Abstract
Background:
Dentin is a permeable tubular composite and complex structure, and in weight, it is composed of 20% organic matrix, 10% water, and 70% hydroxyapatite crystalline matrix. Demineralization of dentin with gradient concentrations of ethylene diamine tetraacetic acid, 0.6 N hydrochloric acid, or 2% nitric acid removes a major part of the crystalline apatite and maintains a majority of collagen type I and non-collagenous proteins, which creates an osteoinductive scaffold containing numerous matrix elements and growth factors. Therefore, demineralized dentin should be considered as an excellent naturally-derived bioactive material to enhance dental and alveolar bone tissues regeneration.
Method:
The PubMed and Midline databases were searched in October 2021 for the relevant articles on treated dentin matrix (TDM)/demineralized dentin matrix (DDM) and their potential roles in tissue regeneration.
Results:
Several studies with different study designs evaluating the effect of TDM/DDM on dental and bone tissues regeneration were found. TDM/DDM was obtained from human or animal sources and processed in different forms (particles, liquid extract, hydrogel, and paste) and different shapes (sheets, slices, disc-shaped, root-shaped, and barrier membranes), with variable sizes measured in micrometers or millimeters, demineralized with different protocols regarding the concentration of demineralizing agents and exposure time, and then sterilized and preserved with different techniques. In the act of biomimetic acellular material, TDM/DDM was used for the regeneration of the dentin-pulp complex through direct pulp capping technique, and it was found to possess the ability to activate the odontogenic differentiation of stem cells resident in the pulp tissues and induce reparative dentin formation. TDM/DDM was also considered for alveolar ridge and maxillary sinus floor augmentations, socket preservation, furcation perforation repair, guided bone, and bioroot regenerations as well as bone and cartilage healing.
Conclusion:
To our knowledge, there are no standard procedures to adopt a specific form for a specific purpose; therefore, future studies are required to come up with a well-characterized TDM/DDM for each specific application. Likely as decellularized dermal matrix and prospectively, if the TDM/DDM is supplied in proper consistency, forms, and in different sizes with good biological properties, it can be used efficiently instead of some widely-used regenerative biomaterials.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13770-022-00438-4.
Keywords: Dentin, Treated dentin matrix, Demineralized dentin matrix, Bone regeneration, Dental tissue regeneration
Introduction
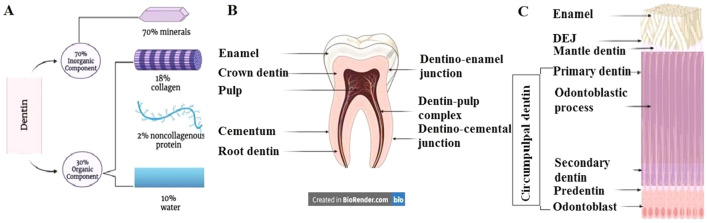
Dentin is chemically composed of approximately 70% mineral phase (40%–45% in vol), 20% organic matrix (30% in vol), and 10% water (20–25% in vol). Additionally, the organic component of dentin consists of 18% collagen and 2% noncollagenous proteins (NCP), proteoglycans, growth factors, phospholipids, and enzymes (Fig. 1A). The matrix is a repository for growth factors, such as basic fibroblast growth factor, insulin-like growth factor, transforming growth factor-β, and bone morphogenetic proteins (BMP). Several NCPs, such as osteopontin and osteocalcin, are common in dentin and bone; however, dentin phosphoprotein is an NCP found specifically in dentin [1]. The inorganic components of dentin are calcium and phosphate ions that form hydroxyapatite crystals that are larger compared with those found in bone and much smaller than those in enamel [2].
Fig. 1.
Representative diagrams created with BioRender.com for the chemical composition of A dentin, B component parts of the tooth and C histological structure of dentin
Regarding the histological structure of dentin, it is considered as a specialized mineralized avascular connective tissue that forms the main bulk of the tooth. It is covered by enamel on the crown and cementum on the root and surrounds the entire pulp tissue (Fig. 1B). Beneath the enamel, dentin has an outer mantle layer of 15–30 µm thickness whereas underneath the cementum, Tomes granular and/or the hyaline Hopewell-Smith layers are identified, and each of them represents approximately 15–30 µm thickness. The circumpulpal dentin forms the main bulk of the dentin, and its thickness continuously increases by about 4 µm/day at the expense of dental pulp space. Circumpulpal dentin includes the intertubular dentin and peritubular (intratubular) one. Compared with intertubular dentin, peritubular dentin has a relatively higher proportion of sulfated proteoglycans and minerals with lesser collagen fibrils; therefore, it is considered harder than intertubular dentin. Intertubular dentin results from the transformation of predentin into dentin and it is a composite consisting of collagen fibrils discontinuously reinforced with nanoplates of carbonated hydroxyapatite [3].
Dentin is highly permeable as it contains numerous dentinal tubules running from the pulp tissue to the dentino-enamel junction (DEJ) in the crown and till the dentino-cemental junction (DCJ) in the root. Dentin exhibits regional differences in tubule density and diameter wherein tubule diameter can vary from 0.9 µm peripherally to 2.5 µm at the pulp side. In the meantime, density is approximately 59,000–76,000 tubules/mm2 at the pulp side whereas the number of these tubules decreases to half of such quantity at the area close to the DEJ. Dentinal tubules have collateral branches measuring 1 μm in diameter that form a three-dimensional network as they extended at specific angles crisscrossing intertubular dentin [4].
The dentinal tubule contains an odontoblast cell process, which is an extension of an odontoblast cell, and serum-like fluid which contains a mixture of proteoglycans, tenascin, transferrin, and albumin. Interestingly, odontoblast processes were seen only in the tubules near the pulp. Further, odontoblasts differentiate from ectomesenchymal cells of the dental papilla and are organized at the periphery of the pulp as a cellular palisade. They form the dentin matrix (predentin) by synthesizing collagen types I, III, and V; noncollagenous proteins as integrin-binding sialoprotein, matrix extracellular phosphoglycoprotein, osteopontin, dentin matrix protein 1, and sialophosphoprotein; glycoproteins as dermatan sulfate, keratan sulfate, heparan sulfate, and chondroitin sulfate [5]. They are also responsible for the deposition of minerals in the dentin matrix, which is not simply restricted in the mineralization front at the edge of predentin and dentin but occurs along the whole length of the odontoblast process [6].
During tooth development and organogenesis, odontoblasts form the primary dentin which comprises the main bulk of the circumpulpal dentin. After root completion and throughout the life of the tooth, odontoblasts form secondary dentin bordering the pulp at a slow rate (Fig. 1C). This type of dentin contains fewer dentinal tubules than primary dentin and there is usually a bend in the tubules at the interface between primary and secondary dentin. In response to environmental conditions and according to the severity of the stimuli, tertiary dentin (reactionary/reparative) is formed as odontoblasts forming the reactionary dentin while the dental pulp stem cells form the reparative one. Sometimes, the word synonyms of tertiary, reactionary, and reparative terms are used interchangeably [7].
At the micron length scale, crown dentin is similar to root dentin; however, unlike root dentin, the proportion of the tubular area is higher and tubules follow a gentle S-shaped curve in the crown part while they are straight in the root area. The dentinal tubules are surrounded by a 2–6 µm dense cuff of peritubular dentin, which suggests that the dentin mineral density is higher in the crown than in the root and the predentin is significantly wider in the crown than in the root [8].
Considering the physical and biological properties of dentin, the thickness is approximately 3–10 mm or even more, and this thickness differs in the regional parts of the same tooth, among different teeth and as a result of aging. The color of dentin greatly affects the color of the tooth due to the translucency of enamel as dentin is a yellowish-hued material that becomes darker with age. In radiographic images, dentin is more radiolucent than enamel due to its lower mineral content whereas it is more radiopaque than cementum and bone. Dentin has no replacement mechanism for biologic turnover but it can be remodeled to a certain degree as odontoblasts and pulpal resident stem cells can produce secondary and tertiary dentin when it is damaged by excessive tooth wear, carious lesion, trauma, or through iatrogenic insult, such as accidental exposure. Dentin is a bone-like matrix that is a vital, sensitive and porous tissue, capable of responding to mechanical, thermal, chemical, evaporative, and osmotic environmental stimuli. Dentin can be regarded as both a barrier and permeable structure, depending upon its thickness, age, and other variables. The permeability to fluid flow through the dentinal tubules, as well as a directional design of these tubules, suggests that dentin has a sensory hydrodynamic function. Micropore-sized dentinal tubules that measure 0.9–2.5 µm diameter provide micropore spaces of 3.70%–5.88% porosity that increases the surface contact area of dentin [3].
Regarding the mechanical properties of dentin, the elastic modulus and hardness gradually increase from the pulp side toward DEJ and DCJ. The poorly mineralized intertubular dentin has a lower Young's modulus than the highly mineralized peritubular dentin. A hydrated environment affects the mechanical behavior of dentin, as the elastic modulus decreases by 35% and hardness decreases by 30% [9]. Young dentin has higher initial toughness and stable toughness values than aged dentin. The fatigue crack growth exponent is associated with the direction of the dentinal tubules [10]. Unlike enamel, dentin is less brittle and somewhat has viscoelastic properties. This elasticity is important to provide the flexibility that is required to support the overlying enamel and prevent its fracture. The tensile strength of dentin is attributed to the fibrous arrays of collagen type I, while high compressive strength and rigidity are provided by the crystals of the mineralized phase deposited within the collagen fibers [7].
