Abstract
Objective:
The present study examined device-measured physical activity levels and sedentary behavior participation during different times of the day (i.e., morning, midday, and evening) in adults with multiple sclerosis (MS) who differed in fatigue status.
Design:
Cross-sectional, survey.
Setting:
Remote survey study managed by a university-based research laboratory.
Participants:
A population-based sample of 1,000 participants with MS were sent recruitment materials by the North American Research Committee on Multiple Sclerosis.
Main Outcome Measures:
Participants (N=218) completed the Fatigue Severity Scale (FSS) as a measure of fatigue severity and were divided into subgroups of fatigued (FSS score ≥4) and non-fatigued (FSS score <4). Participants wore an ActiGraph GT3X+ on the non-dominant hip for seven days for measuring physical activity(i.e., light physical activity[LPA], moderate-to-vigorous physical activity[MVPA], steps) and sedentary behavior.
Results:
Fatigued participants engaged in less MVPA(F(1,216)=18.5, p<0.001), fewer steps(F(1,216)=27.8, p<0.001) and more sedentary time (F(1,216)=8.2, p=0.005) than non-fatigued participants. Regardless of group, the highest levels of LPA(F(1.7,355.7)=72.9, p<0.001) and MVPA(F(1.8,395.3)=23.0,p<0.001) occurred in the morning and middle of the day, with the lowest levels in the evening. Regardless of group, the highest levels of sedentary behavior occurred in the evening with similar levels in the morning and evening(F(1.6,354.5)=84.3,p<0.001). Regardless of group, participants had more steps in the middle of the day followed by the morning and then the evening(F(1.8,383.9)=84.7,p<0.001).
Conclusions:
Our results suggest that physical activity timing should be considered in future development and delivery of behavior interventions that focus on increasing physical activity and reducing sedentary behavior among adults with MS who have fatigue.
Keywords: fatigue, multiple sclerosis, physical activity, sedentary behavior
Introduction
Multiple sclerosis (MS) is an inflammatory, neurodegenerative disease of the central nervous system with a prevalence of nearly 1 million adults in the United States1 and 2.8 million people worldwide.2 Fatigue is reported by 81% of adults with MS3 making it one of the most common consequences of this disease. Fatigue is further burdensome based on its association with outcomes of physical and cognitive function, anxiety, depression, sleep dysfunction, and quality of life.4–6 This makes fatigue a common target of symptom management in MS,7 and physical activity represents a promising approach for managing fatigue in adults with MS,8–11 yet the presence of fatigue likely influences engagement in approaches for symptom management such as physical activity.
Adults with severe MS-related fatigue participate in lower levels of physical activity and higher levels of sedentary behavior than people with MS without fatigue.12 This is alarming as adults with MS participate in significantly lower levels of physical activity than healthy controls, wherein one study estimated that only 20% of adults with MS meet public health guidelines for daily rates of health-promoting physical activity.13 Overall, adults with MS, particularly those with severe MS-related fatigue, may not be engaging in sufficient levels of physical activity necessary for managing the symptom of fatigue.
The low rates of physical activity participation in adults with fatigue may be associated with a reactionary reduction of activity levels resulting from or in anticipation of fatigue (i.e., those with higher levels of fatigue may avoid or limit activity to prevent an increase in fatigue symptoms). Adults with MS-related fatigue are often encouraged to utilize energy conservation strategies, and these focus on prioritization and optimization of available energy for more important tasks/activities. Although energy conservation methods may provide short-term benefits for fatigue symptoms,14 one study reported that use of activity pacing strategies in adults with MS-related fatigue was associated with lower physical activity levels overall.15 Perhaps people with MS who use energy conservation strategies may be limiting physical activity participation in response to the anticipation of increased fatigue, and this may reduce the likelihood of experiencing the beneficial effects of physical activity on MS-related fatigue.
The timing of physical activity participation may further be an important consideration, as fatigue may increase throughout the course of the day. One study indicated that fatigue levels are highest in the middle of the day (i.e., 12:00–13:00) and afternoon (i.e., 15:00–16:00) compared with in the morning; however, that study reported no significant effect of time of day on walking capacity, and this suggests that while fatigue levels may increase during the day, walking capacity remains stable.16 To date, we are unaware of a study that has examined rates of physical activity and sedentary behavior across different time periods of day in persons with MS who have elevated levels of fatigue. The examination of physical activity patterns throughout the day in those with and without MS-related fatigue may inform the design and delivery of behavioral interventions targeting an increase in physical activity and a reduction in sedentary behavior in adults with MS, particularly for managing fatigue.
The present study examined device-measured physical activity levels and sedentary behavior participation during different times of the day (i.e., morning, midday, and evening) among adults with MS who differed in levels of fatigue. Based on the aforementioned literature, we expected that participants with fatigue would participate in less daily physical activity and more daily sedentary behavior than non-fatigued participants.12 We further anticipated lower physical activity in the middle of the day and afternoon when fatigue may be higher than in the morning when fatigue levels may be lower.16
Methods
Participants
The present study consisted of a secondary analysis of data from a larger study examining patterns of sedentary behavior in adults with MS.17 That study recruited persons with MS nationally through the North American Research Committee on Multiple Sclerosis (NARCOMS) patient registry. NARCOMS sent recruitment materials to a random sample of 1,000 people who had completed the fall 2017 survey. Interested persons (N=296) contacted the research team and were assessed for the inclusion criteria: 18 years of age or older, diagnosis of MS, and member of the NARCOMS registry. Of those who returned materials (N=275), 218 persons with MS completed all relevant study procedures and were included in the current study. No formal sample size calculations were performed.
