Abstract
A potential correlation between polycystic ovary syndrome (PCOS) and asthma, used to be identified as diseases originating from two independent systems, has been supported by increasing evidence. From an epidemiological perspective, mounting studies have confirmed that women suffering from PCOS exhibit increased susceptibility to asthma. Meanwhile, PCOS and asthma seem to share several mutual pathological conditions, such as metabolic disorders, hormonal fluctuation, proinflammatory state, etc. Here, we further elucidate the correlation between asthma and PCOS by focusing on the internal common pathophysiology and adverse influences on women’s health. Understanding the internal connection between PCOS and asthma may shed light on developing new prevention and control strategies to fight against these conditions.
Keywords: polycystic ovary syndrome, asthma, metabolic syndrome, chronic inflammation, reproductive health
Introduction
As a common endocrinopathy among women of childbearing age, polycystic ovary syndrome (PCOS) poses a considerable threat to the health of women throughout the whole world with a prevalence of 15%-20% (1, 2). According to the revised 2003 Rotterdam criteria, a final diagnosis of PCOS can be made if two of the following indicators are met: hyperandrogenism, ovulatory dysfunction, and polycystic changes in the ovary (3). In addition to reproductive health issues (4), PCOS carries an important responsibility for female metabolic disorders and mental problems (5, 6). Notably, chronic systemic and local ovarian inflammation has emerged as a vital focus for the study of PCOS pathophysiology (7–9). To be more specific, previous studies have indicated that PCOS women usually exhibit abnormally elevated levels of inflammatory cytokines (e.g. TNF-α, interleukins, CRP) (9), as well as DNA single-nucleotide polymorphisms of relevant inflammatory mediators (10).
Asthma, a chronic airway inflammatory disease with recurrence and reversibility, is characterized by paroxysmal wheezing, shortness of breath, chest tightness, and cough. To date, approximately 235 million people worldwide suffer from asthma, causing a considerable burden of disease to both families and societies (11). The pathogenesis of asthma involves complex interplay of multiple factors, such as airway hyperresponsiveness (AHR), chronic low-grade inflammation, and airway structural changes (12). In particular, intensive investigations have proposed a potential causal relationship between hormonal fluctuation and the occurrence or severity of asthma, and extensively highlighted the gender disparities that can change with age, which probably accounts for the reproductive disorders in asthmatic women. Intriguingly, as indicated by epidemiological studies, females with metabolic syndrome tend to exhibit an elevation in susceptibility to asthma attacks, whereas the mechanisms underlying the pathophysiology are poorly understood.
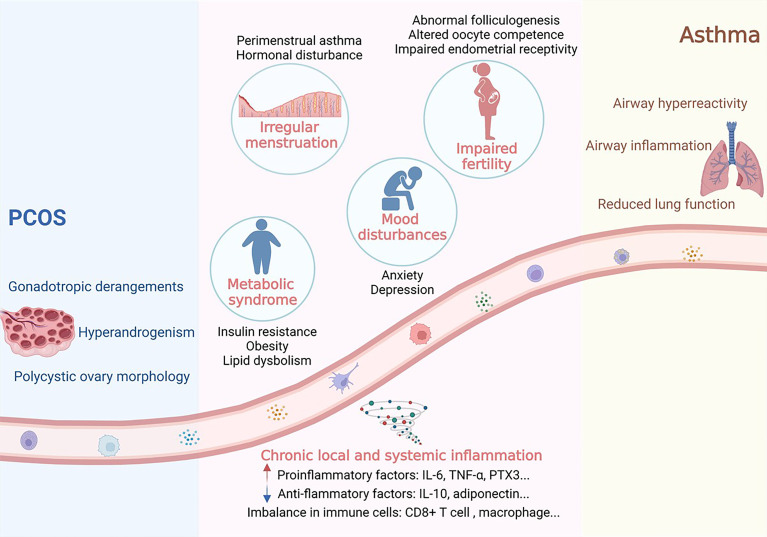
Following the similar risk factors as well as the intersection of potential pathogenesis, several lines of evidence have gradually revealed a non-negligible association between asthma and PCOS in metabolic syndrome, impaired fertility, irregular menstruation, and mood disturbances. And chronic local and systemic inflammation may act as one of the common molecular mechanisms behind these phenomena. Therefore, by literature retrieval and inductive analysis, we further expounded the correlation between asthma and PCOS by focusing on the internal common pathophysiology and insults on female health, to provide unique insights for further exploration of disease prevention and therapy ( Figure 1 ).
Figure 1.
Potential associations between PCOS and asthma. Women with PCOS or asthma are characterized by several common disease phenotypes, including metabolic syndrome, irregular menstruation, impaired fertility and mood disturbances. And chronic local and systemic inflammation may act as one of the common molecular mechanisms behind these phenomena. Image was created with Biorender®.
Concomitance of Asthma and PCOS
PCOS is comorbid with respiratory diseases with a considerable frequency, and asthma in particular. A retrospective cohort study in 2015, which collected and mined the data from Australian statewide hospitals, reported that the prevalence of respiratory disease in women with PCOS was about 22.8%, compared with 14.2% in the control group; among them, 10.6% of the former were admitted to hospital for asthma, while only 4.5% of the latter, suggesting that women with PCOS were more prone to develop asthma (13). And other epidemiological studies of different populations have reached similar conclusions (4, 12–16). In support of the above findings, Underdal et al. propose a potential link between PCOS and asthma from the perspective of lung function, reporting a combined obstructive (forced expiratory volume in one second (FEV1) % predicted, 93.7 vs 102.0) and restrictive (forced vital capacity (FVC) % predicted, 94.5 vs 103.7) respiratory impairment in PCOS compared with controls (17). Meanwhile, a historical cohort study reported that irregular period was associated with higher odds of atopic asthma (18), which once again verified the potential connection between asthma and ovulatory dysfunction. Recent studies have further explored whether confounding factors alter the association between asthma and PCOS, and indicate that the incidence of asthma remained higher even after adjusting for heterogeneity of body mass index (BMI), smoking status, and dietary intake (12, 16). Furthermore, Sun H et al. (19) performed a meta-analysis that systematically evaluated the prevalence of asthma as related to PCOS, and determined PCOS as an independent risk factor for asthma.
Although the link between PCOS and asthma incidence is well established, results from different studies on asthma severity among women with or without PCOS are conflicting. To be specific, Zierau et al. investigated the severity of asthma based on the use of anti-asthma medication, and indicated that PCOS status is not a contributing factor to exacerbation of asthma (14). However, other studies report opposite results, which are verified by a higher hospital admission rate for asthma and increased doses of anti-asthma medication in PCOS women (13, 15). In combination, the researches mentioned above suggest an important correlation between PCOS and asthma attack and provide a reliable perspective to investigate the corresponding pathogenesis ( Table 1 ).
Table 1.
General characteristics of key studies.
| Author | Year | Country | Study design | PCOS criteria | Asthma criteria | Characteristics of the subjects | Asthma incidence % (n) | P value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PCOS | Control | PCOS | Control | |||||||
| Hart et al. (13) |
2015 | Australia | Retrospective cohort study | ICD-10 | Medical record |
Median age: 27.9 | Not available | 10.56% (271/2566) |
4,52% (1160/25660) |
<0.01 |
| Underdal et al. (17) | 2020 | Norway | Prospective cohort study | Rotterdam criteria |
Medical record |
Mean age: 38 Mean BMI: 28.5 |
Mean age: 38 Mean BMI: 25.7 |
19.31% (28/145) |
8.74% (38/435) |
<0.01 |
| Doherty et al. (4) |
2015 | Australia | Retrospective cohort study | ICD-10 | Medical record |
Not available | Not available | 13.64% (478/3505) |
9.90% (3450/34856) |
<0.01 |
| Zierau et al. (14) | 2018 | Denmark | Prospective cohort study | Rotterdam criteria |
Medical record |
Mean age: 34 BMI: 25.8 ± 5.3 |
Mean age: 34 BMI: 24.3 ± 4.9 |
19.59% (266/1358) |
14.48% (786/5430) |
<0.001 |
| Glintborg et al. (15) |
2015 | Denmark | Prospective cohort study | ICD-10 | Medical record |
Mean age: 30.6 | Mean Age: 30.6 | 3,05% (586/19199) |
2.21% (1270/57483) |
<0.001 |
| Htet et al. (16) |
2017 | Australia | Prospective cohort study | Questionnaire | Questionnaire | Age: 30.5 ± 0.1 BMI: 28.0 ± 0.3 |
Age: 30.6 ± 0.02 BMI: 25.1 ± 0.1 |
16.11% (77/478) |
10.52% (856/8134) |
0.004 |
| Grieger et al. (12) | 2020 | Australia | Prospective cohort study | Questionnaire | Questionnaire | Age: 33.5 ± 1.4 BMI: 28.5 ± 7.4 |
Age: 33.7 ± 1.5 BMI: 25.6 ± 5.8 |
13.64% (478/3505) |
9.90% (3450/34856) |
0.004 |
Overlap Between Features of PCOS and Asthma
Metabolic Syndrome
Metabolic syndrome (MS) is a cluster of complex disorders characterized by abnormal glucose metabolism, dyslipidemia, overweight or obesity, and hypertension, and has been recognized as the most critical long-term health problem associated with PCOS. Both obese and lean women with PCOS are reported to have a much higher risk of MS compared with the control group (20–22). So far, numerous pathologic changes have been identified in exploring the pathogenesis of PCOS, such as hyperandrogenemia, hyperinsulinemia, dysfunction of the hypothalamic-pituitary-ovarian axis, and disturbances of adipokines secretion. These specific alterations interact with each other in fat tissues, liver, muscle, and ovary, resulting in diverse phenotypes of MS.
