetic capacity of some species (Payne, 1964; Payne et al., 1972). However, the failure to report these compounds to date does not entirely reflect their absence. The detection of secondary metabolites depends on the isolation, separation, and identification techniques, as well as their selectivity and sensitivity, which have significantly improved in recent decades.
5. Biological activities of Ambrosia sesquiterpenes
Sesquiterpene compounds isolated from different Ambrosia species exert various bioactivities, including antiproliferative, cytotoxic, anti-inflammatory, antimicrobial, molluscicidal, and allelopathic activities, which are discussed below.
5.1. Antiproliferative and cytotoxic activity
In the search for new anticancer and cytotoxic compounds in the genus Ambrosia, cell death, cell proliferation, micronuclei formation, cell cycle phase distribution, or cell migration were measured (Table 10). The IC50 values were obtained from MTT assays, whereas the cell cycle distribution, cell death analyzis, and cell surface markers were identified via flow cytometry. Cytotoxic and antiproliferative activities are the key characteristic bioactivities of the naturally occurring sesquiterpene lactones. Nearly all known cytotoxic sesquiterpenes in the genus Ambrosia have been shown to contain a lactone function, which is α,β-unsaturated and the α-methylenic linkage which are exocyclic. Among the isolated sesquiterpene lactones, the presence of a C-11 or C-13 exocyclic double bound conjugated to the γ-lactone is crucial for their cytotoxicity. Several studies have noted that the structures and reactivities of Ambrosia sesquiterpenes could be associated with the observation that these compounds alkylate the nucleophilic groups of enzymes controlling cell division (Orofino Kreuger et al., 2012; Sotillo et al., 2017; Sturgeon et al., 2005; Wu et al., 2017; Konaklieva and Plotkin, 2005). Meanwhile, the presence of functional groups, such as the epoxide, hydroxyl, unsaturated ketone, or O-acyl adjacent to the α-CH2 of γ-lactone, can enhance the reactivity of these compounds toward biological nucleophiles.
Table 10.
Antiproliferative, cytotoxic and antimicrobial activity of the genus Ambrosia.
| Extract/compound | Biological assay | Bacterial, fungal strain/cell lines | Effective concentration | Taxa | Ref. |
|---|---|---|---|---|---|
| MeOH extract | Alamar Blue redox bioassay | M. tuberculosis (H37Rv) | MIC (790 μg/mL) | A. ambrosioides | (Robles-Zepeda et al., 2013) |
| MeOH extract | Alamar Blue redox bioassay | M. tuberculosis (H37Rv) | MIC (200 μg/mL) | A. confertiflora | (Robles-Zepeda et al., 2013) |
| CHCl3 extract | Alamar Blue redox bioassay | M. tuberculosis (H37Rv) | MIC (90 μg/mL) | ||
| CH2Cl2 extract | Alamar Blue redox bioassay | M. tuberculosis (H37Rv) | MIC (120 μg/mL) | ||
| EtOAc extract | Alamar Blue redox bioassay | M. tuberculosis (H37Rv) | MIC (160 μg/mL) | ||
| MeOH extract | Alamar Blue redox bioassay | M. tuberculosis (H37Rv) | MIC (200 μg/mL) | ||
| damsin | MTT assay | MCF-7 (HTB-22) | IC50: 3.7 μM | A. arborescens | (Sotillo et al., 2017) |
| HCC1937 (CRL-2336) | IC50: 6.8 μM | ||||
| MCF-10A (CRL-10317) | IC50 (μM): 8.1 | ||||
| JIMT-1 | IC50 (μM): 3.3 | ||||
| coronopilin | MCF-7 (HTB-22) | IC50 (μM): 16 | |||
| HCC1937 (CRL-2336) | IC50 (μM): 16 | ||||
| MCF-10A (CRL-10317) | IC50 (μM): 15 | ||||
| JIMT-1 | |||||
| EtOH extract | 3-step multi parametric phenotypic assay | Hep3B | IC50 (μg/mL): 49.6 | (Carraz et al., 2015) | |
| HepG2 | IC50 (μg/mL): >50 | ||||
| PLC PFR 5 | IC50 (μg/mL): 27.2 | ||||
| SNU-182 | IC50 (μg/mL): >50 | ||||
| coronopilin | MTT assay | Jurkat | IC50 (μM) 24 h: 16 | (Cotugno et al., 2012) | |
| IC50 (μM) 48 h: 5 | |||||
| U937 | IC50 (μM) 24 h: 19.3 | ||||
| IC50 (μM) 24 h: 11 | |||||
| damsin | cell proliferative assay | Caco-2 and HeLa | IC50 (μM): 7.2, 12.4 | A. arborescens | (Villagomez et al., 2013) |
| coronopilin | IC50 (μM): 10.1, 18.3 | ||||
| psilostachyin | MTT assay | MCF-7 mp 53 | IC50 (μM): 20 | A. artemisiifolia | (Sturgeon et al., 2005) |
| psilostachyin C | MCF-7 mp 53 | IC50 (μM): 10.6 | |||
| psilostachyin B | HCT116 p53−/− | - | |||
| paulitin | MCF-7 mp 53 | IC50 (μM): 1.5 | |||
| isopaulitin | MCF-7 mp 53 | IC50 (μM): 4 | |||
| isabelin | agar diffusion test | S. aureus 533R4 | CFU:1.33 × 106 CFU/mL | A. artemisiifolia | (Molinaro et al., 2018) |
| C. albicans BSMY 212 | CFU: 1.32 × 107 CFU/mL | ||||
| reynosin | MTT assay | M. tuberculosis (H37Rv, 366-2009, 104-2010) | MBC: 128 μg/mL | A. confertiflora | (Coronado-Aceves et al., 2016) |
| M. tuberculosis H37Rv | MIC (μg/mL): 64 | ||||
| M. tuberculosis 104-2010 | MIC (μg/mL): 64 | 4.3. | |||
| M. tuberculosis 63-2009 | MIC (μg/mL): 128 | 5.3. | |||
| M. tuberculosis 366-2009 | MIC (μg/mL): 128 | 6.3. | |||
| M. tuberculosis 430-2010 | MIC (μg/mL): 128 | 7.3. | |||
| santamarine | MTT assay | M. tuberculosis H37Rv | MBC (μg/mL): 128 | 8.3. | |
| M. tuberculosis 104-2010 | MBC (μg/mL): 128 | ||||
| M. tuberculosis H37Rv | MIC (μg/mL): 128 | ||||
| M. tuberculosis 366-2009 | MIC (μg/mL): 218 | ||||
| M. tuberculosis 104-2010 | MIC (μg/mL): 128 | ||||
| 1,10-epoxyparthenolide | MTT assay | M. tuberculosis H37Rv | MIC (μg/mL): 128 | ||
| santamarine | MTT assay | L929 | IC50 (μg/mL): 20.2 | ||
| SI: 0.16 | |||||
| reynosine | IC50 (μg/mL): 33.2 | ||||
| SI: 0.52 | |||||
| 1,10-epoxyparthenolide | IC50 (μg/mL): 8.7 | ||||
| SI: 0.07 | |||||
| santamarine | MTT assay | NSCLC (A549) | IC50 (μM): 45 | A. confertiflora | (Wu et al., 2017) |
| NSCLC (NCI-H1650) | IC50 (μM): 43 | ||||
| NL-20 | IC50 (μM): 85 | ||||
| neoambrosin | MIC assay | A. tumefaciens | MIC (mg/mL): 150 | A. maritima | (Badawy et al., 2014) |
| E. carotovora | MIC (mg/mL): 90 | ||||
| damsinic acid | A. tumefaciens | MIC (mg/mL): 500 | |||
| E. carotovora | MIC (mg/mL): 200 | ||||
| damsin | A. tumefaciens | MIC (mg/mL): 175 | |||
| E. carotovora | MIC (mg/mL): 160 | ||||
| ambrosin | A. tumefaciens | MIC (mg/mL): 510 | |||
| E. carotovora | MIC (mg/mL): 215 | ||||
| hymenin | A. tumefaciens | MIC (mg/mL): 520 | |||
| E. carotovora | MIC (mg/mL): 310 | ||||
| neoambrosin | Mycelial radial growth inhibition assay | B. cinerea | EC50 (mg/L): 316.6 | A. maritima | (Abdelgaleil et al., 2011) |
| 9.3. | F. oxysporum | EC50 (mg/L): 204.3 | |||
| damsinic acid | 10.3. | B. cinerea | EC50 (mg/L): 488 | ||
| 11.3. | F. oxysporum | EC50 (mg/L): 464.8 | |||
| damsin | B. cinerea | EC50 (mg/L): 332.5 | |||
| F. oxysporum | EC50 (mg/L): 230.2 | ||||
| ambrosin | B. cinerea | EC50 (mg/L): 413.9 | |||
| F. oxysporum | EC50 (mg/L): 246.6 | ||||
| hymenin | B. cinerea | EC50 (mg/L): 495.3 | |||
| F. oxysporum | EC50 (mg/L): 444.1 | ||||
| neoambrosin | resazurin assay | CCRF-CEM | IC50 (μM): 4.5 | A. maritima | (Saeed et al., 2015) |
| MDA-MB-231- pcDNA3 | IC50 (μM): 29.9 | ||||
| HEK293 | IC50 (μM): 4.5 | ||||
| HCT116 p53+/+ | IC50 (μM): 26.5 | ||||
| U87MG | IC50 (μM): 132.2 | ||||
| CEM/ADR5000 | IC50 (μM): 4.8 | ||||
| MDA-MB-231-BCRP | IC50 (μM): 43.3 | ||||
| HEK293/ABCB5 | IC50 (μM): 4.8 | ||||
| HCT116 P53−/− | IC50 (μM): 39.4 | ||||
| U87ΔEGFR | IC50 (μM): 24.1 | ||||
| damsin | resazurin assay | CCRF-CEM | IC50 (μM): 4.8 | ||
| MDA-MB-231- pcDNA3 | IC50 (μM): 33.8 | ||||
| HEK293 | IC50 (μM): 4.5 | ||||
| HCT116 P53+/+ | IC50 (μM): 32.5 | ||||
| U87MG | IC50 (μM): 154.4 | ||||
| CEM/ADR5000 | IC50 (μM): 4.7 | ||||
| MDA-MB-231-BCRP | IC50 (μM): 46.4 | ||||
| HEK293/ABCB5 | IC50 (μM): 4.6 | ||||
| HCT116 P53−/− | IC50 (μM): 133.6 | ||||
| U87ΔEGFR | IC50 (μM): 24.1 | ||||
| damsin | cytotoxicity assay | 3T3 | GI (μM): 11.2 | A. peruviana | (Aponte et al., 2010) |
| H460 | GI (μM): 15.7 | ||||
| DU145 | GI (μM): 7.6 | ||||
| MCF-7 | GI (μM): 12 | ||||
| M-14 | GI (μM): 10.8 | ||||
| HT-29 | GI (μM): 14 | ||||
| K562 | GI (μM): 14 | ||||
| VERO | GI (μM): 11.2 | ||||
| U937 | GI50 (μM): 8.1 | ||||
| DU145 | GI50 (μM): 10.3 | ||||
| LNCaP | GI50 (μM): 21.3 | ||||
| HCT116 | GI50 (μM): 11.7 | ||||
| A549 | GI50 (μM): 23.4 | ||||
| HeLa | GI50 (μM): 16.1 | ||||
| confertin | cytotoxicity assay | 3T3 | GI50 (μM): 6.4 | ||
| H460 | GI50 (μM): 7.2 | ||||
| DU145 | GI50 (μM): 5.6 | ||||
| MCF-7 | GI50 (μM): 11.2 | ||||
| M-14 | GI50 (μM): 9.2 | ||||
| HT-29 | GI50 (μM): 7.6 | ||||
| K562 | GI50 (μM): 6.8 | ||||
| VERO | GI50 (μM): 6 | ||||
| U937 | GI50 (μM): 3.6 | ||||
| DU145 | GI50 (μM): 7.2 | ||||
| LCCaP | GI50 (μM): 8.1 | ||||
| HCT116 | GI50 (μM): 5.8 | ||||
| A549 | GI50 (μM): 9.1 | ||||
| HeLa | GI50 (μM): 17.1 | ||||
| cumanin | sulforhodamine B (SRB) assay | A549 | GI50 (μM): 19 | A. tenuifolia | (Beer et al., 2019) |
| HBL100 | GI50 (μM): 21 | ||||
| HeLa | GI50 (μM): 19 | ||||
| SW1573 | GI50 (μM): 14 | ||||
| T47-D | GI50 (μM): 30 | ||||
| WiDr | GI50 (μM): 36 | ||||
| A549 | CC50 (μM): 1.5 | ||||
| HBL100 | CC50 (μM): 1.4 | ||||
| HeLa | CC50 (μM): 1.5 | ||||
| SW1573 | CC50 (μM): 2.1 | ||||
| T47-D | CC50 (μM): 1 | ||||
| WiDr | CC50 (μM): 0.8 |
