Abstract
Background:
Selective serotonin reuptake inhibitor (SSRI) is believed to accelerate wound healing, and thus expected to have a positive effect on rotator cuff repair. We hypothesized that SSRI has a positive effect on the healing of the bone-tendon interface (BTI), and improved rotator cuff tear healing would be confirmed by mechanical strength measurements and histological assessment of the restored tendon.
Methods:
The study used 40 adult male Sprague–Dawley wild-type rats. The animals were divided into two groups: group-SSRI, the supraspinatus repair with SSRI injection group, and group-C, conventional supraspinatus repair only without SSRI. Biomechanical and histological analyses were performed 8 weeks after index rotator cuff surgery.
Results:
The ultimate load (N) was significantly higher in group-SSRI than in group-C (54.8 ± 56.9 Vs 25.1 ± 11.1, p = .031). In the histological evaluation, the Bonar score confirmed significant differences in collagen fiber density (group-C: 0.6 ± 0.5, group-SSRI: 1.1 ± 0.6, p = .024), vascularity (group-C: 0.1 ± 0.2, group-SSRI: 0.3 ± 0.4, p = .024) and cellularity (group-C: 1.7 ± 0.4, group-SSRI: 2.0 ± 0.0, p = .023) between the groups. Based on the total score, group-SSRI was significantly better compared with group-C (6.3 ± 2.7 Vs 4.3 ± 1.9, p = .019).
Conclusion:
Our study demonstrated that SSRI could facilitate improved biomechanical and histological outcomes 8 weeks after rotator cuff repair in a rat model. Consequently, SSRI may improve healing after rotator cuff repair.
Keywords: Serotonin uptake inhibitors, Anti-inflammatory agents, Histology, Biomechanics, Rotator cuff healing
Introduction
As the incidence of rotator cuff tears (RCT) increases due to aging and sporting activities, it is hoped that the injury inflicted can be repaired through surgical treatment. However, despite the implementation of novel surgical interventions for rotator cuff repair, failure rates remain quite high [1]. Serotonin (5-hydroxytryptamine, 5-HT) is a well-known key mood modulator, strongly associated with the etiology of depression [2]. Based on the role of 5-HT, selective serotonin reuptake inhibitors (SSRIs) have been used worldwide as a first-line of treatment for major depressive disorder (MDD) [3]. The major function of SSRIs is to increase the amount of serotonin in the synaptic cleft by down-regulating post-synaptic beta receptors and serotonin receptors in the brain [4, 5]. Several studies have suggested that innate immune activation during chronic medical illnesses (e.g., cancer, heart disease, type 2 diabetes mellitus (DM), and autoimmune disorders), which is characterized by elevated levels of inflammatory cytokines, may contribute to high rates of depression [6]. Recent studies have shown that adult patients with MDD have elevated levels of proinflammatory cytokines (IL-1β, IL-6, IFN-γ, and TNF-α) and decreased levels of anti-inflammatory cytokines (IL-4 and IL-10), leading to an imbalance of inflammatory factors [7]. In particular, antidepressant treatment has been reported to significantly reduce IL-6 and TNF-α levels [8]. Many studies have proved the effectiveness of SSRIs in treating depression, and it is believed that there is a link between SSRIs and certain inflammatory cytokines involved in depression. SSRIs exhibit an anti-inflammatory effect by significantly decreasing the levels of proinflammatory cytokines, including IL-1β, IL-6, TNF-α, and by significantly increasing the levels of the anti-inflammatory cytokine IL-10 [9, 10]. However, there have not been any studies on administration for rotator cuff tendon‐to‐bone healing. This study aimed to investigate the effect of SSRI on BTI healing following rotator cuff repair in a rat model. We hypothesized that SSRI has a positive effect on BTI healing, and mechanical strength and histological findings of the restored tendon support the observed beneficial effects.