Remarkably, insulin resistance (IR) and hyperandrogenism stand out as pivotal factors in the development of PCOS. Approximately 50-80% of PCOS females exhibit varying degrees of IR, which possibly accounts for the higher annual conversion rate to impaired glucose tolerance (IGT) or diabetes mellitus type 2 (T2DM) in them (23, 24). Previous studies have identified several abnormal or dysfunctional cells and molecules involved in the IR status of PCOS women, such as glucose transporter 4 (GLUT4), insulin receptor β subunit, pancreatic β-cells, and so on (25, 26). In PCOS, adipose tissue dysfunction, possibly leading to excessive visceral fat depots, is thought to be involved in determining both IR and dysregulated androgen metabolism (27). When exposed to excessive androgen, adipocytes seem to be prone to hypertrophy, releasing superabundant adipokines and inflammatory mediators. TNF-α, for instance, can block the tyrosine kinase phosphorylation of insulin receptors (28), and affect glucose transport by inhibiting GLUT-4 activity (29), thus inducing the obesity-related IR or hyperinsulinemia. Functionally, insulin acts as an essential factor in the development of hyperandrogenism (30). Insulin not only directly stimulates follicular theca cells to produce androgen and encourages the development of stromal cells in the ovary, but also induces the secretion of insulin-like growth factor 1 (IGF-1) so as to promote androgen synthesis in adrenal and gonads (31). Thus, adipose tissue dysfunction, inflammation, IR, and hyperandrogenism form a vicious cycle that leads to different phenotypes in PCOS patients.
In recent years, growing evidence also supports MS is a potential risk factor for asthma. Multiple studies have linked MS to asthma attacks but the correlation is still under dispute. Lee et al. (32) put forward that patients with MS are more likely to develop symptoms of wheeze and dyspnea at rest or after exercise. This is consistent with a large retrospective cohort study from Norway showing that excessive waist circumference and diabetes increase the susceptibility to asthma in adults (33). What’s more, cohort studies in Japan and China also revealed that overweight or obesity can dramatically aggrandize susceptibility to asthma in females but not in males (21, 34). The sexual heterogeneity makes it reasonable to presume that specific gonadal hormones and metabolic disorders may complement each other to exert promoting effect on the pathogenesis of asthma. However, whether every element of MS can induce female asthma attacks is still controversial. Assad et al. pointed out that the association between abdominal adiposity, elevated blood pressure, impaired fasting glucose or diabetes and asthma incidence is no longer statistically significant after adjustment for BMI (35). This raises the possibility that BMI or obesity is a stronger predictor of asthma than MS. In turn, so far little is known about the influence of asthma on obesity incidence. Several studies have shown that early-life of asthma may result in a higher susceptibility to obesity in later childhood and adolescence (36, 37), but further investigations are needed to verify the promoting effect of asthma on obesity.
The underlying mechanism of overweight or obesity prompting asthma remains uncertain, and attention is mainly focused on the mechanical factors and altered inflammation responses. First, obesity is theorized to encourage drastic changes in normal lung physiology. Excessive accumulation of fat in the chest and abdominal cavity contributes to lung compression and reduced lung volume, resulting in a pronounced decrease in functional residual capacity (FRC) and expiratory volume (ERV) (38, 39). Low lung volume leads to AHR through impaired ability to stretch the airway wall (40). Among patients with late-onset non-allergic asthma, obesity induces excessive collapsibility of airways by reducing distal airway wall stiffness (41). Second, it is not unexpected that obesogenic dietary patterns tend to contain much fatty acids and fructose but little fiber and antioxidants. Growing evidence verifies that food rich in saturated fatty acids can encourage pulmonary lymphocyte infiltration, induce congenital AHR and further allergic airway inflammation through the IL-1β pathway (42, 43). Third, inflammatory effects of obesity on the lung involve dysregulation of proinflammatory adipokines (e.g. excess leptin) and anti-inflammatory adipokines (e.g. adiponectin deficiency). Excessive adipose tissue secrets redundant pro-inflammatory cytokines, such as TNF-α, interleukin, and leptin, which further trigger systemic inflammation and AHR. Meanwhile, the downregulated level of bronchoalveolar surface-active protein A (SPA) in obese subjects with asthma can cause airway eosinophil aggregation (44), thereby increasing the risk of allergic airway inflammation. In combination, MS may then act as a link between PCOS and asthma, with a co-existence of MS and PCOS aggravating asthma.
Impaired Fertility
It is well established that PCOS status threatens fertility in women of childbearing age. Although women with PCOS are typically characterized by an increase in the quantity of preantral follicles, the quality of oocytes is usually diminished, resulting in impaired fertilization, cleavage as well as embryo implantation. Wild et al. (45) demonstrated that 17.5% of PCOS women, 11 times that of the control group, reported fertility problems, of which 66% of PCOS women suffered from infertility and 24% relied on ovulation induction. Consistently, other researchers also observed remarkable growths in the non-fertility rate, gestational age, and the utilization rate of assisted reproductive technology in PCOS women (46, 47). Hormonal imbalance is the most striking feature of PCOS, accounting for diverse reproductive problems. In PCOS, hypersecretion of LH during follicle formation significantly impairs oocyte maturation, fertilization, and embryo quality (48, 49). Part of the cellular and molecular abnormalities caused by excessive LH have been identified, including suppressed FSH function, dysfunctional granulosa cell (GC), damaged oocyte nucleus, and impaired extrusion of the first polar body (50, 51). Excessive insulin can cooperate with LH to stimulate androgen production (52), which thereby retards the development of dominant follicles, impacts endometrial receptivity, and increases the risk of various obstetric complications (53–55).
Recently, much more attention has been paid to the similar association between allergic diseases and infertility. Gade et al. (56) revealed that asthma was responsible for a prolonged time to pregnancy (TTP), with TTP of about 27% of asthmatic women extended beyond one year, and specific treatment for asthma could effectively abolish the extension of TTP (57). Hansen et al. (58) explored the association between asthma and the need for fertility treatment among women with life births, and pointed out that the association remained significant after adjusting for age, BMI, and smoking habits. What’s worse, despite fertility treatment, asthma is still associated with unfavorable pregnancy outcomes (59). Rocklin et al. reported that maternal asthma may increase the risk of preterm birth, low birth weight, and perinatal mortality (60). Overall, the vast majority of evidence points toward the possibility that asthma in women has an inextricable link with delayed conception, lowered genitality, and poor pregnancy outcomes.
Obesity and inflammation response are two pathologic conditions closely related to both PCOS and asthma, which may provide insights into the impaired fertility associated with these two conditions. Obesity is partially responsible for poor oocyte quality through a variety of pathways, including follicular development retardation, oocyte maturation problems, meiosis abnormalities, as well as mitochondrial dynamics disorder (61–64). Excessive free fatty acids are presumed to exert toxic effects on reproductive tissues through oxidative stress, leading to damage or apoptosis of female gametes (64). Recently, studies have demonstrated that several adipokines may carry metabolic responsibilities for female reproductive function, especially in ovarian physiology. Through the interaction with LH and insulin, adiponectin encourages the expression of genes related to periovulatory maturation of ovarian follicles (65). Lower adiponectin levels were reported in the plasma of either PCOS (66) or asthma (67) women, as well as in the follicles of PCOS women (68). These suggest its possible role in the follicular arrest and ovulatory dysfunction. In combination, obesity, independently or jointly with endocrine disruption, impair female fertility in women with PCOS or asthma.
What’s more, abundant available evidence supports chronic systematic inflammation as the common cornerstone for the internal pathogenesis of impaired fertility in both PCOS and asthma. The immune system plays a pivotal part in the reproductive process. Concretely speaking, Th2 immunity supports the pregnancy process by reducing the rejection of fetal tissue, while Th1 immunity is thought to be unfavorable to the “foreign” fetus (56). Based on this recognition, researchers speculate that asthma might cause female fertility impairment and poor pregnancy outcomes by inducing an imbalance of the adaptive immune system. The systemic inflammation induced by asthma may increase the infiltration of inflammatory cells and alter cytokine profiles in the ovary and uterus exerting a negative effect on fertility. For example, TNF-α is regarded as a potential regulator of follicular development, ovulation, and oocyte apoptosis, playing an important role in reproductive failure (69–71). Imbalanced IL-6 is associated with recurrent miscarriages and failed implantation by regulating the proliferation, differentiation, and survival of germ cells (56, 72). Intriguingly, growing evidence supports that PCOS is also closely related to chronic systemic inflammation. The amount of immune cells in the peripheral blood of PCOS women dramatically increases with high androgen levels, inducing the secretion of inflammatory factors such as CRP, TNF-α, and IL-6 (73). In addition, previous studies revealed the altered immune cell profiles (e.g. macrophages, dendritic cells, and CD8+ T cells) in the ovary and endometrium of PCOS women (74, 75), which possibly compromise ovarian function, normal implantation, and endometrial health in the long run. For example, pentraxin-3 (PTX3), a crucial humoral innate immunity component secreted by macrophages, which stands out as a structural constituent of the cumulus oophorus extracellular matrix essential for female fertility, was reported abnormally high both in plasma and ovary in PCOS women (76–78). And higher PTX3 is associated with hyperandrogenism (79), which might perturb interactions of the sperm with the matrix during in vivo fertilization (80). Low-grade systemic inflammation, therefore, carries pivotal responsibility for impaired female fertility, which may build a bridge connecting asthma and PCOS.