Damsin (8) and coronopilin (11) are pseudoguaianolide sesquiterpenes isolated from A. arborescens. The anticancer activities of these compounds were evaluated on treatment-resistant CSCs especially on breast cancer cell lines (MCF-7, HCC1937, and JIMT-1) and the normal-like breast epithelial cell line MCF-10A (Sotillo et al., 2017). Both compounds inhibited cell proliferation. Damsin (8) was cytotoxic at single-digit micromolar ranges, whereas for the same effect, higher concentrations of coronopilin (11) were required. Sesquiterpenes were also shown to inhibit tumor necrosis factor-α-induced translocation of NF-κB from the cytoplasm to the nucleus. The effects of coronopilin (11) isolated from A. arborescens on two leukemia cell lines demonstrated that it could exert cell population growth-inhibitory activity (IC50 20 μM) on Jurkat cells, mainly by triggering caspase-dependent apoptosis, whereas its primary effect on U937 cells was a robust arrest in G2/M (Cotugno et al., 2012). This finding was consistent with that obtained using sesquiterpene costunolide (89), which induced DNA damage, resulting in arrested G2/M interface (Lohberger et al., 2013). Accordingly, nucleophilic sites makes DNA a potential target for the α,β-unsaturated carbonyl functional group of SLs. Thus, increased levels of γH2AX DNA damaged marker were observed in both the Jurkat and U937 cells, which demonstrates that DNA damage might be the initiating factor in both cell lines following coronopilin (11) exposure. After DNA damage, different molecular pathways trigger early or mitotic arrest/delayed apoptotic cell death. However, coronopilin (11) exhibited poor cytotoxicity to normal white blood cells. Both damsin (8) and coronopilin (11) had antiproliferative effects, which inhibited DNA biosynthesis as well as the formation of cytoplasmic DNA histone complexes in Caco-2 cells (Villagomez et al., 2013). Damsin (8) exposure inhibited the IFNγ-induced expression of STAT3 and TNFα-induced expression of NF-κB more remarkably than coronopilin (11). Both compounds inhibited the expression of the signal transducer, activator of transcription-3 (STAT3), and nuclear factor-κB (NF-κB). Interestingly, the ethanol extract of A. arborescens exhibited antiproliferative activity against the human cancer cell line Hep3B at IC50 50 μg/mL (Carraz et al., 2015).
The bioassay-guided fractionation of methanol extract from the common ragweed led to the identification of psilostachyin C (62) and A (63) as novel checkpoint inhibitors (Sturgeon et al., 2005). Both compounds could arrest cell mitosis and cause the formation of aberrant microtubule spindles in MCF-7 mp53 cells. The α,β-unsaturated carbonyl group is necessary for their activity, thus suggesting that the molecules covalently bind to target proteins through the Michael-type addition. However, psilostachyin B (66), altamisic acid (79), paulitin (67), and isopaulitin (68) showed no checkpoint inhibitory activity despite having one or two α,β-unsaturated carbonyl groups. This suggested that although the moiety is required for the G2 checkpoint inhibitory activity, its presence is not sufficient to convey activity to these types of compound.
Wu et al. (2017) investigated the molecular mechanism of anticancer activities of santamarine (145) isolated from A. confertiflora. Santamarine (145) induced apoptosis and inhibited the growth of A549 lung adenocarcinoma cells by generating reactive oxygen species (ROS), inhibiting TrxR activity, and depleting GSH level, thereby increasing oxidative stress (Table 10). The cytotoxic effect of santamarine (145) was evaluated in a mechanistic study via a microscopic analyzis of the cell morphology, and the results indicated that the compound induced potent morphological changes, such as junction breaks and rounding up of cells, which are associated with cell death both in the A549 and NCI-H1650 cells. Two pseudoguaianolides, neoambrosin (35) and damsin (8), were isolated from A. maritima, and their cytotoxic activities have been evaluated in the multidrug-resistant cells using the resazurin assay. The ABC transporters, P-glycoprotein, BCRP, and ABCB5, overexpressing MDR cells did not convey cross-resistance toward these pseudoguaianolides, indicating that these compounds are not the ideal substrates for analyzing these ABC transporters (Saeed et al., 2015).
The pseudoguaianolide cumanin (17), isolated from the aerial parts of A. tenuifolia, exhibited potent antiproliferative and cytotoxic activities against several cancer cell lines, including A549, HBL100, HeLa, SW1573, T47-D, and WiDr. Mouse splenocytes were used to evaluate its cytotoxic activity, and the results were expressed as CC50. Cumanin (17) was less toxic on splenocytes (CC50: 29.4 μM) compared with helenalin (5) and hymenin (38). The presence of two hydroxyl groups in the structure of cumanin (17) enabled the preparation of its acetylated, silylated, and triazole derivatives. The modified derivatives had improved activities, with most showing higher cytotoxic activity and selectivity indices than the natural parent compound (Beer et al., 2019).
The ethanolic extracts of A. peruviana exerted notable cytotoxic activity in vitro. The bioactivity-guided purification of the extract led to the isolation of two pseudoguaianolide sesquiterpene lactones, damsin (8) and confertin (10). Both compounds had cytotoxic activity against a panel of six human cancer and non-tumorigenic cell lines (Table 10). The enzyme-alkylating abilities of damsin (8) and confertin (10) are derived from the α-methylene-γ-lactone group; thus, their active moiety can undergo Michael-type addition reactions toward biological nucleophiles, such as cytochrome cofactors and a few Krebs cycle dehydrogenases (Konaklieva and Plotkin, 2005). Damsin (8) demonstrated selectivity for DU145 prostate cancer cells among solid tumor cell lines (Aponte et al., 2010).
5.2. Antimicrobial activity
The redox Alamar Blue microplate bioassay is commonly used to evaluate the antibacterial activity of isolated compounds. The cell viability was evaluated via an MTT reduction assay. The anti-mycobacterial analyzis revealed that the methanolic extracts of A. confertiflora and A. ambrosioides had the most significant in vitro activities compared with other seven medicinal plants (Robles-Zepeda et al., 2013). The chloroform, dichloromethane, and ethyl acetate extracts of A. confertiflora exhibited antimicrobial activities with MICs of 90, 120, and 160 μg/mL, respectively. Isabelin (86) compound isolated from A. artemisiifolia exerted a remarkable antimicrobial activity against the soil bacterium Paenibacillus sp. and the multidrug-resistant Staphylococcus aureus (Table 10). Similarly, isabelin (86) inhibited the growth of C. albicans in the same MIC range (Molinaro et al., 2018), and the authors hypothesized that its growth-inhibitory activity, which is similar to that of cnicin, could be due to the alkylation of thiol groups of cysteine residues of the UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) associated with the biosynthesis of bacterial cell wall (Bachelier et al., 2006).
Reynosin (144) and santamarine (145) compounds isolated from A. confertiflora had potent inhibitory activity against Mycobacterium tuberculosis strains. The MBC and MIC values indicated that reynosin (144) was the most active compound against the H37Rv, 366-2009, and 104-2010 M. tuberculosis strains (Table 10). Santamarine (145), reynosine (144), and 1,10-epoxyparthenolide (101) had low selectivity index (<1) toward the L929 normal cell line, whereas santamarine (145) and reynosin (144) were about six and four times more toxic to the normal cell line than M. tuberculosis, respectively. As aforementioned, the anti-mycobacterial activity of these compounds was correlated with the presence ofα-methylene γ-lactone moiety (Picman, 1986), and their activity could be improved by different chemical modifications (Cantrell et al., 2001).
Badawy et al. (2014) isolated five pseudoguaianolides, including neoambrosin (35), damsinic acid (16), damsin (8), ambrosin (20), and hymenin (38), from the aerial parts of A. maritima, and their antibacterial activities were tested on two plant pathogenic bacteria, Erwinia carotovora and Agrobacterium tumefaciens. The results indicated that neoambrosin (35) was the most active, with MIC values of 90–150 mg/L, whereas hymenin (38) was the least effective, with MIC values of 520 and 310 mg/L against A. tumefaciens and E. carotovora, respectively (Table 10). Sesquiterpene lactones appeared to be less effective against Gram-negative than Gram-positive bacteria. The low activity against Gram-negative bacteria could be attributed to the presence of their outer membrane, which prevents the diffusion of hydrophobic molecules (Badawy et al., 2014). The study also observed the highest in vitro antibacterial activity and sulfhydryl functional group reduction in neoambrosin (35), damsinic acid (16), and damsin (8), relative to hymenin (38), which showed least effectiveness. These results indicate that sesquiterpenes might exert their antibacterial activity by irreversibly binding to the SH of vital enzymes. The antibacterial activity of damsin (8) is determined by its α-methylene-γ-lactone molecule; thus, it was more effective than damsinic acid (16), in which the open lactone ring formed a carboxyl group. The neoambrosin (35) compound with C1-C-2 double bond was more potent than ambrosin (20) with C2-C3 double bond. The lipophilic nature of sesquiterpenes also has an influence on their antibacterial activity, for example, the presence of a hydroxyl moiety at the C-1 position in hymenin (38) caused a less remarkable antibacterial activity compared with ambrosin (20). Due to the high lipophilic nature of bacterial cell walls, a moderate to high lipophilicity is crucial for antibacterial activity.
The evaluation of antifungal activity using isolated compound against two plant pathogenic fungi, Fusarium oxysporum and Botrytis cinerea, was conducted by (Abdelgaleil et al., 2011). The study observed the highest inhibitory activity against mycelial radial growth in neoambrosin (35) and damsin (8), whereas damsinic acid (16) and hymenin (38) were less effective. In addition, the spore germination in both fungi was significantly decreased by exposure to compounds tested at 50–200 mg/L, with a general trend showing greater inhibitory effect on spore germination than on mycelial growth. Neoambrosin (35), damsin (8), and ambrosin (20) harboring the α-methylene-γ-lactone were more active than damsinic acid (16), in which the open lactone ring formed a carboxyl group. Conversely, the introduction of hydroxyl group at the C-1 position in hymenin (38) decreased its antifungal activity relative to ambrosin (20). Notably, the least polar compounds exhibited the greatest activity. The relatively low polarity of neoambrosin (35), damsin (8), and ambrosin (20) is countered by the optimum lipophilicity for entering the fungal cell wall (Barrero et al., 2000).