Materials and methods
Animal model
All animal procedures were approved by the institutional animal commission at the author’s institution. The G*Power software, version 3.01 (Franz Faul, Christian-Albrechts-Universität Kiel, Kiel, Germany) was used to calculate the sample size required for the comparison between two independent means in a 1:1 allocation ratio. Based on previous studies that used power analysis, it was determined that 20 specimens per group (comparison between two groups, α error = 0.05, power = 0.95, dropout rate = 20%) were required for the detection of significant differences in the ultimate fracture load [11]. We used 12-week-old male Sprague–Dawley rats that were housed in a specific pathogen-free facility. Prior to the experimental process, the rats were acclimated to a 12 h/12 h light/dark cycle at 22 °C ± 2 °C for 1 week, and they were allowed unlimited access to food and water. The rat’s right shoulder was used for the subsequent biomechanical evaluation, including assessment of ultimate failure load at 8 weeks, whereas the left shoulder was used for histological analysis. A total of 40 rats were randomly allocated into two groups (20 each): group-C, repair only (control), and group-SSRI (SSRI treatment group).
Surgical procedure
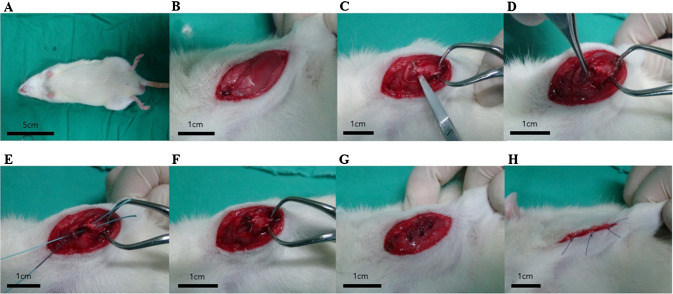
All animals were anesthetized using zolazepam (0.05–0.3 ml/kg, Zoletil®, Virbac S.A., Carros Cedex, France) and xylazine hydrochloride (0.15 ml/kg, Rompun®, Bayer HealthCare, Leverkusen, Germany). This offered pain relief and muscle relaxation that helped these animals to maintain a sleep state. The right shoulder of the rats was shaved and sterilized to maintain aseptic conditions. Consequently, rotator cuff incisions were performed. Briefly, palpate the scapular spine and made a 3-cm longitudinal incision. The deltoid muscle was devided bluntly, and the supraspinatus tendon was confirmed. The supraspinatus tendon was seperated with metzembaum and cut at the end of the tendon insertion site using a blade. Exposure the greater tuberosity widely and two parallel bone tunnels were made using a drill. The supraspinatus tendon was repaired with a single row technique through the tunnel using 3–0 Ethibond (Ethicon, Somerville, NJ, USA) [11]. (Fig. 1) Rats in the control group were subjected to surgical repair and had no other interventions. In the experimental group, sertraline was injected intraperitoneally after repair. Sertraline is the most commonly used SSRI class drug along with fluoxetine and paroxetine. Sertraline is known to have a greater dose effect than other drugs. Sertraline (Hanmi Pharm. Co., Suwon, South Korea) was prepared at a dose of 10 mg/kg/day and intraperitoneally injected with 0.2 ml each time for a period of 2 weeks (five times/week). Drug dose and regimen were referred to the existing rat model experiment using SSRI. [12–14]. Eight weeks after repair, all rats were sacrificed, and the tendon tissue was harvested.
Fig. 1.