Irregular Menstruation
Irregular menstruation refers to oligomenorrhea, amenorrhea, abnormal uterine bleeding, etc. Numerous studies have revealed that PCOS women tend to exhibit a higher prevalence of irregular menstruation, which is often initially onset at a younger age and is positively correlated to BMI (81, 82). Compared with the age-matched controls, PCOS females reported more frequent hospitalization for menstrual abnormalities (20.3% vs 4.9%), endometriosis (26.4% vs 4.4%), endometrial hyperplasia (1.8% vs 0.1%) and other gynecological diseases (13). Based on the available evidence, lifestyle interventions, insulin sensitizers, and anti-androgen drugs are thought to be able to improve the reproductive health of PCOS women by inhibiting IR or declining androgen levels.
Abnormal regulation of steroidogenesis and ovarian regulation is a pivotal part of the pathophysiology of PCOS. Hypersecretion of LH and IGF-1, anti-Müllerian hormone (AMH) interact with each other to induce hyperandrogenism (83). For one thing, excessive androgen enhances the initial recruitment of primordial follicles into the growth pool, thus regulating the growth of small antral follicles (84). For another, hypersecretion of androgen encourages premature luteinization, thus inhibiting the selection of the dominant follicle and hindering normal ovulation (49, 85). Ovarian follicular arrest results in oligo-ovulation or anovulation, characterized by delayed menstruation, amenorrhea, or abnormal uterine bleeding (85).
Meanwhile, growing evidence demonstrates a strong correlation between menstruation and asthma attacks. Earlier menarche or irregular menstruation seems to predict worse lung function and increased susceptibility to asthma in adulthood (86–88). Women with abnormal or irregular menstrual cycles tend to exhibit significantly lowered FVC and elevated prevalence of asthma attacks (89). Furthermore, the exacerbation of asthma during menstruation, known as perimenstrual asthma (PMA), affects 30-40% of women with asthma. Compared with normal asthma attacks, PMA is characterized by more severity, increased drug use, lower vital capacity and PEF, as well as higher airway responsiveness (90). The association between deviations in levels of sex hormones and PMA has been suggested. Compared to non-PMA asthmatics, PMA subjects exhibit elevated estradiol levels in the luteal phase of the cycle (91). Rubio et al. reported that on the 5th and 21st days of the menstrual cycle, approximately 80% of asthmatic women exhibit abnormal hormonal changes (92). These studies indicate that sex hormone fluctuations may act as a risk factor for severe asthma attacks.
Indeed, more pronounced incidence and severity of asthma starting around puberty usually occur in females but not in males (86), suggesting the important role of sex hormones in the development of female airway pathology. Estrogen is the most widely studied ovarian hormone in airway inflammation. Mouse models have suggested the role of estrogen receptor (ER) signaling in allergic airway inflammation by increasing the function of dendritic cells, M2 macrophages, and mast cells (93–95). Besides, 17β-estradiol and progesterone promote TH17 cell differentiation and subsequently enhance IL-17A-mediated airway inflammation (96). From the perspective of neurological factors, as the parasympathetic nerve pathway is an essential determinant of bronchial tension, estradiol can indirectly cause bronchial muscle contraction and bronchial mucosal swelling by regulating the activity of acetylcholine and cholinesterase (97, 98). Generally speaking, given the commonly harbored hormonal disorders in PCOS women, these studies provide a new insight to investigate the correlation between PCOS and asthma by elucidating the effect of sex hormones.
Mood Disturbances
Clear evidence from epidemiological studies corroborates that women with PCOS have an increased incidence of depression, anxiety, sleep disorders, or other psychological disorders (46, 99, 100). Since the presence of overweight or obesity and infertility may exacerbate depression and anxiety reported in the general population, Damone et al. indicated that the associations between PCOS and depression or anxiety remain statistically significant, although weakened after adjusting for BMI, infertility, and sociodemographic variables (101). This confirms the hypothesis that the PCOS state itself may have an independent effect on mental functioning. When shown negative emotional images, PCOS women with IR exhibited increased activation in the left prefrontal cortex and the ventral anterior cingulate region compared with controls. These affected areas are responsible for the integration and regulation of cognitive and emotion-related information (102). In addition, PCOS women with IR were found to have greater marginal activation in emotional tasks than controls, leading to the aggravation of anxiety symptoms (103).
Meanwhile, numerous epidemiological studies have determined mental disorders as one of the important comorbidities of asthma. Adolescents with asthma are more prone to develop anxiety unipolar depressive disorder and bipolar disorder than the control group (104, 105). However, Slattery et al. have reached conflicting conclusions, demonstrating depressive symptoms were not significantly associated with a lifetime history of asthma (106). Different research methodology and diagnostic criteria, as well as a lack of adjustment for other co-existing allergic disorders, may explain the inconsistency. In recent years, magnetic resonance imaging (MRI) has been applied to investigate brain regions that may play a mechanistic role in asthma and mood disorders. Compared with the healthy controls, asthmatic patients showed abnormal structural connectivity in the bilateral frontal gyrus, right temporo-parietal cortex, and limbic regions, as well as decreased globus pallidus volume, suggesting changes in the function of brain regions involved in emotional regulation (107, 108).
Several common pathophysiological alterations may carry responsibility for mood disturbances in both PCOS and asthma, such as hypothalamic-pituitary-adrenal (HPA) dysfunction and inflammatory responses. HPA axis dysfunction is a potential mechanism underlying a variety of mental health conditions, especially anxiety and depression (109, 110). Cortisol, a steroid hormone with diurnal fluctuations, is widely recognized as a highly sensitive biomarker of stress-related changes in the maintenance of metabolic homeostasis (111). Flattening the circadian rhythm of cortisol results in dysregulation of several downstream biological and behavioral systems, including immunity, metabolism, energy, as well as appetite. Interestingly, chronic HPA axis disorders are common in asthmatic children (109). Compared with the healthy controls, asthmatic children had lower cortisol levels in hair samples, reduced cortisol reactivity in saliva samples, and a blunted cortisol awakening response (CAR) (112, 113). Further study indicated that adrenal insufficiency is not simply a consequence caused by inhaled corticosteroid treatments for asthma, it may also be a feature of asthma itself (114). Likewise, abnormal HPA activity in PCOS women has also been widely reported, but results vary. Some studies indicated that women with PCOS or different hyperandrogenic states are characterized by HPA-axis overactivity (115, 116). Nevertheless, Prelevic et al. (117) observed significantly lower cortisol levels at nighttime in PCOS women. Taken together, as abnormal cortisol levels are observed among mental disorders, asthma, and PCOS, HPA dysfunction could be an underlying mechanism that predisposes patients with asthma or PCOS to develop mood disturbances.
Inflammation responses could also explain this co-association between asthma or PCOS and multiple mental disorders. Increasing evidence shows that the levels of pro-inflammatory cytokines (e.g. IL-6, TNF-α, CRP) in peripheral blood of patients with mental problems are dramatically increased (118–121). Given that both asthma and PCOS are characterized by chronic systemic inflammation, these findings provide a basis for such association. The oversecreted pro-inflammatory cytokines can penetrate the blood-brain barrier during allergic reactions (122). On the one hand, it activates abnormal neuroimmune mechanisms in certain neural circuits involved in emotion regulation; On the other hand, it leads to decreased synthesis and increased uptake of 5-hydroxytryptamine, a neurotransmitter associated with various psychiatric disorders (123–125). Meanwhile, elevated levels of peripheral cytokines can activate microglia (126), which are involved in neuronal cell apoptosis, neurogenesis, and synaptic interactions (127). Inflammation-induced hyperactivation of microglia can inhibit neuronal activity through increased phagocytosis of dendritic spines, thus encouraging the development of mood disturbances (128). So far, the role of inflammatory response in the pathogenesis of mood disorders has not been fully elucidated, but it possibly becomes one of the promising targets to protect asthma or PCOS patients from mood disorders.