5.3. Anti-inflammatory activity
Antiphlogistic activities were evaluated using the effects of sesquiterpenes on cytokine production, NF-κB activity, and in vivo experiments. For anti-inflammatory activity assays, the cell morphology was investigated via phase-contrast microscopy. The coronopilin (11) and damsin (8) compounds isolated from A. arborescens reduced the NF-κB phosphorylation in human cell lines (Svensson et al., 2018). Both coronopilin (11) and damsin (8) downregulated pro-inflammatory cytokine production in HDFa cells and prevented the LPS-induced MCP-1 expression more potently than dexamethasone. Damsin (8) also interacted with the NF-κB signaling steps causing a reduction in the levels of phosphorylated NF-κB p65 and p105 subunits and upregulating IκBα. Meanwhile, coronopilin (11) reduced LPS-stimulated cytokine expression without affecting the IκBα expression or the phosphorylated NF-κB subunit, suggesting that other mechanisms might be involved in its activity to downregulate cytokine expression. The study also hypothesized that most SLs with anti-inflammatory effects act as Michael acceptors owing to their α-methylene-γ-lactone functionalities. These compounds might interact and inactivate certain thiol-containing proteins, with NF-κB as the main target along with other pro-inflammatory transcription factors (Orofino Kreuger et al., 2012). However, Chib et al. synthesized psilostachyin analogues, and their anti-inflammatory potential has been evaluated. Interestingly, their results indicated that compounds without α-methylene moiety exhibited better anti-inflammatory potential against the in vitro expression of TNF-α, IL-1β, and IL-6 in murine neutrophils compared with naturally occurring sesquiterpenes, which harbor the α-methylene-γ-lactone group (Chib et al., 2011). Moreover, the semi-synthesized derivatives had significantly improved anti-inflammatory activity compared with the parent molecules, for example, a dipolar cycloaddition on exocyclic double bond and its migration during catalytic hydrogenation increased the activity of the derivative molecules. The study argued that the presence of α-methylene-γ-lactone moiety is not essential for the TNF-α activity or the inactivation of other pro-inflammatory transcription factors in the tested compounds.
The alcoholic extracts of A. artemisiifolia leaves have been investigated for anti-inflammatory activity using several experimental models, including carrageenan-induced edema, cotton pellet granuloma, croton oil ear edema, and formaldehyde-induced arthritis (Pérez, 1996). The results were compared with those of phenylbutazone and betamethasone, which are standard anti-inflammatory drugs. The alcoholic extract exhibited significant inhibitory effects on acute edema induced by croton oil or carrageenan in mice and rats at doses of 50, 100, 150 mg/kg. The A. artemisiifolia extracts had marked activity in both granuloma croton oil model and cotton pellet granuloma model for subacute arthritis, and the extract activities were more comparable with those of phenylbutazone and betamethasone. The study also observed that alcoholic extract could inhibit formaldehyde-induced arthritis (52.04%) in the early stage. The extracts effectively reduced both swelling of paw injected with phlogistic agents in acute inflammatory process and inflammatory exudate formation in croton oil granuloma in rats. Notably, the topical application of alcoholic extracts produced more active effects than when used orally in the cotton pellet granuloma model.
5.4. Larvicidal activity
The larvicidal activity of plant extracts was determined according to the protocols proposed by the WHO. Aedes aegypti is an African mosquito species responsible for the spread of several vector-borne diseases, such as dengue, Chikungunya, and Zika. There are no effective vaccines or drugs available for the prevention or treatment of these diseases; thus, controlling the population of A. aegypti remains the primary goal. Due to climate change and population growth, the distribution area of this mosquito species is increasing in the subtropical and tropical regions of the world. The constant and, in some cases, indiscriminate use of pesticides has caused the emergence of resistant A. aegypti and other adverse nontargeted effects. In vitro studies demonstrated that A. arborescens contains water-soluble larvicidal compounds against A. aegypti larvae, which can be augmented when administered in combination with silver nanoparticles (Morejón et al., 2018). The aqueous extract of A. arborescens has an LC50 value of 1,844.61 ppm; however, an LC50 value of 0.28 ppm was obtained when the same extract was used to synthesize AgNPs, thus representing an exponential potentiation of the lethal effect (Arjunan et al., 2012; Subramaniam et al., 2015). Geerts et al. (1994) evaluated the effects of Ambrosia maritima extract on A. aegypti and Anopheles stephensi (Geerts et al., 1994). The dry leaf extracts were applied on the water surface at a concentration of up to 2,000 mg/L, but only a negligible A. stephensi larvae mortality of 38% was observed.
5.5. Molluscicidal activity
Schistosomiasis is still one of the most widespread tropical diseases in Africa, South America, and southeast Asia. Controlling snail population is one of the most essential elements of the integrated approach to preventing schistosomiasis (Vos et al., 2016). A. maritima is a promising molluscicidal plant and the only studied species from the genus Ambrosia. The plant is used in traditional medicine both in Egypt and Senegal as a drug against urinary schistosomiasis. The A. maritima leaves and flower organs are generally used to prepare an infusion or a decoction, and the plant has been demonstrated to control the snail vectors of both schistosomiasis and distomatosis (Kloos et al., 1982). Several studies have demonstrated the activity of A. maritima against Biomphalaria, Bulinus, and Lymnaea species, with most experiments being conducted with the dried plant tissues, especially the leaves and flowers, which exhibit higher molluscicidal activity than the stems or roots (Shoeb and El-Emam, 1976; El-Sawy et al., 1981).
The molluscicidal properties of A. maritima were evaluated using a slow-release test, where molluscs were exposed to plant extract for up to 4 days followed by a 1-day recovery period (Belot et al., 1991). The molluscicidal activity of A. maritima was assayed against the indigenous Senegalese snails, namely, Lymnaea natalensis, Bulinus globosus, Bulinus forskalii, and Biomphalaria pfeifferi. As a result, the observed LC50 value for Lymnaea natalensis was 108.3 mg/L, whereas the LC50 values for B. forskalii and B. globulus were 165.4 and 148.5 mg/L, respectively. Contrarily, a slightly higher LC50 value of 227.4 mg/L was observed against B. pfeifferi. Furthermore, in this study, field experiments were conducted using dried A. maritima leaf extracts (400 mg/L) to be applied in the creeks of the Lampsar River, and the results indicated reduced B. globosus population by up to 54%–56% 2 weeks after treatment. Similarly, treatment with 150–300 mg/L dried A. maritima extract in a closed irrigation canal caused a 77% reduction in B. pfeifferi density 2 weeks after treatment. However, the population of these snail species recovered post field trials (Belot et al., 1993).
Analysis of the molluscicidal activities of the damsin (8) and ambrosin (20) compounds isolated from A. maritima against Biomphalaria havanensis revealed no snail mortality 24 h after exposure (Yoke Marchant et al., 1984). The study concluded that the result was due to the very low damsin (8) and ambrosin (20) solubility in water, rendering the compounds poor control agents of schistosomiasis snail vectors. The effects of A. maritima ethanol extracts on the DNA and protein contents of the digestive glands from schistosomiasis vectors were evaluated by Abdel-Haleem (2013). The digestive glands of Biomphalaria alexandrina and Bulinus truncates vectors were selected as target organs based on the fact that these tissues are affected of the molluscs (Bode et al., 1996). Analysis of the two treated snails with SDS-PAGE revealed a reduction in the band profiles of targeted protein from nine to eight and five, following exposure to A. maritima. An increase in the DNA concentration in the treated and infected animals was observed partly due to the S. mansoni and S. haematobium infection. In addition, treatment with ethanolic A. maritima extracts enhanced mollusc DNA replication to synthesize mutant genes in the lymphocytes of the immune system.
5.6. Schistosomicidal activity
A. maritima is one of the most promising molluscicidal plants in the Ambrosia genus (Geerts et al., 1991). It has no toxic effects on aquatic and other non-target organisms, making it an ideal treatment agent for slack water bodies (Alard et al., 1991). Other than its molluscicidal activity, A. maritima is a popular traditional medicine used in urinary schistosomiasis treatment among at least 40% of the northern Egyptian population (Kloos et al., 1982). The schistomicidal activity of ethanolic extracts from dried A. maritima leaves was studied in two mice groups infected with S. mansoni, with one group receiving a single dose of 15 g leaves/kg body weight while the other group receiving a similar dose for 5 consecutive days (Abadome et al., 1994). The schistosomicidal activity, determined based on the worm burden, motility of worms, number of eggs in the intestinal wall and liver, and oogram of the plant extract was evaluated 1 week after treatment. A single dose did not have any effect; however, the ratio of immature eggs in the intestinal wall decreased compared with that of the control group in mice group treated with five doses, suggesting a degree of plant extract inhibitory activity. On the other hand, this observation was compensated for by an increase in the number of eggs in the liver of this group of mice due to the hepatic shift of the parasites. A similar phenomenon after treatment with several substances containing schistosomicidal activity was also confirmed by Delgado et al. (1992). The dislocation of schistosomes is often a reversible phenomenon that occurs after administration of a drug at a very low dose or for a short period (Popiel and Erasmus, 1984; Jordan and Webbe, 1982). In this case, A. maritima extract was used at a high dose of 15 g leaves/kg for 5 consecutive days; however, even at a high dosage, oral administration of the plant extract only had negligible effect on adult S. mansoni.
5.7. Antiprotozoal activity
The antiprotozoal activity has only been investigated in A. scabra and A. tenuifolia from the genus Ambrosia. To determine the IC50 of isolated compounds, the parasite number was counted using a Neubauer chamber, and their viability was measured using the trypan blue exclusion method. Trypanosoma cruzi, which is responsible for trypanosomiasis, is restricted to Latin America, where it endangers 25–90 million people (Díaz-Chiguer et al., 2012). In contrast, leishmaniasis, which is transmitted by the monoflagellate Leishmania species, is a widely occurring disease (Barrera et al., 2013). Both trypanosomiasis and leishmaniasis are widespread neglected protozoal diseases that mostly affect poor and marginalized populations in Latin America and some African and Asian regions. There are no modern drugs for the treatment of these diseases, and the available therapeutic options, such as nifurtimox and benznidazole for trypanosomiasis and pentavalent antimony derivatives, pentamidine, and amphotericin B for leishmaniasis, have unfavorable side effects (Soeiro and de Castro, 2009). However, some Leishmania species have already developed resistance to pentavalent antimony drugs, thus requiring the use of paromomycin or allopurinol drugs, which have serious toxicity and teratogenicity drawbacks (Mishra et al., 2007). Moreover, trypanosomiasis and leishmaniasis might appear as co-infections (Chiaramonte et al., 1999; Frank et al., 2003; Mendes et al., 2007). The discovery of antimalarial drugs, such as sesquiterpene lactone artemisinin isolated from Artemisia annua and quinine, has been a major breakthrough in the field of parasitic disease treatment, thus prompting investigation of similar natural compounds. The discovery of the two antimalarial drugs, which are currently being used, has subserved the evaluation of other medicinal plant in the search for new antiprotozoal agents with potential activities against drug- and multidrug-resistant strains of T. cruzi and Leishmania species.