Fatigue Severity
The Fatigue Severity Scale (FSS) is a nine-item questionnaire assessing the impact of fatigue on motivation, exercise, physical function, completing tasks, and interference with work, family or social life over the past four weeks.18 The items are rated on a Likert scale from 1 (strongly disagree) to 7 (strongly agree) and individual item scores are averaged into an overall severity score, whereby lower scores indicate less fatigue in everyday life. Of note, FSS scores of four or above are indicative of severe MS-related fatigue,4, 19 and participants were divided into subgroups of fatigued (FSS≥ 4) and non-fatigued (FSS< 4).
Device-measured Activity Participation
An ActiGraph GT3X+ accelerometer worn on a belt around the waist during waking hours for a seven-day period provided device-measured outcomes of physical activity and sedentary behavior. The GT3X+ model of ActiGraph accelerometer has acceptable accuracy in MS across a range of walking speed and levels of neurological disability, including persons who walk with canes or rollators.20 Raw data were processed and scored by trained staff with the ActiLife software as band-pass filtered and vector magnitudes of the x, y, and z axes were digitally integrated as counts per minute and presented as an average of the valid days.21 Daily wear time was estimated using the Troiano 2007 algorithm in the ActiLife software, and participants recorded the times they wore the monitor in a diary daily for verifying wear time. A day of wear was considered valid if there was a minimum of 10 hours (i.e., 600 minutes) of total wear time without continuous zeros exceeding 30 minutes, and participants with 1 or more valid days of data in each time period (i.e., morning, midday, evening) were included in the analyses. We further processed data per hour of each valid day of wear time and aggregated the total of each hour into time period buckets of morning (i.e., 6:00 AM-11:59 AM), midday (i.e., 12:00 PM-5:59 PM), and evening (i.e., 6:00 PM-11:59 PM),22 wherein an hour of wear was included in analysis if the monitor registered a minimum wear time of 30 minutes within the given hour.
Step counts.
Step count data were processed without the low frequency extension (LFE) available in ActiLife, as there is evidence to suggest that steps in free-living conditions are overestimated using the LFE filter with this model of ActiGraph.23 Step counts are presented as the daily average (steps/day) and total steps in each time-of-day period averaged for the seven-day period.
Physical activity and sedentary behavior.
Data from the accelerometers were processed with LFE and quantified as average minutes per day of sedentary time, light physical activity (LPA), and moderate-to-vigorous physical activity (MVPA), based on MS-specific cut-points.24 We further quantified the total minutes of LPA, MVPA, and sedentary time in each time-of-day period and then calculated the percentage of time spent in each time-of-day period (i.e., morning, midday, evening) as a function of wear time by dividing the total minutes in each time period by the total wear time in that time period (e.g., percentage of morning LPA = minutes of morning LPA / minutes of morning wear time × 100).
Demographics and Clinical Characteristics
Participants completed a questionnaire for demographic and clinical characteristics (i.e., age, gender, race, employment status, MS type, and disease duration) and provided a list of medications, including any MS-related disease-modifying treatments (DMTs). Participants further completed the single-item, self-reported Patient Determined Disease Steps (PDDS) scale as a valid measure of disability status.25
Procedure
The procedure was approved by the University’s Institutional Review Board and all participants provided written informed consent. Participants were mailed a packet containing the informed consent document, questionnaires, accelerometer and wear time log, as well as a pre-stamped and pre-addressed envelope for return service. Participants completed the questionnaires and wore the monitor for a seven-day period. Participants returned a copy of the signed informed consent along with the study materials through the United States Postal Service. All participants were remunerated upon completion of the study.
Statistical Analyses
All data were analyzed in SPSS Version 26 (IBM Corporation, Armonk, NY), and all analyses were interpreted with an a priori p-value of 0.05. Descriptive statistics are reported as mean and standard deviation (SD) for continuous variables, median and interquartile range[IQR] for ordinal variables, and number and percentage for dichotomous variables. We examined frequency distributions and conducted Shapiro-Wilks analysis for establishing normality of the variables, whereby a p-value of >0.05 was indicative of a normal distribution. There were no missing data. We examined differences in demographic and clinical characteristics between fatigue severity subgroups (i.e., non-fatigued and fatigued) with independent samples t-tests for normally distributed continuous variables, Mann Whitney-U tests for non-normally distributed continuous variables, and Chi-squared tests for dichotomous variables, whereby an a priori alpha level of 0.05 indicated a significant difference between groups. We further conducted two-way mixed-factor analysis of variance (ANOVA) with group (i.e., fatigued and non-fatigued) as the between-subjects factor and time (i.e., morning, midday, and evening) as the within-subjects factor on the dependent variables of wear time, LPA, MVPA, steps, and sedentary time.