Discussion
Both PCOS and asthma are common diseases in adult females, causing severe damage to women’s physical and mental health as well as their quality of life. Clarifying the full landscape of pathogenesis and development of these two diseases under physiological conditions can elucidate the complex negative effects on female health from a whole-life perspective. Previous studies mainly focus on inheritance, molecular cytology, epigenetics, or other aspects, and have already made significant breakthroughs. In recent years, growing epidemiological studies have demonstrated that PCOS, to a certain degree, correlates to asthma in several aspects. Specifically, women with PCOS are prone to develop asthma, and in return, asthma patients tend to exhibit increased susceptibility to metabolic syndrome, impaired fertility, irregular menstruation, and other clinical symptoms similar to PCOS. Furthermore, in the context of the coronavirus disease 2019 (COVID-19) pandemic, emerging data link the risk of severe COVID-19 with both PCOS and asthma. Researchers have identified potential overlap between common PCOS features and key risk factors of COVID-19, including cardio-metabolic comorbidity, hyperandrogenism, hyper-inflammation, etc. (129). Studies that have addressed the issue of asthma patients’ susceptibility to the disease have come to discrepant conclusions, possibly due to complex interplay between numerous factors such as geographical differences, asthma phenotypes, and asthma medication (130–133). Of note, patients suffering from Th2-low asthma, especially the subjects with concomitant MS, are at a higher risk for progression to severe COVID-19 (134, 135). This provides new insights into the association between PCOS and asthma and requires further research. Interestingly, PCOS and asthma have been confirmed to share common physiological and pathological changes, such as specific hormonal abnormalities, insulin resistance as well as chronic systemic inflammation, whereas the potential mechanisms linking asthma to PCOS remain poorly investigated. It is important to mention that both conditions were recognized to originate in the early stages of life (136, 137).
PCOS is considered to have a strong correlation with autoimmune and chronic systematic inflammation related to IR, T2DM, or other metabolic syndromes, suggesting a possible mechanism that may occur when it comes to airway inflammation or even asthma in PCOS women. Given that numerous studies have reported an increased incidence of asthma-related pathological changes in PCOS females, further longitudinal studies and more precise elucidation of pathogenesis are required to examine the deeper connection between PCOS and asthma. To date, most data support a negative impact of asthma on female reproductive health through inducing irregular menstruation and reduced fertility. Based on the available evidence, it could be speculated that the imbalance of sex hormones and metabolic disorders behind impaired female reproductive health is likely to be one of the potential explanations for this correlation.
Currently, most evidence comes from observational studies, making it hard to establish a causal relationship between PCOS and asthma. The pathophysiology of the two conditions may involve diverse factors, and further prospective studies are of great significance to elucidate the complex cross-influence of inflammatory, metabolic disturbance, as well as endocrine disorders, and to provide broader screening and treatment strategies for women suffering from PCOS, asthma, or related complications.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author Contributions
H-FH and J-XP conceived of the study; YX and Z-YZ wrote the manuscript; J-XP and YX revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Key R&D Program of China (2021YFC2700603), the National Nature Science Foundation of China (82088102, 82171688, 82192873), Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01), CAMS Innovation Fund for Medical Sciences (2019- I2M-5-064), Clinical Research Plan of SHDC (SHDC2020CR1008A), Shanghai Frontiers Science Center of Reproduction and Development and Research Project of Shanghai Municipal Health Commission (20204Y0234).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Image was created with Biorender®.
Abbreviations
PCOS, polycystic ovary syndrome; T2DM, diabetes mellitus type 2; CRP, C-reactive protein; AHR, hyperresponsiveness; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; BMI, body mass index; MS, metabolic syndrome; IR, insulin resistance; IGT, impaired glucose tolerance; GLUT4, glucose transporter 4; IGF-1, insulin-like growth factor 1; FRC, functional residual capacity; ERV, expiratory volume; SPA, surface-active protein A; GC, granulosa cell; TTP, time to pregnancy; PTX3, pentraxin-3; PMA, perimenstrual asthma; MRI, magnetic resonance imaging; HPA, hypothalamic-pituitary-adrenal; CAR, cortisol awakening response; AMH, anti-Müllerian hormone; COVID-19, coronavirus disease 2019.
References
- 1. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The Prevalence and Phenotypic Features of Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum Reprod Oxf Engl (2016) 31(12):2841–55. doi: 10.1093/humrep/dew218 [DOI] [PubMed] [Google Scholar]
- 2. Sirmans SM, Pate KA. Epidemiology, Diagnosis, and Management of Polycystic Ovary Syndrome. Clin Epidemiol (2013) 6:1–13. doi: 10.2147/CLEP.S37559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group . Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome. Fertil Steril (2004) 81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004 [DOI] [PubMed] [Google Scholar]
- 4. Doherty DA, Newnham JP, Bower C, Hart R. Implications of Polycystic Ovary Syndrome for Pregnancy and for the Health of Offspring. Obstet Gynecol (2015) 125(6):1397–406. doi: 10.1097/AOG.0000000000000852 [DOI] [PubMed] [Google Scholar]
- 5. Ollila MME, Piltonen T, Puukka K, Ruokonen A, Järvelin MR, Tapanainen JS, et al. Weight Gain and Dyslipidemia in Early Adulthood Associate With Polycystic Ovary Syndrome: Prospective Cohort Study. J Clin Endocrinol Metab (2016) 101(2):739–47. doi: 10.1210/jc.2015-3543 [DOI] [PubMed] [Google Scholar]
- 6. Cooney LG, Lee I, Sammel MD, Dokras A. High Prevalence of Moderate and Severe Depressive and Anxiety Symptoms in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum Reprod Oxf Engl (2017) 32(5):1075–91. doi: 10.1093/humrep/dex044 [DOI] [PubMed] [Google Scholar]
- 7. Rudnicka E, Suchta K, Grymowicz M, Calik-Ksepka A, Smolarczyk K, Duszewska AM, et al. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int J Mol Sci (2021) 22(7):3789. doi: 10.3390/ijms22073789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abraham Gnanadass S, Divakar Prabhu Y, Valsala Gopalakrishnan A. Association of Metabolic and Inflammatory Markers With Polycystic Ovarian Syndrome (PCOS): An Update. Arch Gynecol Obstet (2021) 303(3):631–43. doi: 10.1007/s00404-020-05951-2 [DOI] [PubMed] [Google Scholar]
- 9. Samy N, Hashim M, Sayed M, Said M. Clinical Significance of Inflammatory Markers in Polycystic Ovary Syndrome: Their Relationship to Insulin Resistance and Body Mass Index. Dis Markers (2009) 26(4):163–70. doi: 10.3233/DMA-2009-0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hesampour F, Namavar Jahromi B, Tahmasebi F, Gharesi-Fard B. Association Between Interleukin-32 and Interleukin-17a Single Nucleotide Polymorphisms and Serum Levels With Polycystic Ovary Syndrome. Iran J Allergy Asthma Immunol (2019) 18(1):91–9. doi: 10.18502/ijaai.v18i1.634 [DOI] [PubMed] [Google Scholar]
- 11. Moraes TJ, Sears MR, Subbarao P. Epidemiology of Asthma and Influence of Ethnicity. Semin Respir Crit Care Med (2018) 39(1):3–11. doi: 10.1055/s-0037-1618568 [DOI] [PubMed] [Google Scholar]