To discover new trypanocidal lead compounds, dichloromethane-methanolic (1:1) extracts of plants used in folk medicine were tested for antiparasitic effects (Sülsen et al., 2006). In this study, the trypanocidal activity was assayed on T. cruzi epimastigotes, and the results indicated that A. scabra and A. tenuifolia extracts had >70% trypanocidal activity at 100 μg/mL concentration, suggesting that the two extracts were effective and promising sources of lead compounds for the development of trypanosomiasis treatment drugs. Bioassay-guided fractionation of A. tenuifolia extract led to the isolation of pseudogauiane peruvin (18) and seco-pseudoguaiane psilostachyin (63) (Sülsen et al., 2008). Both compounds were tested via in vitro assay on the epimastigote and trypomastigote developmental stages of T. cruzi. The IC50 values of both compounds were similar for the noninfective form of T. cruzi, with psilostachyin (63) and peruvin (18) displaying IC50 values of 1.22 and 1.65 μg/mL, respectively, after 72 h. Psilostachyin (63) was more active on the trypomastigote forms than the epimastigote stage, with IC50 values of 0.76 and 52.8 μg/mL, respectively. The in vitro leishmanicidal activity of psilostachyin (63) and peruvin (18) was also tested on Leishmania mexicana promastigotes (MNYC/BZ/62/M strain), and IC50 values of 0.12 and 0.39 μg/mL were obtained, respectively. In addition, the cytotoxicity of these compounds was assessed on mammalian murine T lymphocyte cells, and the 50% cytotoxic concentrations of 25.7 and 35.0 μg/mL were observed for psilostachyin (63) and peruvin (18), respectively. The calculated selectivity index for psilostachyin (63) was 33.8 on the trypomastigotes of T. cruzi, indicating good selectivity, whereas peruvin (18) was not selective (SI: 0.7). The in vivo evaluation of trypanocidal activity in C3H/HeN mice infected with T. cruzi trypomastigotes showed 100% mice survival from acute infection and low parasitemia in those treated with psilostachyin (63) for 5 days relative to the control group.
The antiproliferative effect of psilostachyin (63) obtained from A. tenuifolia was tested on the Tulahuen strain, which is a noninfective form of T. cruzi (Sülsen et al., 2010). Psilostachyin (63) inhibited parasitic growth dose-dependently with an IC50 value of 0.3 μg/mL (1.1 μM), and this effect was irreversible with an IC50 value above 1.0 μg/mL. A trypan blue exclusion method was employed to evaluate parasite viability, and high mortality, which represents the cytotoxic or cytostatic activity of psilostachyin (63), was observed on the second day after its addition to the culture (2.5 μg/mL). The cytotoxicity of psilostachyin (63) was also assessed on T-lymphocytes from BALB/c mice using the trypan blue dye exclusion method, and a CC50 value of 3.0 μg/mL was observed after 72 h. A selectivity index of 10 was observed for psilostachyin (63), which indicated its more pronounced toxicity on parasites than mammalian cells. In the presence of GHS, the effects of psilostachyin (63) on T. cruzi epimastigotes was partially blocked, suggesting that the α-methylene-γ-lactone functionality is not exclusively responsible for its activity. Psilostachyin (63) caused a dose-dependent (0.5–2.5 μg/mL) cytoplasmic vacuolization in T. cruzi epimastigotes after 24-h treatment and induced malformations in the kinetoplast.
The bioassay-guided fractionation of A. tenuifolia extract resulted in the isolation of peruvin (18) and psilostachyin (63) SLs (Sülsen et al., 2011). The in vitro evaluation of the antiplasmodial activity of these SLs against chloroquine-sensitive (F32) and chloroquine-resistant (W2) Plasmodium falciparum strains revealed their positive activities on both parasite strains, with a higher activity on the F32 strain. Peruvin (18) was the most active SL with an IC50 value of 1.1 μM, whereas psilostachyin (63) exhibited activity on both the F32 and W2 strains with IC50 values of 2.1 and 6.4 μM, respectively. In addition, the cytotoxicity assay of psilostachyin (63) and peruvin (18) on lymphoid T-cells revealed CC50 values of 24.3 and 37.9 μM, respectively, after 48 h.
The thymidine uptake assay was employed to evaluate the in vitro antiprotozoal activity of psilostachyin C (62) against L. mexicana promastigotes (MNYC/BZ/62/M strain), T. cruzi epimastigotes (RA strain), and Leishmania amazonensis promastigotes (IFLA/BR67/PH8 strain) (Sülsen et al., 2007). The results indicated that protozoan growth was inhibited by 86.7% and 81.2% after 72- and 120-h exposure to 10 μg/mL concentration, respectively. The IC50 values of 0.6 and 0.8 μg/mL were obtained for psilostachyin C (62) activity against epimastigotes after 72 and 120 h of incubation, respectively, whereas its activity on trypomastigotes was less remarkable, revealing an IC50 value of 3.5 μg/mL. Long-term psilostachyin C (62) treatment for over 24 h with 2.5 μg/mL decreased the rate of DNA replication, whereas treatment for 72 h achieved IC50 values of 1.5 and 1.2 μg/mL for L. amazonensis and L. mexicana, respectively. In addition, more epimastigotes were observed when T. cruzi was incubated with GHS and psilostachyin C (62) than when treated only with psilostachyin C (62).). The α-methylene-γ-lactone moiety is highly reactive with thiol groups, which reduces their toxicity (Schmidt et al., 2009).
An in vitro cytotoxicity test on mammalian cells using the MTT method to assess the specificity of the trypanocidal activity indicated that the psilostachyin C (62)-treated peritoneal macrophages had CC50 values of 100 and 87.5 μg/mL at 3 and 48 h, respectively. The calculated selectivity indices of psilostachyin C (62) were 25.0 and 97.2 for T. cruzi trypomastigotes and amastigotes, respectively, which suggested that the selectivity of psilostachyin C (62) for parasites is greater than that for mammalian cells. An in vivo assay using groups of five female C3H/HeN mice infected with bloodstream T. cruzi trypomastigotes revealed that animals treated with psilostachyin C (62) had a lower level of circulating parasites and a 20% survival rate 20 days after treatment. In contrast, untreated mice infected with T. cruzi exhibited a high level of parasitemia and 100% mortality 20 days after infection. Transmission electron microscopy images revealed alterations in the ultrastructure of T. cruzi epimastigotes treated with 2.5 μg/mL psilostachyin C (62) for 24 h, including vacuolized cytoplasm, multilamellar structures, and abnormal presence of multiple kinetoplasts and flagella. In addition, studies conducted by Izumi et al. (2008) and Sülsen et al. (2010) observed that psilostachyin C (62) did not cause mitochondrial swelling, whereas swelling occurred when it was used with other compounds that block parasite metabolism, indicating that psilostachyin C (62) might acts by other mechanisms.
Both the in vitro and in vivo assays of A. tenuifolia psilostachyin (63) activities on the acute model of T. cruzi (Y strain) revealed low trypanocidal EC50 activity of 33 μg/mL after 24 h against bloodstream trypomastigotes (da Silva et al., 2013). The in vivo assay to explore the acute toxicity of psilostachyin (63) on Swiss female mice treated with 25–400 mg/kg of the compound revealed no adverse effects 45 h after treatment. In addition, the in vivo efficacy analyzis of psilostachyin C (62) in different T. cruzi acute infection models (Y and Colombiana strains) revealed that the compound decreased the parasitemia levels by about 40% and 70% at 0.5 and 50 mg/kg doses, respectively, but with no change in animal mortality.
Barrera et al. (2013) evaluated the effects of psilostachyin (63) and psilostachyin C (62) isolated from A. tenuifolia and A. scabra on the ameliorating mechanism of L. mexicana subsp. mexicana promastigotes against oxidative stress. The antiproliferative activity of psilostachyin (63) and, to a lesser extent, psilostachyin C (62) was blocked by 1.5 mM GSH. In addition, psilostachyin (63) but not psilostachyin C (62) reduced the concentration of endogenous GSH. On the other hand, the psilostachyin (63)-treated L. mexicana subsp. mexicana promastigotes exhibited a significant increase in ROS generation. The generation of free radicals in Leishmania promastigotes by the psilostachyin compounds is potentially harmful to the trypanosomatids. Chawla and Madhubala (2010) concluded that SLs could inhibit the activity of key enzymes that are vital against oxidative stress by reacting with sulfhydryl groups via the Michael-type addition.
The determination of psilostachyin (63) and psilostachyin C (62) affinity to hemin using the hemin binding assay revealed their remarkable interaction with hemin, with higher affinity being observed for psilostachyin C (62) than for psilostachyin (63) under non-reducing conditions (Sülsen et al., 2016). Contrarily, the affinity of psilostachyin (63) to hemin increased, whereas that of psilostachyin C (62) decreased under reducing conditions. In the T. cruzi growth inhibition assay, psilostachyin C (62) was the most active in the absence of hemin, whereas in the presence of hemin, psilostachyin (63) was characterized by a low IC50 value of 10 μM. The addition of psilostachyin (63) and psilostachyin C (62) to the culture medium induced inhibitory effects of 87% and 72%, respectively; the compounds were shown to have the binding ability to protoporphyrin IX-Fe(III) (hemin) and protoporphyrin IX-Fe(II) (heme). The inhibition of hemin metabolism in parasitic vectors leads to oxidative stress. Notably, Sülsen et al. (2016) showed that psilostachyin C (62) had higher affinity for hemin than psilostachyin (63) (Sülsen et al., 2016). Subsequently, T. cruzi epimastigotes treated with 35 μM of both compounds were used to evaluate the induced intracellular oxidative stress via flow cytometry. As a result, a 4.5-fold increase in the fluorescence of H2DCFDA-loaded epimastigotes was observed after 4 h of treatment with psilostachyin (63), whereas little change was observed in treatments with psilostachyin C (62) after 4 and 8 h. In addition, the TryR and CP enzymes were obtained from the cell-free extract of T. cruzi epimastigotes. TryR and CP are only present in trypanosomatids but absent in mammalian cells. The former is a trypanosomatid-specific oxidoreductase, whereas the latter is a cysteine protease relevant for parasite metabolism (Duschak and Couto, 2009). Both enzymes could be considered as potential targets for drug design. However, psilostachyin (63) and psilostachyin C (62) could not inhibit TryR and CP at concentrations of 10, 20, and 50 μM (Sülsen et al., 2016).
The biosynthesis of ergosterol is essential for the growth and development of T. cruzi. The squalene/ergosterol ratio of 8.5 was observed in psilostachyin C (62) treated epimastigotes representing a 4-fold increase than that of untreated control. In contrast, the squalene/ergosterol ratio in positive-control treatments of terbinafine-treated parasites at a concentration of 50 μM was 4.10. The differences in the lipid profiles of psilostachyin (63)- and psilostachyin C (62)-treated T. cruzi suggest that psilostachyin C (62) probably inhibited the squalene epoxidase activity; thus, the biosynthesis of ergosterol could be a target for this compound. The antiprotozoal activities of psilostachyin (63) and psilostachyin C (62) were evaluated in T. cruzi after treatment with 35 μM for 8, 24, and 48 h, followed by the detection of apoptotic cells by annexin-V FITC/PI staining. The results indicated that apoptosis increased in both SLs in a time-dependent manner, and the ratios of early apoptotic cells were 42% and 38% in psilostachyin (63) and psilostachyin C (62), respectively, after 48 h of treatment. Similarly, the ratio of late apoptotic cells also increased, reaching 10.7% and 9.0% in psilostachyin (63) and psilostachyin C (62), respectively. Nuclear fragmentation was observed in 82.9% (psilostachyin C (62)) and 72.2% (psilostachyin (63)) of parasites treated with 350 μM solutions. The combined effects of both psilostachyin (63) and psilostachyin C (62) were investigated in drug interaction experiments, and a FICI of 1.11 was observed. These findings indicated an additive trypanocidal effect, suggesting that these molecules exert their antiparasitic activity through different targets (Sülsen et al., 2016).
