Highlights
-
•
We analyzed the number of normal variants in a SCORE database of 3050 EEG recordings.
-
•
The most common normal variant was sharp transients.
-
•
We present typical examples and detailed characterization of the normal variants.
Keywords: EEG, Epilepsy, Normal variant, Benign epileptiform variants
Abstract
Objective
To determine the prevalence and characteristics of normal variants in EEG recordings in a large cohort, and provide readers with typical examples of all normal variants for educational purposes.
Methods
Using the SCORE EEG system (Standardized Computer-Based Organized Reporting of EEG), we prospectively extracted EEG features in consecutive patients. In this dataset, we analyzed 3050 recordings from 2319 patients (mean age 38.5 years; range: 1–89 years).
Results
The distribution of the normal variants was as follows: sharp transients 19.21% (including wicket spikes), rhythmic temporal theta of drowsiness 6.03%, temporal slowing of the old 2.89%, slow fused transients 2.59%, 14-and 6-Hz bursts 1.83%, breach rhythm 1.25%, small sharp spikes 1.05%, 6-Hz spike and slow wave 0.69% and SREDA 0.03%.
Conclusions
The most prevalent normal variants are the sharp transients, which must not be over-read as epileptiform discharges.
Significance
EEG readers must be familiar with the normal variants to avoid misdiagnosis and misclassification of patients referred to clinical EEG recordings.
1. Introduction
“Primum non nocere – First, do no harm” is part of the original Hippocratic oath. This is also important for clinical EEG reading, as over-diagnosing patients can do great harm. In addition to the considerable economic burden, over-diagnosing epilepsy has detrimental consequence for the patients, such as exposure to the unnecessary side effects of a futile treatment and limiting the patients’ mobility and career choices (Juarez-Garcia et al., 2006, Nowack, 1997).
When reading EEG, it is important to distinguish normal from abnormal patterns, and to avoid over-reading of normal patterns that share features resembling the abnormal patterns. This is crucial as the same patterns can be seen in healthy individuals too. These benign (normal) variants can resemble both epileptiform waveforms and rhythmic patterns (Tatum et al., 2006). With untrained EEGers, these patterns may lead to misdiagnosis, as they are often over-interpreted by readers lacking proper training (Benbadis and Tatum, 2003). Rathore et al. looked at 282 EEG studies in which none of these normal variants were even mentioned and were probably misdiagnosed as epileptiform (Rathore et al., 2021). Over-diagnosis of epilepsy is a common problem and often caused by unreliable history and misinterpretation of EEG. Approximately 25–30% of the patients with diagnosed epilepsy, who did not respond to initial antiepileptic drug treatment, were, in fact, incorrectly diagnosed with epilepsy. When first diagnosed with epilepsy, it is quite difficult to reverse the diagnosis even though several follow up EEGs are completely normal (Amin and Benbadis, 2019).
SCORE is a standardized EEG terminology, developed by a working group of the International Federation of Clinical Neurophysiology (IFCN) and the International League Against Epilepsy (ILAE) terms (Beniczky et al., 2013, Beniczky et al., 2017). Using the SCORE EEG system (Holberg EEG AS, Norway), patterns identified in the EEG are annotated with the predefined, standardized terms, clinical reports are generated and the extracted features are stored in the SCORE database. The standardized feature extraction of SCORE EEG seems to be an ideal tool to evaluate the occurrence and types of normal variants. Here we report the normal findings identified using the SCORE EEG system. There is considerable heterogeneity in the literature concerning what is considered a physiologic pattern, and what is normal variant (aka. pattern of uncertain significance). We chose the list of normal variants as listed in the SCORE standard, as patterns of uncertain significance. In addition to detailed description of these phenomena, we provide screenshot examples of the identified typical normal variants in order to convey an educational value to the paper.
2. Methods
EEGs were recorded as part of the diagnostic workup of consecutive patients in the Danish Epilepsy Center (Dianalund, Denmark) and the EEG laboratory in Nuuk (Greenland) between May 1, 2019 and February 8, 2021.The EEGs were recorded with the NicoletOne EEG system (Natus Neuro, USA), using the IFCN electrode array (Seeck et al., 2017). All recordings were carried out by certified EEG technicians or by EEG technicians in training under supervision of certified technicians. The duration of the recordings was 30 min for the routine EEG recordings, one hour for the sleep-EEG recordings, and up to 4 h for the short-term video EEG recordings. The recordings included different provocation techniques, such as intermittent photic stimulation, hyperventilation, sleep and specific provocation protocols (patient-tailored for reflex epilepsy and reflex epileptic traits).
Recordings were first evaluated by the clinical neurophysiology technicians and then by physicians with board-certification in clinical neurophysiology. Observed EEG features were annotated and logged in the database using the SCORE EEG system (Beniczky et al., 2013, Beniczky et al., 2017). SCORE (Standardized Computer-based Organized reporting of EEG) uses pre-defined terms to document the EEG-features annotated in the recording. The data for this study were prospectively recorded, with special emphasis on the presence of any normal variant or pattern of uncertain significance (Beniczky et al., 2013, Beniczky et al., 2017, Klass and Westmoreland, 1985). In addition to the EEG features, we also extracted demographic data (age and sex), diagnosis, and type of benign variants. All recordings were evaluated by at least two experts. However, determining inter-rater agreement in this large prospective dataset was beyond the scope and limitations of this study. For each recording, the diagnostic gold standard was derived from the EEG in combination with all available clinical and para-clinical data. This was coded in the SCORE system as the entry “diagnostic significance”.
3. Results
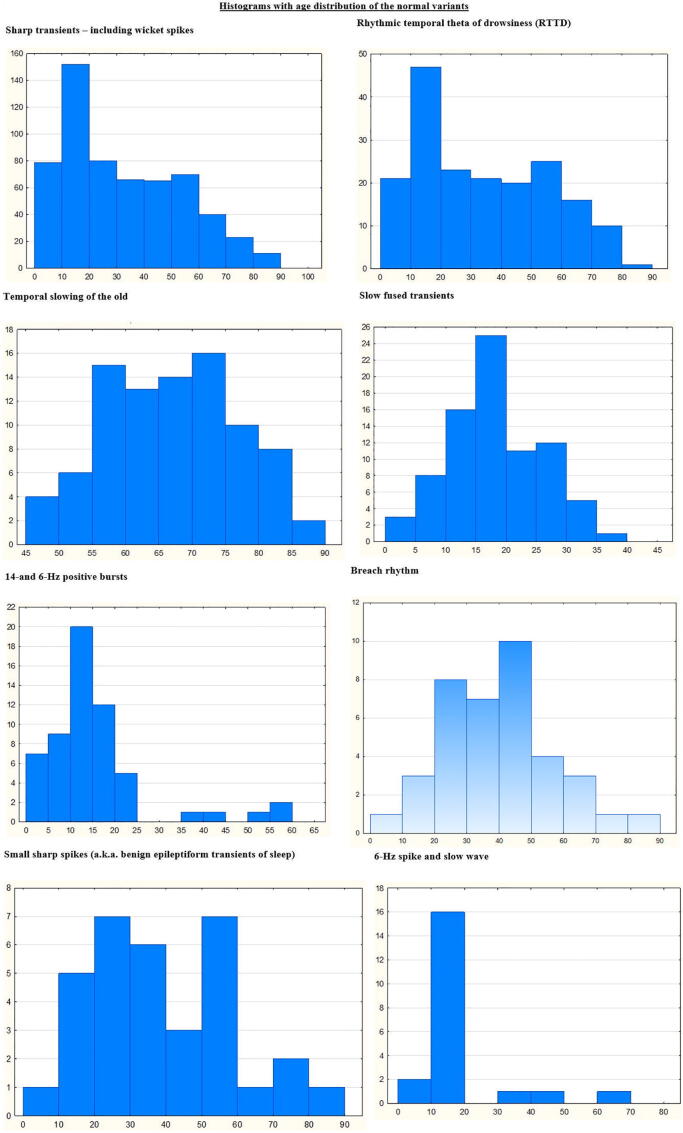
We analyzed 3050 consecutive EEG recordings from 2319 patients (1196 female, 1092 male, and 31 recordings where the sex was not available in the system). The mean age at time of the recording was 38 years (range from 6 months to 89 years). Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11 show typical examples of the normal variants, along with a description of their characteristics. Fig. 12 shows the histograms of the age distribution of the normal variants.
