Abstract
In recent years, there has been increasing recognition of the diversity of autoimmune neurological diseases affecting all levels of the nervous system. A growing understanding of disease pathogenesis has enabled us to better target specific elements of the immune system responsible for the cell dysfunction and cell destruction seen in these diseases. This is no better demonstrated than in the development of complement directed therapies for the treatment of complement mediated autoimmune neurological conditions. Herein, we describe the basic elements of the complement cascade, provide an overview of select autoimmune neurological diseases whose pathogenesis is mediated by complement, the effector system of autoantigen bound autoantibodies, and discuss the complement directed therapies trialed in the treatment of these diseases. Several complement-directed therapies have demonstrated benefit in the treatment of autoimmune neurological diseases; we also review the trials resulting in the approval of these therapies for the treatment of AChR Ab-positive myasthenia gravis (MG) and neuromyelitis spectrum disorder. Finally, on the heels of the recent successes described, we discuss possibilities for the future, including additional targeted therapies with greater ease of administration, improved risk profiles, and other possible uses for therapeutics targeting elements of the complement cascade.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01223-w.
Keywords: Anti-complement agents, NMOSD, Myasthenia gravis, Autoimmune neurologic disease
Introduction
Autoimmune neurological diseases encompass a wide range of disorders affecting the entirety of the nervous system from the muscle to the cortex and can often be severe with significant associated morbidity and mortality. Unlike many neurological diseases, however, there is significant potential for reversibility [1]. Over the last decade, there have been significant advancements in the treatment of autoimmune neurological diseases, largely driven by an increasing understanding of their pathophysiology and the subsequent development of targeted therapies [2].
The treatment of autoimmune neurological diseases has a rich history of trialing targeted therapies based on an evolving understanding of the pathogenesis of a disease. The evolution of treatments for multiple sclerosis (MS) spanning nearly 30 years is emblematic [3]. This began with the development of interferon beta (IFN-β) therapies [4]. Early trials demonstrated that there is an increased number of interferon-gamma secreting cells in the CSF of patients with MS [5]. Early trials also demonstrated that interferon-gamma provoked acute exacerbations of MS, which ceased when the drug was removed [4]. Given a recognition that IFN-β was an inhibitor of interferon-gamma, IFN-β 1B was trialed and ultimately became the first therapy approved for the treatment of relapsing remitting MS [6].
The era of monoclonal antibodies has significantly advanced our approach to therapy development. Substantial advancements in the understanding of the innate and adaptive immune systems and of the elements of the immune system integral to disease pathogenesis have made it possible to develop therapies targeted to specific elements of the immune system. The development of therapies targeting complement is an elegant example of how a better understanding of disease pathogenesis can lead to the development of a targeted treatment with significant efficacy and minimal ill effects [2].
Several complement therapies have now been studied or are being studied for use in a range of neurological diseases. There are a variety of possible therapeutic targets at various levels of the complement cascade including enzymatic blockade, supplementing or increasing endogenous complement regulators, antibodies against components of the complement cascade, smaller non-antibody molecules that block components of the complement cascade, or blockade of the binding to complement receptors [7]. Herein, we will focus on those targets for which therapeutics have been developed and are being tested or have been approved. Similarly, we will only discuss neurological diseases in which complement-targeted therapies have been trialed or are in trial, recognizing that there are additional neurological diseases in which complement has been demonstrated to play a role in pathogenesis. For a more inclusive review of complement in neurological disease and neurological therapeutics, we refer the reader to a recent review by Dalakas et al. [8].
History of Complement and Its Consideration in Therapeutics
The complement system was first described in 1888 by Nuttall, and the term complement was later coined by Ehrlich in 1899, reflecting a perception that it played a supportive role in the immune system [7]. The complement system has subsequently been investigated with waxing and waning enthusiasm, with much controversy and notable disagreements, likely related to the complexity of the system and the evolution of laboratory techniques over time [9].
The earliest indication that manipulation of the complement system may be of therapeutic benefit was in 1902 when the anti-complement activity of cobra venom was first described [10]. Cobra venom works through a series of steps eventually producing a stable C3 convertase that consumes all plasma C3, eliminating all functional complement. The effect however is short lived, as an immune response directed at the venom results in neutralizing antibodies. Due in part to this discovery, there is a history of public enthusiasm for the use of snake venom in the treatment of MS [11, 12].
Overview of the Complement System
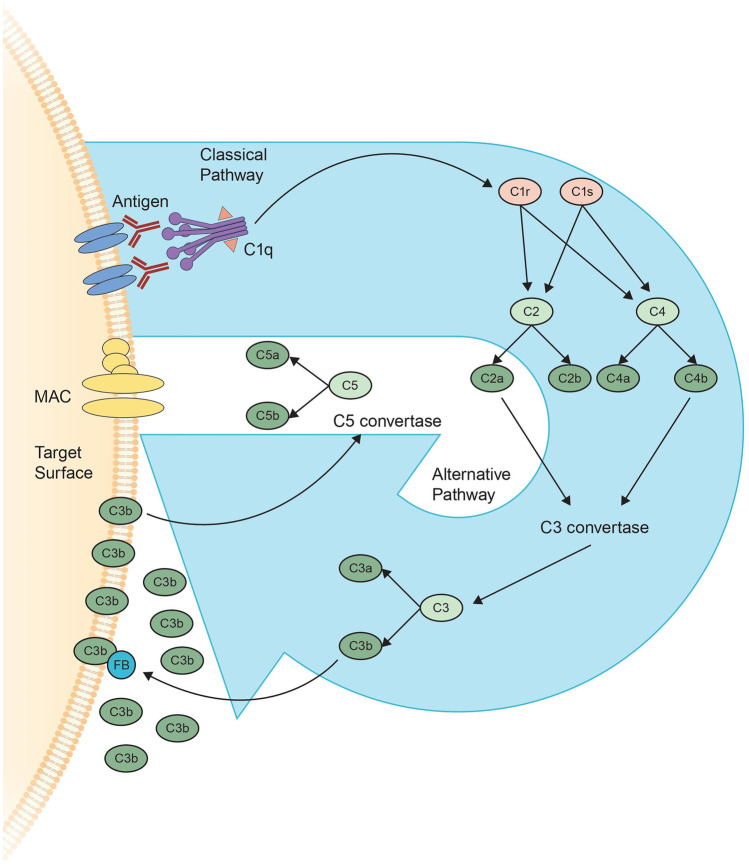
Interest in the complement system has increased again in recent years and has been extensively reviewed [8, 13]. What follows is a brief overview (Fig. 1).
Fig. 1.
The complement cascade consists of 3 pathways; the classical pathway, the alternative pathway and the lectin pathway (not depicted). The classical pathway begins with binding of the antibody to its target antigen resulting in the generation of active forms of C1r and C1s. These 2 proteases cleave C2 and C4 resulting in the formation of the C3 convertase (which consists of C2a and C4b). C3 convertase cleaves C3, generating C3a and C3b. C3b interacts with C3 convertase forming C5 convertase which cleaves C5, generating C5a and C5b, the latter of which forms part of the membrane attack complex (MAC). In the alternative pathway, C3b interacts with factor B (FB), which through an amplification loop results in increased C3b generating additional C5 convertase and ultimately MAC. Note is made that some of the elements of the complement cascade are surface associated although are not depicted as such in the figure, for simplicity of the illustration
The complement system is made up of more than 30 proteins produced by the liver, which work together allowing for the recognition, tagging, and elimination of intruders and foreign or apoptotic cells, resulting in downstream immune responses. The complement system is a key player of the innate immune system, is integral in the maintenance and regulation of immune reactions, and is essential in supporting the actions of antibodies. Pathologically, any process that results in a tip of the fine balance between activation and regulation can result in self-attack. An error in activation or insufficient regulation of the complement cascade can turn its destructive action against the host’s cells. As many autoimmune diseases are thought to be caused or supported by unchecked complement, inhibition or modulation of complement activity is becoming an important therapeutic strategy [14].
Complement proteins (Table 1) circulate through the blood in native form and together contribute to a series of steps known as the complement cascade. The end products of this cascade are membrane attack complexes (MACs) which are inserted into cells walls, leading to cell death.
Table 1.
Selected proteins involved in the complement cascade
| Recognition and binding | |
| Binds to antigen–antibody complexes | C1q |
| Recognize2 non-antibody associated structure | Lectins, ficolins |
| Effector components | |
| Activating enzymes | C1r, C1s, C2b, Factor B, MASPs |
| Membrane-binding opsonins | C4b, C3b |
| Membrane attack | C5b, C6, C7, C8, C9 |
| Complement receptors | CR1, CR2, C5aR1 |
| Regulator components | |
| Regulatory proteins | CR1, MCP, DAF, factor H, CD59, C4BP, FH, FHL-1, CD55, CF1 |
Mannose-associated serine proteases (MASPs); complement receptor 1 and 2 (CR1, CR2); C5a receptor 1 (C5aR1), membrane cofactor protein (MCP); decay accelerating factor (DAF), C4 binding protein (C4BP); factor H (FH); factor H like protein 1 (FHL-1); complement factor 1 (CF1)
There are three complement activation pathways. The classical pathway, so named because it was described first, relies on the binding of the C1q protein by an antibody made in response to an antigen. It is strongly initiated by IgM or IgG1, IgG2, or IgG3 clusters. The surface binding of C1q results in the activation of proteases C1r and C1s; C1s is primarily responsible for cleaving C4 into C4a and C4b, resulting in opsonization of the antigen by C4b. The C1s protease also cleaves C2 into C2a and C2b and mediates the formation of classical C3 convertase (C2aC4b) which cleaves C3 ultimately resulting in the formation of MAC.