Results
Participants
The summary of participant demographic and clinical characteristics for the overall sample (N=218) and fatigue severity subgroups is presented in Table 1. The sample had an average age of 59.3 ± 10.1 years and was primarily female (82%), Caucasian (96%), and unemployed (61%). Regarding clinical characteristics, the sample primarily had relapsing-remitting MS (70%) with an average disease duration of 19.9 ± 9.3 years, a median PDDS score of 3 [IQR=4], and 84% of participants reported taking a DMT. Regarding fatigue, 67% (n=146) of the sample classified as fatigued (FSS≥ 4) and the fatigued group had significantly higher disability (p<0.001) compared with the non-fatigued group (n=72); there were no other significant differences between groups in demographic or clinical variables. We did not control for PDDS (disability) in the analyses, as fatigue and disability were strongly correlated and the control of disability would likely attenuate the differences in fatigue groups, as the control of FSS (fatigue) would attenuate differences in physical activity between disability groupings.
Table 1:
Participant characteristics for the overall sample and fatigue severity subgroups
| Variable | Overall Sample (N=218) |
Non-Fatigued (n=72) |
Fatigued (n=146) |
p-value |
|---|---|---|---|---|
| Age (years) | 59.3 ± 10.1 | 60.5 ± 9.1 | 58.7 ± 10.6 | 0.297* |
| Gender (n (%) Female) | 179 (82%) | 58 (81%) | 121 (83%) | 0.674 |
| Race (n (%)) | 0.458 | |||
| Caucasian | 209 (96%) | 69 (96%) | 140 (96%) | — |
| Latino/Latina | 4 (2%) | 1 (1%) | 3 (2%) | — |
| Black/African American | 2 (1%) | 0 (0%) | 2 (1%) | — |
| Employed (n (%)) | 84 (39%) | 34 (47%) | 50 (35%) | 0.082 |
| MS Type (n (%)) | 0.339 | |||
| Relapsing-remitting MS | 153 (70%) | 53 (74%) | 100 (69%) | — |
| Secondary Progressive MS | 38 (17%) | 12 (17%) | 26 (18%) | — |
| Primary Progressive MS | 24 (11%) | 5 (7%) | 19 (13%) | — |
| MS Disease Duration (years) | 19.9 ± 9.3 | 20.4 ± 8.5 | 19.7 ± 9.7 | 0.515* |
| PDDS (median [IQR]) | 3 [4.0] | 1.0 [3.0] | 3.0 [4.0] | <0.001 |
| DMT Use (n (%)) | 184 (84%) | 60 (83%) | 124 (85%) | 0.935 |
| FSS Score | 4.6 ± 1.7 | 2.5 ± 0.9 | 5.6 ± 0.8 | <0.001* |
Notes: Values are presented as mean ± standard deviation unless otherwise specified; Non-fatigued based on an FSS score of < 4; Fatigued based on an FSS score ≥ 4; *] Mann-Whitney U test; MS Multiple Sclerosis; PDDS Patient Determined Disease Status; DMT Disease-modifying Therapy; FSS Fatigue Severity Scale.
Accelerometer Wear Time
The summary of accelerometer wear time during different times of the day for the overall sample and by fatigue severity subgroups is presented in Table 2. There was no significant group by time-of-day interaction (F(1.3,278.5)=0.2,p=0.690), but there were significant effects of time-of-day (F(1.3,278.5)=97.9,p<0.001) and group (F(1,216)=5.2,p=0.024) for accelerometer wear time (Figure 1a). The fatigued group wore the monitor for significantly less time than the non-fatigued group, and both groups wore the monitor for significantly more time in the middle of the day than in the morning and evening periods. We therefore performed additional analyses of physical activity and sedentary time outcomes based on percentages of wear time, as this accounts for differences in wear time between groups and across time-of-day periods.
Table 2:
Physical activity and sedentary behavior participation overall and by time of day in the overall sample and by fatigue severity subgroups.