Rotator cuff tear (RCT) surgical procedure in a rat model. A–D Creation of the RCT model and E–H reattachment of the supraspinatus tendon
Biomechanical evaluation
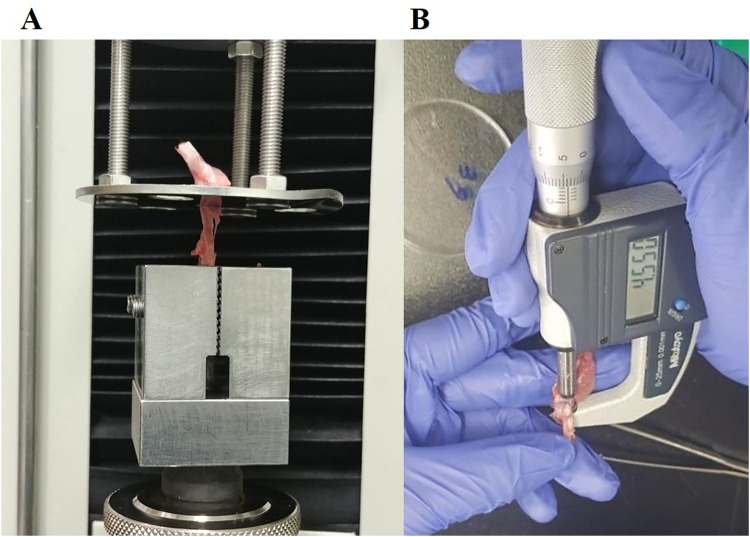
The supraspinatus tendon of the right shoulder was adequately harvested. Using a Digimatic Micrometer (MDC-25SB; Mitutoyo Co., Kanagawa, Japan), the cross-sectional area was evaluated at the mid-portion of the suprasupinatus tendon, and using optical methods its width was calculated [11, 15]. Load to failure, mode of failure (insertional tear or mid substance tear), and ultimate stress measured at a rate of 10 mm/min with a 20-kg load cell using a universal test machine (OTT-03; Oriental TM, Siheung, South Korea) and a custom fixture clamping system were included for the mechanical evaluation parameters. An insertional tear suggests relatively weak tendon-bone healing, whereas a mid substance tear suggests strong tendon-bone healing. [16]. Allowing the supraspinatus tendon to be fixed to this system along its anatomic direction led to tensile loading and tendon-to-be interface, consequently forming a right angle [11]. (Fig. 2).
Fig. 2.
Biomechanical evaluation. A Mode of failure, load to failure, and ultimate stress measurements. B Cross-sectional area measurements of the supraspinatus tendon
Histological evaluation
All specimens from each group were histologically analyzed to determine the extent of regeneration. First, the left shoulder of each rat was harvested. Specimens were fixed in neutral buffered 10% formalin (pH 7.4) and paraffin blocks were made. Those were cut into 4-μm thick sections and then deparaffinized and rehydrated [17]. Second, sample slides were randomly selected and stained with hematoxylin and eosin (H&E), picrosirius red, and safranin-O. The analyses were performed by two investigators who were blinded to the different animal groups. Bonar scoring with an H&E stained slide was utilized for the histological analyses [11, 18]. The score was set to 0–3, with 0 being the worst result. The greater the healing observed, the higher the score. The slides were interpreted with the scoring scale, and various features of the tendon tissue were evaluated. The whole slide was used for the assessment of areas of increased cellularity and vascularity, proportion of collagen fibers, and level of maturation of the BTI structure. The scored items included (1) continuity of collagen fiber, (2) orientation of collagen fiber, (3) density of collagen fiber, (4) maturation of the tendon-to-bone interface structure, (5) vascularity, and (6) cellularity. The four-point scoring system was applied one more time. The histological findings for each of these items were graded semi-quantitatively into four stages (grade [G] 0, 1, 2, and 3), wherein 0 indicated the poorest ruptured tendon appearance, 1 indicated poorer appearance, 2 indicated better appearance, and 3 indicated noticeably regenerated appearance, respectively. Overall, the total score could vary between 0 (a ruptured tendon) to 18 (greatest degrees of regeneration). With regard to the collagen fiber continuity and parallel collagen fiber orientation items, we divided their stages using a percentage value: present at < 1/4 proportion (grade 0), 1/4–1/2 proportion (grade 1), 1/2–3/4 proportion (grade 2), and > 3/4 proportion (grade 3). Collagen fiber density was graded as very loose (grade 0), loose (grade 1), dense (grade 2), or very dense (grade 3). Each tissue slide was photographed under a microscope (Leica DM IL LED; Leica Microsystems, Wetzlar, Germany) using the LAS V4.8 software (Leica Microsystems) imaging system. Three observations were made at the rotator cuff tissue at the same location and area at × 40 magnification [19]. In addition, the area of discoloration with safranin-O staining was measured and quantified (in square millimeters) using the ImageJ software (National Institutes of Health, Bethesda, MD, USA) [20]. All images were obtained with the same illumination and magnification parameters. After a photomicrograph was captured, 8-bit digitization was performed by the ImageJ software. Images were converted into black and white, and they were processed by using the same thresholds set for areas of metachromasia. Areas of metachromasia within the standardized field were measured automatically [20].