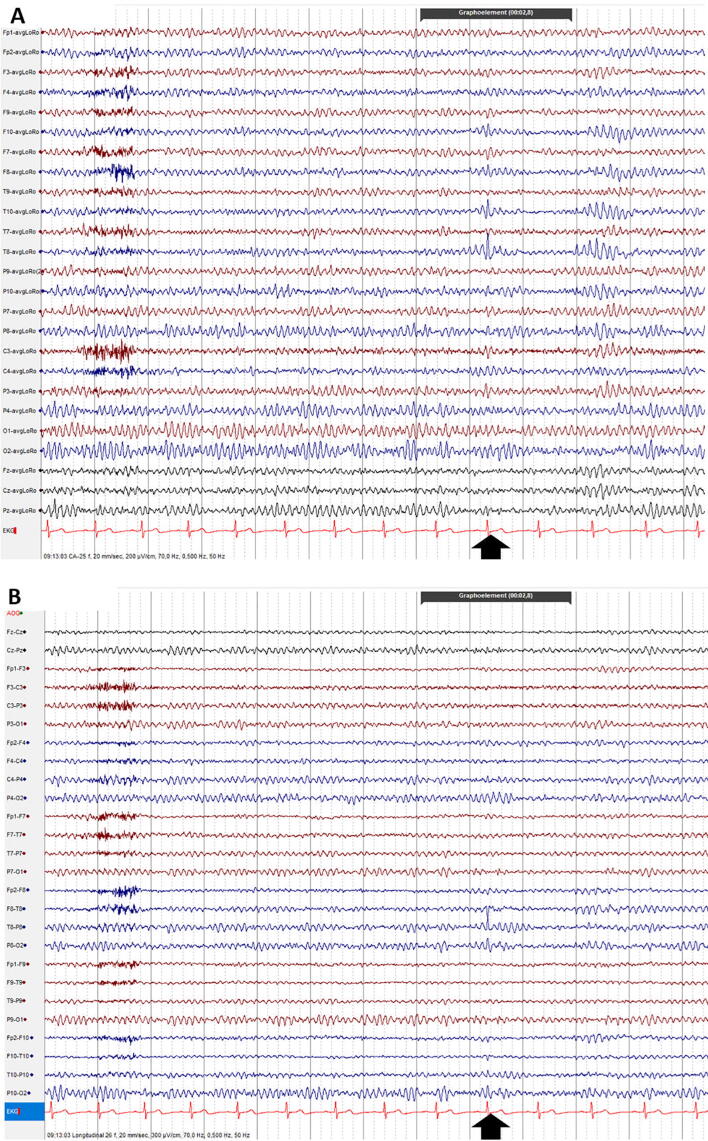
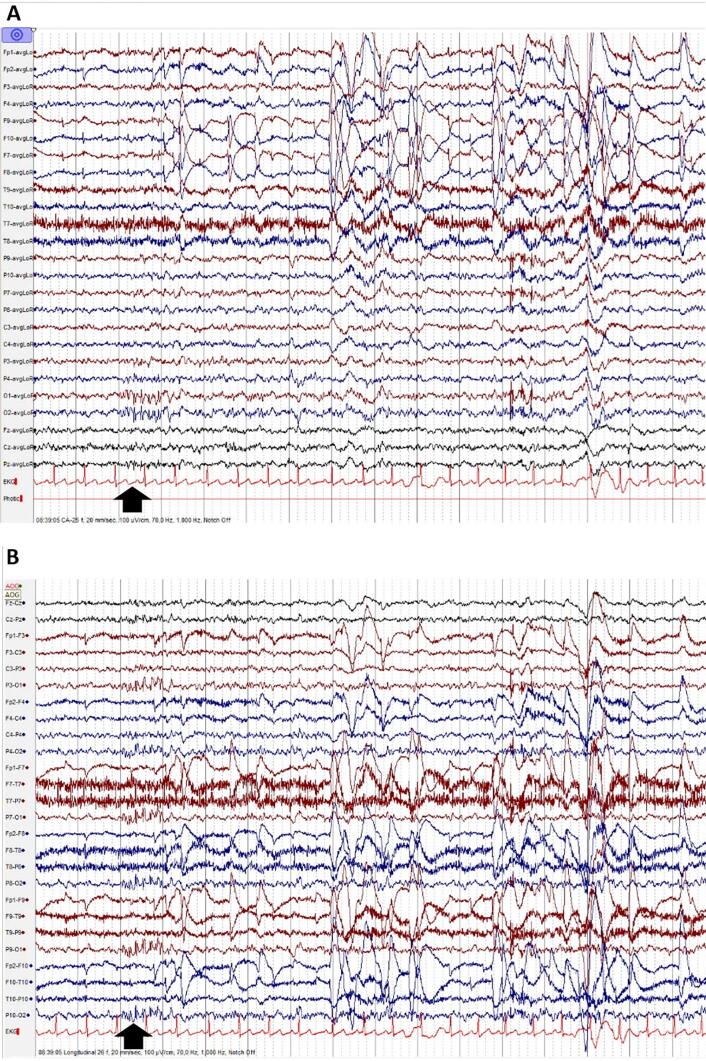
Fig. 1.
Sharp transients: fluctuations of the background activity which do not fulfill the operational criteria for epileptic discharges defined by the IFCN. Amplitude is usually higher than the rest of the background activity and they have a pointed peak. This normal variant is often misinterpreted as an epileptiform discharge. Observe the sharp transient at electrodes P10, P8, O2, at the time-point indicated by the black vertical arrow (A: Common Average montage; B: Longitudinal Bipolar montage).
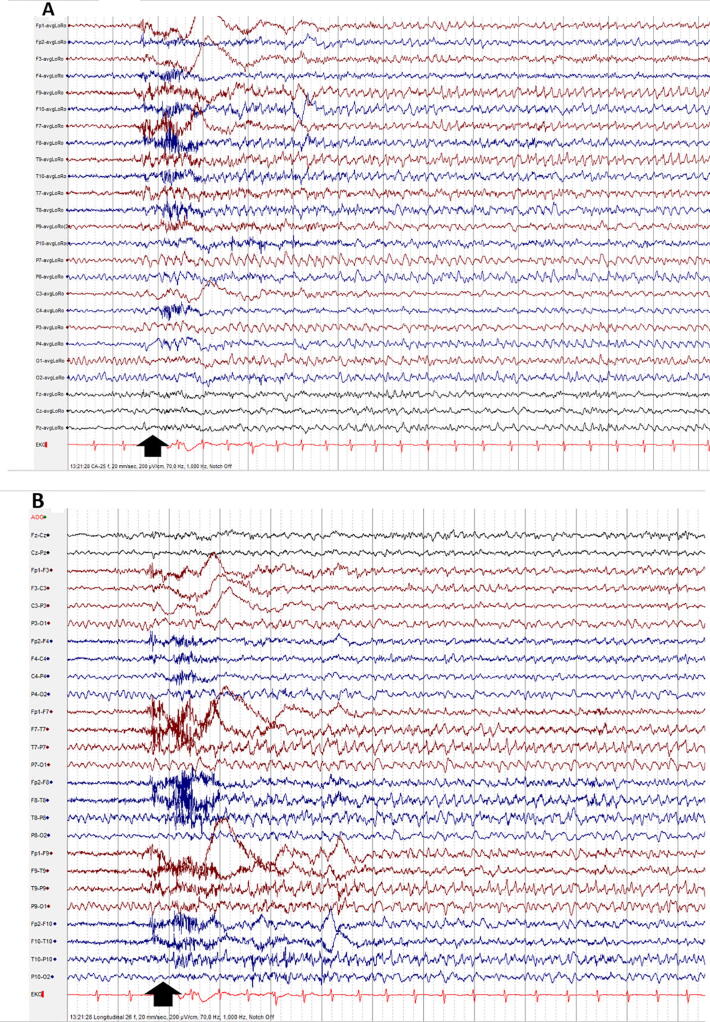
Fig. 2.