The alternative pathway involves the direct binding of complement components to the surface of pathogens without involvement of antibodies. The central molecule of the alternative pathway is C3. C3 is cleaved into C3a and C3b. C3b attaches to components of the target surface (opsonization) which is then amplified in foreign cells and regulated on human cells. Opsonization by C3b leads to the binding of factor B and conversion into the alternative pathway’s C3 convertase C3bBbP, which cleaves more C3 into C3b and amplifies the complement response. The alternative pathway also involves a molecule termed properdin, which binds to the C3bBb complex on the pathogen’s surface and propagates the complement response by attracting more C3b and stabilizing the C3 convertase complexes. The alternative pathway through its amplification loop, is thought to account for up to 80% of total complement activation. Ultimately, the activation of the alternative pathway results in an increased density of deposited C3b gradually leading to the formation of convertases that contain an additional C3b molecule resulting in a shift from C3 convertases to C5 convertases which cleave C5 to C5a and C5b. C5b associates with C6 and C7 and inserts into cell membranes where it interacts with C8 and several units of C9 forming MAC. C5a (as well as C3a) are anaphylatoxins that induce inflammation by attracting additional immune system components, and activate multiple other immune cells, including neutrophils. The pro-inflammatory actions of C5a occur primarily via binding to C5a receptor 1.
The third pathway, termed the lectin pathway, involves the binding of other complement proteins, termed lectins and ficolins, directly to components on the surface of pathogens, without the involvement of antibodies. Lectins and ficolins predominantly recognize carbohydrate patterns and assemble with proteases that are structurally similar to C1s (termed mannose-associated serine proteases or MASPs) resulting in the cleavage of C4 and C2, generating the same C3 convertase as the classical pathway.
All three of these pathways converge on the cleavage of C3 into its active fragments C3a and C3b by the C3 convertases and result in the generation of MAC. In addition, opsonization by C1q and C3b, as well as other proteins, facilitates the action of phagocytic cells expressing complement receptors, which helps mobilize innate defense mechanisms. On B cells, binding of antigens decorated by C3 breakdown products to the CR2/CD19 co-receptor complex significantly lowers activation threshold and has an important role in B cell maturation. Binding of complement to complement receptors CR1 and CR2 on follicular dendritic cells helps with the selection of memory B cells with the highest affinity for the target antigen. Complement receptors and regulators also contribute to modulating IL-12 in antigen presenting cells and influence the activation and differentiation of T cells. For example, activated macrophages require intact C5aR signaling to promote the differentiation of CD4 + T cells into TH17 cells; in vivo, this can produce experimental autoimmune encephalitis and depends on synergistic induction of IL-6 by C5aR and several Toll-like receptors (another class of pattern recognition receptors) [15].
In addition to these activation pathways (and additional activation pathways not described), the complement system also contains regulatory proteins that aid in limiting and terminating the process. On healthy human cells, complement activation and amplification is attenuated by soluble and surface-bound regulators that accelerate the decay of convertases (CR1, DAF, factor H, CD55), cleave cell bound or soluble C3b and C4b (CF1), act as cofactors for the degradation of C3b and C4b (CR1, MCP, factor H), or prevent the formation of the MAC (CD59). Soluble regulators such as C4BP, FH, and FHL-1 also recognize self components and further impair activation.
Complement in Neurological Autoimmune Disease
As noted in a recent review of complement in neurological disorders by Dalakas et al., there are a growing number of neurological diseases which are demonstrated to be mediated by complement activation [8]. Of these, there are a number which are autoimmune in etiology, including dermatomyositis (Fig. 2A) and necrotizing autoimmune myositis (Fig. 2B), AChR Ab-positive myasthenia gravis (MG) (Fig. 2C), Guillain–Barre syndrome (GBS) and chronic inflammatory demyelinating polyneuropathy variants (Fig. 2D), and neuromyelitis optic spectrum disorders (NMOSD) (Fig. 2E).
Fig. 2.
Complement is demonstrated to be involved in the immunopathogenesis of several neurological autoimmune diseases. In dermatomyositis, initiation of the complement cascade results in MAC being deposited in capillary endothelium resulting in destruction of capillaries and muscle fiber ischemia. In immune mediated necrotizing myopathy, MAC is deposited directly into the muscle cell wall resulting in muscle fiber necrosis. In AChR antibody positive myasthenia gravis, binding of antibodies to the acetylcholine receptor results in MAC formation with resultant damage to the muscle endplate as well as increased internalization and degradation of acetylcholine receptors. In Guillain Barre Syndrome, MAC is deposited on Schwann cells whereas in acute motor axonal neuropathy and multifocal motor neuropathy, MAC is deposited on nodal and paranodal regions of the axon. In AQP4 positive neuromyelitis spectrum disorder, MAC is deposited on astrocytes with bystander injury resulting in MAC deposited on neuronal cell membranes as well as generation of C5a resulting in activation and recruitment of neutrophils to the site, an increase in reactive astrocytes, and further immune mediated destruction of nearby cells
Dermatomyositis
It has been recognized for over 100 years that the pathophysiology of dermatomyositis involves the infiltration of perimysial blood vessels in muscle by lymphocytes [16]. Further elucidation of the pathophysiology of this disease was achieved with the demonstration of MAC in the intramuscular microvasculature of patients with dermatomyositis [17]. Later studies revealed MAC deposits in muscle capillaries early in the disease, suggesting that a complement mediated vasculopathy is what drives disease pathogenesis [18, 19]. Recent studies proposed immunopathogenesis involving activation of the complement cascade by distinct autoantibodies, resulting in MAC deposition in endothelial cells of capillaries and ischemia of muscle fibers, and further triggering of down-stream immune processes [16, 20]. In contrast, polymyositis and inclusion-body myositis are T cell mediated [20] and as such, are not amenable to treatment by complement directed therapies.
Necrotizing Autoimmune Myositis
In 2003, immune mediated necrotizing myopathy (IMNM) was first defined as distinct from other inflammatory myopathies [21]. IMNMs are most often associated with antibodies against signal recognition particle (SRP) or against 3-hydroxy-3- methylglutaryl–coenzyme A reductase (HMGCR) [20, 22], both of which are pathogenic antibodies [23, 24]. Analysis of biopsies from patients with anti-SRP + and anti-HMGCR + IMNM reveals MAC in sarcolemma of muscle fibers as well as C1q and IgG deposits, supporting the assertion that activation of the classical complement pathway is involved in muscle fiber necrosis in these 2 diseases [25].
AChR Ab-Positive MG
The activation of complement via disease-specific autoantibodies to the acetylcholine receptor has been known for over four decades and is well-studied. As early as 1960, changes in serum complement activity were demonstrated in patients with MG [26]. In rats depleted of complement with cobra venom factor, there was no destruction of AChR seen after passive transfer of antibodies, demonstrating that complement was critical in the pathogenicity of AChR antibodies [27]. Evidence from human studies have demonstrated aggregation of MAC in muscle endplates of human patients [28]. Acetylcholine receptor antibodies bind C1q, activate the complement cascade, and lead to MAC generation. AchR-specific autoantibodies can activate the complement cascade as they are IgG1 and IgG3 isotypes. However, a role for complement is not demonstrated in MG associated with other autoantibodies. Anti-muscle-specific kinase antibodies, for example, are of the IgG4 isotype which do not bind complement [29]. As such, complement directed treatment trials have only included AChR Ab positive patients.
GBS, CIDP, and Related Disorders
Many subtypes of GBS have been described with some subtypes clearly associated with specific autoantibodies. Specific antiganglioside antibodies can predict clinical manifestations of the disease and disease course [30, 31]. Pathological studies have demonstrated MAC on Schwann cells in early AIDP [32]. In AMAN, IgG and complement are deposited on nodal and internodal regions of the axon [33]. Similarly, a growing number of specific autoantibodies are being discovered in CIDP with antibody and complement mediated pathophysiologies described [34, 35]. Multifocal motor neuropathy (MMN) is a rare immune mediated neuropathy commonly associated with anti-GM1 antibodies with complement mediated damage at nodal and paranodal regions leading to axonal degeneration [36]. Some of the antibodies found in association with CIDP, however, such as contactin 1, contactin-associated protein 1 and neurofascin 155, are of the IgG4 subtype and therefore unable to activate complement [37]. As such, similar to myasthenia gravis, complement therapies may not be efficacious in all subtypes of these CIDP clinical syndromes.
NMOSD
AQP4 was the first autoantibody discovered for CNS demyelinating disease [38, 39]. Further clarification of the pathogenic outcomes of the interaction of the antibody and its target revealed that IgG1 is predominantly involved, which triggers the complement cascade [40]. Additionally, attacks of NMO are associated with a rise in complement activation values in the sera of patients compared to controls, which demonstrates the role of complement in disease pathogenesis [41]. It has been demonstrated that both AQP4 IgG and complement are necessary to produce the CNS injury characteristic of NMO [42, 43]. Evidence of the deposition of the complement MAC on neurons, but not C1q which was only seen on astrocytes, supported a mechanism for complement bystander injury to neurons following AQP4 IgG targeted astrocyte death [44, 45]. AQP4-IgG mediated astrocyte destruction is dependent on the assembly of cell-surface AQP4 tetramers into multimeric autoantibody complexes where denser AQP4-IgG binding enhances interactions with C1q, resulting in classical complement activation and overwhelming complement inhibition [46]. But this only accounts for some of the pathology seen in NMOSD; release of C5a results in preactivation of neutrophils which are recruited to the astrocyte resulting in destabilization and reversal of the glutamate pump, and ultimately an increase in extracellular glutamate concentrations [47]. C5a induced neutrophil activation also results in a shift of remaining astrocytes to reactive astrocytes which produce multiple pro-inflammatory cytokines and chemokines, perpetuating the immune-mediated destruction of surrounding cells [47].