| Variable | Overall Sample (N=218) |
Non-Fatigued (n=72) |
Fatigued (n=146) |
|---|---|---|---|
| Wear Time (min/day) | 802.2 ± 204.5 | 846.7 ± 281.2 | 780.2 ± 149.7 |
| Morning Wear Time (min) | 229.7 ± 92.3 | 244.8 ± 100.8 | 222.3 ± 87.2 |
| Midday Wear Time (min) | 339.8 ± 86.3 | 358.7 ± 119.9 | 330.5 ± 62.1 |
| Evening Wear Time (min) | 232.6 ± 117.7 | 243.2 ± 160.1 | 227.4 ± 90.0 |
| Steps | 3975.4 ± 2946.1 | 5388.0 ± 3780.4 | 3278.8 ± 2124.2 |
| Morning Step Counts | 1313.0 ± 1235.9 | 1852.1 ± 1582.6 | 1047.2 ± 918.6 |
| Midday Step Counts | 1869.0 ± 1441.2 | 2484.0 ± 1857.9 | 1565.7 ± 1066.6 |
| Evening Step Counts | 793.4 ± 929.4 | 1051.9 ± 1297.6 | 665.9 ± 647.2 |
| LPA (min/day) | 241.7 ± 94.7 | 267.9 ± 119.3 | 228.8 ± 77.2 |
| Morning LPA (min) | 74.3 ± 39.3 | 82.3 ± 42.9 | 70.4 ± 36.9 |
| Midday LPA (min) | 108.5 ± 45.5 | 119.2 ± 58.2 | 103.2 ± 36.9 |
| Evening LPA (min) | 58.9 ± 39.8 | 66.4 ± 50.8 | 55.2 ± 32.5 |
| MVPA (min/day) | 15.9 ± 21.3 | 25.0 ± 29.3 | 11.5 ± 14.2 |
| Morning MVPA (min) | 5.5 ± 9.1 | 9.5 ± 13.3 | 3.6 ± 5.1 |
| Midday MVPA (min) | 7.8 ± 11.1 | 11.6 ± 15.0 | 5.9 ± 7.9 |
| Evening MVPA (min) | 2.6 ± 6.6 | 3.9 ± 9.4 | 2.0 ± 4.6 |
| Sedentary Time (min/day) | 540.5 ± 159.0 | 541.6 ± 188.9 | 539.9 ± 142.7 |
| Morning Sedentary (min) | 148.2 ± 66.6 | 148.2 ± 61.9 | 148.3 ± 69.1 |
| Midday Sedentary (min) | 221.9 ± 64.6 | 222.8 ± 83.1 | 221.4 ± 53.5 |
| Evening Sedentary (min) | 170.3 ± 90.3 | 170.6 ± 118.2 | 170.2 ± 73.1 |
Notes: Values are presented as mean ± standard deviation; Non-fatigued based on an FSS score of < 4; Fatigued based on an FSS score ≥ 4; LPA light physical activity; MVPA moderate-to-vigorous physical activity.
Figure 1: Wear time and step counts during different times of day in adults with multiple sclerosis based on fatigue severity groups. Values are mean and standard error of the mean.
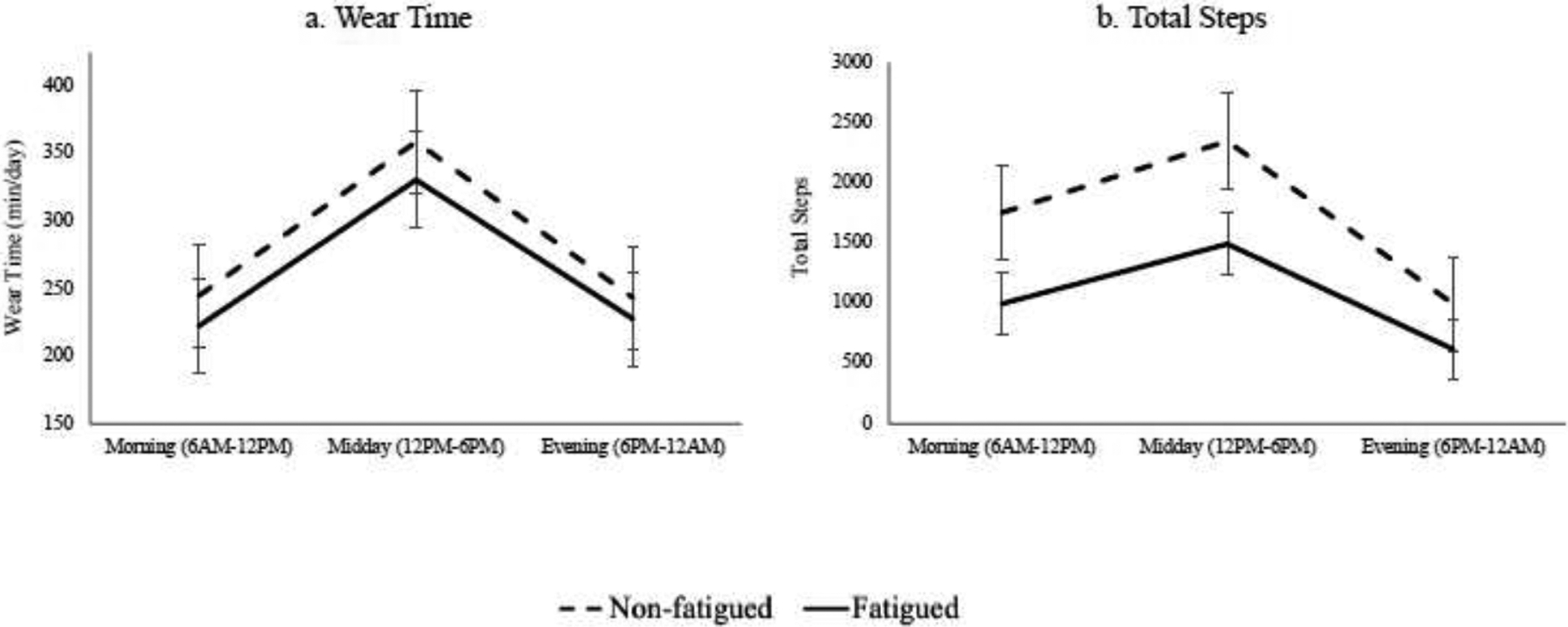
Accelerometer data during different times of day in adults with multiple sclerosis separated into fatigued (solid line) and non-fatigued (dashed line) groups. Figure 1a represents the total accelerometer wear time in minutes and Figure 1b represents total step counts during each time of day. Values are mean and standard error of the mean.