Wicket spikes / rhythm: subtype of sharp transients, with arciform waveforms, which are simple monophasic with surface negativity. They can be observed as single discharges or runs. Observe the wicket spike at electrode T8, at the time-point indicated by the black vertical arrow (A: Common Average montage; B: Longitudinal Bipolar montage).
Fig. 3.
Rhythmic Temporal Theta of Drowsiness (RTTD): trains of theta activity, usually in the mid-temporal region, during drowsiness. It can be observed independently over one side or bilaterally. The typical configuration is arch-shaped and often notched. Observe the RTTD in the left temporal area, at the time-point indicated by the black vertical arrow (A: Common Average montage; B: Longitudinal Bipolar montage).
Fig. 4.
Temporal slowing of the old: short runs of theta activity or delta potentials, mixed with the background activity of the same area, in age groups ≥ 50 years, without clinical abnormalities. Observe the delta potential in the left temporal area, at the time-point indicated by the black vertical arrow (A: Common Average montage; B: Longitudinal Bipolar montage).
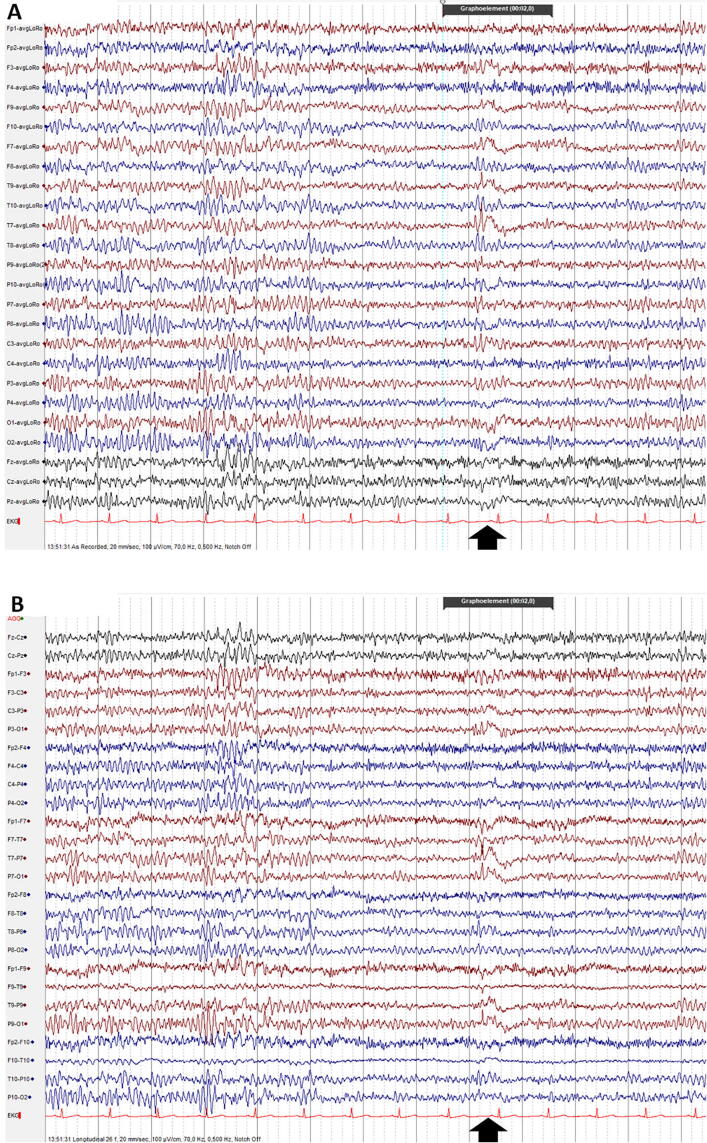
Fig. 5.
Slow-fused transients: by coincidence, a non-epileptiform sharp transient precedes a posterior slow-wave of youth or a temporal slowing of the old, giving the false impression of a spike-wave. Browsing the entire recording the two phenomena (sharp transients and slow-wave) are usually observed several times, dissociated from each other. Observe the slow-fused transient in the left temporal area, at the time-point indicated by the black vertical arrow. In addition, wicket rhythm is seen in the left frontal leads, in the 4th second. (A: Common Average montage; B: Longitudinal Bipolar montage).
Fig. 6.
14-and 6-Hz positive bursts: 0.5–1 s bursts of arciform, 14 or 6 Hz activity with pointed positive peaks, recorded during light sleep and drowsiness, with shifting side-predominance and maximum amplitude mostly over the posterior temporal regions. Observe 14 Hz positive bursts in the left temporal area, at the time-point indicated by the black vertical arrow. In addition, vertex sharp wave is seen in the 14th second (A: Common Average montage; B: Longitudinal Bipolar montage).
Fig. 7.
Breach rhythm: which consists of Focal / asymmetric high amplitude beta activity, due to an underlying skull defect. Observe the breach rhythm at F8 electrode. In addition, vertex sharp wave is seen in the 3rd second (A: Common Average montage; B: Longitudinal Bipolar montage).
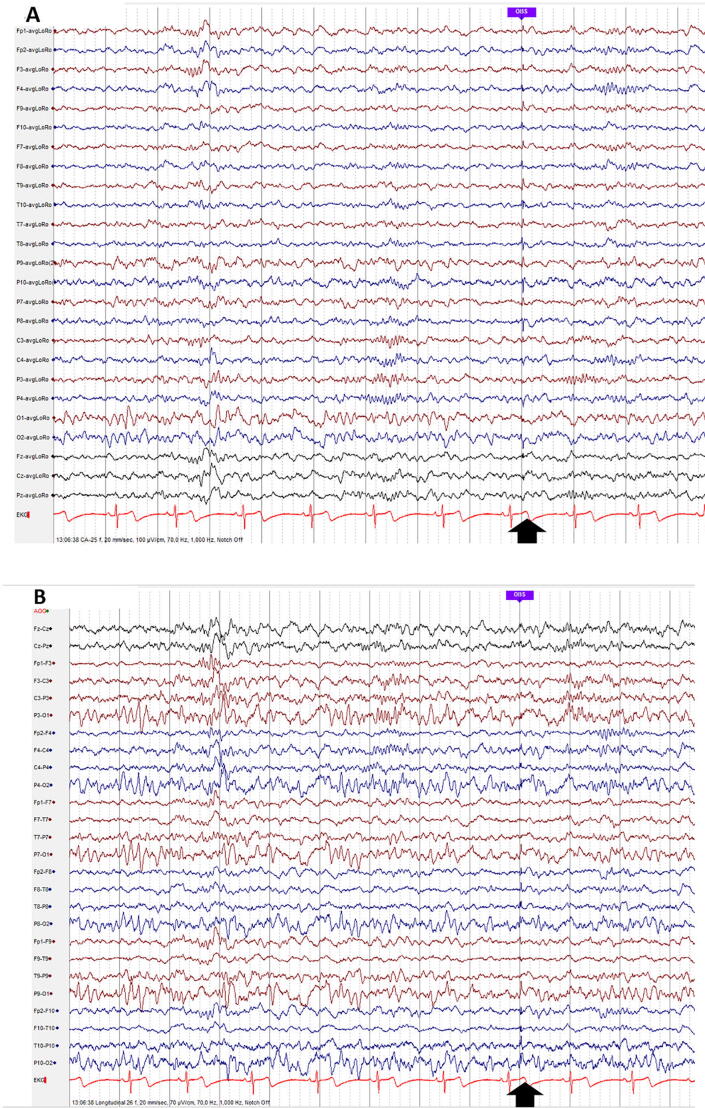
Fig. 8.