- 12. Grieger JA, Hodge A, Mishra G, Joham AE, Moran LJ. The Association Between Dietary Intake, Asthma, and PCOS in Women From the Australian Longitudinal Study on Women’s Health. J Clin Med (2020) 9(1):E233. doi: 10.3390/jcm9010233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hart R, Doherty DA. The Potential Implications of a PCOS Diagnosis on a Woman’s Long-Term Health Using Data Linkage. J Clin Endocrinol Metab (2015) 100(3):911–9. doi: 10.1210/jc.2014-3886 [DOI] [PubMed] [Google Scholar]
- 14. Zierau L, Cortes R, Thomsen SF, Jimenez-Solem E, Lindenberg S, Backer V. Asthma Severity and Fertility Outcome in Women With Polycystic Ovary Syndrome: A Registry-Based Study. ERJ Open Res (2018) 4(4):00138–2017. doi: 10.1183/23120541.00138-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glintborg D, Hass Rubin K, Nybo M, Abrahamsen B, Andersen M. Morbidity and Medicine Prescriptions in a Nationwide Danish Population of Patients Diagnosed With Polycystic Ovary Syndrome. Eur J Endocrinol (2015) 172(5):627–38. doi: 10.1530/EJE-14-1108 [DOI] [PubMed] [Google Scholar]
- 16. Htet TD, Teede HJ, de Courten B, Loxton D, Real FG, Moran LJ, et al. Asthma in Reproductive-Aged Women With Polycystic Ovary Syndrome and Association With Obesity. Eur Respir J (2017) 49(5):1601334. doi: 10.1183/13993003.01334-2016 [DOI] [PubMed] [Google Scholar]
- 17. Underdal MO, Salvesen Ø, Henriksen AH, Andersen M, Vanky E. Impaired Respiratory Function in Women With PCOS Compared With Matched Controls From a Population-Based Study. J Clin Endocrinol Metab (2020) 105(1):dgz053. doi: 10.1210/clinem/dgz053 [DOI] [PubMed] [Google Scholar]
- 18. Galobardes B, Patel S, Henderson J, Jeffreys M, Smith GD. The Association Between Irregular Menstruations and Acne With Asthma and Atopy Phenotypes. Am J Epidemiol (2012) 176(8):733–7. doi: 10.1093/aje/kws161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun H, Li D, Jiao J, Liu Q, Bian J, Wang X. A Potential Link Between Polycystic Ovary Syndrome and Asthma: A Meta-Analysis. Reprod Sci Thousand Oaks Calif (2022) 29(1):312–9. doi: 10.1007/s43032-021-00662-8 [DOI] [PubMed] [Google Scholar]
- 20. Dokras A, Bochner M, Hollinrake E, Markham S, Vanvoorhis B, Jagasia DH. Screening Women With Polycystic Ovary Syndrome for Metabolic Syndrome. Obstet Gynecol (2005) 106(1):131–7. doi: 10.1097/01.AOG.0000167408.30893.6b [DOI] [PubMed] [Google Scholar]
- 21. Zhang J, Fan P, Liu H, Bai H, Wang Y, Zhang F. Apolipoprotein A-I and B Levels, Dyslipidemia and Metabolic Syndrome in South-West Chinese Women With PCOS. Hum Reprod Oxf Engl (2012) 27(8):2484–93. doi: 10.1093/humrep/des191 [DOI] [PubMed] [Google Scholar]
- 22. Sanchez-Garrido MA, Tena-Sempere M. Metabolic Dysfunction in Polycystic Ovary Syndrome: Pathogenic Role of Androgen Excess and Potential Therapeutic Strategies. Mol Metab (2020) 35:100937. doi: 10.1016/j.molmet.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in Glucose Tolerance Over Time in Women With Polycystic Ovary Syndrome: A Controlled Study. J Clin Endocrinol Metab (2005) 90(6):3236–42. doi: 10.1210/jc.2004-1843 [DOI] [PubMed] [Google Scholar]
- 24. Celik C, Tasdemir N, Abali R, Bastu E, Yilmaz M. Progression to Impaired Glucose Tolerance or Type 2 Diabetes Mellitus in Polycystic Ovary Syndrome: A Controlled Follow-Up Study. Fertil Steril (2014) 101(4):1123–1128.e1. doi: 10.1016/j.fertnstert.2013.12.050 [DOI] [PubMed] [Google Scholar]
- 25. Seow KM, Juan CC, Hsu YP, Hwang JL, Huang LW, Ho LT. Amelioration of Insulin Resistance in Women With PCOS via Reduced Insulin Receptor Substrate-1 Ser312 Phosphorylation Following Laparoscopic Ovarian Electrocautery. Hum Reprod Oxf Engl (2007) 22(4):1003–10. doi: 10.1093/humrep/del466 [DOI] [PubMed] [Google Scholar]
- 26. Panidis D, Macut D, Farmakiotis D, Rousso D, Kourtis A, Katsikis I, et al. Indices of Insulin Sensitivity, Beta Cell Function and Serum Proinsulin Levels in the Polycystic Ovary Syndrome. Eur J Obstet Gynecol Reprod Biol (2006) 127(1):99–105. doi: 10.1016/j.ejogrb.2005.12.016 [DOI] [PubMed] [Google Scholar]
- 27. Spritzer PM, Lecke SB, Satler F, Morsch DM. Adipose Tissue Dysfunction, Adipokines, and Low-Grade Chronic Inflammation in Polycystic Ovary Syndrome. Reprod Camb Engl (2015) 149(5):R219–227. doi: 10.1530/REP-14-0435 [DOI] [PubMed] [Google Scholar]
- 28. Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced Tyrosine Kinase Activity of the Insulin Receptor in Obesity-Diabetes. Central Role of Tumor Necrosis Factor-Alpha. J Clin Invest (1994) 94(4):1543–9. doi: 10.1172/JCI117495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stephens JM, Pekala PH. Transcriptional Repression of the GLUT4 and C/EBP Genes in 3T3-L1 Adipocytes by Tumor Necrosis Factor-Alpha. J Biol Chem (1991) 266(32):21839–45. doi: 10.1016/S0021-9258(18)54714-1 [DOI] [PubMed] [Google Scholar]
- 30. Zeng X, Xie YJ, Liu YT, Long SL, Mo ZC. Polycystic Ovarian Syndrome: Correlation Between Hyperandrogenism, Insulin Resistance and Obesity. Clin Chim Acta Int J Clin Chem (2020) 502:214–21. doi: 10.1016/j.cca.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 31. Delitala AP, Capobianco G, Delitala G, Cherchi PL, Dessole S. Polycystic Ovary Syndrome, Adipose Tissue and Metabolic Syndrome. Arch Gynecol Obstet (2017) 296(3):405–19. doi: 10.1007/s00404-017-4429-2 [DOI] [PubMed] [Google Scholar]
- 32. Lee EJ, In KH, Ha ES, Lee KJ, Hur GY, Kang EH, et al. Asthma-Like Symptoms are Increased in the Metabolic Syndrome. J Asthma Off J Assoc Care Asthma (2009) 46(4):339–42. doi: 10.1080/02770900802660931 [DOI] [PubMed] [Google Scholar]
- 33. Brumpton BM, Camargo CA, Romundstad PR, Langhammer A, Chen Y, Mai XM. Metabolic Syndrome and Incidence of Asthma in Adults: The HUNT Study. Eur Respir J (2013) 42(6):1495–502. doi: 10.1183/09031936.00046013 [DOI] [PubMed] [Google Scholar]
- 34. Tomita Y, Fukutomi Y, Irie M, Azekawa K, Hayashi H, Kamide Y, et al. Obesity, But Not Metabolic Syndrome, as a Risk Factor for Late-Onset Asthma in Japanese Women. Allergol Int Off J Jpn Soc Allergol (2019) 68(2):240–6. doi: 10.1016/j.alit.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 35. Assad N, Qualls C, Smith LJ, Arynchyn A, Thyagarajan B, Schuyler M, et al. Body Mass Index is a Stronger Predictor Than the Metabolic Syndrome for Future Asthma in Women. The Longitudinal CARDIA Study. Am J Respir Crit Care Med (2013) 188(3):319–26. doi: 10.1164/rccm.201303-0457OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Contreras ZA, Chen Z, Roumeliotaki T, Annesi-Maesano I, Baïz N, von Berg A, et al. Does Early Onset Asthma Increase Childhood Obesity Risk? A Pooled Analysis of 16 European Cohorts. Eur Respir J (2018) 52(3):1800504. doi: 10.1183/13993003.00504-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen Z, Salam MT, Alderete TL, Habre R, Bastain TM, Berhane K, et al. Effects of Childhood Asthma on the Development of Obesity Among School-Aged Children. Am J Respir Crit Care Med (2017) 195(9):1181–8. doi: 10.1164/rccm.201608-1691OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ding DJ, Martin JG, Macklem PT. Effects of Lung Volume on Maximal Methacholine-Induced Bronchoconstriction in Normal Humans. J Appl Physiol Bethesda Md 1985 (1987) 62(3):1324–30. doi: 10.1152/jappl.1987.62.3.1324 [DOI] [PubMed] [Google Scholar]
- 39. Miethe S, Karsonova A, Karaulov A, Renz H. Obesity and Asthma. J Allergy Clin Immunol (2020) 146(4):685–93. doi: 10.1016/j.jaci.2020.08.011 [DOI] [PubMed] [Google Scholar]
- 40. Chapman DG, Berend N, Horlyck KR, King GG, Salome CM. Does Increased Baseline Ventilation Heterogeneity Following Chest Wall Strapping Predispose to Airway Hyperresponsiveness? J Appl Physiol Bethesda Md 1985 (2012) 113(1):25–30. doi: 10.1152/japplphysiol.01582.2011 [DOI] [PubMed] [Google Scholar]
- 41. Al-Alwan A, Bates JHT, Chapman DG, Kaminsky DA, DeSarno MJ, Irvin CG, et al. The Nonallergic Asthma of Obesity. A Matter of Distal Lung Compliance. Am J Respir Crit Care Med (2014) 189(12):1494–502. doi: 10.1164/rccm.201401-0178OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wood LG, Garg ML, Gibson PG. A High-Fat Challenge Increases Airway Inflammation and Impairs Bronchodilator Recovery in Asthma. J Allergy Clin Immunol (2011) 127(5):1133–40. doi: 10.1016/j.jaci.2011.01.036 [DOI] [PubMed] [Google Scholar]