Statistical analysis
All statistics were analyzed using SPSS 12.0 software (SPSS Inc. Chicago, IL, USA), and statistical significance was set to p-value < 0.05. Chi-Square test, followed by student’s t-test, was used to evaluate the biomechanical and histological differences between groups. Data are presented as the mean and standard deviation.
Results
Gross inspection and biomechanical evaluation
In total, we evaluated 20 rats per group. No evidence of retear was identified in any experimental group. Comparison of failure modes after supraspinatus repair, bone-to-tendon failure, and midsubstance failure did not show any significant differences between the two groups (p = 0.736) (Table 1). Biomechanical evaluation showed that the ultimate load (N) was significantly higher in group-SSRI (54.8 ± 56.9) than in group-C (25.1 ± 11.1) (p = 0.031). However, there were no significant differences in the cross-sectional area (mm2) and ultimate stress between the groups (p = 0.932, p = 0.249) (Table 2).
Table 1.
Comparison of failure mode after supraspinatus repair between groups
| Group | Group-C | Group-SSRI | p-value |
|---|---|---|---|
| Bone-to-tendon failure | 14 | 13 | 0.736 |
| Midsubstance failure | 6 | 7 |
*Statistically significant difference between groups (p < 0.05)
Table 2.
Comparison of the biomechanical characteristics between groups
| Variables | Group-C | Group-SSRI | p-value |
|---|---|---|---|
| Cross-section area (mm2) | 6.3 ± 2.0 | 6.4 ± 1.9 | 0.932 |
| Ultimate load (N) | 25.1 ± 11.1 | 54.8 ± 56.9 | 0.031* |
| Ultimate stress (Mpa) | 5.6 ± 6.7 | 8.4 ± 5.5 | 0.249 |
*Statistically significant difference between groups (p < 0.05)
Histological evaluation
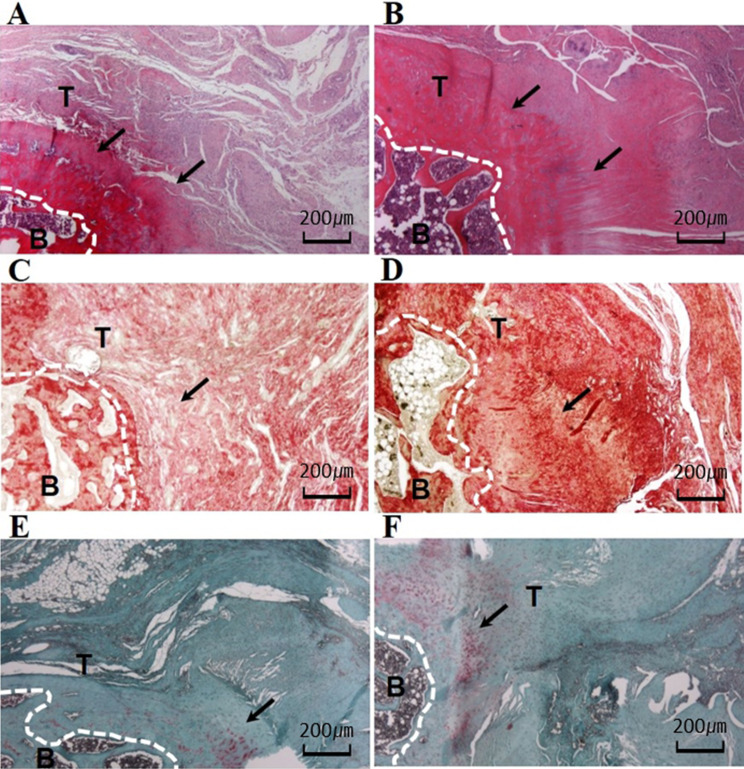
Histologically, the group-SSRI showed an organized bone tendon interface structure with higher collagen fiber density and vascularity than the control group. Furthermore, the group-SSRI (1.1 ± 0.6) demonstrated a higher density of collagen fibers than group-C (group-C: 0.6 ± 0.5) (p = 0.024). However, there were no differences in collagen fiber continuity (p = 0.130) and collagen fiber orientation (p = 0.108) between the groups. The group-SSRI exhibited significantly increased vascularity and cellularity compared with group-C (p = 0.024, p = 0.023). However, there were no differences in tendon-to-bone maturation (p = 0.449). Finally, the group-SSRI (6.3 ± 2.7,) showed a higher total score that was significantly different from group-C (4.3 ± 1.9) (p = 0.019) (Fig. 3; Table 3). We measured the areas of metachromasia in safranin-O staining to confirm fibrocartilage at the tendon-to-bone junction. Metachromasia in group-SSRI (0.2 ± 0.1, p = 0.000) appeared stronger and higher compared with group-C (0.1 ± 0.0) (Table 4).