Small sharp spikes are a mono- or diphasic sharp transients with a short duration (usually < 50 ms) and low amplitude (usually < 50 µV), recorded in drowsiness/light sleep (N1, N2). Observe the small sharp spike in the temporal areas at the time-point indicated by the black vertical arrow. Other graphoelements in this figure correspond to N2 sleep: spindles, K complex and positive occipital sharp transients of sleep (A: Common Average montage; B: Longitudinal Bipolar montage).
Fig. 9.
6-Hz spike and slow wave (aka phantom spike-waves) are short bursts of spike-and-waves of 5–7 Hz, of brief duration (<30 ms). The amplitude is mostly higher over the fronto-central regions – WHAM (Wake, High amplitude > 45 µV, Anterior, Male). Observe the bi-frontal, 6 Hz phantom spike-waves at the time-point indicated by the black vertical arrow (A: Common Average montage; B: Longitudinal Bipolar montage).
Fig. 10.
6-Hz spike and slow wave (aka phantom spike-waves) are short bursts of spike-and-waves of 5–7 Hz, with low-voltage-spikes (<25 µV) of brief duration (<30 ms). It is seen in the occipital derivations and the amplitude is low – FOLD (Female, Occipital, Low amplitude < 25 µV, most often during Drowsiness). Observe the bi-occipital, 6 Hz phantom spike-waves at the time-point indicated by the black vertical arrow (A: Common Average montage; B: Longitudinal Bipolar montage).
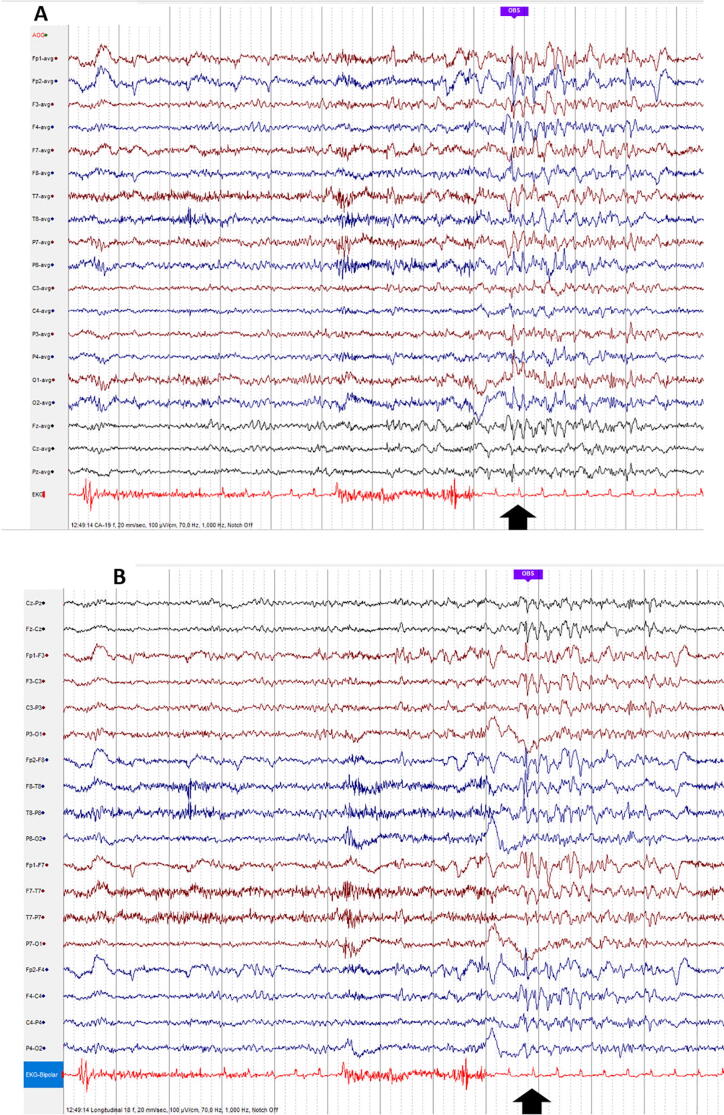
Fig. 11.
Subclinical rhythmic electrographic discharges in adults (SREDA): sharply contoured rhythm with theta frequency, usually bilateral, with shifting side-preponderance, in the temporal and parietal regions, with duration from 15 s to several minutes. Observe SREDA in the left temporal areas, starting at the time-point indicated by the black vertical arrow, and continuing throughout the page. Muscle artifacts in the 2nd and 3rd second (A: Common Average montage; B: Longitudinal Bipolar montage).
Fig. 12.
Histograms of the age distribution of the normal variants evaluated in this study.
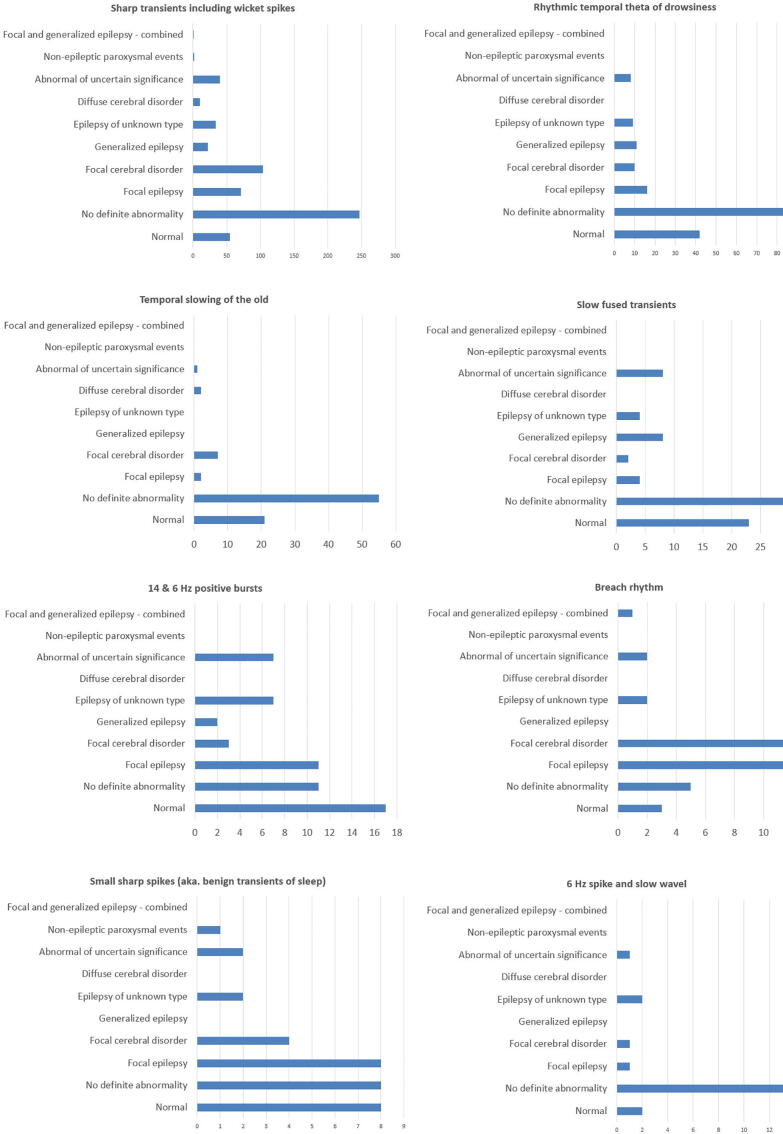
Fig. 13 shows the distribution of the clinical context of the EEG recordings, separately for each normal variant, according to the categories of diagnostic significance, as listed in the SCORE standard. 858 individual recordings showed 1085 normal variants, 143 (16.67%) of these examinations were normal recordings, 320 (37.30%) did not have definite abnormal findings, and 395 (46.03%) had clearly abnormal findings. When recordings were considered abnormal, this was not related the observed normal variants, and it was based on other, unequivocal EEG abnormalities, along with the clinical context.