5.8. Other activities
A previous study investigated plant sensitizing capacity using diethyl ether extracts from 20 Asteraceae species in female albino guinea pigs of the Pirbright White strain (Zeller et al., 1984). For each extract, 10 animals were used. In the open epicutaneous test (OET), 0.05 mL of 10% diluted acetone extract was applied daily to a 2 cm2 test area of the clipped and shaved flank of each animal. The MR of 10% concentrations obtained from the average of 24-, 48-, and 72-h readings were calculated using the following formula: MR = sum of reactions/number of animals. As a result, the extracts from A. trifida produced medium sensitization mean responses of 11.2, 1.05, and 0.85 at 24, 48, and 72 h, respectively, whereas the extracts from A. ambrosioides produced weak sensitization mean responses of 0.1, 0.15, and 0.05 at 24, 48, and 72 h, respectively. The MR values were classified as described by Magnusson and Kligman (1971), where 0–1, 1–2, and >2 represent weak, medium strong, and strong responses, respectively.
Allergic reaction from contact with the common ragweed was tested in sesquiterpene lactone-sensitive patients in southern Sweden using samples harvested in the summer and autumn in Arkansas, USA, and Sweden (Moller et al., 2002). A previous patch testing conducted on 17 patients revealed positive results of sesquiterpene lactone mix. The plant extracts were assayed in 10-fold serial dilutions (1.0%–0.0001%) and applied on the back of participants using Finn Chambers on Scanpor tape and then removed after 48 h. All the 17 patients had pompholyx hand eczema (vesicular palmar eczema) before the study. As a result, a flare-up reaction characterized by increased itching sensation and new pompholyx vesicles was observed in five patients. Subsequently, new patch testing with the sesquiterpene lactone mix was positive in all cases. Positive results were obtained for 14, 15, and 8 patients treated with Swedish summer extract, Swedish autumn extract, and American extract, respectively. The difference in the response rates was attributed to the different extraction methods as the American samples were extracted using diethyl ether, whereas acetone/diethyl ether was used for Swedish samples.
An in vivo experiment was used to determine the protective potential of a 50% aqueous-methanolic A. maritima extract on acetaminophen-induced liver damage in two groups of rats pretreated with 100- and 200-mg/kg doses (Ahmed and Khater, 2001). The acetaminophen-treated rats exhibited significant hepatocellular damage, which was confirmed by a 12-, 13-, and 16-fold increase in the levels of ALT, AST, and ALP, respectively. In contrast, the elevated levels of these enzymes were decreased after pretreatment with A. maritima extracts. Notably, acetaminophen also caused an increase in hepatic MDA concentration, which was consequently decreased to normal levels after treatment with A. maritima extracts. The pretreatment of rats with A. maritima extracts caused 89% and 124% elevation in hepatic GSH levels.
The acute and subchronic toxicity of aqueous A. maritima extract as well as that of ambrosin (20) was evaluated on Albino Wistar rats and the mutagenic activity tested on Salmonella typhimurium strains TA97, TA98, TA100, TA1538, and TA1535 (Alard et al., 1991). Acute toxicity assay was conducted with 5–5.6 g/kg A. maritima powder, whereas subchronic toxicity test in rats was conducted with doses of 3,125, 12,500, and 50,000 ppm of leaf powder. As a result, the weight gains in the control and treated animals showed no significant difference. Similarly, no adverse effects were observed, and no macroscopic lesions were found in animal organs. In addition, no differences in urine, serum chemistry, and hematological values were observed between the two groups. Contrarily, slight toxicity of ambrosin (20) to Salmonella typhimurium at a concentration of 1000 μg/plate was detected; however, its concentration of up to 1000 μg/plate was not mutagenic on the TA98 and TA100 strains. Metabolic activation did not alter the mutagenic potential of ambrosin (20), and no mutagenicity was observed when the strains TA1538, TA100, TA97, TA98, and TA1535 were treated with A. maritima water extracts with or without metabolic activation.
In an experiment on dissociated jugular or nodose neurons, parthenin (40) and coronopilin (11) increased [Ca2+]I in 37% (n = 372) and 32% (n = 207) of cinnamaldehyde-responsive (CA+) neurons and in 6% (n = 311) and 7% (n = 197) of cinnamaldehyde-unresponsive (CA−) neurons (Božičević et al., 2017). Coronopilin (11) and parthenin (40) mixed in the ratio of 7:3 induced relaxation of murine tracheal ring samples isolated from C57BL/6 mice strain. The methacholine-constricted tracheal rings treated with the coronopilin/parthenin mixture exhibited significant relaxation, likely due to the activation of bitter receptors (T2Rs) via the Ca2+-mediated activation of the large-conductance Ca2+-dependent K+ channel (BKCa), and subsequent hyperpolarization and smooth muscle relaxation (Devillier et al., 2015).
A study on the determination of in vitro bioactivity of cumanin (17) isolated from aerial parts of A. psilostachya revealed a dose-dependent inhibition of nitrite production in a NO production assay, with an IC50 value of 9.38 μM (Lastra et al., 2004). The MTT assay corroborated that macrophages treated with cumanin (17) retained their cellular viability with all the tested doses, which suggested that the influence on nitrite production was not a result of cytotoxicity.
6. Conclusions and future prospects
In the present review, we summarize the phytochemistry and biological activities of sesquiterpenes isolated from the genus Ambrosia. A total of 158 different sesquiterpenes, including guaiane, pseudoguaiane, seco-pseudoguaiane, daucane, germacrane, eudesmane, oplopane, clavane, and aromadendrane-type secondary metabolites, have been isolated from 23 species since 1950. Among these, pseudoguaianes are the most characteristic and abundant subclass of terpenoids, representing 57 compounds, whereas eudesmanes are the most diverse group that contains several types of compound derivatives. In contrast, clovane and the aromadendrane-type sesquiterpenes are rare in the genus, both represented by a single compound detected in two different species. Similarly, only a few dimeric and glycosylated derivatives have so far been identified from the genus Ambrosia. Most sesquiterpene compounds were isolated from the aerial parts of plants, which corroborates the observation that the key enzymes of the sesquiterpene biosynthesis are localized in the smooth endoplasmic reticulum of secretory cells of capitate glandular trichomes located on the leaf surface (Champagne and Boutry, 2016; Padilla-Gonzalez et al., 2016). Few or no phytochemical investigations on sesquiterpene content have been conducted on root samples. Most of the phytochemical analyses have been widely conducted on A. artemisiifolia and A. psilostachya species, and a high number of compounds as well as nearly all representative sesquiterpene carbon skeleton families have been isolated from these plants.
The current pharmacological experiments with Ambrosia sesquiterpenes encompass the assessment of their antiproliferative, cytotoxic, antimicrobial, anti-inflammatory, larvicidal, molluscicidal, schistomicidal, and antiprotozoal activities. An investigation of Ambrosia SLs is very promising owing to their antiproliferative and cytotoxic activities. Numerous studies have reported that the cytotoxic effects of these compounds can be associated with the presence of a C-11/C-13 exocyclic double bond conjugated to the γ-lactone ring, which can be enhanced by the presence of other functional groups, such as epoxide, hydroxyl, unsaturated ketone, or O-acyl. Moreover, Ambrosia SLs are effective against T. cruzi, S. mansoni, P. falciparum, and L. amazonensis and can therefore be used as model compounds in the drug development against tropical parasitic diseases. A. maritima is considered as one of the most effective molluscicidal and schistomicidal plants and the only studied species from the genus Ambrosia.
The centers of diversity and origin of the Ambrosia genus are the northwestern Mexico and the Sonoran Desert. However, only a few species were used by native Americans for medicinal purposes, whereas the genus is not part of the folk medicine in Europe. Although the Ambrosia SLs and their antiproliferative effects have been widely examined on several tumor cell lines, only half of the species have been studied phytochemically and pharmacologically. Other bioactivities, such as antiprotozoal and anti-inflammatory effects, might also be promising. Future studies should focus on the extensive phytochemical discovery in the genus, and pharmacological analyzes should be accompanied by the elucidation of mechanisms of action and determination of the relationships between the structures and their activities.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the University of Szeged Open Access Fund.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abadome F., Geerts S., Kumar V. Evaluation of the activity of Ambrosia maritima L. against Schistosoma mansoni infection in mice. J. Ethnopharmacol. 1994;44:195–198. doi: 10.1016/0378-8741(94)01186-9. [DOI] [PubMed] [Google Scholar]
- Abdel Salam N.A., Mahmoud Z.F., Ziesche J., Jakupovic J. Sesquiterpene lactones from Ambrosia maritima (Damssissa) Phytochemistry. 1984;23:2851–2853. [Google Scholar]
- Abdelgaleil S.A.M., Badawy M.E.I., Suganuma T., Kitahara K. Antifungal and biochemical effects of pseudoguaianolide sesquiterpenes isolated from Ambrosia maritima L. Afr. J. Microbiol. Res. 2011;5:3385–3392. [Google Scholar]
- Abdel-Haleem A.A. Molluscicidal impacts of some Egyptian plant extracts on protein and DNA-contents of two snail-vectors of schistosomiasis, using electrophoresis. J. Basic Appl. Zoology. 2013;66:34–40. [Google Scholar]
- Abu-Shady H., Soine T.O. The chemistry of Ambrosia maritima L. J. Am. Pharm. Assoc. 1953;42:387–395. doi: 10.1002/jps.3030420702. [DOI] [PubMed] [Google Scholar]
- Adekenov S.M. Sesquiterpene lactones with unusual structure. Their biogenesis and biological activity. Fitoterapia. 2017;121:16–30. doi: 10.1016/j.fitote.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Ahmed M.B., Khater M.R. Evaluation of the protective potential of Ambrosia maritima extract on acetaminophen-induced liver damage. J. Ethnopharmacol. 2001;75:169–174. doi: 10.1016/s0378-8741(00)00400-1. [DOI] [PubMed] [Google Scholar]