Fig. 3.
H&E, picrosirius red, and safranin-O staining for supraspinatus tendon repair site. A, B H&E staining after 8 weeks of SSRI treatment. C, D picrosirius red staining after 8 weeks of SSRI treatment. E, F safranin-O staining after 8 weeks of SSRI treatment. Arrows indicate the collagen feature in each group. B, bone; T, tendon. (magnification × 40). A, C, E; Group C, B, D, F; Group SSRI
Table 3.
Histological results according to the Bonar score
| Group | Group-C | Group-SSRI | p-value |
|---|---|---|---|
| Collagen fiber continuity | 0.3 ± 0.4 | 0.6 ± 0.8 | 0.130 |
| Collagen fiber orientation | 0.4 ± 0.6 | 0.8 ± 0.8 | 0.108 |
| Collagen fiber density | 0.6 ± 0.5 | 1.1 ± 0.6 | 0.024* |
| Maturation of the tendon-to-bone | 1.1 ± 0.5 | 1.3 ± 0.6 | 0.449 |
| Vascularity | 0.1 ± 0.2 | 0.3 ± 0.4 | 0.024* |
| Cellularity | 1.7 ± 0.4 | 2.0 ± 0.0 | 0.023* |
| Total score | 4.3 ± 1.9 | 6.3 ± 2.7 | 0.019* |
*Statistically significant difference between groups (p < 0.05)
Table 4.
Comparison of the areas of metachromasia between groups
| Variables | Group-C | Group-SSRI | p-value |
|---|---|---|---|
| Area of metachromasia, mm2/mm2 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.000* |
*Statistically significant difference between groups
Discussion
Our primary finding is that SSRI enhanced rotator cuff healing in the rat model 8 weeks postoperatively as confirmed with biomechanical and histological examinations. The ultimate load was verified by the biomechanical characteristics, and we confirmed that the group treated with SSRIs showed better healing than the control group. Furthermore, histological comparisons revealed that collagen fiber density, vascularity, and cellularity were markedly better in group-SSRI than in the control group.
A nationwide population-based retrospective study revealed that patients with depression had a significantly higher risk of RCT, thus requiring a subsequent repair surgery, compared with patients without depression [21]. However, the mechanism of action that associates depression with RCT remains unclear. We speculate that SSRIs play an essential role in the development of RCT through a mechanism that inhibits post-surgical stress-mediated increased expression of proinflammatory cytokines and glucocorticoids.
First, SSRIs have an anti-inflammatory effect, and they act by significantly decreasing the levels of proinflammatory cytokines, including IL-1β, IL-2, IL-6, TNF-α, and IFN-γ, and by significantly increasing the levels of anti-inflammatory cytokines IL-4 and IL-10 [7, 8, 22]. However, IL-6 displays pleiotropic biological effects by virtue of its pro- and anti-inflammatory nature. Therefore, IL-6 appears to signal differently through different receptors. IL-6 knock-out mice experiments have shown that IL-6 is involved in tendon healing. IL-6 also promotes angiogenesis through vascular endothelial growth factor(VEGF) expression [23, 24]. Many studies have highlighted the role of different cytokines on tendons. IL-1β and TNF-α strongly stimulate tenocytes to upregulate pro- and anti-inflammatory cytokines, such as IL-1β, TNF-α, IL-6, and IL-10 through auto/paracrine action. They induce MMPs, which in turn promote the degradation of tendon extracellular matrix, resulting in the loss of the biomechanical properties of tendon [25]. In this study, SSRIs are thought to regulate cytokines levels, affect collagen synthesis, and eventually influence the rotator cuff healing process.