Fig. 13.
Distribution of the clinical context of the EEG recordings, separately for each normal variant, according to the categories of diagnostic significance, as listed in the SCORE standard.
Table 1 shows the distribution of the normal variants in our cohort. The most frequent normal variants were the sharp transients (including wicket spikes), 19.21%, followed by the rhythmic temporal theta of drowsiness (RTTD) 6.03%. In our prospective dataset of 3050 routine EEGs, we only found one patient with rudimentary spike-wave complex (male, 19 years old), four patients with Ciganek rhythm (2 female, 2 male, age: 1–13 years) and none with needle-like occipital spikes of the blind.
Table 1.
Overview of normal variants.
| Normal variant | Nr in total | % of all recordings | Gender |
Median age (years) (range) |
Observed in recordings considered |
|||
|---|---|---|---|---|---|---|---|---|
| Male (%) | Female (%) | Normal (%) |
No definite abnormality (%) | Abnormal *(%) | ||||
| Sharp transients incl. wicket spikes | 586 | 19.21 | 39.77 | 60.23 | 28 (1–89) |
55 (9.39) |
247 (42.15) |
284 (48.46) |
| RTTD | 184 | 6.03 | 42.39 | 57.61 | 30 (3–80) |
88 (47.83) |
42 (22.83) |
5 (29.34) |
| Temporal slowing of the old | 88 | 2.89 | 48.86 | 51.14 | 68 (49–86) |
21 (23.86) |
55 (62.50) |
12 (13.64) |
| Slow fused transients | 79 | 2.59 | 44.30 | 55.70 | 16 (1–37) |
23 (29.11%) |
30 (37.97%) |
26 (32.92) |
| 14-and 6-Hz bursts | 56 | 1.83 | 53.57 | 46.43 | 13 (3–59) |
17 (30.36) |
11 (19.64) |
28 (50) |
| Breach rhythm | 38 | 1.25 | 36.84 | 63.16 | 40 (2–82) |
3 (7.89) |
5 (13.16) |
30 (78.95) |
| Small sharp spikes | 32 | 1.05 | 56.25 | 43.75 | 38 (10–80) |
8 (25.00) |
8 (25.00) |
16 (50.00) |
| 6-Hz spike and slow wave | 21 | 0.69 | 33.33 | 66.67 | 16 (1–66) |
2 (9.52) |
14 (66.67) |
5 (23.81) |
| SREDA | 1 | 0.03 | 100.00 | 0 | 39 (39) |
0 (0%) |
1 (100%) |
0 (0%) |
Recordings containing abnormal EEG findings other than the normal variants.
4. Discussion
Misinterpretation of normal patterns resembling abnormal EEG findings leads to erroneous diagnosis in normal patients and may lead to erroneous classification in patients with epilepsy. Therefore, it is essential that experts reading clinical EEG are familiar with the characteristics of the various normal variants. In this large prospectively recorded dataset, we extracted normal variants using the SCORE EEG system. Here, we describe them in order of their prevalence in our cohort.
4.1. Sharp transients
Sharp transients (Fig. 1) are sharp EEG changes/fluctuations of the background activity which do not fulfill the operational criteria for epileptic discharges defined by the IFCN (Kane et al., 2017, Kural et al., 2020). These fluctuations in the EEG background activity have a sharp morphology and a higher amplitude and can easily be misinterpreted as epileptic discharges (Benbadis, 2007, Benbadis and Lin, 2008, Benbadis and Tatum, 2003). In this study, sharp transients were the most often described normal variant with 19.25%. To our knowledge, there is no study describing the prevalence of sharp transients. However, several studies point out that these spiky fluctuations of the background activity are the most common cause of misinterpreting (over-reading) EEGs (Benbadis, 2007, Benbadis and Lin, 2008, Benbadis and Tatum, 2003).
Wicket spikes (Fig. 2) are a special subtype of the sharp transients, with arciform waveforms, which are simple monophasic with surface negativity. They can be seen in awake and sleep stages (Reiher and Lebel, 1977) and can be best recognized during the initial sleep stages but also be seen in REM sleep (Gélisse et al., 2003, Reiher and Lebel, 1977, Serafini et al., 2014). These waves can be observed as single discharges or runs. When seen in runs, the frequency is usually 7–11 Hz. The amplitude can range between 60 and 210 µV and their maximum is located over the temporal regions (Klass and Westmoreland, 1985, Reiher and Lebel, 1977, Tatum et al., 2006). Their appearance can shift from side to side, but mostly one side is dominant. Some studies suggest that wicket spikes are more often lateralized to the left side (Reiher and Lebel, 1977). The incidence ranges from 0.037 to 0.96% overall and in adults over 30 years up 2.9% (Monin et al., 2018, Radhakrishnan et al., 1999, Reiher and Lebel, 1977, Santoshkumar et al., 2009), while one of the newer studies describes it in 6.8% (Rathore et al., 2021) and up to 15% (Macorig et al., 2021). More specifically Santoshkumar et al. (2009) described them in 0.037%, mean age 55.36 (range 32–76), 38.46% male, followed by the numbers of Radhakrishnan et al. (1999) 0.96%, mean age 34.82 (range 20–52), 70.59% male and 6.80%, mean age 34.1 (range 7–75), 57% male, 72.4% normal EEG and 29% non-epilepsy (Rathore et al., 2021).
In our dataset, sharp transients including wicket spikes occurred in 19.21% of all cases, with a median age 28 (1–89), 39.77% male, 9.39% normal, and 42.15% without definite abnormality. The combined numbers of prevalence of sharp transients and wicket spikes in our study are quite consistent with the prevalence in the literature especially regarding the studies of Beun et al. (1998), who observed only sleep and Macorig et al. (2021), who observed their participants with long term EEG.
4.2. Rhythmic temporal theta of drowsiness (RTTD)
RTTD (Fig. 3) formerly known as psychomotor variant, is mostly seen during drowsiness and light sleep, even though it can be occasionally seen in awake patients as well. The maximum of the 5–7 Hz trains is usually mid-temporal but may sometimes spread to the parasagittal or occipito-temporal regions. RTTD can be observed independently over one side or bilaterally. The typical configuration is arch-shaped and often notched. Therefore, it can sometimes be misinterpreted as sharp waves. Sometimes, these arches are flat topped (Chatrian et al., 1974, Gibbs and Gibbs, 1941, Lipman and Hughes, 1969). The runs of theta activity can last from mere seconds to several minutes (Beiske et al., 2016). As other normal rhythms, this one is also clearly monomorphic, monorhythmic, and there is no evolution (da Silva, 2018).