Complement Therapies in Trial or in Clinical Use for the Treatment of Autoimmune Neurological Disease
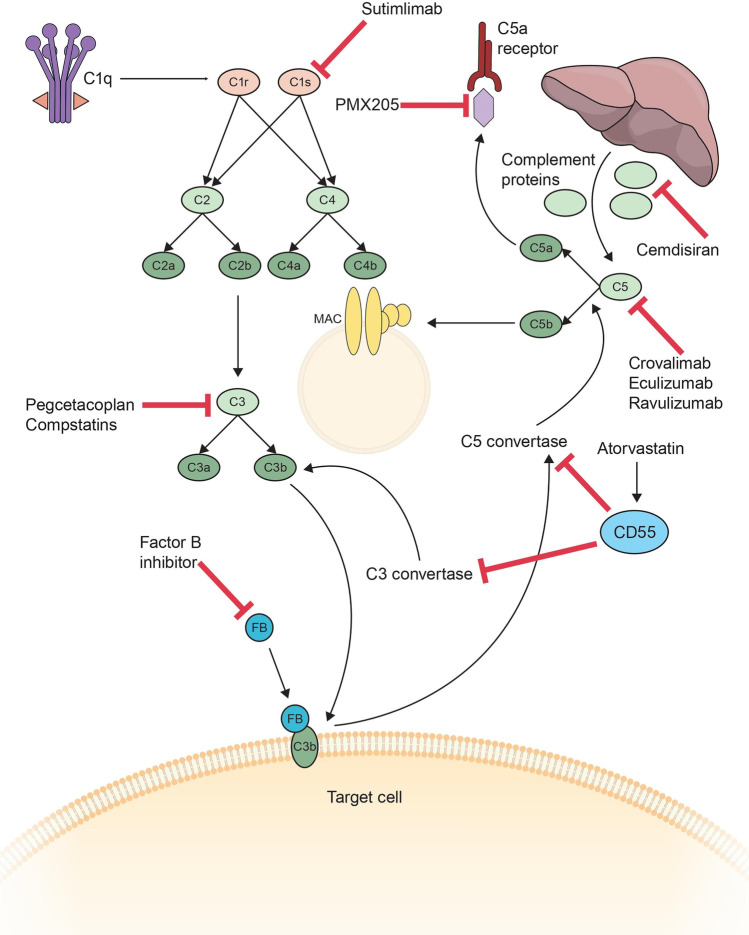
Several therapies have been developed, directed at a handful of targets in the complement cascade (Fig. 3). Table 2 summarizes the mechanism of action and key aspects of the therapeutics previously or currently being studied or approved for use in the treatment of autoimmune neurological diseases. Eculizumab was the first complement directed therapy approved for use in neurological disease. As discussed below, it has been demonstrated to be highly effective in the treatment of both NMOSD and AChR Ab positive MG, its use is limited by its high cost and necessity for frequent dosing. Ravulizumab is a next-generation C5 inhibitor which requires less frequent dosing, which was achieved by altering the Fc domain and engineering the antigen-binding region to release C5 at pH6 [48]. Neonatal Fc receptor (FcRn) allows for the transfer of IgG across the placenta to the fetus. FcRn only interacts with IgG in an acidic environment, allowing for it to be recycled back to the cell surface where exposure to a neutral pH allows the IgG to be released from the receptor. Ravulizumab has increased affinity for the FcRn as compared to eculizumab; as such, there is more rapid degradation of C5, increased recycling of ravulizumab, resulting in a longer half-life compared to eculizumab [49]. Ravulizumab only needs to be administered every 8 weeks as opposed to every 2, making it more feasible for patients to maintain, mitigating at one of the factors that limits patient access and adherence to the therapy.
Fig. 3.
Multiple therapeutic targets are being explored at all levels of the complement cascade. Cemdisiran suppresses the production of complement proteins by the liver. Sutimlimab blocks C1s in the proximal portion of the classical pathway. Pegcetacoplan and compstatins block C3, and factor B inhibitors block factor B and ultimately its interaction with C3b and the resultant amplification loop. Atorvastatin increases endogenous CD55 which inhibits C3 convertase and C5 convertase. Cravalimab, eculizumab and ravulizumab block C5, whereas PMX205 inhibits the C5a receptor C5aR1
Table 2.
Characteristics of complement therapies studied in trial for neurological autoimmune disease
| Medication | Mechanism of action | Dosing | Significant and common adverse effects | Important considerations |
|---|---|---|---|---|
| Eculizumab | Humanized monoclonal antibody that binds C5 preventing its cleavage |
IV: Induction: 900 mg weekly for 4 doses Maintenance: 1,200 mg at week 5, then 1,200 mg every 2 weeks thereafter |
Increased risk of meningococcal infection (vaccination or prophylaxis necessary) Headache, URTI and nausea |
Rebound with acute withdrawal Convenience: frequent IV dosing Very high cost |
| Ravulizumab | Humanized monoclonal antibody that binds C5 preventing its cleavage |
IV: Induction: Weight based dosing at day 1 Maintenance: Weight based dosing every 8 weeks starting 2 weeks after loading dose |
Increased risk of meningococcal infection (vaccination or prophylaxis necessary) |
Convenience: Less frequent IV dosing Very high cost |
| Zilucoplan | Synthetic, macrocyclic peptide that binds to a unique site on C5, preventing its cleavage | SC: 0.3 mg/kg daily | Injection site reactions |
Convenience: self-administration Cost unknown Extended release formulation in development |
| ANX005 | Humanized monoclonal antibody against C1q that inhibits its function as the initiating molecule of the classical complement cascade |
IV: Single dose Optimal dose still being determined |
Still under investigation | Still under investigation |
| Cinryze | Purified human C1-esterase inhibitor binds to C1r and C1s causing disassociation from C1q; regulates the alternative pathway by inhibiting C3b and the lectin pathway by inhibiting MASP1 and MASP2 | IV: 3 daily infusions of 2000 Units (for acute attack) | No significant adverse effects noted in a small trial evaluating its use in 10 patients [77] | In vivo and in vitro studies did not demonstrate significant effect [78] |
Eculizumab
Dermatomyositis
A small trial comparing eculizumab to placebo for the treatment of dermatomyositis was reported in abstract form in 2012 [50]. The trial included 13 patients, 10 of whom received eculizumab at a dose of 8 mg/kg IV weekly for 5 weeks followed by every second week for 2 additional doses. Results demonstrated no effect in the treatment group as compared to placebo. Of note, the trialed dose was much smaller than is currently used in other neurological diseases. Subsequently, a case report described using eculizumab in a 19-year-old woman with severe disease refractory to steroids, IVIg and plasma exchange [51]. Eculizumab 900 mg weekly for 4 doses followed by 1200 mg every 2 weeks resulted in stabilization of the patient. Given the paucity of studies and equivocal results, adequately powered clinical trials are needed to determine eculizumab’s efficacy in treating dermatomyositis.
Anti-acetylcholine Receptor Antibody-Positive Generalized Myasthenia Gravis
Phase 2 Data
A phase 2 clinical trial was the first indication of the efficacy of eculizumab in the treatment of MG [52]. This multicenter, randomized, placebo-controlled trial utilized a crossover design in 14 patients with severe, refractory MG. All trial participants were AChR ab positive generalized MG patients with persisting weakness despite treatment with at least 2 other agents. Patients were randomized in a 1:1 ratio to receive either eculizumab for 16 weeks followed by a 5-week washout and then placebo, or placebo for 16 weeks followed by washout and then eculizumab. Patients in the eculizumab portion of the treatment trial received eculizumab 600 mg IV weekly for 4 weeks followed by 900 mg IV every 2 weeks for 11 weeks. Patients continued their other MG treatments for the length of the trial. Eleven patients completed the study; two did not due to early termination of the trial by the sponsor and a third did not as they had a myasthenic crisis while on placebo during the 2nd portion of the treatment arm. Results demonstrated that 6 of 7 patients who received eculizumab during the first period of treatment met the primary endpoint. There was also a suggestion of carry-over effect as the eculizumab treated patients did not return to baseline despite the 5-week washout period. As a likely consequence, the treatment effect size for the primary endpoint at the end of the trial did not reach statistical significance (P = 0.0577). Further examination of the effect during the first treatment period in those patients on eculizumab revealed an improvement in clinical measures as early as 1 week after receiving the first dose of drug. Given positive signals, a phase 3 clinical trial was pursued.
Phase 3 Data: REGAIN
A multicenter, randomized, double-blind, placebo-controlled phase 3 trial (REGAIN) was completed after the phase 2 trial [53]. This trial included 125 patients with AChR ab positive refractory generalized MG. Patients had to have previously received treatment with at least 2 immunotherapies to be included. Patients were randomized in a 1:1 ratio to 26 weeks of either eculizumab or placebo. Patients in the eculizumab arm received eculizumab 600 mg iv weekly for 4 weeks followed by 1200 mg iv every 2 weeks thereafter. One hundred eighteen patients completed the trial. Serious adverse events did not differ between the groups. The trial though did not reach statistical significance for the primary endpoint, change in MG-ADL score from baseline to week 26 (P = 0.0698). However, prespecified sensitivity and secondary analyses did reach significance demonstrating rapid improvement in the eculizumab treated patients (improvement in MG-ADL score by week 1, P = 0.0125) as well as a significant reduction in rate of relapse (10% in the eculizumab group compared to 24% in the placebo group).
Data from an interim analysis of the open-label extension of the REGAIN study were also published revealing significant and sustained improvements in patients treated with eculizumab [54]. The interim data included 117 of the 118 patients who completed the REGAIN trial and who were followed for a median duration of 22.7 months. Safety data revealed that 19% of patients experienced an infectious event of interest, in keeping with what was observed during the REGAIN trial. No patients developed meningococcal infection, the hallmark of improper MAC formation. Compared with the year prior to entry into the trial, exacerbation rate was reduced by 75.2% (P < 0.0001) and the rate of MG-related hospitalizations was reduced by 80% (P < 0.0001).