Step Counts
The summary of total step counts daily and during different times of day for the overall sample and by fatigue severity subgroups is presented in Table 2. There was a significant group by time-of-day interaction for daily steps (F(1.8,383.9)=4.9,p=0.010), whereby there was a steeper decrease in steps from midday to evening for the non-fatigued group compared with the fatigued group (Figure 1b). There was a significant effect of time-of-day on daily steps (F(1.8,383.9)=84.7,p<0.001), whereby both groups had more steps in the middle of the day followed by in the morning than in the evening. There was a significant effect of group for steps (F(1,216)=27.8,p<0.001), whereby the fatigue group had significantly fewer steps compared with the non-fatigued group.
Physical Activity Timing
The summaries of physical activity participation in total minutes and as a percentage of wear time for the overall sample and by fatigue subgroups are presented in Table 2 and Table 3, respectively. There was no significant group by time-of-day interaction for percentage of time in LPA (F(1.7,355.7)=0.7, p=0.461), but there was a significant interaction for percentage of time in MVPA (F(1.8,395.3)=6.2, p=0.003), whereby there was a progressive decrease in MVPA across the day in the non-fatigued group compared with more consistent levels in the fatigued group. There was a significant effect of time-of-day for percentage of time in LPA (F(1.7,355.7)=72.9, p<0.001) and percentage of time in MVPA (F(1.8,395.3)=23.0, p<0.001), whereby both groups spent significantly more time in LPA and MVPA in the morning and middle of the day than in the evening. There was no significant effect of group for percentage of time in LPA (F(1,216)=2.7, p=0.100), but a significant effect of group for percentage of time in MVPA (F(1,216)=18.5, p<0.001), whereby the fatigued group spent significantly less time in MVPA compared with the non-fatigued group. Percentage of time spent in LPA and MVPA during different times of day for the fatigued group and non-fatigued group are visually presented in Figure 2a–b.
Table 3:
Physical activity and sedentary behavior as a function of wear time overall and by time of day in the overall sample and by fatigue severity subgroups.
| Variable | Overall Sample (N=218) |
Non-Fatigued (n=72) |
Fatigued (n=146) |
|---|---|---|---|
| Daily LPA | 29.9 ± 9.4 | 31.3 ± 10.3 | 29.1 ± 8.9 |
| Morning LPA | 32.6 ± 11.3 | 33.6 ± 11.3 | 32.1 ± 11.3 |
| Midday LPA | 31.8 ± 10.4 | 33.3 ± 11.7 | 31.1 ± 9.6 |
| Evening LPA | 25.2 ± 10.6 | 27.2 ± 11.1 | 24.2 ± 10.2 |
| Daily MVPA | 1.9 ± 2.5 | 2.8 ± 3.4 | 1.4 ± 1.7 |
| Morning MVPA | 2.3 ± 3.8 | 3.8 ± 5.7 | 1.5 ± 2.0 |
| Midday MVPA | 2.2 ± 3.2 | 3.3 ± 4.3 | 1.7 ± 2.3 |
| Evening MVPA | 1.1 ± 2.3 | 1.4 ± 2.9 | 0.9 ± 1.9 |
| Daily Sedentary | 68.1 ± 10.8 | 65.1 ± 12.5 | 69.5 ± 9.6 |
| Morning Sedentary | 64.9 ± 12.9 | 62.0 ± 14.2 | 66.4 ± 12.0 |
| Midday Sedentary | 65.7 ± 12.2 | 62.7 ± 14.5 | 67.2 ± 10.6 |
| Evening Sedentary | 73.5 ± 11.8 | 70.7 ± 12.9 | 74.9 ± 11.0 |
Notes: Values are presented as mean ± standard deviation of the percentage of wear time. Fatigue groups were based on an FSS score of < 4 as non-fatigued and FSS score ≥ 4 as fatigued; LPA light physical activity; MVPA moderate-to-vigorous physical activity.
Figure 2: Physical activity and sedentary behavior as a function of wear time during different times of day in adults with multiple sclerosis based on fatigue severity groups. Values are mean scores and standard error of the mean.