To obtain tooth-derived substances, demineralization is required before clinical adaptation to open the dentinal tubules and release BMPs. Demineralization is the process of removing some of the highly crystalline inorganic substances from the dental hard tissues, which results in the loss of its structural integrity with the collapse and degradation of the supporting collagen matrix. Enamel and dentin contain calcium-deficient carbonate-rich hydroxyapatite crystallites with enamel having much larger crystals compared with dentin. The crystal sizes comprise 85 vol% of enamel structure compared to about 50 vol% in dentin. The larger surface area of the dentin apatite increases its dissolution susceptibility when exposed to acids and this susceptibility is further increased because of the higher carbonate content of the dentin mineral. Thus, the dentin minerals are dissolved more rapidly than the enamel ones, and because there is less total mineral in dentin than in enamel, the acid attack proceeds more quickly in dentin [11].
By preferentially removing peritubular dentin, acid-etching agents used during dental restorative procedures and ethylenediamine tetraacetic acid (EDTA) used in endodontic treatments enlarge the openings of the dentinal tubules, making the dentin more permeable. Specific acids and chelating agents as 17% EDTA, sodium hypochlorite, and citric acid were used during root canal instrumentation to remove the smear layer and clean dentinal walls [11]. Moreover, bacterial acids (lactic acid), dietary acids (acetic acid, phosphoric acid, and citric acid), and gastric acid {hydrochloric acid (HCl)}, all demineralized the dentin through the processes of caries and acid-erosion. It has become standard to use laboratory acids, such as formic acid [12, acetic and lactic acids [13], or chelating agents such as EDTA [14], to produce demineralized dentin models for use in in vitro remineralization studies. It is well known that each demineralizing agent has a unique effect as chelating agents, strong acids, and weak acids affecting both mineral and organic phases of dentin in significantly different ways. For example, the demineralizing agents caused some degree of collagen denaturation, citric acid caused the most damage and varying the concentrations of EDTA and citric acid affected collagen in a dissimilar manner [15].
In the past decade, teeth as graft material have been proposed with fascinating outcomes. Therefore, this study aimed to review evidence on treated dentin matrix (TDM)/demineralized dentin matrix (DDM) for dental and bone tissues regeneration and summarize the in vitro and in vivo animal and human studies using TDM/DDM as an osteoinductive material for clinical applications.
Materials and methods
Study design
We conducted a scoping review and searching for articles on TDM/DDM for tissue regeneration. For our scoping review, a five-stage framework was adopted following Arskey and O'Malley's design [16]. The five stages were: specifying research questions; identifying relevant studies; studies selection; extraction, mapping and charting the data; collating, summarizing, synthesizing and reporting results.
Stage I: identification of research questions
We aimed to answer the following questions; (1) is there is a difference between TDM and DDM, (2) with respect to species and tooth parts, what are the different sources of obtaining TDM/DDM, (3) what is the conventional forms and shapes of TDM/DDM and their sizes, (4) what is the most widely-used demineralizing agents and what is the optimal exposure time, and (5) what are the most common preclinical and clinical applications of TDM/DDM in tissue regeneration.
Stage II: identification of relevant studies
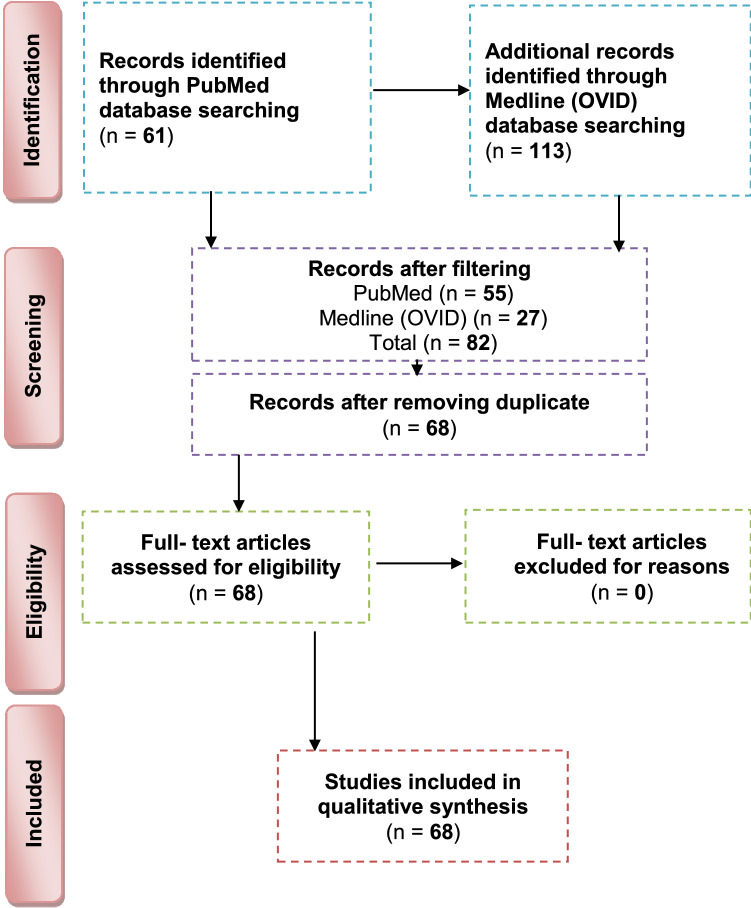
PubMed and Medline (OVID) databases were searched in October 2021 for the relevant articles performed on TDM/DDM using the following strategy for PubMed search {(treated dentin matrix [Title/Abstract]) AND (regeneration [Title/Abstract])/(demineralized dentin matrix [Title/Abstract]) AND (regeneration [Title/Abstract])} and the following one for Medline (OVID) databases)treated dentin matrix and regeneration).af (all fields) and (demineralized dentin matrix and regeneration).af. Figure 2 demonstrates the studies’ search procedure using the PRISMA flowchart.
Fig. 2.
Flowchart for article selection according to preferred reporting items for systematic reviews and meta-analyses guidelines
Stage III: selection of studies
The relevant studies were selected according to the inclusion and exclusion criteria. The searches were not restricted by language type, however, were limited to original researches including in vitro and in vivo animal and human studies and excluding narrative reviews, systematic reviews, and meta-analyses.
Stage IV: extraction, mapping, and charting the data
A template was established and reviewed by each author. The authors were calibrated to extract the following data; authors, publication year, country of origin, study design, source of TDM/DDM (human or animal), tooth part (crown or root), matrix form, matrix size, demineralization protocol, ways of sterilization, and preservation and the outcome.
Stage V: collating, summarizing, synthesizing, and reporting results
Meta-synthesis and integration of findings from qualitative studies were performed to provide a new and more comprehensive interpretation of the findings.
Statistical analysis
Degree of chance–adjusted agreement (kappa coefficient value) was used to determine the inter-reviewer reliability.
Results
Studies' selection and distribution of relevant articles according to date of publication, country origin and study designs
The initial search identified 113 unique references. No additional studies were recognized through hand searching. After filtering, 82 references were recorded and screened. After the eligibility criteria were applied and duplicates were removed, 68 in vitro, in vivo animal and human studies were obtained and were included in the present review (Fig. 2). The kappa value for inter-reviewer agreement was 0.85. The distribution of relevant articles according to the date of publication, distribution of relevant articles according to country of origin, study designs of relevant articles, teeth involved in study designs, tooth part involved in study designs, forms and sizes, demineralization protocol, methods of sterilization, and preservation and outcome are presented in Supplementary Tables 1, 2, 3, and 4.
Considering publication year, the highest number of articles was published in 2021 (19.11%) whereas the lowest ones were published in 2014 (1.47%), 2008 (1.47%), 2005 (1.47%), 2002 (1.47%), and 2001 (1.47%). The percentage of articles published in 2020, 2019, 2018, 2017, 2016, 2015, 2013, 2012, 2011, and 2009 were 8.82%, 5.88%, 8.82%, 10.29%, 10.29%, 13.23%, 5.88%, 4.41%, 2.94%, and 2.49%, respectively. No articles were published in 2010, 2007, 2006, 2004, and 2003. Regarding the country of origin, most of the studies were performed in China (50%), Republic of Korea (16.17%), Japan (10.29%), Brazil (7.35%), Iran (4.41%), Egypt (2.94%), Sweden (2.49%), Taiwan (1.47%), UK (1.47%), USA (1.47%), and Thailand (1.47%). The study designs were in vitro, in vivo animal studies, case reports, in vivo human studies, randomized controlled clinical trials, and split-mouth randomized controlled clinical trials.
Answering research questions
-
Is there a difference between TDM and DDM?