The open-label extension study was completed in January 2019 [55]; 87 patients completed the study. After 130 weeks of eculizumab treatment, 60% of the patients had achieved minimal manifestations status, defined as having no symptoms of MG resulting in functional limitations, 90% of patients were improved and 2 patients achieved pharmacological remission. The safety profile was similar with what had been described in over 10 years of this medication’s use in other clinical indications. Table 3 highlights important outcomes demonstrated in the pivotal trials.
Table 3.
Pivotal trials of eculizumab in MG and NMOSD
| Trial | Efficacy (primary endpoint) | Efficacy (select secondary endpoints) | Open-label extension |
|---|---|---|---|
| REGAIN | Change from baseline to week 26 in MG-ADL total score; rank based treatment difference − 11.7 (− 24.3 to 0.96); P = 0.0698 | Pre-specified sensitivity ANCOVA, change from baseline in total score at week 26; MG-ADL − 1.4 (− 2.8, − 0.1) P = 0·0390; QMG 2.5 (− 4.2, − 0.9); P = 0.0032 |
Exacerbation rate reduced by 75.2% (prestudy, 102.4 exacerbations per 100 patient-years; open-label study, 25.4 exacerbations per 100 patient-years; P < 0.0001 Rate of MG-related hospitalizations reduced by over 80% (prestudy, 81.3 hospitalizations per 100 patient-years; open-label study, 13.7 hospitalizations per 100 patient-years; P < 0.0001 Mean change from open-label baseline was statistically significant as early as the first visit after eculizumab administration (assessed at week 1 for MG-ADL (−1.6), QMG (−2.3), and MGC (−3.0); all P < 0.0001 22 (18.8%) patients experienced an infectious event of special interest, including 5 cases of sepsis, septic shock, or pseudomonas sepsis and 1 case each of aspergillus, cytomegalovirus, and pseudomonas infection |
| PREVENT | First adjudicated relapse; relapses occurred in 3 of 96 patients (3%) in the eculizumab group and 20 of 47 (43%) in the placebo group (HR, 0.06; CI 0.02 to 0.20; P < 0.001) | Adjudicated annualized relapse rate was 0.02 in the eculizumab group and 0.35 in the placebo group (rate ratio, 0.04; CI, 0.01 to 0.15; P < 0.001) |
At 3.7 years, 94.4% (CI, 88.6–97.3) of patients remained adjudicated relapse-free 49/119 (41.2%) open-label extension participants changed their other immunotherapies: 17 (14.3%) stopped using other immunotherapies (corticosteroids, azathioprine, mycophenolate mofetil, or cyclophosphamide), 27 (22.7%) decreased their use, 1 (0.8%) started an immunotherapy, and 4 (3.4%) had multiple immunotherapy changes; (27/119, 22.7%) used no additional immunotherapies through the open label extension The rates of serious infections were 15.1 events/100 PY in the PREVENT placebo group (6/47 patients, 12.8%), 9.3 events/100 PY in the PREVENT eculizumab group (11/96 patients, 11.5%), and 10.2 Events/100 PY in the combined eculizumab group (25/137 patients, 18.2%); serious infections that occurred in more than one patient in the combined eculizumab group were pneumonia (5 patients), urinary tract infection (4 patients), cellulitis (2 patients), and sepsis (2 patients) |
Data from the REGAIN trial generated multiple additional reports. Patients treated with eculizumab: (1) showed significant reductions in fatigue as measured by the Neuro-QOL Fatigue score [56]; (2) were nearly 20% more likely to achieve sustained “minimal symptom expression” [57]; (3) demonstrated improved disease control, including those patients on chronic IVIg [58]; and (4) showed improvements in muscle strength throughout the body and in the associated activities of daily living [59]. Additionally, during the open-label extension, a significant proportion of patients either stopped or decreased their other immunosuppressive therapy (IST). In patients who decreased or stopped ≥ 1 IST, mean daily doses of their other immunosuppressive agents decreased between baseline and last assessment by 60.8% for prednisone (P < 0.0001), 89.1% for azathioprine (P < 0.0001), and 56.0% for mycophenolate mofetil (P < 0.0001) [60]. Efficacy was specifically evaluated in the subgroup of Japanese patients included in the study [61] demonstrating safety and efficacy in line with what was reported in the entire trial populations.
Real-World Data
Post-marketing analysis in 34 Japanese patients not included in the open-label extension [62] demonstrated safety and efficacy results similar to those reported in the REGAIN trial. A retrospective chart review of 15 patients with refractory AChR ab-positive MG in the USA described real-world patient experience over 12 months of eculizumab therapy. The chart review revealed clinically significant improvements at 3 months in all patients which was sustained over the course of the 12 months of therapy [63]; the mean number of exacerbations per patient fell by 2.33 (SD 0.98; P < 0.001) over 12 months, compared with the 12 months before eculizumab treatment; all 15 patients decreased their other immunotherapies; and the medication was well tolerated.
Ongoing Trials
There are ongoing studies looking at the safety and efficacy of eculizumab in pediatric patients (ClinicalTrials.gov Identifier: NCT03759366 and NCT04155424) and a registry recruiting patients with MG, who have received complement C5 inhibition therapy and who were not a part of the original trial, to accumulate additional real-world efficacy and safety data (ClinicalTrials.gov Identifier: NCT04202341).
Guillain–Barré Syndrome
A small phase 2 trial of eculizumab in GBS was reported in 2017 [64]. In this single-center study, 8 patients were randomized 2:1 to receive eculizumab for 4 weeks together with standard IVIg treatment, compared to placebo and IVIg. There were difficulties with recruitment as a significant number of patients declined for fear of meningococcal infection. The trial was double blind and patients were evaluated for 26 weeks. Patients also received concomitant antibiotics as there was no opportunity to vaccinate patients for meningococcal infection in advance of receiving the therapy. Patients included had severe GBS (were unable to walk 10 m independently) with onset of symptoms within 2 weeks of enrollment. Seven patients completed the trial. Five patients were randomized to receive eculizumab. One patient receiving eculizumab died of septic shock. Two patients receiving eculizumab did not receive the full dosing regimen as they developed infections necessitating withholding of therapy. Both the placebo patients and 2 of the 5 eculizumab treated patients met the primary end point, predefined improvement at 4 weeks.
A second phase 2 trial of eculizumab in GBS was completed in 2018 [65]. This multicenter, double-blind, randomized placebo-controlled trial accepted patients with severe GBS who could not walk independently, enrolled within 2 weeks of symptom onset. Thirty-four patients were randomized in a 2:1 ratio. Patients in the treatment arm received eculizumab 900 mg IV weekly for 4 weeks, in addition to the standard IVIg treatment, and were evaluated for 24 weeks. The results found that 61% of the patients in the eculizumab group and 45% in the placebo group met the primary end point, predefined improvement of symptoms at week 4, a difference that did not reach statistical significance. The secondary outcome measures did not reach statistical significance, save for one: the ability to run at week 24, which was significantly more likely in the eculizumab treated patients.
A phase 3, prospective, multicenter, placebo-controlled, double-blind randomized clinical trial investigating the safety and efficacy of eculizumab in GBS in Japan, is currently registered (ClinicalTrials.gov Identifier: NCT04752566). The anticipated recruitment is 57 patients. Eculizumab will be administered weekly for 4 weeks. The primary outcome measure is the time to improvement to a pre-determined score on a validated outcome scale with several prespecified secondary outcome measures.
Multifocal Motor Neuropathy
In an open-label study, 13 patients with MMN were treated with eculizumab for 14 weeks [66]. No requirement for the presence of specific autoantibodies was required for inclusion in the study. Ten of the patients were also being treated with IVIg. The primary outcomes of the study were all safety related. Secondary outcomes included IVIg inter-treatment interval, as well as clinical and electrophysiological measures. Although there was no significant difference in IVIg dosing intervals when comparing the run-in period to the treatment and run out period, there was a statistically significant improvement in IVIg interval when comparing to the historical pre-trial IVIg interval. There were some statistically significant improvements in objective measures of strength although not all reached significance. There were improvements in subjective scores of functioning as well as a small yet significant decrease in conduction block on electrophysiological studies. Given these modest improvements over only a short interval of treatment, more study is needed; however, there is some indication that eculizumab may prove efficacious in the treatment of patients with MMN.
Neuromyelitis Optic Spectrum Disorder
The possibility that eculizumab may be of benefit in NMOSD was first suggested in an open-label pilot study of 14 patients [67]. Over 12 months of treatment only 2 patients suffered possible attacks. One patient developed meningococcal sepsis although recovered fully. Five patients suffered 8 attacks in the year after withdrawal of the medication. As a result of this small study, a larger randomized clinical trial was pursued.
Phase 3 Data: PREVENT
The landmark study demonstrating the efficacy and safety of eculizumab for the treatment of NMOSD (PREVENT) was published in 2019 [68]. This trial randomized in a 2:1 ratio 143 adult patients with NMOSD to either eculizumab 900 mg iv weekly for 4 weeks followed by 1200 mg iv every 2 weeks beginning at week 5, or placebo. The continued use of immunosuppressive therapy was permitted. The trial demonstrated a significant effect size in the primary endpoint, which was time to first relapse with a prespecification of a maximum of 24 relapses. Relapses occurred in 3 of 96 patients in the eculizumab group compared to 20 of 47 patients in the placebo group (HR 0.06; 95% CI 0.02 to 0.2; P < 0.001). Higher rates of URTI and headache were reported in the eculizumab group. One patient in the eculizumab group died due to a pulmonary empyema. No patients developed meningococcal infection.
Post-hoc analysis of clinically relevant subgroups, including those with concomitant immunotherapy use and prior rituximab use, demographic and disease characteristics, as well as autoimmune co-morbidity, revealed that eculizumab was safe and efficacious for a wide range of patients with NMOSD regardless of age, sex, additional use of other immunotherapies, autoimmune comorbidities, disease duration, or disease activity [69]. Notably, eculizumab consistently reduced relapse rate in patients who were concomitantly using other immunotherapies including corticosteroids, azathioprine, mycophenolate mofetil, and previous exposure to rituximab (although those treated within 3 months were excluded). Eculizumab was also demonstrated to be safe when added to these other immunotherapies.