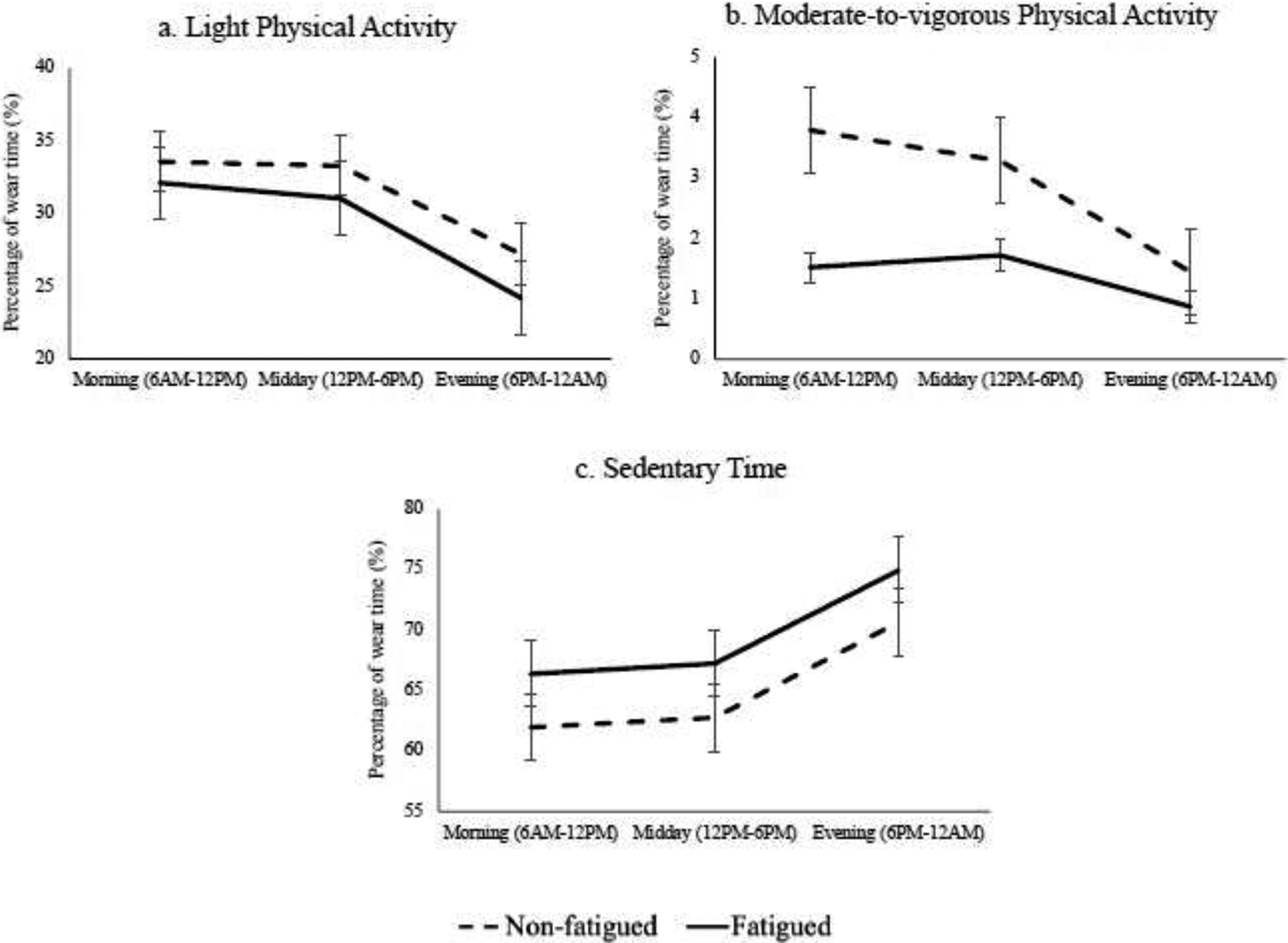
Physical activity and sedentary behavior as a function of wear time during different times of day in adults with multiple sclerosis separated into fatigued (solid line) and non-fatigued (dashed line) groups; (a) percentage of time spent in light physical activity, (b) percentage of time spent in moderate-to-vigorous physical activity, and (c) percentage of time spent sedentary. Values are mean scores and standard error of the mean.
Sedentary Behavior Timing
The summaries of sedentary time in total minutes and as a percentage of wear time for the overall sample and by fatigue subgroups are presented in Table 2 and Table 3, respectively. There was no significant group by time-of-day interaction for percentage of time in sedentary time (F(1.6,354.5)=0.01, p=0.980). There was a significant effect of time-of-day (F(1.6,354.5)=84.3, p<0.001), whereby both groups spent significantly more time in sedentary behavior in the evening than in the morning and in the middle of the day. There was a significant effect of group for percentage of time in sedentary time (F(1,216)=8.2, p=0.005), whereby the fatigued group spent significantly more time sedentary than the non-fatigued group. Percentage of sedentary time during different times of day for the fatigued group and non-fatigued group is visually presented in Figure 2c.
Discussion
The present study examined device-measured physical activity and sedentary behavior in adults with MS who were fatigued and non-fatigued as a function of time of day. Our primary results indicate: (a) fatigued participants engaged in less MVPA, more sedentary time and took fewer steps than non-fatigued participants; (b) the highest levels of LPA and MVPA occurred in the morning and middle of the day, with the lowest levels in the evening; (c) the highest levels of sedentary behavior occurred in the evening with similar levels in the morning and middle of the day; and (d) participants had more steps in the middle of the day followed by the morning and then the evening. These results suggest that physical activity and sedentary behavior timing should be considered in future development and delivery of physical activity interventions as the timing of physical activity may be impactful for improving this behavior in adults with MS who present with fatigue.