The search strategy of PubMed and Medline (OVID) databases using the appropriate search terms yielded 55 articles for PubMed database and 13 articles for Midline (OVID). Among them, there were 32 articles for TDM and 36 articles for DDM. Both terms were used interchangeably despite that the matrices were fabricated with the same protocols using gradient concentrations of EDTA, 0.6 N-HCl or 2% HNO3. In our opinion, it is devisable to use the term DDM for the dentin matrices prepared for regenerative purposes that obtained from different sources, fabricated in different forms and shapes, and demineralized with different protocols as the term TDD could mean physical treatment using laser therapy or mechanical one by means of abrasives.
-
Teeth and tooth parts involved in study designs
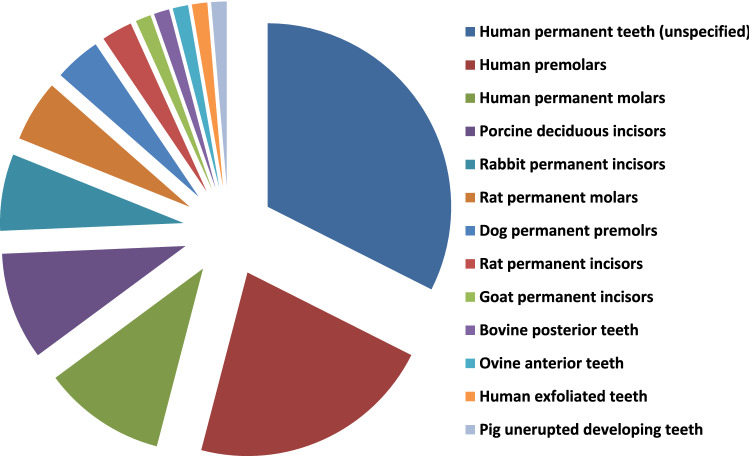
The majority of the published studies relied on harvesting vital and nonvital human permanent teeth. The human teeth were maxillary and mandibular premolars extracted for orthodontic reasons, nonfunctional third molars requiring removal for clinical reasons and exfoliated deciduous teeth. In addition, teeth obtained from other animal species were involved in a few study designs such as porcine deciduous incisors, rabbit permanent incisors, rat permanent molars, dog permanent premolars and rat permanent incisors, that were frequently selected whereas goat incisors, bovine posterior teeth, ovine lower anterior teeth and pig unerupted developing teeth were selected to less comparatively (Fig. 3).
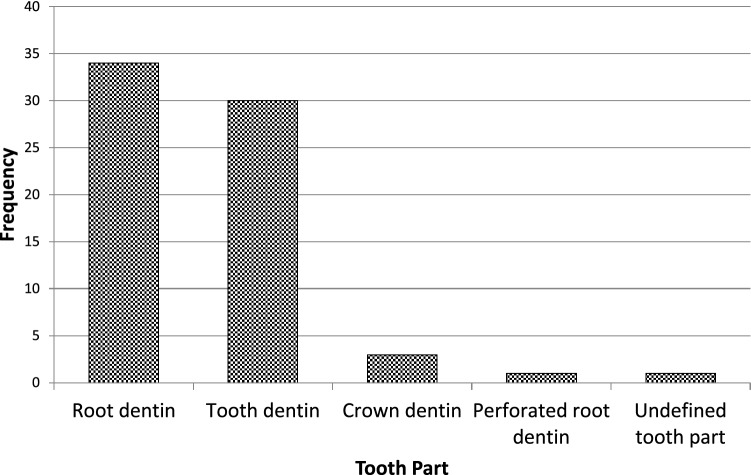
Primarily and with the use of proper dental instruments, enamel, cementum, and periodontal tissues of the extracted teeth were completely removed from the outer tooth surface, and pulp tissue with predentin was completely removed from the internal one. Root dentin is the tooth part that is commonly used whereas crown dentin was used to a lesser degree (Fig. 4).
-
Forms and sizes
The TDM/DDM were produced in different forms likely as particles, liquid extract, hydrogel, and paste, and in different shapes such as sheets, slices, chips, blocks, discs, root-shaped, barrier membranes, and press-fitted according to the defect shape.
Dentin grinder and ball mill machines were used for preparing particulate dentin [17, 19, 22, 25, 26, 45, 49–52, 55, 56–60, 62, 65, 68, 73, 74, 76, 77, 80]. The diameter of the particle size was measured in either µm or mm. The minimum diameter was less than 40 µm [74] whereas the maximum was 2 mm [45, 58, 59]. In certain instances, the particles were atelopeptidized [25], decorated with carboxymethyl chitosan [49], loaded with recombinant human bone morphogenic protein-2 [52], encapsulated into liposomes [54], or used as particle-based bio-ink [47] (Fig. 5). An extract [19, 22, 54, 63, 74] was obtained through soaking particles of 1 g in 5 ml saline [19], adding particles of 20 g to 100 ml DMEM/F12 and filtered using a 0.22 μm filter [22], reconstituting particles of 0.5 g to 1 mg/ml tris-buffered saline and sterile filtered through a 0.22-µm filter [54], or by adding granules of 10 mg to 1 ml α-MEM and then the extract was diluted to 1 mg/ml, or used as 10 mg/ml [63]. Hydrogel [21] was prepared by dispersing particles measuring 350–500 µm in 0.125 g sodium alginate solution in a 1:1 ratio and then dripped into 5% (w/v) sterile CaCl2 solution. The paste form [29] was prepared by mixing a powder of DDM with particle size less than 76 µm to aqueous extract of DDM with a volume ratio of 1:1.
Sheets [19, 23, 42, 44, 78] were sized in 2 × 6 × 6 mm matrices [23, 42, 44] or fabricated in a porous shape of 100 µm thickness [78], and in certain instances, the sheets were autoclaved [23]. Slices [61, 69–71, 82] were 8 µm thickness [61, 69–71], chips were 1 mm thickness [75], and blocks [79, 81, 83] were 5 mm diameter and 2 mm thickness [79] or 5 mm diameter and 2 mm height [81] or 2–3 mm thickness and 4 mm diameter [83] whereas discs were 5 mm thickness [24]. The sizes of the root-shaped matrix [18, 20, 27, 28, 30, 32, 34–38, 41, 43, 48] for human premolars were 10 mm in length and 1.0 mm in thickness [18, 32, 41] or 10 mm in length and 3-5 in mm diameter [38]. Regarding molars, the average length and width were 13.08 and 8.41 mm, respectively, and then perforated with uniformly distributed thirty pores measuring 1 mm in diameter [48]. Root-shaped prepared from porcine deciduous incisors [18, 28, 30, 34] were also prepared with the following sets of dimensions: 10 mm length and 1.0 mm thickness [18], 10 mm length and 3–5 mm diameter [28, 30], 2 mm length and 1 mm diameter [28] or in 9.4 mm length with 4.9 mm upper diameter and 3.4 mm bottom diameter [34], and in addition, tubes of 8 mm length were prepared from rat incisors [84]. Barrier membrane size was 300–800 µm thick slice with 0.2–0.3 mm diameter holes [46] or it was produced in the form of semi-rigid cubic shape with 2 mm × 2 mm × 8 mm [64]. Finally, the DDM was also manufactured to be press-fitted to the premolar furcation defect with a 2-mm diameter scaffold [31].
-
Methods of demineralization, sterilization and preservation
To our knowledge, no standard protocol for demineralization was established for the different forms and shapes of dentin matrices used for tissue regeneration. Therefore, the protocol requires optimization of the concentration and pH of the demineralizing agent, and the exposure time needs to be properly adjusted for each form and shape. Before demineralization, it is necessary to prepare dentin matrix to remove the debris resulting from mechanical instrumentation. Accordingly, the dentin matrices were soaked in deionized water for 5–6 h and a cleaning cycle of 5–20 min was performed every hour using an ultrasonic cleaner [34, 40, 43]. There are several demineralizing protocols tested with different study designs. Excluding the size parameter of the dentin matrix, the particles obtained from human permanent teeth were demineralized with consecutive gradient concentrations of 17%, 10%, and 5% EDTA [17, 26, 49] with different time frames. Correspondingly they were 10 min, 5 min, and 10 min [17], 10 min, 10 min, and 10 min [26], and 5 min, 5 min, and 10 min [49]. In addition, 0.6 N HCl [45, 50, 52, 57, 65, 68, 73, 77, 80] was used for demineralization and the exposure time was considered once and varied between 15 min [77], 30 min [52, 73] and 12 h [50]. The particles obtained from human exfoliated deciduous teeth were demineralized once with 0.6 N HCl and exposure time was varied between 10 min, 15 min, 20 min, 25 min, 30 min, 60 min, and 90 min [80]. Moreover, 2% HNO3 [51, 53, 55, 62] was also used for demineralization with two period's time frame, 10 min [55] or 20 min [53]. Similarly, particles obtained from bovine posterior teeth were demineralized using gradient concentrations of 17% EDTA for 1, 7 and 13 days [25] each. Alternatively, particles obtained from rabbit mandibular incisors [65] and ovine lower anterior teeth [68] were demineralized with 0.6 N HCl once and the exposure time was 7 days for the whole tooth before pulverization to small particles whereas particles obtained from rat incisors were demineralized using 17% EDTA for 10 min [67].