An analysis of patients in the Asian subgroup was performed and revealed similar efficacy and safety profile to the overall trial population [70].
Sustained benefit of eculizumab treatment was demonstrated in an interim analysis of PREVENT’s open-label extension with 94.4% of patients remaining relapse free at 3.7 years, with 37% of patients having stopped or decreased background immunosuppressive therapy use [71]. Additionally, serious infection rates remained lower than in the PREVENT placebo group and there were no patients who developed a meningococcal infection. A pre-specified analysis of patients receiving monotherapy with eculizumab or placebo alone demonstrated that 96% of these patients were free from adjudicated relapses and 95% of the 21 patients receiving eculizumab monotherapy had no disability worsening [72]. Table 3 highlights important outcomes demonstrated in the pivotal trials.
Safety
Although complement directed therapies are new in the treatment of neurological disease, they have been used for several years for hematologic indications; as such, we have safety data over 10 years of use [73]. Of special interest is the risk of meningococcal infection associated with C5 inhibition. Formation of MAC is the primary defense against Gram-negative bacteria, particularly Neisseria meningitidis. Over 10 years of eculizumab use in paroxysmal nocturnal hemoglobinuria (PNH), 76 cases of meningococcal infection have been reported representing a rate of 0.25 cases per 100 patient years. There have been 8 fatalities reported in patients vaccinated for meningococcal infection, although these patients were not vaccinated against all subtypes. The rate of meningococcal infection has decreased over time with a rate of 0.57 cases per 100 patient years up to 2007 and 0.16 cases per 100 patient years from 2007 to 2016. Most of the meningococcal infections were serogroup B, even since the introduction of the MenB vaccine in 2013. This may be because MenB vaccination does not seem to result in protection, as demonstrated by a lack of phagocytic killing of meningitis B using whole blood killing assays [74]. As such, many countries including England and France require patients on eculizumab to take antimicrobial prophylaxis in addition to vaccination, a practice that clinicians can consider based on statements by the CDC (https://www.cdc.gov/meningococcal/clinical/eculizumab.html). Table 4 outlines the recommended vaccinations as per the CDC. Other serious infections in patients treated over 10 years with eculizumab were only seen in patients with significant comorbidities or other immune compromising condition.
Table 4.
Vaccination recommendations prior to eculizumab and ravalizumab (CDC)
| Meningococcal vaccine | Recommended at least 2 weeks before administration of first dose with revaccination as needed depending on length of therapy |
| Tetravalent vaccines against serogroups A, C, W, and Y |
Menactra Primary vaccination: Two 0.5 mL doses, given 8 weeks apart Note: Doses may be administered ≥ 4 weeks apart if accelerated vaccination is needed If previously vaccinated: Administer single dose prior to therapy and every 5 years thereafter as needed depending on length of therapy |
| Vaccines against the B serogroup (MenB vaccines) | Bexsero: 2-dose series, second dose given at least 8 weeks after first dose |
| S. pneumoniae (PCV13/PPSV23) | Highly recommended for adults, required for children |
| H. influenza (Hib, PEDVAX HIB) | Highly recommended for adults, required for children |
Malignancies have been reported at a rate of 2.6 cases per 100 patient years, with a mean time of diagnosis of 2.2 years after the first dose of eculizumab; this is likely similar to what would be expected in this population [73].
Sub-group analyses of open label extensions of both PREVENT and REGAIN also demonstrate safety in the setting of concomitant immunosuppressive use as well as exposure to rituximab [60, 69, 75]. This is of significant clinical importance given that real-world use will undoubtably result in exposure to overlapping treatments with other immunosuppressants used in the treatment of these diseases.
In terms of safety during pregnancy, eculizumab does not appear to accumulate in fetal blood or affect complement system activation of the newborn. Eculizumab is not found in breast milk [73].
Finally, there was some suggestion of rebound disease seen in the phase 2 eculizumab trial in MG [52]. One of the patients who was initially in the therapeutic arm suffered a severe relapse during the second phase of the trial, while receiving placebo. As there is more widespread use with necessary cessations of drug due to infection, side effects or other comorbidities, experience will accrue and we will see if rebound disease is truly an issue.
Ravulizumab
Dermatomyositis
A multicenter phase 2/3 randomized, double-blind, placebo-controlled trial to evaluate the safety and efficacy, as well as pharmacokinetics and pharmacodynamics of ravulizumab in the treatment of dermatomyositis is anticipated to start soon. It is anticipated to enroll approximately 180 patients and open label extension is planned. Participants will receive a loading dose followed by maintenance dosing every 8 weeks; the treatment is administered iv. The primary outcome measure is a predetermined degree of improvement on a myositis assessment tool at weeks 26 and 50. There are also several pre-specified secondary outcome measures (ClinicalTrials.gov Identifier: NCT04999020).
Anti-acetylcholine Receptor Antibody-Positive Generalized Myasthenia Gravis
A multicenter phase 3, randomized, double-blind, placebo-controlled trial to evaluate the safety and efficacy of ravulizumab in the treatment of patients with MG is currently underway. One hundred seventy-five patients have been enrolled. The primary outcome measure in the change from baseline in MG-ADL score for patients with MG, at week 26. There are also a small number of prespecified secondary outcome measures (ClinicalTrials.gov Identifier: NCT03920293).
Neuromyelitis Optic Spectrum Disorder
A multicenter, phase 3, external placebo-controlled, open-label study to evaluate the safety and efficacy of ravulizumab in adult patients with NMOSD is currently ongoing. Fifty-eight patients have been enrolled. Patients receive a weight-based loading dose on day 1 followed by weight-based maintenance doing after 2 weeks and every 8 weeks after that. The primary outcome measure is the time to first relapse with limited predetermined secondary outcome measures also being evaluated (ClinicalTrials.gov Identifier: NCT04201262).
Zilucoplan
Necrotizing Myopathy
A phase 2, multicenter, randomized, double-blind, placebo control trial has been completed looking at the safety and efficacy of zilucoplan in the treatment of immune-mediated necrotizing myopathy (ClinicalTrials.gov Identifier: NCT04025632). It included patients with positive serology for HMGCR or SRP antibodies with evidence of weakness and elevated CK. This trial was terminated after enrollment of 27 patients with no safety differences noted comparing drug to placebo. Study results are yet to be reported.
Anti-acetylcholine Receptor Antibody-Positive Generalized Myasthenia Gravis
A phase 2 study was the first indication of the efficacy of zilucoplan in the treatment of MG [76]. This was a multicenter, randomized, double-blind, placebo-controlled trial of 44 patients over 12 weeks. Patients were randomized 1:1:1 to either 0.1 mg/kg zilucoplan SC daily or 0.3 mg/kg zilucoplan SC daily or placebo. Results demonstrated a significant response in the primary outcome measure (change from baseline to week 12 in QMG score) in both dosing groups, with a slower onset of action and less pronounced effect seen in the lower dose group. Zilucoplan at a dose of 0.3 mg/kg SC daily resulted in a mean reduction from baseline of 6.0 points in the QMG score (placebo-corrected change, –2.8; P = 0.05) and 3.4 points in the MG-ADL score (placebo-corrected change, –2.3; P = 0.04). Many of the secondary outcome measures also demonstrated a statistically significant improvement in the treatment groups.
A phase 3 trial (RAISE) is currently underway using the 0.3 mg/kg SC daily dosing (ClinicalTrials.gov Identifier: NCT04115293). An open-label extension of the phase 2 clinical trial is also ongoing (ClinicalTrials.gov Identifier: NCT04225871).
ANX005
Guillain–Barré Syndrome
A phase 1b study to evaluate the safety, tolerability and drug-drug interactions of ANX005 and IVIg in patients with GBS was completed in May 2021 though results have yet to be reported (ClinicalTrials.gov Identifier: NCT04035135). The study, completed in Bangladesh and in Denmark, enrolled 14 patients presenting within 14 days of symptom onset and employed an open label design. Patients received a single dose of ANX005 75 mg/kg together with the standing IVIg treatment.
A phase 2/3 trial evaluating the pharmacological properties, safety, and efficacy of ANX005 in the treatment of patients with GBS is currently underway (ClinicalTrials.gov Identifier: NCT04701164). It is a randomized, double-blind, placebo-controlled study anticipated to enroll approximately 180 patients. Patients receive only a single iv infusion on day 1; two distinct doses of the medication are being evaluated. Only patients presenting with onset within 10 days will be included. Two primary outcome measures have been stipulated: one evaluating the safety of the medication and the other evaluating efficacy. There are also a small number of pre-specified secondary outcome measures.
CINRYZE
Neuromyelitis Optic Spectrum Disorder
An open-label phase 1b safety and proof-of-concept trial was completed in 10 patients with NMOSD who presented with acute relapse (acute transverse myelitis and/or optic neuritis) [77]. The investigational therapy was given in addition to conventional treatment with high dose iv methylprednisolone. There were no serious adverse effects noted in any of the patients. EDSS scores improved in all patients with most patients returning to pre-attack EDSS on subsequent follow-up and only 2 patients requiring escalation to plasmapheresis.
Subsequent in vivo data from studies in rats and in vitro data with human serum demonstrated that complement dependent cytotoxicity was not sufficiently reduced to confer clinical benefit, even when extremely large doses were utilized [78]. As such, no further trials utilizing this therapeutic strategy have been completed.
Neurological Disease Associated with Genetic Abnormalities of the Complement System
Rarely, genetic diseases resulting in deficiencies or defective control of the complement system can result in immune mediated neurological disease. One such example is fulminant CNS inflammation resembling acute disseminated encephalomyelitis (ADEM) occurring in patients with complement factor I (CFI) deficiency [79]. Another example of this are mutations resulting in deficiencies of CD59; one such patient developed recurrent aseptic meningitis which ceased after the administration of eculizumab [80] and in another report 4 patients with recurrent demyelinating neuropathy were effectively managed with eculizumab [81].