In the literature, the incidence has been reported in 0.4% in patients between the age of 15–19 years (Gibbs and Gibbs, 1964) overall between 0.1 and 2.1% (Gibbs et al., 1963, Macorig et al., 2021, Maulsby, 1979, Radhakrishnan et al., 1999, Rathore et al., 2021, Santoshkumar et al., 2009), and in up to 10% in adults over 60 years old (Kang and Krauss, 2019). More specifically, Santoshkumar et al. (2009) have seen RTTD in 0.12% of their cases, mean age 27.5 (range 9–80), 44% male, while Rathore et al. (2021) have described it in 0.4% of their recordings with a mean age of 21.8 (range 11–52), 57% male, 43% with normal EEG and 42.8% non-epilepsy. Even a higher number of 0.79% was described with mean age 31.36 (range 6–58), 71.43 male (Radhakrishnan et al., 1999).
In our dataset, this normal variant was seen in 6.03% with a median of age of 30 (range 3–80), 42.39% male, while 47.83% of the recordings were normal, or, in 22.83% of all cases with no definite abnormality. This means that our prevalence is higher than most of the overall numbers of the studies, but still in the range of all reported numbers with a rising prevalence with age. In accordance with previous studies, we have also observed a little predominance of females.
4.3. Temporal slowing of the old
Temporal slowing of the old (Fig. 4) is a mostly unilateral normal variant (mainly on the left or both sides) showing short runs of theta or delta activity mixed with the background activity of the same area. It is normal in age groups ≥ 50 years, without clinical abnormalities. Furthermore, it can be accentuated during hyperventilation or drowsiness (Kane et al., 2019).
In our cohort, this normal variant was seen in 2.89%, median age 68 (range 49–86), 48.86% male, while 23.86% were described as normal and 62.50% with definite abnormality. To our knowledge, this variant has not previously been described in larger studies. This pattern may have been included into RTTD in previous studies, since both phenomena include theta activity in the temporal areas (Kang and Krauss, 2019).
4.4. Slow fused transients
In patients with posterior slow waves of the youth (Fig. 5), or in a patient with temporal slowing of the old, a sharp transient can precede a slow wave. This can give the false impression of a spike and slow wave (Kane et al., 2019). To the best of our knowledge, there are no specific numbers in the literature for this phenomenon. It was seen in our study in 2.59% of all cases, with a median age of 16 (range 1–37). 29.11% of the recordings were normal and 37.97% were without any definite abnormality.
4.5. 14-and 6-Hz positive bursts
The typical bursts last between 0.5 and 1 s (Fig. 6). They occur during light sleep and drowsiness; however, there are reported cases during REM sleep. These bursts can be seen unilaterally or bilaterally, but mainly with shifting predominance in the same patient. The laterality can shift but the maximum amplitude is mostly over the posterior temporal regions (Ebersole and Pedley, 2003, Klass and Westmoreland, 1985, Tatum et al., 2006).
The numbers in the literature range from about 16% at the age of five to nine; 20% between the age of ten to 14; 8–10% between the age of 20 and 24; 1–2% between 25 and 29 years of age (Eeg-Olofsson et al., 1971, Gibbs and Gibbs, 1964). Another study described this normal variant in 58% in boys of the age between 13 and 15 years of age (Lombroso et al., 1966). The incidence is falling with increasing age (Klass and Westmoreland, 1985).
More recent studies have described 14-and 6-Hz positive bursts in 0.53% (Santoshkumar et al., 2009), 0.6% (Rathore et al., 2021) and to 5,68% (Radhakrishnan et al., 1999) and 8.3% (Macorig et al., 2021) of their EEGs looking at a wide age range of prevalence.
When having a closer look at the numbers, 0.53% was the lowest prevalence where extended data was available mean age 23.23 (range 4–67), 44.32% male (Santoshkumar et al., 2009). A little higher prevalence was described with 0.6%, mean age 28.1 (range 11–50), 36% male, 90% normal EEG and 45.5% non-epileptic. While it was seen in 5.68%, mean age 14.72 (range 6.5–31.0), 49.50% male by Rathore et al. (2021). In our study it was seen in 1.83% of all cases, 53.57% male, the median age was 13, 30.36% were normal and 19.64% without definite abnormality. These numbers are quite consistent with the detailed numbers in the literature.
4.6. Breach rhythm
A skull defect can lead to a breach rhythm, which consists of focal, asymmetric high amplitude beta activity (Fig. 7). This is described as normal as long as it is not associated with spikes or focal slowing (Tatum et al., 2006).
To the best of our knowledge, there are no specific reports about the incidence in a standard population. This is probably due to the fact that this normal variant appears only symptomatically if caused by a lesion of the skull. The numbers can vary depending on the examined population and can be influenced by a selection bias of patients after neurosurgery. Already in 1979, this was described in 21 cases of 33 patients (63.64%) with iatrogenic skull defect (Cobb et al., 1979), which is a high number in patients with skull defect. In our study, this normal variant was seen in 1.25% of all studies, which includes several patients with follow-up after epilepsy surgery. The median age was 40, while the range was 2–82 years. Of these cases, 7.89% were classified as normal and 13.16% without any definite abnormality, while the rest of the recordings were abnormal. Since we do not have any information in this study about skull defects, we cannot perform a more detailed subgroup analysis.
4.7. Small sharp spikes (a.k.a. benign epileptiform transients of sleep)
Small sharp spikes (Fig. 8) are sharp transients with a short duration (usually < 50 ms) and low amplitude (usually < 50 µV). Their simple shape consists of a mono- or diphasic spike with a steep descending arm. If present, the following slow wave is not prominent and of low amplitude. The background is not disturbed and they can be seen in drowsiness/light sleep (N1, N2) (Ebersole and Pedley, 2003, Klass and Westmoreland, 1985, Olson and Hughes, 1970, Tatum et al., 2006). Small spikes in patients with temporal lobe epilepsy may resemble small sharp spikes. To avoid over-reading (which is potentially more harmful than under-reading) it is advisable to opt for a conservative interpretation in these cases.
The incidence is described in up to 25% of the normal population (Tatum et al., 2006, White et al., 1977), but the numbers in the literature range from 1.85% (Santoshkumar et al., 2009)¸ through 3.3% (Macorig et al., 2021), 3.7% (Rathore et al., 2021) and, in more recent studies, to up to 8.16% (Radhakrishnan et al., 1999). Small sharp spikes are almost never seen in children younger than 10 years (Koshino and Niedermeyer, 1975). When having a closer look at the data (where available) Santoshkumar et al. (2009) described the lowest prevalence with 1.85%, mean age 39.75 (range 4–94), 52.91% male. This number is followed by 3.7% (Rathore et al., 2021), mean age 27.6 (range 3–60), 72% male, 65.2% normal, 21% non-epileptic. While Radhakrishna et al. have seen in 9.16% of their recordings, mean age 33.83 (range 18–58), 72.41% male (Radhakrishnan et al., 1999). In our study it was seen in 1.05%, 56.25% were male, the median age was 38 (range 10–80), 25% normal, 25% no definite abnormality.
The prevalence in our dataset is lower than previously reported, which is probably due to the fact that our staff is trained to spot sharp elements and then distinguish between epileptic or non-epileptic discharges. When this decision is made, most of the non-epileptic phenomes are often described as sharp transients. That may explain why by far the most normal variant in our data set is sharp transients incl. wicket spikes. Regarding the gender difference, we also see a little predominance in male patients, and our results are quite close the previous described age distribution.