Human-derived particles used for preparing liquid extracts were demineralized with 17%, 10%, and 5% EDTA with two different time frames as 5 min, 5 min, 10 min [22], 30 min, 30 min, 30 min [74] and in addition, porcine-derived particles used for the same purpose were exposed for 30 min each [74]. Hydrogel was prepared by incorporating human-derived particles demineralized with 17%, 10%, and 5% EDTA for 10 min, 10 min and 5 min [21], respectively, in sodium alginate solution. Moreover, human and porcine-derived particles used to prepare paste form were demineralized with 17%, 10%, and 5% EDTA for 10 min, 10 min, and 5 min [29], respectively.
Human-derived sheet scaffolds were demineralized with 17% and 5% with two different time frames as 5 min, 5 min [23] and 4 min, 2 min [42, 44] or alternatively demineralized with 0.6 N HCl for 2 weeks [78] while human-derived slices were demineralized with 10% EDTA for approximately 3 months [82]. The human-derived dentin chips were demineralized with 17%, 10%, and 5% for 5 min, 5 min, and 10 min [75], respectively. Also, human-derived dentin blocks were demineralized with 24% EDTA for 12 h [79] and blocks derived from unerupted developing pigs' teeth were demineralized with 24% EDTA for 2 min, 6 min, and 12 min [83], respectively. The human-derived disc-shaped scaffolds were demineralized with 10% EDTA for 3 days, and then successively soaked in 17%, 10%, and 5% for 20 min, 20 min, and 20 min [24], respectively. Demineralization of the root-shaped matrix obtained from human sources was performed with 17%, 10%, and 5% EDTA for 5 min, 5 min, and 10 min [18, 32, 35, 38, 41, 43] respectively, or for 12 min, 12 min, 20 min [27], for 30 min, 30 min, 30 min, respectively, or demineralized with 0.34 N HNO3 for 30 min [48] whereas those obtained from porcine deciduous incisors were demineralized with 17%, 10%, and 5% EDTA for 20 min, 18 min, and 15 min [18, 28, 30] or for 20 min, 20 min, 10 min [34], or for 10 min, 10 min, 5 min [36], respectively, and those obtained from dogs were demineralized with 17%, 10%, and 5% for 12 min, 12 min, and 20 min [20], or for 8 min, 8 min, 12 min [37], respectively. Dentin tubes obtained from rat incisors were demineralized with 0.6 N HCl for 3 h [84]. Human-derived barrier membranes were demineralized with 0.6 N HCl [46]. Scaffolds prepared to be press-fitted into dogs' furcation perforation were demineralized with 17%, 10%, 5% for 5 min, 5 min, 10 min [31], respectively. Figure 6 presents DDM with opened dentinal tubules after demineralization.
Sterilization was performed by maintaining the matrices in sterile phosphate-buffered saline (PBS) with 100 units/ml of penicillin and 100 μg/ml of streptomycin at 37 °C, for 72 h and then washed in sterilized deionized water for 5 min [17, 21, 22, 24, 28, 30–36, 38–44], socking into penicillin and streptomycin [72] only, preservation in 5% peracetic acid and 75% ethanol for 10 min [53, 80, 81], immersion in 5 ml alcohol/2 ml of gentamicin [61, 69–71], rinsing with sterile saline for 10 min [75], washing in sterilized deionized water for 10 min [82], rinsing twice in 0.1 M Tris-HCl (pH 7.4) for 10 min [55, 62] and using gradient ethanol concentrations [47]. Other methods of sterilization were gamma irradiation processing using Cobalt 60 radiation with a dose of 5 kGy [66], ethylene oxide gas sterilization at low temperatures [19, 45, 49, 52, 57, 58, 60, 67, 68, 76], steam sterilization at 121°C and a pressure of 1 bar for 15 min [23], lyophilization and freeze-drying [46], or mixing was accomplished in a sterile container [65].
The matrices were preserved in α-MEM media containing 50 units/ml of penicillin and 50 μg/ml of streptomycin or in 0.1 x PBS and kept in refrigerator at 4 °C [18, 20, 24, 27, 28, 30–32, 34, 36, 39–42, 44, 47] cryopreserved at -196°C [38], −80 °C [26], −18 °C [83], and -20°C [25] or stored at room temperature [45, 52, 65]. Other methods of preservation included syringes for hydrogel [21], package for barrier membranes [46] and particles [58–60], or freshly prepared during operation [53, 55, 63]. In addition, slices were stored at 2°C until implantation [61, 69–71].
-
Outcome
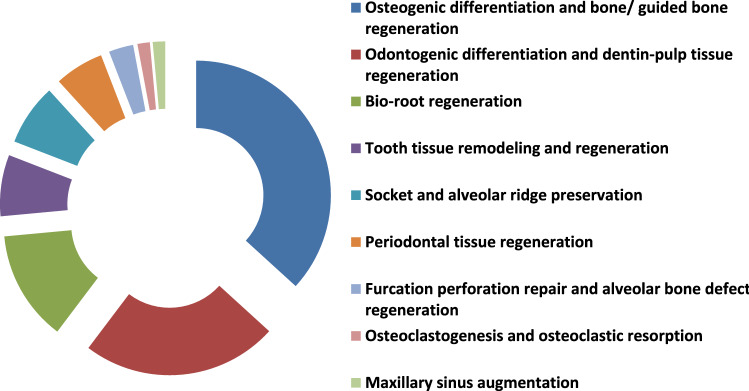
The TDM/DDM were considered for osteogenic differentiation [39, 49, 50] and bone/guided bone regeneration [45, 46, 49, 51, 53, 55–62, 66, 69–71, 80–82, 84], odontogenic differentiation [17, 40], and dentin-pulp tissue regeneration [25, 38] (Fig. 7), bio-root regeneration [18, 24, 26, 27, 34], tooth tissue remodeling and regeneration [30], socket preservation and alveolar ridge augmentation [52, 55, 73, 76, 82], periodontal tissue regeneration [32, 64, 78], furcation perforation repair [31] and alveolar bone defect regeneration [57], osteoclastogenesis and osteoclastic resorption [28], and maxillary sinus augmentation [55, 77] (Fig. 8).
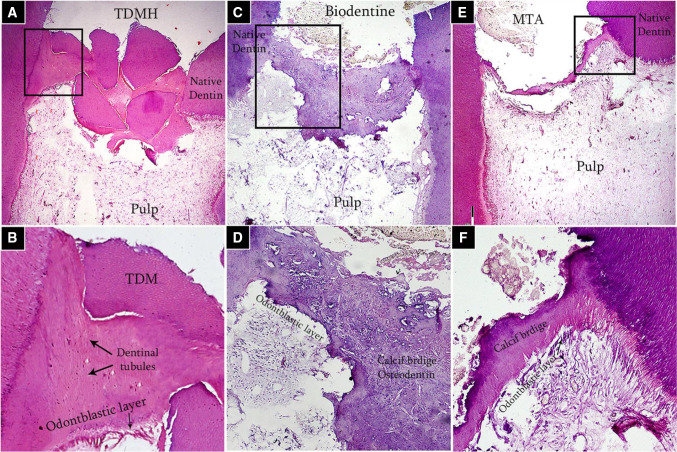
Regarding dentin-pulp complex regeneration, Holiel et al. [21] evaluated clinically the regenerative potential of DDM hydrogel as a direct pulp capping material in comparison with Biodentine and mineral trioxide aggregate (MTA). The study was performed on thirty intact fully erupted premolars scheduled to be extracted for orthodontic reasons and they found that hydrogel could achieve dentin regeneration and conserve pulp vitality and might serve as a reasonable natural substitute for Biodentine and MTA in restoring in vivo dentin defects. In addition, Mehrvarzfar et al. [75] compared in a clinical trial of thrity-three intact third molars of eleven healthy volunteers the pulpal responses to MTA and combination therapy of MTA and DDM as a pulp-dressing agent s for partial pulpotomy. They found that the dentin bridge was significantly thicker in MTA/DDM group than MTA group alone.
Considering alveolar bone regeneration, Um et al. [52] evaluated in a case series study the efficacy of DDM loaded with recombinant human bone morphogenetic protein-2 (rhBMP-2) on ten experimental sites for socket preservation. They suggested that DDM may be a potential carrier for rhBMP-2 and it may be conceivable to reduce the concentration of rhBMP-2 to 0.2 mg/ml. In addition, Li et al. [53] conducted a clinical prospective study on forty patients and concluded that autogenous granules of DDM prepared at the chair side after extractions could act as an outstanding readily available alternative to bone graft material in guided bone regeneration, even for implantation of severe periodontitis cases. Moreover, Minamizato et al. [55] evaluated in a pilot study of sixteen patients underwent dental implant placement the clinical application of autogenous partially DDM prepared immediately after extraction for alveolar bone regeneration in implant dentistry and they considered partially DDM as an efficient, safe, and reasonable bone substitute. Furthermore, Pang et al. [57] conducted a prospective randomized clinical trial of a total 33thrity-three graft sites in twenty-four patients and they suggested that autogenous DDM is a viable option for alveolar bone augmentation following dental extraction, in comparison with anorganic bovine bone. Likewise, Elfana et al. [73] conducted a randomized controlled clinical trial to evaluate autogenous whole-tooth versus DDM grafts for alveolar ridge preservation and they concluded that the two grafts have similar clinical effects but histologically autogenous DDM grafts seems to demonstrate better graft remodeling, integration, and osteoinductive properties. Recently, Ouyyamwongs et al. [76] assessed clinically in a split-mouth randomized controlled clinical trial the potential of using autologous DDM in combination with platelet-rich fibrin (PRF) membrane or PRF membrane alone to preserve the ridge dimension. They concluded that the combination therapy reduced the horizontal ridge collapse, and promoted bone healing as shown clinically and radiographically.