A Special Note on IVIg
Over the last few years, partly motivated by the shortage of IVIg and the effort to synthesize a rationally designed alternative, IVIg’s mechanism of action has been better elucidated. Studies demonstrate that IVIg’s mechanism of action is complex and differs depending on the disease state being treated [82]. The mechanism of action targets myriad different parts of the innate and adaptive immune systems. From the perspective of complement, IVIg binds to complement proteins and sequesters them away from autoantibodies. Decreases in C1q and C4 concentrations as well as classical pathway activity have been demonstrated, as has the elimination of complement fixation [83].
Future Directions
There are several additional complement therapies being considered, investigated, and trialed. Some of these target different proteins in the complement cascade while others represent novel modes of administration or dosing regimens.
Crovalimab is a monoclonal antibody directed against C5 and engineered for self-administered subcutaneous dosing of small volumes (680 mg; 4 mL, administered once every 4 weeks) [84]. Crovalimab can bind to the antigen repeatedly. A phase 1/2 trial has demonstrated safety and preliminary efficacy in patients with PNH [84]. This would be an important modification of the therapy making it significantly more accessible to patients unable to receive iv infusions in their home or travel to an infusion site.
Cemdisiran is currently under development for the treatment of complement-mediated diseases by suppressing liver production of complement 5 (C5) protein [85]. In a phase 1/2 trial, Cemdisiran plasma concentrations declined rapidly while showing rapid C5 suppression maintained up to 13 months following single and multiple doses. The long duration of action allows for low, infrequent SC dosing making it a promising therapeutic candidate in complement-mediated diseases as monotherapy or in combination with a C5 inhibitor antibody [85].
In terms of novel targets, PMX205 is a noncompetitive inhibitor of complement C5a receptor 1 [86]. PMX205 has greater than 90% bioavailability when given subcutaneously and is more efficient than similar molecules in this class at entering the intact CNS [86]. A therapy targeting the action of C5a carries significant promise as it may eliminate the increased risk of meningococcal infection associated with C5 inhibition, given it would not result in disruption of MAC formation. Whether this results in a significant decline in efficacy will need to be evaluated. Compstatins are a family of proteins that inhibit C3. They bind to C3 and prevent its cleavage by C3 convertases [87]. The first compstatin-based therapeutic has recently been approved for the treatment of PNH after demonstration of its superiority as compared to eculizumab in these patients [88]. The FDA has also approved complement protein C3 inhibitor pegcetacoplan for the treatment PNH. In patients with deficiencies in C3, we see increased risk of infections with encapsulated bacteria as well as increased risk for autoimmune disease. While initial studies demonstrate acceptable safety [88], it remains to be seen what will occur over the long term.
CD55 is a complement regulatory protein that when upregulated has been shown to reduce NMOSD pathology (89). Using a cell-based ELISA developed to screen for pharmacological upregulators of endogenous CD55, atorvastatin was identified as a potential therapeutic candidate [89]. Atorvastatin at 10–20 mg/kg/day for 3 days was found to strongly increase CD55 immunofluorescence in mouse brain and spinal cord, and reduced NMO pathology following intracerebral AQP4-IgG injection. Sutimlimab is a first-in-class, humanized monoclonal antibody designed to target C1s [90], which is responsible for activating the classic complement pathway. A phase 3 trial demonstrating its efficacy in Cold Agglutinin Disease has been reported (90). It is dosed as an intravenous infusion of sutimlimab on day 0 (the first day of treatment) and day 7, followed by an infusion every 2 weeks. Other targets being explored include Factor B and Factor D inhibitors [91].
In addition to alternative proteins or receptors in the complement cascade being targeted, another potential role for complement mediated therapies is in the treatment of acute attacks. Studies using surrogate markers of complement inhibition demonstrate complete inhibition within 15 min of eculizumab administration [92]. Given in vitro demonstrations of the necessity of complement in driving NMOSD pathology [42, 43, 47], it seems highly possible that if given acutely, eculizumab could limit the degree of inflammatory injury which occurs during acute attack. This, in addition to the question of whether chronic complement inhibition results in less severe relapse, remains to be seen.
Finally, as the pathogenesis of additional autoimmune diseases is elucidated, more disease will be identified that may benefit from complement directed therapy. For example, 2 of the lesser common autoimmune disorders, namely CASPR2 associated encephalitis and glycine receptor antibody associated autoimmunity, have both been demonstrated to involve complement activation [93, 94]. Complement directed therapies may prove to be effective in the management of diseases such as these and may allow for the rational choice of therapeutics in diseases where rarity precludes the ability to develop evidence-based treatment protocols.
Conclusions
More and more we are discovering ways to manage and treat devastating neurological conditions that historically resulted in significant morbidity and mortality. These therapies have been demonstrated to be effective and safe over the short and medium term; however, it remains to be seen what effect they might have over the long term, and to what risks we may be exposing our patients. Recognizing that these therapies will likely need to be continued over the long term, it is imperative that they be not only well tolerated, but also convenient to administer, as well as affordable and accessible to all patients. In addition, we need to remain vigilant to unintended outcomes of our manipulation of the immune system in general, and complement cascade in particular, in the long-term management of disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sechi E, Flanagan EP. Antibody-mediated autoimmune diseases of the CNS: challenges and approaches to diagnosis and management. Front Neurol. 2021;12:673339. doi: 10.3389/fneur.2021.673339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pittock SJ, Zekeridou A, Weinshenker BG. New hope for patients with neuromyelitis optica: From biomarker discovery to completion of phase 3 trials. Submitted. 2022.
- 3.Tintore M, Vidal-Jordana A, Sastre-Garriga J. Treatment of multiple sclerosis - success from bench to bedside. Nat Rev Neurol. 2019;15(1):53–58. doi: 10.1038/s41582-018-0082-z. [DOI] [PubMed] [Google Scholar]
- 4.Lublin F. History of modern multiple sclerosis therapy. J Neurol. 2005;252(Suppl 3):iii3–iii9. doi: 10.1007/s00415-005-2010-6. [DOI] [PubMed] [Google Scholar]
- 5.Olsson T. Cytokines in neuroinflammatory disease: role of myelin autoreactive T cell production of interferon-gamma. J Neuroimmunol. 1992;40(2–3):211–218. doi: 10.1016/0165-5728(92)90135-8. [DOI] [PubMed] [Google Scholar]
- 6.The IFNB Multiple Sclerosis Study Group Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43(4):655–661. doi: 10.1212/WNL.43.4.655. [DOI] [PubMed] [Google Scholar]
- 7.Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25(11):1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalakas MC, Alexopoulos H, Spaeth PJ. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat Rev Neurol. 2020;16(11):601–617. doi: 10.1038/s41582-020-0400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepow IH. Presidential address to American Association of Immunologists in Anaheim, California, April 16, 1980. Louis Pillemer, Properdin, and scientific controversy. J Immunol. 1980;125(2):471–475. [PubMed] [Google Scholar]
- 10.Flexner S, Noguchi H. Snake venom in relation to haemolysis, bacteriolysis and toxicity. J Exp Med. 1902;6(3):277–301. doi: 10.1084/jem.6.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holden C. Flurry over venom. Science. 1980;207(4427):161. doi: 10.1126/science.7350649. [DOI] [PubMed] [Google Scholar]
- 12.Morgan BP, Harris CL. Complement therapeutics; history and current progress. Mol Immunol. 2003;40(2–4):159–170. doi: 10.1016/S0161-5890(03)00111-1. [DOI] [PubMed] [Google Scholar]
- 13.Reis ES, Mastellos DC, Hajishengallis G, Lambris JD. New insights into the immune functions of complement. Nat Rev Immunol. 2019;19(8):503–516. doi: 10.1038/s41577-019-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Lin F, Strainic MG, An F, Miller RH, Altuntas CZ, et al. IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J Immunol. 2008;180(9):5882–5889. doi: 10.4049/jimmunol.180.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahoria R, Selcen D, Engel AG. Microvascular alterations and the role of complement in dermatomyositis. Brain. 2016;139(Pt 7):1891–1903. doi: 10.1093/brain/aww122. [DOI] [PubMed] [Google Scholar]
- 17.Kissel JT, Mendell JR, Rammohan KW. Microvascular deposition of complement membrane attack complex in dermatomyositis. N Engl J Med. 1986;314(6):329–334. doi: 10.1056/NEJM198602063140601. [DOI] [PubMed] [Google Scholar]
- 18.Emslie-Smith AM, Engel AG. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol. 1990;27(4):343–356. doi: 10.1002/ana.410270402. [DOI] [PubMed] [Google Scholar]