- Alard F., Stievenart C., Vanparys Ph, et al. Toxicity and mutagenicity of the molluscicidal plant Ambrosia maritima L. Drug Chem. Toxicol. 1991;14:353–373. doi: 10.3109/01480549109011639. [DOI] [PubMed] [Google Scholar]
- Amrehn E., Heller A., Spring O. Capitate glandular trichomes of Helianthus annuus (Asteraceae): ultrastructure and cytological development. Protoplasma. 2014;251:161–167. doi: 10.1007/s00709-013-0534-7. [DOI] [PubMed] [Google Scholar]
- An J.-P., Ha T.K.Q., Kim H.W., et al. Eudesmane glycosides from Ambrosia artemisiifolia (common ragweed) as potential neuroprotective agents. J. Nat. Prod. 2019;82:1128–1138. doi: 10.1021/acs.jnatprod.8b00841. [DOI] [PubMed] [Google Scholar]
- Aponte J., Yang H., Vaisberg A., et al. Cytotoxic and anti-infective sesquiterpenes present in Plagiochila disticha (plagiochilaceae) and Ambrosia peruviana (Asteraceae) Planta Med. 2010;76:705–707. doi: 10.1055/s-0029-1240681. [DOI] [PubMed] [Google Scholar]
- Arjunan N.K., Murugan K., Rejeeth C., et al. Green synthesis of silver nanoparticles for the control of mosquito vectors of malaria, filariasis, and dengue. Vector Borne Zoonotic Dis. 2012;12:262–268. doi: 10.1089/vbz.2011.0661. [DOI] [PubMed] [Google Scholar]
- Athar A., Courtney D., Chibuike C.U. New chemical constituents of Ambrosia psilostachya. Arkivoc. 2007 [Google Scholar]
- Bachelier A., Mayer R., Klein C.D. Sesquiterpene lactones are potent and irreversible inhibitors of the antibacterial target enzyme MurA. Bioorg. Med. Chem. Lett. 2006;16:5605–5609. doi: 10.1016/j.bmcl.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Badawy M.E.I., Abdelgaleil S.A.M., Suganuma T., Fuji M. Antibacterial and biochemical activity of pseudoguaianolide sesquiterpenes isolated from Ambrosia maritima against plant pathogenic bacteria. Plant Protect. Sci. 2014;50:64–69. [Google Scholar]
- Barrera P., Sülsen V.P., Lozano E., et al. Natural sesquiterpene lactones induce oxidative stress in Leishmania mexicana. Evid. base Compl. Alternative Med. 2013;2013:1–6. doi: 10.1155/2013/163404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero A.F., Oltra J.E., Álvarez M., et al. New sources and antifungal activity of sesquiterpene lactones. Fitoterapia. 2000;71:60–64. doi: 10.1016/s0367-326x(99)00122-7. [DOI] [PubMed] [Google Scholar]
- Beechan C.M., Djerassi C., Eggert H. Terpenoids-LXXIV. Tetrahedron. 1978;34:2503–2508. [Google Scholar]
- Beer M.F., Bivona A.E., Sánchez Alberti A., et al. Preparation of sesquiterpene lactone derivatives: cytotoxic activity and selectivity of action. Molecules. 2019;24:1113. doi: 10.3390/molecules24061113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belot J., Bornarel P., Diouf M., Polderman A.M. Ambrosia maritima L: molluscicidal effects on the local snails Lymnea natalensis, Bulinus forskalii, Bulinus globosus and Biomphalaria pfeifferi from Senegal. Plant Sci. 1991;74:167–170. [Google Scholar]
- Belot J., Geerts S., Sarr S., Polderman A.M. Field trials to control schistosome intermediate hosts by the plant molluscicide Ambrosia maritima L. in the Senegal River Basin. Acta Trop. 1993;52:275–282. doi: 10.1016/0001-706x(93)90012-z. [DOI] [PubMed] [Google Scholar]
- Bianchi E., Culvenor C., Loder J. Psilostachyin, a cytotoxic constituent of Ambrosia artemsisiifolia L. Aust. J. Chem. 1968;21:1109. [Google Scholar]
- Bick J.A., Lange B.M. Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Arch. Biochem. Biophys. 2003;415:146–154. doi: 10.1016/s0003-9861(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Błoszyk E., Rychłewska U., Szczepanska B., et al. Sesquiterpene Lactones of Ambrosia artemisiifolia L. and Ambrosia trifida L. Species. Collect. Czech. Chem. Commun. 1992;57:1092–1102. [Google Scholar]
- Bode A.U.D., Adewunmi C.O., Dörfler G., Becker W. The effects of extracts from Tetrapleura tetraptera (Taub.) and Bayluscide® on cells and tissue structures of Biomphalaria glabrata (Say) J. Ethnopharmacol. 1996;50:103–113. doi: 10.1016/0378-8741(95)01341-5. [DOI] [PubMed] [Google Scholar]
- Borges J., Manresa M.T., Martín J.L., et al. Altamisin, a new sesquiterpene lactone from H.B.K. Tetrahedron Lett. 1978;19:1513–1514. [Google Scholar]
- Borges-del-Castillo J., Bradley-Delso A., Manresa-Ferrero Ma T., et al. A sesquiterpenoid lactone from Ambrosia cumanensis. Phytochemistry. 1983;22:782–783. [Google Scholar]
- Božičević A., De Mieri M., Nassenstein C., et al. Secondary metabolites in allergic plant pollen samples modulate afferent neurons and murine tracheal rings. J. Nat. Prod. 2017;80:2953–2961. doi: 10.1021/acs.jnatprod.7b00495. [DOI] [PubMed] [Google Scholar]
- Cantrell C., Franzblau S., Fischer N. Antimycobacterial plant terpenoids. Planta Med. 2001;67:685–694. doi: 10.1055/s-2001-18365. [DOI] [PubMed] [Google Scholar]
- Carraz M., Lavergne C., Jullian V., et al. Antiproliferative activity and phenotypic modification induced by selected Peruvian medicinal plants on human hepatocellular carcinoma Hep3B cells. J. Ethnopharmacol. 2015;166:185–199. doi: 10.1016/j.jep.2015.02.028. [DOI] [PubMed] [Google Scholar]
- Champagne A., Boutry M. Proteomics of terpenoid biosynthesis and secretion in trichomes of higher plant species. Biochim. Biophys. Acta, Proteins Proteomics. 2016;1864:1039–1049. doi: 10.1016/j.bbapap.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Chawla B., Madhubala R. Drug targets in Leishmania. J. Parasit. Dis. 2010;34:1–13. doi: 10.1007/s12639-010-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Bean M.F., Chambers C., et al. Arrivacins, novel pseudoguaianolide esters with potent angiotensin II binding activity from Ambrosia psilostachya. Tetrahedron. 1991;47:4869–4878. [Google Scholar]
- Chiaramonte M.G., Frank F.M., Furer G.M., et al. Polymerase chain reaction reveals Trypanosoma cruzi infection suspected by serology in cutaneous and mucocutaneous leishmaniasis patients. Acta Trop. 1999;72:295–308. doi: 10.1016/s0001-706x(99)00005-4. [DOI] [PubMed] [Google Scholar]
- Chib R., Shah B.A., Anand N., et al. Psilostachyin, acetylated pseudoguaianolides and their analogues: preparation and evaluation of their anti-inflammatory potential. Bioorg. Med. Chem. Lett. 2011;21:4847–4851. doi: 10.1016/j.bmcl.2011.06.037. [DOI] [PubMed] [Google Scholar]
- Coronado-Aceves E.W., Velázquez C., Robles-Zepeda R.E., et al. Reynosin and santamarine: two sesquiterpene lactones from Ambrosia confertiflora with bactericidal activity against clinical strains of Mycobacterium tuberculosis. Pharm. Biol. 2016;54:2623–2628. doi: 10.3109/13880209.2016.1173067. [DOI] [PubMed] [Google Scholar]
- Cotugno R., Fortunato R., Santoro A., et al. Effect of sesquiterpene lactone coronopilin on leukaemia cell population growth, cell type-specific induction of apoptosis and mitotic catastrophe: effect of sesquiterpene lactone coronopilin on leukaemia. Cell Prolif. 2012;45:53–65. doi: 10.1111/j.1365-2184.2011.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva C.F., Batista D. da GJ., De Araújo J.S., et al. Activities of psilostachyin A and cynaropicrin against trypanosoma cruzi in vitro and in vivo. Antimicrob. Agents Chemother. 2013;57:5307–5314. doi: 10.1128/AAC.00595-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J.P., de O. Santos A.J., da S. Guedes M.L., et al. Sesquiterpene Lactones from Ambrosia artemisiaefolia (Asteraceae) Pharm. Biol. 1999;37:165–168. [Google Scholar]
- De Leo M., Saltos M.B.V., Puente B.F.N., et al. Sesquiterpenes and diterpenes from Ambrosia arborescens. Phytochemistry. 2010;71:804–809. doi: 10.1016/j.phytochem.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Delgado G., Chavez M.I., Alvarez L. New seco-pseudoguaianolides and other terpenoids from a population of Ambrosia confertiflora. J. Nat. Prod. 1988;51:83–87. [Google Scholar]
- Delgado V.S., Suarez D.P., Cesari I.M., Incani R.N. Experimental chemotherapy of Schistosoma mansoni with praziquantel and oxamniquine: differential effect of single or combined formulations of drugs on various strains and on both sexes of the parasite. Parasitol. Res. 1992;78:648–654. doi: 10.1007/BF00931515. [DOI] [PubMed] [Google Scholar]
- Devillier P., Naline E., Grassin-Delyle S. The pharmacology of bitter taste receptors and their role in human airways. Pharmacol. Ther. 2015;155:11–21. doi: 10.1016/j.pharmthera.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Díaz-Chiguer D.L., Márquez-Navarro A., Nogueda-Torres B., et al. In vitro and in vivo trypanocidal activity of some benzimidazole derivatives against two strains of Trypanosoma cruzi. Acta Trop. 2012;122:108–112. doi: 10.1016/j.actatropica.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Ding W., Huang R., Zhou Z., Li Y. New sesquiterpenoids from Ambrosia artemisiifolia L. Molecules. 2015;20:4450–4459. doi: 10.3390/molecules20034450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doskotch R.W., Hufford C.D. Damsin, the cytotoxic principle of Ambrosia ambrosioides (Cav.) payne. J. Pharm. Sci. 1969;58(2):186–188. doi: 10.1002/jps.2600580208. [DOI] [PubMed] [Google Scholar]
- Duschak V., Couto A. Cruzipain, the major cysteine protease of Trypanosoma cruzi: a sulfated glycoprotein antigen as relevant candidate for vaccine development and drug target. A Review. CMC. 2009;16:3174–3202. doi: 10.2174/092986709788802971. [DOI] [PubMed] [Google Scholar]
- El-Sawy M.F., Bassiouny H.K., El-Magdoub A.I. Biological combat of schistosomiasis Ambrosia maritima (Damsissa) for snail control. J. Egypt. Soc. Parasitol. 1981;11:99–117. [PubMed] [Google Scholar]
- Fischer N.H., Mabry T.J., Kagan H.B. The structure of tamaulipin-A. Tetrahedron. 1968;24:4091–4097. [Google Scholar]
- Fischer N.H., Olivier E.J., Fischer H.D. Progress in the Chemistry of Organic Natural Products. Springer-Verlag; Wien: 1979. The biogeneisis and chemistry of sesquiterpene lactones; pp. 48–114. [Google Scholar]
- Frank F.M., Fernández M.M., Taranto N.J., et al. Characterization of human infection by Leishmania spp. in the Northwest of Argentina: immune response, double infection with Trypanosoma cruzi and species of Leishmania involved. Parasitology. 2003;126:31–39. doi: 10.1017/s0031182002002585. [DOI] [PubMed] [Google Scholar]
- Frederick C.S. The Botanica Review. The New York Botanical Garden; 1982. Sesquiterpene lactones as taxonomic characters in the Asteracea; pp. 121–592. [Google Scholar]