4.8. 6-Hz spike and slow wave
6-Hz spike and slow wave (Fig. 9, Fig. 10) is also called phantom spike-and-wave. These are bursts of spike-and-wave of 5 to 7 Hz (typically duration of 1–2 s) with low-voltage-spikes (<25 µV) of brief duration (<30 ms). The amplitude is mostly higher over the fronto-central regions. Even though the bursts are usually diffuse, they can have anterior or posterior predominance. They can be seen during wakefulness but mostly occur during drowsiness and light sleep (Marshall, 1955, Tharp, 1966, Tharp, 1967).
The incidence is described between from 0.1% (Macorig et al., 2021) over 0.9% to 2.76% (Hughes, 1980, Marshall, 1955, Olson and Hughes, 1970, Radhakrishnan et al., 1999, Rathore et al., 2021, Santoshkumar et al., 2009, Tharp, 1966). One of the lowest numbers with detailed data is 0.9%, mean age 25.5 (range 15–60), 56% male, 75% normal, 22.5% non-epilepsy (Rathore et al., 2021). This is followed by 1.02%, mean age 23.23 (range 4–67), 44.32 male (Santoshkumar et al., 2009) and 2.76%, mean age 20.84 (range 6–31), 48.98% male (Radhakrishnan et al., 1999).
In our population the results showed a prevalence of 0.69%, median age 16 (range 1–66), 33.33% male, 9.52% normal and 66.67% without definite abnormality. Both prevalence and age are close to the numbers in the literature. We have seen it in less males, which deviates from the gender distribution in the literature where sex is described quite even.
4.9. Subclinical rhythmic electrographic discharges in adults (SREDA)
SREDA (Fig. 11) can be seen mostly in relaxed awake patients or during drowsy states but also in sleep (Fleming et al., 2004, Westmoreland and Klass, 1981). The phenomenon is described as diffuse, sharply contoured rhythm with theta frequency. This pattern is usually bilateral in the temporal and parietal regions (it may be asymmetric). It can run from 15 s to a minute and even more (Westmoreland and Klass, 1981).
The incidence ranges from none (Macorig et al., 2021, Radhakrishnan et al., 1999), through 0.07% (Santoshkumar et al., 2009) to 0.2% (Rathore et al., 2021). Santoshkumar et al. described SREDA in their study with af prevalence of 0.07% with a mean age of 52.1 (range 9–80), male 34.62% (Santoshkumar et al., 2009), Rathore et al. (2021) found this variant in 0.2% patients with mean age of 58.7 (range 41–72), 67% male, 67% with a normal EEG and 67% with non-epilepsy.
In our dataset, we have only one documented case (0.03%) age 39, male, no definite abnormality. This is consistent with the other studies and its rarity. Comparing our data set with gender distribution and the age is therefore difficult. Nonetheless, it is important to be aware of SREDA and not to confuse it with an epileptic (electrographical) seizure.
5. Conclusion
Our prospective study of a large number of EEGs gives an overview of the various normal variants in EEG, sharp transients being the most often identified pattern (19.21%). This emphasizes the importance of the knowledge of this normal variant and the criteria for distinguishing it from epileptiform discharges (Kural et al., 2020, Kural et al., 2021). The numbers in our large prospective dataset were consistent with previous studies, for most types of normal variants. In our population, only the incidence of small sharp spikes and 6-Hz spike and slow wave were somewhat lower than previously reported.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Harald Aurlien is Chief Medical Officer and minority shareholder in Holberg EEG AS. The remaining authors do not have conflicts of interest related to this work.
References
- Amin U., Benbadis S.R. The role of EEG in the erroneous diagnosis of epilepsy. J. Clin. Neurophysiol. 2019;36(4):294–297. doi: 10.1097/WNP.0000000000000572. [DOI] [PubMed] [Google Scholar]
- Beiske K.K., Kostov K.H., Kostov H. Rhythmic midtemporal discharge in a youth during light sleep. Neurodiagn. J. 2016;56(1):32–36. doi: 10.1080/21646821.2015.1119579. [DOI] [PubMed] [Google Scholar]
- Benbadis S.R. Errors in EEGs and the misdiagnosis of epilepsy: importance, causes, consequences, and proposed remedies. Epilepsy Behav. 2007;11(3):257–262. doi: 10.1016/j.yebeh.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Benbadis S.R., Lin K. Errors in EEG interpretation and misdiagnosis of epilepsy. Which EEG patterns are overread? Eur. Neurol. 2008;59(5):267–271. doi: 10.1159/000115641. [DOI] [PubMed] [Google Scholar]
- Benbadis S.R., Tatum W.O. Overintepretation of EEGs and misdiagnosis of epilepsy. J. Clin. Neurophysiol. 2003;20(1):42–44. doi: 10.1097/00004691-200302000-00005. [DOI] [PubMed] [Google Scholar]
- Beniczky S., Aurlien H., Brøgger J.C., Fuglsang-Frederiksen A., Martins-da-Silva A., Trinka E., et al. Standardized computer-based organized reporting of EEG: SCORE. Epilepsia. 2013;54(6):1112–1124. doi: 10.1111/epi.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beniczky S., Aurlien H., Brøgger J.C., Hirsch L.J., Schomer D.L., Trinka E., et al. Standardized computer-based organized reporting of EEG: SCORE - Second version. Clin. Neurophysiol. 2017;128(11):2334–2346. doi: 10.1016/j.clinph.2017.07.418. [DOI] [PubMed] [Google Scholar]
- Beun A., van Emde B.W., Dekker E. Sharp transients in the sleep EEG of healthy adults: a possible pitfall in the diagnostic assessment of seizure disorders. Electroencephalogr. Clin. Neurophysiol. 1998;106(1):44–51. doi: 10.1016/s0013-4694(97)00083-7. [DOI] [PubMed] [Google Scholar]
- Chatrian G.E., Bergamini L., Dondey M., Klass D.W., Lennox-Buchthal L., Petersén I. A glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogr. Clin. Neurophysiol. 1974;37(5):538–548. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- Cobb W.A., Guiloff R.J., Cast J. Breach rhythm: the EEG related to skull defects. Electroencephalogr. Clin. Neurophysiol. 1979;47(3):251–271. doi: 10.1016/0013-4694(79)90278-5. [DOI] [PubMed] [Google Scholar]
- da F.H.L., Silva . Oxford University Press; 2018. Niedermeyer's Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; pp. 319–330. [Google Scholar]
- Ebersole J.S., Pedley T.A. Lippincott Williams & Wilkins; 2003. Current practice of clinical electroencephalography. [Google Scholar]
- Eeg-Olofsson O., Petersén I., Selldén U. The development of the electroencephalogram in normal children from the age of 1 through 15 years. Paroxysmal activity. Neuropadiatrie. 1971;2(4):375–404. doi: 10.1055/s-0028-1091791. [DOI] [PubMed] [Google Scholar]
- Fleming W.E., Avidan A., Malow B.A. Subclinical rhythmic electrographic discharge of adults (SREDA) in REM sleep. Sleep Med. 2004;5(1):77–81. doi: 10.1016/j.sleep.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Gélisse P., Kuate C., Coubes P., Baldy-Moulinier M., Crespel A. Wicket spikes during rapid eye movement sleep. J. Clin. Neurophysiol. 2003;20(5):345–350. doi: 10.1097/00004691-200309000-00006. [DOI] [PubMed] [Google Scholar]