Fig. 3.
Exploded pie chart showing analytical data of the frequencies regarding source of teeth selected in study designs from the relevant articles
Fig. 4.
Bar chart showing analytical data of the frequencies regarding tooth part selected in study designs from the relevant articles
Fig. 5.

SEM images showing DDM particle size ranging from 350-500lm. Courtesy provided by the staff members of Oral Biology, Faculty of Dentistry, Mansoura University, Mansoura, Egypt
Fig. 6.
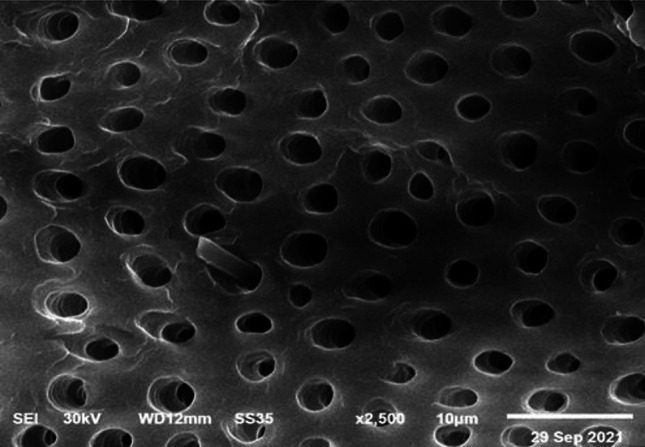
SEM images showing the basic dentin micro-texture after demineralization. Structurally, dentinal tubules are enlarged. Courtesy provided by the staff members of Oral Biology, Faculty of Dentistry, Mansoura University, Mansoura, Egypt.
Fig. 7.
Decalcified sections of human pulp capped with A, B DDM hydrogel, C, D Biodentine, and E, F MTA after 2-mon examination period showing complete dentin bridge formation and absence of inflammatory pulp response. a, b, c × 40 and a1, b1, c1 are higher magnification of boxed areas × 200. Courtesy provided by the staff members of Conservative Dentistry, Faculty of Dentistry, Alexandria University, Alexandria, Egypt
Fig. 8.
Exploded doughnut chart showing outcomes from the relevant articles
Discussion
Regenerative dentistry has widely been recognized as a promising field in the provision of functional and biocompatible dental tissues as alternatives for conventional materials. Current progress in tissue engineering has offered new methods and technologies for dentin-pulp complex and bone regeneration. As there are several reasons for dental extraction, including caries, mobility, orthodontic tooth reasons, and trauma, DDM can be prepared with low risks of infection and rejection with non-invasive attainability; thus, it should be considered as a natural resource to be used to full advantage for other applications.
DDM is autogenous tooth dentin that has osteoconductive and osteoinductive potential since dentin contains extracellular COL-1 and various growth factors. Based on the demineralization process, the factors remain available to the host environment; however, extracting proper concentrations of collagen and bioactive molecules from the extracted teeth is a challenging task and requires meticulous preparation of the tooth dentin. DDM is used in dental surgery in the treatment of extraction socket preservation and guided bone regenerations. It functions in a dual capacity: First as a scaffold to support bone regeneration and second as a carrier for bone morphogenic protein (BMP-2). When DDM serves as a carrier, it combines the properties of the grafting material with those of the delivered substances [38].
The type of demineralizing agents and time frames used to prepare DDM are dissimilar in different studies, and therefore they affect the amount of the mineral percentage remaining in the DDM. The DDM components have different inorganic/organic ratios compared to those originally found in the dentin matrix. Approximately, the powder form has mineral content of about 5%–10%, whereas block form has a mineral content of about 10%–30% [1]. ELISA performed on dentin particles showed a slightly higher amount of COL-1 in demineralized samples, as compared with untreated ones, although no significant difference was found and such a finding provides compelling evidence that the demineralization process didn't damage the extracellular matrix of dentin [85]. COL-1 was identified by electrophoresis approximately at 110–120 kDa [86]. Minor bands at 76–102 kDa were detected by electrophoresis, corresponding to dentin matrix protein-1, osteocalcin, osteopontin, proteoglycan, glycoprotein, sialoprotein, and phosphophoryn [87]. In vivo, osteonectin was found in the dentinal tubules of DDM [88]. Besides, the demineralization process is required for freeing the various growth factors and proteins which are essential for tissue regeneration and repair. Consequently, demineralization process is believed to induce release an abundant amount of transforming growth factor-β; an intermediate abundance of BMP-2, fibroblast growth factor-2, vascular endothelial growth factor, platelet-derived growth factor and insulin-like growth factor-1 with a lower abundance from BMP-4 and BMP-7 [89].
The degree of demineralization is critical for optimal dentin regeneration; the partially demineralized dentin matrix (PDDM) is thought to have optimal conditions for dentin regeneration. Partial demineralization results in the elimination of the major part of the mineral phase and immunogenic components while retaining a very low fraction of minerals (5–10 wt%), providing an osteoconductive and osteoinductive scaffold containing several growth factors [62]. On the other hand, the complete DDM (CDDM) showed bone resorption in the early stage of bone regeneration, probably because of the enzymatic digestion of exposed collagen [90]. PDDM probably promotes more osteogenic effects than CDDM does since several noncollagenous proteins were released from the dentin matrix during the process of demineralization. This may account for the more prominent bone formation in PDDM than CDDM in most previous studies.
In terms of particle size, the dentinogenic properties of DDM were greatly affected by the size and shape of dentin matrix particles. The larger-sized particles of the DDM, whose sizes ranged between 350 μm and 800 μm, were found to have better bone regeneration results than the smaller-sized particles that had more resorbability in the defect site before the initiation of new bone formation [89]. PDDM with larger particle sizes induced prominent bone regeneration, probably because PDDM possessed a suitable surface for cell attachment. There might be an exquisite balance between its resorption and bone formation on it. PDDM could be considered as a potential bone substitute.
Dentin and bone are mineralized tissues known to be an organic–inorganic hybrid. They are almost similar.
n their biochemical components but dentin is acellular matrix, while bone includes osteocytes. Autogenous bone is an ideal bone graft material as it has osteoconductive, osteoinductive and osteogenic capabilities but the major drawbacks of autogenous bone are that it requires a secondary donor site with an increase in the susceptibility risk of infection, therefore clinicians prefer commercially available non-autogenous graft materials. Allogenic and xenogeneic bone grafts have osteoinductive ability, but risk of viral infection still remains a considerable problem. Alloplastic bone grafts are clinically used, but they have disadvantages such as limited osteoinductivity and high cost [91]. Among these, demineralized freeze-dried bone allografts have been widely used for bone augmentation [92]. Demineralized bone matrix (DBM) likely as DDM is predominantly composed of COL-1 (95%) with the remainder comprising of NCPs with a small amount of growth factors. Consequently, DDM and DBM can be defined as acid-insoluble collagen bind with BMPs, which are members of the TGF-β super-family that enhances bone formation [93]. The quality and effectiveness of commercial DBM varies with processing techniques and several donor dependent factors likely as gender and age as they have an effect on osteoinductivity of DBM [94]. Therefore, differences in preparation and processing methods for bone can impact properties and clinical performance of DBM. In our opinion, the superiority of autogenous DDM over other graft materials should be confined to the dental applications facilitating the release of BMPs to induce the differentiation of undifferentiated mesenchymal cells into osteogenic and odontogenic cells, which have the potential to stimulate bone and dentin formation [95]. DDM is biocompatible, does not induce foreign body reactions and can be prepared by a standard treatment with very low cost.
It is beneficial to utilize extracted teeth as bone substitutes in implant dentistry, even though there are limited cases with available teeth, in addition to a limited volume. However, there is no risk of transmitting diseases as it is autogenous tissue and no additional surgery is needed to harvest tissues since unwanted teeth are utilized and this raises the need for tooth banking. The Korea Tooth Bank, which was established in Seoul in 2009, is one such tooth-banking facility that can procure and store teeth, and then process them into bone graft substitutes. The Hospital Tooth Bank at Seoul National University Bundang Hospital, which was established in 2010 for performing storage and grafting of auto-tooth bone grafts based on experimental and clinical research.