- Geerts S., Belot J., Sabbe F., et al. Ambrosia maritima: effects on molluscs and non-target organisms. J. Ethnopharmacol. 1991;33:1–12. doi: 10.1016/0378-8741(91)90153-5. [DOI] [PubMed] [Google Scholar]
- Geerts S., Van Blerk K., Triest L. Effect of Ambrosia maritima on Anopheles stephensi and Aedes aegypti. J. Ethnopharmacol. 1994;42:7–11. doi: 10.1016/0378-8741(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Geissman T.A., Griffin S., Waddell T.G., Chen H.H. Sesquiterpene lactones. Some new constituents of Ambrosia species: A. psilostachya and A. acanthicarpa. Phytochemistry. 1969;8:145–150. [Google Scholar]
- Geissman T.A., Levy J. Sesquiterpenoid lactones. Phytochemistry. 1967;6:899–900. [Google Scholar]
- Geissman T.A., Lucas A.J., Saitoh T., Payne W.W. Sesquiterpene lactones of Ambrosia chamissonis. Biochem. Syst. Ecol. 1973;1:13–20. [Google Scholar]
- Geissman T.A., Matsueda S. Sesquiterpene lactones. Constituents of diploid and polyploid Ambrosia dumosa Gray. Phytochemistry. 1968;7:1613–1621. [Google Scholar]
- Geissman T.A., Turley R.J. Sesquiterpene lactones. Coronopilic acid. J. Org. Chem. 1964;29:2553–2555. [Google Scholar]
- Gerber E., Schaffner U., Gassmann A., et al. Prospects for biological control of Ambrosia artemisiifolia in Europe: learning from the past: biological control of Ambrosia in Europe. Weed Res. 2011;51:559–573. [Google Scholar]
- Goldsby G., Burke B.A. Sesquiterpene lactones and a sesquiterpene diol from Jamaican Ambrosia peruviana. Phytochemistry. 1987;26:1059–1063. [Google Scholar]
- Herz W. In: Natural Product Chemistry. Rahman A., editor. Springer Berlin Heidelberg; Berlin, Heidelberg: 1986. Biogenetic aspects of sesquiterpene lactone chemistry; pp. 154–174. [Google Scholar]
- Herz W., Anderson G., Gibaja S., Raulais D. Sesquiterpene lactones of some Ambrosia species. Phytochemistry. 1969;8:877–881. [Google Scholar]
- Herz W., Chikamatsu H., Tether L.R. Constituents of Ambrosia ilicifolia (gray) Payne. J. Org. Chem. 1966;31:1632–1634. [Google Scholar]
- Herz W., Fitzhenry B., Anderson G.D. 5,4′-Dihydroxy-3,7-dimethoxyflavone from Ambrosia eriocentra. Phytochemistry. 1973;12:1181–1182. [Google Scholar]
- Herz W., Gage D., Kumar N. Damsinic acid and ambrosanolides from vegetative Ambrosia hispida. Phytochemistry. 1981;20:1601–1604. [Google Scholar]
- Herz W., Högenauer G. Isolation and structure of coronopilin, a new sesquiterpene lactone. J. Org. Chem. 1961;26:5011–5013. [Google Scholar]
- Heywood V.H., Harborne J.B., Turner B.L., editors. The biology and chemistry of the Compositae. Academic Press; London: 1977. [Google Scholar]
- Huang R., Ding W., Zhou Z., Li Y. A novel eudesmane sesquiterpene glycoside from Ambrosia artemisiifolia L. Biochem. Syst. Ecol. 2014;57:363–366. [Google Scholar]
- Iamonico D. Invasive Species Compendium. 2016. Ambrosia trifida (giant ragweed)https://www.cabi.org/isc/datasheet/4693#50C56CDA-ED19-467D-A403-9D9DE62545D5 [Google Scholar]
- Inayama S., Ohkura T., Kawamata T., et al. Ambrosic acid, a new irritant principle isolated from Ambrosia arthemisiifolia. Toxicon. 1975;13:99. [Google Scholar]
- Inayama S., Ohkura T., Kawamata T., Yanagita M. Ambrosic acid, a new irritant principle from Ambrosia arthemisiifolia. Chem. Pharm. Bull. 1974;22:1435–1437. [Google Scholar]
- Iskander G.M., Modawi B.M., Ahmed H.E., et al. Crystal and molecular structure of ambrosin and damsin, sesquiterpene lactone isolates of Ambrosia maritima L. J. Prakt. Chem. 1988;330:182–190. [Google Scholar]
- Izumi E., Morello L.G., Ueda-Nakamura T., et al. Trypanosoma cruzi: antiprotozoal activity of parthenolide obtained from Tanacetum parthenium (L.) Schultz Bip. (Asteraceae, Compositae) against epimastigote and amastigote forms. Exp. Parasitol. 2008;118:324–330. doi: 10.1016/j.exppara.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Jakupovic J., Jaensch M., Bohlmann F., Dillon M.O. Eudesmanolides, 5,10-bis-epi-eudesmanes and oplopanone derivatives from Ambrosia artemisioides. Phytochemistry. 1988;27:3551–3556. [Google Scholar]
- Jakupovic J., Sun H., Geerts S., Bohlmann F. New pseudoguaianolides from Ambrosia maritima. Planta Med. 1987;53:49–51. doi: 10.1055/s-2006-962617. [DOI] [PubMed] [Google Scholar]
- Jean B. second ed. Lavoisier Publishing; Paris: 1999. Pharmacognosy Phytochemistry Medicinal Plants. [Google Scholar]
- Jimenez-Usuga N. del S., Malafronte N., Cotugno R., et al. New sesquiterpene lactones from Ambrosia cumanensis Kunth. Fitoterapia. 2016;113:170–174. doi: 10.1016/j.fitote.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Jordan P., Webbe G. Pitman Press; Bath, UK: 1982. Schistosomiasis: Epidemiology, treatment and control. [Google Scholar]
- Joseph-Nathan P., Romo J. Isolation and structure of peruvin. Tetrahedron. 1966;22:1723–1728. [Google Scholar]
- Klayman D.L. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228:1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- Kloos H., Sidrak W., Michael A.A., et al. Disease concepts and treatment practices relating to Schistosomiasis haematobium in Upper Egypt. J. Trop. Med. Hyg. 1982;85:99–107. [PubMed] [Google Scholar]
- Knoche H., Ourisson G., Perold G.W., et al. Allergenic component of a liverwort: a sesquiterpene lactone. Science. 1969;166:239–240. doi: 10.1126/science.166.3902.239. [DOI] [PubMed] [Google Scholar]
- Koji N., Toshio G., Sho I., et al., editors. Natural Product Chemistry. Kodansha Scientific Books; Tokyo: 1974. [Google Scholar]
- Konaklieva M.I., Plotkin B.J. Lactones: generic inhibitors of enzymes? Mini Rev. Med. Chem. 2005;5:73–95. doi: 10.2174/1389557053402828. [DOI] [PubMed] [Google Scholar]
- Lastra A.L., Ramírez T.O., Salazar L., et al. The ambrosanolide cumanin inhibits macrophage nitric oxide synthesis: some structural considerations. J. Ethnopharmacol. 2004;95:221–227. doi: 10.1016/j.jep.2004.07.020. [DOI] [PubMed] [Google Scholar]
- León de la Luz J.L., Rebman J.P. Una nueva ambrosia (Asteraceae) de la Península de Baja California, México. Bot. Sci. 2019;86:65. [Google Scholar]
- Lh́omme M.F., Geissman T.A., Yoshioka H., et al. The structure of chamissonin. Tetrahedron Lett. 1969;10:3161–3163. [Google Scholar]
- Li Z., Howell K., Fang Z., Zhang P. Sesquiterpenes in grapes and wines: occurrence, biosynthesis, functionality, and influence of winemaking processes. Compr. Rev. Food Sci. Food Saf. 2020;19:247–281. doi: 10.1111/1541-4337.12516. [DOI] [PubMed] [Google Scholar]
- Lohberger B., Rinner B., Stuendl N., et al. Sesquiterpene lactones downregulate G2/M cell cycle regulator proteins and affect the invasive potential of human soft tissue sarcoma cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabry T.J., Miller H.E., Kagan H.B., Renold W. The structure of psilostachyin, a new sesquiterpene dilactone from Ambroisia psilostachya. Tetrahedron. 1966;22:1139–1146. [Google Scholar]
- Mabry T.J., Renold W., Miller H.E., Kagan H.B. The structure of ambrosiol. A new sesquiterpene lactone from Ambrosia psilostachya. J. Org. Chem. 1966;31:681–684. [Google Scholar]
- Magnusson B., Kligman A. Allergic contact Dermatitis in the Guinea pig. Identifications of contact allergens. Ann. Intern. Med. 1971;74:468. [Google Scholar]
- Mahmoud A.A., Ahmed A.A., Bassuony A.A.E. A new chlorosesquiterpene lactone from Ambrosia maritima. Fitoterapia. 1999;70:575–578. [Google Scholar]
- Mendes D.G., Lauria-Pires L., Nitz N., et al. Exposure to mixed asymptomatic infections with Trypanosoma cruzi, Leishmania braziliensis and Leishmania chagasi in the human population of the greater Amazon: human exposure to mixed kinetoplastid infections. Trop. Med. Int. Health. 2007;12:629–636. doi: 10.1111/j.1365-3156.2007.01831.x. [DOI] [PubMed] [Google Scholar]
- Miller H.E., Kagan H.B., Renold W., Mabry T.J. Psilostachyin, a new type of sesquiterpene lactone. Tetrahedron Lett. 1965;6:3397–3403. [Google Scholar]
- Miller H.E., Mabry T.J. 3-Hydroxydamsin, a new pseudoguaianolide from Ambrosia psilostachya. J. Org. Chem. 1967;32:2929–2931. [Google Scholar]
- Mishra J., Saxena A., Singh S. Chemotherapy of leishmaniasis: past, present and future. Comput. Mater. Continua (CMC) 2007;14:1153–1169. doi: 10.2174/092986707780362862. [DOI] [PubMed] [Google Scholar]
- Molinaro F., Monterumici C.M., Ferrero A., et al. Bioherbicidal activity of a germacranolide sesquiterpene dilactone from Ambrosia artemisiifolia L. J. Environ. Sci. Health (Part B) 2016;51:847–852. doi: 10.1080/03601234.2016.1208466. [DOI] [PubMed] [Google Scholar]
- Molinaro F., Tyc O., Beekwilder J., et al. The effect of isabelin, a sesquiterpene lactone from Ambrosia artemisiifolia on soil microorganisms and human pathogens. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 2018;365 doi: 10.1093/femsle/fny001. [DOI] [PubMed] [Google Scholar]
- Moller H., Spiren A., Svensson A., et al. Contact allergy to the Asteraceae plant Ambrosia artemisiifolia L. (ragweed) in sesquiterpenelactone-sensitive patients in southern Sweden. Contact Dermatitis. 2002;47:157–160. doi: 10.1034/j.1600-0536.2002.470306.x. [DOI] [PubMed] [Google Scholar]
- Morejón B., Pilaquinga F., Domenech F., et al. Larvicidal activity of silver nanoparticles synthesized using extracts of Ambrosia arborescens (Asteraceae) to control Aedes aegypti L. (Diptera: Culicidae) J. Nanotechnol. 2018;2018:1–8. [Google Scholar]
- Oberti J.C., Silva G.L., Sosa V.E., et al. Ambrosanolides and secoambrosanolides from Ambrosia tenuifolia. Phytochemistry. 1986;25:1355–1358. [Google Scholar]
- Ohmoto T., Ikeda K., Nomura S., et al. Studies on the sesquiterpenes from Ambrosia elatior Linne. Chem. Pharm. Bull. 1987;35:2272–2279. [Google Scholar]
- Orofino Kreuger M.R., Grootjans S., Biavatti M.W., et al. Sesquiterpene lactones as drugs with multiple targets in cancer treatment: focus on parthenolide. Anti Cancer Drugs. 2012;23:883–896. doi: 10.1097/CAD.0b013e328356cad9. [DOI] [PubMed] [Google Scholar]
- Padilla-Gonzalez G.F., dos Santos F.A., Da Costa F.B. Sesquiterpene lactones: more than protective plant compounds with high toxicity. Crit. Rev. Plant Sci. 2016;35:18–37. [Google Scholar]
- Payne W.W. Notes on the ragweeds of South America with the description of two new species: Ambrosia pannosa and A. parvifolia (Compositae) Brittonia. 1966;18:28. [Google Scholar]
- Payne W.W. A re-evaluation of the genus Ambrosia (Compositae) J. Arnold Arboretum. 1964;45:401–438. [Google Scholar]