- Gibbs F.A., Gibbs E.L. Lew A. Cummings Co.; Cambridge, MA: 1941. Atlas of Electroencephalography. [Google Scholar]
- Gibbs F.A., Gibbs E.L. Addison-Wesley; 1964. Atlas of Electroencephalography. Volume 3. Neurological and Psychiatric Disorders. [Google Scholar]
- Gibbs F.A., Rich C.L., Gibbs E.L. Psychomotor variant type of seizure discharges. Neurology. 1963;13:991–998. doi: 10.1212/wnl.13.12.991. [DOI] [PubMed] [Google Scholar]
- Hughes J.R. Two forms of the 6/sec spike and wave complex. Electroencephalogr. Clin. Neurophysiol. 1980;48(5):535–550. doi: 10.1016/0013-4694(80)90289-8. [DOI] [PubMed] [Google Scholar]
- Juarez-Garcia A., Stokes T., Shaw B., Camosso-Stefinovic J., Baker R. The costs of epilepsy misdiagnosis in England and Wales. Seizure. 2006;15(8):598–605. doi: 10.1016/j.seizure.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Kane N., Acharya J., Benickzy S., Caboclo L., Finnigan S., Kaplan P.W., et al. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin. Neurophysiol. Pract. 2017;2:170–185. doi: 10.1016/j.cnp.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane N., Acharya J., Beniczky S., Caboclo L., Finnigan S., Kaplan P.W., et al. Corrigendum to “A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017” [Clin. Neurophysiol. Practice 2 (2017) 170-185] Clin. Neurophysiol. Pract. 2019;4:133. doi: 10.1016/j.cnp.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.Y., Krauss G.L. Normal variants are commonly overread as interictal epileptiform abnormalities. J. Clin. Neurophysiol. 2019;36(4):257–263. doi: 10.1097/WNP.0000000000000613. [DOI] [PubMed] [Google Scholar]
- Klass D.W., Westmoreland B.F. Nonepileptogenic epileptiform electroencephalographic activity. Ann. Neurol. 1985;18(6):627–635. doi: 10.1002/ana.410180602. [DOI] [PubMed] [Google Scholar]
- Koshino Y., Niedermeyer E. The clinical significance of small sharp spikes in the electroencephalogram. Clin. Electroencephalogr. 1975;6(3):131–140. [Google Scholar]
- Kural M.A., Duez L., Sejer Hansen V., Larsson P.G., Rampp S., Schulz R., et al. Criteria for defining interictal epileptiform discharges in EEG: A clinical validation study. Neurology. 2020;94(20):e2139–e2147. doi: 10.1212/WNL.0000000000009439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kural M.A., Qerama E., Johnsen B., Fuchs S., Beniczky S. The influence of the abundance and morphology of epileptiform discharges on diagnostic accuracy: How many spikes you need to spot in an EEG. Clin. Neurophysiol. 2021;132(7):1543–1549. doi: 10.1016/j.clinph.2021.03.045. [DOI] [PubMed] [Google Scholar]
- Lipman I.J., Hughes J.R. Rhythmic mid-temporal discharges. An electro-clinical study. Electroencephalogr. Clin. Neurophysiol. 1969;27(1):43–47. doi: 10.1016/0013-4694(69)90107-2. [DOI] [PubMed] [Google Scholar]
- Lombroso C.T., Schwartz I.H., Clark D.M., Muench H., Barry P.H., Barry J. Ctenoids in healthy youths. Controlled study of 14- and 6-per-second positive spiking. Neurology. 1966;16(12):1152–1158. doi: 10.1212/wnl.16.12.1152. [DOI] [PubMed] [Google Scholar]
- Macorig G., Crespel A., Nilo A., Tang N.P.L., Valente M., Gigli G.L., et al. Benign EEG variants in the sleep-wake cycle: A prospective observational study using the 10–20 system and additional electrodes. Neurophysiol. Clin. 2021;51(3):233–242. doi: 10.1016/j.neucli.2021.03.006. [DOI] [PubMed] [Google Scholar]
- Marshall C. Some clinical correlates of the wave and spike phantom. Electroencephalogr. Clin. Neurophysiol. 1955;7(4):633–636. doi: 10.1016/0013-4694(55)90090-0. [DOI] [PubMed] [Google Scholar]
- Maulsby R.L. In: Current Practice of Clinical Electroencephalography. Klass D.W., Daly D.D., editors. New York; Raven Press: 1979. EEG patterns of uncertain diagnostic significance; pp. 411–449. [Google Scholar]
- Monin J., Pruvost-Robieux E., Huiban N., Marchi A., Crepon B., Dubourdieu D., et al. Prevalence of benign epileptiform variants during initial EEG examination in French military aircrew. Neurophysiol. Clin. 2018;48(3):171–179. doi: 10.1016/j.neucli.2018.04.001. [DOI] [PubMed] [Google Scholar]
- Nowack W.J. Epilepsy: a costly misdiagnosis. Clin. Electroencephalogr. 1997;28(4):225–228. doi: 10.1177/155005949702800407. [DOI] [PubMed] [Google Scholar]
- Olson S.F., Hughes J.R. The clinical symptomatology associated with the 6 c-sec spike and wave complex. Epilepsia. 1970;11(4):383–393. doi: 10.1111/j.1528-1157.1970.tb03904.x. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan K., Santoshkumar B., Venugopal A. Prevalence of benign epileptiform variants observed in an EEG laboratory from South India. Clin. Neurophysiol. 1999;110(2):280–285. doi: 10.1016/s1388-2457(98)00002-9. [DOI] [PubMed] [Google Scholar]
- Rathore C., Prakash S., Rana K., Makwana P. Prevalence of benign epileptiform variants from an EEG laboratory in India and frequency of their misinterpretation. Epilepsy Res. 2021;170:106539. doi: 10.1016/j.eplepsyres.2020.106539. [DOI] [PubMed] [Google Scholar]
- Reiher J., Lebel M. Wicket spikes: clinical correlates of a previously undescribed EEG pattern. Can. J. Neurol. Sci. 1977;4(1):39–47. [PubMed] [Google Scholar]
- Santoshkumar B., Chong J.J., Blume W.T., McLachlan R.S., Young G.B., Diosy D.C., et al. Prevalence of benign epileptiform variants. Clin. Neurophysiol. 2009;120(5):856–861. doi: 10.1016/j.clinph.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Seeck M., Koessler L., Bast T., Leijten F., Michel C., Baumgartner C., et al. The standardized EEG electrode array of the IFCN. Clin. Neurophysiol. 2017;128(10):2070–2077. doi: 10.1016/j.clinph.2017.06.254. [DOI] [PubMed] [Google Scholar]
- Serafini A., Crespel A., Velizarova R., Gélisse P. Activation of wicket spikes by REM sleep. Neurophysiol. Clin. 2014;44(3):245–249. doi: 10.1016/j.neucli.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Tatum W.O.T., Husain A.M., Benbadis S.R., Kaplan P.W. Normal adult EEG and patterns of uncertain significance. J. Clin. Neurophysiol. 2006;23(3):194–207. doi: 10.1097/01.wnp.0000220110.92126.a6. [DOI] [PubMed] [Google Scholar]
- Tharp B.R. The 6-per-second spike and wave complex. The wave and spike phantom. Arch. Neurol. 1966;15(5):533–537. doi: 10.1001/archneur.1966.00470170087009. [DOI] [PubMed] [Google Scholar]
- Tharp B.R. The six per second spike and wave complex (the wave and spike phantom) Electroencephalogr. Clin. Neurophysiol. 1967;23(3):291. [PubMed] [Google Scholar]
- Westmoreland B.F., Klass D.W. A distinctive rhythmic EEG discharge of adults. Electroencephalogr. Clin. Neurophysiol. 1981;51(2):186–191. doi: 10.1016/0013-4694(81)90008-0. [DOI] [PubMed] [Google Scholar]
- White J.C., Langston J.W., Pedley T.A. Benign epileptiform transients of sleep. Clarification of the small sharp spike controversy. Neurology. 1977;27(11):1061–1068. doi: 10.1212/wnl.27.11.1061. [DOI] [PubMed] [Google Scholar]