The primary novel findings of the present study suggest that the highest levels of physical activity (i.e., LPA, MVPA, and step counts) occur in the morning and middle of the day and, correspondingly, the highest levels of sedentary behavior occurred in the evening. Interestingly, adults without fatigue had a steeper decrease in MVPA and daily steps across the day, whereas adults with fatigue had similar levels of MVPA in the morning and midday and then a progressive decrease in the evening. These results suggest that those with fatigue may be practicing activity pacing (i.e., prioritizing available energy for tasks/activities) during the day and minimizing activity in the evening. This is consistent with the notion that fatigue worsens as the day progresses.16, 26 Thus, if fatigue levels are highest in the middle of day and evening, participants may be less likely to engage in physical activity and more likely to engage in sedentary behavior in an effort to conserve energy. On the other hand, participants may have increased fatigue in the evening and may not have enough energy to engage in activity later in the day after engaging in higher levels of activity in the morning and middle of the day. Indeed, a recent study reported that adults with MS respond to an increase in perceived fatigue by reducing physical activity levels.27 That study further examined the association between physical activity and fatigue severity within different times of the day and reported that higher levels of physical activity in the morning and evening were associated with lower fatigue severity during those time points, but higher levels of physical activity in the middle of the day were associated with higher fatigue severity.27 Collectively, these findings suggest that the physical activity levels and fatigue severity across different times of the day have a dynamic, bi-directional relationship. Importantly, the pattern of decreased activity and higher levels of sedentary behavior in the evenings was observed in both participants with and without fatigue, suggesting that reduced activity in the evening may not be related to fatigue severity alone. Future research should examine the relationship between physical activity timing and fatigue severity in adults with MS as increasing physical activity, particularly in the evenings when participation is lower, may be an important target for managing fatigue severity.
Our results further suggest that fatigued participants engage in less MVPA and more sedentary time with fewer total daily steps than non-fatigued participants. These findings are consistent with a previous study that demonstrated significant differences in the proportion of time spent in physical activity and sedentary between groups of fatigued and non-fatigued adults with MS.12 However, that study further indicated a diminution of significance between groups when controlling for disability status and employment status. Therefore, significant differences between fatigued and non-fatigued adults with MS may be related to the significant difference in disease severity between groups, whereby those with a higher disability status may have more impaired physical function and participate in less activity throughout the day. Future research should examine the role of other MS-related symptoms and consequences in the relationship among physical activity, sedentary behavior and fatigue as a function of time-of-day for a more comprehensive understanding of fatigue in MS.
Study Limitations
The present study is not without limitations. The cross-sectional study design precludes any inferences on causality and longitudinal studies are necessary to determine the directionality of the relationship between fatigue and physical activity and sedentary behavior across the day. There was no formal sample size calculation and a relatively small proportion of potential participants (i.e., 218 of 1000 recruited) completed data collection that may increase the risk of participation or outcome bias. We did not include additional outcomes that may be associated with physical activity participation as a result of higher disability status (e.g., physical function), and we did not control for disability in the analyses based on the dynamic, bidirectional association between disability and fatigue. Our measure of fatigue severity was obtained over the previous month and physical activity and sedentary behavior was measured over the previous week; this may limit our ability to compare these outcomes. Additionally, fatigue severity averaged over the previous month may not provide important detail regarding fatigue severity fluctuation from day-to-day or fluctuation within a day; this could be overcome in future research using experience sampling methodology along with accelerometry. We did not exclude participants who were using medications to manage fatigue, which may influence variability in fatigue throughout the day.
Conclusions
The present study describes physical activity and sedentary behavior participation in adults with MS as a function of fatigue over the course of the day. Our primary novel findings suggest that, regardless of fatigue group, the highest levels of physical activity are in the morning and middle of the day and the highest levels of sedentary behavior occur in the evening. These results suggest that physical activity timing may be an important target for behavioral interventions that focus on increasing physical activity and reducing sedentary behavior in adults with MS with severe fatigue. Clinicians and researchers utilizing behavioral interventions for increasing physical activity and reducing sedentary behavior may consider encouraging an increase in physical activity and step count levels during different time points throughout the day, particularly when levels of physical activity are low and sedentary time is high.
Highlights.
Fatigued adults engaged in less MVPA, more sedentary time and took fewer steps
The highest levels of LPA and MVPA occurred in the morning and middle of the day
The highest levels of sedentary behavior occurred in the evening
Participants had the most steps in the middle of the day
Financial Support:
This work was supported, in part, by a pilot grant [PP 1412] and a mentor-based post-doctoral fellowship from the National Multiple Sclerosis Society [MB 0011], and by the National Heart, Lung, and Blood Institute of the National Institutes of Health [T32HL110952] and the Eunice Kennedy Shriver National Institute Of Child Health & Human Development [F31HD101281]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ANOVA
analysis of variance
- DMTs
disease-modifying therapies
- FSS
Fatigue Severity Scale
- IQR
interquartile range
- LFE
low frequency extension
- LPA
light physical activity
- MS
multiple sclerosis
- MVPA
moderate-to-vigorous physical activity
- NARCOMS
North American Research Committee on Multiple Sclerosis
- PDDS
Patient Determined Disease Steps
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: The authors have no competing interests to disclose.
References
- 1.Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 2019;92(10):e1029–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Multiple Sclerosis International Federation. Atlas of MS: Mapping multiple sclerosis around the world 2020. September 24, 2020]. Available from: URL: https://www.atlasofms.org/map/global/epidemiology/number-of-people-with-ms.