- Payne W.W. Biochemistry and species problems in ambrosia (Asteraceae-Ambrosieae) Plant Systemat. Evol. 1976;125:169–178. [Google Scholar]
- Parkhomenko A.Yu., Andreeva O.A., Oganesyan E.T., Ivashev M.N. Ambrosia artemisiifolia as a source of biologically active substances. Pharm. Chem. J. 2005;39:149–153. [Google Scholar]
- Payne W.W. The morphology of the inflorescence of ragweeds (Ambrosia-Franseria : Compositae) Am. J. Bot. 1963;50:872–880. [Google Scholar]
- Payne W.W., Geissman T.A., Lucas A.J., Saitoh T. Chemosystematics and taxonomy of Ambrosia chamissonis. Biochem. Syst. Ecol. 1973;1:21–33. [Google Scholar]
- Payne W.W., Scora R.W., Kumamoto J. The volatile oils of Ambrosia (Compositae: Ambrosieae) Brittonia. 1972;24:189. [Google Scholar]
- Perera W.H., Meepa K.M., Fronczek F.R., et al. Bioassay-guided isolation and structure elucidation of fungicidal and herbicidal compounds from Ambrosia salsola (Asteraceae) Molecules. 2019;24 doi: 10.3390/molecules24050835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez G.R.M. Anti-inflammatory activity of Ambrosia artemisiaefolia and Rhoeo spathacea. Phytomedicine. 1996;3:163–167. doi: 10.1016/S0944-7113(96)80030-4. [DOI] [PubMed] [Google Scholar]
- Picman A.K. Biological activities of sesquiterpene lactones. Biochem. Systemat. Ecol. 1986;14:255–281. [Google Scholar]
- Popiel I., Erasmus D.A. Schistosoma mansoni: ultrastructure of adults from mice treated with oxamniquine. Exp. Parasitol. 1984;58:254–262. doi: 10.1016/0014-4894(84)90042-0. [DOI] [PubMed] [Google Scholar]
- Porter T.H., Mabry T.J. Sesquiterpene lactones. Constituents of Ambrosia artemisiifolia L. (Compositae) Phytochemistry. 1969;8:793–794. [Google Scholar]
- Porter T.H., Mabry T.J., Yoshioka H., Fischer N.H. The isolation and structure determination of artemisiifolin, a new germacranolide from Ambrosia artemisiifolia L. (Compositae) Phytochemistry. 1970;9:199–204. [Google Scholar]
- Potter J.L., Mabry T.J. Origin of the Texas gulf coast island populations of Ambrosia psilostachya: a numerical study using terpenoid data. Phytochemistry. 1972;11:715–723. [Google Scholar]
- Raszeja W., Gill St. Isolation and identification of psilostachyin B from Ambrosia artemisiifolia L. Planta Med. 1977;32:319–322. doi: 10.1055/s-0028-1097606. [DOI] [PubMed] [Google Scholar]
- Raven P.H., Kyhos D.W., Breedlove D.E., Payne W.W. Polyploidy in Ambrosia dumosa (Compositae: Ambrosieae) Brittonia. 1968;20:205. [Google Scholar]
- Robles-Zepeda R.E., Coronado-Aceves E.W., Velázquez-Contreras C.A., et al. In vitro anti-mycobacterial activity of nine medicinal plants used by ethnic groups in Sonora, Mexico. BMC Compl. Alternative Med. 2013;13:329. doi: 10.1186/1472-6882-13-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo J., Rodríguez-Hahn L. Canambrin, a new sesquiterpene dilactone from Ambrosia canescens. Phytochemistry. 1970;9:1611–1614. [Google Scholar]
- Romo J., Romo de Vivar A., Díaz E. The guaianolides of Ambrosia cumanensis HBK. Tetrahedron. 1968;24:5625–5631. [Google Scholar]
- Saeed M., Jacob S., Sandjo L.P., et al. Cytotoxicity of the sesquiterpene lactones neoambrosin and damsin from Ambrosia maritima against multidrug-resistant cancer cells. Front. Pharmacol. 2015;6 doi: 10.3389/fphar.2015.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T., Nour A., Khalid S., et al. Quantitative structure ‒ antiprotozoal activity relationships of sesquiterpene lactones. Molecules. 2009;14:2062–2076. doi: 10.3390/molecules14062062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman F.C., Mabry T.J. Sesquiterpene lactone patterns in diploid and polyploid Ambrosia dumosa. Biochem. Systemat. Ecol. 1979;7:7–12. [Google Scholar]
- Seaman F.C., Mabry T.J. Sesquiterpene lactones of diploid and tetraploid Ambrosia camphorata. Biochem. Systemat. Ecol. 1979;7:3–6. [Google Scholar]
- Shoeb H.A., El-Emam M.A. The molluscicidal properties of natural products from Ambrosia maritima. Egypt. J. Bilharz. 1976;3:157–167. [PubMed] [Google Scholar]
- Silva G.L., Oberti J.C., Herz W. Sesquiterpene lactones and other constituents of argentine Ambrosia species. Phytochemistry. 1992;31:859–861. [Google Scholar]
- Slacanin I., Vargas D., Marston A., Hostettmann K. Determination of molluscicidal sesquiterpene lactones from Ambrosia maritima (Compositae) J. Chromatogr. A. 1988;457:325–331. doi: 10.1016/s0021-9673(01)82080-x. [DOI] [PubMed] [Google Scholar]
- Soeiro M.N.C., de Castro S.L. Trypanosoma cruzi targets for new chemotherapeutic approaches. Expert Opin. Ther. Targets. 2009;13:105–121. doi: 10.1517/14728220802623881. [DOI] [PubMed] [Google Scholar]
- Solujić S., Sukdolak S., Vuković N., et al. Chemical composition and biological activity of the acetone extract of Ambrosia artemisiifolia L. pollen. J. Serb. Chem. Soc. 2008;73:1039–1049. [Google Scholar]
- Sotillo W.S., Villagomez R., Smiljanic S., et al. Anti-cancer stem cell activity of a sesquiterpene lactone isolated from Ambrosia arborescens and of a synthetic derivative. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanović M., Aljančić-Solaja I., Milosavljević S. A 34-seco-ambrosanolide from Ambrosia artemisiifolia. Phytochemistry. 1987;26:850–852. [Google Scholar]
- Sturgeon C.M., Craig K., Brown C., et al. Modulation of the G 2 cell cycle checkpoint by sesquiterpene lactones psilostachyins A and C isolated from the common ragweed Ambrosia artemisiifolia. Planta Med. 2005;71:938–943. doi: 10.1055/s-2005-873109. [DOI] [PubMed] [Google Scholar]
- Subramaniam J., Murugan K., Panneerselvam C., et al. Eco-friendly control of malaria and arbovirus vectors using the mosquitofish Gambusia affinis and ultra-low dosages of Mimusops elengi-synthesized silver nanoparticles: towards an integrative approach? Environ. Sci. Pollut. Res. 2015;22:20067–20083. doi: 10.1007/s11356-015-5253-5. [DOI] [PubMed] [Google Scholar]
- Sülsen V., Barrera P., Muschietti L., et al. Antiproliferative effect and ultrastructural alterations induced by psilostachyin on Trypanosoma cruzi. Molecules. 2010;15:545–553. doi: 10.3390/molecules15010545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sülsen V., Güida C., Coussio J., et al. In vitro evaluation of trypanocidal activity in plants used in Argentine traditional medicine. Parasitol. Res. 2006;98:370–374. doi: 10.1007/s00436-005-0060-4. [DOI] [PubMed] [Google Scholar]
- Sülsen V., Gutierrez Yappu D., Laurella L., et al. In vitro antiplasmodial activity of sesquiterpene lactones from Ambrosia tenuifolia. Evid. base Compl. Alternative Med. 2011;2011:1–4. doi: 10.1155/2011/352938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sülsen V.P., Frank F.M., Cazorla S.I., et al. Trypanocidal and leishmanicidal activities of sesquiterpene lactones from Ambrosia tenuifolia Sprengel (Asteraceae) AAC. 2008;52:2415–2419. doi: 10.1128/AAC.01630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sülsen V.P., Muschietti L.V., Martino V.S., et al. Trypanocidal and leishmanicidal activities of flavonoids from Argentine medicinal plants. Am. J. Trop. Med. Hyg. 2007;77:654–659. [PubMed] [Google Scholar]
- Sülsen V.P., Puente V., Papademetrio D., et al. Mode of action of the sesquiterpene lactones psilostachyin and psilostachyin C on Trypanosoma cruzi. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson D., Lozano M., Almanza G.R., et al. Sesquiterpene lactones from Ambrosia arborescens Mill. inhibit pro-inflammatory cytokine expression and modulate NF-κB signaling in human skin cells. Phytomedicine. 2018;50:118–126. doi: 10.1016/j.phymed.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Taglialatela-Scafati O., Pollastro F., Minassi A., et al. Sesquiterpenoids from common ragweed (Ambrosia artemisiifolia L.), an invasive biological polluter. Eur. J. Org. Chem. 2012;2012:5162–5170. [Google Scholar]
- Tiansheng L., Parodi F.J., Vargas D., et al. Sesquiterpenes and thiarubrines from Ambrosia trifida and its transformed roots. Phytochemistry. 1993;33:113–116. [Google Scholar]
- Vargas D., Fronczek F.R., Fischer N.H., Hostettmann K. The chemistry of confertiflorin and the molecular structure of confertiflorin and allodesacetylconfertiflorin, two Molluscicidal sesquiterpene lactones. J. Nat. Prod. 1986;49:133–138. [Google Scholar]
- Vichnewski W., Shuhama I.K., Rasanske R.C., Herz W. Granilin and ivasperin from Ambrosia polystachya. 13C-NMR spectra of hydroxylated isoalantones. Phytochemistry. 1976;15:1531–1532. [Google Scholar]
- Vidotto F., Tesio F., Ferrero A. Allelopathic effects of Ambrosia artemisiifolia L. in the invasive process. Crop Protect. 2013;54:161–167. [Google Scholar]
- Villagomez R., Rodrigo G.C., Collado I.G., et al. Multiple anticancer effects of damsin and coronopilin isolated from Ambrosia arborescens on cell cultures. Anticancer Res. 2013;33:3799–3805. [PubMed] [Google Scholar]
- Vos T., Allen C., Arora M., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W.H., Beals T.F. Perennial ragweeds (ambrosia) in Michigan, with the description of a new, intermediate taxon. Rhodora. 1958;60:177–204. [Google Scholar]
- Wu X., Zhu H., Yan J., et al. Santamarine inhibits NF- κ B activation and induces mitochondrial apoptosis in A549 lung adenocarcinoma cells via oxidative stress. BioMed Res. Int. 2017;2017:1–11. doi: 10.1155/2017/4734127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoke Marchant Y., Balza F., Abeysekera B.F., Towers G.H.N. Molluscicidal activity of sesquiterpene lactones. Biochem. Systemat. Ecol. 1984;12:285–286. [Google Scholar]
- Yoshioka H., Mabry T.J. The structure and chemistry of isabelin. Tetrahedron. 1969;25:4767–4779. [Google Scholar]
- Yoshioka H., Mabry T.J., Miller H.E. Isabelin, a novel germacranolide dilactone from Ambrosia psilostachya DC. Chem. Commun. (London) 1968:1679–1681. [Google Scholar]
- Yoshioka H., Renold W., Fischer N.H., et al. Sesquiterpene lactones from Ambrosia confertiflora (compositae) Phytochemistry. 1970;9:823–832. [Google Scholar]
- Zeller W., de Gols M., Hausen B.M. The sensitizing capacity of Compositae plants: VI. Guinea pig sensitization experiments with ornamental plants and weeds using different methods. Arch. Dermatol. Res. 1984;277:28–35. doi: 10.1007/BF00406478. [DOI] [PubMed] [Google Scholar]
- Zhang P., Fuentes S., Siebert T., et al. Terpene evolution during the development of Vitis vinifera L. cv. Shiraz grapes. Food Chem. 2016;204:463–474. doi: 10.1016/j.foodchem.2016.02.125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.