Second, another possible mechanism might be related to stress response to surgery. SSRIs may help limit the adverse effects of steroids by lowering the systemic steroid concentration and reducing the expression of proinflammatory cytokines. The stress response is related to the hypothalamic–pituitary–adrenal (HPA) axis. The endocrine response that is activated from the surgical site is transmitted to the hypothalamus via the spinal cord and medulla [26]. Corticotropin-releasing hormone (CRH) released from the hypothalamus stimulates adrenocorticotropic hormone (ACTH) secretion in the anterior pituitary. In response to ACTH stimulation, cortisol (the stress hormone) is produced by the adrenal glands. The HPA axis is regulated by a negative feedback mechanism through which cortisol inhibits CRH and ACTH release [27]. Chronic exposure to postoperative stress may cause HPA axis abnormalities that may not adequately reduce elevated cortisol levels through negative feedback mechanisms [28]. It is believed that continuous stress after surgery influences the release of proinflammatory cytokines and control of negative feedback, thus causing hypercortisolemia owing to hyperactivity of the HPA axis. In addition, glucocorticoids prevent angiogenesis by VEGF suppression, decreased production of factors necessary for healing, such as the insulin-like growth factor and transforming growth factor, and suppression of collagen production [29–31]. However, SSRIs also improve microvascular circulation by affecting the thrombocytes and increasing nitric oxide (NO) synthesis and angiogenesis [32]. This latter point is expected to have a positive effect on rotator cuff repair.
Although our study is the first to analyze the effect of SSRI on tendon-bone healing after rotator cuff repair, there are some limitations in interpreting the results. First, the differences of anatomical features and mechanism of healing process between rats and human are not fully identified on account of the limitation of animal testing itself. Second, the limited evaluation period of 8 weeks does not take into account all possible subsequent events and may not be sufficient for the final analysis. Also, although a common rotator cuff tear is a chronic disease, our experimental design is only for selected acute injury model. Third, there is a limitation that histological evaluation was semi-quantitative, and we did not use a histological system specific to tendon-bone healing. To overcome this, we tried to evaluate collagen fiber arrangement, continuity and maturity as quantitatively as possible. In addition, several additional methods were used to improve the accuracy of the maturity of BTI. Metachromasia is a measure for the degree of proteoglycan in safranin-O staining and is used as an indicator of maturity in the BTI [20]. In this study, we have found some favorable results by metachromasia evaluation, but it will be more helpful in evaluating the maturity of BTI to use IHC of specific cytokines in future study. Forth, our study was confirmed to have no significant difference in failure mode. While more precise measurements are being tried with the strain gauge and optical tracker, in recent animal biomechanical experiments, we used a conventional measurement system [33]. Therefore, if our test results had analyzed using the recently developed precision measuring equipment, they might have resulted in significantly different findings.
In conclusion, our study demonstrated that SSRI could facilitate improved biomechanical and histological outcomes 8 weeks after rotator cuff repair in a rat model. Consequently, SSRI may improve healing after rotator cuff repair.
Acknowledgements
None
Declarations
Conflict of interest
All authors of this article declare that they have no conflict of interest.