Concluding remarks and future perspectives
Before clinical application and for each specific purpose, optimizing protocols of demineralization, characterization, developing a proper consistency, and applying a proper handling technique are necessary for standardization. Further studies are required to determine the most suitable conditions of demineralization and particle sizes for clinical application in implant dentistry.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
The original online version of this article was revised: In this article the graphics relating to Figs 3, 4, 5 and 6 captions had been interchanged; they have been corrected now.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/20/2022
A Correction to this paper has been published: 10.1007/s13770-022-00463-3
References
- 1.Um IW, Kim YK, Mitsugi M. Demineralized dentin matrix scaffolds for alveolar bone engineering. J Indian Prosthodont Soc. 2017;17:120–127. doi: 10.4103/jips.jips_62_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pajor K, Pajchel L, Kolmas J. Hydroxyapatite and fluorapatite in conservative dentistry and oral implantology-A review. Materials (Basel) 2019;12:2683. doi: 10.3390/ma12172683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: structure, composition and mineralization. Front Biosci (Elite Ed) 2011;3:711–735. doi: 10.2741/e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kontakiotis EG, Tsatsoulis IN, Filippatos CG, Agrafioti A. A quantitative and diametral analysis of human dentinal tubules at pulp chamber ceiling and floor under scanning electron microscopy. Aust Endod J. 2015;41:29–34. doi: 10.1111/aej.12068. [DOI] [PubMed] [Google Scholar]
- 5.Kawashima N, Okiji T. Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit Anom (Kyoto) 2016;56:144–153. doi: 10.1111/cga.12169. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Jing Y, Wang K, Ren Y, Liu X, Wang X, et al. Dentinal mineralization is not limited in the mineralization front but occurs along with the entire odontoblast process. Int J Biol Sci. 2018;14:693–704. doi: 10.7150/ijbs.25712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almushayt A, Narayanan K, Zaki AE, George A. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther. 2006;13:611–620. doi: 10.1038/sj.gt.3302687. [DOI] [PubMed] [Google Scholar]
- 8.Harrán Ponce E, Canalda Sahli C, Vilar Fernandez JA. Study of dentinal tubule architecture of permanent upper premolars: evaluation by SEM. Aust Endod J. 2001;27:66–72. doi: 10.1111/j.1747-4477.2001.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 9.Senawongse P, Otsuki M, Tagami J, Mjör I. Age-related changes in hardness and modulus of elasticity of dentine. Arch Oral Biol. 2006;51:457–463. doi: 10.1016/j.archoralbio.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Zhang YR, Du W, Zhou XD, Yu HY. Review of research on the mechanical properties of the human tooth. Int J Oral Sci. 2014;6:61–69. doi: 10.1038/ijos.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandolfi MG, Taddei P, Pondrelli A, Zamparini F, Prati C, Spagnuolo G. Demineralization, collagen modification and remineralization degree of human dentin after EDTA and citric acid treatments. Materials (Basel) 2018;12:25. doi: 10.3390/ma12010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barón M, Morales V, Fuentes MV, Linares M, Escribano N, Ceballos L. The influence of irrigation solutions in the inorganic and organic radicular dentine composition. Aust Endod J. 2020;46:217–225. doi: 10.1111/aej.12395. [DOI] [PubMed] [Google Scholar]
- 13.Besinis A, van Noort R, Martin N. Remineralization potential of fully demineralized dentin infiltrated with silica and hydroxyapatite nanoparticles. Dent Mater. 2014;30:249–262. doi: 10.1016/j.dental.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Lippert F, Churchley D, Lynch RJ. Effect of Lesion Baseline Severity and Mineral Distribution on Remineralization and Progression of Human and Bovine Dentin Caries Lesions. Caries Res. 2015;49:467–476. doi: 10.1159/000431039. [DOI] [PubMed] [Google Scholar]
- 15.Miller CA, Ashworth E, Deery C, El Sharkasi L, Moorehead RD, Martin N. Effect of demineralising agents on organic and inorganic components of dentine. Caries Res. 2021;55:521–33. [DOI] [PubMed]
- 16.Arksey H, O'Malley L. Scoping studies: Towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 17.Liu S, Sun J, Yuan S, Yang Y, Gong Y, Wang Y, et al. Treated dentin matrix induces odontogenic differentiation of dental pulp stem cells via regulation of Wnt/β-catenin signaling. Bioact Mater. 2021;7:85–97. doi: 10.1016/j.bioactmat.2021.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Ma B, Yang H, Qiao J, Tian W, Yu R. Xenogeneic dentin matrix as a scaffold for biomineralization and induced odontogenesis. Biomed Mater. 2021;16:045020. [DOI] [PubMed]
- 19.Zhang J, Lan T, Han X, Xu Y, Liao L, Xie L, et al. Improvement of ECM-based bioroot regeneration via N-acetylcysteine-induced antioxidative effects. Stem Cell Res Ther. 2021;12:202. doi: 10.1186/s13287-021-02237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu J, Chen J, Li W, Yang X, Yang J, Quan H, et al. Laminin-modified dental pulp extracellular matrix for dental pulp regeneration. Front Bioeng Biotechnol. 2021 doi: 10.3389/fbioe.2020.595096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holiel AA, Mahmoud EM, Abdel-Fattah WM, Kawana KY. Histological evaluation of the regenerative potential of a novel treated dentin matrix hydrogel in direct pulp capping. Clin Oral Investig. 2021;25:2101–2112. doi: 10.1007/s00784-020-03521-z. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, Zhang W, Li Y, Ren L, Deng H, Yin X, et al. Human umbilical cord mesenchymal stem cell differentiation into odontoblast-like cells and endothelial cells: A Potential cell source for dental pulp tissue engineering. Front Physiol. 2020;11:593. doi: 10.3389/fphys.2020.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CC, Lin TA, Wu SY, Lin CP, Chang HH. Regeneration of tooth with allogenous, autoclaved treated dentin matrix with dental pulpal stem cells: An in vivo study. J Endod. 2020;46:1256–1264. doi: 10.1016/j.joen.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Meng H, Hu L, Zhou Y, Ge Z, Wang H, Wu CT, et al. A Sandwich structure of human dental pulp stem cell sheet, treated dentin matrix, and matrigel for tooth root regeneration. Stem Cells Dev. 2020;29:521–532. doi: 10.1089/scd.2019.0162. [DOI] [PubMed] [Google Scholar]
- 25.Bakhtiar H, Mazidi A, Mohammadi-Asl S, Hasannia S, Ellini MR, Pezeshki-Modaress M, et al. Potential of treated dentin matrix xenograft for dentin-pulp tissue engineering. J Endod. 2020;46:57–64.e1. doi: 10.1016/j.joen.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Li J, Hu Y, Sun J, Guo W, Li H, et al. Treated dentin matrix particles combined with dental follicle cell sheet stimulate periodontal regeneration. Dent Mater. 2019;35:1238–1253. doi: 10.1016/j.dental.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Ma Y, Guo W, Yang B, Tian W. Stem cells from human exfoliated deciduous teeth as an alternative cell source in bio-root regeneration. Theranostics. 2019;9:2694–2711. doi: 10.7150/thno.31801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun J, Li J, Li H, Yang H, Chen J, Yang B, et al. tBHQ suppresses osteoclastic resorption in xenogeneic-treated dentin matrix-based scaffolds. Adv Healthc Mater. 2017;6:1700127. [DOI] [PubMed]
- 29.Chen J, Cui C, Qiao X, Yang B, Yu M, Guo W, et al. Treated dentin matrix paste as a novel pulp capping agent for dentin regeneration. J Tissue Eng Regen Med. 2017;11:3428–3436. doi: 10.1002/term.2256. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Sun J, Li J, Yang H, Luo X, Chen J, et al. Xenogeneic bio-root prompts the constructive process characterized by macrophage phenotype polarization in rodents and nonhuman primates. Adv Healthc Mater. 2017;6:1601112. [DOI] [PubMed]
- 31.Bakhtiar H, Mirzaei H, Bagheri MR, Fani N, Mashhadiabbas F, Baghaban Eslaminejad M, et al. Histologic tissue response to furcation perforation repair using mineral trioxide aggregate or dental pulp stem cells loaded onto treated dentin matrix or tricalcium phosphate. Clin Oral Investig. 2017;21:1579–1588. doi: 10.1007/s00784-016-1967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang ZS, Feng ZH, Wu GF, Bai SZ, Dong Y, Chen FM, et al. The use of platelet-rich fibrin combined with periodontal ligament and jaw bone mesenchymal stem cell sheets for periodontal tissue engineering. Sci Rep. 2016;6:28126. doi: 10.1038/srep28126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian Y, Bai D, Guo W, Li J, Zeng J, Yang L, et al. Comparison of human dental follicle cells and human periodontal ligament cells for dentin tissue regeneration. Regen Med. 2015;10:461–479. doi: 10.2217/rme.15.21. [DOI] [PubMed] [Google Scholar]