- 43. Everaere L, Ait-Yahia S, Molendi-Coste O, Vorng H, Quemener S, LeVu P, et al. Innate Lymphoid Cells Contribute to Allergic Airway Disease Exacerbation by Obesity. J Allergy Clin Immunol (2016) 138(5):1309–1318.e11. doi: 10.1016/j.jaci.2016.03.019 [DOI] [PubMed] [Google Scholar]
- 44. Lugogo N, Francisco D, Addison KJ, Manne A, Pederson W, Ingram JL, et al. Obese Asthmatic Patients Have Decreased Surfactant Protein A Levels: Mechanisms and Implications. J Allergy Clin Immunol (2018) 141(3):918–926.e3. doi: 10.1016/j.jaci.2017.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lindner PG. Long-Term Health of Offspring of Women With Polycystic Ovarian Syndrome. Clin Obstet Gynecol (2021) 64(1):48–54. doi: 10.1097/GRF.0000000000000598 [DOI] [PubMed] [Google Scholar]
- 46. Hung JH, Hu LY, Tsai SJ, Yang AC, Huang MW, Chen PM, et al. Risk of Psychiatric Disorders Following Polycystic Ovary Syndrome: A Nationwide Population-Based Cohort Study. PloS One (2014) 9(5):e97041. doi: 10.1371/journal.pone.0097041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Joham AE, Teede HJ, Ranasinha S, Zoungas S, Boyle J. Prevalence of Infertility and Use of Fertility Treatment in Women With Polycystic Ovary Syndrome: Data From a Large Community-Based Cohort Study. J Womens Health 2002 (2015) 24(4):299–307. doi: 10.1089/jwh.2014.5000 [DOI] [PubMed] [Google Scholar]
- 48. Santos MA, Kuijk EW, Macklon NS. The Impact of Ovarian Stimulation for IVF on the Developing Embryo. Reprod Camb Engl (2010) 139(1):23–34. doi: 10.1530/REP-09-0187 [DOI] [PubMed] [Google Scholar]
- 49. Franks S, Stark J, Hardy K. Follicle Dynamics and Anovulation in Polycystic Ovary Syndrome. Hum Reprod Update (2008) 14(4):367–78. doi: 10.1093/humupd/dmn015 [DOI] [PubMed] [Google Scholar]
- 50. Qiao J, Feng HL. Extra- and Intra-Ovarian Factors in Polycystic Ovary Syndrome: Impact on Oocyte Maturation and Embryo Developmental Competence. Hum Reprod Update (2011) 17(1):17–33. doi: 10.1093/humupd/dmq032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Teissier MP, Chable H, Paulhac S, Aubard Y. Comparison of Follicle Steroidogenesis From Normal and Polycystic Ovaries in Women Undergoing IVF: Relationship Between Steroid Concentrations, Follicle Size, Oocyte Quality and Fecundability. Hum Reprod Oxf Engl (2000) 15(12):2471–7. doi: 10.1093/humrep/15.12.2471 [DOI] [PubMed] [Google Scholar]
- 52. Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, et al. A Direct Effect of Hyperinsulinemia on Serum Sex Hormone-Binding Globulin Levels in Obese Women With the Polycystic Ovary Syndrome. J Clin Endocrinol Metab (1991) 72(1):83–9. doi: 10.1210/jcem-72-1-83 [DOI] [PubMed] [Google Scholar]
- 53. Billig H, Furuta I, Hsueh AJ. Estrogens Inhibit and Androgens Enhance Ovarian Granulosa Cell Apoptosis. Endocrinology (1993) 133(5):2204–12. doi: 10.1210/endo.133.5.8404672 [DOI] [PubMed] [Google Scholar]
- 54. Hillier SG. Current Concepts of the Roles of Follicle Stimulating Hormone and Luteinizing Hormone in Folliculogenesis. Hum Reprod Oxf Engl (1994) 9(2):188–91. doi: 10.1093/oxfordjournals.humrep.a138480 [DOI] [PubMed] [Google Scholar]
- 55. de Wilde MA, de Ruiter ML, Veltman-Verhulst SM, Kwee A, Laven JS, Lambalk CB, et al. Increased Rates of Complications in Singleton Pregnancies of Women Previously Diagnosed With Polycystic Ovary Syndrome Predominantly in the Hyperandrogenic Phenotype. Fertil Steril (2017) 108(2):333–40. doi: 10.1016/j.fertnstert.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 56. Juul Gade E, Thomsen SF, Lindenberg S, Backer V. Female Asthma has a Negative Effect on Fertility: What is the Connection? ISRN Allergy (2014) 2014:131092. doi: 10.1155/2014/131092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sunyer J, Antó JM, Plana E, Janson C, Jarvis D, Kony S, et al. Maternal Atopy and Changes in Parity. Clin Exp Allergy J Br Soc Allergy Clin Immunol (2005) 35(8):1028–32. doi: 10.1111/j.1365-2222.2005.02300.x [DOI] [PubMed] [Google Scholar]
- 58. Vejen Hansen A, Ali Z, Malchau SS, Blafoss J, Pinborg A, Ulrik CS. Fertility Treatment Among Women With Asthma: A Case-Control Study of 3689 Women With Live Births. Eur Respir J (2019) 53(2):1800597. doi: 10.1183/13993003.00597-2018 [DOI] [PubMed] [Google Scholar]
- 59. Gade EJ, Thomsen SF, Lindenberg S, Backer V. Fertility Outcomes in Asthma: A Clinical Study of 245 Women With Unexplained Infertility. Eur Respir J (2016) 47(4):1144–51. doi: 10.1183/13993003.01389-2015 [DOI] [PubMed] [Google Scholar]
- 60. Rocklin RE. Asthma, Asthma Medications and Their Effects on Maternal/Fetal Outcomes During Pregnancy. Reprod Toxicol Elmsford N (2011) 32(2):189–97. doi: 10.1016/j.reprotox.2011.05.023 [DOI] [PubMed] [Google Scholar]
- 61. Metwally M, Li TC, Ledger WL. The Impact of Obesity on Female Reproductive Function. Obes Rev Off J Int Assoc Study Obes (2007) 8(6):515–23. doi: 10.1111/j.1467-789X.2007.00406.x [DOI] [PubMed] [Google Scholar]
- 62. Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, et al. High Fat Diet Induced Developmental Defects in the Mouse: Oocyte Meiotic Aneuploidy and Fetal Growth Retardation/Brain Defects. PloS One (2012) 7(11):e49217. doi: 10.1371/journal.pone.0049217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-Induced Obesity Model: Abnormal Oocytes and Persistent Growth Abnormalities in the Offspring. Endocrinology (2010) 151(8):4039–46. doi: 10.1210/en.2010-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Silvestris E, de Pergola G, Rosania R, Loverro G. Obesity as Disruptor of the Female Fertility. Reprod Biol Endocrinol RBE (2018) 16(1):22. doi: 10.1186/s12958-018-0336-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Campos DB, Palin MF, Bordignon V, Murphy BD. The ‘Beneficial’ Adipokines in Reproduction and Fertility. Int J Obes 2005 (2008) 32(2):223–31. doi: 10.1038/sj.ijo.0803719 [DOI] [PubMed] [Google Scholar]
- 66. Li S, Huang X, Zhong H, Peng Q, Chen S, Xie Y, et al. Low Circulating Adiponectin Levels in Women With Polycystic Ovary Syndrome: An Updated Meta-Analysis. Tumour Biol J Int Soc Oncodevelopmental Biol Med (2014) 35(5):3961–73. doi: 10.1007/s13277-013-1595-0 [DOI] [PubMed] [Google Scholar]
- 67. Zhang MY, Dini AA, Yang XK, Li LJ, Wu GC, Leng RX, et al. Association Between Serum/Plasma Adiponectin Levels and Immune-Mediated Diseases: A Meta-Analysis. Arch Dermatol Res (2017) 309(8):625–35. doi: 10.1007/s00403-017-1755-y [DOI] [PubMed] [Google Scholar]
- 68. Bongrani A, Mellouk N, Rame C, Cornuau M, Guérif F, Froment P, et al. Ovarian Expression of Adipokines in Polycystic Ovary Syndrome: A Role for Chemerin, Omentin, and Apelin in Follicular Growth Arrest and Ovulatory Dysfunction? Int J Mol Sci (2019) 20(15):E3778. doi: 10.3390/ijms20153778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Morrison LJ, Marcinkiewicz JL. Tumor Necrosis Factor Alpha Enhances Oocyte/Follicle Apoptosis in the Neonatal Rat Ovary. Biol Reprod (2002) 66(2):450–7. doi: 10.1095/biolreprod66.2.450 [DOI] [PubMed] [Google Scholar]
- 70. Nakayama M, Manabe N, Inoue N, Matsui T, Miyamoto H. Changes in the Expression of Tumor Necrosis Factor (TNF) Alpha, TNFalpha Receptor (TNFR) 2, and TNFR-Associated Factor 2 in Granulosa Cells During Atresia in Pig Ovaries. Biol Reprod (2003) 68(2):530–5. doi: 10.1095/biolreprod.102.004820 [DOI] [PubMed] [Google Scholar]
- 71. lan CL, Yang G, Pan J, Zhang C. Tumor Necrosis Factor α Knockout Increases Fertility of Mice. Theriogenology (2011) 75(5):867–76. doi: 10.1016/j.theriogenology.2010.10.029 [DOI] [PubMed] [Google Scholar]
- 72. Eddie SL, Childs AJ, Jabbour HN, Anderson RA. Developmentally Regulated IL6-Type Cytokines Signal to Germ Cells in the Human Fetal Ovary. Mol Hum Reprod (2012) 18(2):88–95. doi: 10.1093/molehr/gar061 [DOI] [PubMed] [Google Scholar]
- 73. Choi YS, Yang HI, Cho S, Jung JA, Jeon YE, Kim HY, et al. Serum Asymmetric Dimethylarginine, Apelin, and Tumor Necrosis Factor-α Levels in non-Obese Women With Polycystic Ovary Syndrome. Steroids (2012) 77(13):1352–8. doi: 10.1016/j.steroids.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 74. Rostamtabar M, Esmaeilzadeh S, Tourani M, Rahmani A, Baee M, Shirafkan F, et al. Pathophysiological Roles of Chronic Low-Grade Inflammation Mediators in Polycystic Ovary Syndrome. J Cell Physiol (2021) 236(2):824–38. doi: 10.1002/jcp.29912 [DOI] [PubMed] [Google Scholar]