Ethical statement
The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in Kyungpook Natinal University (IACUC approval No. 2021–138).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Park JH, Oh KS, Kim TM, Kim J, Yoon JP, Kim JY, et al. Effect of smoking on healing failure after rotator cuff repair. Am J Sports Med. 2018;46:2960–2968. doi: 10.1177/0363546518789691. [DOI] [PubMed] [Google Scholar]
- 2.Robson MJ, Quinlan MA, Blakely RD. Immune system activation and depression: roles of serotonin in the central nervous system and periphery. ACS Chem Neurosci. 2017;8:932–942. doi: 10.1021/acschemneuro.6b00412. [DOI] [PubMed] [Google Scholar]
- 3.Gualano MR, Bert F, Mannocci A, La Torre G, Zeppegno P, Siliquini R. Consumption of antidepressants in Italy: recent trends and their significance for public health. Psychiatr Serv. 2014;65:1226–1231. doi: 10.1176/appi.ps.201300510. [DOI] [PubMed] [Google Scholar]
- 4.Leonard BE. Pharmacological differences of serotonin reuptake inhibitors and possible clinical relevance. Drugs. 1992;43:3–10. doi: 10.2165/00003495-199200432-00003. [DOI] [PubMed] [Google Scholar]
- 5.Eison AS, Mullins UL. Regulation of central 5-HT2A receptors: a review of in vivo studies. Behav Brain Res. 1996;73:177–181. doi: 10.1016/0166-4328(96)00092-7. [DOI] [PubMed] [Google Scholar]
- 6.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Li P, Zhang Y, Zhang C, Li K, Song C. Cytokine changes in different types of depression: specific or general? Neurol Psychiatry Brain Res. 2020;36:39–51. doi: 10.1016/j.npbr.2020.02.009. [DOI] [Google Scholar]
- 8.Yoshimura R, Hori H, Ikenouchi-Sugita A, Umene-Nakano W, Ueda N, Nakamura J. Higher plasma interleukin-6 (IL-6) level is associated with SSRI-or SNRI-refractory depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:722–726. doi: 10.1016/j.pnpbp.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Kubera M, Simbirtsev A, Mathison R, Maes M. Effects of repeated fluoxetine and citalopram administration on cytokine release in C57BL/6 mice. Psychiatry Res. 2000;96:255–266. doi: 10.1016/S0165-1781(00)00184-0. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Wang R, Liu L, Qiao D, Baldwin DS, Hou R. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: a systematic review and meta-analysis. Brain Behav Immun. 2019;79:24–38. doi: 10.1016/j.bbi.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Yoon JP, Chung SW, Jung JW, Lee YS, Kim KI, Park GY, et al. Is a local administration of parathyroid hormone effective to tendon-to-bone healing in a rat rotator cuff repair model? J Orthop Res. 2020;38:82–91. doi: 10.1002/jor.24452. [DOI] [PubMed] [Google Scholar]
- 12.Sell SL, Craft RM, Seitz PK, Stutz SJ, Cunningham KA, Thomas ML. Estradiol-sertraline synergy in ovariectomized rats. Psychoneuroendocrinology. 2008;33:1051–1060. doi: 10.1016/j.psyneuen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Cryan JF, Lucki I. Antidepressant-like behavioral effects mediated by 5-hydroxytryptamine2C receptors. J Pharmacol Exp Ther. 2000;295:1120–6. [PubMed]
- 14.Bilge S, Bozkurt A, Bas DB, Aksoz E, Savli E, Ilkaya F, et al. Chronic treatment with fluoxetine and sertraline prevents forced swimming test-induced hypercontractility of rat detrusor muscle. Pharmacol Rep. 2008;60:872–9. [PubMed]
- 15.Gigante A, Cesari E, Busilacchi A, Manzotti S, Kyriakidou K, Greco F, et al. Collagen I membranes for tendon repair: effect of collagen fiber orientation on cell behavior. J Orthop Res. 2009;27:826–32. [DOI] [PubMed]
- 16.Trudel G, Ramachandran N, Ryan SE, Rakhra K, Uhthoff HK. Supraspinatus tendon repair into a bony trough in the rabbit: mechanical restoration and correlative imaging. J Orthop Res. 2010;28:710–715. doi: 10.1002/jor.21045. [DOI] [PubMed] [Google Scholar]
- 17.Laudier D, Schaffler MB, Flatow EL, Wang VM. Novel procedure for high-fidelity tendon histology. J Orthop Res. 2007;25:390–395. doi: 10.1002/jor.20304. [DOI] [PubMed] [Google Scholar]