- 3.Broch L, Simonsen CS, Flemmen H, Berg-Hansen P, Skardhamar Å, Ormstad H et al. High prevalence of fatigue in contemporary patients with multiple sclerosis. Mult Scler J Exp Transl Clin 2021;7(1):2055217321999826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayache SS, Chalah MA. Fatigue in multiple sclerosis - Insights into evaluation and management. Neurophysiol Clin 2017;47(2):139–71. [DOI] [PubMed] [Google Scholar]
- 5.Braley TJ, Chervin RD. Fatigue in multiple sclerosis: mechanisms, evaluation, and treatment. Sleep 2010;33(8):1061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Induruwa I, Constantinescu CS, Gran B. Fatigue in multiple sclerosis - a brief review. J Neurol Sci 2012;323(1–2):9–15. [DOI] [PubMed] [Google Scholar]
- 7.Nourbakhsh B, Revirajan N, Morris B, Cordano C, Creasman J, Manguinao M et al. Safety and efficacy of amantadine, modafinil, and methylphenidate for fatigue in multiple sclerosis: a randomised, placebo-controlled, crossover, double-blind trial. Lancet Neurol 2021;20(1):38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asano M, Finlayson ML. Meta-analysis of three different types of fatigue management interventions for people with multiple sclerosis: exercise, education, and medication. Mult Scler Int 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heine M, van de Port I, Rietberg MB, van Wegen EE, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev 2015(9):CD009956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilutti LA, Greenlee TA, Motl RW, Nickrent MS, Petruzzello SJ. Effects of exercise training on fatigue in multiple sclerosis: a meta-analysis. Psychosom Med 2013;75(6):575–80. [DOI] [PubMed] [Google Scholar]
- 11.Latimer-Cheung AE, Pilutti LA, Hicks AL, Martin Ginis KA, Fenuta AM, MacKibbon KA et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: A systematic review to inform guideline development. Arch Phys Med Rehabil 2013;94(9):1800–28. e3. [DOI] [PubMed] [Google Scholar]
- 12.Neal WN, Cederberg KL, Jeng B, Sasaki JE, Motl RW. Is Symptomatic Fatigue Associated With Physical Activity and Sedentary Behaviors Among Persons With Multiple Sclerosis? Neurorehabil Neural Repair 2020;34(6):505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klaren RE, Motl RW, Dlugonski D, Sandroff BM, Pilutti LA. Objectively quantified physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil 2013;94(12):2342–8. [DOI] [PubMed] [Google Scholar]
- 14.Blikman LJ, Huisstede BM, Kooijmans H, Stam HJ, Bussmann JB, van Meeteren J. Effectiveness of energy conservation treatment in reducing fatigue in multiple sclerosis: a systematic review and meta-analysis. Arch Phys Med Rehabil 2013;94(7):1360–76. [DOI] [PubMed] [Google Scholar]
- 15.Abonie US, Saxton J, Baker K, Hettinga FJ. Objectively-assessed physical activity and self-reported activity pacing in adults with multiple sclerosis: A pilot study. Clin Rehabil 2021:2692155211024135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feys P, Gijbels D, Romberg A, Santoyo C, Gebara B, de Noordhout BM et al. Effect of time of day on walking capacity and self-reported fatigue in persons with multiple sclerosis: a multi-center trial. Mult Scler 2012;18(3):351–7. [DOI] [PubMed] [Google Scholar]
- 17.Motl RW, Sasaki JE, Cederberg KL, Jeng B. Social-cognitive theory variables as correlates of sedentary behavior in multiple sclerosis: Preliminary evidence. Disabil Health J 2019;12(4):622–7. [DOI] [PubMed] [Google Scholar]
- 18.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46(10):1121–3. [DOI] [PubMed] [Google Scholar]
- 19.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46(10):1121–3. [DOI] [PubMed] [Google Scholar]
- 20.Sandroff BM, Motl RW, Pilutti LA, Learmonth YC, Ensari I, Dlugonski D et al. Accuracy of StepWatch and ActiGraph accelerometers for measuring steps taken among persons with multiple sclerosis. PLoS One 2014;9(4):e93511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motl RW, Zhu W, Park Y, McAuley E, Scott JA, Snook EM. Reliability of scores from physical activity monitors in adults with multiple sclerosis. Adapt Phys Activ Q 2007;24(3):245–53. [DOI] [PubMed] [Google Scholar]
- 22.Reimers AK, Heidenreich V, Bittermann HJ, Knapp G, Reimers CD. Accelerometer-measured physical activity and its impact on sleep quality in patients suffering from restless legs syndrome. BMC Neurol 2021;21(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feito Y, Garner HR, Bassett DR. Evaluation of ActiGraph’s low-frequency filter in laboratory and free-living environments. Med Sci Sports Exerc 2015;47(1):211–7. [DOI] [PubMed] [Google Scholar]
- 24.Sandroff BM, Riskin BJ, Agiovlasitis S, Motl RW. Accelerometer cut-points derived during over-ground walking in persons with mild, moderate, and severe multiple sclerosis. J Neurol Sci 2014;340(1–2):50–7. [DOI] [PubMed] [Google Scholar]
- 25.Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol 2013;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell DJH, Liossi C, Schlotz W, Moss-Morris R. Tracking daily fatigue fluctuations in multiple sclerosis: ecological momentary assessment provides unique insights. J Behav Med 2017;40(5):772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kratz AL, Fritz NE, Braley TJ, Scott EL, Foxen-Craft E, Murphy SL. Daily Temporal Associations Between Physical Activity and Symptoms in Multiple Sclerosis. Ann Behav Med 2019;53(1):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]