- 75. Liu S, Hong L, Mo M, Xiao S, Chen C, Li Y, et al. Evaluation of Endometrial Immune Status of Polycystic Ovary Syndrome. J Reprod Immunol (2021) 144:103282. doi: 10.1016/j.jri.2021.103282 [DOI] [PubMed] [Google Scholar]
- 76. Pan J, Zhou C, Zhou Z, Yang Z, Dai T, Huang H, et al. Elevated Ovarian Pentraxin 3 in Polycystic Ovary Syndrome. J Assist Reprod Genet (2021) 38(5):1231–7. doi: 10.1007/s10815-021-02105-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wyskida K, Franik G, Choręza P, Pohl N, Markuszewski L, Owczarek A, et al. Pentraxin 3 Levels in Young Women With and Without Polycystic Ovary Syndrome (PCOS) in Relation to the Nutritional Status and Systemic Inflammation. Int J Endocrinol (2020) 2020:1380176. doi: 10.1155/2020/1380176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tosi F, Di Sarra D, Bonin C, Zambotti F, Dall’Alda M, Fiers T, et al. Plasma Levels of Pentraxin-3, an Inflammatory Protein Involved in Fertility, are Reduced in Women With Polycystic Ovary Syndrome. Eur J Endocrinol (2014) 170(3):401–9. doi: 10.1530/EJE-13-0761 [DOI] [PubMed] [Google Scholar]
- 79. Jin C, Zou K, Xu Y, Yang H, Pan J. Elevated Plasma Pentraxin-3 in Polycystic Ovary Syndrome is Associated With Hyperandrogenism: A Case-Control Study. BMC Endocr Disord (2021) 21(1):240. doi: 10.1186/s12902-021-00886-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, et al. PTX3 Plays a Key Role in the Organization of the Cumulus Oophorus Extracellular Matrix and in In Vivo Fertilization. Dev Camb Engl (2004) 131(7):1577–86. doi: 10.1242/dev.01056 [DOI] [PubMed] [Google Scholar]
- 81. Teede HJ, Joham AE, Paul E, Moran LJ, Loxton D, Jolley D, et al. Longitudinal Weight Gain in Women Identified With Polycystic Ovary Syndrome: Results of an Observational Study in Young Women. Obes Silver Spring Md. (2013) 21(8):1526–32. doi: 10.1002/oby.20213 [DOI] [PubMed] [Google Scholar]
- 82. Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, et al. Polycystic Ovary Syndrome: The Spectrum of the Disorder in 1741 Patients. Hum Reprod Oxf Engl (1995) 10(8):2107–11. doi: 10.1093/oxfordjournals.humrep.a136243 [DOI] [PubMed] [Google Scholar]
- 83. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JSE, Legro RS, et al. Polycystic Ovary Syndrome. Nat Rev Dis Primer (2016) 2(1):1–18. doi: 10.1038/nrdp.2016.57 [DOI] [PubMed] [Google Scholar]
- 84. Jonard S, Dewailly D. The Follicular Excess in Polycystic Ovaries, Due to Intra-Ovarian Hyperandrogenism, may be the Main Culprit for the Follicular Arrest. Hum Reprod Update (2004) 10(2):107–17. doi: 10.1093/humupd/dmh010 [DOI] [PubMed] [Google Scholar]
- 85. Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr Rev (2016) 37(5):467–520. doi: 10.1210/er.2015-1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yung JA, Fuseini H, Newcomb DC. Hormones, Sex, and Asthma. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol (2018) 120(5):488–94. doi: 10.1016/j.anai.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Svanes C, Real FG, Gislason T, Jansson C, Jögi R, Norrman E, et al. Association of Asthma and Hay Fever With Irregular Menstruation. Thorax (2005) 60(6):445–50. doi: 10.1136/thx.2004.032615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gill D, Sheehan NA, Wielscher M, Shrine N, Amaral AFS, Thompson JR, et al. Age at Menarche and Lung Function: A Mendelian Randomization Study. Eur J Epidemiol (2017) 32(8):701–10. doi: 10.1007/s10654-017-0272-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Real FG, Svanes C, Omenaas ER, Antò JM, Plana E, Janson C, et al. Menstrual Irregularity and Asthma and Lung Function. J Allergy Clin Immunol (2007) 120(3):557–64. doi: 10.1016/j.jaci.2007.04.041 [DOI] [PubMed] [Google Scholar]
- 90. Vrieze A, Postma DS, Kerstjens HA. Perimenstrual Asthma: A Syndrome Without Known Cause or Cure. J Allergy Clin Immunol (2003) 112(2):271–82. doi: 10.1067/mai.2003.1676 [DOI] [PubMed] [Google Scholar]
- 91. Semik-Orzech A, Skoczyński S, Pierzchała W. Serum Estradiol Concentration, Estradiol-to-Progesterone Ratio and Sputum IL-5 and IL-8 Concentrations are Increased in Luteal Phase of the Menstrual Cycle in Perimenstrual Asthma Patients. Eur Ann Allergy Clin Immunol (2017) 49(4):161–70. doi: 10.23822/eurannaci.1764-1489.09 [DOI] [PubMed] [Google Scholar]
- 92. Rubio Ravelo L, Gago Rodríguez B, Almirall Collazo JJ, Bell Heredia L, Fernández Fernández L. Comparative Study of Progesterone, Estradiol and Cortisol Concentrations in Asthmatic and non-Asthmatic Women. Allergol Immunopathol (Madr) (1988) 16(4):263–6. [PubMed] [Google Scholar]
- 93. Carey MA, Card JW, Bradbury JA, Moorman MP, Haykal-Coates N, Gavett SH, et al. Spontaneous Airway Hyperresponsiveness in Estrogen Receptor-Alpha-Deficient Mice. Am J Respir Crit Care Med (2007) 175(2):126–35. doi: 10.1164/rccm.200509-1493OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Keselman A, Fang X, White PB, Heller NM. Estrogen Signaling Contributes to Sex Differences in Macrophage Polarization During Asthma. J Immunol Baltim Md 1950 (2017) 199(5):1573–83. doi: 10.4049/jimmunol.1601975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen Preferentially Promotes the Differentiation of CD11c+ CD11b(intermediate) Dendritic Cells From Bone Marrow Precursors. J Immunol Baltim Md 1950 (2004) 172(3):1426–36. doi: 10.4049/jimmunol.172.3.1426 [DOI] [PubMed] [Google Scholar]
- 96. Newcomb DC, Cephus JY, Boswell MG, Fahrenholz JM, Langley EW, Feldman AS, et al. Estrogen and Progesterone Decrease Let-7f microRNA Expression and Increase IL-23/IL-23 Receptor Signaling and IL-17A Production in Patients With Severe Asthma. J Allergy Clin Immunol (2015) 136(4):1025–1034.e11. doi: 10.1016/j.jaci.2015.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Abdul-Karim RW, Drucker M, Jacobs RD. The Influence of Estradiol-17 Beta on Cholinesterase Activity in the Lung. Am J Obstet Gynecol (1970) 108(7):1098–101. doi: 10.1016/0002-9378(70)90459-x [DOI] [PubMed] [Google Scholar]
- 98. Boulet LP, Boulay MÈ. Asthma-Related Comorbidities. Expert Rev Respir Med (2011) 5(3):377–93. doi: 10.1586/ers.11.34 [DOI] [PubMed] [Google Scholar]
- 99. Deeks AA, Gibson-Helm ME, Paul E, Teede HJ. Is Having Polycystic Ovary Syndrome a Predictor of Poor Psychological Function Including Anxiety and Depression? Hum Reprod Oxf Engl (2011) 26(6):1399–407. doi: 10.1093/humrep/der071 [DOI] [PubMed] [Google Scholar]
- 100. Rowlands IJ, Teede H, Lucke J, Dobson AJ, Mishra GD. Young Women’s Psychological Distress After a Diagnosis of Polycystic Ovary Syndrome or Endometriosis. Hum Reprod Oxf Engl (2016) 31(9):2072–81. doi: 10.1093/humrep/dew174 [DOI] [PubMed] [Google Scholar]
- 101. Damone AL, Joham AE, Loxton D, Earnest A, Teede HJ, Moran LJ. Depression, Anxiety and Perceived Stress in Women With and Without PCOS: A Community-Based Study. Psychol Med (2019) 49(9):1510–20. doi: 10.1017/S0033291718002076 [DOI] [PubMed] [Google Scholar]
- 102. De Silva A, Salem V, Matthews PM, Dhillo WS. The Use of Functional MRI to Study Appetite Control in the CNS. Exp Diabetes Res (2012) 2012:764017. doi: 10.1155/2012/764017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Marsh CA, Berent-Spillson A, Love T, Persad CC, Pop-Busui R, Zubieta JK, et al. Functional Neuroimaging of Emotional Processing in Women With Polycystic Ovary Syndrome: A Case-Control Pilot Study. Fertil Steril (2013) 100(1):200–207.e1. doi: 10.1016/j.fertnstert.2013.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen MH, Su TP, Chen YS, Hsu JW, Huang KL, Chang WH, et al. Higher Risk of Developing Major Depression and Bipolar Disorder in Later Life Among Adolescents With Asthma: A Nationwide Prospective Study. J Psychiatr Res (2014) 49:25–30. doi: 10.1016/j.jpsychires.2013.10.015 [DOI] [PubMed] [Google Scholar]