- 34.Luo X, Yang B, Sheng L, Chen J, Li H, Xie L, et al. CAD based design sensitivity analysis and shape optimization of scaffolds for bio-root regeneration in swine. Biomaterials. 2015;57:59–72. doi: 10.1016/j.biomaterials.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Sun Q, Xie L, Jiang Z, Feng L, Yu M, et al. Comparison of the odontogenic differentiation potential of dental follicle, dental papilla, and cranial neural crest cells. J Endod. 2015;41:1091–1099. doi: 10.1016/j.joen.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Chen G, Chen J, Yang B, Li L, Luo X, Zhang X, et al. Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials. 2015;52:56–70. doi: 10.1016/j.biomaterials.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Ji B, Sheng L, Chen G, Guo S, Xie L, Yang B, et al. The combination use of platelet-rich fibrin and treated dentin matrix for tooth root regeneration by cell homing. Tissue Eng Part A. 2015;21:26–34. doi: 10.1089/ten.tea.2014.0043. [DOI] [PubMed] [Google Scholar]
- 38.Jiao L, Xie L, Yang B, Yu M, Jiang Z, Feng L, et al. Cryopreserved dentin matrix as a scaffold material for dentin-pulp tissue regeneration. Biomaterials. 2014;35:4929–4939. doi: 10.1016/j.biomaterials.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Ge Y, Chen G, Yan Z, Yu M, Feng L, et al. Hertwig's epithelial root sheath cells regulate osteogenic differentiation of dental follicle cells through the Wnt pathway. Bone. 2014;63:158–165. doi: 10.1016/j.bone.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Guo L, Li J, Qiao X, Yu M, Tang W, Wang H, et al. Comparison of odontogenic differentiation of human dental follicle cells and human dental papilla cells. PLoS ONE. 2013;8:e62332. doi: 10.1371/journal.pone.0062332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang B, Chen G, Li J, Zou Q, Xie D, Chen Y, et al. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix - based scaffold. Biomaterials. 2012;33:2449–2461. doi: 10.1016/j.biomaterials.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 42.Guo W, Gong K, Shi H, Zhu G, He Y, Ding B, et al. Dental follicle cells and treated dentin matrix scaffold for tissue engineering the tooth root. Biomaterials. 2012;33:1291–1302. doi: 10.1016/j.biomaterials.2011.09.068. [DOI] [PubMed] [Google Scholar]
- 43.Li R, Guo W, Yang B, Guo L, Sheng L, Chen G, et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials. 2011;32:4525–4538. doi: 10.1016/j.biomaterials.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Guo W, He Y, Zhang X, Lu W, Wang C, Yu H, et al. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials. 2009;30:6708–6723. doi: 10.1016/j.biomaterials.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 45.Kim BJ, Kim SK, Lee JH. Bone regeneration of demineralized dentin matrix with platelet-rich fibrin and recombinant human bone morphogenetic protein-2 on the bone defects in rabbit calvaria. Maxillofac Plast Reconstr Surg. 2021;43:34. doi: 10.1186/s40902-021-00320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ku JK, Um IW, Jun MK, Kim IH. Dentin-derived-barrier membrane in guided bone regeneration: A Case Report. Materials (Basel) 2021;14:2166. doi: 10.3390/ma14092166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han J, Jeong W, Kim MK, Nam SH, Park EK, Kang HW. Demineralized dentin matrix particle-based bio-ink for patient-specific shaped 3D dental tissue regeneration. Polymers (Basel) 2021;13:1294. doi: 10.3390/polym13081294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kabir MA, Murata M, Shakya M, Yamada K, Akazawa T. Bio-absorption of human dentin-derived biomaterial in sheep critical-size Iliac defects. Materials (Basel) 2021;14:223. doi: 10.3390/ma14010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jing X, Xie B, Li X, Dai Y, Nie L, Li C. Peptide decorated demineralized dentin matrix with enhanced bioactivity, osteogenic differentiation via carboxymethyl chitosan. Dent Mater. 2021;37:19–29. doi: 10.1016/j.dental.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 50.Gao X, Qin W, Chen L, Fan W, Ma T, Schneider A, et al. Effects of targeted delivery of metformin and dental pulp stem cells on osteogenesis via demineralized dentin matrix under high glucose conditions. ACS Biomater Sci Eng. 2020;6:2346–2356. doi: 10.1021/acsbiomaterials.0c00124. [DOI] [PubMed] [Google Scholar]
- 51.Umebayashi M, Ohba S, Kurogi T, Noda S, Asahina I. Full regeneration of maxillary alveolar bone using autogenous partially demineralized dentin matrix and particulate cancellous bone and marrow for implant-supported full arch rehabilitation. J Oral Implantol. 2020;46:122–127. doi: 10.1563/aaid-joi-D-19-00315. [DOI] [PubMed] [Google Scholar]
- 52.Um IW, Kim YK, Park JC, Lee JH. Clinical application of autogenous demineralized dentin matrix loaded with recombinant human bone morphogenetic-2 for socket preservation: A case series. Clin Implant Dent Relat Res. 2019;21:4–10. doi: 10.1111/cid.12710. [DOI] [PubMed] [Google Scholar]
- 53.Li P, Zhu H, Huang D. Autogenous DDM versus Bio-Oss granules in GBR for immediate implantation in periodontal postextraction sites: A prospective clinical study. Clin Implant Dent Relat Res. 2018;20:923–928. doi: 10.1111/cid.12667. [DOI] [PubMed] [Google Scholar]
- 54.Melling GE, Colombo JS, Avery SJ, Ayre WN, Evans SL, Waddington RJ, et al. Liposomal delivery of demineralized dentin matrix for dental tissue regeneration. Tissue Eng Part A. 2018;24:1057–1065. doi: 10.1089/ten.tea.2017.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Minamizato T, Koga T, Takashi I, Nakatani Y, Umebayashi M, Sumita Y, Ikeda T, et al. Clinical application of autogenous partially demineralized dentin matrix prepared immediately after extraction for alveolar bone regeneration in implant dentistry: a pilot study. Int J Oral Maxillofac Surg. 2018;47:125–132. doi: 10.1016/j.ijom.2017.02.1279. [DOI] [PubMed] [Google Scholar]
- 56.Kabir MA, Murata M, Akazawa T, Kusano K, Yamada K, Ito M. Evaluation of perforated demineralized dentin scaffold on bone regeneration in critical-size sheep iliac defects. Clin Oral Implants Res. 2017;28:e227–e235. doi: 10.1111/clr.13000. [DOI] [PubMed] [Google Scholar]
- 57.Pang KM, Um IW, Kim YK, Woo JM, Kim SM, Lee JH. Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: a prospective randomized clinical trial in comparison with anorganic bovine bone. Clin Oral Implants Res. 2017;28:809–815. doi: 10.1111/clr.12885. [DOI] [PubMed] [Google Scholar]
- 58.Nam JW, Kim MY, Han SJ. Cranial bone regeneration according to different particle sizes and densities of demineralized dentin matrix in the rabbit model. Maxillofac Plast Reconstr Surg. 2016;38:27. doi: 10.1186/s40902-016-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SK, Huh CK, Lee JH, Kim KW, Kim MY. Histologic study of bone-forming capacity on polydeoxyribonucleotide combined with demineralized dentin matrix. Maxillofac Plast Reconstr Surg. 2016;38:7. doi: 10.1186/s40902-016-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim YK, Lee JH, Um IW, Cho WJ. Guided bone regeneration using demineralized dentin matrix: Long-term follow-up. J Oral Maxillofac Surg. 2016;74:e1–9. doi: 10.1016/S0278-2391(15)01481-0. [DOI] [PubMed] [Google Scholar]
- 61.Gomes MF, Valva VN, Vieira EM, Giannasi LC, Salgado MA, Vilela-Goulart MG. Homogenous demineralized dentin matrix and platelet-rich plasma for bone tissue engineering in cranioplasty of diabetic rabbits: biochemical, radiographic, and histological analysis. Int J Oral Maxillofac Surg. 2016;45:255–266. doi: 10.1016/j.ijom.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Koga T, Minamizato T, Kawai Y, Miura K, Takashi I, Nakatani Y, Sumita Y, et al. Bone regeneration using dentin matrix depends on the degree of demineralization and particle size. PLoS ONE. 2016;11:e0147235. doi: 10.1371/journal.pone.0147235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu G, Xu G, Gao Z, Liu Z, Xu J, Wang J, et al. Demineralized dentin matrix induces odontoblastic differentiation of dental pulp stem cells. Cells Tissues Organs. 2016;201:65–76. doi: 10.1159/000440952. [DOI] [PubMed] [Google Scholar]