- 19.Kissel JT, Halterman RK, Rammohan KW, Mendell JR. The relationship of complement-mediated microvasculopathy to the histologic features and clinical duration of disease in dermatomyositis. Arch Neurol. 1991;48(1):26–30. doi: 10.1001/archneur.1991.00530130034016. [DOI] [PubMed] [Google Scholar]
- 20.Dalakas MC. Inflammatory muscle diseases. N Engl J Med. 2015;372:1734–1747. doi: 10.1056/NEJMra1402225. [DOI] [PubMed] [Google Scholar]
- 21.Hoogendijk JE, Amato AA, Lecky BR, Choy EH, Lundberg IE, Rose MR, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden. The Netherlands Neuromuscul Disord. 2004;14(5):337–345. doi: 10.1016/j.nmd.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Stenzel W, Goebel HH, Aronica E. Review: immune-mediated necrotizing myopathies–a heterogeneous group of diseases with specific myopathological features. Neuropathol Appl Neurobiol. 2012;38(7):632–646. doi: 10.1111/j.1365-2990.2012.01302.x. [DOI] [PubMed] [Google Scholar]
- 23.Anquetil C, Boyer O, Wesner N, Benveniste O, Allenbach Y. Myositis-specific autoantibodies, a cornerstone in immune-mediated necrotizing myopathy. Autoimmun Rev. 2019;18(3):223–230. doi: 10.1016/j.autrev.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Allenbach Y, Benveniste O, Stenzel W, Boyer O. Immune-mediated necrotizing myopathy: clinical features and pathogenesis. Nat Rev Rheumatol. 2020;16(12):689–701. doi: 10.1038/s41584-020-00515-9. [DOI] [PubMed] [Google Scholar]
- 25.Allenbach Y, Arouche-Delaperche L, Preusse C, Radbruch H, Butler-Browne G, Champtiaux N, et al. Necrosis in anti-SRP. Neurology. 2018;90(6):e507–e517. doi: 10.1212/WNL.0000000000004923. [DOI] [PubMed] [Google Scholar]
- 26.Nastuk WL, Plescia OJ, Osserman KE. Changes in serum complement activity in patients with myasthenia gravis. Proc Soc Exp Biol Med. 1960;105:177–184. doi: 10.3181/00379727-105-26050. [DOI] [PubMed] [Google Scholar]
- 27.Lennon VA, Seybold ME, Lindstrom JM, Cochrane C, Ulevitch R. Role of complement in the pathogenesis of experimental autoimmune myasthenia gravis. J Exp Med. 1978;147(4):973–983. doi: 10.1084/jem.147.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fazekas A, Komoly S, Bózsik B, Szobor A. Myasthenia gravis: demonstration of membrane attack complex in muscle end-plates. Clin Neuropathol. 1986;5(2):78–83. [PubMed] [Google Scholar]
- 29.Albazli K, Kaminski HJ, Howard JF. Complement inhibitor therapy for myasthenia gravis. Front Immunol. 2020;11:917. doi: 10.3389/fimmu.2020.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cutillo G, Saariaho AH, Meri S. Physiology of gangliosides and the role of antiganglioside antibodies in human diseases. Cell Mol Immunol. 2020;17(4):313–322. doi: 10.1038/s41423-020-0388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shahrizaila N, Lehmann HC, Kuwabara S. Guillain-Barré syndrome. Lancet. 2021;397(10280):1214–1228. doi: 10.1016/S0140-6736(21)00517-1. [DOI] [PubMed] [Google Scholar]
- 32.Hafer-Macko CE, Sheikh KA, Li CY, Ho TW, Cornblath DR, McKhann GM, et al. Immune attack on the Schwann cell surface in acute inflammatory demyelinating polyneuropathy. Ann Neurol. 1996;39(5):625–635. doi: 10.1002/ana.410390512. [DOI] [PubMed] [Google Scholar]
- 33.Hafer-Macko C, Hsieh ST, Li CY, Ho TW, Sheikh K, Cornblath DR, et al. Acute motor axonal neuropathy: an antibody-mediated attack on axolemma. Ann Neurol. 1996;40(4):635–644. doi: 10.1002/ana.410400414. [DOI] [PubMed] [Google Scholar]
- 34.Pascual-Goñi E, Martín-Aguilar L, Querol L. Autoantibodies in chronic inflammatory demyelinating polyradiculoneuropathy. Curr Opin Neurol. 2019;32(5):651–657. doi: 10.1097/WCO.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 35.Guo X, Tang L, Huang Q, Tang X. A systematic review and meta-analysis of autoantibodies for diagnosis and prognosis in patients with chronic inflammatory demyelinating polyradiculoneuropathy. Front Neurosci. 2021;15:637336. doi: 10.3389/fnins.2021.637336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wanleenuwat P, Iwanowski P, Kozubski W. Antiganglioside antibodies in neurological diseases. J Neurol Sci. 2020;15(408):116576. doi: 10.1016/j.jns.2019.116576. [DOI] [PubMed] [Google Scholar]
- 37.Uncini A, Vallat JM. Autoimmune nodo-paranodopathies of peripheral nerve: the concept is gaining ground. J Neurol Neurosurg Psychiatry. 2018;89(6):627–635. doi: 10.1136/jnnp-2017-317192. [DOI] [PubMed] [Google Scholar]
- 38.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 39.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202(4):473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinson SR, Pittock SJ, Lucchinetti CF, Roemer SF, Fryer JP, Kryzer TJ, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69(24):2221–2231. doi: 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed] [Google Scholar]
- 41.Hinson SR, McKeon A, Fryer JP, Apiwattanakul M, Lennon VA, Pittock SJ. Prediction of neuromyelitis optica attack severity by quantitation of complement-mediated injury to aquaporin-4-expressing cells. Arch Neurol. 2009;66(9):1164–1167. doi: 10.1001/archneurol.2009.188. [DOI] [PubMed] [Google Scholar]
- 42.Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133(Pt 2):349–361. doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Bennett JL, Verkman AS. Ex vivo spinal cord slice model of neuromyelitis optica reveals novel immunopathogenic mechanisms. Ann Neurol. 2011;70(6):943–954. doi: 10.1002/ana.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan T, Smith AJ, Verkman AS. Complement-dependent bystander injury to neurons in AQP4-IgG seropositive neuromyelitis optica. J Neuroinflammation. 2018;15(1):294. doi: 10.1186/s12974-018-1333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hinson SR, Romero MF, Popescu BF, Lucchinetti CF, Fryer JP, Wolburg H, et al. Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes. Proc Natl Acad Sci U S A. 2012;109(4):1245–1250. doi: 10.1073/pnas.1109980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soltys J, Liu Y, Ritchie A, Wemlinger S, Schaller K, Schumann H, et al. Membrane assembly of aquaporin-4 autoantibodies regulates classical complement activation in neuromyelitis optica. J Clin Invest. 2019;129(5):2000–2013. doi: 10.1172/JCI122942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piatek P, Domowicz M, Lewkowicz N, Przygodzka P, Matysiak M, Dzitko K, Lewkowicz P. C5a-preactivated neutrophils are critical for autoimmune-induced astrocyte dysregulation in neuromyelitis optica spectrum disorder. Front Immunol. 2018;23(9):1694. doi: 10.3389/fimmu.2018.01694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris CL, Pouw RB, Kavanagh D, Sun R, Ricklin D. Developments in anti-complement therapy; from disease to clinical trial. Mol Immunol. 2018;102:89–119. doi: 10.1016/j.molimm.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Keller CW, Pawlitzki M, Wiendl H, Lünemann JD. Fc-receptor targeted therapies for the treatment of Myasthenia gravis. Int J Mol Sci. 2021;22(11):5755. doi: 10.3390/ijms22115755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon PA, Winer JB, Hoogendijk JE, Choy EH. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev. 2012;8:CD003643. doi: 10.1002/14651858.CD003643.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faguer S, Belliere J, Ribes D. Complement C5-blocking agent in refractory dermatomyositis. J Rheumatol. 2018;45(12):1710–1711. doi: 10.3899/jrheum.180060. [DOI] [PubMed] [Google Scholar]
- 52.Howard JF, Barohn RJ, Cutter GR, Freimer M, Juel VC, Mozaffar T, et al. A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve. 2013;48(1):76–84. doi: 10.1002/mus.23839. [DOI] [PubMed] [Google Scholar]
- 53.Howard JF, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16(12):976–986. doi: 10.1016/S1474-4422(17)30369-1. [DOI] [PubMed] [Google Scholar]
- 54.Muppidi S, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, et al. Long-term safety and efficacy of eculizumab in generalized myasthenia gravis. Muscle Nerve. 2019;60(1):14–24. doi: 10.1002/mus.26447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mantegazza R, Wolfe GI, Muppidi S, Wiendl H, Fujita KP, O'Brien FL, et al. Post-intervention status in patients with refractory myasthenia gravis treated with eculizumab during REGAIN and its open-label extension. Neurology. 2021;96(4):e610–e618. doi: 10.1212/WNL.0000000000011207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andersen H, Mantegazza R, Wang JJ, O'Brien F, Patra K, Howard JF, et al. Eculizumab improves fatigue in refractory generalized myasthenia gravis. Qual Life Res. 2019;28(8):2247–2254. doi: 10.1007/s11136-019-02148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vissing J, Jacob S, Fujita KP, O'Brien F, Howard JF, Group Rs 'Minimal symptom expression' in patients with acetylcholine receptor antibody-positive refractory generalized myasthenia gravis treated with eculizumab. J Neurol. 2020;267(7):1991–2001. doi: 10.1007/s00415-020-09770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacob S, Murai H, Utsugisawa K, Nowak RJ, Wiendl H, Fujita KP, et al. Response to eculizumab in patients with myasthenia gravis recently treated with chronic IVIg: a subgroup analysis of REGAIN and its open-label extension study. Ther Adv Neurol Disord. 2020;13:1756286420911784. doi: 10.1177/1756286420911784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mantegazza R, O'Brien FL, Yountz M, Howard JF, Group Rs Consistent improvement with eculizumab across muscle groups in myasthenia gravis. Ann Clin Transl Neurol. 2020;7(8):1327–1339. doi: 10.1002/acn3.51121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nowak RJ, Muppidi S, Beydoun SR, O'Brien FL, Yountz M, Howard JF. Concomitant immunosuppressive therapy use in eculizumab-treated adults with generalized myasthenia gravis during the. Front Neurol. 2020;11:556104. doi: 10.3389/fneur.2020.556104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murai H, Uzawa A, Suzuki Y, Imai T, Shiraishi H, Suzuki H, et al. Long-term efficacy and safety of eculizumab in Japanese patients with generalized myasthenia gravis: A subgroup analysis of the REGAIN open-label extension study. J Neurol Sci. 2019;407:116419. doi: 10.1016/j.jns.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 62.Murai H, Suzuki S, Hasebe M, Fukamizu Y, Rodrigues E, Utsugisawa K. Safety and effectiveness of eculizumab in Japanese patients with generalized myasthenia gravis: interim analysis of post-marketing surveillance. Ther Adv Neurol Disord. 2021;14:17562864211001995. doi: 10.1177/17562864211001995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katyal N, Narula N, Govindarajan R. Clinical experience with eculizumab in treatment-refractory acetylcholine receptor antibody-positive generalized myasthenia gravis. J Neuromuscul Dis. 2021;8(2):287–294. doi: 10.3233/JND-200584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davidson AI, Halstead SK, Goodfellow JA, Chavada G, Mallik A, Overell J, et al. Inhibition of complement in Guillain-Barré syndrome: the ICA-GBS study. J Peripher Nerv Syst. 2017;22(1):4–12. doi: 10.1111/jns.12194. [DOI] [PubMed] [Google Scholar]