- 105. Dudeney J, Sharpe L, Jaffe A, Jones EB, Hunt C. Anxiety in Youth With Asthma: A Meta-Analysis. Pediatr Pulmonol (2017) 52(9):1121–9. doi: 10.1002/ppul.23689 [DOI] [PubMed] [Google Scholar]
- 106. Slattery MJ, Essex MJ. Specificity in the Association of Anxiety, Depression, and Atopic Disorders in a Community Sample of Adolescents. J Psychiatr Res (2011) 45(6):788–95. doi: 10.1016/j.jpsychires.2010.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gao X, Xiao Y, Lv P, Zhang W, Gong Y, Wang T, et al. Altered Brain Network Integrity in Patients With Asthma: A Structural Connectomic Diffusion Tensor Imaging Study. Respir Physiol Neurobiol (2019) 266:89–94. doi: 10.1016/j.resp.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 108. Ritz T, Kroll JL, Aslan S, Janssens T, Khan DA, Pinkham AE, et al. Subcortical Gray Matter Volumes in Asthma: Associations With Asthma Duration, Control, and Anxiety. Brain Imaging Behav (2020) 14(6):2341–50. doi: 10.1007/s11682-019-00188-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE. Diurnal Cortisol Slopes and Mental and Physical Health Outcomes: A Systematic Review and Meta-Analysis. Psychoneuroendocrinology (2017) 83:25–41. doi: 10.1016/j.psyneuen.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dieleman GC, Huizink AC, Tulen JHM, Utens EMWJ, Creemers HE, van der Ende J, et al. Alterations in HPA-Axis and Autonomic Nervous System Functioning in Childhood Anxiety Disorders Point to a Chronic Stress Hypothesis. Psychoneuroendocrinology (2015) 51:135–50. doi: 10.1016/j.psyneuen.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 111. McEwen BS. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol Rev (2007) 87(3):873–904. doi: 10.1152/physrev.00041.2006 [DOI] [PubMed] [Google Scholar]
- 112. Kamps AWA, Molenmaker M, Kemperman R, van der Veen BS, Bocca G, Veeger NJGM. Children With Asthma Have Significantly Lower Long-Term Cortisol Levels in Their Scalp Hair Than Healthy Children. Acta Paediatr Oslo Nor 1992 (2014) 103(9):957–61. doi: 10.1111/apa.12685 [DOI] [PubMed] [Google Scholar]
- 113. Kroll JL, Brown ES, Ritz T. Cortisol Awakening Response and Fractional Exhaled Nitric Oxide in Asthma. Clin Exp Allergy J Br Soc Allergy Clin Immunol (2019) 49(8):1150–3. doi: 10.1111/cea.13446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Caulfield JI, Cavigelli SA. Individual Differences in Glucocorticoid Regulation: Does it Relate to Disease Risk and Resilience? Front Neuroendocrinol (2020) 56:100803. doi: 10.1016/j.yfrne.2019.100803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Basu BR, Chowdhury O, Saha SK. Possible Link Between Stress-Related Factors and Altered Body Composition in Women With Polycystic Ovarian Syndrome. J Hum Reprod Sci (2018) 11(1):10–8. doi: 10.4103/jhrs.JHRS_78_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mezzullo M, Fanelli F, Di Dalmazi G, Fazzini A, Ibarra-Gasparini D, Mastroroberto M, et al. Salivary Cortisol and Cortisone Responses to Short-Term Psychological Stress Challenge in Late Adolescent and Young Women With Different Hyperandrogenic States. Psychoneuroendocrinology (2018) 91:31–40. doi: 10.1016/j.psyneuen.2018.02.022 [DOI] [PubMed] [Google Scholar]
- 117. Prelević GM, Würzburger MI, Balint-Perić L. 24-Hour Serum Cortisol Profiles in Women With Polycystic Ovary Syndrome. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol (1993) 7(3):179–84. doi: 10.3109/09513599309152500 [DOI] [PubMed] [Google Scholar]
- 118. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A Meta-Analysis of Cytokines in Major Depression. Biol Psychiatry (2010) 67(5):446–57. doi: 10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- 119. Goldsmith DR, Rapaport MH, Miller BJ. A Meta-Analysis of Blood Cytokine Network Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder and Depression. Mol Psychiatry (2016) 21(12):1696–709. doi: 10.1038/mp.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gao T, Li B, Hou Y, Luo S, Feng L, Nie J, et al. Interleukin-4 Signalling Pathway Underlies the Anxiolytic Effect Induced by 3-Deoxyadenosine. Psychopharmacol (Berl) (2019) 236(10):2959–73. doi: 10.1007/s00213-019-5186-7 [DOI] [PubMed] [Google Scholar]
- 121. Costello H, Gould RL, Abrol E, Howard R. Systematic Review and Meta-Analysis of the Association Between Peripheral Inflammatory Cytokines and Generalised Anxiety Disorder. BMJ Open (2019) 9(7):e027925. doi: 10.1136/bmjopen-2018-027925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yarlagadda A, Alfson E, Clayton AH. The Blood Brain Barrier and the Role of Cytokines in Neuropsychiatry. Psychiatry Edgmont Pa Townsh (2009) 6(11):18–22. [PMC free article] [PubMed] [Google Scholar]
- 123. Shelton RC, Miller AH. Inflammation in Depression: Is Adiposity a Cause? Dialogues Clin Neurosci (2011) 13(1):41–53. doi: 10.31887/DCNS.2011.13.1/rshelton [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 Receptor Activation by Systemic Lipopolysaccharide Induces Behavioral Despair Linked to MAPK Regulation of CNS Serotonin Transporters. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol (2010) 35(13):2510–20. doi: 10.1038/npp.2010.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kolhe JV, Chhipa AS, Butani S, Chavda V, Patel SS. PCOS and Depression: Common Links and Potential Targets. Reprod Sci Thousand Oaks Calif (2021). doi: 10.1007/s43032-021-00765-2 [DOI] [PubMed] [Google Scholar]
- 126. Caulfield JI, Schopf KJ, Cavigelli SA. Peri-Adolescent Asthma: Acute Impacts on Innate Immune Response, Corticosterone, and Microglia in Mice. J Neuroimmunol (2020) 350:577450. doi: 10.1016/j.jneuroim.2020.577450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Brites D, Fernandes A. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front Cell Neurosci (2015) 9:476. doi: 10.3389/fncel.2015.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Cao P, Chen C, Liu A, Shan Q, Zhu X, Jia C, et al. Early-Life Inflammation Promotes Depressive Symptoms in Adolescence via Microglial Engulfment of Dendritic Spines. Neuron (2021) 109(16):2573–2589.e9. doi: 10.1016/j.neuron.2021.06.012 [DOI] [PubMed] [Google Scholar]
- 129. Kyrou I, Karteris E, Robbins T, Chatha K, Drenos F, Randeva HS. Polycystic Ovary Syndrome (PCOS) and COVID-19: An Overlooked Female Patient Population at Potentially Higher Risk During the COVID-19 Pandemic. BMC Med (2020) 18(1):220. doi: 10.1186/s12916-020-01697-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Eger K, Bel EH. Asthma and COVID-19: Do We Finally Have Answers? Eur Respir J (2021) 57(3):2004451–4. doi: 10.1183/13993003.04451-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Johnston SL. Asthma and COVID-19: Is Asthma a Risk Factor for Severe Outcomes? Allergy (2020) 75(7):1543–5. doi: 10.1111/all.14348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Adir Y, Saliba W, Beurnier A, Humbert M. Asthma and COVID-19: An Update. Eur Respir Rev (2021) 30(162):210152. doi: 10.1183/16000617.0152-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Izquierdo JL, Almonacid C, González Y, Del Rio-Bermudez C, Ancochea J, Cárdenas R, et al. The Impact of COVID-19 on Patients With Asthma. Eur Respir J (2021) 57(3):2003142. doi: 10.1183/13993003.03142-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Skevaki C, Karsonova A, Karaulov A, Xie M, Renz H. Asthma-Associated Risk for COVID-19 Development. J Allergy Clin Immunol (2020) 146(6):1295–301. doi: 10.1016/j.jaci.2020.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet Lond Engl (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chen X, Lin H, Yang D, Xu W, Liu G, Liu X, et al. Early-Life Undernutrition Reprograms CD4+ T-Cell Glycolysis and Epigenetics to Facilitate Asthma. J Allergy Clin Immunol (2019) 143(6):2038–2051.e12. doi: 10.1016/j.jaci.2018.12.999 [DOI] [PubMed] [Google Scholar]
- 137. Mimouni NEH, Paiva I, Barbotin AL, Timzoura FE, Plassard D, Le Gras S, et al. Polycystic Ovary Syndrome is Transmitted via a Transgenerational Epigenetic Process. Cell Metab (2021) 33(3):513–530.e8. doi: 10.1016/j.cmet.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]