- 64.Qin X, Zou F, Chen W, Xu Y, Ma B, Huang Z, et al. Demineralized dentin as a semi-rigid barrier for guiding periodontal tissue regeneration. J Periodontol. 2015;86:1370–1379. doi: 10.1902/jop.2015.150271. [DOI] [PubMed] [Google Scholar]
- 65.Bakhshalian N, Hooshmand S, Campbell SC, Kim JS, Brummel-Smith K, Arjmandi BH. Biocompatibility and microstructural analysis of osteopromotive property of allogenic demineralized dentin matrix. Int J Oral Maxillofac Implants. 2013;28:1655–1662. doi: 10.11607/jomi.2833. [DOI] [PubMed] [Google Scholar]
- 66.Li J, Yang J, Zhong X, He F, Wu X, Shen G. Demineralized dentin matrix composite collagen material for bone tissue regeneration. J Biomater Sci Polym Ed. 2013;24:1519–1528. doi: 10.1080/09205063.2013.777227. [DOI] [PubMed] [Google Scholar]
- 67.Zhang H, Liu S, Zhou Y, Tan J, Che H, Ning F, et al. Natural mineralized scaffolds promote the dentinogenic potential of dental pulp stem cells via the mitogen-activated protein kinase signaling pathway. Tissue Eng Part A. 2012;18:677–691. doi: 10.1089/ten.tea.2011.0269. [DOI] [PubMed] [Google Scholar]
- 68.Yagihashi K, Miyazawa K, Togari K, Goto S. Demineralized dentin matrix acts as a scaffold for repair of articular cartilage defects. Calcif Tissue Int. 2009;84:210–220. doi: 10.1007/s00223-008-9205-7. [DOI] [PubMed] [Google Scholar]
- 69.Gomes MF, Destro MF, Banzi EC, Vieira EM, Morosolli AR, Goulart MD. Optical density of bone repair after implantation of homogenous demineralized dentin matrix in diabetic rabbits. Braz Oral Res. 2008;22:275–280. doi: 10.1590/S1806-83242008000300015. [DOI] [PubMed] [Google Scholar]
- 70.Gomes MF, dos Anjos MJ, Nogueira Tde O, Catanzaro Guimarães SA. Autogenous demineralized dentin matrix for tissue engineering applications: radiographic and histomorphometric studies. Int J Oral Maxillofac Implants. 2002;17:488–497. [PubMed] [Google Scholar]
- 71.Gomes MF, dos Anjos MJ, Nogueira TO, Guimarães SA. Histologic evaluation of the osteoinductive property of autogenous demineralized dentin matrix on surgical bone defects in rabbit skulls using human amniotic membrane for guided bone regeneration. Int J Oral Maxillofac Implants. 2001;16:563–571. [PubMed] [Google Scholar]
- 72.Xu X, Liang C, Gao X, Huang H, Xing X, Tang Q, et al. Adipose Tissue-derived Microvascular Fragments as Vascularization Units for Dental Pulp Regeneration. J Endod. 2021;47:1092–1100. doi: 10.1016/j.joen.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 73.Elfana A, El-Kholy S, Saleh HA, Fawzy E-S. Alveolar ridge preservation using autogenous whole-tooth versus demineralized dentin grafts: A randomized controlled clinical trial. Clin Oral Implants Res. 2021;32:539–548. doi: 10.1111/clr.13722. [DOI] [PubMed] [Google Scholar]
- 74.Wen B, Huang Y, Qiu T, Huo F, Xie L, Liao L, et al. Reparative dentin formation by dentin matrix proteins and small extracellular vesicles. J Endod. 2021;47:253–262. doi: 10.1016/j.joen.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 75.Mehrvarzfar P, Abbott PV, Mashhadiabbas F, Vatanpour M, Tour SS. Clinical and histological responses of human dental pulp to MTA and combined MTA/treated dentin matrix in partial pulpotomy. Aust Endod J. 2018;44:46–53. doi: 10.1111/aej.12217. [DOI] [PubMed] [Google Scholar]
- 76.Ouyyamwongs W, Leepong N, Suttapreyasri S. Alveolar ridge preservation using autologous demineralized tooth matrix and platelet-rich fibrin versus platelet-rich fibrin alone: A split-mouth randomized controlled clinical trial. Implant Dent. 2019;28:455–462. doi: 10.1097/ID.0000000000000918. [DOI] [PubMed] [Google Scholar]
- 77.Xu X, Sohn DS, Kim HG, Lee SJ, Moon YS. Comparative histomorphometric analysis of maxillary sinus augmentation with deproteinized bovine bone and demineralized particulate human tooth graft: An experimental study in rabbits. Implant Dent. 2018;27:324–331. doi: 10.1097/ID.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 78.Feng G, Wu Y, Yu Y, Huang L, An S, Hu B, et al. Periodontal ligament-like tissue regeneration with drilled porous decalcified dentin matrix sheet composite. Oral Dis. 2018;24:429–441. doi: 10.1111/odi.12734. [DOI] [PubMed] [Google Scholar]
- 79.Al-Asfour A, Farzad P, Al-Musawi A, Dahlin C, Andersson L. Demineralized xenogenic dentin and autogenous bone as onlay grafts to rabbit tibia. Implant Dent. 2017;26:232–237. doi: 10.1097/ID.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 80.Park M, Mah YJ, Kim DH, Kim ES, Park EJ. Demineralized deciduous tooth as a source of bone graft material: its biological and physicochemical characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:307–314. doi: 10.1016/j.oooo.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 81.Park SM, Kim DH, Pang EK. Bone formation of demineralized human dentin block graft with different demineralization time: In vitro and in vivo study. J Craniomaxillofac Surg. 2017;45:903–912. doi: 10.1016/j.jcms.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 82.de Oliveira GS, Miziara MN, Silva ER, Ferreira EL, Biulchi AP, Alves JB. Enhanced bone formation during healing process of tooth sockets filled with demineralized human dentine matrix. Aust Dent J. 2013;58:326–332. doi: 10.1111/adj.12088. [DOI] [PubMed] [Google Scholar]
- 83.Mordenfeld A, Hallman M, Lindskog S. Tissue reactions to subperiosteal onlays of demineralized xenogenous dentin blocks in rats. Dent Traumatol. 2011;27:446–451. doi: 10.1111/j.1600-9657.2011.01026.x. [DOI] [PubMed] [Google Scholar]
- 84.Koike Y, Murakami S, Matsuzaka K, Inoue T. The effect of Emdogain on ectopic bone formation in tubes of rat demineralized dentin matrix. J Periodontal Res. 2005;40:385–394. doi: 10.1111/j.1600-0765.2005.00819.x. [DOI] [PubMed] [Google Scholar]
- 85.Bono N, Tarsini P, Candiani G. Demineralized dentin and enamel matrices as suitable substrates for bone regeneration. J Appl Biomater Funct Mater. 2017;15(3):e236–e243. doi: 10.5301/jabfm.5000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahn GJ, Kim YK, Um IW, Kim JY. Evaluation of prognosis of autogenous tooth bone graft material according to the condition of donor tooth. J Dent Implant Res. 2015;341:7–11. [Google Scholar]
- 87.Kim YK, Lee JH, Kim KW, Um IW, Murata M, Ito K. Analysis of organic components and osteoinductivity in autogenous tooth bone graft material. J Korean Assoc Maxillofac Plast Reconstr Surg. 2013;35:353–359. [Google Scholar]
- 88.Kim YK, Um IW, An HJ, Kim KW, Hong KS, Murata M. Effects of demineralized dentin matrix used as an rhBMP-2 carrier for bone regeneration. J Hard Tissue Biol. 2014;23:415–422. doi: 10.2485/jhtb.23.415. [DOI] [Google Scholar]
- 89.Gao X, Qin W, Wang P, Wang L, Weir MD, Reynolds MA, et al. Nano-structured demineralized human dentin matrix to enhance bone and dental repair and regeneration. Appl Sci. 2019;9:1013. doi: 10.3390/app9051013. [DOI] [Google Scholar]
- 90.Murata M. Collagen biology for bone regenerative surgery. J Korean Assoc Oral Maxillofac Surg. 2012;38:321–325. doi: 10.5125/jkaoms.2012.38.6.321. [DOI] [Google Scholar]
- 91.Kim SY, Kim YK, Park YH, Park JC, Ku JK, Um IW, et al. Evaluation of the healing potential of demineralized dentin matrix fixed with recombinant human bone morphogenetic protein-2 in bone grafts. Materials (Basel) 2017;10:1049. doi: 10.3390/ma10091049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cammack GV, 2nd, Nevins M, Clem DS, 3rd, Hatch JP, Mellonig JT. Histologic evaluation of mineralized and demineralized freeze-dried bone allograft for ridge and sinus augmentations. Int J Periodontics Restorative Dent. 2005;25(3):231–237. [PubMed] [Google Scholar]
- 93.Kim YK, Bang K, Murata M, Mitsugi M, Um IW. Retrospective clinical study of allogenic demineralized dentin matrix for alveolar bone repair. J Hard Tissue Biol. 2017;26:95–102. doi: 10.2485/jhtb.26.95. [DOI] [Google Scholar]
- 94.Traianedes K, Russell JL, Edwards JT, Stubbs HA, Shanahan IR, Knaack D. Donor age and gender effects on osteoinductivity of demineralized bone matrix. J Biomed Mater Res B Appl Biomater. 2004;70(1):21–29. doi: 10.1002/jbm.b.30015. [DOI] [PubMed] [Google Scholar]
- 95.Bessho K, Tanaka N, Matsumoto J, Tagawa T, Murata M. Human dentin-matrix-derived bone morphogenetic protein. J Dent Res. 1991;70:171–175. doi: 10.1177/00220345910700030301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.