- 65.Misawa S, Kuwabara S, Sato Y, Yamaguchi N, Nagashima K, Katayama K, et al. Safety and efficacy of eculizumab in Guillain-Barré syndrome: a multicentre, double-blind, randomised phase 2 trial. Lancet Neurol. 2018;17(6):519–529. doi: 10.1016/S1474-4422(18)30114-5. [DOI] [PubMed] [Google Scholar]
- 66.Fitzpatrick AM, Mann CA, Barry S, Brennan K, Overell JR, Willison HJ. An open label clinical trial of complement inhibition in multifocal motor neuropathy. J Peripher Nerv Syst. 2011;16(2):84–91. doi: 10.1111/j.1529-8027.2011.00328.x. [DOI] [PubMed] [Google Scholar]
- 67.Pittock SJ, Lennon VA, McKeon A, Mandrekar J, Weinshenker BG, Lucchinetti CF, et al. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot study. Lancet Neurol. 2013;12(6):554–562. doi: 10.1016/S1474-4422(13)70076-0. [DOI] [PubMed] [Google Scholar]
- 68.Pittock SJ, Berthele A, Fujihara K, Kim HJ, Levy M, Palace J, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(7):614–625. doi: 10.1056/NEJMoa1900866. [DOI] [PubMed] [Google Scholar]
- 69.Palace J, Wingerchuk DM, Fujihara K, Berthele A, Oreja-Guevara C, Kim HJ, et al. Benefits of eculizumab in AQP4+ neuromyelitis optica spectrum disorder: Subgroup analyses of the randomized controlled phase 3 PREVENT trial. Mult Scler Relat Disord. 2021;47:102641. doi: 10.1016/j.msard.2020.102641. [DOI] [PubMed] [Google Scholar]
- 70.Kim HJ, Nakashima I, Viswanathan S, Wang KC, Shang S, Miller L, et al. Eculizumab in Asian patients with anti-aquaporin-IgG-positive neuromyelitis optica spectrum disorder: A subgroup analysis from the randomized phase 3 PREVENT trial and its open-label extension. Mult Scler Relat Disord. 2021;50:102849. doi: 10.1016/j.msard.2021.102849. [DOI] [PubMed] [Google Scholar]
- 71.Wingerchuk DM, Fujihara K, Palace J, Berthele A, Levy M, Kim HJ, et al. Long-Term Safety and Efficacy of Eculizumab in Aquaporin-4 IgG-Positive NMOSD. Ann Neurol. 2021;89(6):1088–1098. doi: 10.1002/ana.26049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pittock SJ, Fujihara K, Palace J, Berthele A, Kim HJ, Oreja-Guevara C, et al. Eculizumab monotherapy for NMOSD: Data from PREVENT and its open-label extension. Mult Scler. 2021:13524585211038291. [DOI] [PMC free article] [PubMed]
- 73.Lee SE, Lee JW. Safety of current treatments for paroxysmal nocturnal hemoglobinuria. Expert Opin Drug Saf. 2021;20(2):171–179. doi: 10.1080/14740338.2021.1857723. [DOI] [PubMed] [Google Scholar]
- 74.Langereis JD, van den Broek B, Franssen S, Joosten I, Blijlevens NMA, de Jonge MI, Langemeijer S. Eculizumab impairs Neisseria meningitidis serogroup B killing in whole blood despite 4CMenB vaccination of PNH patients. Blood Adv. 2020;4(15):3615–3620. doi: 10.1182/bloodadvances.2020002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siddiqi ZA, Nowak RJ, Mozaffar T, O'Brien F, Yountz M, Patti F, REGAIN Study Group. Eculizumab in refractory generalized myasthenia gravis previously treated with rituximab: subgroup analysis of REGAIN and its extension study. Muscle Nerve. 2021. [DOI] [PubMed]
- 76.Howard JF, Nowak RJ, Wolfe GI, Freimer ML, Vu TH, Hinton JL, et al. Clinical effects of the self-administered subcutaneous complement inhibitor zilucoplan in patients with moderate to severe generalized myasthenia gravis: results of a phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. JAMA Neurol. 2020;77(5):582–592. doi: 10.1001/jamaneurol.2019.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levy M, Mealy MA. Purified human C1-esterase inhibitor is safe in acute relapses of neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2014;1(1):e5. doi: 10.1212/NXI.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tradtrantip L, Asavapanumas N, Phuan PW, Verkman AS. Potential therapeutic benefit of C1-esterase inhibitor in neuromyelitis optica evaluated in vitro and in an experimental rat model. PLoS ONE. 2014;9(9):e106824. doi: 10.1371/journal.pone.0106824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Altmann T, Torvell M, Owens S, Mitra D, Sheerin NS, Morgan BP, Kavanagh D, Forsyth R. Complement factor I deficiency: a potentially treatable cause of fulminant cerebral inflammation. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e689. doi: 10.1212/NXI.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawamoto M, Murakami Y, Kinoshita T, Kohara N. Recurrent aseptic meningitis with PIGT mutations: a novel pathogenesis of recurrent meningitis successfully treated by eculizumab. BMJ Case Rep. 2018;2018:bcr2018225910. [DOI] [PMC free article] [PubMed]
- 81.Mevorach D, Reiner I, Grau A, Ilan U, Berkun Y, Ta-Shma A, Elpeleg O, Shorer Z, Edvardson S, Tabib A. Therapy with eculizumab for patients with CD59 p.Cys89Tyr mutation. Ann Neurol. 2016;80(5):708–717. doi: 10.1002/ana.24770. [DOI] [PubMed] [Google Scholar]
- 82.Arumugham VB, Rayi A. Intravenous Immunoglobulin (IVIG). 2021. In: StatPearls. [PubMed]
- 83.Norris PAA, Kaur G, Lazarus AH. New insights into IVIg mechanisms and alternatives in autoimmune and inflammatory diseases. Curr Opin Hematol. 2020;27(6):392–398. doi: 10.1097/MOH.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 84.Röth A, Nishimura JI, Nagy Z, Gaàl-Weisinger J, Panse J, Yoon SS, et al. The complement C5 inhibitor crovalimab in paroxysmal nocturnal hemoglobinuria. Blood. 2020;135(12):912–920. doi: 10.1182/blood.2019003399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Badri P, Jiang X, Borodovsky A, Najafian N, Kim J, Clausen VA, et al. Pharmacokinetic and pharmacodynamic properties of cemdisiran, an RNAi therapeutic targeting complement component 5, in healthy subjects and patients with paroxysmal nocturnal hemoglobinuria. Clin Pharmacokinet. 2021;60(3):365–378. doi: 10.1007/s40262-020-00940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumar V, Lee JD, Clark RJ, Noakes PG, Taylor SM, Woodruff TM. preclinical pharmacokinetics of complement C5a receptor antagonists PMX53 and PMX205 in mice. ACS Omega. 2020;5(5):2345–2354. doi: 10.1021/acsomega.9b03735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mastellos DC, Ricklin D, Sfyroera G, Sahu A. From discovery to approval: a brief history of the compstatin family of complement C3 inhibitors. Clin Immunol. 2021:108785. [DOI] [PubMed]
- 88.Hillmen P, Szer J, Weitz I, Röth A, Höchsmann B, Panse J, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021;384(11):1028–1037. doi: 10.1056/NEJMoa2029073. [DOI] [PubMed] [Google Scholar]
- 89.Tradtrantip L, Duan T, Yeaman MR, Verkman AS. CD55 upregulation in astrocytes by statins as potential therapy for AQP4-IgG seropositive neuromyelitis optica. J Neuroinflammation. 2019;16(1):57. doi: 10.1186/s12974-019-1448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Röth A, Barcellini W, D'Sa S, Miyakawa Y, Broome CM, Michel M, et al. Sutimlimab in cold agglutinin disease. N Engl J Med. 2021;384(14):1323–1334. doi: 10.1056/NEJMoa2027760. [DOI] [PubMed] [Google Scholar]
- 91.Barratt J, Weitz I. Complement factor D as a strategic target for regulating the alternative complement pathway. Front Immunol. 2021;12:712572. doi: 10.3389/fimmu.2021.712572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wijnsma KL, Ter Heine R, Moes DJAR, Langemeijer S, Schols SEM, Volokhina EB, van den Heuvel LP, Wetzels JFM, van de Kar NCAJ, Brüggemann RJ. Pharmacology, pharmacokinetics and pharmacodynamics of eculizumab, and possibilities for an individualized approach to eculizumab. Clin Pharmacokinet. 2019;58(7):859–874. doi: 10.1007/s40262-019-00742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Körtvelyessy P, Bauer J, Stoppel CM, Brück W, Gerth I, Vielhaber S, Wiedemann FR, Heinze HJ, Bartels C, Bien CG. Complement-associated neuronal loss in a patient with CASPR2 antibody-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. 2015;2(2):e75. doi: 10.1212/NXI.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carvajal-González A, Leite MI, Waters P, Woodhall M, Coutinho E, Balint B, Lang B, Pettingill P, Carr A, Sheerin UM, Press R, Press R, Lunn MP, Lim M, Maddison P, Meinck HM, Vandenberghe W, Vincent A. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain. 2014;137(Pt 8):2178–2192. doi: 10.1093/brain/awu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.