- 18.Yoon JP, Lee CH, Jung JW, Lee HJ, Lee YS, Kim JY, et al. Sustained delivery of transforming growth factor β1 by use of absorbable alginate scaffold enhances rotator cuff healing in a rabbit model. Am J Sports Med. 2018;46:1441–1450. doi: 10.1177/0363546518757759. [DOI] [PubMed] [Google Scholar]
- 19.Chung SW, Park H, Kwon J, Choe GY, Kim SH, Oh JH. Effect of hypercholesterolemia on fatty infiltration and quality of tendon-to-bone healing in a rabbit model of a chronic rotator cuff tear: Electrophysiological, biomechanical, and histological analyses. Am J Sports Med. 2016;44:1153–1164. doi: 10.1177/0363546515627816. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa H, Morihara T, Fujiwara H, Kabuto Y, Sukenari T, Kida Y, et al. Effect of footprint preparation on tendon-to-bone healing: A histologic and biomechanical study in a rat rotator cuff repair model. Arthroscopy. 2017;33:1482–1492. doi: 10.1016/j.arthro.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 21.Kuo LT, Chen HM, Yu PA, Chen CL, Hsu WH, Tsai YH, et al. Depression increases the risk of rotator cuff tear and rotator cuff repair surgery: a nationwide population-based study. PLoS One. 2019;14:e0225778. doi: 10.1371/journal.pone.0225778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin TW, Cardenas L, Glaser DL, Soslowsky LJ. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J Biomech. 2006;39:61–69. doi: 10.1016/j.jbiomech.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Schulze-Tanzil G, Al-Sadi O, Wiegand E, Ertel W, Busch C, Kohl B, et al. The role of pro-inflammatory and immunoregulatory cytokines in tendon healing and rupture: new insights. Scand J Med Sci Sports. 2011;21:337–351. doi: 10.1111/j.1600-0838.2010.01265.x. [DOI] [PubMed] [Google Scholar]
- 24.Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–27. [DOI] [PubMed]
- 25.Postlethwaite AE, Holness MA, Katai H, Raghow R. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Invest. 1992;90:1479–1485. doi: 10.1172/JCI116015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desborough J. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–117. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 27.Finnerty CC, Mabvuure NT, Ali A, Kozar RA, Herndon DN. The surgically induced stress response. JPEN J Parenter Enteral Nutr. 2013;37:21S–9. [DOI] [PMC free article] [PubMed]
- 28.Guilliams TG, Edwards L. Chronic stress and the HPA axis. The standard. 2010;9:1–12. [Google Scholar]
- 29.Song C-Z, Tian X, Gelehrter TD. Glucocorticoid receptor inhibits transforming growth factor-β signaling by directly targeting the transcriptional activation function of Smad3. Proc Natl Acad Sci U S A. 1999;96:11776–11781. doi: 10.1073/pnas.96.21.11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delany AM, Durant D, Canalis E. Glucocorticoid suppression of IGF I transcription in osteoblasts. Mol Endocrinol. 2001;15:1781–1789. doi: 10.1210/mend.15.10.0704. [DOI] [PubMed] [Google Scholar]
- 31.Logie JJ, Ali S, Marshall KM, Heck MM, Walker BR, Hadoke PW. Glucocorticoid-mediated inhibition of angiogenic changes in human endothelial cells is not caused by reductions in cell proliferation or migration. PLoS One. 2010;5:e14476. doi: 10.1371/journal.pone.0014476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozturk MB, Egemen O, Basat SO, Bozdağ E, Sakız D, Akan M. Histologic and biomechanical evaluation of the effects of social stress and the antidepressant fluoxetine on tendon healing in rats. J Hand Microsurg. 2015;7:294–299. doi: 10.1007/s12593-015-0204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duffy DJ, Chang YJ, Fisher MB, Moore GE. Biomechanical evaluation of a novel barbed suture pattern with epitendinous suture augmentation in a canine flexor tendon model. Vet Surg. 2021;50:1128–1136. doi: 10.1111/vsu.13653. [DOI] [PubMed] [Google Scholar]